Abstract
Several clinical trials in women with endometriosis demonstrated that dienogest reduces endometrial lesions and improves health-related quality of life (HRQoL). To assess HRQoL in dienogest-treated patients in real-world setting, we conducted a prospective, non-interventional study in 6 Asian countries. Women aged ≥18 years with clinical or surgical diagnosis of endometriosis, presence of endometriosis-associated pelvic pain (EAPP) and initiating dienogest therapy were enrolled. The primary objective was to evaluate HRQoL using the Endometriosis Health Profile-30 (EHP-30) questionnaire. The secondary objectives included analysis of EAPP, satisfaction with dienogest, endometriosis symptoms and bleeding patterns. 887 patients started dienogest therapy. Scores for all EHP-30 scales improved with the largest mean changes at month 6 and 24 in scale pain (−28.9 ± 27.5 and − 34 ± 28.4) and control and powerlessness (−23.7 ± 28.2 and − 28.5 ± 26.2). Mean EAPP score change was −4.6 ± 3.0 for both month 6 and 24 assessments. EAPP decrease was similar in surgically and only clinically diagnosed patients. From baseline to month 24, rates of normal bleeding decreased (from 85.8% to 17.5%) while rates of amenorrhea increased (from 3.5% to 70.8%). Majority of patients and physicians were satisfied with dienogest. Over 80% of patients reported symptoms improvement. 39.9% of patients had drug-related treatment-emergent adverse events, including vaginal hemorrhage (10.4%), metrorrhagia (7.3%) and amenorrhea (6.4%). In conclusion, dienogest improves HRQoL and EAPP in the real-world setting in women with either clinical or surgical diagnosis of endometriosis. Dienogest might be a promising first-line treatment option for the long-term management of debilitating endometriosis-associated symptoms.
NCT02425462, 24 April 2015.
Keywords: Endometriosis, Health-related quality of life, Pain, Clinical diagnosis
Introduction
Endometriosis affects approximately 10% of women in the reproductive age, with a prevalence of up to 50% in infertile women [1, 2]. The prevalence of endometriosis varies by race and ethnicity with Asian women (particularly in East Asian countries) more likely to be affected by this disease than Caucasians [1, 3–5]. The most common clinical signs of endometriosis are menstrual irregularities, chronic pelvic pain, dysmenorrhea, dyspareunia and infertility [6]. Symptoms of endometriosis often affect psychological and social functioning of patients. For this reason, endometriosis is considered as a disabling condition that may significantly reduce health-related quality of life (HRQoL). Previous studies demonstrated that endometriosis has a negative impact on social relationships, sexuality, mental health, and work productivity [7–10]. Furthermore, poor HRQoL is observed particularly in patients suffering from a more severe pelvic pain [10–12]. Importantly, hormonal therapy and surgical treatment may improve HRQoL and reduce pelvic pain in patients with endometriosis [13].
Dienogest is an oral progestin approved for endometriosis treatment in 157 countries, including 15 countries in Asia [7]. Dienogest is an attractive option for prolonged treatment due to a moderate suppression of estrogen levels and a low androgenic, mineralocorticoid, or glucocorticoid activity [14]. Long-term studies in Europe and Japan demonstrated that dienogest is efficacious in reducing pelvic pain, well tolerated and has a good safety profile [15–17]. Although several clinical trials demonstrated improvements in HRQoL following the dienogest therapy, the real-world evidence is scarce [18–24]. Our interim analysis of non-interventional study ENVISIOeN demonstrated an improved HRQoL and reduced endometriosis-associated pelvic pain (EAPP) after 6 months of dienogest therapy in clinically or surgically diagnosed Asian women with endometriosis [25]. In this manuscript, we report the final analysis of HRQoL, EAPP and safety in patients receiving dienogest for up to 24 months within the ENVISIOeN study.
Methods
Study Design
The ENVISIOeN study (NCT02425462) was a prospective, multicenter, international, noninterventional cohort study performed in 6 Asian countries at 36 sites (Republic of Korea: 12 sites, Indonesia: 10 sites, Thailand: 5 sites, Malaysia: 4 sites, Philippines: 3 sites, Singapore: 2 sites). The study was conducted in accordance with the Declaration of Helsinki and in compliance with Good Clinical Practice.
The primary objective of the study was to determine the change in pain dimension of Endometriosis Health Profile-30 (EHP-30) after 6-month therapy with dienogest. The secondary objectives included change in EHP-30 scores at month 6 and 24, efficacy of dienogest in reducing EAPP at month 6, 12 and 24, patients’ and clinicians’ satisfaction with dienogest, assessment of endometriosis symptoms and bleeding pattern, and rates of dienogest discontinuation and repeated surgery. Data were collected during study visits at basline, and at months 1, 3, 6, 12, and 24 after start of dienogest therapy; all study visits occurred on site and within the routine clinical practice.
Patients
Asian women aged ≥18 years with clinical diagnosis (by suggestive symptoms and positive finding of chocolate cyst on imaging) or surgical diagnosis of endometriosis and presence of EAPP were eligible to participate in the study. In this study, EAPP was defined as pain at menstruation, chronic pelvic pain irrelevant to menstruation, and/or dyspareunia. Further inclusion criteria were physician’s independent decision to newly prescribe dienogest and written informed consent provided by patient. Exclusion criteria were contraindication for dienogest use as per the summary of product characteristics (active venous thromboembolic disorder, arterial and cardiovascular disease, diabetes mellitus with vascular involvement, severe hepatic disease, liver tumors, known or suspected sex hormone-dependent malignancies, undiagnosed vaginal bleeding and hypersensitivity to the active substance or to any of the excipients) and participation in an investigational program with interventions outside of routine clinical practice. Patient were treated according to the respective national guidelines and in line with the American Society for Reproductive Medicine and/or European Society of Human Reproduction and Embryology guidelines.
Assessments
Enrolled patients were observed over the 24-month period, irrespective of whether they remained or discontinued dienogest, unless lost to follow up. Patients filled out the EHP-30 questionnaire at baseline and at month 6 and month 24. EHP-30 consists of the core instrument scales addressing pain, control and powerlessness, social support, emotional well-being, and self-image [26–28]. Additionally, supplementary module scales covering areas of work, relationship with children, sexual relationship, treatment, and infertility were used. Items for each scale were summed and transformed on a range from 0 (best possible HRQoL) to 100 (worst possible HRQoL).
Patients evaluated EAPP using the numeric rating scale with scores ranging from 0 (no pain) to 10 (unbearable pain) at baseline and after 6 and 24 months of therapy with a 4-week recall period.
Bleeding profile was analyzed across the following categories: normal bleeding (regular bleeding with normal flow and duration), irregular bleeding cycle (intermenstrual interval < 21 or > 35 days), amenorrhea (no menstruation during last 90 days), and intermenstrual spotting/bleeding (irregular episodes of bleeding, often light and short, occurring between otherwise fairly normal menstrual periods).
Further assessments included the rate and reason for dienogest discontinuation, patients’ and physicians’ satisfaction with treatment, and pain recurrence and repeated surgery rates.
Treatment-emergent adverse events (TEAE) and serious adverse events (SAE) were documented at each study visit.
Statistical Analysis
Analysis of efficacy was performed on the efficacy analysis set (EFF) that included all patients with evaluable primary outcome, i.e., EHP-30 questionnaires filled out at baseline and between week 12 and 36 after start of treatment (6-month visit) by patients who continued dienogest therapy. Measurements after dienogest discontinuation were excluded. Safety analyses were performed on the full analysis set (FAS) that comprised of all patients with at least one dose of dienogest.
Data were analyzed using SAS release 9.4 (SAS Institute, Cary, NC, USA) and described by visit (baseline, 6- and 24-month visit) and by change from baseline. Continuous variables were summarized by mean (± standard deviation), median, minimum, maximum; and categorical variables as a number and percentage. 95% confidence intervals (CI) were provided for changes in EHP-30 scores. Missing data were not imputed. Incidence of TEAE and SAE was presented as the Medical Dictionary for Regulatory Activities (MedDRA) preferred terms by system organ class.
Results
Patient Disposition and Characteristics
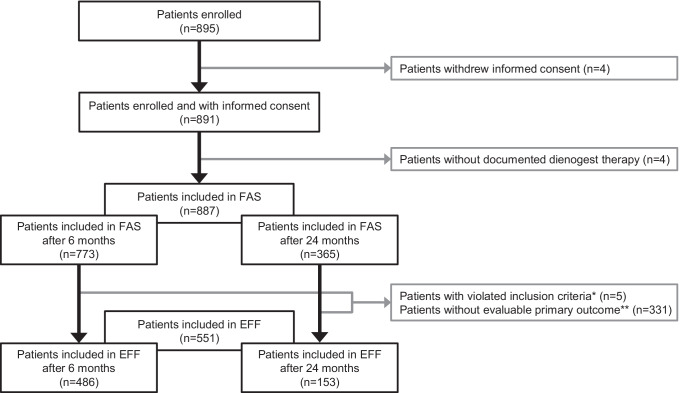
From April 2015 to August 2016, 895 patients at 36 sites were enrolled in the study (Fig. 1). 887 patients were included in FAS; 551 patients were included in EFF set. The mean duration of newly prescribed dienogest treatment in EFF set was 16.9 ± 7.6 months and the main reasons for end of observation were regular end of study (64.4%), lost to follow-up (23.1%) and relief of symptoms (6.7%). At month 6, the majority of patients continued treatment with dienogest (85.3%); 13.3% of patients discontinued the therapy, most often due to relief of symptoms (38.4%), adverse event (AE, 35.6%) or wish to conceive (9.6%). At month 24, dienogest therapy was continued by 44.2% of patients whereas 28.8% discontinued the dienogest, most frequently due to relief of symptoms (53.6%), physician decision (17.9%) or AE (14.3%).
Fig. 1.
CONSORT diagram. *One patient violated inclusion criteria: clinical or surgical diagnosis of endometriosis and endometriosis-associated pelvic pain, and four patients violated inclusion criterion decision taken by the physician to newly prescribe dienogest. **EHP-30 pain score was not evaluable at baseline and/or at study visit at month 6. EFF, efficacy analysis set; FAS, full analysis set
536 patients (60.4%) received endometriosis treatment prior to study start (Table 1); 454 (84.7%) among them underwent prior surgery and 186 women (34.7%) had previous hormonal treatment, most often gonadotropin-releasing hormone agonist (55.4%), followed by progestin plus estrogen-progestin combination (29.6%) and progestin alone (19.9%); 3.2% of patients received dienogest. Pain recurred in 28.2% of women with surgery (within 17.6 ± 21.2 months), and in 56.5% of patients with prior hormonal treatment (within 8.0 ± 11.9 months). Pain medication was given to 18.3% of patients (n = 98/536); mean time to pain recurrence was 9.7 ± 33.5 days.
Table 1.
Baseline characteristics
| Parameter | ||
|---|---|---|
| Demography | N | years, mean ± SD |
| Age at registration | 887 | 34.4 ± 7.6 |
| History of endometriosis | N | % |
| Onset of first symptomsa | ||
| <1 year ago | 407 | 45.9 |
| Between 1 and 5 years ago | 273 | 30.8 |
| >5 years ago | 206 | 23.2 |
| Missing | 1 | 0.1 |
| Most common symptoms of endometriosisb | ||
| Dysmenorrhea | 720 | 81.2 |
| Chronic pelvic pain | 304 | 34.3 |
| Dyspareunia | 55 | 6.2 |
| Subfertility | 44 | 5.0 |
| Time point of first diagnosisa | ||
| <1 year ago | 651 | 73.4 |
| Between 1 and 5 years ago | 145 | 16.4 |
| >5 years ago | 91 | 10.3 |
| Method of diagnosisb | ||
| Surgical diagnosis | 621 | 70.0 |
| Clinical diagnosis | 560 | 63.1 |
| Endometriosis lesions (n = 777 evaluable patients) | ||
| Single | 425 | 54.7 |
| Multi | 352 | 45.3 |
| Endometriosis localization (n = 737 evaluable patients)b | ||
| Ovary | 650 | 88.2 |
| Pelvic organ | 287 | 38.9 |
| Extra pelvic | 21 | 2.9 |
| Prior endometriosis treatment | N | % |
| Patients without prior treatment | 351 | 39.6 |
| Patients with prior treatment | 536 | 60.4 |
| Type of treatment (n = 536) | N | % |
| Surgery | 454 | 84.7 |
| Hormonal treatment | 186 | 34.7 |
| Pain therapy | 98 | 18.3 |
| rASRM stage of endometriosis (n = 471)b,c | N | % |
| Stage I (minimal) | 17 | 3.7 |
| Stage II (mild) | 25 | 5.5 |
| Stage III (moderate) | 92 | 20.3 |
| Stage IV (severe) | 130 | 28.6 |
| Unknown | 207 | 45.6 |
| Most common previous diseases (n = 145)b | N | % |
| Uterine leiomyoma | 71 | 49.0 |
| Endometrial polyp | 36 | 24.8 |
| Ovarian cysts | 26 | 17.9 |
| Pelvic inflammatory disease | 20 | 13.8 |
| Most common concomitant diseases (n = 154)b | N | % |
| Adenomyosis | 51 | 33.1 |
| Uterine leiomyoma | 42 | 27.3 |
| Anemia | 18 | 11.7 |
aPartially missing dates were imputed by the earliest possible time point: In case that only the day was missing, the date was imputed as the first day of the month. In case that the day and the month were missing, i.e., only the year was available, the day and month was imputed by January 1st
bMultiple answers possible
crASRM was assessed for each individual surgery (n = 471 surgeries) in 454 patients with a prior surgical treatment for endometriosis. rASRM, revised American Society for Reproductive Medicine score
EHP-30 Scores
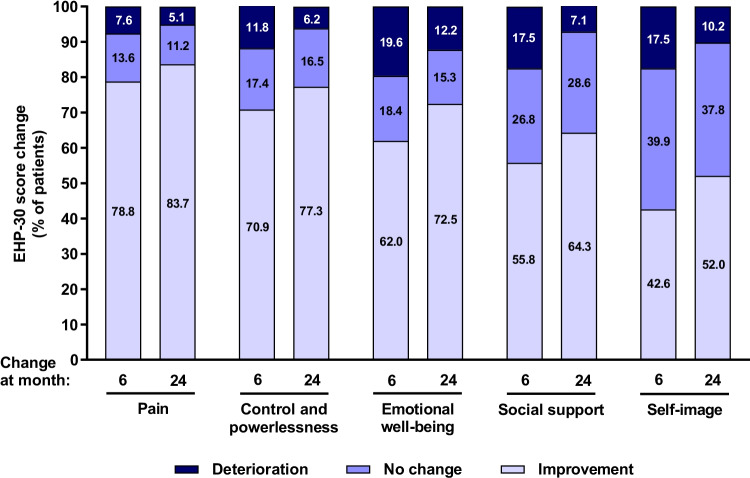
For 6-month and 24-month assessments, EHP-30 core instrument questionnaires were filled out on average at 178 ± 22.5 days (n = 486) and 713.2 ± 30.9 days (n = 98) after baseline visit, respectively, and EHP-30 modular instrument questionnaires were filled out on average 178 ± 22.2 days (n = 482) and 713.7 ± 30.4 days (n = 100) after baseline visit, respectively. Scores for all EHP-30 core and modular instrument scales improved during the first 6 months of dienogest therapy and continued to improve until month 24 (Tables 2 and 3, respectively). Percentage of patients with improvement in EHP-30 scores was highest for scales Pain, and Control and powerlessness (Fig. 2).
Table 2.
EHP-30 core scores and changes from baseline
| Dimension | N | Mean | SD | Median | Min; max | 95%CI | Nmiss |
|---|---|---|---|---|---|---|---|
| Pain | |||||||
| Baseline | 551 | 37.5 | 28.1 | 38.6 | 0; 100 | – | 0 |
| Month 6 | 486 | 8.4 | 13.5 | 0 | 0; 65.9 | – | 0 |
| Month 24 | 98 | 3.9 | 8.4 | 0 | 0; 38.6 | – | 55 |
| Change from baseline at month 6 | 486 | −28.9 | 27.5 | −25 | −100; 36.4 | −31.35; −26.45 | 0 |
| Change from baseline at month 24 | 98 | −34 | 28.4 | −30.7 | −100; 29.6 | −39.66; −28.29 | 55 |
| Control and powerlessness | |||||||
| Baseline | 550 | 35.3 | 28.1 | 33.3 | 0; 100 | – | 1 |
| Month 6 | 485 | 11.4 | 16.2 | 4.2 | 0; 87.5 | – | 1 |
| Month 24 | 98 | 5.7 | 9.9 | 0 | 0; 58.3 | – | 55 |
| Change from baseline at month 6 | 484 | −23.7 | 28.2 | −18.8 | −100; 58.3 | −26.23; −21.19 | 2 |
| Change from baseline at month 24 | 97 | −28.5 | 26.2 | −29.2 | −100; 25 | −33.81; −23.23 | 56 |
| Emotional well-being | |||||||
| Baseline | 549 | 32.3 | 25.9 | 29.2 | 0; 100 | – | 2 |
| Month 6 | 485 | 17.3 | 20.3 | 8.3 | 0; 91.7 | – | 1 |
| Month 24 | 98 | 9.8 | 16.8 | 0 | 0; 75 | – | 55 |
| Change from baseline at month 6 | 484 | −15 | 26.7 | −10.4 | −100; 79.2 | −17.35; −12.57 | 2 |
| Change from baseline at month 24 | 98 | −20.6 | 22.8 | −20.8 | −70.8; 33.3 | −25.19; −16.05 | 55 |
| Social support | |||||||
| Baseline | 550 | 27.7 | 25.8 | 25 | 0; 100 | – | 1 |
| Month 6 | 486 | 14.4 | 19.8 | 6.3 | 0; 87.5 | – | 0 |
| Month 24 | 98 | 8.4 | 18 | 0 | 0; 100 | – | 55 |
| Change from baseline at month 6 | 487 | −13.2 | 27 | −6.3 | −100; 87.5 | −15.56; −10.75 | 0 |
| Change from baseline at month 24 | 98 | −17.9 | 24.3 | −12.5 | −87.5; 43.8 | −22.79; −13.05 | 55 |
| Self-image | |||||||
| Baseline | 550 | 20.6 | 24.9 | 8.3 | 0; 100 | – | 1 |
| Month 6 | 486 | 11.7 | 18.9 | 0 | 0; 91.7 | – | 0 |
| Month 24 | 98 | 7.1 | 17.3 | 0 | 0; 100 | – | 55 |
| Change from baseline at month 6 | 486 | −8.4 | 26.4 | 0 | −100; 91.7 | −10.76; −6.05 | 0 |
| Change from baseline at month 24 | 98 | −13.9 | 23.2 | −8.3 | −83.3; 50 | −18.52; −9.20 | 55 |
Table 3.
EHP-30 modular scores and changes from baseline
| Dimension | N | Mean | SD | Median | Min; max | 95%CI | Nmiss | N/A* |
|---|---|---|---|---|---|---|---|---|
| Work | ||||||||
| Baseline | 475 | 30.4 | 27.1 | 30 | 0; 100 | – | 13 | 63 |
| Month 6 | 431 | 7.5 | 13.6 | 0 | 0; 80 | – | 7 | 46 |
| Month 24 | 86 | 2.8 | 8.8 | 0 | 0; 60 | – | 54 | 14 |
| Change from baseline at month 6 | 395 | −22.4 | 25.4 | −15 | −90; 40 | −24.88; −19.86 | 89 | |
| Change from baseline at month 24 | 81 | −25.4 | 25 | −20 | −90; 35 | −30.89; −19.85 | 73 | |
| Relationship with children | ||||||||
| Baseline | 189 | 25.5 | 30.4 | 0 | 0; 100 | – | 27 | 335 |
| Month 6 | 188 | 6.7 | 14.7 | 0 | 0; 75 | – | 29 | 267 |
| Month 24 | 41 | 2.4 | 7 | 0 | 0; 25 | – | 17 | 96 |
| Change from baseline at month 6 | 140 | −18.5 | 29.4 | 0 | −100; 50 | −23.39; −13.57 | 344 | |
| Change from baseline at month 24 | 30 | −25.8 | 28.6 | −25 | −100; 12.5 | −36.51; −15.15 | 124 | |
| Sexual intercourse | ||||||||
| Baseline | 353 | 28.1 | 26.8 | 25 | 0; 100 | – | 79 | 119 |
| Month 6 | 303 | 15 | 20.5 | 5 | 0; 95 | – | 36 | 145 |
| Month 24 | 66 | 11.1 | 18.3 | 0 | 0; 75 | – | 62 | 26 |
| Change from baseline at month 6 | 258 | −13.1 | 26.4 | −5 | −90; 75 | −16.29; −9.82 | 226 | |
| Change from baseline at month 24 | 52 | −17.8 | 27.5 | −10 | −75; 40 | −25.45; −10.17 | 102 | |
| Treatment | ||||||||
| Baseline | 383 | 19.9 | 22.7 | 16.7 | 0; 100 | – | 31 | 137 |
| Month 6 | 447 | 11.2 | 16.3 | 0 | 0; 83.3 | – | 6 | 31 |
| Month 24 | 92 | 6.9 | 13 | 0 | 0; 75 | – | 54 | 8 |
| Change from baseline at month 6 | 322 | −7.2 | 22.4 | 0 | −91.7; 50 | −10.43; −3.96 | 162 | |
| Change from baseline at month 24 | 70 | −11.6 | 25.2 | 0 | −91.7; 50 | −17.56; −5.53 | 84 | |
| Infertility | ||||||||
| Baseline | 317 | 29 | 28.8 | 25 | 0; 100 | – | 36 | 198 |
| Month 6 | 308 | 20.2 | 24.1 | 12.5 | 0; 100 | – | 23 | 153 |
| Month 24 | 59 | 14.2 | 18.3 | 6.3 | 0; 81.3 | – | 57 | 38 |
| Change from baseline at month 6 | 235 | −8.9 | 21.9 | 0 | −100; 56.3 | −11.69; −6.07 | 249 | |
| Change from baseline at month 24 | 47 | −11.4 | 19.4 | −12.5 | −75; 31.3 | −17.14; −5.73 | 107 | |
*Not applicable to the respective issue during the last 4 weeks
Fig. 2.
Changes in core EHP-30 scores from baseline to month 6 and to month 24. Results demonstrate proportions of patients with deterioration, no change and improvement defined as >0, 0 and < 0 difference in EHP-30 scores between baseline and visit at month 6 and 24. Data were analyzed in patients with evaluable score changes after 6 (n = 486, scales pain, social support, self-image; n = 484, scales control and powerlessness and emotional well-being) and after 24 months of therapy (n = 98, scales pain, emotional well-being, social support and self-image; n = 97, scale control and powerlessness). EHP-30, Endometriosis Health Profile-30 questionnaire
EAPP
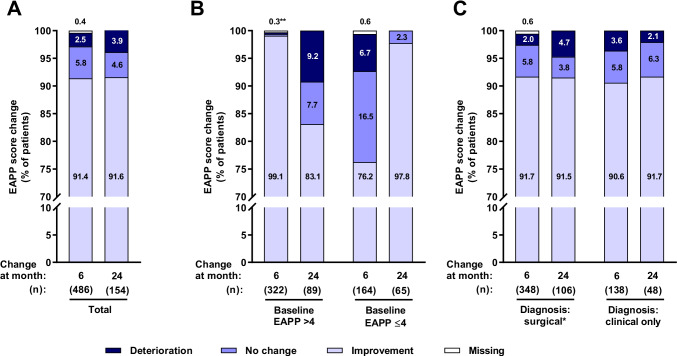
EAPP assessment at month 6 and month 24 was performed on average 175.6 ± 22.6 days (n = 484) and 701.1 ± 41 days (n = 154) after baseline visit. EAPP score improved during the first 6 months of therapy and remained stable thereafter (Table 4). Patients with baseline EAPP >4 had a greater mean EAPP score change than those with baseline EAPP ≤4 during therapy. Patients not taking rescue medication (defined as any medication for pain management used while on treatment with dienogest) showed a tendency towards an improved EAPP compared to those taking the rescue medication (note that only a few patients were included in the latter category). Type of diagnosis (surgical vs only clinical) and prior surgical or hormonal treatment had no impact on efficacy of dienogest to alleviate EAPP (Table 4). More than 90% of patients had an improvement in EAPP at both post-baseline study visits (Fig. 3). Amelioration of EAPP was more often experienced at month 6 by women with a higher baseline EAPP severity (>4), and at month 24 by those with a lower baseline EAPP (≤4).
Table 4.
EAPP scores and changes from baseline
| Parameter | N | Mean | SD | Median | Min, max | Nmiss | |
|---|---|---|---|---|---|---|---|
| Total | Baseline | 551 | 5.6 | 2.8 | 6 | 0; 10 | 0 |
| Month 6 | 484 | 1 | 1.4 | 0 | 0; 7 | 2 | |
| Month 24 | 154 | 0.7 | 1.3 | 0 | 0; 9 | 0 | |
| Change from baseline at month 6 | 484 | −4.6 | 3 | −5 | −10; 5 | 2 | |
| Change from baseline at month 24 | 154 | −4.6 | 3 | −5 | −10; 3 | 0 | |
| Surgical diagnosis* | Baseline | 396 | 5.3 | 2.8 | 6 | 0; 10 | 0 |
| Month 6 | 346 | 1 | 1.4 | 0 | 0; 7 | 2 | |
| Month 24 | 106 | 0.8 | 1.3 | 0 | 0; 5 | 0 | |
| Change from baseline at month 6 | 346 | −4.3 | 2.8 | −4 | −10; 4 | 2 | |
| Change from baseline at month 24 | 106 | −4.5 | 3 | −5 | −10; 3 | 0 | |
| Clinical diagnosis only | Baseline | 155 | 6.3 | 2.7 | 7 | 0; 10 | 0 |
| Month 6 | 138 | 1 | 1.5 | 0 | 0; 6 | 0 | |
| Month 24 | 48 | 0.6 | 1.4 | 0 | 0; 9 | 0 | |
| Change from baseline at month 6 | 138 | −5.3 | 3.2 | −6 | −10; 5 | 0 | |
| Change from baseline at month 24 | 48 | −4.8 | 3.1 | −5 | −10; 2 | 0 | |
| Baseline severity of EAPP ≤4 | Baseline | 185 | 2.3 | 1.2 | 2 | 0; 4 | 0 |
| Month 6 | 163 | 0.8 | 1.3 | 0 | 0; 6 | 1 | |
| Month 24 | 65 | 0.6 | 1.1 | 0 | 0; 5 | 0 | |
| Change from baseline at month 6 | 163 | −1.5 | 1.7 | −1 | −4; 5 | 1 | |
| Change from baseline at month 24 | 65 | −1.8 | 1.6 | −2 | −4; 3 | 0 | |
| Baseline severity of EAPP >4 | Baseline | 366 | 7.3 | 1.6 | 7 | 5; 10 | 0 |
| Month 6 | 321 | 1 | 1.5 | 0 | 0; 7 | 1 | |
| Month 24 | 89 | 0.8 | 1.5 | 0 | 0; 9 | 0 | |
| Change from baseline at month 6 | 321 | −6.2 | 2.1 | −6 | −10; 1 | 1 | |
| Change from baseline at month 24 | 89 | −6.6 | 2 | −7 | −10; 0 | 0 | |
| Previous surgical or hormonal treatment | Baseline | 329 | 5.3 | 2.7 | 6 | 0; 10 | 0 |
| Month 6 | 283 | 0.9 | 1.4 | 0 | 0; 7 | 2 | |
| Month 24 | 93 | 0.6 | 1.1 | 0 | 0; 5 | 0 | |
| Change from baseline at month 6 | 283 | −4.4 | 2.8 | −5 | −10; 3 | 2 | |
| Change from baseline at month 24 | 93 | −4.3 | 2.7 | −4 | −9; 1 | 0 | |
| No previous surgical or hormonal treatment | Baseline | 222 | 6 | 2.8 | 6 | 0; 10 | 0 |
| Month 6 | 201 | 1 | 1.4 | 0 | 0; 6 | 0 | |
| Month 24 | 61 | 0.9 | 1.6 | 0 | 0; 9 | 0 | |
| Change from baseline at month 6 | 201 | −4.9 | 3.2 | −5 | −10; 5 | 0 | |
| Change from baseline at month 24 | 61 | −5 | 3.5 | −5 | −10; 3 | 0 | |
| Use of rescue medication | Baseline | 55 | 6.7 | 2.6 | 8 | 0; 10 | 0 |
| Month 6 | 13 | 2.9 | 1.8 | 3 | 0; 5 | 0 | |
| Month 24 | 3 | 2.3 | 1.2 | 3 | 1; 3 | 0 | |
| Change from baseline at month 6 | 13 | −3.2 | 3.4 | −3 | −10; 4 | 0 | |
| Change from baseline at month 24 | 3 | −2.7 | 5.1 | −4 | −7; 3 | 0 | |
| No use of rescue medication | Baseline | 496 | 5.5 | 2.8 | 6 | 0; 10 | 0 |
| Month 6 | 471 | 0.9 | 1.4 | 0 | 0; 7 | 0 | |
| Month 24 | 151 | 0.7 | 1.3 | 0 | 0; 9 | 0 | |
| Change from baseline at month 6 | 471 | −4.7 | 3 | −5 | −10; 5 | 0 | |
| Change from baseline at month 24 | 151 | −4.6 | 3 | −5 | −10; 3 | 0 |
*Includes patients, who only had surgical diagnosis as well as surgical + clinical diagnosis. EAPP, endometriosis-associated pelvic pain
Fig. 3.
Changes in EAPP from baseline to month 6 and to month 24 among all patients included in efficacy analysis set (a) and according to type of diagnosis (b) and baseline EAPP severity (c). Data shown are proportions of patients with deterioration, no change and improvement defined as > 0, 0 and < 0 difference in EAPP scores between baseline and visits at months 6 and 24. *Including patients with surgical diagnosis only and surgical and clinical diagnosis. **0.3% of patients each had no change, improvement or missing data on change of EAPP. EAPP, endometriosis-associated pelvic pain
Pain recurrence rate in EFF set was approximately three times higher in patients discontinuing (8.3%, n = 8/97) than among women continuing dienogest treatment for up to 24 months (2.7%, n = 4/146). Median time from dienogest discontinuation to pain recurrence was 22.2 months (Q1–Q3: 22.2–22.2).
Bleeding Pattern
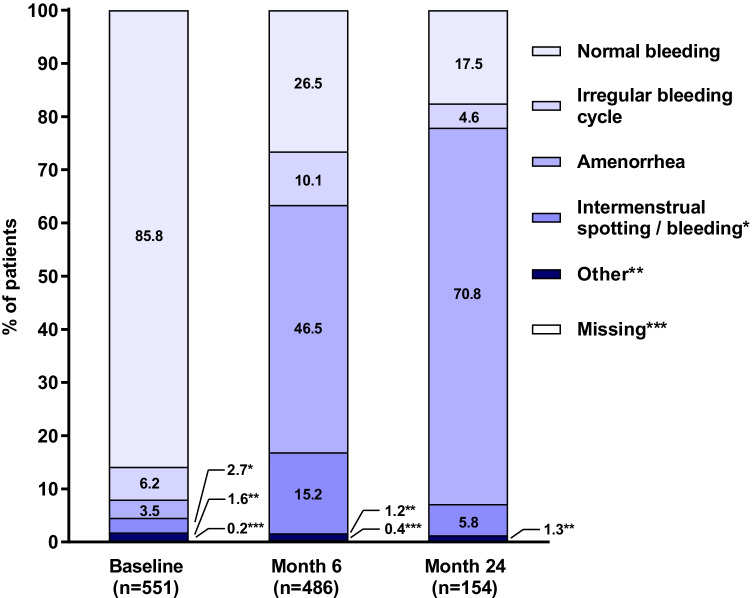
Share of patients with normal bleeding decreased while the rate of amenorrhea increased during the 24-month therapy period (Fig. 4). The percentage of patients with irregular bleeding and intermenstrual spotting/bleeding increased during the first 6 months of therapy and then returned to baseline levels at month 24.
Fig. 4.
Bleeding patterns at the baseline, month 6 and month 24. The bleeding patterns were defined as follows: normal bleeding: regular bleeding with normal flow and duration; irregular bleeding cycle: bleeding cycle less than 21 days or more than 35 days; amenorrhea: no menstruation during last 90 days; intermenstrual spotting/bleeding: irregular episodes of bleeding, often light and short, occurring between otherwise fairly normal menstrual periods
Evaluation of Satisfaction and Symptoms
66.6% of patients (n = 515/773 of FAS) and 67.7% (n = 523/773) of physicians at month 6 and 52.3% (n = 191/365) of both patients and physicians at month 24 were very satisfied or somewhat satisfied with dienogest. At month 6 and month 24, only 4.5% and 2.5% of patients, and 2.1% and 1.9% of physicians were dissatisfied with treatment. 83.7% (n = 407/486 of EFF) and 87.0% of patients (n = 134/154) reported improved of symptoms at month 6 and month 24, respectively. Only 1.0% and 1.3% of patients reported that their symptoms worsened at month 6 and month 24, respectively. The proportion of patients receiving another or no treatment increased from 1.1% at month 6 to 27.1% at month 24. Surgery was repeated in two out of 273 patients that received a surgery prior to dienogest, including one patient (1.3%) among the 77 patients who stopped dienogest and one patient (0.5%) in the group of 196 patients that continued therapy until month 24.
Safety Analysis
TEAEs, predominantly of mild-to-moderate intensity, were reported by 45.9% of patients (n = 407/887). TEAEs occurring in at least 1% of patients are presented in Table 5.
Table 5.
Treatment-emergent adverse events observed in at least 1% of patients
| TEAE (MedDRA PT) | N | % |
|---|---|---|
| Any TEAE | 407 | 45.9 |
| Vaginal hemorrhage | 95 | 10.7 |
| Metrorrhagia | 75 | 8.5 |
| Amenorrhea | 65 | 7.3 |
| Weight increased | 41 | 4.6 |
| Menstruation irregular | 39 | 4.4 |
| Headache | 33 | 3.7 |
| Acne | 23 | 2.6 |
| Alopecia | 23 | 2.6 |
| Depression | 16 | 1.8 |
| Menorrhagia | 16 | 1.8 |
| Insomnia | 13 | 1.5 |
| Abdominal pain | 12 | 1.4 |
| Dizziness | 12 | 1.4 |
| Uterine hemorrhage | 10 | 1.1 |
| Breast tenderness | 9 | 1 |
MedDRA Medical Dictionary for Regulatory Activities, PT preferred term, TEAE Treatment-emergent adverse event
A total of 616 drug-related TEAEs were documented in 39.9% of patients (n = 354/887), most often vaginal hemorrhage (defined as any bloody discharge outside of normal menstrual cycle, including prolonged or irregular bleeding/spotting, in 10.4% of patients), metrorrhagia (defined as abnormal bleeding between regular menstrual periods, in 7.3%) and amenorrhea (6.4%). 63 patients (7.1%) discontinued dienogest therapy due to drug-related TEAEs. Abnormal uterine bleeding (MedDRA preferred terms: metrorrhagia, menorrhagia, vaginal or uterine hemorrhage) was the most prominent reason for discontinuation (2.0%, n = 18/887).
Eleven SAEs were reported in nine patients (Table 6). Anemia and menorrhagia (two events each) were the most common SAEs. Ten events were classified as serious because they required hospitalization and one case of anemia was an important medical event. All SAEs were recovered or resolved.
Table 6.
All documented serious adverse events
| SAE (MedDRA PT) | N | % |
|---|---|---|
| Any SAE | 9 | 1 |
| Anemia | 2 | 0.2 |
| Menorrhagia | 2 | 0.2 |
| Vomiting* | 1 | 0.1 |
| Asthenia* | 1 | 0.1 |
| Bartholin’s abscess | 1 | 0.1 |
| Leptospirosis | 1 | 0.1 |
| Peritonitis | 1 | 0.1 |
| Dysmenorrhea* | 1 | 0.1 |
| Ovarian cyst | 1 | 0.1 |
*Drug-related SAE. MedDRA Medical Dictionary for Regulatory Activities, PT preferred term, SAE serious adverse event
Discussion
Almost half of women with endometriosis are dissatisfied with their medical treatment [29, 30]. This indicates that there is an unmet need for therapeutic approaches effective in alleviating disease symptoms and maintaining good HRQoL. Although surgery is efficacious in alleviating endometriosis symptoms and improving HRQoL, 40% to 50% of patients have symptom recurrence, with 47% needing reoperation [31–34]. For these reasons, several guidelines recommend empirical long-term medicinal treatment and taking into account specific needs and expectations of the patient [2, 7, 35–37]. In our study, we found that dienogest improved scores for all EHP-30 scales already at month 6 of treatment, with continuous improvement until month 24. Similarly, continuous improvements in EHP-30 scores were noted in adolescents over the 12-month dienogest therapy [22]. In long-term, dienogest improved all EHP-30 core scores in patients with rectosigmoid endometriosis treated for 36 months [38]. Furthermore, 6-month treatment with dienogest increased HRQoL in patients who had a pain persistence and were unsatisfied with norethisterone acetate therapy [39]. In addition to EHP-30 questionnaire, Caruso and colleagues reported an improved HRQoL as assessed by SF-36 questionnaire in patients treated with dienogest for 6 months [20]. That study also demonstrated an increased sexual function and reduced sexual distress following the therapy. Collectively, these data indicate that dienogest rapidly improves HRQoL and is effective in a long-term. Moreover, EAPP scores improved during 6 months of dienogest therapy, and this effect was maintained over the entire observation period. Thus, our results confirm data on EAPP from other observational studies investigating long-term dienogest therapy. For example, in the study by Park et al., EAPP decreased by 33.5 mm on visual analog scale during 12 months of therapy [40] while Römer reported a decrease in EAPP of 50 mm after 60 months of treatment [41]. Furthermore, in a large open-label extension study following a 12-week placebo-controlled trial, EAPP decrease amounted to 43.2 mm at week 65 [15]. Rate of patients with EAPP reduction in our study (over 91% of patients) was slightly lower than previously reported in clinical trials (96.7% to 97.1%) [40, 42]. Furthermore, patients with baseline EAPP score > 4 had a more pronounced pain reduction than those with baseline EAPP score ≤ 4. Interestingly, percentage of patients with improvement in pain scores decreased from month 6 to month 24 among those with baseline EAPP score > 4 and increased in those with score ≤ 4. On the one hand, these data indicate that a longer duration of dienogest therapy is required to alleviate EAPP in patients with low baseline EAPP score. On the other hand, some patients with a higher initial EAPP severity might require rescue medication during therapy. The latter notion is supported by the fact that only a few patients had documented use of rescue medication. A lower EAPP improvement in that group compared with patients not taking rescue medication stresses the need for appropriate pain management in patients with severe EAPP. Furthermore, prior treatment (surgery or hormonal therapy) and type of diagnosis (surgical or clinical) had no impact on magnitude of EAPP improvement. Similarly, no difference in EAPP reduction between patients with or without surgery was reported in the study by Römer et al. [41]. This suggests that dienogest is efficacious in reducing EAPP in both first- and later lines of therapy and it is an optimal choice when access to surgical diagnostic techniques like laparoscopy is limited. Several guidelines now emphasize the value of non-invasive clinical diagnosis based on clinical symptoms and patient’s history, especially in low-resource setting [2, 35, 37]. Collectively, our results support a new paradigm that the diagnosis of endometriosis does not always require histological data, but with appropriate clinical approaches and empirical therapy, we can induce long-term improvements in quality of life in [2, 7, 35–37]. This approach would be even better if we succeeded in having non-invasive endometriosis diagnostic markers [43–45].
Reduction in severity of EAPP potentially contributed to the fact that 66.6% of patients after month 6 and 52.3% after month 24 were satisfied with dienogest therapy. Similar or even higher rates of satisfaction with dienogest therapy were reported by European patients [22, 41, 46]. Considering that 40% to 45.5% of women with endometriosis are dissatisfied with their current treatment, data accumulated thus far suggest that dienogest largely meets expectations of patients regarding their treatment [29, 30].
Analysis of TEAE revealed that rate of patients with depression was similar or lower than previously reported (1.8% vs 2.0% to 82.9%, [18, 22, 38, 41, 42]). Furthermore, fewer patients reported headache than in other studies (3.7% vs 9.0% to 12.5%, [18, 22, 41, 42]). Rates of weight gain, acne and alopecia were slightly lower or similar as previously reported [22, 38, 42]. Only 1% of patients had SAE, mostly anemia and menorrhagia. 39.9% of patients had drug-related TEAE while previous studies reported either higher or lower rates of drug-related AE (15.3% to 100%, [15, 22, 46, 47]). It should be noted however, that dienogest was used at various doses in these studies and most of reported drug-related AE were non-serious. Yu et al. reported lower rates for dienogest-related vaginal hemorrhage and amenorrhea (1.8%, both, vs 10.4% and 6.4%, respectively, in our study [46]). Conversely, Harada et al. demonstrated a higher frequency of genital bleeding (95%, [47]). Overall, the share of patients with normal bleeding profile decreased while proportion of patients with amenorrhea increased during the study which is in line with current evidence on dienogest profile [7, 15, 17, 18, 42, 48]. Therefore, physicians should inform patients about potential abnormal uterine bleedings which could occur during long-term treatment with progestins.
The key strengths of our study are the large sample size spanning 6 Asian countries and the long duration of follow-up. Additionally, by using disease-specific patient-reported outcome measures, we captured the patients’ perspective on changes in HRQoL during therapy. Furthermore, broad inclusion criteria ensured collection of real-world evidence in unselected population of patients treated with dienogest. Our study has also several limitations. The number of patients included in the efficacy analysis decreased from month 6 to month 24, therefore, results from the latter time-point may not be representative to all investigated patients. Furthermore, due to international setting of this study, differences between the healthcare systems could affect the diagnosis or type of rescue treatment of endometriosis. Finally, the observational nature of this study precluded the comparison of efficacy and safety between dienogest and other medicines for endometriosis.
Conclusion
The results of the ENVISIOeN study indicate that dienogest improves patient-reported HRQoL and EAPP in the real-life setting in Asian women with endometriosis diagnosed either clinically or surgically. Given that observed safety profile was consistent with the previous results and that satisfaction with the treatment was high, dienogest might be a promising first-line treatment option for the long-term management of only clinically diagnosed patients with debilitating endometriosis-associated symptoms.
Acknowledgements
The authors would like to thank all sites and patients who participated in the study as well as their responsible investigators. We thank Stephen Christopher and Marco Serrani, Bayer AG, for insightful discussions relating to this work. Medical writing and editorial support were provided by Lukasz Wujak and Yvonne Holighaus, Alcedis GmbH, Giessen, Germany.
Availability of Data and Material
The datasets analyzed are available according to Bayer’s policy on sharing clinical study data (https://www.clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Bayer.aspx).
Code Availability
Not applicable.
Authors’ Contributions
SoYoung Shin developed the study protocol, conceptualized, and designed the study, was responsible for study supervision, contributed to the data analysis and interpretation, and critically reviewed the manuscript. Ling Cai contributed to the study design, data analysis and interpretation, and critically reviewed the manuscript. Kitirat Techatraisak, Andon Hestiantoro, Ruey Soon, Maria Jesusa Banal-Silao, Mee-Ran Kim, Seok Ju Seong, Syarief Thaufik Hidayat and Byung Seok Lee were responsible for data collection and implementation of the study at the respective sites and helped to draft the manuscript. Byung Seok Lee was the principal investigator who helped to conceptualize the study and was responsible for supervision of the study. All authors were involved in revising the manuscript for important intellectual content and approved the final manuscript.
Funding
This study, including its design, and the collection, analysis, and interpretation of the data, as well as medical writing and editorial assistance, was funded by Bayer AG.
Declarations
Competing Interests
LC is a current employee of Bayer AG, Germany and SS is a former employee of Bayer AG. All other authors declare no competing interests.
Ethics Approval
The Food and Drug Administration, Department of Health, Republic of the Philippines approved the study protocol and all relevant study documents prior to study start. Ethical approval was obtained from each participating site of the Philippines (St. Luke’s Medical Center; University of the East Ramon Magsaysay Memorial Medical Center; University of Santo Tomas Hospital), Thailand (Faculty of Medicine Siriraj Hospital; Faculty of Medicine Vajra Hospital; Faculty of Medicine Chiang Mai University; Faculty of Medicine Ramathibodi Hospital, Mahidol University; Maharat Nakhon Ratchasima Hospital) and Korea (CHA Gangnam Medical Center, CHA University; Keimyung University Dongsan Hospital; ChungNam National University Hospital; Inha University Hospital; The Catholic University Of Korea, Seoul St. Mary’s Hospital; Seoul National University Hospital; Korea University Anam Hospital Clinical Trial Center; Ajou University Hospital; Severance Hospital Clinical Trial Center; ChonNam National University Hospital; Pusan National University Yangsan Hospital; GangNam Severance Hospital). Centralized ethical approval for participating sites was obtained for Singapore (SingHealth), Malaysia (Ministry of Health) and Indonesia (Faculty of Medicine University of Indonesia).
Consent to Participate
All enrolled patients gave written informed consent prior to any study-related procedure.
Consent for Publication
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gerlinger C, Faustmann T, Hassall JJ, Seitz C. Treatment of endometriosis in different ethnic populations: a meta-analysis of two clinical trials. BMC Womens Health. 2012;12:9. doi: 10.1186/1472-6874-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunselman GA, Vermeulen N, Becker C, Calhaz-Jorge C, D'Hooghe T, De Bie B, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29:400–412. doi: 10.1093/humrep/det457. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto A, Johnstone EB, Bloom MS, Huddleston HG, Fujimoto VY. A higher prevalence of endometriosis among Asian women does not contribute to poorer IVF outcomes. J Assist Reprod Genet. 2017;34:765–774. doi: 10.1007/s10815-017-0919-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arumugam K, Templeton AA. Endometriosis and race. Aust N Z J Obstet Gynaecol. 1992;32:164–165. doi: 10.1111/j.1479-828X.1992.tb01932.x. [DOI] [PubMed] [Google Scholar]
- 5.Sangi-Haghpeykar H, Poindexter AN., 3rd Epidemiology of endometriosis among parous women. Obstet Gynecol. 1995;85:983–992. doi: 10.1016/0029-7844(95)00074-2. [DOI] [PubMed] [Google Scholar]
- 6.Sachedina A, Todd N. Dysmenorrhea, endometriosis and chronic pelvic pain in adolescents. J Clin Res Pediatr Endocrinol. 2020;12:7–17. doi: 10.4274/jcrpe.galenos.2019.2019.S0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schindler AE. Dienogest in long-term treatment of endometriosis. Int J Women's Health. 2011;3:175–184. doi: 10.2147/IJWH.S5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lagana AS, La Rosa VL, Rapisarda AMC, Valenti G, Sapia F, Chiofalo B, et al. Anxiety and depression in patients with endometriosis: impact and management challenges. Int J Women's Health. 2017;9:323–330. doi: 10.2147/IJWH.S119729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lagana AS, Condemi I, Retto G, Muscatello MR, Bruno A, Zoccali RA, et al. Analysis of psychopathological comorbidity behind the common symptoms and signs of endometriosis. Eur J Obstet Gynecol Reprod Biol. 2015;194:30–33. doi: 10.1016/j.ejogrb.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 10.Tripoli TM, Sato H, Sartori MG, de Araujo FF, Girao MJ, Schor E. Evaluation of quality of life and sexual satisfaction in women suffering from chronic pelvic pain with or without endometriosis. J Sex Med. 2011;8:497–503. doi: 10.1111/j.1743-6109.2010.01976.x. [DOI] [PubMed] [Google Scholar]
- 11.Souza CA, Oliveira LM, Scheffel C, Genro VK, Rosa V, Chaves MF, et al. Quality of life associated to chronic pelvic pain is independent of endometriosis diagnosis--a cross-sectional survey. Health Qual Life Outcomes. 2011;9:41. doi: 10.1186/1477-7525-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Facchin F, Barbara G, Saita E, Mosconi P, Roberto A, Fedele L, et al. Impact of endometriosis on quality of life and mental health: pelvic pain makes the difference. J Psychosom Obstet Gynaecol. 2015;36:135–141. doi: 10.3109/0167482X.2015.1074173. [DOI] [PubMed] [Google Scholar]
- 13.Jia SZ, Leng JH, Shi JH, Sun PR, Lang JH. Health-related quality of life in women with endometriosis: a systematic review. J Ovarian Res. 2012;5:29. doi: 10.1186/1757-2215-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harada T, Taniguchi F. Dienogest: a new therapeutic agent for the treatment of endometriosis. Womens Health (Lond) 2010;6:27–35. doi: 10.2217/whe.09.72. [DOI] [PubMed] [Google Scholar]
- 15.Petraglia F, Hornung D, Seitz C, Faustmann T, Gerlinger C, Luisi S, et al. Reduced pelvic pain in women with endometriosis: efficacy of long-term dienogest treatment. Arch Gynecol Obstet. 2012;285:167–173. doi: 10.1007/s00404-011-1941-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Momoeda M, Harada T, Terakawa N, Aso T, Fukunaga M, Hagino H, et al. Long-term use of dienogest for the treatment of endometriosis. J Obstet Gynaecol Res. 2009;35:1069–1076. doi: 10.1111/j.1447-0756.2009.01076.x. [DOI] [PubMed] [Google Scholar]
- 17.Strowitzki T, Faustmann T, Gerlinger C, Schumacher U, Ahlers C, Seitz C. Safety and tolerability of dienogest in endometriosis: pooled analysis from the European clinical study program. Int J Women's Health. 2015;7:393–401. doi: 10.2147/IJWH.S77202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strowitzki T, Faustmann T, Gerlinger C, Seitz C. Dienogest in the treatment of endometriosis-associated pelvic pain: a 12-week, randomized, double-blind, placebo-controlled study. Eur J Obstet Gynecol Reprod Biol. 2010;151:193–198. doi: 10.1016/j.ejogrb.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Granese R, Perino A, Calagna G, Saitta S, De Franciscis P, Colacurci N, et al. Gonadotrophin-releasing hormone analogue or dienogest plus estradiol valerate to prevent pain recurrence after laparoscopic surgery for endometriosis: a multi-center randomized trial. Acta Obstet Gynecol Scand. 2015;94:637–645. doi: 10.1111/aogs.12633. [DOI] [PubMed] [Google Scholar]
- 20.Caruso S, Iraci M, Cianci S, Casella E, Fava V, Cianci A. Quality of life and sexual function of women affected by endometriosis-associated pelvic pain when treated with dienogest. J Endocrinol Investig. 2015;38:1211–1218. doi: 10.1007/s40618-015-0383-7. [DOI] [PubMed] [Google Scholar]
- 21.Caruso S, Iraci M, Cianci S, Fava V, Casella E, Cianci A. Comparative, open-label prospective study on the quality of life and sexual function of women affected by endometriosis-associated pelvic pain on 2 mg dienogest/30 microg ethinyl estradiol continuous or 21/7 regimen oral contraceptive. J Endocrinol Investig. 2016;39:923–931. doi: 10.1007/s40618-016-0460-6. [DOI] [PubMed] [Google Scholar]
- 22.Ebert AD, Dong L, Merz M, Kirsch B, Francuski M, Bottcher B, et al. Dienogest 2 mg daily in the treatment of adolescents with clinically suspected endometriosis: the VISanne study to assess safety in ADOlescents. J Pediatr Adolesc Gynecol. 2017;30:560–567. doi: 10.1016/j.jpag.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Leonardo-Pinto JP, Benetti-Pinto CL, Cursino K, Yela DA. Dienogest and deep infiltrating endometriosis: the remission of symptoms is not related to endometriosis nodule remission. Eur J Obstet Gynecol Reprod Biol. 2017;211:108–111. doi: 10.1016/j.ejogrb.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 24.Luisi S, Parazzini F, Angioni S, Arena S, Berretta P, Candiani M, et al. Dienogest treatment improves quality of life in women with endometriosis. J Endometriosis Pelvic Pain Disord. 2015;7:124–128. doi: 10.5301/je.5000232. [DOI] [Google Scholar]
- 25.Techatraisak K, Hestiantoro A, Ruey S, Banal-Silao MJ, Kim MR, Seong SJ, et al. Effectiveness of dienogest in improving quality of life in Asian women with endometriosis (ENVISIOeN): interim results from a prospective cohort study under real-life clinical practice. BMC Womens Health. 2019;19:68. doi: 10.1186/s12905-019-0758-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones G, Jenkinson C, Kennedy S. Evaluating the responsiveness of the endometriosis health profile questionnaire: the EHP-30. Qual Life Res. 2004;13:705–713. doi: 10.1023/B:QURE.0000021316.79349.af. [DOI] [PubMed] [Google Scholar]
- 27.Jones G, Jenkinson C, Taylor N, Mills A, Kennedy S. Measuring quality of life in women with endometriosis: tests of data quality, score reliability, response rate and scaling assumptions of the endometriosis health profile questionnaire. Hum Reprod. 2006;21:2686–2693. doi: 10.1093/humrep/del231. [DOI] [PubMed] [Google Scholar]
- 28.Jones G, Kennedy S, Barnard A, Wong J, Jenkinson C. Development of an endometriosis quality-of-life instrument: the endometriosis health Profile-30. Obstet Gynecol. 2001;98:258–264. doi: 10.1016/s0029-7844(01)01433-8. [DOI] [PubMed] [Google Scholar]
- 29.Lukas I, Kohl-Schwartz A, Geraedts K, Rauchfuss M, Wolfler MM, Haberlin F, et al. Satisfaction with medical support in women with endometriosis. PLoS One. 2018;13:e0208023. doi: 10.1371/journal.pone.0208023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bernuit D, Ebert AD, Halis G, Strothmann A, Gerlinger C, Geppert K, et al. Female perspectives on endometriosis: findings from the uterine bleeding and pain women’s research study. J Endometriosis. 2011;3:73–85. doi: 10.5301/JE.2011.8525. [DOI] [Google Scholar]
- 31.Vercellini P, Crosignani PG, Abbiati A, Somigliana E, Vigano P, Fedele L. The effect of surgery for symptomatic endometriosis: the other side of the story. Hum Reprod Update. 2009;15:177–188. doi: 10.1093/humupd/dmn062. [DOI] [PubMed] [Google Scholar]
- 32.Shakiba K, Bena JF, McGill KM, Minger J, Falcone T. Surgical treatment of endometriosis: a 7-year follow-up on the requirement for further surgery. Obstet Gynecol. 2008;111:1285–1292. doi: 10.1097/AOG.0b013e3181758ec6. [DOI] [PubMed] [Google Scholar]
- 33.Guo SW. Recurrence of endometriosis and its control. Hum Reprod Update. 2009;15:441–461. doi: 10.1093/humupd/dmp007. [DOI] [PubMed] [Google Scholar]
- 34.Cheong Y, Tay P, Luk F, Gan HC, Li TC, Cooke I. Laparoscopic surgery for endometriosis: how often do we need to re-operate? J Obstet Gynaecol: J Inst Obstet Gynaecol. 2008;28:82–85. doi: 10.1080/01443610701811761. [DOI] [PubMed] [Google Scholar]
- 35.Johnson NP. Hummelshoj L, world endometriosis society Montpellier C. Consensus on current management of endometriosis. Hum Reprod. 2013;28:1552–1568. doi: 10.1093/humrep/det050. [DOI] [PubMed] [Google Scholar]
- 36.Practice Committee of the American Society for Reproductive M Treatment of pelvic pain associated with endometriosis: a committee opinion. Fertil Steril. 2014;101:927–935. doi: 10.1016/j.fertnstert.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 37.Hwang H, Chung YJ, Lee SR, Park HT, Song JY, Kim H, et al. Clinical evaluation and management of endometriosis: guideline for Korean patients from Korean Society of Endometriosis. Obstet Gynecol Sci. 2018;61:553–564. doi: 10.5468/ogs.2018.61.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barra F, Scala C, Maggiore ULR, Ferrero S. Long-term Administration of Dienogest for the treatment of pain and intestinal symptoms in patients with Rectosigmoid endometriosis. J Clin Med. 2020;9. 10.3390/jcm9010154. [DOI] [PMC free article] [PubMed]
- 39.Morotti M, Sozzi F, Remorgida V, Venturini PL, Ferrero S. Dienogest in women with persistent endometriosis-related pelvic pain during norethisterone acetate treatment. Eur J Obstet Gynecol Reprod Biol. 2014;183:188–192. doi: 10.1016/j.ejogrb.2014.10.036. [DOI] [PubMed] [Google Scholar]
- 40.Park SY, Kim SH, Chae HD, Kim CH, Kang BM. Efficacy and safety of dienogest in patients with endometriosis: a single-center observational study over 12 months. Clin Exp Reprod Med. 2016;43:215–220. doi: 10.5653/cerm.2016.43.4.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romer T. Long-term treatment of endometriosis with dienogest: retrospective analysis of efficacy and safety in clinical practice. Arch Gynecol Obstet. 2018;298:747–753. doi: 10.1007/s00404-018-4864-8. [DOI] [PubMed] [Google Scholar]
- 42.Strowitzki T, Marr J, Gerlinger C, Faustmann T, Seitz C. Dienogest is as effective as leuprolide acetate in treating the painful symptoms of endometriosis: a 24-week, randomized, multicentre, open-label trial. Hum Reprod. 2010;25:633–641. doi: 10.1093/humrep/dep469. [DOI] [PubMed] [Google Scholar]
- 43.Agarwal SK, Foster WG, Groessl EJ. Rethinking endometriosis care: applying the chronic care model via a multidisciplinary program for the care of women with endometriosis. Int J Women's Health. 2019;11:405–410. doi: 10.2147/IJWH.S207373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agarwal SK, Chapron C, Giudice LC, Laufer MR, Leyland N, Missmer SA, et al. Clinical diagnosis of endometriosis: a call to action. Am J Obstet Gynecol. 2019;220:354.e1–354e12. doi: 10.1016/j.ajog.2018.12.039. [DOI] [PubMed] [Google Scholar]
- 45.Fraser MA, Agarwal S, Chen I, Singh SS. Routine vs. expert-guided transvaginal ultrasound in the diagnosis of endometriosis: a retrospective review. Abdom Imaging. 2015;40:587–594. doi: 10.1007/s00261-014-0243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu Q, Zhang S, Li H, Wang P, Zvolanek M, Ren X, et al. Dienogest for treatment of endometriosis in women: a 28-week, open-label, extension study. J Women's Health (Larchmt) 2019;28:170–177. doi: 10.1089/jwh.2018.7084. [DOI] [PubMed] [Google Scholar]
- 47.Harada T, Momoeda M, Taketani Y, Aso T, Fukunaga M, Hagino H, et al. Dienogest is as effective as intranasal buserelin acetate for the relief of pain symptoms associated with endometriosis--a randomized, double-blind, multicenter, controlled trial. Fertil Steril. 2009;91:675–681. doi: 10.1016/j.fertnstert.2007.12.080. [DOI] [PubMed] [Google Scholar]
- 48.Kohler G, Faustmann TA, Gerlinger C, Seitz C, Mueck AO. A dose-ranging study to determine the efficacy and safety of 1, 2, and 4mg of dienogest daily for endometriosis. Int J Gynaecol Obstet. 2010;108:21–25. doi: 10.1016/j.ijgo.2009.08.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed are available according to Bayer’s policy on sharing clinical study data (https://www.clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Bayer.aspx).