Abstract
A murine model of intratracheally induced histoplasmosis was used to evaluate a new triazole antifungal agent, Schering (SCH) 56592, for treatment of histoplasmosis. MICs were determined for SCH 56592, amphotericin B, and itraconazole by testing yeast-phase isolates from 20 patients by a macrobroth dilution method. The MICs at which 90% of the isolates are inhibited were for 0.019 μg/ml for SCH 56592, 0.5 μg/ml for amphotericin B, and ≤0.019 μg/ml for itraconazole. Survival studies were done on groups of 10 B6C3F1 mice with a lethal inoculum of 105. All mice receiving 5, 1, or 0.25 mg of SCH 56592 per kg of body weight per day, 2.5 mg of amphotericin B per kg every other day (qod), or 75 mg of itraconazole per kg per day survived to day 29. Only 44% of mice receiving 5 mg of itraconazole/kg/day survived to day 29. Fungal burden studies done in similar groups of mice with a sublethal inoculum of 104 showed a reduction in CFUs and Histoplasma antigen levels in lung and spleen tissue in animals treated with 2 mg of amphotericin B/kg qod, 1 mg of SCH 56592/kg/day, and 75 mg of itraconazole/kg/day, but not in those treated with lower doses of the study drugs (0.2 mg of amphotericin B/kg qod, 0.1 mg of SCH 56592/kg/day, or 10 mg of itraconazole/kg/day). Serum drug concentrations were measured 3 and 24 h after the last dose in mice (groups of five to seven mice), each treated for 7 days with SCH 56592 (10 and 1 mg/kg/day) and itraconazole (75 and 10 mg/kg/day). Mean levels measured by bioassay were as follows: SCH 56592, 10 mg/kg/day (2.15 μg/ml at 3 h and 0.35 μg/ml at 24 h); SCH 56592, 1 mg/kg/day (0.54 μg/ml at 3 h and none detected at 24 h); itraconazole, 75 mg/kg/day (22.53 μg/ml at 3 h and none detected at 24 h); itraconazole, 10 mg/kg/day (1.33 μg/ml at 3 h and none detected at 24 h). Confirmatory results were obtained by high-pressure liquid chromatography assay. These studies show SCH 56592 to be a promising candidate for studies of treatment of histoplasmosis in humans.
Histoplasmosis is a common infection in areas of the United States and Latin America where it is endemic. Histoplasmosis causes progressive infection in immunocompromised individuals and those with underlying chronic lung disease. Treatment is indicated in patients with chronic pulmonary or disseminated histoplasmosis and in those with more severe forms of acute pulmonary illnesses following primary infection. Itraconazole offers an effective alternative to amphotericin B for treatment of patients with mild to moderately severe manifestations of histoplasmosis and for completion of therapy in those who have improved following a short course of amphotericin B (5, 21, 22).
Itraconazole’s pharmacokinetic and drug interaction profiles limit its usefulness in certain situations. Absorption is variable in patients with AIDS, leading to treatment failures because of the inability to achieve therapeutic concentrations in blood in some patients (21). Medications which impair itraconazole solubilization and absorption by reduction of gastric acidity (17) or which accelerate hepatic metabolism by stimulation of hepatic cytochrome P450 (CYP 3A4) enzymes (19) reduce blood itraconazole levels. Itraconazole also inhibits CYP 3A4 enzymes, delaying the metabolism and causing adverse effects of many other medications, complicating its use in patients with other medical problems.
Fluconazole offers pharmacological advantages over itraconazole but is less active in vitro and less effective for treatment (24). Fluconazole is at least 50 times less active than itraconazole in vitro, with MICs at which 90% of the isolates are inhibited (MIC90s) of 1.25 and ≤0.019 μg/ml, respectively (24). In one study, 34% of patients relapsed while receiving fluconazole for chronic maintenance therapy of disseminated histoplasmosis (24). Resistance to fluconazole developed in about half of the isolates from patients who failed induction therapy or relapsed during chronic maintenance therapy (23). Oral agents with improved antifungal activity and more favorable pharmacokinetics and drug interaction profiles are needed for the treatment of histoplasmosis.
Studies of experimentally induced histoplasmosis in mice have been useful for identifying antifungal agents for trials in humans. Itraconazole at dosages of 10 to 25 mg/kg of body weight/day was as effective as amphotericin B at 1 mg/kg/day at preventing death (100% survival) and more effective than comparable doses of fluconazole (70 to 80% survival) (14). Several other agents have been shown to be effective in animal models but have not been studied in humans (3, 8, 9).
A new triazole antifungal agent, Schering (SCH) 56592, is highly active in vitro against a broad spectrum of fungal pathogens. SCH 56592 was ≥200-fold more active than fluconazole and ≥50-fold more active than itraconazole at reducing fungal burden and 20-fold more effective at preventing mortality in an intravenous murine model of coccidioidomycosis (12). In an intranasal inoculation model of blastomycosis, SCH 56592 at 25 mg/kg/day and amphotericin B at 1 mg/kg/day were highly effective at preventing mortality and sterilizing lung tissue while itraconazole at 150 mg/kg/day showed poor survival results and was unable to lower colony counts below 104 CFU/lung (18). Interestingly, although SCH 56592 failed to achieve detectable levels in the cerebrospinal fluid, both SCH 56592 and fluconazole, at doses of 80 mg/kg/day, were equally effective at reducing the fungal burden in the cerebrospinal fluid (15). SCH 56592 was more effective than itraconazole at preventing mortality in a murine model of pulmonary aspergillosis and more effective than fluconazole in an intravenous model of candidiasis (2). Excellent bioavailability has been demonstrated in several animal species including mice (13) and humans (11). Negligible amounts of unmetabolized drug are found in the urine, suggesting that the major route of elimination is metabolic (Schering-Plough Research Institute, Kenilworth, N.J.). In this report, we compare the activities of SCH 56592, itraconazole, and amphotericin B in vitro and in a pulmonary challenge model of histoplasmosis.
MATERIALS AND METHODS
Antifungal susceptibility testing.
Suspensions of 20 clinical isolates of Histoplasma capsulatum var. capsulatum yeasts that were grown for 4 days on brain heart infusion (BHI) agar containing 5% sheep blood were adjusted to equal a McFarland standard of 5 at 530 nm. Each suspension was diluted (1:100) in RPMI 1640 medium and added to tubes containing a series of doubling drug dilutions. Itraconazole (Janssen Pharmaceutica, Beerse, Belgium) was diluted in polyethylene glycol 200. SCH 56592 (Schering-Plough Research Institute) and amphotericin B (Bristol-Myers Squibb, Princeton, N.J.) were diluted in dimethyl sulfoxide. Macrobroth suspensions were incubated at 37°C, and results were determined at 120 to 144 h by visual inspection. Candida parapsilosis ATCC 90018 was used as a control to ensure that the drug activity of the dilutions fell in the expected range. The MICs for the azoles were defined as the dilutions at which the turbidity was equal to or less than that of an 80% dilution of the no-drug control, and the MIC for amphotericin B was defined as the lowest drug dilution with no visible growth, as recommended by the National Committee for Clinical Laboratory Standards (NCCLS) (20).
Preparation of H. capsulatum yeast inoculum for animal studies.
The yeast phase of a single clinical isolate, IU-CT, of H. capsulatum was grown in HMM medium (25) in a 37°C incubator with shaking at 150 rpm for 48 h. The yeast culture was centrifuged and washed with Hanks’ balanced salt solution containing 20 mM HEPES. The inoculum was adjusted with a hemacytometer. The MICs for this isolate were 0.5 μg/ml for amphotericin B, 0.002 μg/ml for itraconazole, and 0.0095 μg/ml for SCH 56592.
Intratracheal mouse inoculation model.
Six-week-old B6C3F1 mice (Harlan Sprague Dawley) were anesthetized with 4.5% halothane at an oxygen flow rate of 0.9 liter/min. A 20-gauge, 1.25-inch Angiocath (Becton Dickinson) was passed through the mouth into the trachea, and 25 μl of the H. capsulatum inoculum was administered by a stylet passed to the bifurcation of the trachea (7, 10).
Survival following lethal inoculum.
Mice received a lethal inoculum of 105 H. capsulatum yeasts intratracheally. Treatment began 4 days after infection and continued for 10 days. Mice received amphotericin B (Fungizone) at 2.5 mg/kg intraperitoneally every other day (qod). Itraconazole (10-mg/ml solution in hydroxypropyl-β-cyclodextrin, gift of Janssen Pharmaceutica) was given once daily by gavage at 75 and 5 mg/kg/day. SCH 56592 was suspended in 0.4% methylcellulose and given by gavage at 5, 1, and 0.25 mg/kg/day. Control mice were treated with 0.4% methylcellulose alone. Mice were monitored for 1 month at which time survivors were sacrificed.
Fungal burden assessment following sublethal infection.
Treatment began 4 days after inoculation with 104 H. capsulatum yeasts and continued for 10 days. Mice received amphotericin B at 2.0 and 0.2 mg/kg qod, itraconazole at 75 and 10 mg/kg/day, and SCH 56592 at 1 and 0.1 mg/kg/day. Control mice received 0.4% methylcellulose alone. Approximately 24 h after the last dose of antifungal drug, mice were sacrificed, and lungs and spleens were removed aseptically. Organs were weighed and then ground in Ten Broeck tissue grinders containing 2.0 ml of RPMI 1640 medium. Homogenates were diluted and plated on BHI agar containing 10% sheep blood. Plates were incubated for 10 days at 30°C, and colony counts were determined.
A 0.1-ml aliquot of the 2.0 ml of undiluted homogenate sample was cultured, representing a detection limit of 20 CFU/organ. Quantitative culture data were expressed as CFU per gram by dividing the CFU per organ by the organ weights, ranging from about 0.171 to 0.357 g for lungs and 0.079 to 0.202 g for spleens for treated versus untreated mice, respectively. Histoplasma antigen was measured in organ homogenates by enzyme immunoassay (EIA) (6). Dilutions of the organ homogenates (1:10 for spleen and 1:100 for lung) were made to fall within the working range of the assay. The EIA results are expressed as EIA units (EU) and were determined by dividing the mean value obtained for each organ by 1.5 times the mean value of the negative controls. Results of ≥1.0 are considered positive.
Pharmacokinetics of itraconazole and SCH 56592.
The mice in the groups of five to seven healthy mice were each given itraconazole at 75 or 10 mg/kg/day or SCH 56592 at 10 or 1 mg/kg/day for 7 days by gavage. Animals were sacrificed, and blood was collected 3 and 24 h after the last dose to determine steady-state peak and trough serum drug concentrations by using a bioassay methodology (1). Serum samples from animals were run in duplicate against the study drugs at concentrations ranging from 20 to 0.62 μg/ml for itraconazole and 2.5 to 0.155 μg/ml for SCH 56592. Zones of inhibition for each pair were determined in duplicate, and the mean of those four readings was compared to the standard curve to calculate serum drug concentrations. When no zone of inhibition was observable, results were reported as none detected. High-pressure liquid chromatography (HPLC) analysis was performed on these same samples to detect concentrations below the lower limit of detection of the bioassay (0.62 μg/ml for itraconazole and 0.155 μg/ml for SCH 56592). The detection limit for the HPLC assay was 50 ng for both itraconazole and SCH 56592. HPLC results for itraconazole represent the parent compound only, not the hydroxy-itraconazole metabolite, and thus underestimate the total microbiologically active compound.
Statistical analysis.
A one-way analysis of variance was performed on the ranks of the antigen levels and quantitative cultures (4). Pairwise comparisons of each treatment group to the control group were adjusted by Dunnett’s multiple comparison procedure. Survival times among the treatment groups were compared by a Wilcoxon test for survival analysis. An overall significance level of alpha equals 0.05 was used to test all hypotheses.
RESULTS
Antifungal susceptibility.
MICs were determined for SCH 56592, itraconazole, and amphotericin B by testing 20 patient isolates of H. capsulatum following NCCLS guidelines for yeasts with modifications made to suit H. capsulatum (16). The median MIC for SCH 56592 was 0.012 μg/ml, with a range of ≤0.0047 to 0.019 μg/ml and a MIC90 of 0.019 μg/ml. For this experiment, 0.019 μg/ml was the lowest concentration of itraconazole tested, and the median MIC and MIC90 were both ≤0.019 μg/ml. The median MIC for amphotericin B was 0.5 μg/ml, with a range of 0.25 to 1.0 μg/ml and a MIC90 of 0.5 μg/ml (Fig. 1). In a subsequent experiment, MIC testing was performed for 12 of these isolates with lower concentrations of itraconazole. MICs ranged from 0.002 to 0.008 μg/ml, with a median of 0.004 μg/ml and an MIC90 of 0.008 μg/ml.
FIG. 1.
MICs of amphotericin B (Am B), SCH 56592 (Schering), and itraconazole (Itra) for 20 isolates of H. capsulatum. The lowest concentrations tested for the antifungal agents were as follows: amphotericin B, 0.031 μg/ml; itraconazole, 0.019 μg/ml; and SCH 56592, 0.0047 μg/ml.
Survival following a lethal inoculum.
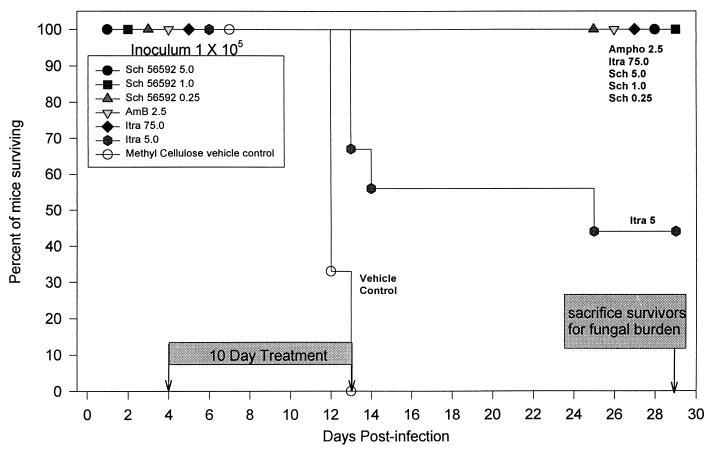
Survival studies used a lethal inoculum of 105 yeasts. All mice receiving SCH 56592 at 5, 1, and 0.25 mg/kg/day, itraconazole at 75 mg/kg/day, and amphotericin B at 2.5 mg/kg qod survived to 4 weeks (Fig. 2). At day 13, mice receiving itraconazole at 5 mg/kg/day began to die. Only 44% survived to day 29. No mice receiving vehicle alone survived past day 13. Wilcoxon’s test for survival analysis showed that of these survival curves, there was a significant difference from the control curve (P < 0.0001). In a second experiment, identical results were observed with amphotericin B at 5 mg/kg qod and SCH 56592 at 5 mg/kg/day: all mice receiving these regimens survived until day 29 when they were sacrificed. Results with itraconazole at 75 mg/kg/day differed somewhat from those seen in the first experiment. In the experiment shown in Fig. 2, all animals receiving 75 mg of itraconazole/kg/day survived until the conclusion of the experiment, whereas in this second experiment, 3 of the 10 receiving 75 mg of itraconazole/kg/day died, 2 on day 6 and 1 on day 10. The deaths of the two animals that died on day 6 occurred earlier than the deaths of the untreated controls (all control animals died between days 10 and 13) and were thought to have resulted from trauma during gavage rather than histoplasmosis. There were no gavage-related deaths in the experiment shown in Fig. 2.
FIG. 2.
Survival following intratracheal infection with 105 H. capsulatum yeasts. Therapy was given from days 4 to 13. The no-drug control group received daily gavage with the methylcellulose vehicle used to dilute SCH 56592. There were nine animals in each group. Survivors were sacrificed on day 29.
Fungal burden analysis at day 29 in mice that survived following infection with a lethal inoculum (105).
Mice that survived challenge with 105 yeasts shown in Fig. 2 were sacrificed 15 days after completion of antifungal therapy to determine if 10 days of treatment had sterilized the tissues. The following groups were included in this analysis: mice given amphotericin B at 2.5 mg/kg qod, itraconazole at 75 and 5 mg/kg/day, and SCH 56592 at 5 mg/kg/day. Untreated animals could not be included as controls because they all died before day 29. Amphotericin B at 2.5 mg/kg qod had the greatest effect on fungal burden: median of 0 CFU/g in the lung and spleen tissues. Cultures were sterile in eight of nine (89.0%) lung tissue samples and in seven of nine (77.8%) spleen tissue samples. Mice treated with SCH 56592 at 5 mg/kg/day had median CFU per gram in the lung of 1.11 × 104 and spleen of 1.69 × 104 (Table 1). For mice treated with 75 mg of itraconazole/kg/day, median counts (in CFU per gram) were 1.34 × 104 in the lung and 3.88 × 104 in the spleen, while those in the four mice which survived to day 29 in the group given itraconazole at 5 mg/kg/day were 2.31 × 105 in the lung and 5.06 × 105 in the spleen. The median colony count in the lung in the group given amphotericin B at 2.5 mg/kg qod was lower than that in the group given itraconazole at 75 or 5 mg/kg/day (P < 0.05 for each). In the spleen, the median colony count in the group given amphotericin B at 2.5 mg/kg qod was lower than those for itraconazole given at 75 and 5 mg/kg/day (P < 0.05). The group given SCH 56592 at 5 mg/kg/day was not statistically significantly different from the group given amphotericin B at 2.5 mg/kg qod.
TABLE 1.
Antigen and culture results from fungal burden experiment in animals remaining in a survival study that were sacrificed at day 29 following challenge with a 105 inoculum
| Regimena (no. of mice) | Median fungal burden (mean ± SD)
|
|||
|---|---|---|---|---|
| Antigen level (EU)
|
Quantitative culture (CFU/g of organ)
|
|||
| Lung | Spleen | Lung | Spleen | |
| Ampho 2.5 (n = 9) | 2.7 (2.9 ± 1.4) | 1.2 (1.5 ± 1.2) | 0 (1.07 × 101 ± 3.22 × 101) | 0 (3.39 × 101 ± 6.75 × 101) |
| Itra 75 (n = 9) | 1.0 (2.0 ± 1.6) | 5.8b (4.8 ± 2.2) | 1.34 × 104b (1.41 × 104 ± 4.72 × 103) | 3.99 × 104b (3.99 × 104 ± 1.09 × 104) |
| Itra 5.0 (n = 4) | 7.7b (7.9 ± 0.8) | 7.1b (7.3 ± 0.5) | 2.31 × 105b (1.38 × 105 ± 2.05 × 105) | 5.06 × 105 (2.86 × 105 ± 3.43 × 105) |
| SCH 56592 5.0 (n = 9) | 4.0 (3.7 ± 0.9) | 1.7 (1.7 ± 0.6) | 1.11 × 104 (8.33 × 103 ± 5.10 × 103) | 1.69 × 104 (1.51 × 104 ± 6.91 × 103) |
Abbreviations: Ampho 2.5, amphotericin B at 2.5 mg/kg qod; Itra 75 and 5.0, itraconazole at 75 and 5 mg/kg/day, respectively; SCH 56592 5.0, SCH 56592 at 5 mg/kg/day.
Statistically significantly different from (higher than) the value for the group of mice given amphotericin B at 2.5 mg/kg qod (P < 0.05).
The effects of treatments on antigen levels were also studied in survivors following lethal challenge. Mice treated with amphotericin B at 2.5 mg/kg qod had median antigen levels of 2.7 EU in the lung and 1.2 EU in the spleen, those treated with SCH 56592 at 5 mg/kg/day had median antigen levels in the lung of 4.0 EU and in the spleen of 1.7 EU, while those treated with itraconazole at 75 mg/kg/day had median antigen levels in the lung of 1.0 EU and in the spleen of 5.8 EU. Antigen levels in the lungs and spleen were higher, 7.7 and 7.1 EU, respectively, for mice treated with itraconazole at 5 mg/kg/day than with amphotericin B, 2.7 and 1.2 EU, respectively (P < 0.05) (Table 1). Antigen levels in the spleens of mice treated with itraconazole at 75 mg/kg/day (5.8 EU) were higher than in those treated with amphotericin B at 2.5 mg/kg qod (1.2 EU) (P < 0.05) and with SCH 56592 at a dose of 5 mg/kg/day (1.7 EU) (P < 0.05).
Fungal burden analysis at day 14 following infection with a 104 inoculum.
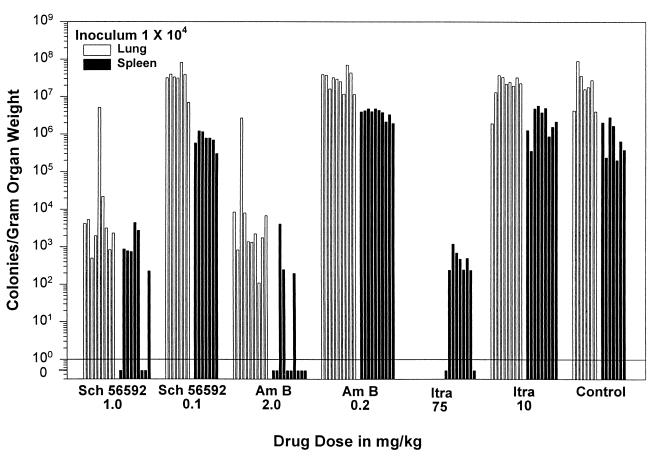
Treatment with amphotericin B at 2 mg/kg qod, itraconazole at 75 mg/kg/day, and SCH 56592 at 1.0 mg/kg/day reduced fungal burden in the lungs and spleens of mice infected with an inoculum of 104 (Table 2). The group receiving amphotericin B at 2.0 mg/kg qod had median quantitative culture results of 1.97 × 103 CFU/g of organ in the lung and 0 CFU/g in the spleen, with a P of <0.0001 for both compared to untreated controls, which had median quantitative culture results of 1.81 × 107 CFU/g in the lung and 6.37 × 105 CFU/g in the spleen (Fig. 3). The mice receiving 75 mg of itraconazole/kg/day had a median of 247 CFU/g in the spleen, with a P of <0.0001 compared to vehicle controls. The lung cultures were not evaluable because of overdilution of the homogenates (the lowest dilution tested, 1:1,000, yielded negative results). SCH 56592 administered at 1 mg/kg/day resulted in median values of 3.15 × 103 CFU/g in the lung and 741 CFU/g in the spleen, with a P of <0.0001 for each compared to the vehicle control animals.
TABLE 2.
Antigen and culture results in fungal burden experiment in animals sacrificed at day 14 following challenge with a 104 inoculum
| Regimena (no. of mice) | Median fungal burden (mean ± SD)
|
|||
|---|---|---|---|---|
| Antigen level (EU)
|
Quantitative culture (CFU/g of organ)
|
|||
| Lung | Spleen | Lung | Spleen | |
| Ampho 2.0 (n = 10) | 5.0b (6.4 ± 4.7) | 2.5b (4.0 ± 4.1) | 1.97 × 103b (2.68 × 105 ± 8.37 × 105) | 0b (4.51 × 102 ± 1.27 × 103) |
| Ampho 0.2 (n = 10) | 22.7 (22.2 ± 1.6) | 20.0 (19.7 ± 1.5) | 3.09 × 107 (3.15 × 107 ± 1.74 × 107) | >3.94 × 106c (3.68 × 106 ± 9.81 × 105) |
| Itra 75 (n = 9) | 5.5b (5.3 ± 2.1) | 1.5b (1.7 ± 0.6) | Not evaluable | 2.47 × 102b (3.99 × 102 ± 3.75 × 102) |
| Itra 10 (n = 9) | 24.8 (24.6 ± 2.3) | 19.8 (18.8 ± 3.3) | 2.25 × 107 (2.27 × 107 ± 1.08 × 107) | >2.13 × 106c (2.81 × 106 ± 1.99 × 106) |
| SCH 56592 1.0 (n = 9) | 5.6b (6.9 ± 3.1) | 1.5b (2.3 ± 1.2) | 3.15 × 103b (5.68 × 105 ± 1.69 × 106) | 7.41 × 102b (1.08 × 103 ± 1.51 × 103) |
| SCH 56592 0.1 (n = 7) | 21.2 (22.2 ± 1.9) | 20.0 (19.1 ± 2.5) | 3.31 × 107 (3.29 × 107 ± 2.41 × 107) | 7.70 × 105 (6.76 × 105 ± 3.93 × 105) |
| None (Untreated control) (n = 8) | 23.9 (23.8 ± 2.6) | 19.7 (19.7 ± 1.2) | 1.81 × 107 (2.79 × 107 ± 2.97 × 107) | 6.37 × 105 (1.14 × 106 ± 1.03 × 106) |
Abbreviations: Ampho 2.0 and 0.2, amphotericin B at 2.0 and 0.2 mg/kg qod, respectively; Itra 75 and 10, itraconazole at 75 and 10 mg/kg/day, respectively; SCH 56592 1.0 and 0.1, SCH 56592 at 1.0 and 0.1 mg/kg/day, respectively.
Statistically significantly different from (lower than) the value for the untreated controls (P < 0.0001).
Statistically significantly different from (greater than) the value for the untreated controls (P < 0.05).
FIG. 3.
Quantitative culture results of lung and spleen tissues at day 14 in mice receiving a sublethal inoculum (104). Mice were sacrificed 24 h after the last dose of therapy. Each bar represents one animal. There were 7 to 10 mice in each study group. The minimum detection limit was 20 CFU/organ, representing about 50 to 250 CFU/g of tissue. In mice receiving itraconazole at 75 mg/kg/day, the lung cultures were not evaluable because of overdilution of the homogenates; the lowest dilution tested, 1:1,000, yielded negative results. The groups given amphotericin (Am B) at 0.2 mg/kg qod and itraconazole (Itra) at 10 mg/kg/day had some individual platings of the spleen that were too numerous to count. Thus, for statistical analysis, results for animals with confluent growth were assigned a colony count of 500/plate, based on the largest number of colonies which were individually counted on evaluable plates.
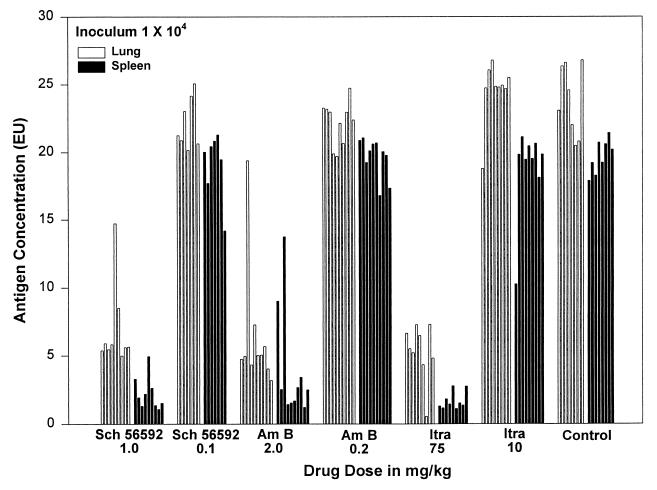
Antigen levels were also lower in the treated animals than in the control animals which received the vehicle alone (Fig. 4). Animals receiving amphotericin B at 2 mg/kg qod had median antigen levels of 5.0 EU in the lung and 2.5 EU in the spleen, compared to 23.9 and 19.7 EU, respectively, in the controls (P < 0.0001 for each) (Table 2). At 75 mg/kg/day, itraconazole resulted in median values of 5.5 and 1.5 EU in the lung and spleen, respectively (P < 0.0001 for each in comparison to the vehicle controls). Treatment with SCH 56592 at 1 mg/kg/day resulted in median antigen levels of 5.6 EU in the lung and 1.9 EU in the spleen (P < 0.0001 for each compared to control animals).
FIG. 4.
Quantitative Histoplasma antigen levels in lung and spleen tissues of mice sacrificed on day 14 following sublethal infection (104 inoculum). Antigen levels in homogenized tissues measured by EIA are expressed as EIA units (EU), and results above 1.0 are positive. Lung homogenates were diluted 1:100, and spleen homogenates were diluted 1:10. Each bar represents one animal, and the sequence of results is identical to that shown for cultures in Fig. 3. Drug abbreviations: Am B, amphotericin B; Itra, itraconazole.
The lower drug doses studied were not effective at reducing fungal burden as assessed both by culture and by antigen in both lungs and spleens. Mice treated with amphotericin B at 0.2 mg/kg qod had a median of 3.09 × 107 CFU/g in the lung (P = 0.5915) and >3.94 × 106 in the spleen (P = 0.0009) (Table 2). Those treated with itraconazole at 10 mg/kg/day had a median of 2.25 × 107 (P = 1.0) and >2.13 × 106 CFU/g (P = 0.03) in the lung and spleen, respectively. The quantitative cultures in the spleens from the groups given amphotericin B at 0.2 mg/kg qod and itraconazole at 10 mg/kg/day were statistically significantly higher than the untreated controls (6.37 × 105 CFU/g) (Table 2). Animals treated with SCH 56592 at 0.1 mg/kg/day had a median CFU/gram of organ of 3.31 × 107 in the lung (P = 0.2601) and 7.70 × 105 (P = 0.9988) in the spleen compared to untreated controls. The median antigen levels for the low dose groups of all three study drugs ranged from 21.2 to 24.9 EU in the lung (versus 23.9 EU in the untreated controls) and 19.8 to 20.1 EU in the spleen (versus 19.7 EU in the untreated control animals) (P > 0.05 for the lung and spleen EU in all three treatment groups compared to the control values).
In a second experiment evaluating the effect of therapy on fungal burden using amphotericin B at 2.0 and 0.2 mg/kg qod, itraconazole at 10 and 1 mg/kg/day, and SCH 56592 at 1, 0.1, and 0.01 mg/kg/day for 10 days, amphotericin B at 2 mg/kg qod, and SCH 56592 at 1 mg/kg/day markedly reduced the numbers of organisms and antigen concentrations in lung and spleen tissues (Table 3). Spleen cultures were negative in five of the seven mice given amphotericin B at 2 mg/kg qod and six of the seven mice that received SCH 56592 at 1 mg/kg/day. Amphotericin B at 0.2 mg/kg qod, SCH 56592 at 0.01 mg/kg/day, and itraconazole at 10 and 1 mg/kg/day had no effect on fungal burden. In this experiment, amphotericin B at 0.2 mg/kg qod and SCH 56592 at 0.1 mg/kg/day also reduced the organism burden in the spleen by culture and antigen concentration from those of untreated control animals (Table 3).
TABLE 3.
Antigen and culture results in a second fungal burden experiment in animals sacrificed at day 14 following challenge with a 104 inoculum
| Regimena (no. of mice) | Median fungal burden (mean ± SD)
|
|||
|---|---|---|---|---|
| Antigen level (EU)
|
Quantitative culture (CFU/g of organ)
|
|||
| Lung | Spleen | Lung | Spleen | |
| Ampho 2.0 (n = 7) | 3.1b (3.6 ± 1.1) | 1.2b (1.4 ± 0.4) | 1.03 × 103 (2.66 × 103 ± 4.44 × 103) | 0b (3.17 × 102 ± 6.65 × 102) |
| Ampho 0.2 (n = 7) | 16.8 (16.2 ± 1.1) | 14.4 (14.4 ± 1.4) | 2.55 × 106 (2.60 × 106 ± 4.72 × 105) | 2.34 × 105 (3.19 × 105 ± 2.63 × 105) |
| Itra 10 (n = 6) | 16.3 (16.7 ± 0.8) | 16.0 (15.7 ± 0.8) | 2.80 × 106 (2.83 × 106 ± 1.47 × 105) | 3.20 × 105 (4.21 × 105 ± 3.11 × 105) |
| Itra 1.0 (n = 7) | 16.9 (17.0 ± 0.5) | 16.3 (16.5 ± 0.8) | 2.54 × 106 (2.53 × 106 ± 1.08 × 105) | 1.15 × 106 (9.75 × 105 ± 5.05 × 105) |
| SCH 56592 1.0 (n = 7) | 2.6b (2.6 ± 0.5) | 1.1b (1.1 ± 0.1) | 1.14 × 102b (1.15 × 102 ± 1.59 × 102) | 0b (3.7 × 101 ± 9.8 × 101) |
| SCH 56592 0.1 (n = 7) | 17.5 (16.2 ± 2.9) | 15.5 (13.9 ± 5.0) | 3.42 × 106 (3.16 × 106 ± 8.74 × 105) | 1.13 × 105b (1.38 × 105 ± 9.62 × 104) |
| SCH 56592 0.1 (n = 7) | 19.0 (18.7 ± 1.1) | 18.2 (18.4 ± 0.6) | 2.66 × 106 (2.65 × 106 ± 3.62 × 105) | 1.03 × 106 (1.43 × 106 ± 9.05 × 105) |
| None (untreated control) (n = 7) | 17.7 (18.0 ± 1.3) | 17.1 (17.1 ± 0.7) | 2.48 × 106 (2.35 × 106 ± 9.50 × 105) | 2.51 × 106 (2.62 × 106 ± 1.15 × 106) |
Abbreviations: Ampho 2.0 and 0.2, amphotericin B at 2.0 and 0.2 mg/kg qod, respectively; Itra 10 and 1.0, itraconazole at 10 and 1.0 mg/kg/day, respectively; SCH 56592 1.0 and 0.1, SCH 56592 at 1.0 and 0.1 mg/kg/day, respectively.
Statistically significantly different from (lower than) the value for the untreated controls (P < 0.0001).
Pharmacokinetics.
Results of drug concentrations in blood 3 and 24 h after the last dose were determined in groups of mice that received itraconazole or SCH 56592 for 7 days before sacrifice. Itraconazole at 75 mg/kg/day produced the highest mean peak serum drug concentration, 22.53 μg/ml by bioassay and 7.53 μg/ml by HPLC, but the levels were not sustained throughout the 24-h dose interval, resulting in none detected by either method at 24 h (Table 4). SCH 56592 at 10 mg/kg/day produced higher peak concentrations than itraconazole at 10 mg/kg/day, 2.15 μg/ml by bioassay and 2.92 μg/ml by HPLC versus 1.33 μg/ml by bioassay and 0.25 μg/ml by HPLC, respectively. SCH 56592 at 10 mg/kg/day could still be detected 24 h following the last gavage treatment, 0.35 μg/ml by bioassay and 0.33 μg/ml by HPLC, whereas itraconazole at 10 mg/kg/day had undetectable levels by both methods at 24 h. SCH 56592 given at a dose of 1 mg/kg/day produced detectable peak serum drug concentrations, 0.54 μg/ml by bioassay and 0.70 μg/ml by HPLC, and was still detected at 24 h (0.023 μg/ml), but only by HPLC.
TABLE 4.
Mean SCH 56592 and itraconazole concentrations in serum at day 7 following once-daily therapya
| Method and sampling time | Drug concn in serum (mean ± SD)
|
|||
|---|---|---|---|---|
| Itraconazole
|
SCH 56592
|
|||
| 75 mg/kg/day | 10 mg/kg/day | 10 mg/kg/day | 1 mg/kg/day | |
| Bioassay | ||||
| 3 h | l22.53 ± 6.26 (n = 7) | 1.33 ± 0.65 (n = 7) | 2.15 ± 0.28 (n = 6) | 0.54 ± 0.13 (n = 5) |
| 24 h | 0 (n = 7) | 0 (n = 7) | 0.35 ± 0.15 (n = 6) | 0 (n = 6) |
| HPLC | ||||
| 3 h | 7.53 ± 2.08 (n = 6) | 0.25 ± 0.14 (n = 7) | 2.92 ± 0.35b (n = 3) | 0.70 ± 0.09b (n = 3) |
| 24 h | 0 (n = 7) | 0 (n = 7) | 0.33 ± 0.06b (n = 3) | 0.02 ± 0.04b (n = 3) |
The detection limits for the different drugs and tests were as follows: for bioassay, 0.62 μg/ml for itraconazole and 0.155 μg/ml for SCH 56592; for HPLC, 50 ng/ml for itraconazole and SCH 56592.
Samples were pooled into three separate pools in these groups.
DISCUSSION
This murine model of intratracheally induced histoplasmosis resembles natural infection following inhalation exposure. Animals develop a diffuse pulmonary infection followed by hematogenous dissemination to the liver and spleen (7). The severity of the infection is determined by the size of the inoculum and the immune status of the host. Immunocompetent mice infected with 103 yeasts recover spontaneously and develop immunity to H. capsulatum, while those receiving larger inocula experience progressive infection. The 104 dose was chosen to measure fungal burden so that the untreated control mice would be available at day 14, when the fungal burden peaks in the lungs and spleen in this inhalation model.
SCH 56592 demonstrated greater in vitro activity against H. capsulatum than did amphotericin B. The MIC90 for SCH 56592 was 0.019 μg/ml compared to 0.5 μg/ml for amphotericin B. SCH 56592 was as effective as amphotericin B in preventing death following exposure to a lethal inoculum. All mice treated with 5, 1, and 0.25 mg of SCH 56592 per kg per day or with 2.5 mg of amphotericin B 2.5 per kg qod survived over a 29-day period of observation. Both drugs were equally effective at lowering fungal burden during the course of a sublethal infection following exposure to an inoculum of 104 organisms (Fig. 3 and 4 and Tables 2 and 3); moreover, no statistically significant difference was found in the ability of SCH 56592 to reduce tissue burden compared to amphotericin B in mice that survived to day 29 following a lethal challenge with 105 yeasts (Table 1). Whether a higher dose or longer course of SCH 56592 treatment would have sterilized the tissues is unknown. Amphotericin B at 2 mg/kg qod and SCH 56592 at 1 mg/kg/day markedly reduced the fungal burden in lung and spleen tissues, as measured by both quantitative culture and antigen detection in the sublethal challenge experiment with 104 yeasts. Amphotericin B and SCH 56592 at lower doses of 0.2 mg/kg qod and 0.1 mg/kg/day, respectively, did not reduce fungal burdens.
Although the MIC90s for itraconazole and SCH 56592 were similar, SCH 56592 was considerably more effective than itraconazole for the treatment of experimental Histoplasma infection. SCH 56592 at a dose of 0.25 mg/kg/day totally prevented mortality following lethal exposure; however, more than half the animals treated with itraconazole at 5 mg/kg/day died. SCH 56592 also was at least 10 times more effective than itraconazole in reducing fungal burdens in lung and spleen tissues following exposure to a lower inoculum. The concentrations of itraconazole in blood were somewhat lower than those of SCH 56592, and itraconazole appears to have been cleared more rapidly, perhaps contributing to the superior efficacy of SCH 56592 over itraconazole. Improved efficacy may have been demonstrated if itraconazole had been administered twice daily. Importantly, the hydroxypropyl-β-cyclodextrin oral solution formulation of itraconazole was used in this study and therefore solubility was not a factor. Itraconazole powder resuspended in polyethylene glycol 400 was even less effective than SCH 56592 for treatment of experimental blastomycosis, possibly because of the poorer solubility and absorption of that formulation (18). The murine model may favor SCH 56592 over itraconazole because of pharmacokinetic or other unknown factors.
In conclusion, SCH 56592 was as effective as amphotericin B and more effective than itraconazole in preventing death and as effective as amphotericin B in reducing fungal burdens in this model of intratracheally induced histoplasmosis. Assuming that it has a favorable toxicity profile, it is an excellent candidate for clinical trials for treatment of histoplasmosis in humans. Studies in immunocompromised animals with histoplasmosis are needed to support its use in patients with immunosuppressive disorders since SCH 56592 may not be as effective as amphotericin B in sterilizing the tissues. These studies are ongoing.
ACKNOWLEDGMENTS
This work was supported by a grant from Schering-Plough Research Institute and was done with a model developed in a project sponsored by The Department of Veterans’ Affairs.
REFERENCES
- 1.Bodet C A, III, Jorgensen J H, Drutz D J. Simplified bioassay method for measurement of flucytosine or ketoconazole. J Clin Microbiol. 1985;22:157–160. doi: 10.1128/jcm.22.2.157-160.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cacciapuoti A, Parmegiani R, Loebenberg D, Antonacci B, et al. Program and abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. Efficacy of SCH 56592 in pulmonary aspergillosis and candidiasis in mice, abstr. F66; p. 124. [Google Scholar]
- 3.Clemons K V, Stevens D A. Efficacy of the triazole D0870 in a murine model of systemic histoplasmosis. Antimicrob Agents Chemother. 1995;39:778–780. doi: 10.1128/AAC.39.3.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conover W J, Iman R L. Rank transformations as a bridge between parametric and nonparametric statistics. Am Stat. 1981;35:124–129. [Google Scholar]
- 5.Dismukes W E, Bradsher R W, Jr, Cloud G C, Kauffman C A, Chapman S W, George R B, Stevens D A, Girard W M, Bowles-Patton C NIAID Mycoses Study Group. Itraconazole therapy for blastomycosis and histoplasmosis. Am J Med. 1992;93:489–497. doi: 10.1016/0002-9343(92)90575-v. [DOI] [PubMed] [Google Scholar]
- 6.Durkin M M, Connolly P A, Wheat L J. Comparison of radioimmunoassay and enzyme-linked immunoassay methods for detection of Histoplasma capsulatum var. capsulatum antigen. J Clin Microbiol. 1997;35:2252–2255. doi: 10.1128/jcm.35.9.2252-2255.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fojtasek M F, Sherman M R, Garringer T, Blair R, Wheat L J, Schnizlein-Bick C T. Local immunity in lung-associated lymph nodes in a murine model of pulmonary histoplasmosis. Infect Immun. 1993;61:4607–4614. doi: 10.1128/iai.61.11.4607-4614.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hector R F, Zimmer B L, Pappagianis D. Evaluation of nikkomycins X and Z in murine models of coccidioidomycosis, histoplasmosis, and blastomycosis. Antimicrob Agents Chemother. 1990;34:587–593. doi: 10.1128/aac.34.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobayashi G S, Travis S J, Rinaldi M G, Medoff G. In vitro and in vivo activities of SCH 39304, fluconazole, and amphotericin B against Histoplasma capsulatum. Antimicrob Agents Chemother. 1990;34:524–528. doi: 10.1128/aac.34.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohler S, Blair R, Schnizlein-Bick C, Fojtasek M, Connolly-Stringfield P, Wheat J. Clearance of Histoplasma capsulatum variety capsulatum antigen is useful for monitoring treatment of experimental histoplasmosis. J Clin Lab Anal. 1994;8:1–3. doi: 10.1002/jcla.1860080102. [DOI] [PubMed] [Google Scholar]
- 11.Laughlin M, Pai S, Menon S, Nomeir A, Colucci R, Affrime M, Kosoglou T. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. SCH 56592: rising multiple-dose safety, tolerance, and pharmacokinetic evaluation in healthy volunteers, abstr. A87; p. 18. [Google Scholar]
- 12.Lutz J E, Clemons K V, Aristizabal B H, Stevens D A. Activity of the triazole SCH 56592 against disseminated murine coccidioidomycosis. Antimicrob Agents Chemother. 1997;41:1558–1561. doi: 10.1128/aac.41.7.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nomeir A, Kumari P, Hilbert M J, Loebenberg D, et al. Program and abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. Comparative pharmacokinetics of a new triazole antifungal agent SCH 56592, in mice, rats, rabbits, dogs and cynomolgus monkeys, abstr. F68; p. 124. [Google Scholar]
- 14.Pappagianis D, Zimmer B L, Theodoropoulos G, Plempel M, Hector R F. Therapeutic effect of the triazole bay R 3783 in mouse models of coccidioidomycosis, blastomycosis, and histoplasmosis. Antimicrob Agents Chemother. 1990;34:1132–1138. doi: 10.1128/aac.34.6.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perfect J R, Cox G M, Dodge R K, Schell W A. In vitro and in vivo efficacies of the azole SCH56592 against Cryptococcus neoformans. Antimicrob Agents Chemother. 1996;40:1910–1913. doi: 10.1128/aac.40.8.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smedema M, Connolly P, Wheat J. Abstracts of the 96th General Meeting of the American Society for Microbiology, 1996. Washington, D.C: American Society for Microbiology; 1996. Antifungal susceptibility testing on isolates of Histoplasma capsulatum obtained from a clinical trial evaluating fluconazole treatment in patients with AIDS, abstr. F65; p. 85. [Google Scholar]
- 17.Stein A G, Daneshmend T K, Warnock D W, Bhaskar N, Burke J, Hawkey C J. The effects of H2-receptor antagonists on the pharmacokinetics of itraconazole, a new oral antifungal. Br J Clin Pharmacol. 1989;27:105P–106P. [Google Scholar]
- 18.Sugar A M, Liu X P. In vitro and in vivo activities of SCH 56592 against Blastomyces dermatitidis. Antimicrob Agents Chemother. 1996;40:1314–1316. doi: 10.1128/aac.40.5.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tucker R M, Denning D W, Hanson L H, Rinaldi M G, Graybill J R, Sharkey P K, Pappagianis D, Stevens D A. Interaction of azoles with rifampin, phenytoin, and carbamazepine: in vitro and clinical observations. Clin Infect Dis. 1992;14:165–174. doi: 10.1093/clinids/14.1.165. [DOI] [PubMed] [Google Scholar]
- 20.Waitz J A, Bartlett M S, Ghannoum M A, Espinel-Ingroff A, Lancaster M V, Odds F C, Pfaller M A, Rex J H, Rinaldi M G, Walsh T J, Galgiani J N. Report M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. Reference method of broth dilution antifungal susceptibility testing of yeasts; approved standard; pp. 1–29. [Google Scholar]
- 21.Wheat J, Hafner R, Korzun A H, Limjoco M T, Spencer P, Larsen R A, Hecht F M, Powderly W AIDS Clinical Trial Group. Itraconazole treatment of disseminated histoplasmosis in patients with the acquired immunodeficiency syndrome. Am J Med. 1995;98:336–342. doi: 10.1016/s0002-9343(99)80311-8. [DOI] [PubMed] [Google Scholar]
- 22.Wheat J, Hafner R, Wulfson M, Spencer P, Squires K, Powderly W, Wong B, Rinaldi M, Saag M, Hamill R, Murphy R, Connolly-Stringfield P, Briggs N, Owens S NIAID Clinical Trials and Mycoses Study Group Collaborators. Prevention of relapse of histoplasmosis with itraconazole in patients with the acquired immunodeficiency syndrome. Ann Intern Med. 1993;118:610–616. doi: 10.7326/0003-4819-118-8-199304150-00006. [DOI] [PubMed] [Google Scholar]
- 23.Wheat J, Marichal P, Vanden Bossche H, Le Monte A, Connolly P. Hypothesis on the mechanism of resistance to fluconazole in Histoplasma capsulatum. Antimicrob Agents Chemother. 1997;41:410–414. doi: 10.1128/aac.41.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wheat J, Mawhinney S, Hafner R, McKinsey D, Chen D F, Korzun A, Shakan K J, Johnson P, Hamill R, Bamberger D, Pappas P, Stansell J, Koletar S, Squires K, Larsen R A, Cheung T, Hyslop N, Lai K K, Schneider D, Kauffman C, Saag M, Dismukes W, Powderly W. Treatment of histoplasmosis with fluconazole in patients with acquired immunodeficiency syndrome. Am J Med. 1997;103:223–232. doi: 10.1016/s0002-9343(97)00151-4. [DOI] [PubMed] [Google Scholar]
- 25.Worsham P L, Goldman W E. Quantitative plating of Histoplasma capsulatum without addition of conditioned medium or siderophores. J Med Vet Mycol. 1988;26:137–143. [PubMed] [Google Scholar]