Abstract
Background
Healthcare-associated coronavirus disease 2019 (COVID-19) has significant implications for patients, their companions and healthcare workers (HCWs). Controlling transmission in healthcare settings is critical to reduce deaths due to COVID-19.
Aim
To describe the epidemiology and characteristics of healthcare-associated COVID-19 outbreaks and outbreak-related cases.
Methods
The investigation data for each healthcare-associated outbreak that occurred between 15th January 2020 and 31st July 2021 in Taiwan were analysed retrospectively. Confirmed outbreak-associated cases were categorized as HCW cases, patient companion cases or patient cases, and the characteristics of the confirmed cases were compared between these categories.
Findings
In total, 54 healthcare-associated COVID-19 outbreaks including 512 confirmed cases were reported. The median number of affected cases per outbreak was six [interquartile range (IQR) 2–12], and the median outbreak duration was 12 days (IQR 4.3–17.0). Only 5.7% and 0.2% of all confirmed cases were partially and fully vaccinated, respectively. Most outbreaks (90%, 48/54) occurred in May and June 2021. HCW cases, companion cases and patient cases accounted for 19.5%, 41.2% and 39.3% of the total cases. Patient cases were significantly older (median age 72 years, IQR 61–83) and had higher 30-day all-cause mortality (37.4%) than HCW cases (median age 41 years, IQR 28–58, 0%) and companion cases (median age 52 years; IQR 42–62, 1%).
Conclusion
Healthcare-associated COVID-19 outbreaks have a critical impact on patients. Nevertheless, two-thirds of cases in the healthcare-associated outbreaks in this study comprised HCWs and companions. In order to effectively mitigate COVID-19 transmission in healthcare settings, multi-pronged infection prevention and control measures should be implemented and tailored for these three groups.
Keywords: Healthcare-associated infection, Outbreak, COVID-19
Introduction
During the coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), healthcare-associated transmission has emerged as a significant concern worldwide, accounting for up to 40% of all confirmed cases of COVID-19 according to early reports [1]. Healthcare-associated COVID-19 outbreaks pose significant threats to both patients and healthcare workers (HCWs) [[2], [3], [4], [5]], and place enormous pressure on the daily operation of healthcare systems. This underscores the importance of infection prevention and control (IPC) practices and mitigation measures to prevent healthcare-associated outbreaks and contain the spread of such outbreaks once they emerge in healthcare settings. However, prevention of healthcare-associated COVID-19 outbreaks is a unique challenge due to the infectivity of SARS-CoV-2 prior to the onset of symptoms [5,6]. Multi-faceted infection prevention measures should be implemented, such as symptom screening, universal masking, maintaining physical distances, appropriate contact tracing, quarantine practices and isolation of confirmed cases.
From the start of the pandemic in early 2020 until mid-May 2021, community transmission of COVID-19 remained at a very low level in Taiwan, with 100 cumulative confirmed domestic cases and three healthcare-associated COVID-19 outbreaks reported [[7], [8], [9]]. Subsequently, community transmission increased rapidly, and the Central Epidemic Command Centre (CECC) raised the nationwide epidemic alert to level 2 on 11th May 2021 and level 3 on 15th May 2021 [10]. Between 11th May 2021 and 31st July 2021, the cumulative number of confirmed domestic cases increased to >14,000, mainly in metropolitan areas in northern Taiwan, with the alpha variant representing the major circulating strain [11]. Thereafter, the number of healthcare-associated outbreaks increased concomitantly with widespread community transmission. The aims of this study were to describe the epidemiology of healthcare-associated COVID-19 outbreaks in Taiwan; compare the characteristics of infected HCWs, patients and their companions; and identify IPC practices.
Methods
Study design and setting
This was a nationwide retrospective population-based cohort study of all healthcare-associated COVID-19 outbreaks between 15th January 2020 and 31st July 2021 in Taiwanese hospitals, including 25 medical centres, 82 regional hospitals and 366 community hospitals. COVID-19 has been classified as a notifiable disease in Taiwan since 15th January 2020. Healthcare providers must report all suspected patients through the National Infectious Disease Reporting System (NIDRS) within 24 h, and collect respiratory specimens for testing of SARS-CoV-2 by reverse transcription polymerase chain reaction (RT-PCR). When a laboratory-confirmed case was identified in any hospital, public health departments, in collaboration with stakeholders, conducted a case investigation and contact tracing in accordance with the guidelines [7] established by the Taiwan Centers for Disease Control (CDC). When a healthcare-associated COVID-19 outbreak was identified in the initial investigation, the hospital immediately launched the contingency protocol according to the guidance [7] and under the supervision of the public health department to contain transmission within the hospital. Briefly, the contingency protocol comprises multi-disciplinary detailed investigations and containment measures such as periodic surveillance testing of all contact persons, closure of outbreak-affected units, isolation and quarantine of confirmed cases and close contacts, and environmental cleaning and disinfection. The end of a healthcare-associated COVID-19 outbreak was declared by the public health department when no further confirmed cases were identified for 14 days after the last confirmed case was isolated or discharged from the unit. Finally, an outbreak investigation report was completed by the public health department.
Ethics statement
The study protocol (No. TwCDCIRB109206) was reviewed and approved by the institutional review board (IRB) of Taiwan CDC. The IRB waived the requirement for informed consent due to the retrospective nature of the study.
Definitions
A confirmed case was defined as an individual with a positive RT-PCR test result for SARS-CoV-2. Confirmed cases were categorized into three groups: HCWs; patient companions; and patients who were seeking medical attention or hospitalized for diseases other than COVID-19, according to their diagnostic status. A healthcare-associated COVID-19 outbreak was defined as two or more infections with a likely epidemiological link, and the acquisition of at least one infection in the healthcare setting was assumed by the investigation team. A confirmed case was regarded as the primary case when the case was suspected to be the one that initiated the chain of healthcare-associated transmission. Other confirmed cases involved in the healthcare-associated outbreak with epidemiological links were deemed as secondary cases. The delayed period of identification for a symptomatic case was defined as the duration between the date of symptom onset and the date of the first positive RT-PCR test result. Thirty-day all-cause mortality was defined as death occurring within 30 days after the date of symptom onset, or the date of the first positive RT-PCR test result for asymptomatic patients. The outbreak duration was defined as the duration between the dates of the first positive RT-PCR test result in the primary case and the date of the first positive RT-PCR test result of the last confirmed case in the same outbreak. A person was deemed to be fully vaccinated 14 days after completion of the full series of a COVID-19 vaccine. A person was deemed to be partially vaccinated 14 days after receiving the first dose of the COVID-19 vaccine in a two-dose series.
Data collection
In this study, the demographics, characteristics and test results of confirmed cases of COVID-19 were obtained from NIDRS. Information regarding symptoms, occupation and extent of the outbreaks was retrieved from the outbreak investigation reports. The vaccination status of the confirmed cases was acquired from the National Immunization Information System [12]. The date of death was obtained from the investigation report or by linking the unique identification number of cases with the NIDRS, National Register of Deaths, and National Health Insurance databases.
Statistical analysis
Categorical variables were compared using Chi-squared test, and continuous variables were compared using parametric or non-parametric tests. Survival curves were estimated using the Kaplan–Meier method and compared with the log-rank test. Both univariate and multi-variate analyses were performed using the Cox regression model to determine hazard ratios and 95% confidence intervals for 30-day all-cause mortality. Statistical significance was set at P<0.05. Statistical analyses were performed using R Version 4.1.0 (R Core Team, R Foundation for Statistical Computing, Vienna, Austria).
Results
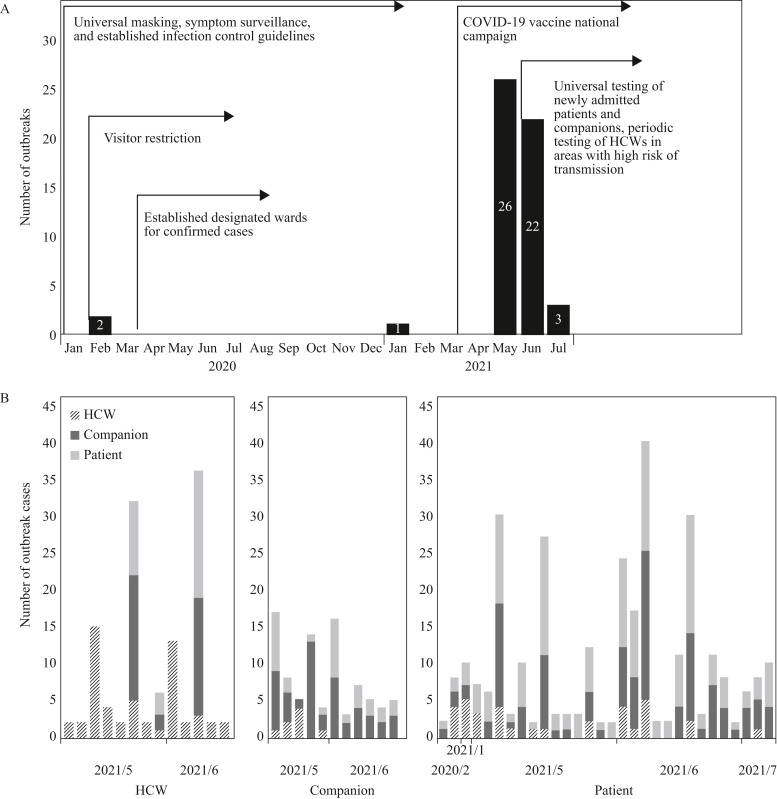
From 15th January 2020 to 31st July 2021, 26 of 495 hospitals in Taiwan reported 54 healthcare-associated outbreaks of COVID-19, among which 512 cases were confirmed. Considering the dates of RT-PCR confirmation in chronological order, the number of confirmed cases in Taiwan peaked in May 2021, mainly in northern Taiwan. Among the 48 (90%) healthcare-associated outbreaks that occurred in May and June 2021, 44 (92%) were reported by hospitals in northern Taiwan (Figure 1 ). Multiple national infection control policies were established and implemented in 2020 by CECC; however, in response to the progressing COVID-19 epidemic in Taiwan in May 2021, further infection control measures and policies were initiated, such as universal testing for all newly admitted patients and their companions, and periodic testing for HCWs working in high-risk areas.
Figure 1.
Healthcare-associated coronavirus disease 2019 (COVID-19) outbreaks by category and diagnosis month. (a) Epi curve of healthcare-associated COVID-19 outbreaks by month of primary case diagnosis and (b) number of healthcare-associated COVID-19 outbreaks by category and month of primary case diagnosis in Taiwan from 15th January 2020 to 31st July 2021. Each bar represents a single outbreak. (Left) Thirteen healthcare-associated outbreaks with healthcare workers (HCWs) as the primary cases. (Middle) Eleven healthcare-associated outbreaks with companions as the primary cases. (Right) Thirty healthcare-associated outbreaks with patients as the primary cases.
The 54 healthcare-associated outbreaks included 512 cases of COVID-19, with a median of six cases [interquartile range (IQR) 2–12] per outbreak. There were 13 (24.1%), 11 (20.4%) and 30 (55.5%) outbreaks initiated by HCWs, companions and patients, respectively. Outbreaks initiated by HCWs, companions and patients were linked to 120 (23.4%, median 2, IQR 2.0–13.0), 88 (17.2%, median 5, IQR 4.5–10.5) and 304 (59.4%, median 7.5, IQR 3.0–11.0) cases per outbreak, respectively. The proportion of secondary cases categorized as HCWs in outbreaks with HCW primary cases was 39.2% (42/107), which was significantly higher than the proportion of HCW secondary cases in outbreaks with companion (10.4%, 8/77) and patient primary cases (13.5%, 37/274; P<0.001). The median duration of outbreaks with patient primary cases was 12.0 days (IQR 4.3–17.0 days), which was longer than that of outbreaks with companion (9.0 days, IQR 1.0–10.5) and HCW (2.0 days, IQR 1.0–5.0 days) primary cases; however, the difference was not significant.
The median age of 512 patients in the outbreak-associated cases was 59 years (IQR 45–71 years), and 334 (65.2%) were female. The demographic characteristics of the confirmed cases are shown in Table I . Among the 100 cases involving HCWs, nurses accounted for 50%, followed by hospital support staff (12%, all infected in the same outbreak) and cleaners (11%). The median age was 72 years (IQR 61–83 years), 41 years (IQR 28–58 years) and 52 years (IQR 42–62 years) among patient cases, HCW cases and companion cases, respectively (P<0.05). The proportions of females among HCW cases (72/100, 72.0%) and companion cases (162/201, 80.6%) were significantly higher than in patient cases (96/211, 45.5%). For 272 (53.1%) patients who had symptoms before a confirmed diagnosis, the median period of delayed identification was 1 day (IQR 0–3 days).
Table I.
Demographic characteristics of coronavirus 2019 cases in healthcare-associated outbreaks in Taiwan from 15th January 2020 to 31st July 2021
| Cases |
Total (N=512) | |||
|---|---|---|---|---|
| HCWs (N=100) | Companions (N=201) | Patients (N=211) | ||
| Age | 41 (28–58) | 52 (42–62) | 72 (61–83) | 59 (45–71) |
| Sex, female | 72 (72.0) | 162 (80.6) | 100 (47.4) | 334 (65.2) |
| Symptom statusa, asymptomatic | 36 (36.0) | 108 (53.7) | 96 (45.5) | 240 (46.9) |
| Delayed period of identificationb | 1.0 (0.0–4.0) | 1.0 (0.0–2.0) | 1.0 (0.0–3.0) | 1.0 (0.0–3.0) |
| 30-day all-cause mortality | 0 (0) | 2 (1.0) | 79 (37.4) | 81 (15.8) |
| Hospital type | ||||
| Medical centre | 50 (50.0) | 97 (48.3) | 78 (37.0) | 225 (43.9) |
| Regional hospital | 46 (46.0) | 98 (48.8) | 118 (55.9) | 262 (51.2) |
| Community hospital | 4 (4.0) | 6 (3.0) | 15 (7.1) | 25 (4.9) |
| COVID-19 vaccine statusc | ||||
| Fully vaccinated | 1 (1.0) | 0 (0.0) | 0 (0.0) | 1 (0.2) |
| Partially vaccinated | 14 (14.0) | 12 (6.0) | 3 (1.4) | 29 (5.7) |
| Unvaccinated | 85 (85.0) | 189 (94.0) | 208 (98.6) | 482 (94.1) |
COVID-19, coronavirus disease 2019; HCW, healthcare worker.
Values are presented as median (interquartile range) or N (%).
Symptom status at the time of reverse transcription polymerase chain reaction (RT-PCR) test.
Duration from the date of symptom onset to the date of the first positive RT-PCR result.
A person was deemed to be fully vaccinated 14 days after the completion of the COVID-19 vaccination series, or partially vaccinated 14 days after receiving the first dose of a COVID-19 vaccine in a two-dose series.
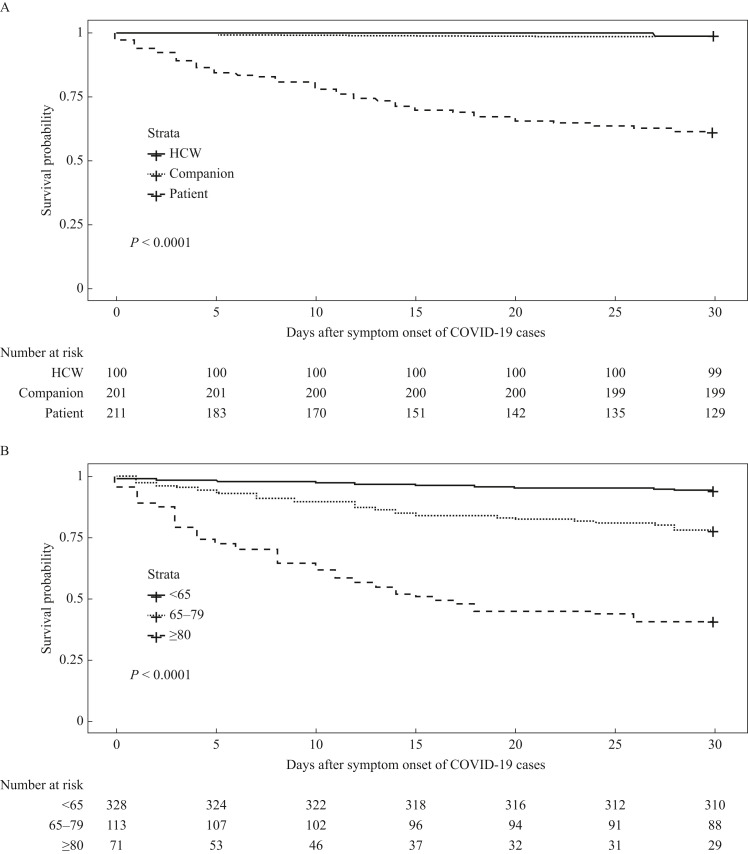
Thirty-day all-cause mortality of patients was 37.4% (79/211), which was significantly higher than that of HCWs (0%, 0/100) and companions (1.0%, 2/201) (P<0.05). Thirty-day all-cause mortality stratified by age showed that the mortality of patients aged ≥80 years was 59.2% (42/71), which was significantly higher than that of patients aged 65–79 years (22.1%, 25/113) and <65 years (5.5%, 18/328) (P<0.05) (Figure 2 ). The multi-variable Cox regression analyses revealed that 30-day all-cause mortality in healthcare-associated COVID-19 outbreaks was significantly associated with age ≥80 years, classification as a patient case, and female sex (Table II ). Among 15,674 confirmed cases of COVID-19 and 787 deaths reported as of 31st July 2021 in Taiwan [13], healthcare-associated outbreak cases accounted for 3.3% of all confirmed cases and 10.3% of all deaths.
Figure 2.
Kaplan–Meier survival curves for 30-day all-cause mortality of cases of coronavirus disease 2019 (COVID-19) in healthcare-associated outbreaks in Taiwan from 15th January 2020 to 31st July 2021. (a) Categories and (b) age groups of confirmed cases. HCW, healthcare worker.
Table II.
Multi-variable analyses for 30-day all-cause mortality of coronavirus disease 2019 cases in healthcare-associated outbreaks in Taiwan from 15th January 2020 to 31st July 2021
| N | Deceased (%) | Crude HR |
Adjusted HRa |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |||
| Hospital type | ||||||
| Medical centre | 225 | 36 (16.0) | Reference | Reference | ||
| Regional hospital | 262 | 39 (14.9) | 0.93 (0.59–1.46) | 0.75 | 0.70 (0.44–1.12) | 0.13 |
| Community hospital | 25 | 6 (24.0) | 1.59 (0.67–3.76) | 0.30 | 1.12 (0.45–2.81) | 0.81 |
| Type of case | ||||||
| Worker/companion | 301 | 2 (0.7) | Reference | Reference | ||
| Patient | 211 | 79 (37.4) | 70.25 (17.26–285.9) | <0.001 | 50.05 (11.74–213.44) | <0.001 |
| Age (years) | ||||||
| <65 | 328 | 18 (5.5) | Reference | Reference | ||
| 65–79 | 113 | 21 (18.6) | 3.68 (1.96–6.90) | <0.001 | 1.36 (0.71–2.60) | 0.35 |
| ≥80 | 71 | 42 (59.2) | 16.34 (9.38–28.46) | <0.001 | 3.93 (2.21–7.01) | <0.001 |
| Sex | ||||||
| Male | 178 | 31 (17.4) | Reference | Reference | ||
| Female | 334 | 50 (15.0) | 0.85 (0.54–1.33) | 0.47 | 1.92 (1.21–3.06) | 0.005 |
CI, confidence interval; HR, hazard ratio.
Multi-variable mixed-effects Cox regression was adjusted for hospital type, date of diagnosis confirmation, type of case, age group and sex.
Discussion
To the best of the authors’ knowledge, this is the first nationwide report of the demographics, characteristics and extent of healthcare-associated COVID-19 outbreaks in Taiwan. The majority of healthcare-associated COVID-19 outbreaks were identified in northern Taiwan, which was the epicentre of community transmission during the study period [10]. The wide spread of COVID-19 community transmission could increase presymptomatic or asymptomatic infections among HCWs and patients, and consequently lead to outbreaks of healthcare-associated COVID-19 [14]. The proportion of healthcare-associated COVID-19 in Taiwan was lower than that reported in previous studies [1,15], but the results of studies conducted in different countries and during different time periods might not be directly comparable. In addition, only cases attributed to healthcare-associated outbreaks were included in this study. Nevertheless, there were several advantages of Taiwanese hospitals responding to healthcare-associated COVID-19, including a lengthy period without community transmission of SARS-CoV-2 in 2020, increased experience and knowledge regarding the transmission of SARS-CoV-2 and containment of healthcare-associated infections acquired from other countries, and a longer period for preparedness for healthcare resources given the late peak of the COVID-19 epidemic in Taiwan.
The impacts of healthcare-associated COVID-19 on the prognosis of patients were significantly more critical than those for HCWs and companions, especially in wards caring for vulnerable groups [15,16]. Thirty-day all-cause mortality of the patients in this study was approximately 40%, which is similar to rates reported in other studies [2,3]. Aging is a known risk factor for death in patients with COVID-19 [15,17]. In a previous cohort study, patients with SARS-CoV-2 infection aged ≥75 years without other risk factors were at four-fold higher risk of death compared with patients aged <65 years [17]. Although the underlying conditions of the confirmed cases were not assessed in this study, it can be anticipated that 30-day all-cause mortality in patient cases was higher than that in HCW cases or companion cases, considering the more advanced ages of the patients. Therefore, implementation of appropriate infection control measures to prevent COVID-19 outbreaks in hospitals is of utmost importance [2].
The exact reason for the higher proportions of symptoms present among HCWs at diagnosis is unclear, but work-related stress [18] and vigilance and knowledge for recognizing COVID-19 symptoms early may partially explain this phenomenon. Therefore, during the management of a healthcare-associated COVID-19 outbreak, clear and easily understandable educational messages and instructions should be delivered to close contacts of companions and patients to raise their awareness of COVID-19 symptoms, and prompt them to report their symptoms early.
Although it was not significant and was probably related to the lower outbreak numbers in this study, there was a trend observed in outbreaks initiated by patients in that they involved a broader extent, with more cases and longer durations, than outbreaks initiated by HCWs or companions. This might be attributed to the frequent movement of patients between units for various medical and non-medical reasons. In addition, given the advanced age and increased vulnerability to severe infection in patient cases, higher viral loads and longer viral shedding durations may occur in patient cases [19], which could reasonably facilitate the spread of SARS-CoV-2 in hospitals. Nevertheless, HCWs and companions are usually the interface between hospitals and the community, and could play a role in initiating outbreaks when there is significant transmission in the community, which accounted for approximately 60% of outbreaks in this study. In Taiwanese hospitals, almost all hospitalized patients had companions with them 24 h/day to provide assistance in daily activities and emotional support [20]. These companions may flow in more extensive areas in hospitals than inpatients due to their relatively good health status. Hence, IPC strategies should include measures tailored for all three groups to reduce the transmission of SARS-CoV-2 in healthcare settings.
COVID-19 presents considerable challenges for infection control. Due to the significant infectivity of SARS-CoV-2 prior to the onset of symptoms, isolating infected persons once symptoms are recognized is not sufficient to prevent transmission [5,21,22]. In this study, 46.9% of patients were asymptomatic at the time of their first positive RT-PCR test result for COVID-19. In a cohort study conducted in England [23], 43.1% of patients infected with the alpha variant, which was the major circulating SARS-CoV-2 variant during the study period in the present study [11], were asymptomatic at the time of specimen collection. Testing asymptomatic people with potential exposure has been proposed as a measure to contain healthcare-associated COVID-19 outbreaks [2,21], in addition to basic infection control practices, such as universal source control, adequate hand hygiene and physical distancing. From 16th May 2021, CECC implemented several testing strategies, including universal testing for all newly admitted patients and their companions, and periodic testing for HCWs working in high-risk areas, such as emergency departments, intensive care units and designated COVID-19 units. Although healthcare-associated COVID-19 outbreaks decreased in July 2021, the level of community transmission also declined at the same time [24], which could have led to the downward trend of healthcare-associated outbreaks [14]. Although it is acknowledged that it is impossible to attribute the subsiding COVID-19 epidemic to a specific intervention, which could be the effect of multi-pronged measures implemented concomitantly, the strategies of universal screening for newly admitted patients and companions, and periodic testing for HCWs working in hospital areas of high-risk COVID-19 transmission may play a role in the prevention of nosocomial COVID-19 transmission.
Outbreaks initiated by HCWs involved a higher proportion of HCW secondary cases. Universal masking for source control in hospitals was implemented in early 2020 in Taiwan, and HCWs typically wear personal protective equipment appropriately during patient care. Nevertheless, the breakdown of universal masking for HCWs could occur in workrooms and dining areas during other non-medical activities or mealtimes, and adequate physical distancing might not be maintained [4,25]. Therefore, although HCWs are in contact with many suspected patients daily, HCW-to-HCW transmission can be a potential amplifying factor in COVID-19 outbreaks in healthcare settings, and may even outweigh the risks incurred through patient contact in some cases [17]. Hence, hospital IPC teams should address this issue by developing measures to maintain physical distance among HCWs in situations where continuous facemask wearing is difficult to maintain, such as providing well-ventilated dedicated spaces for eating meals and staggered break times [4,25,26].
This study had several limitations. First, given the high incidence of community transmission in May and June 2021 in Taiwan, the number of healthcare-associated infections may have been overestimated because participants with healthcare-associated infections with symptom onset within 14 days after admission could have had community-acquired infections. In addition, as contact tracing was difficult during the spread of the epidemic in the community, it was difficult to determine the source of infection or clarify the transmission chain. Second, individual causes of death were not assessed, but it is reasonable to assume that COVID-19 was the cause of death for those who died within 30 days after diagnosis was confirmed.
In conclusion, patients in healthcare settings are the most vulnerable group to healthcare-associated COVID-19 outbreaks; if infected, they have a poor prognosis and may contribute to broader extended outbreaks. Nevertheless, HCWs and companions accounted for two-thirds of the cases in the healthcare-associated COVID-19 outbreaks in this study. Therefore, multi-pronged IPC practices tailored for these three groups should be a priority, including universal testing and adapted physical distancing measures for HCWs, patients and their companions, in order to prevent healthcare-associated COVID-19 outbreaks.
Acknowledgements
The authors wish to thank all public health workers and healthcare workers who participated in COVID-19 control and prevention in Taiwan. The outbreak investigation report was kindly provided by the branches of the Taiwan CDC.
Data availability
The study data are available from the corresponding author on reasonable request.
Author contributions
Conceptualization and methodology: S-C. Chang, H-H. Wu, C-H. Su, L-J. Chien, S-H. Tseng. Investigation, data curation and formal analysis: S-C. Chang, H-H. Wu, C-H. Su. Writing –original draft: S-C. Chang, H-H. Wu, C-H. Su. Writing – review and editing: S-C. Chang, H-H. Wu, C-H. Su, L-J. Chien, S-H. Tseng. All authors read and approved the final manuscript.
Conflict of interest statement
None declared.
Funding sources
This study was supported by the Taiwan CDC (Grant No. TwCDCIRB109206). The funder had no role in the study design; in the collection, analysis or interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
References
- 1.Zhou Q., Gao Y., Wang X., Liu R., Du P., Wang X., et al. COVID-19 Evidence and Recommendations Working Group. Nosocomial infections among patients with COVID-19, SARS and MERS: a rapid review and meta-analysis. Ann Transl Med. 2020;8:629. doi: 10.21037/atm-20-3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rickman H.M., Rampling T., Shaw K., Martinez-Garcia G., Hail L., Coen P., et al. Nosocomial transmission of coronavirus disease 2019: a retrospective study of 66 hospital-acquired cases in a London teaching hospital. Clin Infect Dis. 2021;72:690–693. doi: 10.1093/cid/ciaa816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ponsford M.J., Jefferies R., Davies C., Farewell D., Humphreys I.R., Jolles S., et al. Burden of nosocomial COVID-19 in Wales: results from a multicentre retrospective observational study of 2508 hospitalised adults. Thorax. 2021;76:1246–1249. doi: 10.1136/thoraxjnl-2021-216964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harada S., Uno S., Ando T., Iida M., Takano Y., Ishibashi Y., et al. Control of a nosocomial outbreak of COVID-19 in a university hospital. Open Forum Infect Dis. 2020;22 doi: 10.1093/ofid/ofaa512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abbas M., Robalo Nunes T., Martischang R., Zingg W., Iten A., Pittet D., et al. Nosocomial transmission and outbreaks of coronavirus disease 2019: the need to protect both patients and healthcare workers. Antimicrob Resist Infect Control. 2021;10:7. doi: 10.1186/s13756-020-00875-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kimball A., Hatfield K.M., Arons M., James A., Taylor J., Spicer K., et al. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility – King County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:377–381. doi: 10.15585/mmwr.mm6913e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chien L.J., Su C.H., Wu H.H. Recommendations on contingency operations for hospitals in response to COVID-19 cases identified in inpatients – Taiwan. J Formos Med Assoc. 2020;119:1572–1574. doi: 10.1016/j.jfma.2020.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taiwan Centers for Disease Control . Taiwan CDC; Taipei: 2021. CECC confirms 1 more indigenous COVID-19 case; a contact of case #838 confirmed to have COVID-19.https://www.cdc.gov.tw/En/Bulletin/Detail/MzCxFCd-7JLpdNfad6KPzA?typeid=158 Available at: [last accessed February 2022] [Google Scholar]
- 9.Taiwan Centers for Disease Control . 2021. CECC confirms 2 more indigenous COVID-19 cases; discharged patient linked to hospital cluster infection and his family member found to have COVID-19. Taipei: Taiwan CDC.https://www.cdc.gov.tw/En/Bulletin/Detail/wNNl-KkvdrvT7I27_ia7ZA?typeid=158 Available at: [last accessed February 2022] [Google Scholar]
- 10.Taiwan Centers for Disease Control . Taiwan CDC; Taipei: 2021. CECC raises epidemic alert level for Taipei City and New Taipei City to level 3 and strengthens national restrictions and measures, effective from May 15 to May 28, in response to increasing level of community transmission.https://www.cdc.gov.tw/En/Bulletin/Detail/R1K7gSjoYa7Wojk54nW7fg?typeid=158 Available at: [last accessed February 2022] [Google Scholar]
- 11.Tsai S.C., Chang W.W., Lee W.S. Analysis of an outbreak of COVID-19 (alpha-variant) with rapid progression to mortality in Taipei, Taiwan. J Infect. 2022;84:e33–e34. doi: 10.1016/j.jinf.2021.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu D.P., Wang E.T., Pan Y.H., Cheng S.H. Innovative applications of immunisation registration information systems: example of improved measles control in Taiwan. Euro Surveill. 2014;19:20994. doi: 10.2807/1560-7917.es2014.19.50.20994. [DOI] [PubMed] [Google Scholar]
- 13.Taiwan Centers for Disease Control . Taiwan CDC; Taipei: 2021. CECC confirms 12 more COVID-19 cases, including 11 domestic cases and 1 imported case.https://www.cdc.gov.tw/En/Bulletin/Detail/C_JNUnmHQv5dS3SFK_FSuQ?typeid=158 Available at: [last accessed 21 February 2022] [Google Scholar]
- 14.Sei H, Shunsuke U, Takayuki A, Miho I, Yaoko T, Yoshiki I, et al. Control of a nosocomial outbreak of COVID-19 in a university hospital. Open Forum Infect Dis 22;7:ofaa512. [DOI] [PMC free article] [PubMed]
- 15.Carter B., Collins J.T., Barlow-Pay F., Rickard F., Bruce E., Verduri A., et al. Nosocomial COVID-19 infection: examining the risk of mortality. The COPE-Nosocomial Study (COVID in Older PEople) J Hosp Infect. 2020;106:376–384. doi: 10.1016/j.jhin.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bestilleiro R.S., Señaris D.M., Rodríguez M.J.P., Vázquez R.G., Rodríguez R.G., Rodriguez M.T.G., et al. Nosocomial infection outbreak due to SARS-COV-2 in a hospital unit of particularly vulnerable patients. Int J Med Sci. 2021;18:2146–2154. doi: 10.7150/ijms.53270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho F.K., Petermann-Rocha F., Gray S.R., Jani B.D., Katikireddi S.V., Niedzwiedz C.L., et al. Is older age associated with COVID-19 mortality in the absence of other risk factors? General population cohort study of 470,034 participants. PLoS One. 2020;15 doi: 10.1371/journal.pone.0241824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vimercati L., De Maria L., Quarato M., Caputi A., Stefanizzi P., Gesualdo L., et al. COVID-19 hospital outbreaks: protecting healthcare workers to protect frail patients. An Italian observational cohort study. Int J Infect Dis. 2021;102:532–537. doi: 10.1016/j.ijid.2020.10.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng S., Fan J., Yu F., Feng B., Lou B., Zou Q., et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January–March 2020: retrospective cohort study. BMJ. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tzeng H.M., Yin C.Y. Family involvement in inpatient care in Taiwan. Clin Nurs Res. 2008;17:297–311. doi: 10.1177/1054773808324655. [DOI] [PubMed] [Google Scholar]
- 21.US Centers for Disease Control and Prevention . CDC; Atlanta, GA: 2021. Overview of testing for SARS-CoV-2 (COVID-19)https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing-overview.html Available at: [last accessed February 2022] [Google Scholar]
- 22.Provincial Infectious Diseases Advisory Committee . Toronto: PHO; 2021. Best practices for managing COVID-19 outbreaks in acute care settings.https://www.publichealthontario.ca/-/media/documents/ncov/ipac/2021/03/covid-19-pidac-outbreaks-acute-care.pdf?sc_lang=en Available at: [last accessed February 2022] [Google Scholar]
- 23.Twohig K.A., Nyberg T., Zaidi A., Thelwall S., Sinnathamby M.A., Shirin A., et al. Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern. Lancet Infect Dis. 2022;22:35–42. doi: 10.1016/S1473-3099(21)00475-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taiwan Centers for Disease Control . Taiwan CDC; 2021. CECC extends nationwide level 3 epidemic alert until July 26 to safeguard disease prevention efforts in the community; CECC to partially relax restrictions starting July 13. Taipei.https://www.cdc.gov.tw/En/Bulletin/Detail/vlmAORqyqEntz1Tr_Ls7DQ?typeid=158 Available at: [last accessed February 2022] [Google Scholar]
- 25.Richterman A., Meyerowitz E.A., Cevik M. Hospital-acquired SARS-CoV-2 infection: lessons for public health. JAMA. 2020;324:2155–2156. doi: 10.1001/jama.2020.21399. [DOI] [PubMed] [Google Scholar]
- 26.Schneider S., Piening B., Nouri-Pasovsky P.A., Krüger A.C., Gastmeier P., Aghdassi S.J.S. SARS-coronavirus-2 cases in healthcare workers may not regularly originate from patient care: lessons from a university hospital on the underestimated risk of healthcare worker to healthcare worker transmission. Antimicrob Resist Infect Control. 2020;9:192. doi: 10.1186/s13756-020-00848-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The study data are available from the corresponding author on reasonable request.