Abstract
In this retrospective, multicenter study, we determined the predictive value of imaging biomarkers in diabetic macular edema (DME) outcomes following dexamethasone (DEX) implant(s). Sixty-seven eyes of 47 patients’ best-corrected visual acuity (BCVA) and central foveal thickness (CFT) on optical coherence tomography (OCT) before and after intravitreal DEX implants were evaluated. Baseline imaging biomarkers were graded using fundus photography and OCT, and the predictive value of biomarkers for significant treatment effects at six months was analyzed. Six months after 2.0 ± 0.8 (mean ± SD) DEX implants, 35 (52%) and 16 (24%) eyes had CFT reduction ≥ 10% from baseline and decreased to < 300 µm, respectively. BCVA improved ≥ 3 lines in 15 (22%) and remained stable in 38 (57%) eyes. At six months, eyes with severe intraretinal cyst (IRC), abundant hyperreflective dots (HRD), and moderate or severe hard exudate had a significantly higher chance of CFT reduction ≥ 10%. Eyes with abundant HRD at baseline and those underwent three DEX implants were more likely to achieve CFT < 300 µm. Eyes with DME and severe IRC, abundant HRD, or moderate-to-severe hard exudate at baseline were more likely to show a significant reduction in CFT six months after DEX implant.
Subject terms: Biological techniques, Biomarkers
Introduction
Diabetes is a metabolic disease that affects over 422 million people worldwide1. Among the main complications of diabetes, diabetic retinopathy and diabetic macular edema (DME) are the leading causes of visual impairment in working-age people.
Anti-vascular endothelial growth factor (anti-VEGF) drugs have been widely used as a treatment for DME and improved visual outcomes. However, some patients do not respond to anti-VEGF. A post hoc analysis showed that nearly 40% of the eyes resulted in suboptimal best-corrected visual acuity (BCVA) improvement of less than five letters after three months of anti-VEGF treatment2. In addition, a study showed that after six months of a monthly injection, 32–66% of the treated eyes did not respond to anti-VEGF, resulting in persisting edema and reduced visual acuity (VA)3. Since inflammation is tightly involved in the development and worsening of DME4, the use of steroids has emerged as an alternative treatment5.
Intravitreal dexamethasone (DEX) implants (Ozurdex, Allergan, Irvine, CA) have been applied since 2009 to treat different ocular diseases. After the injection, the implant slowly dissolves in the vitreous cavity and provides a sustained release of DEX for up to six months. It is indicated for macular edema associated with retinal vein occlusions, noninfectious uveitis6, macular edema associated with diabetes7,8, and diabetic tractional retinal detachment9.
Studies have shown that DEX implants are effective in naive eyes as well as those that responded poorly to anti-VEGF10 and that several non-invasive imaging characteristics, or so-called imaging biomarkers, could predict these outcomes11–14. Although some of the earlier studies were either limited by patient number15–17, or short-term follow-up14,18, later real-life multicenter studies with bigger samples have shown the importance of the use of imaging biomarkers to ascertain outcomes in DME patients following DEX implants19. Our study aimed to evaluate the predictive value of imaging biomarkers for outcomes in Asian DME patients following DEX implants in a real-world, multicenter scenario.
Materials and methods
A retrospective multicenter, single-arm study was conducted between June 2017 and February 2020 in four branch hospital sites in Taiwan. The inclusion criteria were patients with diabetes type 1 or 2 who presented a vision decline and DME and were treated with intravitreal DEX implant(s). DME was diagnosed based on a central foveal thickness (CFT) over 300 µm by spectral-domain optical coherence tomography (SD-OCT; Spectralis; Heidelberg Engineering, Heidelberg, Germany) measured with the built-in software. The Institutional Review Board approved the study (No. 201600962A3C101 from the Chang Gung Memorial Hospital) and adhered to the tenets of the Declaration of Helsinki. The Chang Gung Memorial Hospital Institutional Review Board also waived the requirement for informed consent due to the retrospective nature of this study.
The exclusion criteria were patients who received anti-VEGF for less than one month or macular grid laser/intravitreal corticosteroids for less than three months before the baseline (i.e., the time a patient received the first DEX implant), had concurrent macular pathologies or prior intraocular surgeries except for cataract extraction, or were followed for less than six months. Patients who received treatments for DME other than DEX implant and vitrectomies during the six-month follow-up period were also excluded.
Demographics, diabetic status, and systemic and ocular conditions were obtained from medical records. HbA1c levels were documented if measured less than three months before the baseline. Ophthalmic examinations, including BCVA, intraocular pressure (IOP), slit-lamp biomicroscopy, color fundus photographs, and SD-OCT images, were collected at baseline and six months after the first DEX implant.
DME and imaging biomarkers grading
Four unmasked graders (HDC, CHW, WYC, NNC) graded the imaging biomarkers. For each biomarker, two graders were needed to reach a consensus, and a third one to arbitrate in case of discrepancy between the first two graders. Color fundus photographs were used to determine whether the eye underwent pan-retinal photocoagulation (PRP) and grade hard exudate (HE) status according to the Early Treatment Diabetic Retinopathy Study (ETDRS)20. Tomographic qualitative and quantitative OCT parameters were graded according to a recently described DME grading protocol21, which includes CFT, intraretinal cysts (IRC), subretinal fluid (SRF), the ellipsoid zone (EZ) status, disorganization of the inner retinal layers (DRIL), hyperreflective dots (HRD), and vitreoretinal relationship. Early, advanced, severe, and atrophic were the four distinct stages of DME, based on the four previously mentioned parameters (CFT, IRC, EZ, and DRIL).
Study outcomes and endpoints
Snellen BCVA measurements were converted to logarithm of the minimum angle of resolution for statistical analysis. The main outcomes were evaluated six months after the first DEX implant. The three endpoints of the study were: (1) decrease in CFT greater than 10% from baseline; (2) decrease in CFT to less than 300 µm; and (3) improvement in BCVA of three or more ETDRS lines.
Statistical analysis
Categorical and continuous variables is presented as No. (%) and mean ± standard deviation, respectively. The interrater reliability (interclass correlation coefficient) of the biomarker grading among the initial 2 graders were calculated by using the two-way mixed effects model for all pooled biomarkers gradings. The pre-and post-treatment conditions were compared by the Wilcoxon signed-rank test. A univariate logistic regression analysis was performed under the generalized estimating equations (GEE) framework to assess factors related to outcomes, considering that both eyes from an individual could be enrolled. For the GEE models with multivariate adjustments, age, sex, HbA1c level, and predictors with a P < 0.1 from the univariate models were included. All the analyses were performed using SAS Enterprise Guide Version 7.1. Statistical significance was considered with P < 0.05 for two-tailed tests.
Results
Demographic and baseline characteristics of the study population are shown in Table 1. The study included 67 eyes of 47 patients, with a mean age of 67 and a mean HbA1c of 7.6%. Half of the eyes underwent prior PRP, and 11 eyes (16%) received anti-VEGF intravitreal injections more than one month before the DEX implant. The rest of the eyes (56 eyes, 84%) were all anti-VEGF naïve. The mean time since last anti-VEGF injection was 954 days (range, 42–3137 days) and the mean duration of DME was 109 days. Five eyes (8%) had glaucoma and were receiving IOP-lowering medication at the study baseline.
Table 1.
Demographic and baseline characteristics.
| Characteristics | Baseline |
|---|---|
| No. of patients | 47 |
| No. of eyes | 67 |
| Age, mean ± SD, y | 66.6 ± 8.2 |
| Male, No. (%) | 29 (62) |
| Under renal dialysis, No. (%) | 1 (2) |
| Hypertension, No. (%) | 19 (40) |
| Prior myocardial infarction, No. (%) | 6 (13) |
| Prior stroke, No. (%) | 2 (4) |
| Diabetis mellitus duration, No. (%) | |
| 5 y or less | 8 (12) |
| 5–15 y | 19 (29) |
| More than 15 y | 6 (9) |
| Not recorded | 34 (51) |
| HbA1c (%), mean ± SD | 7.6 ± 1.3 |
| Diabetis retinopathy grading, No. (%) | |
| Mild or moderate NPDR | 5 (8) |
| Severe or very severe NPDR | 17 (25) |
| PDR | 11 (16) |
| High-risk PDR | 9 (13) |
| Cannot gradea | 25 (37) |
| Diabetis retinopathy durationb, mean ± SD, y | 5.1 ± 6.2 |
| Diabetic macular edema duration, mean ± SD, d | 109 ± 134 |
| Prior PRP, No. (%) | 33 (50) |
| Intravitreal anti-VEGF treated, No. (%) | 11 (16) |
| Last intravitreal anti-VEGF injection time before DEX implant, mean ± SD, d | 954 (1124) |
| Glaucoma status, No. (%) | 5(8) |
aEyes with prior PRP were non-gradable except for those with neovascularization.
bValid n = 48.
Abbreviation: anti-VEGF anti-vascular endothelial growth factor, DEX implant dexamethasone intravitreal implant, NPDR nonproliferative diabetic retinopathy, PDR proliferative diabetic retinopathy, PRP panretinal photocoagulation.
Imaging biomarker grades at study baseline
Details of the baseline imaging biomarkers are listed in Table 2. The interrater reliability for biomarker gradings was moderate (interclass correlation coefficient: 0.56, 95% CI, 0.44–0.66). Forty-one eyes (62%) had CFT over 390 µm, 20 (30%) had severe IRC, and 14 (21%) had SRF in the fovea. The majority (63%) of the eyes had an intact EZ; however, DRIL was present in 74%. HRD was graded as abundant (higher than 30 dots) in nearly half of the eyes (45%). Half of the eyes (49%) had an epiretinal membrane. Combining the CFT, IRC, EZ, and DRIL status, the resulting DME staging was: early (17%), advanced (57%), and severe (12%) DME. Fundus photographic grading showed that 22 eyes (33%) had moderate or severe HE at baseline.
Table 2.
Baseline DME imaging biomarkers.
| DME imaging biomarkers | Baseline |
|---|---|
| Central foveal thickness, No. (%) | |
| 300–329 µm | 10 (15) |
| 330–389 µm | 16 (24) |
| ≥ 390 µm | 41 (62) |
| Intraretinal cysts, No. (%) | |
| Absent | 6 (9) |
| Mild | 15 (23) |
| Moderate | 26 (39) |
| Severe | 20 (30) |
| Presence of subretinal fluid, No. (%) | 14 (21) |
| Ellipsoid zone status, No. (%) | |
| Intact | 42 (63) |
| Disrupted | 15 (23) |
| Absent | 8 (12) |
| Cannot grade | 2 (3) |
| Presence of DRIL, No. (%) | 49 (74) |
| Hyperreflective dots, No. (%) | |
| Absent or scarce (< 30) | 37 (56) |
| Abundant (> 30) | 30 (45) |
| Vitreoretinal relationship | |
| Attached vitreous cortex in the fovea | 19 (28) |
| Detached vitreous cortex in the fovea | 10 (15) |
| Epiretinal membrane | 33 (49) |
| Cannot grade | 5 (8) |
| Hard exudates, No. (%) | |
| Absent | 30 (45) |
| Mild | 12 (18) |
| Moderate | 12 (18) |
| Severe | 10 (15) |
| Cannot grade | 2 (3) |
| DME stagea, No. (%) | |
| Early DME | 11 (17) |
| Advanced DME | 38 (57) |
| Severe DME | 8 (12) |
| Atrophic maculopathy | 8 (12) |
| Cannot grade | 2 (3) |
aDME stage was graded according to Panozzo et al.’s21 study.
Abbreviation: DME diabetic macular edema, DRIL disorganized retinal inner layers.
Anatomical and functional outcomes at six months
At six months, the eyes received an average of 2.0 ± 0.8 DEX implants (Table 3). Anatomically, CFT showed a significant reduction from pre-operative 459.9 ± 146.3 µm to post-operative 360.5 ± 127.4 µm (P < 0.001), and 35 eyes (52%) surmount the endpoint of CFT reduction ≥ 10% from baseline, with 16 eyes (24%) achieving CFT ≤ 300 µm after six months. Although the functional outcome showed no overall significant improvement in BCVA (P = 0.35), 15 eyes (22%) improved three lines or more, and 38 (57%) remained stable vision. Four eyes (6%) showed cataract progression over two grades after six months, but none underwent cataract surgery. The mean IOP through the study period significantly increased from 14 to 17 mmHg (P = 0.003); nevertheless, only five eyes (9%) exceeded 25 mmHg. Notably, two eyes (3%) had persistently elevated IOP despite maximal medical treatment and underwent transscleral cyclophotocoagulation.
Table 3.
Six-month outcomes in eyes with diabetic macular edema treated by DEX implants.
| Values | p-valuea | |
|---|---|---|
| No. of eyes | 67 | – |
| DEX implant number mean ± SD | 2.0 ± 0.8 | – |
| CFT, mean ± SD, µm | < 0.001 | |
| Baseline | 459.9 ± 146.3 | |
| 6-moa | 360.5 ± 127.4 | |
| CFT change at 6-mob | – | |
| Decreased 10% or more from baseline | 35 (52) | |
| Decreased to < 300 µm | 16 (24) | |
| BCVA, mean ± SD, logMAR | 0.35 | |
| Baseline | 0.80 ± 0.37 | |
| 6-moc | 0.79 ± 0.49 | |
| BCVA change at 6-mo | – | |
| Improved 15 ETDRS letters or more | 15 (22) | |
| Decreased 15 ETDRS letters or more | 8 (12) | |
| Stable | 38 (57) | |
| Missing | 6 (9) | |
| Lens status, phakic, No. (%) | 1.0 | |
| Baseline | 22 (33) | |
| 6-moc | 22 (33) | |
| Cataract progression | 4 (6) | |
| IOP, mean ± SD, mmHg | 0.003 | |
| Baseline | 14.2 ± 3.7 | |
| 6-moc | 17.7 ± 5.1 | |
| Elevated IOPb, No. (%) | – | |
| > 25 mmHg | 5 (9) | |
| > 35 mmHg | 2 (3) | |
| Uncontrolled glaucoma needing intervention, No. (%) | 2 (3)d | – |
aBetween the baseline and the 6-mo values.
bValid n = 58.
cValid n = 61.
dTwo eyes from the same patient with persistent elevated IOP and needed argon laser trabeculoplasty.
Abbreviation: BCVA best-corrected visual acuity, CFT central foveal thickness, DEX implant dexamethasone intravitreal implant, IOP intraocular pressure, logMAR logarithm of the minimum angle of resolution.
Univariate analysis of the biomarkers related to outcomes after DEX implants
To simplify the analysis, the grades of certain biomarkers, including IRC, EZ, and HE, were combined. Then, univariate logistic analysis of biomarkers was performed to select those that predict the outcomes after DEX implants (Table 4). A reduction in CFT ≥ 10% from baseline values could be predicted by DEX implants number = 3 at six months (OR, 1.44; 95% CI, 1.10–1.88; P < 0.01) and baseline biomarkers including a CFT ≥ 390 µm (OR, 1.3; 95% CI, 1.05–1.83; P = 0.02), total absence or partial disruption of EZ (OR, 1.35; 95% CI, 1.05–1.75; P = 0.02), presence of abundant HRD (OR, 1.42; 95% CI, 1.14–1.77; P < 0.01), and moderate or severe HE (OR, 1.34; 95% CI, 1.07–1.68; P = 0.01). Additionally, severe DME stage was associated with a decrease in CFT ≥ 10% at six months.
Table 4.
Univariate analysis for predictors of 6-month outcomes in diabetic macular edema treatment with DEX implantsa.
| Variables | Endpoint 1: CFT decreased ≥ 10% from baseline | Endpoint 2: CFT decreased to < 300 µm | Endpoint 3: BCVA improved ≥ 15 ETDRS letters | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Beta | OR (95% CI) | p-value | Beta | OR (95% CI) | p-value | Beta | OR (95% CI) | p-value | |
| Age | − 0.02 | 0.99 (0.98–1.01) | 0.10 | − 0.01 | 1.00 (0.98–1.01) | 0.37 | − 0.01 | 1.00 (0.98–1.02) | 0.49 |
| Male | − 0.08 | 0.94 (0.72–1.23) | 0.61 | 0.07 | 1.07 (0.83–1.38) | 0.61 | 0.07 | 1.08 (0.85–1.36) | 0.57 |
| HbA1c | − 0.04 | 0.97 (0.87–1.08) | 0.49 | 0.02 | 1.02 (0.90–1.15) | 0.82 | 0.06 | 1.06 (0.97–1.15) | 0.25 |
| Prior PRP | − 0.25 | 0.79 (0.62–1.01) | 0.05 | − 0.11 | 0.91 (0.71–1.16) | 0.42 | − 0.24 | 0.79 (0.64–0.98) | 0.03* |
| Prior anti-VEGF | 0.24 | 1.27 (0.95–1.71) | 0.11 | 0.25 | 1.29 (0.90–1.84) | 0.17 | 0.19 | 1.21 (0.83–1.75) | 0.34 |
| DEX implants No. at 6-mo | |||||||||
| DEX implant = 1 | Reference | Reference | Reference | ||||||
| DEX implants = 2 | 0.22 | 1.24 (0.93–1.65) | 0.14 | 0.24 | 1.28 (1.02–1.60) | 0.04* | − 0.08 | 0.93 (0.66–1.30) | 0.65 |
| DEX implants = 3 | 0.37 | 1.44 (1.10–1.88) | < 0.01** | 0.32 | 1.38 (1.04–1.84) | 0.03* | − 0.08 | 0.92 (0.66–1.30) | 0.64 |
| Phakic lens status | 0.03 | 1.03 (0.77–1.37) | 0.87 | -0.02 | 0.99 (0.77–1.27) | 0.93 | 0.14 | 1.15 (0.89–1.48) | 0.31 |
| CFT ≥ 390 µm | 0.33 | 1.39 (1.05–1.83) | 0.02* | 0.06 | 1.06 (0.83–1.36) | 0.66 | 0.08 | 1.08 (0.87–1.34) | 0.51 |
| Severe IRC (reference: moderate or less IRC) | 0.28 | 1.32 (0.99–1.75) | 0.06 | 0.09 | 1.09 (0.84–1.41) | 0.53 | 0.02 | 1.02 (0.78–1.33) | 0.91 |
| Disrupted or absent EZ (reference: intact EZ) | 0.3 | 1.35 (1.05–1.75) | 0.02* | 0.18 | 1.20 (0.94–1.54) | 0.16 | 0.11 | 1.11 (0.88–1.4) | 0.39 |
| DRIL present | 0.19 | 1.21 (0.88–1.66) | 0.26 | -0.06 | 0.95 (0.71–1.27) | 0.72 | 0.15 | 1.17 (0.95–1.44) | 0.17 |
| Abundant HRD | 0.35 | 1.42 (1.14–1.77) | < 0.01** | 0.33 | 1.39 (1.11–1.76) | < 0.01** | 0.14 | 1.15 (1.13–1.49) | 0.29 |
| Subretinal fluid present | 0.25 | 1.28 (0.97–1.68) | 0.09 | 0.18 | 1.20 (0.87–1.66) | 0.28 | 0.06 | 1.06 (0.78–1.43) | 0.74 |
| Detached vitreous cortex in fovea (reference: attached vitreous cortex in fovea) | − 0.3 | 0.75 (0.49–1.14) | 0.17 | − 0.29 | 0.76 (0.54–1.06) | 0.10 | − 0.37 | 0.70 (0.50–0.98) | 0.03* |
| Presence of epiretinal membrane | − 0.16 | 0.86 (0.65–1.10) | 0.22 | − 0.27 | 0.77 (0.59–0.99) | 0.04* | − 0.13 | 0.88 (0.68–1.14) | 0.34 |
| Moderate or severe HE (reference: absent or mild HE) | 0.3 | 1.34 (1.07–1.68) | 0.01* | 0.04 | 1.04 (0.82–1.31) | 0.77 | − 0.01 | 1.00 (0.80–1.25) | 0.96 |
| DME stageb | 0.13 | 1.13 (0.81–1.59) | 0.49 | − 0.18 | 0.84 (0.58–1.22) | 0.35 | 0.21 | 1.23 (0.94–1.61) | 0.14 |
| Early DME | Reference | Reference | Reference | ||||||
| Advanced DME | 0.13 | 1.13 (0.81–1.59) | 0.49 | − 0.18 | 0.84 (0.58–1.22) | 0.35 | 0.21 | 1.23 (0.94–1.61) | 0.14 |
| Severe DME | 0.45 | 1.56 (1.13–2.16) | < 0.01** | 0.06 | 1.06 (0.67–1.68) | 0.81 | 0.14 | 1.15 (0.79–1.69) | 0.48 |
*P < 0.05; **P < 0.01.
aUnivariate logistic regression analysis.
bDME stage was graded according to a previous study20.
Abbreviation: anti-VEGF anti-vascular endothelial growth factor, BCVA best-corrected visual acuity, CFT central foveal thickness, DEX implant dexamethasone intravitreal implant, DME diabetic macular edema, DRIL disorganized retinal inner layers, ETDRS Early Treatment Diabetic Retinopathy Study, EZ ellipsoid zone, HE hard exudates, HRD Hyperreflective dot, IRC intraretinal cyst, PRP = panretinal photocoagulation.
For the second anatomical endpoint, CFT reduction to less than 300 µm was significantly associated with two and three DEX implants at six months (OR, 1.28; 95% CI, 1.02–1.60; P = 0.04 and OR, 1.38; CI, 1.04–1.84; P = 0.03, respectively), and with the biomarker of abundant HRD at baseline (OR, 1.39; 95% CI, 1.16–1.7; P < 0.01). An epiretinal membrane at baseline had a negative effect on the CFT < 300 µm endpoint (Table 4).
As for the functional endpoint, two negative predictors were identified, namely eyes with prior PRP and a detached vitreous cortex in the fovea (OR, 0.79; 95% CI, 0.64–0.98; P = 0.03 and OR, 0.70; 95% CI, 0.50–0.98; P = 0.03, respectively). The eyes with these factors were less likely to improve BCVA to more than three lines at six months after DEX implants.
Multivariate analysis of the biomarkers related to outcomes after DEX implants
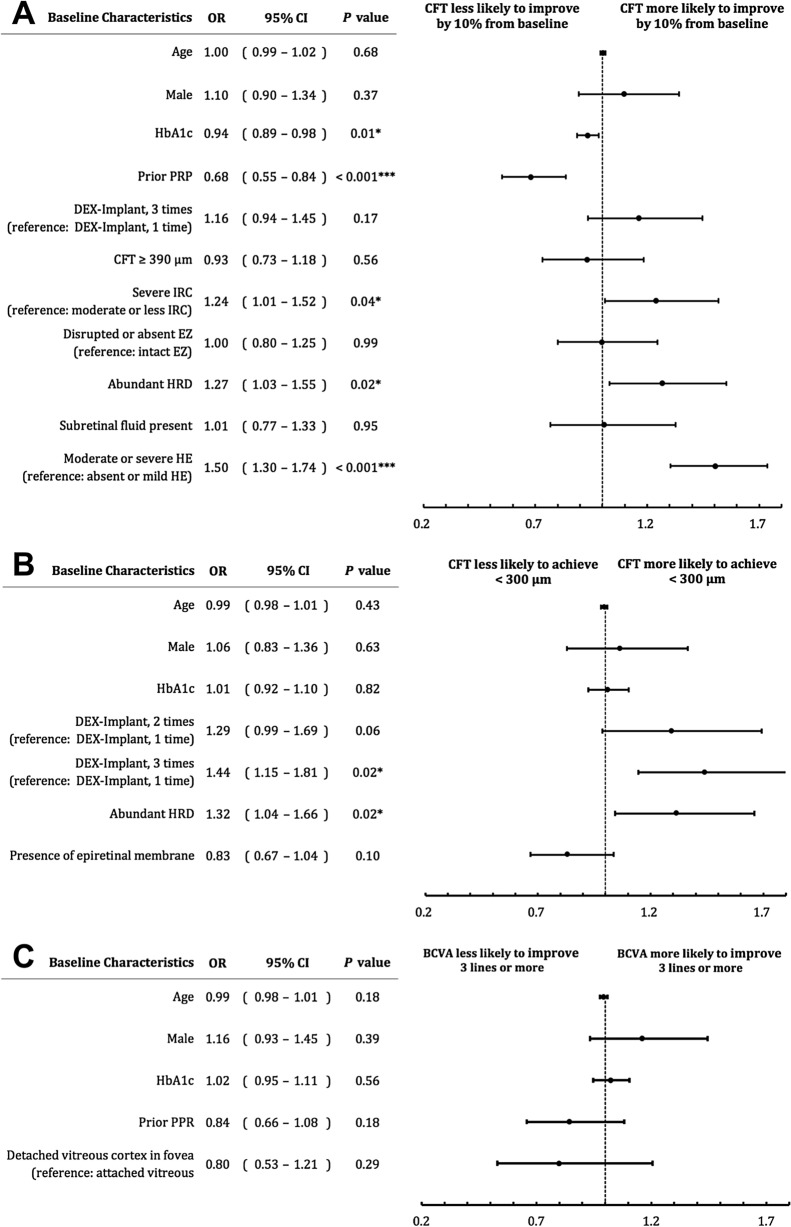
Further multivariate analysis of the factors that correlated with six-month outcomes was performed. The results demonstrate that patients with high levels of HbA1c and eyes with prior PRP are less likely to have a CFT reduction ≥ 10% from baseline (OR, 0.94; 95% CI, 0.89–0.98; P = 0.01 and OR, 0.68; 95% CI, 0.55–0.84; P < 0.001, respectively; Fig. 1A). Biomarkers including severe IRC, abundant HRD, and moderate or severe HE had a significantly higher chance of improving CFT ≥ 10% (OR, 1.24; 95% CI, 1.01–1.52; P = 0.04; OR, 1.27; 95% CI, 1.03–1.55; P = 0.02 and OR, 1.50; 95% CI, 1.30–1.74; P < 0.001, respectively). Furthermore, eyes with abundant HRD at baseline were more likely to achieve the endpoint of CFT < 300 µm (OR, 1.32; 95% CI, 1.04–1.66; P = 0.02), as well as eyes that underwent three DEX implants at six months (OR, 1.44; 95% CI, 1.15–1.81; P = 0.02; Fig. 1B). Nevertheless, none of the above predictors found in the univariate analysis was significantly correlated with the functional outcome of BCVA improvement ≥ three lines in the multivariate model (Fig. 1C). Although severe DME stage was a significant factor in the univariate analysis, it was not included in the multivariate analysis since it was composed of some other factors included in the model.
Figure 1.
Multivariate analysis of imaging biomarkers and outcomes after dexamethasone (DEX) implant treatment in patients with diabetic macular edema (DME). (A) Endpoint 1: CFT reduction of 10% or more from baseline. (B) Endpoint 2: CFT < 300 µm at 6-month. (C) Endpoint 3: BCVA improvement of 3 lines or more from baseline. (BCVA best-corrected visual acuity CFT central foveal thickness, CI confidence interval, DEX dexamethasone, EZ ellipsoid zone, HE hard exudates, HRD Hyperreflective dot, IRC intraretinal cyst, OR odds ratio, PRP panretinal photocoagulation).
Discussion
In this retrospective, multicenter Asian study, DME patients who were treated with DEX implants had significant anatomical improvement after six months, and their vision improved or stabilized in 79%. Several baseline imaging characteristics including HE, HRD, and IRC were found to be predictive of the significant treatment outcomes. Since OCT is widely available in most clinical settings and is non-invasive, these results provide physicians instant guidance for deciding the individualized treatment options.
For several decades, the assessment of diabetic retinopathy and DME was made by color fundus photography or invasive imaging by fluorescein angiography. The advances in OCT have contributed to understanding the morphological changes, pathophysiology, and classification of DME11,19,21. With this technology, the evaluation of various non-invasive imaging biomarkers of DME can be easily performed. Biomarkers such as CFT, choroidal thickness, HRD, HE, SRF, IRC, EZ or external limiting membrane disruption, and DRIL have been shown as general prognostic biomarkers of anatomical or functional outcomes11,19,21,22.
Although the pathogenesis of DME is still not completely understood and thought to be multifactorial, there is growing evidence that inflammation plays an important role in the development and worsening of DME4. Therefore, biomarkers related to inflammation and DME outcomes are emerging as treatment predictors.
The nature of HRD remained unclear despite several proposed inflammatory origins, including lipid extravasation from a compromised vasculature, microglia proliferation, or retinal pigmented epithelium migration23. After anti-VEGF treatment for DME, the results on the relationship between HRD and treatment responses are conflicting. While some studies have reported better VA after anti-VEGF in eyes with more HRD23, many others showed no such associations, or even a poorer VA improvement or final VA in eyes with HRD24.
Similarly, for the outcomes of treating DME with DEX implants, HRD had different predictive values. Zur et al.14 and Chatziralli et al.15 reported that the presence of HRD at baseline was related to a worse VA or VA gain after DEX implants. Conversely, in eyes with more HRD, greater improvement in retinal sensitivity was found one month after DEX implant18. Additionally, more HRD was found in anti-VEGF non-responders. After switching to DEX implant, a significant reduction in retinal thickness or better VA outcomes were observed in eyes with more HRD14,16. A recent report also found that the number of HRD decreased significantly in eyes treated with DEX when compared to those treated with anti-VEGF25. Since HRD might be related to inflammation, intravitreal DEX implants may be more effective than anti-VEGF agents in eyes with more HRD12. This explains the results from the current study, in which abundant HRD at baseline were associated with a significant reduction in CFT and increased likelihood of achieving a dry macula at six months after DEX implants.
HE develops due to protein and lipid extravasation as a result of increased vascular permeability, vasodilatation, inflammation, and a breakdown of the inner blood-retina barrier; and, if left untreated, might lead to retinal fibrosis26. Studies have shown that the presence of HE was associated with improvement of CFT and correlated with VA improvement after anti-VEGF27, while other studies reported an absence of association between HE and VA outcomes in anti-VEGF treated eyes28. Compared to bevacizumab29, DEX implants provided a more rapid regression of HE, and a recent study showed that a decrease in HE area after DEX implants correlated with VA improvement30. The current study also showed that eyes with moderate or severe HE have a significantly higher chance of improving CFT > 10%. These effects can be explained by the different mechanisms of action between anti-VEGFs and DEX implants. While anti-VEGF only exhibits anti-vascular permeability effects, DEX is involved in anti-vascular permeability, vasoconstriction, and anti-inflammatory effects31.
Lobulated retinal edema including SRF and IRC may also serve as a biomarker for DME. A study showed that the concentrations of interleukin (IL)-6 and IL-8 were significantly higher in eyes with SRF than those without SRF32. The inflammatory nature of IRC and the involved cytokines have also been extensively studied and include IL-6, IL-8, monocyte chemoattractant protein (MCP)-1, intercellular adhesion molecule (ICAM)-1, and VEGF among others33. An improvement in IRC after DEX implant was reported in previous studies8,11, in the current study, we found that severe IRC at baseline was related to a significant reduction in CFT. The association between IRC and inflammatory cytokines may explain why there is a significant response after DEX treatment in the current study, and our results suggest this biomarker as a predictor of anatomical outcomes.
The current study also identified significant negative prognostic biomarkers that predict a worse outcome after DEX treatment. After six months, eyes with prior PRP or patients who had higher baseline HbA1c levels showed worse morphological outcomes, evidenced by a lack of CFT reduction. In line with this, previous studies have shown that patients with prior PRP tended to have poorer final BCVA after DEX implants34, and that higher HbA1c levels were correlated with a worse visual outcome in DME patients15,25. In our study, 50% of the patients had prior PRP. Nevertheless, DEX implants significantly reduced DME and improved vision in 22% of the patients while significant worsening of vision was noted only in 12% of the patients after six months of DEX implant treatment.
It might be worth noting that no overall mean VA improvement was observed in the current study since this is a real-world study and that half of the patients had prior PRP. In fact, many of the published studies showed a positive functional effect in certain DME subgroups, not in the overall study population. A meta-analysis of 4 randomized trials also demonstrated that DEX implant achieved anatomical improvement but not functional enhancement at 12 months31. In recent years, studies utilizing newer imaging modalities including ultra-widefield or OCT angiography showed that DME severity or functional outcomes was related to biomarkers such as retinal vascular bed area or foveal avascular zone of the deep vascular plexus35,36. Further studies utilizing those modalities will further elucidate the response of DME to DEX implants. Nevertheless, in eyes affected by DME, morphological improvement paves the road to functional recovery, especially in those poorly responding to anti-VEGF agents.
Finally, this study has several limitations that require consideration. It was a non-randomized retrospective single-arm study with a relatively small number of eyes. The treatments were arranged using a non-unified protocol, and patients received different injections of DEX implants over the period of six months. Also, the higher proportion of patients with previous PRP (> 50%) and the longer mean duration of DME (109 days) indicates that the patient cohort leaned to the more severe and chronic end of diabetic retinopathy, which makes the results of this study less generalizable to the overall DME patients. Still, the strength lies in studying an Asian population from different medical centers. We observed that the number of DEX implants was significantly related to anatomical outcomes; nevertheless, after adjusting the number of DEX implants in the multivariate analyses, we found that other biomarkers are still significantly related to the outcomes.
In conclusion, we identified the predictive value of imaging biomarkers of anatomical outcomes in DME patients following a DEX implant in a real-world, multicenter scenario. Biomarkers including abundant HRD, moderate-to-severe HE, or severe IRC at baseline were more likely to exhibit anatomical improvements after DEX implant, while eyes with prior PRP and underlying higher HbA1C were less likely to improve. In line with this, a recent guideline for DME treatment suggested that eyes with more abundant HRD or HE should consider using DEX implant as the first-line treatment instead of anti-VEGF37. Given the anti-inflammatory nature and the ability to target more components of DME pathophysiology, DEX implant should be considered in eyes presenting these inflammatory biomarkers.
Acknowledgements
The work was funded by Allergan plc, Dublin, Ireland. All authors met the ICMJE authorship criteria. Neither honoraria nor payments were made for authorship. Writing and editorial assistance was provided to the authors by Nova Journal Experts.
Author contributions
Conceptualization: W.C.W., C.C.L.; Formal analysis: H.D.C., C.H.W., W.Y.C., N.N.C.; Investigation: H.D.C., S.C.S., C.H.W., W.Y.C., N.N.C.; Writing (original draft): H.D.C.; Writing (review and editing): W.C.W., Y.S.H., K.J.C., C.H.L., P.C.W., Y.H.C., L.Y.; Validation: H.D.C., S.C.S., C.H.W., W.Y.C., N.N.C.; Supervision: W.C.W., Y.S.H., K.J.C., C.H.L., P.C.W., Y.H.C., L.Y., C.C.L.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet387, 1513–1530 (2016). [DOI] [PMC free article] [PubMed]
- 2.Gonzalez VH, et al. Early and long-term responses to anti-vascular endothelial growth factor therapy in diabetic macular edema: analysis of protocol I data. Am. J. Ophthalmol. 2016;172:72–79. doi: 10.1016/j.ajo.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Sun JK, Jampol LM. The diabetic retinopathy clinical research network (drcr.net) and its contributions to the treatment of diabetic retinopathy. Ophthalmic Res. 2019;62:225–230. doi: 10.1159/000502779. [DOI] [PubMed] [Google Scholar]
- 4.Daruich A, et al. Mechanisms of macular edema: beyond the surface. Prog. Retin. Eye Res. 2018;63:20–68. doi: 10.1016/j.preteyeres.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Zur D, Iglicki M, Loewenstein A. The role of steroids in the management of diabetic macular edema. Ophthalmic Res. 2019;62:231–236. doi: 10.1159/000499540. [DOI] [PubMed] [Google Scholar]
- 6.London NJS, Chiang A, Haller JA. The dexamethasone drug delivery system: indications and evidence. Adv. Ther. 2011;28:351–366. doi: 10.1007/s12325-011-0019-z. [DOI] [PubMed] [Google Scholar]
- 7.Filho PM, et al. Effectiveness and safety of intravitreal dexamethasone implant (ozurdex) in patients with diabetic macular edema: a real-world experience. Ophthalmologica. 2019;241:9–16. doi: 10.1159/000492132. [DOI] [PubMed] [Google Scholar]
- 8.Rosenblatt A, et al. A collaborative retrospective study on the efficacy and safety of intravitreal dexamethasone implant (ozurdex) in patients with diabetic macular edema: the European DME registry study. Ophthalmology. 2020;127:377–393. doi: 10.1016/j.ophtha.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Iglicki M, et al. TRActional DIabetic reTInal detachment surgery with co-adjuvant intravitreal dexamethasONe implant: the tradition study. Acta Diabetol. 2019;56:1141–1147. doi: 10.1007/s00592-019-01357-y. [DOI] [PubMed] [Google Scholar]
- 10.Iglicki M, et al. Dexamethasone implant for diabetic macular edema in naive compared with refractory eyes: The International Retina Group Real-Life 24-Month Multicenter Study. The IRGREL-DEX Study. Retina (Philadelphia, Pa) 2019;39:44–51. doi: 10.1097/IAE.0000000000002196. [DOI] [PubMed] [Google Scholar]
- 11.Meduri A, et al. Optical coherence tomography predictors of favorable functional response in naïve diabetic macular edema eyes treated with dexamethasone implants as a first-line agent. J. Ophthalmol. 2021;2021:6639418. doi: 10.1155/2021/6639418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vujosevic S, et al. Diabetic macular edema with neuroretinal detachment: OCT and OCT-angiography biomarkers of treatment response to anti-VEGF and steroids. Acta Diabetol. 2020;57:287–296. doi: 10.1007/s00592-019-01424-4. [DOI] [PubMed] [Google Scholar]
- 13.Iglicki M, Loewenstein A, Barak A, Schwartz S, Zur D. Outer retinal hyperreflective deposits (ORYD): a new OCT feature in naïve diabetic macular oedema after PPV with ILM peeling. Br. J. Ophthalmol. 2020;104:666–671. doi: 10.1136/bjophthalmol-2019-314523. [DOI] [PubMed] [Google Scholar]
- 14.Zur D, et al. OCT biomarkers as functional outcome predictors in diabetic macular edema treated with dexamethasone implant. Ophthalmology. 2018;125:267–275. doi: 10.1016/j.ophtha.2017.08.031. [DOI] [PubMed] [Google Scholar]
- 15.Chatziralli IP, Sergentanis TN, Sivaprasad S. Hyperreflective foci as an independent visual outcome predictor in macular edema due to retinal vascular diseases treated with intravitreal dexamethasone or ranibizumab. Retina (Philadelphia, Pa) 2016;36:2319–2328. doi: 10.1097/IAE.0000000000001070. [DOI] [PubMed] [Google Scholar]
- 16.Hwang HS, Chae JB, Kim JY, Kim DY. Association between hyperreflective dots on spectral-domain optical coherence tomography in macular edema and response to treatment. Invest. Ophthalmol. Vis. Sci. 2017;58:5958–5967. doi: 10.1167/iovs.17-22725. [DOI] [PubMed] [Google Scholar]
- 17.Cavalleri M, et al. Prognostic role of optical coherence tomography after switch to dexamethasone in diabetic macular edema. Acta Diabetol. 2020;57:163–171. doi: 10.1007/s00592-019-01389-4. [DOI] [PubMed] [Google Scholar]
- 18.Vujosevic S, et al. Imaging retinal inflammatory biomarkers after intravitreal steroid and anti-VEGF treatment in diabetic macular oedema. Acta Ophthalmol. 2017;95:464–471. doi: 10.1111/aos.13294. [DOI] [PubMed] [Google Scholar]
- 19.Zur D, et al. Disorganization of retinal inner layers as a biomarker in patients with diabetic macular oedema treated with dexamethasone implant. Acta Ophthalmol. 2020;98:e217–e223. doi: 10.1111/aos.14230. [DOI] [PubMed] [Google Scholar]
- 20.Chew EY, et al. Association of elevated serum lipid levels with retinal hard exudate in diabetic retinopathy. Early treatment diabetic retinopathy study (ETDRS) Report 22. Arch. Ophthalmol. 1996;114:1079–1084. doi: 10.1001/archopht.1996.01100140281004. [DOI] [PubMed] [Google Scholar]
- 21.Panozzo G, et al. An optical coherence tomography-based grading of diabetic maculopathy proposed by an international expert panel: The European school for advanced studies in ophthalmology classification. Eur. J. Ophthalmol. 2020;30:8–18. doi: 10.1177/1120672119880394. [DOI] [PubMed] [Google Scholar]
- 22.Chen N-N, et al. Optical coherence tomographic patterns as predictors of structural outcome after intravitreal ranibizumab in diabetic macula edema. Clin. Ophthalmol. 2020;14:4023–4030. doi: 10.2147/OPTH.S264669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang C-H, et al. Hyperreflective foci in predicting the treatment outcomes of diabetic macular oedema after anti-vascular endothelial growth factor therapy. Sci. Rep. 2021;11:5103. doi: 10.1038/s41598-021-84553-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang J-W, Chung H, Kim HC. Correlation of optical coherence tomographic hyperreflective foci with visual outcomes in different patterns of diabetic macular edema. Retina (Philadelphia, Pa) 2016;36:1630–1639. doi: 10.1097/IAE.0000000000000995. [DOI] [PubMed] [Google Scholar]
- 25.Ceravolo, I. et al. The application of structural retinal biomarkers to evaluate the effect of intravitreal ranibizumab and dexamethasone intravitreal implant on treatment of diabetic macular edema. Diagnostics (Basel)10, (2020). [DOI] [PMC free article] [PubMed]
- 26.Ota M, et al. Optical coherence tomographic evaluation of foveal hard exudates in patients with diabetic maculopathy accompanying macular detachment. Ophthalmology. 2010;117:1996–2002. doi: 10.1016/j.ophtha.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 27.Srinivas S, et al. Effect of Intravitreal ranibizumab on intraretinal hard exudates in eyes with diabetic macular edema. Am. J. Ophthalmol. 2020;211:183–190. doi: 10.1016/j.ajo.2019.11.014. [DOI] [PubMed] [Google Scholar]
- 28.Domalpally A, Ip MS, Ehrlich JS. Effects of intravitreal ranibizumab on retinal hard exudate in diabetic macular edema: findings from the RIDE and RISE phase III clinical trials. Ophthalmology. 2015;122:779–786. doi: 10.1016/j.ophtha.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 29.Mehta H, et al. Efficacy of dexamethasone versus bevacizumab on regression of hard exudates in diabetic maculopathy: data from the BEVORDEX randomised clinical trial. Br. J. Ophthalmol. 2016;100:1000–1004. doi: 10.1136/bjophthalmol-2015-307797. [DOI] [PubMed] [Google Scholar]
- 30.Yoon CK, et al. Title: efficacy of intravitreal dexamethasone implant on hard exudate in diabetic macular edema. BMC Ophthalmol. 2021;21:41. doi: 10.1186/s12886-020-01786-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He Y, Ren X-J, Hu B-J, Lam W-C, Li X-R. A meta-analysis of the effect of a dexamethasone intravitreal implant versus intravitreal anti-vascular endothelial growth factor treatment for diabetic macular edema. BMC Ophthalmol. 2018;18:121. doi: 10.1186/s12886-018-0779-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sonoda S, et al. Retinal morphologic changes and concentrations of cytokines in eyes with diabetic macular edema. Retina (Philadelphia, Pa) 2014;34:741–748. doi: 10.1097/IAE.0b013e3182a48917. [DOI] [PubMed] [Google Scholar]
- 33.Spaide RF. Retinal vascular cystoid macular edema: review and new theory. Retina (Philadelphia, Pa) 2016;36:1823–1842. doi: 10.1097/IAE.0000000000001158. [DOI] [PubMed] [Google Scholar]
- 34.Lai I-A, Hsu W-C, Yang C-M, Hsieh Y-T. Prognostic factors of short-term outcomes of intravitreal ranibizumab in diabetic macular edema. Int. J. Ophthalmol. 2017;10:765–771. doi: 10.18240/ijo.2017.05.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsai ASH, et al. Diabetic macular ischemia: influence of optical coherence tomography angiography parameters on changes in functional outcomes over one year. Invest. Ophthalmol. Vis. Sci. 2021;62:9. doi: 10.1167/iovs.62.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fan W, et al. Severity of diabetic macular edema correlates with retinal vascular bed area on ultra-wide field fluorescein angiography: dave Study. Retina (Philadelphia, Pa) 2020;40:1029–1037. doi: 10.1097/IAE.0000000000002579. [DOI] [PubMed] [Google Scholar]
- 37.Kodjikian L, et al. First-line treatment algorithm and guidelines in center-involving diabetic macular edema. Eur. J. Ophthalmol. 2019;29:573–584. doi: 10.1177/1120672119857511. [DOI] [PubMed] [Google Scholar]