Abstract
To improve the potency of Heptamethine cyanines (Hcyanines) in cancer research, we designed and synthesized two novel Hcyanines based theranostic probes, IR794-Morph and IR794-Morph-Mpip, to enhance cancer cell internalization and targeting. In acidic conditions that resemble to tumour environment, both IR794 derivatives exhibited broad NIR absorption band (704‒794 nm) and fluorescence emission (798‒828 nm) that is suitable for deep seated tumour imaging. Moreover, in vitro study revealed that IR794-Morph-Mpip exhibited better cancer targetability towards various cancer cell lines under physiological and slightly acidic conditions compared to normal cells. IR794-Morph-Mpip was fast internalized into the cancer cells within the first 5 min and mostly localized in lysosomes and mitochondria. In addition, the internalized signal was brighter when the cells were in the hypoxic environment. Furthermore, cellular uptake mechanism of both IR794 dyes, investigated via flow cytometry, revealed that endocytosis through OATPs receptors and clathrin-mediated endocytosis were the main routes. Moreover, IR794-Morph-Mpip, displayed anti-cancer activity towards all tested cancer cell types with IC50 below 7 μM (at 6 h incubation), which is approximately three times lower than that of the normal cells. Therefore, increasing protonated cites in tumour environment of Hcyanines together with incorporating morpholine in the molecule can enhance structure-inherent targeting of these dyes.
Subject terms: Chemistry, Chemical biology, Medicinal chemistry, Organic chemistry
Introduction
Heptamethine cyanines (Hcyanines) are near-infrared (NIR) fluorescent dyes that can absorb and emit light in a range 700–1000 nm, which is suitable for tumour detection at millimetre depth due to less background fluorescence from endogenous molecules1,2. Hcyanines were used extensively as tools for cancer imaging because of their bright fluorescence in NIR region and good biocompatibility3,4. Moreover, some Hcyanines with a meso-chloride and a cyclohexenyl skeleton were found to accumulate and persist in solid tumors for several days5–9. Although there is no clear reason why they tend to accumulate in solid tumors, uptake into cancer cells via organic anion transporting polypeptides (OATPs)10–12 and roles of albumin in Hcyanines accumulation and persistence in solid tumor were discussed6,13,14. The uptake of Hcyanines in cancer cells was concerted actions exerted by hypoxia and activation of HIF1alpha/OATPs signalling leading to enhance dye uptake9,15. In general, cancer cells behave differently in comparison with normal cells16,17. For example, the lack of oxygen in tumour creates strong hypoxic condition leading to lactic acid build-up and lower extracellular pH level in tumour environment (pH 6.2–6.9)18,19. In addition, the lysosomal pH in cancer cells (pHlys 3.8 – 4.7) expresses higher acidity than that in the normal cells (pHlys 4.5–6.0)20.
Recently, an idea of developing structure–inherent targeting (SIT) NIR fluorescent dyes was proposed5,21,22. This offers a new opportunity to realize targeting delivery with no need of extra conjugations. In this work, we took an advantage of Hcyanines structures that can be recognized by OATPs on tumour cell surface. Additionally, we added more protonation sites into the structures to turn them to cationic probes when they reach tumour environment. Generally, particles carrying positive charges tend to have high cell-membrane binding affinity due to attractive electrostatic forces between cationic probe and anionic cellular membrane23,24. Therefore, we expected that our cationic dyes would internalize cancer cells faster than the neutral dyes.
Besides the charges, morpholine was also conjugated to the dyes aiming at lysosome targeting, which lysosomes emerged as an attractive target for cancer diagnosis and therapy25,26. Morpholine is a pharmacophore that involves in wide range of biological activities, including anti-cancer27,28. Altering pH in lysosomes might cause the organelle swelling and disruption, leading to cell death. Hence, if the particles could enter the cells via endocytosis, it has a high possibility to accumulate at lysosomes and trigger cellular cascades that can be harmful to the cells29.
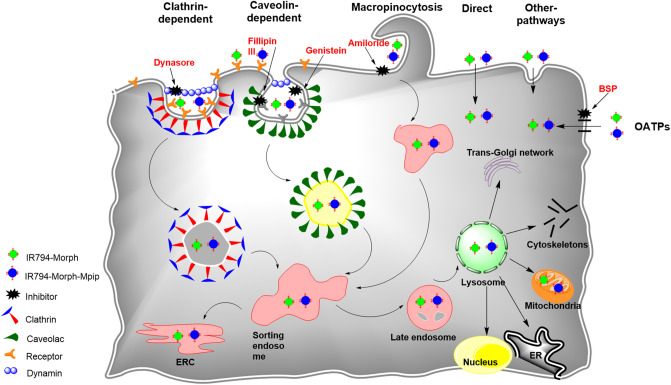
Endocytosis is an energy-dependent process that cells transport substances from outside by engulfing them in a vesicle30. Large particles tend to be internalized by phagocytosis, whereas small molecules that suspended in extracellular fluid enter the cells via pinocytosis31. Pinocytosis including macropinocytosis, and clathrin- or caveolin-dependent endocytosis have been extensively studied32,33. Clathrin-mediated endocytosis plays a key role in cell signalling through the trafficking of membrane receptors34. After endocytosis, the uncoated vesicles will fuse with early endosomes and transport to Golgi for signal processing or direct to lysosomes for degradation while some of the receptors will recycle back to the membrane35. The clathrin-independent endocytosis, however, is much less understood, except caveolar endocytosis that is mediated by bulb-shaped plasma membrane named caveolae33,36.
Therefore, in this work, we developed a Hcyanine based theranostic probe (IR794), with NIR fluorescence and anti-cancer effect, that was intended to target tumour environment by selective internalization to cancer cells via OATPs. Our experimental data revealed that IR794-Morph and IR794-Morph-Mpip increased HepG2 cells uptake via clathrin-mediated endocytosis and offered high photocytotoxic efficacies. Moreover, IR794-Morph-Mpip (more positive charge) exhibited intense NIR absorbance in acidic pH and bright fluorescence in tumour cells especially under hypoxic environment. In addition, cytotoxicity profiles of these probes were also investigated in various cell lines.
Results and discussion
Synthetic procedures
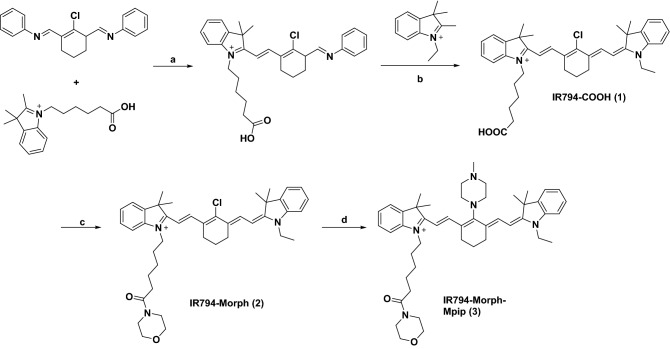
Synthesis of asymmetrical Hcyanines IR794-COOH (1) involves the condensation of alkyl indoleninium salts in steps a and b (Fig. 1). IR794-Morph (2) was further synthesized via an amide coupling reaction between IR794-COOH (1) and morpholine using EDC.HCl as a coupling reagent and DMAP as a catalyst. IR794-Morph-Mpip (3) was synthesized by conjugation addition of Hcyanine 2 with N-methylpiperazine. These compounds were fully characterized by 1H and 13C NMR spectroscopy and high-resolution mass spectrometry (HRMS) (data are available in ESI).
Figure 1.
Syntheses of IR794 derivatives. Reagents: (a, b) NaOAc, EtOH, 80 °C, 2 h37. (c) Morpholine, EDC.HCl, DMAP, CH2Cl2, 0 °C, under N2, 2 h38. (d) N-methylpiperazine, DMF, 25 °C, under N2, 3 h39.
Photophysical properties
We expected that substitution of N-methylpiperazine group on the cyclohexenyl position of 2 yielded 3 with pH responsive ability. At neutral pH, the fluorescence of 3 is expected to be quenched by the effect from the nitrogen lone pair electrons of N-methylpiperazine moiety through a photoinduced electron transfer (PeT) process. While in acidic environments, protonation of the nitrogen atoms will block the PeT process causing the increased fluorescence signal. To explore this phenomenon, optical properties of IR794 have been systematically investigated.
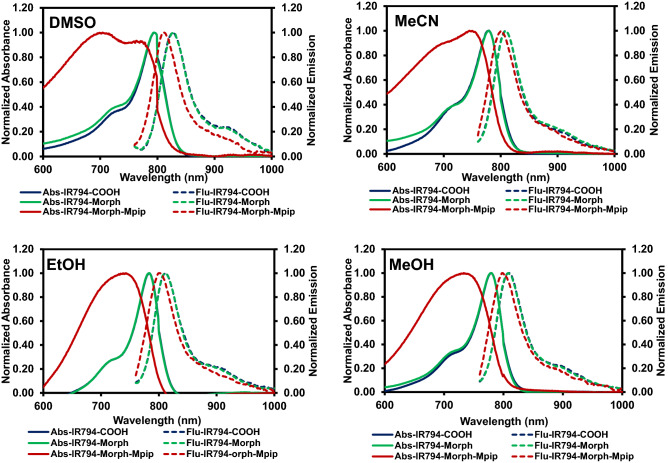
The absorbance and fluorescence spectra of IR794 (1–3) were first measured in various organic solvents including, DMSO, MeCN MeOH, and EtOH. The photophysical properties listed in Table 1 showed that all three IR794 compounds exhibited absorption maxima at the range from 704 to 794 nm while possessed emission maxima at the range from 798 to 828 nm. Interestingly, most of the stokes shifts of these compounds are in the scope from 28 to 65 nm, except that of IR794-Morph-Mpip in DMSO that displays a large stoke shift of 108 nm which could be the result from unique intermolecular forces between the methylpiperazine moiety and DMSO molecules. The fluorescent quantum yields (Φf) of these compounds were obtained in the range between 0.1 and 0.2 which are typical among Hcyanines. However, it is worth to note that the probes in polar aprotic solvents (i.e. DMSO and MeCN) exhibited higher Φf than those in polar protic solvents (i.e. MeOH and EtOH).
Table 1.
The photophysical properties of IR794 (1–3) in the different solvents.
| Dye | Solvent | aλmax (nm) | bλemiss (nm) | c∆ʋ(nm) | dΦf | eε (M−1 cm−1) |
|---|---|---|---|---|---|---|
| IR794-COOH | DMSO | 794 | 827 | 33 | 0.192 | 5.1 × 104 |
| MeCN | 779 | 808 | 29 | 0.202 | 3.4 × 104 | |
| EtOH | 784 | 812 | 28 | 0.164 | 5.0 × 104 | |
| MeOH | 779 | 809 | 30 | 0.119 | 6.7 × 104 | |
| IR794-Morph | DMSO | 794 | 828 | 34 | 0.176 | 7.8 × 104 |
| MeCN | 779 | 810 | 31 | 0.149 | 7.5 × 104 | |
| EtOH | 783 | 812 | 29 | 0.166 | 7.9 × 104 | |
| MeOH | 779 | 808 | 29 | 0.120 | 8.6 × 104 | |
| IR794-Morph-Mpip | DMSO | 704 | 812 | 108 | 0.206 | 2.2 × 104 |
| MeCN | 745 | 801 | 56 | 0.167 | 3.3 × 104 | |
| EtOH | 743 | 800 | 57 | 0.131 | 3.5 × 104 | |
| MeOH | 733 | 798 | 65 | 0.101 | 3.7 × 104 |
aλabs = absorption maximum wavelength, bλem = emission maximum wavelength (Excitation wavelength = 750 nm), c∆λ = stokes shifts (λem − λabs), dΦf = fluorescence quantum yields calculated by using Indocyanine green (ICG) was used as a standard (Φ = 0.13 in DMSO), eε = molar absorptivity.
Based on the absorption and emission spectra demonstrated in Fig. 2, IR794-COOH and IR794-Morph displayed inherent spectral characteristics of the typical Hcyanines in all solvents. Notably, the absorption spectra of IR794-Morph-Mpip showed a broad appearance which could be the effect from N-methylpiperazine moiety as seen in our previous report39. The excitation spectra of IR794-Morph-Mpip in organic solvents also demonstrated similar appearance with the absorption spectra as presented in Fig. S1.
Figure 2.
Vis–NIR absorption and fluorescent spectra of IR794 derivatives (1 µM) excited at 750 nm in DMSO, MeCN, EtOH and MeOH.
pH effects of IR794 by vis–NIR and fluorescence spectroscopic analysis
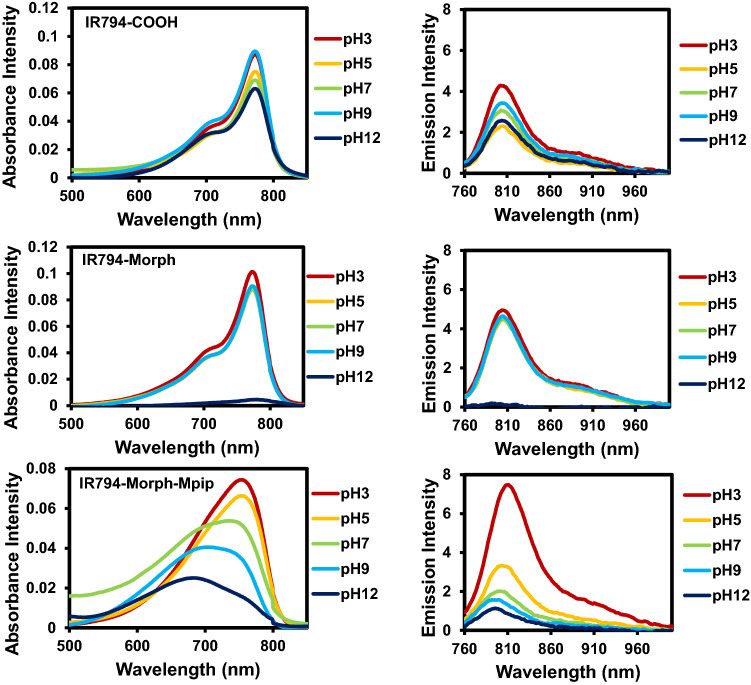
Since the IR794 derivatives contain different functional groups in the structures (‒COOH, ‒Morph and –Morph-Mpip), it is possible that they could form different charge states at different pH (3–12), leading to the alteration of photophysical properties of our IR794. As shown in Fig. 3, there is no significant changes in IR794-COOH absorption and emission spectra under either acidic or basic conditions. In contrast, IR794-Morph, shows hypochromic effect of the spectra where the absorption and emission decreasing the intensity in highly basic condition (pH 12).
Figure 3.
Vis–NIR absorption and fluorescent spectra of IR794 in different pH 3.0–12.0.
IR794-Morph-Mpip, on the other hand, displays bathochromic shifts of absorbance and fluorescence spectra in acidic conditions. In addition, the emission signals are enhanced as the acidity of the media increases. These phenomena might cause by the effect of proton exchange of N-methylpiperazine moiety (pKa = 3.81 and 8.38)40 that alter optical properties of the dye subjectable to the pH of the solutions. IR794-Morph-Mpip absorbs light from visible to NIR region (500–920 nm), peaking at 750 nm under neutral pH. When two nitrogen atoms of N-methylpiperazine on IR794-Morph-Mpip are fully protonated under acidic environments (pH 3, 5), absorption spectra are red shifted. Whereas, once IR794-Morph-Mpip exposes to basic environments (pH 9, 12), absorption spectra are blue shifted. These could be explained by an intramolecular charge transfer (ICT) within the molecule. Since the IR794-Morph-Mpip contains both electron donor (amine) and acceptor (Hcyanine), a charge separation is obtained within the fluorophore. The electron-donating ability of the donor at the meso-position in a cyanine scaffold would cause absorption and/or fluorescence spectra shift as seen in the previous study39.
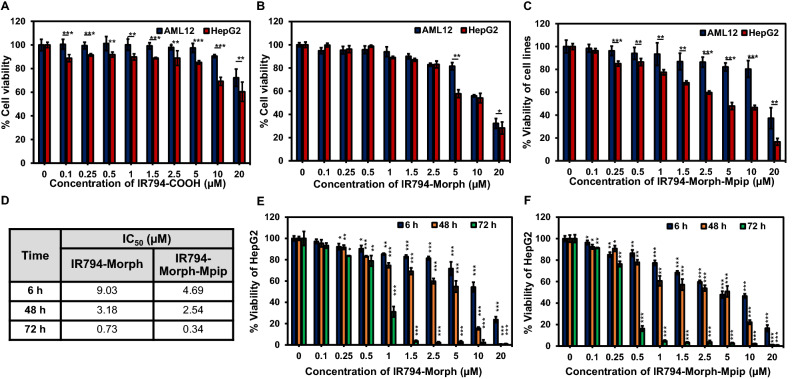
To discover applications of IR794 derivatives in living cells, series of in vitro experiments were performed. First, cytotoxicity profile of IR794 on liver cells including normal, alpha mouse liver 12 (AML12), and cancer, human hepatoma (HepG2), cells were evaluated at various concentrations of IR794 (0‒5.0 μM) for 6 h. The comparative cell viability was determined by standard MTT assays39,41. As shown in Fig. 4A–C, at concentrations up to 5 μM, the normal cells maintained more than 80% viability when they exposed to IR794 derivatives. However, the compounds started to cause toxicity to the normal cells when the dose were higher than 5 μM. Remarkably, among the series, IR794-Morph-Mpip exhibited the best cancer selectivity with significant anti-cancer effect towards HepG2 with IC50 4.69 μM (IC50 = 8.88 μM for IR794-Morph and IC50 > 20 μM for IR794-COOH, Table 2 and Fig. S2).
Figure 4.
Relative viabilities of AML12 cells (blue bars) and HepG2 cells (red bars) after exposure to IR794-COOH (A), IR794-Morph (B) and IR794-Morph-Mpip (C) at various concentrations (0‒20 μM) for 6 h. (D) IC50 values of IR794-Morph and IR794-Morph-Mpip treated with HepG2 for 6, 48 and 72 h. Relative viabilities of HepG2 cells treated with IR794-Morph (E) and IR794-Morph-Mpip (F) for 6 h (blue bars), 48 h (orange bars) and 72 h (green bars). Statistical analysis is based on Paired Student’s T-test analysis (*P < 0.05, **P < 0.01, ***P < 0.001) where (A–C) is the comparison between AML-2 and HepG2 at the same concentration; (E,F) is the comparison between the treatment and the control.
Table 2.
IC50 of IR794 treated with AML12 and HepG2 cells for 6 h.
| Cells | IC50 (µM) | ||
|---|---|---|---|
| IR794-COOH | IR794-Morph | IR794-Morph-Mpip | |
| AML12 | > 20 | 12.01 | 19.68 |
| HepG2 | > 20 | 8.88* | 4.69* |
Moreover, to better understand the effect of the compounds on cell viability, HepG2 cells were incubated with IR794-Morph and IR794-Morph-Mpip for extended period (48 and 72 h). As expected, a prolonged incubation resulted in reducing cell viability (Fig. 4D–F). After 72 h treatment, HepG2 cell viability reduced dramatically when exposed to IR794-Morph and IR794-Morph-Mpip with IC50 = 0.73 and 0.34 μM, respectively (Figs. 4D and S2). However, the cytotoxicity of these two probes is still not as good as that of an anti-cancer agent, i.e. doxorubicin, which has IC50 = 2.16 and 0.02 μM when the cells were treated for 6 and 24 h, respectively (Fig. S3). Nevertheless, IR794-Morph-Mpip displayed superior anti-cancer effect compared to IR794 analogues.
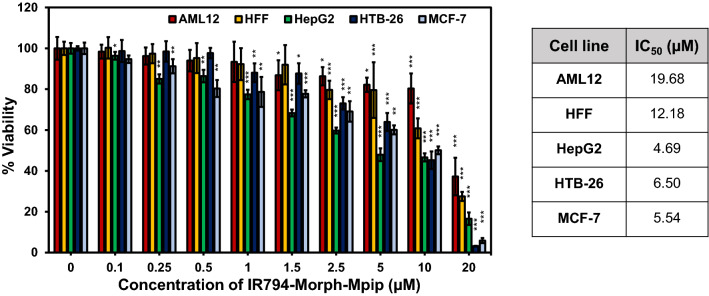
As IR794-Morph-Mpip showed some cancer selectivity, its activity towards other cell lines including human foreskin fibroblasts (HFF), and human breast cancer cells (HTB-26 or MDA-MB-231 and MCF-7) was also investigated. After 6 h incubation, IR794-Morph-Mpip exhibited selective cancer eradicating with IC50 values below 7 μM for all cancer lines, whereas IC50 values are higher than 12 μM for the normal lines (Fig. 5). However, after extended incubation time, no significant cancer cell selectivity was observed.
Figure 5.
Relative viabilities of various cell lines after exposure to IR794-Morph-Mpip at various concentrations (0‒20 μM) for 6 h. Statistical analysis is based on Pair Student’s T-test analysis (*P < 0.05, **P < 0.01, ***P < 0.001) which compared between the treatment and the control of each cell line. Table shows IC50 of IR794-Morph-Mpip treated on different cell lines.
Cell internalization
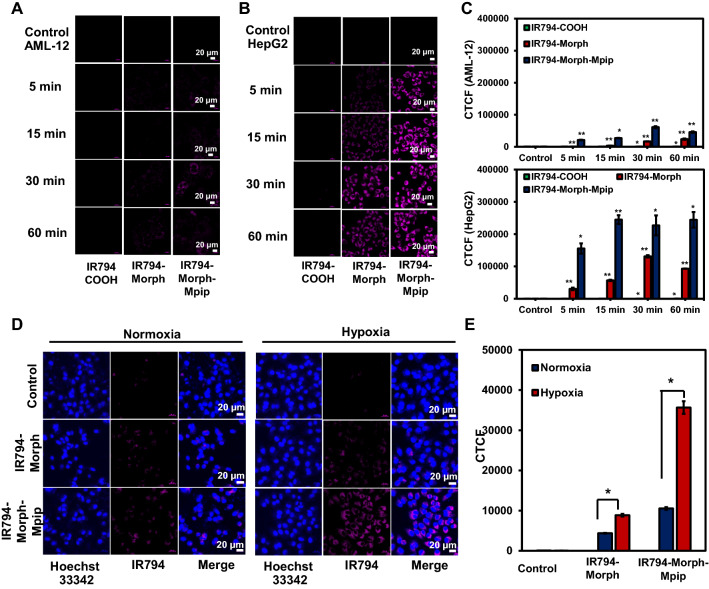
Subsequent, time dependent internalization of IR794 in normal cells (AML12) and cancer cells (HepG2) was monitored to investigate how fast the probes can enter cells. At the first hour, there was little to no internalized signals of all three probes from AML12 cells (Figs. 6A–C and S4). In contrast, the fast uptake of IR794-Morph-Mpip in HepG2 cells was obviously detected within the first 5 min of incubation (Figs. 6B,C and S5). In case of IR794-Morph, the signal was clearly observed from HepG2 cells after 15 min incubation, but the intensity was not as bright as the fluorescence from IR794-Morph-Mpip. Interestingly, there is no signal of IR794-COOH from both normal and cancer cells, implying no uptake of this dye at the first hour of incubation. In addition, IR794-Morph-Mpip was found to be fast internalized in all tested cancer cell lines (MCF-7, HTB26 and HepG2), Fig. S6.
Figure 6.
Confocal images of (A) AML12 and (B) HepG2 cells obtained using a laser scanning confocal microscope (Nikon A1Rsi, 63 × oil immersed optics) incubated with 1 μM of IR794 for 5–60 min. (C) Quantitative fluorescent intensity represented as corrected total cell fluorescence (CTCF), which were quantified using ImageJ and represent the mean ± SD (from three independent experiments, 30 cells/set). (D) Confocal images of HepG2 cells incubated with 0.5 μM of IR794-Morph and IR794-Morph-Mpip for 5 min under normoxia and hypoxia conditions. (E) Quantitative CTCF quantified using ImageJ and represent the mean ± SD (n = 3, 30 cells/set). Statistical analysis is based on T-test (*P < 0.05, **P < 0.01, ***P < 0.001) where the comparison between the control and different incubation duration (for each compound) is marked in C and the comparison between normoxia and hypoxia is marked in (D). Scale bar = 20 μm.
To further verify that IR794-Morph-Mpip is a pH-sensitive theranostic reagent and the increasing of protonation sites could improve the targeting of cyanine dyes to tumour cells, cellular uptake under hypoxia condition was investigated. As depict in Fig. 6D and E, compared to the normoxic condition, the fluorescent signals significantly enhance when IR794-Morph-Mpip treated with hypoxic cells only for 5 min at low dose (0.5 μM). Nevertheless, IR794-Morph did not show the same effect under hypoxia. Tumour hypoxia is a common feature in solid tumour that arises when oxygen supply is deficient, resulting in an acidic environment inside tumour micro-environment19. Therefore, when IR794-Morph-Mpip reaches hypoxic environment, it is highly protonated leading to fluorescence enhancement.
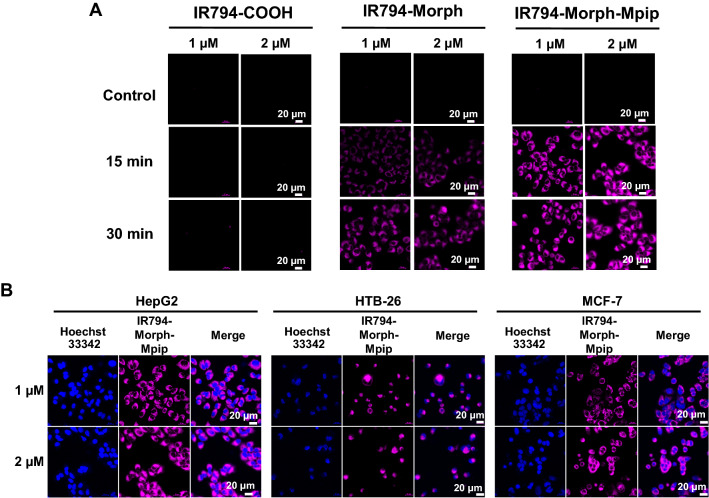
Furthermore, in HepG2 cells, the fluorescence signal was found to be in a dose-dependent manner (Fig. 7A). When concentrations of IR794-Morph-Mpip and IR794-Morph increased from 1 to 2 µM, the HepG2 cells uptake was also improved. After incubation time was increased to 30 min, the enhancement of the signal was also observed. The difference is more noticeable in the case of IR794-Morph (15 min vs 30 min), while signal from IR794-Morph-Mpip seems to be saturated since 15 min incubation. Moreover, the signal from IR794-Morph-Mpip in other cancer cells (MCF-7 and HTB-26) was slightly higher when the dose increased (Fig. 7B); quantitative fluorescent signals are shown in Fig. S7. From this result, 1 µM of IR794-Morph-Mpip and IR794-Morph was enough for cell internalization and could be used as an indicator to distinguish cancer from normal cells.
Figure 7.
(A) Confocal images of dose-dependent effect of IR794 (1 and 2 µM) in HepG2 cells, which were incubated for 15 and 30 min. (B) Confocal images of cancer cells (HepG2, HTB-26 and MCF-7) after treated with IR794-Morph-Mpip (1 and 2 µM) for 15 min.
Intracellular localization study
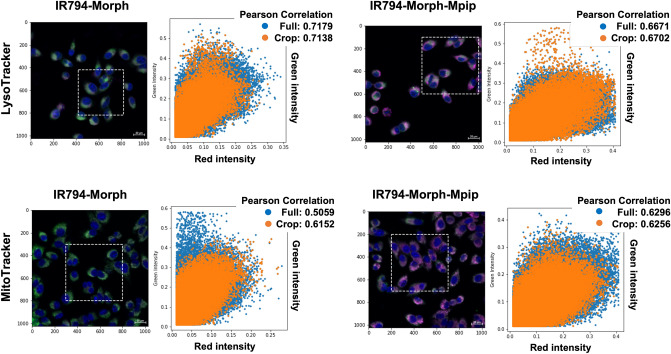
As IR794-Morph and IR794-Morph-Mpip were fast uptake in cancer cells, intracellular localization of both probes was studied. Both dyes contain morpholine moiety in the structure, which is known to direct a molecule to lysosomes42–44. Moreover, some reported Hcyanines with net positive charge on the structures tend to localize in mitochondria45,46. Therefore, Hoechst 33342 (for nuclei), LysoTracker (for lysosomes) and MitoTracker (for mitochondria) were used as markers to pinpoint the intracellular localization of IR794-Morph and IR794-Morph-Mpip. The confocal images in Fig. 8 reveals magenta fluorescence of IR794-Morph and IR794-Morph-Mpip overlaps with LysoTracker (Pearson’s R value = 0.72 for IR794-Morph and 0.67 for IR794-Morph-Mpip) and MitoTracker (Pearson’s R value = 0.51 for IR794-Morph and 0.63 for IR794-Morph-Mpip). From these results, IR794-Morph prefers to localize in lysosome rather than mitochondria. Whereas IR794-Morph-Mpip localizes in both organelles with a similar amount. This could be because extra positive charge on the N-methylpiperazine makes the molecule favours import across the inner mitochondrial membrane.
Figure 8.
Confocal images of HepG2 cells incubated with 1 µM of IR794-Morph and IR794-Morph-Mpip for 15 min and colocalization of the probes with LysoTracker green (Pearson’s R value = 0.72 for IR794-Morph and 0.67 for IR794-Morph-Mpip) and MitoTracker (Pearson’s R value = 0.51 for IR794-Morph and 0.63 for IR794-Morph-Mpip). Pearson’s correlation coefficient between the two intensities was performed in python, using a built-in function from scipy statistical package.
Cellular trafficking study
Furthermore, we would like to understand the uptake pathways of both dyes, IR794-Morph and IR794-Morph-Mpip. We hypothesized that the extra positive charges of IR794-Morph-Mpip might alter the uptake mechanism of the dye.
In general, cellular uptake of macromolecules occurs via ATP-dependent endocytosis and this process can be attenuated at low temperature. Therefore, the effect of temperature on cellular uptake was studied by incubating IR794-Morph and IR794-Morph-Mpip with HepG2 cells at 4 °C for 30 min and the cell uptake was quantified using flow cytometry. At 4 °C, percent medium fluorescence intensity (% MFI) of both compounds is about 50% (Fig. 9) compared to the uptake at 37 °C. This indicates that the endocytosis of both dyes is energy dependent.
Figure 9.
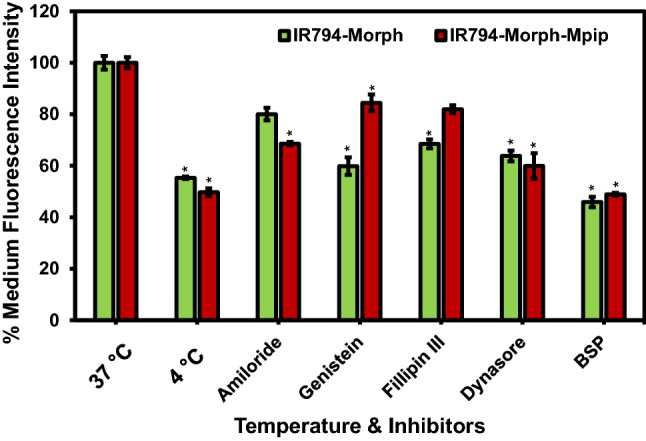
Flow cytometry of HepG2 cells incubated with 1 μM of IR794 for 15 min in culture media. Cellular trafficking pathway of IR794-Morph and IR794-Morph-Mpip characterised by various inhibitors and at 4 °C condition. The endocytosis inhibitors used include amiloride (micropinocytosis), genistein (caveolin-mediated), Fillipin III, dynasore (clathrin-mediated) and BSP. Data reports as % Medium fluorescence intensity and represents the mean ± SD (n = 3, independent experiment). Statistical analysis is based on Pair Student’s T-test analysis (*P < 0.05, **P < 0.01, ***P < 0.001) where the comparison between the uptake at 37 °C and the uptake in the presence of different inhibitors is marked.
To further explore cellular trafficking, a panel of endocytosis inhibitors were selected to inhibit specific endocytic pathways47. These include Filipin III and genistein, which inhibit caveolae-mediated endocytosis, amiloride which is against micropinocytosis, and dynasore which blocks clathrin-mediated endocytosis. Quantitative fluorescence from flow cytometry displayed that genistein, Filipin III, and dynasore had significant effect on the internalization of IR794-Morph by reducing the cell uptake to lower than 70%. However, amiloride and dynasore showed significant inhibition of IR794-Morph-Mpip uptake (< 70%). These findings suggested that both dyes follow endocytosis through clathrin-mediated pathway. In addition, caveolae-mediated endocytosis is a predominant pathway for IR794-Morph uptake whereas micropinocytosis is another main route for IR794-Morph-Mpip uptake.
It was reported that some Hcyanine dyes containing cyclohexyl ring in the conjugation system localize in solid tumor48 but not in normal tissue12,45,49–51 which the organic anion transporter proteins (OATPs) was suggested to be the main uptake channel. OATPs are the cell surface receptors that overexpressed on solid tumors but not on the normal tissue52. Therefore, in this study, we treated a nonspecific OATPs inhibitor, bromosulfophthalein (BSP), with HepG2 cells prior to add IR794-Morph and IR794-Morph-Mpip to determine if the dyes uptake and accumulation were dependent upon OATPs. From Fig. 8, after blocking OATPs, the uptake of both dyes was significantly reduced, confirming the OATP-dependent trafficking. Schematic representation of possible internalization pathways of our probes and inhibitors is displayed in Fig. 10.
Figure 10.
Schematic representation of the possible internalization pathways of IR794-Morph and IR794-Morph-Mpip.
Conclusions
In summary, IR794-Morph and IR794-Morph-Mpip were successfully developed as pH-sensitive theranostic agents for fluorescent imaging and anti-cancer. IR794-Morph-Mpip absorbs slightly red-shifted in NIR region under acidic conditions and emit strong fluorescence at low pH, which is suitable to visualize cells in tumour environment, especially in hypoxia. Moreover, IR794-Morph and IR794-Morph-Mpip selectively internalized cancer cells and the fast uptake was observed in dose- and time-dependence manners. Cytotoxicity profiles confirmed selectivity of both probes toward cancer cells where the derivative containing morpholine, IR794-Morph-Mpip, offered superior anti-cancer activity with IC50 below 7 μM for all tested cancer cells. Intracellular trafficking of both probes was proved to be endocytosis via transporters (OATPs) and clathrin-dependent pathway. Therefore, adding morpholine moiety and increasing protonation sites of cyanine dyes enhance cancer cells selectivity and cytotoxicity.
Methods
General details for vis–NIR and fluorescence measurements
Stock solutions (1000 µM) of IR794 probes were prepared in DMSO then diluted to 1 µM in a 3 mL quartz cuvette in various solvents (DMSO, MeCN, EtOH, MeOH and buffers pH 3–12). Vis–NIR absorption spectra were recorded on a UV–vis Spectrophotometer (Agilent Technologies Cary 300). The fluorescence spectra were recorded by PTI QuantaMaster 500—Near Infra-Red Photoluminescence System (HORIBA Scientific), using the following parameters: excitation wavelengths = 750 nm, excitation slit widths = 10 nm, and emission slit widths = 10 nm.
Cell culture
Human liver hepatocellular carcinoma (HepG2), human breast cancer (MCF-7), human breast adenocarcinoma (HTB-26 or MDA-MB-231), human foreskin fibroblasts (HFF) and alpha mouse liver 12 (AML12) cell lines were purchased from the American Type Culture Collection (ATCC) and cultured according to the company procedure. All the cells were incubated at 37 °C in a humidified 95% air, 5% CO2 atmosphere.
Cell viability assay
The cells were seeded roughly 7 × 104 cells per well on 96-well plate and incubated in completed media for 24 h. Thereafter, the cells were treated with 0, 0.1, 0.25, 0.50, 1.0, 1.5, 2.5, 5.0, 10 and 20 µM of IR794 series and continued culturing for 6, 48 and 72 h. After washing with PBS, the cell viability was determined using the standard MTT protocol39,41.
Relative viability of cell is calculated by: % Viability = (Atreatment − Ablank)/(Acontrol − Ablank) × 100% (where, A = absorbance at λ = 560 nm).
Time- and dose-dependent internalization
Cells, approximately 1 × 104 cells, were seeded on an 8-well chambered coverglass (LabTek, Nunc) and incubated in completed media for 24 h. For time dependent experiment, the cells were treated with 1 µM of IR794 for 0, 5, 15, 30 and 60 min. For dose dependent experiment, the cells were treated with 1.0 and 2.0 µM of IR794 probes for 15 and 30. Before imaging with Laser Scanning Confocal Microscope (Nikon A1Rsi), all the cells were washed with PBS and stained with Hoechst 33342. Excitation lasers: 641 nm (IR794 probes); 405 nm (Hoechst 33342). A 60 × oil immersion objective lens was used. All data were analyzed by ImageJ and presented quantitative fluorescent intensity as corrected total cell fluorescence (CTCF), mean ± SD (n = 30).
For co-localization study, after cells were incubated with IR794 probes, they were stained with Lysotracker Green DND 26 or Mitotracker Green FM (Thermo Fisher Scientific) for 20 min. Before visualization under LSCM, the cells were washed and stained with Hoechst 33342. To quantify the colocalization intensities of the red and green channels, each pixel of the confocal image, corresponding to the excitations of the probes and organelles trackers, was used in the calculation of Pearson’s correlation coefficient between the two intensities using python with a built-in function from scipy statistical package.
Flow cytometry
Experiment was performed according to the previous protocol39. Briefly, the cells were seeded into 6-well cell culture plates density of 3 × 105 cells/ well and incubated for 24 h. After media were removed, solutions of IR794 in DMEM with final concentrations of 1 µM were added. After 1 h incubation, the cells were thoroughly washed with PBS and harvested by trypsinization then transferred into Eppendorf tubes (1.5 mL). Non-uptake dye was removed by washing with ice cold PBS and centrifugation at 800×g, 4 °C for 5 min, then resuspended and repeat washing for 3 times. Trypan blue (0.2%, 1 mL) in PBS was added to the cells to quench non-internalized signal before analysed by flow cytometry (Attune NxT Flow Cytometer, Life Technologies) using red excitation laser 637 nm and emission filter 780/60 nm.
Cellular trafficking study
Temperature’s effect on cellular uptake was examined by incubating HepG2 cells treated with IR794-Morph and IR794-Morph-Mpip (1 µM) at 4 °C for 30 min. The endocytosis pathway was further investigated using different endocytosis inhibitors, including amiloride hydrochloride (final concentration 10 µM), genistein (100 µM), filipin III (2.5 µM) and dynasore (80 µM)47. HepG2 cells (3 × 105 cells per well) in 6-well plate were pre-incubated with inhibitors individually for 30 min at 37 °C. Afterwards, treated cells were incubated with 1 µL of IR794-Morph and IR794-Morph-Mpip for 15 min. IR794-Morph and IR794-Morph-Mpip without inhibitors was used as controls. Data were analysed as mentioned above in “Flow cytometry” method.
Statistical analysis
Data are expressed of three independent experiments (n = 3) and presented as the mean of at least four individual observations with the standard deviation (mean ± SD). The statistical analysis was performed using Paired Student’s T-test analysis. P-values < 0.05 were considered to indicate significance (*P < 0.05, **P < 0.01, ***P < 0.001).
Supplementary Information
Acknowledgements
This work was supported by Suranaree University of Technology (SUT) and by Thailand Science Research and Innovation (TSRI) and the National Research Council of Thailand (N41A640150). Flow cytometry facility was supported by Thailand Science Research and Innovation (TSRI) (Grant Number RTA6180012) and Ministry of Higher Education, Science, Research and Innovation (MHESI) (Grant Number 256101A3040017).
Author contributions
S.W. synthesize the compounds and conceived all the cell experiments and drew fig. 10. K.C. and P.P. conducted the photophysical properties and analyzed all spectroscopic data. O.W. performed preliminary cell viability assay. T.S. and M.Y. supported flow cytometry facility and data analysis. P.N. provided funding on cell culture facilities and data analysis. R.-Y.L. and A.K. validated the results. S.W. and A.K. wrote the main manuscript text. All authors reviewed the manuscript.
Data availability
All data generated or analysed during this study are included in this published article and Supplementary Information file.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-07533-5.
References
- 1.Luo S, Zhang E, Su Y, Cheng T, Shi C. A review of NIR dyes in cancer targeting and imaging. Biomaterials. 2011;32:7127–7138. doi: 10.1016/j.biomaterials.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 2.Weissleder R. A clearer vision for in vivo imaging. Nat. Biotechnol. 2001;19:316–317. doi: 10.1038/86684. [DOI] [PubMed] [Google Scholar]
- 3.Shi C, Wu JB, Pan D. Review on near-infrared heptamethine cyanine dyes as theranostic agents for tumor imaging, targeting, and photodynamic therapy. J. Biomed. Opt. 2016;21:50901. doi: 10.1117/1.JBO.21.5.050901. [DOI] [PubMed] [Google Scholar]
- 4.Sun W, Guo S, Hu C, Fan J, Peng X. Recent development of chemosensors based on cyanine platforms. Chem. Rev. 2016;116:7768–7817. doi: 10.1021/acs.chemrev.6b00001. [DOI] [PubMed] [Google Scholar]
- 5.Owens EA, Hyun H, Tawney JG, Choi HS, Henary M. Correlating molecular character of NIR imaging agents with tissue-specific uptake. J. Med. Chem. 2015;58:4348–4356. doi: 10.1021/acs.jmedchem.5b00475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang E, Luo S, Tan X, Shi C. Mechanistic study of IR-780 dye as a potential tumor targeting and drug delivery agent. Biomaterials. 2014;35:771–778. doi: 10.1016/j.biomaterials.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 7.Zhao X, et al. A near-infrared multifunctional fluorescent probe with an inherent tumor-targeting property for bioimaging. Chem. Commun. 2015;51:11721–11724. doi: 10.1039/C5CC03878B. [DOI] [PubMed] [Google Scholar]
- 8.Zhao X, et al. A tumor-targeting near-infrared heptamethine cyanine photosensitizer with twisted molecular structure for enhanced imaging-guided cancer phototherapy. J. Am. Chem. Soc. 2021 doi: 10.1021/jacs.1c09155. [DOI] [PubMed] [Google Scholar]
- 9.Usama SM, Lin C-M, Burgess K. On the mechanisms of uptake of tumor-seeking cyanine dyes. Bioconjugate Chem. 2018;29:3886–3895. doi: 10.1021/acs.bioconjchem.8b00708. [DOI] [PubMed] [Google Scholar]
- 10.Buxhofer-Ausch V, et al. Tumor-specific expression of organic anion-transporting polypeptides: Transporters as novel targets for cancer therapy. J. Drug. Deliv. 2013;2013:863539. doi: 10.1155/2013/863539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu T, Li Q. Organic anion-transporting polypeptides: A novel approach for cancer therapy. J. Drug Targeting. 2014;22:14–22. doi: 10.3109/1061186X.2013.832767. [DOI] [PubMed] [Google Scholar]
- 12.Tan X, et al. A NIR heptamethine dye with intrinsic cancer targeting, imaging and photosensitizing properties. Biomaterials. 2012;33:2230–2239. doi: 10.1016/j.biomaterials.2011.11.081. [DOI] [PubMed] [Google Scholar]
- 13.Usama SM, Burgess K. Hows and whys of tumor-seeking dyes. Acc Chem. Res. 2021;54:2121–2131. doi: 10.1021/acs.accounts.0c00733. [DOI] [PubMed] [Google Scholar]
- 14.Usama SM, et al. Role of albumin in accumulation and persistence of tumor-seeking cyanine dyes. Bioconjug. Chem. 2020;31:248–259. doi: 10.1021/acs.bioconjchem.9b00771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, et al. Characterization of HIF-1α/glycolysis hyperactive cell population via small-molecule-based imaging of mitochondrial transporter activity. Adv. Sci. 2018;5:1700392. doi: 10.1002/advs.201700392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao N, et al. Optical imaging of gastric cancer with near-infrared heptamethine carbocyanine fluorescence dyes. Oncotarget. 2016;7:57277–57289. doi: 10.18632/oncotarget.10031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu JB, et al. Near-infrared fluorescence imaging of cancer mediated by tumor hypoxia and HIF1α/OATPs signaling axis. Biomaterials. 2014;35:8175–8185. doi: 10.1016/j.biomaterials.2014.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hao G, Xu ZP, Li L. Manipulating extracellular tumour pH: an effective target for cancer therapy. RSC Adv. 2018;8:22182–22192. doi: 10.1039/C8RA02095G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Persi E, et al. Systems analysis of intracellular pH vulnerabilities for cancer therapy. Nat. Commun. 2018;9:2997. doi: 10.1038/s41467-018-05261-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kroemer G, Jaattela M. Lysosomes and autophagy in cell death control. Nat. Rev. Cancer. 2005;5:886–897. doi: 10.1038/nrc1738. [DOI] [PubMed] [Google Scholar]
- 21.Hyun H, et al. Structure-inherent targeting of near-infrared fluorophores for parathyroid and thyroid gland imaging. Nat. Med. 2015;21:192–197. doi: 10.1038/nm.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li M, et al. De novo design of phototheranostic sensitizers based on structure-inherent targeting for enhanced cancer ablation. J. Am. Chem. Soc. 2018;140:15820–15826. doi: 10.1021/jacs.8b09117. [DOI] [PubMed] [Google Scholar]
- 23.Fröhlich E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012;7:5577–5591. doi: 10.2147/IJN.S36111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jensen TJ, et al. Effect of overall charge and charge distribution on cellular uptake, distribution and phototoxicity of cationic porphyrins in HEp2 cells. J. Photochem. Photobiol. B. 2010;100:100–111. doi: 10.1016/j.jphotobiol.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Izumi H, et al. Cellular pH regulators: Potentially promising molecular targets for cancer chemotherapy. Cancer Treat Rev. 2003;29:541–549. doi: 10.1016/s0305-7372(03)00106-3. [DOI] [PubMed] [Google Scholar]
- 26.Lagadic-Gossmann D, Huc L, Lecureur V. Alterations of intracellular pH homeostasis in apoptosis: Origins and roles. Cell Death Differ. 2004;11:953–961. doi: 10.1038/sj.cdd.4401466. [DOI] [PubMed] [Google Scholar]
- 27.Kourounakis AP, Xanthopoulos D, Tzara A. Morpholine as a privileged structure: A review on the medicinal chemistry and pharmacological activity of morpholine containing bioactive molecules. Med. Res. Rev. 2020;40:709–752. doi: 10.1002/med.21634. [DOI] [PubMed] [Google Scholar]
- 28.Kumari A, Singh RK. Morpholine as ubiquitous pharmacophore in medicinal chemistry: Deep insight into the structure-activity relationship (SAR) Bioorg. Chem. 2020;96:103578. doi: 10.1016/j.bioorg.2020.103578. [DOI] [PubMed] [Google Scholar]
- 29.Vitner EB, Platt FM, Futerman AH. Common and uncommon pathogenic cascades in lysosomal storage diseases. J. Biol. Chem. 2010;285:20423–20427. doi: 10.1074/jbc.R110.134452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Donaldson, J. G. in Encyclopedia of Biological Chemistry (Second Edition) (eds William J. Lennarz & M. Daniel Lane) 197–199 (Academic Press, 2013).
- 31.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 32.Marsh M, McMahon H. The structural era of endocytosis. Science. 1999;285:215–220. doi: 10.1126/science.285.5425.215. [DOI] [PubMed] [Google Scholar]
- 33.Parton RG, Simons K. The multiple faces of caveolae. Nat. Rev. Mol. Cell Biol. 2007;8:185–194. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- 34.Benmerah A, Lamaze C. Clathrin-coated pits: Vive la difference? Traffic. 2007;8:970–982. doi: 10.1111/j.1600-0854.2007.00585.x. [DOI] [PubMed] [Google Scholar]
- 35.Maxfield FR, McGraw TE. Endocytic recycling. Nat. Rev. Mol. Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 36.Cheng Z-J, Singh RD, Marks D, Pagano R. Membrane microdomains, caveolae, and caveolar endocytosis of sphingolipids. Mol. Membr. Biol. 2006;23:101–110. doi: 10.1080/09687860500460041. [DOI] [PubMed] [Google Scholar]
- 37.Atchison J, et al. Iodinated Cyanine Dyes: A New Class of Sensitisers for use in NIR Activated Photodynamic Therapy (PDT) Springer; 2020. [DOI] [PubMed] [Google Scholar]
- 38.Wang B, et al. A Nile blue based infrared fluorescent probe: Imaging tumors that over-express cyclooxygenase-2. Chem. Commun. 2014 doi: 10.1039/c4cc08915d. [DOI] [PubMed] [Google Scholar]
- 39.Siriwibool S, et al. Near-infrared fluorescent pH responsive probe for targeted photodynamic cancer therapy. Sci. Rep. 2020;10:1283. doi: 10.1038/s41598-020-58239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khalili F, Henni A, East ALL. pKa values of some piperazines at (298, 303, 313, and 323) K. J. Chem. Eng. Data. 2009;54:2914–2917. doi: 10.1021/je900005c. [DOI] [Google Scholar]
- 41.Pewklang T, Chansaenpak K, Lai R-Y, Noisa P, Kamkaew A. Aza-BODIPY probe for selective visualization of cyclooxygenase-2 in cancer cells. RSC Adv. 2019;9:13372–13377. doi: 10.1039/c9ra01948k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choi N-E, Lee J-Y, Park E-C, Lee J-H, Lee J. Recent advances in organelle-targeted fluorescent probes. Molecules. 2021 doi: 10.3390/molecules26010217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu J-L, Xu Z, Yang Y, Xu L. Small-molecule fluorescent probes for specific detection and imaging of chemical species inside lysosomes. Chem. Commun. 2019 doi: 10.1039/C9CC03299A. [DOI] [PubMed] [Google Scholar]
- 44.Saminathan A, Zajac M, Anees P, Krishnan Y. Organelle-level precision with next-generation targeting technologies. Nat. Rev. Mater. 2021 doi: 10.1038/s41578-021-00396-8. [DOI] [Google Scholar]
- 45.Yang X, et al. Near IR heptamethine cyanine dye-mediated cancer imaging. Clin. Cancer Res. 2010;16:2833–2844. doi: 10.1158/1078-0432.CCR-10-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang C, Long L, Shi C. Mitochondria-targeting IR-780 dye and its derivatives: Synthesis, mechanisms of action, and theranostic applications. Adv. Ther. 2018;1:1800069. doi: 10.1002/adtp.201800069. [DOI] [Google Scholar]
- 47.Ng SY, et al. Active targeted ligand-aza-BODIPY conjugate for near-infrared photodynamic therapy in melanoma. Int. J. Pharm. 2020;579:119189. doi: 10.1016/j.ijpharm.2020.119189. [DOI] [PubMed] [Google Scholar]
- 48.Usama SM, Thavornpradit S, Burgess K. Optimized heptamethine cyanines for photodynamic therapy. ACS Appl. Biol. Mater. 2018;1:1195–1205. doi: 10.1021/acsabm.8b00414. [DOI] [PubMed] [Google Scholar]
- 49.Gao M, Yu F, Lv C, Choo J, Chen L. Fluorescent chemical probes for accurate tumor diagnosis and targeting therapy. Chem. Soc. Rev. 2017;46:2237–2271. doi: 10.1039/c6cs00908e. [DOI] [PubMed] [Google Scholar]
- 50.Luo S, Yang X, Shi C. Newly emerging theranostic agents for simultaneous cancertargeted imaging and therapy. Curr. Med. Chem. 2016;23:483–497. doi: 10.2174/0929867323666151223095718. [DOI] [PubMed] [Google Scholar]
- 51.Zhang C, et al. A near-infrared fluorescent heptamethine indocyanine dye with preferential tumor accumulation for in vivo imaging. Biomaterials. 2010;31:6612–6617. doi: 10.1016/j.biomaterials.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 52.Thakkar N, Lockhart AC, Lee W. Role of organic anion-transporting polypeptides (OATPs) in cancer therapy. AAPS J. 2015;17:535–545. doi: 10.1208/s12248-015-9740-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article and Supplementary Information file.