Abstract
NorA is a membrane-associated multidrug efflux protein that can decrease susceptibility to fluoroquinolones in Staphylococcus aureus. To determine the effect of NorA inhibition on the pharmacodynamics of fluoroquinolones, we evaluated the activities of levofloxacin, ciprofloxacin, and norfloxacin with and without various NorA inhibitors against three genetically related strains of S. aureus (SA 1199, the wild-type; SA 1199B, a NorA hyperproducer with a grlA mutation; and SA 1199-3, a strain that inducibly hyperproduces NorA) using susceptibility testing, time-kill curves, and postantibiotic effect (PAE) methods. Levofloxacin had the most potent activity against all three strains and was minimally affected by addition of NorA inhibitors. In contrast, reserpine, omeprazole, and lansoprazole produced 4-fold decreases in ciprofloxacin and norfloxacin MICs and MBCs for SA 1199 and 4- to 16-fold decreases for both SA 1199B and SA 1199-3. In time-kill experiments reserpine, omeprazole, or lansoprazole increased levofloxacin activity against SA 1199-3 alone by 2 log10 CFU/ml and increased norfloxacin and ciprofloxacin activities against all three strains by 0.5 to 4 log10 CFU/ml. Reserpine and omeprazole increased norfloxacin PAEs on SA 1199, SA 1199B, and SA 1199-3 from 0.9, 0.6, and 0.2 h to 2.5 to 4.5, 1.1 to 1.3, and 0.4 to 1.1 h, respectively; similar effects were observed with ciprofloxacin. Reserpine and omeprazole increased the levofloxacin PAE only on SA 1199B (from 1.6 to 5.0 and 3.1 h, respectively). In conclusion, the NorA inhibitors dramatically improved the activities of the more hydrophilic fluoroquinolones (norfloxacin and ciprofloxacin). These compounds may restore the activities of these fluoroquinolones against resistant strains of S. aureus or may potentially enhance their activities against sensitive strains.
The fluoroquinolones are a class of synthetic, broad-spectrum antimicrobials with potent activities against a variety of gram-positive and -negative organisms. When first introduced into clinical practice, these agents offered an alternative for the treatment of infections caused by both methicillin-sensitive and methicillin-resistant Staphylococcus aureus. However, the rapid emergence of resistance both in vitro and in the clinical setting has now significantly impacted the use of these agents (6, 12–14, 16, 25).
Fluoroquinolone resistance expression by S. aureus has been an area of intense research, and at least three mechanisms of resistance have been described. Mutations in the grlA gene can lead to an alteration of topoisomerase IV, the primary target site for fluoroquinolones in S. aureus (3, 6). Mutation of the gyrA gene is a second mechanism of resistance and results in an alteration of DNA gyrase and high-level fluoroquinolone resistance when it is combined with topoisomerase IV mutations in S. aureus (3, 6, 25). The third mechanism of resistance involves the membrane-associated NorA efflux pump (11–14, 16, 19, 20). NorA has been compared to a number of other drug efflux systems such as TetA, Bmr, and the mammalian multidrug efflux transporter P-glycoprotein (Pgp), but the greatest degree of homology (44%) has been found between NorA and Bmr (13, 18, 19). NorA is present in wild-type S. aureus, is the product of the norA gene, and confers a baseline low level of intrinsic resistance to fluoroquinolones and other structurally unrelated compounds considered toxic to the bacterial cell such as chloramphenicol, ethidium bromide, rhodamine, and puromycin (13, 18, 19). Some fluoroquinolone-resistant strains of S. aureus have increased quantities of NorA that appear to result from either increased transcription of norA or an increased stability of its mRNA (12). There is some evidence that suggests that hydrophilic fluoroquinolones are removed more efficiently than hydrophobic agents, but the exact reasons for this preference are not yet clear (13).
The efflux mechanism of fluoroquinolone resistance has received substantial attention since the demonstration that NorA activity could be inhibited by compounds such as the protonophore carbonyl cyanide m-chlorophenylhydrazone (CCCP) and the competitive pump blocker reserpine (12–14, 19, 20, 26, 27). These findings raise the interesting possibility of inhibition or modulation of efflux such that the activities of fluoroquinolones are restored or preserved. [3H]norfloxacin uptake studies using whole cells or everted membrane vesicles (where drug uptake is equivalent to drug efflux by whole cells) have demonstrated a restoration of drug accumulation in fluoroquinolone-resistant isolates (S. aureus and cloned Escherichia coli mutants) to the levels of accumulation in wild-type fluoroquinolone-susceptible isolates by the addition of CCCP (13, 14, 20). Earlier work had demonstrated that the plant alkaloid reserpine reversed Bmr-conferred fluoroquinolone resistance, and a similar effect on NorA-induced resistance has been observed (12, 13, 19, 20). Kaatz and Seo reported that reserpine produced a 12-fold reduction in norfloxacin MICs for strains of S. aureus that constitutively and inducibly hyperproduce NorA (12). Recently, verapamil (a calcium channel blocker) was also shown to decrease the effects of NorA on fluoroquinolone resistance (20). The latest types of compounds to be investigated for their potential role as inhibitors of NorA-mediated efflux are the H+ and K+ ATPase pump inhibitors such as omeprazole and lansoprazole (9). These compounds presumably affect the activity of NorA by affecting the cell proton gradient in a manner analagous to that of CCCP.
Fluoroquinolone resistance in S. aureus has recently been described to occur in a stepwise fashion by Ferrero et al. (6). In this investigation, constitutive hyperproduction of NorA was not documented until the second or third mutational step and was not universal but results supported the hypothesis that development of high-level fluoroquinolone resistance needs the concerted effect of two or three independent resistance mechanisms (3). An intriguing possible effect of NorA inhibition involves the delay, prevention, or reduction of fluoroquinolone resistance in susceptible strains of S. aureus. A brief report by Markham and Neyfakh described reduced growth of norfloxacin-resistant mutants of S. aureus with the addition of reserpine to the norfloxacin-containing agar (15). Thus, while NorA hyperproduction may not be a stable initial fluoroquinolone resistance mechanism, it may play a role as a promoter for the more common initial grlA mutations.
In most of the studies performed to date, the effects of NorA and its inhibition have focused on describing fluoroquinolone uptake over a period of minutes or effects on simple fluoroquinolone bacteriostatic activity. It is important to consider whether NorA inhibition can be sustained over a prolonged period and whether it can affect such pharmacodynamic parameters as bactericidal activity or the postantibiotic effect (PAE). The objective of this study was to evaluate the in vitro activities of three fluoroquinolones in the presence and absence of various potential NorA inhibitors. The fluoroquinolones used were chosen to represent a range of hydrophobic compounds (levofloxacin) and hydrophilic compounds (ciprofloxacin and norfloxacin). Inhibitors that may have potential clinical application in combination with fluoroquinolones were chosen. Three genetically related strains of S. aureus that produce NorA either constitutively, inducibly, or at wild-type levels were tested. Evaluations of activities were performed by MIC and MBC analyses, concentration–time-kill curve experiments, and PAE methods.
MATERIALS AND METHODS
Bacterial strains.
The strains of S. aureus used included SA 1199 (a wild-type clinical isolate), SA 1199B (a posttreatment fluoroquinolone-resistant derivative that constitutively produces NorA and that harbors a grlA mutation [11]), and SA 1199-3 (a laboratory-derived mutant of SA 1199 that inducibly NorA hyperproduces (12). Prior to each experiment, NorA induction for SA 1199-3 was accomplished by overnight growth on Mueller-Hinton medium (Difco Laboratories, Detroit, Mich.) containing 0.25× MICs of cetrimide (lot 36H04421; Sigma Chemical Co., St. Louis, Mo.) (12).
Media and antibiotics.
Mueller-Hinton broth (Difco) supplemented with calcium (25 mg/liter) and magnesium (12.5 mg/liter) (SMHB) was used for all susceptibility testing, time-kill curve experiments, and PAE experiments. Tryptic soy agar (Difco) plates were used for counting colonies in samples. Ciprofloxacin was obtained from Bayer (lot 7BF1), levofloxacin was supplied by R. W. Johnson Pharmaceutical Research Institute (lots N8017 and N8018), and norfloxacin was commercially purchased (lot 83H0921; Sigma). The NorA inhibitors reserpine (lot 16H1177), verapamil (lot 56H0925), diltiazem (lot 106H0981), and lansoprazole (lot 66H0259) were obtained from Sigma. Omeprazole (lot E6828) was obtained from Astra Merck (Södertälje, Sweden). Cyclosporine was commercially purchased as the oral suspension formulation (lot 243; Sandoz, East Hanover, N.J.). All stock solutions of compounds were prepared with sterile water, with the exceptions of reserpine, omeprazole, lansoprazole, and cyclosporine. For these compounds an initial stock solution in dimethyl sulfoxide was prepared and then further diluted to desired concentrations with water or broth.
In vitro antibiotic susceptibility tests.
For each organism MICs and MBCs of each fluoroquinolone and NorA inhibitor alone and in combinations were determined by broth microdilution according to the guidelines of the National Committee for Clinical Laboratory Standards (17). A starting inoculum of 105.5 to 106 CFU/ml was used, and combinations of fluoroquinolones and inhibitors were initially tested with doubling serial dilutions of the antibiotic and each NorA inhibitor. Preliminary results from these MICs revealed a range of NorA inhibitor concentrations that had either no effect or a maximal effect on fluoroquinolone MICs. A fixed concentration of 0.1 μg/ml was chosen as a low inhibitor concentration to evaluate any effects not detected by changes in MICs. Because of solubility considerations, a high inhibitor concentration of 100 μg/ml was chosen to evaluate the effects of maximal MIC reductions on killing and PAEs. The two exceptions to these higher concentrations (for reasons of poor solubility) were reserpine, which was tested at a high concentration of 20 μg/ml, and cyclosporine, which was tested at a high concentration of 10 μg/ml.
Time-kill curves.
An initial bacterial inoculum of 106 CFU/ml was prepared by diluting 1 ml of a 0.5 dilution of MacFarland suspension into 9 ml of SMHB and then adding 0.8 to 7.2 ml of SMHB containing the antibiotic to be tested. Samples (0.1 ml) were taken at 0 (inoculum control), 4, 8, and 24 h for each organism. These samples were serially diluted with cold normal saline, and aliquots (20 μl) were plated in triplicate on tryptic soy agar to allow for bacterial enumeration. Initial time-kill curve experiments were performed with 0.25, 0.5, 1, 2, and 4× MICs of each fluoroquinolone to determine the best concentration for the evaluation of NorA inhibitor effects on killing activity. At fluoroquinolone concentrations of ≥1× the MIC, significant killing activity occurred, making it difficult to discern any additional effect of NorA inhibitors. Based on these initial time-kill curves, subsequent experiments used 0.25× MICs of the fluoroquinolones alone or in combinations with the previously determined standard low and high concentrations of the NorA inhibitors. Because of the initial 1:10 dilution of all samples, the concentrations of drug were such that any effect of antibiotic carryover would be minimal (≤0.05× MICs).
PAE.
The PAEs of the fluoroquinolones alone and in combination with the most potent NorA inhibitors were measured by methods described by Craig and Gudmundsson (4). Antibiotics were added at the MICs to test tubes containing 106 CFU of each S. aureus isolate per ml. NorA inhibitors, reserpine and omeprazel, were used in fixed concentrations of 20 and 100 μg/ml, respectively, as stated above under “In vitro antibiotic susceptibility tests.” After exposure to the antibiotics with or without the NorA inhibitors for 1 h, samples were diluted to 1:1,000 to effectively remove the drugs. Samples were taken every hour until visual cloudiness was noted. The PAE was calculated by the following equation: PAE = T − C, where T represents the time required for the count in the test culture to increase 1 log10 CFU/ml above the count observed immediately after drug removal and C represents the time required for the count of the untreated control tube to increase by 1 log10 CFU/ml.
Statistical analyses.
Mean bacterial inocula (log10 CFU/ml) at the 8- and 24-h time points were compared between regimens by analysis of variance followed by Tukey’s test for multiple comparisons. The time required to achieve 99.9% killing and the PAE were determined by linear regression (if r > 0.95) or visual inspection of the kill-growth curves. For all statistical tests a P value of <0.05 was considered significant. All statistical analyses were performed with SPSS (Chicago, Ill.) statistical software (release 6.1.3).
RESULTS
Susceptibility testing.
The MIC and MBC results are summarized in Table 1. The MICs of all of the potential NorA inhibitors against these strains of S. aureus when tested alone were >128 μg/ml. For the two calcium channel blockers tested (verapamil and diltiazem) and for cyclosporine, reductions in the MICs and MBCs were minimal (≤1 twofold dilution) for all isolates. On average, omeprazole and lansoprazole provided a fourfold decrease in the MICs and MBCs of both ciprofloxacin and norfloxacin for SA 1199; no effects on levofloxacin MICs and MBCs were observed. Reserpine produced eightfold decreases in the MIC and MBC of norfloxacin, fourfold decreases in those of ciprofloxacin, and minimal changes in those of levofloxacin for SA 1199. We observed much greater effects on fluoroquinolone MICs and MBCs by the NorA inhibitors with SA 1199B and 1199-3. Reserpine, omeprazole, and lansoprazole all produced 8- to 16-fold decreases in the MICs and MBCs of norfloxacin, 4- to 16-fold decreases in those of ciprofloxacin, and 2- to 4-fold decreases in those of levofloxacin. All three compounds when used at 1- and 10-μg/ml concentrations also reduced fluoroquinolone MICs, but reductions were only two- to fourfold.
TABLE 1.
MICs and MBCs of test compounds for S. aureus strains
| Strain | Regimena | MIC/MBC (μg/ml)
|
||
|---|---|---|---|---|
| Nor-floxacin | Cipro-floxacin | Levo-floxacin | ||
| SA 1199 | Fluoroquinolone alone | 0.5/1 | 0.25/0.25 | 0.125/0.125 |
| Fluoroquinolone plus: | ||||
| Cyclosporine (10 μg/ml) | 1/1 | 0.25/0.25 | 0.125/0.125 | |
| Reserpine (20 μg/ml) | 0.63/0.125 | 0.063/0.125 | 0.063/0.125 | |
| Omeprazole | 0.125/0.25 | 0.125/0.25 | 0.125/0.125 | |
| Lansoprazole | 0.125/0.5 | 0.063/0.125 | 0.125/0.5 | |
| Verapamil | 0.25/0.5 | 0.125/0.125 | 0.125/0.25 | |
| Diltiazem | 0.25/0.5 | 0.125/0.125 | 0.125/0.5 | |
| SA 1199B | Fluoroquinolone alone | 32/64 | 4/16 | 1/2 |
| Fluoroquinolone plus: | ||||
| Cyclosporine | 32/64 | 8/8 | 1/1 | |
| Reserpine | 2/4 | 0.5/1 | 0.25/0.5 | |
| Omeprazole | 4/16 | 1/2 | 0.5/1 | |
| Lansoprazole | 4/8 | 1/1 | 0.5/2 | |
| Verapamil | 8/16 | 2/4 | 0.5/1 | |
| Diltiazem | 16/32 | 4/8 | 0.5/2 | |
| SA 1199-3 (induced) | Fluoroquinolone alone | 8/16 | 4/8 | 0.5/0.5 |
| Fluoroquinolone plus: | ||||
| Cyclosporine | 16/16 | 8/8 | 0.5/0.5 | |
| Reserpine | 0.5/1 | 0.25/0.5 | 0.125/0.25 | |
| Omeprazole | 1/2 | 0.5/1 | 0.125/0.25 | |
| Lansoprazole | 1/2 | 0.5/1 | 0.125/0.25 | |
| Verapamil | 2/2 | 1/2 | 0.25/0.5 | |
| Diltiazem | 2/4 | 2/4 | 0.5/0.5 | |
Unless otherwise noted, the NorA inhibitor concentration was 100 μg/ml.
Time-kill curve results.
Regrowth after 8 h of incubation commonly occurred during the time-kill curve experiments. It could not be clearly determined whether this phenomenon was related to compound degradation or insolubility, to organism adaptation, or to a combination of both. Slight cloudiness of the SMHB was often noted when only minimal bacterial inocula were present (less than 4 log10 CFU/ml), which suggested degradation or insolubility. However, repeat susceptibility testing of the colonies recovered at 24 h from samples with the ciprofloxacin and norfloxacin combinations with NorA inhibitors revealed MICs that were four to eight times higher than baseline (determined in the absence of any NorA inhibitors).
Each NorA inhibitor produced no appreciable effects on organism growth at both the high (100 μg/ml) and low (0.1 μg/ml) concentrations tested. For all NorA inhibitors the addition of the low (0.1 μg/ml) concentration produced no noticeable synergistic, additive, or antagonistic effects on the time-kill curves of each fluoroquinolone (data not shown). For both diltiazem and verapamil, the minimal changes in susceptibility were associated with no augmentation of fluoroquinolone activity in the time-kill curves. Similarly, because of the lack of effect on MICs and MBCs, time-kill curves were not determined for cyclosporine. Neither high nor low concentrations of any NorA inhibitor had any effects on levofloxacin killing curves against SA 1199 or 1199B. However, reserpine, omeprazole, and lansoprazole all caused levofloxacin to inhibit SA 1199-3 growth by 1 to 1.5 log10 CFU/ml over the 24-h test period (graphs not shown).
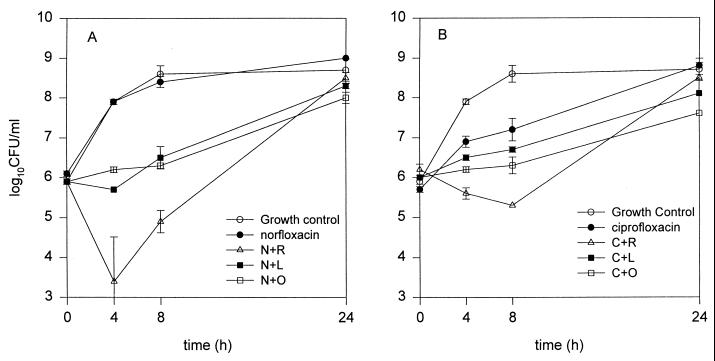
The time-kill curves for norfloxacin and ciprofloxacin against SA 1199 are shown in Fig. 1. Inclusion of either omeprazole or lansoprazole resulted in a significantly greater inhibition of growth at the 4- and 8-h time points than that with norfloxacin alone (Fig. 1A). When added to norfloxacin, reserpine appeared the most potent inhibitor when results were compared to results of all other regimens (P < 0.05), providing ∼3.5 log10 CFU/ml additional antibacterial activity at the 4-hour time point, but its activity was quite variable. For all NorA inhibitor-norfloxacin combinations, the residual bacterial counts became similar to that with norfloxacin alone at the 24-h time point. Similar results were observed for ciprofloxacin versus SA 1199. Addition of omeprazole and lansoprazole produced significantly lower bacterial counts at all time points than norfloxacin alone, while reserpine produced much more dramatic reductions in bacterial counts at the 4- and 8-h time points only (Fig. 1B). At 4 and 8 h, this combination was significantly more potent than all other regimens except ciprofloxacin plus omeprazole.
FIG. 1.
Time-kill curves for norfloxacin alone or combined with reserpine (N+R), lansoprazole (N+L), or omeprazole (N+O) (A) and for ciprofloxacin alone or combined with reserpine (C+R), lansoprazole (C+L), or omeprazole (C+O) (B) versus SA 1199. The fluoroquinolones were tested at 0.25× MICs, and inhibitor concentrations were 100 μg/ml for omeprazole and lansoprazole and 20 μg/ml for reserpine.
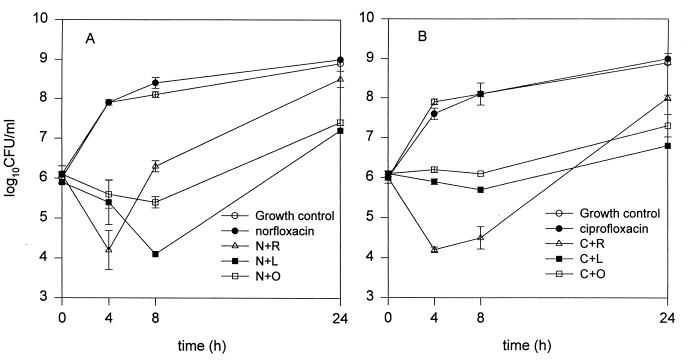
The time-kill curves for norfloxacin and ciprofloxacin against SA 1199B are shown in Fig. 2. The growth curves of all regimens except that of ciprofloxacin and norfloxacin alone were significantly different from the control growth curve. For both drugs, addition of the same three NorA inhibitors produced trends in activity similar to those observed against SA 1199, but activity was significantly greater against SA 1199B. Norfloxacin plus lansoprazole was significantly better than the other combinations at the 8- and 24-hour time points, while ciprofloxacin plus reserpine was significantly more active than any other regimen at both the 4 and 8-h time points.
FIG. 2.
Time-kill curves for norfloxacin alone or combined with reserpine (N+R), lansoprazole (N+L), or omeprazole (N+O) (A) and for ciprofloxacin alone or combined with reserpine (C+R), lansoprazole (C+L), or omeprazole (C+O) (B) versus SA 1199B. The fluoroquinolones were tested at 0.25× MICs, and inhibitor concentrations were 100 μg/ml for omeprazole and lansoprazole and 20 μg/ml for reserpine.
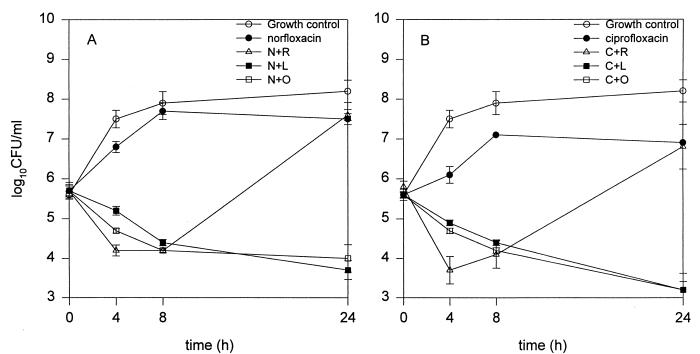
The time-kill curves for norfloxacin and ciprofloxacin against SA 1199-3 are shown in Fig. 3. Reserpine, omeprazole, and lansoprazole combined with norfloxacin all produced killing activity of ∼1.5 to 2.5 log10 CFU/ml at the 8-h time point; additional killing at 24 h occurred with both omeprazole and lansoprazole, but regrowth occurred with reserpine (Fig. 3A).
FIG. 3.
Time-kill curves for norfloxacin alone or combined with reserpine (N+R), lansoprazole (N+L), or omeprazole (N+O) (A) and for ciprofloxacin alone or combined with reserpine (C+R), lansoprazole (C+L), or omeprazole (C+O) (B) versus SA 1199-3. The fluoroquinolones were tested at 0.25× MICs, and inhibitor concentrations were 100 μg/ml for omeprazole and ansoprazole and 20 μg/ml for reserpine.
PAEs.
A summary of the results from PAE experiments is shown in Table 2. As verapamil, diltiazem, and cyclosporine produced marginal effects on inhibitor and killing activities, and because results with lansoprazole were nearly identical to those with omeprazole, these compounds were not evaluated for their PAEs. Levofloxacin PAEs were significantly greater than norfloxacin PAEs on all three isolates and were significantly greater than ciprofloxacin PAEs on SA 1199B. Reserpine or omeprazole produced no numerically or statistically significant changes in the PAEs of levofloxacin on SA 1199 or SA 1199-3. However, against SA 1199B, reserpine increased levofloxacin PAEs by approximately threefold (from 1.6 to 5.0 h) and omeprazole nearly doubled levofloxacin’s PAEs (from 1.6 to 3.1 h).
TABLE 2.
PAEs of fluoroquinolones (1× MIC) alone and in combination with NorA inhibitors
| Regimena | Mean PAE (h) ± SD on:
|
||
|---|---|---|---|
| SA 1199 | SA 1199B | SA 1199-3 | |
| Norfloxacin | 0.9 ± 0.1 | 0.6 ± 0.0 | 0.2 ± 0.4 |
| Norfloxacin + reserpine | 4.5 ± 0.9 | 1.1 ± 0.1 | 0.4 ± 0.5 |
| Norfloxacin + omeprazole | 2.5 ± 0.6 | 1.3 ± 0.1 | 1.1 ± 0.1 |
| Ciprofloxacin | 1.4 ± 0.2 | 0.5 ± 0.1 | 1.6 ± 0.2 |
| Ciprofloxacin + reserpine | 2.8 ± 0.1 | 3.2 ± 0.0 | 3.5 ± 2.2 |
| Ciprofloxacin + omeprazole | 3.8 ± 0.1 | 2.4 ± 0.2 | 3.6 ± 1.1 |
| Levofloxacin | 1.4 ± 0 | 1.6 ± 0.1 | 1.3 ± 0.2 |
| Levofloxacin + reserpine | 1.7 ± 0.2 | 5.0 ± 0.1 | 1.0 ± 0.2 |
| Levofloxacin + omeprazole | 1.5 ± 0 | 3.1 ± 0.1 | 1.0 ± 0.1 |
Reserpine and omeprazole concentrations were 20 and 100 μg/ml, respectively.
Norfloxacin PAEs on SA 1199B (0.6 h) and SA 1199-3 (0.2 h) were significantly smaller than those on SA 1199 (0.9 hours). Ciprofloxacin PAEs on SA 1199B (0.5 h) were significantly smaller than those on either SA 1199 (1.4 h) or SA 1199-3 (1.6 h). Both norfloxacin and ciprofloxacin PAEs were appreciably affected by the addition of either reserpine or omeprazole with all strains. With norfloxacin, reserpine and omeprazole increased PAEs on SA 1199 by ∼4.5- and ∼2.5-fold, respectively. Reserpine doubled the norfloxacin PAEs on both SA 1199B and 1199-3, while omeprazole doubled PAEs on SA 1199B and increased PAEs on SA 1199-3 by approximately fivefold. Similar two- to sixfold increases in PAEs were observed when the NorA inhibitors were added to ciprofloxacin.
DISCUSSION
Efflux systems are one of several mechanisms of resistance described for a variety of bacterial species, including S. aureus. The NorA protein has received considerable attention since its function can be altered by direct competitive inhibitors or protonophores (3, 13, 14, 18, 20, 26, 27). These initial investigations of efflux pump inhibition have measured MICs as well as the intracellular accumulation of fluoroquinolones in both the presence and the absence of NorA inhibition. The impact of NorA inhibition on the pharmacodynamics of fluoroquinolones (such as killing activity or PAE) has not been previously studied.
The S. aureus isolates selected represented a wild-type strain (SA 1199), a posttreatment fluoroquinolone-resistant mutant (SA 1199B), and a laboratory-produced strain that inducibly hyperproduces NorA (SA 1199-3) (12). Based on the current knowledge of NorA efflux activity, we expected to see a greater impact of the inhibitory compounds on the activities of the more hydrophilic fluoroquinolones (ciprofloxacin and norfloxacin) than on those of levofloxacin (12–14, 16). As anticipated, levofloxacin had the greatest activity (as measured by MICs and MBCs) against all three isolates, and minimal changes in activity versus SA 1199 and SA 1199B were demonstrated with all inhibitors, supporting previous findings with other hydrophobic fluoroquinolones such as sparfloxacin (12, 24). Interestingly, appreciable decreases in MICs and MBCs and greater killing of SA 1199-3 occurred when levofloxacin was combined with the NorA inhibitors. The exact reasons for the increased levofloxacin activity against one NorA hyperproducer (SA 1199-3) but no change against the other (SA 1199B) are currently unknown but might be related to the more dramatic expression of NorA in SA 1199-3 following induction (as measured by RNA transcripts with significant homology to norA) than in SA 1199B (12) such that efflux of a more hydrophobic fluoroquinolone such as levofloxacin became appreciable.
Although the static concentrations of drugs in the test tubes precluded a true pharmacodynamic analysis (incorporating parameters such as the peak concentration/MIC or area under the curve/MIC ratios), it was interesting to note that the NorA inhibitors increased killing activity in conjunction with a reduction in the MIC (improving the peak concentration/MIC ratio). In this context, our findings support those from previous studies of pharmacodynamic predictors of fluoroquinolone activity against S. aureus (2, 5).
The potential NorA inhibitors were chosen based upon current NorA literature and research on other drug efflux transporters such as Pgp (1, 8, 9, 12, 23). In our experiments the proton pump inhibitors omeprazole and lansoprazole displayed moderate activities, having less than reserpine but significantly more than verapamil, diltiazem, and cyclosporine. We did not find that one proton pump inhibitor was consistently more potent than the other. Diltiazem was included in our investigations to determine whether other calcium channel blockers besides verapamil possess NorA inhibitory activity. Based on our susceptibility and time-kill curve results, further studies with this compound do not appear to be warranted. Many compounds are capable of inhibiting both NorA and Pgp (reserpine and verapamil, for example), even though no significant homology exists between the two proteins (1, 8, 23). As cyclosporine possesses potent Pgp inhibitory activity (many times higher than that of reserpine), we chose to test the anti-NorA activity of this compound, but cross-activity of cyclosporine against NorA did not occur.
Significant improvements in fluoroquinolone activity were often limited to the 4- and 8-h time points followed by regrowth within 24 h. This observation was made most commonly with reserpine and to a lesser extent with both lansoprazole and omeprazole. The solubility of reserpine in aqueous solutions is poor, and during the experiments many test tubes containing reserpine contained a faint precipitate starting at the 4-h time point (suggesting loss of activity due to removal of the compound from solution). Time-kill curve experiments repeated with 25% dimethyl sulfoxide in SMHB (in an attempt to improve solubility) caused stunted bacterial growth and abolished the effects of reserpine. Other methods to improve the solubility of reserpine may help to better assess its effects over extended test periods.
However, the development or selection of resistance via the increased production of NorA or the emergence of strains with topoisomerase mutations may also have caused bacterial regrowth. Colonies recovered from the 24-h time point commonly required MICs of the test drugs that were four to eight times above baseline. Further study of NorA expression and sequencing of the grlA and gyrA genes in the bacteria from various time points of the time-kill curve would be a way to investigate these possibilities (12).
The PAE is a phenomenon that represents the continued suppression of bacterial growth after a brief exposure to an antimicrobial agent. Although the exact mechanisms causing the PAE are unknown, many different hypotheses such as persistence at the intracellular site(s) of action, slow recovery from nonlethal cellular damage, and a lag time for the synthesis of new proteins and/or enzymes have been proposed (4). The SOS response and the repair of DNA lesions may also contribute to the fluoroquinolone PAE in S. aureus (10, 21, 22). We observed impressive increases in the PAEs of both norfloxacin and ciprofloxacin with the addition of potent NorA inhibitors against all three strains of S. aureus. Based on proposed mechanisms for fluoroquinolone PAEs, the increases may be due to increases in intracellular accumulation of the drugs, resulting in more significant DNA damage.
In conclusion, we showed that reserpine, omeprazole, and lansoprazole can improve fluoroquinolone (especially hydrophilic fluoroquinolone) activity against strains expressing different levels of NorA. The results of our study are consistent with the reported effects of NorA inhibitors on fluoroquinolone MICs and MBCs and cellular uptake (12, 13, 16, 19, 20). However, a limitation of the present study is that we cannot state with certainty that the observed improvements in fluoroquinolone activity were due solely to the modulation of NorA. Stronger evidence for a pure effect of the tested compounds on NorA might be provided from future studies of strains devoid of NorA. Additional research into such issues as the longevity of NorA inhibitory effects, the impact of fluctuating fluoroquinolone drug concentrations on NorA inhibitor activity, and the emergence of fluoroquinolone resistance is needed to determine whether these improvements in activities can be carried over into the clinical setting. In addition, more potent inhibitors of NorA need to be discovered, as the currently used compounds require concentrations beyond those achievable in humans for significant anti-NorA activity to be observed.
REFERENCES
- 1.Barnes K M, Dickstein B, Cutler G B, Fojo T, Bates S E. Steroid transport, accumulation, and antagonism of p-glycoprotein in multidrug-resistant cells. Biochemistry. 1996;35:4820–4827. doi: 10.1021/bi952380k. [DOI] [PubMed] [Google Scholar]
- 2.Blaser J, Stone B B, Groner M C, Zinner S H. Comparative study with enoxacin and netilmicin in a pharmacodynamic model to determine importance of ratio of antibiotic peak concentration to MIC for bactericidal activity and emergence of resistance. Antimicrob Agents Chemother. 1987;31:1054–1060. doi: 10.1128/aac.31.7.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cambau, E., and L. Gutman. 1993. Mechanisms of resistance to quinolones. Drugs 45(Suppl. 3):15–23. [DOI] [PubMed]
- 4.Craig W A, Gudmundsson S. Postantibiotic effect. In: Lorian V, editor. Antibiotics in laboratory medicine. 4th ed. Baltimore, Md: The Williams and Wilkins Co.; 1996. pp. 296–329. [Google Scholar]
- 5.Dudley, M. N., H. D. Mandler, D. Gilbert, J. Ericson, K. H. Mayer, and S. H. Zinner. 1987. Pharmacokinetics and pharmacodynamics of intravenous ciprofloxacin. Am. J. Med. 82(Suppl. 4A):363–368. [PubMed]
- 6.Ferrero L, Cameron B, Crouzet J. Analysis of gyrA and grlA mutations in stepwise-selected ciprofloxacin-resistant mutants of Staphylococcus aureus. Antimicrob Agents Chemother. 1995;39:1554–1558. doi: 10.1128/aac.39.7.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher G A, Lum B L, Hausdorff J, Sikic B I. Pharmacological considerations in the modulation of multidrug resistance. Eur J Cancer. 1996;32A:1082–1088. doi: 10.1016/0959-8049(96)00111-6. [DOI] [PubMed] [Google Scholar]
- 8.Ford J M. Experimental reversal of P-glycoprotein-mediated multidrug resistance by pharmacological chemosensitisers. Eur J Cancer. 1996;32A:991–1001. doi: 10.1016/0959-8049(96)00047-0. [DOI] [PubMed] [Google Scholar]
- 9.Fraimow H S, Esposito M. Program and abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Effects of omeprazole (Om) and lansoprazole (Lan) on fluoroquinolone (FQ) and aminoglycoside (AG) activity in Staphylococcus aureus (SA), abstr. C36. [Google Scholar]
- 10.Gottfredsson M, Erlendsdottir H, Gudmundsson A, Gudmundsson S. Different patterns of bacterial DNA synthesis during the postantibiotic effect. Antimicrob Agents Chemother. 1995;39:1314–1319. doi: 10.1128/aac.39.6.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaatz G W, Seo S M. Mechanisms of fluoroquinolone resistance in genetically related strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1997;41:2733–2737. doi: 10.1128/aac.41.12.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaatz G W, Seo S M. Inducible NorA-mediated multidrug resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1995;39:2650–2655. doi: 10.1128/aac.39.12.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaatz G W, Seo S M, Ruble C A. Efflux-mediated fluoroquinolone resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1993;37:1086–1094. doi: 10.1128/aac.37.5.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaatz G W, Seo S M, Ruble C A. Mechanisms of fluoroquinolone resistance in Staphylococcus aureus. J Infect Dis. 1991;163:1080–1086. doi: 10.1093/infdis/163.5.1080. [DOI] [PubMed] [Google Scholar]
- 15.Markham P N, Neyfakh A A. Inhibition of the multidrug transporter NorA prevents emergence of norfloxacin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1996;40:2673–2674. doi: 10.1128/aac.40.11.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakanishi N, Yoshida S, Wakebe H, Inoue M, Yamaguchi T, Mitsuhashi S. Mechanisms of clinical resistance to fluoroquinolones in Staphylococcus aureus. Antimicrob Agents Chemother. 1991;35:2562–2576. doi: 10.1128/aac.35.12.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 3rd ed. Approved standard M7-A3. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1993. [Google Scholar]
- 18.Neyfakh A A. The multidrug efflux transporter of Bacillus subtilis is a structural and functional homolog of the Staphylococcus NorA protein. Antimicrob Agents Chemother. 1992;36:484–485. doi: 10.1128/aac.36.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neyfakh A A, Borsch C M, Kaatz G W. Fluoroquinolone resistance protein NorA of Staphylococcus aureus is a multidrug efflux transporter. Antimicrob Agents Chemother. 1993;37:128–129. doi: 10.1128/aac.37.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng E Y, Trucksis M, Hooper D C. Quinolone resistance mediated by norA: physiologic characterization and relationship to flqB, a quinolone resistance locus on the Staphylococcus aureus chromosome. Antimicrob Agents Chemother. 1994;38:1343–1355. doi: 10.1128/aac.38.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piddock L J V, Wise R. Induction of the SOS response in Escherichia coli by 4-quinolone antimicrobial agents. FEMS Microbiol Lett. 1987;41:918–932. [Google Scholar]
- 22.Salles E, Defrais M. Signal induction of recA protein in E. coli. Mutat Res. 1984;131:53–59. doi: 10.1016/0167-8817(84)90011-7. [DOI] [PubMed] [Google Scholar]
- 23.Sonneveld P. Reversal of multidrug resistance in acute myeloid leukemia and other haematological malignancies. Eur J Cancer. 1996;32A:1062–1069. doi: 10.1016/0959-8049(96)00054-8. [DOI] [PubMed] [Google Scholar]
- 24.Takenouchi T, Tabata F, Iwata Y, Hanzawa H, Sugawara M, Ohya S. Hydrophilicity of quinolones is not an exclusive factor for decreased activity in efflux-mediated resistant mutants of Staphylococcus aureus. Antimicrob Agents Chemother. 1996;40:1835–1842. doi: 10.1128/aac.40.8.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka M, Zhang Y X, Ishida H, et al. Mechanisms of 4-quinolone resistance in quinolone-resistant and methicillin-resistant Staphylococcus aureus. J Med Microbiol. 1995;42:214–219. doi: 10.1099/00222615-42-3-214. [DOI] [PubMed] [Google Scholar]
- 26.Wiedemann, B., and P. Heisig. 1994. Mechanisms of quinolone resistance. Infection 22(Suppl. 2):73–79. [DOI] [PubMed]
- 27.Yoshida S, Kojima T, Inoue M, Mitsuhashi S. Uptake of sparfloxacin and norfloxacin by clinical isolates of Staphylococcus aureus. Antimicrob Agents Chemother. 1991;35:368–370. doi: 10.1128/aac.35.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]