Abstract
Acute bacterial infections are often treated empirically, with the choice of antibiotic therapy updated during treatment. The effects of such rapid antibiotic switching on the evolution of antibiotic resistance in individual patients are poorly understood. Here we find that low-frequency antibiotic resistance mutations emerge, contract, and even go to extinction within days of changes in therapy. We analyzed Pseudomonas aeruginosa populations in sputum samples collected serially from 7 mechanically ventilated patients at the onset of respiratory infection. Combining short- and long-read sequencing and resistance phenotyping of 420 isolates revealed that while new infections are near-clonal, reflecting a recent colonization bottleneck, resistance mutations could emerge at low frequencies within days of therapy. We then measured the in vivo frequencies of select resistance mutations in intact sputum samples with resistance-targeted deep amplicon sequencing (RETRA-Seq), which revealed that rare resistance mutations not detected by clinically used culture-based methods can increase by nearly 40-fold over 5–12 days in response to antibiotic changes. Conversely, mutations conferring resistance to antibiotics not administered diminish and even go to extinction. Our results underscore how therapy choice shapes the dynamics of low-frequency resistance mutations at short time scales, and the findings provide a possibility for driving resistance mutations to extinction during early stages of infection by designing patient-specific antibiotic cycling strategies informed by deep genomic surveillance.
Subject terms: Antibiotics, Molecular evolution, Infectious-disease diagnostics
It remains unclear how rapid antibiotic switching affects the evolution of antibiotic resistance in individual patients. Here, Chung et al. combine short- and long-read sequencing and resistance phenotyping of 420 serial isolates of Pseudomonas aeruginosa collected from the onset of respiratory infection, and show that rare resistance mutations can increase by nearly 40-fold over 5–12 days in response to antibiotic changes, while mutations conferring resistance to antibiotics not administered diminish and even go to extinction.
Introduction
Antibiotic treatment selects for resistance mutations, posing a major threat to effective treatment of bacterial infections1. The selection of resistance mutations during chronic infections as a result of antibiotic treatment over months to years is well-known2–9. However, it is not well-understood how short-term changes in antibiotic therapy affect the dynamics of resistance mutations in acute infections, especially in a newly colonizing infection that is thought to start from a clonal population10.
Emerging resistance is of particular concern in the treatment of acute respiratory tract infections that are common in intensive care units (ICUs) worldwide, particularly in mechanically ventilated patients who are at high risk for ventilator-associated pneumonia (VAP), septic shock, and infection-associated mortality11–15. VAP and other lower respiratory tract infections are of major concern in the SARS-CoV-2 pandemic given the large number of hospitalized COVID-19 patients requiring ventilation16–19. Pseudomonas aeruginosa is one of the most common bacterial pathogens causing respiratory infections in ventilated patients, and is associated with increased mortality and low treatment efficacy due to the high rates of antibiotic resistance that can occur within days of antibiotic treatment10,20–26.
Shallow profiling of pathogen populations using cultured isolates have shown that the frequencies of antibiotic resistance mutations can fluctuate over days to weeks during infection10,27, but whether these changes reflect drift, sampling bias, or treatment-induced selection at short timescales is unknown. Current clinical methods for detecting resistance variants are largely culture-based, where isolates with visually distinct morphology (by size, shape, and color) are selected for resistance phenotyping. However, these methods are susceptible to bias from culture-based growth and are limited in their sampling resolution, especially for detecting low-frequency mutations. While molecular surveillance methods such as rapid PCR tests and real-time genome sequencing can identify the presence of known resistance genes28–31, e.g., efflux pumps, to identify resistant strains, they are not suitable for monitoring within-population pathogen diversity. Furthermore, it is not well-understood whether resistance mutations can contract and be reversed during the course of treatment in acute infections. A molecular, culture-free diagnostic could determine the role of low-frequency resistance variants at short time scales, and possibly inform which antibiotics should be avoided.
Here we combine whole-genome sequencing with resistance-targeted deep amplicon sequencing (RETRA-Seq) to show that resistance mutations, either pre-existing or de novo, expand and contract rapidly within days of changes in therapy. By conducting a deep sampling study of P. aeruginosa populations, and using long-read sequencing to construct patient-specific reference genomes in order to maximize the detection of within-population mutations, we construct a high-resolution view of pathogen evolution during acute respiratory infection. We then relate how changes in empirically administered antibiotics impact resistance in individual patients, and discover that resistance mutation frequencies change within days, depending on the duration and type of antibiotic therapy.
Results
Prospective study of P. aeruginosa populations during acute respiratory infections
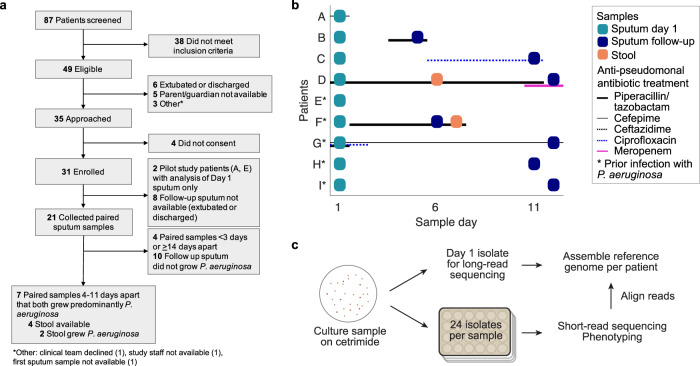
We conducted a prospective study of mechanically ventilated patients with clinical evidence of acute respiratory tract infection in the pediatric or cardiac intensive care unit at Boston Children’s Hospital. Eighty-seven patients were screened to identify 49 patients that met the inclusion criteria, of which 31 patients consented to enrollment (Fig. 1a; Methods). Endotracheal or tracheal aspirates (referred here throughout as sputum samples) were collected at the onset of symptoms (‘sputum day 1’), with serial samples (‘sputum follow-up’) collected when possible. We first conducted a small pilot study to assess the genomic diversity of P. aeruginosa in two patients, patients A and E*, who were sampled only at day 1. After confirming population growth and detectable diversity, we focused on studying short-term infection dynamics in 7 patients whose serial samples were collected 4–11 days after day 1 and exhibited P. aeruginosa growth at both time points as the predominant pathogen (Fig. 1b; Supplementary Fig. 1; Methods; Supplementary Table 1). In addition, as GI tract carriage is thought to be a source of intrapatient infection32, stool was also collected if available, of which only 2 of 4 available samples exhibited P. aeruginosa growth. Among the 9 patients (7 serially sampled and 2 pilot study patients), 4 had no history of P. aeruginosa infection (patients A-D) while 5 had a documented history of prior P. aeruginosa infection (denoted by an asterisk, patients E*-I*). In total, we collected 18 sputum and 2 stool samples across 9 patients from the onset of infection.
Fig. 1. Prospective study of P. aeruginosa populations from mechanically ventilated patients during acute respiratory tract infection.
a Prospective study design describing the enrollment strategy of mechanically ventilated patients in the ICU. Of 87 patients screened, 49 eligible patients were identified, from which 31 consented to enrollment. We focused on 2 pilot patients sampled at only day 1, and 7 patients sampled serially across 4–11 days that exhibited predominant P. aeruginosa growth in both samples. b Sampling sputum and stool across patients (y-axis) over time (x-axis) from the onset of symptoms. Day 1 sputum sample (teal box) were collected in all patients, and a follow-up sputum (dark blue box) were collected in 7 patients between day 5 and day 12, i.e. 4–11 days after day 1. Stool samples (brown box) with confirmed P. aeruginosa growth were collected in 2 patients. Asterisk: patients with prior P. aeruginosa infection. Treatment with anti-pseudomonal antibiotics are indicated by horizontal lines: piperacillin/tazobactam (weighted black), cefepime (thin black), ceftazidime (dotted black), ciprofloxacin (dotted blue), meropenem (weighted pink). c Samples (sputum or stool) were cultured on cetrimide agar in serial dilutions. A single isolate from day 1 sputum of each patient was used to construct a patient-specific reference genome using long-read sequencing. From each sample, 24 isolates were randomly selected for short-read whole-genome sequencing and phenotyping.
Maximizing the detection of genomic diversity by constructing patient-specific reference genomes
To capture the full extent of genomic diversity in pathogens, we used both long-read and short-read sequencing to characterize the P. aeruginosa populations in each patient. P. aeruginosa has a flexible pangenome with variations in gene content across strains by up to 50%33. A poor choice in the reference genome would impact the alignment rate of short reads and therefore, the fraction of usable reads for identifying within-patient polymorphisms. We thus assembled a complete patient-specific reference genome using long-read sequencing of a single P. aeruginosa colony per patient10,34,35 (Fig. 1c), which supported that each patient was infected with a unique strain based on gene content (Supplementary Fig. 2a; Methods). To capture within-patient diversity, we collected 24 additional cultured isolates from each sputum or stool (n = 420 total), sequenced their whole genomes with short reads, and aligned these reads to the patient-specific reference genomes (average alignment rate > 99%, Supplementary Fig. 2b) in order to identify within-patient single nucleotide polymorphisms (SNPs) and short insertions and deletions (indels) (Supplementary Fig. 2c; Supplementary Data 1). We then used the within-patient variants (SNPs and indels) to construct patient-specific phylogenies of P. aeruginosa populations and to infer the most recent common ancestor (MRCA) in each patient (Methods).
New infections start with clonal founders
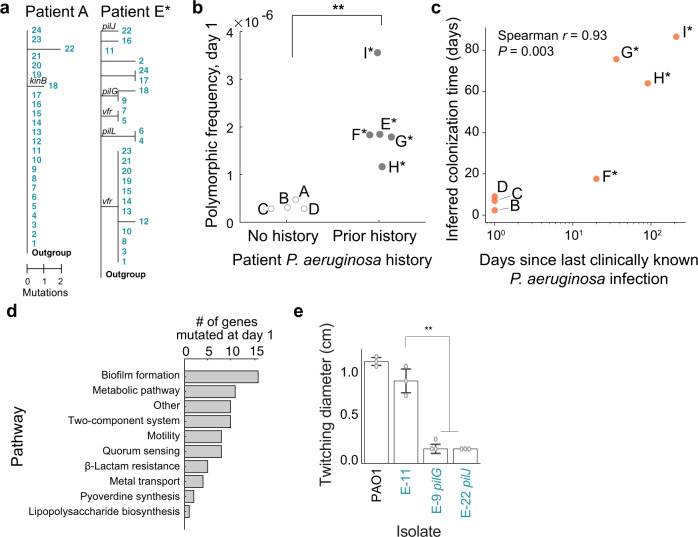
The diversity of pathogens at the onset of infection depended on the infection history of patients. In the case of a presumed new infection in patient A, the population was nearly clonal, which was consistent with a recent colonization by a single founder36–38 (Fig. 2a, left). In contrast, the day 1 population was polymorphic in patient E*, who had a documented history of P. aeruginosa infection (Fig. 2a, right). Testing whether initial pathogen diversity—defined as the frequency of polymorphisms at day 1—differed by infection history across patients indeed revealed that patients with prior infection had higher initial diversity (Fig. 2b; P = 0.007, two-sided t-test). Suspecting that populations from prior infections were maintained in these patients, we compared the inferred colonization time of each patient based on genomic data (Methods) to the time since the last clinically documented P. aeruginosa infection, which were significantly associated (Fig. 2b; Spearman r = 0.93, P = 0.003), providing evidence that pathogen reservoirs were maintained between symptomatic episodes that resembles a chronic infection6,7. Consistently, mutations at day 1 were found in genes and pathways important for pathogen colonization6, such as biofilm formation (Fig. 2d; Supplementary Data 2) and impairment in motility (Fig. 2e, P < 0.0001). Altogether, these results show that new infections are colonized by a single clonal founder, and that once colonized, pathogen reservoirs can be maintained in patients between symptomatic episodes that resembles subchronic infection.
Fig. 2. Patient infection history impacts genomic diversity of P. aeruginosa at the onset of infection.
a Phylogenetic trees of P. aeruginosa populations in pilot patients A and E* rooted with an Outgroup (Methods). Numbers (rows) correspond to tree leaves (teal) representing an isolate. Scale: mutational events (single nucleotide polymorphisms (SNPs) and indels) from the most recent common ancestor (MRCA) in each patient. Select branches are labeled with mutated genes. b Comparing the initial pathogen diversity of patients (dots) based on prior history of P. aeruginosa infection. Frequency of polymorphic loci (y-axis; calculated as number of unique SNP or indel positions divided by genome size) in patients with no prior P. aeruginosa history vs. in patients with clinically documented infection history (x-axis). Significance: P = 0.007, two-sided t-test. c Relation between the estimated colonization time of the pathogen in each patient (days, y-axis; Methods) and time to the last clinically documented infection (days, x-axis). Spearman correlation (two-sided), r = 0.93, P = 0.003. d Pathways (y-axis) found in mutations of coding regions at day 1 (x-axis) across all patients. e Altered twitching phenotype in isolates with single point mutations in genes of the pil locus. Isolates (x-axis) assayed for twitching diameter (cm, y-axis; Methods), from left to right: PAO1 reference strain, E-11 isogenic control, E-9 singleton pilG mutant, E-22 pilJ singleton mutant. Each assay was conducted across 3 technical replicates (dots), representative of 3 biologically independent replicates. Bars show median; error bars, standard error (s.e.). Significance: Tukey’s multiple comparisons test (E-11 vs. E-9, P = 0.001; E-11 vs. E-22, P = 0.001; adjusted P-values).
Mutations that alter clinical phenotypes are accrued over days
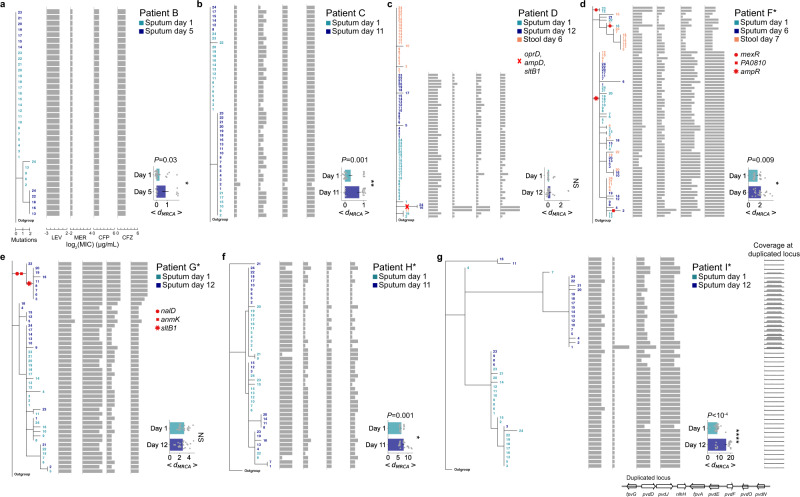
Pathogen populations diversified within all patients by the emergence of single point mutations. In most patients, mutations accumulated significantly over days as quantified by the increase in distance to the most recent common ancestor (dMRCA; Fig. 3, horizontal bar plots insets, permutation test of <dMRCA>; Methods), although the frequencies of individual mutations could both increase or decrease (Supplementary Fig. 3a). Notably, stool and sputum populations within each patient, where observed, were indistinguishable (Fig. 3c, d), indicating either gut carriage as the source of respiratory colonization10,32, or more simply, that stool samples reflect the passage of ingested sputum through the gastrointestinal tract.
Fig. 3. Phylogenetic analyses of P. aeruginosa populations within each patient and their corresponding antibiotic resistance profiles.
a–g Left: Phylogenetic trees of P. aeruginosa populations in serially sampled patients: patient B (a), patient C (b), patient D (c), patient F* (d), patient G* (e), patient H* (f), patient I* (g). Numbers (rows) correspond to tree leaves that represent an isolate (day 1 sputum in teal, follow-up sputum in dark blue, stool in brown). Scale: mutational events (single nucleotide polymorphisms (SNPs) and indels) from the most recent common ancestor (MRCA) in each patient. A subset of branches associated with antibiotic resistance are marked with red symbols. Middle: Antibiotic resistance profiles (horizontal gray bars) in units of minimum inhibitory concentration (log2(MIC); µg/mL) of individual isolates (rows) aligned to the isolate’s position on the tree, shown for levofloxacin (LEV), meropenem (MER), cefepime (CFP), and ceftazidime (CFZ). Right: distance to the MRCA (<dMRCA>, x-axis) of isolates (gray dots) at each time point (y-axis, days of infection). Mean (horizontal bars) and standard error (error bars) calculated over n = 24 biologically independent isolates per sputum or stool sample (exception: n = 12 isolates in Day 5 sputum of patient B (a)). Significance, one-tailed permutation test: P = 0.03 (a), P = 0.001 (b), P = 0.009 (d), P = 0.001 (f), P < 10−4 (g). NS not significant. g Far right, bottom: schematic showing the relative copy number (y-axis) of a ~34 kb duplicated chromosomal region (x-axis) that encodes, among others, genes of the pyoverdine pathway (bottom).
To assess whether mutations could reflect diversifying selection, we characterized clinically relevant phenotypes of a subset of non-synonymous mutations that increased in frequency (Supplementary Fig. 3a) or those that occurred recurrently in genes, which we interpret as under selection2,3,5,8 (Supplementary Fig. 3b). To estimate a genotype-phenotype relation, we focused on singleton mutations and compared isolates with and without only that mutation that were otherwise genetically identical, i.e., comparing mutants to estimated isogenic controls (based on >99% alignment rate of isolate genomes, Supplementary Fig. 2b). Point mutations impacted a wide range of clinically important phenotypes, including those in wbpL and wzy that altered lipopolysaccharide (LPS) and O-antigen presentation thereby affecting sensitivity to human serum (Supplementary Fig. 3c–f; Methods), and those in biofilm-related genes encoding BifA and KinB that impacted swarming, biofilm formation, and alginate production (Supplementary Fig. 3g–k). Altogether, our findings show that the evolution of P. aeruginosa over days leads to the diversification of clinically important phenotypes.
Measuring the in vivo frequencies of resistance mutations
We identified mutations associated with resistance by mapping the antibiotic resistance profiles of isolates to their genomes (Fig. 3, red symbols; Supplementary Fig. 4). The frequencies of these mutations changed across days, based on cultured isolates, with some resistant mutants observed only in later time points. For example, nalD (a repressor of MexAB-OprM39), anmK (involved in peptidoglycan recycling40), and sltB1 (a lytic transglycosylase41) were found in a sublineage of patient G* that appeared to emerge after day 1 (Fig. 3e). Another set of linked mutations—oprD, ampD, and sltB1 in patient D—conferring resistance to meropenem and ceftazidime (Fig. 3c) were found at low frequencies in only the second time point.
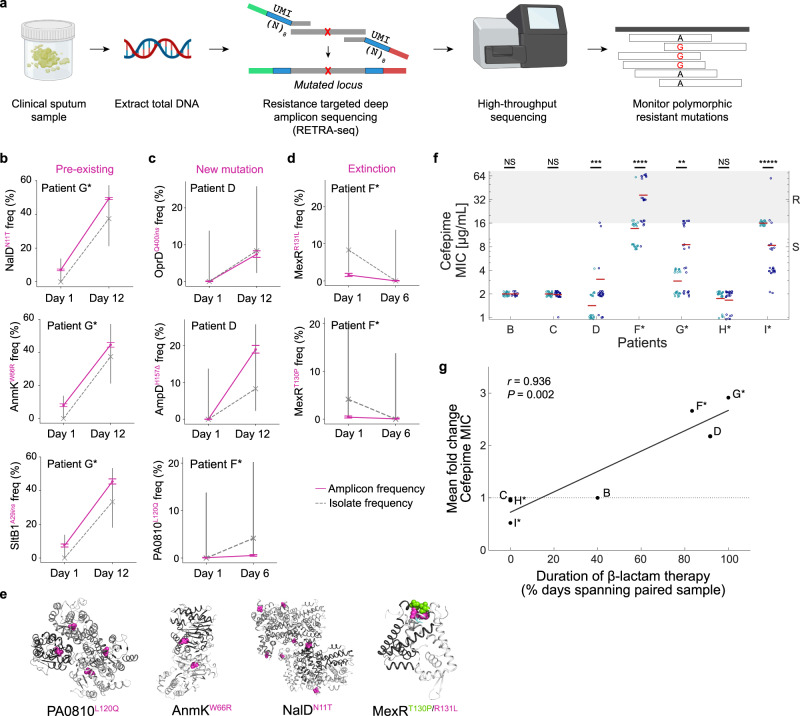
To accurately capture the dynamics of resistance mutations in patients, without culture-based growth bias, we designed a scheme to measure the mutation frequencies directly from intact sputum samples by developing “resistance-targeted deep amplicon sequencing” (RETRA-Seq), in which we amplify the mutated loci from total DNA extracted from sputum for deep amplicon sequencing (Fig. 4a). In order to control for amplification bias and reliably measure the number of unique genomes across thousands of single cells that correspond to each allele, we incorporate unique molecular identifiers (UMIs) in the primers, and sequence at a saturating depth such that allele frequencies are resolved to the sequencing error rate (Supplementary Fig. 5; Supplementary Data 3; Methods).
Fig. 4. Treatment-associated dynamics of low-frequency resistance mutations.
a Workflow of resistance-targeted deep amplicon sequencing (RETRA-Seq) as a diagnostic for monitoring resistance mutation frequencies. Total DNA is extracted from clinical sputum and prepared as sequencing libraries via PCR using primers that contain sequencing adapters (green, red) and unique molecular identifiers (UMIs; blue) composed of 8 degenerate nucleotides (N). Amplicon libraries are sequenced on a next-generation sequencing platform and aligned to a reference genome to determine polymorphic frequencies. Images created with BioRender.com. b–d Mutation frequencies (y-axis) across time (x-axis) of select resistance loci within each patient, measured by RETRA-Seq (solid pink) and by the fraction of culture-based isolates (dashed gray). Axis labels (y-axis) indicate the gene name and the mutation type (pink superscript): non-synonymous substitution, insertion (ins), or deletion (Δ). Error bars: Wilson Score interval of UMI counts (amplicon sequencing) or discrete counts (isolate sampling; Methods). Three types of changes in resistance frequencies: expansion of pre-existing mutations that were undetected by culture-based assay (b), expansion of presumed de novo mutations (c), and extinction of mutations (d). e Select non-synonymous mapped on protein structures of homologs of PA0810 (Protein Data Bank ID: 3UMC), AnmK (3QBW), NalD (5DAJ), and MexR (3ECH). Shades of gray indicate distinct monomers and pink/green spheres indicate mutated residues. f Distribution of cefepime MIC (µg/mL; y-axis) in individual isolates (dots) from day 1 (teal) and follow-up (dark blue) sputum samples, with mean value (horizontal read line). Ranges of resistance/intermediate susceptibility (R; gray) and sensitive (S; white) shown on the right and by background color, according to breakpoints defined by the Clinical Laboratory Standards Institute (CLSI). Significance (two-sided Mann–Whitney test): P = 7.5 × 10−4 (patient D), P = 2.2 × 10−5 (patient F), P = 0.004 (patient G), P < 10−5 (patient I). NS – not significant. g Relationship between cefepime resistance and clinical history of patient therapy. Fold change in mean cefepime MIC (y-axis) vs. the duration of β-lactam antibiotics administered to each patient during the study period (x-axis), shown for serially sampled patients (dots). Pearson’s correlation (two-sided), r = 0.936, P = 0.002.
RETRA-Seq of select resistance mutations (Fig. 3c-e, red symbols on branches of trees) revealed three types of in vivo mutation dynamics: (i) ‘pre-existing’ mutations that expanded from low frequencies at day 1 that were initially undetected by culture-based assay, (ii) presumed ‘de novo’ mutations within sequencing error, and (iii) mutations that went to ‘extinction’ (Fig. 4b–d). Some of these mutations impacted key residues at the interface of multimers, suggesting a loss-of-function (Fig. 4e). The observed magnitudes of expansions that occurred in vivo were striking: for instance, pre-existing mutations in nalD, anmK, and sltB1 started at 7–8% allele frequency and increased to 44–49%, and a presumed de novo mutation in ampD increased to 19%, all over 11 days. Conversely, two independent mexR mutations conferring levofloxacin resistance went to extinction in <5 days. Altogether, our findings show that low-frequency resistance mutations can expand or contract over large magnitudes within days, suggesting that RETRA-Seq could be utilized during acute infection to accurately survey the in vivo dynamics of resistance mutations.
Relating dynamics of low-frequency resistance mutations with antibiotic therapy
The expansion and contraction of low-frequency resistance mutations coincided with changes in antibiotic therapy. In several patients, population-wide resistance to β-lactams—cefepime, ceftazidime, piperacillin-tazobactam—changed significantly over time (Fig. 4f, Supplementary Fig. 4; two-sided Mann–Whitney U-test). Furthermore, increased cefepime resistance over time was associated with longer duration of β-lactam therapy, which was calculated as the fraction of days between serial samples that patients were treated with at least one β-lactam (Fig. 4g, Pearson’s r = 0.936, P = 0.002), that were driven in part by expansions of low-frequency mutations (patients D, F*, G*; Fig. 4b-d). Of note, oprD mutants may have emerged after meropenem therapy10, which was administered to patient D one day prior to mutant detection (Fig. 1b).
Conversely, changes in therapy also coincided with the contraction of resistance mutations. Patient I* was treated with ceftazidime for several days before day 1 but not during the study period (Supplementary Fig. 1), which coincided with a decrease in cefepime resistance over time (Fig. 4g; Supplementary Fig. 4d, f). In the case of the aforementioned extinction of levofloxacin-resistant mexR mutations in patient F* (Fig. 4d), the patient had received ciprofloxacin 6 months earlier but was not treated with fluoroquinolones during the study period. Altogether, our findings show that population resistance can shift within days based on prior and ongoing choice of antibiotic therapy, in part by the expansion or contraction of low-frequency resistance mutations.
Discussion
Our study shows that the frequencies of resistance mutations change rapidly with antibiotic therapy, highlighting a potential for deep sequencing-guided, short-term cycling of antibiotics within patients as a possible future therapeutic strategy. As resistance mutations can persist in the population for months following treatment42, monitoring low-frequency mutations by deep population profiling can inform which antibiotics should be avoided, or conversely, should be actively used in the case of compounds that select against a specific type of resistance43,44. While antibiotic cycling has been proposed as a strategy to limit the selective advantage of resistance mutations based on mathematical modeling and experimental evolution studies45–49, to date, there are limited data on its clinical efficacy50. Our study suggests a possible approach in acute infections, by cycling drugs over days within individual patients over short timescales, which requires further study.
To inform patient-specific antibiotic cycling strategies, molecular diagnostics that deeply and accurately monitor pathogen diversity throughout infection, particularly at the start of infection, are needed. Current culture-based clinical microbiology practice risks missing low-frequency resistant variants51. Furthermore, culture-based assays introduce growth bias that differs from the native context of the human lung, where spatial selection is known to occur on pathogens across different niches8. Specific alleles encoding resistance could be detected with next-generation molecular assays, e.g., CRISPR-based diagnostics52. To monitor known hotspots of mutated genes, we propose resistance-targeted deep amplicon sequencing (RETRA-Seq), using primers that are designed to be suitable across multiple strains, as a highly sensitive method to monitor numerous loci across pathogen genomes.
Methods
Patient enrollment
The clinical research conducted in this study complies with all relevant ethical regulations, and the study protocol was approved by the Institutional Review Board of Boston Children’s Hospital. Informed consent was obtained for sample use/collection and medical record review. For paediatric patients, consent was obtained from legal guardians of each patient. Mechanically ventilated patients in the pediatric ICU (via endotracheal tube (ETT) or tracheostomy tube (trach)) were enrolled in the study at the time of suspected infection, defined as when respiratory samples (sputum obtained via endotracheal aspirate or trach aspirate) were ordered by the clinical team for evaluation of suspected infection, with subsequent confirmation of P. aeruginosa growth in the clinical microbiology lab. Patients typically experienced fever or hypothermia, increase in ventilator settings or oxygen requirement, and/or increase in quantity and/or change in color or thickness of respiratory secretions (Supplementary Table 1). Patients were classified as having pneumonia if they met these criteria and there was a new and persistent infiltrate on chest radiograph (CXR). Patients were classified as tracheitis if CXR showed no evidence of pneumonia but sputum obtained via ETT aspirate or tracheal aspirate showed few, moderate, or abundant polymorphonuclear leukocytes (PMN) on Gram stain. None of the patients met criteria for a ventilator-associated event (VAE). None of the patients had bacteremia, and all recovered from their infection.
Sample collection
Sputum and stool samples were processed within 24–48 h of collection from the patient, and solubilized with 10 mM dithiothreitol, frozen in 15% glycerol, and stored at −80 °C until further processing.
Whole-genome sequencing of P. aeruginosa isolates
solates were cultured from sputum and stool samples as previously described8. Serial dilutions (100–10−4) of each sample in PBS were plated onto cetrimide agar (BD) to identify a dilution plate with growth of 50–300 colonies in total to use for colony picking in order to maximize diversity while minimizing competition between isolates. Colonies (24) were randomly picked by taping a paper pre-marked with 24 random “x” marks to the back of each Petri dish using a clean toothpick, which were placed into 1 mL of LB broth in 96 deep-well plates, then grown overnight at 37 °C with shaking. Half of the saturated cultures were used to make glycerol stocks and the rest were used for DNA extraction (Invitrogen PureLink Pro 96 Genomic DNA Purification Kit). Sequencing libraries of the genomes were prepared as previously described53 and sequenced using paired-end 100 bp reads on the Illumina HiSeq 2000 platform, targeting an average sequencing coverage of 40X per isolate.
Constructing patient-specific reference genomes with long reads
A single colony was isolated from a cetrimide agar plate streaked with each patient’s day 1 sputum sample, grown overnight at 37 °C, and cultured overnight in LB broth with shaking, from which genomes were extracted (Invitrogen PureLink Pro 96 Genomic DNA Purification Kit). Genomes were sequenced on both the PacBio platform (long reads) and on the Illumina HiSeq 2500 platform (short reads) to enable error-correction of assembled contigs. Illumina reads were filtered (min Phred score 15) then trimmed for adapter sequences and assembled de novo using Newbler (v2.7), with minimum contig size 100 bp and minimum coverage at 50×. PacBio reads were assembled de novo using default HGAP 2.0/HGAP 3.0 parameters in the SMRT Analysis Portal (v. 2.3.0). Overlapping contig ends were removed to circularize individual PacBio contigs, and Illumina data was mapped to circularized contigs to detect/correct errors. Comparative genomic analyses were performed using Geneious54.
Constructing a pangenome of coding sequences across reference genomes
A pangenome of all coding sequences found across the patient reference genomes, and two published strains PAO1 and PA14, was constructed with Roary55 3.8.0 (-i 80; minimum percentage identity for blastp). Serotypes were predicted using the web server of PAst56.
Identifying within-patient mutations and short indels
Short reads (Illumina platform) of individual isolate genomes were adapter trimmed (cutadapt v1.8.3), filtered (sickle, quality cutoff 25, length cutoff 50), and aligned to the corresponding patient-specific reference genome (bowtie2 v2.2.4 paired-end, maximum fragment length 2000 bp, no-mixed, dovetail, very-sensitive, n-ceil 0, 0.01). Within-patient single nucleotide polymorphisms (SNPs) were determined by first identifying variant positions of individual isolates with respect to patient-specific references (SAMtools v1.357, FQ ≤ −30), combining the list of variant positions across all isolates of a patient, which were then filtered to high-quality SNP positions. High-quality SNPs were defined as nucleotides at which any two isolates disagreed in the called nucleotide, with both calls meeting a patient-specific FQ threshold that was set based on the distribution of all FQ scores within each patient2. Short insertions and deletions (indels) were identified with platypus58 (v0.8.1, getVariantsFromBAMs = 1, genSNPs = 0, genIndels = 1, minMapQual = 30), using a QD (ratio of variant quality to read depth) threshold set for each patient based on the distribution of all QD values. All short indels were confirmed by visual inspection of the aligned reads. A genotype matrix (isolates by positions) based on SNPs and indels were constructed for each patient’s pathogen population used for downstream analysis.
Within-patient phylogenetic trees
A maximum parsimony phylogenetic tree was constructed for each patient, using the genotype matrix of within-patient SNPs and indels, with dnapars v3.696 (PHYLIP package)59. Indels were treated as a mutational event, with “I” or “D” designating an insertion or deletion. To root the tree, an “Outgroup” for each patient was created by using the most likely ancestral nucleotide state at each polymorphic locus; this was identified by querying a 101 bp sequence (50 bp upstream and downstream from each mutated locus) against all Pseudomonas aeruginosa genomes in the NCBI database with BLASTN. For all polymorphic loci, only one state was found in the database, which was designated as the ancestral state based on its prior observation, while the other state was interpreted as a de novo mutation. All phylogenetic trees were plotted with Toytree v2.0.160.
Estimating patient colonization time
Bayesian phylogenetic analysis (BEAST 1.10.461) was conducted on the genotype matrix of each patient to estimate the time to the ancestral node in days. Input files were generated with BEAUTi v.10.4, and BEAST 1.10.4 was run under a tree prior of coalescent expansion growth model and otherwise default parameters. Analyses were run using CIPRES62.
Pathway analysis of day 1 mutations
Mutations within day 1 pathogen populations across all patients that were found in annotated coding genes (50 of 81 mutations total) were used to identify associated KEGG pathways on The Pseudomonas Genome Database63.
Twitching motility assay
Assay was conducted as previously reported64. Frozen isolates were streaked onto LB-agar plates and grown at 37 °C o/n. Individual colonies were selected with a toothpick and stabbed to the bottom of the twitching assay plate (1% tryptone (Sigma–Aldrich), 0.5% yeast extract (Sigma–Aldrich), 0.5% NaCl (Sigma)); plates were incubated at 37 °C for 20 h. Agar was carefully removed, then plates were stained with 0.1% of Crystal Violet (Sigma) in DI water for 15 min and rinsed with DI water once, then dried. The diameter of the circle was measured in cm.
Permutation test for shift in < dMRCA > over time
The distance to the most recent common ancestor (dMRCA), inferred by the maximum parsimony tree of each patient, was calculated for each isolate within a patient population. Mean <dMRCA> of each sputum sample, <dMRCA> t1 for day 1 and <dMRCA> t2 for follow-up sputum, was calculated within each patient. To test whether the observed difference in means, <dMRCA> t2 − <dMRCA> t1 was significant, we constructed a null model by permuting the sputum sample assignment across all sputa isolates and recalculating the difference in means across 1000 permutations, from which a one-tailed p-value was calculated.
Pro-Q gel for lipopolysaccharide
Colonies from an overnight grown Luria Agar plate were resuspended in Luria Broth, normalized to an OD600 of 2.0, then pelleted. LPS was prepared as previously documented65, and 15 µL of each LPS sample was loaded into each well, then separated by SDS-PAGE in a 10% Mini-PROTEAN TGX gel (Bio-Rad) along with CandyCane glycoprotein ladder (Thermo Fisher). LPS was stained using Pro-Q Emerald 300 LPS Gel Stain (Thermo Fisher) according to the manufacturer’s instructions with slight modifications (the initial fixation step was repeated twice and each washing step was repeated three times).
O6 serotype Western blot
Colonies from an overnight grown Luria Agar plate were resuspended in Luria Broth, normalized to an OD600 of 2.0, then pelleted. LPS was prepared as previously documented65, and 15 µL of each LPS sample was loaded into each well, then separated by SDS-PAGE in a 10% Mini-PROTEAN TGX gel (Bio-Rad) along with Precision Plus All Blue Protein ladder (Bio-Rad). The LPS was then transferred to a PVDF membrane and blocked for 1 h, at room temperature, in PBST-5% milk. O6 primary antibody was incubated in a 1:2500 dilution (Group G, Accurate Chemical & Scientific) in PBST-3% BSA overnight at 4 °C. Secondary α-rabbit-HRP IgG (Sigma) was incubated in a 1:10,000 dilution in PBST-3% BSA for 1 h at room temperature. Blot was visualized using Pierce ECL Western Blotting Substrate (Thermo) according to the manufacturer’s instructions.
Serum killing assay
Isolates were streaked onto TSA plates and incubated at 37 °C o/n, then resuspended in 10 mL PBS + (PBS, 1% proteose peptone, 1 mM CaCl2, 1 mM MgCl2) to an OD600 of 0.25, and diluted 1:23 fold to a final concentration of 5 × 105 CFU/100 µL. Hundred microliters of the diluted culture was mixed with 50% serum (Human Serum, male AB plasma, Sigma–Aldrich H4522; diluted 1:2 with PBS+) in a 96-well round bottom plate in triplicate. Serum assay plates were incubated at 37 °C with shaking at 100 r.p.m. for 1 h, then plated onto TSA, incubated at 37 °C o/n, and quantified for colony forming units (CFU). The PAO1 strain was used as a negative control (not serum sensitive) and PAO1 galU mutant66 was used as a positive control (serum sensitive).
Swarming motility assay
Swarming assays were performed as previously reported67. Swarming medium contained 0.52% agar with M8 medium supplemented with casamino acids (0.5%), glucose (0.2%) and MgSO4 (1 mM). Swarming plates were inoculated with 2.5 µL of an overnight culture grown in LB at 37 °C. Plates were incubated at 37 °C for 16 h. The “Total Swarm Area” is a measure of the number of pixels calculated using ImageJ by first selecting the swarm area, converting images to grayscale (Image → Type → 8-bit), thresholding the image (converting to a black and white image where swarm area is black), and analyzing the particles in the swarm (the number of pixels).
Biofilm and Psl assay
Biofilm assays were performed as previously described68. Overnight cultures (1.5 µL) were inoculated in 100 µL swarming medium and incubated at 37 °C for 24 h. Plates were then stained with 0.1% crystal violet. Absorbance was read at OD550. Psl ELISA was conducted following published methods69. Briefly, 96-well flat-bottom ELISA plates were coated with bacteria overnight at 4 °C. Diluted anti-Psl monoclonal antibody69 (Cam-003; gift from Antonio DiGiandomenico) was added to PBS + 1% BSA (PBS-B)–blocked plates for 1 h, washed with PBS supplemented with 0.1% Tween 20 (PBST), and treated with alkaline phosphatase-conjugated anti–human IgG secondary antibodies (Sigma #A1543) at 1:1000 for 1 h, followed by development with PNP substrate (Sigma).
AlgD promoter activity assay
Strains carrying the lacZ fusion were streaked on PIA or PIA supplemented with 0.1 mM uracil at 37 °C for 24 h. The colonies were then scraped into 4 mL 1× PBS and then diluted to OD600 0.3–0.7. Triplicates of 100 µL of the sample were added to 900 µL of Z-Buffer and 20 µL toluene in a 1.5 mL elution tube. After mixing by inverting 4–5 times tubes were placed with tops open in a shaking incubator at 37 °C for 40 min. After, 200 µL of ortho-Nitrophenyl-β-galactoside (ONPG) (4 mg/mL) (Thermo Scientific, Waltham, MA) was added and the time of color change was recorded the reaction was stopped by adding 500 µL of 1 M Na2CO3 (Fisher Scientific, Waltham, MA) after 20 min. OD420 and OD550 were measured using a SpectraMax i3x (Molecular Devices, Downingtown, PA) plate reader. Miller units were calculated using the following formula: 1000 × [OD420 − (1.75 × OD550)]/[color change time (min.) × Sample volume × OD600]. In-frame deletion of kinB in strain PA14 was conducted using pEX100T-NotI-ΔkinB through a two-step allelic exchange procedure70. Single-crossover merodiploid strains were selected based on sensitivity to sucrose (sacB) and resistance to carbenicillin. Selected merodiploid strains were then grown in LB broth at 37 °C. Double-crossover strains were selected based on sensitivity to carbenicillin and confirmed through PCR amplification of the flanking region of target gene.
Antibiotic susceptibility measurements
Minimum inhibitory concentrations (MICs) or zones of inhibition were measured for each isolate in the Infectious Diseases Diagnostic Laboratory at Boston Children’s Hospital, using the Vitek-2 instrument (liquid culture assay) or disk diffusion assay, respectively.
Preparation of amplicon sequencing library
Total genomic DNA was extracted from each sputum following previously published methods71. Briefly, sputum was mixed with 1 mM dithiothreitol (DTT), incubated at 30 °C for 30 min with 0.18 mg/mL lysostaphin and 3.6 mg/mL lysozyme. DNA was purified using the High Pure PCR Template Preparation Kit (Roche) according to the manufacturer’s instructions and eluted in 30 µL of sterile water. A two-step PCR reaction was used to amplify select loci and add adapter sequences as previously documented72. First PCR. PCR mix was the following: 2 µL DNA template, 10 µL Q5 Hot-Start High-Fidelity 2X Master Mix, 1 µL (NEB #M0494S), 1 µL locus-specific forward primer with UMIs, 1 µL locus-specific reverse primer with UMIs (primers in Supplementary Data 3), 6 µL PCR grade sterile water. Cycling program: hot-start 30 s at 98 °C, 20× cycles of [10 s at 98 °C, 15 s at 67 °C, 15 s at 72 °C], then final extension 2 min at 72 °C. Dilute PCR1 products 1:10 in PCR grade water. Second PCR. PCR mix was the following: 2 µL 1:10 diluted PCR1 product, 10 µL Q5 Hot-Start High-Fidelity 2X Master Mix, 1 µL universal forward primer, 1 µL sample-specific barcoded reverse primer, 6 µL PCR grade sterile water. Cycling program: hot-start 30 s at 98 °C, 20× cycles of [10 s at 98 °C, 30 s at 72 °C], then final extension 2 min at 72 °C. Pool and clean up PCR reaction using a column (Zymo Research #D4013). Amplicon libraries were assessed for correct fragment sizes (350–400 bp) on a 2% agarose gel and quantified using Qubit. Libraries were sequenced on a MiSeq v2 300 cycle kit (Illumina #MS-102-2002) with Read 1: 150 cycles, Index 1: 8 cycles, Read 2: 150 cycles, sequenced at a minimum saturating depth defined as 1/Illumina sequencing error rate, estimated as 0.5%73.
Analysis of amplicon sequencing data
Paired-end reads were trimmed for adapter sequences and filtered with cutadapt (pair-filter q30), then merged across overlapping regions of Read 1 and Read 2 with vsearch v2.15.2, and aligned to the coding sequence of mutated genes (bowtie2–local). From each merged and aligned read, we extracted both the sequence at the profiled locus (wild-type vs. mutant) and the unique UMI sequence (from both forward and reverse), which were used to count the number of unique UMI corresponding to each allele type. Uncertainty of each allele frequency was calculated using the Wilson Score interval based on UMI counts using the statsmodels package (proportion_confint).
Mapping mutations onto protein structure
Protein sequences of mutated genes were queried in the Protein Data Bank (PDB) to find the closes homolog structures: NalD (PDB ID: 5DAJ, 94% identity), AnmK (3QBW, 99% identity), MexR (1LNW, 99% identity), AmpR (5MMH, 100% identity), PA0810 (3UMC, 93% identity).
Statistical analyses
Statistical analyses using Mann–Whitney U-test (ranksum) and Kolmogorov–Smirov test (kstest2) were conducted using built-in packages in MATLAB (R2017b). ANOVA tests for phenotype assays were conducted in Prism (GraphPad). Permutation test for <dMRCA> were conducted in python, with code available at GitHub https://github.com/hattiechung/Paeruginosa_acute_infection.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
This work was funded in part by the Richard A. and Susan F. Smith President’s Innovation Award (to G.P.P.) and by funds for the Translational Research for Infection Prevention in Pediatric Anesthesia and Critical Care (TRIPPACC) Program of the Department of Anesthesiology, Critical Care and Pain Medicine at Boston Children’s Hospital (to G.P.P.), U.S. National Institutes of Health grant R01 GM081617 (to R.K.) and R37 AI83256 (to G.A.O.), The Ernest and Bonnie Beutler Research Program of Excellence in Genomic Medicine (to R.K.), and European Research Council FP7 grant 281891 (to R.K.).
Source data
Author contributions
H.C., K.B.F., R.K., and G.P.P. designed the study. K.B.F., J.B., E.H., M.A., and G.P.P. enrolled patients and collected samples. H.C. and R.L. prepared isolates and sequencing libraries. E.C.S., P.M., J.A.S., and M.H. conducted PacBio sequencing and constructed patient-specific reference genomes. C.M., S.M., S.S.W., A.R.C., R.A.A., H.Y., J.B.G., G.O., and A.J.M. conducted phenotypic assays. H.C. and M.M.S. developed and conducted amplicon sequencing. H.C. developed and conducted all analyses and interpreted results. H.C. wrote the manuscript with N.S.M., G.P.P. and R.K. All authors provided feedback on the manuscript.
Peer review
Peer review information
Nature Communications thanks Marc Bonten and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Data availability
The patient-specific reference genomes constructed from PacBio sequencing in this study have been deposited to Sequence Read Archive (SRA) under accession code PRJNA638217. The raw FASTQ files of Illumina sequencing of the 420 isolates generated in this study have been deposited to SRA under accession code PRJNA622605. The list of all within-patient pathogen variants is available in Supplementary Data 1. The processed data of genomic variants used to construct phylogenetic trees and the data on antibiotic resistance susceptibility profiles of all 420 isolates are available on GitHub [https://github.com/hattiechung/Paeruginosa_acute_infection]. Protein structure data are available at the Protein Data Bank under the following IDs: 5DAJ, 3QBW, 1LNW, 5MMH, 3UMC. Source data are provided with this paper.
Materials availability
All 420 clinical isolates of P. aeruginosa used for whole-genome sequencing on the Illumina platform and for antibiotic susceptibility measurements are available upon request. Source data are provided with this paper.
Code availability
Code used for analyses are available on GitHub [https://github.com/hattiechung/Paeruginosa_acute_infection].
Competing interests
H.C., M.M.S., R.K., and G.P.P. are co-inventors on a provisional patent application filed on RETRA-Seq.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors equally supervised this work: Roy Kishony, Gregory P. Priebe.
Contributor Information
Roy Kishony, Email: rkishony@technion.ac.il.
Gregory P. Priebe, Email: gregory.priebe@childrens.harvard.edu
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-28188-w.
References
- 1.Levy SB, Marshall B. Antibacterial resistance worldwide: causes, challenges and responses. Nat. Med. 2004;10:S122–S129. doi: 10.1038/nm1145. [DOI] [PubMed] [Google Scholar]
- 2.Lieberman TD, et al. Parallel bacterial evolution within multiple patients identifies candidate pathogenicity genes. Nat. Genet. 2011;43:1275–1280. doi: 10.1038/ng.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lieberman TD, et al. Genetic variation of a bacterial pathogen within individuals with cystic fibrosis provides a record of selective pressures. Nat. Genet. 2014;46:82–87. doi: 10.1038/ng.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Markussen T, et al. Environmental heterogeneity drives within-host diversification and evolution of Pseudomonas aeruginosa. MBio. 2014;5:e01592–14. doi: 10.1128/mBio.01592-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marvig RL, Sommer LM, Molin S, Johansen HK. Convergent evolution and adaptation of Pseudomonas aeruginosa within patients with cystic fibrosis. Nat. Genet. 2015;47:57–64. doi: 10.1038/ng.3148. [DOI] [PubMed] [Google Scholar]
- 6.Winstanley C, O’Brien S, Brockhurst MA. Pseudomonas aeruginosa evolutionary adaptation and diversification in cystic fibrosis chronic lung infections. Trends Microbiol. 2016;24:327–337. doi: 10.1016/j.tim.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hilliam Y, et al. Pseudomonas aeruginosa adaptation and diversification in the non-cystic fibrosis bronchiectasis lung. Eur. Respir. J. 2017;49:1602108. doi: 10.1183/13993003.02108-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung H, et al. Global and local selection acting on the pathogen Stenotrophomonas maltophilia in the human lung. Nat. Commun. 2017;8:14078. doi: 10.1038/ncomms14078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossi E, et al. Pseudomonas aeruginosa adaptation and evolution in patients with cystic fibrosis. Nat. Rev. Microbiol. 2020 doi: 10.1038/s41579-020-00477-5. [DOI] [PubMed] [Google Scholar]
- 10.Wheatley R, et al. Rapid evolution and host immunity drive the rise and fall of carbapenem resistance during an acute Pseudomonas aeruginosa infection. Nat. Commun. 2021;12:2460. doi: 10.1038/s41467-021-22814-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rello J, et al. Risk factors for ventilator-associated pneumonia by Pseudomonas aeruginosa in presence of recent antibiotic exposure. Anesthesiology. 2006;105:709–714. doi: 10.1097/00000542-200610000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Ong DSY, et al. Antibiotic exposure and resistance development in Pseudomonas aeruginosa and Enterobacter species in intensive care units. Crit. Care Med. 2011;39:2458–2463. doi: 10.1097/CCM.0b013e318225756d. [DOI] [PubMed] [Google Scholar]
- 13.Kollef MH, et al. Global prospective epidemiologic and surveillance study of ventilator-associated pneumonia due to Pseudomonas aeruginosa. Crit. Care Med. 2014;42:2178–2187. doi: 10.1097/CCM.0000000000000510. [DOI] [PubMed] [Google Scholar]
- 14.Noteboom Y, et al. Antibiotic-induced within-host resistance development of gram-negative bacteria in patients receiving selective decontamination or standard care. Crit. Care Med. 2015;43:2582–2588. doi: 10.1097/CCM.0000000000001298. [DOI] [PubMed] [Google Scholar]
- 15.Vincent J-L, et al. Prevalence and outcomes of infection among patients in intensive care units in 2017. JAMA. 2020;323:1478–1487. doi: 10.1001/jama.2020.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luyt C-E, et al. Ventilator-associated pneumonia in patients with SARS-CoV-2-associated acute respiratory distress syndrome requiring ECMO: a retrospective cohort study. Ann. Intensive Care. 2020;10:158. doi: 10.1186/s13613-020-00775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Razazi K, et al. Risks of ventilator-associated pneumonia and invasive pulmonary aspergillosis in patients with viral acute respiratory distress syndrome related or not to Coronavirus 19 disease. Crit. Care. 2020;24:699. doi: 10.1186/s13054-020-03417-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rouzé A, et al. Relationship between SARS-CoV-2 infection and the incidence of ventilator-associated lower respiratory tract infections: a European multicenter cohort study. Intensive Care Med. 2021;47:188–198. doi: 10.1007/s00134-020-06323-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gragueb-Chatti I, et al. Impact of dexamethasone on the incidence of ventilator-associated pneumonia and blood stream infections in COVID-19 patients requiring invasive mechanical ventilation: a multicenter retrospective study. Ann. Intensive Care. 2021;11:87. doi: 10.1186/s13613-021-00876-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crouch Brewer S, Wunderink RG, Jones CB, Leeper KV., Jr Ventilator-associated pneumonia due to Pseudomonas aeruginosa. Chest. 1996;109:1019–1029. doi: 10.1378/chest.109.4.1019. [DOI] [PubMed] [Google Scholar]
- 21.Kang C-I, et al. Pseudomonas aeruginosa bacteremia: risk factors for mortality and influence of delayed receipt of effective antimicrobial therapy on clinical outcome. Clin. Infect. Dis. 2003;37:745–751. doi: 10.1086/377200. [DOI] [PubMed] [Google Scholar]
- 22.Garnacho-Montero J, et al. Optimal management therapy for Pseudomonas aeruginosa ventilator-associated pneumonia: An observational, multicenter study comparing monotherapy with combination antibiotic therapy. Crit. Care Med. 2007;35:1888–1895. doi: 10.1097/01.CCM.0000275389.31974.22. [DOI] [PubMed] [Google Scholar]
- 23.Ramírez-Estrada S, Borgatta B, Rello J. Pseudomonas aeruginosa ventilator-associated pneumonia management. Infect. Drug Resist. 2016;9:7–18. doi: 10.2147/IDR.S50669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pachori P, Gothalwal R, Gandhi P. Emergence of antibiotic resistance Pseudomonas aeruginosa in intensive care unit; a critical review. Genes Dis. 2019;6:109–119. doi: 10.1016/j.gendis.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang K, et al. The rapid in vivo evolution of Pseudomonas aeruginosa in ventilator-associated pneumonia patients leads to attenuated virulence. Open Biol. 2017;7:170029. doi: 10.1098/rsob.170029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Persyn E, et al. Rapid genetic and phenotypic changes in Pseudomonas aeruginosa clinical strains during ventilator-associated pneumonia. Sci. Rep. 2019;9:4720. doi: 10.1038/s41598-019-41201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diaz Caballero J, et al. Selective sweeps and parallel pathoadaptation drive Pseudomonas aeruginosa evolution in the cystic fibrosis lung. MBio. 2015;6:e00981–15. doi: 10.1128/mBio.00981-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Didelot X, Bowden R, Wilson DJ, Peto TEA, Crook DW. Transforming clinical microbiology with bacterial genome sequencing. Nat. Rev. Genet. 2012;13:601–612. doi: 10.1038/nrg3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baker S, Thomson N, Weill F-X, Holt KE. Genomic insights into the emergence and spread of antimicrobial-resistant bacterial pathogens. Science. 2018;360:733–738. doi: 10.1126/science.aar3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Břinda K, et al. Rapid inference of antibiotic resistance and susceptibility by genomic neighbour typing. Nat. Microbiol. 2020;5:455–464. doi: 10.1038/s41564-019-0656-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pincus NB, et al. A genome-based model to predict the virulence of Pseudomonas aeruginosa isolates. MBio. 2020;11:e01527–20. doi: 10.1128/mBio.01527-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorrie CL, et al. Gastrointestinal carriage is a major reservoir of Klebsiella pneumoniae infection in intensive care patients. Clin. Infect. Dis. 2017;65:208–215. doi: 10.1093/cid/cix270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freschi L, et al. The Pseudomonas aeruginosa pan-genome provides new insights on its population structure, horizontal gene transfer, and pathogenicity. Genome Biol. Evol. 2019;11:109–120. doi: 10.1093/gbe/evy259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bush SJ, et al. Genomic diversity affects the accuracy of bacterial single-nucleotide polymorphism-calling pipelines. Gigascience. 2020;9:giaa007. doi: 10.1093/gigascience/giaa007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vargas R, et al. In-host population dynamics of Mycobacterium tuberculosis complex during active disease. Elife. 2021;10:e61805. doi: 10.7554/eLife.61805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hannan TJ, et al. Host-pathogen checkpoints and population bottlenecks in persistent and intracellular uropathogenic Escherichia coli bladder infection. FEMS Microbiol. Rev. 2012;36:616–648. doi: 10.1111/j.1574-6976.2012.00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Thompson CM, Trzciński K, Lipsitch M. Within-host selection is limited by an effective population of Streptococcus pneumoniae during nasopharyngeal colonization. Infect. Immun. 2013;81:4534–4543. doi: 10.1128/IAI.00527-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aidley J, et al. Nonselective bottlenecks control the divergence and diversification of phase-variable bacterial populations. MBio. 2017;8:e02311–16. doi: 10.1128/mBio.02311-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morita Y, Cao L, Gould VC, Avison MB, Poole K. nalD encodes a second repressor of the mexAB-oprM multidrug efflux operon of Pseudomonas aeruginosa. J. Bacteriol. 2006;188:8649–8654. doi: 10.1128/JB.01342-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ertesvåg H, et al. Identification of genes affecting alginate biosynthesis in Pseudomonas fluorescens by screening a transposon insertion library. BMC Genomics. 2017;18:11. doi: 10.1186/s12864-016-3467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lamers RP, Nguyen UT, Nguyen Y, Buensuceso RNC, Burrows LL. Loss of membrane-bound lytic transglycosylases increases outer membrane permeability and β-lactam sensitivity in Pseudomonas aeruginosa. Microbiology Open. 2015;4:879–895. doi: 10.1002/mbo3.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yelin I, et al. Personal clinical history predicts antibiotic resistance of urinary tract infections. Nat. Med. 2019;25:1143–1152. doi: 10.1038/s41591-019-0503-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gonzales PR, et al. Synergistic, collaterally sensitive β-lactam combinations suppress resistance in MRSA. Nat. Chem. Biol. 2015;11:855–861. doi: 10.1038/nchembio.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stone LK, et al. Compounds that select against the tetracycline-resistance efflux pump. Nat. Chem. Biol. 2016;12:902–904. doi: 10.1038/nchembio.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szybalski W, Bryson V. Genetic studies on microbial cross resistance to toxic agents i. J. Bacteriol. 1952;64:489–499. doi: 10.1128/jb.64.4.489-499.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Imamovic L, Sommer MOA. Use of collateral sensitivity networks to design drug cycling protocols that avoid resistance development. Sci. Transl. Med. 2013;5:204ra132. doi: 10.1126/scitranslmed.3006609. [DOI] [PubMed] [Google Scholar]
- 47.Kim S, Lieberman TD, Kishony R. Alternating antibiotic treatments constrain evolutionary paths to multidrug resistance. Proc. Natl Acad. Sci. USA. 2014;111:14494–14499. doi: 10.1073/pnas.1409800111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baym M, Stone LK, Kishony R. Multidrug evolutionary strategies to reverse antibiotic resistance. Science. 2016;351:aad3292. doi: 10.1126/science.aad3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barbosa C, et al. Alternative evolutionary paths to bacterial antibiotic resistance cause distinct collateral effects. Mol. Biol. Evol. 2017;34:2229–2244. doi: 10.1093/molbev/msx158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Duijn PJ, et al. The effects of antibiotic cycling and mixing on antibiotic resistance in intensive care units: a cluster-randomised crossover trial. Lancet Infect. Dis. 2018;18:401–409. doi: 10.1016/S1473-3099(18)30056-2. [DOI] [PubMed] [Google Scholar]
- 51.Andersson DI, Nicoloff H, Hjort K. Mechanisms and clinical relevance of bacterial heteroresistance. Nat. Rev. Microbiol. 2019;17:479–496. doi: 10.1038/s41579-019-0218-1. [DOI] [PubMed] [Google Scholar]
- 52.Ackerman CM, et al. Massively multiplexed nucleic acid detection with Cas13. Nature. 2020;582:277–282. doi: 10.1038/s41586-020-2279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baym M, et al. Inexpensive multiplexed library preparation for megabase-sized genomes. PLoS ONE. 2015;10:e0128036. doi: 10.1371/journal.pone.0128036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kearse M, et al. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Page AJ, et al. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics. 2015;31:3691–3693. doi: 10.1093/bioinformatics/btv421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thrane SW, Taylor VL, Lund O, Lam JS, Jelsbak L. Application of whole-genome sequencing data for O-specificantigen analysis and in silico serotyping of Pseudomonas aeruginosa isolates. J. Clin. Microbiol. 2016;54:1782–1788. doi: 10.1128/JCM.00349-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li H, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rimmer A, et al. Integrating mapping-, assembly- and haplotype-based approaches for calling variants in clinical sequencing applications. Nat. Genet. 2014;46:912–918. doi: 10.1038/ng.3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baum BR. PHYLIP: Phylogeny inference package. Version 3.2. Joel Felsenstein. Q. Rev. Biol. 1989;64:539–541. [Google Scholar]
- 60.Eaton DAR. Toytree: A minimalist tree visualization and manipulation library for Python. Methods Ecol. Evol. 2020;11:187–191. [Google Scholar]
- 61.Bouckaert R, et al. BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2019;15:e1006650. doi: 10.1371/journal.pcbi.1006650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miller, M. A., Pfeiffer, W. & Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. in 2010 Gateway Computing Environments Workshop (GCE) (IEEE, 2010).
- 63.Winsor GL, et al. Enhanced annotations and features for comparing thousands of Pseudomonas genomes in the Pseudomonas genome database. Nucleic Acids Res. 2016;44:D646–D653. doi: 10.1093/nar/gkv1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O’May C, Tufenkji N. The swarming motility of Pseudomonas aeruginosa is blocked by cranberry proanthocyanidins and other tannin-containing materials. Appl. Environ. Microbiol. 2011;77:3061–3067. doi: 10.1128/AEM.02677-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Davis, M. R., Jr & Goldberg, J. B. Purification and visualization of lipopolysaccharide from Gram-negative bacteria by hot aqueous-phenol extraction. J. Vis. Exp. 10.3791/3916 (2012). [DOI] [PMC free article] [PubMed]
- 66.Priebe GP, et al. The galU gene of Pseudomonas aeruginosa is required for corneal infection and efficient systemic spread following pneumonia but not for infection confined to the lung. Infect. Immun. 2004;72:4224–4232. doi: 10.1128/IAI.72.7.4224-4232.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ha D-G, Kuchma SL, O’Toole GA. Plate-based assay for swarming motility in Pseudomonas aeruginosa. Methods Mol. Biol. 2014;1149:67–72. doi: 10.1007/978-1-4939-0473-0_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O’Toole, G. A. Microtiter dish biofilm formation assay. J. Vis. Exp. 10.3791/2437 (2011). [DOI] [PMC free article] [PubMed]
- 69.DiGiandomenico A, et al. Identification of broadly protective human antibodies to Pseudomonas aeruginosa exopolysaccharide Psl by phenotypic screening. J. Exp. Med. 2012;209:1273–1287. doi: 10.1084/jem.20120033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Damron FH, Qiu D, Yu HD. The Pseudomonas aeruginosa sensor kinase KinB negatively controls alginate production through AlgW-dependent MucA proteolysis. J. Bacteriol. 2009;191:2285–2295. doi: 10.1128/JB.01490-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Terranova L, et al. How to process sputum samples and extract bacterial DNA for microbiota analysis. Int. J. Mol. Sci. 2018;19:3256. doi: 10.3390/ijms19103256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kelsic ED, et al. RNA structural determinants of optimal codons revealed by MAGE-seq. Cell Syst. 2016;3:563–571.e6. doi: 10.1016/j.cels.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stoler N, Nekrutenko A. Sequencing error profiles of Illumina sequencing instruments. NAR Genom Bioinform. 2021;3:lqab019. doi: 10.1093/nargab/lqab019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The patient-specific reference genomes constructed from PacBio sequencing in this study have been deposited to Sequence Read Archive (SRA) under accession code PRJNA638217. The raw FASTQ files of Illumina sequencing of the 420 isolates generated in this study have been deposited to SRA under accession code PRJNA622605. The list of all within-patient pathogen variants is available in Supplementary Data 1. The processed data of genomic variants used to construct phylogenetic trees and the data on antibiotic resistance susceptibility profiles of all 420 isolates are available on GitHub [https://github.com/hattiechung/Paeruginosa_acute_infection]. Protein structure data are available at the Protein Data Bank under the following IDs: 5DAJ, 3QBW, 1LNW, 5MMH, 3UMC. Source data are provided with this paper.
All 420 clinical isolates of P. aeruginosa used for whole-genome sequencing on the Illumina platform and for antibiotic susceptibility measurements are available upon request. Source data are provided with this paper.
Code used for analyses are available on GitHub [https://github.com/hattiechung/Paeruginosa_acute_infection].