Abstract
Objectives
Transfusion with washed packed red blood cells (PRBCs) may be associated with reduced transfusion‐related pro‐inflammatory cytokine production. This may be because of alterations in recipient immune responses.
Methods
This randomised trial evaluated the effect of transfusion with washed compared with unwashed PRBCs on pro‐inflammatory cytokines and endothelial activation in 154 preterm newborns born before 29 weeks’ gestation. Changes in plasma cytokines and measures of endothelial activation in recipient blood were analysed after each of the first three transfusions.
Results
By the third transfusion, infants receiving unwashed blood had an increase in IL‐17A (P = 0.04) and TNF (P = 0.007), whereas infants receiving washed blood had reductions in IL‐17A (P = 0.013), TNF (P = 0.048), IL‐6 (P = 0.001), IL‐8 (P = 0.037), IL‐12 (P = 0.001) and IFN‐γ (P = 0.001). The magnitude of the post‐transfusion increase in cytokines did not change between the first and third transfusions in the unwashed group but decreased in the washed group for IL‐12 (P = 0.001), IL‐17A (P = 0.01) and TNF (P = 0.03), with the difference between the groups reaching significance by the third transfusion (P < 0.001 for each cytokine).
Conclusion
The pro‐inflammatory immune response to transfusion in preterm infants can be modified when PRBCs are washed prior to transfusion. Further studies are required to determine whether the use of washed PRBCs for neonatal transfusion translates into reduced morbidity and mortality.
Keywords: immunomodulation, preterm, red blood cells, transfusion
This study found that the pro‐inflammatory response to transfusion in very preterm infants is modified when packed red blood cells (PRBCs) are washed prior to transfusion. Further studies are required to determine whether the routine use of washed PRBCs for neonatal transfusion will reduce morbidity and mortality.
![]()
Introduction
Packed red blood cell (PRBC) transfusion is an independent predictor of death in critically ill adults and children. In addition, transfusion increases the incidence of multiple‐organ system failure, length of hospital stay, nosocomial infection and long‐term immune modulation. 1 , 2 Although this association is well described, the underlying mechanism(s) remain poorly understood. One contributing pathway may be the recipient’s immune response to the transfusion itself, a process termed transfusion‐related immunomodulation (TRIM). TRIM is characterised by both adverse pro‐inflammatory and immunosuppressive responses and is likely a ‘two‐insult’ process. 3 Initial sensitisation to inflammatory processes primes host neutrophils with subsequent exposure to biological response mediators that accumulate during PRBC storage resulting in an amplified immune response in the recipient. 4 , 5
Extremely premature infants are susceptible to so‐called ‘foetal inflammatory syndrome’, the association of intra‐amniotic infection in spontaneous preterm labour with systemic inflammation in the newborn. 6 It is associated with one or more potentially fatal or severely disabling morbidities, including necrotising enterocolitis (NEC) and bronchopulmonary dysplasia (BPD). These conditions are characterised by systemic and local tissue inflammation and endothelial activation, associated with elevated peripheral blood plasma pro‐inflammatory cytokines. 7 , 8 Transfusion of blood products, an essential intervention for many critically ill preterm infants, is notoriously associated with the promotion of an inflammatory state in the recipient. 9 , 10 , 11 Modifications in blood processing, such as pre‐storage leukodepletion, have resulted in reductions in the incidence of these morbidities. 12 However, blood products remain biologically active, with increases in both pro‐inflammatory cytokines and measures of endothelial activation seen post‐transfusion, effects that increase with repeated transfusion exposure. 9 , 10 , 11
Pre‐transfusion washing of PRBCs removes proteins, extracellular potassium, inflammatory cytokines and chemokines, as well as red blood cell microparticles, 11 reducing their adverse immunomodulatory potential. In adult patients, transfusion with washed PRBCs is associated with reduced morbidity and mortality, 13 while in paediatric cardiac surgical patients, it is associated with reduced transfusion‐related pro‐inflammatory cytokine production. 14 The aim of this study was to investigate whether transfusion with washed compared to unwashed leucodepleted PRBCs in extremely preterm infants results in an amelioration of both the pro‐inflammatory cytokine and endothelial activation responses following transfusion.
Results
Baseline clinical characteristics are shown in Table 1. No differences were seen for antenatal characteristics, gestational age at birth, sex or birthweight. The transfusion characteristics at the three PRBC transfusion exposures are shown in Table 2, with no significant differences seen between the groups. Long‐term clinical outcomes for both groups are shown in Table 3. No unexpected serious adverse events occurred in either treatment arm.
Table 1.
Baseline antenatal and neonatal characteristics
| Unwashed PRBCs (n = 77) | Washed PRBCs (n = 77) | P‐value | |
|---|---|---|---|
| Maternal | |||
| Age, years | 30 (26–35) | 32 (27–36) | 0.18 |
| Parity | 1 (0–1) | 1 (0–2) | 0.24 |
| Mode of delivery | |||
| SVD | 29 (38) | 26 (34) | 0.74 |
| LSCS | 48 (62) | 51 (66) | |
| Steroids | |||
| None | 6 (8) | 5 (7) | 0.8 |
| Incomplete | 13 (17) | 15 (20) | |
| Complete | 48 (63) | 49 (64) | |
| Repeat | 9 (12) | 7 (9) | |
| Antibiotics | 31 (40) | 31 (40) | 1.0 |
| Magnesium sulphate | 48 (62) | 52 (67) | 0.15 |
| Chorioamnionitis, histological | 30 (39) | 23 (30) | 0.3 |
| High vaginal swab | 20 (26) | 19 (24) | 1.0 |
| Diabetes, any | 3 (4) | 6 (8) | 0.5 |
| Premature rupture of membranes | 22 (29) | 28 (36) | 0.3 |
| Antepartum haemorrhage | 26 (34) | 20 (26) | 0.38 |
| Preeclampsia | 2 (3) | 5 (6) | 0.27 |
| Multiple birth | 22 (29) | 17 (22) | 0.46 |
| Neonatal | |||
| Gestation, weeks | 26 (24–27) | 26 (25–27) | 0.72 |
| Male | 50 (65) | 47 (61) | 0.74 |
| Birth weight, g | 800 (660–966) | 830 (650–990) | 0.8 |
| IUGR | 9 (12) | 15 (19) | 0.27 |
| Intubated at delivery | 31 (40) | 40 (52) | 0.2 |
| APGAR 5 min | 7 (6–8) | 7 (6–8) | 0.9 |
IUGR, intrauterine growth restriction; LSCS, lower segment caesarean section; PRBCs, packed red blood cells; SVD, spontaneous vaginal delivery.
Data are presented as median (IQR) or N (%). The Kruskal–Wallis test and the Pearson chi‐squared test were used for comparison between groups.
Table 2.
Transfusion exposure
| Transfusion 1 | Transfusion 2 | Transfusion 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| UW (n = 77) | W (n = 77) | P‐value | UW (n = 59) | W (n = 62) | P‐value | UW (n = 47) | W (n = 50) | P‐value | |
| Postnatal age (days) | 4 (2–11) | 3 (2–7) | 0.64 | 6 (3–11) | 7 (3–18) | 0.88 | 13 (6–26) | 19 (7–28) | 0.58 |
| Pre‐transfusion Hb (g L−1) | 122 (103–135) | 122 (102–131) | 0.54 | 115 (97–126) | 111 (95–123) | 0.49 | 105 (89–126) | 100 (84–115) | 0.16 |
| Post‐transfusion Hb (g L−1) | 133 (122–149) | 135 (124–146) | 0.89 | 133 (119–144) | 131 (115–144) | 0.75 | 126 (118–141) | 127 (114–137) | 0.82 |
| Transfusion out of protocol | 22 (28) | 17 (22) | 0.7 | 15 (25) | 8 (13) | 0.4 | 13 (28) | 11 (23) | 0.84 |
Hb, haemoglobin; UW, unwashed group; W, washed group.
Data are presented as median (IQR). The Kruskal–Wallis test and the Pearson chi‐squared test were used for comparison between groups.
Table 3.
Long‐term clinical outcomes
| Unwashed PRBCs (n = 77) | Washed PRBCs (n = 77) | P‐value | |
|---|---|---|---|
| RDS | 74 (96) | 72 (94) | 0.72 |
| Surfactant | 64 (83) | 70 (91) | 0.23 |
| Sepsis, culture positive | 20 (26) | 12 (16) | 0.5 |
| Early (< 48 h) | 1 | 3 | |
| Late | 19 | 9 | |
| Total transfusions | 3 (2–6) | 3 (2–6) | 1.0 |
| Emergency transfusion | 21 (27) | 21 (27) | 1.0 |
| Received platelets or plasma | 23 (31) | 21 (29) | 0.9 |
| SIP | 5 (6) | 2 (3) | 0.44 |
| NEC | 5 (6) | 2 (3) | 0.44 |
| PVL | 2 (3) | 4 (5) | 0.7 |
| IVH | 9 (12) | 11 (14) | 0.8 |
| ROP | 21 (27) | 18 (23) | 0.7 |
| BPD | 45 (58) | 45 (58) | 1.0 |
| Length of ventilation, days | 10 (2–22) | 11 (4–18) | 0.9 |
| Postnatal steroids | 38 (49) | 40 (52) | 0.87 |
| Length of stay, days | 91 (78–110) | 92 (68–111) | 0.49 |
| Death | 5 (6) | 8 (10) | 0.4 |
BPD, bronchopulmonary dysplasia; IVH, intraventricular haemorrhage; NEC, necrotising enterocolitis; PRBC, packed red blood cells; PVL, periventricular leucomalacia; RDS, respiratory distress syndrome; ROP, retinopathy of prematurity; SIP, spontaneous intestinal perforation.
Data are presented as median (IQR) or N (%). The Kruskal–Wallis test and the Fisher exact test or the Pearson chi‐squared test were used for comparison between groups.
A random selection of transfusion pack samples across the three transfusions was carried out (n = 36 per group) to determine whether detectable levels of cytokines and measures of endothelial activation were present. IFN‐γ, IL‐1β, IL‐10, IL‐12 and IL‐17A were undetectable in both the unwashed and washed packs. However, IL‐6 was detectable in the unwashed packs and TNF, IL‐8, sICAM, sVCAM, PAI and MIF were detectable in both pack types (Table 4).
Table 4.
Transfusion pack concentrations of cytokines and markers of endothelial activation (pg mL−1)
| Unwashed (n = 34) | Washed (n = 34) | P‐value | |
|---|---|---|---|
| IFN‐γ | UD | UD | – |
| IL‐6 | 5.5 (1.2–13.8) | 0 | < 0.001 |
| IL‐8 | 6.5 (2.7–8.6) | 3.7 (2.1–8.3) | 0.36 |
| IL‐12 | UD | UD | – |
| IL‐17A | UD | UD | – |
| TNF | 4.5 (3.9–5.2) | 3.3 (3.2–3.9) | 0.01 |
| MIF | 100.2 (67.7–158.9) | 199.5 (104.5–290.6) | 0.002 |
| sICAM | 83 (53–116) | 37 (29–105) | 0.32 |
| sVCAM | 1097 (789–1424) | 198 (144–608) | < 0.001 |
| PAI1 | 61 (48–100) | 26 (14–43) | 0.005 |
UD, undetectable.
Data are presented as median (IQR). The Mann–Whitney U‐test was used for comparison between groups.
Transfusion‐related changes in plasma cytokines
Neither washed nor unwashed PRBCs resulted in significant post‐transfusion changes in IL‐1β, IL‐12, IL‐17A, IFN‐γ or TNF following the first transfusion exposure. However, transfusion with washed PRBCs was associated with a significant post‐transfusion reduction in IL‐6 (P = 0.008) and IL‐10 (P = 0.014) and both washed and unwashed PRBCs resulted in a reduction in IL‐8 (washed P = 0.027, unwashed P = 0.024). Following the second PRBC exposure, no changes were seen for IL‐1β, IL‐6 or IL‐17A for either washed or unwashed PRBCs. However, transfusion of washed PRBCs resulted in a reduction in IFN‐γ (P = 0.043), TNF (P = 0.041), IL‐8 (P = 0.004) and IL‐10 (P = 0.003) and an increase in IL‐12 (P = 0.01). By the third transfusion, significant post‐transfusion reductions were seen for IL‐17A (P = 0.013), TNF (P = 0.048), IL‐6 (P = 0.001), IL‐8 (P = 0.037), IL‐12 (P = 0.001) and IFN‐γ (P = 0.001) in the washed group, with IL‐17A (P = 0.04) and TNF (P = 0.007) increased in the unwashed group (Figure 1).
Figure 1.

Plasma cytokine response following three transfusion exposures. (a) IL‐17A, (b) TNF, (c) IL‐6, (d) IL‐8, (e) IL‐12 and (f) IFN‐γ. Median (IQR). *P‐value < 0.05, **P‐value < 0.01 and ***P‐value < 0.001.
Within‐subject changes in the pre‐ to post‐transfusion change in plasma cytokines for both groups across the three transfusion exposures were analysed by mixed linear models with gestation, sex, age at first transfusion and pre‐transfusion haemoglobin as covariates. Tests for fixed effects demonstrated a significant interaction effect between PRBC group and transfusion exposure for IL‐12 (F 1,307 = 4.0, P = 0.04), IL‐17A (F 1,307 = 4.9, P = 0.03) and TNF (F 1,206 = 2.4, P = 0.046) (Figure 2). For newborns transfused with washed PRBCs, the pre‐ to post‐transfusion reduction in IL‐12 (P = 0.001), IL‐17A (P = 0.01) and TNF (P = 0.03) became progressively greater between the first and third transfusion exposures. No such changes were seen in newborns transfused with unwashed PRBCs. By the third transfusion, the pre‐ to post‐transfusion change between the groups was significantly different for IL‐12, IL‐17A and TNF (P < 0.001 for each). In addition, a fixed effect for transfusion exposure was demonstrated for IFN‐γ (F 1,236 = 4.9, P = 0.028) with the change being greatest after the third transfusion irrespective of the type of PRBCs transfused.
Figure 2.

Mixed models analysis of the pre‐ to post‐transfusion change in plasma cytokines at transfusions 1–3. (a) IL‐12, (b) IL‐17A and (c) TNF. *P‐value < 0.05, **P‐value < 0.01 and ***P‐value < 0.001.
No baseline differences were seen in cytokine concentrations between the groups for the first transfusion exposure. However, newborns receiving washed PRBCs had higher IL‐1β (P = 0.03), IL‐6 (P = 0.01) and IFN‐γ (P = 0.05) prior to their second transfusion and higher IL‐1β (P = 0.03) and IL‐6 (P = 0.01) prior to the third transfusion. For those newborns receiving unwashed blood, the pre‐transfusion concentrations did not vary between the first and third transfusions. However, for newborns transfused with washed PRBCs, significant changes in baseline cytokine concentrations were seen for IFN‐γ (P < 0.001), IL‐12 (P = 0.012), IL‐17A (P = 0.002) and IL‐1β (P = 0.008) (Table 5). The post hoc tests demonstrated increases in transfusion from 1 to 3 for IFN‐γ (P = 0.002), IL‐12 (P < 0.001) and IL‐17A (P = 0.01).
Table 5.
Baseline cytokines and markers of endothelial activation
| Transfusion 1 | Transfusion 2 | Transfusion 3 | ||||
|---|---|---|---|---|---|---|
| UW | W | UW | W | UW | W | |
| IFN‐γ | 0.9 (0–13.9) | 1.32 (0–4.9) | 0 (0–15.2) | 4.2* (0.4–6.7) | 2.58 (0–15.4) | 5.5 (0.8–19) |
| IL‐10 | 13 (1.4–40.1) | 16 (4–32.1) | 11.9 (1.7–66.3) | 12.8 (3.6–49.8) | 11.3 (1.7–25.1) | 12 (5.1–25.1) |
| IL‐12 | 1.8 (0–8.8) | 1.7 (0.8–6.4) | 3.5 (0–9) | 2.7 (1–8) | 2.7 (0–6.3) | 4 (1.3–12.5) |
| IL‐17A | 3.5 (0–14.8) | 2.1 (0.1–7) | 5.1 (0–11.9) | 3.4 (0.4–9.6) | 7.4 (1.2–16.7) | 5.9 (0.6–22) |
| IL‐1β | 0 (0–6.4) | 0.5 (0–2.8) | 0 (0–1.2) | 0.8* (0–5.4) | 0 (0–2.1) | 1.2* (0.3–5.3) |
| IL‐6 | 17.58 (0–54.6) | 28.1 (6.4–103) | 10.9 (0–42.8) | 29.9** (7.4–87.2) | 8.2 (0–29.3) | 23.8** (8.9–73.4) |
| IL‐8 | 191 (64–314) | 252 (118–527) | 75.4 (37.5–242.5) | 259.6 (97.5–455) | 89.2 (35.3–274.4) | 285.3 (67.4–396.2) |
| TNF | 50.2 (31.8–81) | 40.2 (28.5–66) | 40.8 (26–93.9) | 44.2 (30.2–71.6) | 46.5 (29.9–66.9) | 55.5 (32.7–73.6) |
| MIF | 126.5 (61.7–296.2) | 149 (62–349) | 121.8 (98.9–210.2) | 237.3** (126.5–628.2) | 132.8 (70.2–332.9) | 126.8 (50.8–404.9) |
| sICAM1a | 19.8 (14.3–31.4) | 20.4 (11–32.6) | 22.9 (16.0–34.7) | 26.4 (13.3–41.7) | 29.0 (23.8–42.9) | 23.6 (11.5–32.9) |
| sFasL | 17.2 (6.7–28.7) | 15.1 (8.6–30.6) | 15.4 (7.1–25.6) | 17.4 (11.3–37.2) | 15.2 (8.5–26.5) | 18.9 (5.2–33.4) |
| sFas | 245 (173–380) | 322 (261–442) | 257.8 (168.7–392) | 343* (242–439) | 247 (194–506) | 318 (221–422) |
| sVCAM1a | 227 (171–389) | 226 (19–446) | 251 (160–367) | 246 (184–420) | 190 (113–375) | 259 (114–397) |
| PAI1a | 12.2 (7.0–16.6) | 9.3 (4.2–17.1) | 9.5 (4.3–13.0) | 14.1 (4.1–25.5) | 6.9 (3.8–13.9) | 10.5 (7.6–17.5) |
UW, unwashed; W, washed.
Data are presented as median (IQR) and pg mL−1 unless ang mL−1. The Mann–Whitney U‐test was used for comparison between groups.
*P < 0.05 and **P < 0.01.
Transfusion‐related changes in measures of endothelial activation
No changes were observed for any of the measures of endothelial activation following the first transfusion in either group. Following the second PRBC transfusion, unwashed PRBCs resulted in an increase in sICAM1 (P = 0.023), while transfusion with washed PRBCs was associated with an increase in sFas (P = 0.026). At the third transfusion, there was an increase in MIF (P = 0.005) and PAI1 (P = 0.05) in those transfused with unwashed PRBCs. However, no significant fixed effects in either washed or unwashed group were seen on mixed linear model analysis. Pre‐transfusion concentrations of the measures of endothelial activation did not differ between the first, second and third transfusions for either group.
Discussion
Preterm neonates are particularly susceptible to inflammatory injury, characterised by elevated pro‐inflammatory cytokines in peripheral blood plasma. 15 , 16 This is consistent with a mild‐to‐moderate inflammatory response attributable to the insult of premature delivery, and often, especially in spontaneous preterm birth, exposure to pro‐inflammatory infectious or sterile stimuli in utero. 17 This inflammatory state, related to exposure to intra‐amniotic infection, is associated with a greater incidence of inflammatory pathologies, particularly affecting the lungs, gastrointestinal tract and brain. 18 , 19 , 20 Therefore, interventions that improve or limit further inflammation are likely to improve clinical outcomes and reduce neonatal morbidity and mortality. In the current study, repeated transfusion exposures to washed leucodepleted PRBCs elicited a decrease in plasma pro‐inflammatory cytokines and chemokines, an effect not seen in newborns transfused with standard unwashed leucodepleted PRBCs. As a result, by the third transfusion exposure, the post‐transfusion levels of plasma IL‐12, IL‐17A and TNF were significantly lower than those in infants exposed to unwashed PRBCs. In addition, newborns transfused with unwashed PRBCs also had increased MIF and PAI1, markers of endothelial activation, an effect not seen with washed PRBCs.
Extremely preterm newborns are a heavily transfused population with the greatest exposure in the first days to weeks following birth. 21 Despite this, previous studies have focused on single exposures, often weeks after birth. 10 , 22 Observational studies in this high‐risk patient group suggest an association between increasing transfusion exposure and cumulative volume of blood transfused with a greater risk of mortality 23 and morbidities such as BPD and NEC. 5 , 24 , 25 We have previously shown that repeated transfusion with unwashed leucodepleted PRBCs results in a greater magnitude of the post‐transfusion increases in several pro‐inflammatory cytokines. 9 The current data suggest that the strength and nature of transfusion‐related alterations in circulating pro‐inflammatory cytokines changes over the course of repeated transfusion exposure, but importantly, this can be modified by washing PRBCs prior to transfusion. If TRIM underlies the association between transfusion exposure and neonatal morbidity and mortality, transfusion with washed PRBCs could reduce its impact, ultimately contributing to improved clinical outcomes.
The current observations are consistent with emerging evidence that suggests washing alters the immunomodulatory potential of PRBCs. This is proposed to be secondary to the removal of cell‐free haemoglobin, microparticles, eicosanoids and pro‐inflammatory cytokines and chemokines, all of which accumulate during storage and stimulate the production of pro‐inflammatory cytokines in the transfusion recipient. 11 , 26 , 27 In vitro data support a beneficial effect of PRBC washing, with exposure to supernatant from unwashed PRBCs resulting in increased endothelial permeability and higher pro‐inflammatory cytokine and chemokine release, an effect not induced by supernatant from washed PRBCs. 11 , 28 In the sole clinical study comparing the effect of transfusion with washed to unwashed PRBCs in paediatric patients, transfusion with washed PRBCs was associated with a reduction in post‐transfusion increases in IL‐6 and IL‐10. 14 It would appear from the current data that the beneficial effect of PRBC washing is also evident in the extremely preterm newborn. Washing may, however, result in post‐washing increases in potassium and haemolysis, 29 and as a result, washed PRBCs have a reduced shelf life of 28 days compared with that of 35 for standard unwashed leucodepleted PRBCs. In addition, while animal data report conflicting effects on survival and end‐organ injury, 30 , 31 a recent review concludes that it is unclear how these data relate to humans. 29
While a ‘two‐insult’ hypothesis has been proposed to underlie TRIM with long‐term or permanent alteration to immune function compounded by repeat transfusion exposure, 32 the exact mechanism remains poorly understood. PRBCs have been shown to prime mononuclear cells and neutrophils resulting in increased pro‐inflammatory cytokines such as IL‐8, while altering the chemotactic properties of red blood cells. 33 , 34 PRBCs may also activate vascular endothelial cells and platelets, cells that are highly sensitive to inflammatory signals, with subsequent release of additional toxic bioactive mediators. 4 Here, we show that post‐transfusion levels of pro‐inflammatory cytokines and markers of endothelial activation can be modified by washing PRBCs before transfusion. However, the characterisation of the immunomodulatory effects of PRBC products in individual patients is challenging. 35 We propose that the increases in post‐transfusion cytokines, even if modest, contribute to an amplified inflammatory response, which represents the ‘second hit’ in the proposed two‐insult model of transfusion‐related immunomodulation. The development of morbidities linked to transfusion exposure in the very preterm newborn is not the result of a single event but rather cumulative events in nature. Indeed, multiple risk factors for these morbidities have been identified, including gestational age, requirement for mechanical ventilation and growth restriction. As such, the removal of biological response mediators that accumulate during storage by the washing process prevents the ‘second insult’ and amplification of the inflammatory response with beneficial consequences for the transfused newborn.
Repeat transfusion with washed versus unwashed PRBCs was associated with significantly different changes in both pro‐inflammatory cytokines and markers of endothelial activation. The interaction between pro‐inflammatory cytokines and the endothelium may play an important role in linking transfusion exposure to adverse outcomes. For instance, TNF and IL‐1β activate endothelial cells resulting in the recruitment of leucocytes to sites of cellular damage 36 and IL‐8 promotes leucocyte emigration from the vasculature. 37 Central to these processes is MIF, which was only increased following transfusion with unwashed PRBCs. MIF is important in the regulation of host inflammatory and immune responses and is produced by monocytes/macrophages upon stimulation with various pro‐inflammatory stimuli including TNF and interferon‐γ. 38 Similarly, transfusion with unwashed PRBC alone resulted in increased concentrations of PAI1. PAI1 has been proposed to play a role in the pathogenesis of BPD. 39 Produced by a number of cells including macrophages, it is strongly influenced by inflammatory cytokines such as TNF and IL‐6. 40 The current data suggest a potential link between TRIM and endothelial activation or damage, with the third transfusion exposure to unwashed but not washed PRBCs associated with increases in pro‐inflammatory cytokines and measures of endothelial activation. This could contribute to organ dysfunction and morbidity, for instance post‐transfusion lung injury. 41
Previous studies, focusing solely on unwashed PRBCs, report conflicting evidence for the presence of detectable levels of cytokines and markers of endothelial activation in the transfusion packs. 10 , 22 , 42 The current data are consistent with those reported by Keir et al., 10 but contradict findings by Dani and Locke. 22 , 42 While the age of the PRBCs and the assay methodology are similar between these studies, in the current study and that of Keir et al., the blood pack additive solution was SAG‐M rather than AS‐5 Optisol. 10 While some studies have reported increased cytokine levels, microparticle production and endothelial adhesion 43 in packs containing SAG‐M, 44 others have failed to show a difference. 45 We contend that the presence of detectable cytokines in both the unwashed and washed PRBC packs is of questionable significance. As SAG‐M was the blood product additive for both the unwashed and washed PRBCs in the current study, it does not explain the observed differences between the groups. Further, if any post‐transfusion increase was the result of an infusion of cytokines along with the PRBCs, one would expect consistent post‐transfusion changes following each transfusion exposure rather than the transfusion‐specific changes observed.
The current study has several strengths and limitations. Transfusion requirement and the age of PRBCs were controlled using a defined transfusion threshold and a maximum 14‐day shelf life as per routine practice. By investigating repeat transfusion exposure, the current data have greater clinical relevance in extremely preterm newborns who are typically serially transfused. 21 However, as post‐transfusion cytokines and measures of endothelial activation were only determined at a single time point, it is unknown whether the changes are transient or sustained, an important consideration given that there is evidence for time‐related changes in specific cytokines and adhesion molecules. 22 In addition, data on temporal changes in cytokines during early life in extremely preterm infants are limited and conflicting, 46 , 47 with reports of both increases and decreases. 48 , 49 These descriptive studies are confounded by small sample sizes 50 and exposure to intrauterine inflammation, 51 or limited by a focus on predicting a specific morbidity. 52 As a result, it is difficult to interpret the significance of the pre‐transfusion increases in IFN‐γ, IL‐1β, IL‐12 and IL‐17A across the three transfusion exposures, an effect only observed in the washed transfusion group. Further, this difference between the washed and unwashed groups cannot be explained by differences in clinical characteristics. In addition, as the post‐transfusion concentrations were decreased rather than being increased for each cytokine where the baseline changed, we contend that these baseline differences have no impact on the post‐transfusion differences observed between the groups.
While all newborns only received PRBCs as per study allocation, it is important to acknowledge that a significant number of newborns were transfused outside the transfusion protocol. This was a result of clinical decisions made to transfuse above the predefined threshold. This is an inherent difficulty in studying this high‐risk population, one that is prone to cardiovascular instability particularly during the early postnatal period. Importantly, the rate of transfusions out of protocol was similar between the unwashed and washed groups and across the three transfusion exposures. In addition, pre‐transfusion haemoglobin was included as a covariate in the mixed model analysis. Finally, as the sample size in both groups fell as transfusion exposure increased, resulting in a loss of power, we concede that this should be considered when interpreting the observed differences between the groups.
Conclusion
The current data support the potential for TRIM to be present early following preterm birth, influenced by repeat transfusion exposure and, critically, to be modifiable when PRBCs are washed before transfusion. These data indicate that PRBC washing may mitigate the adverse consequences of PRBC transfusion in neonates and provide important mechanistic insights into the potential of washed PRBCs to be associated with improved neonatal outcome. However, to conclusively determine whether transfusion with washed PRBCs results in improved survival and reduced postnatal morbidity requires an adequately powered randomised controlled trial to compare clinical outcomes after transfusion with washed and unwashed PRBCs.
Methods
This multicentre, double‐blinded, parallel randomised clinical trial was conducted in the two level III NICUs located in South Australia (The Women’s and Children’s Hospital and Flinders Medical Centre) from September 2015 to December 2019. The Women’s and Children’s Human Research Ethics Committee approved the study protocol (REC 2498/9/15, HREC/12/WCHN/5, SSA/12/WCHN/56, SSA/17/SAC/230). In addition, ethics approval was also gained from the Australian Red Cross Lifeblood Human Research Ethics Committee (2013#06). The study was prospectively registered in the Australia and New Zealand Clinical Trial Registry (ACTRN12613000237785).
Randomisation
A computer‐generated randomisation schedule using a balanced variable block design was generated by an independent statistician not involved with the trial participants or data analysis. Infants were randomised into washed and unwashed groups with a 1:1 allocation ratio, stratified by gestational age (23+0–25+6 and 26+0–28+6 weeks). Randomisation was completed by the Women’s and Children’s Hospital transfusion laboratory when notified of an enrolled infant reaching the transfusion threshold. Study investigators, clinical staff and parents were blinded to study allocation, whereas transfusion laboratory staff were unblinded to comply with hospital ordering and Australian Red Cross Lifeblood safety protocols.
Participants
Infants with < 29 weeks’ gestation were screened prior to participation, and written informed consent was obtained from parents before enrolment (Figure 3). Those infants with major congenital malformations or requiring emergency transfusion prior to consent were excluded. For enrolled infants, the decision to transfuse was based on the haemoglobin concentration determined by complete blood picture with the transfusion threshold based on the restrictive arm of the Premature Infants in Need of Transfusion (PINT) study (Table 6). 21 The contribution of enteral feeding during transfusion on significant morbidity, specifically NEC, remains contentious. For the current study, infants were fasted 4 h before, during and 4 h following transfusion, according to standard nursery practice. A fixed transfusion volume of 15 mL kg−1 was given over 3 h via a peripheral intravenous cannula. Exposure to other transfusion products (platelets, fresh frozen plasma and cryoprecipitate) was recorded.
Figure 3.
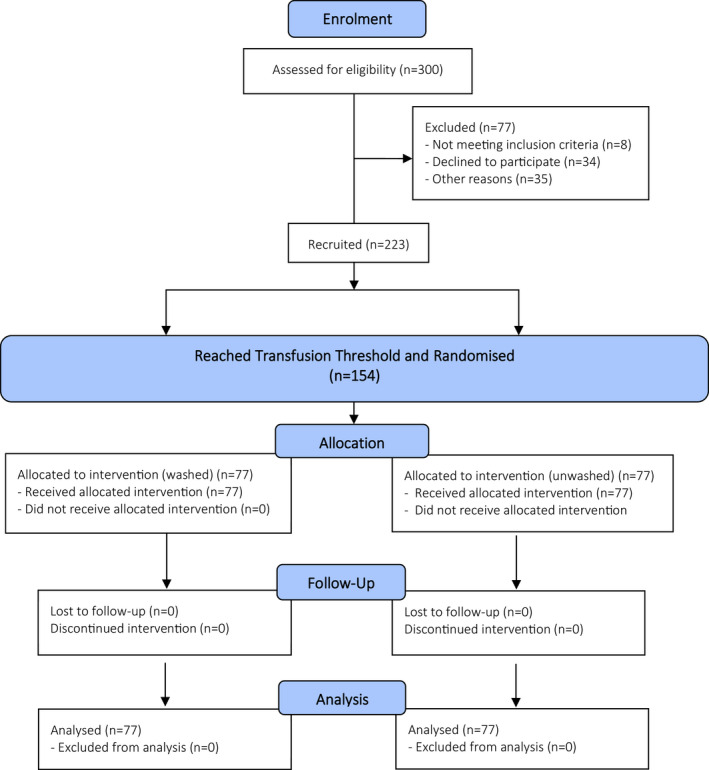
Study consort diagram.
Table 6.
Transfusion algorithm based on modified transfusion threshold employed in the PINT study
| Age (days) | Blood sampling | Respiratory support a | No respiratory support a |
|---|---|---|---|
| 1–7 | Capillary | ≤ 135 | ≤ 120 |
| Central | ≤ 122 | ≤ 109 | |
| 8–14 | Capillary | ≤ 120 | ≤ 100 |
| Central | ≤ 109 | ≤ 90 | |
| ≥ 15 | Capillary | ≤ 100 | ≤ 85 |
| Central | ≤ 90 | ≤ 77 |
Hb threshold levels (g L−1).
Intervention
Australian Red Cross Lifeblood supplied both washed and unwashed PRBC packs for the duration of the study period. Unwashed PRBC packs were group O rhesus negative, CMV negative, non‐irradiated and leucodepleted (quad packs). Each unwashed pack had a mean (SD) volume of 60 ± 4 mL unit−1 and a mean (SD) haematocrit of 61 ± 4. 53 The PRBCs were washed according to established protocols and divided into quad packs. 53 Each washed, leucodepleted red cell pack had a mean (SD) volume of 65 ± 6 mL unit−1 and a mean (SD) haematocrit of 55 ± 3. 53 Both washed and unwashed packs contained the storage additive SAG‐M (adenine, 0.169 g L−1; glucose, 9.0 g L−1; mannitol, 5.25 g L−1; sodium chloride, 8.77 g L−1). The shelf life of both washed and unwashed leucodepleted PRBC quad packs was limited to less than 14 days to control for the risk of storage lesion.
Outcomes
We, and others, have investigated the potential inflammatory effect of PRBC transfusions mediated by increases in plasma pro‐inflammatory cytokines, though the targets of interest and post‐transfusion differences have varied. 9 , 10 , 22 , 41 The primary outcome therefore was the change from pre‐ to post‐transfusion plasma cytokine concentrations in the recipient, specifically IFN‐γ, IL‐1β, IL‐6, IL‐8, IL‐10, IL‐12, IL‐17A and TNF. Secondary outcomes included the pre‐ to post‐transfusion change from baseline in plasma markers of endothelial activation, specifically macrophage inhibitory factor (MIF), soluble intercellular adhesion molecule (sICAM), soluble vascular cell adhesion molecule (sVCAM), soluble Fas ligand (sFasL), soluble Fas (sFas) and endothelial plasminogen activator inhibitor (PAI1). These cytokines, chemokines and markers on endothelial activation were chosen based on our previous studies investigating the response to single and repeated exposure to standard unwashed PRBC transfusions in preterm newborns. 9 , 10
Blood samples (0.4 mL−1) were collected immediately prior to and 4–6 h following transfusion to determine the effect of transfusion on recipient responses as previously described. 9 , 10 , 14 A further sample was collected from each transfusion pack. Samples were centrifuged at 3500 g, and plasma was aliquoted and stored at −80°C. Pre‐ to post‐transfusion changes in cytokines and markers of endothelial activation were analysed by MILLIPLEX Human Cytokine/Chemokine and Human Sepsis Panel multiplex ELISA, respectively (Merck Millipore, Billerica, MA, USA).
Sample size
Significant post‐transfusion changes in IFN‐γ, IL‐6, IL‐8 and IL‐17A have been reported following exposure to allogeneic leucodepleted unwashed PRBCs, 9 , 10 , 22 with in vitro data showing blunted responses following exposure to supernatant from washed PRBCs. 11 However, there is a lack of consistency in the timing of post‐transfusion cytokine measurements. Given that IL‐17A increases at multiple post‐transfusion time points, 22 the current sample size was based on the Δ (the difference between pre‐ and post‐transfusion concentrations) in IL‐17A derived from our previous study in extremely preterm infants. 9 With the mean (SD) ΔIL‐17 in this previous study of 46 infants being 15.3 (13.1) pg mL−1, we determined that a sample size of 62 newborns per group would be required to detect a group difference of 6.6 pg mL−1 (one‐half of a SD) in ΔIL‐17A between the groups with 80% power and an α = 0.05. Since it could not be determined prospectively which newborns would be transfused, enrolment exceeded the necessary sample size to ensure adequate power for the primary aim.
Statistical analysis
In keeping with the hypothesis that repeated exposure results in an amplified inflammatory response in the transfusion recipient and with reports of immunomodulation following exposure to 3–4 PRBC transfusions, 54 , 55 , 56 , 57 an a priori decision was made to determine the effect of washed compared with unwashed PRBC transfusion on the first three transfusions. As pre‐ and post‐transfusion cytokine concentrations were not normally distributed, the Friedman test was used to assess differences between baseline concentrations prior to each transfusion exposure, with an alpha level of 0.01 to adjust for multiple comparisons. Pre‐ and post‐transfusion levels of cytokines and measures of endothelial activation are presented as median (IQR), with differences assessed using the Wilcoxon signed rank test.
Within‐subject temporal differences in the pre‐ to post‐transfusion change in cytokines and measures of endothelial activation across the three transfusion exposures for the two groups were analysed using mixed linear models. Heterogeneous compound symmetry was used as the repeated covariance type and LSD as the confidence interval adjustment to compare changes within treatment groups. To investigate whether transfusion‐related changes were influenced by gestation, sex, age at first transfusion and pre‐transfusion haemoglobin, these variables were included as covariates. Data were analysed using the Statistical Package for the Social Sciences (SPSS v26; IBM SPSS, Chicago, IL, USA).
Conflict of interest
The authors declare no conflict of interest.
Author contributions
Tara M Crawford: Data curation; Formal analysis; Funding acquisition; Investigation; Project administration; Visualization; Writing – original draft; Writing – review & editing. Chad C Andersen: Conceptualization; Methodology; Supervision; Writing – original draft; Writing – review & editing. Nicolette A Hodyl: Conceptualization; Formal analysis; Supervision; Writing – review & editing. Sarah A Robertson: Resources; Supervision; Writing – original draft; Writing – review & editing. Michael J Stark: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Writing – original draft; Writing – review & editing.
Acknowledgments
We acknowledge the significant contribution of Adam Dichiera, Transfusion Laboratory, SA Pathology, the Women’s and Children’s Hospital, Adelaide, Dr Scott Morris, Neonatal Medicine, Flinders Medical Centre, Adelaide, and Associate Professor Denese Marks, Australian Red Cross Lifeblood. Sample analysis was supported by a National Blood Authority Grant, ID413.
References
- 1. Glance LG, Dick AW, Mukamel DB et al. Association between intraoperative blood transfusion and mortality and morbidity in patients undergoing noncardiac surgery. Anesthesiology 2011; 114: 283–292. [DOI] [PubMed] [Google Scholar]
- 2. Rajasekaran S, Kort E, Hackbarth R et al. Red cell transfusions as an independent risk for mortality in critically ill children. J Intensive Care 2016; 4: 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Karam O, Tucci M, Toledano BJ et al. Length of storage and in vitro immunomodulation induced by prestorage leukoreduced red blood cells. Transfusion 2009; 49: 2326–2334. [DOI] [PubMed] [Google Scholar]
- 4. Sparrow RL. Red blood cell storage and transfusion‐related immunomodulation. Blood Transfus 2010; 8: 26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blau J, Calo JM, Dozor D, Sutton M, Alpan G, La Gamma EF. Transfusion‐related acute gut injury: necrotizing enterocolitis in very low birth weight neonates after packed red blood cell transfusion. J Pediatr 2011; 158: 403–409. [DOI] [PubMed] [Google Scholar]
- 6. Jung E, Romero R, Yeo L et al. The fetal inflammatory response syndrome: the origins of a concept, pathophysiology, diagnosis, and obstetrical implications. Semin Fetal Neonatal Med 2020; 25: 101146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Menden H, Tate E, Hogg N, Sampath V. LPS‐mediated endothelial activation in pulmonary endothelial cells: role of Nox2‐dependent IKK‐β phosphorylation. Am J Physiol Lung Cell Mol Physiol 2013; 304: 445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yazji I, Sodhi CP, Lee EK et al. Endothelial TLR4 activation impairs intestinal microcirculatory perfusion in necrotizing enterocolitis via eNOS‐NO‐nitrite signaling. Proc Natl Acad Sci USA 2013; 110: 9451–9456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crawford TM, Andersen CC, Stark MJ. Effect of repeat transfusion exposure on plasma cytokine and markers of endothelial activation in the extremely preterm neonate. Transfusion 2020; 60: 2217–2224. [DOI] [PubMed] [Google Scholar]
- 10. Keir AK, McPhee AJ, Andersen CC, Stark MJ. Plasma cytokines and markers of endothelial activation increase after packed red blood cell transfusion in the preterm infant. Pediatr Res 2013; 73: 75–79. [DOI] [PubMed] [Google Scholar]
- 11. Loh YS, Tan S, Kwok M, Stark MJ, Marks DC. Reduction of biological response modifiers in the supernatant of washed paediatric red blood cells. Vox Sang 2016; 111: 365–373. [DOI] [PubMed] [Google Scholar]
- 12. Fergusson D, Hébert PC, Lee SK et al. Clinical outcomes following institution of universal leukoreduction of blood transfusions for premature infants. JAMA 2003; 289: 1950–1956. [DOI] [PubMed] [Google Scholar]
- 13. Blumberg NHJ, Rowe JM. A randomized trial of washed red blood cell and platelet transfusions in adult acute leukemia. BMC Blood Disord 2004; 4: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cholette JM, Henrichs KF, Alfieris GM et al. Washing red blood cells and platelets transfused in cardiac surgery reduces postoperative inflammation and number of transfusions: results of a prospective, randomized, controlled clinical trial. Pediatric Crit Care Med 2012; 13: 290–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Humberg A, Fortmann I, Siller B et al. Preterm birth and sustained inflammation: consequences for the neonate. Semin Immunopathol 2020; 42: 451–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dammann O, Allred EN, Fichorova RN et al. Duration of systemic inflammation in the first postnatal month among infants born before the 28th week of gestation. Inflammation 2016; 39: 672–677. [DOI] [PubMed] [Google Scholar]
- 17. Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol 1998; 179: 194–202. [DOI] [PubMed] [Google Scholar]
- 18. Yoon BH, Romero R, Kim KS et al. A systemic fetal inflammatory response and the development of bronchopulmonary dysplasia. Am J Obstet Gynecol 1999; 181: 773–779. [DOI] [PubMed] [Google Scholar]
- 19. Pilypienė I, Drazdienė N, Dumalakienė I et al. The significance of fetal inflammatory response syndrome in early and later adaptation of premature infants. Arch Gynecol Obstet 2015; 291: 67–72. [DOI] [PubMed] [Google Scholar]
- 20. Yoon BH, Romero R, Yang SH et al. Interleukin‐6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am J Obstet Gynecol 1996; 174: 1433–1440. [DOI] [PubMed] [Google Scholar]
- 21. Kirpalani H, Whyte RK, Andersen C et al. The Premature Infants in Need of Transfusion (PINT) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr 2006; 149: 301–307. [DOI] [PubMed] [Google Scholar]
- 22. Dani C, Poggi C, Gozzini E et al. Red blood cell transfusions can induce proinflammatory cytokines in preterm infants. Transfusion 2017; 57: 1304–1310. [DOI] [PubMed] [Google Scholar]
- 23. dos Santos AMN, Guinsburg R, de Almeida MFB et al. Red blood cell transfusions are independently associated with intra‐hospital mortality in very low birth weight preterm infants. J Pediatr 2011; 159: 371–376. [DOI] [PubMed] [Google Scholar]
- 24. Collard KJ. Is there a causal relationship between the receipt of blood transfusions and the development of chronic lung disease of prematurity? Med Hypotheses 2006; 66: 355–364. [DOI] [PubMed] [Google Scholar]
- 25. Valieva OA, Strandjord TP, Mayock DE, Juul SE. Effects of transfusions in extremely low birth weight infants: a retrospective study. J Pediatr 2009; 155: 331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jacobi KE, Wanke C, Jacobi A, Weisbach V, Hemmerling TM. Determination of eicosanoid and cytokine production in salvaged blood, stored red blood cell concentrates, and whole blood. J Clin Anesth 2000; 12: 94–99. [DOI] [PubMed] [Google Scholar]
- 27. Olofsson KE, Andersson L, Nilsson J, Bjorkbacka H. Nanomolar concentrations of lysophosphatidylcholine recruit monocytes and induce pro‐inflammatory cytokine production in macrophages. Biochem Biophys Res Commun 2008; 370: 348–352. [DOI] [PubMed] [Google Scholar]
- 28. Rao RS, Howard CA, Teague TK. Pulmonary endothelial permeability is increased by fluid from packed red blood cell units but not by fluid from clinically‐available washed units. J Trauma 2006; 60: 851–858. [DOI] [PubMed] [Google Scholar]
- 29. Cardigan R, New HV, Tinegate H, Thomas S. Washed red cells: theory and practice. Vox Sang 2020; 115: 606–616. [DOI] [PubMed] [Google Scholar]
- 30. Cortés‐Puch I, Wang D, Sun J et al. Washing older blood units before transfusion reduces plasma iron and improves outcomes in experimental canine pneumonia. Blood 2014; 123: 1403–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wozniak MJ, Qureshi S, Sullo N et al. A comparison of red cell rejuvenation versus mechanical washing for the prevention of transfusion‐associated organ injury in swine. Anesthesiology 2018; 128: 375–385. [DOI] [PubMed] [Google Scholar]
- 32. Taylor RW, Manganaro L, O'Brien J, Trottier SJ, Parkar N, Veremakis C. Impact of allogenic packed red blood cell transfusion on nosocomial infection rates in the critically ill patient. Crit Care Med 2002; 30: 2249–2254. [DOI] [PubMed] [Google Scholar]
- 33. Collard KJ, White DL. On the source of the non‐transferrin‐bound iron which accumulates in packed red blood cell units during storage. Blood Transfus 2014; 12: 527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sparrow RL, Patton KA. Supernatant from stored red blood cell primes inflammatory cells: influence of prestorage white cell reduction. Transfusion 2004; 44: 722–730. [DOI] [PubMed] [Google Scholar]
- 35. Remy KE, Hall MW, Cholette J et al. Mechanisms of red blood cell transfusion‐related immunomodulation. Transfusion 2018; 58: 804–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Petri B, Phillipson M, Kubes P. The physiology of leukocyte recruitment: an in vivo perspective. J Immunol 2008; 180: 6439–6446. [DOI] [PubMed] [Google Scholar]
- 37. Zarbock A, Ley K. Neutrophil adhesion and activation under flow. Microcirculation 2009; 16: 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Calder PC. Polyunsaturated fatty acids, inflammatory processes and inflammatory bowel diseases. Mol Nutr Food Res 2008; 52: 885–897. [DOI] [PubMed] [Google Scholar]
- 39. Ince DA, Atac FB, Ozkiraz S et al. The role of plasminogen activator inhibitor‐1 and angiotensin‐converting enzyme gene polymorphisms in bronchopulmonary dysplasia. Genet Test Mol Biomarkers 2010; 14: 643–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cesari M, Pahor M, Incalzi RA. Plasminogen activator inhibitor‐1 (PAI‐1): a key factor linking fibrinolysis and age‐related subclinical and clinical conditions. Cardiovasc Ther 2010; 28: 72–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rashid N, Al‐Sufayan F, Seshia MM, Baier RJ. Post transfusion lung injury in the neonatal population. J Perinatol 2013; 33: 292–296. [DOI] [PubMed] [Google Scholar]
- 42. Locke R, Paul D, Touch S, Mackley A, Maduskuie V, Fawcett P. Cytokine load in prestorage leukoreduced PRBC transfusions in premature infants. J Perinatol 2005; 25: 526–530. [DOI] [PubMed] [Google Scholar]
- 43. Veale MF, Healey G, Sparrow RL. Effect of additive solutions on red blood cell (RBC) membrane properties of stored RBCs prepared from whole blood held for 24 hours at room temperature. Transfusion 2011; 51: 25–33. [DOI] [PubMed] [Google Scholar]
- 44. Cardigan R, Sutherland J, Wadhwa M, Dilger P, Thorpe R. The influence of platelet additive solutions on cytokine levels and complement activation in platelet concentrates during storage. Vox Sang 2003; 84: 28–35. [DOI] [PubMed] [Google Scholar]
- 45. Weisbach V, Wanke C, Zingsem J, Zimmermann R, Eckstein R. Cytokine generation in whole blood, leukocyte‐depleted and temporarily warmed red blood cell concentrates. Vox Sang 1999; 76: 100–106. [PubMed] [Google Scholar]
- 46. Rizos D, Protonotariou E, Malamitsi‐Puchner A, Sarandakou A, Trakakis E, Salamalekis E. Cytokine concentrations during the first days of life. Eur J Obstet Gynecol Reprod Biol 2007; 131: 32–35. [DOI] [PubMed] [Google Scholar]
- 47. Matoba N, Yu Y, Mestan K et al. Differential patterns of 27 cord blood immune biomarkers across gestational age. Pediatrics 2009; 123: 1320–1328. [DOI] [PubMed] [Google Scholar]
- 48. Dammann O, Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res 1997; 42: 1–8. [DOI] [PubMed] [Google Scholar]
- 49. Schelonka RL, Maheshwari A, Carlo WA et al. T cell cytokines and the risk of blood stream infection in extremely low birth weight infants. Cytokine 2011; 53: 249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lusyati S, Hulzebos CV, Zandvoort J, Sauer PJ. Levels of 25 cytokines in the first seven days of life in newborn infants. BMC Res Notes 2013; 6: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nishimaki S, Shima Y, Sato M, An H, Kadota K, Yokota S. Postnatal changes of cytokines in premature infants with or without funisitis. J Matern Fetal Neonatal Med 2014; 27: 1545–1549. [DOI] [PubMed] [Google Scholar]
- 52. Paananen R, Husa AK, Vuolteenaho R, Herva R, Kaukola T, Hallman M. Blood cytokines during the perinatal period in very preterm infants: relationship of inflammatory response and bronchopulmonary dysplasia. J Pediatr 2009; 154: 39–43. [DOI] [PubMed] [Google Scholar]
- 53. Australian Red Cross Lifeblood . Blood component information 2020 [Web page on Internet]. Commonwealth of Australia [updated June 2020; cited 2020 24th November, Online Resource]. Available from: https://transfusion.com.au/BCI
- 54. Bernard AC, Davenport DL, Chang PK, Vaughan TB, Zwischenberger JB. Intraoperative transfusion of 1 U to 2 U packed red blood cells is associated with increased 30‐day mortality, surgical‐site infection, pneumonia, and sepsis in general surgery patients. J Am Coll Surg 2009; 208: 931–937. [DOI] [PubMed] [Google Scholar]
- 55. Murphy GJ, Reeves BC, Rogers CA, Rizvi SI, Culliford L, Angelini GD. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation 2007; 116: 2544–2552. [DOI] [PubMed] [Google Scholar]
- 56. Santos AA, Sousa AG, Piotto RF, Pedroso JC. Mortality risk is dose‐dependent on the number of packed red blood cell transfused after coronary artery bypass graft. Rev Bras Cir Cardiovasc 2013; 28: 509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Scott BH, Seifert FC, Grimson R. Blood transfusion is associated with increased resource utilisation, morbidity and mortality in cardiac surgery. Ann Card Anaesth 2008; 11: 15–19. [DOI] [PubMed] [Google Scholar]


