Abstract
Purpose of Review
Chimeric antigen receptor (CAR) T-cell therapy is a relatively new, innovative treatment strategy to manage refractory hematological cancers, including some types of leukemia, lymphoma, and multiple myeloma. This article outlines the CAR T-cell therapy process, toxicity, and complications, along with an overview of the currently known short- and long-term physical and functional sequelae that will be helpful for general or oncology rehabilitation specialists caring for these patients.
Recent Findings
There is a dearth of literature on the topic of rehabilitation of patients receiving CAR T-cell therapy. Rehabilitation practices can be extrapolated from the limited functional information on patients who have completed treatment for lymphoma and multiple myeloma. Patients present with cognitive impairment, muscle weakness, reduced exercise capacity, neuropathy, and cancer-related fatigue. Physical activity and rehabilitation programs may be beneficial to address fatigue, psychological symptoms, and quality of life.
Summary
There is limited rehabilitation research in patients receiving CAR T-cell therapy. These patients may present with general deconditioning and neurological complications which translate to neuromuscular and cognitive impairment that benefit from multidisciplinary rehabilitation intervention prior to, during, and after treatment. Studies measuring the impairments at baseline and evaluation of the impact of rehabilitation practices are much needed to support this.
Keywords: Cancer rehabilitation, CAR T-cell rehabilitation, CAR T-cell, Exercise oncology, Lymphoma rehabilitation, Multiple myeloma rehabilitation
Introduction
Chimeric antigen receptor (CAR) T-cell therapy is a relatively new, effective immunotherapy for hematologic malignancies. This pivotal treatment has become a standard-of-care for certain malignancies, such as relapsed non-Hodgkins lymphoma, relapsed or refractory acute lymphoblastic leukemia, and relapsed refractory multiple myeloma, with some studies showing at least 80% of the patients with positive response to treatment.[1, 2] In CAR T-cell therapy, autologous or allogenic T-cells are leukapheresed and genetically modified ex vivo to express a CAR, and these CAR T-cells are then expanded. The patient undergoes lymphocyte-depleting chemotherapy followed by infusion of the CAR T-cells.[3] When CAR T cells confront target cells expressing the matching cell surface antigen, signaling from the CAR induces CAR T cell growth and expansion, cytokine secretion, and subsequently target cell lysis.[4]
Tisagenlecleucel (tisa-cel) was the first CAR T-cell therapy approved by the US Food and Drug Administration (FDA) in 2017.[5] Since that time, four additional CAR T-cell therapies have been approved by the FDA: axicabtagene ciloleucel (axi-cel), lisocabtagene maraleucel (liso-cel), brexucabtagene autoleucel (brexu-cel), and idecabtagene vicleucel (ide-cel).[6] These therapies each have been FDA approved for varying diagnoses including B-Cell precursor acute lymphoblastic leukemia (ALL), diffuse large B-cell lymphoma (DLBCL), follicular lymphoma, mantle cell lymphoma, and multiple myeloma (Table 1).[7] While there continue to be advances in CAR T-cell therapy research in solid tumors, there are no current FDA-approved CAR T-cell treatments for solid tumors.
Table 1.
CAR T-cell therapies approved by the US Food and Drug Administration (FDA).[7] Abbreviations: ALL, acute lymphoblastic leukemia; DLBCL, diffuse large B-cell lymphoma
| CAR T-cell therapy | FDA-approved treatment |
|---|---|
| Tisagenlecleucel (tisa-cel) |
Patients up to 25 years of age with B-cell precursor ALL that is refractory, or in second or later relapse. Adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy including DLBCL not otherwise specified, high-grade B-cell lymphoma, and DLBCL arising from follicular lymphoma. |
| Axicabtagene ciloleucel (axi-cel) |
Adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy, including DLBCL not otherwise specified, primary mediastinal large B-cell lymphoma, high-grade B-cell lymphoma, and DLBCL arising from follicular lymphoma. Adult patients with relapsed or refractory follicular lymphoma after two or more lines of systemic therapy. |
| Lisocabtagene maraleucel (liso-cel) | Adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy, including DLBCL not otherwise specified (including DLBCL arising from indolent lymphoma), high-grade B-cell lymphoma, primary mediastinal large B-cell lymphoma, and follicular lymphoma Grade 3B. |
| Brexucabtagene autoleucel (brexu-cel) | Adult patients with relapsed or refractory mantle cell lymphoma. |
| Idecabtagene vicleucel (ide-cel) | Adult patients with relapsed or refractory multiple myeloma after four or more prior lines of therapy, including an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 monoclonal antibody. |
CAR T-cell therapies can be administered safely in the outpatient setting; however, most patients still receive treatment in the inpatient setting.[8] Barriers to outpatient treatment include specific institutional training and safeguards, monitoring for patient complications, poor understanding of predictive risk factors for the likelihood of CRS or ICANS, and variation in reimbursement policies.[8] Additionally, due to the COVID-19 pandemic, CAR T-cell therapy has been administered in the inpatient setting to ensure bed availability for possible complications.[8] After a series of screening evaluations for CAR T-cell therapy, patients are sent for apheresis, wherein blood is drawn and the T cells are extracted. These T cells are sent to a special laboratory where they are genetically engineered to express the chimeric antigen receptors. These cells are then expanded (multiplied) and sent back to the treating facility; this process of cell expansion followed by quality checks usually takes 3–4 weeks. Most patients receive lymphodepletion chemotherapy outpatient and then are admitted for infusion of the CAR T-cells. Patients are monitored for complications (as outlined below) daily for 7–10 days post infusion. Multidisciplinary care with bedside nursing, case management, social worker support, and if specific functional concerns arise during the hospitalization, PT, OT, and SLP are essential to ensure comprehensive psychosocial support, optimization or preservation of function, and safety for adequate discharge. Metrics for discharge include adequate counts, lack of uncontrolled infection or acute organ dysfunction, and ability for self-care.
Case Presentation
A 74-year-old Caucasian male with a history of atrial fibrillation, diabetes, coronary artery disease, and refractory stage 4 diffuse large B-cell lymphoma status post two lines of chemotherapy treatment was referred to prehabilitation clinic 6 weeks prior to planned chimeric antigen receptor (CAR) T-cell therapy. He was evaluated by the multidisciplinary team through the Enhanced Recovery Cellular Therapy (ERCT) program, a novel early supportive care program integrating multiple specialties to care for older adults requiring allogeneic hematopoietic stem cell transplantation (SCT), which evolved to include CAR T-cell therapy patients. Functional scores obtained during his occupational therapy (OT) and physical therapy (PT) outpatient sessions included Montreal Cognitive Assessment (MoCA) score of 23/30, 6-min walk test (6MWT) distance 309 meters(m) without an assistive device, five times sit-to-stand (5xSTS) in 15.73 seconds(s), gait speed of 1.24 meters per second(m/s), and Timed Up and Go test (TUG) 11.81 s.
The patient was admitted to acute care, received medical clearance, and underwent CAR T-cell therapy. After lymphodepletion with cyclophosphamide and fludarabine, the patient received lisocabtagene maraleucel (liso-cel). His 35-day hospital course was complicated by Grade 1 cytokine release syndrome (CRS) with fever and Grade 2 immune effector cell–associated neurotoxicity syndrome (ICANS) on Day 4 after cell infusion, for which he received a one-time dose of tocilizumab and was started on high-dose corticosteroids. On Day 5, the CRS resolved; however, the neurotoxicity progressed to Grade 3 ICANS. He developed nonconvulsive status epilepticus on Day 7. Magnetic resonance imaging (MRI) of the brain was negative for acute abnormalities. The corticosteroid dose was further increased due to persistent Grade 3 ICANS in the subsequent days. His ICANS began to improve on Day 11 and corticosteroids were tapered. CRS And ICANS resolved by Day 14 after cell infusion. He was treated for neutropenic fever due to pseudomonas aeruginosa in the urine. Patient developed anasarca and significant deconditioning. Around the time of discharge, his functional independence mobility scores included supervision for bed mobility, minimal assistance required for transfers, gait distance of 750 feet with minimal assistance with a rolling walker (RW), moderate assistance for stairs, modified independent for self-feeding and grooming, contact guard assist for toilet transfers, and supervision level for toileting. He was discharged to home with recommendations for outpatient PT and OT.
Upon follow-up in the clinic 1 month after CAR T-cell therapy, the patient complained of word-finding difficulty and difficulty with memory, which had worsened after the CAR T-cell treatment. He now had a MoCA score of 13, Immune Effector Cell Associated Encephalopathy (ICE) score of 7, and ICANS Grade 1. MRI brain revealed no acute findings and positron emission tomography/computed tomography showed a good response to treatment. Lumbar puncture (LP) was negative for infection. Electroencephalography (EEG) revealed triphasic waves suggestive of a diffuse disturbance of cerebral activity and no seizure activity. His physical function outcome measures included ambulated 265 m for the 6MWT with a RW, 5xSTS in 19.71 s, gait speed 0.78 m/s, and TUG in 17.45 s with use of a RW. The patient continued with outpatient PT to work on transfers, gait, balance, and activity tolerance and with OT for instrumental activities of daily living training, cognitive impairment, and energy conservation strategies. He was referred to outpatient speech language pathology (SLP) to address his impaired cognition.
CAR T-Cell Therapy Complications with Functional Implications
While CAR T-cell therapy has led to significant advances in the treatment of hematologic malignancies, it also presents a set of unique toxicities. The two most common acute adverse effects are cytokine release syndrome (CRS) and neurological toxicity known as immune effector cell–associated neurotoxicity syndrome (ICANS), respectively.[9].
CRS is a systemic inflammatory response due to the release of cytokines following the activation of CAR T-cells upon tumor recognition in vivo.[10] CAR T-cells may also activate bystander immune cells causing the release of further inflammatory cytokines.[9] This supraphysiologic inflammatory state can manifest as fever, fatigue, myalgias, rigors, and anorexia, but can rapidly progress to hypotension, tachycardia, tachypnea, hypoxia, and multiorgan dysfunction.[9, 11] Risk factors associated with CRS include cancer type, high disease burden, high number of CAR T-cells administered, and high peak of CAR T-cell expansion in vivo.[11] The median time to onset varies based on the CAR T-cell therapy product and the targeted disease.[11] The onset can range from a few days to several weeks after infusion.[12] Fever is a key clinical sign that should raise suspicion for CRS. Fever tends to be the first symptom of CRS. Patients should be frequently reassessed for worsening signs of CRS, and outpatients should be considered for admission to the hospital.[12].
The American Society of Transplantation and Cellular Therapy (ASTCT), formerly known as the American Society for Blood and Marrow Transplantation (ASBMT), developed the most recent grading system for CRS and ICANS in 2019.[13] CRS grading is based on 3 vital signs: temperature, blood pressure, and oxygen saturation (Table 2).[13] CRS-mediated fever is a diagnosis of exclusion and an infectious workup including, but not limited to, laboratory tests, blood cultures, and imaging should be completed, including the initiation of empiric antibiotics as patients are likely neutropenic.[11, 13].
Table 2.
Grading scale for cytokine release syndrome (CRS), a common complication with CAR T-cell therapy. Reproduced with permission [13] ASTCT CRS consensus grading
| CRS parameter | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Fever* | Temperature ≥ 38 °C | Temperature ≥ 38 °C | Temperature ≥ 38 °C | Temperature ≥ 38 °C |
| With | ||||
| Hypotension | None | Not requiring vasopressors | Requiring a vasopressor with or without vasopressin | Requiring multiple vasopressors (excluding vasopressin) |
| And/or† | ||||
| Hypoxia | None | Requiring low-flow nasal cannula‡ or blow-by | Requiring high-flow nasal cannula‡, facemask, nonrebreather mask, or Venturi mask | Requiring positive pressure (e.g., CPAP, BiPAP, intubation, and mechanical ventilation) |
Abbreviations: Organ toxicities associated with CRS may be graded according to Common Terminology Criteria for Adverse Events (CTCAE) v5.0 but they do not influence CRS grading
*Fever is defined as temperature ≥ 38°C not attributable to any other cause. In patients who have CRS then receive antipyretic or anticytokine therapy such as tocilizumab or steroids, fever is no longer required to grade subsequent CRS severity. In this case, CRS grading is driven by hypotension and/or hypoxia
†CRS grade is determined by the more severe event: hypotension or hypoxia not attributable to any other cause. For example, a patient with temperature of 39.5°C, hypotension requiring 1 vasopressor, and hypoxia requiring low-flow nasal cannula is classified as grade 3 CRS
‡Low-flow nasal cannula is defined as oxygen delivered at ≤6 L/min. Low flow also includes blow-by oxygen delivery, sometimes used in pediatrics. High-flow nasal cannula is defined as oxygen delivered at >6 L/min
CRS management for Grade 1 or 2 CRS is primarily supportive with antipyretics, intravenous fluids, and/or oxygen (Table 2).[13] Serum interleukin-6 (IL-6) have been shown to correlate with severity of CRS.[14] Tocilizumab is an anti-IL-6 receptor antibody which is approved for the treatment of adults and pediatric patients 2 years of age and older with CAR T cell–induced severe or life-threatening cytokine release syndrome (CRS).[7, 14] For patients with clinical deterioration despite at least two doses of tocilizumab and increased risk of severe CRS and neurotoxicity, additional treatment with corticosteroids should be considered.[11] For Grades 3 and 4 CRS, patients require vasopressors and should receive tocilizumab and corticosteroids.[11, 13] With early intervention, CRS can be reversible.[15].
Other encephalopathic-like changes including disorientation, impaired memory with preserved alertness, impulsivity, emotional lability, and agitation may be present while physical exam may detect frontal release signs (snout, grasp, palmomental reflexes).[4] ICANS can occur with CRS, independent of CRS, or after resolution of CRS.[9, 11] ICANS can present 4–5 days after CAR T-cell therapy, but delayed ICANS can also occur up to 4 weeks later.[11, 16] Expressive aphasia, impairment of attention, and confusion are typical presenting symptoms, but ICANS can progress to depressed level of consciousness, coma, seizures, motor weakness, and cerebral edema.[13, 17] Headaches frequently occur with mild ICANS and typically present as tension or pressure type of pain along the occiput. In some patients, tremors, asterixis, and myoclonus are noted, which wax and wane and may worsen during febrile moments. Generalized muscle weakness and balance impairment are excluded from ICANS assessments as they may occur due to deconditioning from intensive chemotherapy and transplantation.[13] The pathophysiology of ICANS is currently not well understood.[9, 11] CAR T-cell trafficking to the central nervous system (CNS), diffusion of cytokines into the CNS, endothelial activation, blood–brain barrier disruption (BBB), and myeloid cell activation have all been suggested to possibly contribute to ICANS.[9, 11] ICANS grading is based on five neurotoxicity domains: the Immune Effector Cell Associated Encephalopathy (ICE) assessment tool, level of consciousness, seizures, motor weakness, and elevated intracranial pressure/cerebral edema (see Tables 3 and 4). Grade 1 ICANS is managed by supportive care and close monitoring.[11] Other etiologies, such as infection, malignancy, stroke, or hemorrhage, need to be excluded. Workup can include EEG, imaging, and lumbar puncture. Corticosteroids can be considered for Grade 2 ICANS and are indicated for Grade 3 and 4 ICANS.[11] The role and timing of anti-epileptic prophylaxis have yet to be determined.[11] If prophylaxis is chosen, levetiracetam is the agent of choice.[9, 17] Similar to CRS, ICANS can be reversible.[9] Tocilizumab can be administered if there is concurrent CRS. However, tocilizumab has been shown to have poor BBB penetration and is not recommended in the absence of CRS.[9, 18].
Table 3.
Immune effector cell–associated encephalopathy (ICE) scoring that is included in the total grade for immune effector cell–associated neurotoxicity syndrome (ICANS). Reproduced with permission [13]
| ICE |
|---|
| • Orientation: orientation to year, month, city, hospital: 4 points |
| • Naming: ability to name 3 objects (e.g., point to clock, pen, button): 3 points |
| • Following commands: ability to follow simple commands (e.g., “Show me 2 fingers” or “Close your eyes and stick out your tongue”): 1 point |
| • Writing: ability to write a standard sentence (e.g., “Our national bird is the bald eagle”): 1 point |
| • Attention: ability to count backwards from 100 by 10: 1 point |
Table 4.
Grading of immune effector cell–associated neurotoxicity syndrome (ICANS). Reproduced with permission [13] ASTCT ICANS consensus grading for adults
| Neurotoxicity domain | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| ICE Score* | 7–9 | 3–6 | 0–2 | 0 (patient is unarousable and unable to perform ICE) |
| Depressed level of consciousness† | Awakens spontaneously | Awakens to voice | Awakens only to tactile stimulus | Patient is unarousable or requires vigorous or repetitive tactile stimuli to arouse. Stupor or coma |
| Seizure | N/A | N/A | Any clinical seizure focal or generalized that resolves rapidly or nonconvulsive seizures on EEG that resolve with intervention | Life-threatening prolonged seizure (> 5 min); or repetitive clinical or electrical seizures without return to baseline in between |
| Motor findings‡ | N/A | N/A | N/A | Deep focal motor weakness such as hemiparesis or paraparesis |
| Elevated ICP/cerebral edema | N/A | N/A | Focal/local edema on neuroimaging§ | Diffuse cerebral edema on neuroimaging; decerebrate or decorticate posturing; or cranial nerve VI palsy; or papilledema; or Cushing’s triad |
ICANS grade is determined by the most severe event (ICE score, level of consciousness, seizure, motor findings, raised ICP/cerebral edema) not attributable to any other cause; for example, a patient with an ICE score of 3 who has a generalized seizure is classified as grade 3 ICANS
N/A indicates not applicable
*A patient with an ICE score of 0 may be classified as grade 3 ICANS if awake with global aphasia, but a patient with an ICE score of 0 may be classified as grade 4 ICANS if unarousable
†Depressed level of consciousness should be attributable to no other cause (e.g., no sedating medication)
‡Tremors and myoclonus associated with immune effector cell therapies may be graded according to CTCAE v5.0, but they do not influence ICANS grading
§Intracranial hemorrhage with or without associated edema is not considered a neurotoxicity feature and is excluded from ICANS grading. It may be graded according to CTCAE v5.0
Other delayed toxicities of CAR T-cell therapy can include B-cell aplasia and hypogammaglobulinemia due to on-target off-tumor effects and Grade 3 or 4 cytopenias.[9] While long-term outcomes of CAR T-cell therapy are awaited, the management and prophylaxis will continue to evolve.
Rehabilitation Interventions for Functional Decline
Prior to CAR T-cell therapy, patients with refractory disease can present with variable functional and/or neurologic deficits based on cancer type, disease burden, and prior failed systemic therapies. These deficits can be exacerbated, or new deficits can develop after CAR T-cell therapy. Fatigue is a frequent complication of cancer and treatments and is commonly reported by patients receiving CAR T-cell therapy. In multi-center trial among patients with refractory diffuse large B-cell lymphoma, mediastinal B-cell lymphoma, or transformed follicular lymphoma treated with axi-cel, 51% of patients reported fatigue after treatment.[19] Similarly, 96% of patients with hematological malignancies treated with axi-cel reported fatigue with 84% reporting moderate to severe fatigue.[20] In a study comparing patient-reported quality of life and symptom burden after CAR T-cell therapy and other forms of cellular therapy, 44% of patients in the CAR T-cell group reported fatigue after treatment.[21] Furthermore, the conditioning chemotherapy that precedes CAR T-cell therapy may cause side effects such as pancytopenia, weakness, nausea, vomiting, poor appetite, and in severe cases neuropathy in the hands and feet, affecting physical function.[22]
Certain lymphomas and their treatments are associated with neurological deficits, translating to impairment in physical function, activities of daily living, psychosocial realms, and behavior.[23–25] In a prospective trial evaluating physical fitness before, during, and after treatment for lymphoma, exercise capacity and lower limb muscle strength were impaired at baseline.[26] As these patients received treatment, there were declines in hemoglobin, fatigue, and declines in exercise capacity and muscle strength. While it has not been yet been determined in CAR T-cell patient populations, exercise has been shown to improve fatigue in patients with hematologic malignancies.[27–29] A systemic review and meta-analysis by Oberoi et al. showed aerobic, neuromotor, resistance, and combination exercises were all effective in reducing fatigue in patients with cancer and hematopoietic stem cell transplant recipients.[30]
Physical activity programs have been shown to impact psychological symptoms, fatigue, and quality of life.[26, 31] A systemic review published in 2021 showed beneficial effects of exercise for fatigue, psychological symptoms, and quality of life.[32] More specifically, aerobic exercise improves physical functioning, quality of life, fatigue, general health, happiness, depression, cardiovascular fitness, and lean body mass in patients with lymphoma who are either on or off treatment.[33] On the other hand, multimodal exercise program that includes endurance and strength training positively affects physical performance, function, and fatigue in leukemia patients undergoing autologous hematopoietic stem cell transplantation (HSCT).[34]
It is important to identify functional deficits before and after CAR T-cell therapy. Cardiorespiratory fitness or submaximal exercise capacity, as measured by the 6MWT, is an important element to measure during survivorship, as a reduction in 6MWT distance strongly predicts an increase in cancer mortality.[35, 36] A prospective study assessing pre- and post-HSCT physical function showed that a decline in the 6MWT distance is highly associated with worsened overall survival and non-relapse mortality.[37] Limbach et al. found that older female patients with low physical activity and who received cardiotoxic therapy prior to allogeneic HSCT are at risk of decreased cardiorespiratory function.[38] Also, higher BMI and lower physical activity were significant predictors for low cardiorespiratory fitness. As seen in our case, the patient had a decline in distance by 44 m and required a RW. His 5xSTS time worsened by 3.98 s. His gait speed as measured by the 10-m walk test declined by 0.46 m/s. His TUG worsened by 5.64 s.
As outlined above, corticosteroids may be used to manage CRS and ICANS. Corticosteroids induce muscle weakness that is significantly worse in lower limb muscles than upper limb muscles.[39] Although there are no studies on steroid myopathy in patients who received CAR T-cell therapy, in patients undergoing HSCT, total corticosteroid dose is negatively correlated with handgrip strength and knee extensor strength, with greater overall decline in strength in knee extensors than muscles of the hand.[40, 41] In a small cohort of HSCT patients receiving corticosteroids for acute graft versus host disease, weakness occurred as early as 14 days after starting treatment, with reduced 5xSTS and knee extensor strength.[42] As muscle weakness and exercise tolerance occur in patients preparing for HSCT,[43] a further decrease in strength after HSCT could result in decreased ability to participate in household and community level activities safely and independently. This decrease in strength after HSCT persists and affects health-related quality of life.[44]
Baseline cognitive function should be collected from the patient and their caregivers. A baseline cognitive evaluation with the MoCA or Saint Louis University Mental Status Examination should be administered. The same baseline test should be administered after CAR T-cell therapy. In our case, impairment was documented at baseline with serial abnormal MoCA exams. Once etiologies, such as infection, malignancy, stroke, and/or hemorrhage are excluded, ICANS should be graded. In an inpatient or outpatient setting, PT, OT, and SLP referrals should be made accordingly. Based on the patient’s prior level of function and cognitive baseline, outpatient neuropsychiatric testing may be appropriate.
Prehabilitation for CAR T-cell Therapy—Is It Possible?
Prehabilitation in oncology care is the process of identifying a baseline functional level, anticipated cancer-associated or cancer therapy-related impairments and to intervene early before or early during the treatment process. There is a paucity of literature on rehabilitation prior to, during, or after CAR T-cell therapy. One recent study showed that 58 patients receiving CAR T-cell therapy had worse physical function (based on patient-reported outcomes) compared with the general population.[45] However, the research on physical fitness and the effects of physical activity on muscle strength, cardiorespiratory endurance, fatigue, and health-related quality of life in patients undergoing HSCT can be used to inform the design and implementation of prehabilitation in patients undergoing CAR T-cell therapy.
The importance of optimization of strength and endurance prior to HSCT is underscored by a retrospective study on patients who received HSCT and participated in an exercise program before, during, and after HSCT. There is a strong association between pre-HSCT and post-HSCT knee extension torque and peak VO2, suggesting that a higher physical function before HSCT results in a higher physical function after HSCT.[46] This highlights the importance of improving muscular strength and cardiopulmonary fitness before receiving HSCT.
The 2019 American College of Sports Medicine’s (ACSM) exercise guidelines for cancer survivors recommend moderate-intensity aerobic exercises for at least 30 min, for at least three times per week, for at least 8 to 12 weeks to address health-related outcomes stemming from cancer diagnosis and cancer-related treatment.[47] Recommendations also include resistance training using at least two sets of 8 to 15 repetitions at a load of at least 60% repetition maximum for at least two times per week.[47] Exercise intensity can be measured through heart rate reserve (HRR), maximal heart rate (HR max), or maximal oxygen consumption (VO2 max). ACSM guidelines for healthy individuals recommend the following ranges for moderate-intensity exercise training: 40–59% HRR, 64–76% HRmax, or 46–63% VO2 max.[48] Walking at around 100 steps per minute for 30 min or 3000 steps in 30 min on 5 days each week meets this guideline.[49] The Australian Association for Exercise and Sport Science guidelines for exercise in cancer patient recommend training at 50–75% HRR or VO2 max and 60–80% HR max to achieve moderate-intensity exercise.[50]
Intensive chemotherapy potentially may affect the cardiovascular response to acute exercise. Cancer patients may exhibit a significant reduction in cardiorespiratory fitness as a result of anticancer therapy and physical inactivity.[51, 52] In an exercise prescription, a sufficient training stimulus without overexertion is necessary to achieve favorable effects. This dose–response relationship between exercise intensity and favorable effects may be absent in cancer patients. A study including patients who underwent allogeneic HSCT showed HRR in these patients was considerably lower than recommended percentages.[53] Based on the results of this study, the application of ACSM guidelines may result in a prescription of higher intensity than intended.[53] In patients who are unable to tolerate moderate- or high-intensity exercises, low-intensity exercises performed at high frequency could also improve physical function.[54]
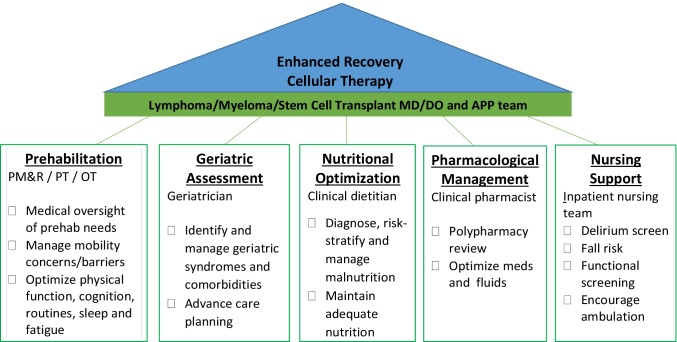
Exercises for patients with cancer could be done with or without supervision of a qualified rehabilitation specialist. Results of a systematic review with meta-analysis showed that supervised exercise interventions result in statistically significant beneficial effects on self-reported quality of life and physical function.[40] However, supervised exercise is often challenging at large cancer centers that receive patients from different geographic locations, as they may not stay locally for lengthy periods or they may out of network for rehabilitation services. At our cancer center, we are currently exploring the role of prehabilitation for patients undergoing CAR T-cell therapy, as discussed in our case. Patients are referred at the time of CAR T-cell therapy work up, typically 3 weeks prior to hospital admission, as part of Enhanced Recovery Cellular Therapy, a supportive care program incorporating multiple disciplines (medical oncologists, physical medicine and rehabilitation [PM&R], PT, OT, geriatrics, pharmacist, clinical dietitian, nursing) to optimize patients prior to treatment (Fig. 1).[55] For the physical prehabilitation, patients are seen by a senior PT and PM&R physician to assess for any neuromuscular, functional, or medical concerns that may interfere with physical activity. They are evaluated by OT to screen for and manage fatigue, sleep hygiene, and cognitive concerns, and to establish a routine conducive to recovery. They are prescribed a home-based exercise program; however, if they exhibit significant functional impairment at these initial visits, they are often referred to outpatient PT and OT. Patients are evaluated and managed by most of the Enhanced Recovery team during their inpatient stay and then again followed as outpatient in order to facilitate rehabilitation. This program has demonstrated favorable benefits for older patients who receive allogeneic HSCT; feasibility and outcomes in patients receiving CAR T-cell therapy will be studied going forward. Despite feasibility, smaller cancer centers may have difficulty duplicating this supportive care model due to limited resources (geriatricians, PM&R physicians, oncology-specialized physical and occupational therapists, clinical pharmacist, and a dedicated clinical dietitian).
Fig. 1.
Elements of the Enhanced Recovery Cellular Transplant (ER-CT) program, a multidisciplinary supportive care approach. Abbreviations: MD/DO, medical doctor/doctor of osteopathic medicine; APP, advanced practice provider; PM&R, Physical Medicine and Rehabilitation physicians; PT, physical therapists; OT, occupational therapists; RN, registered nurse
Summary of Recommendations
CAR T-cell therapy remains a relatively new, effective immunotherapy for hematologic malignancies. There are currently five FDA-approved CAR T-cell therapies with the most common acute adverse effects being CRS and ICANS. Although there is limited data on physical function of patients prior to, during, and after CAR T-cell therapy treatment, rehabilitation concerns and management can be derived from similar patient populations such as from patients who have received HSCT. There should be an emphasis on prehabilitation soon after initiating the workup process for CAR T-cell, maintaining function during active treatment, and close follow-up with rehabilitation especially if neurological sequelae persist.
Declarations
Conflict of Interest
Dr. Obada Obaisi, Dr. Rhodora Fontillas, and Dr. An Ngo-Huang declare they have no financial interests. Dr. Krina Patel served on the advisory boards and received research funding from Janssen, Bristol Meyers Squibb; served on the advisory board for Arcellx and Pfizer; and completed clinical trials with Cellectis, Poseida, Takeda, and Prescisionbio.
Footnotes
This article is part of the Topical Collection on Cancer Rehabilitation
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.June CH, Sadelain M. Chimeric antigen receptor therapy. N Engl J Med. 2018;379(1):64–73. doi: 10.1056/NEJMra1706169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Landry K, Thomas AA. Neurological complications of CAR T cell therapy. Curr Oncol Rep. 2020;22(8):83. doi: 10.1007/s11912-020-00935-6. [DOI] [PubMed] [Google Scholar]
- 3.Tallantyre EC, Evans NA, Parry-Jones J, Morgan MPG, Jones CH, Ingram W. Neurological updates: neurological complications of CAR-T therapy. J Neurol. 2021;268(4):1544–1554. doi: 10.1007/s00415-020-10237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruff MW, Siegler EL, Kenderian SS. A concise review of neurologic complications associated with chimeric antigen receptor T-cell immunotherapy. Neurol Clin. 2020;38(4):953–963. doi: 10.1016/j.ncl.2020.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Sermer D, Brentjens R. CAR T-cell therapy: full speed ahead. Hematol Oncol. 2019;37(Suppl 1):95–100. doi: 10.1002/hon.2591. [DOI] [PubMed] [Google Scholar]
- 6.Society AC. CAR T-cell therapy and its side effects [Available from: https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/immunotherapy/car-t-cell1.html. Accessed 1 Oct 2021.
- 7.Society LaL. Chimeric antigen receptor (CAR) T-cell therapy [Available from: https://www.lls.org/treatment/types-treatment/immunotherapy/chimeric-antigen-receptor-car-t-cell-therapy. Accessed 1 Oct 2021
- 8.Myers GD, Verneris MR, Goy A, Maziarz RT. Perspectives on outpatient administration of CAR-T cell therapy in aggressive B-cell lymphoma and acute lymphoblastic leukemia. J Immunother Cancer. 2021;9(4):1-10. [DOI] [PMC free article] [PubMed]
- 9.Neelapu SS. Managing the toxicities of CAR T-cell therapy. Hematol Oncol. 2019;37(Suppl 1):48–52. doi: 10.1002/hon.2595. [DOI] [PubMed] [Google Scholar]
- 10.Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schubert ML, Schmitt M, Wang L, Ramos CA, Jordan K, Müller-Tidow C, et al. Side-effect management of chimeric antigen receptor (CAR) T-cell therapy. Ann Oncol. 2021;32(1):34–48. doi: 10.1016/j.annonc.2020.10.478. [DOI] [PubMed] [Google Scholar]
- 12.Shimabukuro-Vornhagen A, Gödel P, Subklewe M, Stemmler HJ, Schlößer HA, Schlaak M, et al. Cytokine release syndrome. J Immunother. Cancer. 2018;6(1):56. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with ummune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625–638. doi: 10.1016/j.bbmt.2018.12.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varadarajan I, Lee DW. Management of T-cell engaging immunotherapy complications. Cancer J. 2019;25(3):223–230. doi: 10.1097/PPO.0000000000000377. [DOI] [PubMed] [Google Scholar]
- 16.Hunter BD, Jacobson CA. CAR T-cell associated neurotoxicity: mechanisms, clinicopathologic correlates, and future directions. J Natl Cancer Inst. 2019;111(7):646–654. doi: 10.1093/jnci/djz017. [DOI] [PubMed] [Google Scholar]
- 17.Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15(1):47–62. doi: 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nellan A, McCully CML, Cruz Garcia R, Jayaprakash N, Widemann BC, Lee DW, et al. Improved CNS exposure to tocilizumab after cerebrospinal fluid compared to intravenous administration in rhesus macaques. Blood. 2018;132(6):662–666. doi: 10.1182/blood-2018-05-846428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoogland AI, Jayani RV, Collier A, Irizarry-Arroyo N, Rodriguez Y, Jain MD, et al. Acute patient-reported outcomes in B-cell malignancies treated with axicabtagene ciloleucel. Cancer Med. 2021;10(6):1936–1943. doi: 10.1002/cam4.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sidana S, Thanarajasingam G, Griffin J, Thompson CA, Burtis M, Warsame R, et al. Patient experience of chimeric antigen receptor (CAR)-T cell therapy vs. stem cell transplant: longitudinal patient reported adverse events, cognition and quality of life. Blood. 2019;134:794. doi: 10.1182/blood-2019-121715. [DOI] [Google Scholar]
- 22.Acharya UH, Dhawale T, Yun S, Jacobson CA, Chavez JC, Ramos JD, et al. Management of cytokine release syndrome and neurotoxicity in chimeric antigen receptor (CAR) T cell therapy. Expert Rev Hematol. 2019;12(3):195–205. doi: 10.1080/17474086.2019.1585238. [DOI] [PubMed] [Google Scholar]
- 23.Oerlemans S, Issa DE, van den Broek EC, Nijziel MR, Coebergh JW, Mols F, et al. Impact of therapy and disease-related symptoms on health-related quality of life in patients with follicular lymphoma: results of the population-based PHAROS-registry. Eur J Haematol. 2014;93(3):229–238. doi: 10.1111/ejh.12335. [DOI] [PubMed] [Google Scholar]
- 24.Oerlemans S, Mols F, Nijziel MR, Lybeert M, van de Poll-Franse LV. The impact of treatment, socio-demographic and clinical characteristics on health-related quality of life among Hodgkin’s and non-Hodgkin’s lymphoma survivors: a systematic review. Ann Hematol. 2011;90(9):993–1004. doi: 10.1007/s00277-011-1274-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oerlemans S, Nijziel MR, van de Poll-Franse LV. Age-related differences in quality of life among patients with diffuse large B-cell lymphoma. Cancer. 2015;121(16):2857–2858. doi: 10.1002/cncr.29427. [DOI] [PubMed] [Google Scholar]
- 26.Vermaete N, Wolter P, Verhoef G, Gosselink R. Physical activity and physical fitness in lymphoma patients before, during, and after chemotherapy: a prospective longitudinal study. Ann Hematol. 2014;93(3):411–424. doi: 10.1007/s00277-013-1881-3. [DOI] [PubMed] [Google Scholar]
- 27.Nakano J, Hashizume K, Fukushima T, Ueno K, Matsuura E, Ikio Y, et al. Effects of aerobic and resistance exercises on physical symptoms in cancer patients: a meta-analysis. Integr Cancer Ther. 2018;17(4):1048–1058. doi: 10.1177/1534735418807555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Persoon S, Kersten MJ, van der Weiden K, Buffart LM, Nollet F, Brug J, et al. Effects of exercise in patients treated with stem cell transplantation for a hematologic malignancy: a systematic review and meta-analysis. Cancer Treat Rev. 2013;39(6):682–690. doi: 10.1016/j.ctrv.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 29.van Haren IE, Timmerman H, Potting CM, Blijlevens NM, Staal JB, Nijhuis-van der Sanden MW. Physical exercise for patients undergoing hematopoietic stem cell transplantation: systematic review and meta-analyses of randomized controlled trials. Phys Ther. 2013;93(4):514–28. doi: 10.2522/ptj.20120181. [DOI] [PubMed] [Google Scholar]
- 30.Oberoi S, Robinson PD, Cataudella D, Culos-Reed SN, Davis H, Duong N, et al. Physical activity reduces fatigue in patients with cancer and hematopoietic stem cell transplant recipients: a systematic review and meta-analysis of randomized trials. Crit Rev Oncol Hematol. 2018;122:52–59. doi: 10.1016/j.critrevonc.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 31.Vermaete N, Wolter P, Verhoef G, Gosselink R. Physical activity, physical fitness and the effect of exercise training interventions in lymphoma patients: a systematic review. Ann Hematol. 2013;92(8):1007–1021. doi: 10.1007/s00277-013-1689-1. [DOI] [PubMed] [Google Scholar]
- 32.Amatya B, Khan F, Lew TE, Dickinson M. Rehabilitation in patients with lymphoma: an overview of Systematic Reviews. J Rehabil Med. 2021;53(3):jrm00163. doi: 10.2340/16501977-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Courneya KS, Sellar CM, Stevinson C, McNeely ML, Peddle CJ, Friedenreich CM, et al. Randomized controlled trial of the effects of aerobic exercise on physical functioning and quality of life in lymphoma patients. J Clin Oncol. 2009;27(27):4605–4612. doi: 10.1200/JCO.2008.20.0634. [DOI] [PubMed] [Google Scholar]
- 34.Oechsle K, Aslan Z, Suesse Y, Jensen W, Bokemeyer C, de Wit M. Multimodal exercise training during myeloablative chemotherapy: a prospective randomized pilot trial. Support Care Cancer. 2014;22(1):63–69. doi: 10.1007/s00520-013-1927-z. [DOI] [PubMed] [Google Scholar]
- 35.Åhlund K, Ekerstad N, Bäck M, Karlson BW, Öberg B. Preserved physical fitness is associated with lower 1-year mortality in frail elderly patients with a severe comorbidity burden. Clin Interv Aging. 2019;14:577–586. doi: 10.2147/CIA.S198591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee K, Zhou J, Norris MK, Chow C, Dieli-Conwright CM. Prehabilitative exercise for the enhancement of physical, psychosocial, and biological outcomes among patients diagnosed with cancer. Curr Oncol Rep. 2020;22(7):71. doi: 10.1007/s11912-020-00932-9. [DOI] [PubMed] [Google Scholar]
- 37.Mishra A, Pidala J, Thapa R, Betts BC, Fernandez H, Locke FL, et al. Objective and subjective physical function in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2021;56(12):2897–2903. doi: 10.1038/s41409-021-01428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Limbach M, Kuehl R, Dreger P, Luft T, Rosenberger F, Kleindienst N, et al. Influencing factors of cardiorespiratory fitness in allogeneic stem cell transplant candidates prior to transplantation. Support Care Cancer. 2021;29(1):359–367. doi: 10.1007/s00520-020-05485-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Minetto MA, Qaisar R, Agoni V, Motta G, Longa E, Miotti D, et al. Quantitative and qualitative adaptations of muscle fibers to glucocorticoids. Muscle Nerve. 2015;52(4):631–639. doi: 10.1002/mus.24572. [DOI] [PubMed] [Google Scholar]
- 40.Morishita S, Kaida K, Yamauchi S, Sota K, Ishii S, Ikegame K, et al. Relationship between corticosteroid dose and declines in physical function among allogeneic hematopoietic stem cell transplantation patients. Support Care Cancer. 2013;21(8):2161–2169. doi: 10.1007/s00520-013-1778-7. [DOI] [PubMed] [Google Scholar]
- 41.Takekiyo T, Dozono K, Mitsuishi T, Murayama Y, Maeda A, Nakano N, et al. Effect of exercise therapy on muscle mass and physical functioning in patients undergoing allogeneic hematopoietic stem cell transplantation. Support Care Cancer. 2015;23(4):985–992. doi: 10.1007/s00520-014-2425-7. [DOI] [PubMed] [Google Scholar]
- 42.Ngo-Huang A, Yadav R, Bansal S, Williams J, Wu J, Fu JB, et al. An exploratory study on physical function in stem cell transplant patients undergoing corticosteroid treatment for acute graft-versus-host-disease. Am J Phys Med Rehabil. 2021;100(4):402–406. doi: 10.1097/PHM.0000000000001660. [DOI] [PubMed] [Google Scholar]
- 43.Morishita S, Kaida K, Ikegame K, Yoshihara S, Taniguchi K, Okada M, et al. Impaired physiological function and health-related QOL in patients before hematopoietic stem-cell transplantation. Support Care Cancer. 2012;20(4):821–829. doi: 10.1007/s00520-011-1156-2. [DOI] [PubMed] [Google Scholar]
- 44.Inoue J, Kai M, Doi H, Okamura A, Yakushijin K, Makiura D, et al. Association between physical function and health-related quality of life in survivors of hematological malignancies undergoing hematopoietic stem cell transplantation. Trends Transplant. 2020;14:1-5.
- 45.Mullane E, Jones T, Voutsinas J, Wu QV, Loggers E, Fann J, et al. Patient-reported outcomes at time of CAR-T cell therapy. Blood. 2020;136:35–36. doi: 10.1182/blood-2020-134646. [DOI] [Google Scholar]
- 46.Ishikawa A, Otaka Y, Kamisako M, Suzuki T, Miyata C, Tsuji T, et al. Factors affecting lower limb muscle strength and cardiopulmonary fitness after allogeneic hematopoietic stem cell transplantation. Support Care Cancer. 2019;27(5):1793–1800. doi: 10.1007/s00520-018-4433-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Campbell KL, Winters-Stone KM, Wiskemann J, May AM, Schwartz AL, Courneya KS, et al. Exercise guidelines for cancer survivors: consensus statement from International Multidisciplinary Roundtable. Med Sci Sports Exerc. 2019;51(11):2375–2390. doi: 10.1249/MSS.0000000000002116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334–59. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 49.Marshall SJ, Levy SS, Tudor-Locke CE, Kolkhorst FW, Wooten KM, Ji M, et al. Translating physical activity recommendations into a pedometer-based step goal: 3000 steps in 30 minutes. Am J Prev Med. 2009;36(5):410–415. doi: 10.1016/j.amepre.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 50.Hayes SC, Spence RR, Galvao DA, Newton RU. Australian Association for Exercise and Sport Science position stand: optimising cancer outcomes through exercise. J Sci Med Sport. 2009;12(4):428–434. doi: 10.1016/j.jsams.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 51.Jones LW, Eves ND, Haykowsky M, Freedland SJ, Mackey JR. Exercise intolerance in cancer and the role of exercise therapy to reverse dysfunction. Lancet Oncol. 2009;10(6):598–605. doi: 10.1016/S1470-2045(09)70031-2. [DOI] [PubMed] [Google Scholar]
- 52.Lakoski SG, Eves ND, Douglas PS, Jones LW. Exercise rehabilitation in patients with cancer. Nat Rev Clin Oncol. 2012;9(5):288–296. doi: 10.1038/nrclinonc.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuehl R, Scharhag-Rosenberger F, Schommer K, Schmidt ME, Dreger P, Huber G, et al. Exercise intensity classification in cancer patients undergoing allogeneic HCT. Med Sci Sports Exerc. 2015;47(5):889–895. doi: 10.1249/MSS.0000000000000498. [DOI] [PubMed] [Google Scholar]
- 54.Fukushima T, Nakano J, Ishii S, Natsuzako A, Sakamoto J, Okita M. Low-intensity exercise therapy with high frequency improves physical function and mental and physical symptoms in patients with haematological malignancies undergoing chemotherapy. Eur J Cancer Care (Engl) 2018;27(6):e12922. doi: 10.1111/ecc.12922. [DOI] [PubMed] [Google Scholar]
- 55.Szewczyk NA, Ngo-Huang A, Soones TN, Adekoya LM, Fontillas RC, Ferguson JK, et al. Feasibility and implementation of a multimodal supportive care program to improve outcomes in older patients undergoing allogeneic stem cell transplantation. Transplant Cell Ther. 2021;27(12):1008–1014. doi: 10.1016/j.jtct.2021.09.002. [DOI] [PubMed] [Google Scholar]