Abstract
Purpose of Review
Diabetes mellitus is a complex, chronic illness characterized by elevated blood glucose levels that occurs when there is cellular resistance to insulin action, pancreatic β-cells do not produce sufficient insulin, or both. Diabetes prevalence has greatly increased in recent decades; consequently, it is considered one of the fastest-growing public health emergencies globally. Poor blood glucose control can result in long-term micro- and macrovascular complications such as nephropathy, retinopathy, neuropathy, and cardiovascular disease. Individuals with diabetes require continuous medical care, including pharmacological intervention as well as lifestyle and dietary changes.
Recent Findings
The most common form of diabetes mellitus, type 2 diabetes (T2DM), represents approximately 90% of all cases worldwide. T2DM occurs more often in middle-aged and elderly adults, and its cause is multifactorial. However, its incidence has increased in children and young adults due to obesity, sedentary lifestyle, and inadequate nutrition. This high incidence is also accompanied by an estimated underdiagnosis prevalence of more than 50% worldwide. Implementing successful and cost-effective strategies for systematic screening of diabetes mellitus is imperative to ensure early detection, lowering patients' risk of developing life-threatening disease complications. Therefore, identifying new biomarkers and assay methods for diabetes mellitus to develop robust, non-invasive, painless, highly-sensitive, and precise screening techniques is essential.
Summary
This review focuses on the recent development of new clinically validated and novel biomarkers as well as the methods for their determination that represent cost-effective alternatives for screening and early diagnosis of T2DM.
Keywords: T2DM, Biomarkers, Glycemia, Enzymatic methods, Screening
Introduction
Diabetes mellitus is characterized by hyperglycemia resulting from defects in insulin production and secretion by pancreatic β-cells, development of insulin resistance in tissues, or both. According to its pathophysiology, diabetes can be classified as type 1 diabetes (T1DM), type 2 diabetes (T2DM), hyperglycemia in pregnancy (including gestational diabetes), and diabetes that has a specific etiology (including genetic or secondary to drugs, pancreatic factors, or other illnesses) [1]. T2DM accounts for 90% of diabetes cases and could be prevented to a great extent by adopting a healthy lifestyle [2].
T2DM is considered a multifactorial, chronic, and complex metabolic disease in which family medical history, age, lifestyle, diet, genetics, and environmental factors play a role. T2DM usually develops gradually—the early stages of the disease may be asymptomatic and undetected for several years. Initial symptoms commonly include polydipsia, polyuria, polyphagia, and eventually, weight loss. This chronic disease triggers a series of complications with a high degree of morbidity and mortality, resulting in a significant number of medical consultations, hospitalizations, disabilities, and deaths. Examples of these multisystemic complications include microvascular events, such as retinopathy, nephropathy, and neuropathy, and macrovascular events, including ischemic heart disease, stroke, and peripheral vascular disease [3]. A significant fraction of T2DM patients often present advanced complications that can be difficult to manage and costly to treat. In this context, the high incidence of T2DM presents a heavy burden on worldwide public health systems. Screening strategies have a positive impact on the quality of life and reduction of health costs since they allow early diagnosis lowering the prevalence of underdiagnosis, thus reducing the generation of complications, which in the long run decreases the pressure on health systems [4, 5]. Therefore, developing strategies focused on prevention, diagnosis, control, and treatment will be a priority in the next years. This review focuses on the recent development of new biomarkers and methods that represent cost-effective alternatives for screening and early diagnosis of T2DM, which could be widely implemented in apparently healthy people.
Standards of Medical Care in Diabetes
According to the “Standards of Medical Care in Diabetes” published by the American Diabetes Association (ADA) [6] and the World Health Organization (WHO) guidelines [5], diabetes may be diagnosed based on the concentration of plasma glucose—either fasting plasma glucose (FPG) or two-hour plasma glucose during a 75 g oral glucose tolerance test (OGTT)—or based on glycated hemoglobin A1c (HbA1c) concentration [5–7]. Prediabetes is an intermediate hyperglycemic state in which glycemic markers such as blood glucose and HbA1c are above the threshold considered healthy but below the diagnostic criteria for diabetes. This state constitutes a high risk for the development of diabetes and complications associated with the loss of glycemic control [8]. The diagnostic reference values for prediabetes and diabetes have not been universally standardized. However, the vast majority of clinical guidelines are based on the WHO [5] and ADA [6] criteria (Table 1).
Table 1.
Prediabetes and T2DM diagnostic reference values
| FPG | HbA1c | OGTT, 2 h | |
|---|---|---|---|
| Prediabetes | |||
| ADA | 100–125 mg/dL | 5.7–6.4% | 140–199 mg/dL |
| WHO | 110–125 mg/dL | Not recommended | |
| Diabetes | |||
| ADA | ≥ 126 mg/dL | ≥ 6.5% | ≥ 200 mg/dL |
| WHO | |||
References: Global Report on Diabetes[5] and Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes[6]
Abbreviations: FPG, fasting plasma glucose; HbA1c, glycated hemoglobin A1c; OGTT, oral glucose tolerance test; ADA, American Diabetes Association; WHO, World Health Organization.
Each of the currently used diagnostic T2DM diagnostics possess advantages and disadvantages. Fasting plasma glucose has been the gold standard diagnostic criterion for T2DM and is still the most widely accepted due to its availability, low cost, and compatibility with automated clinical chemistry analyzers [9, 10]. Among the disadvantages of FPG are that it requires at least 8-h fasting, shows substantial biological and diurnal variability, reflects only a single point in time, and the samples involved present stability issues [10]. Despite this, FPG is still widely used individually and as part of blood chemistry panels [11].
The classification and diagnosis of diabetes historically relied solely on plasma glucose concentration and patient symptomatology until HbA1c emerged as a useful glycemic biomarker [9]. Because it is directly related to long-term average blood glucose levels, HbA1c level is strongly correlated with the development of complications due to hyperglycemia [9]. In 2009, an international expert committee recommended HbA1c as a precise measure of chronic glycemic levels [9] which the WHO subsequently implemented [9, 12, 13]. Currently, HbA1c measurement is a crucial part of the international guidelines for the diagnosis of T2DM [9, 12, 13]. HbA1c testing is more clinical-workflow convenient than FPG and OGTT, as it does not require fasting, samples may be obtained at any time, and is a better predictor of long-term complications. Another significant advantage is that blood samples used for HbA1c testing present high stability and low short-term variability—one sample reflects average blood glucose concentrations over three months [9, 12, 14]. HbA1c may also be used as a monitoring test and guide for T2DM treatment. It is essential that the test is performed by a National Glycohemoglobin Standardization Program (NGSP) certified method and standardized to the Diabetes Control and Complications Trial (DCCT) assay to avoid misdiagnosis or missed diagnosis [14].
Despite its advantages over FPG and OGTT, HbA1c presents some inconveniences, such as lower clinical sensitivity at the designated diagnostic threshold (Table 1). Moreover, age, race, ethnicity, and any clinical condition that alters the lifetime of erythrocytes or hemoglobin levels can alter HbA1c independent of glucose concentration. Additionally, the limited availability and expense of HbA1c testing make it infeasible for routine use in some regions of the world [9, 14, 15, 16]. According to the US National Health and Nutrition Examination Survey (NHANES), HbA1c testing using the ≥ 6.5% diagnostic threshold only diagnoses 30% of the total T2DM cases identified through HbA1c, FPG, and OGTT [10, 17]. Also, there is a low correlation between HbA1c and FPG, insulin resistance, and insulin secretion [18].
As a marker of early impaired glucose homeostasis, OGTT is a more sensitive method of prediabetes and diabetes diagnosis than FPG and HbA1c [9, 10, 19]. Abnormally high plasma glucose concentration in OGTT is a proven indicator of prediabetes and diabetes [10, 19]. However, OGTT is relatively costly, can be complicated, and have low reproducibility in some settings. The test protocol requires that the patient ingest an oral load of 75g of glucose and undergo multiple blood draws over a two-hour period, which can be inconvenient and invasive for the patient. The need for timed samples creates logistical and analytical constraints [10, 16]. Despite its indication for T2DM screening by the ADA [6], OGTT is not usually performed on non-pregnant adults [11].
FPG, OGTT, and HbA1c are not always perfectly concordant [9, 14]. This discordance can be partly explained because different physiological stages of glucose metabolism are measured; the same occurs between HbA1c and glucose-based tests [14, 20]. OGTT and HbA1c tests are not routinely performed in middle-to-low income countries due to time and cost constraints. In these countries, FPG is still a valuable test for the screening, diagnosis, and monitoring of T2DM [16, 21].
In Mexico, clinical practice guidelines recommend that diabetes diagnosis is established when patients present polyuria, polydipsia, polyphagia, and weight loss accompanied by a fasting glucose concentration ≥ 200 mg/dL independent of time elapsed since the last meal [22]. These symptoms represent a typical case of diabetes with evident hyperglycemia. However, some patients with T2DM may present with few of these symptoms or may be completely asymptomatic. Per ADA recommendations, two abnormal test results from the same sample or two different samples must be obtained to confirm a diagnosis [23].
On the other hand, HbA1c is a more reliable marker for assessing the presence and severity of the disease [9]. Therefore, it is recommended by the ADA [6] and WHO [9] as an appropriate test for diabetes screening and diagnosis [24]. The final objective in the guidelines for the screening, diagnosis, and monitoring of T2DM should not be to seek a perfect concordance between biomarkers. Rather, their determination should identify individuals with altered glycemic levels at risk of suffering long-term complications.
Developments in Diabetes Screening and Diagnosis
More than half of individuals with diabetes, mainly T2DM, are undiagnosed; cases are frequently not diagnosed until severe complications appear [7]. However, even early diagnosis is not enough to slow the rise in the incidence of T2DM and its complications. Even in diagnosed patients, the disease’s progression may be accelerated by aggravating factors such as the lack of rigorous glycemic monitoring, under-treatment, inadequate treatment adherence, and omission of lifestyle changes. In addition to the inability of biomarkers to reflect glycemic status accurately. Because early diabetes is largely asymptomatic, many patients are at risk of developing life-threatening complications due to hyperglycemia. In low- and medium-income countries, patients present with an even higher risk of complications due to inadequate healthcare.
Currently, there are several challenges in the management of T2DM that need to be addressed. On the technical side, there is a need for novel, more comprehensive strategies for optimal screening, early diagnosis, and adequate management of T2DM. Approaches combining the use of resources for risk assessment, such as Finnish Diabetes Risk Score (FINDRISC) [25], along with more effective biomarkers for screening and progression T2DM, have a higher probability of success in managing the global diabetes epidemic. It will also positively impact the prevention of complications caused by hyperglycemic episodes in individuals diagnosed with diabetes and prediabetes by reducing the under-diagnosis and under-treatment of diabetes.
Glucose in Unconventional Samples
Recently, non-conventional biological fluids such as saliva have been explored to develop non-invasive, cost-effective, and sensitive methods that can be applied to T2DM screening, diagnosis, and monitoring [26, 27]. Despite this, a method for glucose quantification in saliva has not been clinically validated, nor have reference values been established. In a 2012 report, Abikshyeet et al. concluded that it was possible to detect glucose in saliva by the glucose oxidase-peroxidase method; in this report, salivary glucose was significantly higher in individuals with diabetes, and a highly significant correlation coefficient was shown between salivary glucose level and serum glucose level [28]. However, in a previous study, it was reported that it was not possible to detect glucose in normoglycemic individuals saliva with the enzymatic colorimetric test kit, GOD-PAP, which is one of the primary methods for glucose determination in serum at clinical laboratories [29]. Glucose concentrations in the saliva of normoglycemic patients may be below the detection limit of the method used, or interfering compounds in the saliva samples may hinder assay performance and result in low accuracy. In a 2014 meta-analysis [30], it was observed that the reported values of salivary glucose and its correlation with the conventional markers FPG and HbA1c show inconsistencies. The low correlation in the group of subjects without diabetes may be due to the low permeability of the salivary glands in healthy individuals and the high detection limit of the technique used (GOD-POD/GOD-PAP). If the technique is responsible, saliva-based testing with this method would be limited to monitoring glycemic control in already diagnosed patients. This evidences the need to validate specific techniques using more accurate and sensitive techniques for determining glucose in unconventional fluids, like saliva [31, 32]. In 2015, Liu et al. described a dual-enzyme biosensor composed of glucose oxidase (GOx) and pistol-like DNAzyme (PLDz) with the ability to quantify glucose in tears and saliva [33]. Lee et al. in 2017 presented a wearable monitoring device for glucose in sweat, including a transdermal drug delivery module [34]. The study concluded that the device would require improvements for long-term stability and uniformity of sensors. More in-depth studies of the relationship between blood glucose levels and sweat in healthy and individuals with diabetes are also needed. The same year, Soni et al. reported a portable, non-invasive optical glucose biosensor using a smartphone application to measure glucose in saliva and its clinical validation [35]. The use of a smartphone as the only device necessary for the determination makes it an easily adaptable technology; the smartphone-based test allows the patient to monitor salivary glucose at home inexpensively, making it highly attractive for its use in low- and medium-income countries [35]. In 2019, de Castro et al. gave proof of concept for a wearable glucose monitor based on a microfluidic paper platform integrated into a mouth guard for point-of-care testing (POCT) [36]. However, this prototype had some technical limitations that must be addressed, such as a long-run time and high sample volume required [36]. With the development of more sensitive platforms capable of detecting the low concentration of glucose present in the saliva of normoglycemic subjects, it would be possible to generate a non-invasive method for screening and monitoring glycemia [30, 31, 32]. Before a potential biomarker is introduced into the clinic successfully, adequate validation must be carried out [37]. To achieve this, it is vital to design adequate clinical assays to obtain reliable results in significant samples of the population. Nevertheless, the need to propose robust, sensible, and cost-effective methods to evaluate those markers should not be overlooked. The analysis of biomarkers in unconventional biological fluids is an area of opportunity that will surely attract even more interest from the scientific community in the coming years.
Clinically Validated Biomarkers
Traditional glycemic markers, such as glucose and HbA1c, present several limitations that can lead to under-diagnosis and poor disease prognosis in people with T2DM. As previously stated, HbA1c concentration cannot measure transitory hyperglycemic changes and is altered by patient characteristics (medical conditions and ethnicity). Furthermore, fasting glucose alone does not give enough information to fully understand the glycemic state of the patient [38, 39]. Evidence from studies comparing the performance of new glycemic markers such as glycated albumin (GA), fructosamine (FA), and 1,5-anhydroglucitol (1,5-AHG) have shown that they provide independent clinical information and can improve the prognostic value of conventional markers [40–45]. Previous studies in the Atherosclerosis Risk in Communities (ARIC) Study framework have confirmed that FA, GA, and 1,5-AHG markers are strongly related to the risk of developing diabetes. These intermediate markers can be used to determine the risk of T2DM and its complications independently of fasting blood glucose and HbA1c values [40, 41, 46, 47]. The moderate correlation and clinical variations between non-traditional markers such as GA, FA, and 1,5-AHG and conventional markers might be due to the fact that they are more strongly influenced by postprandial excursions than HbA1c, which is more affected by long-term glycemia as well as by the differential effect of oxidative stress [40]. The ability to evaluate blood glucose in the short-, intermediate-, and long-term is critical to face the health challenges posed by T2DM. The selective and combined use of these tools will allow access to more timely diabetes prevention, early diagnosis, and timely management of T2DM [48]. As presented in the following sections, efforts have been made to clinically validate new biomarkers for short and intermediate-term glycemic control in different populations and to further explore more sensitive and less invasive methods than those currently available. The following sections present relevant evidence of the clinical applications, advantages, and disadvantages of the three primary clinically validated, less-utilized markers of T2DM: FA, GA, and 1,5-AHG. Characteristics of these markers are summarized in Table 2. Also, the characteristics of these three clinically validated markers are presented in Table 2, a comparison of their diagnostic performance in Table 3, and a graphical representation of the mechanism by which they correlate to hyperglycemia and progression of T2DM is shown in Fig. 1.
Table 2.
Comparison of the three clinically validated biomarkers
| Fructosamine (FA) | Glycated albumin (GA) | 1,5-Anhydroglucitol (1,5-AHG) | |
|---|---|---|---|
| Time required for significant change | 1–2 weeks | 1–2 weeks | 24–72 h |
| Length of glycemic observation | 2–3 weeks | 2–3 weeks | 1–2 weeks |
| Reflection of fasting glucose levels | + | + | + |
| Reflection of postprandial glucose and glucose excursions | + | + | + |
| Correlation to diabetes complications | + | + | + |
| Determination by enzymatic methods | Available | Available | Available |
| Optimal detection range | Medium to high hyperglycemia | Medium to high hyperglycemia | Modest hyperglycemia to near normoglycemia |
| Point of care testing status | Biosensors and paper-based platforms have been evaluated | ||
| Paper-based platforms [138] |
Paper-based platforms [143] |
||
| Most common sources of error | Falsely low levels: hypothyroidism and liver cirrhosis | Falsely high levels: chronic kidney disease stages 4–5 | |
| Falsely high levels: hypoalbuminemia, hyperthyroidism, hyperuricemia, hypertriglyceridemia, nonalcoholic fatty liver disease | Falsely low levels: pregnancy, chronic liver disease, glucokinase-maturity-onset diabetes of the young | ||
| References | [48, 54, 55, 60, 63, 145, 146, 147] | [48, 65, 76, 147, 148, 149, 150] | [84, 85, 87, 95, 99, 147, 151, 152, 153, 154] |
Table 3.
Comparison of diagnostic performance of the three clinically validated biomarkers
| Biomarkers | Cohort/country | Description | AUC | Cutoff | Sensitivity (%) | Specificity (%) | Reference |
|---|---|---|---|---|---|---|---|
| 1,5-AHG (µg/mL), FA (mmol/L) and GA (%) |
ARIC/USA N = 1719 |
Diabetes defined by HbA1c (1,5-AHG) | 0.74 | - | - | - | [41] |
| Diabetes defined by FPG (1,5-AHG) | 0.70 | ||||||
| Diabetes defined by HbA1c (FA) | 0.83 | ||||||
| Diabetes defined by FPG (FA) | 0.83 | ||||||
| Diabetes defined by HbA1c (GA) | 0.87 | ||||||
| Diabetes defined by FPG (GA) | 0.86 | ||||||
| 1,5-AHG (µg/mL) |
NA/China N = 64 |
Predictor of remission after insulin therapy | 0.85 | 8.9 | 78.6 | 83.3 | [42] |
| FA (mmol/L) | AMORIS/Sweden | Fasting n = 5590 | 0.91 | 2.5 | 61 | 97 | [45] |
| Non-fasting n = 5397 | 0.95 | 82 | 94 | ||||
| GA (%) |
Taiwan Lifestyle Study/Taiwan n = 1559 |
Diagnosis of diabetes by the OGTT | 0.86 | 15 | 74 | 85 | [77] |
| GA < 14 to exclude and ≥ 17 to diagnose T2DM | 83.3 | 98.2 | |||||
| 1,5-AHG (µg/mL) and HbA1c (%) |
NA/China n = 3098 |
Diabetes (only 1,5-AHG) | 0.781 | 15.9 | 69.2 | 66.8 | [44] |
| Diabetes (1,5-AHG + FPG) | 0.912 | 82.5 | 83.5 | ||||
| Diabetes (HbA1c + FPG) | 0.911 | - | - | - | |||
| GA (%) |
NA/Brazil n = 242 |
Diagnosis of T2DM by OGTT | 0.703 | 14.8 | 64.9 | 65.5 | [79] |
| Diagnosis of T2DM by OGTT and/or HbA1c | 0.708 | 14.7 | 64.0 | 64.1 | |||
| GA (%) |
VMH/South Africa n = 1294 |
Prediabetes | 0.873 | 12.75 | 64.8 | 93.5 | [43] |
| Diabetes | - | 14.90 | 67.3 | 51.8 |
Abbreviations: AMORIS, Apolipoprotein-related Mortality RISk; ARIC, Atherosclerosis Risk in Communities; AUC, area under the curve; FA, fructosamine; FPG, fasting plasma glucose; GA, glycated albumin; HbA1c, glycated hemoglobin; OGTT, oral glucose tolerance test; VMH, Cape Town Vascular and Metabolic Health
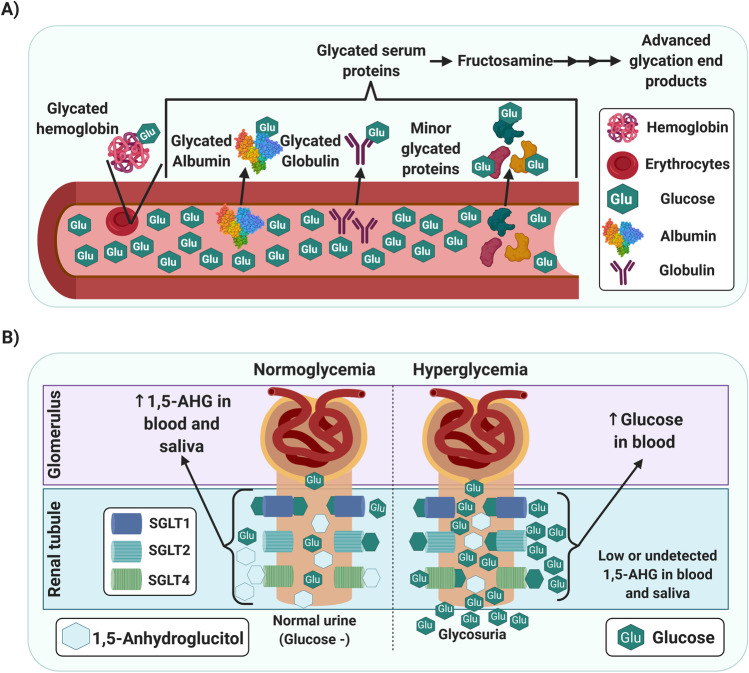
Fig. 1.
A Graphical representation of the mechanism by which glycated proteins and fructosamine correlate to hyperglycemia. B Kidney reuptake of 1,5-anhydroglucitol and glucose under normoglycemia and hyperglycemia. Abbreviations: 1,5-AHG, 1,5-anhydroglucitol; SGLT, sodium-glucose linked transporter. (Created with BioRender)
Fructosamine
Fructosamine (FA) refers to all stable ketoamines produced through the non-enzymatic glycation of circulating serum proteins (albumins, globulins, and other minority proteins) (Fig. 1A) [49, 50]. After a complex cascade of reactions, the early glycation products generate irreversible conjugates, called advanced glycation end products (AGEs) [51, 52, 53]. The concentration of FA in serum increases in T2DM due to the higher sugar concentration in the blood. Therefore, it could be useful as a glycemic marker that allows discrimination between normoglycemic and individuals with diabetes [54, 55]. Also, its application as a biomarker for screening or diagnosis of gestational diabetes mellitus compared against OGTT has been reported [56]. Unlike the determination of HbA1c, which measures long-term changes because of the longer circulating lifetime of hemoglobin, FA reflects glucose levels over 2 to 3 weeks.
Furthermore, FA assays are more affordable and less complicated than HbA1c [54, 55, 57]. The most widely employed methods for assessing FA are colorimetric-based, which are fast, technically easy, inexpensive, and available for automation [49]. Several studies have shown strong correlations between FA and HbA1c in T2DM with high sensitivity and specificity to distinguish between normoglycemic and individuals with diabetes [45, 58••]. Also, FA does not require fasting [41, 45]. In addition to its clinical application as a marker for diagnosing and monitoring hyperglycemia, high FA levels are associated with an increased incidence of vascular complications associated with T2DM, and persistently high FA levels indicate a more aggressive disease progression [38].
The FA assay can be applied to detect and monitor T2DM, although it is currently only used in combination with the traditional markers [54, 58••]. In a clinical study, 43 normoglycemic subjects were divided into two groups, with and without a family history of diabetes, and compared with 23 individuals with diabetes. The use of FA as a risk predictor for the development of T2DM was evaluated in these three groups [59]. The serum FA values of subjects with a family history were significantly higher with fasting plasma glucose and HbA1c values near the borderline, suggesting that protein glycation occurs at lower glycemic levels in these subjects. On the contrary, the group of subjects with diabetes showed near-normal FA values and high FPG and HbA1c. As expected, a positive correlation between FPG and HbA1c was observed in all groups, while the positive correlation between FPG and FA was only observed in subjects with diabetes [59]. Preoperative hyperglycemia can be a risk factor for postoperative complications. Despite its relevance, there is no consensus on the marker that should be used to evaluate it. A 2017 study involving 829 patients undergoing primary total joint arthroplasty reported that FA is a better choice as a preoperative marker and is a better predictor of adverse outcomes than HbA1c in patients with previously diagnosed diabetes and those newly diagnosed T2DM or glycemic dysregulation [60]. FA seems to have its application as a risk biomarker rather than a diagnostic or monitoring marker. FA as an intermediate marker is particularly beneficial for monitoring the glycemic status in patients with poor glycemic control or those starting a new therapeutic regimen [55, 61]. The assessment of salivary FA levels has been proposed as a possible biomarker that can be measured non-invasively, but more evidence is needed before its clinical application is established [59, 62, 63]. The focus of future studies should observe the relationship between FA and the onset of diabetes complications to assess the marker’s potential as a risk indicator in already diagnosed patients.
Glycated Albumin
Human serum albumin, the major circulating protein in blood, can undergo increased glycation due to hyperglycemia [64, 65]. Glycation is the non-enzymatic addition of reducing sugars, in this case, glucose, to amine groups in proteins. This addition creates an intermediate product that subsequently undergoes a rearrangement to create a more stable derivative, either an Amadori product or a ketoamine (Fig. 1A) [66, 67]. There is a direct relationship between hyperglycemic states and the generation of glycated albumin; this, combined with the half-life of albumin, is the reason why the GA ratio can be used as an intermediate-term biomarker of glycemic control [38, 65, 68]. The generation of AGEs from albumin and other serum proteins is directly related to the development and progression of diabetic complications. Thus, measurement of FA and GA can assess not only glycemic status for screening and diagnostics of T2DM but for its progression [66, 69, 70]. The main methods for the isolation and quantification of GA are boronate affinity chromatography, ion-exchange chromatography, high-performance liquid chromatography, immunoassays, and more recently, a two-steps enzymatic assay; the first step is AGEs and peroxide elimination by ketoamine oxidase (KO) then hydrolyzation (proteinase) and oxidation by KO to produce hydrogen peroxide measured by a colorimetric method, the latter being the most reported [49, 68, 71, 72, 73].
Additionally, GA as a biomarker of glycemic control has shown higher sensitivity and specificity than the gold standard for long-term monitoring, HbA1c [49, 68, 71, 72]. Furthermore, GA has been explored as a measure of risk for developing nephropathy and cardiovascular diseases [72, 74]. The possibility of supplementing self-monitoring through capillary glucose measurements with a GA test on a POCT platform has been evaluated with great interest since it would reduce health expenses and improve patients’ quality of life. However, there is still a need to develop and validate a robust method adapted for a home test that does not require specialized personnel or equipment [49, 68]. In 2018, Jagadeeshaprasad et al. recommended the combined measurement of glycated hemoglobin and GA to manage T2DM [13]. This combination has proven especially useful in patients on hemodialysis and chronic kidney disease (CKD) [71, 73]. Also, it has been demonstrated that GA is stable for up to 23 years when stored at − 80 °C [75]. Measurement of GA is especially relevant for controlling postprandial hyperglycemia and glycemic fluctuations. Because of its atherogenic potential, GA is a marker of cardiovascular risk, as well as a glycemic marker [76].
GA has demonstrated similar diagnostic performance to HbA1c, with areas under the curve (AUC) in the receiver operating characteristic curve analysis (ROC) of 0.86 and 0.90, respectively [77]. Both FA and GA performed similarly to FPG with AUCs of 0.83, 0.87, and 0.83, respectively, for identifying undiagnosed cases of diabetes when HbA1c was used to define T2DM. Both markers could be used in addition to HbA1c or FPG when HbA1c is not recommended [41]. The ability of GA to measure postprandial glycemia has been evaluated using a steamed bread meal test, demonstrating that while 1,5-AHG increased after the test, both HbA1c and GA remained stable, making them reliable markers even in non-fasting conditions [78]. In contrast, they confirmed the potential of 1,5-AHG as a marker to measure postprandial variability. In a study with Brazilian subjects, it was observed that GA with a cut-off of 16.8% showed a similar performance to HbA1c in detecting T2DM [79]. In a South African population, the diagnostic performance of GA was evaluated for prediabetes and T2DM, observing an optimal cut-off of 12.7% for prediabetes and 14.9% for diabetes. HbA1c performed better than GA in this population, with an AUC of 0.899 and 0.873 for diabetes. In prediabetes, the behavior was similar, although GA was more sensitive but less specific [43]. It is important to mention that despite their promising results, the conventional markers HbA1c and OGTT and the new clinically validated marker GA do not detect T2DM in the same individuals strengthening the hypothesis that the best approach is the combined use of the available markers. However, GA has an advantage over HbA1c in that GA can be measured in both plasma and serum, so unlike HbA1c, it could be measured with the rest of the biochemical biomarker panel from a single blood sample [79]. Despite evidence showing GA's clinical utility and superior efficiency over FA and HbA1c in a broad range of clinical settings [41, 49], there are no commercial GA assays currently available. However, due to the high incidence of T2DM, healthcare professionals recognize the need for auxiliary indicators for their screening and early diagnosis that can add to traditional tools or, in an ideal case, a new biomarker that can improve prevention schemes and patient care. This need can be an impetus for a unified GA quantification method and comprehensive studies regarding its clinical application.
1,5-Anhydroglucitol
The glycemic biomarker 1,5-anhydroglucitol (1,5-AHG), or 1-deoxyglucose, is a six-carbon monosaccharide which also is known as 1-deoxyglucose. As one of the major polyols in the human body, 1,5-AHG was first isolated from the Polygala amara plant in 1888, and its structure was defined in 1943 [80]. This biomarker is metabolically stable, originates mainly from the diet (where it is found in low concentrations) and is well absorbed intestinally [81, 82, 83, 84]. Also, its tissue concentrations reach steady-state levels due to the absence of a metabolic pathway for 1,5-AHG degradation and its renal reabsorption. Therefore, its levels in different biological fluids are stable and correlated with blood glucose [85, 86]. The relationship between 1,5-AHG and glucose levels was reported in 1973 by Pitkänen [81]. In 1988, the mechanism of 1,5-AHG as a marker of glycemic control was elucidated [41, 85, 87, 88, 89, 90]. Soon after, 1,5-AHG was proposed as a novel biomarker for diabetes [85]. The enzymatic kit GlycoMark® is the most popular assay for blood 1,5-AHG determination; this kit was developed and widely used in Japan. In 2003, the US FDA approved this serum-based assay for evaluating short-term glycemic control [61, 91, 92].
The concentration of systemic 1,5-AHG is kept in balance by urine excretion. In normoglycemic individuals, about 99.9% of 1,5-AHG is renally absorbed, competing with glucose at the sodium-glucose linked transporters (SGLT) for kidney reuptake; thus, it is retained in detectable concentrations in blood and saliva [41, 61, 93]. Under hyperglycemia, the glucose transporters are monopolized by the excess glucose (Fig. 1B). The 1,5-AHG is not reabsorbed at the tubular level, reducing its concentration in serum and saliva [41, 61, 93]. 1,5-AHG in serum decreases while glucose levels rise above the renal glucose threshold; thus, it has been reported that 1,5-AHG represents postprandial hyperglycemia in individuals with diabetes more robustly than HbA1c or FA [61, 94, 95]. Low concentrations of 1,5-AHG reflect poor glycemic control in the preceding 1–2 weeks [95, 96, 97].
Most studies report 1,5-AHG levels measured by GC-MS, HPLC-MS, or the commercially available enzymatic kit, GlycoMark® [95, 98]. GlycoMark® is the most popular assay and is FDA approved [92]. Widespread implementation of 1,5-AHG as a glycemic control marker presents several advantages over traditional tests [85, 94]. For instance, low 1,5-AHG has been reported to have a stronger correlation with high HbA1c than FPG [41, 95]. The serum level of 1,5-AHG reflects postprandial glucose variations and, in combination with FPG, improves its potential for early detection of T2DM with a cutoff of 15.9 µg/mL in a Chinese population. The combination of FPG with 1,5-AHG had an AUC of 0.912 with a sensitivity of 82.5% and a specificity of 83.5%, while the combined use of HbA1c with FPG generated an AUC of 0.911. In addition, it reduced the need for an OGTT by 75.8% [44], decreasing costs since OGTT would be reserved for those patients who need it. 1,5-AHG can differentiate between patients with similar HbA1c levels but differing degrees of glycemic control [61]. Also, its tissue levels reach steady-state levels due to the absence of a metabolic pathway for its degradation and the low amount taken from the diet compared with the total body pool. Therefore, its levels in different fluids are stable and correlated with blood glucose [85, 86]. As mentioned above, 1,5-AHG is a better marker to measure postprandial variability than GA. However, a 2017 study reported that serum 1,5-AHG levels increase slightly after consuming a 75 g dose of sugar as glucose increases regardless of metabolism status, gender, or body mass index (BMI). This may be due to the transport of 1,5-AHG from the compartments where it is stored. The maximum sampling time was 180 min [99]; observations over a more extended period could provide more information on the mechanism involved. 1,5-AHG is affected by a family history of diabetes, with significantly lower levels reported in individuals with normal glucose tolerance who have first-degree relatives with T2DM. Furthermore, 1,5-AHG showed greater sensitivity than HbA1c or GA as a marker for early detection of abnormalities in glucose metabolism [100].
1,5-AHG acts not only as a glycemic marker but can also be integrated into a model that considers other risk factors or combined with conventional markers to improve its T2DM diagnostic potential [97]. It has been proven that 1,5-AHG levels increase as glycemic control is achieved independent of body weight, sex, age, treatment, and diabetes evolution among non-insulin-dependent diabetes patients [85]. Controlled glycemia in individuals with diabetes due to combined treatment schemes (pharmacological and non-pharmacological) generates a sustained increase in 1,5-AHG values up to the expected range for normoglycemic individuals. This ability allows 1,5-AHG to exert a differential function in the different stages of T2DM management, screening, diagnosis, and monitoring [84]. Notably, a study published in 2012 reports that 1,5-AHG is more useful for monitoring hyperglycemic states in individuals with diabetes than FA, GA, and HbA1c [41]. It has been reported that 1,5-AHG is a useful and accurate index for monitoring subjects with prediabetes and well-controlled children with diabetes and obesity in the HbA1c range of 5.5 to 8% [101]. This makes it of particular interest considering the increasing incidence of obesity and early onset of diabetes.
Studies in mice have shown that changes in 1,5-AHG levels are responsive to the development of diabetes due to the loss of function of pancreatic beta cells [102]. Blood 1,5-AHG quantification of at-risk individuals could provide a targeted screening strategy to prevent the development of T2DM or identify those with asymptomatic diabetes [102]. The glycemic biomarker 1,5-AHG not only has a diagnostic application for diabetes, but it has the potential to evaluate the risk of long-term complications, including the most documented association with cardiovascular diseases and mortality in people with T2DM [96, 102, 103]. There is also evidence of its prognostic value for microvascular complications such as retinopathy and CKD [97]. Furthermore, there is evidence that 1,5-AHG is a valuable marker of diabetes progression for individuals affected by diabetic nephropathy in which the determination of HbA1c is not recommended [72, 97, 104]. Sodium-glucose cotransporter-2 inhibitors (SGLT-2i) are a class of drugs that lower glucose concentrations by an insulin-independent mechanism, reducing renal reabsorption of glucose and increasing its urinary elimination. They have shown to be effective for treating T2DM, and there is also evidence of their potential to decrease the risk of cardiovascular events [105, 106]. Different mechanisms have been proposed for their cardioprotective effect, and evidence has shown that this effect goes beyond their hypoglycemic activity [105, 107]. It has been reported that SGLT-2i treatment can interfere with the measurement of 1,5-AHG, resulting in a falsely low value in patients with controlled blood glucose levels [108]. The interaction between SGs and 1,5-AHG has also been confirmed in a metabolomic study by Kappel et al., where they report that the observed decrease in 1,5-AHG may be due to a decrease in its renal reabsorption [109]. Because of this phenomenon, its use as a glycemic marker in individuals treated with SGLT-2i is not recommended [108, 109]. Furthermore, 1,5-AHG can be used as a marker of remission in patients with T2DM treated with insulin. Although it failed to predict remission immediately after insulin treatment after a 1-month follow-up, 1,5-AHG was an independent predictor for remission (odds ratio 1.56, 95% confidence interval [CI] 1.15–2.12, P = 0.004). The AUC of 1,5-AHG in the ROC was 0.85 (95% CI 0.75–0.96, P < 0.001), this can be explained due to the nature of 1,5-AHG as a glycemic marker since its main source is the diet, it takes time with controlled glycemic values to manifest an increase in this marker [42].
Salivary 1,5-AHG has been previously shown to be strongly correlated with serum 1,5-AHG and inversely correlated with fasting glucose, OGTT, and HbA1c [95]. A clear advantage of using saliva as a sample is that its collection is non-invasive compared to traditional blood collection [28, 32, 110]. 1,5-AHG concentrations are significantly lower in unconventional biological fluids, such as saliva, tears, and sweat, than in plasma. Therefore, highly sensitive testing methods are required for accurate measure 1,5-AHG in these fluids. The most promising candidates are enzymatic-based assays, as they are less expensive and easier to adapt to the clinical field [98]. Also, given that sample-drawing can be done outside of a clinical laboratory, methods may even be adapted to portable devices that the patient can use at home [88]. In their 2020 study, Jian et al. reported a positive correlation between salivary and serum 1,5-AHG and a negative correlation with FPG and HbA1c. Furthermore, this study showed that salivary 1,5-AHG, combined with fasting glucose or HbA1c, has been shown to enhance the efficiency of diabetes screening and reduce the necessity of OGTT [95]. In 2018, Furusawa et al. reported the detection of glucose and 1,5-AHG, two diabetes biomarkers, using an electrochemical sensor with a Prussian blue (PB) electrode, modified with glucose oxidase (GOx) and pyranose oxidase (POx) in a device fabricated on a flexible plastic film; however, this was not tested with actual biological samples [111]. However, although methods based on POx for quantifying 1,5-AHG have been reported, it must be considered that pyranoses are not selective so that the presence of galactose and glucose in saliva can interfere with the measurement of 1,5-AHG. Hence, it is crucial to consider a clearance step that reduces or eliminates the interferences [72]. More studies are required on 1,5-AHG in saliva in patients with T2DM. But, with further validation, salivary 1,5-AHG may become a promising, non-invasive, and convenient tool for T2DM screening in the future [95].
Novel Biomarkers
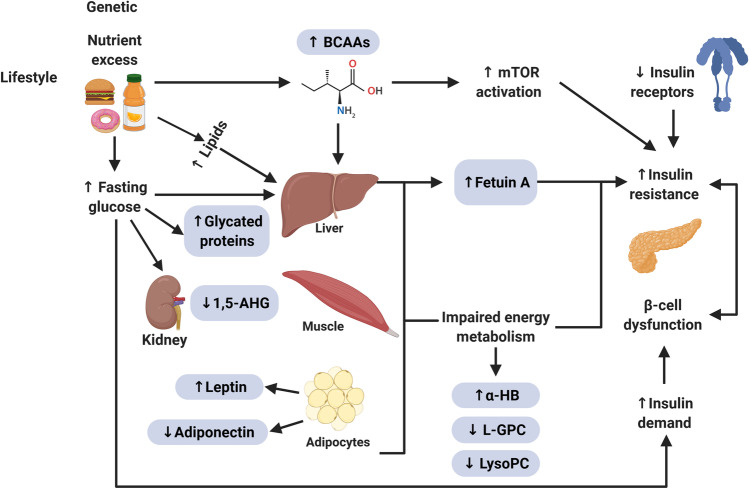
Metabolomics is especially useful in identifying biomarkers of T2DM because of the metabolic basis of its etiology and the fact that its development is strongly related to lifestyle and environmental factors [112, 113, 114]. Several studies have been carried out to evaluate novel biomarkers in conventional fluids such as blood and urine. Some examples of these studies are presented in the sections “Clinically Validated Biomarkers” and “Novel Biomarkers.” Few novel biomarkers have shown significant advantages over those already established and validated, such as FPG, OGTT, and HbA1c. However, it is expected that more extensive studies will lead to new resources in the management of the T2DM epidemic [115]. No biomarker studied so far is the perfect marker for all T2DM patients in all conditions. The most effective approach to search for new biomarkers and exploit their differences with conventional ones is to stop looking for a perfect marker that achieves the status of a universal gold standard for glycemic control and work on multivariable panels that consider the combination of biomarkers, anthropometric characteristics, and lifestyle habits that allow from risk assessment to continuous monitoring of individuals with diabetes [116, 117]. More in-depth studies involving subpopulations of interest are also required, considering pathological conditions and underlying diseases of high incidence to define which biomarkers are the best option for each case. A pivotal approach in the search for new markers of T2DM is the use of metabolomics to generate profiles by monitoring the various metabolic pathways for evidence of deregulated metabolites whose study may lead to potential biomarkers for screening, diagnosis, and monitoring [117, 118]. These metabolomic profiles may also provide insight into potential therapeutic targets and contribute to generating risk profiles for complications [114, 119, 120]. Currently, the search for novel biomarkers for T2DM is primarily based on metabolomic studies. Identifying novel biomarkers that predict the risk, incidence, or complications associated with T2DM usually starts with non-targeted metabolomic analyses [121]. Metabolites that show strong correlations with the diagnosis of diabetes, its validated risk factors [122], or its complications [123] are later analyzed by targeted metabolomic analyses [124]. Usually, these metabolomic strategies must be carried out in large populations to increase the results’ significance and validity—sometimes over long periods [125••]. Several metabolomic studies have found characteristic patterns and specific biomarkers associated with the deregulation of energy metabolism in T2DM (Fig. 2) [39, 118]. The most reported metabolic alterations in the profiling of patients with metabolic disorders and specifically individuals with diabetes are high levels of branched-chain amino acids (BCAAs) and aromatic amino acids (AAAs), as well as the ketosis marker β-hydroxybutyrate (β-HB) [112, 125••, 126, 127, 128, 129, 130, 131]. Altered amino acid metabolism appears to be a link between T2DM and the development of cardiovascular disease. It is known that the physiological mechanism of this alteration is the relationship of amino acid metabolism with insulin secretion and tolerance, so monitoring the amino acid profile can provide information about cardiometabolic health [125••, 129, 130]. In contrast, the metabolites 1,5-AHG, lysophosphatidylcholine (LysoPC), Linoleoylglyglycerophosphocholine (L-GPC), glutamine (Gln), and glutamine/glutamate ratio present a decrease [112, 125••, 126, 127, 128, 129, 130, 131]. In these studies, the roles of clinically validated biomarkers such as 1,5-AHG have been confirmed, in addition to new markers such as fetuin-A [132], BCAAs [133], adipokines [2], L-GPC [134], LysoPC [124], among others (Table 4). Despite the substantial data generated by metabolomic studies, before a potential biomarker is successfully introduced into the clinic, the marker must undergo rigorous clinical validation for its predictive power independently and in conjunction with traditional risk assessment tools [37, 126]. Clinical evaluation of biochemical markers should focus primarily on individuals identified as high risk, as this will allow greater coverage with less expense, which is crucial in resource-limited settings. Identification of these high-risk individuals can be made on a mass scale with the help of standardized tools such as risk assessment questionnaires that usually consider the most recognized risk factors such as age, sex, family history, level of physical activity, as well as family history of hypertension [135, 136]. In some more extensive studies, such as FINDRISC, fruit and vegetable consumption and anthropometric measures such as BMI and waist circumference are also included [137]. These parameters can be used in combination with conventional biomarkers such as FPG and HbA1c in predictive models, and cardiovascular risk markers such as triglycerides and HDL cholesterol can be added to improve their performance and assess the risk of developing complications [136]. It has been shown that using a multi-metabolite score consisting of phenylalanine, non-esterified cholesterol in large HDL, and the ratio of cholesteryl ester to total lipid in large VLDL allows the determination of long-term risk for T2DM in young adults with better performance than any individual metabolite [125••]. It is important to consider that most risk assessment models reported have been developed and validated in the USA, Europe, and Asia. Before being used in a new population, they should be adequately validated in that population and must not be simply assumed that their relevance can be extrapolated [48, 136]. The information from these risk assessment tools is crucial for making decisions based on a cost-benefit analysis and generating screening and monitoring programs with greater coverage [39, 118, 124, 127]. Nevertheless, further studies are needed to evaluate whether the newly reported biomarkers can be included in multianalyte panels to improve the sensitivity of current models and that more focused approaches can be generated to meet the goals of personalized medicine. The field of metabolomics is an emerging area and is still at a basic level of research. Both measurement protocols and data analysis still suffer from a lack of standardization, making it difficult to compare information obtained from independent studies [118, 128, 129]. This was evidenced in a study focused on a sample of adult men over 54 years of age from the KORA F3 cohort study [118]. Metabolite detection and quantification were conducted by three metabolomics providers, two of them using mass spectrometry and one based on nuclear magnetic resonance, where a different profile of significantly altered metabolites was observed in individuals with diabetes [118]. However, metabolomics studies are equipment- and personnel-intensive. One cost-saving alternative is the development of immunological or enzyme-based methods for clinical validation. Validated assays are imperative in obtaining reliable results in diverse populations so that factors affecting the metabolome, such as age, gender, ethnicity, and medical history, can be observed. Nevertheless, the need to propose robust, sensitive, and cost-effective methods to evaluate those markers should not be overlooked. It will mostly be of great interest to evaluate new clinically validated biomarkers in unconventional fluids like saliva to develop non-invasive methods. These new tests and platforms will generate more effective risk assessment schemes for earlier and more accurate T2DM diagnosis and prevention in healthy and subjects with prediabetes. It is possible that a combination of some of the biomarkers mentioned above, rather than a single biomarker, will be utilized to assess the risk for T2DM development accurately.
Fig. 2.
Model representing the relationship between impaired energy metabolism and biomarkers for the screening and diagnosis of type 2 diabetes mellitus. Abbreviations: 1,5-AHG, 1,5-anhydroglucitol; BCAAs, branched-chain amino acid; α-HB, α-hydroxybutyrate; L-GPC, linoleoylglycerophosphocholine; LysoPC, lysophosphatidylcholine; mTOR, mammalian target of rapamycin. (Created with BioRender)
Table 4.
Main novel biomarkers for type 2 diabetes mellitus (T2DM)
| Biomarker | Findings | Reference |
|---|---|---|
| Amino acids | A different amino acid profile was found for patients with impaired fasting glucose and T2DM compared to a control population. In subjects with impaired FPG and T2DM, the fasting levels of BCAAs, glutamic acid, lysine, phenylalanine, arginine, alanine, tyrosine, and aspartic acid increased as glycemic control was lost. The concentration of these amino acids correlates significantly with FPG and HbA1c classical markers of T2DM and pro-inflammatory cytokines TNF-α and IL-6. These amino acids demonstrated the ability to discriminate normoglycemic subjects from those with impaired FPG or T2DM. | [130] |
| Amino acids | The ability of amino acid levels, including BCAAs (isoleucine, leucine, valine) and aromatic amino acids (tyrosine and phenylalanine), to predict prediabetes risk was evaluated. Levels of aspartic acid, asparagine, and histidine significantly predicted the incidence of prediabetes, with the increased risk differing between African Americans and European Americans. The evidence observed in prediabetes suggests that changes in the amino acid profile occur in the transition from normoglycemia to the development of T2DM. | [155] |
| α-HB | In this metabolomic study, the α-HB was the biomarker with the best performance to identify individuals with insulin resistance. This behavior was consistent in both screening and targeted assays. α-HB predictive potential can be explained both by its metabolic relevance and that its synthesis is stimulated by the elevation of the NADH/NAD+ ratio due to increased lipid oxidation. | [156] |
| L-GPC | The potential of L-GPC values as a biomarker of insulin resistance during fasting and a five-point OGTT was evaluated. Despite not showing a linear correlation with classic risk markers such as BMI, fat tissue distribution, lipids, fasting glucose, and HbA1c, subjects with high L-GPC showed higher glycemic excursions during a five-point OGTT. L-GPC has a strong negative correlation with glucose disposal and is negatively associated with insulin sensitivity, showing that it may be used as a biomarker for insulin resistance, especially in patients who do not present the classic risk factors. | [157] |
| Leptin | The relationship between leptin and microvascular complications caused by diabetes progression in a population of T2DM patients was explored. Leptin serum values showed a positive correlation with duration of diabetes, BMI, waist circumference, blood pressure, fasting glucose, HbA1c, serum insulin levels, cholesterol, triglycerides, and LDL cholesterol, consistent with previous reports identifying leptin as a marker of insulin resistance and a possible diagnostic marker for T2DM. Regarding its potential as a predictor of microvascular complications, leptin concentration is positively correlated with urinary albumin-creatinine ratio, peripheral neuropathy, and retinopathy. eGFR showed a negative correlation with serum leptin. | [158] |
| Adiponectin and fetuin-A | A case-control study was conducted to assess the association between fetuin-A levels and the risk of T2DM in the Chinese population. High values of fetuin-A were associated with an increased risk of T2DM; no significant interaction with adiponectin levels on T2DM risk was observed. High fetuin-A, regardless of adiponectin levels, was associated with an increased risk of diabetes. The mechanism by which fetuin-A participates in the development of T2DM may not be directly related to adiponectin. | [159] |
| Adiponectin and leptin | The relationship between plasma leptin levels and biomarkers associated with energy and hormone metabolism was explored. The untargeted metabolomics analysis showed that 64 metabolite features were associated with fasting leptin levels. The profile of metabolites associated with leptin levels varied by gender—the leptin level was approximately three times higher in women. A positive correlation was found between leptin and adiponectin, and a negative correlation with caloric intake, serum triglyceride levels, and VLDL. The evidence supports the role of leptin as a mediator of energy and hormone metabolism. | [160] |
| BCAAs and LysoPC | The role of BCAAs and LysoPCs in the progression from prediabetes to T2DM was evaluated using a targeted metabolomic approach. It was demonstrated that BCAAs (isoleucine, leucine, and valine) and LysoPCs, especially LysoPCs acyl C28:1, contribute to the progression of diabetes. Regression analysis established a significant association of HbA1c with LysoPCs acyl 28:1, age, and FPG. | [161] |
| Fetuin-A and pFet-A | The role of phosphorylated fetuin-A (pFet-A) in insulin action in cell lines, primary cultures, animal models, and humans was examined. In obese individuals, serum levels of fetuin-A and pFet-A were significantly higher than in those of normal weight. pFet-A demonstrated a strong positive association with fasting glucose and fasting insulin. This study showed that the role of fetuin-A in inhibiting insulin-mediated glucose uptake and glycogen synthesis depends on its phosphorylation status. | [162] |
| α-HB and L-GPC | The ability of the metabolites α-HB and L-GPC as markers of insulin resistance and glucose intolerance was evaluated. The levels of α-HB increase, and L-GPC levels decrease with high insulin resistance. This high α-HB and low L-GPC stage can be interpreted as a metabolic imbalance with a high NADH to NAD+ ratio and low glucose metabolism. | [134] |
| Adiponectin, BCAAs and Leptin | The relation between altered levels of adipokines, leptin, and adiponectin with BCAAs and insulin resistance in subjects with different degrees of glucose tolerance was assessed. A strong relationship was observed between insulin resistance and BCAAs. The levels of BCAAs show a clear differentiation between groups with different insulin resistance. Moreover, this relationship is independent of adipokine levels, suggesting that variations in BCAA levels occur by a different mechanism than adipose tissue deregulation. | [163] |
| Adiponectin, CRP and Leptin | A direct relationship was observed between high CRP and leptin concentrations and increased insulin resistance. Increased adiponectin concentration was associated with decreased insulin resistance. These relationships were not affected by gender. The relationship between abdominal subcutaneous adipose tissue and insulin resistance is mediated by leptin and not CRP or adiponectin. For the visceral adipose tissue, insulin resistance was partially mediated by the adipokines, and the inflammation marker CRP does not affect this relationship. | [164] |
| BCAAs, carnitines and LysoPC | A non-targeted metabolomics study comparing normoglycemic, prediabetic, and diabetic phenotypes was carried out in five tissues relevant to T2DM: serum, visceral adipose tissue, liver, skeletal muscle, and pancreatic islets. The levels of BCAAs correlated with HbA1c in various tissues. Carnitines and LysoPC levels in plasma could be used to discriminate patients with T2DM from controls. The compounds identified in the differential profile are part of glycerophospholipid metabolism, fatty acid biosynthesis, arachidonic acid metabolism, linoleic acid metabolism, sphingolipid metabolism, fatty acid elongation in mitochondria, alpha-linolenic acid metabolism, and fatty acid metabolism. | [165] |
| BCAAs, α-HB, β-HB, and lactate | α-HB, β-HB, lactate, and BCAAs were analyzed as biomarkers of glycemic control in young adults with and without diabetes during an OGTT. High α-HB values were observed during fasting, and α-HB and BCAA levels increased after glucose ingestion. BCAAs, β-HB, and lactate did not show differential profiles during OGTT related to the subjects' insulin resistance. The concentration of α-HB is negatively related to insulin sensitivity in young people. Levels during fasting and following glucose ingestion differentiate those subjects at risk of developing glucose metabolism deregulation. | [166] |
| AAAs, BCAAs, Gln/Glu ratio, 25(OH)D, and LysoPC | The association of different serum metabolites with the loss of glycemic control leading to T2DM and their correlation with metabolic disease risk was evaluated. Serum BCAA levels were higher in the hyperglycemic group, while Gln/Glu ratio and unsaturated LysoPC levels were lower in this group. LysoPC, BCAAs, and AAAs correlate strongly with traditional metabolic risk factors. In contrast, the Gln/Glu ratio, unsaturated LysoPC, and 25-hydroxyvitamin D correlated with protective factors such as HDL-C and apoAI. | [126] |
| Adiponectin, CRP, gGT, IL-1b, IL-6, leptin, and TNF-α | The predictive capacity of CRP, IL-1b, IL-6, TNF-α, leptin, adiponectin, and gGT for T2DM diagnosis were evaluated individually and in conjunction with Kahn's risk score. High levels of IL-6, CRP, leptin, and gGT, and low adiponectin levels were observed in patients who developed T2DM; there were no significant differences in IL-1b and TNF-α. In the adjusted multivariate analysis, only the positive association with gGT and the negative association with adiponectin retained their significance. The use of these biomarkers did not significantly improve the already validated risk assessment tool's predictive ability. | [167] |
| α-HB, β-HB, KL, L-GPC, oleic acid, serine, and vitamin B5 | Multivariate models were tested for their ability to discriminate normoglycemia from impaired glucose tolerance based on a series of metabolites and risk factors previously reported. It was found that the metabolites α-HB and L-GPC predicted impaired glucose tolerance with a performance similar to FPG. A model based on FPG, including α-HB, β-HB, KL, L-GPC, oleic acid, serine, and vitamin B5, was developed. This inclusive metabolite-based includes biomarkers involved in a diversity of metabolic pathways representing the patient’s current glycemic status as FPG and the subject’s ability to react to a glucose load, the same as in the OGTT test. This test demonstrated its ability to discriminate patients having impaired glucose tolerance (IGT). | [168] |
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; BCAAs, branched-chain amino acid; BMI, body mass index; CRP, C-reactive protein; eGFR, epidermal growth factor receptor; FPG, fasting plasma glucose; gGT, gamma-glutamyl transpeptidase; Glu, glutamic acid; Gln, glutamine; HbA1c, glycated hemoglobin A1c; α-HB, α-hydroxybutyrate; β-HB, β-hydroxybutyrate; IL-1b, interleukin 1 beta; IL-6, interleukin 6; KL, 4-methyl-2- oxopentanoic acid; LDL, low-density lipoproteins; L-GPC, linoleoylglycerophosphocholine; LysoPC, lysophosphatidylcholine; NAD+, nicotinamide adenine dinucleotide; NADH, reduced nicotinamide adenine dinucleotide; OGTT, oral glucose tolerance test; pFet-A, phosphorylated fetuin-A; TNF-α, tumor necrosis factor-α; T2DM, type 2 diabetes mellitus; VLDL, very low-density lipoprotein
Conclusions
Although significant advances have been made in the search for new biomarkers for T2DM, more research is needed for further advancement. Developments in this area can reduce the incidence of T2DM and improve disease prognosis by addressing the current under-diagnosis and under-treatment of diabetes. The biomarker candidates described in this review require further study to be clinically validated. With further validation, these novel biomarkers can be used with or even replace conventional markers of diabetes. However, performing current metabolomic techniques requires significant resources and time, hindering novel biomarkers’ potential discovery. Enzymatic methods are a viable alternative for developing affordable assays, allowing the clinical validation and application of these novel biomarkers. Furthermore, accessibility to these tests can be improved by developing POCT platforms. A POCT could be used in communities with limited access to health services or at home by the patient, which would allow for prevention, early diagnosis, and health maintenance of persons with T2DM. Effective approaches for diabetes screening, monitoring, and diagnosis are essential to decrease the disease’s prevalence, prevent the onset of complications and improve the quality of life. It is crucial to decrease the burden on health systems caused by T2DM and its complications. Better management of T2DM will also reduce the impact of other public health threats such as the SARS-CoV-2 pandemic.
Acknowledgements
The authors would like to thank the Bioengineering and Regenerative Medicine and the Pathophysiology of Metabolic and Emerging Diseases Strategic Focus Groups, as well as the Bioengineering and Medical Devices Unit of Tecnologico de Monterrey and Fundación FEMSA for their financial support.
Declarations
Conflict of Interest
The authors declare no competing interests.
Footnotes
This article is part if the Topical Collection on Pathogenesis of Type 2 Diabetes and Insulin Resistance
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
- 1.Forouhi NG, Wareham NJ. Epidemiology of diabetes. Medicine (Baltimore) 2018;47:22–7. doi: 10.1016/j.mpmed.2018.10.004. [DOI] [Google Scholar]
- 2.DeFronzo RA, Ferrannini E, Groop L, Henry RR, Herman WH, Holst JJ, et al. Type 2 diabetes mellitus. Nat Rev Dis Prim [Internet]. Macmillan Publishers Limited; 2015;1:15019. Available from: http://www.nature.com/articles/nrdp201519 [DOI] [PubMed]
- 3.Adler AI. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ [Internet]. 2000;321:412–9. doi: 10.1136/bmj.321.7258.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.International Diabetes Federation. IDF Diabetes Atlas [Internet]. 10th edition. Boyko EJ, Magliano DJ, Karuranga S, Piemonti L, Riley P, Saeedi P, et al., editors. Brussels, Belgium: International Diabetes Federation; 2021. Available from: https://diabetesatlas.org/atlas/tenth-edition/
- 5.World Health Organization. Global Report on Diabetes [Internet]. Glob. Rep. Diabetes. Geneva; 2016 Dec. Available from: https://www.who.int/publications/i/item/9789241565257
- 6.American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2020. Diabetes Care [Internet]. 2020;43:S14–31. Available from: http://care.diabetesjournals.org/lookup/doi/10.2337/dc20-S002 [DOI] [PubMed]
- 7.International Diabetes Federation. IDF Diabetes Atlas [Internet]. Ninth edit. Karuranga S, Malanda B, Saeedi P, Salpea P, editors. IDF Diabetes Atlas. Brussels, Belgium: International Diabetes Federation; 2019. Available from: https://www.idf.org/e-library/epidemiology-research/diabetes-atlas/159-idf-diabetes-atlas-ninth-edition-2019.html
- 8.Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for diabetes development. Lancet. Elsevier Ltd; 2012. p. 2279–90. [DOI] [PMC free article] [PubMed]
- 9.Nathan DM, Balkau B, Bonora E, Borch-Johnsen K, Buse JB, Colagiuri S, et al. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care [Internet]. 2009;32:1327–34. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sacks DB. A1C versus glucose testing: a comparison. Diabetes Care [Internet]. 2011;34:518–23. doi: 10.2337/dc10-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evron JM, Herman WH, McEwen LN. Changes in screening practices for prediabetes and diabetes since the recommendation for hemoglobin A 1c testing. Diabetes Care [Internet]. 2019;42:576–84. doi: 10.2337/dc17-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karnchanasorn R, Huang J, Ou H-Y, Feng W, Chuang L-M, Chiu KC, et al. Comparison of the current diagnostic criterion of HbA1c with fasting and 2-hour plasma glucose concentration. J Diabetes Res [Internet]. Hindawi Publishing Corporation; 2016;2016:1–11. Available from: http://www.hindawi.com/journals/jdr/2016/6195494/ [DOI] [PMC free article] [PubMed]
- 13.Jagadeeshaprasad MG, Venkatasubramani V, Unnikrishnan AG, Kulkarni MJ. Albumin abundance and its glycation status determine hemoglobin glycation. ACS Omega. 2018;3:12999–3008. doi: 10.1021/acsomega.8b01702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2019. Diabetes Care. 2019;42:S13–28. 10.2337/dc19-S002 [DOI] [PubMed]
- 15.Herman WH, Dungan KM, Wolffenbuttel BHR, Buse JB, Fahrbach JL, Jiang H, et al. Racial and ethnic differences in mean plasma glucose, hemoglobin A1c, and 1,5-anhydroglucitol in over 2000 patients with type 2 diabetes. J Clin Endocrinol Metab. 2009;94:1689–94. Available from: https://academic.oup.com/jcem/article/94/5/1689/2598400 [DOI] [PubMed]
- 16.Katulanda GW, Katulanda P, Dematapitiya C, Dissanayake HA, Wijeratne S, Sheriff MHR, et al. Plasma glucose in screening for diabetes and pre-diabetes: how much is too much? Analysis of fasting plasma glucose and oral glucose tolerance test in Sri Lankans. BMC Endocr Disord. BMC Endocrine Disorders. 2019;19:4–8. doi: 10.1186/s12902-018-0332-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cowie CC, Rust KF, Byrd-Holt DD, Gregg EW, Ford ES, Geiss LS, et al. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988–2006. Diabetes Care. 2010;33:562–568. doi: 10.2337/dc09-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gómez-Pérez FJ. Glycated Hemoglobin, fasting, two-hour post-challenge and postprandial glycemia in the diagnosis and treatment of diabetes mellitus: are we giving them the right interpretation and use? Rev Invest Clin [Internet]. 2015;67:76–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25938839 [PubMed]
- 19.Meijnikman AS, De Block CEM, Dirinck E, Verrijken A, Mertens I, Corthouts B, et al. Not performing an OGTT results in significant underdiagnosis of (pre)diabetes in a high risk adult Caucasian population. Int J Obes. 2017;41:1615–20. Available from: http://www.nature.com/articles/ijo2017165 [DOI] [PubMed]
- 20.Abdul-Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the veterans administration genetic epidemiology study. Diabetes. 2006;55:1430–5. doi: 10.2337/db05-1200. [DOI] [PubMed] [Google Scholar]
- 21.López-Jaramillo P, Nieto-Martínez RE, Aure-Fariñez G, Mendivil CO, Lahsen RA, Silva-Filho RL, et al. Identification and management of prediabetes: results of the Latin America Strategic Prediabetes Meeting. Rev Panam Salud Pública [Internet]. 2017;41:1. Available from: http://iris.paho.org/xmlui/handle/123456789/34531 [DOI] [PMC free article] [PubMed]
- 22.Secretaria de salud (SS). GCP-RR. Tratamiento de la DIABETES MELLITUS TIPO 2 en el primer nivel de atención. IMSS-718-14 [Internet]. Guia Pract. Clin. 2012 [cited 2020 Aug 25]. p. 189–92. Available from: http://www.cenetec.salud.gob.mx/descargas/gpc/CatalogoMaestro/718_GPC_Tratamiento_de_diabetes_mellitus_tipo_2_/718GRR.pdf
- 23.Selvin E, Wang D, Matsushita K, Grams ME, Coresh J. Prognostic implications of single-sample confirmatory testing for undiagnosed diabetes. Ann Intern Med [Internet]. 2018;169:156. Available from: http://annals.org/article.aspx?doi=10.7326/M18-0091 [DOI] [PMC free article] [PubMed]
- 24.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care [Internet]. 2011;34:S62–9. Available from: http://care.diabetesjournals.org/cgi/doi/10.2337/dc11-S062
- 25.Lim HM, Chia YC, Koay ZL. Performance of the Finnish Diabetes Risk Score (FINDRISC) and Modified Asian FINDRISC (ModAsian FINDRISC) for screening of undiagnosed type 2 diabetes mellitus and dysglycaemia in primary care. Prim Care Diabetes [Internet]. Primary Care Diabetes Europe; 2020; Available from: 10.1016/j.pcd.2020.02.008 [DOI] [PubMed]
- 26.Caixeta DC, Aguiar EMG, Cardoso-Sousa L, Coelho LMD, Oliveira SW, Espindola FS, et al. Salivary molecular spectroscopy: A sustainable, rapid and non-invasive monitoring tool for diabetes mellitus during insulin treatment. Fürnsinn C, editor. PLoS One. 2020;15:e0223461. 10.1371/journal.pone.0223461 [DOI] [PMC free article] [PubMed]
- 27.Rao PV, Reddy AP, Lu X, Dasari S, Krishnaprasad A, Biggs E, et al. Proteomic identification of salivary biomarkers of type-2 diabetes. J Proteome Res. 2009;8:239–45. doi: 10.1021/pr8003776. [DOI] [PubMed] [Google Scholar]
- 28.Abikshyeet P, Venkatapathy R, Nirima O. Glucose estimation in the salivary secretion of diabetes mellitus patients. Diabetes, Metab Syndr Obes Targets Ther [Internet]. 2012;5:149. Available from: http://www.dovepress.com/glucose-estimation-in-the-salivary-secretion-of-diabetes-mellitus-pati-peer-reviewed-article-DMSO [DOI] [PMC free article] [PubMed]
- 29.Amer S, Yousuf M, Siddqiui PQ, Alam J. Salivary glucose concentrations in patients with diabetes mellitus-a minimally invasive technique for monitoring blood glucose levels. Pak J Pharm Sci [Internet]. 2001;14:33–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16414850 [PubMed]
- 30.Mascarenhas P, Fatela B, Barahona I. Effect of diabetes mellitus type 2 on salivary glucose - a systematic review and meta-analysis of observational studies. PLoS One. 2014;9. [DOI] [PMC free article] [PubMed]
- 31.Chiappin S, Antonelli G, Gatti R, De Palo EF. Saliva specimen: a new laboratory tool for diagnostic and basic investigation. Clin Chim Acta. 2007;383:30–40. doi: 10.1016/j.cca.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 32.Ilea A, Andrei V, Feurdean CN, Bǎbtan AM, Petrescu NB, Câmpian RS, et al. Saliva, a magic biofluid available for multilevel assessment and a mirror of general health-a systematic review. Biosensors. 2019;9:1–22. doi: 10.3390/bios9010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu C, Sheng Y, Sun Y, Feng J, Wang S, Zhang J, et al. A glucose oxidase-coupled DNAzyme sensor for glucose detection in tears and saliva. Biosens Bioelectron. 2015;70:455–61. doi: 10.1016/j.bios.2015.03.070. [DOI] [PubMed] [Google Scholar]
- 34.Lee H, Song C, Hong YS, Kim MS, Cho HR, Kang T, et al. Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Sci Adv [Internet]. 2017;3:e1601314. Available from: https://advances.sciencemag.org/lookup/doi/10.1126/sciadv.1601314 [DOI] [PMC free article] [PubMed]
- 35.Soni A, Jha SK. Smartphone based non-invasive salivary glucose biosensor. Anal Chim Acta. 2017;996:54–63. doi: 10.1016/j.aca.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 36.de Castro LF, de Freitas S V., Duarte LC, de Souza JAC, Paixão TRLC, Coltro WKT. Salivary diagnostics on paper microfluidic devices and their use as wearable sensors for glucose monitoring. Anal Bioanal Chem [Internet]. Analytical and Bioanalytical Chemistry; 2019;411:4919–28. Available from: http://link.springer.com/10.1007/s00216-019-01788-0 [DOI] [PubMed]
- 37.Roberts LD, Koulman A, Griffin JL. Towards metabolic biomarkers of insulin resistance and type 2 diabetes: progress from the metabolome. Lancet Diabetes Endocrinol [Internet]. 2014;2:65–75. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2213858713701438 [DOI] [PubMed]
- 38.Dorcely B, Katz K, Jagannathan R, Chiang SS, Oluwadare B, Goldberg IJ, et al. Novel biomarkers for prediabetes, diabetes, and associated complications. Diabetes, Metab Syndr Obes Targets Ther [Internet]. 2017;Volume 10:345–61. Available from: https://www.dovepress.com/novel-biomarkers-for-prediabetes-diabetes-and-associated-complications-peer-reviewed-article-DMSO [DOI] [PMC free article] [PubMed]
- 39.Long J, Liu L, Jia Q, Yang Z, Sun Z, Yan C, et al. Integrated biomarker for type 2 diabetes mellitus and impaired fasting glucose based on metabolomics analysis using ultra‐high performance liquid chromatography quadrupole‐Orbitrap high‐resolution accurate mass spectrometry. Rapid Commun Mass Spectrom [Internet]. 2020;34:1–12. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/rcm.8779 [DOI] [PubMed]
- 40.Selvin E, Francis LMA, Ballantyne CM, Hoogeveen RC, Coresh J, Brancati FL, et al. Nontraditional Markers of Glycemia: Associations with microvascular conditions. Diabetes Care [Internet]. 2011;34:960–7. Available from: http://care.diabetesjournals.org/cgi/doi/10.2337/dc10-1945 [DOI] [PMC free article] [PubMed]
- 41.Juraschek SP, Steffes MW, Selvin E. Associations of alternative markers of glycemia with hemoglobin A1c and fasting glucose. Clin Chem [Internet]. 2012;58:1648–55. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3624763/pdf/nihms412728.pdf [DOI] [PMC free article] [PubMed]
- 42.Liu L, Wan X, Liu J, Huang Z, Cao X, Li Y. Increased 1,5-anhydroglucitol predicts glycemic remission in patients with newly diagnosed type 2 diabetes treated with short-term intensive insulin therapy. Diabetes Technol Ther [Internet]. 2012;14:756–61. Available from: http://www.liebertpub.com/doi/10.1089/dia.2012.0055 [DOI] [PMC free article] [PubMed]
- 43.Zemlin AE, Barkhuizen M, Kengne AP, Erasmus RT, Matsha TE. Performance of glycated albumin for type 2 diabetes and prediabetes diagnosis in a South African population. Clin Chim Acta. 2019;488:122–8. doi: 10.1016/j.cca.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 44.Ying L, He X, Ma X, Shen Y, Su H, Peng J, et al. Serum 1,5-anhydroglucitol when used with fasting plasma glucose improves the efficiency of diabetes screening in a Chinese population. Sci Rep [Internet]. Springer US; 2017;7:11968. Available from: http://dx.doi.org/10.1038/s41598-017-12210-z [DOI] [PMC free article] [PubMed]
- 45.Malmström H, Walldius G, Grill V, Jungner I, Gudbjörnsdottir S, Hammar N. Fructosamine is a useful indicator of hyperglycaemia and glucose control in clinical and epidemiological studies – cross-sectional and longitudinal experience from the AMORIS cohort. Hribal ML, editor. PLoS One [Internet]. 2014;9:e111463. Available from: https://dx.plos.org/10.1371/journal.pone.0111463 [DOI] [PMC free article] [PubMed]
- 46.Juraschek SP, Steffes MW, Miller ER, Selvin E. Alternative markers of hyperglycemia and risk of diabetes. Diabetes Care. 2012;35:2265–70. doi: 10.2337/dc12-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Selvin E, Rawlings AM, Grams M, Klein R, Sharrett AR, Steffes M, et al. Fructosamine and glycated albumin for risk stratification and prediction of incident diabetes and microvascular complications: a prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol [Internet]. 2014;2:279–88. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2213858713701992 [DOI] [PMC free article] [PubMed]
- 48.Adepoyibi T, Weigl B, Greb H, Neogi T, McGuire H. New screening technologies for type 2 diabetes mellitus appropriate for use in tuberculosis patients. Public Heal Action [Internet]. 2013;3:10–7. Available from: http://www.ingentaconnect.com/content/10.5588/pha.13.0036 [DOI] [PMC free article] [PubMed]
- 49.Danese E, Montagnana M, Nouvenne A, Lippi G. Advantages and pitfalls of fructosamine and glycated albumin in the diagnosis and treatment of diabetes. J Diabetes Sci Technol [Internet]. 2015;9:169–76. Available from: http://journals.sagepub.com/doi/10.1177/1932296814567227 [DOI] [PMC free article] [PubMed]
- 50.Selvin E, Rawlings AM, Lutsey PL, Maruthur N, Pankow JS, Steffes M, et al. Fructosamine and glycated albumin and the risk of cardiovascular outcomes and death. Circulation [Internet]. 2015;132:269–77. Available from: https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.115.015415 [DOI] [PMC free article] [PubMed]
- 51.Cohen MP. Intervention strategies to prevent pathogenetic effects of glycated albumin. Arch Biochem Biophys. 2003;419:25–30. doi: 10.1016/j.abb.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 52.Cohen MP, Shea E, Chen S, Shearman CW. Glycated albumin increases oxidative stress, activates NF-κB and extracellular signal-regulated kinase (ERK), and stimulates erk-dependent transforming growth factor-β1 production in macrophage RAW cells. J Lab Clin Med. 2003;141:242–9. doi: 10.1067/mlc.2003.27. [DOI] [PubMed] [Google Scholar]
- 53.Nedić O, Rattan SIS, Grune T, Trougakos IP. Molecular effects of advanced glycation end products on cell signalling pathways, ageing and pathophysiology. Free Radic Res. 2013;47:28–38. doi: 10.3109/10715762.2013.806798. [DOI] [PubMed] [Google Scholar]
- 54.Armbruster D a. Fructosamine: structure, analysis, and clinical usefulness. Clin Chem [Internet]. 1987;33:2153–63. Available from: http://www.ncbi.nlm.nih.gov/pubmed/3319287 [PubMed]
- 55.Austin GE, Wheaton R, Nanes MS, Rubin J, Mullins RE. Usefulness of fructosamine for monitoring outpatients with diabetes. Am J Med Sci [Internet]. Elsevier Masson SAS; 1999;318:316–23. Available from: http://dx.doi.org/10.1016/S0002-9629(15)40645-7 [DOI] [PubMed]
- 56.Peiris, D. S. Weerasekera H. The value of serum fructosamine in comparison with oral glucose tolerance test (OGTT) as a screening test for detection of gestational diabetes mellitus. J Obstet Gynaecol (Lahore) [Internet]. 2000;20:136–8. Available from: http://www.tandfonline.com/doi/full/10.1080/01443610062878 [DOI] [PubMed]
- 57.Lee J-E. Alternative biomarkers for assessing glycemic control in diabetes: fructosamine, glycated albumin, and 1,5-anhydroglucitol. Ann Pediatr Endocrinol Metab [Internet]. 2015;20:74. Available from: http://e-apem.org/journal/view.php?doi=10.6065/apem.2015.20.2.74 [DOI] [PMC free article] [PubMed]
- 58.Selvin E, Warren B, He X, Sacks DB, Saenger AK. Establishment of community-based reference intervals for fructosamine, glycated albumin, and 1,5-anhydroglucitol. Clin Chem. 2018;64:843–50. . doi: 10.1373/clinchem.2017.285742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Manjrekar PA, Hegde A, Shrilaxmi, Dsouza F, Kaveeshwar V, Jose A, et al. Fructosamine in non-diabetic first degree relatives of type 2 diabetes patients: Risk assessor. J Clin Diagnostic Res. 2012;6:770–3.
- 60.Shohat N, Tarabichi M, Tischler EH, Jabbour S, Parvizi J. Serum fructosamine: a simple and inexpensive test for assessing preoperative glycemic control. J Bone Jt Surg [Internet]. 2017;99:1900–7. Available from: https://journals.lww.com/00004623-201711150-00005 [DOI] [PubMed]
- 61.Dungan KM. 1,5-Anhydroglucitol (GlycoMarkTM) as a marker of short-term glycemic control and glycemic excursions. Expert Rev Mol Diagn. 2008;8:9–19. doi: 10.1586/14737159.8.1.9. [DOI] [PubMed] [Google Scholar]
- 62.Kandavel S, Kumar P. M. Association between salivary fructosamine, plasma glycated hemoglobin, and plasma glucose levels among type II diabetes mellitus and nondiabetic individuals—a cross-sectional study. Eur J Dent [Internet]. 2019;13:310–7. Available from: http://www.thieme-connect.de/DOI/DOI?10.1055/s-0039-1695080 [DOI] [PMC free article] [PubMed]
- 63.Khoury ZH, Illesca P, Sultan AS. Salivary fructosamine as a noninvasive glycemic biomarker: a systematic review. JDR Clin Transl Res. 2020;XX:1–8. [DOI] [PubMed]
- 64.Evans TW. Review article: Albumin as a drug - Biological effects of albumin unrelated to oncotic pressure. Aliment Pharmacol Ther Suppl. 2002;16:6–11. doi: 10.1046/j.1365-2036.16.s5.2.x. [DOI] [PubMed] [Google Scholar]
- 65.Rondeau P, Bourdon E. The glycation of albumin: Structural and functional impacts. Biochimie [Internet]. Elsevier Masson SAS; 2011;93:645–58. 10.1016/j.biochi.2010.12.003 [DOI] [PubMed]
- 66.Anguizola J, Matsuda R, Barnaby OS, Hoy KS, Wa C, DeBolt E, et al. Review: Glycation of human serum albumin. Clin Chim Acta [Internet]. 2013;425:64–76. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0009898113002817 [DOI] [PMC free article] [PubMed]
- 67.Baynes JW, Thorpe SR, Murtiashaw MH. Nonenzymatic glucosylation of lysine residues in albumin. Methods Enzymol [Internet]. 1984. p. 88–98. Available from: https://linkinghub.elsevier.com/retrieve/pii/0076687984060109 [DOI] [PubMed]
- 68.Roohk HV, Zaidi AR. A review of glycated albumin as an intermediate glycation index for controlling diabetes. J Diabetes Sci Technol [Internet]. 2008;2:1114–21. Available from: http://journals.sagepub.com/doi/10.1177/193229680800200620 [DOI] [PMC free article] [PubMed]
- 69.Singh VP, Bali A, Singh N, Jaggi AS. Advanced glycation end products and diabetic complications. Korean J Physiol Pharmacol [Internet]. 2014;18:1. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16415936 [DOI] [PMC free article] [PubMed]
- 70.Negre-Salvayre A, Salvayre R, Augé N, Pamplona R, Portero-Otín M. Hyperglycemia and glycation in diabetic complications. Antioxid Redox Signal [Internet]. 2009;11:3071–109. Available from: http://www.liebertpub.com/doi/10.1089/ars.2009.2484 [DOI] [PubMed]
- 71.Inaba M, Okuno S, Kumeda Y, Yamada S, Imanishi Y, Tabata T, et al. Glycated albumin is a better glycemic indicator than glycated hemoglobin values in hemodialysis patients with diabetes: effect of anemia and erythropoietin injection. J Am Soc Nephrol [Internet]. 2007;18:896–903. Available from: https://jasn.asnjournals.org/lookup/doi/10.1681/ASN.2006070772 [DOI] [PubMed]
- 72.Gan T, Liu X, Xu G. Glycated albumin versus HbA1c in the evaluation of glycemic control in patients with diabetes and CKD. Kidney Int Reports. 2018;3:542–54. doi: 10.1016/j.ekir.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim IY, Kim MJ, Lee DW, Lee SB, Rhee H, Song SH, et al. Glycated albumin is a more accurate glycaemic indicator than haemoglobin A 1c in diabetic patients with pre-dialysis chronic kidney disease. Nephrology [Internet]. 2015;20:715–20. Available from: https://onlinelibrary.wiley.com/doi/10.1111/nep.12508 [DOI] [PubMed]
- 74.Pu LJ, Lu L, Shen WF, Zhang Q, Zhang RY, Zhang JS, et al. Increased serum glycated albumin level is associated with the presence and severity of coronary artery disease in type 2 diabetic patients. Circ J [Internet]. 2007;71:1067–73. Available from: https://www.jstage.jst.go.jp/article/circj/71/7/71_7_1067/_article [DOI] [PubMed]
- 75.Nathan DM, Steffes MW, Sun W, Rynders GP, Lachin JM. Determining stability of stored samples retrospectively: the validation of glycated albumin. Clin Chem. 2011;57:286–90. doi: 10.1373/clinchem.2010.150250. [DOI] [PubMed] [Google Scholar]
- 76.Furusyo N, Hayashi J. Glycated albumin and diabetes mellitus. Biochim Biophys Acta - Gen Subj [Internet]. Elsevier B.V.; 2013;1830:5509–14. Available from: http://dx.doi.org/10.1016/j.bbagen.2013.05.010 [DOI] [PubMed]
- 77.Wu W-C, Ma W-Y, Wei J-N, Yu T-Y, Lin M-S, Shih S-R, et al. Serum glycated albumin to guide the diagnosis of diabetes mellitus. Eckel J, editor. PLoS One [Internet]. 2016;11:e0146780. Available from: https://dx.plos.org/10.1371/journal.pone.0146780 [DOI] [PMC free article] [PubMed]
- 78.Su H, Wang Y, Ma X, He X, Ying L, Tang J, et al. Comparative agreement analysis of differences in 1,5-anhydroglucitol, glycated albumin, and glycated hemoglobin A1c levels between fasting and postprandial states in steamed bread meal test. Int J Endocrinol [Internet]. 2017;2017:1–8. Available from: https://www.hindawi.com/journals/ije/2017/5917293/ [DOI] [PMC free article] [PubMed]
- 79.Chume FC, Kieling MH, Correa Freitas PA, Cavagnolli G, Camargo JL. Glycated albumin as a diagnostic tool in diabetes: an alternative or an additional test? Bjornstad P, editor. PLoS One [Internet]. 2019;14:e0227065. Available from: https://dx.plos.org/10.1371/journal.pone.0227065 [DOI] [PMC free article] [PubMed]
- 80.Pitkänen E. 1,5-Anhydro-D-glucitol—A novel type of sugar in the human organism. Scand J Clin Lab Invest [Internet]. 1990;50:55–62. Available from: http://www.tandfonline.com/doi/full/10.1080/00365519009085801 [PubMed]
- 81.Pitkanen E. Occurrence Of 1,5-anhydroglucitol in human cerebrospinal fluid. Clin Chim Acta. 1973;48:159–66. doi: 10.1016/0009-8981(73)90361-6. [DOI] [PubMed] [Google Scholar]
- 82.Yamanouchi T, Tachibana Y, Akanuma H, Minoda S, Shinohara T, Moromizato H, et al. Origin and disposal of 1,5-anhydroglucitol, a major polyol in the human body. Am J Physiol Metab [Internet]. 1992;263:E268–73. Available from: https://www.physiology.org/doi/10.1152/ajpendo.1992.263.2.E268 [DOI] [PubMed]
- 83.Yamanouchi T, Akanuma Y. Serum 1,5-anhydroglucitol (1,5 AG): new clinical marker for glycemic control. Diabetes Res Clin Pract [Internet]. 1994;24:S261–8. Available from: https://linkinghub.elsevier.com/retrieve/pii/0168822794902593 [DOI] [PubMed]
- 84.Buse JB, Freeman JLR, Edelman S V., Jovanovic L, McGill JB. Serum 1,5-Anhydroglucitol (GlycoMarkTM): a short-term glycemic marker. Diabetes Technol Ther [Internet]. 2003;5:355–63. Available from: http://www.liebertpub.com/doi/10.1089/152091503765691839 [DOI] [PubMed]
- 85.Yamanouchi T, Minoda S, Yabuuchi M, Akanuma Y, Akanuma H, Miyashita H, et al. Plasma 1,5-anhydro-D-glucitol as new clinical marker of glycemic control in NIDDM patients. Diabetes. 1989;38:723–9. doi: 10.2337/diab.38.6.723. [DOI] [PubMed] [Google Scholar]
- 86.Stickle D, Turk J. A kinetic mass balance model for 1,5-anhydroglucitol: applications to monitoring of glycemic control. Am J Physiol Metab [Internet]. 1997;273:E821–30. Available from: https://www.physiology.org/doi/10.1152/ajpendo.1997.273.4.E821 [DOI] [PubMed]
- 87.Pitkänen E. Serum 1,5-anhydroglucitol in normal subjects and in patients with insulin-dependent diabetes mellitus. Scand J Clin Lab Invest. 1982;42:445–8. doi: 10.1080/00365518209168111. [DOI] [PubMed] [Google Scholar]
- 88.Halama A, Kulinski M, Kader SA, Satheesh NJ, Abou-Samra AB, Suhre K, et al. Measurement of 1,5-anhydroglucitol in blood and saliva: from non-targeted metabolomics to biochemical assay. J Transl Med [Internet]. BioMed Central; 2016;14:140. Available from: http://translational-medicine.biomedcentral.com/articles/10.1186/s12967-016-0897-6 [DOI] [PMC free article] [PubMed]
- 89.Akanuma Y, Morita M, Fukuzawa N, Yamanouchi T, Akanuma H. Urinary excretion of 1,5-anhydro-D-glucitol accompanying glucose excretion in diabetic patients. Diabetologia [Internet]. 1988;31:831–5. Available from: http://link.springer.com/10.1007/BF00277486 [DOI] [PubMed]
- 90.Yamanouchi T, Akanuma H, Nakamura T, Akaoka I, Akanuma Y. Reduction of plasma 1,5-anhydroglucitol (1-deoxyglucose) concentration in diabetic patients. Diabetologia. 1988;31:41–5. doi: 10.1007/BF00279131. [DOI] [PubMed] [Google Scholar]
- 91.Nowatzke W, Sarno MJ, Birch NC, Stickle DF, Eden T, Cole TG. Evaluation of an assay for serum 1,5-anhydroglucitol (GlycoMarkTM) and determination of reference intervals on the Hitachi 917 analyzer. Clin Chim Acta [Internet]. 2004;350:201–9. Available from: https://linkinghub.elsevier.com/retrieve/pii/S000989810400419X [DOI] [PubMed]
- 92.Welter M, Boritza KC, Anghebem-Oliveira MI, Henneberg R, Hauser AB, Rego FGM, et al. Reference intervals for serum 1,5-anhydroglucitol in children, adolescents, adults, and pregnant women. Clin Chim Acta [Internet]. Elsevier; 2018;486:54–8. 10.1016/j.cca.2018.07.018 [DOI] [PubMed]
- 93.Koga M. 1,5-Anhydroglucitol and glycated albumin in glycemia. Adv Clin Chem [Internet]. 1st ed. Kawanishi: Elsevier Inc.; 2014. p. 269–301. 10.1016/B978-0-12-800263-6.00007-0 [DOI] [PubMed]
- 94.Dungan KM, Buse JB, Largay J, Kelly MM, Button EA, Kato S, et al. 1,5-Anhydroglucitol and postprandial hyperglycemia as measured by continuous glucose monitoring system in moderately controlled patients with diabetes. Diabetes Care [Internet]. 2006;29:1214–9. Available from: http://care.diabetesjournals.org/cgi/doi/10.2337/dc06-1910 [DOI] [PubMed]
- 95.Jian C, Zhao A, Ma X, Ge K, Lu W, Zhu W, et al. Diabetes Screening: Detection and Application of Saliva 1,5-Anhydroglucitol by Liquid Chromatography–Mass Spectrometry. J Clin Endocrinol Metab [Internet]. 2020;105:1759–69. Available from: https://academic.oup.com/jcem/article/105/6/1759/5805160 [DOI] [PubMed]
- 96.Selvin E, Rawlings A, Lutsey P, Maruthur N, Pankow JS, Steffes M, et al. Association of 1,5-anhydroglucitol with cardiovascular disease and mortality. Diabetes [Internet]. 2015;65:db150607. Available from: http://diabetes.diabetesjournals.org/lookup/doi/10.2337/db15-0607 [DOI] [PMC free article] [PubMed]
- 97.Selvin E, Rawlings AM, Grams M, Klein R, Steffes M, Coresh J. Association of 1,5-anhydroglucitol with diabetes and microvascular conditions. Clin Chem [Internet]. 2014;60:1409–18. Available from: https://academic.oup.com/clinchem/article/60/11/1409/5621578 [DOI] [PMC free article] [PubMed]
- 98.Onorato JM, Langish RA, Shipkova PA, Sanders M, Wang J, Kwagh J, et al. A novel method for the determination of 1,5-anhydroglucitol, a glycemic marker, in human urine utilizing hydrophilic interaction liquid chromatography/MS3. J Chromatogr B [Internet]. 2008;873:144–50. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1570023208006065 [DOI] [PubMed]
- 99.Su H, Ma X, Yin J, Wang Y, He X, Bao Y, et al. Serum 1,5-anhydroglucitol levels slightly increase rather than decrease after a glucose load in subjects with different glucose tolerance status. Acta Diabetol [Internet]. Springer Milan; 2017;54:463–70. Available from: http://link.springer.com/10.1007/s00592-017-0968-z [DOI] [PubMed]
- 100.Hu X, He X, Ma X, Su H, Ying L, Peng J, et al. A decrease in serum 1,5-anhydroglucitol levels is associated with the presence of a first-degree family history of diabetes in a Chinese population with normal glucose tolerance. Diabet Med [Internet]. 2018;35:131–6. Available from: https://onlinelibrary.wiley.com/doi/10.1111/dme.13534 [DOI] [PubMed]
- 101.Yoo HY, Kwak BO, Son JS, Kim KS, Chung S. Value of serum 1,5-anhydroglucitol measurements in childhood obesity in the continuum of diabetes. Ann Pediatr Endocrinol Metab [Internet]. 2015;20:192. Available from: http://e-apem.org/journal/view.php?doi=10.6065/apem.2015.20.4.192 [DOI] [PMC free article] [PubMed]
- 102.Li L, Krznar P, Erban A, Agazzi A, Martin-Levilain J, Supale S, et al. Metabolomics identifies a biomarker revealing in vivo loss of functional β-cell mass before diabetes onset. Diabetes [Internet]. 2019;68:2272–86. Available from: http://diabetes.diabetesjournals.org/lookup/doi/10.2337/db19-0131 [DOI] [PubMed]
- 103.Su G, Gao M-X, Shi G-L, Dai X-X, Yao W-F, Zhang T, et al. Effect of 1,5-anhydroglucitol levels on culprit plaque rupture in diabetic patients with acute coronary syndrome. Cardiovasc Diabetol [Internet]. 2020;19:71. Available from: https://cardiab.biomedcentral.com/articles/10.1186/s12933-020-01045-0 [DOI] [PMC free article] [PubMed]
- 104.Zhang K, Xue B, Yuan Y, Wang Y. Correlation of serum 1,5-AG with uric acid in type 2 diabetes mellitus with different renal functions. Int J Endocrinol [Internet]. 2019;2019:1–7. Available from: https://www.hindawi.com/journals/ije/2019/4353075/ [DOI] [PMC free article] [PubMed]
- 105.Santos-Ferreira D, Gonçalves-Teixeira P, Fontes-Carvalho R. SGLT-2 inhibitors in heart failure and type-2 diabetes: hitting two birds with one stone? Cardiology [Internet]. 2020;145:311–20. Available from: https://www.karger.com/Article/FullText/504694 [DOI] [PubMed]
- 106.Lupsa BC, Inzucchi SE. Use of SGLT2 inhibitors in type 2 diabetes: weighing the risks and benefits. Diabetologia [Internet]. Diabetologia; 2018;61:2118–25. Available from: http://link.springer.com/10.1007/s00125-018-4663-6 [DOI] [PubMed]
- 107.Gyimesi G, Pujol-Giménez J, Kanai Y, Hediger MA. Sodium-coupled glucose transport, the SLC5 family, and therapeutically relevant inhibitors: from molecular discovery to clinical application. Pflügers Arch - Eur J Physiol [Internet]. Pflügers Archiv - European Journal of Physiology; 2020;472:1177–206. Available from: https://link.springer.com/10.1007/s00424-020-02433-x [DOI] [PMC free article] [PubMed]
- 108.Balis DA, Tong C, Meininger G. Effect of canagliflozin, a sodium–glucose cotransporter 2 inhibitor, on measurement of serum 1,5‐anhydroglucitol. J Diabetes [Internet]. 2014;6:378–80. Available from: https://onlinelibrary.wiley.com/doi/10.1111/1753-0407.12116 [DOI] [PubMed]
- 109.Kappel BA, Moellmann J, Thiele K, Rau M, Artati A, Adamski J, et al. Human and mouse non‐targeted metabolomics identify 1,5‐anhydroglucitol as SGLT2‐dependent glycemic marker. Clin Transl Med [Internet]. 2021;11:1–7. Available from: https://onlinelibrary.wiley.com/doi/10.1002/ctm2.470 [DOI] [PMC free article] [PubMed]
- 110.Dame ZT, Aziat F, Mandal R, Krishnamurthy R, Bouatra S, Borzouie S, et al. The human saliva metabolome. Metabolomics. Springer US; 2015;11:1864–83.
- 111.Furusawa H, Ichimura Y, Nagamine K, Shiwaku R, Matsui H, Tokito S. Detection of 1,5-anhydroglucitol as a biomarker for diabetes using an organic field-effect transistor-based biosensor. Technologies [Internet]. 2018;6:77. Available from: http://www.mdpi.com/2227-7080/6/3/77
- 112.Chen Z-Z, Gerszten RE. Metabolomics and proteomics in type 2 diabetes. Circ Res [Internet]. 2020;126:1613–27. Available from: https://www.ahajournals.org/doi/10.1161/CIRCRESAHA.120.315898 [DOI] [PMC free article] [PubMed]
- 113.Yousri NA, Mook-Kanamori DO, Selim MMEE-D, Takiddin AH, Al-Homsi H, Al-Mahmoud KAS, et al. A systems view of type 2 diabetes-associated metabolic perturbations in saliva, blood and urine at different timescales of glycaemic control. Diabetologia [Internet]. 2015;58:1855–67. Available from: http://link.springer.com/10.1007/s00125-015-3636-2 [DOI] [PMC free article] [PubMed]
- 114.Suhre K. Metabolic profiling in diabetes. J Endocrinol [Internet]. 2014;221:R75–85. Available from: https://joe.bioscientifica.com/view/journals/joe/221/3/R75.xml [DOI] [PubMed]
- 115.Prasad RB, Groop L. Precision medicine in type 2 diabetes. J Intern Med [Internet]. 2019;285:40–8. Available from: http://doi.wiley.com/10.1111/joim.12859 [DOI] [PubMed]
- 116.Cohen RM, Sacks DB. Comparing multiple measures of glycemia: how to transition from biomarker to diagnostic test? Clin Chem [Internet]. 2012;58:1615–7. Available from: https://academic.oup.com/clinchem/article/58/12/1615/5620825 [DOI] [PubMed]
- 117.Barnes VM, Kennedy AD, Panagakos F, Devizio W, Trivedi HM, Jönsson T, et al. Global metabolomic analysis of human saliva and plasma from healthy and diabetic subjects, with and without periodontal disease. Yilmaz Ö, editor. PLoS One [Internet]. 2014;9:e105181. Available from: https://dx.plos.org/10.1371/journal.pone.0105181 [DOI] [PMC free article] [PubMed]
- 118.Suhre K, Meisinger C, Döring A, Altmaier E, Belcredi P, Gieger C, et al. Metabolic footprint of diabetes: A multiplatform metabolomics study in an epidemiological setting. PLoS One. 2010;5. [DOI] [PMC free article] [PubMed]
- 119.Kohler I, Hankemeier T, van der Graaf PH, Knibbe CAJ, van Hasselt JGC. Integrating clinical metabolomics-based biomarker discovery and clinical pharmacology to enable precision medicine. Eur J Pharm Sci. 2017;109:S15–S21. doi: 10.1016/j.ejps.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 120.Kammer M, Heinzel A, Willency JA, Duffin KL, Mayer G, Simons K, et al. Integrative analysis of prognostic biomarkers derived from multiomics panels helps discrimination of chronic kidney disease trajectories in people with type 2 diabetes. Kidney Int. 2019;96:1381–8. doi: 10.1016/j.kint.2019.07.025. [DOI] [PubMed] [Google Scholar]
- 121.Peddinti G, Cobb J, Yengo L, Froguel P, Kravić J, Balkau B, et al. Early metabolic markers identify potential targets for the prevention of type 2 diabetes. Diabetologia [Internet]. 2017;60:1740–50. Available from: http://link.springer.com/10.1007/s00125-017-4325-0 [DOI] [PMC free article] [PubMed]
- 122.Libert DM, Nowacki AS, Natowicz MR. Metabolomic analysis of obesity, metabolic syndrome, and type 2 diabetes: amino acid and acylcarnitine levels change along a spectrum of metabolic wellness. PeerJ [Internet]. 2018;6:e5410. Available from: https://peerj.com/articles/5410 [DOI] [PMC free article] [PubMed]
- 123.Huth C, Bauer A, Zierer A, Sudduth-Klinger J, Meisinger C, Roden M, et al. Biomarker-defined pathways for incident type 2 diabetes and coronary heart disease—a comparison in the MONICA/KORA study. Cardiovasc Diabetol [Internet]. 2020;19:32. Available from: https://cardiab.biomedcentral.com/articles/10.1186/s12933-020-01003-w [DOI] [PMC free article] [PubMed]
- 124.Fikri AM, Smyth R, Kumar V, Al-Abadla Z, Abusnana S, Munday MR. Pre-diagnostic biomarkers of type 2 diabetes identified in the UAE’s obese national population using targeted metabolomics. Sci Rep [Internet]. 2020;10:17616. Available from: http://www.nature.com/articles/s41598-020-73384-7 [DOI] [PMC free article] [PubMed]
- 125.Ahola-Olli AV, Mustelin L, Kalimeri M, Kettunen J, Jokelainen J, Auvinen J, et al. Circulating metabolites and the risk of type 2 diabetes: a prospective study of 11,896 young adults from four Finnish cohorts. Diabetologia. 2019;62:2298–309 . doi: 10.1007/s00125-019-05001-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang S, Yu X, Zhang W, Ji F, Wang M, Yang R, et al. Association of serum metabolites with impaired fasting glucose/diabetes and traditional risk factors for metabolic disease in Chinese adults. Clin Chim Acta. 2018;487:60–5. doi: 10.1016/j.aca.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 127.Lowe WL, Bain JR. “Prediction is very hard, especially about the future”: new biomarkers for type 2 diabetes? Diabetes [Internet]. 2013;62:1384–5. Available from: http://diabetes.diabetesjournals.org/cgi/doi/10.2337/db13-0057 [DOI] [PMC free article] [PubMed]
- 128.Sun Y, Gao H-Y, Fan Z-Y, He Y, Yan Y-X. Metabolomics signatures in type 2 diabetes: a systematic review and integrative analysis. J Clin Endocrinol Metab [Internet]. 2020;105:1000–8. Available from: https://academic.oup.com/jcem/article/105/4/1000/5645632 [DOI] [PubMed]
- 129.Klein MS, Shearer J. Metabolomics and type 2 diabetes: translating basic research into clinical application. J Diabetes Res. 2016;2016. [DOI] [PMC free article] [PubMed]
- 130.Lee S-G, Yim YS, Lee Y, Lee B-W, Kim H-S, Kim K-S, et al. Fasting serum amino acids concentration is associated with insulin resistance and pro-inflammatory cytokines. Diabetes Res Clin Pract [Internet]. Elsevier B.V.; 2018;140:107–17. 10.1016/j.diabres.2018.03.028 [DOI] [PubMed]
- 131.Nilsen MS, Jersin RÅ, Ulvik A, Madsen A, McCann A, Svensson P-A, et al. 3-Hydroxyisobutyrate, a strong marker of insulin resistance in type 2 diabetes and obesity that modulates white and brown adipocyte metabolism. Diabetes. 2020;69:1903–16. doi: 10.2337/db19-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Stefan N, Häring HU. The role of hepatokines in metabolism. Nat Rev Endocrinol 2013;9:144–52 10.1038/nrendo.2012.258 [DOI] [PubMed]
- 133.Holeček M. Why are branched-chain amino acids increased in starvation and diabetes? Nutrients [Internet]. 2020;12:1–15. Available from: http://www.ncbi.nlm.nih.gov/pubmed/33050579 [DOI] [PMC free article] [PubMed]
- 134.Ferrannini E, Natali A, Camastra S, Nannipieri M, Mari A, Adam K-P, et al. Early metabolic markers of the development of dysglycemia and type 2 diabetes and their physiological significance. Diabetes [Internet]. 2013;62:1730–7. Available from: http://diabetes.diabetesjournals.org/cgi/doi/10.2337/db12-0707 [DOI] [PMC free article] [PubMed]
- 135.American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2021. Diabetes Care [Internet]. 2021;44:S15–33. Available from: http://care.diabetesjournals.org/lookup/doi/10.2337/dc21-S002 [DOI] [PubMed]
- 136.Buijsse B, Simmons RK, Griffin SJ, Schulze MB. Risk assessment tools for identifying individuals at risk of developing type 2 diabetes. Epidemiol Rev [Internet]. 2011;33:46–62. Available from: https://academic.oup.com/epirev/article-lookup/doi/10.1093/epirev/mxq019 [DOI] [PMC free article] [PubMed]
- 137.Lindstrom J, Tuomilehto J. The Diabetes Risk Score: a practical tool to predict type 2 diabetes risk. Diabetes Care [Internet]. 2003;26:725–31. Available from: http://care.diabetesjournals.org/cgi/doi/10.2337/diacare.26.3.725 [DOI] [PubMed]
- 138.Boonyasit Y, Laiwattanapaisal W. A microfluidic paper-based analytical device for the assay of albumin-corrected fructosamine values from whole blood samples. Bioanalysis [Internet]. 2015;7:79–90. Available from: https://www.future-science.com/doi/10.4155/bio.14.199 [DOI] [PubMed]
- 139.Ki H, Jang H, Oh J, Han G-R, Lee H, Kim S, et al. Simultaneous detection of serum glucose and glycated albumin on a paper-based sensor for acute hyperglycemia and diabetes mellitus. Anal Chem [Internet]. 2020;92:11530–4. Available from: https://pubs.acs.org/doi/10.1021/acs.analchem.0c02940 [DOI] [PubMed]
- 140.Belsare S, Coté G. Development of a colorimetric paper fluidic dipstick assay for measurement of glycated albumin to monitor gestational diabetes at the point-of-care. Talanta [Internet]. 2021;223:121728. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0039914020310195 [DOI] [PubMed]
- 141.Hatada M, Tsugawa W, Kamio E, Loew N, Klonoff DC, Sode K. Development of a screen-printed carbon electrode based disposable enzyme sensor strip for the measurement of glycated albumin. Biosens Bioelectron [Internet]. Elsevier; 2017;88:167–73. Available from: http://dx.doi.org/10.1016/j.bios.2016.08.005 [DOI] [PubMed]
- 142.Hatada M, Loew N, Okuda-Shimazaki J, Khanwalker M, Tsugawa W, Mulchandani A, et al. Development of an interdigitated electrode-based disposable enzyme sensor strip for glycated albumin measurement. Molecules [Internet]. 2021;26:734. Available from: https://www.mdpi.com/1420-3049/26/3/734 [DOI] [PMC free article] [PubMed]
- 143.Jang H, Oh J, Ki H, Kim M-G. Paper-based 1,5-anhydroglucitol quantification using enzyme-based glucose elimination. Analyst [Internet]. Royal Society of Chemistry; 2020;145:5740–3. Available from: http://xlink.rsc.org/?DOI=D0AN00905A [DOI] [PubMed]
- 144.Adamson TL, Cook CB, LaBelle JT. Detection of 1,5-anhydroglucitol by electrochemical impedance spectroscopy. J Diabetes Sci Technol [Internet]. 2014;8:350–5. Available from: http://journals.sagepub.com/doi/10.1177/1932296814523874 [DOI] [PMC free article] [PubMed]
- 145.Baker JR, Johnson RN, Scott DJ. Serum fructosamine concentrations in patients with type II (non-insulin-dependent) diabetes mellitus during changes in management. BMJ [Internet]. 1984;288:1484–6. Available from: https://www.bmj.com/lookup/doi/10.1136/bmj.288.6429.1484 [DOI] [PMC free article] [PubMed]
- 146.Allgrove J, Cockrill BL. Fructosamine or glycated haemoglobin as a measure of diabetic control? Arch Dis Child. 1988;63:418–22. doi: 10.1136/adc.63.4.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wright LA-C, Hirsch IB. The challenge of the use of glycemic biomarkers in diabetes: reflecting on hemoglobin A1C, 1,5-anhydroglucitol, and the glycated proteins fructosamine and glycated albumin. Diabetes Spectr [Internet]. 2012;25:141–8. Available from: http://spectrum.diabetesjournals.org/cgi/doi/10.2337/diaspect.25.3.141
- 148.Koga M, Murai J, Saito H, Kasayama S. Glycated albumin and glycated hemoglobin are influenced differently by endogenous insulin secretion in patients with type 2 diabetes. Diabetes Care. 2010;33:270–2. doi: 10.2337/dc09-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Paroni R, Ceriotti F, Galanello R, Leoni GB, Panico A, Scurati E, et al. Performance characteristics and clinical utility of an enzymatic method for the measurement of glycated albumin in plasma. Clin Biochem [Internet]. 2007;40:1398–405. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0009912007002962 [DOI] [PubMed]
- 150.Yoshiuchi K, Matsuhisa M, Katakami N, Nakatani Y, Sakamoto K, Matsuoka T, et al. Glycated albumin is a better indicator for glucose excursion than glycated hemoglobin in type 1 and type 2 diabetes. Endocr J. 2008;55:503–7. doi: 10.1507/endocrj.K07E-089. [DOI] [PubMed] [Google Scholar]
- 151.Yamanouchi T, Ogata N, Tagaya T, Kawasaki T, Sekino N, Funato H, et al. Clinical usefulness of serum 1,5-anhydroglucitol in monitoring glycaemic control. Lancet [Internet]. 1996;347:1514–8. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673696906728 [DOI] [PubMed]
- 152.Ma X, Hao Y, Hu X, Luo Y, Deng Z, Zhou J, et al. 1,5-Anhydroglucitol is associated with early-phase insulin secretion in Chinese patients with newly diagnosed type 2 diabetes mellitus. Diabetes Technol Ther [Internet]. 2015;17:320–6. Available from: http://www.liebertpub.com/doi/10.1089/dia.2014.0346 [DOI] [PubMed]
- 153.Stettler C, Stahl M, Allemann S, Diem P, Schmidlin K, Zwahlen M, et al. Association of 1,5-anhydroglucitol and 2-h postprandial blood glucose in type 2 diabetic patients. Diabetes Care [Internet]. 2008;31:1534–5. Available from: http://care.diabetesjournals.org/cgi/doi/10.2337/dc08-0385 [DOI] [PMC free article] [PubMed]
- 154.Kim WJ, Park C-Y. 1,5-Anhydroglucitol in diabetes mellitus. Endocrine [Internet]. 2013;43:33–40. Available from: http://link.springer.com/10.1007/s12020-012-9760-6 [DOI] [PubMed]
- 155.Owei I, Umekwe N, Stentz F, Wan J, Dagogo-Jack S. Amino acid signature predictive of incident prediabetes: A case-control study nested within the longitudinal pathobiology of prediabetes in a biracial cohort. Metabolism. 2019;98:76–83. doi: 10.1016/j.metabol.2019.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gall WE, Beebe K, Lawton KA, Adam K-P, Mitchell MW, Nakhle PJ, et al. α-Hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. Federici M, editor. PLoS One 2010;5:e10883. 10.1371/journal.pone.0010883 [DOI] [PMC free article] [PubMed]
- 157.Pérez-Matos MC, Morales-Álvarez MC, Toloza FJK, Ricardo-Silgado ML, Mantilla-Rivas JO, Pinzón-Cortes JA, et al. The phospholipid linoleoylglycerophosphocholine as a biomarker of directly measured insulin resistance. Diabetes Metab J [Internet]. 2017;41:466. Available from: http://e-dmj.org/journal/view.php?doi=10.4093/dmj.2017.41.6.466 [DOI] [PMC free article] [PubMed]
- 158.Aaty TAA, Rezk MM, Megallaa MH, Yousseif ME, Kassab HS. Serum leptin level and microvascular complications in type 2 diabetes. Clin Diabetol [Internet]. 2020;9:239–44. Available from: https://journals.viamedica.pl/clinical_diabetology/article/view/67524
- 159.Wang Y, Koh W-P, Jensen MK, Yuan J-M, Pan A. Plasma fetuin-a levels and risk of type 2 diabetes mellitus in a Chinese population: a nested case-control study. Diabetes Metab J [Internet]. 2019;43:474. Available from: http://e-dmj.org/journal/view.php?doi=10.4093/dmj.2018.0171 [DOI] [PMC free article] [PubMed]
- 160.Kumar AA, Satheesh G, Vijayakumar G, Chandran M, Prabhu PR, Simon L, et al. Plasma leptin level mirrors metabolome alterations in young adults. Metabolomics. 2020;16:87. doi: 10.1007/s11306-020-01708-9. [DOI] [PubMed] [Google Scholar]
- 161.Chailurkit L, Paiyabhroma N, Sritara P, Vathesatogkit P, Yamwong S, Thonmung N, et al. Independent and opposite associations between branched-chain amino acids and lysophosphatidylcholines with incident diabetes in Thais. Metabolites [Internet]. 2020;10:76. Available from: https://www.mdpi.com/2218-1989/10/2/76 [DOI] [PMC free article] [PubMed]
- 162.Ren G, Kim T, Papizan JB, Okerberg CK, Kothari VM, Zaid H, et al. Phosphorylation status of fetuin-A is critical for inhibition of insulin action and is correlated with obesity and insulin resistance. Am J Physiol Metab [Internet]. 2019;317:E250–60. Available from: https://www.physiology.org/doi/10.1152/ajpendo.00089.2018 [DOI] [PubMed]
- 163.Connelly MA, Wolak-Dinsmore J, Dullaart RPF. Branched chain amino acids are associated with insulin resistance independent of leptin and adiponectin in subjects with varying degrees of glucose tolerance. Metab Syndr Relat Disord [Internet]. 2017;15:183–6. Available from: http://www.liebertpub.com/doi/10.1089/met.2016.0145 [DOI] [PubMed]
- 164.Noordam R, Boersma V, Verkouter I, le Cessie S, Christen T, Lamb HJ, et al. The role of C-reactive protein, adiponectin and leptin in the association between abdominal adiposity and insulin resistance in middle-aged individuals. Nutr Metab Cardiovasc Dis. 2020;30:1306–14. doi: 10.1016/j.numecd.2020.04.021. [DOI] [PubMed] [Google Scholar]
- 165.Diamanti K, Cavalli M, Pan G, Pereira MJ, Kumar C, Skrtic S, et al. Intra- and inter-individual metabolic profiling highlights carnitine and lysophosphatidylcholine pathways as key molecular defects in type 2 diabetes. Sci Rep [Internet]. 2019;9:9653. Available from: http://www.nature.com/articles/s41598-019-45906-5 [DOI] [PMC free article] [PubMed]
- 166.Tricò D, Prinsen H, Giannini C, de Graaf R, Juchem C, Li F, et al. Elevated α-Hydroxybutyrate and Branched-Chain Amino Acid Levels Predict Deterioration of Glycemic Control in Adolescents. J Clin Endocrinol Metab [Internet]. 2017;102:2473–81. Available from: https://academic.oup.com/jcem/article-lookup/doi/10.1210/jc.2017-00475 [DOI] [PMC free article] [PubMed]
- 167.Marques-Vidal P, Schmid R, Bochud M, Bastardot F, von Känel R, Paccaud F, et al. Adipocytokines, hepatic and inflammatory biomarkers and incidence of type 2 diabetes. The CoLaus Study. Herder C, editor. PLoS One [Internet]. 2012;7:e51768. Available from: https://dx.plos.org/10.1371/journal.pone.0051768 [DOI] [PMC free article] [PubMed]
- 168.Cobb J, Eckhart A, Perichon R, Wulff J, Mitchell M, Adam K-P, et al. A novel test for IGT utilizing metabolite markers of glucose tolerance. J Diabetes Sci Technol [Internet]. 2015;9:69–76. Available from: http://journals.sagepub.com/doi/10.1177/1932296814553622 [DOI] [PMC free article] [PubMed]