Abstract
STUDY QUESTION
What is the scope of literature regarding women’s reproductive span in terms of definitions, trends and determinants?
SUMMARY ANSWER
The scoping review found a wide variation in definitions, trends and determinants of biological, social and effective women’s reproductive span.
WHAT IS KNOWN ALREADY
A woman’s reproductive span refers to her childbearing years. Its span influences a woman’s reproductive decisions.
STUDY DESIGN, SIZE, DURATION
A systematic scoping review was conducted. We searched MEDLINE, PubMed, JSTOR, CINAHL, Web of Science and Scopus electronic databases from inception to January 2021 without imposing language or date restrictions. We searched unpublished sources including the Global Burden of Disease, Demographic and Health Surveys, and National Health and Nutrition Examination Surveys. The list of relevant references was searched by hand. Sixty-seven reports on women’s reproductive span were included in this review.
PARTICIPANTS/MATERIALS, SETTING, METHODS
This scoping systematic review followed an established framework. The reporting of this scoping review followed the reporting requirements provided in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses, Extension for Scoping Reviews. Identified records were independently screened and data were extracted. We performed conceptual synthesis by grouping the studies by available concepts of reproductive span and then summarized definitions, measures used, temporal trends, determinants, and broad findings of implications on population demographics and assisted reproduction. Structured tabulation and graphical synthesis were used to show patterns in the data and convey detailed information efficiently, along with a narrative commentary.
MAIN RESULTS AND THE ROLE OF CHANCE
A total of 67 relevant reports on women’s reproductive span were published between 1980 and 2020 from 74 countries. Most reports (42/67) were cross-sectional in design. Literature on reproductive span was conceptually grouped as biological (the interval between age at menarche and age at menopause), effective (when a woman is both fertile and engaging in sexual activity) and social (period of exposure to sexual activity). We summarized the working definitions, trends and determinants of each concept. Few articles addressed implications on demographics and assisted reproduction.
LIMITATIONS, REASONS FOR CAUTION
A formal assessment of methodological quality of the included studies was not performed because the aim of this review was to provide an overview of the existing evidence base regardless of quality.
WIDER IMPLICATIONS OF THE FINDINGS
The review produced a comprehensive set of possible definitions of women’s reproductive span, trends, and potential determinants. Further advancement of these findings will involve collaboration with relevant stakeholders to rate the importance of each definition in relation to demography and fertility care, outline a set of core definitions, identify implications for policy, practice or research and define future research opportunities to explore linkages between reproductive spans, their determinants, and the need for assisted reproduction.
STUDY FUNDING/COMPETING INTEREST(S)
This work received funding from the UNDP-UNFPA-UNICEF-WHO-World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), a cosponsored programme executed by the World Health Organization (WHO). The authors had no competing interests.
STUDY REGISTRATION NUMBER
N/A.
Keywords: reproductive span, menarche, menopause, demography, assisted reproduction, infertility, humans, female
WHAT DOES THIS MEAN FOR PATIENTS?
A woman’s ‘reproductive span’ is an important concept that includes her childbearing years and therefore has an impact on her decision making, including when to try for a pregnancy, spacing between pregnancies, desired family size and, finally, when to have the last baby. There have been notable changes in recent decades, with women choosing to delay marriage, not to marry at all, postpone childbearing or limit the number of births. This study searched for all published research on women’s reproductive span. Studies were grouped as biological (the interval between the beginning and end of menstruation), effective (when a woman is both fertile and sexually active) and social (period of exposure to sexual activity). Currently, the biological reproductive span of women ranges from 30.9 to 39.3 years, while the effective reproductive span was found to vary, with a steady decline worldwide. A wide variety of determinants of the reproductive span were reported in the literature, but limited studies reported the implications of contemporary trends in reproductive span on population demographics or assisted reproduction. Trends in women’s reproductive span may have an impact on the need or utilization of fertility care services, including medically assisted reproduction.
Introduction
Globally, infertility is considered a major public health issue, affecting ∼8–12% of couples or 186 million people (Inhorn and Patrizio, 2015; Vander Borght and Wyns, 2018). Infertility remains a woman’s social burden (Inhorn and Patrizio, 2015), affecting 8% of women aged 19–26 years, 13–14% of women aged 27–34 years and 18% of women aged 35–39 years (Dunson et al., 2004). Although advances in reproductive medicine continuously provide additional solutions and interventions for those who desire to conceive, an important challenge that remains is that women have a finite reproductive lifespan (Inhorn and Patrizio, 2015).
A woman’s reproductive span is an important concept that encompasses childbearing years and therefore has an impact on women’s reproductive decisions including when to get pregnant, spacing between pregnancies, desired family size and, finally, when to have the last birth. With notable changes in social-economic contexts over the past decades, more women may choose to delay marriage, not to marry at all, postpone childbearing to an older age or limit the number of births. Since women’s fertility declines with age owing to a decline in the number and quality of oocytes, the propensity to delay childbearing has a significant impact on fertility because it reduces the number of reproductive years, particularly the most fertile years (Velde and Pearson, 2002).
Under most demographic circumstances, reproduction during this period in a woman’s life is the most important determinant of population dynamics and growth (Vitzthum, 2021). Therefore, advancing our understanding of women’s reproductive span and its determinants and trends is critical for making future directions for policy, practice and research (Carey and Roach, 2020).
The rationale to conduct this scoping review was based on the absence of any publication examining the scope of literature on women’s reproductive span.
The aim of this systematic scoping review was, therefore, to determine the scope of literature and to synthesize what is known about women’s reproductive span in terms of definitions, trends and determinants, and the impact that contemporary trends in reproductive span have on population demographics and assisted reproduction.
Materials and methods
A scoping review approach was chosen as the appropriate method, given the broad and complex nature of the concept of women’s reproductive span. To confirm that no other similar scoping reviews existed, Medline and Prospero databases were searched, and the results indicated an absence of systematic scoping articles related to women’s reproductive span. The review was conducted based on the methods that were pre-specified in the protocol. The review protocol was prospectively registered in the Open Science Framework platform (https://osf.io/wysru; Nabhan et al., 2020).
The methods for this scoping review were guided by the framework developed by Arksey and O’Malley (2005), subsequently adapted by Levac et al. (2010), Colquhoun et al. (2014) and by the Joanna Briggs Institute guidelines (Peters et al., 2015), as described below, in five stages.
Stage 1: Identifying research questions. The following questions guided the scoping review: What are the definitions of the reproductive span? What are the trends in the reproductive span? What are the determinants of the reproductive span? What are the effects of the reproductive span on population demography? What are the effects of the reproductive span on fertility services?
Stage 2: Identifying relevant studies. We conducted a systematic search to identify both published and unpublished sources relevant to the concept of women’s reproductive span.
As a first step, an initial limited search of one bibliographic database was performed. We analyzed the text words contained in the titles, abstracts and index terms in the retrieved articles. In the second step, all identified text words and index terms were used to develop the search strategy by an experienced author [A.F.N.]. The search strategy was further refined through team discussion. The strategy for searching bibliographic databases included the following terms ‘menopause/statistics and numerical data’ [MeSH Terms] OR ‘menarche/statistics and numerical data’ [MeSH Terms] OR ‘age at menarche’ [Text Word] OR ‘age at menopause’ [Text Word] OR ‘age at natural menopause’ [Text Word] OR ‘reproductive span’ [Text Word]. The search strategy for different databases can be found in Supplementary Data. We searched MEDLINE, PubMed, JSTOR, CINAHL, Web of Science and Scopus electronic databases from inception to January 2021. The search was updated in December 2021. We also searched the Fertility Estimates 1950–2019 and Population Estimates 1950–2019 of the Global Burden of Disease Study 2019, Organization for Economic Co-operation and Development Database, Demographic and Health Surveys data sets and the National Health and Nutrition Examination Survey data sets. We did not impose any language or date restriction. In the third step, for all relevant articles, we hand-searched the list of references and explored the cited-by logs.
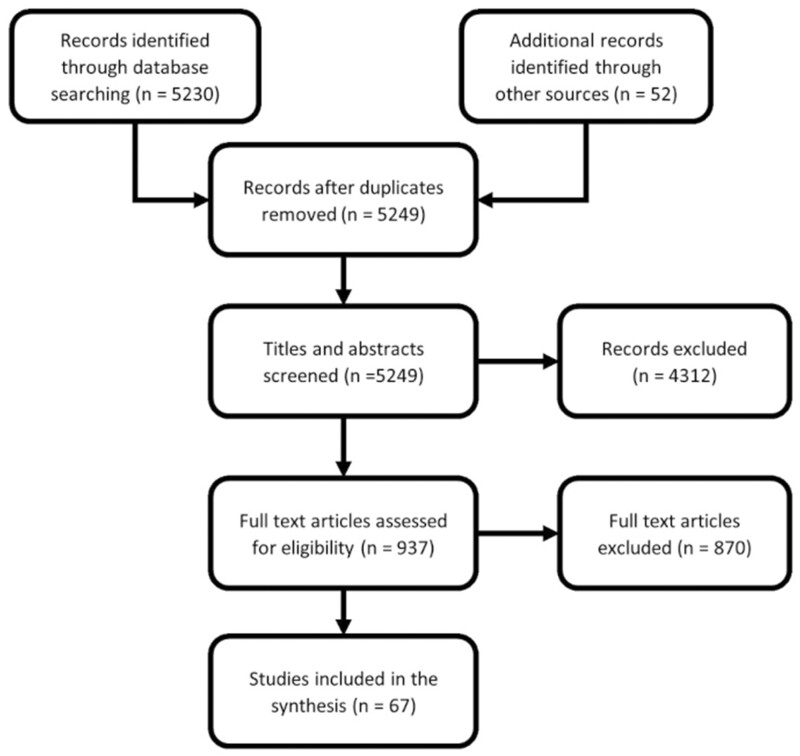
Stage 3: Study selection. Inclusion criteria were studies that reported on women’s (population) reproductive span (concept) and from any country globally (context). All study designs were eligible. The titles and abstracts of the records identified by electronic search were independently screened by two authors. This was followed by reviewing the full text of potentially relevant articles. If an agreement for inclusion could not be reached between the two authors, an opinion was requested from a third author. Figure 1 shows the process of study selection.
Stage 4: Data charting process. A data extraction form was developed a priori to capture relevant data from included studies. It was piloted and refined based on feedback from the team during regular meetings. The team regularly discussed the data and continuously updated the data-charting form in an iterative process. Two authors independently extracted the following data items: report data (title (TI), publication date (DP), first author (FAU), language (LA), publication type (PT), article identifier (AID)), methodological data (research design, participants, sample if applicable, study period, countries), definitions of reproductive span, data used for estimating the reproductive span, temporal trends and implications on population demographics and assisted reproduction. We did not plan to perform a formal critical appraisal of studies for this scoping review.
Stage 5: Collating and summarizing results. We performed conceptual synthesis by grouping the studies by concepts and then summarized definitions, measures used, temporal trends, determinants and broad findings of implications on population demographics and assisted reproduction. Structured tabulation and graphical synthesis were used to show patterns in the data and convey detailed information efficiently along with a narrative commentary.
Figure 1.
PRISMA flowchart. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses, Extension for Scoping Reviews.
The review was reported in accordance with the reporting guidance provided in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses, Extension for Scoping Reviews (PRISMA-ScR) (Tricco et al., 2018).
R software v4 was used for text mining, data wrangling and data visualization (R Core Team, 2020).
Results
Literature search results
The electronic search yielded 5230 records and an additional 52 records from hand searches. Screening titles and abstracts identified 937 potentially relevant records. These potentially relevant full-length articles were assessed, and 67 sources were included in this scoping review as depicted in the PRISMA flowchart (Fig. 1). We further explored two data sets (‘UK Biobank,’ 2021; ‘Centers for Disease Control and Prevention (CDC).’ n.d.) and one dissertation (Mulder, 1987) for additional data related to the included publications. Reports were excluded if they did not contain data on women’s reproductive span.
Mapping of research findings
Study design
The literature included studies with different methodologies. The majority (42/67; 62.69%) used a cross-sectional study design (Table I). The publication date of the included studies extended from 1980 to 2020.
Table I.
Different methodologies used in the literature on women’s reproductive span.
| Design | Count |
|---|---|
| Case–control | 4 |
| Cohort | |
| Ambidirectional | 1 |
| Prospective | 3 |
| Retrospective | 6 |
| Cross-sectional | 42 |
| Reviews | |
| Meta-analysis | 3 |
| Narrative Review | 5 |
| Systematic Review | 1 |
| Systematic review and meta-analysis | 2 |
Participants
The extent of the literature on women’s reproductive span encompassed participants across all races, ethnic groups, ancestries, religions, socioeconomic status, residence, marital status, educational levels and occupations. The age of participants ranged from 3 to 89 years, with birth cohorts and women born as early as 1900.
Context
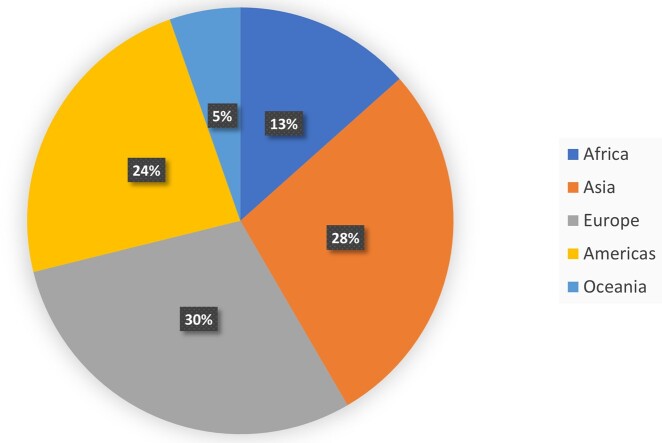
All continents contributed data to the literature on women’s reproductive span with 44 data sets from Europe, 42 from Asia, 35 from Americas, 20 from Africa and 8 from Oceania (Table II), (Figure 2). Data were available from 74 countries. USA, India and China contributed the largest number of studies on women’s reproductive span.
Table II.
Regions and sub-regions contributing to the literature on women’s reproductive span.
| Region | Sub-region | Data sets |
|---|---|---|
| Africa | Northern Africa | 5 |
| Sub-Saharan Africa | 15 | |
| Americas | Latin America and the Caribbean | 16 |
| Northern America | 19 | |
| Asia | Eastern Asia | 16 |
| South-eastern Asia | 7 | |
| Southern Asia | 13 | |
| Western Asia | 6 | |
| Europe | Eastern Europe | 6 |
| Northern Europe | 18 | |
| Southern Europe | 8 | |
| Western Europe | 12 | |
| Oceania | Australia and New Zealand | 5 |
| Melanesia | 2 | |
| Polynesia | 1 |
Figure 2.
Available literature identified by this scoping review on women’s reproductive span, shown as percentage of available datasets from each continent.
Concept
Conceptual synthesis of reproductive span included biological, effective and social (Table III).
Table III.
Mapping different concepts and working definitions used in the literature on women’s reproductive span.
Definitions and measures of reproductive span
Biological reproductive span
Studies used different terms for the ‘biological reproductive span’ (Beall, 1983; Menken, 1987; Padmadas et al., 2004; Barlow, 2011), including ‘reproductive period’ (Riener et al., 2004; Liu et al., 2010; Cerne et al., 2011; Yunus et al., 2014; Bjelland et al., 2018), ‘menstruation span’ (Chen et al., 2010), ‘reproductive years’ (Nichols et al., 2006; Dorjgochoo et al., 2008; Forman et al., 2013), ‘fertile span’ (Goodman et al., 1985), ‘total fertility span’ (Kapoor and Kapoor, 1986), ‘years of menstruation’ (Long et al., 2006), ‘reproductive life’ (Morabia et al., 1996; Morabia and Costanza, 1998), ‘potential span’ (Singh and Ahuja, 1980; Padmadas et al., 2004; Singh et al., 2020), ‘span of fertility’ (Shi et al., 2016), ‘natural reproductive period’ (Thomas et al., 2001; Sinha et al., 2021), ‘fertile period’ (Tea et al., 2013), ‘total years of fertility’ (Zerbetto et al., 2008) and ‘menstrual life’ (Singh and Ahuja, 1980).
The biological reproductive span broadly constitutes the interval between age at menarche and age at menopause (Singh and Ahuja, 1980; Beall, 1983; Goodman et al., 1985; Kapoor and Kapoor, 1986; Menken, 1987; Wood and Weinstein, 1988; Thomas et al., 2001; Padmadas et al., 2004; Riener et al., 2004; Aydos et al., 2005; Kalichman et al., 2007; Dorjgochoo et al., 2008; Liu et al., 2010; Lu et al., 2010; Cerne et al., 2011; Fukuda et al., 2011; Forman et al., 2013; Tea et al., 2013; Pyun et al., 2014; Duarte et al., 2017; Bjelland et al., 2018; Shaw et al., 2018; Demakakos et al., 2019; Gottschalk et al., 2020; Singh et al., 2020; Sinha et al., 2021). The end of the biological span might be age at natural menopause (Pavia et al., 1994; Morabia et al., 1996; Morabia and Costanza, 1998; Johnston, 2001; Hefler et al., 2002; Worda et al., 2004; Bartmann et al., 2005; Long et al., 2006; Nichols et al., 2006; He et al., 2007, 2009b; Kalichman et al., 2007; Kevenaar et al., 2007; Dorjgochoo et al., 2008; Mitchell et al., 2008; Zerbetto et al., 2008; Hartge, 2009; He et al., 2009a, 2010; Chen et al., 2010; Liu et al., 2010; Barlow, 2011; Cerne et al., 2011; Fukuda et al., 2011; Chen et al., 2012; Carty et al., 2013; Lewington et al., 2014; Pyun et al., 2014; Yunus et al., 2014; Duan et al., 2015; Ruth et al., 2016; Shi et al., 2016; Mishra et al., 2017; Bjelland et al., 2018; Fernández-Rhodes et al., 2018; Huang et al., 2018; Sharma and Bansal, 2018; Demakakos et al., 2019; InterLACE Study Team, 2019; Gottschalk et al., 2020; Sinha et al., 2021) or surgically-, hormonally-, chemotherapy- or radiation-induced menopause (Chow et al., 1997; Snieder et al., 1998; Nichols et al., 2006; Barlow, 2011; Chen et al., 2012; Carty et al., 2013; Bjelland et al., 2018).
Social reproductive span
The social reproductive span is the period of exposure to sexual activity, defined as the duration between marriage or entry into a union in which sexual relations take place regularly and final marriage dissolution or permanent abstinence (Menken, 1987; Wood and Weinstein, 1988; Padmadas et al., 2004; Singh et al., 2020). While marriage dissolution entails separation of a couple or widowhood, permanent abstinence may be culturally dictated (Menken, 1987). In some cultures, the social reproductive span starts when both partners co-habit (approximately a year after marriage) and ends at widowhood, as there is no divorce once the first child is born (Wood et al., 1985).
Effective reproductive span
The effective or behavioral (Singh et al., 2020) reproductive span, during which a woman is both fertile and engaging in sexual activity, represents the overlap of the biological and social reproductive spans (Menken, 1987).
Effective span extends from the age at marriage or entry into a union in which sexual relations take place regularly to the age at menopause (Padmadas et al., 2004; Singh and Singh, 2014; Singh et al., 2020), from marriage until sterilization (Wood et al., 1985; Padmadas et al., 2004; Murthy, 2012a,b; Singh and Singh, 2014; Singh et al., 2020), whether sterilization of either partner (Padmadas et al., 2004; Murthy, 2012a,b; Singh et al., 2020) or sterilization of the woman (Singh and Singh, 2014).
Other definitions included the years from the first marriage or menarche, whichever occurs last, to menopause or marriage dissolution, whichever occurs first (Menken, 1987), from marriage to last birth (Singh et al., 2020) or from first birth to last birth (Horne, 1989; Stevenson et al., 1989; Singh et al., 2020). One study derived the effective reproductive span by two methods both having age at last livebirth as the endpoint, while the start point was either the age at marriage or an estimated age at menarche (Mulder, 1989).
Temporal trends in women’s reproductive span
Data from recent datasets indicate that the duration of the biological reproductive span, worldwide, ranges from 30.94 to 39.30 years, with a mean (SD) of 35.85 (2.02) years.
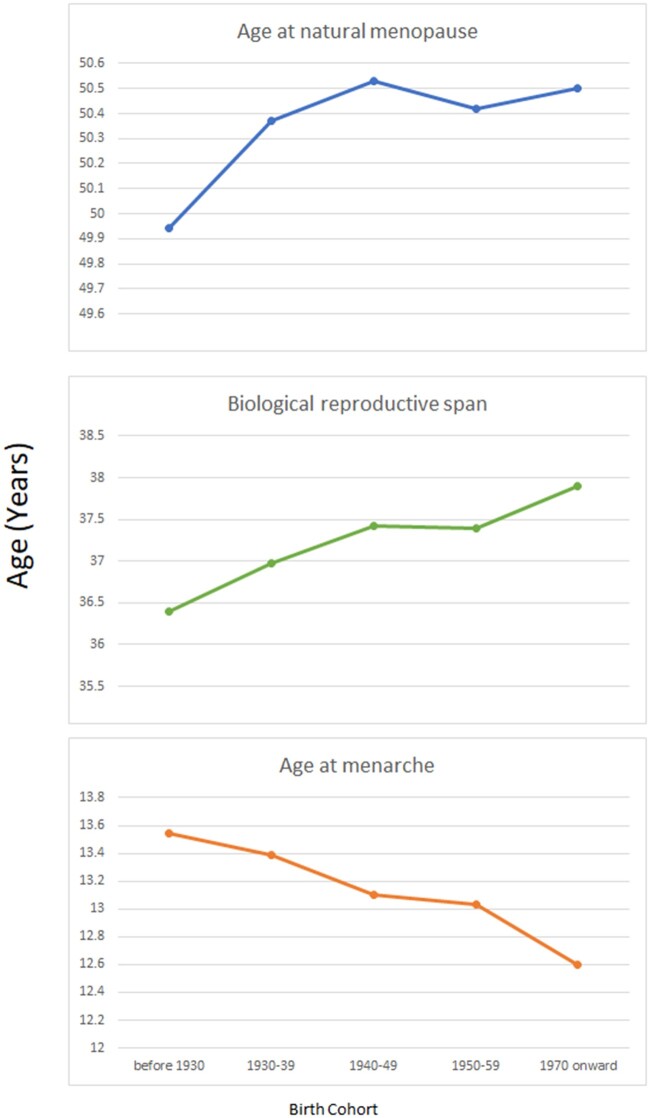
Data from 23 studies across 10 countries (Australia, Demark, Sweden, Norway, UK, USA, Japan, Lebanon, Spain and Morocco) contributed to the estimates of age at menarche, age at first birth and age at menopause in women born between 1900 and 1984 (InterLACE Study Team, 2019) (Table IV). The mean age at menarche declined steadily from women born before 1930 to those born after 1970 (13.5 versus 12.6 years), the age at menopause remained steady with no significant change, the age at first birth, however, showed an initial decline from 1900 to 1949 (27.2 versus 24.8 years) followed by a progressive rise to 27.3 years for women born after 1970. The mean values for biological span increased from 36.4 to 37.9 years in women born before 1930 and those born after 1970, respectively. The mean values for effective span followed a trend, with an initial increase for women born between 1900 and 1949 (22.69 versus 25.25) followed by a decline for women born in 1970 onward (mean 23.12 years) (InterLACE Study Team, 2019) (Fig. 3).
Table IV.
Temporal trend of women’s reproductive span: pooled data from 10 countries.
| Birth cohort | Age at menarche | Age at natural menopause | Biological span | Age at first birth | Effective Span |
|---|---|---|---|---|---|
| Before 1930 | 13.54 | 49.94 | 36.40 | 27.25 | 22.69 |
| 1930–1939 | 13.39 | 50.37 | 36.98 | 26.26 | 24.11 |
| 1940–1949 | 13.10 | 50.53 | 37.43 | 25.25 | 25.28 |
| 1950–1959 | 13.03 | 50.42 | 37.39 | 25.81 | 24.61 |
| 1970 onward | 12.60 | 50.50 | 37.90 | 27.38 | 23.12 |
All data are in years.
Figure 3.
Temporal trend of women’s biological reproductive span: pooled data from 23 studies across 10 countries. Data points are mean values. The 10 countries are Australia, Demark, Sweden, Norway, UK, USA, Japan, Lebanon, Spain and Morocco.
China: data included 45 birth cohorts (born before 1930 to after 1970) in socially diverse urban and rural regions of China. The mean increased from 47.9 to 49.3 years. Mean age at menarche decreased steadily from 16.1 to 14.3 years. The biological reproductive span showed an increasing trend from 31.8 to 35 years (Lewington et al., 2014).
Norway: data included women born in Norway during the years 1936–1964. The mean age at menarche decreased from 13.42 years among women born during 1936–1939 to 13.24 years among women born during 1960–1964. The mean age at menopause increased from 50.31 years among women born during 1936–1939 to 52.73 years among women born during 1960–1964. The mean biological reproductive span increased from 36.83 years to 40.22 years (Gottschalk et al., 2020).
Russia: in a rural population, the mean values of age at menopause increased from 47.0 years (women born 1920–1925) to 49.7 years (women born 1940–1945) and 49.3 years (women born 1945–1950). Mean values of the biological reproductive span increased from 30.7 (women born 1920–1925) to 34.1 (women born during 1940–1945) and then slightly decreased to 33.7 years (women born 1945–1950) (Kalichman et al., 2007).
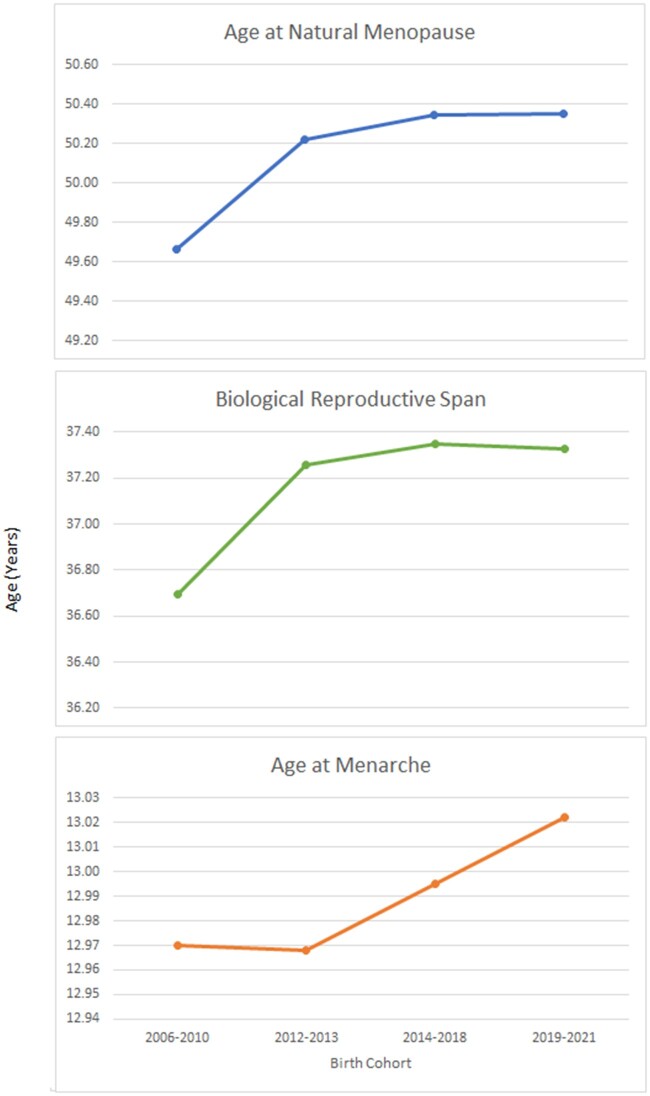
UK: for this review, we extracted available data from the UK Biobank (‘UK Biobank,’ 2021) from 2006 to 2019 (Table V). The biological and effective reproductive span remained stable from 2006 to 2019 onward (Fig. 4).
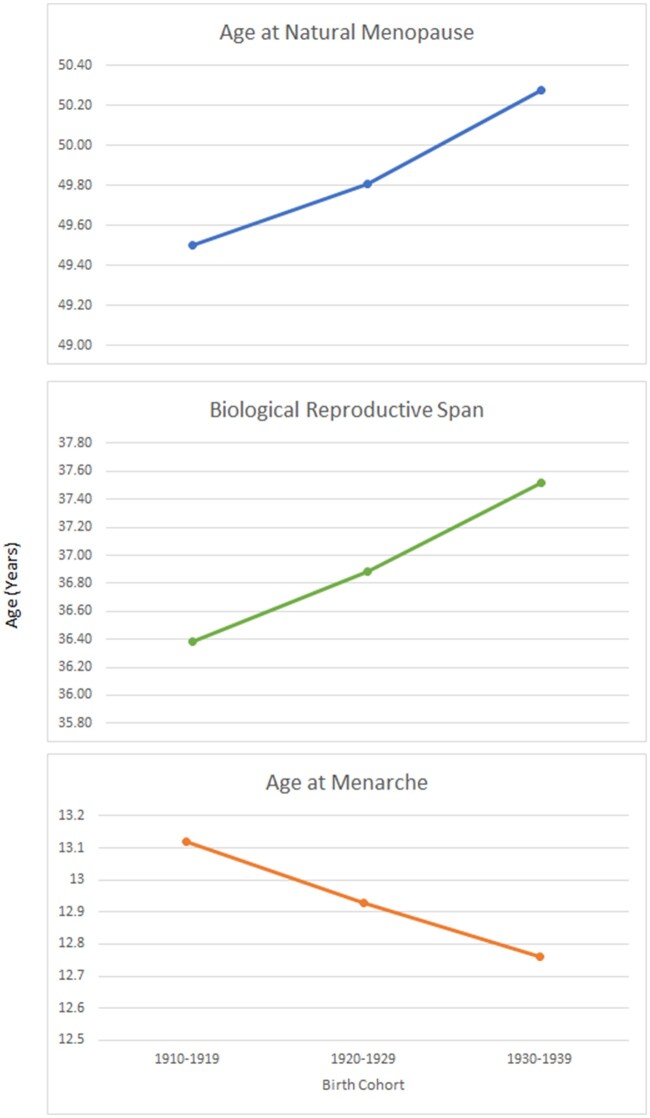
USA: data collected between 1988 and 2001 included women born between 1910 and 1969. Birth cohorts were created using 5- and 10-year periods. The mean age at menarche decreased for those born between 1910 and 1939 (13.12 versus 12.76 years), with a subsequent increase to 13.0 years among women born between 1960 and 1969. Among naturally menopausal women aged 60 or more years, there was an increase in the mean age at menopause for those born between 1910 and 1939 (49.51 versus 50.28 years). Mean values of the biological reproductive span (subtracting age at menarche from age at menopause), increased from 36.4 years among women born between 1910 and 1919 to 37.5 years among the 1930–1939 cohort (Nichols et al., 2006) (Table VI, Fig. 5).
India: the effective reproductive spans, defined as the time between age at marriage and age at sterilization, of successive cohorts of women decreased from 22 years among those who married during the 1960s to 15 years among those who married in the 1970s, to 10 years among those who married in the 1980s and 5 years among those who married in 1990–1996 (Padmadas et al., 2004; Murthy, 2012a).
Table V.
Temporal trend of women’s reproductive span: UK data.
| Year | Menarche | Menopause | Biological span |
|---|---|---|---|
| 2006–2010 | 12.9698 | 49.6646 | 36.6948 |
| 2012–2013 | 12.9681 | 50.2232 | 37.2551 |
| 2014–2018 | 12.9953 | 50.3452 | 37.3499 |
| 2019–2021 | 13.0222 | 50.3512 | 37.3290 |
All data are in years.
Figure 4.
Temporal trend of women’s biological reproductive span: UK data. Data points are mean values.
Table VI.
Temporal trend of women’s reproductive span: USA data.
| Year | Menarche | Menopause | Biological span |
|---|---|---|---|
| 1910–1919 | 13.12 | 49.505 | 36.385 |
| 1920–1929 | 12.93 | 49.810 | 36.880 |
| 1930–1939 | 12.76 | 50.280 | 37.520 |
All data are in years.
Figure 5.
Temporal trend of women’s biological reproductive span: US data. Data points are mean values.
Determinants of women’s reproductive span
A myriad of factors has been investigated as determinants of women’s reproductive span (Table VII). A word cloud depicts the determinants of women’s reproductive span (Fig. 6).
Table VII.
Mapping potential determinants of women’s reproductive span.
AAM, age at menarche; ANM, age at natural menopause; DDT, dichlorodiphenyltrichloroethane; SES, socio-economic status; DES, diethylstilboestrol; SNP, single nucleotide polymorphisms.
Figure 6.

Word cloud of determinants of women's reproductive span. All factors shown in the image have been assessed or found to be determinants of women’s reproductive span.
Biological reproductive span
Hereditary factors
Twenty-two studies analyzed genotypic determinants of biological reproductive span. Several genes and intergenic single nucleotide polymorphisms were associated with biological span through age at menarche, age at menopause or both (Table VII).
One study found an association between telomere length and the length of biological span (Aydos et al., 2005).
Other studies investigated the association between biological span and zygosity (Snieder et al., 1998), handedness (Pavia et al., 1994) and mothers of trisomy babies (Bartmann et al., 2005).
Ethnicity and racial factors
Japanese women probably have a longer biological reproductive span than Caucasians (InterLACE Study Team, 2019). Gainj women may have a short biological reproductive span (Wood et al., 1985), while Agta Negritos (in the Philippines) may have a longer biological reproductive span than the Dobe Kung (hunter-gatherer women of the Kalahari Desert in Africa) despite a later age at menarche (Goodman et al., 1985). age at menarche was reported to be earlier among the US Black race (Menken, 1987). Asian and African countries have increased age at menarche compared to western countries (Morabia and Costanza, 1998).
Environmental factors
Season of birth was not associated with span in one study (Si et al., 2017), while the effect of year of birth varied among studies (Kalichman et al., 2007; Duarte et al., 2017; InterLACE Study Team, 2019).
Changes in body mass index (BMI) were associated with a change in the duration of span in eight studies (Hefler et al., 2002; Riener et al., 2004; Worda et al., 2004; Nichols et al., 2006; Dorjgochoo et al., 2008; Forman et al., 2013; Bjelland et al., 2018; Sinha et al., 2021), while four studies reported no association(Johnston, 2001; He et al., 2007; Kalichman et al., 2007; Cerne et al., 2011). Age at menopause and hence the biological reproductive span was neither associated with skin-fold thickness (Johnston, 2001) nor a woman’s height (Johnston, 2001; He et al., 2007). Psychosocial stress decreases both age at menarche and age at menopause (Forman et al., 2013).
Arsenic exposure was associated with a decrease in biological reproductive span by increasing age at menarche and decreasing age at menopause (Yunus et al., 2014) and higher urinary levels of some types of polycyclic aromatic hydrocarbons is associated with earlier age at menopause (Huang et al., 2018).
The association between the age at menarche and age at menopause was inconsistent (Snieder et al., 1998; He et al., 2007; Kalichman et al., 2007; Dorjgochoo et al., 2008; Chen et al., 2010; Liu et al., 2010; Mishra et al., 2017).
Studies reported inconsistent associations between the duration of biological span and breastfeeding (Johnston, 2001; Long et al., 2006; Dorjgochoo et al., 2008; Liu et al., 2010; Cerne et al., 2011; Forman et al., 2013; Sinha et al., 2021), parity (Johnston, 2001; Thomas et al., 2001; Long et al., 2006; Nichols et al., 2006; Kalichman et al., 2007; Dorjgochoo et al., 2008; Chen et al., 2010; Mishra et al., 2017; Sinha et al., 2021), marital status (Johnston, 2001; Dorjgochoo et al., 2008; Sinha et al., 2021), the age at first birth (Johnston, 2001; Thomas et al., 2001; Dorjgochoo et al., 2008; Sharma and Bansal, 2018), gravidity (Worda et al., 2004; Liu et al., 2010), weight gain in pregnancy (Forman et al., 2013), birthweight (Forman et al., 2013) and the use of contraceptive methods including oral contraceptives and intrauterine device (Johnston, 2001; Long et al., 2006; Dorjgochoo et al., 2008; Liu et al., 2010).
A study suggested that a longer interval between age at menarche and first livebirth may be associated with an increased biological span and that menstrual irregularities maybe associated with changes in biological reproductive span (Dorjgochoo et al., 2008).
The age at last birth (Dorjgochoo et al., 2008; Sharma and Bansal, 2018) and age at first and last pregnancies (Sinha et al., 2021) might be associated with changes in biological reproductive span.
Neither abortions (Long et al., 2006; Kalichman et al., 2007; Dorjgochoo et al., 2008) nor stillbirths (Dorjgochoo et al., 2008) showed an association with biological reproductive span.
Several studies reported an association between smoking and biological reproductive span (Hefler et al., 2002; Worda et al., 2004; Long et al., 2006; Nichols et al., 2006; Dorjgochoo et al., 2008; Liu et al., 2010; Cerne et al., 2011; Fukuda et al., 2011; Forman et al., 2013; Bjelland et al., 2018; Sinha et al., 2021). Most of these studies reported that smoking decreases biological reproductive span (Hefler et al., 2002; Worda et al., 2004; Long et al., 2006; Nichols et al., 2006; Dorjgochoo et al., 2008; Cerne et al., 2011; Fukuda et al., 2011; Forman et al., 2013; Bjelland et al., 2018). Both in utero exposure to smoking and paternal periconceptional smoking were associated with earlier age at menopause in offspring who were not actively smoking (Fukuda et al., 2011; Forman et al., 2013).
Three studies reported no association between alcohol and biological reproductive span (Long et al., 2006; Dorjgochoo et al., 2008; Cerne et al., 2011).
Diethylstilboestrol exposure in utero decreases both age at menarche and age at menopause, as reported by one study (Forman et al., 2013).
Physical exercise showed a variable association with biological reproductive span. Two studies showed a longer span by increasing age at menopause (Long et al., 2006; Dorjgochoo et al., 2008), while vigorous exercise might shorten the span by delaying age at menarche (Menken, 1987).
Increased total intake of calories, fruits, protein and long-term tea consumption were associated with increased biological reproductive span, while an increased intake of vegetables, soy, fiber, red meat, carbohydrates and fats was probably not associated with changes in biological reproductive span (Dorjgochoo et al., 2008).
Data are inconsistent for the association between low socioeconomic status and biological span (Menken, 1987; Forman et al., 2013). Improved living conditions (increased vegetable intake, decreased illiteracy and decreased child labor) decrease age at menarche, thus increasing biological span (Thomas et al., 2001). Three studies found that higher family income increases biological reproductive span (Johnston, 2001; Long et al., 2006; Dorjgochoo et al., 2008). Current employment was described to have a positive correlation with biological reproductive span (Johnston, 2001).
Two studies report parenting as a determinant of span. One study (Demakakos et al., 2019) found that maternal care, paternal care and maternal over protection are not associated with span, while paternal over protection decreases span. Another study (Forman et al., 2013) reported that paternal absence is associated with early age at menarche.
Higher education might extend the biological reproductive span (Long et al., 2006; Nichols et al., 2006; Dorjgochoo et al., 2008; Lewington et al., 2014; InterLACE Study Team, 2019).
Urban residence might be associated with a longer biological reproductive span (Lewington et al., 2014), while another study found no association (Duarte et al., 2017). Living in high altitude was also investigated in a few studies (Beall, 1983; Kapoor and Kapoor, 1986; Shaw et al., 2018).
Effective reproductive span h4
Higher educational level (Horne, 1989; Padmadas et al., 2004; Murthy, 2012a,b; Singh and Singh, 2014), increased age at menarche (Wood et al., 1985; Mulder, 1989), younger women (Padmadas et al., 2004; Murthy, 2012b), experiencing pre-marital hardships (Singh and Singh, 2014), lack of interspousal communication about family planning (Padmadas et al., 2004), offspring sex composition (Padmadas et al., 2004), sterilization (Menken, 1987) and marital dissolution without remarriage (Horne, 1989) were found to decrease effective reproductive span.
Child deaths (Padmadas et al., 2004; Murthy, 2012a), fetal loss (Padmadas et al., 2004), termination of pregnancy (Murthy, 2012a,b), increased age at last livebirth (Horne, 1989; Mulder, 1989), the use of contraceptives (Padmadas et al., 2004; Singh and Singh, 2014) and marital dissolution with remarriage (Horne, 1989) were found to increase effective reproductive span.
The level of a partner’s education (Murthy, 2012a,b) and household structure (nuclear versus non-nuclear families) (Murthy, 2012a) were reported as not associated with effective reproductive span.
The effect of increased age at first marriage was variable among studies. Three studies (Menken, 1987; Horne, 1989; Singh and Singh, 2014) reported that it decreases the effective reproductive span, while one study (Murthy, 2012a) reported the contrary.
Three studies reported that urban residence decreases effective reproductive span (Horne, 1989; Padmadas et al., 2004; Murthy, 2012b), while only one study (Murthy, 2012a) found no association.
Employment (Murthy, 2012a,b) and parity (Horne, 1989; Murthy, 2012a) were also reported to have variable effects on effective reproductive span.
Cultural patterns (Menken, 1987), birth interval (Padmadas et al., 2004; Murthy, 2012a), ideal number and sex of offspring (Murthy, 2012a) and wealth (Mulder, 1989; Murthy, 2012a) are all associated with changes in effective reproductive span.
Concerning ethnicity, Kipsigis (tribe in Kenya) were reported to be associated with a shorter effective reproductive span than Netherlands and US samples, and a comparable effective reproductive span with non-industrialized countries (Mulder, 1989).
Muslims and Christians, compared to Hindus, had a shorter effective reproductive span because of accepting sterilization at a younger age than Hindus (Padmadas et al., 2004; Murthy, 2012a).
In China, the effective reproductive span decreased because of population policies (Lewington et al., 2014).
Social reproductive span
We found no studies reporting the determinants of social reproductive span.
Effects on population demography
Twelve studies reported the effect of reproductive span on demography (Wood et al., 1985; Menken, 1987; Stevenson et al., 1989; Padmadas et al., 2004; Kalichman et al., 2007; Hartge, 2009; Murthy, 2012b; Lewington et al., 2014; Singh and Singh, 2014; Shaw et al., 2018; Gottschalk et al., 2020; Singh et al., 2020). These included six studies of biological reproductive span (Wood et al., 1985; Kalichman et al., 2007; Hartge, 2009; Lewington et al., 2014; Shaw et al., 2018; Gottschalk et al., 2020), five studies of effective reproductive span (Menken, 1987; Padmadas et al., 2004; Murthy, 2012b; Singh and Singh, 2014; Singh et al., 2020) and one study of social reproductive span (Wood et al., 1985).
Two studies reported that the increase in biological span had no effect on the number of births (Kalichman et al., 2007; Gottschalk et al., 2020). In China, an increase in biological span between 1930 and the end of the 20th century occurred, while during a similar period, parity decreased (Lewington et al., 2014).
A systematic review showed that women living at high altitude, compared to those living at low altitude, have a delayed age at menarche and a shorter biological span and this was associated with a lower total fertility (Shaw et al., 2018).
Differences among populations in patterns and dissolution of marriage were associated with changes in total fertility rate. Women with decreased effective reproductive span had a lower fertility rate (Menken, 1987). Four studies reported the impact of effective reproductive span on fertility rate in India (Padmadas et al., 2004; Murthy, 2012b; Singh and Singh, 2014; Singh et al., 2020). The effective reproductive span has decreased in India owing to the rise in legal age of marriage in 1978 and acceptance of earlier sterilization as a method of permanent contraception (Padmadas et al., 2004; Singh et al., 2020). During the same period, fertility rate dropped (Singh and Singh, 2014).
Effects on fertility services
The available literature lacks primary data examining the impact of reproductive span on the need or utilization of fertility services, including medically assisted reproduction. One narrative review suggested, based on data from the Human Fertilization and Embryology Authority of the UK, that the trend of women being interested in postponing pregnancy to a later age is consistent with the average age of women undergoing IVF or donor insemination in the UK (Barlow, 2011). The narrative review enumerated different approaches that might help to extend the reproductive span, including ovarian tissue cryopreservation and transplantation, oocyte cryopreservation, oocyte donation, embryo cryopreservation, surgical ovarian transposition and suppression of ovarian activity during cancer treatment, modulation of the primordial follicle–primary follicle transition and the possible use of adult somatic cells in the generation of artificial gametes for reproductive use (Barlow, 2011).
Discussion
This systematic scoping review is the first and most comprehensive attempt to map the extent of research regarding women’s reproductive span. On its own, the review will serve to inform readers on the extent and nature of existing literature in this area, as well as the working definitions, determinants, trends, impact on demographics and assisted reproduction. We identified 67 relevant reports, spanning 120 years, and involving women from 74 countries. We grouped the reproductive span into three concepts, namely biological, social and effective. We summarized key milestones in a woman’s reproductive span which mark the changing life stages. Knowing the typical ages at such events contributes to understanding the changes in family and population. It also helps inform the needs for assisted and other reproductive health services. The review revealed wide variation among reports in the definitions of the start and end of both the biological and the effective reproductive span concepts.
While the extent of the literature on the duration of biological span is sizable and shows minimal trend over decades, the scope of research on the effective reproductive span remains modest despite the considerable trend toward a shorter span.
Several factors have been investigated as determinants of reproductive span with substantial variations in the reported association with women’s reproductive span. This landscape of literature should be read with caution since most of the included literature is cross-sectional, therefore the direction of the association is unknown. Based on this map, rigorous research is warranted to find answers to several questions, for example:
What are the hypotheses that could be based on these associations?
What could be the underlying mechanisms of significant associations, if any?
There is insufficient literature on the effect of the current trends in reproductive span on population demographics or assisted reproductive services.
This review has several strengths. These include the extensive search including searching for gray literature. A major challenge that we anticipated as part of this scoping review was that a proportion of the evidence may not be in the bibliographic databases of peer-reviewed journals. For this reason, we also searched the gray and non-bibliographic sources. However, it remains a probability that we may not have captured all relevant sources. Further strengths include adherence to rigorous methods of scoping reviews and the broad inclusion criteria of eligible reports, without restriction by study type, publication status, date or language.
The review has some limitations. A formal assessment of methodological quality of the included studies was not performed because the aim of this review was to provide an overview of the existing evidence base regardless of quality (Peters et al., 2015). Also, the review process did not include a thematic analysis. While we understand the importance of producing a quantitative summary of the association between various determinants and reproductive span, this was neither our aim nor in our planned scoping review methods. Although a comprehensive search was made for existing literature regardless of date, language and peer review status, it is possible that some data were not captured.
This scoping review produced a comprehensive map of the existing literature on women’s reproductive spans. The findings open a window of opportunity to construct clear definitions, generate hypotheses and conduct suitable study designs regarding the determinants of women’s reproductive span, to understand the underlying mechanisms of associations. The wide array of determinants summarized in this scoping review can provide a building block for further research to better understand which of these play a role in the temporal trends of either the biological or the effective reproductive span.
Supplementary data
Supplementary data are available at Human Reproduction Open online.
Data availability
All data generated or analyzed during this study are included in the published scoping review article and is available upon request from the corresponding author.
Authors’ roles
A.F.N., G.M. and J.K. conceived the idea for this review. A.F.N. designed the scoping review methods. AF.N., F.E., R.M., M.K., M.E., Y.G.A., Mo.G., M.H.A., M.G., P.E., A.M., M.N., N.A., F.E., A.A.H., N.E., E.S., Y.T., Y.D., N.F., A.A., Y.S. and M.D. collaborated in searching, screening and selecting studies. F.E., R.M., M.K., M.E., Y.G.A., Mo.G., M.H.A., M.G., P.E., A.M., M.N., N.A., F.E., A.A.H., N.E., E.S., Y.T., Y.D., N.F., A.A., M.F.G., M.M., Y.S. and M.D. collaborated in data extraction and synthesis. A.F.N., Y.G.A., F.E., R.M. and M.K. collaborated in writing the first draft of the manuscript. All authors critically reviewed the manuscript resulting in a revision of several drafts. All authors read and approved the final version of the manuscript. G.M and J.K are staff members of the World Health Organization. Views expressed in this manuscript are their own; they do not necessarily represent the views, decisions or policies of the World Health Organization.
Funding
This work received funding from the UNDP-UNFPA-UNICEF-WHO-World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), a cosponsored programme executed by the World Health Organization (WHO). Grant number 2020/1073913-0.
Conflict of interest
The authors have no competing interests.
Supplementary Material
Contributor Information
A F Nabhan, Department of Obstetrics and Gynecology, Faculty of Medicine, Ain Shams University, Cairo, Egypt.
G Mburu, The UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP Research), World Health Organization, Geneva, Switzerland.
F Elshafeey, Egyptian Center for Evidence Based Medicine, Cairo, Egypt.
R Magdi, Egyptian Center for Evidence Based Medicine, Cairo, Egypt.
M Kamel, Egyptian Center for Evidence Based Medicine, Cairo, Egypt.
M Elshebiny, Egyptian Center for Evidence Based Medicine, Cairo, Egypt.
Y G Abuelnaga, Egyptian Center for Evidence Based Medicine, Cairo, Egypt.
M Ghonim, Egyptian Center for Evidence Based Medicine, Cairo, Egypt.
M H Abdelhamid, Egyptian Center for Evidence Based Medicine, Cairo, Egypt.
Mo Ghonim, Egyptian Center for Evidence Based Medicine, Cairo, Egypt.
P Eid, Egyptian Center for Evidence Based Medicine, Cairo, Egypt.
A Morsy, Egyptian Center for Evidence Based Medicine, Cairo, Egypt.
M Nasser, Egyptian Center for Evidence Based Medicine, Cairo, Egypt.
N Abdelwahab, Egyptian Center for Evidence Based Medicine, Cairo, Egypt.
F Elhayatmy, Egyptian Center for Evidence Based Medicine, Cairo, Egypt.
A A Hussein, Egyptian Center for Evidence Based Medicine, Cairo, Egypt.
N Elgabaly, Egyptian Center for Evidence Based Medicine, Cairo, Egypt.
E Sawires, Egyptian Center for Evidence Based Medicine, Cairo, Egypt.
Y Tarkhan, Egyptian Center for Evidence Based Medicine, Cairo, Egypt.
Y Doas, Egyptian Center for Evidence Based Medicine, Cairo, Egypt.
N Farrag, Egyptian Center for Evidence Based Medicine, Cairo, Egypt.
A Amir, Egyptian Center for Evidence Based Medicine, Cairo, Egypt.
M F Gobran, Egyptian Center for Evidence Based Medicine, Cairo, Egypt.
M Maged, Egyptian Center for Evidence Based Medicine, Cairo, Egypt.
M Abdulhady, Egyptian Center for Evidence Based Medicine, Cairo, Egypt.
Y Sherif, Egyptian Center for Evidence Based Medicine, Cairo, Egypt.
M Dyab, Egyptian Center for Evidence Based Medicine, Cairo, Egypt.
J Kiarie, The UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP Research), World Health Organization, Geneva, Switzerland.
References
- Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol 2005;8:19–32. [Google Scholar]
- Aydos SE, Elhan AH, Tükün A. Is telomere length one of the determinants of reproductive life span? Arch Gynecol Obstet 2005;272:113–116. [DOI] [PubMed] [Google Scholar]
- Barlow DH. Interventions in the prolongation of reproductive life in women. The prolongation of reproductive life in women. Ann N Y Acad Sci 2011;1221:1–9. [DOI] [PubMed] [Google Scholar]
- Bartmann AK, Araújo FM, Iannetta O, Paneto JCC, Martelli L, Ramos ES. Down syndrome and precocious menopause. J Assist Reprod Genet 2005;22:129–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall CM. Ages at menopause and menarche in a high-altitude Himalayan population. Ann Hum Biol 1983;10:365–370. [DOI] [PubMed] [Google Scholar]
- Bjelland EK, Hofvind S, Byberg L, Eskild A. The relation of age at menarche with age at natural menopause: a population study of 336 788 women in Norway. Human Reprod 2018;33:1149–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey JR, Roach DA. Biodemography: An Introduction to Concepts and Methods. Princeton University Press, 2020, 84–112. http://www.jstor.org/stable/j.ctvkjb4n8.9 Accessed on April 1, 2021 [Google Scholar]
- Carty CL, Spencer KL, Setiawan VW, Fernandez-Rhodes L, Malinowski J, Buyske S, Young A, Jorgensen NW, Cheng I, Carlson CS et al. Replication of genetic loci for ages at menarche and menopause in the multi-ethnic population architecture using genomics and epidemiology (PAGE) study. Hum Reprod 2013;28:1695–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. https://www.cdc.gov/nchs/nhanes/index.htm Accessed on April 1, 2021
- Cerne J-Z, Pohar-Perme M, Cerkovnik P, Gersak K, Novakovic S. Age at menarche and menopause is not associated with two common genetic variants in the methylenetetrahydrofolate reductase (MTHFR) gene. Eur J Contracept Reprod Health Care 2011;16:241–247. [DOI] [PubMed] [Google Scholar]
- Chen CTL, Fernández-Rhodes L, Brzyski RG, Carlson CS, Chen Z, Heiss G, North KE, Woods NF, Rajkovic A, Kooperberg C et al. Replication of loci influencing ages at menarche and menopause in Hispanic women: the women’s health initiative SHARE study. Hum Mol Genet 2012;21:1419–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Feng Y, Shu H, Lu T, Zhu H, Yang B, Xiong M. [Survey on menopausal age and menstruation span in women in Pudong district of Shanghai]. Zhonghua fu Chan Ke Za Zhi 2010;45:415–419. [PubMed] [Google Scholar]
- Chow SN, Huang CC, Lee YT. Demographic characteristics and medical aspects of menopausal women in Taiwan. J Formos Med Assoc 1997;96:806–811. [PubMed] [Google Scholar]
- Colquhoun HL, Levac D, O’Brien KK, Straus S, Tricco AC, Perrier L, Kastner M, Moher D. Scoping reviews: time for clarity in definition, methods, and reporting. J Clin Epidemiol 2014;67:1291–1294. [DOI] [PubMed] [Google Scholar]
- Demakakos P, Pashayan N, Chrousos G, Linara-Demakakou E, Mishra GD. Childhood experiences of parenting and age at menarche, age at menopause and duration of reproductive lifespan: evidence from the English longitudinal study of ageing. Maturitas 2019;122:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorjgochoo T, Kallianpur A, Gao Y-T, Cai H, Yang G, Li H, Zheng W, Shu XO. Dietary and lifestyle predictors of age at natural menopause and reproductive span in the Shanghai women’s health study. Menopause 2008;15:924–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan P, Wang Z-M, Liu J, Wang L-N, Yang Z, Tu P. Gene polymorphisms in RANKL/RANK/OPG pathway are associated with ages at menarche and natural menopause in Chinese women. BMC Womens Health 2015;15:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte E, de Sousa B, Cadarso-Suárez C, Klein N, Kneib T, Rodrigues V. Studying the relationship between a woman’s reproductive lifespan and age at menarche using a Bayesian multivariate structured additive distributional regression model. Biom J 2017;59:1232–1246. [DOI] [PubMed] [Google Scholar]
- Dunson DB, Baird DD, Colombo B. Increased infertility with age in men and women. Obstet Gynecol 2004;103:51–56. [DOI] [PubMed] [Google Scholar]
- Fernández-Rhodes L, Malinowski JR, Wang Y, Tao R, Pankratz N, Jeff JM, Yoneyama S, Carty CL, Setiawan VW, Le Marchand L et al. The genetic underpinnings of variation in ages at menarche and natural menopause among women from the multi-ethnic population architecture using genomics and epidemiology (PAGE) study: a trans-ethnic meta-analysis. PloS One 2018;13:e0200486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman MR, Mangini LD, Thelus-Jean R, Hayward MD. Life-course origins of the ages at menarche and menopause. Adolesc Health Med Ther 2013;4:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Fukuda K, Shimizu T, Nobunaga M, Andersen EW, Byskov AG, Andersen CY. Paternal smoking habits affect the reproductive life span of daughters. Fertil Steril 2011;95:2542–2544. [DOI] [PubMed] [Google Scholar]
- Goodman MJ, Estioko-Griffin A, Griffin PB, Grove JS. Menarche, pregnancy, birth spacing and menopause among the Agta women foragers of Cagayan province, Luzon, the Philippines. Ann Hum Biol 1985;12:169–177. [DOI] [PubMed] [Google Scholar]
- Gottschalk MS, Eskild A, Hofvind S, Gran JM, Bjelland EK. Temporal trends in age at menarche and age at menopause: a population study of 312 656 women in Norway. Hum Reprod 2020;35:464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartge P. Genetics of reproductive lifespan. Nat Genet 2009;41:637–638. [DOI] [PubMed] [Google Scholar]
- He C, Kraft P, Chasman DI, Buring JE, Chen C, Hankinson SE, Paré G, Chanock S, Ridker PM, Hunter DJ. A large-scale candidate gene association study of age at menarche and age at natural menopause. Hum Genet 2010;128:515–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Kraft P, Chen C, Buring JE, Paré G, Hankinson SE, Chanock SJ, Ridker PM, Hunter DJ, Chasman DI. Genome-wide association studies identify loci associated with age at menarche and age at natural menopause. Nat Genet 2009a;41:724–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L-N, Recker RR, Deng H-W, Dvornyk V. A polymorphism of apolipoprotein e (APOE) gene is associated with age at natural menopause in Caucasian females. Maturitas 2009b;62:37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L-N, Xiong D-H, Liu Y-J, Zhang F, Recker RR, Deng H-W. Association study of the oestrogen signaling pathway genes in relation to age at natural menopause. J Genet 2007;86:269–276. [DOI] [PubMed] [Google Scholar]
- Hefler LA, Worda C, Huber JC, Tempfer CB. A polymorphism of the Nos3 gene and age at natural menopause. Fertil Steril 2002;78:1184–1186. [DOI] [PubMed] [Google Scholar]
- Horne AD. The span of reproduction in Egypt. Soc Biol 1989;36:255–261. [DOI] [PubMed] [Google Scholar]
- Huang Y, Guo J, Lv N, Li S, Wu Y, Bai R, Shen J, Chen G, Zhang D. Associations of urinary polycyclic aromatic hydrocarbons with age at natural menopause in U.S. Women aged 35-65, NHANES 2003-2012. Environ Pollut 2018;243:1878–1886. [DOI] [PubMed] [Google Scholar]
- Inhorn MC, Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update 2015;21:411–426. [DOI] [PubMed] [Google Scholar]
- InterLACE Study Team. Variations in reproductive events across life: a pooled analysis of data from 505 147 women across 10 countries. Hum Reprod 2019;34:881–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston SL. Associations with age at natural menopause in Blackfeet women. Am J Hum Biol 2001;13:512–520. [DOI] [PubMed] [Google Scholar]
- Kalichman L, Malkin I, Kobyliansky E. Time-related trends of age at menopause and reproductive period of women in a Chuvashian rural population. Menopause 2007;14:135–140. [DOI] [PubMed] [Google Scholar]
- Kapoor AK, Kapoor S. The effects of high altitude on age at menarche and menopause. Int J Biometeorol 1986;30:21–26. [DOI] [PubMed] [Google Scholar]
- Kevenaar ME, Themmen APN, Rivadeneira F, Uitterlinden AG, Laven JSE, van Schoor NM, Lips P, Pols HAP, Visser JA. A polymorphism in the AMH type II receptor gene is associated with age at menopause in interaction with parity. Hum Reprod 2007;22:2382–2388. [DOI] [PubMed] [Google Scholar]
- Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implement Sci 2010;5:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewington S, Li L, Murugasen S, Hong L-S, Yang L, Guo Y, Bian Z, Collins R, Chen J, He H et al. ; China Kadoorie Biobank Study Oration. Temporal trends of main reproductive characteristics in ten urban and rural regions of China: the China Kadoorie biobank study of 300 000 women. Int J Epidemiol 2014;43:1252–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Lu Y, Recker RR, Deng H-W, Dvornyk V. Association analyses suggest multiple interaction effects of the methylenetetrahydrofolate reductase polymorphisms on timing of menarche and natural menopause in white women. Menopause 2010;17:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J-R, Shu X-O, Cai Q, Cai H, Gao Y-T, Jin F, Zheng W. Polymorphisms of the CYP1B1 gene may be associated with the onset of natural menopause in Chinese women. Maturitas 2006;55:238–246. [DOI] [PubMed] [Google Scholar]
- Lu Y, Liu P, Recker RR, Deng H-W, Dvornyk V. TNFRSF11A and TNFSF11 are associated with age at menarche and natural menopause in white women. Menopause 2010;17:1048–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menken J. Proximate determinants of fertility and mortality: a review of recent findings. Sociol Forum 1987;2:697–717. [Google Scholar]
- Mishra GD, Pandeya N, Dobson AJ, Chung H-F, Anderson D, Kuh D, Sandin S, Giles GG, Bruinsma F, Hayashi K et al. Early menarche, nulliparity and the risk for premature and early natural menopause. Hum Reprod 2017;32:679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell ES, Farin FM, Stapleton PL, Tsai JM, Tao EY, Smith-DiJulio K, Woods NF. Association of estrogen-related polymorphisms with age at menarche, age at final menstrual period, and stages of the menopausal transition. Menopause 2008;15:105–111. [DOI] [PubMed] [Google Scholar]
- Morabia A, Costanza MC. International variability in ages at menarche, first livebirth, and menopause. World health organization collaborative study of neoplasia and steroid contraceptives. Am J Epidemiol 1998;148:1195–1205. [DOI] [PubMed] [Google Scholar]
- Morabia A, Khatchatrian N, Bernstein M, Walker DM, Campana A. Reproductive characteristics of a population of urban Swiss women. Acta Obstet Gynecol Scand 1996;75:838–842. [DOI] [PubMed] [Google Scholar]
- Mulder MB. Menarche, menopause and reproduction in the Kipsigis of Kenya. J Biosoc Sci 1989;21:179–192. [DOI] [PubMed] [Google Scholar]
- Mulder MB. Marriage and reproduction in the Kipsigis of Kenya. ProQuest Dissertations and Theses. 1987, 375. https://www.proquest.com/dissertations-theses/marriage-reproduction-kipsigis-kenya/docview/303592172/se-2?accountid=178282 [Google Scholar]
- Murthy MSR. Determinants of reproductive spans among Muslims in India: a study. J Sociol Soc Anthropol 2012b;3:63–71. [Google Scholar]
- Murthy MSR. Determinants of reproductive duration among women of Jharkhand state: a study. J Hum Ecol 2012a;37:111–117. [Google Scholar]
- Nabhan AF, Mburu G, Elshafeey F, Kamel M, Magdi R, Elshebiny M, Abuelnaga YG, Ghonim M, Abdelhamid MHS, Ghonim M et al. Woman’s reproductive span: a protocol of a systematic scoping review 2020, 10.31222/osf.io/pj7qu [DOI] [PMC free article] [PubMed]
- Nichols HB, Trentham-Dietz A, Hampton JM, Titus-Ernstoff L, Egan KM, Willett WC, Newcomb PA. From menarche to menopause: trends among US women born from 1912 to 1969. Am J Epidemiol 2006;164:1003–1011. [DOI] [PubMed] [Google Scholar]
- Padmadas SS, Hutter I, Willekens F. Compression of women’s reproductive spans in Andhra Pradesh, India. Int Fam Plan Perspect 2004;30:12–19. [DOI] [PubMed] [Google Scholar]
- Pavia M, Hsieh CC, Ekbom A, Adami HO, Trichopoulos D. Handedness, age at menarche, and age at menopause. Obstet Gynecol 1994;83:579–582. [DOI] [PubMed] [Google Scholar]
- Peters MDJ, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc 2015;13:141–146. [DOI] [PubMed] [Google Scholar]
- Pyun J-A, Kim S, Cho NH, Koh I, Lee J-Y, Shin C, Kwack K. Genome-wide association studies and epistasis analyses of candidate genes related to age at menarche and age at natural menopause in a Korean population. Menopause 2014;21:522–529. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2020. https://www.R-project.org/ [Google Scholar]
- Riener E-K, Keck C, Worda C, Hefler LA, Tempfer CB. Body mass index but not a polymorphism of the interleukin-1 receptor antagonist (IL-1 RA) gene is associated with age at natural menopause. Gynecol Obstet Invest 2004;58:117–120. [DOI] [PubMed] [Google Scholar]
- Ruth KS, Beaumont RN, Tyrrell J, Jones SE, Tuke MA, Yaghootkar H, Wood AR, Freathy RM, Weedon MN, Frayling TM et al. Genetic evidence that lower circulating FSH levels lengthen menstrual cycle, increase age at menopause and impact female reproductive health. Hum Reprod 2016;31:473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K, Bansal M. Association of age at menopause with post-menopausal symptoms, menarche age and other reproductive factors among rural females in Shimla, Himachal Pradesh. J Biosoc Sci 2018;50:19–25. [DOI] [PubMed] [Google Scholar]
- Shaw S, Ghosh D, Kumar U, Panjwani U, Kumar B. Impact of high altitude on key determinants of female reproductive health: A review. Int J Biometeorol 2018;62:2045–2055. [DOI] [PubMed] [Google Scholar]
- Shi J, Zhang B, Choi J-Y, Gao Y-T, Li H, Lu W, Long J, Kang D, Xiang Y-B, Wen W et al. Age at menarche and age at natural menopause in east Asian women: a genome-wide association study. Age (Dordr) 2016;38:513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si JH, Meng RR, Lyu J, Guo Y, Bian Z, Yu CQ, Yang L, Tan YL, Pei P, Chen JS et al. [Associations between season of birth and age both at menarche and at menopause]. Zhonghua Liu Xing Bing Xue za Zhi 2017;38:877–882. [DOI] [PubMed] [Google Scholar]
- Singh BP, Singh G, Singh K. An application of life table and survival model to study the reproductive span. J Crit Revi 2020;7:2020. [Google Scholar]
- Singh HB, Singh NS. Covariates of women reproductive span in Manipur: a life table approach. Int J Sci Res 2014;10:235–239. [Google Scholar]
- Singh L, Ahuja S. An estimation of reproductive performance in the women of Punjab. Anthropol Anz 1980;37:266–270. [PubMed] [Google Scholar]
- Sinha I, Tigga P, Mondal N, Sen J. Association between age at menarche and age at menopause among women of an indigenous population of north Bengal, India. J Biosoc Sci 2021;53:319–325. [DOI] [PubMed] [Google Scholar]
- Snieder H, MacGregor AJ, Spector TD. Genes control the cessation of a woman’s reproductive life: a twin study of hysterectomy and age at menopause. J Clin Endocrinol Metab 1998;83:1875–1880. [DOI] [PubMed] [Google Scholar]
- Stevenson JC, Everson PM, Crawford MH. Changes in completed family size and reproductive span in anabaptist populations. Hum Biol 1989;61:100–115. [PubMed] [Google Scholar]
- Tea M-KM, Weghofer A, Wagner K, Singer CF. Association of BRCA1/2 mutations with FMR1 genotypes: effects on menarcheal and menopausal age. Maturitas 2013;75:148–151. [DOI] [PubMed] [Google Scholar]
- Thomas F, Renaud F, Benefice E, T de M, Guegan JF. International variability of ages at menarche and menopause: patterns and main determinants. Hum Biol 2001;73:271–290. [DOI] [PubMed] [Google Scholar]
- Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, Moher D, Peters MDJ, Horsley T, Weeks L et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018;169:467–473. [DOI] [PubMed] [Google Scholar]
- UK Biobank. https://biobankndphoxacuk 2021. Accessed on April 1, 2021
- Vander Borght M, Wyns C. Fertility and infertility: definition and epidemiology. Clin Biochem 2018;62:2–10. [DOI] [PubMed] [Google Scholar]
- Velde E. T, Pearson PL. The variability of female reproductive ageing. Hum Reprod Update 2002;8:141–154. [DOI] [PubMed] [Google Scholar]
- Vitzthum VJ. Field methods and strategies for assessing female reproductive functioning. Am J Hum Biol 2021;33:e23513. [DOI] [PubMed] [Google Scholar]
- Wood JW, Johnson PL, Campbell KL. Demographic and endocrinological aspects of low natural fertility in Highland New Guinea. J Biosoc Sci 1985;17:57–79. [DOI] [PubMed] [Google Scholar]
- Wood JW, Weinstein M. A model of age-specific fecundability. Popul Stud 1988;42:85–113. [Google Scholar]
- Worda C, Walch K, Sator M, Eppel W, Tempfer CB, Schneeberger C, Huber JC, Hefler LA. The influence of Nos3 polymorphisms on age at menarche and natural menopause. Maturitas 2004;49:157–162. [DOI] [PubMed] [Google Scholar]
- Yunus FM, Rahman MJ, Alam MZ, Hore SK, Rahman M. Relationship between arsenic skin lesions and the age of natural menopause. BMC Public Health 2014;14:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbetto I, Gromoll J, Luisi S, Reis FM, Nieschlag E, Simoni M, Petraglia F. Follicle-stimulating hormone receptor and DAZL gene polymorphisms do not affect the age of menopause. Fertil Steril 2008;90:2264–2268. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in the published scoping review article and is available upon request from the corresponding author.