Abstract
Purpose
Our understanding of breast cancer in very young women (≤35 years old) remains limited. We aimed to assess the clinicopathological characteristics, molecular subtype, and treatment distribution and prognosis of these young patients compared with patients over 35 years.
Methods
We retrospectively analyzed non-metastatic female breast cancer cases treated at three Chinese academic hospitals between January 1, 2008, and December 31, 2018. Local recurrence-free survival (LRFS), disease-free survival (DFS), and overall survival (OS) were compared between different age groups and stratified with distinct molecular subtypes.
Results
A total of 11,671 women were eligible for the final analyses, and 1,207 women (10.3%) were ≤35 years at disease onset. Very young breast cancer women were more likely to be single or childless, have higher-grade disease, have more probability of lymphovascular invasion (LVI) in tumor and triple-negative subtype, and be treated by lumpectomy, chemotherapy especially more anthracycline- and paclitaxel-based chemotherapy, endocrine therapy plus ovarian function suppression (OFS), anti-HER2 therapy, and/or radiotherapy than older women (P < 0.05 for all). Very young women had the lowest 5-year LRFS and DFS among all age groups (P < 0.001 for all). When stratified by molecular subtype, very young women had the worst outcomes vs. women from the 35~50-year-old group or those from >50-year-old group for hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2−) subtype, including LRFS, DFS, and OS (P < 0.05 for all). In terms of LRFS and DFS, multivariate analyses showed similar results among the different age groups.
Conclusion
Our study demonstrated that very young women with breast cancer had higher-grade tumors, more probability of LVI in tumor, and more triple-negative subtype, when compared with older patients. They had less favorable survival outcomes, especially for patients with the HR+/HER2− subtype.
Keywords: breast cancer, very young, multi-institutional, characteristics and prognosis, comparison
Introduction
Although the incidence of breast carcinoma in China is lower compared with that in European countries or America, yet, the newly diagnosed breast cancer cases have been increasing in China, particularly in urban areas (1, 2). Nowadays, in China, the most frequent, newly diagnostic cancer is breast cancer, which is also the fifth cause for cancer-related deaths in women (3). A report from the Chinese Cancer Center showed that China had 3.8 million new patients with malignant tumor in 2014, including new 1.69 million female malignant tumor patients, in which the number of newly diagnosed breast cancer accounted for 16.51% (4). The median age at diagnosis of Chinese female patients with breast cancer is 48 years old, which is 14 years younger than that of breast cancer women (62 years old) reported by the Surveillance Epidemiology and End Results (SEER) database (5).
Young breast cancer is usually defined as patients <40 years at diagnosis (6). Prior studies showed that young breast cancer patients were more likely to have adverse tumor characteristics (for instance, higher grade, higher fraction in tumor proliferation, higher probability in lymph vascular invasion, and hormone receptor negative) and worse prognosis than older patients (7–10). Among these women, there is a special group of patients who have been diagnosed at a very young age (≤35 years old). These very young patients may have special needs and meet more challenges, for example, career break, reproductive barriers, sexual dysfunction, unexpected changes in body image, and psychosocial stress. It is of great importance to address the feature and progress of very young breast cancer for guiding clinical treatment.
Because of the much more younger average age, breast cancer women of very young age account for 6.99%~7.43% among all female cases in China (11, 12); therefore, the incidence is higher than that in the United States (<4%) (13). Very few studies to date have focused on very young breast cancer patients; moreover, these limited studies all had small sample size and no data of tumor molecular subtypes (8, 9, 14, 15). Our study aimed to explore the distribution of clinicopathological features, molecular subtypes, and treatment characteristics in non-metastatic breast cancer women with very young age (≤35 years old) in China and to assess the survival differences between age groups.
Materials and Methods
Participants
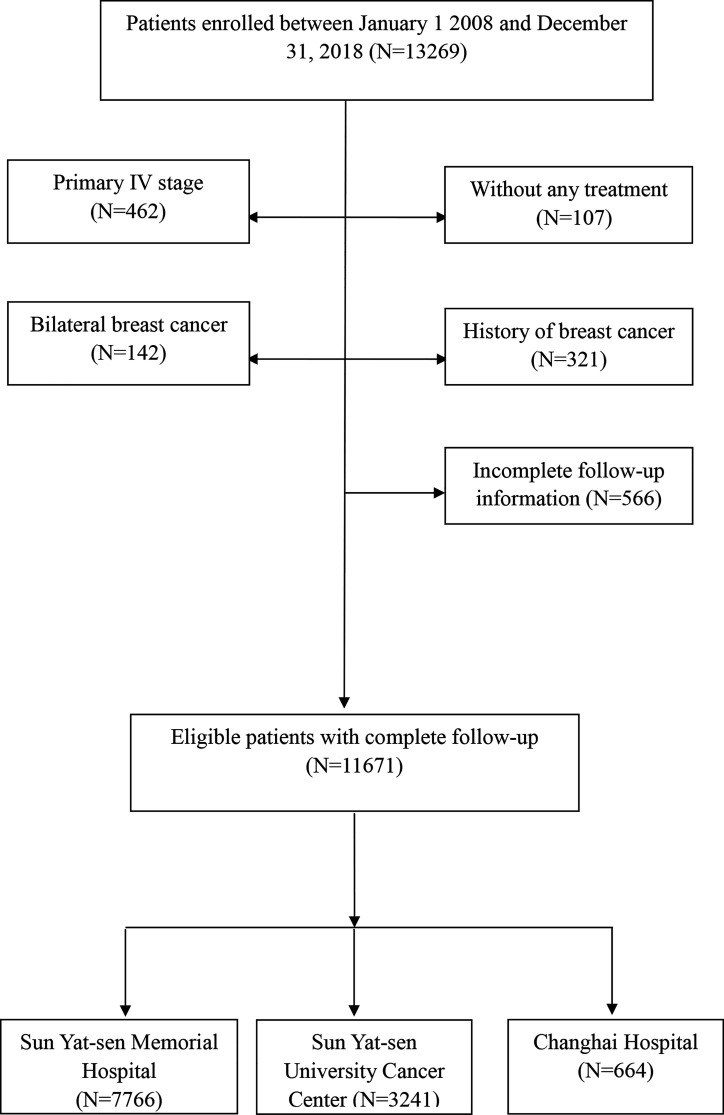
This study reviewed the medical records of all female breast cancer cases who were diagnosed with stage 0 to III according to the American Joint Committee on Cancer Staging Manual (AJCC), 8th edition, and treated between January 1, 2008, and December 31, 2018 at three hospitals, retrospectively. A total of 11,671 women with complete follow-up were included in the current study, consisting of 7,766 patients treated at Sun Yat-sen Memorial Hospital (SYSMH) from Guangzhou, 3,241 patients treated at Sun Yat-sen University Cancer Center from Guangzhou, and 664 patients from Changhai Hospital at Shanghai ( Figure 1 ). Patients with previous malignancies in 5 years, history of breast cancer, and stage IV or bilateral breast cancer and who did not receive any treatment were excluded. Approximately 10.3% (1,207/11,671) female breast cancer patients were ≤35 years old during this period of time. The demographic and clinicopathological data and treatment variables were collected from a multicenter online database, “Yixian Database” (16), with strict privacy standards. Survival data were obtained from the follow-up registry of each center and follow-up data were censored on January 31, 2021. This study was approved by Sun Yat-sen Memorial Hospital (SYSMH) Ethics Committee. As this is a retrospective study, informed consent from the study participants can be exempted, and a participant who was newly diagnosed to have breast cancer enrolled into the online “Yixian Database” is a default option at the time of diagnosis.
Figure 1.
Flowchart of enrollment in the study.
Variable Definitions
Clinicopathological features and treatment variables were compared between age groups: ≤35-year-old group, 35~50-year-old group, and >50-year-old group. The information collected included family history, marriage status, reproductive history, histological type, breast surgery, axillary surgery, tumor grade, tumor–node–metastasis (TNM) stage, molecular subtype, and estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) statuses. Adjuvant therapies included chemotherapy, anti-HER2 therapy, endocrine therapy, and radiation therapy.
Definition of Survival Endpoints
Local recurrence-free survival (LRFS) was defined as the time from the date of diagnosis and first event of local invasive breast recurrence and regional invasive recurrence. Disease-free survival (DFS) was defined as the time between diagnosis and tumor first recurrence or metastasis or death from any cause. Overall survival (OS) was defined as the time between diagnosis and death from any cause.
Statistical Analysis
Descriptive statistics were reported as frequency with percentage for categorical variables, and these variables were compared with chi-square tests or Fisher’s exact test according to age groups (≤35 years, 35~50 years, and >50 years). Survival analyses (LRFS, DFS, and OS) were performed with the Kaplan–Meier method and compared using the log-rank test. Univariate Cox regression was analyzed for LRFS, DFS, and OS, respectively. The significant variables in univariate Cox regression were then included in the multivariate Cox regression analyses, and the forward conditional method was used to select independent survival factors. It was two sided in all statistical tests and P <0.05 was considered statistically significant. All the statistical analysis of this study was performed with STATA 13.0 (StataCorp, College Station, TX, USA).
Results
Baseline Clinicopathological Data
A total of 11,671 (median age: 48 years old; range: 17~95) women with breast cancer were included. Characteristic features and treatment characteristics are outlined in Table 1 . Among these patients, 1,207 women (10.3%, median age: 32 years) were in the ≤35-year-old group, 5,675 women (48.6%, median age: 44 years) were in the 35~50-year-old group, and 4,789 women (41.0%, median age: 58 years) were in the over 50-year-old group. Overall, the median of follow-up time was 52 months in this study. Compared with older women, very young breast cancer women were more likely to be single and childless (P < 0.001) when they were diagnosed. Very young patients had more high-grade disease, more probability of lymphovascular invasion (LVI) in tumor, and more triple-negative subtype (P < 0.05). Moreover, very young patients were more likely to receive lumpectomy, chemotherapy especially more anthracycline- and paclitaxel-based chemotherapy, endocrine therapy plus ovarian function suppression (OFS), anti-HER2 therapy, or radiotherapy (P < 0.05) ( Table 1 ). Women in the ≤35-year-old group had the highest proportion (43.7%) of OFS in HR-positive patients than the other age groups ( Table 1 ).
Table 1.
Descriptive characteristics and treatments of breast cancer patients by age at diagnosis.
| Age group | TotalN (%) | P-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ≤35 (n = 1,207)N (%) | 35–50 (n = 5,675)N (%) | >50 (n = 4,789)N (%) | ||||||||
| Family history of cancer | No | 1,165 | 96.5 | 5,481 | 96.6 | 4,606 | 96.2 | 11,252 | 96.4 | 0.531 |
| Yes | 42 | 3.5 | 194 | 3.4 | 183 | 3.8 | 419 | 3.6 | ||
| Marital status | Single | 146 | 12.1 | 143 | 2.5 | 108 | 2.3 | 397 | 3.4 | <0.001 |
| Married | 1,061 | 87.9 | 5,532 | 97.5 | 4,681 | 97.7 | 11,274 | 96.6 | ||
| Pregnancy | No | 265 | 22.0 | 468 | 8.2 | 305 | 6.4 | 1,038 | 8.9 | <0.001 |
| Yes | 942 | 78.0 | 5,207 | 91.8 | 4,484 | 93.6 | 10,633 | 91.1 | ||
| Histological type | IDC | 1,007 | 83.5 | 4,818 | 85.0 | 4,145 | 86.6 | 9,970 | 85.5 | <0.001 |
| ILC | 9 | 0.7 | 156 | 2.8 | 111 | 2.3 | 276 | 2.4 | ||
| DCIS | 94 | 7.8 | 354 | 6.2 | 162 | 3.4 | 610 | 5.2 | ||
| Others | 96 | 8.0 | 354 | 6.0 | 368 | 7.7 | 807 | 6.9 | ||
| Tumor grade | 1 | 57 | 4.7 | 361 | 6.4 | 257 | 5.4 | 675 | 5.8 | <0.001 |
| 2 | 639 | 52.9 | 3,134 | 55.2 | 2,600 | 54.3 | 6,373 | 54.6 | ||
| 3 | 449 | 37.2 | 1,913 | 33.7 | 1,760 | 36.8 | 4,122 | 35.3 | ||
| Unknown | 62 | 5.1 | 267 | 4.7 | 172 | 3.6 | 501 | 4.3 | ||
| ER | Negative | 242 | 20.1 | 1,073 | 19.0 | 1,076 | 22.6 | 2,391 | 20.6 | <0.001 |
| Positive | 962 | 79.9 | 4,578 | 81.0 | 3,688 | 77.4 | 9,228 | 79.4 | ||
| PR | Negative | 322 | 26.7 | 1,441 | 25.5 | 1,683 | 35.3 | 3,446 | 29.7 | <0.001 |
| Positive | 882 | 73.3 | 4,210 | 74.5 | 3,081 | 64.7 | 8,173 | 70.3 | ||
| HER2 | Negative | 903 | 75.3 | 4,326 | 76.6 | 3,533 | 74.2 | 8,762 | 75.5 | 0.023 |
| Positive | 297 | 24.8 | 1,323 | 23.4 | 1,228 | 25.8 | 2,848 | 24.5 | ||
| Ki67 | <15 | 309 | 26.5 | 1,480 | 26.8 | 1,193 | 25.5 | 2,982 | 26.2 | 0.334 |
| ≥15 | 857 | 73.5 | 4,041 | 73.2 | 3,481 | 74.5 | 8,379 | 73.8 | ||
| LVI | Negative | 914 | 78.7 | 4,228 | 80.3 | 3,417 | 82.0 | 8,559 | 80.8 | 0.023 |
| Positive | 247 | 21.3 | 1,035 | 19.7 | 752 | 18.0 | 2,034 | 19.2 | ||
| Molecular subtype | ER+/HER2− | 774 | 64.5 | 3,783 | 67.0 | 3,047 | 64.0 | 7,604 | 65.5 | <0.001 |
| ER+/HER2+ | 226 | 18.8 | 962 | 17.0 | 775 | 16.3 | 1,963 | 16.9 | ||
| ER−/HER2+ | 71 | 5.9 | 361 | 6.4 | 453 | 9.5 | 885 | 7.6 | ||
| Triple-negative | 129 | 10.8 | 543 | 9.6 | 486 | 10.2 | 1,158 | 10.0 | ||
| T stage | 0–1 | 416 | 34.5 | 1,837 | 32.4 | 1,442 | 30.1 | 3,695 | 31.6 | <0.001 |
| 2 | 523 | 43.3 | 2,867 | 50.5 | 2,602 | 54.3 | 5,992 | 51.3 | ||
| 3–4 | 166 | 13.8 | 668 | 11.8 | 594 | 12.4 | 1,428 | 12.2 | ||
| Unknown | 102 | 8.5 | 303 | 5.3 | 151 | 3.2 | 556 | 4.8 | ||
| N stage | 0 | 713 | 59.1 | 3,429 | 60.4 | 2,826 | 59.0 | 6,968 | 59.7 | 0.721 |
| 1 | 290 | 24.0 | 1,328 | 23.4 | 1,135 | 23.7 | 2,753 | 23.6 | ||
| 2–3 | 203 | 16.8 | 909 | 16.0 | 820 | 17.1 | 1,932 | 16.6 | ||
| Unknown | 1 | 0.1 | 9 | 0.2 | 8 | 0.2 | 18 | 0.2 | ||
| Stage | 0–1 | 307 | 25.4 | 1,413 | 24.9 | 1,095 | 22.9 | 2,815 | 24.1 | 0.004 |
| 2 | 582 | 48.2 | 2,933 | 51.7 | 2,592 | 54.1 | 6,107 | 52.3 | ||
| 3 | 206 | 17.1 | 1,009 | 17.8 | 942 | 19.7 | 2,157 | 18.5 | ||
| Unknown | 112 | 9.3 | 320 | 5.6 | 160 | 3.3 | 592 | 5.1 | ||
| Chemotherapy | Yes | 1,101 | 91.2 | 5,000 | 88.1 | 4,019 | 83.9 | 10,120 | 86.7 | <0.001 |
| No | 106 | 8.8 | 675 | 11.9 | 770 | 16.1 | 1,551 | 13.3 | ||
| Chemotherapy treatment | Anthracycline- and paclitaxel-based | 709 | 64.4 | 2,872 | 57.4 | 1,932 | 48.1 | 5,513 | 54.5 | <0.001 |
| Paclitaxel-based | 115 | 10.4 | 682 | 13.6 | 736 | 18.3 | 1,533 | 15.1 | ||
| Anthracycline-based | 149 | 13.5 | 702 | 14.0 | 433 | 10.8 | 1,284 | 12.7 | ||
| Other therapy | 29 | 2.6 | 161 | 3.2 | 142 | 3.5 | 332 | 3.3 | ||
| Unknown | 99 | 9.0 | 583 | 11.7 | 776 | 19.3 | 1,458 | 14.4 | ||
| Surgical type | Mastectomy | 632 | 52.4 | 3,494 | 61.6 | 3,441 | 71.9 | 7,567 | 64.8 | <0.001 |
| Breast-conserving surgery | 575 | 47.6 | 2,181 | 38.4 | 1,348 | 28.1 | 4,104 | 35.2 | ||
| Nodal surgery | ALND | 745 | 61.7 | 3,605 | 63.5 | 3,364 | 70.2 | 7,714 | 66.1 | <0.001 |
| SLNB | 462 | 38.3 | 2,070 | 36.5 | 1,425 | 29.8 | 3,957 | 33.9 | ||
| Endocrine therapy | AI | 0 | 0 | 180 | 4.0 | 2,374 | 65.6 | 2,554 | 28.0 | <0.001 |
| TAM | 447 | 47.1 | 2,987 | 65.6 | 823 | 22.7 | 4,257 | 46.7 | ||
| AI+OFS | 140 | 17.1 | 429 | 9.4 | 25 | 0.7 | 616 | 6.8 | ||
| TAM+OFS | 253 | 26.6 | 487 | 10.7 | 15 | 0.4 | 755 | 8.3 | ||
| Unknown | 88 | 9.3 | 473 | 10.4 | 381 | 10.5 | 942 | 10.3 | ||
| Anti-HER2 therapy | No | 106 | 35.9 | 586 | 44.8 | 553 | 46.3 | 1,245 | 44.5 | 0.006 |
| Yes | 189 | 64.1 | 723 | 55.2 | 641 | 53.7 | 1,553 | 55.5 | ||
| Radiotherapy | No | 466 | 38.6 | 2,661 | 46.9 | 2,781 | 58.1 | 5,908 | 50.6 | <0.001 |
| Yes | 741 | 61.4 | 3,014 | 53.1 | 2,008 | 41.9 | 5,763 | 49.4 | ||
LVI, lymphovascular invasion; IDC, invasive ductal carcinoma; DCIS, ductal carcinoma in situ; ILC, invasive lobular carcinoma; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; PR, progesterone receptor; SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection; OFS, ovarian function suppression; AI, aromatase inhibitor; TAM, tamoxifen.
Survival Differences Between Age Groups
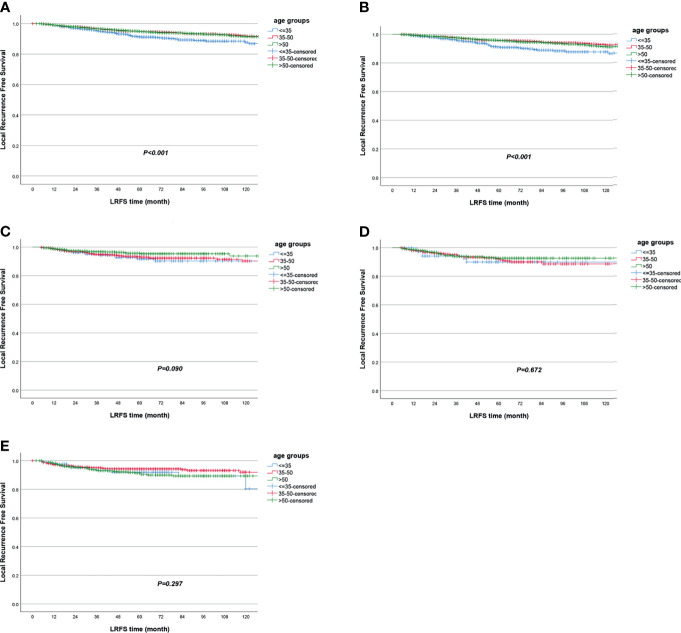
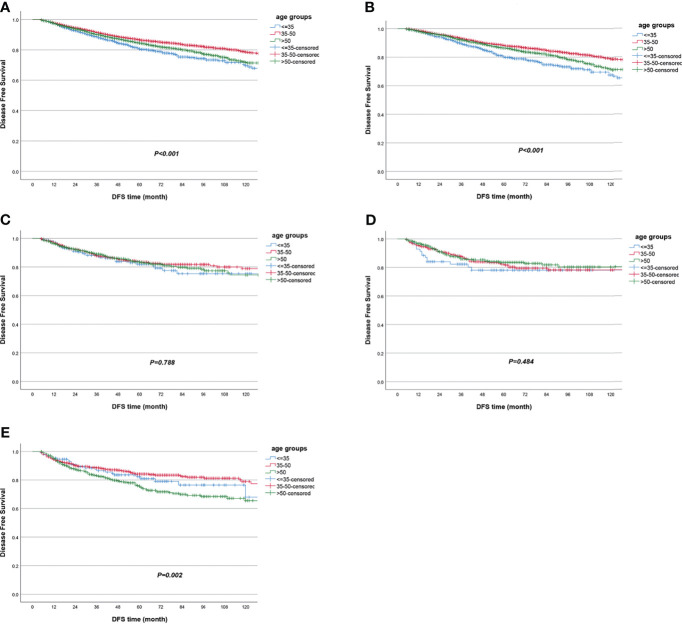
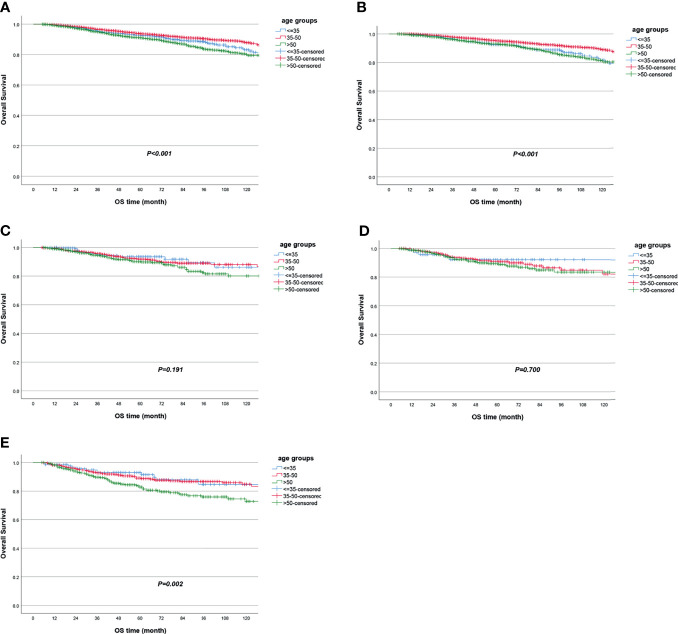
The LRFS of all patients was 94.5% [95% confidence interval (95% CI) 94.0%–95.0%] in 5 years. Women in the ≤35-year-old group had the lowest LRFS rate, which was 91.3% (95% CI 89.3%–93.2%) in 5 years. When univariate Cox regression was conducted between age groups, the ≤35-year-old group served as reference, and the HR was 0.63 (95% CI 0.50–0.80, P < 0.001) for the 35~50-year-old group and 0.64 (95% CI 0.50–0.82, P < 0.001) for the >50-year-old group ( Table 2 , Figure 2A and Supplemental Table 1 ). The molecular subtype of HR−/HER2+ had the lowest LRFS rate, which was 92.1% (95% CI 90.0%–94.2%) in 5 years (P < 0.001) ( Supplemental Table 1 ). The DFS of all patients was 85.0% (95% CI 84.2%–85.8%) in 5 years. Women in the ≤35-year-old group had the lowest DFS rate, which was 80.5% (95% CI 77.8%–83.2%) in 5 years. When univariate Cox regression was conducted between age groups, the ≤35-year-old group served as reference, and the HR was 0.68 (95% CI 0.59–0.79, P < 0.001) for the 35~50-year-old group and 0.86 (95% CI 0.74–1.00, P = 0.054) for the >50-year-old group ( Table 2 and Figure 3A ). The OS of all patients was 92.5% (95% CI 91.9%–93.1%) in 5 years. Women in the >50-year-old group had the lowest OS rate, which was 91.0% (95% CI 90.0%–92.0%) in 5 years. When univariate Cox regression was conducted between age groups, the ≤35-year-old group served as reference, and the HR was 0.80 (95% CI 0.64–0.99, P = 0.048) for the 35~50-year-old group and 1.30 (95% CI 1.04–1.61, P = 0.021) for the >50-year-old group ( Table 2 , Supplemental Table 1 and Figure 4A ). Patients with triple-negative breast tumor had the lowest OS rate, which was 86.9% (95% CI 84.7%–89.1%) in 5 years (P < 0.001) ( Supplemental Table 1 ).
Table 2.
Analysis of the association between age and survival, adjusted for tumor molecular subtype.
| Age | LRFS | DFS | OS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value | ||
| ALL | ≤35 | Ref | Ref | Ref | ||||||
| 35~50 | 0.63 | 0.50~0.80 | <0.001 | 0.68 | 0.59~0.79 | <0.001 | 0.80 | 0.64~0.99 | 0.048 | |
| >50 | 0.64 | 0.50~0.82 | <0.001 | 0.86 | 0.74~1.00 | 0.054 | 1.30 | 1.04~1.61 | 0.021 | |
| ≤35 | Ref | Ref | Ref | |||||||
| HR+/HER2− | 35~50 | 0.52 | 0.39~0.71 | <0.001 | 0.60 | 0.50~0.72 | <0.001 | 0.63 | 0.48~0.83 | 0.001 |
| >50 | 0.57 | 0.42~0.78 | <0.001 | 0.78 | 0.65~0.94 | 0.010 | 1.12 | 0.85~1.46 | 0.433 | |
| ≤35 | Ref | Ref | Ref | |||||||
| HR+/HER2+ | 35~50 | 0.91 | 0.52~1.61 | 0.747 | 0.88 | 0.61~1.27 | 0.498 | 1.17 | 0.66~2.08 | 0.590 |
| >50 | 0.58 | 0.31~1.08 | 0.087 | 0.92 | 0.63~1.34 | 0.657 | 1.52 | 0.85~2.70 | 0.156 | |
| ≤35 | Ref | Ref | Ref | |||||||
| HR−/HER2+ | 35~50 | 0.89 | 0.37~2.16 | 0.799 | 0.77 | 0.43~1.37 | 0.370 | 1.25 | 0.49~3.21 | 0.644 |
| >50 | 0.73 | 0.30~1.77 | 0.482 | 0.70 | 0.39~1.26 | 0.233 | 1.42 | 0.56~3.59 | 0.457 | |
| ≤35 | Ref | Ref | Ref | |||||||
| Triple negative | 35~50 | 0.67 | 0.34~1.34 | 0.260 | 0.83 | 0.53~1.30 | 0.412 | 1.17 | 0.63~2.17 | 0.618 |
| >50 | 0.95 | 0.48~1.85 | 0.869 | 1.36 | 0.88~2.11 | 0.172 | 1.98 | 1.08~3.63 | 0.027 | |
HR+, hormone receptor positive; HR−, hormone receptor negative; HER2, human epidermal growth factor receptor 2; LRF, local recurrence-free survival; DFS, disease-free survival; OS, overall survival; 95% CI, 95% confidence interval.
Figure 2.
Local recurrence-free survival according to age groups in (A) all patients and in subgroup analyses stratified by (B) HR+/HER2−, (C) HR+/HER2+, (D) HR−/HER2+, and (E) triple negative.
Figure 3.
Disease-free survival according to age groups in (A) all patients and in subgroup analyses stratified by (B) HR+/HER2−, (C) HR+/HER2+, (D) HR−/HER2+, and (E) triple negative.
Figure 4.
Overall survival according to age groups in (A) all patients and in subgroup analyses stratified by (B) HR+/HER2−, (C) HR+/HER2+, (D) HR−/HER2+, and (E) triple negative.
When stratified by tumor molecular subtype, women in the ≤35-year-old group had the worst survival outcomes vs. the 35~50-year-old group and the >50-year-old group for the HR+/HER2− subtype, including LRFS (≤35-year-old group as reference; HR = 0.52, 95% CI 0.39–0.71, P < 0.001 for the 35~50-year-old group; HR = 0.57, 95% CI 0.42–0.78, P < 0.001 for the >50-year-old group), DFS (≤35-year-old group as reference; HR = 0.60, 95% CI 0.50–0.72, P < 0.001 for the 35~50-year-old group; HR = 0.78, 95% CI 0.65–0.94, P = 0.010 for the >50-year-old group), and OS (≤35-year-old group as reference; HR = 0.63, 95% CI 0.48–0.83, P = 0.001 for the 35~50-year-old group) ( Table 2 and Figures 2B , 3B , 4B ). In the triple-negative subtype, patients in the >50-year-old group also got the worst OS compared with the ≤35-year-old group (HR = 1.98, 95% CI 1.08–3.63, P = 0.027) ( Table 2 and Figure 4E ). In the other molecular subtype, no differences were found between women in the ≤35-year-old group, 35–50-year-old group, and over 50-year-old group ( Table 2 and Figures 2C–E , 3C–E , 4C, D ).
Univariate and Multivariate Survival Analyses Stratified by Age
Risk factors of survival were selected by univariate analysis. There were several clinicopathological and treatment factors associated with breast cancer survival, including age, family history of cancer, TNM stage, histological type, tumor grade, Ki67, LVI, ER, PR, HER2 status, and receiving of adjuvant therapy (such as endocrine therapy, anti-HER2 therapy, and chemotherapy treatment) (P < 0.05) ( Table 3 ). Patients with higher stage, higher T/N stage, higher tumor grade, higher Ki67, negative ER/PR status, positive HER2 status, invasive ductal carcinoma (IDC) type, and LVI would get worse survival outcomes (LRFS, DFS, and OS) (P < 0.05) ( Table 3 ). Moreover, patients who received breast-conserving surgery (BCS), sentinel lymph node biopsy (SLNB), hormone treatment, anti-HER2 therapy, radiotherapy, and chemotherapy would get better survival outcomes (LRFS, DFS, and OS) (P < 0.05) ( Table 3 ). HER2-positive patients who received anti-HER2 therapy would get better DFS (HR = 0.78, 95% CI 0.64~0.95, P = 0.012) and OS (HR = 0.57, 95% CI 0.43~0.76, P = 0.012) compared with HER2-positive patients with no anti-HER2 therapy, especially in breast cancer patients diagnosed after 50 years old (DFS: HR = 0.64, P = 0.006; OS: HR = 0.51, P = 0.002) ( Supplemental Table 2 ). However, there were no differences for patients diagnosed ≤35 years old (LRFS: HR = 1.05, P = 0.915; DFS: HR = 1.08, P = 0.792; OS: HR = 0.51, P = 0.159) and 35~50 years old (LRFS: HR = 0.91, P = 0.671; DFS: HR = 0.82, P = 0.189; OS: HR = 0.68, P = 0.068) ( Supplemental Table 2 ).
Table 3.
Univariable survival analyses for age and other clinicopathological factors.
| Characters | Subgroups | LRFS | DFS | OS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value | ||
| Age group | ≤35 | Ref | Ref | Ref | ||||||
| 35~50 | 0.63 | 0.50~0.80 | <0.001 | 0.68 | 0.59~0.79 | <0.001 | 0.80 | 0.64~1.00 | 0.048 | |
| >50 | 0.64 | 0.50~0.82 | <0.001 | 0.86 | 0.74~1.00 | 0.054 | 1.30 | 1.04~1.61 | 0.021 | |
| Family history of cancer | Yes vs. no | 0.95 | 0.61~1.48 | 0.817 | 0.81 | 0.61~1.08 | 0.146 | 0.72 | 0.48~1.08 | 0.115 |
| Marital status | Married vs. single | 0.76 | 0.52~1.12 | 0.169 | 0.86 | 0.68~1.10 | 0.234 | 1.01 | 0.71~1.42 | 0.977 |
| Pregnancy | Yes vs. no | 1.12 | 0.85~1.49 | 0.429 | 1.14 | 0.96~1.34 | 0.127 | 1.32 | 1.05~1.66 | 0.018 |
| Surgical type | BCS vs. mastectomy | 0.96 | 0.84~1.14 | 0.616 | 0.58 | 0.52~0.65 | <0.001 | 0.46 | 0.40~0.54 | <0.001 |
| Nodal surgery | SLNB vs. ALND | 0.79 | 0.66~0.95 | 0.010 | 0.48 | 0.43~0.54 | <0.001 | 0.34 | 0.28~0.411 | <0.001 |
| T stage | 0–1 | Ref | Ref | Ref | ||||||
| 2 | 1.39 | 1.15~1.69 | <0.001 | 1.68 | 1.49~1.90 | <0.001 | 2.04 | 1.73~2.42 | <0.001 | |
| 3 | 2.11 | 1.631~2.73 | <0.001 | 3.40 | 2.94~3.95 | <0.001 | 4.57 | 3.73~5.59 | <0.001 | |
| N stage | 0 | Ref | Ref | Ref | ||||||
| 1 | 1.34 | 1.09~1.63 | 0.005 | 1.84 | 1.63~2.08 | <0.001 | 2.25 | 1.89~2.66 | <0.001 | |
| 2–3 | 2.26 | 1.86~2.74 | <0.001 | 4.10 | 3.67~4.59 | <0.001 | 5.50 | 4.72~6.41 | <0.001 | |
| Stage | 0–1 | Ref | Ref | Ref | ||||||
| 2 | 1.26 | 1.01~1.57 | 0.043 | 1.72 | 1.48~2.00 | <0.001 | 2.35 | 1.86~2.96 | <0.001 | |
| 3 | 2.33 | 1.83~2.96 | <0.001 | 4.91 | 4.21~5.73 | <0.001 | 7.72 | 6.12~9.73 | <0.001 | |
| Histological type | IDC | Ref | Ref | Ref | ||||||
| ILC | 0.41 | 0.19~0.92 | 0.031 | 1.06 | 0.78~1.43 | 0.722 | 1.02 | 0.67~1.54 | 0.936 | |
| DCIS | 0.90 | 0.61~1.32 | 0.580 | 0.51 | 0.38~0.69 | <0.001 | 0.26 | 0.14~0.45 | <0.001 | |
| Others | 0.58 | 0.40~0.85 | 0.005 | 0.61 | 0.49~0.76 | <0.001 | 0.65 | 0.49~0.85 | 0.002 | |
| Tumor grade | 1 | Ref | Ref | Ref | ||||||
| 2 | 1.17 | 0.80~1.70 | 0.419 | 1.38 | 1.09~1.734 | 0.007 | 1.21 | 0.89~1.62 | 0.220 | |
| 3 | 1.61 | 1.10~2.36 | 0.014 | 1.70 | 1.34~2.15 | <0.001 | 1.64 | 1.21~2.21 | 0.001 | |
| ER status | Positive vs. negative | 0.67 | 0.56~0.80 | <0.001 | 0.75 | 0.67~0.83 | <0.001 | 0.62 | 0.53~0.71 | <0.001 |
| PR status | Positive vs. negative | 0.65 | 0.55~0.77 | <0.001 | 0.70 | 0.63~0.77 | <0.001 | 0.58 | 0.51~0.67 | <0.001 |
| HER2 status | Positive vs. negative | 1.28 | 1.06~1.53 | 0.009 | 1.15 | 1.03~1.28 | 0.016 | 1.19 | 1.02~1.38 | 0.024 |
| Ki67 | ≥15 vs. <15 | 1.69 | 1.37~2.09 | <0.001 | 1.44 | 1.28~1.62 | <0.001 | 1.57 | 1.33~1.85 | <0.001 |
| LVI | Positive vs. negative | 1.71 | 1.44~2.09 | <0.001 | 1.68 | 1.49~1.90 | <0.001 | 1.49 | 1.25~1.78 | <0.001 |
| Hormone treatment | Yes vs. no | 0.50 | 0.35~0.71 | <0.001 | 0.63 | 0.51~0.79 | <0.001 | 0.66 | 0.48~0.91 | 0.010 |
| TAM | Ref | Ref | Ref | |||||||
| AI | 0.81 | 0.63~1.04 | 0.103 | 1.07 | 0.93~1.24 | 0.359 | 1.42 | 1.15~1.74 | 0.001 | |
| Endocrine therapy | AI+OFS | 1.71 | 1.15~2.54 | 0.008 | 1.48 | 1.13~1.93 | 0.004 | 1.53 | 1.00~2.34 | 0.048 |
| TAM+OFS | 1.59 | 1.15~2.20 | 0.005 | 1.55 | 1.26~1.91 | <0.001 | 1.19 | 0.82~1.71 | 0.361 | |
| Anti-HER2 therapy | Yes vs. no | 0.85 | 0.62~1.17 | 0.330 | 0.78 | 0.64~0.95 | 0.012 | 0.57 | 0.43~0.76 | <0.001 |
| Radiotherapy | Yes vs. no | 1.16 | 0.99~1.36 | 0.076 | 0.99 | 0.90~1.09 | 0.850 | 0.74 | 0.65~0.84 | <0.001 |
| Chemotherapy | Yes vs. no | 1.01 | 0.79~1.30 | 0.919 | 0.59 | 0.49~0.71 | <0.001 | 0.43 | 0.32~0.57 | <0.001 |
| Chemotherapy treatment | Other treatment | Ref | Ref | Ref | ||||||
| Anthracycline- and paclitaxel-based | 0.86 | 0.58~1.29 | 0.478 | 1.51 | 1.13~2.02 | 0.006 | 1.96 | 1.27~3.01 | 0.002 | |
| Paclitaxel-based | 0.60 | 0.37~0.95 | 0.030 | 0.88 | 0.63~1.22 | 0.441 | 1.09 | 0.68~1.77 | 0.715 | |
| Anthracycline-based | 0.75 | 0.48~1.17 | 0.206 | 1.03 | 0.75~1.42 | 0.859 | 1.22 | 0.77~1.95 | 0.399 | |
BCS, breast-conserving surgery; IDC, invasive ductal carcinoma; DCIS, ductal carcinoma in situ; ILC, invasive lobular carcinoma; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; PR, progesterone receptor; LVI, lymphovascular invasion; SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection; LRFS, local recurrence-free survival; DFS, disease-free survival; OS, overall survival; 95% CI, 95% confidence interval; AI, aromatase inhibitor; TAM, tamoxifen.
In the multivariable model, clinicopathological characteristics and treatment were controlled for LRFS, DFS, and OS. In the adjusted models, we found that women diagnosed between 35 and 50 years old or diagnosed after 50 years old remained about 35% less likely to develop local recurrence than women diagnosed at ages ≤35 years old (HR = 0.62, 95% CI 0.48–0.81, P < 0.001; HR = 0.65, 95% CI 0.50–0.85, P = 0.002, respectively) ( Table 4 ). For DFS, women diagnosed between 35 and 50 years old were 31% less likely to relapse or develop distant metastasis than those diagnosed at ages ≤35 years old (HR = 0.69, 95% CI 0.59–0.81, P < 0.001), and women diagnosed after 50 years old were 20% less likely to relapse or develop distant metastasis than women diagnosed at ages ≤35 years old (HR = 0.80, 95% CI 0.68–0.95, P = 0.010) ( Table 4 ).
Table 4.
Multivariable survival analyses stratified by age, adjusted for clinicopathological factors.
| LRFS | DFS | OS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value | ||
| Age | ≤35 | Ref | Ref | Ref | ||||||
| 35~50 | 0.62 | 0.48~0.81 | <0.001 | 0.69 | 0.59~0.81 | <0.001 | 0.80 | 0.63~1.03 | 0.082 | |
| >50 | 0.65 | 0.50~0.85 | 0.002 | 0.80 | 0.68~0.95 | 0.010 | 1.13 | 0.88~1.44 | 0.345 | |
| T stage | 0–1 | Ref | Ref | Ref | ||||||
| 2 | 1.23 | 0.99~1.53 | 0.060 | 1.32 | 1.15~1.51 | <0.001 | 1.48 | 1.22~1.80 | <0.001 | |
| 3–4 | 1.49 | 1.12~1.99 | 0.007 | 2.00 | 1.69~2.38 | <0.001 | 2.65 | 2.10~3.34 | <0.001 | |
| N stage | 0 | Ref | Ref | Ref | ||||||
| 1 | 1.23 | 0.98~1.54 | 0.078 | 1.66 | 1.45~1.90 | <0.001 | 2.34 | 1.93~2.83 | <0.001 | |
| 2–3 | 1.96 | 1.55~2.47 | <0.001 | 3.32 | 2.91~3.78 | <0.001 | 5.87 | 4.87~7.08 | <0.001 | |
| LVI | Positive vs. negative | 1.35 | 1.09~1.69 | 0.007 | / | / | / | / | / | / |
| Ki67 | ≥15 vs. <15 | 1.52 | 1.24~1.91 | <0.001 | 1.31 | 1.15~1.48 | <0.001 | 1.38 | 1.15~1.65 | <0.001 |
| ER | Positive vs. negative | 0.62 | 0.51~0.75 | <0.001 | / | / | / | 0.81 | 0.66~1.00 | 0.048 |
| PR | Positive vs. negative | / | / | / | 0.80 | 0.71~0.89 | <0.001 | 0.77 | 0.63~0.94 | 0.009 |
| Surgical type | BCS vs. mastectomy | / | / | / | 0.80 | 0.71~0.91 | 0.001 | / | / | / |
| Chemotherapy | Yes vs. no | 0.68 | 0.51~0.91 | 0.010 | / | / | / | / | / | / |
| Radiotherapy | Yes vs. no | / | / | / | / | / | / | 0.50 | 0.43~0.59 | <0.001 |
ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; PR, progesterone receptor; LVI, lymphovascular invasion; BCS, breast-conserving surgery; LRFS, local recurrence-free survival; DFS, disease-free survival; OS, overall survival; 95% CI, 95% confidence interval.
Discussion
This study demonstrated that very young women (age group ≤35 years) with breast cancer had more higher-grade tumors, more probability of LVI in tumor, and more triple-negative subtype and received more lumpectomy, more chemotherapy especially more anthracycline- and paclitaxel-based chemotherapy, and more endocrine therapy plus OFS, anti-HER2 therapy, and adjuvant radiotherapy when compared with older patients. Moreover, very young women with breast cancer had the lowest 5-year LRFS and DFS among all age groups. When stratified by molecular subtype, very young women with breast cancer had the worst outcomes vs. women in the 35~50-year-old group or those in the >50-year-old group for the HR+/HER2− subtype, including LRFS, DFS, and OS.
The recent European Society of Breast Cancer Specialists (EUSOMA) and the ESO-ESMO fourth international consensus guidelines defined young breast cancer women as those who were diagnosed at or before age 40 (6). Very young breast cancer women were described as those diagnosed at age ≤35 years old, consisting of a unique group of patients that may need further investigation. In this study, very young (≤35 years old) women with breast cancer account for 10.3% of all female breast cancer cases that were diagnosed as stage 0 to III in China; this incidence was similar to the reports from South Korea (9) and Egypt (14), but much higher than the studies from Greece (17) and other European countries (8). This is the largest report from China focusing on very young non-metastatic breast cancer patient cohorts (n = 1,207) and, globally, the second largest study so far for these special breast cancer populations. This study reported the clinicopathological characteristics, tumor molecular subtype, and treatment distributions of these special very young onset breast cancer women. Furthermore, the survival differences between age groups, especially stratified by molecular subtype, were explored as well.
We observed that female patients diagnosed with breast cancer at a very young age (≤35 years old) were more likely to be single and childless than older patients, so this group of patients may need to face this life-threatening disease alone and meet more problems, such as stress of establishment of family, inability of childbearing, and the negative impact of distinct treatment on sexuality or body image. They also had more high-grade tumors, which was consistent with prior reports (8, 9, 14). Moreover, we found that very young breast cancer women had more probability of LVI in tumor and more triple-negative disease than older women. For treatment options, we observed that very young breast cancer patients were less willing to receive mastectomy compared with older female patients in China, which was in agreement with a prior Korean study (9). Our study is the only research with available data of chemotherapy, OFS, and adjuvant radiation therapy, and we found that very young patients might receive more chemotherapy especially more anthracycline- and paclitaxel-based chemotherapy, and more endocrine therapy plus OFS, anti-HER2 therapy, and/or adjuvant radiotherapy than older patients. This may be due to the situation that these young women had more aggressive tumors (higher grade, more LVI in tumor, or triple-negative subtype) and preferred breast-conserving surgery because of higher cosmetic requirements.
Not surprisingly, we have found that the younger age group (women ≤35 years old) was an independent risk factor of LRFS and DFS. Moreover, we demonstrated that very young breast cancer women with HR+/HER2− subtype showed the most unfavorable survival outcomes including LRFS, DFS, and OS when compared with older patients. This finding was similar to the results of prior studies which demonstrated that HR+ very young breast cancer women had worse outcomes than older women (7–9). Patients diagnosed after 50 years old had the lowest OS rate and had similar result in patients with triple-negative breast tumor. A study showed that in ER-negative patients, there were no differences between the ≤35-year-old group and the >35-year-old group (8) for OS. Other reports found that triple-negative breast cancer patients ≤50 years of age had worse OS and BCS than the 51–60 age group patients (7). Hence, in patients with triple-negative breast cancer, the effect of age on survival outcomes was unclear, which needs further investigation.
There were several studies reporting that very young breast cancer patients more frequently expressed a high proliferation cell surface marker, and these young patients more frequently had endocrine-resistance features compared with older women (18–21). These findings may partially explain our results of the unfavorable prognosis of HR+/HER− subtype among patients at a very young age. Further studies are required to assess the underlying mechanisms why these younger patients have such molecular profile. The TEXT/SOFT trial demonstrated that compared with tamoxifen alone, OFS plus exemestane or tamoxifen was correlated with much more favorable survival outcomes among HR+ breast cancer women diagnosed at age ≤35 years old (22). However, there were reports that women with younger age had lower adherence and were more likely to give up halfway to endocrine therapy including OFS (23–25). Efforts such as providing social support, establishing good patient–physician relationship, and minimizing the side effects of therapy should be made to address this problem for these very young HR+/HER2− breast cancer patients. This study was based on the multicenter online database “Yixian Database” (5), and 11,671 women with breast cancer were included, which was a large cohort to explore the very young breast cancer patients’ characteristics and prognosis. Because of the higher-grade tumors and more triple-negative subtype for these very young patients, they also had the worst survival outcomes than the other age groups. We should pay more attention to these very young patients with breast cancer.
We acknowledged that there were some limitations in this study. Firstly, as the detailed information of adjuvant endocrine therapy’s treatment duration was not investigated in our study, the findings should be interpreted carefully. Furthermore, hormone receptor and HER2 statuses were used instead of the molecular subtype classification in this study, although this was commonly applied by several population-based studies on breast cancer. The lack of genetic testing results of BRCA1/2 mutations for these very young female breast cancer patients and the lack of family support and economic situation were other limitations.
Conclusion
In summary, this multi-institutional study demonstrated that very young women with breast cancer in China had more high-grade tumors and more triple-negative subtype and were more likely to receive lumpectomy, neoadjuvant chemotherapy, and radiotherapy than older patients. Moreover, they had less favorable survival outcomes, especially for patients with HR+/HER2− tumors. Further studies are required to focus on this special subpopulation of female breast cancer patients with very young age and to specifically address some clinical characteristics and prognostic outcomes.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.
Ethics Statement
This study was approved by the Ethics Committee of Sun Yat-sen Memorial Hospital, Sun Yat-sen University, with approval number (2015-BA-041). As this was a retrospective study, written informed consent to participate in this study was excused by the institutional review board.
Author Contributions
JL and YY made main contributions to the study conception and design. YY, HL, WW, HP, HH, SS, ZL, and MW contributed to the data collection, analysis, and interpretation. LJ and JL drafted the article and revised it. All authors agreed to submit the study to the current journal and gave final approval of the version to be published.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Grant No. 82003311, recipient: YY; 82072906, recipient: JL), Sun Yat-sen Memorial Hospital Cultivation Project for Clinical Research (Grant No. SYS-Q-202004, recipient: YY), Sun Yat-sen Memorial Hospital Yat-sen Scientific Research Launch Project (Grant No. YXQH201920, recipient: YY), Medical Science and Technology Research Fund of Guangdong Province (No. A2020391, recipient: YY), and Guangzhou Science and Technology Program (No. 202102010272, recipient: YY).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We appreciate the assistance from the Follow-up Registry Department of Sun Yat-sen Memorial Hospital, Sun Yat-sen University; Sun Yat-sen University Cancer Center; and Changhai Hospital.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.783487/full#supplementary-material
Differences of 5 years survival outcomes between age groups and molecular subtypes in breast cancer patients. HR+, Hormone receptor positive; HR-, Hormone receptor negative; HER2, human epidermal growth factor receptor 2; LRFS, Local recurrence free survival; DFS, Disease free survival; OS, Overall survival; 95%CI, 95% confidence interval.
Analysis of the association between therapy, age and survival for different tumor molecular subtypes. HER2, human epidermal growth factor receptor 2; LRFS, Local recurrence free survival; DFS, Disease free survival; OS, Overall survival; 95%CI, 95% confidence interval.
Abbreviations
LRFS, local recurrence-free survival; DFS, disease-free survival; OS, overall survival; SEER, Surveillance Epidemiology and End Results database; AJCC, American Joint Committee on Cancer Staging Manual; TNM, tumor–node–metastasis stage; HR+, hormone receptor positive; HR−, hormone receptor negative; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; EUSOMA, European Society of Breast Cancer Specialists; OFS, ovarian function suppression; LVI, lymphovascular invasion; IDC, invasive ductal carcinoma; DCIS, ductal carcinoma in situ; ILC, invasive lobular carcinoma; AI, aromatase inhibitor; TAM, tamoxifen; BCS, breast-conserving surgery; SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection; 95% CI, 95% confidence interval.
References
- 1. Sun, Kexin ZR, Gu X, Zhang S, Zeng H, Zou X, et al. Incidence Trend and Change in the Age Distribution of Female Breast Cancer in Cancer Registration Areas of China From 2000 to 2014. Chin J Prev Med (2018) 52(6):567–72. doi: 10.3760/cma.j.issn.0253-9624.2018.06.003 [DOI] [PubMed] [Google Scholar]
- 2. Fan L, Strasser-Weippl K, Li JJ, St Louis J, Finkelstein DM, Yu KD, et al. Breast Cancer in China. Lancet Oncol (2014) 15(7):e279–89. doi: 10.1016/s1470-2045(13)70567-9 [DOI] [PubMed] [Google Scholar]
- 3. Zheng Rongshou SK, Zhang S, Zeng H, Zou X, Chen R, Gu X, et al. Report of Cancer Epidemiology in China, 2015. Chin J Oncol (2019) 41(1):19–28. doi: 10.3760/cma.j.issn.0253-3766.2019.01.008 [DOI] [PubMed] [Google Scholar]
- 4. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer Statistics in China, 2015. CA Cancer J Clin (2016) 66(2):115–32. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 5. Yang Y, Liu J, Peng M, Su F, Xie X, Liu Z, et al. Introduction of a Multicenter Online Database for Non-Metastatic Breast Cancer in China. Sci China Life Sci (2020) 63(9):1–4. doi: 10.1007/s11427-019-1625-1 [DOI] [PubMed] [Google Scholar]
- 6. Paluch-Shimon S, Cardoso F, Partridge AH, Abulkhair O, Azim HA , Jr., Bianchi-Micheli G, et al. ESO-ESMO 4th International Consensus Guidelines for Breast Cancer in Young Women (BCY4). Ann Oncol (2020) 31(6):674–96. doi: 10.1016/j.annonc.2020.03.284 [DOI] [PubMed] [Google Scholar]
- 7. Partridge AH, Hughes ME, Warner ET, Ottesen RA, Wong YN, Edge SB, et al. Subtype-Dependent Relationship Between Young Age at Diagnosis and Breast Cancer Survival. J Clin Oncol (2016) 34(27):3308–14. doi: 10.1200/JCO.2015.65.8013 [DOI] [PubMed] [Google Scholar]
- 8. Colleoni M, Rotmensz N, Peruzzotti G, Maisonneuve P, Orlando L, Ghisini R, et al. Role of Endocrine Responsiveness and Adjuvant Therapy in Very Young Women (Below 35 Years) With Operable Breast Cancer and Node Negative Disease. Ann Oncol (2006) 17(10):1497–503. doi: 10.1093/annonc/mdl145 [DOI] [PubMed] [Google Scholar]
- 9. Ahn SH, Son BH, Kim SW, Kim SI, Jeong J, Ko SS, et al. Poor Outcome of Hormone Receptor-Positive Breast Cancer at Very Young Age Is Due to Tamoxifen Resistance: Nationwide Survival Data in Korea–A Report From the Korean Breast Cancer Society. J Clin Oncol (2007) 25(17):2360–8. doi: 10.1200/JCO.2006.10.3754 [DOI] [PubMed] [Google Scholar]
- 10. Anders CK, Johnson R, Litton J, Phillips M, Bleyer A. Breast Cancer Before Age 40 Years. Semin Oncol (2009) 36(3):237–49. doi: 10.1053/j.seminoncol.2009.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hu Y, Yang Y, Gu R, Jin L, Shen S, Liu F, et al. Does Patient Age Affect the PPV3 of ACR BI-RADS Ultrasound Categories 4 and 5 in the Diagnostic Setting? Eur Radiol (2018) 28(6):2492–8. doi: 10.1007/s00330-017-5203-3 [DOI] [PubMed] [Google Scholar]
- 12. Wang Y, Yang Y, Chen Z, Zhu T, Wu J, Su F, et al. Development and Validation of a Novel Nomogram for Predicting Distant Metastasis-Free Survival Among Breast Cancer Patients. Ann Transl Med (2019) 7(20):537. doi: 10.21037/atm.2019.10.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. American Cancer Society . Breast Cancer Facts &Figures 2019-2020. Atlanta, GA: American Cancer Society, 2020; (2019). [Google Scholar]
- 14. Darwish AD, Helal AM, Aly El-Din NH, Solaiman LL, Amin A. Breast Cancer in Women Aging 35 Years Old and Younger: The Egyptian National Cancer Institute (NCI) Experience. Breast (2017) 31:1–8. doi: 10.1016/j.breast.2016.09.018 [DOI] [PubMed] [Google Scholar]
- 15. Farouk O, Ebrahim MA, Senbel A, Emarah Z, Abozeed W, Seisa MO, et al. Breast Cancer Characteristics in Very Young Egyptian Women </=35 Years. Breast Cancer (Dove Med Press) (2016) 8:53–8. doi: 10.2147/BCTT.S99350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang Y, Liu J, Peng M, Su F, Xie X, Liu Z, et al. Introduction of a Multicenter Online Database for Non-Metastatic Breast Cancer in China. Sci China Life Sci (2020) 63(9):1417–20. doi: 10.1007/s11427-019-1625-1 [DOI] [PubMed] [Google Scholar]
- 17. Zouzoulas D, Tsolakidis D, Gitas G, Zafrakas M, Goulis DG, Douganiotis G, et al. Breast Cancer in Women Younger Than 35 Years Old. Arch Gynecol Obstet (2020) 302(3):721–30. doi: 10.1007/s00404-020-05695-z [DOI] [PubMed] [Google Scholar]
- 18. Choi DH, Kim S, Rimm DL, Carter D, Haffty BG. Immunohistochemical Biomarkers in Patients With Early-Onset Breast Carcinoma by Tissue Microarray. Cancer J (2005) 11(5):404–11. doi: 10.1097/00130404-200509000-00008 [DOI] [PubMed] [Google Scholar]
- 19. Colleoni M, Rotmensz N, Robertson C, Orlando L, Viale G, Renne G, et al. Very Young Women (<35 Years) With Operable Breast Cancer: Features of Disease at Presentation. Ann Oncol (2002) 13(2):273–9. doi: 10.1093/annonc/mdf039 [DOI] [PubMed] [Google Scholar]
- 20. Azim HA, Jr., Nguyen B, Brohee S, Zoppoli G, Sotiriou C. Genomic Aberrations in Young and Elderly Breast Cancer Patients. BMC Med (2015) 13:266. doi: 10.1186/s12916-015-0504-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Azim HA, Jr., Michiels S, Bedard PL, Singhal SK, Criscitiello C, Ignatiadis M, et al. Elucidating Prognosis and Biology of Breast Cancer Arising in Young Women Using Gene Expression Profiling. Clin Cancer Res (2012) 18(5):1341–51. doi: 10.1158/1078-0432.CCR-11-2599 [DOI] [PubMed] [Google Scholar]
- 22. Saha P, Regan MM, Pagani O, Francis PA, Walley BA, Ribi K, et al. Treatment Efficacy, Adherence, and Quality of Life Among Women Younger Than 35 Years in the International Breast Cancer Study Group TEXT and SOFT Adjuvant Endocrine Therapy Trials. J Clin Oncol (2017) 35(27):3113–22. doi: 10.1200/JCO.2016.72.0946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Llarena NC, Estevez SL, Tucker SL, Jeruss JS. Impact of Fertility Concerns on Tamoxifen Initiation and Persistence. J Natl Cancer Inst (2015) 107(10):djv202. doi: 10.1093/jnci/djv202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Francis PA, Pagani O, Fleming GF, Walley BA, Colleoni M, Lang I, et al. Tailoring Adjuvant Endocrine Therapy for Premenopausal Breast Cancer. N Engl J Med (2018) 379(2):122–37. doi: 10.1056/NEJMoa1803164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW. Adherence to Adjuvant Hormonal Therapy Among Breast Cancer Survivors in Clinical Practice: A Systematic Review. Breast Cancer Res Treat (2012) 134(2):459–78. doi: 10.1007/s10549-012-2114-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Differences of 5 years survival outcomes between age groups and molecular subtypes in breast cancer patients. HR+, Hormone receptor positive; HR-, Hormone receptor negative; HER2, human epidermal growth factor receptor 2; LRFS, Local recurrence free survival; DFS, Disease free survival; OS, Overall survival; 95%CI, 95% confidence interval.
Analysis of the association between therapy, age and survival for different tumor molecular subtypes. HER2, human epidermal growth factor receptor 2; LRFS, Local recurrence free survival; DFS, Disease free survival; OS, Overall survival; 95%CI, 95% confidence interval.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.