Abstract
Vitamin K is traditionally connected with blood coagulation, since it is needed for the posttranslational modification of 7 proteins involved in this cascade. However, it is also involved in the maturation of another 11 or 12 proteins that play different roles, encompassing in particular the modulation of the calcification of connective tissues. Since this process is physiologically needed in bones, but is pathological in arteries, a great deal of research has been devoted to finding a possible link between vitamin K and the prevention of osteoporosis and cardiovascular diseases. Unfortunately, the current knowledge does not allow us to make a decisive conclusion about such a link. One possible explanation for this is the diversity of the biological activity of vitamin K, which is not a single compound but a general term covering natural plant and animal forms of vitamin K (K1 and K2) as well as their synthetic congeners (K3 and K4). Vitamin K1 (phylloquinone) is found in several vegetables. Menaquinones (MK4–MK13, a series of compounds known as vitamin K2) are mostly of a bacterial origin and are introduced into the human diet mainly through fermented cheeses. Current knowledge about the kinetics of different forms of vitamin K, their detection, and their toxicity are discussed in this review.
Keywords: bioanalysis, coagulation, diet, menaquinone, phylloquinone
INTRODUCTION
Vitamin K is well known as an essential factor in blood coagulation. Hence its name, vitamin K, which is derived from the German term for coagulation (Koagulation). Its importance was recognized by the Nobel Committee for Physiology or Medicine, since the Nobel Prize in Physiology or Medicine for 1943 was awarded to Henrik Carl Peter Dam and Edward Adelbert Doisy for their discoveries of vitamin K and its chemical nature, respectively.1
Vitamin K is not a single compound but is a term for many similar compounds that have the physiological function of this vitamin (Fig. S1 in the Supporting Information online). They share a common structure, the 2-methyl-1,4-naphthoquinone core, also known as menadione. The simplest form, which contains only this core, is known as vitamin K3. In contrast to the natural forms, K3 is hydrophilic and is not obtained through the diet. However, it acts as an intermediate in human metabolism.2 Vitamin K obtained from the diet originates either from plant sources (in the form of vitamin K1, known as phylloquinone [phytomenadione, phytonadione]) or more commonly from animal sources in the form of vitamin K2 (menaquinone, generally abbreviated as MK, see Fig. S1 in the Supporting Information online). Vitamin K4 also exists, and this term is associated with other synthetic forms of vitamin K. It may be a reduced form of vitamin K3 (menadiol) or its ester forms (eg, diacetate vitamin K3).
DIETARY SOURCES OF VITAMIN K
Vitamin K1 is a single compound found in photosynthetic organisms like cyanobacteria, algae, and green plants.3–5 Due to the high vitamin K1 content in the green parts of plants, phylloquinone was originally thought to be present only in chloroplasts, but further research has confirmed that it is also present in peroxisomes and plasma membranes. It is even present in some non-photosynthetic parasitic plants. Its function in plants is best seen in the chloroplasts, where it is tightly bound to the thylakoid membrane. Here, it serves as an electron acceptor during photosynthesis, forming part of the electron transport chain of photosystem I. The function of vitamin K1 in other cellular compartments is also associated with its redox properties.5–7 In absolute amounts, it constitutes approximately 75%–90% of all vitamin K in our diet, but dietary K1 has low bioavailability,8–11 as will be discussed in more detail below. Green cruciferous vegetables (broccoli, brussels sprouts, cabbage, kale, kai-lan, etc.) are rich sources of vitamin K1, but spinach, chard, parsley, and various types of lettuce also have considerable phylloquinone content .8,12–14 In general, all edible green parts of plants can be considered an important source of vitamin K1. Of course, this also applies to wild edible plants. Examples include stinging nettles (Urtica dioica L.), wild garlic (Allium ursinum L.), dandelion leaves (Taraxacum campylodes G.E.Haglund), and ground elder (Aegopodium podagraria L.), a relative of parsley whose vitamin content is comparable with that of cultivated vegetables.15 Edible greens also include culinary herbs, of which marjoram (Origanum majorana L.), mint (Mentha × piperita L.), and savoury (Satureja hortensis L.) have the highest content of vitamin K1, reaching values of up to 1250μg/100 g when fresh and over 3000 μg/100 g in dried herb samples. The seeds of some species of Apiaceae plants that are used as spices also contain significant amounts of the vitamin K1.16 Data on their vitamin K1 content differ significantly from study to study because it can be affected by a number of factors, such as cultivar, cultivation method, cultivation site, climatic conditions, plant maturation, and the method of determination.17–19 The richest vegetable sources of vitamin K1, kale and spinach, are good examples of this. Its content in these vegetables appears to vary greatly: 250–1139 μg/100 g and 240–1220 μg/100 g, respectively.12,15,20 In most cases, neither boiling nor microwaving affects the vitamin K1 content of vegetables.12 Certain vegetable oils are also important dietary sources of phylloquinone for humans. Of these, soybean oil is the most significant source, followed by rapeseed oil and olive oil, containing on average about 180, 130, and 55 μg/100 g of phylloquinone, respectively.8,21,22 Since these oils are frequently used when cooking vegetables, they can enrich the food with additional vitamin K1. Its content in vegetable oils is relatively stable when heated, reaching a maximum decrease of 15% after being heated for 40 minutes at 185–190°C. On the other hand, vitamin K1 is extremely sensitive to daylight and fluorescent light, and these light sources decrease the vitamin content by 46% and 87% after only 2 days of exposure, respectively. Therefore, it is recommended to store oils in dark containers.22
Vitamin K1 is the predominant form of vitamin K used in food supplements and drugs indicated in vitamin K deficiency, and therefore production of a large amount is required. Synthetic vitamin K1 is manufactured by condensing naphthoquinone with isoprenoid precursors.4,23 Demand for vitamins of a natural origin and their sustainable production is increasing, so research is underway seeking efficient production strategies. One promising option is the use of microalgae and cyanobacteria, which can be grown in bioreactors under highly controlled conditions and which produce a significant amount of vitamin K1. This is true even when the algae are cultivated using conventional aquaculture techniques.3,4,24,25
Vitamin K2 is produced by certain obligate and facultative anaerobic bacteria. The form of vitamin K2 generated depends on the strain of bacteria. The various forms principally differ in the number of 5-carbon prenyl units, which ranges from 4 to 13 (Fig. S1B in the Supporting Information online). The number of these units is given as a suffix(-n), ie, they are named MK-4 to MK-13. It should be also pointed out that there can be differences between natural and synthetic vitamin K2, as was demonstrated in the case of MK-7, for which the synthetic mono-cis form (2Z) was inactive or had little activity compared with the natural all-E form. Some bacteria also produce one or more saturated prenyl units, and this is indicated by an additional suffix, eg, MK-8(2H) in Fig. S1C in the Supporting Information online or MK-9(4H).2,26–28 MK-4 (menatetrenone) is not produced by bacteria, but instead is formed in human and animal organisms from K1. It can hence be both consumed in the diet or formed in the human body. The enzyme responsible for this conversion is UbiA prenyltransferase domain–containing protein-1 (also known as transitional epithelial response protein 1, TERE1 ). This enzyme can cleave the side chain to release vitamin K3 and then prenylate it with geranylgeranyl pyrophosphate to produce MK-4. There is still some controversy as to whether or not vitamin K3 is produced in the gastrointestinal tract (GIT), and whether the enzyme TERE1 catalyzes only prenylation. It is possible that both the production of K3 in the GIT from human vitamin K by, eg, microflora, with subsequent metabolism to MK-4 by TERE1, and direct conversion of K1 to MK-4 by TERE1 could be occurring.2,8,29–32 Major sources of vitamin K2 in the human diet include dairy products. Cheeses are a particularly rich source, due to the bacterial fermentation that occurs when they are produced. In fact, about one-half of all vitamin K2 ingested by humans comes from cheese.33,34 Other important sources of MKs are fermented vegetables such as sauerkraut, and in Japan, nattō.27 The concentrations of MK-4 to MK-12 can reach high values (eg, 5–50 µg/100 g), with MK-8, MK-9, MK-10, and MK-11 being the major forms in many different cheeses (Fig. S2 in the Supporting Information online8,27,35–37). It should be stressed, however, that apparently discrepant data have been reported in relation to MK-10 content. Its levels can be even higher than that of MK-8 in some cheeses.36,37 The type of vitamin K2 is largely dependent on which bacterial species (eg, Lactococcus, Propionibacterium) is used in production. Ripening results in an increase in vitamin K2 content.37 Reduced-fat or fat-free cheeses contain less than one-quarter of the vitamin K2 compared with their full-fat equivalents.36 Sauerkraut is a less rich source of vitamin K2 (total MKs content is approximately 5 µg/100 g), but it contains a substantial quantity of vitamin K1,8,27 while nattō is by far the richest source of vitamin K2 (the content of MK-7 is approximately 900 µg/100 g). Nattō is produced from soybeans through fermentation with Bacillus subtilis var. natto, which is responsible for the production of MK-7. Other important dietary sources of vitamin K2 are bovine and pork liver, whereas vitamin K2 is barely present in most fish, with the important exception of eel.27,37 Fermented beverages like beer and wine do not contain detectable amounts of MKs, because yeasts, in contrast to bacteria, do not produce MKs.2,8,27 These theoretical nutritional data fit nicely into the assessment of human vitamin K2 consumption in Europe. In a large epidemiological study, the intake of vitamin K2 was more than 95% comprised of MK-4, MK-8, and MK-9; dietary MK-5 to MK-7 were detected in only low amounts.10 It should, however, be pointed out that vitamin K consumption assessment based on a dietary questionnaire is not very precise.38 Even though a large amount of research on vitamin K has been conducted, no precise daily requirement level for vitamin K (likely due to the wide range of vitamin K forms) has been established as yet. It has been suggested that the mean intake of vitamin K ranges from 70 µg/day to 300 µg/day,10,11,31,39–41 and the current recommendation by the European Food Safety Authority indicates 1 µg/kg per day is an adequate intake of total vitamin K in both children and adults, including pregnant women.31 Interestingly, the current average human intake of vitamin K in developed countries is in general above this recommendation, notwithstanding the low frequency of use of vitamin K supplements.28
An early study did not find substantial differences in the levels of vitamin K1, MK-7, and MK-8 between young and older persons.42 However, regarding what constitutes adequate vitamin K level and how usable vitamin K is in the elderly, current knowledge is unsatisfactory. Other research rather suggests a level of insufficient vitamin K status in the elderly.29,43,44
THE KINETICS OF VITAMIN K
Absorption
There are apparent differences in the absorption of the various forms of vitamin K. In general, bile salts enable the formation of mixed micelles, which allow the uptake of natural lipophilic forms of vitamin K into the enterocytes. The vitamin is further packaged inside the enterocytes into the chylomicrons, and it then enters systemic circulation directly via the lymphatic system31 (Fig. 1A). The absorption of vitamin K2, in particular long-chain MKs, is excellent and may even be complete due to the co-presence of fat, eg, in dairy products.8,9 Vitamin K1 is much less easily absorbed from the diet than dietary MK-7 or pure MK-4 are.8,45 However, pure vitamin K1 has better bioavailability than dietary vitamin K1, pure MK-4, or pure MK-9, but lower than pure MK-7.8,23,45,46 Dietary vitamin K1 is tightly bound to plant tissue, as mentioned, so pure vitamin K1 is better absorbed than the dietary form. We note that there is no difference in the bioavailability of vitamin K1 from various vegetables, and that cooking does not improve its absorption.47 Fat, however, increases vitamin K1 bioavailability from plant sources by about 3 times, but its bioavailability is still much lower than that of commercially solubilized vitamin K1.45 One likely reason is that fat stimulates bile secretion. There are also differences in vitamin K1 bioavailability between people with different types of diet. Diet involving fast-food eating resulted surprisingly in better absorption of vitamin K1 than other diets with the same amount of fat ingested. It is possible that vegetable oil offers more easily absorbable vitamin K1 than whole vegetables.48 Surprisingly, at least according to in vitro everted intestinal sac experiments, unsaturated fatty acids can decrease the extent of vitamin K1 absorption.49 There is also substantial interindividual variability in vitamin K1 absorption.45,48 Linear pharmacokinetics, ie, a linear relationship between dose and serum levels, were observed for both MK-7 and K1.23 Long-term administration of MK-7 results in a marked increase in its serum levels, in contrast to vitamin K1, for which serum levels are only slightly above placebo.23 This is because MK-7 has an elimination half-life of approximately 3 days, while that of vitamin K1 is about 1–2 hours.8,23 Further, MK-9 has been shown to have a long elimination half-life (about 60 hours).46 This is, however, less than that of MK-7, which suggests that prolongation of the side chain is not directly associated with increase in half-life. In contrast, MK-4 has a short half-life in systemic circulation, and, similarly to vitamin K1, its level dropped virtually to zero 24 hours after administration.45 In rats, the oral administration of various forms of vitamin K resulted in increased plasma and liver levels of vitamin K3, and MK-4 to MK-11; MK-1 to MK-3 and MK-12 to MK-14, however, were apparently not absorbed. There was no correlation between plasma and liver levels since, surprisingly, the highest plasma levels were observed after the administration of vitamin K3 and MK-8; MK-6 reached its highest levels in the liver.50 Furthermore, vitamin K absorption can be decreased by certain drugs: cholestyramine can decrease vitamin K absorption likely due to the binding of bile acids; rifampicin can decrease vitamin K absorption due to the induction of metabolism; and orlistat forces the patient to decrease their intake of fat since it is associated with unpleasant GIT side effects.51–54
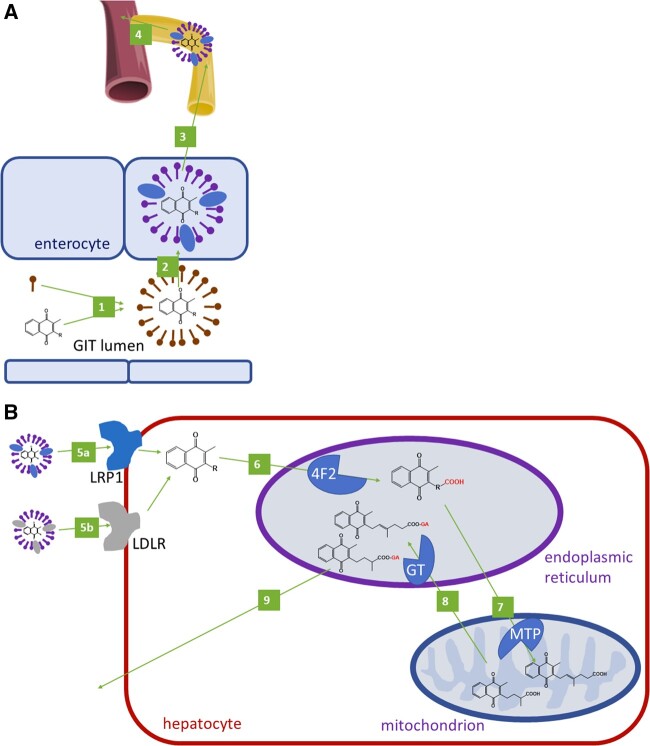
Figure 1.
Absorption and elimination of vitamin K. A: Oral absorption of vitamin K. 1: Formation of micelles from vitamin K and bile acids. 2: Uptake of vitamin K from a micelle to an enterocyte. 3: Formation and release of a chylomicron containing vitamin K. 4: Through lymphatic circulation, vitamin K in the chylomicron enters systemic circulation (through the vena cava inferior). B: Metabolism of vitamin K in the hepatocyte. A chylomicron loaded with vitamin K binds to low density lipoprotein receptor-related protein 1 (LRP1, 5a) or LDL particles loaded with vitamin K bind to LDL-receptors (LDLRs, 5 b), and this results in the uptake of vitamin K into the hepatocyte. A similar process can also be observed with VLDL in the peripheral tissues (not shown). 6: Vitamin K is ω-hydroxylated by cytochrome 4F2 in the endoplasmic reticulum. 7: This metabolite is subsequently β-oxidized by mitochondrial trifunctional protein (MTP) to 5 C or 7 C or 10 C (not shown), which are subjected to glucuronidation by UDP-glucuronosyltransferase (GT, 8) in the endoplasmic reticulum. 9: Excretion of glucuronides in feces and urine
As mentioned, human microflora produces MKs. Many bacterial species are able to synthesize vitamin K2, and the form synthesized is species specific: Bacteroides produce mainly MK-10 and MK-11, Eubacterium lentum MK-6, Veillonella MK-7, and Enterobacter and Escherichia coli MK-8.9,27,55,56 There has been some discussion about the extent of absorption of MKs produced by human microflora.9,27,31,57 One study demonstrated that they are able to be absorbed and reach systemic circulation, at least on a small scale.58 Furthermore, it is known that large-spectrum antibiotics can inhibit the growth of some vitamin K–producing bacteria and increase the risk of vitamin K deficiency.59,60 It is unlikely that the vitamin K2 forms with large side chains are absorbed in the distal colon, but they can be absorbed in the terminal ileum, where microflora is still present, and the absorption is further supported by the bile salts there. These large MKs are tightly bound to bacterial inner cytoplasmic membranes, and bile salts are hence required for their solubilization.27,61
After intestinal absorption, vitamin K1 appears to be taken up primarily by the liver. The reason for the predominant clearance of vitamin K1 in the liver might lie in the fact that vitamin K1 is transported mainly by chylomicrons, which are taken up by the liver, whereas vitamin K2 forms are also present in the very-low-density lipoprotein/low-density lipoprotein (VLDL/LDL) particles that are transported from the liver to the extrahepatic tissue. However, vitamin K2 is also apparently taken up by the liver, where it can be used for carboxylation of proteins.2,31,46,62 Cellular uptake of vitamin K is managed via lipoprotein receptors.2 In humans, plasma and serum levels of vitamin K1, MK-4, MK-5, MK-6, and MK-8 are low, and are expressed in units of nM or even in lower concentrations.42,46,48,63–68 The plasma concentrations of vitamin K1 and MK-4 in mice and rats are apparently similar to those in humans.32,69 There is, as expected, a large difference in plasma levels of MK-7 between European and certain Japanese populations due to the Japanese consumption of nattō. In Europe, the plasma levels of MK-7 are below 1 nM or in the low nM range,42,65 while in one recent Japanese study, the mean plasma levels of MK-7 were 15.6 nM.63 To enable a better comparison to be made, adjustments to triglyceride levels were recommended, but the results were essentially similar.31,65 The total body pool of vitamin K1 is roughly 0.55 µg/kg, but no such data are as yet available for vitamin K2.31
DISTRIBUTION AND ELIMINATION
Experimental data has shown that vitamin K1 and MK-4 are distributed differently. In rats, the same oral dose resulted in much higher levels in the liver of vitamin K1 than of MK-4, while the opposite was found in the aorta.70 This was also confirmed in human post-mortem liver analyses, in which the amount of vitamin K1 was always higher than of MK-4.71 Surprisingly, the sum of the MK-7 to MK-11 levels in the human liver was mostly much higher than that of vitamin K1.71,72 One study found that there were no differences in the liver vitamin K1 content and MK-7 to MK-9 content between non-cancerous liver samples and hepatitis or cirrhotic liver samples; however, a clear difference in the MK-10 to MK-13 content was observed. In that study, the subcellular localization of MK-10 and MK-11 was found to be mainly in the mitochondria.72 The catabolism of vitamin K1 and of vitamin K2 shares common mechanisms, beginning with initial ω-hydroxylation mediated by CYP4F2, followed by shortening of the polyisoprenoic side chain via β-oxidation to carboxylic acids (in 5 C, 7 C or 10 C metabolites, Fig. 1B), which are glucuronidated and excreted in urine and bile.2,31,73 Hence, the previously mentioned localization of vitamin K2 forms in the mitochondria could be linked to their metabolism via β-oxidation. The urinary excretion of these metabolites can be used as a marker of vitamin K body status.74
THE PHYSIOLOGICAL FUNCTION OF VITAMIN K
γ-carboxylation process
The only well-known function of vitamin K in humans is its involvement in the γ-carboxylation of a number of proteins (Fig. 2). Vitamin K is a crucial coenzyme for the posttranslational γ-carboxylation of glutamic acid residues on the luminal side of the rough endoplasmic reticulum (Fig. 375–78). This process requires 2 enzymes [γ-glutamyl carboxylase (vitamin K–dependent carboxylase) and vitamin K epoxide reductase (vitamin K epoxide reductase complex subunit 1, VKORC1), which are likely located in close proximity to one another in the membrane of the endoplasmic reticulum62,79–81] as well as carbon dioxide and oxygen. Proteins that are undergoing γ-carboxylation contain a homologous sequence of about 18 amino acids long located immediately upstream of the carboxylated domain. This domain binds to the γ-glutamyl carboxylase and markedly facilitates the enzymatic reactions catalyzed by this enzyme. Interestingly, there are different affinities between the various vitamin K–dependent proproteins and the enzyme, which might explain some differences in their processing.75,78,82 VKORC1 must first reduce vitamin K, which is found in food in its quinone form. The reduced form of vitamin K (hydroquinone) is modified by the carboxylase, by deprotonation, and by the subsequent incorporation of oxygen into the alkoxide. This form is a strong base that reacts with the targeted protein and forms a carbanion in the γ-position of glutamic acid. This enables carboxylation by γ-glutamyl carboxylase. The vitamin K epoxide must then be reduced again into quinone and subsequently hydroquinone by VKORC1, and the reaction continues.75,76 Through this process, vitamin K is recycled, so the physiological requirements of vitamin K are relatively low.83 Formed γ-carboxylated proteins are then likely transported via the classical secretory pathway through the Golgi apparatus, where the prosequence is mostly cleaved, as has been demonstrated for prothrombin by a Ca2+-dependent serine proteinase.84 There are, however, some differences: (1) matrix Gla-protein is exceptional, since the proprotein sequence is also maintained in the mature protein;85 and (2) the impact of carboxylation on secretion is different (Factor II and Factor X can be secreted both in the uncarboxylated and carboxylated forms, while proteins C and Z are secreted only in the carboxylated forms).86,87 In the case of protein C, the uncarboxylated form is decomposed by proteasome, which explains its inability to be excreted.87 Although mutated γ-glutamic acid carboxylated proteins can be cleft intracellularly,87 secreted γ-carboxyglutamated proteins cannot be reused and are excreted in urine.61 As a result, they have attracted great interest as being potentially useful for diagnostic purposes, as will be discussed later in this article.
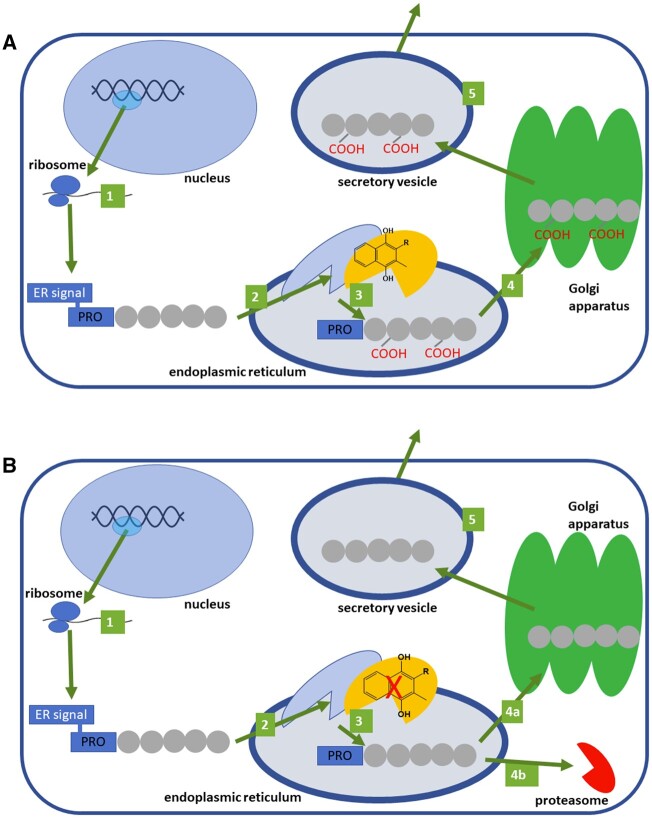
Figure 2.
The general fate of vitamin K–dependent proteins at the site of production. A: Under normal conditions. B: Under vitamin K depletion. 1: Preproprotein is synthesized from mRNA by ribosomes. 2: Preproprotein is targeted to the endoplasmic reticulum (ER), where the ER signaling sequence is removed and the protein is processed by (vitamin K–dependent) γ-glutamyl carboxylase. 3: Proprotein is carboxylated when vitamin K is present. 4: Carboxylated or 4a: uncarboxylated proprotein is transported to the Golgi apparatus, where the PRO-sequence is mostly cleaved (some proteins are also glycosylated there, not shown). 4b: In some cases under vitamin K deficiency, the uncarboxylated protein is cleft by proteasome. 5: Mature protein is extruded via a secretory vesicle into the extracellular space
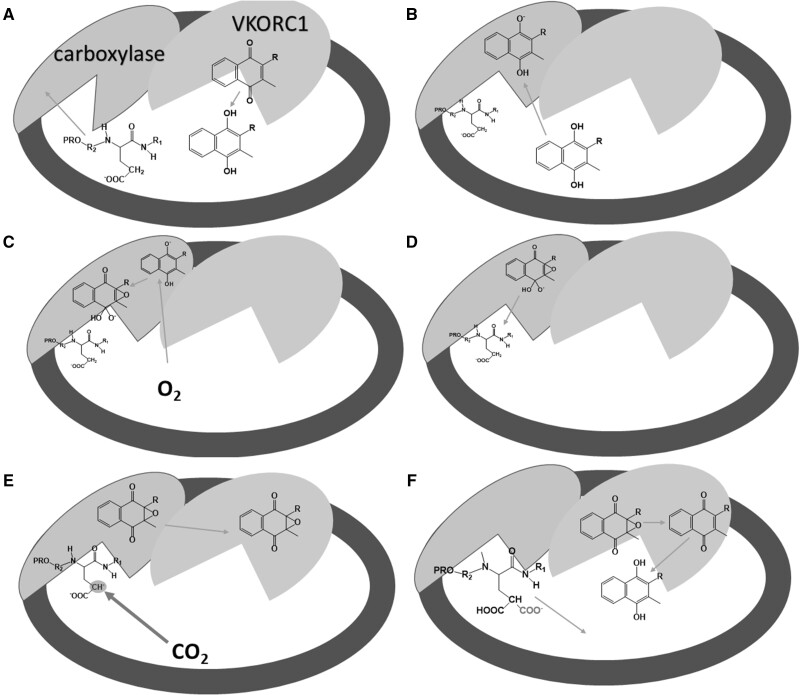
Figure 3.
Probable steps in the carboxylation process mediated by vitamin K in the endoplasmic reticulum. A protein that contains a PRO-sequence is targeted and subsequently bound to the carboxylase in the first step (A). This binding markedly increases the enzymatic function of the carboxylase. The quinone form of vitamin K is reduced to hydroquinone by VKORC1. Hydroquinone is deprotonated by carboxylase in the next step (B). Oxygen reacts with deprotonated vitamin K hydroquinone to produce alkoxide (C). This strong base deprotonates the γ-carbon of glutamyl residue to form a carbanion, which reacts with carbon dioxide (D–E). At the same time, vitamin K epoxide is formed (E). γ-glutamyl carboxylation is accomplished and the formed protein is released from the enzyme and further transported to the Golgi apparatus (not shown), while vitamin K epoxide is converted first to vitamin K quinone and then to vitamin K hydroquinone (F) by VKORC1. Data for this figure were taken from Rishavy et al (2004),75 Down et al (1995),76 Ayombil et al (2020)77 and Berkner (2000)78Abbreviations: carboxylase, vitamin K–dependent γ-glutamyl carboxylase; VKORC1, vitamin K epoxide reductase
There are some differences in the carboxylation process related to the form of vitamin K. Vitamin K3 (MK-0) is inactive and vitamin K1 is less active than MK-4; with increasing numbers of isoprene units, from MK-4 to MK-10, the carboxylation activity decreases.88 Also, MK-7 has been shown to be more active than vitamin K1 in the recovery of vitamin K–dependent synthesis of coagulation factors.23 In accordance with this, the best catalytic effects on carboxylation in rats were observed for MK-4 to MK-7. Another experiment showed MK-9 also to be active, but the lag time between administration and the effect was longer. Interestingly, vitamin K3 is not significantly active, even after intravenous administration.50 This information fits the reported trend in enzymatic vitamin K reduction, since vitamin K3 was again here inactive. At a concentration of 10 µM, there was no difference between vitamin K1 and MK-4 reduction, but at 100 µM, vitamin K1 was a better substrate. MK-7 was much less reduced than either vitamin K1 or MK-4.89 Interestingly, there was little difference between the epoxide, hydroquinone, and quinone forms, suggesting that the reduction process is more rapid than carboxylation itself.88
In the absence of vitamin K, or with a blockade of VKORC-1 by coumarin anticoagulants, vitamin K quinone can be reduced to vitamin K hydroquinone by cytosolic NAD(P)H–dependent oxidoreductase (flavoprotein DT-diaphorase), but vitamin K epoxide is not a substrate for this enzyme, so vitamin K recycling is not enabled.61 Moreover, at least in rats, DT-diaphorase has very low activity in the arterial wall compared with the liver.90 This seems to explain why the effects of vitamin K anticoagulants on blood coagulation are easily reversed by the administration of vitamin K1, which is rapidly taken up by the liver, whereas the extrahepatic inhibition of protein γ-carboxylation is not so easily reversed. Contrarily, MK-4 is able to normalize blood coagulation and reverse aortic calcification.70 It should be mentioned that, in extrahepatic tissues, vitamin K epoxide reductase like 1 (VKORL1) can also be involved in vitamin K recovery; however, its importance under physiological conditions seems to be low.2,81
γ-carboxyglutamate proteins
Currently, there are at least 18 or 19 human physiological proteins and 1 pathological protein for which posttranslational maturation is enabled by vitamin K–dependent γ-glutamyl carboxylation29,91 (Table 1). These proteins are known as Gla proteins (from γ-carboxyglutamic acid). They can be tentatively subclassified into a few categories according to their major (or more precisely their most well-known) effects. Many of these proteins have, however, more complex effects. There are 7 proteins involved in blood coagulation (coagulation factors II, VII, IX, and X and proteins C, S, and Z), 4 or 5 proteins primarily have effects on connective tissue mineralization or have a closely related role (4 well-documented Gla proteins: matrix Gla protein (MGP), osteocalcin, Gla-rich protein, and nephrocalcin; if the fifth, periostin, is a Gla protein is disputable), 4 are transmembrane receptors (proline-rich Gla proteins 1–4), 1 is a growth-factor-like signaling molecule, 1 binds to hyaluronic acid in the extracellular matrix, and the last is γ-glutamyl carboxylase itself.92 Given the necessity of this enzyme for other Gla protein formation, it is not surprising that in contrast to other Gla proteins, it does not necessitate the proprotein activation of the enzyme. The individual vitamin K–dependent enzymes will be briefly characterized in Table 1 and the following text.
Table 1.
Vitamin K–dependent Gla proteins and their available basic characteristics
| Protein | Year of discovery | Size of final protein | Number of Gla sequences | Function |
|---|---|---|---|---|
| Factor VII (proconvertin) | 1951 | 50 kDa | 10 | Zymogen, procoagulant |
| Factor IX (Christmas factor) | 1952 | 56 kDa | 12 | Zymogen, procoagulant |
| Factor X (Stuart–Prower factor) | 1953 | 56 kDa | 11 | Zymogen, procoagulant |
| Gas6 | 1988 (murine fibroblasts) | 75 kDa (678 AAs) | 11 | Likely a growth factor |
| γ-glutamyl carboxylase | 1975 | 758 AA (94 kDa) | Integral membrane enzyme | |
| Prothrombin | 1894 | 72 kDa | 10 | Zymogen, procoagulant |
| Protein C (autoprothrombin II-a) | 1960 | 56 kDa | 9 | Zymogen, anticoagulant |
| Protein S | 1977 | 80 kDa (635 AAs) | 11 | Anticoagulant, cofactor of protein C, immune and vascular system regulation |
| Protein Z | 1977 (bovine plasma), 1984 (human plasma) | 62 kDa (360 AAs) | 13 | Anticoagulant, with PZI blocks factor Xa |
| Matrix Gla protein (MGP) | 1983 | 11 kDa | 5 | Extracellular matrix protein |
| Nephrocalcin | 1981 | 14 kDa (110 AAs) | 2–3 | Inhibitor of the formation of calcium renal stones |
| Osteocalcin (BGP, bone Gla protein) | 1977 | 5.6 kDa (49 AAs) | 3 | Carboxylated – an extracellular matrix protein, uncarboxylated – a hormone |
| Periostin (osteoblastic-specific factor 2, OSF-2) | 1993 | 90 kDa (836) | 0 up to 24b | Extracellular matrix protein influencing the growth and repair of connective tissues |
| Proline-rich Gla proteins (PRGP1, PRGP2) | 1997 | PRGP1: 23 kDa (198 AAs) | ? | Transmembrane receptors likely involved in signaling pathways |
| PRGP2: 17 KDa (153 AAs) | ||||
| Inter-alpha-trypsin inhibitor heavy chain H2 (ITIH2) | 1988 | 72 kDa (648 AAs) | 2 | Heavy chain of inter-α-trypsin inhibitor |
| Transmembrane Gla proteins 3 and 4 (TGM3, TGM4)a | 2000 | TGM3: 23.7 kDa (212 AAs) | TGM3: 15 | Transmembrane receptors |
| TGM4: 19.9 kDa (177 AAs) | TGM4: 9 | |||
| Gla-rich protein (GRP) | 2008 | 15 | Modulator of tissue calcification, anti-inflammatory activity | |
| Transthyretin in Moyamoya disease | 2008 | 27.5 kDa (dimer) | 1 | Unclear; not present in healthy patients |
Also known as PRGP3 and PRGP4.
There is a report showing that periostin is not a Gla protein, and it is suggested that it can contain up 24 Gla residues (it has 28 glutamyl amino acid residues).
Abbreviations: AAs, amino acids ; PZI, Protein Z–dependent protease inhibitor
The first well-described proteins dependent on vitamin K were 7 players in the coagulation cascade, including (pro)coagulatory factors II (thrombin), VII, IX, and X, as well as anticoagulant proteins C, S, and Z.93–97 Coagulation factors VII, IX, and X, and protein C have high structural homology both in gene and protein structure and organization.94,97 In addition to γ-glutamyl carboxylation, they also undergo another specific posttranslational modification – the β-hydroxylation of aspartic acid or asparagine.94 In general, we can classify them as proenzymes (the majority being zymogens) or co-factors (protein S and Z). Proenzymes act as serine proteases upon activation. In fact, they share several features with the digestive enzymes chymotrypsinogen/chymotrypsin and trypsinogen/trypsin. In contrast to these nonselective enzymes, the coagulation factors with enzymatic activity mentioned here have an additional polypeptide chain, which procures narrower substrate specificity.94,97 All of these anticoagulation/coagulation factors, with the exception of protein S,98 are synthesized mainly in the liver. The Gla content ranges between 9 and 13. Binding to calcium leads to modifications in these Gla proteins, and the resultant structural changes cause the exposure of the phospholipid-binding domain, which anchors them in membranes, exposing in particular phosphatidylserine.61,94,97,99–101 This is apparently the crucial step, since in absence or inhibition of vitamin K regeneration, the coagulation process is inhibited. The roles of the vitamin K–dependent coagulation factors are shown in Fig. 4. Since protein Z is the newest member, it will be discussed here briefly. This anticoagulant protein shares similarities with other vitamin K–dependent proteins involved in the coagulation cascade, but lacks a functional serine protease activity.100,102 Its complex with protein Z–dependent protease inhibitor (PZI) binds to factor Xa on the phospholipid surface and blocks it. It seems to be important during pregnancy, since low levels of protein Z are associated with several pregnancy complications.103
Figure 4.
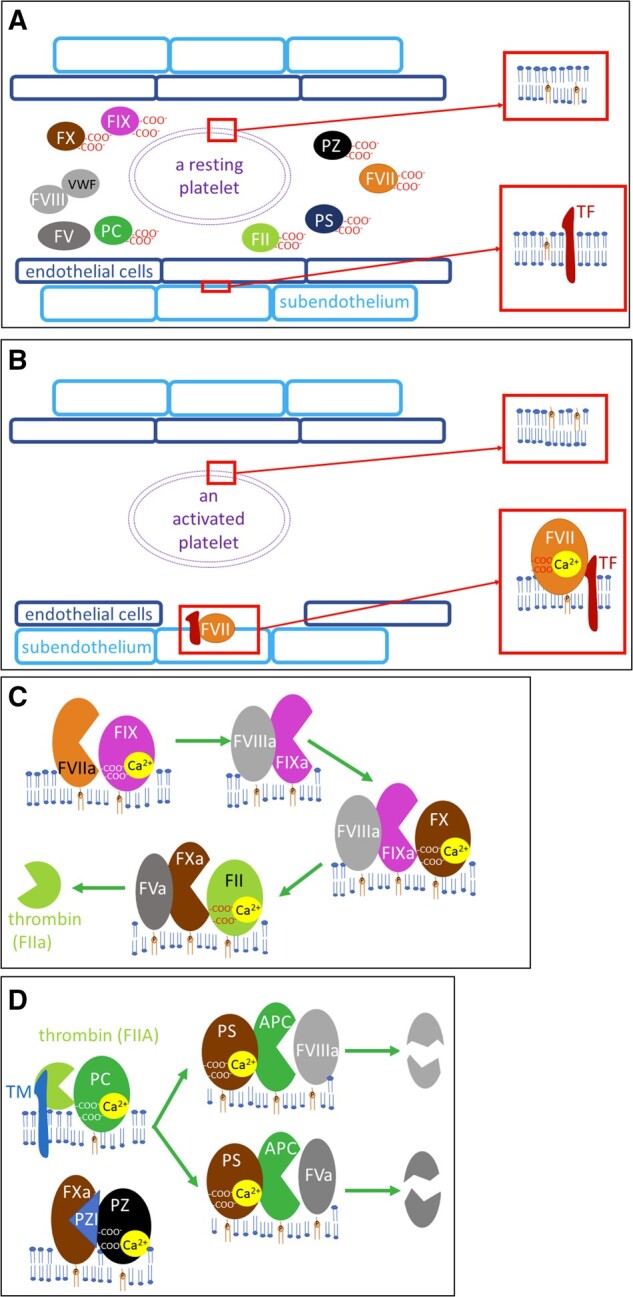
Vitamin K (pro)coagulatory and anticoagulatory factors. A: Resting state: coagulation factors are found in inactive forms in circulation, phosphatidylserine is not at the surface of the platelets (see the upper magnification), and tissue factor (TF) is not in direct contact with the blood (see the lower magnification). B: Activation of blood coagulation: in case of vascular damage, TF on subendothelial cells is now available for factor VII (FVII), which is activated. Platelet activation leads to modifications in the structure of the plasmatic membrane, with the exposure of phosphatidylserine on its surface. C: Activation of vitamin K–dependent coagulation factor: activated factor VII (FVIIa) cleaves factor IX (FIX) into an active enzyme (factor IXa, FIXa), which needs activated factor VIII (FVIIIa) for its activity. This complex activates factor X (FX) into an active enzyme (FXa), which needs activated factor V (FVa) for its activity. The whole FXa, FVa, calcium and phospholipid complex is also known as prothrombinase, and it activates thrombin (factor II, FII). Factor V (FV) or factor VIII (FVIII) are activated either by FXa or thrombin (not shown). D: Regulatory anticoagulant vitamin K–dependent factors: thrombin with thrombomodulin (TM) cleaves inactive plasma protein C (PC) into the active enzyme APC. For its enzymatic activity, APC also needs protein S (PS). PS does not require proteolysis in order to be active in PC-catalyzed lysis. This complex cleaves both FVa and FVIIIa. Protein Z (PZ) is a cofactor of PZ-dependent protease inhibitor (PZI), which blocks the enzymatic activity of FXa
Another important γ-carboxyglutamic acid protein, osteocalcin (also known as BGP and bone Gla protein, Fig. 5), has been largely associated with its effect on bones. Its physiological role is apparently more complex and clearly not fully understood. Most research has been carried out in mice, which however, have 2 genes for osteocalcin, together with 1 osteocalcin-related gene. This is an important difference from rats and humans, which have only 1 osteocalcin gene. For this reason, caution should be taken when interpreting animal studies. Some, but not all, results have been confirmed in limited number of human studies.104–109 Osteocalcin is synthesized mainly in osteoblasts, with a lesser contribution from odontoblasts and hypertrophic chondrocytes. Three glutamic acid residues are γ-carboxylated,104–106 and the signal sequence is removed by peptidases.77 This carboxylated protein is secreted from the intracellular vesicles into the bone matrix. This form has, as is the case for coagulation factors, a high affinity for calcium ions, and hence it binds to these ions in hydroxyapatite. It should be mentioned that it represents about 15%–20% of the noncollagen proteins in the matrix, which renders it the most frequent noncollagen protein in bones.108 It was once thought that carboxylated osteocalcin initiated hydroxyapatite formation, whereas the current data rather point out that it regulates the hydroxyapatite size and is crucial for the alignment of apatite crystals to collagen fibers. This ability is considered to be the reason for osteocalcin’s effect on bone strength and its protective properties against bone fractures.104,107 The data on other systemic effects of osteocalcin are less conclusive. Regardless, during bone resorption, the pH in bones decreases, which leads to osteocalcin decarboxylation and its release into the circulation.105,106 Un(der)carboxylated osteocalcin is considered by most authors to be a hormone.104,105,107,108 It has been shown, at least in animal studies, to affect glucose and lipid metabolism and to modulate fertility; it even has central nervous system effects, and could be involved in some types of cancer.104–106,108 The largest data are on the cross-link between osteocalcin and insulin, and un(der)carboxylated osteocalcin has been shown to improve insulin sensitivity and glucose tolerance. It causes the release of insulin, mainly via incretin glucagon-like peptide-1. Insulin, on the other hand, stimulates bone resorption and promotes the decarboxylation of osteocalcin, enabling its systemic effects. However, these data have been only partly confirmed in human studies; apparently, various factors such as gender, concomitant disease, and age influence the final effect.105–109 In addition, the information available on un(der)carboxylated osteocalcin receptors is not fully conclusive. Un(der)carboxylated osteocalcin has been shown to bind to GPRC6A, a G-protein-coupled receptor family C group 6-member A, in most tissues, and many of the above-mentioned effects are likely mediated by its binding to this receptor.108 At least in mice, another receptor mediates its effects in the brain.104,105 One candidate, Gpr158, has recently been suggested.110 In addition, un(der)carboxylated osteocalcin is considered to be a marker of bone quality, since its levels correlate with bone mineral density and are increased in bone fractures.111,112 Supplementation with vitamins K1 or K2 decreases the serum un(der)carboxylated osteocalcin level or the ratio of un(der)carbocylated to total osteocalcin68,109,113–116; hence, un(der)carboxylated osteocalcin is considered one of the markers of vitamin K deficiency.74
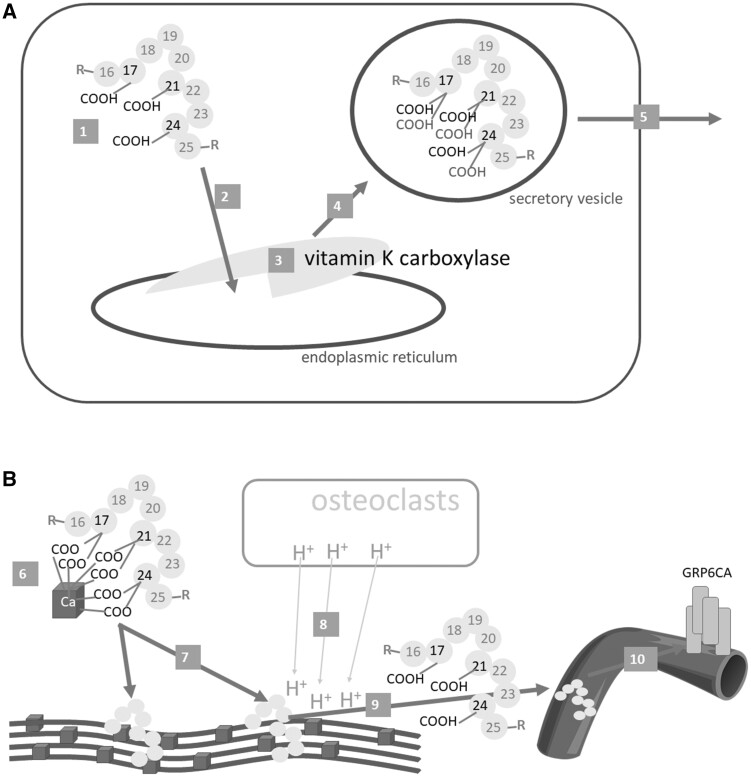
Figure 5.
Osteocalcin. A: Synthesis and release from the osteoblast. B: Effect on bones and systemic release. Osteocalcin (1) is synthesized as preproprotein, then travels to the endoplasmic reticulum (2), where 3 glutamic acid residues at positions 17, 21 and 24 are γ-carboxylated by γ-glutamyl carboxylase (vitamin K carboxylase) (3). The carboxylated osteocalcin is further processed (eg, see Figure 4), transported in the vesicles (4), and released into the bone matrix (5), where it binds calcium ions in hydroxyapatite (6). This is a crucial step in bone formation – the correct alignment of collagen fibers with hydroxyapatite (7). When osteoresorption takes place, osteoclasts decrease the pH level in the bones (8); this can lead to the decarboxylation of osteocalcin to form un(der)carboxylated osteocalcin, which is released into the systemic circulation (9), where it could exert its hormonal effect, in particular by binding to the GRP6CA receptor (10)
As carboxylated osteocalcin modulates extracellular matrix mineralization in bones, another protein that is dependent on vitamin K, MGP, blocks this process in other tissues where it would be pathological. In fact, it is synthesized in vascular smooth muscles and chondrocytes. It protects arteries and cartilage against mineralization.85,117 Apparently, both carboxylated osteocalcin and activated MGP act at the local level, ie, at the site where they are produced.85,118 In contrast to uncarboxylated osteocalcin, it is not known whether uncarboxylated MGP has any systemic effects. Moreover, MGP is not only carboxylated in 5 of 9 glutamate residues, it also undergoes serine phosphorylation in 3 of 5 serine residues (Fig. S3 in the Supporting Information online85). Carboxylation is again carried out by γ-glutamyl carboxylase, while phosphorylation occurs by the Golgi casein kinase.85,119 The function of phosphorylation is currently unknown, but it might be responsible for regulating trafficking. The inhibition of soft tissue calcification might be accomplished by its binding both to calcium and to bone morphogenic protein-2 (BMP-2).85,117 Solid evidence of the importance of MGP as an inhibitor of soft tissue calcification can be derived from MGP-deficient animals, which generally die within 2 months due to the calcification and subsequent rupture of the thoracic or abdominal aorta.120 Extensive arterial calcification is also observed in patients with Keutel syndrome, in which mutation of the MGP gene is present.85,121 Since the MGP can be presented as uncarboxylated-unphosphorylated, or uncarboxylated-phosphorylated and vice versa, or carboxylated-phosphorylated, it has been tested for diagnostic purposes. The results are ambiguous, but high levels of uncarboxylated-unphosphorylated MGP is considered to be a marker of vitamin K deficiency. It decreases after administration of vitamin K, but is dependent on age .68,83,85,91,117,122
Periostin: Periostin is another suggested γ-carboxyglutamyl protein, or more precisely glycoprotein, with an effect on bones. It was initially discovered in a mouse osteoblastic cell line and was formerly named osteoblastic-specific factor 2 (OSF-2).123 The current name derives from its presence in the periosteum of long bones. It is, however, widely expressed both in the fetus and in adults, and its effects are clearly not limited to the bones. It plays an important role in the growth and repair of damaged connective tissues (eg, bones, teeth, skin, tendons, and valves).124–127 It stimulates inflammatory reactions (eg, by the activation of NF-κB),125,126,128 which is likely linked to the healing process. On the other hand, it is also associated with fibrosis.125,127,128 On the molecular level, it is secreted into the extracellular matrix, where it can bind to several integrins and initiate signaling cascades associated with protein kinases.124,126 Interestingly, the γ-carboxylation of the possible 28 glutamyl residues has not been widely investigated, and there is a report indicating that gamma-glutamyl residues were not detected in periostin, either in lungs from patients suffering from idiopathic fibrosis or in a cell culture.129 It is, hence, not clear whether it is a Gla protein. Periostin has been suggested to be a marker of type II immune reactions in chronic inflammatory diseases such as asthma and atopic dermatitis, as well as a predictor of the effect of biologic drugs acting on this level. It is also thought to be a useful marker when making risk assessments of developing fractures and when investigating left ventricular function after myocardial infarction and chronic kidney disease.124–127,130 Given the diversity of these processes, it cannot be considered a selective marker. Moreover, it is highly expressed in youth, most likely due to its impact on bone growth.125 Contrarily, smoking decreases its expression.126 There have been some attempts to develop drugs that influence periostin or its receptors.125,127,131 Periostin has 4 isoforms designated 1–4, which differ in the presence/absence of particular exons, leading to varied (sometimes opposite) effects.91 This can complicate the deciphering of its physiological function and its possible use as a therapeutic target.
Nephrocalcin: Nephrocalcin is another acidic glycoprotein containing a Gla sequence. It is produced in the human kidneys. Nephrocalcin has 4 similar forms that differ in Gla content. Normal form A contains 3 Gla residues, while B and C only contain 2, and D none. In particular, normal form A binds 4 atoms of calcium and has a high affinity to calcium oxalate. It inhibits calcium oxalate crystallization. Interestingly, uncarboxylated nephrocalcin has been isolated from the urine and even from the kidney stones of patients suffering from them. Increased nephrocalcin has been detected in the urine of patients with renal cell carcinoma.132–136
Gas6: Gas6, a product of growth arrest–specific gene 6, shares 43% homology with protein S and has the same protein organization.137,138 In contrast to protein S, plasma Gas6 levels are very low, in subnanomolar concentrations, and it is not produced by the liver.98,138 Its target is the so-called TMA, three tyrosine kinase receptors that gave name to this family—Tyro3 (Sky), MerTK (c-mer), and Axl. Gla residues bind to calcium ions, and this causes a structural modification, which likely enables the binding of Gas6/protein S to the TMA receptors. Protein S binds only to the former 2 receptors and contains a thrombin-sensitive cleavage site, in contrast to Gas6.98,138 In fact, Gas6 is likely not involved in coagulation cascade-like protein S, but on the other hand it is involved in platelet aggregation.139,140 The pathophysiological function of Gas6 is extensive.138,139,141,142 It inhibits inflammatory reactions and plays a role as a growth factor, which may reflect its impact on cancer growth. Gas6 overexpression is observed in several cancers, and in general its expression predicts a poor prognosis.142 In addition to the aforementioned receptor binding, the Gla residues (via calcium and phosphatidylserine) are attached to damaged tissues in line with its role as a growth factor.138,139 It is also involved in tissue fibrosis.141 Gas6 has also been suggested to affect vascular calcification. However, this has recently been refuted.143 Since protein S binds to the same 2 receptors as Gas6, there may be an overlap between some of their functions, but decisive data on this are missing. The crucial involvement of protein S in blood coagulation is undisputable.98 One good example is the clear risk of venous thromboembolism in humans with rare cases of protein S deficiency.144 Interestingly, no other apparent important symptoms in these patients are described, which belies the other physiological role(s) of protein S. In any case, protein S is involved in immune and vascular system regulation. It should be emphasized that approximately 60% of protein S circulates with complement component C4b. It has been speculated that the C4b–protein S complex can bind via its Gla domain to the surface of certain membranes and in this way regulate the complement activity.98
Heavy chain 2 of inter-α-trypsin inhibitors: Two γ-glutamic acid carboxylated residues have also been discovered in heavy chain 2 of inter-α-trypsin inhibitors (gene ITIH2). The function of heavy chains of the inter-α-trypsin inhibitor family is interaction with hyaluronic acid, with a possible impact on maintaining the integrity of the extracellular matrix. Originally, the inter-α-trypsin inhibitor was designated a protein inhibitor. Its enzyme inhibition properties are rather weak, especially when compared with other physiological inhibitors. The structure is specific. It is composed of a common light chain (known as bikunin) to which 1 or 2 heavy chains are attached by a unique ester bond between the carboxyl group of the aspartic acid terminal residue and a C6-hydroxy group of the N-acetyl galactosamine residue of the chondroitin sulphate of the light chain. The 6 heavy chains that are currently known are structurally related. The function of the Gla residues of heavy chain 2 is unknown, since 3 other characterized heavy chains (1, 3, and 4) do not contain them .145–149
Proline-rich Gla proteins and transmembrane Gla proteins: Targeted amino acid sequence research resulted in the discovery of other Gla proteins. First, 2 proteins with specific proline-rich cytoplasmic regions were found. They were, hence, named proline-rich Gla proteins 1 and 2 (PRGP1 and PRGP2). They are widely distributed, with the highest expression of PRGP1 being in the spinal cord and of PRGP2 being in the thyroid gland.150 PRGP1 expression is limited to some tissues, whereas PRGP2 expression is more extensive.151 Further research showed that carboxylated PRGP2 is localized on the cell surface, and it was suggested that it has an intracellular binding partner YAP (Yes-associated protein).152 Later, other structurally similar members of the vitamin K protein family were discovered. They were initially named transmembrane Gla proteins 3 and 4 (TMG3 and TMG4). They are also known as PRGP3 and PRGP4. In fact, TMG3 is similar to PRGP1 and TMG4 to PRGP2. Both TMG3 and TMG4 are widely expressed in both fetal and adult human tissue.151
Gla-rich protein: The last-discovered member of the vitamin K–dependent proteins is Gla-rich protein (GRP). In comparison with other Gla proteins, it contains the highest number of γ-glutamic acid moieties, which span over the entire protein. It was initially discovered in the calcified cartilage of Adriatic sturgeon. Its ortholog is found in many species, including humans.153 In humans, GRP is widely expressed. Carboxylated GRP apparently acts as an inhibitor of tissue calcification, in particular of vascular and chondrocyte calcification, possibly by direct interaction with calcium.154–158 Its anti-inflammatory properties have also been documented.157 Given its similar function to osteocalcin and MGP, it is not surprising that it has a similar expression pattern, at least in some pathologies.154,157 Higher GRP accumulation has been observed in calcified aortic valves and atheromatous regions. Interestingly, foam cells contain lower levels of carboxylated and higher levels of uncarboxylated GRP.154 Similarly, higher GRP gene expression but only uncarboxylated GRP were detected in fibroblast-like synoviocytes from osteoarthritic patients. At the same time, lower levels of γ-glutamyl carboxylase and VKOR1 were found in these samples.157
Transthyretin: There is one other Gla protein that is contrarily considered to be pathological. Physiologically, transthyretin, a protein involved in the transport of the thyroid hormone thyroxine and retinol, is not a Gla protein. But in rare cerebral steno-occlusive Moyamoya disease, its γ-glutamic acid carboxylated counterpart was observed in cerebrospinal fluid. It has been observed in 71% of patients suffering from this disease.159–161
Some researchers have suggested that vitamin K might play an additional role(s) in the human body, such as directly binding to intracellular receptors, interfering with enzymes, or having antioxidant and/or anti-inflammatory activities.2,44,162
VITAMIN K DEFICIENCY
The symptoms of vitamin K deficiency are spontaneous cutaneous purpura, epistaxis, gastrointestinal, genitourinary, gingival, or other bleeding. Although vitamin K deficiency is not common in adults, it can occur for several reasons: (a) poor vitamin K dietary content, (b) many pathological states (eg, liver disease, cholestasis, cystic fibrosis, alcoholism, malabsorption states (including inflammatory bowel disease), and bariatric surgical intervention), and (c) pharmacotherapy with several drugs (mainly coumarin-based anticoagulants, but also the drugs previously mentioned that interfere with the absorption, metabolism, and synthesis of vitamin K, such as rifampicin and antibiotics).2,29,54,111,163,164 In new-born babies, there is also a risk of hypovitaminosis, since (a) they have poor vitamin K stores, (b) vitamin K levels are low in breast milk, and (c) immature GIT microflora is likely not a source of the vitamin. In line with these findings, exclusively breast-fed infants are more susceptible to the development of vitamin K hypovitaminosis than formula-fed children.31,165,166
DETECTION OF VITAMIN K AND OTHER MARKERS OF VITAMIN K STATUS
Making an accurate bioanalysis of vitamin K deficiency is not an easy task. Because it is among the most lipophilic and least abundant fat-soluble vitamins, it is extremely difficult to determine in human biological samples, even when using the most modern instrumentation. It can be measured directly in plasma, but there are many forms of vitamin K, and an abnormal lipid profile may also affect the result.74
Chromatographic methods have become dominant for directly measuring vitamin K. An example of such analysis is shown in Fig. S4 in the Supporting Information online.167 Unfortunately, the complex preparation of the samples also relates to the detection methods, such as mass spectrometry (MS), which has become the detection method of choice in vitamin K determination. There are many methods used for measuring systemic vitamin K levels.167–187 Some of them are briefly characterized in Table 2.167–187 Vitamin K deficiency will lead to a state in which Gla proteins cannot be carboxylated and are hence mostly released in their uncarboxylated form.61 Uncarboxylated vitamin K proteins can thus be alternative targets for quantitation, but these assays are not yet standardized, and some suffer from important drawbacks 74,163 or there are other aspects to consider. These proteins were formerly known as PIVKA (proteins induced by vitamin K absence). However, not all uncarboxylated proteins are secreted.86,87 Furthermore, elevated expression of proteins can overwhelm the intrinsic carboxylation system and lead to the production of uncarboxylated proteins, as was documented with PRGP2.152 Additionally, in contrast to coagulation Gla proteins, other Gla proteins are apparently not fully carboxylated, even in healthy organisms.28,109,156 The time aspect should also be considered, since some coagulation factors have shorter or longer half-lives.188 PIVKA II (des-carboxyprothrombin) is an earlier-known marker of vitamin K status with low sensitivity.68 It is (1) increased in many infants, and (2) its normalization takes months. In adults, it is clearly diagnostically better than prothrombin time or partial thromboplastin time, which are also affected by liver diseases, hematological diseases, and some other diseases, and their sensitivity in mild vitamin K deficiency is also low.31,74,163 Factor VII has also been tested, due to its short half-life, but it is not a sensitive marker.31 Another tested marker of poor vitamin K content in the body, and in particular in the bones, is uncarboxylated osteocalcin. The levels of total osteocalcin vary, so the percentage of uncarboxylated osteocalcin seems to be a more suitable marker. However, the available data do not fully support this, and estrogen levels might influence it.31,163 Uncarboxylated-unphosphorylated MGP as a marker of vitamin K deficiency was mentioned above. A sensitive marker of vitamin K1 dietary depletion is the urinary concentration of Gla residues. However, data on vitamin K2 deficiency in relation to this marker are not available, no parameter cut-off for vitamin K deficiency has yet been established, and the measurement usually requires 24 h urinary collection.31,68,74 Moreover, supplementation of vitamin K did not modify urinary Gla residues, in contrast to the clear increment in the carboxylated:total osteocalcin ratio.64 Another possibility is the measurement of urinary menadione (vitamin K3) excretion, which also changes in response to vitamin K depletion or repletion.68
Table 2.
Advantages and disadvantages of various analytical techniques for the determination of vitamin K in human biological matrices
| Technique | Sensitivity (nM if not specified otherwise) | Advantages | Disadvantages | References |
|---|---|---|---|---|
| LC-PDA/UV | Commonly available technique |
|
Zhang et al (2019)169; Chatzimichalakis et al (2004)168 | |
| LC-FLD |
|
|
Ahmed et al (2015)172; Kishikawa and Kuroda (2014)170; Klapkova et al (2018)171; Zhang et al (2019)169 | |
| LC-EC | Low limits of detection |
|
Zhang et al (2019)169; Harrington et al (2005)173; Kamao et al (2005)174 | |
| LC-CL |
|
|
Ahmed et al (2011)177; Zhang et al (2019)169 | |
| LC-MS |
|
|
Gentili et al (2014)176; Dunovska et al (2019)179; Abro et al (2014)183; Kamao et al (2007)175; Karl et al (2014)167; Levêque et al (2019)182; Hu et al (2018)180 | |
| SFC-MS | K1: 0.22a,b |
|
|
Górská (2019)184; Sandvik et al (2017)178 |
| Immunochemical assays |
|
|
MyBioSource185–187 | |
| Electrochemical sensors | K2: 9 pg/mLa (0.02)d | Simplicity, cost effectiveness, reproducibility, easy handling, miniaturization and high sensitivity |
|
Jedlinska et al (2018)181 |
LOD (limit of detection).
LOQ (limit of quantification).
LLOQ (lower limit of quantification).
Recalculated for MK-4.
Abbreviations:K1, Vitamin K1 (phylloquinone); K2, Vitamin K2 (either the methodology does not allow differentiation between individual K2 forms or not specified); LC-PDA/UV, liquid chromatography with photodiode/ultraviolet detection; LC-FLD, liquid chromatography with fluorescence detection; LC-EC, liquid chromatography with electrochemical detection; LC-CL, liquid chromatography with chemiluminescence detection; CL, chemiluminescence; LC-MS, liquid chromatography with mass spectrometry detection; SFC-MS, supercritical fluid chromatography with mass spectrometry detection
THE PROPHYLACTIC AND THERAPEUTIC POTENTIAL OF VITAMIN K
Approved indications of vitamin K administration are not extensive. Vitamin K is indicated for the prevention of hemorrhagic disease in newborns or as an antidote to correct vitamin K antagonist overshooting or poisoning, since vitamin K antagonists are also used as rodenticides. Oral administration is preferred in most cases. Prophylaxis with vitamin K markedly decreased the vitamin K deficiency in new-born babies due to their above-mentioned poor vitamin K stores.31,165,166 It has been suggested that preterm infants have an even greater risk of vitamin K deficiency, but there are currently insufficient data in relation to the prevention of this deficiency.189 Importantly, the effect of vitamin K administration on coagulation normalization is very rapid.166
Analyzing the 18 or 19 Gla proteins formed by presence of vitamin K, it is apparent that the effects of vitamin K are not limited to blood coagulation. First, their effects on bone and vascular calcification are becoming more apparent. It is, hence, not surprising that a large amount of research has been dedicated to investigating whether increased intake and/or supplementation with vitamin K can positively affect osteoporosis, fractures, and cardiovascular diseases.29,31,163,165 An effect on cancer has also been suggested, and there are reports on a possible relationship between vitamin K and cognitive function, highlighting its role in brain physiology.190
There are solid background data justifying testing of the possible use of vitamin K in the treatment of osteoporosis and the prevention of bone fractures: (1) there is the above-described positive effect of osteocalcin on bones, (2) a number of studies have reported that low serum vitamin K1 levels or low vitamin K1 intake and high un(der)carboxylated osteocalcin levels are associated with an increased risk of fractures, (3) the pharmacological inhibition of vitamin K reduction due to binding to VKORC1 by coumarin anticoagulants such as warfarin may affect bone quality, (4) similarly, the absence of VKORC1 is associated with calcification abnormalities, and (5) vitamin K was a successful treatment in many animal studies (eg, in the tail-suspension rat model of bone volume loss, or in a sciatic neurectomized rat model of osteoporosis).2,111,163,191–195 Most epidemiological as well as clinical studies have demonstrated that the intake of vitamin K can have a positive effect on the bones. On the other hand, this was not found in all studies. Additionally, 2 of the positive effect trials were retracted , so the real effect of vitamin K on osteoporosis remains rather elusive.196 A meta-analysis of 4 prospective cohort studies and 1 nested control study, including a total of almost 81 000 subjects, suggested that the population with the highest intake of dietary vitamin K1 had a 22% lower risk of fractures. A subgroup analysis documented that the effect was observed after 10 years. The authors also observed that an increase of daily vitamin K1 intake by 50 µg was associated with a 3% lower risk of fracture.197 There are also meta-analyses reporting the effect of vitamin K supplementation. The first meta-analysis of 17 studies showed that vitamin supplementation had a positive effect on bone mineral density in the lumbar spine, but not at the femoral neck. Moreover, the net effect on the lumbar spine was questioned by the authors, since it was observed only in Asian and not in Western studies. It was also limited to women and to vitamin K2.198 The latter is not so surprising, given the observed differences in the pharmacokinetics and pharmacodynamics between these forms. The authors speculated that a greater effect might be observed in secondary bone loss caused, eg, by pharmacotherapy with glucocorticoids.198 Additionally, in another meta-analysis, it was shown that the effect of vitamin K2 is observed mainly in postmenopausal women with osteoporosis.113 This investigation of 19 randomized controlled trials with 6759 participants reported the improvement of lumbar and forearm bone mineral density, in particular, after long-term use by osteoporotic women. There was no significant improvement in non-osteoporotic women. The doses in the analyzed studies were mostly 45–90 mg/day of MK-4 or 100–180 µg/day of MK-7 . The overall effect on fractures was numerically high (a decrease in risk by 36%), but not significant. However, a significant decrease of 53% was reported after rejecting 1 study that induced heterogeneity. Interestingly, an older meta-analysis, which included mainly trials with MK-4, showed a massive and even more potent reduction of risk of hip, vertebral, and non-vertebral fractures.199 This analysis was, however, apparently influenced by 2 retracted trials.196 Also, the newest meta-analyses did not clarify the issue, since when clinical trials with different vitamin K forms were assessed, vitamin K supplementation decreased the risk of clinical fractures but not vertebral fractures, although, when assessing the effect of randomized studies with solely MK-4, there was no significant effect on any fracture incidence. Both meta-analyses, however, confirmed a significant effect of vitamin K supplementation on lumbar bone mineral density.116,200 The net effect could also have been influenced by different concomitantly used drugs and comparators (eg, vitamin D, bisphosphonates). Summing up the available information, some positive effect of vitamin K on osteoporosis and decreased frequency of bone fractures seems to be possible, but apparently a long period of treatment is needed to achieve this effect. In addition, vitamin K may not be able to stop a reduction in bone mineral density or content in some parts of the skeleton, but may slow down its progression. Again, this effect may require long-term treatment. The effect of MK-7 supplementation on femoral bone is one example. It was not present after 1 or 2 years of treatment; 3 years were needed to document its positive influence.115 Based on this information, long-term supplementation with vitamin K in postmenopausal women with osteoporosis might have some potential utility, in particular, given its negligible risk of serious side effects. This is also supported by the fact that MK-4 in relatively high doses is registered in Japan for postmenopausal osteoporosis.61,111
The main reason for speculating that vitamin K has positive cardiovascular effects stems from its involvement in the synthesis of MGP. As has been described here in detail, MGP blocks the calcification of arteries. It need not be emphasized that the calcification of arteries is an independent risk factor for the development of coronary heart disease and coronary events.201,202 In addition, several clinical trials have reported that vitamin K antagonists promote atherosclerotic calcification.29 There has been a lot of discussion about whether there is subclinical hypovitaminosis in certain population groups. A substantial under-γ-carboxylation of vitamin K–dependent proteins has been found in postmenopausal women,64,114 and about one-quarter of a cohort ranging from 42 years to 89 years had very low plasma vitamin K1 levels.66 If this theory is correct, then vitamin K supplementation should reduce the risk of arterial calcification and coronary artery disease. Epidemiological studies from the Netherlands have found that vitamin K2, but not vitamin K1, limits coronary calcification and decreases the incidence of and mortality due to coronary artery disease.10,11,203 Also, a very recent Norwegian study has found that higher intake of vitamin K2 decreased the incidence of coronary artery disease, but vitamin K1 had no effect, notwithstanding the fact that higher quartiles of vitamin K1 intake, compared with vitamin K2 intake, were associated with higher physical activity.204 The lack of effectiveness of vitamin K1 was confirmed by the extension of the Dutch EPIC study with a median follow-up of 16.8 years. The same study, however, also reported only a tendency of vitamin K2, particularly its long chain forms, to reduce cardiovascular mortality.205 Considering the differences between the pharmacokinetic and pharmacodynamic data on vitamins K1 and K2 encompassing: the forms of vitamin K2 having longer half-lives, a more potent effect, and higher concentrations in extrahepatic tissues, a stronger effect of vitamin K2 over vitamin K1 would be expected. On the other hand, a similarly designed Spanish epidemiological study found a different outcome. Both forms of vitamin K decreased cardiovascular mortality, but the decreases were insignificant. However, an increased intake of vitamin K1, but not vitamin K2, during the study significantly attenuated cardiovascular mortality.206 Explaining this difference is not easy. One should emphasize that the intake of vitamin K1 was apparently much higher in the Spanish study, which is quite logical, given the difference in vegetable consumption between these two countries. Although the populations were different, this was likely not the reason: on the one hand, a higher consumption of vitamin K1 was linked with a healthier lifestyle in the Spanish study, but in the Dutch EPIC study, it was linked with hypertension, hypercholesterolemia, and diabetes. On the other hand, higher vitamin K2 consumers in the Rotterdam Study had a rather healthier lifestyle. There is also an American epidemiological study in which the average consumption of vitamin K1 was even lower. This study reported that vitamin K1 decreased the incidence of coronary heart disease, but had no effect on mortality due to this disease or on the risk of stroke.39 Interestingly, the authors themselves reported that a positive impact was seen in connection with dietary patterns associated with a high phylloquinone diet. Indeed, higher intake of vitamin K1 generally reflects healthier diets and lifestyles.68
Another study found that patients suffering from chronic kidney disease with inadequate total vitamin K intake (K1 + K2) had higher cardiovascular and all-cause mortality than those with adequate intake.40 A recent meta-analysis of 21 observational studies found that higher intake of either vitamin K1 or vitamin K2 was associated with a decrease in incidence of coronary artery diseases, with the impact of vitamin K2 being numerically higher. Regardless, neither form of the vitamin impacted the cardiovascular fatalities.207 Another meta-analysis confirmed that neither form was found to have an impact on cardiovascular mortality.208 It should also be emphasized that all of these studies were epidemiological studies using questionnaires to assess dietary habits with its known limits38,68; for a definite conclusion on the effect of vitamin K on cardiovascular diseases, prospective clinical studies are needed. The current clinical studies have not brought decisive data: supplementation with vitamin K1 slightly reduced coronary artery calcification in older persons, while supplementation with MK-7 did not influence femoral arterial calcification in diabetic patients.67,209 A meta-analysis of 3 controlled trials also found that vitamin K supplementation decreased vascular calcification. It should, however, be mentioned that this effect was largely driven by one single study.122 The most recent systematic review was not conclusive about the possible impact of vitamin K on calcification of major vessels.210 On the other hand, higher levels of uncarboxylated dephosphorylated MGP, as the above-mentioned marker of vitamin K deficiency, were found to be associated with higher incidence of coronary artery disease and even cardiovascular mortality.207 This was, however, not fully confirmed in another meta-analysis.122 It should also be mentioned that higher levels of uncarboxylated dephosphorylated MGP can be a consequence of administration of vitamin K antagonists and can, therefore, simply reflect that these patients are more ill.207,211,212 Hence, positive cardiovascular effects are not clearly documented for either form of vitamin K, and clinical long-term studies are definitely needed. Based on their negligible toxicity, vitamin K1 and/or vitamin K2 might be potentially administered to patients suffering from coronary artery disease in cases where there is an assumption of low vitamin K levels. They should not, however, be treated with vitamin K anticoagulants.28
There are additional data concerning the effects of vitamin K on all-cause mortality, cancer, and diabetes. Higher levels of uncarboxylated dephosphorylated MGP are associated with all-cause mortality, and this supports the possible impact of vitamin K.207,208,212 The Spanish PREDIMED epidemiological study found that a higher intake of vitamin K1 or vitamin K2 decreased both all-cause mortality and cancer mortality.206 The effect of vitamin K1 on all-cause mortality was contrarily not observed in the most recent study data obtained from the extension of the Dutch EPIC study, which also reported a positive influence of long-chain vitamin K2 forms only, and then only in 1 of 3 models.205 Also, a recent meta-analysis did not find an impact from either form of vitamin K on all-cause mortality.208 The EPIC-Heidelberg study did not find any relationship between vitamin K1 and cancer incidence or mortality, but it did document that vitamin K2 decreased mortality due to cancer. This finding was likely driven by the effect of vitamin K2 on lung cancer. Vitamin K2 also decreased the risk of developing lung and prostate cancer, but had no effect on colorectal or breast cancer.33 The existing literature has some data on the anticancer effects of vitamin K. Most data relate to synthetic vitamin K3, which (on a molecular basis) is apparently a much more active anticancer drug than either vitamin K1 or vitamin K2. Vitamin K3 can redox-cycle, and this represents the major difference from the natural forms of vitamin K, which have a side chain blocking this pro-oxidation mechanism. Interestingly, although the natural forms of vitamin K are less active, a few clinical phase I/II studies have reported some promising data for both.213
Higher vitamin K2 has been suggested to slightly decrease the risk of developing diabetes mellitus type II, while vitamin K1 had only a tendency in this respect. Vitamin K2 also very slightly increased HDL cholesterol and decreased systemic inflammation.34 Since the scientific evidence is limited in such cases, no recommendations can be given at the moment.
An emerging issue is the impact of vitamin K on age-related diseases. Low vitamin K levels are suggested to be associated with functional decline, osteoarthrosis, sarcopenia, mobility limitation and disability, and frailty in older persons. These conditions might be at least partly related to low-grade inflammation and pathological calcification. Both of these processes can be related. Vitamin K can, hence, bring some benefit when they occur. The mechanism has not been elucidated, but it might be associated with inhibition of soft tissue mineralization, anti-inflammatory effects, and impact on the mitochondria.29,44
Very recent research on vitamin K has brought a surprising discovery: vitamin K2 (form not specified) was found in a docking study to be the best ligand for free fatty acid binding pockets in the SARS-CoV-2 spike protein, and hence could potentially impact its binding to human cells via the ACE2 receptor.214
TOXICITY OF VITAMIN K
There have been almost no reported cases of natural vitamin K systemic toxicity, either in animals or in humans. There has been some concern that a high intake of vitamin K could result in overcoagulation. However, this has not been observed, possibly due to the limited sites for γ-carboxylation. Contrarily, very high doses of vitamin K can paradoxically cause hypoprothrombinemia, as has been documented in rare human case reports. In animals, massive doses have led to hemorrhages and anemia.215 Available data in humans show that 10 mg/day of vitamin K1 given for 1 month is not associated with any adverse effects. This is in line with the findings from animal experiments, in which even 2 g/kg for the same period of time was safe.31 In general, the reported side effects of vitamin K have only been local, eg, minor gastrointestinal complaints and skin rashes after vitamin K2, which disappear after discontinuing administration.9,28,113,116 Clinically, intramuscular administration of vitamin K1 is not very convenient due to pain at the site of injection and skin bruising. Therefore, more modern approaches such as microneedles are being examined.29 It should be mentioned, however, that vitamin K3 in high doses can lead, in a dose-dependent manner, to a toxic reaction, including hemolytic anemia, particularly in new-born infants. This reaction is likely associated with the redox-cycling of this vitamin, which does not occur in natural vitamins K1 and K2 due to the absence of unsubstituted position 3, which is highly reactive and binds thiol groups to form thioethers.2,216 Furthermore, preparations of vitamin K1 available for intravenous administration are sometimes associated with anaphylactoid reactions. Because of the lipid solubility of vitamin K, the preparations are aqueous colloidal suspensions that can induce anaphylactoid reactions. However, liposomal preparations can avoid this adverse reaction.217
CONCLUSIONS
Vitamin K has been traditionally linked with coagulation. Although this connection is correct, vitamin K has many other roles in human physiology. Vitamin K is necessary for the posttranslational modification of 18 or 19 proteins, among which 11 or 12 are not related to coagulation. In particular, its involvement in connective tissue calcification has stimulated intense research, although the data are still inconclusive. Vitamin K is not a single compound. Indeed, the diversity and the varied biological properties of the various forms of vitamin K can potentially explain the failure of research to date to clearly establish the role vitamin K plays in human beings. Apparently, additional (and particularly prospective clinical) studies are needed in order to clarify its non–coagulation-related effects in humans. Regardless, supplementation with natural forms of vitamin K is largely safe and hence is acceptable under specific conditions, such as in postmenopausal osteoporosis.
Supplementary Material
Acknowledgments
English expression in this article was revised by the agency “theBESTtranslation.”
Author contributions. P.M. and A.C. wrote about kinetic, physiological, and pharmacological aspects, Ka.M. wrote about the sources of vitamin K, L.K.K., L.J., Kr.M., M.P., and L.N. prepared the chemical and analytical parts of the article, and F.R. prepared the toxicological section. All authors participated in the critical revision of the article. P.M., L.N., Kr.M., A.C., L.J., and L.K.K. obtained funding for the open access publication.
Funding. This open-access review paper was supported by the Erasmus+ Programme of the European Union, Key Action 2: Strategic Partnerships (Project no. 2020–1-CZ01-KA203-078218). The authors also acknowledge the support of the EFSA-CDN (Efficiency and Safety Improvement of Current Drugs and Nutraceuticals: Advanced Methods – New Challenges) project (CZ.02.1.01/0.0/0.0/16_019/0000841) co-funded by the European Regional Development Fund. Kr.M. acknowledges support from Charles University (Project SVV No. 260 548, GA UK No. 360221), A.C. and L.J. to the Czech Health Research Council (NU21J-02–00021), and L.K.K. to MHCZ-DRO (Ministry of Health Czech Republic – Development Research Organisation, University Hospital Hradec Králové, 00179906).
Declaration of interest. The authors have no relevant interests to declare.
Supporting Information
The following Supporting Information is available through the online version of this article at the publisher’s website.
Figure S1 Chemical structures of vitamin K
Figure S2 Vitamin K content in selected food
Figure S3 Structure of matrix Gla protein (MGP)
Figure S4 An HPLC analysis of vitamin K standards and a spiked human fecal sample
REFERENCES
- 1. Raju TNK. The Nobel Chronicles. Lancet .1999;353:761. [DOI] [PubMed] [Google Scholar]
- 2. Shearer M, Newman P.. Recent trends in the metabolism and cell biology of vitamin K with special reference to vitamin K cycling and MK-4 biosynthesis. J Lipid Res .2014;55:345–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tarento TDC, McClure DD, Dehghani F, et al. Pilot-scale production of phylloquinone (vitamin K1) using a bubble column photo-bioreactor. Biochem Eng J. 2019;150:107243. [Google Scholar]
- 4. Tarento TDC, McClure DD, Talbot AM, et al. A potential biotechnological process for the sustainable production of vitamin K1. Crit Rev Biotechnol .2019;39:1–19. [DOI] [PubMed] [Google Scholar]
- 5. Manzotti P, Nisi P, Zocchi G.. Vitamin K in plants. Funct Plant Sci Biotechnol. 2008;2:29–35. [Google Scholar]
- 6. Reumann S. Biosynthesis of vitamin K1 (phylloquinone) by plant peroxisomes and its integration into signaling molecule synthesis pathways. Subcell Biochem .2013;69:213–229. [DOI] [PubMed] [Google Scholar]
- 7. Gu X, Harding SA, Nyamdari B, et al. A role for phylloquinone biosynthesis in the plasma membrane as revealed in a non-photosynthetic parasitic plant. bioRxiv. 2018;257519. [Google Scholar]
- 8. Schurgers LJ, Vermeer C.. Determination of phylloquinone and menaquinones in food. Effect of food matrix on circulating vitamin K concentrations. Haemostasis. 2000;30:298–307. [DOI] [PubMed] [Google Scholar]
- 9. Beulens JWJ, Booth SL, van den Heuvel EGHM, et al. The role of menaquinones (vitamin K2) in human health. Br J Nutr .2013;110:1357–1312. [DOI] [PubMed] [Google Scholar]
- 10. Gast GC, de Roos NM, Sluijs I, et al. A high menaquinone intake reduces the incidence of coronary heart disease. Nutr Metab Cardiovasc Dis .2009;19:504–510. [DOI] [PubMed] [Google Scholar]
- 11. Geleijnse JM, Vermeer C, Grobbee DE, et al. Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: the Rotterdam Study. J Nutr .2004;134:3100–3105. [DOI] [PubMed] [Google Scholar]
- 12. Damon M, Zhang NZ, Haytowitz DB, et al. Phylloquinone (vitamin K1) content of vegetables. J Food Compos Anal. 2005;18:751–758. [Google Scholar]
- 13. Lee HW, Zhang H, Liang X, et al. Simultaneous determination of carotenoids, tocopherols and phylloquinone in 12 Brassicaceae vegetables. LWT-Food Sci Technol. 2020;130:109649. [Google Scholar]
- 14. Otles S, Cagindi O.. Determination of vitamin K1 content in olive oil, chard and human plasma by RP-HPLC method with UV–Vis detection. Food Chem. 2007;100:1220–1222. [Google Scholar]
- 15. Bügel S, Spagner C, Poulsen SK, et al. Phylloquinone content from wild green vegetables may contribute substantially to dietary intake. Can J Agric Crops. 2016;1:83–88. [Google Scholar]
- 16. Presse N, Potvin S, Bertrand B, et al. Phylloquinone content of herbs, spices and seasonings. J Food Compos Anal. 2015;41:15–20. [Google Scholar]
- 17. Ferland G, Sadowski JA.. Vitamin K1 (phylloquinone) content of green vegetables: effects of plant maturation and geographical growth location. J Agric Food Chem .1992;40:1874–1877. [Google Scholar]
- 18. Lester GE, Makus DJ, Hodges DM, et al. Summer (Subarctic) versus winter (Subtropic) production affects spinach (Spinacia oleracea L.) leaf bionutrients: vitamins (C, E, Folate, K1, provitamin A), lutein, phenolics, and antioxidants. J Agric Food Chem .2013;61:7019–7027. [DOI] [PubMed] [Google Scholar]
- 19. Kim MJ, Chiu Y-C, Ku K-M.. Glucosinolates, carotenoids, and vitamins E and K variation from selected kale and collard cultivars. J Food Qual. 2017;2017:1–8. [Google Scholar]
- 20. Booth SL, Sadowski JA, Weihrauch JL, et al. Vitamin K1 (phylloquinone) content of foods: a provisional table. J Food Compos Anal. 1993;6:109–120. [Google Scholar]
- 21. Shearer MJ, Bolton-Smith C.. The UK food data-base for vitamin K and why we need it. Food Chem. 2000;68:213–218. [Google Scholar]
- 22. Ferland G, Sadowski JA.. Vitamin K1 (phylloquinone) content of edible oils: effects of heating and light exposure. J Agric Food Chem .1992;40:1869–1873. [Google Scholar]
- 23. Schurgers LJ, Teunissen KJF, Hamulyak K, et al. Vitamin K–containing dietary supplements: comparison of synthetic vitamin K-1 and natto-derived menaquinone-7. Blood. 2007;109:3279–3283. [DOI] [PubMed] [Google Scholar]
- 24. Tarento TDC, McClure DD, Vasiljevski E, et al. Microalgae as a source of vitamin K1. Algal Res. 2018;36:77–87. [Google Scholar]
- 25. De Roeck-Holtzhauer Y, Quere I, Claire C.. Vitamin analysis of five planktonic microalgae and one macroalga. J Appl Phycol .1991;3:259–264. [Google Scholar]
- 26. Chen Y, Mu Q, Hu K, et al. Characterization of MK8(H2) from Rhodococcus sp. B7740 and its potential antiglycation capacity measurements. Mar Drugs. 2018;16:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Walther B, Chollet M.. Menaquinones, bacteria, and foods: vitamin K2 in the diet. In: Gordeladze JO, ed. Vitamin K2 – Vital for Health and Wellbeing. London, UK: IntechOpen Limited (eBook); 2017:63–84. [Google Scholar]
- 28. Marles RJ, Roe AL, Oketch-Rabah HA.. US Pharmacopeial Convention safety evaluation of menaquinone-7, a form of vitamin K. Nutr Rev .2017;75:553–578. [DOI] [PubMed] [Google Scholar]
- 29. Simes DC, Viegas CSB, Araújo N, et al. Vitamin K as a diet supplement with impact in human health: current evidence in age-related diseases. Nutrients. 2020;12:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nakagawa K, Hirota Y, Sawada N, et al. Identification of UBIAD1 as a novel human menaquinone-4 biosynthetic enzyme. Nature. 2010;468:117–121. [DOI] [PubMed] [Google Scholar]
- 31.EFSA Panel on Dietetic Products Nutrition, and Allergies; Turck D, Bresson J-L, Burlingame B, et al. Dietary reference values for vitamin K. EFSA J .2017;15:e04780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Okano T, Shimomura Y, Yamane M, et al. Conversion of phylloquinone (vitamin K1) into menaquinone-4 (vitamin K2) in mice: two possible routes for menaquinone-4 accumulation in cerebra of mice. J Biol Chem .2008;283:11270–11279. [DOI] [PubMed] [Google Scholar]
- 33. Nimptsch K, Rohrmann S, Kaaks R, et al. Dietary vitamin K intake in relation to cancer incidence and mortality: results from the Heidelberg cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Heidelberg). Am J Clin Nutr .2010;91:1348–1358. [DOI] [PubMed] [Google Scholar]
- 34. Beulens JW, van der AD, Grobbee DE, et al. Dietary phylloquinone and menaquinones intakes and risk of type 2 diabetes. Diabetes Care .2010;33:1699–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Koivu-Tikkanen TJ, Ollilainen V, Piironen VI.. Determination of phylloquinone and menaquinones in animal products with fluorescence detection after postcolumn reduction with metallic zinc. J Agric Food Chem .2000;48:6325–6331. [DOI] [PubMed] [Google Scholar]
- 36. Fu X, Harshman SG, Shen X, et al. Multiple vitamin K forms exist in dairy foods. Curr Dev Nutr .2017;1:e000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vermeer C, Raes J, Van ’t Hoofd C, et al. Menaquinone content of cheese. Nutrients. 2018;10:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zwakenberg SR, Engelen AIP, Dalmeijer GW, et al. Reproducibility and relative validity of a food frequency questionnaire to estimate intake of dietary phylloquinone and menaquinones. Eur J Clin Nutr .2017;71:1423–1428. [DOI] [PubMed] [Google Scholar]
- 39. Erkkilä AT, Booth SL, Hu FB, et al. Phylloquinone intake as a marker for coronary heart disease risk but not stroke in women. Eur J Clin Nutr .2005;59:196–204. [DOI] [PubMed] [Google Scholar]
- 40. Cheung CL, Sahni S, Cheung BM, et al. Vitamin K intake and mortality in people with chronic kidney disease from NHANES III. Clin Nutr .2015;34:235–240. [DOI] [PubMed] [Google Scholar]
- 41. Ortega Anta RM, González-Rodríguez LG, Lombán BN, et al. Vitamin K adequacy in a representative sample of Spanish adults. Dietary determinants. Nutr Hosp. 2014;29:187–195. [DOI] [PubMed] [Google Scholar]
- 42. Hodges SJ, Pilkington MJ, Shearer MJ, et al. Age-related changes in the circulating levels of congeners of vitamin-K2, menaquinone-7 and menaquinone-8. Clin Sci (Lond) .1990;78:63–66. [DOI] [PubMed] [Google Scholar]
- 43. Booth SL. Vitamin K status in the elderly. Curr Opin Clin Nutr Metab Care .2007;10:20–23. [DOI] [PubMed] [Google Scholar]
- 44. Simes DC, Viegas CSB, Araújo N, et al. Vitamin K as a powerful micronutrient in aging and age-related diseases: pros and cons from clinical studies. Int J Mol Sci .2019;20:4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gijsbers BL, Jie KS, Vermeer C.. Effect of food composition on vitamin K absorption in human volunteers. Br J Nutr .1996;76:223–229. [DOI] [PubMed] [Google Scholar]
- 46. Schurgers LJ, Vermeer C.. Differential lipoprotein transport pathways of K-vitamins in healthy subjects. Biochim Biophys Acta .2002;1570:27–32. [DOI] [PubMed] [Google Scholar]
- 47. Garber AK, Binkley NC, Krueger DC, et al. Comparison of phylloquinone bioavailability from food sources or a supplement in human subjects. J Nutr. 1999;129:1201–1203. [DOI] [PubMed] [Google Scholar]
- 48. Jones KS, Bluck LJ, Wang LY, et al. The effect of different meals on the absorption of stable isotope-labelled phylloquinone. Br J Nutr .2009;102:1195–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hollander D, Rim E.. Factors affecting the absorption of vitamin K-1 in vitro. Gut. 1976;17:450–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Akiyama Y, Hara K, Matsumoto A, et al. Comparison of intestinal absorption of vitamin K2 (menaquinone) homologues and their effects on blood coagulation in rats with hypoprothrombinaemia. Biochem Pharmacol .1995;49:1801–1807. [DOI] [PubMed] [Google Scholar]
- 51. MacWalter RS, Fraser HW, Armstrong KM.. Orlistat enhances warfarin effect. Ann Pharmacother .2003;37:510–512. [DOI] [PubMed] [Google Scholar]
- 52. Vroonhof K, van Rijn HJM, van Hattum J.. Vitamin K deficiency and bleeding after long-term use of cholestyramine. Neth J Med. 2003;61:19–21. [PubMed] [Google Scholar]
- 53. Torres-Fernandez D, de Pazos Azpeitia B, Gijon Mediavilla M, et al. Severe rifampicin-induced vitamin K deficiency coagulopathy in a child. Pediatr Infect Dis J .2020;39:833–834. [DOI] [PubMed] [Google Scholar]
- 54. Brayfield A. Martindale: The Complete Drug Reference. 39th ed.London, UK: Pharmaceutical Press; 2017. [Google Scholar]
- 55. Kong MK, Lee PC.. Metabolic engineering of menaquinone-8 pathway of Escherichia coli as a microbial platform for vitamin K production. Biotechnol Bioeng .2011;108:1997–2002. [DOI] [PubMed] [Google Scholar]
- 56. Ramotar K, Conly JM, Chubb H, et al. Production of menaquinones by intestinal anaerobes. J Infect Dis .1984;150:213–218. [DOI] [PubMed] [Google Scholar]
- 57. Karl JP, Meydani M, Barnett JB, et al. Fecal concentrations of bacterially derived vitamin K forms are associated with gut microbiota composition but not plasma or fecal cytokine concentrations in healthy adults. Am J Clin Nutr .2017;106:1052–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Conly JM, Stein K, Worobetz L, et al. The contribution of vitamin K2 (menaquinones) produced by the intestinal microflora to human nutritional requirements for vitamin K. Am J Gastroenterol. 1994;89:915–923. [PubMed] [Google Scholar]
- 59. Chen LJ, Hsiao FY, Shen LJ, et al. Use of hypoprothrombinemia-inducing cephalosporins and the risk of hemorrhagic events: a nationwide nested case–control study. PLoS One .2016;11:e0158407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Frick PG, Riedler G, Brögli H.. Dose response and minimal daily requirement for vitamin K in man. J Appl Physiol .1967;23:387–389. [DOI] [PubMed] [Google Scholar]
- 61. Vermeer C, Schurgers LJ.. A comprehensive review of vitamin K and vitamin K antagonists. Hematol Oncol Clin North Am .2000;14:339–353. [DOI] [PubMed] [Google Scholar]
- 62. Stafford DW. The vitamin K cycle. J Thromb Haemost .2005;3:1873–1878. [DOI] [PubMed] [Google Scholar]
- 63. Torii S, Ikari Y, Tanabe K, et al. Plasma phylloquinone, menaquinone-4 and menaquinone-7 levels and coronary artery calcification. J Nutr Sci .2016;5:e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bugel S, Sorensen AD, Hels O, et al. Effect of phylloquinone supplementation on biochemical markers of vitamin K status and bone turnover in postmenopausal women. Br J Nutr .2007;97:373–380. [DOI] [PubMed] [Google Scholar]
- 65. Fusaro MVitamin K Italian (VIKI) Dialysis Study InvestigatorsNoale M, Viola V, et al. Vitamin K, vertebral fractures, vascular calcifications, and mortality: Vitamin K Italian (VIKI) dialysis study. J Bone Miner Res .2012;27:2271–2278. [DOI] [PubMed] [Google Scholar]
- 66. Neogi T, Booth SL, Zhang YQ, et al. Low vitamin K status is associated with osteoarthritis in the hand and knee. Arthritis Rheum .2006;54:1255–1261. [DOI] [PubMed] [Google Scholar]
- 67. Shea MK, O’Donnell CJ, Hoffmann U, et al. Vitamin K supplementation and progression of coronary artery calcium in older men and women. Am J Clin Nutr .2009;89:1799–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shea MK, Booth SL.. Concepts and controversies in evaluating vitamin K status in population-based studies. Nutrients. 2016;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Thijssen HH, Drittij-Reijnders MJ, Fischer MA.. Phylloquinone and menaquinone-4 distribution in rats: synthesis rather than uptake determines menaquinone-4 organ concentrations. J Nutr .1996;126:537–543. [DOI] [PubMed] [Google Scholar]
- 70. Spronk H, Soute B, Schurgers L, et al. Tissue-specific utilization of menaquinone-4 results in the prevention of arterial calcification in Warfarin-treated rats. J Vasc Res .2003;40:531–537. [DOI] [PubMed] [Google Scholar]
- 71. Thijssen HH, Drittij-Reijnders MJ.. Vitamin K status in human tissues: tissue-specific accumulation of phylloquinone and menaquinone-4. Br J Nutr .1996;75:121–127. [DOI] [PubMed] [Google Scholar]
- 72. Usui Y, Nishimura N, Kobayashi N, et al. Measurement of vitamin-K in human-liver by gradient elution high-performance liquid-chromatography using platinum-black catalyst reduction and fluorimetric detection. J Chromatogr-Biomed. 1989;489:291–301. [DOI] [PubMed] [Google Scholar]
- 73. Harrington DJ, Booth SL, Card DJ, et al. Excretion of the urinary 5C- and 7C-aglycone metabolites of vitamin K by young adults responds to changes in dietary phylloquinone and dihydrophylloquinone intakes. J Nutr. 2007;137:1763–1768. [DOI] [PubMed] [Google Scholar]
- 74. Card DJ, Gorska R, Harrington DJ.. Laboratory assessment of vitamin K status. J Clin Pathol .2020;73:70–75. [DOI] [PubMed] [Google Scholar]
- 75. Rishavy MA, Pudota BN, Hallgren KW, et al. A new model for vitamin K-dependent carboxylation: the catalytic base that deprotonates vitamin K hydroquinone is not Cys but an activated amine. Proc Natl Acad Sci USA .2004;101:13732–13737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dowd P, Ham SW, Naganathan S, et al. The mechanism of action of vitamin K. Annu Rev Nutr .1995;15:419–440. [DOI] [PubMed] [Google Scholar]
- 77. Ayombil F, Camire RM.. Insights into vitamin K-dependent carboxylation: home field advantage. Haematologica. 2020;105:1996–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Berkner KL. The vitamin K-dependent carboxylase. J Nutr .2000;130:1877–1880. [DOI] [PubMed] [Google Scholar]
- 79. Cain D, Hutson SM, Wallin R.. Assembly of the warfarin-sensitive vitamin K 2,3-epoxide reductase enzyme complex in the endoplasmic reticulum membrane. J Biol Chem .1997;272:29068–29075. [DOI] [PubMed] [Google Scholar]
- 80. Parker CH, Morgan CR, Rand KD, et al. A conformational investigation of propeptide binding to the integral membrane protein γ-glutamyl carboxylase using nanodisc hydrogen exchange mass spectrometry. Biochemistry. 2014;53:1511–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Tie JK, Stafford DW.. Structural and functional insights into enzymes of the vitamin K cycle. J Thromb Haemost .2016;14:236–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Stanley TB, Jin DY, Lin PJ, et al. The propeptides of the vitamin K-dependent proteins possess different affinities for the vitamin K-dependent carboxylase. J Biol Chem .1999;274:16940–16944. [DOI] [PubMed] [Google Scholar]
- 83. Schurgers LJ, Dissel PE, Spronk HM, et al. Role of vitamin K and vitamin K-dependent proteins in vascular calcification. Z Kardiol. 2001;90(suppl 3):57–63. [DOI] [PubMed] [Google Scholar]
- 84. Stanton C, Taylor R, Wallin R.. Processing of prothrombin in the secretory pathway. Biochem J. 1991;277:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Schurgers L, Cranenburg E, Vermeer C.. Matrix Gla-protein: the calcification inhibitor in need of vitamin K. Thromb Haemost .2008;100:593–603. [PubMed] [Google Scholar]
- 86. Souri M, Iwata H, Zhang WG, et al. Unique secretion mode of human protein Z: its Gla domain is responsible for inefficient, vitamin K-dependent and warfarin-sensitive secretion. Blood. 2009;113:3857–3864. [DOI] [PubMed] [Google Scholar]
- 87. Tokunaga F, Takeuchi S, Omura S, et al. Secretion, gamma-carboxylation, and endoplasmic reticulum–associated degradation of chimeras with mutually exchanged Gla domain between human protein C and prothrombin. Thromb Res .2000;99:511–521. [DOI] [PubMed] [Google Scholar]
- 88. Buitenhuis HC, Soute BA, Vermeer C.. Comparison of the vitamins K1, K2 and K3 as cofactors for the hepatic vitamin K-dependent carboxylase. Biochim Biophys Acta .1990;1034:170–175. [DOI] [PubMed] [Google Scholar]
- 89. Chatron N, Hammed A, Benoit E, et al. Structural insights into phylloquinone (vitamin K1), menaquinone (MK4, MK7), and menadione (vitamin K3) binding to VKORC1. Nutrients. 2019;11:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wallin R, Cain D, Sane DC.. Matrix Gla protein synthesis and gamma-carboxylation in the aortic vessel wall and proliferating vascular smooth muscle cells—a cell system which resembles the system in bone cells. Thromb Haemost .1999;82:1764–1767. [PubMed] [Google Scholar]
- 91. Wen L, Chen J, Duan L, et al. Vitamin K–dependent proteins involved in bone and cardiovascular health. Mol Med Rep .2018;18:3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Berkner KL, Pudota BN.. Vitamin K-dependent carboxylation of the carboxylase. Proc Natl Acad Sci USA .1998;95:466–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Davie EW, Fujikawa K, Kisiel W.. The coagulation cascade: initiation, maintenance, and regulation. Biochemistry. 1991;30:10363–10370. [DOI] [PubMed] [Google Scholar]
- 94. Furie B, Furie BC.. The molecular basis of blood coagulation. Cell. 1988;53:505–518. [DOI] [PubMed] [Google Scholar]
- 95. Winter WE, Flax SD, Harris NS.. Coagulation testing in the core laboratory. Lab Med .2017;48:295–313. [DOI] [PubMed] [Google Scholar]
- 96. Mackman N. The role of tissue factor and factor VIIa in hemostasis. Anesth Analg .2009;108:1447–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Mann KG, Nesheim ME, Church WR, et al. Surface-dependent reactions of the vitamin K-dependent enzyme complexes. Blood. 1990;76:1–16. [PubMed] [Google Scholar]
- 98. Dahlbäck B. Vitamin K–dependent protein S: beyond the protein C pathway. Semin Thromb Hemost .2018;44:176–184. [DOI] [PubMed] [Google Scholar]
- 99. Karimi Z, Falsafi-Zade S, Galehdari H.. The role of Ca2+ ions in the complex assembling of protein Z and Z-dependent protease inhibitor: a structure and dynamics investigation. Bioinformation. 2012;8:407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Sengupta T, Manoj N.. Phosphatidylserine and phosphatidylethanolamine bind to protein Z cooperatively and with equal affinity. PLoS One .2016;11:e0161896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Majumder R, Weinreb G, Lentz BR.. Efficient thrombin generation requires molecular phosphatidylserine, not a membrane surface. Biochemistry. 2005;44:16998–17006. [DOI] [PubMed] [Google Scholar]
- 102. Chandrasekaran V, Lee CJ, Duke RE, et al. Computational study of the putative active form of protein Z (PZa): sequence design and structural modeling. Protein Sci .2008;17:1354–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Almawi WY, Al-Shaikh FS, Melemedjian OK, et al. Protein Z, an anticoagulant protein with expanding role in reproductive biology. Reproduction .2013;146:R73–R80. [DOI] [PubMed] [Google Scholar]
- 104. Zoch ML, Clemens TL, Riddle RC.. New insights into the biology of osteocalcin. Bone. 2016;82:42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Mizokami A, Kawakubo-Yasukochi T, Hirata M.. Osteocalcin and its endocrine functions. Biochem Pharmacol .2017;132:1–8. [DOI] [PubMed] [Google Scholar]
- 106. Moser SC, van der Eerden BCJ.. Osteocalcin—a versatile bone-derived hormone. Front Endocrinol. 2019;9:794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Manolagas SC. Osteocalcin promotes bone mineralization but is not a hormone. PLoS Genet .2020;16:e1008714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Diaz-Franco MC, Franco-Diaz de Leon R, Villafan-Bernal JR. Osteocalcin-GPRC6A: an update of its clinical and biological multi-organic interactions. Mol Med Rep. 2019;19:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Booth SL, Centi A, Smith SR, et al. The role of osteocalcin in human glucose metabolism: marker or mediator? Nat Rev Endocrinol .2013;9:43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Khrimian L, Obri A, Ramos-Brossier M, et al. Gpr158 mediates osteocalcin’s regulation of cognition. J Exp Med .2017;214:2859–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Rodríguez-Olleros Rodríguez C, Díaz Curiel M.. Vitamin K and bone health: a review on the effects of vitamin K deficiency and supplementation and the effect of non-vitamin K antagonist oral anticoagulants on different bone parameters. J Osteoporos .2019;2019:2069176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Knapen MH, Nieuwenhuijzen Kruseman AC, Wouters RS, et al. Correlation of serum osteocalcin fractions with bone mineral density in women during the first 10 years after menopause. Calcif Tissue Int .1998;63:375–379. [DOI] [PubMed] [Google Scholar]
- 113. Huang ZB, Wan SL, Lu YJ, et al. Does vitamin K2 play a role in the prevention and treatment of osteoporosis for postmenopausal women: a meta-analysis of randomized controlled trials. Osteoporos Int .2015;26:1175–1186. [DOI] [PubMed] [Google Scholar]
- 114. Binkley NC, Krueger DC, Kawahara TN, et al. A high phylloquinone intake is required to achieve maximal osteocalcin gamma-carboxylation. Am J Clin Nutr. 2002;76:1055–1060. [DOI] [PubMed] [Google Scholar]
- 115. Knapen M, Drummen N, Smit E, et al. Three-year low-dose menaquinone-7 supplementation helps decrease bone loss in healthy postmenopausal women. Osteoporos Int .2013;24:2499–2507. [DOI] [PubMed] [Google Scholar]
- 116. Su S, He N, Men P, et al. The efficacy and safety of menatetrenone in the management of osteoporosis: a systematic review and meta-analysis of randomized controlled trials. Osteoporos Int .2019;30:1175–1186. [DOI] [PubMed] [Google Scholar]
- 117. Epstein M. Matrix Gla-Protein (MGP) not only inhibits calcification in large arteries but also may be renoprotective: connecting the dots. EBioMedicine. 2016;4:16–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Murshed M, Schinke T, McKee MD, et al. Extracellular matrix mineralization is regulated locally; different roles of two gla-containing proteins. J Cell Biol .2004;165:625–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Price PA, Urist MR, Otawara Y.. Matrix Gla protein, a new γ-carboxyglutamic acid-containing protein which is associated with the organic matrix of bone. Biochem Biophys Res Commun. 1983;117:765–771. [DOI] [PubMed] [Google Scholar]
- 120. Luo G, Ducy P, McKee MD, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. [DOI] [PubMed] [Google Scholar]
- 121. Khosroshahi HE, Sahin SC, Akyuz Y, et al. Long term follow-up of four patients with Keutel syndrome. Am J Med Genet .2014;164:2849–2856. [DOI] [PubMed] [Google Scholar]
- 122. Lees JS, Chapman FA, Witham MD, et al. Vitamin K status, supplementation and vascular disease: a systematic review and meta-analysis. Heart. 2019;105:938–945. [DOI] [PubMed] [Google Scholar]
- 123. Takeshita S, Kikuno R, Tezuka K, et al. Osteoblast-specific factor 2: cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem J. 1993;294:271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Bonnet N, Garnero P, Ferrari S.. Periostin action in bone. Mol Cell Endocrinol .2016;432:75–82. [DOI] [PubMed] [Google Scholar]
- 125. Izuhara K, Nunomura S, Nanri Y, et al. Periostin in inflammation and allergy. Cell Mol Life Sci .2017;74:4293–4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Izuhara K, Nunomura S, Nanri Y, et al. Periostin: an emerging biomarker for allergic diseases. Allergy. 2019;74:2116–2128. [DOI] [PubMed] [Google Scholar]
- 127. Landry NM, Cohen S, Dixon IMC.. Periostin in cardiovascular disease and development: a tale of two distinct roles. Basic Res Cardiol .2018;113:1. [DOI] [PubMed] [Google Scholar]
- 128. Prakoura N, Chatziantoniou C.. Periostin in kidney diseases. Cell Mol Life Sci .2017;74:4315–4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Annis DS, Ma H, Balas DM, et al. Absence of vitamin K-dependent γ-carboxylation in human periostin extracted from fibrotic lung or secreted from a cell line engineered to optimize γ-carboxylation. PLoS One .2015;10:e0135374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Rousseau JC, Sornay-Rendu E, Bertholon C, et al. Serum periostin is associated with fracture risk in postmenopausal women: a 7-year prospective analysis of the OFELY study. J Clin Endoc Metab. 2014;99:2533–2539. [DOI] [PubMed] [Google Scholar]
- 131. Naik PK, Bozyk PD, Bentley JK, et al. ; the COMET Investigators. Periostin promotes fibrosis and predicts progression in patients with idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2012;303:L1046–L1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Gupta M, Bhayana S, Sikka S.. Role of urinary inhibitors and promoters in calcium oxalate crystallisation. IJRPC. 2011;1:793–798. [Google Scholar]
- 133. Nakagawa Y. Properties and function of nephrocalcin: mechanism of kidney stone inhibition or promotion. Keio J Med .1997;46:1–9. [DOI] [PubMed] [Google Scholar]
- 134. Nakagawa Y, Abram V, Kézdy FJ, et al. Purification and characterization of the principal inhibitor of calcium oxalate monohydrate crystal growth in human urine. J Biol Chem .1983;258:12594–12600. [PubMed] [Google Scholar]
- 135. Nakagawa Y, Ahmed M, Hall SL, et al. Isolation from human calcium oxalate renal stones of nephrocalcin, a glycoprotein inhibitor of calcium oxalate crystal growth. Evidence that nephrocalcin from patients with calcium oxalate nephrolithiasis is deficient in gamma-carboxyglutamic acid. J Clin Invest .1987;79:1782–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Coe FL, Nakagawa Y, Asplin J, et al. Role of nephrocalcin in inhibition of calcium oxalate crystallization and nephrolithiasis. Miner Electrolyte Metab .1994;20:378–384. [PubMed] [Google Scholar]
- 137. Sasaki T, Knyazev P, Cheburkin Y, et al. Crystal structure of a C-terminal fragment of growth arrest-specific protein Gas6. Receptor tyrosine kinase activation by laminin G-like domains. J Biol Chem .2002;277:44164–44170. [DOI] [PubMed] [Google Scholar]
- 138. Hafizi S, Dahlback B.. Gas6 and protein S. Vitamin K-dependent ligands for the Axl receptor tyrosine kinase subfamily. FEBS J .2006;273:5231–5244. [DOI] [PubMed] [Google Scholar]
- 139. Law LA, Graham DK, Di Paola J, et al. GAS6/TAM pathway signaling in hemostasis and thrombosis. Front Med (Lausanne) 2018;5:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Sachs UJH, Nieswandt B.. In vivo thrombus formation in murine models. Circ Res .2007;100:979–991. [DOI] [PubMed] [Google Scholar]
- 141. Bellan M, Cittone MG, Tonello S, et al. Gas6/TAM system: a key modulator of the interplay between inflammation and fibrosis. Int J Mol Sci .2019;20:5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Wu G, Ma Z, Hu W, et al. Molecular insights of Gas6/TAM in cancer development and therapy. Cell Death Dis .2017;8:e2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Kaesler N, Immendorf S, Ouyang C, et al. Gas6 protein: its role in cardiovascular calcification. BMC Nephrol .2016;17:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. ten Kate MK, van der Meer J.. Protein S deficiency: a clinical perspective. Haemophilia .2008;14:1222–1228. [DOI] [PubMed] [Google Scholar]
- 145. Gebhard W, Schreitmüller T, Hochstrasser K, et al. Complementary DNA and derived amino acid sequence of the precursor of one of the three protein components of the inter-α-trypsin inhibitor complex. FEBS Lett .1988;229:63–67. [DOI] [PubMed] [Google Scholar]
- 146. Schaller J, Gerber S, Kämpfer U, et al. Inhibitors. In: Human Blood Plasma Proteins: Structure and Function. Chichester, UK: John Wiley & Sons, Ltd; 2008:283–316. [Google Scholar]
- 147. Zhuo L, Kimata K.. Structure and function of inter-alpha-trypsin inhibitor heavy chains. Connect Tissue Res .2008;49:311–320. [DOI] [PubMed] [Google Scholar]
- 148. Bost F, Diarra-Mehrpour M, Martin JP.. Inter-alpha-trypsin inhibitor proteoglycan family—a group of proteins binding and stabilizing the extracellular matrix. Eur J Biochem .1998;252:339–346. [DOI] [PubMed] [Google Scholar]
- 149. Zhuo L, Hascall VC, Kimata K.. Inter-alpha-trypsin inhibitor, a covalent protein–glycosaminoglycan–protein complex. J Biol Chem .2004;279:38079–38082. [DOI] [PubMed] [Google Scholar]
- 150. Kulman JD, Harris JE, Haldeman BA, et al. Primary structure and tissue distribution of two novel proline-rich gamma-carboxyglutamic acid proteins. Proc Natl Acad Sci USA .1997;94:9058–9062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Kulman JD, Harris JE, Xie L, et al. Identification of two novel transmembrane gamma-carboxyglutamic acid proteins expressed broadly in fetal and adult tissues. Proc Natl Acad Sci USA .2001;98:1370–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Kulman JD, Harris JE, Xie L, et al. Proline-rich Gla protein 2 is a cell-surface vitamin K-dependent protein that binds to the transcriptional coactivator Yes-associated protein. Proc Natl Acad Sci USA .2007;104:8767–8772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Viegas CS, Simes DC, Laizé V, et al. Gla-rich protein (GRP), a new vitamin K-dependent protein identified from sturgeon cartilage and highly conserved in vertebrates. J Biol Chem .2008;283:36655–36664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Viegas CSB, Rafael MS, Enriquez JL, et al. Gla-rich protein acts as a calcification inhibitor in the human cardiovascular system. Arterioscler Thromb Vasc Biol .2015;35:399–408. [DOI] [PubMed] [Google Scholar]
- 155. Viegas CSB, Simes DC.. A dual role for GRP in cardiovascular disease. Aging (Albany NY) .2019;11:1323–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Viegas CSB, Herfs M, Rafael MS, et al. Gla-rich protein is a potential new vitamin K target in cancer: evidences for a direct GRP–mineral interaction. Biomed Res Int .2014;2014:340216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Cavaco S, Viegas CSB, Rafael MS, et al. Gla-rich protein is involved in the cross-talk between calcification and inflammation in osteoarthritis. Cell Mol Life Sci .2016;73:1051–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Cancela ML, Conceição N, Laizé V.. Gla-rich protein, a new player in tissue calcification? Adv Nutr . 2012;3:174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Vieira M, Saraiva MJ.. Transthyretin: a multifaceted protein. Biomol Concepts .2014;5:45–54. [DOI] [PubMed] [Google Scholar]
- 160. Ruggeberg S, Horn P, Li X, et al. Detection of a gamma-carboxy-glutamate as novel post-translational modification of human transthyretin. Protein Pept Lett .2008;15:43–46. [DOI] [PubMed] [Google Scholar]
- 161. Scott RM, Smith ER.. Moyamoya disease and moyamoya syndrome. N Engl J Med .2009;360:1226–1237. [DOI] [PubMed] [Google Scholar]
- 162. Sultana H, Watanabe K, Rana MM, et al. Effects of vitamin K2 on the expression of genes involved in bile acid synthesis and glucose homeostasis in mice with humanized PXR. Nutrients. 2018;10:982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Palermo A, Tuccinardi D, D’Onofrio L, et al. Vitamin K and osteoporosis: myth or reality? Metabolism .2017;70:57–71. [DOI] [PubMed] [Google Scholar]
- 164. Najmanová I, Doseděl M, Hrdina R, et al. Cardiovascular effects of coumarins besides their antioxidant activity. Curr Top Med Chem .2015;15:830–849. [DOI] [PubMed] [Google Scholar]
- 165. Raspini B, Benvenuto C, Cazzola R.. The role of vitamin K2 in osteoporosis and cardiovascular disease prevention. Agro Food Ind Hi Tech. 2015;26:31–35. [Google Scholar]
- 166. Schulte R, Jordan LC, Morad A, et al. Rise in late onset vitamin K deficiency bleeding in young infants because of omission or refusal of prophylaxis at birth. Pediatr Neurol .2014;50:564–568. [DOI] [PubMed] [Google Scholar]
- 167. Karl JP, Fu XY, Dolnikowski GG, et al. Quantification of phylloquinone and menaquinones in feces, serum, and food by high-performance liquid chromatography–mass spectrometry. J Chromatogr B. 2014;963:128–133. [DOI] [PubMed] [Google Scholar]
- 168. Chatzimichalakis PF, Samanidou VF, Papadoyannis IN.. Development of a validated liquid chromatography method for the simultaneous determination of eight fat-soluble vitamins in biological fluids after solid-phase extraction. J Chromatogr B Analyt Technol Biomed Life Sci .2004;805:289–296. [DOI] [PubMed] [Google Scholar]
- 169. Zhang YN, Bala V, Mao ZH, et al. A concise review of quantification methods for determination of vitamin K in various biological matrices. J Pharm Biomed Anal .2019;169:133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Kishikawa N, Kuroda N.. Analytical techniques for the determination of biologically active quinones in biological and environmental samples. J Pharm Biomed Anal .2014;87:261–270. [DOI] [PubMed] [Google Scholar]
- 171. Klapkova E, Cepova J, Dunovska K, et al. Determination of vitamins K1, MK-4, and MK-7 in human serum of postmenopausal women by HPLC with fluorescence detection. J Clin Lab Anal .2018;32:e22381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Ahmed S, Mahmoud AM.. A novel salting-out assisted extraction coupled with HPLC- fluorescence detection for trace determination of vitamin K homologues in human plasma. Talanta. 2015;144:480–487. [DOI] [PubMed] [Google Scholar]
- 173. Harrington DJ, Soper R, Edwards C, et al. Determination of the urinary aglycone metabolites of vitamin K by HPLC with redox-mode electrochemical detection. J Lipid Res. 2005;46:1053–1060. [DOI] [PubMed] [Google Scholar]
- 174. Kamao M, Suhara Y, Tsugawa N, et al. Determination of plasma vitamin K by high-performance liquid chromatography with fluorescence detection using vitamin K analogs as internal standards. J Chromatogr B Analyt Technol Biomed Life Sci .2005;816:41–48. [DOI] [PubMed] [Google Scholar]
- 175. Kamao M, Tsugawa N, Suhara Y, et al. Quantification of fat-soluble vitamins in human breast milk by liquid chromatography–tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci .2007;859:192–200. [DOI] [PubMed] [Google Scholar]
- 176. Gentili A, Cafolla A, Gasperi T, et al. Rapid, high performance method for the determination of vitamin K1, menaquinone-4 and vitamin K1 2,3-epoxide in human serum and plasma using liquid chromatography–hybrid quadrupole linear ion trap mass spectrometry. J Chromatogr A .2014;1338:102–110. [DOI] [PubMed] [Google Scholar]
- 177. Ahmed S, Kishikawa N, Ohyama K, et al. Selective chemiluminescence method for monitoring of vitamin K homologues in rheumatoid arthritis patients. Talanta. 2011;85:230–236. [DOI] [PubMed] [Google Scholar]
- 178. Sandvik TA, Husa A, Buchmann M, et al. Routine supercritical fluid chromatography tandem mass spectrometry method for determination of vitamin K1 extracted from serum with a 96-well solid-phase extraction method. J Appl Lab Med .2017;1:637–648. [DOI] [PubMed] [Google Scholar]
- 179. Dunovska K, Klapkova E, Sopko B, et al. LC-MS/MS quantitative analysis of phylloquinone, menaquinone-4 and menaquinone-7 in the human serum of a healthy population. PeerJ. 2019;7:e7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Hu K, Li Y, Ding R, et al. A simple, sensitive, and high-throughput LC-APCI-MS/MS method for simultaneous determination of vitamin K1, vitamin K1 2,3-epoxide in human plasma and its application to a clinical pharmacodynamic study of warfarin. J Pharm Biomed Anal .2018;159:82–91. [DOI] [PubMed] [Google Scholar]
- 181. Jedlińska K, Strus M, Baś B.. A new electrochemical sensor with the Refreshable Silver Liquid Amalgam Film multi-Electrode for sensitive voltammetric determination of vitamin K2 (menaquinone). Electrochim Acta. 2018;265:355–363. [Google Scholar]
- 182. Levêques A, Oberson J-M, Tissot EA, et al. Quantification of vitamins A, E, and K and carotenoids in submilliliter volumes of human milk. J AOAC Int. 2019;102:1059–1068. [DOI] [PubMed] [Google Scholar]
- 183. Abro K, Memon N, Bhanger MI, et al. Determination of vitamins E, D3, and K1 in plasma by liquid chromatography—atmospheric pressure chemical ionization–mass spectrometry utilizing a monolithic column. Anal Lett. 2014;47:14–24. [Google Scholar]
- 184. Górska RM. Chapter 5 – Methods for assessment of vitamin K. In: Harrington D, ed. Laboratory Assessment of Vitamin Status. London, United Kingdom: Elsevier; 2019;107–147. [Google Scholar]
- 185.MyBioSource. Human vitamin K1 ELISA. User instruction. Available at: https://cdn.mybiosource.com/tds/protocol_manuals/000000-799999/MBS161267.pdf. Accessed November 9, 2020 [Google Scholar]
- 186.MyBioSource. Human vitamin K1 (VK1) ELISA kit. Available at: https://cdn.mybiosource.com/tds/protocol_manuals/000000-799999/MBS267859.pdf. Accessed November 9, 2020.
- 187.MyBioSource. Human vitamin K2 (VK2) ELISA kit. Available at: https://cdn.mybiosource.com/tds/protocol_manuals/800000-9999999/MBS9302591.pdf. Accessed November 9, 2020.
- 188. Vigano S, Mannucci PM, Solinas S, et al. Decrease in protein C antigen and formation of an abnormal protein soon after starting oral anticoagulant therapy. Br J Haematol .1984;57:213–220. [PubMed] [Google Scholar]
- 189. Ardell S, Offringa M, Ovelman C, et al. Prophylactic vitamin K for the prevention of vitamin K deficiency bleeding in preterm neonates. Cochrane Database Syst Rev .2018;2:CD008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Alisi L, Cao R, De Angelis C, et al. The relationships between vitamin K and cognition: a review of current evidence. Front Neurol. 2019;10:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Hou JW. Fetal warfarin syndrome. Chang Gung Med J .2004;27:691–695. [PubMed] [Google Scholar]
- 192. Starling LD, Sinha A, Boyd D, et al. Fetal warfarin syndrome. BMJ Case Rep. 2012;2012:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Iwamoto J, Sato Y, Takeda T, et al. Bone quality and vitamin K2 in type 2 diabetes: review of preclinical and clinical studies. Nutr Rev .2011;69:162–167. [DOI] [PubMed] [Google Scholar]
- 194. Iwasaki Y, Yamato H, Murayama H, et al. Combination use of vitamin K2 further increases bone volume and ameliorates extremely low turnover bone induced by bisphosphonate therapy in tail-suspension rats. J Bone Miner Metab .2003;21:154–160. [DOI] [PubMed] [Google Scholar]
- 195. Iwamoto J, Matsumoto H, Takeda T, et al. Effects of vitamin K2 on cortical and cancellous bone mass, cortical osteocyte and lacunar system, and porosity in sciatic neurectomized rats. Calcif Tissue Int .2010;87:254–262. [DOI] [PubMed] [Google Scholar]
- 196. Torgerson DJ. Caution to readers about systematic review on vitamin K and prevention of fractures that included problematic trials. JAMA Intern Med .2018;178:863–864. [DOI] [PubMed] [Google Scholar]
- 197. Hao G, Zhang B, Gu M, et al. Vitamin K intake and the risk of fractures: a meta-analysis. Medicine (Baltimore). 2017;96:e6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Fang Y, Hu C, Tao X, et al. Effect of vitamin K on bone mineral density: a meta-analysis of randomized controlled trials. J Bone Miner Metab .2012;30:60–68. [DOI] [PubMed] [Google Scholar]
- 199. Cockayne S, Adamson J, Lanham-New S, et al. Vitamin K and the prevention of fractures: systematic review and meta-analysis of randomized controlled trials. Arch Intern Med .2006;166:1256–1261. [DOI] [PubMed] [Google Scholar]
- 200. Mott A, Bradley T, Wright K, et al. Effect of vitamin K on bone mineral density and fractures in adults: an updated systematic review and meta-analysis of randomised controlled trials. Osteoporos Int .2019;30:1543–1559. [DOI] [PubMed] [Google Scholar]
- 201. Hoffmann U, Massaro JM, D’Agostino RB Sr, et al. Cardiovascular event prediction and risk reclassification by coronary, aortic, and valvular calcification in the Framingham Heart Study. J Am Heart Assoc. 2016;5:e003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Lehmann N, Erbel R, Mahabadi AA, et al. Value of progression of coronary artery calcification for risk prediction of coronary and cardiovascular events: result of the HNR Study (Heinz Nixdorf Recall). Circulation. 2018;137:665–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Beulens JW, Bots ML, Atsma F, et al. High dietary menaquinone intake is associated with reduced coronary calcification. Atherosclerosis. 2009;203:489–493. [DOI] [PubMed] [Google Scholar]
- 204. Haugsgjerd TR, Egeland GM, Nygård OK, et al. Association of dietary vitamin K and risk of coronary heart disease in middle-age adults: the Hordaland Health Study Cohort. BMJ Open .2020;10:e035953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Zwakenberg S, den Braver N, Engelen A, et al. Vitamin K intake and all-cause and cause specific mortality. Clin Nutr .2017;36:1294–1300. [DOI] [PubMed] [Google Scholar]
- 206. Juanola-Falgarona M, Salas-Salvado J, Martinez-Gonzalez MA, et al. Dietary intake of vitamin K is inversely associated with mortality risk. J Nutr .2014;144:743–750. [DOI] [PubMed] [Google Scholar]
- 207. Chen HG, Sheng LT, Zhang YB, et al. Association of vitamin K with cardiovascular events and all-cause mortality: a systematic review and meta-analysis. Eur J Nutr .2019;58:2191–2205. [DOI] [PubMed] [Google Scholar]
- 208. Zhang S, Guo L, Bu C.. Vitamin K status and cardiovascular events or mortality: a meta-analysis. Eur J Prev Cardiol .2019;26:549–553. [DOI] [PubMed] [Google Scholar]
- 209. Zwakenberg SR, de Jong PA, Bartstra JW, et al. The effect of menaquinone-7 supplementation on vascular calcification in patients with diabetes: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr .2019;110:883–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Vlasschaert C, Goss CJ, Pilkey NG, et al. Vitamin K supplementation for the prevention of cardiovascular disease: where is the evidence? A systematic review of controlled trials. Nutrients. 2020;12:2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Delanaye P, Krzesinski JM, Warling X, et al. Dephosphorylated-uncarboxylated Matrix Gla protein concentration is predictive of vitamin K status and is correlated with vascular calcification in a cohort of hemodialysis patients. BMC Nephrol .2014;15:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Delanaye P, Dubois BE, Lukas P, et al. Impact of stopping vitamin K antagonist therapy on concentrations of dephospho-uncarboxylated Matrix Gla protein. Clin Chem Lab Med .2015;53:191–193. [DOI] [PubMed] [Google Scholar]
- 213. Lamson DW, Plaza SM.. The anticancer effects of vitamin K. Altern Med Rev .2003;8:303–318. [PubMed] [Google Scholar]
- 214. Shoemark DK, Colenso CK, Toelzer C, et al. Molecular simulations suggest vitamins, retinoids and steroids as ligands of the free fatty acid pocket of the SARS-CoV-2 spike protein. Angew Chem Int Ed Engl .2021;60:7098–7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Smith AM Jr, Custer RP.. Toxicity of vitamin K: induced hypoprothrombinemia and altered liver function. J Am Med Assoc .1960;173:502–504. [DOI] [PubMed] [Google Scholar]
- 216. Servitja JM, Masgrau R, Pardo R, et al. Effects of oxidative stress on phospholipid signaling in rat cultured astrocytes and brain slices. J Neurochem .2000;75:788–794. [DOI] [PubMed] [Google Scholar]
- 217. de la Rubia J, Grau E, Montserrat I, et al. Anaphylactic shock and vitamin K1. Ann Intern Med .1989;110:943. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.