Abstract
Objectives
The aim of this study was to evaluate the association of pre-existing cardiovascular comorbidities, including hypertension and coronary heart disease, with coronavirus disease 2019 (COVID-19) severity and mortality.
Methods
PubMed, ScienceDirect, and Scopus were searched between January 1, 2020, and July 18, 2020, to identify eligible studies. Random-effect models were used to estimate the pooled event rates of pre-existing cardiovascular disease comorbidities and odds ratio (OR) with 95% confidence intervals (95% CIs) of disease severity and mortality associated with the exposures of interest.
Results
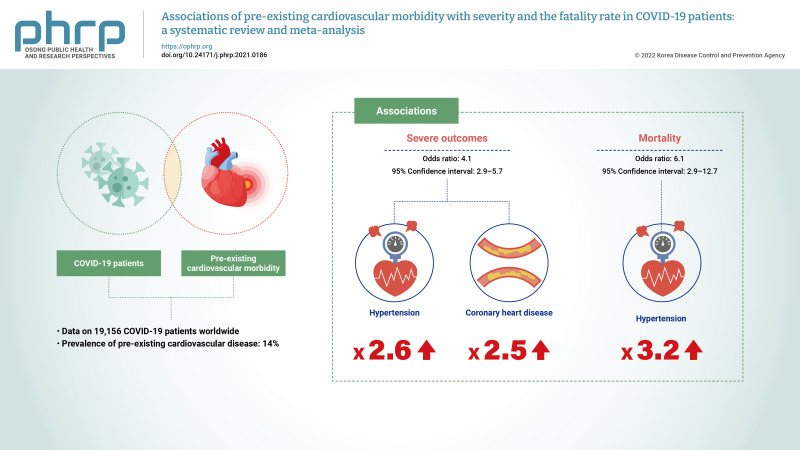
A total of 34 studies involving 19,156 patients with COVID-19 infection met the inclusion criteria. The prevalence of pre-existing cardiovascular disease in the included studies was 14.0%. Pre-existing cardiovascular disease in COVID-19 patients was associated with severe outcomes (OR, 4.1; 95% CI, 2.9 to 5.7) and mortality (OR, 6.1; 95% CI, 2.9 to 12.7). Hypertension and coronary heart disease increased the risk of severe outcomes by 3 times (OR, 3.2; 95% CI, 2.0 to 3.6) and 2.5 times (OR, 2.5; 95% CI, 1.7 to 3.8), respectively. No significant publication bias was indicated.
Conclusion
COVID-19 patients with pre-existing cardiovascular comorbidities have a higher risk of severe outcomes and mortality. Awareness of pre-existing cardiovascular comorbidity is important for the early management of COVID-19.
Keywords: Coronary disease, COVID-19, Hypertension
Graphical abstract
Introduction
The ongoing coronavirus disease 2019 (COVID-19) pandemic poses a significant public health threat to all nations worldwide [1,2]. As of August 23, 2021, COVID-19 has infected approximately 212,763,099 people, including roughly 4,447,912 patients who have died. Regrettably, these numbers have kept increasing worldwide, indicating that the peak is far from over and the global community remains on edge as the number of infected patients continues to escalate.
Several studies from different countries have reported that pre-existing cardiovascular comorbidities are prevalent among COVID-19 patients [3−6]. Understanding the association of cardiovascular comorbidities with the severity and outcomes of COVID-19 may highlight a cohort of patients who require more intensive monitoring during the early phase of infection [7,8]. Epidemiological studies have reported different mortality rates for COVID-19 patients with cardiac manifestations and pre-existing cardiovascular diseases, particularly hypertension and coronary artery disease [8].
Several studies have investigated the association between pre-existing cardiac disease and COVID-19 severity and fatality, and the pooled effects have been estimated in a number of meta-analyses. However, previous reviews varied in how COVID-19 severity was defined; did not report the country of the studies, and reported substantial heterogeneity. Therefore, the present meta-analysis was performed with the following aims: (1) to estimate the overall prevalence rate of pre-existing cardiovascular disease and cardiac manifestations in COVID-19 patients, and (2) to evaluate the association of pre-existing hypertension and coronary heart disease with the severity of COVID-19 and the mortality rate in COVID-19 patients using a random-effect model that incorporates heterogeneity.
Materials and Methods
Data Search
Three databases (PubMed, Science Direct, and Scopus) were searched between January 1, 2020, and July 18, 2020. The following combined keywords were used for searching the databases: cardiovascular and COVID-19; cardiovascular and severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2); cardiovascular, and SARS-CoV-2; cardiovascular, hypertension, and COVID-19. Furthermore, the lists of references of all relevant studies were also manually checked to identify further studies. The protocol for this meta-analysis is registered at PROSPERO CRD42020191768. The meta-analysis was reported following the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement [9].
Study Selection
The study selection was limited to articles in English and studies on adult humans. Case reports, review articles, and editorials were excluded from this analysis. Studies were selected if they provided adequate details on pre-existing cardiovascular disease comorbidities, particularly in patients with positive diagnoses for COVID-19 and hypertension. Studies that did not provide enough details on the number of cases with severe or fatal outcomes were excluded.
Data Abstraction
For studies that met the inclusion criteria, the following data were extracted from each study using a standardized form: the surname of the first author; the design of the study; ratios of clinical characteristics of interest; sample size, country, data relevant to cardiovascular disease comorbidities factor; and pertinent data for arrhythmia and acute cardiac injury as outcomes, and the number of cases with severe and non-severe outcomes, and the number of survivors and non-survivors. As reported in the included studies, severe disease was identified if patients needed to be admitted to the intensive care unit, needed vital life support, or required mechanical ventilation. Non-survivors were defined as cases of death. Two investigators (FA and MA) extracted the relevant data.
Quality Assessment
We used the Joanna Briggs Institute (JBI) critical appraisal checklist for case series to assess the risk of bias [10]. The JBI includes 10 items dealing with confounding, selection, and information bias to assess the internal validity of the case series. The answers for each of the 10 items in the JBI checklist could be “yes,” “no,” “unclear,” or “not applicable.” A detailed description of how to use the JBI tool is provided by Munn et al. in 2020 [10]. It is advised that the results of the quality assessment of the included studies should not be shortened and reported as a score [10]. The quality assessment of the included studies in this meta-analysis was carried out by SA.
Quantitative Data Synthesis and Analysis
Data analysis was carried out using Comprehensive Meta-Analysis V2 (Biostat, Englewood, NJ, USA). A p-value of <0.05 was considered statistically significant. Random-effect models were used to estimate the pooled event rates of pre-existing cardiovascular disease comorbidities as well as the odds ratio (OR) with 95% confidence intervals (95% CIs) of disease severity and mortality associated with the exposures of interest. A random-effect model was used to incorporate heterogeneity among studies [11]. Heterogeneity in any analysis was tested by using the I2 statistic (p<0.1), which estimates the percentage of variation in study results that is explained by between-study heterogeneity rather than sampling error. Usually, an I2 value >50% indicates considerable heterogeneity [11]. Funnel plots and Egger test were used to assess the presence of publication bias.
Results
Search Results and Study Characteristics
A total of 1,601 articles were identified from the 3 databases examined and other sources. After excluding duplicated or overlapping articles and removing reviews and editorials, 169 articles met the primary search criteria. For the quantitative part of our study, 34 studies that reported the event rate of pre-existing cardiovascular disease, arrhythmia, or acute cardiac injury as disease complications were included in the meta-analysis (Figure 1). Most studies were conducted in China (n=21) and the United States of America (n=8), while 4 studies were conducted in Italy and 1 study reported results from different parts of the world. The setting for most of the included studies was the hospital (Table 1) [3−5,12−42].
Figure 1.
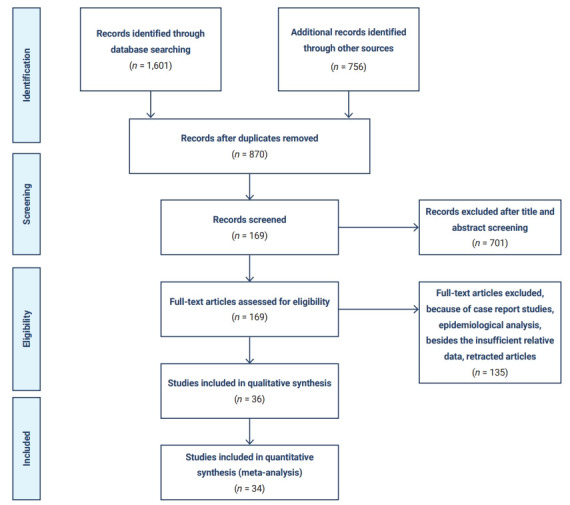
Flow chart of the literature search and study selection.
Table 1.
Number of patients with CVD comorbidities among coronavirus disease 2019 patients
| Study | Country | Condition | Setting | Comorbidities | Sample size (n) | Events (n) | Non-events (n) | Severe cases ratio | Non-severe cases ratio | Non-survivors | Survivors |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wang et al. [3] | China | CVD | Zhongnan Hospital of Wuhan, University in Wuhan, China | HP, CVD, DM, CLD, CRVD, COPD, CKD, Ca, HIV | 138 | 20 | 115 | 9/36 | 11/102 | ||
| Goyal et al. [14] | USA | CAD | An 862-bed quaternary referral center and an affiliated 180-bed nonteaching community hospital in Manhattan | DM, obesity, HP, COPD, asthma, CAD | 393 | 54 | 339 | 25/130 | 29/263 | ||
| Zhang et al. [15] | China | CVD | Zhongnan Hospital of Wuhan University, Wuhan, China | HP, CVD, DM, CLD, CRVD, COPD, CKD, Ca, immunosuppression | 221 | 22 | 199 | 13/55 | 9/166 | ||
| Hu et al. [16] | USA | CVD | Tianyou Hospital, Wuhan University of Science and Technology, China | HP, CVD, DM, CLD, CRVD, COPD, CKD, Ca, cirrhosis | 323 | 34 | 289 | 30/172 | 4/151 | ||
| Guo et al. [17] | China | CHD | The Seventh Hospital of Wuhan City, China | HP, CHD, DM, COPD, CKD, Ca, cardiomyopathy | 187 | 29 | 158 | ||||
| Zhang et al. [18] | China | CVD | The Seventh Hospital of Wuhan City, China | HP, DM, CHD, HL, CG, CVD, CKD, CRVD, COPD, arrhythmia cholelithiasis, fatty liver, thyroid diseases | 140 | 15 | 125 | 10/58 | 5/82 | ||
| Du et al. [19] | China | CHD | Hannan Hospital and Wuhan Union Hospital of Wuhan City, China | HP, DM, CHD, CRVD, CLD, COPD, CKD, Ca | 85 | 10 | 75 | ||||
| Rosenberg et al. [20] | USA | CVDs | 25 hospitals in the New York City, metropolitan region | Obesity, cancer, CKD, COPD, DM, HP, CAD, CHD, dementia | 1,438 | 468 | 1,000 | ||||
| Lei et al. [21] | China | CVDs | Renmin Hospital, Zhongnan Hospital, Tongji Hospital, and Central Hospital in Wuhan | HP, Ca, DM, CVD, CRVD, COPD, CKD | 34 | 7 | 27 | 6/15 | 1/19 | ||
| Mercuro et al. [22] | USA | CVDs | An academic tertiary care center in Boston, Massachusetts | HP, CHF, DM, CAD, AF, COPD, asthma | 90 | 19 | 71 | ||||
| Saleh et al. [23] | USA | CVDs | 14 hospitals of the New York State Northwell Health system | HP, HL, DM, AF, CAD, COPD, CKD, CHF | 201 | 52 | 149 | ||||
| Inciardi et al. [24] | Italy | Cardiac disease | Civil Hospitals of Brescia, Lombardy, Italy | HP, HL, DM, HF, AF, CAD, COPD, CKD, Ca | 99 | 53 | 46 | ||||
| Bhatla et al. [25] | USA | CVDs | The Hospital of the University of Pennsylvania | CHD, HF, HP, AF, DM, COPD, CLD, CKD | 700 | 203 | 497 | 48/79 | 155/621 | ||
| Sala et al. [26] | Italy | CAD | Seven COVID units at a third-level hub center, San Raffaele Hospital, Italy | CAD, COPD, HP, DM, Obesity, AF | 132 | 9 | 123 | ||||
| Enzmann et al. [27] | USA | CVD | Three hospitals in the Dakotas | Asthma, CHF, CVD, DM, RD, CKD, cirrhosis, Ca, immunosuppression | 150 | 93 | 57 | ||||
| Guan et al. [4] | China | CHD | 552 hospitals in 30 provinces, autonomous regions, and municipalities in mainland China | COPD, DM, HP, CHD, CRVD, HBV, CKD, immunosuppression | 1,099 | 27 | 1,072 | 10/173 | 17/926 | ||
| Qin et al. [28] | China | CVD | Tongji Hospital | COPD, HP, CVD, CLD, DM, tuberculosis, Ca, CKD | 452 | 27 | 425 | 24/286 | 3/166 | ||
| Huang et al. [29] | China | CVD | 2 hospitals in the Hubei provinces, China | HP, DM, CHD, Ca | 223 | 13 | 210 | 9/98 | 4/125 | ||
| Huang et al. [30] | China | CVD | Designated hospital in Wuhan | DM, HP, CVD, COPD, Ca, CLD | 41 | 6 | 35 | 3/13 | 3/28 | ||
| Wan et al. [31] | China | CVD | Chongqing University Three Gorges Hospital, | DM, CVD, HP, COPD, Ca, CLD | 135 | 7 | 128 | 6/40 | 1/95 | ||
| Shi et al. [32] | China | CVD | Renmin Hospital of Wuhan University | HP, DM, CAD, CRVD, CHF, CKD, COPD, Ca, HBV | 416 | 61 | 355 | 36/82 | 25/334 | ||
| Zhou et al. [33] | China | CVD | Jinyintan Hospital and Wuhan Pulmonary Hospital | HP, DM, CHD, COPD, Ca, CKD | 191 | 59 | 132 | 41/54 | 18/137 | ||
| Lagi et al. [34] | Italy | CHD | University Hospital, Florence, Italy | DM, COPD, CHD, HP, HBV, CRVD, CKD | 84 | 12 | 72 | 5/16 | 7/68 | ||
| Wang et al. [35] | China | CVD | Zhongnan Hospital of Wuhan University in Wuhan and Xishui Hospital, Hubei Province, China | HP, CVD, DM, CLD, CVD, COPD, CKD | 107 | 13 | 94 | 7/19 | 6/88 | ||
| Chen et al. [36] | China | CVD | Wuhan Tongji Hospital | HP, DM, CVD, CHF, COPD, Ca, HBV, HIV, CRVD, CKD, CG, metabolic arthritis, autoimmune disease | 274 | 23 | 251 | 16/19 | 7/36 | ||
| Yang et al. [37] | China | Chronic cardiac disease | Wuhan Jin Yin-Tan Hospital | CVD, COPD, CRVD, DM, Ca, Dementia | 52 | 5 | 47 | 3/32 | 2/20 | ||
| Chen et al. [12] | China | CVD | Wuhan Jinyintan Hospital | CVD, CRVD, Ca | 99 | 40 | 59 | ||||
| Jin et al. [38] | China | CVD | The Health Commission of Zhejiang province in designated hospitals | HP, DM, CLD, Ca, CKD, CHD COPD, immunosuppression | 651 | 5 | 646 | ||||
| Richardson et al. [13] | USA | CVD | 12 hospitals in New York City, Long Island, and Westchester County, New York | Ca, CVD, HP, CAD, CHF, COPD asthma | 5,700 | 966 | 4,734 | ||||
| Yan et al. [39] | China | CVD | Tongji Hospital, Wuhan, China | HP, CVD, CRVD, CKD, COPD, CLD | 193 | 31 | 162 | 27/108 | 4/85 | ||
| Guan et al. [40] | China | Cardiovascular disease | 575 hospitals in 31 provinces/autonomous regions/provincial municipalities across mainland China | HP, DM, COPD, Ca, CVD | 1,590 | 59 | 1,531 | 20/59 | 234/1,531 | ||
| Gold et al. [41] | USA | CVD | Seven hospitals in metropolitan Atlanta | DM, CVD, CHF, Arrhythmia, COPD, asthma, Obesity, HP, CKD, Ca | 305 | 78 | 227 | ||||
| Grasselli et al. [5] | Italy | CVD | Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan | HP, CVD, HL, DM, Ca, COPD, CKD, CLD | 1,591 | 223 | 1,368 | ||||
| Wang et al. [42] | China | CVD | Tongji Hospital | HP, DM, CVD, COPD | 344 | 40 | 304 | 22/133 | 18/211 | ||
| Wang et al. [3] | China | HP | Zhongnan Hospital of Wuhan University in Wuhan, China | HP, CVD, DM, CLD, CRVD, COPD, CKD, Ca, HIV | 138 | 43 | 95 | 21/36 | 21/102 | ||
| Goyal et al. [14] | USA | HP | An 862-bed quaternary referral center and an affiliated 180-bed nonteaching community hospital in Manhattan | DM, Obesity, HP, COPD, Asthma, CAD | 393 | 197 | 196 | 70/130 | 127/263 | ||
| Zhang et al. [15] | China | HP | Zhongnan Hospital of Wuhan University, Wuhan, China | HP, CVD, DM, CLD, CRVD, COPD, CKD, Ca, immunosuppression | 221 | 55 | 166 | 26/55 | 28/166 | ||
| Hu et al. [16] | USA | HP | Tianyou Hospital, Wuhan University of Science and Technology, China | HP, CVD, DM, CLD, CRVD, COPD, CKD, Ca, cirrhosis | 323 | 105 | 218 | 66/172 | 39/151 | ||
| Guo et al. [17] | China | HP | The Seventh Hospital of Wuhan City, China | HP, CHD, DM, COPD, CKD, Ca, cardiomyopathy | 187 | 61 | 126 | ||||
| Zhang et al. [18] | China | HP | The Seventh Hospital of Wuhan City, China | HP, DM, CHD, HL, CG, CVD, CKD, CRVD, COPD, arrhythmia cholelithiasis, fatty liver, thyroid diseases | 140 | 42 | 98 | 22/58 | 20/82 | ||
| Du et al. [19] | China | HP | Hannan Hospital and Wuhan Union Hospital of Wuhan City, China | HP, DM, CHD, CRVD, CLD, COPD, CKD, Ca | 85 | 32 | 53 | ||||
| Rosenberg et al. [20] | USA | HP | 25 hospitals in the New York City, metropolitan region | Obesity, cancer, CKD, COPD, DM, HP, CAD, CHD, dementia | 1,438 | 816 | 622 | ||||
| Lei et al. [21] | China | HP | Renmin Hospital, Zhongnan Hospital, Tongji Hospital, and Central Hospital in Wuhan, | HP, Ca, DM, CVD,CRVD,COPD, CKD | 34 | 13 | 21 | 9/15 | 4/19 | ||
| Mercuro et al. [22] | USA | HP | An academic tertiary care center in Boston, Massachusetts | HP, CHF, DM, CAD, AF, COPD, asthma | 90 | 48 | 42 | ||||
| Saleh et al. [23] | USA | HP | 14 hospitals of the New York State Northwell Health system | HP, HL, DM, AF, CAD, COPD, CKD, CHF | 201 | 121 | 80 | ||||
| Inciardi et al. [24] | Italy | HP | Civil Hospitals of Brescia, Lombardy, Italy | HP, HL, DM, HF, AF, CAD, COPD, CKD, Ca | 99 | 63 | 36 | ||||
| Bhatla et al. [25] | USA | HP | The Hospital of the University of Pennsylvania | CHD, HF, HP, AF, DM, COPD, CLD, CKD | 700 | 347 | 353 | 62/79 | 285/621 | ||
| Sala et al. [26] | Italy | HP | Seven COVID units at a third-level hub center, San Raffaele Hospital, Italy | CAD, COPD, HP, DM, obesity, AF | 132 | 60 | 72 | ||||
| Guan et al. [4] | China | HP | 552 hospitals in 30 provinces, autonomous regions, and municipalities in mainland China | COPD, DM, HP, CHD, CRVD, HBV, CKD, immunosuppression | 1,099 | 165 | 934 | 41/173 | 124/926 | ||
| Qin et al. [28] | China | HP | Tongji Hospital | COPD, HP, CVD, CLD, DM, tuberculosis, Ca, CKD | 452 | 135 | 317 | 105/286 | 30/166 | ||
| Huang et al. [29] | China | HP | 2 hospitals in the Hubei provinces, China | HP, DM, CHD, Ca | 223 | 40 | 180 | 38/98 | 12/125 | ||
| Huang et al. [30] | China | HP | Designated hospital in Wuhan | DM, HP, CVD, COPD, Ca, CLD | 41 | 6 | 35 | 2/13 | 4/28 | ||
| Wan et al. [31] | China | HP | Chongqing University Three Gorges Hospital | DM, CVD, HP, COPD, Ca, CLD | 135 | 13 | 122 | 4/40 | 9/95 | ||
| Shi et al. [32] | China | HP | Renmin Hospital of Wuhan University | HP, DM, CAD, CRVD, CHF, CKD, COPD, Ca, HBV | 416 | 127 | 289 | 49/82 | 78/334 | ||
| Zhou et al. [33] | China | HP | Jinyintan Hospital and Wuhan Pulmonary Hospital | HP, DM, CHD, COPD, Ca, CKD | 191 | 58 | 133 | 26/54 | 32/137 | ||
| Lagi et al. [34] | Italy | HP | University Hospital, Florence, Italy | DM, COPD, CHD, HP, HBV, CRVD, CKD | 84 | 31 | 53 | 5/16 | 26/68 | ||
| Wang et al. [35] | China | HP | Zhongnan Hospital of Wuhan University in Wuhan and Xishui Hospital, Hubei Province, China | HP, CVD, DM, CLD, CVD, COPD, CKD | 107 | 26 | 81 | 10/19 | 16/88 | ||
| Richardson et al. [13] | USA | HP | 12 hospitals in New York City, Long Island, and Westchester County, New York | Ca, CVD, HP, CAD, CHF, COPD, asthma | 5,700 | 3026 | 2674 | ||||
| Yan et al. [39] | China | HP | Tongji Hospital, Wuhan, China | HP, CVD, CRVD, CKD, COPD, CLD | 193 | 73 | 120 | 57/108 | 16/85 | ||
| Guan et al. [40] | China | HP | 575 hospitals in 31 provinces/autonomous regions/provincial municipalities across mainland China | HP, DM, COPD, Ca, CVD | 1,590 | 269 | 1,321 | ||||
| Gold et al. [41] | USA | HP | Seven hospitals in metropolitan Atlanta | DM, CVD, CHF, Arrhythmia, COPD, asthma, Obesity, HP, CKD, Ca | 305 | 206 | 99 | ||||
| Grasselli et al. [5] | ITALY | Hypertension | Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan | HP, CVD, HL, DM, Ca, COPD, CKD, CLD | 1,591 | 509 | 1,082 | ||||
| Wang et al. [42] | China | Hypertension | Tongji hospital | HP, DM, CVD, COPD | 344 | 141 | 203 | 69/133 | 72/211 |
CVD, cardiovascular disease; HP, hypertension; DM, diabetes mellitus; CLD, chronic liver disease; CRVD, cerebrovascular disease; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; Ca, cancer; HIV, human immunodeficiency virus; CAD, coronary artery disease; CHD, coronary heart disease; HL, hyperlipidemia; CG, chronic gastritis; CHF, congestive heart failure; AF, atrial fibrillation; HF, heart failure; RD, rheumatologic disease; HBV, hepatitis B virus infection.
Quantitative Analysis
The proportions of cardiovascular disease comorbidities and cardiac manifestations in COVID-19 patients
Relevant data regarding the event rate of pre-existing cardiovascular diseases, including hypertension and coronary heart disease, in 19,156 patients with COVID-19 were collected from 34 studies (Table 1) [3−5,12−42]. The pooled prevalence of pre-existing cardiovascular diseases or coronary heart disease among the included studies was 14% (95% CI, 11% to 17%), as is shown in Figure 2.
Figure 2.
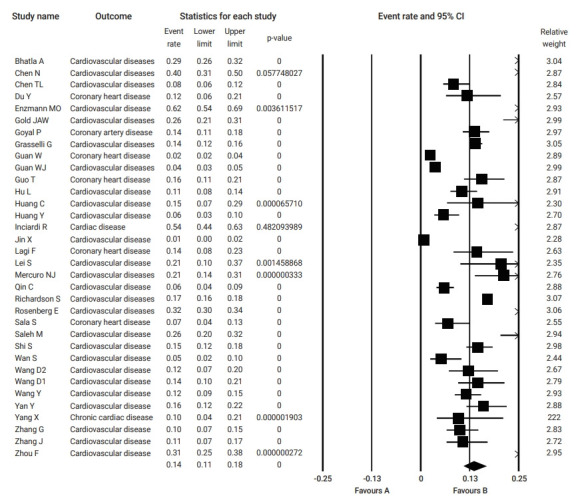
Pooled event rate of pre-existing cardiovascular disease in patients with coronavirus disease 2019. CI, confidence interval.
Pre-existing cardiovascular disease, hypertension, and coronary heart disease and the risk of severity outcomes and mortality in COVID-19
Table 2 summarizes the results of the current analysis. COVID-19 patients with pre-existing cardiovascular comorbidities were 4 times more likely to have severe outcomes (OR, 4.1; 95% CI, 2.9 to 5.7) (Figure 3) or not survive the disease (OR, 6.1; 95% CI; 2.9 to 12.7) (Figure 4), compared to patients with no pre-existing cardiovascular or coronary heart diseases. Severe disease was defined as patients needing to be admitted to the intensive care unit, needing vital life support, or requiring mechanical ventilation. Hypertension as a comorbid factor was associated with 2.6 times higher risk for severe outcomes (OR, 2.6; 95% CI, 1.9 to 3.6) and a 3 times higher fatality rate (OR, 3.2; 95% CI, 2.0 to 5.0) (Figures 5 and 6). However, coronary heart disease was associated with a 2.5 times higher risk for severe outcomes (OR, 2.5; 95% CI, 1.7 to 3.8) (Figure 7).
Table 2.
Effect of cardiovascular comorbidities on severity and mortality outcomes associated with coronavirus disease 2019
| Comorbidity | Severity |
Mortality |
||
|---|---|---|---|---|
| No. of studies | OR (95% CI) | No. of studies | OR (95% CI) | |
| Pre-existing cardiovascular diseases | 14 | 4.1 (2.9 to 5.7) | 7 | 6.1 (2.9 to 12.7) |
| Hypertension | 14 | 2.6 (1.9 to 3.6) | 4 | 3.2 (2.0 to 5.0) |
| Coronary heart disease | 4 | 2.5 (1.7 to 3.8) | - | - |
OR, odds ratio; CI, confidence interval; -, no data available to run analysis.
Figure 3.
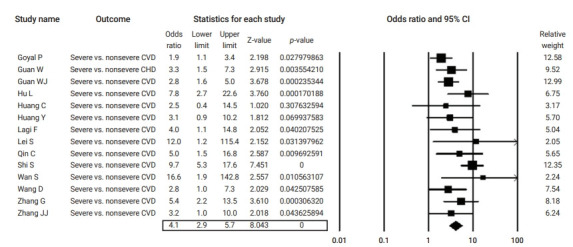
Forest plot of the odds ratios of pre-existing cardiovascular disease (CVD) in severe cases compared to non-severe cases of coronavirus disease 2019.
CI, confidence interval; CHD, coronary heart disease.
Figure 4.
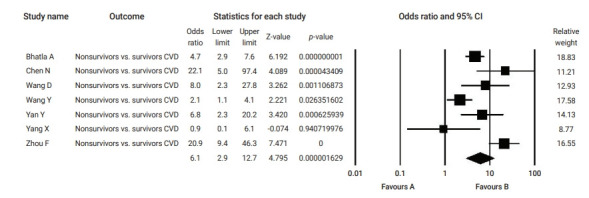
Forest plot of the odds ratios of pre-existing cardiovascular disease (CVD) in non-survivor compared to survivor coronavirus disease 2019 patients. CI, confidence interval.
Figure 5.
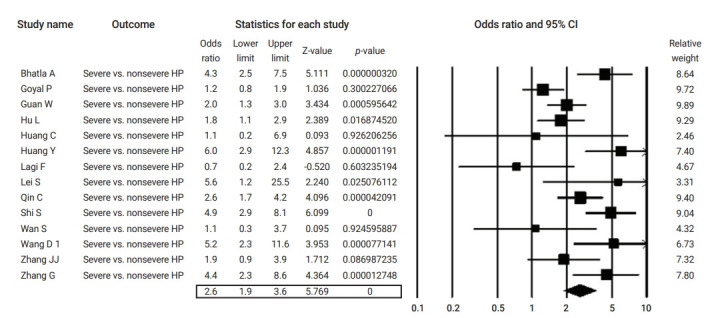
Forest plot of the odds ratios of pre-existing hypertension (HP) in severe compared to non-severe coronavirus disease 2019 cases. CI, confidence interval.
Figure 6.
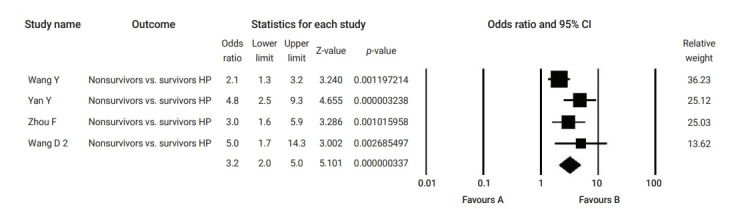
Forest plot of the odds ratios of pre-existing hypertension (HP) non-survivor compared to survivor coronavirus disease 2019 patients. CI, confidence interval.
Figure 7.
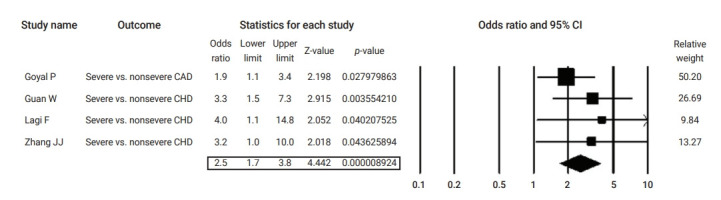
Forest plot of the odds ratios of pre-existing coronary heart disease (CHD) in severe cases compared to non-severe coronavirus disease 2019 cases.
CAD, coronary artery disease; CI, confidence interval.
Quality of the Included Studies
Table S1 shows the quality assessment of the studies on cardiovascular disease as a comorbidity in COVID-19 patients using JBI’s tool [3−5,12−42]. Most of the studies did not define participants’ eligibility criteria. Moreover, most studies were unclear regarding whether they included consecutive participants and whether the inclusion was complete. The majority of the studies diagnosed COVID-19 and the outcomes of interest using valid and reliable methods. All included studies in this analysis reported the demographic and the clinical characteristics, as well as the outcomes of the participants. However, most of the multi-center studies did not present the demographic and the clinical characteristics by site or clinic.
Assessment of Publication Bias
Publication bias was evaluated visually using a funnel plot. As shown in Figure 8 on the event rate of pre-existing cardiovascular comorbidity, a visual symmetry indicates the absence of publication bias. The Egger test also revealed no significant publication bias (Egger test, p=0.09).
Figure 8.
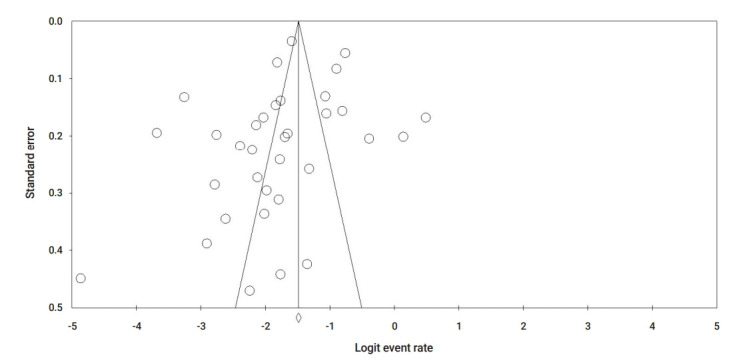
Funnel plot for publication bias based on cardiovascular comorbidity.
Discussion
In the present meta-analysis, we examined 36 independent studies reporting clinical data on 19,156 patients with COVID-19 worldwide. The studies included in this meta-analysis include the latest research available on COVID-19 from January to July 2020. Our pooled analyses indicated that pre-existing cardiovascular diseases, in particular hypertension and coronary heart disease, are prevalent among patients with COVID-19. Our pooled analyses also clearly showed that the presence of pre-existing cardiovascular disease, including hypertension and coronary heart disease, is associated with COVID-19 severity and/or fatality. This association can be confounded by older age, patients with poor outcomes may be older and have more cardiovascular events [43]. In this analysis meta-regression (data are not shown) using the method of moments of the effect of age, reported as mean or median, on association of pre-existing cardiovascular disease with COVID-19 outcomes revealed that age was significantly associated only with estimated OR for severity in patients with pre-existing cardiovascular disease.
In comparison, another meta-analysis of 6 published studies from China including 1,527 patients with COVID-19 that reported a 16.4% prevalence of cardio-cerebrovascular disease [44]. Another analysis of 7 Chinese studies showed that the prevalence of cardiovascular disease and that of hypertension were 21% and 8%, respectively [45]. Our meta-analysis on data from different countries reported a 14% prevalence of cardiovascular disease.
Pre-existing cardiovascular disease was associated with a 4-fold and 6-fold greater risk of disease severity and fatality, respectively. A previous study that analysed data of COVID-19 patients until March 20, 2020 found that cardiovascular disease increased the odds of combined critical/fatal cases of COVID-19 by 5 times [46] and in particular, hypertension was found to increase the odds of combined critical and fatal cases by 2.7 times. The main difference between our analysis and that by Zheng et al. [46] is that we analysed data separately for COVID-19 severity and mortality, while Zheng et al. [46] combined data on COVID-19 critical conditions and mortality. Another previous meta-analysis [44] that included only studies from China reported that comorbid hypertension increased COVID-19 severity by 2-fold, suggesting the prognostic impact of this comorbidity. Our results clearly confirm previous findings and add to them. Li et al. [44] were not able to provide data on cardiovascular comorbidities and death from COVID-19 as data collection was incomplete, and most of the included studies in their analysis did not analyse comorbidities in death cases. Another analysis by Luo et al. [47] included a larger number of studies and found that hypertension was associated with 2.5 times higher odds of mortality; however, considerable heterogeneity was also reported. In this analysis, the relationship between hypertension comorbidity and COVID-19-induced death was pooled using data from China and other countries using a random-effect model to account for heterogeneity. Hypertension was associated with a 3-fold increased fatality rate. The American Heart Association and the American College of Cardiology define hypertension as systolic blood pressure (BP) ≥130 or diastolic BP ≥80 mmHg, and hypertension is a primary risk factor associated with atherosclerotic cardiovascular disease [48]. In line with our analysis, several studies identified high rates of hypertension among severely symptomatic COVID-19 patients [5,12,13]. Roughly half of United States patients with hypertension are prescribed angiotensin-converting enzyme (ACE) inhibitors, aldosterone receptor blockers, and aldosterone antagonists, collectively called renin-angiotensin-aldosterone system (RAAS) inhibitors [49]. The modulator of the RAAS is the ACE2 receptor, which is used by SARS-CoV-2 to bind via its spike (S) protein to allow entry into attached cells. The activation of the RAAS is suggested as a mechanism for severe lung injury, especially in COVID-19 patients [50]. Inhibition of the protective signaling pathways in cardiac myocytes may result in secondary the downregulation of ACE2 expression within the myocardium. Finally, COVID-19 infection induces profound changes in coagulation pathways that create a hypercoagulable state and risk of microvascular thrombosis [51].
A strength of our pooled analysis is that it included more studies than some of the previous ones, and thus a larger sample size from different countries compared to the previous meta-analyses. Hence, our pooled analysis is the most inclusive and up-to-date analysis. The mechanism by which pre-existing cardiovascular disease increases the risk of COVID-19 adverse outcomes is also thought to be through the way that drugs for this disease work [52]. However, studies did not report data on the type of medications prescribed for each comorbidity, and hence we were not able to perform subgroup analyses by medication type. Such analyses are needed in further research. Another strength of this analysis is that visual symmetry in the funnel plot indicates the absence of publication bias. A limitation of this analysis is that most studies did not report the eligibility criteria and whether participants were recruited consecutively. Therefore, selection bias is a likely concern in the included studies. Other biases in the included studies are less likely since all studies sufficiently addressed other points in the JBI tool. Another limitation of this analysis is the possible effect of confounding factors including age, sex, and presence of other comorbidities that contribute to heterogeneity of the included studies. However, we used a random-effect model that addresses heterogeneity.
Conclusion
In summary, the present evidence showed that pre-existing cardiovascular disease in general, as well as hypertension and coronary heart disease, are highly associated with the severity and the mortality rate of COVID-19. Awareness of pre-existing cardiovascular comorbidities is important for the early management of COVID-19.
Footnotes
Ethics Approval
Not applicable.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Funding
None.
Availability of Data
The datasets are not publicly available but are available from the corresponding author upon reasonable request.
Authors’ Contributions
Conceptualization: FA; Data curation: FA, SA, MA; Formal analysis: SA; Investigation: FA; Methodology: FA, SA; Project administration: FA; Software: SA; Supervision: FA; Validation: all authors; Visualization: FA, SA; Writing–original draft: FA, SA; Writing–review & editing: all authors.
Supplementary Material
Table S1. Quality assessment of the studies on cardiovascular disease as a comorbidity in coronavirus disease 2019 patients using the Joanna Briggs Institute’s tool. Supplementary data are available at https://doi.org/10.24171/j.phrp.2021.0186.
Quality assessment of the studies on cardiovascular disease as a comorbidity in coronavirus disease 2019 patients using the Joanna Briggs Institute’s tool
References
- 1.Alzoughool F, Alanagreh L. Coronavirus drugs: using plasma from recovered patients as a treatment for COVID-19. Int J Risk Saf Med. 2020;31:47–51. doi: 10.3233/JRS-201017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alanagreh L, Alzoughool F, Atoum M. The human coronavirus disease COVID-19: its origin, characteristics, and insights into potential drugs and its mechanisms. Pathogens. 2020;9:331. doi: 10.3390/pathogens9050331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–9. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–81. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu Y, Zhu C, Yang L, et al. Identification of risk factors for mortality associated with COVID-19. PeerJ. 2020;8:e9885. doi: 10.7717/peerj.9885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganatra S, Dani SS, Shah S, et al. Management of cardiovascular disease during coronavirus disease (COVID-19) pandemic. Trends Cardiovasc Med. 2020;30:315–25. doi: 10.1016/j.tcm.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mishra AK, Sahu KK, George AA, et al. A review of cardiac manifestations and predictors of outcome in patients with COVID-19. Heart Lung. 2020;49:848–52. doi: 10.1016/j.hrtlng.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munn Z, Barker TH, Moola S, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth. 2020;18:2127–33. doi: 10.11124/JBISRIR-D-19-00099. [DOI] [PubMed] [Google Scholar]
- 11.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 12.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–13. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. 2020;323:2052–9. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of COVID-19 in New York city. N Engl J Med. 2020;382:2372–4. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang G, Hu C, Luo L, et al. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J Clin Virol. 2020;127:104364. doi: 10.1016/j.jcv.2020.104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu L, Chen S, Fu Y, et al. Risk factors associated with clinical outcomes in 323 coronavirus disease 2019 (COVID-19) hospitalized patients in Wuhan, China. Clin Infect Dis. 2020;71:2089–98. doi: 10.1093/cid/ciaa539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–8. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–41. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 19.Du Y, Tu L, Zhu P, et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan: a retrospective observational study. Am J Respir Crit Care Med. 2020;201:1372–9. doi: 10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenberg ES, Dufort EM, Udo T, et al. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York state. JAMA. 2020;323:2493–502. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lei S, Jiang F, Su W, et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine. 2020;21:100331. doi: 10.1016/j.eclinm.2020.100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mercuro NJ, Yen CF, Shim DJ, et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1036–41. doi: 10.1001/jamacardio.2020.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saleh M, Gabriels J, Chang D, et al. Effect of chloroquine, hydroxychloroquine, and azithromycin on the corrected QT interval in patients with SARS-CoV-2 infection. Circ Arrhythm Electrophysiol. 2020;13:e008662. doi: 10.1161/CIRCEP.120.008662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inciardi RM, Adamo M, Lupi L, et al. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur Heart J. 2020;41:1821–9. doi: 10.1093/eurheartj/ehaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhatla A, Mayer MM, Adusumalli S, et al. COVID-19 and cardiac arrhythmias. Heart Rhythm. 2020;17:1439–44. doi: 10.1016/j.hrthm.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sala S, Peretto G, De Luca G, et al. Low prevalence of arrhythmias in clinically stable COVID-19 patients. Pacing Clin Electrophysiol. 2020;43:891–3. doi: 10.1111/pace.13987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Enzmann MO, Erickson MP, Grindeland CJ, et al. Treatment and preliminary outcomes of 150 acute care patients with COVID-19 in a rural health system in the Dakotas. Epidemiol Infect. 2020;148:e124. doi: 10.1017/S0950268820001351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762–8. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang Y, Lyu X, Li D, et al. A cohort study of 223 patients explores the clinical risk factors for the severity diagnosis of COVID-19. medRxiv. 2020 Jan 1; doi: 10.1101/2020.04.18.20070656. [Epub]. [DOI] [Google Scholar]
- 30.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wan S, Xiang Y, Fang W, et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020;92:797–806. doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–10. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lagi F, Piccica M, Graziani L, et al. Early experience of an infectious and tropical diseases unit during the coronavirus disease (COVID-19) pandemic, Florence, Italy, February to March 2020. Euro Surveill. 2020;25:2000556. doi: 10.2807/1560-7917.ES.2020.25.17.2000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang D, Yin Y, Hu C, et al. Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS-CoV-2, discharged from two hospitals in Wuhan, China. Crit Care. 2020;24:188. doi: 10.1186/s13054-020-02895-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen T, Dai Z, Mo P, et al. Clinical characteristics and outcomes of older patients with coronavirus disease 2019 (COVID-19) in Wuhan, China: a single-centered, retrospective study. J Gerontol A Biol Sci Med Sci. 2020;75:1788–95. doi: 10.1093/gerona/glaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–81. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jin X, Lian JS, Hu JH, et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69:1002–9. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yan Y, Yang Y, Wang F, et al. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Res Care. 2020;8:e001343. doi: 10.1136/bmjdrc-2020-001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55:2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gold JA, Wong KK, Szablewski CM, et al. Characteristics and clinical outcomes of adult patients hospitalized with COVID-19: Georgia, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:545–50. doi: 10.15585/mmwr.mm6918e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y, Lu X, Li Y, et al. Clinical course and outcomes of 344 intensive care patients with COVID-19. Am J Respir Crit Care Med. 2020;201:1430–4. doi: 10.1164/rccm.202003-0736LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Z, Xu X, Ni H, et al. Platelet indices are novel predictors of hospital mortality in intensive care unit patients. J Crit Care. 2014;29:885. doi: 10.1016/j.jcrc.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 44.Li B, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109:531–8. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–5. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;81:e16–25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luo L, Fu M, Li Y, et al. The potential association between common comorbidities and severity and mortality of coronavirus disease 2019: a pooled analysis. Clin Cardiol. 2020;43:1478–93. doi: 10.1002/clc.23465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thathapudi S, Kodati V, Erukkambattu J, et al. Association of luteinizing hormone chorionic gonadotropin receptor gene polymorphism (rs2293275) with polycystic ovarian syndrome. Genet Test Mol Biomarkers. 2015;19:128–32. doi: 10.1089/gtmb.2014.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shah SJ, Stafford RS. Current trends of hypertension treatment in the United States. Am J Hypertens. 2017;30:1008–14. doi: 10.1093/ajh/hpx085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vaduganathan M, Vardeny O, Michel T, et al. Renin-angiotensin-aldosterone system inhibitors in patients with COVID-19. N Engl J Med. 2020;382:1653–9. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han H, Yang L, Liu R, et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58:1116–20. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 52.Lang JP, Wang X, Moura FA, et al. A current review of COVID-19 for the cardiovascular specialist. Am Heart J. 2020;226:29–44. doi: 10.1016/j.ahj.2020.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Quality assessment of the studies on cardiovascular disease as a comorbidity in coronavirus disease 2019 patients using the Joanna Briggs Institute’s tool