Abstract
Study Design:
Retrospective case series.
Objective:
Sacral insufficiency fracture is a rare and serious complication following lumbar spine instrumented fusion. The purpose of this study was to describe the patient characteristics, presentation, evaluation, treatment options, and outcomes for patients with sacral insufficiency fracture after short-segment lumbosacral fusion.
Methods:
Six patients from our institutional database and 16 patients from literature review were identified with a sacral insufficiency fracture after short-segment (L4-S1 or L5-S1) lumbar fusion within 1 year of surgery.
Results:
Patients were 55% female with a mean age of 58 years and body mass index of 30 kg/m2. Osteoporosis or osteopenia was the most common comorbidity (85%). Half of patients sustained a sacral fracture after surgery from a posterior approach, while the others had anterior or anterior-posterior surgery. Mean time to fracture was 42 days with patients clinically presenting with new sacral pain (86%), radiculopathy (60%), or neurologic deficit (5%). Ultimately, 73% of patients underwent operative fixation often involving extension of the construct (75%) and fusion to the pelvis (69%). Men (P = .02) and patients with new radicular pain or neurologic deficit (P = .01) were more likely to undergo revision surgical treatment while women over 50 years of age were more likely to be treated conservatively (P = .003).
Conclusions:
Spine surgeons should monitor for sacral insufficiency fracture as a source of new-onset pain in the postoperative period in patients with a short segment fusion to the sacrum. The recognition of this complication should prompt an assessment of bone health and management of underlying bone fragility.
Keywords: S1 fracture, ALIF, L4-S1, L5-S1, osteoporosis
Introduction
One and 2-level posterior decompression and fusion is a well-established treatment for degenerative spinal conditions such as lumbar spinal stenosis with spondylolisthesis resulting in radiculopathy or neurogenic claudication.1-3 More recently, interbody fusions with or without posterolateral spinal fusion (PSF) have been used to create additional surface area for fusion, spinal column height restoration, and indirect decompression of the neural elements.4,5 With the development and maturation of anterior lumbar spine fixation systems and techniques, some authors suggest an anterior lumbar interbody fusion (ALIF)-only approach is sufficient for most cases of short-segment isthmic spondylolisthesis.6-9 However, an ideal method for short-segment lumbosacral fusion is not clearly delineated in the literature, possibly because all approaches have shown the potential to deliver improved patient outcomes with high bony fusion rates.10-14
Although the differing techniques involved with surgical treatment of degenerative spondylolisthesis can lead to improved patient outcomes and have been shown to be cost effective,1,15,16 it is not without risks of complications, including but not exclusive to incidental durotomy, superficial wound or deep implant associated infection, hardware malposition, and adjacent segment disease.17-22 Sacral insufficiency fracture after short fusion construct to the sacrum is a less commonly reported phenomenon. Current data regarding these fractures primarily consists of case reports and small case series. These reports describe risk factors such as osteoporosis, obesity, and gender, but given the low overall incidence, the true incidence and factors remain unknown.23-32
The purpose of this study was to review the current literature and our institutional experience to better describe the patient characteristics, presentation, evaluation, treatment options, and outcomes for these patients with sacral insufficiency fracture after L4-S1 or L5-S1 fusion.
Materials and Methods
Following institutional review board (IRB) approval, we identified all patients diagnosed with a sacral fracture after a short-segment lumbar fusion over a 10-year period (2009-2019). All patients with an atraumatic postoperative sacral fracture within 1 year of a short-segment lumbosacral fusion were included. This was defined as either an L5-S1 or L4-S1 arthrodesis. Patients with a sacral fracture after trauma or caused by a tumor were excluded. Six patients were identified with a mean follow-up of 2 years (range 0.5-6 years). This cohort was reviewed to determine patient demographics, pelvic parameters, clinical presentation, initial lumbar arthrodesis technique, preoperative assessment, medical comorbidities, time to sacral fracture diagnosis, treatment of sacral fracture, and final outcome at last follow-up.
We then completed an electronic literature search in April of 2020 including the PubMed, Medline, and Google Scholar databases. Search keywords included a combination of “sacral fracture,” “lumbar fusion,” “anterior lumbar interbody fusion,” “transforaminal lumbar interbody fusion,” and “posterior lumbar interbody fusion.” Only abstracts in the English language were included for review. These searches resulted in 59 articles of which the title and abstract were reviewed for relevance to sacral fractures after a short-segment lumbosacral arthrodesis. Full texts of 26 relevant articles were then reviewed and only studies which included patient data were included.
Statistical Analysis
Continuous variables between groups were analyzed using the Student’s t test or Wilcoxon test, while categorical variables were compared using Fisher’s exact test and odds ratios calculated when able. A P value <.05 was considered significant for all statistical analyses.
Case Series
Patient 1
A 60 year old male, manual laborer with a past medical history (PMH) of morbid obesity (BMI 41), hypertension (HTN), diabetes mellitus II (DMII), chronic obstructive pulmonary disease (COPD) treated with inhaled steroids, compensated congestive heart failure (CHF), and obstructive sleep apnea (OSA) presented with grade 1 lytic spondylolisthesis with a right L5-S1 disc herniation and right L5 radiculopathy. Plan was for a 2-stage anterior-posterior L5-S1 fusion. The first stage ALIF with bone morphogenetic protein (BMP) was uncomplicated (Figure 1). On postoperative day (POD) 1 he had severe and acute-onset low back pain, when standing at his bedside. On POD2 his pain had resolved and he underwent L5-S1 PSF. Due to difficult intraoperative fluoroscopy, a postoperative CT (computed tomography) was obtained which demonstrated fracture of the superior sacral endplate. He was treated with bed rest for 3 weeks; however, his back and sacral pain continued and CT scan showed subsidence of his anterior cage. At this point the decision was made to proceed with revision L4-pelvis fusion with bilateral iliac screws. At clinic follow-up, a dual-energy X-ray absorptiometry (DEXA) scan showed osteopenia (T score −1.6 at the hip) and he was referred to endocrinology. His back pain improved significantly, and at 6-month follow-up imaging demonstrated solid fusion and a healed fracture. He was diagnosed with a deep vein thrombosis and pulmonary embolism 3 months after surgery and passed away from thromboembolic complications 1 year after surgery.
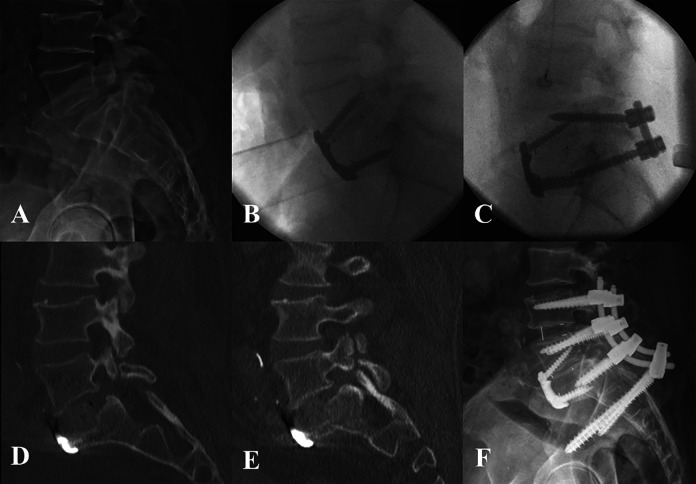
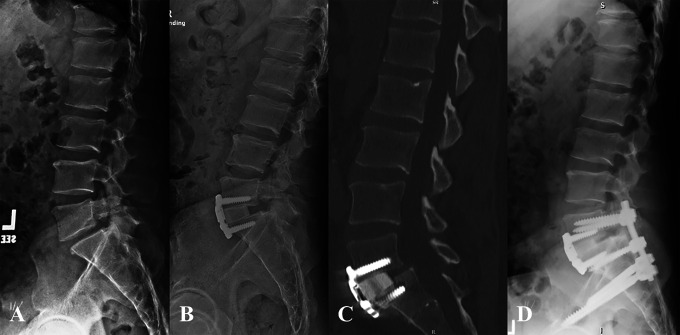
Figure 1.
Patient 1: Preoperative lateral radiograph demonstrating lytic grade I spondylolisthesis (A). Lateral intraoperative radiograph following stage 1 ALIF using PEEK cage with anterior plating (B) and PSF instrumentation after the second stage (C). Postoperative CT scan demonstrating S1 insufficiency fracture (D) and 3 weeks postoperatively showing further subsidence of the ALIF cage (E). Lateral radiograph 6 months after revision L4-pelvis fusion (F).
Patient 2
A 76-year-old overweight (BMI 28) male with a PMH of HTN, OSA, and osteopenia (T score −2.2) presented with L5-S1 stenosis with a right-sided facet cyst causing right radiculopathy and neurogenic claudication. He underwent 2-stage ALIF and PSF at L5-S1 without complication (Figure 2). He was doing well until 4 weeks after surgery when he began to have increased LBP and new left sided radiculopathy and weakness in the L5 and S1 distributions. CT scan on POD53 showed S1 insufficiency fracture and the decision was made to proceed with a L4-pelvis revision arthrodesis. This surgery was uneventful and he recovered well. He was referred to endocrinology and started on bisphosphonate therapy for clinical osteoporosis (osteopenia and insufficiency fracture). At 6 month follow-up his LBP was improved, radiculopathy resolved, and CT scan showed solid arthrodesis.
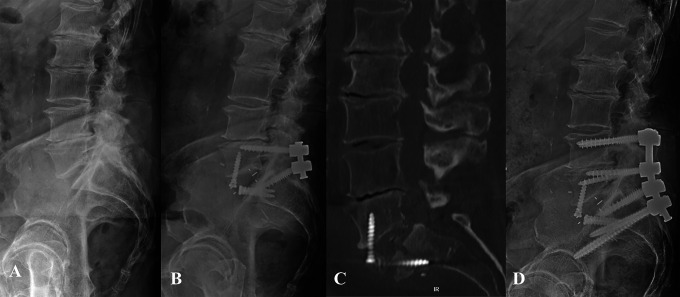
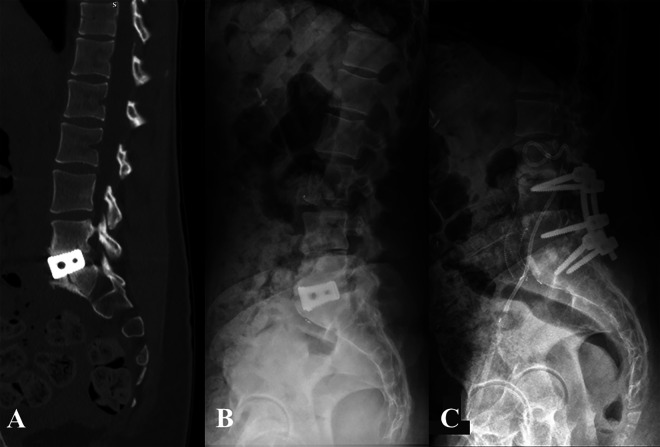
Figure 2.
Patient 2: Preoperative lateral radiograph demonstrating grade I degenerative spondylolisthesis (A) and postoperative radiograph following L5-S1 ALIF using PEEK cage with integrated fixation, and PSF (B). Postoperative day 53 CT scan demonstrating S1 insufficiency fracture (C) and postoperative radiograph 6 months after revision L4-pelvis fusion (D).
Patient 3
A 66-year-old overweight (BMI 29) male with a normal BMD and PMH significant for DMII, peripheral vascular disease (PVD), and cerebrovascular accident (CVA) with residual right-sided weakness, dysphasia, and behavior inhibition presented with grade 2 spondylolisthesis and L5 (R > L) radiculopathy. The decision was made to proceed with 2-stage ALIF and PSF at L5-S1 (Figure 3). He did well until 3 months postoperatively when he began to endorse left-sided radiculopathy and back pain. CT imaging demonstrated an S1 insufficiency fracture with cage subsidence and screw loosening. Nonoperative treatment was attempted with rest and a left-sided transforaminal epidural steroid injection. His pain worsened over the next few months and the decision was made to proceed with L4-pelvis decompression and fusion with L4-L5 transforminal lumbar interbody fusion (TLIF) and iliac screws. At 1-year follow-up his radiculopathy symptoms had resolved and his back pain was much improved but still present and he had a solid arthrodesis and healed fracture.
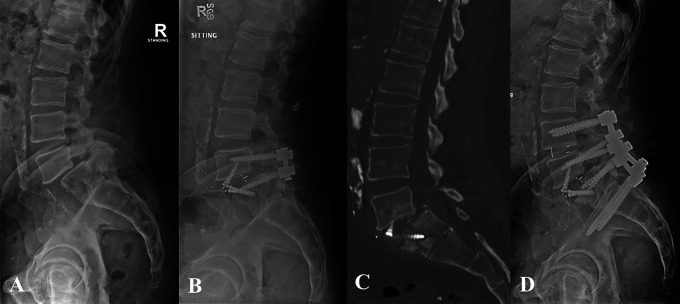
Figure 3.
Patient 3: Preoperative lateral radiograph demonstrating grade II lytic spondylolisthesis (A) and postoperative radiograph following L5-S1 ALIF using PEEK cage with integrated fixation, and PSF (B). Postoperative day 114 CT scan demonstrating S1 insufficiency fracture (C) and postoperative radiograph 6 months after revision L4-pelvis fusion (D).
Patient 4
A 73-year-old female with PMH of HTN, hyperlipidemia (HLD), hypothyroidism, osteopenia (T score −1.5), and DMII presented with right sided-foraminal stenosis with L5 radiculopathy. She underwent an ALIF at L5-S1 and did well for 1 week postoperatively. After this, she began having increased back pain with movement and numbness in the L5 distribution bilaterally. CT scan at 17 days showed an S1 insufficiency fracture and she underwent revision L5-S2AI (S2 sacral alar-iliac) arthrodesis on POD23 (Figure 4). She was referred to endocrinology who continues to monitor her BMD and she takes calcium and vitamin D supplements for her bone health. At 2-year follow-up she was doing well with only mild occasional LBP and was back to playing golf with a solid fusion construct and healed fracture.
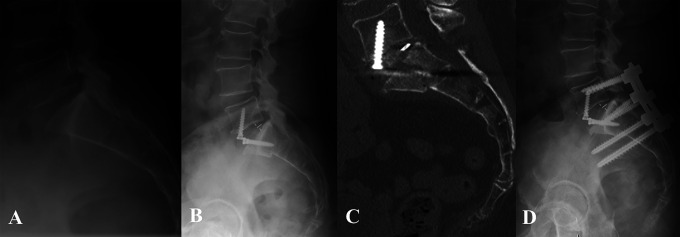
Figure 4.
Patient 4: Preoperative lateral radiograph demonstrating grade I degenerative spondylolisthesis (A) and postoperative radiograph following L5-S1 ALIF with PEEK cage and integrated fixation (B). Postoperative day 17 CT scan demonstrating S1 insufficiency fracture (C) and postoperative radiograph 1 year after revision L5-S2AI fusion (D).
Patient 5
A 53-year-old male with normal BMD presented with a mobile grade 1 spondylolisthesis with right L5 radiculopathy and mechanical LBP. He underwent an uncomplicated L5-S1 ALIF (Figure 5). His radiculopathy improved for 2 weeks, and then he had an episode of significant LBP and recurrence of his radiculopathy when standing up from the toilet. CT scan on POD31 showed an S1 insufficiency fracture and he underwent a revision L5-S2AI on POD56. He recovered well but had some residual posterior sacral pain and his S2AI screws were removed 1.5 years later. At 2-year follow-up his CT showed a solid arthrodesis and healed sacral fracture, while he continues to endorse moderate LBP symptoms.
Figure 5.
Patient 5: Preoperative lateral radiograph demonstrating grade I lytic spondylolisthesis (A) and postoperative radiograph following L5-S1 ALIF using femoral ring allograft with anterior plating (B). Postoperative day 31 CT scan demonstrating S1 insufficiency fracture and graft subsidence (C) and postoperative radiograph 1 year after revision L5-S2AI fusion (D).
Patient 6
A 31-year-old female with depression underwent a stand-alone L5-S1 ALIF for back and leg pain at an outside hospital. Her back and leg pain were improved for 3 days, but then she began to experience progressive back, buttock, and bilateral leg pain. A CT scan on POD85 showed a S1 insufficiency fracture (Figure 6) and she was treated with conservative measures for 3 years. She presented to our institution with continued symptoms (now on methadone). Imaging demonstrated degeneration of the superior adjacent level with L5-S1 pseudarthrosis and subsidence of the ALIF cage. The decision was made to undergo revision L5-S1 ALIF along with L4-5 ALIF and L4-S1 PSF as a 2-stage procedure. Her pain significantly improved; however, her postoperative course was complicated by a DVT requiring anticoagulation and IVC filter and right ureteral obstruction leading to chronic urinary tract infections and ultimately a nephrectomy. She was able to return to work as a nurse, but was not able to return to her emergency department position given continued moderate LBP. At 3-year follow-up, CT showed a stable fusion with degenerative changes superior to her fusion construct. She was offered extension of her fusion but she elected to continue conservative treatment measures.
Figure 6.
Patient 6: CT scan demonstrating S1 insufficiency fracture after stand-alone ALIF using threaded titanium cage, completed at an outside institution 85 days earlier (A). Preoperative lateral radiograph demonstrating L4-L5 disc space collapse (B) and follow-up radiograph from 2.5 years after revision L5-S1 ALIF, L4-L5 ALIF, and L4-S1 PSF (C).
Results
Patient Characteristics
Over the study period, 6 patients at our institution were found to have a sacral insufficiency fracture after short segment lumbosacral fusion. In addition, we reviewed 10 studies with 16 patients from the systematic review. This created a total pooled cohort of 22 patients (Table 1). There were 12 (55%) females with a mean age of 58 ± 12.5 years (range 31-76 years) and BMI of 30 ± 6 kg/m2 (range 23-41). There were 5 (23%) patients with DMII, 4 (18%) patients on steroids preoperatively, 4 (18%) with hypothyroidism, 2 (9%) with COPD, and 1 (5%) active smoker. Bone mineral density was reported in 14 (64%) patients; 7 (50%) had osteoporosis (T score <−2.5), 4 (29%) had osteopenia (T score −1 to −2.5), and 3 (21%) were normal. In our institutional cohort with BMD data, 3 (60%) patients were in the osteopenic range, 2 (40%) were in the normal range, and no patients were in the osteoporotic range (compared to 67% of patients in the reviewed cohort). Similarly, BMI was reported in 12 (55%) patients; 2 (9%) were morbidly obese, 3 (25%) obese, 4 (33%) overweight, and 3 (25%) had a normal BMI. Three patients (14%) had prior lumbar surgery, including 2 patients who underwent previous lumbar decompression, and one who underwent a L5-S1 PLIF (posterior lumbar interbody fusion) prior to the revision L4-S1 decompression and fusion.
Table 1.
Patient Characteristics, Time to Fracture, and Treatment of Sacral Insufficiency Fractures After L5-S1 or L4-S1 Lumbosacral Fusion.
| Case | Age (years), sex | Previous lumbar surgery | Antecedent surgical levels | Procedure | Time to fracture (days) | Sacral fracture treatment | Bone mineral density | Past medical history | Reference article |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 60, male | None | L5-S1 | Staged ALIF (PEEK cage with anterior plating)/PSF | 1 | PSF L4-ilium | Osteopenia | COPD, DMII, HTN, OSA, morbid obesity | — |
| 2 | 76, male | None | L5-S1 | Staged ALIF (PEEK cage with integrated fixation)/PSF | 53 | PSF L4-ilium | Osteopenia | HTN, OSA | — |
| 3 | 66, male | None | L5-S1 | Staged ALIF (PEEK cage with integrated fixation)/PSF | 114 | PSF L4-ilium | Normal | DMII, CVA, PVD | — |
| 4 | 73, female | None | L5-S1 | ALIF (PEEK cage with integrated fixation) | 17 | PSF L5-S2AI | Osteopenia | HTN, HLD, DMII, hypothyroidism | — |
| 5 | 53, male | None | L5-S1 | ALIF (femoral ring allograft with anterior plating) | 31 | PSF L5-S2AI | Normal | Hypothyroidism | — |
| 6 | 31, female | None | L5-S1 | ALIF (titanium treaded cage) | 3 | L4-S1 ALIF/PSF | NA | Depression, chronic pain | — |
| 7 | 41, female | None | L4-S1 | PLIF | 3 months | PSF w/ sacral hooks | Normal | NA | Bose et al 23 |
| 8 | 39, male | None | L5-S1 | ALIF | 34 days | L5-S1 PSF | NA | NA | Lastfogel et al 29 |
| 9 | 36, male | None | L5-S1 | ALIF | 38 days | L5-S1 PSF | NA | NA | Lastfogel et al 29 |
| 10 | 48, male | None | L5-S1 | ALIF | 13 days | L5-S1 PSF | NA | NA | Lastfogel et al 29 |
| 11 | 72, male | None | L5-S1 | ALIF | 9 days | L5-S1 PSF | NA | NA | Phan et al 30 |
| 12 | 70, male | Decompression | L4-S1 | L4-S1 TLIF and PSF | 21 days | L4-ilium | Osteopenia | Pemphigus | Buell et al 31 |
| 13 | 66, male | None | L5-S1 | L5-S1 TLIF and PSF | 28 days | L4-ilium | Osteoporosis | COPD, smoker | Buell et al 31 |
| 14 | 76, female | None | L4-S1 | L4-S1 PSF | 21 days | L4-ilium | Osteoporosis | Hypothyroidism | Buell et al 31 |
| 15 | 69, female | None | L4-S1 | L4-S1 TLIF and PSF | 21 days | L4-ilium | Osteoporosis | Hyperparathyroidism, hypothyroidism, RA on prednisone | Buell et al 31 |
| 16 | 52, female | None | L4-S1 | L4-S1 PSF | Several months | L2-ilium | NA | NA | Hsieh et al 51 |
| 17 | 53, female | Decompression | L4-S1 | PSF/ALIF | 4 months | Brace therapy, osteoporosis tx | Osteoporosis | SLE, long-term prednisone | Elias et al 27 |
| 18 | 61, female | None | L5-S1 | PSF/PLIF | First few days | TLSO, osteoporosis tx | Osteopenia | Breast cancer s/p mastectomy, radiation, chemotherapy; neuromuscular scoliosis; polio | Fourney et al 28 |
| 19 | 57, female | None | L4-S1 | PSF | 2 months | Encouraged activity | NA | Morbid obesity, HTN, OSA | Khan et al 25 |
| 20 | 57, female | L5-S1 PLIF | L4-S1 | PSF | 6 weeks | Water therapy, pain control | NA | DMII, obesity | Khan et al 25 |
| 21 | 70, female | None | L4-S1 | PSF | 2 weeks | Conservative management | Osteoporosis | n/a | Mathews et al 24 |
| 22 | 57, female | None | L4-S1 | PSF/PLIF | 9 days | Short-period immobilization, orthotic | Osteoporosis | DMII, hepatitis C, COPD, long-term cortisone use | Pennekamp et al 26 |
Abbreviations: ALIF, anterior lumbar interbody fusion; PLIF, posterior lumbar interbody fusion; PSF, posterior spinal fusion; S2AI, s2 alar-iliac; HTN, hypertension; DMII, type II diabetes mellitus; OSA, obstructive sleep apnea; SLE, systemic lupus erythematous; COPD, chronic obstructive pulmonary disease.
The 6 patients from our institution had a mean preoperative pelvic incidence (PI) of 54°, sacral slope (SS) of 39°, and pelvic tilt (PT) of 15°, while mean PI-lumbar lordosis (PI-LL) mismatch was 17° (range 5° to 35°). The mean postoperative PI was 54°, SS 34°, PT of 21°, and PI-LL mismatch was 10° (range 5° to 15°). Mean PI-LL correction was 7° while SS decreased a mean of 7° with surgery.
The reason for primary fusion was spinal stenosis with radiculopathy and/or neurogenic claudication with grade 1 spondylolisthesis (50%) and grade 2 spondylolisthesis (23%), while (27%) did not mention spondylolisthesis. In our institutional cohort, 3 patients (50%) had a lytic spondylolisthesis while the other 3 patients (50%) had degenerative spondylolisthesis. Surgery was performed with a stand-alone ALIF in 7 (32%) patients, posterior only approach in 11 (50%) with 55% of these patients receiving a PLIF or TLIF, and anterior-posterior approach in 4 (18%). Therefore, 17 (77%) patients had anterior support at the primary operation. Bone morphogenetic protein-2 (BMP-2) was used in 5 (23%) of the cases while nonstructural iliac crest autograft was used in 4 cases (18%).
Fracture Characteristics
Sacral insufficiency fracture was diagnosed a mean of 42 ± 35 days (range 1-144 days) after surgery. Sacral fracture presentation included new or increased LBP or buttock pain in 86% of patients, new or worsening radiculopathy in 60%, and a new neurologic deficit in 5%. Fracture diagnosis was made plain radiographs in 5 (23%) patients and CT scan in 17 (77%) patients.
Treatment Characteristics
Surgical treatment of the fracture was common with 73% of patients undergoing operative fixation (100% in our institutional cohort). Prior to surgical intervention, nonoperative treatment was attempted in 31% of these patients, which included bedrest, transforaminal epidural steroid injection, activity modification, and pain management. Operative fixation involved extension of the construct in 75% of patients and fusion to the pelvis in 69%. The most common treatment was L4-ilium arthrodesis (44%) followed by L4-S1 PSF (25%), L5-S2AI (25%), L4-S1 with revision ALIF (6%), extension to the sacrum with hooks (6%), and L2-ilium (6%) arthrodesis. All patients went on to heal their fracture and developed a stable fusion.
Six (27%) patients were treated nonoperatively, which included orthotic bracing (50%), activity modification, and pain management. Two patients failed nonoperative treatment and went on to operative treatment. Women over the age of 50 were less likely to receive an operation (P = .003), while men were more likely to require operative intervention (P = .02) as were patients with new radicular pain or neurologic deficit (odds ratio [OR] = 21.67, 95% confidence interval [CI] = 1.80-260.57, P = .01). Similarly, the use of a stand-alone ALIF at the index operation trended toward the need for revision surgery (P = .12; Table 2).
Table 2.
Factors Predictive of the Need for Operative Intervention and Residual Pain at Final Follow-up.
| P value | Odds ratio | 95% Confidence interval | |
|---|---|---|---|
| Factors predictive of need for operative intervention | |||
| Women >50 years | P = .003*,a | NA | NA |
| BMI >30 | P = 1.0 | 0.6 | 0.03-13.58 |
| Stand-alone ALIF | P = .12 | NA | NA |
| Male gender | P = .02* | NA | NA |
| New radicular pain or neurologic deficit | P = .01* | 21.67 | 1.80-260.57 |
| Factors predictive of residual pain | |||
| Operative intervention | P = .62 | 3 | 0.28-32.2 |
| Women >50 years | P = .07 | 0.11 | 0.01-1.17 |
| Male gender | P = .17 | 5 | 0.70-35.50 |
| Fusion to pelvis | P = 1.0 | 0.86 | 0.14-5.22 |
| BMI >30 | P = 1.0 | 1.67 | 0.07-37.7 |
| New radicular pain or neurologic deficit | P = .19 | 5.25 | 0.50-54.91 |
Abbreviations: BMI, body mass index; ALIF, anterior lumbar interbody fusion.
a Women >50 years of age were less likely to require operative treatment.
*P value <.05.
At final follow-up 38% of patients undergoing operative fixation had significant residual low back pain while 17% treated nonoperatively had residual back pain (OR = 3.0, 95% CI = 0.28-32.2, P = .62). Women over the age of 50 trended toward having less pain than men and younger women (OR = 0.11, 95% CI = 0.01-1.17, P = .07); while men trended toward more pain than women (OR = 5, 95% CI = 0.70-35.5, P = .17). Operative intervention and new radicular pain or neurologic symptoms were not predictive of residual pain at final follow-up.
Bone mineral density (BMD) was assessed in 83% (n = 5) of patients treated for a sacral insufficiency fracture after short segment lumbosacral fusion at our institution. Of those patients, 2 patients had a normal bone density scores on DEXA scan and no further treatment recommendations were made. Of the 3 patients with osteopenia, all patients were referred to endocrinology. One patient was treated with bisphosphonates, one with vitamin D and calcium supplementation, and the other died before starting medical treatment (Table 3).
Table 3.
Bone Mineral Density (BMD) Scores and Treatment of Bone Health for Patients With Sacral Insufficiency Fracture After Short-Segment Lumbosacral Fusion.
| Case | Age (years), sex | DEXA score and classification | Treatment for bone health |
|---|---|---|---|
| 1 | 60, male | −1.6 (osteopenia) | Referral to endocrine to begin treatment; however, patient died prior to starting medical treatment |
| 2 | 76, male | −2.2 (osteopenia) | Referral to endocrinology who started bisphosphonate treatment |
| 3 | 66, male | 0.1 (normal) | Normal BMD and therefore no treatment of BMD |
| 4 | 73, female | −1.5 (osteopenia) | Referral to endocrinology who continued to monitor her BMD and was treating with calcium and vitamin D supplementation |
| 5 | 53, male | −0.1 (normal) | Normal BMD and therefore no treatment of BMD |
| 6 | 31, female | NA | No DEXA scan was performed |
Discussion
Adult lumbar spondylolisthesis with neurologic symptoms can be successfully treated with decompression and fusion through a variety of approaches including anterior only, posterior only, or combined anterior and posterior approaches. Complication profiles vary based on treatment approach; however, sacral insufficiency fracture after 1- or 2-level fusion has been described after all approaches. This study evaluated patient characteristics, presentation, evaluation, treatment options, and outcomes of sacral insufficiency fracture after L4-S1 and L5-S1 fusion using a case series from our institution along with cases reported in the literature.
Sacral insufficiency fractures have been commonly reported in elderly women without a history of spinal surgery. These fractures most commonly present after a minor fall or trauma with significant sacral/buttock pain and up to 95% of patients having an underlying diagnosis of osteoporosis.33,34 These fractures are also seen in patients, with or without osteoporosis, undergoing long-segment spinal fusions (>3 segments) as these constructs place abnormally large mechanical forces on the adjacent segments leading to both cranial and caudal fracture patterns.24,27,35-41 Sacral insufficiency fractures have also been reported in patients with a short-segment fusion, but with a significantly lower incidence (0.15% to 2.9% compared to 14.5%).38,42,43 Patients with fractures after short-segment fusion present similarly to those without a history of spine surgery with most complaining of new low back or sacral pain after an atraumatic incident such as stepping off a curb, rolling out of bed, or rising from a chair. However, 60% of patients in this series also reported new radicular symptoms and 5% had a new neurologic deficit.
Some have implicated an increased PI, increased BMI, age, and gender as risk factors for sacral fractures after all lumbosacral spinal fusions. This cohort was younger (mean age 58 years) than reports of sacral fracture after long-segment fusion31,32 and had a similar incidence between men and women (55% female). Many patients were obese or morbidly obese in this series with a mean BMI of 30 kg/m2, which follows previous reports that increased weight may be a risk factor for sacral fracture after lumbosacral fusion. 32 There remains a debate whether bone density predicts sacral fractures after lumbosacral fusion.32,38,39,41,44 In this cohort 85% of patients had diminished bone density scores with a diagnosis of osteoporosis or osteopenia. This highlights the importance of preoperative bone density evaluation and treatment to help prevent any osteoporosis related complications after lumbosacral fusion.45,46 Last, the inherent biomechanics of the L5-S1 level may increase sacral fracture risk. The shear force parallel to the S1 endplate (usually absorbed by the annulus fibrosis and articular facets) and axial compression force perpendicular to the endplate are transferred to the vertebral body or interbody cage (especially in the case of lytic spondylolisthesis with the loss of the posterior tension band) increasing fracture risk.29,30
Treatment strategies for these fractures vary. The majority of osteoporotic insufficiency fractures in patients without a history of spine surgery respond to conservative treatment within a few months.33,34 In this series, 73% of patients were treated with operative fixation. Women older than 50 were all able to be treated without surgery while men and stand-alone ALIF fixation all went on to require surgery. While most cases are treated surgically, surgeon discretion as much as any factor may have played the most significant role in reported cases. One universal finding is when a sacral insufficiency fracture occurs following a stand-alone ALIF, the construct is no longer stable and revision posterior spinal fusion is required. Similarly, patients with new radiculopathy or neurologic deficit were also more likely to undergo operative treatment. Patients without new neurologic symptoms, significant or worsening deformity, and adequate pain control can be treated with nonoperative means including bracing, activity modification, and pain control. Meanwhile patients with new neurologic symptoms, stand-alone ALIF, pseudarthrosis, significant deformity, and failure to tolerate conservative treatment should undergo operative fixation. This is a similar algorithm to that recommended by Buell et al for sacral fractures after spinal fusion including long-segment fusions. 31 To adequately control the lumbosacral junction revision posterior spinal fusion should extend to the pelvis with either iliac screws or S2AI screws; however, revision of a stand-alone ALIF has been reported to be successfully achieved with L5-S1 PSF alone, avoiding implant issues related to extending to the pelvis. Patients with continued pain, but who are poor surgical candidates may benefit from interventional sacroplasty,47-49 but long-term outcomes are sparse and require more research. Most importantly, all of these patients should be referred for DEXA scan and management of their underlying bone fragility by either their primary care provider or a bone health specialist (ie, endocrinologist). Current guidelines state patients with osteopenia who sustain an axial insufficiency fracture have a clinical diagnosis of osteoporosis and should receive medical treatment. 50 Since most of these fractures heal with operative or nonoperative treatment, identifying and treating the osteoporosis underling these fractures may represent the most beneficial impact on the patient’s health.
There are several limitations to this study. First is the overall low number of patients with this complication which limits the conclusions we were able to make. However, we included 6 patients from our institution (largest case series of sacral insufficiency fractures after short-segment lumbar fusion) and all reports of sacral insufficiency fractures in the literature with patient information to help strengthen these conclusions. Unfortunately, not all patients reviewed had bone mineral density information and therefore the incidence of osteoporosis and osteopenia may be lower than reported in this series. Additionally, these patients were treated by a variety of surgeons at multiple institutions with different treatment protocols and strategies; therefore, we were unable to compare efficacy of different surgical treatment options.
In conclusion, atraumatic sacral insufficiency fracture after short-segment lumbar fusion is a rare complication which can lead to new sacral and low back pain, radiculopathy, and neurologic deficits in patients who were previously recovering well after surgery. Nonoperative treatment can be attempted in patients with mild symptoms and no significant spinopelvic deformity, while operative fixation often requires extension of the fusion construct with instrumentation to the pelvis. Many of these patients have clinical osteoporosis and should be referred for appropriate medical treatment. Spine surgeons should have a high index of suspicion for a sacral insufficiency fracture in patients with new-onset postoperative pain even after short-segment lumbosacral fusion.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Joshua M. Kolz, MD, MS  https://orcid.org/0000-0001-8085-3633
https://orcid.org/0000-0001-8085-3633
Brett A. Freedman, MD  https://orcid.org/0000-0002-3408-0163
https://orcid.org/0000-0002-3408-0163
References
- 1.Kornblum MB, Fischgrund JS, Herkowitz HN, Abraham DA, Berkower DL, Ditkoff JS. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective long-term study comparing fusion and pseudarthrosis. Spine (Phila Pa 1976). 2004;29:726–733. doi:10.1097/01.brs.0000119398.22620.92 [DOI] [PubMed] [Google Scholar]
- 2.Fischgrund JS, Mackay M, Herkowitz HN, Brower R, Montgomery DM, Kurz LT. 1997 Volvo Award winner in clinical studies. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective, randomized study comparing decompressive laminectomy and arthrodesis with and without spinal instrumentation. Spine (Phila Pa 1976). 1997;22:2807–2812. doi:10.1097/00007632-199712150-00003 [DOI] [PubMed] [Google Scholar]
- 3.Moller H, Hedlund R. Surgery versus conservative management in adult isthmic spondylolisthesis—a prospective randomized study: part 1. Spine (Phila Pa 1976). 2000;25:1711–1715. doi:10.1097/00007632-200007010-00016 [DOI] [PubMed] [Google Scholar]
- 4.de Kunder SL, van Kuijk SMJ, Rijkers K, et al. Transforaminal lumbar interbody fusion (TLIF) versus posterior lumbar interbody fusion (PLIF) in lumbar spondylolisthesis: a systematic review and meta-analysis. Spine J. 2017;17:1712–1721. doi:10.1016/j.spinee.2017.06.018 [DOI] [PubMed] [Google Scholar]
- 5.Mobbs RJ, Phan K, Malham G, Seex K, Rao PJ. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg. 2015;1:2–18. doi:10.3978/j.issn.2414-469X.2015.10.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim NH, Lee JW. Anterior interbody fusion versus posterolateral fusion with transpedicular fixation for isthmic spondylolisthesis in adults. A comparison of clinical results. Spine (Phila Pa 1976). 1999;24:812–817. [DOI] [PubMed] [Google Scholar]
- 7.Madan S, Boeree N. Comparison of instrumented anterior interbody fusion with instrumented circumferential lumbar fusion. Eur Spine J. 2003;12:567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pradhan BB, Nassar JA, Delamarter RB, Wang JC. Single-level lumbar spine fusion: a comparison of anterior and posterior approaches. J Spinal Disord Tech. 2002;15:355–361. [DOI] [PubMed] [Google Scholar]
- 9.Riouallon G, Lachaniette CHF, Poignard A, Allain J. Outcomes of anterior lumbar interbody fusion in low-grade isthmic spondylolisthesis in adults: a continuous series of 65 cases with an average follow-up of 6.6 years. Orthop Traumatol Surg Res. 2013;99:155–161. [DOI] [PubMed] [Google Scholar]
- 10.Kwon BK, Hilibrand AS, Malloy K, et al. A critical analysis of the literature regarding surgical approach and outcome for adult low-grade isthmic spondylolisthesis. J Spinal Disord Tech. 2005;18(suppl):S30–S40. doi:10.1097/01.bsd.0000133064.20466.88 [DOI] [PubMed] [Google Scholar]
- 11.Jacobs WC, Vreeling A, De Kleuver M. Fusion for low-grade adult isthmic spondylolisthesis: a systematic review of the literature. Eur Spine J. 2006;15:391–402. doi:10.1007/s00586-005-1021-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeWald RL, Faut M, Taddonio R, Neuwirth M. Severe lumbosacral spondylolisthesis in adolescents and children. Reduction and staged circumferential fusion. J Bone Joint Surg Am. 1981;63:619–626. [PubMed] [Google Scholar]
- 13.Smith MD, Bohlman H. Spondylolisthesis treated by a single-stage operation combining decompression with in situ posterolateral and anterior fusion. An analysis of eleven patients who had long-term follow-up. J Bone Joint Surg Am. 1990;72:415–421. [PubMed] [Google Scholar]
- 14.Shim JH, Kim WS, Kim JH, Kim DH, Hwang JH, Park CK. Comparison of instrumented posterolateral fusion versus percutaneous pedicle screw fixation combined with anterior lumbar interbody fusion in elderly patients with L5-S1 isthmic spondylolisthesis and foraminal stenosis. J Neurosurg Spine. 2011;15:311–319. [DOI] [PubMed] [Google Scholar]
- 15.Tosteson AN, Tosteson TD, Lurie JD, et al. Comparative effectiveness evidence from the spine patient outcomes research trial: surgical versus nonoperative care for spinal stenosis, degenerative spondylolisthesis, and intervertebral disc herniation. Spine (Phila Pa 1976). 2011;36:2061–2068. doi:10.1097/BRS.0b013e318235457b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adogwa O, Parker SL, Davis BJ, et al. Cost-effectiveness of transforaminal lumbar interbody fusion for grade I degenerative spondylolisthesis. J Neurosurg Spine. 2011;15:138–143. doi:10.3171/2011.3.Spine10562 [DOI] [PubMed] [Google Scholar]
- 17.Amato V, Giannachi L, Irace C, Corona C. Accuracy of pedicle screw placement in the lumbosacral spine using conventional technique: computed tomography postoperative assessment in 102 consecutive patients. J Neurosurg Spine. 2010;12:306–313. doi:10.3171/2009.9.Spine09261 [DOI] [PubMed] [Google Scholar]
- 18.Patel H, Khoury H, Girgenti D, Welner S, Yu H. Burden of surgical site infections associated with select spine operations and involvement of Staphylococcus aureus. Surg Infect (Larchmt). 2017;18:461–473. doi:10.1089/sur.2016.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi Y, Sato T, Hyodo H, et al. Incidental durotomy during lumbar spine surgery: risk factors and anatomic locations: clinical article. J Neurosurg Spine. 2013;18:165–169. doi:10.3171/2012.10.Spine12271 [DOI] [PubMed] [Google Scholar]
- 20.Ulrich NH, Burgstaller JM, Brunner F, et al. The impact of incidental durotomy on the outcome of decompression surgery in degenerative lumbar spinal canal stenosis: analysis of the Lumbar Spinal Outcome Study (LSOS) data—a Swiss prospective multi-center cohort study. BMC Musculoskelet Disord. 2016;17:170. doi:10.1186/s12891-016-1022-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JY, Ryu DS, Paik HK, et al. Paraspinal muscle, facet joint, and disc problems: risk factors for adjacent segment degeneration after lumbar fusion. Spine J. 2016;16:867–875. doi:10.1016/j.spinee.2016.03.010 [DOI] [PubMed] [Google Scholar]
- 22.Carlson BC, Hines JT, Robinson WA, et al. Implant sonication versus tissue culture for the diagnosis of spinal implant infection. Spine (Phila Pa 1976). 2020;45:E525–E532. doi:10.1097/brs.0000000000003311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bose B.Fracture of S1-2 after L4-S1 decompression and fusion. Case report and review of the literature. J Neurosurg. 2003;99:310–312. doi:10.3171/spi.2003.99.3.0310 [DOI] [PubMed] [Google Scholar]
- 24.Mathews V, McCance SE, O’Leary PF. Early fracture of the sacrum or pelvis: an unusual complication after multilevel instrumented lumbosacral fusion. Spine (Phila Pa 1976). 2001;26:E571–E575. [DOI] [PubMed] [Google Scholar]
- 25.Khan MH, Smith PN, Kang JD. Sacral insufficiency fractures following multilevel instrumented spinal fusion: case report. Spine (Phila Pa 1976). 2005;30:E484–E488. doi:10.1097/01.brs.0000174272.63548.89 [DOI] [PubMed] [Google Scholar]
- 26.Pennekamp PH, Kraft CN, Stutz A, Diedrich O. Sacral fracture as a rare early complication of lumbosacral spondylodesis [in German]. Z Orthop Ihre Grenzgeb. 2005;143:591–593. doi:10.1055/s-2005-836827 [DOI] [PubMed] [Google Scholar]
- 27.Elias WJ, Shaffrey ME, Whitehill R. Sacral stress fracture following lumbosacral arthrodesis. Case illustration. J Neurosurg. 2002;96(1 suppl):135. doi:10.3171/spi.2002.96.1.0135 [DOI] [PubMed] [Google Scholar]
- 28.Fourney DR, Prabhu SS, Cohen ZR, Gokaslan ZL, Rhines LD. Early sacral stress fracture after reduction of spondylolisthesis and lumbosacral fixation: case report. Neurosurgery. 2002;51:1507–1511. [PubMed] [Google Scholar]
- 29.Lastfogel JF, Altstadt TJ, Rodgers RB, Horn EM. Sacral fractures following stand-alone L5-S1 anterior lumbar interbody fusion for isthmic spondylolisthesis: report of 3 cases. J Neurosurg Spine. 2010;13:288–293. doi:10.3171/2010.3.Spine09366 [DOI] [PubMed] [Google Scholar]
- 30.Phan K, Mobbs RJ. Sacrum fracture following L5-S1 stand-alone interbody fusion for isthmic spondylolisthesis. J Clin Neurosci. 2015;22:1837–1839. [DOI] [PubMed] [Google Scholar]
- 31.Buell TJ, Yener U, Wang TR, et al. Sacral insufficiency fractures after lumbosacral arthrodesis: salvage lumbopelvic fixation and a proposed management algorithm. J Neurosurg Spine. Published online March 27, 2020. doi:10.3171/2019.12.Spine191148 [DOI] [PubMed] [Google Scholar]
- 32.Salzmann SN, Miller CO, Carrino JA, et al. BMI and gender increase risk of sacral fractures after multilevel instrumented spinal fusion compared with bone mineral density and pelvic parameters. Spine J. 2019;19:238–245. doi:10.1016/j.spinee.2018.05.021 [DOI] [PubMed] [Google Scholar]
- 33.Gotis-Graham I, McGuigan L, Diamond T, et al. Sacral insufficiency fractures in the elderly. J Bone Joint Surg Br. 1994;76:882–886. [PubMed] [Google Scholar]
- 34.Lourie H.Spontaneous osteoporotic fracture of the sacrum. An unrecognized syndrome of the elderly. JAMA. 1982;248:715–717. [PubMed] [Google Scholar]
- 35.Wood KB, Geissele AE, Ogilvie JW. Pelvic fractures after long lumbosacral spine fusions. Spine (Phila Pa 1976). 1996;21:1357–1362. doi:10.1097/00007632-199606010-00016 [DOI] [PubMed] [Google Scholar]
- 36.Khanna AJ, Kebaish KM, Ozdemir HM, Cohen DB, Gonzales RA, Kostuik JP. Sacral insufficiency fracture surgically treated by fibular allograft. J Spinal Disord Tech. 2004;17:167–173. [DOI] [PubMed] [Google Scholar]
- 37.Koh YD, Kim JO, Lee JJ. Stress fracture of the pelvic wing-sacrum after long-level lumbosacral fusion: a case report. Spine (Phila Pa 1976). 2005;30:E161–E163. doi:10.1097/01.brs.0000155634.12696.02 [DOI] [PubMed] [Google Scholar]
- 38.Meredith DS, Taher F, Cammisa FP, Jr, Girardi FP. Incidence, diagnosis, and management of sacral fractures following multilevel spinal arthrodesis. Spine J. 2013;13:1464–1469. doi:10.1016/j.spinee.2013.03.025 [DOI] [PubMed] [Google Scholar]
- 39.Papadopoulos EC, Cammisa FP, Jr, Girardi FP. Sacral fractures complicating thoracolumbar fusion to the sacrum. Spine (Phila Pa 1976). 2008;33:E699–E707. doi:10.1097/BRS.0b013e31817e03db [DOI] [PubMed] [Google Scholar]
- 40.Scemama C, D’Astorg H, Guigui P. Sacral stress fracture after lumbar and lumbosacral fusion. How to manage it? A proposition based on three cases and literature review. Orthop Traumatol Surg Res. 2016;102:261–268. doi:10.1016/j.otsr.2015.11.012 [DOI] [PubMed] [Google Scholar]
- 41.Wilde GE, Miller TT, Schneider R, Girardi FP. Sacral fractures after lumbosacral fusion: a characteristic fracture pattern. AJR Am J Roentgenol. 2011;197:184–188. doi:10.2214/ajr.10.5902 [DOI] [PubMed] [Google Scholar]
- 42.Gundanna MI, Miller LE, Block JE. Complications with axial presacral lumbar interbody fusion: a 5-year postmarketing surveillance experience. SAS J. 2011;5:90–94. doi:10.1016/j.esas.2011.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lindley EM, McCullough MA, Burger EL, Brown CW, Patel VV. Complications of axial lumbar interbody fusion. J Neurosurg Spine. 2011;15:273–279. doi:10.3171/2011.3.Spine10373 [DOI] [PubMed] [Google Scholar]
- 44.Odate S, Shikata J, Kimura H, Soeda T. Sacral fracture after instrumented lumbosacral fusion: analysis of risk factors from spinopelvic parameters. Spine (Phila Pa 1976). 2013;38:E223–E229. [DOI] [PubMed] [Google Scholar]
- 45.Bjerke BT, Zarrabian M, Aleem IS, et al. Incidence of osteoporosis-related complications following posterior lumbar fusion. Global Spine J. 2018;8:563–569. doi:10.1177/2192568217743727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359–2381. doi:10.1007/s00198-014-2794-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deen HG, Nottmeier EW. Balloon kyphoplasty for treatment of sacral insufficiency fractures: report of three cases. Neurosurg Focus. 2005;18:e7. [PubMed] [Google Scholar]
- 48.Shah RV. Sacral kyphoplasty for the treatment of painful sacral insufficiency fractures and metastases. Spine J. 2012;12:113–120. doi:10.1016/j.spinee.2012.01.019 [DOI] [PubMed] [Google Scholar]
- 49.Choi KM, Song JH, Ahn SK, Choi HC. Therapeutic considerations of percutaneous sacroplasty for the sacral insufficiency fracture. J Korean Neurosurg Soc. 2010;47:58–63. doi:10.3340/jkns.2010.47.1.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wright NC, Saag KG, Dawson-Hughes B, Khosla S, Siris ES. The impact of the new National Bone Health Alliance (NBHA) diagnostic criteria on the prevalence of osteoporosis in the USA. Osteoporos Int. 2017;28:1225–1232. doi:10.1007/s00198-016-3865-3 [DOI] [PubMed] [Google Scholar]
- 51.Hsieh PC, Ondra SL, Wienecke RJ, O’Shaughnessy BA, Koski TR. A novel approach to sagittal balance restoration following iatrogenic sacral fracture and resulting sacral kyphotic deformity. Technical note. J Neurosurg Spine. 2007;6:368–372. doi:10.3171/spi.2007.6.4.15 [DOI] [PubMed] [Google Scholar]