Abstract
Study Design:
A retrospective study.
Objectives:
To determine the association between type-2 diabetes mellitus (T2DM) and the severity of lumbar disc degeneration disease (LDDD).
Methods:
We included 199 patients with low back pain (LBP) who visited our hospital from 2016 to 2018. All patients were divided into 3 groups as per inclusion criteria. Group A, patients without DM (n = 75); group B, patients with controlled DM (n = 72); and group C, patients with uncontrolled DM (n = 52). The patients were further subdivided into group B1, DM duration ≤10 years (n = 38); group B2, DM duration >10 years (n = 34); group C1 DM duration ≤10 years (n = 28); and group C2, DM duration >10 years (n = 24). Sex, age, body mass index, occupation, smoking history, alcohol use, and duration of T2DM were recorded. The severity of LDDD was evaluated using the 5-level Pfirrmann grading system. Operated patients’ disc materials were sent for histological examination.
Results:
Demographic data showed no difference among groups (P > 0.5), except age. Patients with DM showed more severe disc degeneration compared with patients without DM. The average Pfirrmann scores between groups A and B1 had no difference; groups B2, C1, and C2 showed higher average Pfirrmann scores than group A (P < 0.05). Groups B2 and C2 showed higher average Pfirrmann scores than groups B1 and C1 (P < 0.05). Groups C1 and C2 showed higher average Pfirrmann scores than groups B1 and B2 (P < 0.05). The severity of LDDD was significantly related to DM duration in both groups B and C (P < 0.05). DM groups showed increased disc apoptosis and matrix aggrecan fragmentation, disc glycosaminoglycan content and histological analysis were significantly different; the results are similar to Pfirrmann score results.
Conclusions:
DM duration >10 years and uncontrolled DM were risk factors for LDDD.
Keywords: Diabetes mellitus, Lumbar disc degeneration disease, Pfirrmann score, HbA1c
Introduction
In 2014, the World Health Organization estimated the prevalence of diabetes among Indian adults over 18 years of age to be 11.8%. There are an estimated 72.96 million cases of diabetes in the adult population of India. 1 Diabetes mellitus (DM) is associated with high blood glucose levels that result from insulin secretory defects or insulin resistance. There are 2 main types of DM, types 1 and 2. Approximately 90% of all cases of DM are type 2. Diabetes is a multiorgan disease that affects many types of connective tissues, including bone and cartilage. 2 Diabetes is associated with an increased risk of certain musculoskeletal pathologies. Prolonged and frequent complications of diabetes include diabetic neuropathy, with symptoms such as pain and sensory and motor deficits in the legs. 3
Lumbar disc degeneration disease (LDDD) is caused by vertebral space reduction, which can be due to new bone formation or hypertrophic tissue changes. LDDD is a major contributor to back and radicular pain, resulting imbalance in catabolic and anabolic responses leads to the degeneration of intervertebral disc tissues, as well as disc herniation and radicular pain. The process usually begins with water loss or reduction in water content, followed by a hard disc protrusion. 4
The changes in the intervertebral disc play a primary role in the pathophysiology of LDDD. Age-related changes in the cartilage matrix in patients with diabetes are different from the changes in healthy subjects. 5 These changes may lead to faster disc degeneration in patients with diabetes. In some studies, significant differences were observed in the incidence of diabetes in patients who have spinal stenosis compared with the degenerative disc disease. Additionally, diabetes causes the ossification of the posterior longitudinal ligaments and bone, which leads to spinal stenosis and nerve pressure.6,7 Therefore, diabetes can be considered a risk factor for spinal stenosis, although the mechanism of the risk for spinal stenosis in patients with diabetes is not well defined.8,9 Approximately 13% of patients who undergo lumbar disc surgery were diagnosed with diabetes, whereas the prevalence of diabetes in the same population generally was approximately 8%. 10
Diabetic microangiopathy might affect the nutrition of the spine and lead to disc degeneration. 11 With the increasing occurrence of diabetes and the similarity of symptoms of spinal stenosis and diabetic neuropathy, one growing problem in the medical care of these patients is the lack of timely detection of stenosis, which causes several complications. However, the relationship between DM and LDDD remains unclear, and different results have been achieved. In this retrospective study, we determined the prevalence and association of DM with LDDD using the 5-level Pfirrmann scoring system and histopathological evidence.
Methods
This was a retrospective study that was conducted from 2016 to 2018 at a single institution in India. Prior to the study being initiated, approval was obtained from the ethics committee at our institution.
Inclusion Criteria
Age ≥20 and ≤65 years
No previous conservative or surgical treatment history
Spine magnetic resonance imaging scan (no lumbar abnormality other than lumbar degenerative spondylosis)
Nonsmoker/no tobacco chewing
No other comorbidity
Exclusion Criteria
Type 1 DM
History of extreme spinal loading occupation
History of spinal trauma or fracture
A total of 199 consecutive patients were included in this study as per criteria. Group A included patients without DM (n = 75). Patients with DM were divided into 2 groups: group B, a well-controlled group (n = 72) and group C, a poorly controlled group (n = 52). Group B was subdivided into group B1, patients with DM duration ≤10 years (n = 38), group B2, DM duration >10 years (n = 34). Similarly, group C was subdivided into group C1, DM duration ≤10 years (n = 28) and group C2, DM duration >10 years (n = 24). Demographic data, DM duration, and clinical-radiological data were recorded. The criteria for a diagnosis of diabetes were fasting plasma glucose ≥126 mg/dL, or in a patient with classic symptoms of hyperglycemia or hyperglycemic crisis, random plasma glucose ≥200 mg/dL, or HbA1c (glycated hemoglobin) ≥6.5%.
Standing lumbar anteroposterior (AP) and lateral radiographs and magnetic resonance imaging of the lumbar spine were performed for LDDD scoring. Grading of the lumbar disc was performed using T2-weighted sagittal images by a senior radiologist blinded to the patient’s demographic information. The 5-level Pfirmann grading system was used (Table 1). Patients with failed conservative management were managed operatively, various decompression/ spine stabilizing procedure (laminectomy and decompression, transforaminal lumbar interbody fusion) performed according to patients clinical finding. The patients who were operated for LDDD their disc material was sent for histopathological and quantitative immunofluorescence analysis.
Table 1.
Pfirrmann Grades.
| Grade | Structure | Distinction of nucleus and annulus | Signal intensity | Disc height | Sagittal T2 magnetic resonance image |
|---|---|---|---|---|---|
| I | Homogeneous, with bright white band | Clear | Hyperintense | Normal |
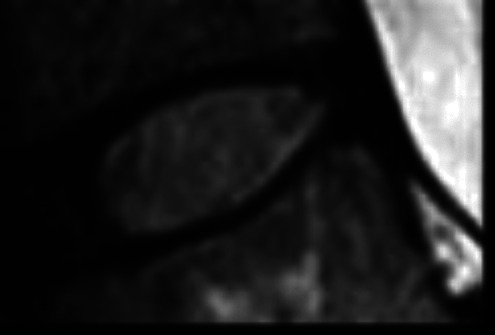
|
| II | Inhomogeneous with or without bright white band | Clear | Hyperintense | Normal |

|
| III | Inhomogeneous with gray band | Unclear | Intermittent | Normal to slightly decreased |

|
| IV | Inhomogeneous with a dark gray band | Lost | Intermittent to hypointense | Slightly or moderately decreased |
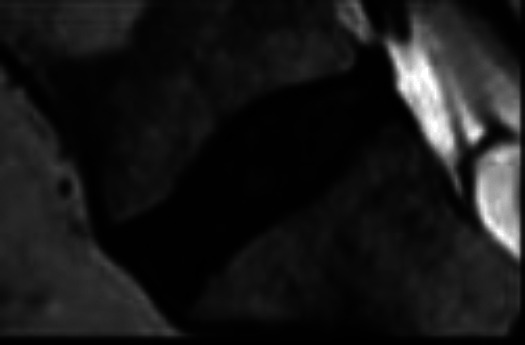
|
| V | Inhomogeneous with a dark gray band | Lost | Hypointense | Disc space is collapsed |

|
Histopathological and Quantitative Immunofluorescence Analysis
For histological analysis, the lumbar disc material was fixed with 2% paraformaldehyde overnight at 48 °C (Tissue Tec processors and Leica embedder). Four-millimeter sections were stained with safranin-O and fast green dyes (Fisher Scientific) by standard procedure and photographed under 40× to 200× magnifications.
Proteoglycan synthesis
Quantitative immunofluorescence
Apoptosis assay
Statistical Analysis
All statistical analyses were performed using SPSS 19.0 (IBM Corp). All data is presented as means ± standard deviations or percentages. The clinical characteristics were compared between patients with and without DM using general linear model analysis for continuous variables and chi-square tests for categorical data. Multinomial logistic regression analysis was adopted to identify the relationship between the DM and severity of LDDD. Estimated average Pfirrmann scores concerning the presence of DM were calculated by analysis of covariance.
Results
A total of 199 adult patients were included with an average age of 55.2 ± 7.9 years (range 20-65 years). There were no significant differences between all groups for sex, body mass index, and other comorbidities (P > 0.05), except for age (P < 0.05) (Table 2). Average Pfirrmann scores from L1/L2 to L5/S1 of patients with good control of DM and DM duration ≤10 years showed no significant difference with patients without DM (P > 0.05) (Table 3). Patients with poor control of DM or DM duration >10 years showed higher average Pfirrmann scores than patients without DM (P < 0.05). Patients with a longer DM duration showed higher average Pfirrmann scores than patients with a shorter disease course (P < 0.05).The average Pfirrmann scores of patients with a poor control of DM were higher than the ones with good control of DM (P < 0.05). By utilizing Spearman correlation analysis to investigate the effect of DM duration on LDDD, a positive trend was observed between DM duration and severity of disc degeneration at L1/2 (r = 0.253), L2/3 (r = 0.456), L3/4 (r = 0.362), L4/5 (r = 0.335), and L5/S1 (r = 0.426) in the group with good DM control and L1/2 (r = 0.210), L2/3 (r = 0.338), L3/4 (r = 0.217), L4/5 (r = 0.239), and L5/S1 (r = 0.328) in the group with poor DM control (P < 0.05).
Table 2.
Demographic Data of Different Groups.
| No DM | Controlled DM | Uncontrolled DM | |||
|---|---|---|---|---|---|
| B1 | B2 | C1 | C2 | ||
| n | 75 | 38 | 34 | 28 | 24 |
| Age, years, mean ± SD | 55.2 ± 7.9 | 58.8 ± 6.9 | 61.2 ± 7.2 | 59.1 ± 7.9 | 61.8 ± 7.2 |
| BMI, kg/m2, mean ± SD | 18.9 ± 2.2 | 20.0 ± 1.7 | 19.1 ± 1.3 | 18.0 ± 1.9 | 19.1 ± 1.6 |
| HbA1c, % | 5.6 ± 0.2 | 6.6 ± 0.4 | 6.7 ± 0.4 | 7.5 ± 0.6 | 7.7 ± 0.5 |
| Duration of DM, years, mean ± SD | — | 6.3 ± 0.7 | 12.1 ± 1.2 | 7.2 ± 1.1 | 13.1 ± 1.5 |
Abbreviations: BMI, body mass index; HbA1c, glycated hemoglobin; DM, diabetes mellitus.
Table 3.
Average Pfirrmann Scores of Different Discs.
| No DM | Controlled DM | Uncontrolled DM | |||
|---|---|---|---|---|---|
| B1 | B2 | C1 | C2 | ||
| n | 75 | 38 | 34 | 28 | 24 |
| L1-L2 | 2.84 ± 0.91 | 2.89 ± 0.73 | 3.28 ± 0.56 | 3.30 ± 0.65 | 3.62 ± 0.80 |
| L2-L3 | 2.85 ± 0.83 | 2.90 ± 0.65 | 3.32 ± 0.73 | 3.33 ± 0.62 | 3.68 ± 0.94 |
| L3-L4 | 2.89 ± 0.12 | 3.01 ± 0.92 | 3.36 ± 0.72 | 3.39 ± 0.78 | 3.71 ± 0.96 |
| L4-L5 | 2.90 ± 0.64 | 3.04 ± 0.75 | 3.39 ± 0.91 | 3.47 ± 0.90 | 3.80 ± 0.71 |
| L5-S1 | 2.93 ± 0.88 | 3.09 ± 0.61 | 3.46 ± 0.71 | 3.48 ± 0.74 | 3.82 ± 0.82 |
Result of Histopathological and Quantitative Immunofluorescence Analysis
Glycosaminoglycan Content
The DMMB (dimethylmethylene blue) assay was performed to measure disc glycosaminoglycan (GAG) content. The GAG amount and level in lumbar discs in all groups was not significantly different after controlling for patient age. This finding was qualitatively confirmed by safranin-O/fast green histological staining.
Proteoglycan Synthesis
Proteoglycan (PG) synthesis was assessed by measuring 35S-sulfate incorporation into disc tissue using a disc organotypic culture system. A significant reduction of PG synthesis was noted in DM patients.
Immunoblot Analysis of Aggrecan Fragments
To investigate if disc matrix degradation was caused by increased aggrecan cleavage, Western blot analysis was performed on disc material of all groups. The amount of disc aggrecan fragments was significantly higher in patients with DM. In particular, the most significant elevation in the number of fragments was noted in patients with a longer duration of diabetes and more poorly controlled diabetes.
Quantitative Immunofluorescence
As ADAMTS seemed to be primarily involved in PG breakdown within the disc, and thus potentially implicated in LDDD, quantitative immunofluorescence with anti-ADAMTS antibodies was performed to identify which isoform could be mainly responsible for aggrecan degradation. More specifically, ADAMTS4 and ADAMTS5 protein expression were investigated in disc sections. These proteins were significantly higher in DM patients, and were noted to be most significantly elevated in patients with longer duration of diabetes and poorly controlled diabetes.
Discussion
Diabetes is one of the common conditions that causes metabolic disturbances in many organs. Degenerative disc disease is a serious health care problem. It can be a cause of moderate to severe pain, affecting the patient’s quality of life as well as increasing health care costs. 10 It is important to clarify the risk factors of LDDD to prevent or delay its onset or progression. This study was the first to use the Pfirrmann grading system to evaluate the association between DM and LDDD with histopathological evidence. In this study, we included patients without DM and patients with different durations and different control effects of DM for comparisons. After removing the effect of age, our study demonstrated that patients with DM tended to develop more severe LDDD than those without DM. The length of DM duration had a positive relationship with severity of LDDD, which meant that the longer the DM duration, the more severe the disc degeneration. Patients with poor control of DM seemed to show more severe disc degeneration than patients with good control. All these demonstrated that DM was a risk factor for LDDD, and such effect was time and control effect dependent.
Frymoyer et al 12 reported that degenerative spondylolisthesis is more prevalent in diabetic patients, while a community-based study by Vogt et al 13 did not find any correlation between a history of DM and the prevalence of L4-5 degenerative spondylolisthesis. In previous studies, one reported that the DM patients had a poorer outcome following lumbar discectomy than controls, and the rates of reoperation and prolonged hospitalization were also significantly higher in DM patients. 10 Sakellaridis et al 14 and Machino et al 15 both reported that high preoperative HbA1c levels and long-term DM were risk factors for poor cervical laminoplasty outcomes in patients with DM and cervical spondylotic myelopathy. Now, we can understand the underlying mechanisms of it and conclude that there is a positive relationship between DM and LDDD. Several studies assumed that hyperglycemia enhances the formation of advanced glycation end products (AGEs) in the nucleus pulposus, which leads to the progression of disc degeneration.16,17 Chen et al 11 found that DM accelerated the degeneration process of the disc by microangiopathy. Autophagy of the nucleus pulposus and annulus fibrosis cells also appears to play an important role in LDDD. Park et al 18 and Kong et al 19 demonstrated that high glucose-induced oxidative stress accelerates premature stress-induced senescence in young rat annulus fibrosus cells in a dose- and time-dependent manner rather than replicative senescence. However, no conclusion has been made. Some studies have investigated methods to slow down the process of LDDD caused by DM. Kong et al 19 suggested that strict blood glucose control is important in preventing or delaying LDDD in older patients with DM.
In our study, we also found that the average Pfirrmann scores of patients with good control of DM and DM duration ≤10 years showed no difference with patients without DM after the removal of the age effect. To the best of our knowledge, this is the first study to investigate the relationship between DM and LDDD using the 5-level Pfirrmann grading system with histopathological evidence. HbA1c was used to detect diabetes that increases the accuracy of our study.
Drawbacks of this study are the short follow-up and smaller data size. Another limitation is the fact that degenerative disc disease, spinal stenosis, and vertebral osteoporotic fractures may simultaneously exist in the same patient. Future prospective comparative studies with larger patient number and longer follow-up are required for confirmation of our results.
Conclusion
There is a positive relationship between DM and LDDD. A longer the DM duration and poor control of DM could aggravate disc degeneration.
Acknowledgements
We thank Prof.Dr Aseem Parekh (Professor & Head, Department of Orthopaedics, Topiwala Medical College & Nair Hospital) for support and guidance.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Ghanshyam Kakadiya, MS  https://orcid.org/0000-0002-0345-7407
https://orcid.org/0000-0002-0345-7407
References
- 1.World Health Organization. Diabetes: key facts. Published June 8, 2020. Accessed July 25, 2020. https://www.who.int/news-room/fact-sheets/detail/diabetes
- 2.Kong CG, Park JB, Kim MS, Park EY. High glucose accelerates autophagy in adult rat intervertebral disc cells. Asian Spine J. 2014;8:543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakellaridis N. The influence of diabetes mellitus on lumbar intervertebral disk herniation. Surg Neurol. 2006;66:152–154. [DOI] [PubMed] [Google Scholar]
- 4.Frymoyer JW. Degenerative spondylolisthesis: diagnosis and treatment. J Am Acad Orthop Surg. 1994;2:9–15. [DOI] [PubMed] [Google Scholar]
- 5.Robinson D, Mirovsky Y, Halperin N, Evron Z, Nevo Z. Changes in proteoglycans of intervertebral disc in diabetic patients. A possible cause of increased back pain. Spine (Phila Pa 1976). 1998;23:849–855. [DOI] [PubMed] [Google Scholar]
- 6.Inamasu J, Guiot BH, Sachs DC. Ossification of the posterior longitudinal ligament: an update on its biology, epidemiology, and natural history. Neurosurgery. 2006;58:1027–1039. [DOI] [PubMed] [Google Scholar]
- 7.Lotan R, Amir O, Anekstein Y, Shalmon E, Mirovsky Y. Lumbar stenosis and systemic diseases: is there any relevance? J Spinal Disord Tech. 2008;21:247–251. [DOI] [PubMed] [Google Scholar]
- 8.Asadian L, Haddadi K, Aarabi M, Zare A. Diabetes mellitus, a new risk factor for lumbar spinal stenosis: a case–control study. Clin Med Insights Endocrinol Diabetes. 2016;9:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anekstein Y, Smorgick Y, Lotan R, et al. Diabetes mellitus as a risk factor for the development of lumbar spinal stenosis. Isr Med Assoc J. 2010;12:16–20. [PubMed] [Google Scholar]
- 10.Mobbs RJ, Fau NR, Chandran KN. Lumbar discectomy and the diabetic patient: incidence and outcome. J Clin Neurosci. 2001;8:10–13. [DOI] [PubMed] [Google Scholar]
- 11.Chen S, Liao M, Li J, Peng H, Xiong M. The correlation between microvessel pathological changes of the endplate and degeneration of the intervertebral disc in diabetic rats. Exp Ther Med. 2013;5:711–717. doi:10.3892/etm.2012.868 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Frymoyer JW. Degenerative spondylolisthesis: diagnosis and treatment. J Am Acad Orthop Surg. 1994;2:9–15. [DOI] [PubMed] [Google Scholar]
- 13.Vogt MT, Rubin D, Valentin RS, et al. Lumbar listhesis and lower back symptoms in elderly white women. Spine (Phila Pa 1976). 1998;23:2640–2647. [DOI] [PubMed] [Google Scholar]
- 14.Sakellaridis N, Androulis A. Influence of diabetes mellitus on cervical intervertebral disc herniation. Clin Neurol Neurosurg. 2008;110:810–812. [DOI] [PubMed] [Google Scholar]
- 15.Machino M, Yukawa Y, Ito K, et al. Risk factors for poor outcome of cervical laminoplasty for cervical spondylotic myelopathy in patients with diabetes. J Bone Joint Surg Am. 2014;96:2049–2055. [DOI] [PubMed] [Google Scholar]
- 16.Illien-Junger S, Lu Y, Qureshi SA, et al. Chronic ingestion of advanced glycation end products induces degenerative spinal changes and hypertrophy in ageing pre-diabetic mice. PLoS One. 2015;10:e0116625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Illien-Junger S, Grosjean F, Laudier DM, Vlassara H, Striker GE, Iatridis JC. Combined anti-inflammatory and anti-AGE drug treatments have a protective effect on intervertebral discs in mice with diabetes. PLoS One. 2013;8:e64302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park JS, Park JB, Park IJ, Park EY. Accelerated premature stress-induced senescence of young annulus fibrosus cells of rats by high glucose-induced oxidative stress. Int Orthop. 2014;38:1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kong JG, Park JB, Lee D, Park EY. Effect of high glucose on stress-induced senescence of nucleus pulposus cells of adult rats. Asian Spine J. 2015;9:155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]


