Abstract
Study Design:
Cross-sectional cohort study.
Objectives:
Cauda equina syndrome (CES) is a neurologic emergency, and delay in diagnosis can result in irreversible impairment. Our purpose was to determine the value of physical examination in diagnosis of CES in patients complaining of bladder and/or bowel complications in the emergency department.
Methods:
Adult patients at one tertiary academic medical center that endorsed bowel/bladder dysfunction, underwent a lumbar magnetic resonance imaging (MRI), and received an orthopedic spine surgery consultation from 2008 to 2017 were included. Patients consulted for trauma or tumor were excluded. A chart and imaging review was performed to collect demographic, physical examination, and treatment data. Sensitivity, specificity, and negative and positive predictive values were calculated, and fast-and-frugal decision trees (FFTs) were generated using R.
Results:
Of 142 eligible patients, 10 were diagnosed with CES. The sensitivity and specificity of the exam findings were highest for bulbocavernosus reflex (BCR) (100% and 100%), followed by rectal tone (80% and 86%), postvoid residual bladder (80% and 59%), and perianal sensation (60% and 68%). The positive predictive value was high for BCR (100%), but low for other findings (13% to 31%). However, negative predictive values were consistently high for all examinations (96% to 100%). Two FFTs utilizing combinations of voluntary rectal tone, perianal sensation, and BCR resulted in no false negatives.
Conclusions:
A combination of physical examination findings of lower sacral function is an effective means of ruling out CES and, with further study, may eliminate the need for MRI in many patients reporting back pain and bowel or bladder dysfunction.
Keywords: cauda equina syndrome, MRI, physical examination, fast-and-frugal decision tree
Introduction
Cauda equina syndrome (CES) is defined as complete or near complete occlusion of the spinal canal resulting in severe compression of the neural elements and loss of lower sacral nerve root function.1-3 Signs and symptoms of CES are variable and can include bilateral radiculopathy and progressive neurologic deficits in the legs. However, to qualify as CES there must be evidence of S2-S4 nerve root dysfunction confirmed by complete or near complete occlusion of the spinal canal on magnetic resonance imaging (MRI). The occlusion can present as impaired perianal sensation, impaired rectal tone, and urinary dysfunction (retention or incontinence).4-7 CES is a neurologic emergency and a delay or missed diagnosis can result in irreversible impairment. The fear of missing CES may drive frontline providers to order MRI studies on all patients presenting with lower back complaints and bowel or bladder dysfunction.4,8,9 Evidence of complete or near complete occlusion of the spinal canal on MRI is considered to be the gold standard in determining the presence of CES.3,8,10
More recent literature suggests that bowel and bladder symptoms are frequent complaints in spine patients without CES. 11 Also related are reports that show patients with high clinical suspicion of CES have a low rate of positive findings on MRI.3,9 In a 2010 study, only 18.8% of patients with clinical features of CES had disc herniation present on MRI. 12 Another study reported that 22% of patients under evaluation for CES had pathologic MRI findings. 9 Despite the published reports of high rates of unremarkable MRIs, the imaging modality persists as a critical step in the algorithm for CES evaluation.4,12-14
The ubiquitous use of MRI for screening for CES has high associated costs when accounting for the low rate of true positive CES. Reduction of expensive imaging has become a major health care initiative. 15 MRI use for lower back symptoms in the United States had increased by 307% from 1994 to 2004 and was on the rise by an annual rate of 9% in the year 2005.16-18 Utilization of spine (cervical, thoracic, and lumbar) MRIs hit a peak rate of 65 per 1000 Medicare beneficiaries in 2009, but dropped to 63.7 per 1000 Medicare beneficiaries in 2010. Despite the slight drop in utilization, spine MRIs still accounted for 37.3% of all Medicare MRIs that year. 19 Studies have shown that the number and timing of imaging for lower back pain has had no effect on quality of life or overall patient outcome.20-22 Therefore, determining a less expensive method for diagnosing or ruling out CES would allow for a significant reduction in costs by decreasing the frequency of spinal imaging.
Our clinical pathway of obtaining MRIs on all patients who complain of bowel and bladder symptoms, along with our standardized physical examination protocol, provides us a unique opportunity to evaluate both the incidence of true CES and the utility of physical examination for determining the presence of CES in this population. Specifically, we assessed the sensitivity and specificity of rectal tone, perianal sensation, bulbocavernosus reflex (BCR), and postvoid residual bladder (PVR) exams with positive and negative MRI findings on patients complaining of bladder or bowel symptoms in the emergency department (ED). We hypothesized that a thorough physical exam may be sufficient to rule out CES during an initial evaluation, which in turn could be associated with a decreased frequency of costly advanced imaging.
Methods
A cross-sectional retrospective cohort study was approved by our institutional review board and conducted over a 10-year period, January 1, 2008 to December 31, 2017 at a single tertiary referral academic medical center. The STROBE criteria for cohort studies was used to report our results. The cohort was composed of adult patients who presented to the ED and received a lumbar MRI for lumbar spine related complaints that also complained of bowel and/or bladder symptoms. Only patients with orthopedic spine consultations were included as they followed a standardized physical examination protocol. Patients who presented with tumor or trauma were excluded. An orthopedic spine physician examined the MRI and determined presence or absence of CES, as well as need for emergent surgery.
Subjective symptoms, including back pain, bowel and bladder involvement (retention/incontinence), physical exam findings (perianal sensation, rectal tone, BCR, and PVR), diagnoses, and the need for emergent surgery as determined by the orthopedic spine attending were collected from electronic medical records. PVR > 300 mL was considered abnormal. Data was collected for the hospitalization period at time of presentation. PVR was assessed via ultrasound or catheter, while BCR was only assessed in patients that received a Foley catheter. Potential biases were addressed by having objective measures collected by research staff that were not part of the treatment team, and by analyzing data in a blinded fashion.
Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and number needed to treat (NNT) were calculated using SAS (SAS Institute) for each major clinical characterization (rectal tone, perianal sensation, BCR, and PVR). As a heuristic for decision making, fast-and-frugal decision trees (FFT) were constructed using R Studio statistical software and the FFTree package by Nathaniel Phillips et al. 23 FFTs are classification trees that are simple in construction and execution, providing a series of dichotomous choices for categorization. These have been used, for example, to categorize high or low risk for acute myocardial injury. 24
Results
A total of 142 patients met all inclusion criteria (Figure 1). Mean (±SD) patient age was 45.3 ± 14.7 years with a range of 18 to 86 years, and 58% were female (Table 1). Ten patients (7%) were diagnosed with CES based on sacral root involvement as determined by MRI. Affected and unaffected patients had similar sex and age distributions. The etiologies of their lesions were disc herniation (90%) and epidural abscess (10%). The affected levels were L4/5 (70%), L2/3 (10%), L3/4 (10%), and L5-S1 (10%). Bladder complaints were reported more often at ED presentation than bowel complaints (n = 131, 92% vs n = 66, 46%, respectively). A combination of bladder and bowel complaints was reported by 55 patients (39%). All 10 patients with CES reported bladder complaints, and 7 of 10 (70%) also reported bowel complaints.
Figure 1.

Flow diagram. Included subjects had a lumbar MRI in the ED, an orthopedic consult, and bowel and/or bladder complaints, without a tumor or trauma diagnosis. ED, emergency department; MRI, magnetic resonance imaging.
Table 1.
Characteristics of Subjects With and Without CES.a
| Overall (n = 142) | CES absent (n = 132) | CES present (n = 10) | P b | |
|---|---|---|---|---|
| Sex | .74 | |||
| Male | 60 (42) | 55 (42) | 5 (50) | |
| Female | 82 (58) | 77 (58) | 5 (50) | |
| Age (years) | .75 | |||
| <30 | 21 (15) | 19 (14) | 2 (20) | |
| 30-39 | 31 (22) | 30 (23) | 1 (10) | |
| 40-49 | 40 (28) | 37 (28) | 3 (30) | |
| 50-59 | 27 (19) | 24 (18) | 3 (30) | |
| ≥60 | 23 (16) | 22 (17) | 1 (10) | |
| MRI etiology | .65 | |||
| Disc herniation | 72 (51) | 63 (48) | 9 (90) | |
| Epidural abscess | 2 (1) | 1 (1) | 1 (10) | |
| Degenerative changes | 41 (29) | 41 (32) | ||
| Osteomyelitis/discitis | 7 (5) | 7 (5) | ||
| No lumbar pathology | 19 (13) | 19 (14) | ||
| Other | 1 (1) | 1 (1) | ||
| Rectal tone | <.01 | |||
| Normal | 109 (77) | 107 (81) | 2 (20) | |
| Abnormal | 26 (18) | 18 (14) | 8 (80) | |
| Missing | 7 (5) | 7 (5) | 0 (0) | |
| Perianal sensation | .09 | |||
| Normal | 91 (64) | 87 (66) | 4 (40) | |
| Abnormal | 47 (33) | 41 (31) | 6 (60) | |
| Missing | 4 (3) | 4 (3) | ||
| BCR | <.01 | |||
| Normal | 30 (21) | 30 (23) | 0 (0) | |
| Abnormal | 3 (2) | 0 (0) | 3 (30) | |
| Missing | 109 (77) | 102 (77) | 7 (70) | |
| PVR | .04 | |||
| Normal | 47 (33) | 45 (34) | 2 (20) | |
| Abnormal | 39 (27) | 31 (23) | 8 (80) | |
| Missing | 56 (39) | 56 (42) |
Abbreviations: BCR, bulbocavernosus reflex; CES, cauda equina syndrome; MRI, magnetic resonance imaging; PVR = postvoid residual.
a Values given as number (%).
b Boldfaced P values indicate statistical significance (P < .05).
Perianal sensation, rectal tone, PVR, and BCR were assessed in 97%, 95%, 61%, and 23% of the entire 142 patient cohort, respectively. Low use of BCR testing can be explained by the examination’s requirement of a Foley catheter. Rectal tone, BCR, and PVR differed significantly between patients diagnosed with CES versus unaffected patients (Table 1). Most notably, rectal tone was normal in 20% of patients with CES versus 81% of unaffected patients (P < .01), and PVR was normal in 20% of patients with CES versus 34% of unaffected patients (P = .04). Perianal sensation was not significantly different in the 2 groups. BCR was normal in zero patients with CES, compared with 23% of unaffected patients (P < .01, Table 1).
The sensitivity and specificity of the exam findings are shown in Table 2. The sensitivity and specificity of the BCR were highest, at 100% each, followed by rectal tone at 80% and 86%, respectively. The PPV and NPV of the physical exam findings were also highest for the BCR, at 100% and 100%, respectively. PPV and NPV for rectal tone were 31% and 98%, for PVR were 21% and 96%, and for perianal sensation were 13% and 96%, respectively (Table 2).
Table 2.
Sensitivity, Specificity, and Predictive Values of Exam Findings, Shown as Percentages.
| Exam finding | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| Rectal tone | 80 | 86 | 31 | 98 |
| Perianal sensation | 60 | 68 | 13 | 96 |
| BCR | 100 | 100 | 100 | 100 |
| PVR | 80 | 59 | 21 | 96 |
Abbreviations: BCR, bulbocavernosus reflex; NPV, negative predictive value; PPV, positive predictive value; PVR, postvoid residual.
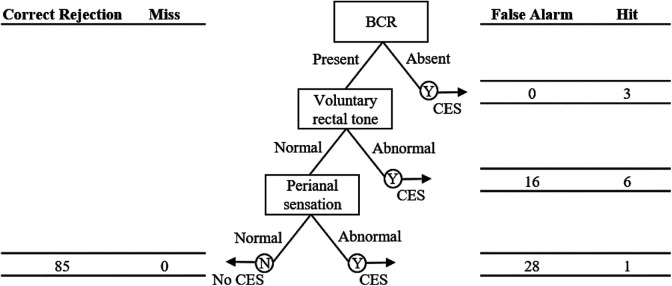
FFT analyses determined 2 pathways utilizing voluntary rectal tone, perianal sensation, and BCR that resulted in no false negatives. One pathway starts with the rectal tone examination, followed by perianal sensation testing and BCR (Figure 2), while the other pathway starts with BCR, followed by the rectal tone examination and perianal sensation testing (Figure 3). In the first model, a lack of voluntary tone triggers concern for CES, and finds that 8 of 24 patients (33%) had CES. Of the remaining patients, 29 patients had a lack of perianal sensation, of which 1 had CES. Finally, an abnormal BCR was seen in 1 remaining patient who had CES; 85 had normal BCR and no CES (60%). This model minimizes Foley placement. In the second FFT model (Figure 3), BCR is the first node and an abnormal exam found 3 of 3 patients with CES. Of the remaining patients, 22 had abnormal voluntary tone, of which 6 had CES. Then, 1 of 28 patients with abnormal sensation had CES and 85 were excluded (60%). Both models have 100% sensitivity and produced no false negatives.
Figure 2.
Fast-and-frugal decision tree, indicating hits, false alarms, misses, and correct rejections at each node. Of 24 patients with abnormal voluntary rectal tone, 8 were diagnosed with CES. Of 29 patients with abnormal perianal sensation, 1 was diagnosed with CES. The 1 patient with an absent BCR was also diagnosed with CES. CES, cauda equina syndrome; BCR, bulbocavernosus reflex.
Figure 3.
Fast-and-frugal decision tree, indicating hits, false alarms, misses, and correct rejections at each node. All 3 patients with absent BCR were diagnosed with CES. Of 22 patients with abnormal voluntary rectal tone, 6 were diagnosed with CES. Of 29 patients with abnormal perianal sensation, 1 was diagnosed with CES. CES, cauda equina syndrome; BCR, bulbocavernosus reflex.
Discussion
Despite the low yield of positive MRI findings of CES, current literature continues to recommend MRIs as part of the diagnostic workup.4,9-11 The often-cited concern for the ubiquitous use of MRIs is the fear of missing or causing a delay in diagnosis, which could result in serious irreversible neurologic loss of lower sacral root function. There is adequate literature to demonstrate that a patient’s subjective complaints of bowel or bladder dysfunction are not highly correlated with the presence of CES, thereby emphasizing the utility of objective physical examination findings as part of the pretest probability prior to obtaining advanced imaging.3,8,11
This study was undertaken to determine whether a series of physical examinations of lower sacral root function can accurately determine the presence or absence of CES. If found to be accurate, we may be able to eliminate the need for MRI in most cases when patients complain of back pain associated with bowel or bladder dysfunction.
Previous research has evaluated a variety of clinical tests for their ability to diagnosis CES. One group found that 100% of tested patients with CES (n = 6) had urinary retention >500 mL. 25 No other assessments, including sensation, bladder, bowel, pain, strength, reflexes, and sexual function, were identified as greater than 75% specific in that analysis. Thus, literature adopted a general definition of CES that requires a minimum of one of the following to be present: bladder or bowel dysfunction, saddle sensation deficits, or sexual dysfunction.5,25,26 A 2015 article by Ahad et al 3 concluded that clinical complaints were not predictive of MRI findings of CES; reported proportions of signs/symptoms of neurologic deficits were <10% in their study population (8.9% bladder incontinence, 8.9% saddle anesthesia, 7.6% decreased anal tone and 7.6% urinary retention). Given the low rates of clinical features, their study concluded that abnormal MRI imaging was the only way to diagnose CES. In contrast, our study population demonstrated higher rates of clinical features present, which therefore enabled us to perform sensitivity, specificity, negative, and positive predictive value analyses. We determined a 96% to 100% negative predictive value of the classic CES clinical features of rectal tone, perianal sensation, BCR, and PVR, and hence conclude that clinical features can be useful in the diagnostic evaluation of CES.
BCR was found to be the clinical examination with the highest positive and negative predictive values. Prior studies focusing on BCR have similarly found this spinal reflex to be associated with CES. Several urodynamic and/or electrophysiologic studies of BCR have determined a high association between absent BCR and CES and other complete lesions of the sacral spinal cord.27-29 This study corroborated these findings, although they were limited by a low cohort size (the 3 patients with abnormal BCR were diagnosed with CES). Only 23% of patients included in the analyses had a documented BCR, which highlights a limitation of this retrospective cohort study. The low rate of BCR testing was because BCR could only be tested in patients with a Foley catheter.
Combining physical examination findings in a fast-and-frugal decision tree resulted in 100% sensitivity and no false negatives, and theoretically could avoid an MRI in 60% of patients presenting with symptoms of bowel and/or bladder dysfunction. While the model needs testing in a prospective manner, it is a simple means by which we may safely lower rates of MRI in this patient population.
The limitations of this study were that it was retrospective in nature and involved a low incidence of CES. Despite these limitations, we found that carefully performed physical examinations may be able to eliminate the necessity of ordering an MRI for every low back pain patient who complains of bowel or bladder dysfunction. The findings of this study are generalizable as it is currently common in EDs for patients with genitourinary dysfunction and back pain to undergo MRI and physical examination maneuvers for CES prior to consultation.
This paper lays a foundation for a larger prospective study in which BCR should be performed on every spine patient admitted to emergency room with bowel or bladder dysfunction. If BCR is as accurate as this article suggests, we may be able to reduce the need for MRI examination in 90-95% of these patients.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Natalie L. Zusman, MD  https://orcid.org/0000-0002-8672-9999
https://orcid.org/0000-0002-8672-9999
Stephanie S. Radoslovich, BA  https://orcid.org/0000-0002-9266-4060
https://orcid.org/0000-0002-9266-4060
Kenneth R. Gundle, MD  https://orcid.org/0000-0003-0451-0561
https://orcid.org/0000-0003-0451-0561
References
- 1.Podnar S. Epidemiology of cauda equina and conus medullaris lesions. Muscle Nerve. 2006;35:529–531. doi:10.1002/mus.20696 [DOI] [PubMed] [Google Scholar]
- 2.Ahn UM, Ahn NU, Buchowski JM, Garrett ES, Sieber AN, Kostuik JP. Cauda equina syndrome secondary to lumbar disc herniation: a meta-analysis of surgical outcomes. Spine (Phila Pa 1976). 2000;25:1515–1522. doi:10.1097/00007632-200006150-00010 [DOI] [PubMed] [Google Scholar]
- 3.Ahad A, Elsayed M, Tohid H. The accuracy of clinical symptoms in detecting cauda equina syndrome in patients undergoing acute MRI of the spine. Neuroradiol J. 2015;28:438–442. doi:10.1177/1971400915598074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Todd NV. Guidelines for cauda equina syndrome. Red flags and white flags. Systematic review and implications for triage. Br J Neurosurg. 2017;31:336–339. doi:10.1080/02688697.2017.1297364 [DOI] [PubMed] [Google Scholar]
- 5.Fraser S, Roberts L, Murphy E. Cauda equina syndrome: a literature review of its definition and clinical presentation. Arch Phys Med Rehabil. 2009;90:1964–1968. doi:10.1016/j.apmr.2009.03.021 [DOI] [PubMed] [Google Scholar]
- 6.Kennedy JG, Soffe KE, McGrath A, Stephens MM, Walsh MG, McManus F. Predictors of outcome in cauda equina syndrome. Eur Spine J. 1999;8:317–322. doi:10.1007/s005860050180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raj D, Coleman N. Cauda equina syndrome secondary to lumbar disc herniation. Acta Orthop Belg. 2008;74:522–527. [PubMed] [Google Scholar]
- 8.Germon T, Ahuja S, Casey ATH, Todd NV, Rai A. British Association of Spine Surgeons standards of care for cauda equina syndrome. Spine J. 2015;15(3 suppl):S2–S4. doi:10.1016/j.spinee.2015.01.006 [DOI] [PubMed] [Google Scholar]
- 9.Bell DA, Collie D, Statham PF. Cauda equina syndrome—what is the correlation between clinical assessment and MRI scanning? Br J Neurosurg. 2009;21:201–203. doi:10.1080/02688690701317144 [DOI] [PubMed] [Google Scholar]
- 10.Crocker M, Fraser G, Boyd E, Wilson J, Chitnavis BP, Thomas NW. The value of interhospital transfer and emergency MRI for suspected cauda equina syndrome: a 2-year retrospective study. Ann R Coll Surg Engl. 2008;90:513–516. doi:10.1308/003588408X301154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lieberman EG, Boone RM, Radoslovich S, et al. Prevalence of preoperative lower urinary tract symptoms in patients undergoing elective lumbar spine surgery. Spine (Phila Pa 1976). 2018;43:E1152–E1156. doi:10.1097/BRS.0000000000002649 [DOI] [PubMed] [Google Scholar]
- 12.Balasubramanian K, Kalsi P, Greenough CG, Seetharam MPK. Reliability of clinical assessment in diagnosing cauda equina syndrome. Br J Neurosurg. 2010;24:383–386. doi:10.3109/02688697.2010.505987 [DOI] [PubMed] [Google Scholar]
- 13.Todd NV, Dickson RA. Standards of care in cauda equina syndrome. Br J Neurosurg. 2016;30:518–522. doi:10.1080/02688697.2016.1187254 [DOI] [PubMed] [Google Scholar]
- 14.Todd NV. An algorithm for suspected cauda equina syndrome. Ann R Coll Surg Engl. 2009;91:358–359. doi:10.1308/003588409X428487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Callaghan BC, De Lot LB, Kerber KA, Burke JF, Skolarus LE. Neurology choosing wisely recommendations: 74 and growing. Neurol Clin Pract. 2015;5:439–447. doi:10.1212/CPJ.0000000000000189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deyo RA, Mirza SK, Turner JA, Martin BI. Overtreating chronic back pain: time to back off? J Am Board Fam Med. 2009;22:62–68. doi:10.3122/jabfm.2009.01.080102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marin BI, Deyo RA, Mirza SK, et al. Expenditures and health status among adults with back and neck problems. JAMA. 2008;299:656–664. doi:10.1001/jama.299.6.656 [DOI] [PubMed] [Google Scholar]
- 18.Gilbert FJ, Grant AM, Gillan MGC, et al. Low back pain: influence of early MR imaging or CR on treatment and outcome—multicenter randomized trial. Radiology. 2004;231:343–351. doi:10.1148/radiol.2312030886 [DOI] [PubMed] [Google Scholar]
- 19.Sharpe RE, Levin DC, Parker L, Rao VM. The recent reversal of the growth trend in MRI: a harbinger of the future? J Am Coll Radiol. 2013;10:599–602. doi:10.1016/j.jacr.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 20.Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 2008;8:8–20. doi:10.1016/j.spinee.2007.10.005 [DOI] [PubMed] [Google Scholar]
- 21.Modic MT, Obuchowski NA, Ross JS, et al. Acute low back pain and radiculopathy: MR imaging findings and their prognostic role and effect on outcome. Radiology. 2005;237:597–604. doi:10.1148/radiol.2372041509 [DOI] [PubMed] [Google Scholar]
- 22.Bansback N, Chiu J, Kerr S, et al. Reducing imaging tests for low back pain: can patients choose wisely? Abstract no. 1146. Published September 28, 2016. Accessed August 4, 2020. https://acrabstracts.org/abstract/reducing-imaging-tests-for-low-back-pain-can-patients-choose-wisely/
- 23.Phillips ND, Woike JK, Gaissmaier W. FFTrees: a toolbox to create, visualize, and evaluate fast-and-frugal decision trees. Judgement Decis Making. 2017;12:344–368. [Google Scholar]
- 24.Green L, Mehr DR. What alters physicians’ decisions to admit to the coronary care unit? J Fam Pract. 1997;45:219–226. [PubMed] [Google Scholar]
- 25.Domen PM, Hofman PA, van Santbrink H, Weber WEJ. Predictive value of clinical characteristics in patients with suspected cauda equina syndrome. Eur J Neurol. 2009;16:416–419. doi:10.1111/j.1468-1331.2008.02510.x [DOI] [PubMed] [Google Scholar]
- 26.Gooding BWT, Higgins MA, Calthorpe DA. Does rectal examination have any value in the clinical diagnosis of cauda equina syndrome? Br J Neurosurg. 2013;27:156–159. doi:10.3109/02688697.2012.732715 [DOI] [PubMed] [Google Scholar]
- 27.Ertekin C, Reel F. Bulbocavernosus reflex in normal men and in patients with neurogenic bladder and/or impotence. J Neurol Sci. 1976;28:1–15. doi:10.1016/0022-510x(76)90044-7 [DOI] [PubMed] [Google Scholar]
- 28.Blaivas JG, Zayeed AA, Labib KB. The bulbocavernosus reflex in urology: a prospective study of 299 patients. J Urol. 1981;126:197–199. doi:10.1016/s0022-5347(17)54445-6 [DOI] [PubMed] [Google Scholar]
- 29.Shi J, Jia L, Yuan W, Shi G, et al. Clinical classification of cauda equina syndrome for proper treatment. Acta Orthop. 2010;81:391–395. doi:10.3109/17453674.2010.483985 [DOI] [PMC free article] [PubMed] [Google Scholar]