Abstract
The characterisation and distribution patterns of key odour-active compounds in head, heart1, heart2, tail, and stillage cuts of freshly distilled brandy were investigated by gas chromatography–olfactometry-mass spectrometry coupled with aroma extract dilution analysis (AEDA) and chemometrics analysis. Results from AEDA showed that there were 50, 61, 48, 25, and 18 odour-active compounds in the head, heart1, heart2, tail, and stillage cuts, respectively. Besides, 19, 22, 11, 5, and 4 quantified compounds with odour activity values ≥ 1, respectively, were considered to be potential contributors to the aroma profile of different distillation cuts. Especially, the chemometrics analysis illustrated the heart1 fraction was characterized by 3-methylbutanol, ethyl hexanoate, 1-hexanol, ethyl octanoate, benzaldehyde, ethyl decanoate, and 2-phenylethyl acetate; (E)-hex-3-en-1-ol, (Z)-hex-3-en-1-ol, and 2-phenylethyl acetate greatly contributed to the characteristics of the heart2 cut. Furthermore, different volatile compounds with a variety of boiling points and solubilities followed diverse distillation rules during the second distillation. Our findings may provide a rational basis for concentrating more pleasant aroma components contributing to brandy.
Abbreviations: AD, aroma descriptor; AEDA, aroma extract dilution analysis; FD, flavor dilution; GC-O-MS, gas chromatography-olfactometry-mass spectrometry; HS-SPME, headspace solid-phase microextraction; MS, mass spectra; OAV, odour activity value; PCA, principal component analysis; PLS-DA, partial least squares discriminant analysis; RI, retention indices; SAFE, solvent-assisted flavour evaporation; Std, standards; VIP, variable importance in projection
Keywords: Distillation cut, Odour-active compounds, Principal component analysis, Partial least squares discriminant analysis, Freshly distilled brandy
Introduction
Brandy, which originated from the French area of Charentes, accounts for one of the representative distilled products from fermented grapes that get maturation within the oak barrels for several years and is also one of the six famous distilled spirits globally (Zhao, Zheng, Song, Sun, & Tian, 2013). Its final quality mainly depends on viticulture and vinification and then on distillation, aging, and blending, which confers specific aromas and tastes to each brandy (Matijašević et al., 2019, Zierer et al., 2016). Distillation is one of the most important processes in brandy production that occurs in various biochemical reactions, which determines the specific and desired volatile compounds to select and concentrate by heating from the distillate mixture (Zhao et al., 2014). In other words, the objective of distillation is to capture the alcohol and agreeable aromas of the underlying fruit and fermentation in the distillate and leave unpleasant odours behind (Arrieta-Garay et al., 2014). The criteria such as boiling point, affinity with water molecules, and solubility in a mixture of alcohol and water are thought to be the reasons for the different distribution of various volatile compounds (Xiang et al., 2020).
Generally, the distillate is further divided into three cuts: the head, which mainly contains low boiling point compounds that impart unpleasant odours (e.g. aldehyde and toluene); the middle cut, called the heart (sometimes also divided into several cuts), which corresponds to the main commercial product (e.g. ethyl esters and higher alcohols); and the tail, which tends to distil compounds with a high molecular weight (e.g. diisopropyl phthalate and dodecanoic acid) (Awad et al., 2017). In the traditional distillation mode without auto-control technology, a critical problem is based on the alcohol content or the smell and taste of the distillate to accurately determine the node of each cut, which depends, to a large extent, on the profound knowledge and professional experience of the distiller (Xiang et al., 2020). Double distillation is indispensable for imparting novel and desirable characteristics concerning brandy, which provides the foundation of the organoleptic quality of wine products (Zhao et al., 2014). To guide the effective instructions of the distillation cut, much attention has been devoted to the subject undergoing variation in volatile substances during the distillation step. In numerous studies, the identification and characterisation of several volatile compounds in freshly distilled brandy have been performed, such as esters, higher alcohols, acids, aldehydes, ketones, furans, terpenes, and aromatic compounds (Malfondet et al., 2016, Xiang et al., 2020). But scarce knowledge is available on the distillation patterns of volatile components during the second distillation. Xiang et al. (2020) identified and provided detailed information on technological parameters for manipulating distillation cuts from head, heart, and tail cuts in Spine grade-derived freshly distilled spirits. However, thus far, the characterisation, distribution patterns, and relationship of key odour-active compounds and unique features of different distillate cuts during second distillation have not been systematically reported.
Because flavour determines the aroma and consumer acceptance, it is generally considered an important index of the quality of beverages, especially alcoholic drinks (Niu et al., 2019, Tian et al., 2021). Integrating multiple technologies with high sensitivity, selectivity, and efficiency and corresponding methods for identifying key odour-active compounds, e.g. solid-phase microextraction (SPME), solvent-assisted flavor evaporation (SAFE), gas chromatography–olfactometry-mass spectrometry (GC-O-MS), odour activity value (OAV), and aroma extract dilution analysis (AEDA), have been widely applied in baijiu (Li et al., 2019), juice (Pang et al., 2019), Chinese soy sauce (Zhao et al., 2020), and meat products (Pu et al., 2020). In this study, two complementary and comparative techniques, SPME and SAFE, were employed to extract volatiles from five cuts to provide data support for the analysis of freshly distilled brandy. Although important odorants can be extracted and identified by applying the aforementioned concepts, only aroma recombination experiments based on the concentrations of the aroma-active compounds in the food are able to address the fact that interactions between the aroma attributes of all odour-active constituents (Fritsch and Schieberle, 2005, Poisson and Schieberle, 2008). Such recombination experiments have recently been successfully applied to a variety of foods, such as porcini mushrooms (Zhang et al., 2018), aged Chinese rice wines (Chen, Wang, Qian, Li, & Xu, 2019), and gingers (Schaller & Schieberle, 2020). However, studies have only reported the concentration of key odour-active compounds in each distillate cut by employing the aforementioned techniques so far; the application of statistical analysis methods to describe the significant difference in the aroma features of different distillation cuts, for instance, principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA), has not yet been presented.
The current work was aimed to recognize and quantify the key odour-active compounds whin head, heart1, heart2, tail, and stillage cuts of freshly distilled brandy by adopting the GC-O-MS method through combining AEDA and OAV to explore their evolution pattern during the second distillation step. Further, PCA and PLS-DA were conducted to model the key odour-active compounds that confer characteristic aromas and odours in different distillate cuts. This study will be helpful to understand potent odorant compounds in freshly distilled brandy, which would provide a valuable and potential reference for winemakers and contribute to the progress of the wine industry.
Materials and methods
Reagents and standards
Analytical grade chemicals (≥95% purity), which included NaOH, NaCl, anhydrous Na2SO4, tartaric acid, and glucose, were provided by China National Pharmaceutical Ground Corporation (Shanghai, China). Solvents of GC grade (≥99% purity), such as ethanol, methanol, and dichloromethane, were provided by Merck Chemical Co. Inc. (Shanghai, China). Pure standards of volatile compounds and C5-C30 n-alkanes were obtained from Sigma-Aldrich (Shanghai, China).
Fermentation
Ugni Blanc grapes with 14.5 °Brix and 6.5 g/L titratable acidity were reaped at the full maturation stage in plantations (latitude, 37°52′N; longitude, 121°39′E; climate, warm temperate continental monsoon; mean altitude, 30 m; mean precipitation, 672 mm; soil type, brunisolic) in the Yantai Region (Shandong, China) on 11 September 2019. In addition, the fruits were manually chosen and transported to the factory of Changyu Pioneer Wine Co. Ltd at 4 °C on the day of collection. The grapes were stemmed and broken, and the crushed grapes were immersed within the 15,000 L fermenter for a 2-day period under 7–10 °C with the addition of 80 mg/L SO2. Then, the must was inoculated with Saccharomyces cerevisiae following the manufacturer’s recommendations (200 g/m3, Lallemand, France) to trigger alcoholic fermentation at 20 °C for 7 days. Fermentation was recorded daily by measuring the total sugar content of wine. The caps of fermenting must were punched down for 10 min at intervals of 8 h. The alcoholic fermentation of the base wine is blocked at < 4 g/L of total sugar content, and Ugni Blanc grape wine had the following basic parameters: alcohol content 8.5 % v/v, total acidity 9.1 g/L, and total sugar content 3.3 g/L.
Distillation
After alcoholic fermentation, Ugni Blanc grape wine was subjected to a double-stage process. We positioned nearly 10,000 L base wine into the pot still, followed by direct steam heating. During the first distillation, base wine was converted to approximately 2000 L with three cuts, including the head (distilled for around 15 min), heart (distilled for around 6 h), and tail (distilled for 1 h) cuts. Besides, the head and tail cuts were re-distilled with successive batches of wine. In the second stage, 2,000 L of the brouillis with an alcohol content of 27 % v/v was then re-distilled into four cuts, including the head (until the alcohol content of the distillate dropped to 70 % v/v, distilled for around 30 min), heart1 (until the alcohol content of the distillate dropped to 58 % v/v, distilled for around 6 h), heart2 (until the alcohol content of the distillate dropped to 20 % v/v, distilled for around 4 h), and tail (until the alcohol content of the distillate dropped to 0 % v/v, distilled for around 1 h) cuts. The mixture left in the boiler after distillation was stillage. The corresponding sample volume and the final alcohol content are shown in Table S1. Subsequently, five cuts were collected for the analysis.
Determination of aroma compounds
Aroma extraction by the solvent-assisted flavour evaporation (SAFE) apparatus
The extracted and analysed methods of volatile compounds in the head, heart1, heart2, tail, and stillage cuts were performed following Xiang et al.’s method with minor modification (Xiang et al., 2020). The sample obtained (200 mL) was adjusted to 10% v/v with distilled water, followed by NaCl saturation. Afterwards, we extracted the saturated solution by 100, 80, and 60 mL dichloromethane at 25 °C and then transferred it to a separating funnel for 25 min each time. Volatile compounds were extracted by employing SAFE (Glasbläserei Bahr, Manching, Germany) at 45 °C and 2 × 10-3 Pa high vacuum. We dried the organic phase through anhydrous Na2SO4, followed by a concentration of 1 mL based on a nitrogen purging device, and finally kept at −20 °C for further analyses.
Gas chromatography–olfactometry-mass spectrometry analysis (GC-O-MS)
This study conducted GC-O-MS analysis with the Thermo Fisher Trace 1310 gas chromatograph-mass spectrometer (Thermo Fisher Scientific, Waltham, USA) equipped with a Gerstel olfactory detection port (ODP, Gerstel, Mülheim, Germany), which operates under the electron ionisation mode (70 eV, ion source temperature 230 °C) over the 32–350 m/z full-scan mode. The sniffing port was equipped with a humidified air makeup and sequentially heated using a laboratory-made rheostat to prevent condensation of high-boiling compounds. The filament current and quadrupole temperature were 150 μA and 250 °C, respectively. Volatiles were separated on a TG-Wax column (30 m × 0.25 mm, 0.25 μm; Thermo Fisher Scientific, Waltham, USA) with helium being the carrier gas at 2 mL/min. In addition, the injector and detector temperatures were set to 230 °C and 260 °C, respectively. Originally, the oven temperature was kept at 40 °C for 2 min, ramped to 100 °C at 6 °C/min and subsequently enhanced to 200 °C at 5 °C/min, eventually getting to 240 °C at 10 °C/min for a 5-min period.
Aroma extract dilution analysis (AEDA)
In this study, the concentrated extract was diluted with dichloromethane at the ratios 1:2, 1:4, 1:8, 1:16, and so on up to 1:1,024. As described earlier, each dilution was performed the GC-O-MS analysis. The five initial distillate cuts were analysed by five experienced panelists, and each was repeated three times to avoid overlooking odour-active compounds.
Panel training and sensory analysis
The samples were tasted by a group of five trained panelists (three males and two females, aged 23–28 years) from the College of Life Sciences, Yantai University, all of whom were part of a sensory group with considerable experience in wine tasting. The training sessions used the definition of the international standards (ISO 8586-1, 1993) as a reference, as follows: (i) a series of 13 supra-threshold aqueous solutions (25 mL within the Teflon vessels) were currently proposed in specific training, which included ethyl acetate (pineapple), 3-methyl butanol (fusel), dimethyl disulfide (onion), ethyl octanoate (pear), linalool (floral), furfural (sweet), 2-phenylethyl acetate (rose), β-damascenone (fruit/floral), acetic acid (vinegar), β-citronellol (cucumber), 2-phenylethanol (floral/rose), octanoic acid (sweaty), and benzaldehyde (almond); (ii) each panellist underwent certain sensory sniffing training, until they could precisely describe, discriminate, and express all the odorants; (iii)the aforementioned standard compounds were added to 1 mL of dichloromethane solution; each panellist also received some sniffing training on GC-O-MS analysis under identical conditions to those of samples extracts when they were able to specifically recognise all the odours.
Sensory evaluation was performed by a well-trained panel of 20 members (10 males and 10 females, ages from 20 to 50 years). The 7 aroma descriptors selected by the panel were e.g. fruit, green/grass, fusel/solvent, sweaty/fatty, sweet, spicy, and flowery. The panel scored the samples according to a 5-point interval scale, and the score ranged from 0 to 5 (0 = weak and hardly detected; 5 = extremely strong) of each attribute. All the samples were presented to the panelists separately and randomly in a sensory evaluation room at 21 ± 1 °C.
Qualification and quantification of aroma compounds
Headspace solid-phase microextraction- gas chromatography–olfactometry-mass spectrometry (HS-SPME-GC–MS)
This study was conducted HS-SPME-GC–MS analysis similarly as reported by Tian et al. (2018). The specimen was diluted with 10 % v/v with distilled water. For this, the sample (5 mL) was mixed with endogenous reference (20 μL, 3-octanol, 50.5 mg/L standard solutions in ethanol), and the mixture was then transferred into a 20 mL glass vial containing 2 g NaCl. Later, we used a 50/30 µm DVB/CAR/PDMS fibre (Supelco, Bellefonte, PA) for capturing the volatile compounds in the prepared glass vial based on its sensitivity and flexibility. After 20 min of equilibration at 50 °C, SPME fiber was inserted in the septum covering the headspace glass vial in order to absorb volatile compounds and maintained for 10 min. Thereafter, the fiber was inserted into the GC injector port for 5 min of analyte desorption at 250 °C.
The GC–MS analysis was conducted using the GC 2010 (Shimadzu, Japan) and DB-Wax capillary column (30 m × 0.25 mm i.d., 0.25 µm film thickness, J&W Scientific, Folsom, CA) according to the aforementioned procedures. All mass spectra were compared with those of the National Institute of Standards and Technology (NIST) 14 database. The retention indices (RI) of unknown compounds were estimated by a modified Kovats method as described by Tian et al. (2021). We obtained standard solutions through the dilution of individual standards in an ethanol/water mixture (10% v/v of ethanol) at seven levels. The calibration curves (7-point calibration curve) were established by drawing the response ratio between the target aroma compounds and the corresponding internal standards against their concentrations.
Calculation of odour activity value (OAV)
The calculation equation of OAV was as follows: OAV = Ci/OTi, where Ci is the concentration of each compound and OTi is its corresponding odour threshold value in a 10 % v/v water/ethanol mixture. The threshold values were acquired from the relevant literature and the GC-O results. Compounds with OAV ≥ 1 had a major contribution to the characteristic aroma of samples (Tian et al., 2021).
Aroma recombination
The aroma reconstitution test was conducted according to the method described by Al-Dalali, Zheng, Sun, and Chen (2020). All volatile compounds with OAV ≥ 1 were added to different alcohol levels with the same as the abovementioned fractions, resulting in Model 1; unlike Model 1, Model 2 included all the detected volatile compounds. The reconstructed samples were balanced at 20–22 °C for 10 min after mixing, and sensory evaluation of the aroma profile was performed, as described previously in Section 2.4.4. The included angle cosine analysis was performed to judge their similarities (Zhang et al., 2018).
Statistical analysis
All analyses were examined thrice. Statistical evaluation was conducted with the use of one-way ANOVA analysis (p < 0.05) by the SPSS package (version 26.0, Chicago, IL, USA). PCA and PLS-DA were conducted using free web-based MetaboAnalyst version 5.0 (https://www.metaboanalyst.ca).
3. Results and Discussion.
Odour-active compounds of the head, heart1, heart2, tail, and stillage cuts
GC-O-MS combined with AEDA is a common method used to rank and identify key odour-active compounds (Niu et al., 2017). Here, we identified 65 odour-active compounds (flavor dilution (FD): ≥ 16) in five distillate cuts (Table 1), i.e. 50, 61, 48, 25, and 18 odour-active compounds from the head, heart1, heart2, tail, and stillage cuts, respectively. More importantly, 4 and 11 compounds were exclusively found in head and heart cuts, respectively, bringing direct proof supporting the difference of aroma characteristics in these two distillate cuts. Among them, 3-methylbutyl acetate, 3-methylbutanol, ethyl hexanoate, ethyl octanoate, as well as ethyl decanoate exhibited the highest FD factors (FD: ≥1024), which is not completely consistent with the results reported in a previous study about the freshly distilled spirit from Spine grape (Xiang et al., 2020). Specifically, these compounds with fruity and fusel descriptors detected by GC-O in the head and heart cuts, such as ethyl acetate, 2-methylbutanal, ethyl butanoate, 2-methylpropanol, 3-methylbutyl acetate, 3-methylbutanol, ethyl hexanoate, hexyl acetate, ethyl decanoate, and ethyl dodecanoate, showed higher FD factors. Similarly, three compounds with sweet descriptors including α-terpineol, β-damascenone, and 2-phenylethanol showed higher FD factors in the tail and stillage cuts.
Table 1.
Odour-active compounds (FD ≥ 16) identified in the second distillation of freshly distilled brandy by AEDA-GC-O-MS.
| NO |
RI | Compound | Aroma descriptiors | FD factora |
Identificationb |
||||
|---|---|---|---|---|---|---|---|---|---|
| Head | Heart1 | Heart2 | Tail | Stillage | |||||
| X1 | <1000 | Acetaldehyde | grass | 64 | 32 | 16 | − | − | AD, RI, Std |
| X2 | <1000 | 2-Methylpropanal | grape | 256 | 128 | 32 | − | − | AD, RI, Std |
| X3 | <1000 | Ethyl formate | pineapple | − | 16 | − | − | − | AD, RI, Std |
| X4 | <1000 | Ethyl acetate | pineapple | 512 | 128 | 64 | 32 | 16 | AD, RI, Std |
| X5 | <1000 | Methanol | alcohol | 16 | − | − | − | − | AD, RI, Std |
| X6 | <1000 | 2-Methylbutanal | grass/sweet | 128 | 16 | − | − | − | AD, RI, Std |
| X7 | <1000 | 3-Methylbutanal | grass/sweet | 64 | 16 | 16 | − | − | AD, RI, Std |
| X8 | <1000 | Ethyl 2-methylpropanoate | fruity/sweet | − | 16 | − | − | − | AD, RI, Std |
| X9 | <1000 | 2-Methylpropyl acetate | flower/fruity | − | 16 | 16 | − | − | AD, RI, Std |
| X10 | 1023 | Ethyl butanoate | sweet | 256 | 64 | 32 | 16 | 16 | AD, RI, Std |
| X11 | 1036 | Ethyl 2-methylbutanoate | apple | 16 | 16 | − | − | − | AD, RI, Std |
| X12 | 1089 | 2-Methylpropanol | solvent | 512 | 256 | 128 | 32 | 16 | AD, RI, Std |
| X13 | 1117 | 3-Methylbutyl acetate | banana | ≥1024 | 512 | 256 | 16 | 16 | AD, RI, Std |
| X14 | 1147 | 1-Butanol | spicy | 32 | 16 | 16 | − | − | AD, RI, Std |
| X15 | 1173 | Heptan-2-one | pear | 32 | 16 | 16 | − | − | AD, RI, Std |
| X16 | 1175 | Methyl hexanoate | flower/fruity | 64 | 32 | 16 | − | − | AD, RI, Std |
| X17 | 1176 | Limonene | pine/vanilla |
16 | 16 | − | − | − | AD, RI, MS |
| X18 | 1208 | 3-Methylbutanol | spicy | ≥1024 | 128 | 64 | 16 | 16 | AD, RI, Std |
| X19 | 1226 | Ethyl hexanoate | fruity | ≥1024 | 512 | 128 | 16 | 16 | AD, RI, Std |
| X20 | 1235 | Octan-3-one | apple | 32 | 16 | 16 | − | − | AD, RI, Std |
| X21 | 1245 | 1-Pentanol | fruity/spicy | 32 | 16 | 16 | − | 16 | AD, RI, Std |
| X22 | 1265 | Hexyl acetate | flower/sweaty | 128 | 32 | − | − | − | AD, RI, MS |
| X23 | 1318 | Heptan-2-ol | oak | 16 | − | − | − | − | AD, RI, Std |
| X24 | 1323 | 3-Methylpentanol | sweet | − | 16 | 32 | − | − | AD, RI, Std |
| X25 | 1342 | Ethyl 2-hydroxypropanoate | fruity | 16 | 16 | 32 | 64 | 16 | AD, RI, Std |
| X26 | 1352 | 1-Hexanol | grass/green | 16 | 128 | 32 | 16 | − | AD, RI, Std |
| X27 | 1362 | (E)-Hex-3-en-1-ol | grass/green | 16 | 32 | 64 | 16 | − | AD, RI, Std |
| X28 | 1386 | Methyl octanoate | fruity | − | 16 | 32 | − | − | AD, RI, Std |
| X29 | 1381 | (Z)-Hex-3-en-1-ol | grass/green | 16 | 32 | 64 | − | − | AD, RI, Std |
| X30 | 1385 | Nonanal | grass | 32 | 16 | 16 | 16 | − | AD, RI, Std |
| X31 | 1427 | Ethyl octanoate | fruity | ≥1024 | 512 | 128 | 32 | 16 | AD, RI, Std |
| X32 | 1462 | Furfural | sweet | 16 | 32 | 16 | − | − | AD, RI, Std |
| X33 | 1451 | 3-Methylbutyl hexanoate | fruity | − | 16 | − | − | − | AD, RI, Std |
| X34 | 1491 | Acetic acid | vinegar | 16 | 32 | 128 | 64 | 32 | AD, RI, Std |
| X35 | 1495 | Decanal | grass/green | 16 | − | − | − | − | AD, RI, Std |
| X36 | 1513 | Benzaldehyde | almond | − | 32 | 16 | 16 | − | AD, RI, Std |
| X37 | 1518 | Ethyl 3-hydroxybutanoate | fruity | 16 | − | − | − | − | AD, RI, MS |
| X38 | 1526 | Ionone | spicy | 64 | 16 | 16 | − | − | AD, RI, Std |
| X39 | 1530 | ethyl nonanoate | fruity | − | 16 | − | − | − | AD, RI, Std |
| X40 | 1550 | Linalool | floral | 32 | 16 | 16 | − | − | AD, RI, Std |
| X41 | 1559 | 1-Octanol | alcoholic | 32 | 16 | 16 | − | − | AD, RI, Std |
| X42 | 1566 | 5-Methylfurfural | sweet | − | 32 | 16 | 16 | − | AD, RI, Std |
| X43 | 1586 | Methyl decanoate | fruity | 16 | 16 | 16 | − | − | AD, RI, MS |
| X44 | 1595 | 4-Terpineol | spicy/soil | 16 | 32 | 64 | − | − | AD, RI, Std |
| X45 | 1621 | Ethyl 2-furoate | spicy | 16 | 16 | 16 | − | − | AD, RI, MS |
| X46 | 1637 | Butanoic acid | sweaty/ pungent | 16 | 16 | 16 | 64 | 32 | AD, RI, Std |
| X47 | 1634 | Ethyl decanoate | fruity | ≥1024 | 512 | 128 | 16 | 16 | AD, RI, Std |
| X48 | 1658 | 3-Methylbutyl octanoate | fruity | − | 16 | − | − | − | AD, RI, Std |
| X49 | 1677 | Diethyl succinate | fruity | − | 16 | 32 | 16 | − | AD, RI, Std |
| X50 | 1694 | α-Terpineol | pine | 16 | 32 | 64 | 128 | 128 | AD, RI, Std |
| X51 | 1720 | Propyl decanoate | fruity | − | 16 | − | − | − | AD, RI, Std |
| X52 | 1751 | 2-Methylpropyl decanoate | fruity | 32 | 16 | 16 | − | 16 | AD, RI, MS |
| X53 | 1768 | β-Citronellol | grass/ cucumber | 32 | 16 | 16 | − | − | AD, RI, Std |
| X54 | 1798 | Nerol | hay | − | 16 | 16 | − | − | AD, RI, Std |
| X55 | 1811 | 2-Phenylethyl acetate | rose | 16 | 256 | 128 | 16 | 16 | AD, RI, Std |
| X56 | 1811 | β-Damascenone | fruity/honey | 16 | 32 | 64 | 128 | 128 | AD, RI, Std |
| X57 | 1840 | Ethyl dodecanoate | fruity | 256 | 64 | 32 | 16 | − | AD, RI, Std |
| X58 | 1862 | Hexanoic acid | sweaty/ pungent | 16 | 16 | 16 | 64 | 32 | AD, RI, MS |
| X59 | 1859 | 3-Methylbutyl decanoate | fruity | 32 | 16 | − | − | − | AD, RI, Std |
| X60 | 1881 | Benzyl alcohol | fragrant | 64 | 32 | 16 | − | − | AD, RI, Std |
| X61 | 1914 | 2-Phenylethanol | rose/honey | 16 | 32 | 64 | 128 | 16 | AD, RI, Std |
| X62 | 2046 | Ethyl tetradecanoate | fruity | 32 | 16 | − | − | − | AD, RI, MS |
| X63 | 2069 | Octanoic acid | fatty/sweaty | − | 16 | 32 | 32 | − | AD, RI, Std |
| X64 | 2252 | Ethyl hexadecanoate | butter/fruity | 16 | 16 | − | − | − | AD, RI, MS |
| X65 | 2270 | Decanoic acid | fatty/sweaty | − | 16 | 32 | 64 | − | AD, RI, Std |
“−” Not detected.
Flavor dilution factors of the head, heart1, heart2, tail, and stillage cuts of freshly distilled brandy.
Identification of odour-active compounds. Positive identification: compounds were identified by comparison of the aroma descriptor (AD), the retention indices (RI), confirmed by authentic standards (Std); Tentative identification: compounds were identified by comparison of the AD, RI, and mass spectra (MS) with compounds in the NIST 2011 Mass Spectral Library or literature.
Three higher alcohol compounds that imparted fusel odour were 2-methylpropanol (FD: 16–512), 3-methylbutanol (FD: 16–1024), and 1-butanol (FD: ≤32); meanwhile, they were also considered the key odour-active compounds in several wine products, such as whisky (Jeleń, Majcher, & Szwengiel, 2019) and baijiu (Wang, Guo, Song, Meng, & Guan, 2020). Correspondingly, these compounds showed increased FD factors within head and heart cuts compared with those in the tail and stillage cuts. Therefore, for instance, 3-methylbutanol presented the greatest FD factor (1024) within head, and no 1-butanol was observed within tail and stillage cuts. Unlike the alcohols mentioned above, three green/grass aroma compounds were detected as grape-derived C6 compounds, which were 1-hexanol, (E)-hex-3-en-1-ol, and (Z)-hex-3-en-1-ol. Among them, 1-hexanol was known as the key odour-active compound of freshly distilled brandy (Malfondet et al., 2016). C6 compounds revealed increased FD factors within heart cut whereas decreased FD factors within head, tail, and stillage cuts, partly illuminated by the presence of greater green/grass aromas within heart cut when compared with those in the other cuts. But C6 compounds showed slightly decreased FD factors (128–512) than the other odour-active compounds within head cut (e.g. ethyl hexanoate, FD ≥ 1024), suggesting that the green/grass odour contributing to the unique features of the heart cut is far less strong than the fruit aroma.
Among the fruity odorous zones, ethyl hexanoate, 3-methylbutyl acetate, ethyl decanoate, and ethyl octanoate presented great FD factors (FD: ≥ 128) within heart and head cuts. Ethyl acetate, ethyl butanoate, hexyl acetate, and ethyl dodecanoate displayed slightly decreased FD factors, while the corresponding FD values within head (FD: 32–512) increased in comparison with heart and tail cuts (FD: 16). In addition, other ester compounds showed lower FD factors (FD: ≤ 32) during the second distillation process. Thus, the head cut typically exhibited greater FD factors compared with tail and heart cuts. During alcoholic fermentation ester compounds are formed by yeast metabolism, and have been considered as key odour-active compounds of brandy, such as apple brandy (He et al., 2020) and jujube brandy (Xia, Liu, Wang, & Shuang, 2020). Notably, however, unlike most ester compounds, ethyl 2-hydroxypropanoate had an increased FD factor within tail cut (FD: 64) relative to other other cuts (FD: 16–32).
Diethyl succinate and 2-phenylethyl acetate, compounds that potentially contribute to sweet/rose fragrances in spirits, were found as key odour-active compounds within heart cut. FD factors of 2-phenylethyl acetate exceeded 128 within heart cut, yet significantly decreased within head (FD: 16) and tail (FD: 16) cut. A parallel finding was observed for diethyl succinate. Albeit these compounds have been considered as important flavour compounds in aged brandy, e.g. apple brandy (Coldea et al., 2020) and Cognac (Thibaud, Courregelongue, & Darriet, 2020), these were primarily documented as key odour-active compounds in freshly distilled brandy because of the high FD factors.
Ionone, linalool, and β-citronellol with floral/spicy odours showed higher FD factors within head cut (FD: 32–64) than heart (FD: 16) and tail (FD: 16) cuts. Another two odour-active compounds, namely, α-terpineol and β-damascenone, which are responsible for soil/hay smell, were also perceived as volatile terpenes, which primarily originated from grapes. Likewise, FD factors of the two terpenes displayed the greatest FD factors within the tail and stillage cuts (FD: 64–128) whereas the smallest FD factors within head cut (FD: 16). This result is supported by the proposed conclusion that yeast can convert some monoterpene alcohols into other substances by fermentation (Awad et al., 2017). Besides, the roles of limonene and nerol, which are described as having a citric, green, or balsamic odour, as varietal aroma compounds in traditional Slovak Tokaj wines have also been reported (Khvalbota, Machyňáková, Čuchorová, Furdíková, & Špánik, 2021).
Two odour-active compounds with sweaty scents were butanoic acid and hexanoic acid, showing the greatest values within tail cut (FD: 64) whereas the smallest values within head cut (FD: 16). Likewise, octanoic acid and decanoic acid, imparting fatty/sweaty odours, could only be observed within heart and tail cuts. Such acids, which have been theoretically generated from yeast and bacterial modulation, were employed as the key odour-active compounds of freshly distilled brandy (Zhao et al., 2014). Nevertheless, these acids are usually undesirable at concentrations > 20 mg/L because the distillate takes on an unpleasant odour (Cao, Chen, Jassbi, & Xiao, 2015).
Three odorous compounds, namely, benzaldehyde, 2-phenylethanol, and 2-phenylethyl acetate, corresponded to floral notes, which, to some extent, were consistent with a previous report by Sánchez et al. (2022), in which the floral smell was partly contributed by 2-phenylethanol and 2-phenylethyl acetate. Except for 2-phenylethyl acetate, benzaldehyde and 2-phenylethanol displayed decreased values within head cut (FD: 16) whereas increased values within tail cut (FD: 128–256). However, apart from the volatile compounds described above, some important compounds were still perceived in the five distillate cuts. Heptan-2-one (pear) and octan-3-one (apple) showed increased values within head cut (FD: 32) when compared with those within heart1 and heart2 cuts (FD: 16). Besides, furfural and 5-methylfurfural, which exhibited a sweet aroma, had higher FD factors in the heart1 cut. Furthermore, some aldehyde compounds, such as 2-methylpropanal, 2-methylbutanal, and 3-methylbutanal (grass/sweet), were detected with increased values (FD: 64–256) within head and heart cuts but not detected in the tail and stillage cuts. These compounds were also known to be important aromatic compounds during distillation process of freshly distilled Cognac (Sánchez et al., 2022). Taken together, the FD factors of most odour-active compounds gradually decreased with the distillation process, and only a few high-boiling compounds, such as β-damascenone, 2-phenylethanol, α-terpineol, and acetic acid showed an upward trend in FD factors in the tail and stillage cuts.
Quantification of odour-active compounds and OAV analysis
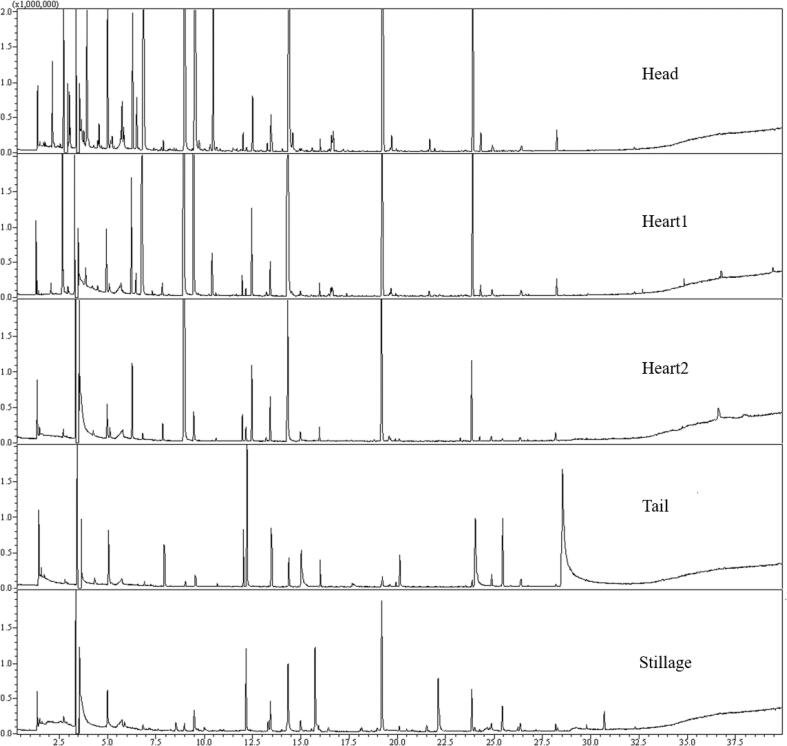
The total level of different classes of compounds and total ion current chromatograms in the five cuts of brandy distillation in this study are shown in Table S2 and Fig. 1. Of 65 compounds, 30 compounds were perceived in amounts equal to or greater than their odour threshold, indicating that they were responsible for freshly distilled brandy (Table 2). In detail, 19, 22, 11, 5, and 4 compounds with OAV ≥ 1 were regarded to be key odour-active compounds within head, heart1, heart2, tail, and stillage cuts, respectively. Within head cut, ethyl decanoate showed the greatest OAV value (OAV: 278.71), then ethyl octanoate (OAV: 62.93), 3-methylbutyl acetate (OAV: 58.87), ethyl hexanoate (OAV: 36.73), ethyl butanoate (OAV: 10.31), and ionone (OAV: 10.24) followed. Although these compounds in the heart1 and heart2 cuts had relatively low concentrations, all their OAV values were ≥ 1, except for 3-methylbutyl acetate (OAV: 0.65) in the heart2, which is in good agreement with the GC-O results. An interesting exception was ethyl acetate, which was also the key odour-active compound (OAV: 4.96) because its detected concentration (16 mg/L) in the head cut exceeded the odour threshold (7.5 mg/L), although it was not smelled during the GC-O analysis. As described in a previous study, ethyl acetate stands for a crucial ester presenting within distilled spirit, which contributes to a favorable and fruity aroma at a low concentration, whereas when its concentration reaches values as high as 200 mg/L, it has sharp and pungent notes (Wei et al., 2021).
Fig. 1.
GC–MS total ion chromatograms of the five cuts in the second distillation obtained on DB-WAX stationary phase.
Table 2.
Concentrations and OAV of key odour-active compounds in the head, heart1, heart2, tail and stillage cuts of freshly distilled brandy.
| Compounda | Concentration (μg/L)b |
Threshold (μg/L)c | OAVd |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Head | Heart1 | Heart2 | Tail | Stillage | Head | Heart1 | Heart2 | Tail | Stillage | ||
| 2-Methylpropanal | 2987.11 ± 56.03a | 407.16 ± 11.23b | 188.72 ± 5.61b | − | − | 8001 | 3.73 | 0.51 | 0.24 | − | − |
| Ethyl acetate | 37164.34 ± 125.03a | 7696.11 ± 187.12b | 228.13 ± 12.36c | 77.54 ± 4.08d | 9.76 ± 0.85e | 75001 | 4.96 | 1.03 | < 0.01 | < 0.01 | < 0.01 |
| 2-Methylbutanal | 2244.45 ± 32.11a | 265.43 ± 11.23b | − | − | − | 10001 | 2.24 | 0.27 | − | − | − |
| Ethyl butanoate | 10313.78 ± 260.94a | 3132.47 ± 202.68b | 1263.34 ± 215.74b | 138.67 ± 11.23c | 93.32 ± 14.20c | 10001 | 10.31 | 3.13 | 1.26 | 0.14 | 0.09 |
| Ethyl 2-methylbutanoate | 114.45 ± 23.05b | 231.21 ± 9.33a | − | − | − | 2002 | 0.57 | 1.16 | − | − | − |
| 2-Methylpropanol | 45740.72 ± 235.21a | 42540.34 ± 356.52a | 21244.45 ± 156.33b | 1115.78 ± 326.89c | 427.41 ± 10.41c | 400001 | 1.43 | 1.06 | 0.53 | 0.03 | 0.01 |
| 3-Methylbutyl acetate | 58870.08 ± 154.32a | 9365.77 ± 214.34b | 652.76 ± 25.05c | 20.05 ± 2.78d | 46.27 ± 8.23d | 10001 | 58.87 | 9.37 | 0.65 | 0.02 | 0.05 |
| 3-Methylbutanol | 52072.23 ± 45.64a | 59138.67 ± 368.32a | 27914.21 ± 351.04b | 15.34 ± 1.64c | 20.15 ± 3.24c | 300001 | 1.74 | 1.97 | 0.93 | < 0.01 | < 0.01 |
| Ethyl hexanoate | 84478.58 ± 210.34a | 14449.87 ± 412.06b | 681.27 ± 14.56c | 35.82 ± 3.10d | 32.24 ± 3.47d | 23001 | 36.73 | 6.82 | 0.30 | 0.02 | 0.01 |
| Hexyl acetate | 8777.64 ± 116.90a | 1701.74 ± 56.08b | 16.78 ± 2.12c | 2.55 ± 0.31d | 4.67 ± 0.54d | 10002 | 8.78 | 1.70 | 0.02 | < 0.01 | < 0.01 |
| Heptan-2-ol | 151.54 ± 8.21a | 30.42 ± 2.32b | 5.23 ± 0.89c | − | − | 702 | 2.16 | 0.43 | 0.07 | − | − |
| Ethyl 2-hydroxypropanoate | 202.73 ± 10.32b | 257.21 ± 5.69b | 528.34 ± 41.20a | 625.56 ± 14.63a | 99.14 ± 6.17c | 4002 | 0.51 | 0.64 | 1.32 | 1.56 | 0.25 |
| 1-Hexanol | 2257.89 ± 113.08b | 3299.75 ± 265.12a | 1774.37 ± 112.11b | 58.25 ± 4.25c | 23.12 ± 3.45c | 20001 | 1.13 | 1.65 | 0.89 | 0.03 | 0.01 |
| (E)-Hex-3-en-1-ol | − | 260.35 ± 9.67b | 460.14 ± 12.33a | 124.50 ± 5.61b | 45.34 ± 3.56c | 4003 | − | 0.65 | 1.15 | 0.31 | 0.11 |
| (Z)-Hex-3-en-1-ol | 289.47 ± 10.23b | 418.58 ± 24.32a | 492.84 ± 21.32a | 56.36 ± 4.32c | 22.28 ± 3.41c | 4003 | 0.72 | 1.05 | 1.23 | 0.14 | 0.06 |
| Ethyl octanoate | 125854.51 ± 116.46a | 58519.23 ± 356.08b | 2890.61 ± 320.53c | 68.65 ± 5.41d | 109.23 ± 9.34d | 20001 | 62.93 | 29.26 | 1.45 | 0.03 | 0.05 |
| Benzaldehyde | − | 1058.72 ± 62.64a | 949.44 ± 13.25a | 45.31 ± 3.45c | 15.45 ± 2.14c | 10001 | − | 1.06 | 0.95 | 0.05 | 0.02 |
| Ionone | 921.26 ± 23.54a | 227.18 ± 8.95b | 17.12 ± 2.31c | 3.53 ± 0.54c | 4.02 ± 0.87c | 903 | 10.24 | 2.52 | 0.19 | 0.04 | 0.04 |
| Methyl decanoate | 580.15 ± 9.65a | 521.01 ± 41.32a | 437.32 ± 12.30b | − | − | 4002 | 1.45 | 1.30 | 1.09 | − | − |
| 4-Terpineol | 370.57 ± 14.25a | 450.38 ± 11.23a | 170.29 ± 11.32b | 17.36 ± 0.87c | − | 3003 | 1.24 | 1.50 | 0.57 | 0.06 | − |
| Butanoic acid | 1338.04 ± 35.11a | 1261.43 ± 32.58a | 856.87 ± 24.35b | 355.54 ± 31.56c | 101.21 ± 8.65d | 12001 | 1.12 | 1.05 | 0.71 | 0.30 | 0.08 |
| Ethyl decanoate | 55742.11 ± 274.09a | 36376.32 ± 298.06a | 4444.67 ± 324.43b | 100.61 ± 10.54c | 181.30 ± 9.12c | 2001 | 278.71 | 181.88 | 22.22 | 0.50 | 0.91 |
| 3-Methylbutyl octanoate | 339.37 ± 35.66b | 522.17 ± 21.35a | 72.20 ± 8.32c | − | − | 4002 | 0.85 | 1.31 | 0.18 | − | − |
| α-Terpineol | − | 58.09 ± 5.61c | 140.24 ± 10.23b | 307.36 ± 12.34a | 302.47 ± 10.26a | 2503 | − | 0.23 | 0.56 | 1.23 | 1.21 |
| β-Citronellol | 113.03 ± 6.58a | 53.12 ± 4.20b | 32.09 ± 3.14b | − | − | 1001 | 1.13 | 0.53 | 0.32 | − | − |
| 2-Phenylethyl acetate | − | 341.06 ± 9.64a | 314.14 ± 12.31a | 33.74 ± 5.41b | 28.23 ± 2.11b | 2501 | − | 1.36 | 1.26 | 0.13 | 0.11 |
| β-Damascenone | 37.21 ± 6.38c | 75.30 ± 8.45b | 101.28 ± 9.45a | 144.31 ± 10.24a | 132.07 ± 9.09a | 1003 | 0.37 | 0.75 | 1.01 | 1.44 | 1.32 |
| Ethyl dodecanoate | 8711.14 ± 165.23a | 3883.12 ± 324.31b | 859.81 ± 20.12c | 29.92 ± 4.13d | 50.34 ± 3.45d | 15001 | 5.81 | 2.59 | 0.57 | 0.02 | 0.03 |
| 2-Phenylethanol | 2443.08 ± 23.54c | 3474.12 ± 56.78c | 4571.12 ± 165.09b | 4764.34 ± 102.23a | 4680.06 ± 155.17a | 26001 | 0.94 | 1.34 | 1.76 | 1.83 | 1.80 |
| Octanoic acid | 445.57 ± 5.61c | 1122.05 ± 6.64b | 1627.10 ± 32.10a | 1750.23 ± 56.14a | 1670.11 ± 30.05a | 5001 | 0.89 | 2.24 | 3.25 | 3.50 | 3.34 |
Compounds with OAV ≥ 1 were identified in the five distillate cuts.
Compounds were quantified by standards; ‘−’ means trace or undetected; different letters in the same row means significant differences according to Duncan test (p < 0.05).
Odour thresholds were taken from 1Willner et al. (2013); 2Gao et al. (2014); and 3Xiang et al. (2020).
Odour activity values (OAVs), ratio of the concentration of certain compound to its odour threshold.
Likewise, 2-methylpropanol and 3-methylbutanol were majorly detected in the head (OAV: 1.43 and 1.74, respectively) and heart1 (OAV: 1.06 and 1.97, respectively) cuts but in lesser amounts in the heart2, tail, and stillage cuts (OAV < 1). Higher alcohols are positively involved in the perceived spirit taste and flavour; they can impart positive attributes and increase complexity to wine quality at low concentrations. However, they always relate to negative sensory descriptors (such as a strong and pungent smell) at great concentrations (>35 mg/L) (Varela, Barker, Tran, Borneman, & Curtin, 2017). These findings explain why some higher alcohols may be important compounds that contribute to the strong and unpleasant odours and tastes of the head cut.
Compared with the head, tail, and stillage cuts, the aforementioned esters, except for ethyl 2-hydroxypropanoate, α-terpineol, β-damascenone, and octanoic acid; higher alcohols, furfurals, terpenes, and aromatic compounds had moderate levels within heart cut but exceeded their respective thresholds. Moreover, higher OAVs of 3-methylbutanol, 1-hexanol, benzaldehyde, 4-terpineol, and 2-phenylethyl acetate were obtained in the heart1 cut than within head and tail cuts, mostly conforming to GC-O results.
The OAVs of α-terpineol (OAV: 1.21–1.23), β-damascenone (OAV: 1.32–1.44), 2-phenylethanol (OAV: 1.80–1.83), and octanoic acid (OAV: 3.34–3.50) were significantly higher within the tail and stillage cuts than in the head, heart1, and heart2 cuts (Table 2). Based on these results, terpenes, acids, and aromatic compounds can be suggested as the important odour-active compounds within tail and stillage cuts, conforming to GC-O data. Moreover, research has claimed that a considerable amount of volatile fatty acids (e.g. 1.75 mg/L for octanoic acid) could present an undesirable smell (Xiang et al., 2020), which might partially account for foul smell within tail cut during distillation.
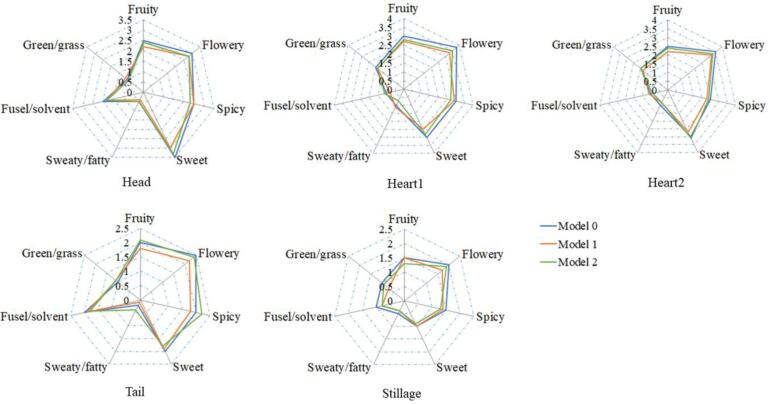
Following aroma descriptors, those key odour-active compounds were usually divided into seven classes, namely, fruity, flowery, fusel/solvent, sweet, sweaty/fatty, spicy, and green/grass aromas (Fig. 2). Esters and higher alcohols, like ethyl butanoate, ethyl hexanoate, 3-methylbutyl acetate, ethyl octanoate, ethyl decanoate, 2-methylpropanol, and 3-methylbutanol, had relatively higher levels within head cut, which imparted stronger fruity, sweet, and fusel/solvent attributes. However, increased aromatic compounds and acid contents, especially 2-phenylethyl acetate, octanoic acid, and decanoic acid in the tail cut resulted in more intense sweaty/fatty odours. In brief, compared with the head, tail, and stillage cuts, the heart1 and heart2 cuts mainly contained fruity and sweet smell with less unwelcome fusel/solvent and sweaty/fatty odours. Besides, slightly higher green/grass notes derived from higher levels of C6 compounds were detected in the heart1 cut. To deeply illustrate how the aroma-active compounds made contributions, we performed included angle cosine analysis to decide the similarities of the two Models to the original samples, showing that the average similarity degree for Model 1 was 0.992 and 0.995 for Model 2. As a result, identifying the key aroma compounds within the distillation samples was considered successful because odorant mixtures exhibited good similarities to the original samples (Fig. 2). Collectivelly, these compounds with OAV ≥ 1were considered to be odour-active compounds, especially 3-methylbutyl acetate, ethyl hexanoate, ethyl octanoate, and ethyl decanoate in the head and heart cuts, which lay the foundation of high quality of the final product. The diversity of boiling point and solubility of volatile compounds resulted in their significantly different concentrations in these cuts, resulting in the different evolution patterns during distillation.
Fig. 2.
Aroma profiles of three models (original Model 0, recombination Model 1, and recombination Model 2) within head, heart1, heart2, tail, and stillage cuts. Model 0 was the sample obtained by second distillation; Model 1 was recombinated from all volatile compounds with OAV ≥ 1; Model 2 was recombinated all the detected volatile compounds.
Combined multivariate analysis of odour-active compounds with OAV ≥ 1
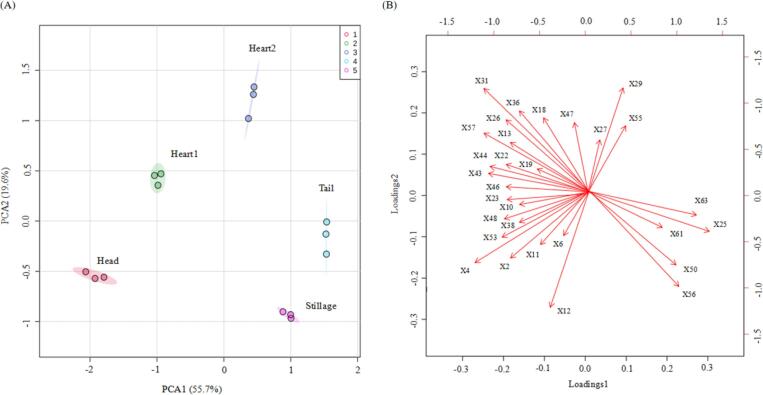
The qualitative and quantitative analyses could identify differences in the aroma compounds present in the head, heart1, heart2, tail, and stillage cuts; however, these methods could not determine the prominent aroma compounds in the samples, e.g. compounds that positively contributed most significantly to the unique flavour of freshly distilled brandy. Therefore, statistical methods were employed to analyse these data. Different combined statistical methods, including PCA and PLS-DA, were pursued to identify the aroma compounds dominating the overall aroma profile of five cuts. In the PCA plot, the eigenvalue of 1st and 2nd principal components remained 19.6 and 11.4, which explained 55.7% and 19.6% variation in the dataset, respectively, and the accumulated variance contribution rate reached 75.3% (Fig. 3). As shown in Fig. 3A, the five cuts were clustered into five spatial regions based on the score plots of the PC1 and PC2. The head cuts were on the left side in the area, which covered the PC1 and PC2 negative axis, and those associated aroma compounds were X4 (ethyl acetate), X6 (2-methylbutanal), X10 (ethyl butanoate), X13 (3-methylbutyl acetate), and X38 (ionone), suggesting that these three compounds accounted for the top aromatic compounds within head cuts (Fig. 3B). The five characteristic compounds in the head cuts belong to the categories of esters, ketones, and aldehydes. The heart1 cuts in the region bottom which covered the PC1 negative axis as well as the PC2 positive axis. Relevant aromatic compounds were X18 (3-methylbutanol), X19 (ethyl hexanoate), X26 (1-hexanol), X31 (ethyl octanoate), X36 (benzaldehyde), X47 (ethyl decanoate), and X55 (2-phenylethyl acetate) (Fig. 3B), implying that these compounds could significantly affect aroma compound profiles in heart1 cuts. Meanwhile, the heart2 cuts on the region top covering PC1 and PC2 positive axes, and the corresponding aroma compounds were X27 ((E)-hex-3-en-1-ol), X29 ((Z)-hex-3-en-1-ol), and X55 (2-phenylethyl acetate) (Fig. 3B), suggesting that these three compounds exerted influence on the aroma characteristics of the heart2 cuts. On the whole, PCA revealed that the distribution of characteristic aroma compounds in the heart1 and heart2 cuts included four esters, four alcohols, one acid, and three aromatics, indicating the unique and complex representative aromatic compounds in the heart cuts.
Fig. 3.
Principal component analysis (PCA) of five distillation cuts and 30 odour-active compounds. (A) Distinction of the samples (scores). The samples (three replicates) are visualised in different colours. (B) Distribution of the 30 odour-active compounds (loadings). The number code in Fig. 2B also corresponds to the volatile code in Table 1.
As shown in Fig. 3A, the tail cuts were mainly distributed in the region right bottom corner which covered the PC1 negative axis together with PC2 positive axis. Those associated aroma compounds were X25 (ethyl 2-hydroxypropanoate), X50 (α-terpineol), X56 (β-damascenone), X61 (2-phenylethanol), and X63 (octanoic acid) (Fig. 3B), implying that these five aroma compounds were the characteristic aroma compounds in the tail cuts. It is worth noting that high levels of X50 (α-terpineol) and X56 (β-damascenone) were detected in both the tail and stillage cuts. Among the five associated aroma compounds, two were terpenes (α-terpineol and β-damascenone), while the remaining three were ester and aromatic and volatile acid; these results implied that the characteristic aroma of the tail and stillage cuts could be attributed to the presence of esters, aromatics, terpenes, and volatile acid, among which the major contributors were esters and terpenes. Based on the PCA, fewer aromatic compounds related to those obtained principal components were found, whereas those present in higher levels, e.g. limonene, ethyl 2-methyibutanoate, heptan-2-ol, and butanoic acid, were not closely associated with the principal components. These results were consistent with the recently published results on Spine grape wine (Xiang et al., 2020). Therefore, these compounds are not the main factors affecting the aroma profiles of freshly distilled brandy.
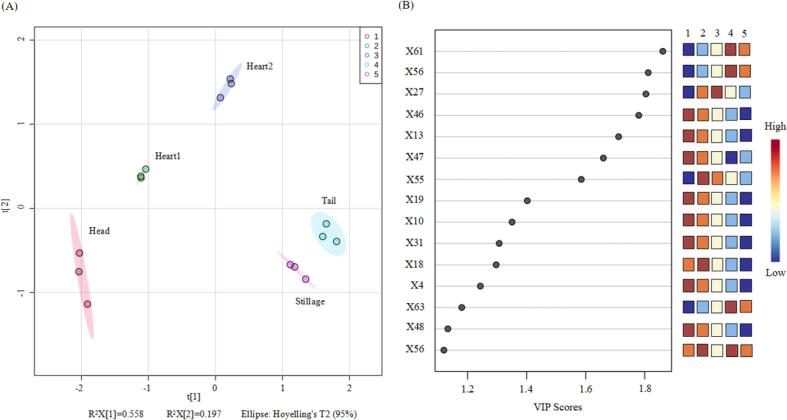
For further better discrimination of the sample characteristics, a PLS-DA model was then constructed. Upon R2 > 0.7 and Q2 > 0.4, PLS-DA was recognized as the favorable model to explore biological data (Dong et al., 2019). According to Fig. 4A, 75.5% of overall variance upon R2Y = 92.7% and Q2 = 86.8% was accounted for, and the five sample groups were smoothly distinguished within the quadrant diagram, and the enhancement classification outcome was similar to the aforementioned PCA model: (a) first, (b) second, (c) third and (d) fourth quadrant-sample dots for heart2, heart1, head and tail/stillage cuts, respectively. To further analyse the odour-active compounds that contributed to discrimination between the two groups, the variable importance in projection (VIP) analysis was conducted (Fig. 4B). In the proposed method, compounds with a ratio value ≥ 1 are regarded to be accountable for the aroma, i.e. the greater their VIP is, the more they contribute to the aroma profile (Ling et al., 2019). As shown in Fig. 4B, the VIP analysis revealed that out of the 30 odour-active compounds, only 12 compounds (VIP ≥ 1) might be considered as key variables that affect the brandy quality. For instance, the heart1 cuts were closely associated with 3-methylbutanol, 1-hexanol, benzaldehyde, ethyl decanoate, and 2-phenylethyl acetate, whereas the heart2 cut was closely associated with (E)-hex-3-en-1-ol, which further confirms the results of PCA. This combined method of PCA and PLS-DA has been broadly applied to investigate the flavour characteristics of citrus fruit (Jandrić & Cannavan, 2017) and Chinese black pork (Li et al., 2021). Moreover, ethyl acetate, ethyl 2-hydroxypropanoate, α-terpineol, 2-phenylethanol, and octanoic acid were associated with the tail and stillage cuts, revealing the close relationship between odour-active compounds and samples. Similarly, volatile compounds like ethyl hexanoate, ethyl acetate, 3-methylbutyl acetate, and hexyl acetate showed a positive correlation with the higher head cuts values. Therefore, we propose that the precise separation of the distillation process may be available to improve the overall quality of brandy.
Fig. 4.
Partial least squares discriminant analysis (PLS-DA) of five distillation cuts and 30 odour-active compounds. (A) PLS-DA of 30 odour-active compounds in five distillation cuts based on a two-dimensional representation of the scores with two PLS components (PLS1 and PLS2). (B) The VIP scores of the odour-active compounds that increase or decrease with changes in five distillation cuts, represented by red squares for higher concentration and blue squares for lower concentration.
Conclusions
We performed a multi-objective study to evaluate the characterisation and distribution patterns of key odour-active compounds in head, heart1, heart2, tail, and stillage cuts of freshly distilled brandy. Among these compounds detected, 19, 22, 11, 5, and 4 compounds with OAVs ≥ 1 were considered to be the most powerful odorants in corresponding distillation cuts, respectively. PCA and PLS-DA models were proven to efficiently reveal five categories of distillation cuts within varieties tested. The heart1 fraction was characterized by 3-methylbutanol, ethyl hexanoate, 1-hexanol, ethyl octanoate, benzaldehyde, ethyl decanoate, and 2-phenylethyl acetate; (E)-hex-3-en-1-ol, (Z)-hex-3-en-1-ol, and 2-phenylethyl acetate greatly contributed to the characteristics of the heart2 cut. Furthermore, as the head and stillage cuts contain respectable volatile compounds, precisely separation of the head and tail cuts from the heart cut could be used to recover these aroma components. Nevertheless, further investigations are required focusing on more brandy samples to discover common distillation patterns. This study provided more detailed knowledge on the distribution characteristic of aroma compounds in freshly distilled brandy, which will provide scientific and modern instructions for the production of high-quality brandy.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are grateful for the financial support of the Yantai City Science and Technology Plan Project Mission Statement (2020XCZX029) and the Start-Up Research Funding from Zhejiang Ocean University (14164060216065).
Contributor Information
Chao Yang, Email: yc52028@hotmail.com.
Hui Cao, Email: hui_cao0830@yahoo.com.
References
- Al-Dalali S., Zheng F.P., Sun B.G., Chen F. Characterization and comparison of aroma profiles and aroma-active compounds between traditional and modern Sichuan vinegars by molecular sensory science. Journal of Agricultural and Food Chemistry. 2020;68(18):5154–5167. doi: 10.1021/acs.jafc.0c00470. [DOI] [PubMed] [Google Scholar]
- Arrieta-Garay Y., Blanco P., López-Vázquez C., Rodríguez-Bencomo J.J., Pérez-Correa J.R., Lopez F., Orriols I. Effects of distillation system and yeast strain on the aroma profile of Albariño (Vitis vinifera L.) grape pomace spirits. Journal of Agricultural and Food Chemistry. 2014;62(43):10552–10560. doi: 10.1021/jf502919n. [DOI] [PubMed] [Google Scholar]
- Awad P., Athes V., Decloux M.E., Ferrari G., Snakkers G., Raguenaud P., Giampaoli P. Evolution of volatile compounds during the distillation of cognac spirit. Journal of Agricultural and Food Chemistry. 2017;65(35):7736–7748. doi: 10.1021/acs.jafc.7b02406. [DOI] [PubMed] [Google Scholar]
- Cao H., Chen X.Q., Jassbi A.R., Xiao J.B. Microbial biotransformation of bioactive flavonoids. Biotechnology Advances. 2015;33(1):214–223. doi: 10.1016/j.biotechadv.2014.10.012. [DOI] [PubMed] [Google Scholar]
- Chen S., Wang C., Qian M., Li Z., Xu Y. Characterization of the key aroma compounds in aged Chinese rice wine by comparative aroma extract dilution analysis, quantitative measurements, aroma recombination, and omission studies. Journal of Agricultural and Food Chemistry. 2019;67(17):4876–4884. doi: 10.1021/acs.jafc.9b01420. [DOI] [PubMed] [Google Scholar]
- Coldea T.E., Socaciu C., Mudura E., Socaci S.A., Ranga F., Pop C.R.…Pasqualone A. Volatile and phenolic profiles of traditional Romanian apple brandy after rapid ageing with different wood chips. Food Chemistry. 2020;320 doi: 10.1016/j.foodchem.2020.126643. [DOI] [PubMed] [Google Scholar]
- Dong W., Guo R.N., Sun X.T., Li H.H., Zhao M.M., Zheng F.P.…Wu J.H. Assessment of phthalate ester residues and distribution patterns in Baijiu raw materials and Baijiu. Food Chemistry. 2019;283:508–516. doi: 10.1016/j.foodchem.2019.01.069. [DOI] [PubMed] [Google Scholar]
- Fritsch H.T., Schieberle P. Identification based on quantitative measurements and aroma recombination of the character impact odorants in a Bavarian Pilsner-type beer. Journal of Agricultural and Food Chemistry. 2005;53(19):7544–7551. doi: 10.1021/jf051167k. [DOI] [PubMed] [Google Scholar]
- Gao W.J., Fan W.L., Xu Y. Characterization of the key odorants in light aroma type Chinese liquor by gas chromatography–olfactometry, quantitative measurements, aroma recombination, and omission studies. Journal of Agricultural and Food Chemistry. 2014;62(25):5796–5804. doi: 10.1021/jf501214c. [DOI] [PubMed] [Google Scholar]
- He W.J., Liu S.X., Heponiemi P., Heinonen M., Marsol-Vall A., Ma X.Y.…Laaksonen O. Effect of Saccharomyces cerevisiae and Schizosaccharomyces pombe strains on chemical composition and sensory quality of ciders made from Finnish apple cultivars. Food Chemistry. 2020;345 doi: 10.1016/j.foodchem.2020.128833. [DOI] [PubMed] [Google Scholar]
- ISO 8586-1. (1993). Sensory analysis-general guidance for selection, training and monitoring of assessors. Ref no. ISO 8586-1:1993. The international Organization for Standardization, GeneÁve.
- Jandrić Z., Cannavan A. An investigative study on differentiation of citrus fruit/fruit juices by UPLC-QToF MS and chemometrics. Food Control. 2017;72:173–180. [Google Scholar]
- Jeleń H.H., Majcher M., Szwengiel A. Key odorants in peated malt whisky and its differentiation from other whisky types using profiling of flavor and volatile compounds. LWT-Food Science and Technology. 2019;107:56–63. [Google Scholar]
- Khvalbota L., Machyňáková A., Čuchorová J., Furdíková K., Špánik I. Enantiomer composition of chiral compounds present in traditional Slovak Tokaj wines. Journal of Food Composition and Analysis. 2021;96 [Google Scholar]
- Li H.H., Qin D., Wu Z.Y., Sun B.G., Sun X.T., Huang M.Q.…Zheng F.P. Characterization of key aroma compounds in Chinese Guojing sesame-flavor Baijiu by means of molecular sensory science. Food Chemistry. 2019;284:100–107. doi: 10.1016/j.foodchem.2019.01.102. [DOI] [PubMed] [Google Scholar]
- Li J., Zhang J., Yang Y., Zhu J., He W., Zhao Q.…Zhang J. Comparative characterization of lipids and volatile compounds of Beijing Heiliu and Laiwu Chinese black pork as markers. Food Research International. 2021;146 doi: 10.1016/j.foodres.2021.110433. [DOI] [PubMed] [Google Scholar]
- Ling M.Q., Xie H., Hua Y.B., Cai J., Li S.Y., Lan Y.B.…Shi Y. Flavor profile evolution of bottle aged rosé and white wines sealed with different closures. Molecules. 2019;24(5):836. doi: 10.3390/molecules24050836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfondet N., Gourrat K., Brunerie P., Le-Quéré J.L. Aroma characterization of freshly-distilled French brandies; their specificity and variability within a limited geographic area. Flavour and Fragrance Journal. 2016;31(5):361–376. [Google Scholar]
- Matijašević S., Popović-Djordjević J., Ristić R., Ćirković D., Ćirković B., Popović T. Volatile aroma compounds of brandy ‘Lozovaa’ produced from muscat table grapevine cultivars (Vitis vinifera L.) Molecules. 2019;24(13):2485. doi: 10.3390/molecules24132485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Y.W., Kong J.L., Xiao Z.B., Chen F., Ma N., Zhu J.C. Characterization of odor-active compounds of various Chinese “Wuliangye” liquors by gas chromatography–olfactometry, gas chromatography–mass spectrometry and sensory evaluation. International Journal of Food Properties. 2017;20(sup1):S735–S745. [Google Scholar]
- Niu Y.W., Wang P.P., Xiao Z.B., Zhu J.C., Sun X.X., Wang R.L. Evaluation of the perceptual interaction among ester aroma compounds in cherry wines by GC–MS, GC-O, odor threshold and sensory analysis: An insight at the molecular level. Food Chemistry. 2019;275:143–153. doi: 10.1016/j.foodchem.2018.09.102. [DOI] [PubMed] [Google Scholar]
- Pang X.L., Zhang Y.Z., Qiu J., Cao J.M., Sun Y.Q., Li H.H., Kong F.Y. Coupled multidimensional GC and odor activity value calculation to identify off-odors in thermally processed muskmelon juice. Food Chemistry. 2019;301 doi: 10.1016/j.foodchem.2019.125307. [DOI] [PubMed] [Google Scholar]
- Poisson L., Schieberle P. Characterization of the key aroma compounds in an American Bourbon whisky by quantitative measurements, aroma recombination, and omission studies. Journal of Agricultural and Food Chemistry. 2008;56(14):5820–5826. doi: 10.1021/jf800383v. [DOI] [PubMed] [Google Scholar]
- Pu D.D., Zhang Y.Y., Zhang H.Y., Sun B.G., Ren F.Z., Chen H.T., Tang Y. Characterization of the key aroma compounds in traditional hunan smoke-cured pork leg (Larou, THSL) by aroma extract dilution analysis (AEDA), odor activity value (OAV), and sensory evaluation experiments. Foods. 2020;9(4):413. doi: 10.3390/foods9040413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez R., Rodríguez-Nogales J.M., Fernández-Fernández E., González M.R., Medina-Trujillo L., Martín P. Volatile composition and sensory properties of wines from vineyards affected by iron chlorosis. Food Chemistry. 2022;369 doi: 10.1016/j.foodchem.2021.130850. [DOI] [PubMed] [Google Scholar]
- Schaller T., Schieberle P. Quantitation of key aroma compounds in fresh, raw ginger (Zingiber officinale Roscoe) from China and roasted ginger by stable isotope dilution assays and aroma profiling by recombination experiments. Journal of Agricultural and Food Chemistry. 2020;68(51):15284–15291. doi: 10.1021/acs.jafc.0c06733. [DOI] [PubMed] [Google Scholar]
- Thibaud F., Courregelongue M., Darriet P. Contribution of volatile odorous terpenoid compounds to aged cognac spirits aroma in a context of multicomponent odor mixtures. Journal of Agricultural and Food Chemistry. 2020;68(47):13310–13318. doi: 10.1021/acs.jafc.9b06656. [DOI] [PubMed] [Google Scholar]
- Tian T.T., Sun J.Y., Wu D.H., Xiao J.B., Lu J. Objective measures of greengage wine quality: From taste-active compound and aroma-active compound to sensory profiles. Food Chemistry. 2021;340 doi: 10.1016/j.foodchem.2020.128179. [DOI] [PubMed] [Google Scholar]
- Tian T.T., Yang H., Yang F., Li B.W., Sun J.Y., Wu D.H., Lu J. Optimization of fermentation conditions and comparison of flavor compounds for three fermented greengage wines. LWT-Food Science and Technology. 2018;89:542–550. [Google Scholar]
- Varela C., Barker A., Tran T., Borneman A., Curtin C. Sensory profile and volatile aroma composition of reduced alcohol Merlot wines fermented with Metschnikowia pulcherrima and Saccharomyces uvarum. International Journal of Food Microbiology. 2017;252:1–9. doi: 10.1016/j.ijfoodmicro.2017.04.002. [DOI] [PubMed] [Google Scholar]
- Wang X.J., Guo M.Y., Song H.L., Meng Q., Guan X.S. Characterization of key odor-active compounds in commercial high-salt liquid-state soy sauce by switchable GC/GC×GC-olfactometry-MS and sensory evaluation. Food Chemistry. 2020;342 doi: 10.1016/j.foodchem.2020.128224. [DOI] [PubMed] [Google Scholar]
- Wei S.H., Xiao X.M., Wei L.J., Li L.S., Li G.C., Liu F.H.…Zhong Y. Development and comprehensive HS-SPME/GC–MS analysis optimization, comparison, and evaluation of different cabbage cultivars (Brassica oleracea L. var. capitata L.) volatile components. Food Chemistry. 2021;340 doi: 10.1016/j.foodchem.2020.128166. [DOI] [PubMed] [Google Scholar]
- Willner B., Granvogl M., Schieberle P. Characterization of the key aroma compounds in Bartlett pear brandies by means of the sensomics concept. Journal of Agricultural and Food Chemistry. 2013;61(40):9583–9593. doi: 10.1021/jf403024t. [DOI] [PubMed] [Google Scholar]
- Xia Y.N., Liu Y.Q., Wang J., Shuang Q. Assessment of key aroma compounds in fresh jujube brandy by GC‐O‐MS and odor activity value. Journal of Food Processing and Preservation. 2020;44(7) e14494. [Google Scholar]
- Xiang X.F., Lan Y.B., Gao X.T., Xie H., An Z.Y., Lv Z.H.…Wu G.F. Characterization of odor-active compounds in the head, heart, and tail fractions of freshly distilled spirit from Spine grape (Vitis davidii Foex) wine by gas chromatography-olfactometry and gas chromatography-mass spectrometry. Food Research International. 2020;137 doi: 10.1016/j.foodres.2020.109388. [DOI] [PubMed] [Google Scholar]
- Zhang H.Y., Pu D.D., Sun B.G., Ren F.Z., Zhang Y.Y., Chen H.T. Characterization and comparison of key aroma compounds in raw and dry porcini mushroom (Boletus edulis) by aroma extract dilution analysis, quantitation and aroma recombination experiments. Food Chemistry. 2018;258:260–268. doi: 10.1016/j.foodchem.2018.03.056. [DOI] [PubMed] [Google Scholar]
- Zhao G.Z., Ding L.L., Hadiatullah H., Li S., Wang X.W., Yao Y.P.…Jiang S.P. Characterization of the typical fragrant compounds in traditional Chinese-type soy sauce. Food Chemistry. 2020;312 doi: 10.1016/j.foodchem.2019.126054. [DOI] [PubMed] [Google Scholar]
- Zhao Y.P., Tian T.T., Li J.M., Zhang B.C., Yu Y., Wang Y.Y., Niu H. Variations in main flavor compounds of freshly distilled brandy during the second distillation. International Journal of Food Engineering. 2014;8(4):809–820. [Google Scholar]
- Zhao Y.P., Zheng X.P., Song P., Sun Z.L., Tian T.T. Characterization of volatiles in the six most well-known distilled spirits. Journal of The American Chemical Society. 2013;71(3):161–169. [Google Scholar]
- Zierer B., Schieberle P., Granvogl M. Aroma-active compounds in bartlett pears and their changes during the manufacturing process of bartlett pear brandy. Journal of Agricultural and Food Chemistry. 2016;64(50):9515–9522. doi: 10.1021/acs.jafc.6b04612. [DOI] [PubMed] [Google Scholar]