Abstract
Jiaogulan (Gynostemma pentaphyllum) is a traditional Chinese medicinal herb that has been widely used in food and supplemental products. In the last 20 years, extensive research has been conducted to investigate the medicinal prospects of jiaogulan, and in this regard, more than 200 compounds have been isolated with various medicinal properties such as anticancer, anti‐obesity, anti‐inflammation, and antioxidation. In respect of potential benefits, jiaogulan market is likely growing, and various food items comprised of jiaogulan (beverage, sport drinks, cola, beer, tea, bread, and noodles) have been commercialized in the United States of America, China, and other Asian countries. More recently, there has been growing interest in the prebiotic potential of jiaogulan, especially at the interface of the gut microbiota. This review focuses on the prebiotic and therapeutic aspects of saponins and polysaccharides of jiaogulan tea by summarizing the literature on cancer, obesity, antioxidant activity, and immune‐modulatory properties.
Keywords: gut microbiota, Gynostemma pentaphyllum (jiaogulan), polysaccharides, saponins
This study summarizes the therapeutic and prebiotic properties of various saponins and polysaccharides from jiaogulan. The study also highlights gut microbiota (GM)‐modulating properties of various compounds from jiaogulan. This review highlights the therapeutic effect of jiaogulan on the diversity and composition of GM.

1. INTRODUCTION
Gynostemma pentaphyllum Makino (Cucurbitaceae; Gp) is a perennial creeping plant and has been used for herbal tea (called jiaogulan) in China. For centuries, the herbal tea made from the aerial part (including stems and leaves) of Gp has been consumed in China as a general tonic. Today, it is progressively popularizing around the world for lowering serum lipid and cholesterol levels (Chen et al., 1991; Lin et al., 2000). Like green tea, jiaogulan tea also holds anticarcinogenic and antioxidative activities (Lin et al., 2000; Razmovski‐Naumovski et al., 2005). Increasing research interest in Gp is evident from a search of the PubMed database (Figure 1).
FIGURE 1.

Bar chart presentation of Gynostemma pentaphyllum reported in PubMed database. These data were generated by including clinical trials, research articles, review articles, and abstracts. Numbers at the top of the bar show G. pentaphyllum reported times per year, ranging from 2000 to 2021. The search was conducted on August 4, 2021
Traditionally, jiaogulan has been broadly applied for the treatment of various illnesses, including hepatitis, diabetes, and cardiovascular disease (Li et al., 2019). However, in the last 20 years, extensive research has been conducted to investigate the medicinal prospects of jiaogulan, which has resulted in the discovery of more than 230 compounds with medicinal properties. These compounds have shown a variety of pharmacological properties, including anti‐inflammatory (Cai et al., 2016; Quan & Qian, 2010), antioxidative (Zhao et al., 2014), antiproliferative (Yan, Wang, Niu, et al., 2014), anxiolytic activities (Choi et al., 2013), anti‐cancer (Hou et al., 1991), lipid metabolism regulation (Qin et al., 2012), anti‐diabetes (Gao et al., 2016), and cardiovascular disease treatment (Circosta et al., 2005). Out of 230 compounds, 189 are saponins, also known as gypenosides (Li et al., 2016). Among these gypenosides, 165 have been classified into 12 types based on the nature of aglycone moiety (Lin, 2011). The details of these gypenosides and their pharmacological properties have been discussed elsewhere (Nguyen et al., 2021). Gypenosides possess several therapeutic properties including anticancer and anti‐obesity (Lee et al., 2019; Lu et al., 2010).
Like other basic research, Chinese medicine research is entering into a new paradigm from one‐gene‐one‐phenotype model toward a much sophisticated and complex model, known as omics strategies, that is based on data‐driven untargeted management, diagnosis, and treatment (Cagan et al., 2013; Yoo et al., 2018). Chinese medicine is holistic in nature and it would be impractical to comprehend it with conventional research tools. Therefore, more recently, researchers have started to evaluate the prebiotic potential of jiaogulan at the interface of the gut microbiota (GM).
The pharmacological properties of jiaogulan at the interface of the GM should be evaluated as commensals that play an important role in human physiology. For instance, the human microbiome constitutes about 47% of our body by cell count and encodes 1000 times more genes than our own body genes (Institute for Genome Sciences, 2017; Knight et al., 2017). It is estimated that the human microbiome encodes 2–20 million genes that surpasses the ~20,000 human genes (Knight et al., 2017). These microbial genes are presented to the host for digestion, metabolism, and immune system maturation (Cani, 2009). Targeted intervention to remodel GM composition has shown encouraging results in disease prevention and treatment. Mounting lines of evidence indicate that various bioactive natural products, such as dietary fibers, phenolic compounds, and undigested carbohydrates, can upregulate beneficial intestinal microbes, improve gut homeostasis, and alleviate disease symptoms (Makki et al., 2018).
In this review, we highlight some key findings by evaluating jiaogulan extracts and the therapeutic nature of purified compounds (gypenosides, polysaccharides, and flavonoids) at the interface of the GM. The study particularly focuses on the anticancer, anti‐obesity, and antidiabetic properties of jiaogulan.
2. ANTICANCER PROPERTIES
Jiaogulan is known to possess potent anticancer abilities. So far, several mechanisms of action have been determined regarding the anticancer activities of jiaogulan, including antioxidant (Li et al., 2015), cell cycle arrest, apoptosis, prevention of invasion and metastasis (Yan, Wang, Niu, et al., 2014), and immunomodulating activities. For instance, G. pentaphyllum saponin (GpS) was reported for the anticancer properties by upregulating Prdx1 and Prdx2 expression and suppressing Ras, RAF/MEK/ERK/STAT, PI3K/AKT/mTOR signaling (Tai et al., 2016). Another in vitro study also showed that GpS revealed the anti‐proliferation effect by arresting cell cycle at the G0/G1 phase, and induced apoptosis of HepG2 cells via death receptor and mitochondrial pathway (Hussain et al., 2020). Yan, Wang, Wang et al. (2014) showed that GpS could significantly upregulate the intracellular ROS level, which induced cell toxicity, apoptosis, and mitochondrial damage in colorectal cancer cells. The anticancer abilities of jiaogulan, both direct and indirect, have been summarized in Figure 2.
FIGURE 2.
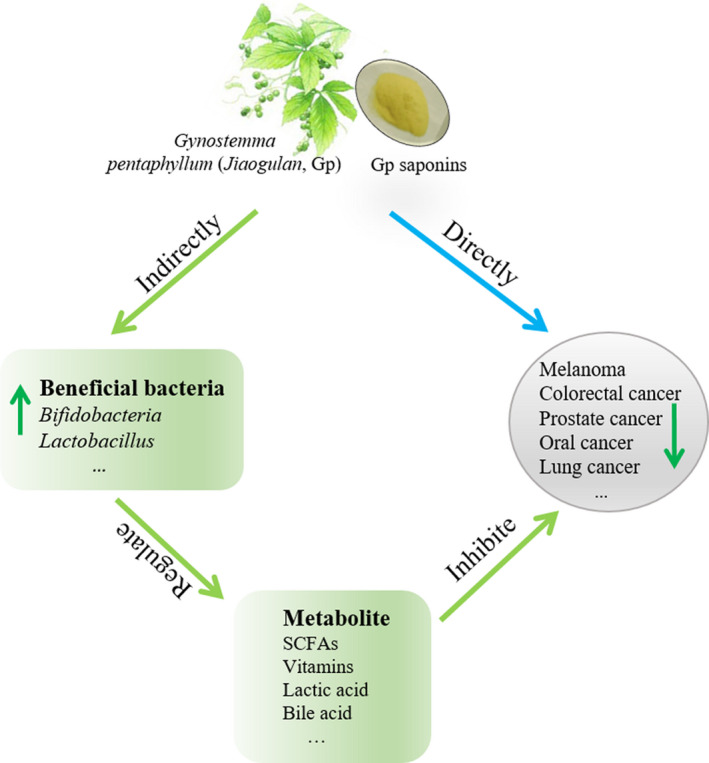
Thematic presentation of the jiaogulan tea's anticancer effects. Through literature review, it is observed that different components of the jiaogulan tea possess anticancer properties that these compounds exert either directly or indirectly. Through indirect approach, jiaogulan tea's component exerts anticancer effects through the interface of the gut microbiota. Here, we summarize that jiaogulan promotes the growth of beneficial bacteria, particularly the short‐chain fatty acid (SCFA) producers. SCFAs eventually exert anticancer properties
2.1. Anticancer properties of jiaogulan's saponin
Unlike the in vitro system, saponins are poorly absorbed and have a long residence time in the intestine when tested in preclinical models (Navarro del Hierro et al., 2018). However, through the recent integration of herbal medicine and GM research, the long residence of saponin in the intestine turned out to improve its efficacy. We and several other studies have demonstrated that GpS improves gut microbial composition by promoting the growth of beneficial bacteria and suppressing potential pathogens (Chen et al., 2015, 2016; Huang et al., 2017, 2018; Khan et al., 2019; Shen, Zhong et al., 2020). While evaluating the anticancer effects of GpS in a Apc Min/+ mouse model, GpS displayed a stimulating effect on the abundance of Lactococcus, Bifidobacterium, Lactobacillus, and short‐chain fatty acids (SCFAs) producing bacteria. However, the growth of potential pathogens, for example, Dysgonomonas spp., Helicobacter spp., sulfate‐reducing bacteria, were suppressed after GpS introduction to mouse gut (Chen et al., 2015, 2016; Huang et al., 2017; Khan et al., 2019; Liao et al., 2020) (Figure 3).
FIGURE 3.

Schematic presentation of GpS' anticancer effects through gut microbiota. (a) In colorectal cancer (CRC), the intestinal track is characterized by polyp formation, imbalanced gut microbiota, reduced mucus layer, suppressed population of goblet and Paneth cells, and inflamed immune milieu. (b) Treated CRC preclinical mouse model with GpS reinstates the inflamed mucosal immunity, promotes goblet and Paneth cell population – that results in mucus layer thickness and higher secretion of lysozyme. Most importantly, the gut microbial composition improves with the prevalence of SCFA producers. (c) At the subcellular level, GpS‐associated increase in SCFAs upregulates fatty acid‐sensing GPCRs that results in the suppression of histone deacetylases and PPARγ, which downstream inhibits PI3K/AKT oncogenic signaling pathways, as well STAT3 and Src. This graph is based on results published by Hsiao's group (Chen et al., 2016; Khan et al., 2019; Liao et al., 2020)
After noticing the stimulating effect of GpS in SCFAs producer in the mouse gut, we proved that GpS could increase the growth of Bifidobacterium animalis, Lactobacillus casei, and Lactobacillus reuteri (Liao et al., 2020). By gavaging B. animalis and butyrate (separately) to a cancer preclinical mouse model and noticing anticancer effects, we proved that the anticancer effect of GpS is partly through stimulating the growth of beneficial bacteria in the gut (Liao et al., 2020). The anticancer efficacy of GpS could be improved in the presence of polysaccharides. We confirmed the enhanced cancer‐preventive properties of GpS when applied in combination with Ganoderma lucidum (Lingzhi) polysaccharides. It was observed that GpS and polysaccharides from lucidum can greatly improve the inflamed gut barrier of Apc Min/+ mice by inhibiting polyp formation, changing colonic M1 to M2 macrophages, stopping the oncogenic signaling molecules, and increasing the E‐cadherin/N‐cadherin ratio (Khan et al., 2019).
2.2. Anticancer properties of jiaogulan's polysaccharides
In addition to the GpS, GP's polysaccharides (GpP) also be reported for anticancer properties. Polysaccharides are potential prebiotic polymers that have been extensively studied. They promote the growth of certain beneficial bacteria (e.g., Bifidobacterium, Lactobacillus) and in the large intestine, metabolize into lactic acid and SCFAs that improve host physiology, particularly gastrointestinal health (Azmi et al., 2012; Devillé et al., 2007; Zaporozhets et al., 2014). Accumulating evidence reports that GpP revealed the anticancer effect in vivo and in vitro. The molecular weight, degree of branching, and solubility of GpP are closely related to its anticancer property. Different molecular weights in the range of 103–106 Da have been found in various GpP using different experimental conditions (Ji et al., 2018). A neutral polysaccharide fraction of GP was found to effectively inhibit the solid tumor growth of H22 hepatocarcinoma transplanted in ICR mice (Liu et al., 2014). Another study showed that GpP improved the proportion and mitochondrial level of T cells, and promoted the secretion of IL‐2 and IFN‐γ in the ascites of mice (He et al., 2020). Yu et al. (2020) reported that a novel acidic polysaccharide from GP exhibited significant apoptotic characteristics, such as cell shrinkage, decreased cell adherence, and the appearance of apoptotic bodies in SPC‐A‐1 and MGC‐803 cells. Chen et al. (2011) isolated a novel GpP and synthesized four sulfated polysaccharides from this GpP using the chlorosulfonic acid method. The results showed that the polysaccharides significantly inhibited the growth of HepG2 cells and Hela cells.
2.3. Anticancer properties of jiaogulan's flavonoid
Flavonoid is a main polyphenolic compound that is widely found in herbal medicines. Flavonoid is also a major constituent of GP, and reveals certain bioactivities, especially anticancer and antioxidant effects. It has been established the flavonoid from GP (GpF) and GpS could equally suppress the growth of prostate cancer PC‐3 cells, with IC50 values of 39.3 and 33.3 μg/ml, respectively. These two GP fractions induced cell cycle arrest at both S and G2/M phases as well as apoptosis (Cheng et al., 2011). Another research reported that GpF induced apoptosis and concomitantly altered the balance of BCL‐2 and BAX expression as well as caspase‐3 expression in both A549 and H469 lung cancer cell lines. However, the authors found that GpF induced cell cycle arrest at both S and G2/M phases and regulated cellular proteins cyclin A, B, p53, and p21 expression in A549, but not H460 (Tsui et al., 2014). Lin et al. (2019) isolated four flavonoids from GP using chromatography and found that the flavonoids could act against AAPH‐induced oxidative damage in LLC‐PK1 cells by suppressing the increase in MDA, and the decrease in SOD and glutathione. Wang, Yang, et al. (2018) showed that GpF exerted antioxidant effect on A549 with H₂O₂‐induced oxidative stress through increasing SOD, GSH, and HO‐1 expression and simultaneously decreasing ROS and MDA expression. Jang et al. (2016) isolated eight flavonoids from GP, including a novel compound, and evaluated the antioxidative effect by the DPPH radical scavenging assay. The results showed that rutin possessed the strongest antioxidative property.
3. HEPATOPROTECTIVE EFFECT
Lipopolysaccharide (LPS) and Toll‐like receptor 4 (TLR4) are significantly increased during the progression of nonalcoholic fatty liver disease (NAFLD). A report showed that GpS improved NAFLD induced by high‐fat diet induced through regulating LPS/TLR4 signaling pathway (Shen, Wang et al., 2020). Hong et al. investigated the underlying mechanisms of GP and its derived compounds on protecting NAFLD through network pharmacology prediction. The authors claimed that GpS, especially gypenoside XL, could target peroxisome proliferator‐activated receptor alpha (PPARα), the expression of which was downregulated in alcoholic fatty liver disease and NAFLD patients in the liver. Further research proved that gypenoside XL could upregulate the expression of acyl‐CoA oxidase and carnitine palmitoyltransferase‐1, which contributed to the anti‐NAFLD effect (Hong et al., 2018). Other related reports showed that GpS protected against NAFLD progression by upregulating the expression of PPARα and downregulating the inflammatory cytokines, oxidative stress indices, and de novo lipogenesis (Gou et al., 2016; He et al., 2015; Qin et al., 2012). Bae et al. (2018) reported that gypenoside UL4 enriched in GP extract exerted the hepatoprotective effect on diet‐induced NAFLD through increasing levels of sirtuin 6 and phase 2 antioxidant enzymes in vivo and in vitro. A recent study showed that GpS could change the GM composition of NAFLD mice to alleviate disease progression. The results showed that GpS reduced the ratio of Firmicutes to Bacteroidetes, elevated GM diversity, and decreased the relative abundance of Fissicatena and Akkermansia, which are enriched in high‐fat and high‐cholesterol‐induced NAFLD mice (Huang et al., 2019). Similar research also showed that GpS alleviated NAFLD by maintaining the gut barrier and reversing gut dysbiosis in a high‐fat diet‐induced NAFLD rat model. Results showed that GpS reduced the ratio of Firmicutes to Bacteroidetes; meanwhile, GpS enriched the abundance of beneficial bacteria (Lactococcus spp.) and inhibited potential pathogens (Shen, Zhong, et al., 2020).
4. ANTI‐OBESITY EFFECT
AMPK is an intracellular energy sensor and regulates the whole‐body and cellular energy balance in response to energy demand and supply. Nguyen et al. (2011) demonstrated that dammarane‐type glucosides from GP were the potential activator of AMPK. Further study from this research team also showed that GP enriched with saponins could improve obesity in ob/ob mice by activating AMPK (Gauhar et al., 2012). Lee et al. demonstrated that GP extract enriched with gypenosides could reduce serum levels of triglyceride, total cholesterol, and LDL‐cholesterol and display the anti‐obesity effect in HFD‐induced obesity. The research mechanism showed that GpS could increase AMPK activation and suppress adipogenesis by decreasing CCAAT/enhancer‐binding protein‐α (C/EBPα), PPARγ, sterol regulatory element‐binding protein‐1c (SREBP1c), PPARγ coactivator‐1α, fatty acid synthase, adipocyte protein 2, and sirtuin 1 (Lee et al., 2019). Inhibiting pancreatic lipase activity is considered one of the treatment strategies for obesity. Previous reports showed that GpS could inhibit pancreatic lipase activity and possibly possess anti‐obesity effect (Bai et al., 2010; Su et al., 2016). Liu et al. found that GpS significantly reduced body weight, plasma total cholesterol, and homeostasis model assessment‐estimated insulin resistance index in a HDF‐induced obese mice model. The authors also showed that GpS could increase brown adipocyte tissue activity and white adipose tissue browning. The gene expression involved in mitochondrial activity and fatty acid β‐oxidation were also increased in both brown and white adipocyte tissues. Moreover, GpS decreased the ratio of Firmicutes to Bacteroidetes, and increased Akkermansia muciniphila abundance in the GM (Liu et al., 2017).
5. ANTIDIABETIC EFFECT
Gynostemma pentaphyllum saponin has been reported for hypoglycemic properties by enhancing the Nrf2 signaling pathway in streptozotocin‐induced diabetic rats (Gao et al., 2016). GP containing standardized gypenosides significantly elevated the plasma insulin concentration and profoundly affected the intraperitoneal insulin tolerance test compared with the control group (Wang, Ha, et al., 2018). In the streptozotocin‐induced diabetic rat model, GpS showed the hypoglycemic effect through enhancing the Nrf2 signaling pathway. Results also showed that GpS increased the level of insulin in the blood, as well as increased SOD and GSH‐px activities (Gao et al., 2016). Norberg et al. (2004) isolated a novel gypenoside from GpS, which was named phanoside, and the results showed that phanoside and its stereoisomers could significantly stimulate insulin secretion. Wang, Ha, et al. (2018) found that two compounds from gypenosides could significantly enhance 2‐deoxy‐2‐[(7‐nitro‐2,1,3‐benzoxadiazol‐4‐yl)amino]‐D‐glucose (2‐NBDG) uptake and glucose transporter 4 (GLUT4) translocation via activating the AMPK and acetyl‐CoA carboxylase signaling pathway. Recently, in a research profiling and screening the active compounds of GP in the diabetic rat model, 27 dammarane‐type triterpenoids were characterized by mass spectrometry and NMR spectroscopy. One of these triterpenoids showed glucose‐dependent insulin secretion activity (Lundqvist et al., 2019). These studies provided the potential candidates for the development of antidiabetic agents of GpS.
Except for the mentioned effects, GpS also possesses other bioactive properties. Research showed that GpS possessed the anti‐fatigue property for exercise‐induced fatigue. GpS could extend the swimming time for the mice, effectively delay the lowering of glucose in the blood, and prevent the increase in lactate (Ding et al., 2010). Aktan et al. (2003) found that GpS could suppress NO synthesis in murine macrophages by inhibiting iNOS enzymatic activity and attenuating NF‐κB‐mediated iNOS protein expression. Tsang et al. (2019) reported that GpS induced melanogenesis and activated cAMP/PKA and Wnt/β‐catenin signaling pathways in both B16 and B16F10 cells. Yang et al. (2013) found that two new saponins from Gp could inhibit lipopolysaccharide (LPS)‐induced IL‐1β, IL‐6, and COX‐2 mRNA expression in RAW 264.7 which showed a prominently anti‐inflammatory effect.
6. ANTIOXIDANT EFFECT
Li et al. (2015) isolated three acid polysaccharides from GP—GPA1 (19.6 kDa), GPA2 (10.6 kDa), and GPA3 (6.7 kDa)—that displayed the antioxidant effect through scavenging 1,1‐diphenyl‐2‐picrylhydrazyl (DPPH) radical and hydroxyl radical, chelating ferrous ion, and reducing ferric ion. GpP increased the scavenging activity of DPPH, hydroxyl radical, superoxide anion, and ABTS radical in vitro (Li et al., 2015). Furthermore, an animal experiment showed that GpP could enhance SOD, CAT, GSH‐Px activities, and decrease MDA activity (Wang et al., 2020). Yu et al. (2020) isolated a novel acid polysaccharide from Gp, and antioxidant assays showed that this GpP could scavenge superoxide radical, ABTS, and DPPH radicals. Chi et al. (2012) showed that GpP significantly prolonged the exercise time to exhaustion of mice, and increased the glycogen level and antioxidant enzymatic activity in the skeletal muscle.
7. IMMUNOMODULATION EFFECT
Mounting evidence indicated that polysaccharides from Chinese herbal medicines usually act as an immunomodulator that provides benefits for the host (Chen et al., 2020; Gan et al., 2004; Khan et al., 2019; Xu et al., 2011; Zhao et al., 2010). GpP activated macrophage phagocytosis and NK cells, and exhibited activity on none or Con A/LPS‐stimulated splenocytes in C57BL/6 mice. GpP also increased CD4+ lymphocyte quantitation and the ratio of CD4+/CD8+, and increased IL‐2 secretion in serum and spleen in immunosuppressed mice (Shang et al., 2016). Ren et al. reported that the acid polysaccharide fraction from Gp could markedly promote the secretion of NO, TNF‐α, IL‐1β, and IL‐6 in murine macrophage RAW264.7. The authors claimed that MAPK, PI3K/Akt, and NF‐κB signaling pathways were involved in these GpP‐induced macrophage activations (Ren et al., 2019). The neutral polysaccharide fraction from GP could modulate the activity of NK cells and cytotoxic T lymphocytes besides increasing the secretion of IL‐2, TNF‐α, and IFN‐γ in tumor‐bearing mice (Liu et al., 2014).
The hepatoprotective effect of GpP was proved by decreasing serum ALT and AST levels, as well as the hepatocyte MDA content and hepatocyte necrosis in the liver‐injured animal model (Song et al., 2013; Zhang, 2013). GpP possessed hypoglycemic and hypolipidemic effects in a streptozotocin‐induced type 2 diabetes rat model (Du et al., 2011). Jia et al. (2015) investigated the neuroprotective effect of GpP and found that GpP could be effective against Aβ (25–35)‐induced neurotoxicity in PC12 cells by inhibiting oxidative stress and suppressing the mitochondrial apoptotic pathway (Jia et al., 2015). Moreover, an associated research also showed that treatment with GpP could markedly increase the exhaustive exercise time of mice through scavenging excessive ROS produced during the exercise regimen (Chi et al., 2012).
8. CONCLUSION
This study summarized the therapeutic and prebiotic properties of various saponins and polysaccharides from jiaogulan. The study also highlighted GM‐modulating properties of various compounds from jiaogulan. This review further highlighted the therapeutic effect of jiaogulan on the diversity and composition of the GM.
ACKNOWLEDEGMENT
We thanks all the authors' contribution to this work. Thanks for the suggestion from Prof. Li Qingnan from Shantou central hospital. Thanks for the foundation of The Hospital Incubation Project from Shantou Central Hospital (2020‐2022).
CONFLICT OF INTEREST
The authors declare no competing financial interest.
ETHICAL APPROVAL
Not applicable.
Huang, G. , Yasir, M. , Zheng, Y. , & Khan, I. (2022). Prebiotic properties of jiaogulan in the context of gut microbiome. Food Science & Nutrition, 10, 731–739. 10.1002/fsn3.2701
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article because no new data were created in this study.
REFERENCES
- Aktan, F. , Henness, S. , Roufogalis, B. D. , & Ammit, A. J. (2003). Gypenosides derived from Gynostemma pentaphyllum suppress NO synthesis in murine macrophages by inhibiting iNOS enzymatic activity and attenuating NF‐κB‐mediated iNOS protein expression. Nitric Oxide, 8, 235–242. 10.1016/S1089-8603(03)00032-6 [DOI] [PubMed] [Google Scholar]
- Azmi, A. F. M. N. , Mustafa, S. , Hashim, D. M. , & Manap, Y. A. (2012). Prebiotic activity of polysaccharides extracted from Gigantochloa levis (Buluh beting) shoots. Molecules, 17, 1635–1651. 10.3390/molecules17021635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae, U.‐J. , Park, E.‐O. , Park, J. , Jung, S.‐J. , Ham, H. , Yu, K.‐W. , Park, Y.‐J. , Chae, S.‐W. , & Park, B.‐H. (2018). Gypenoside UL4‐rich Gynostemma pentaphyllum extract exerts a hepatoprotective effect on diet‐induced nonalcoholic fatty liver disease. American Journal of Chinese Medicine, 46, 1315–1332. 10.1142/S0192415X18500696 [DOI] [PubMed] [Google Scholar]
- Bai, M.‐S. , Gao, J.‐M. , Fan, C. , Yang, S.‐X. , Zhang, G. , & Zheng, C.‐D. (2010). Bioactive dammarane‐type triterpenoids derived from the acid hydrolysate of Gynostemma pentaphyllum saponins. Food Chemistry, 119, 306–310. 10.1016/j.foodchem.2009.06.033 [DOI] [Google Scholar]
- Cagan, R. L. , Justice, M. J. , & Tidmarsh, G. F. (2013). Bridging the gap between basic and applied biology: Towards preclinical translation. Disease Models & Mechanisms, 6, 559–561. 10.1242/dmm.012450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, H. , Liang, Q. , & Ge, G. (2016). Gypenoside attenuates β amyloid‐induced inflammation in N9 microglial cells via SOCS1 signaling. Neural Plasticity, 2016, 6362707. 10.1155/2016/6362707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani, P. D. (2009). Gut microbiota and pregnancy, a matter of inner life. British Journal of Nutrition, 101, 1579–1580. 10.1017/S0007114508111485 [DOI] [PubMed] [Google Scholar]
- Chen, L. , Brar, M. S. , Leung, F. C. C. , & Hsiao, W. L. W. (2016). Triterpenoid herbal saponins enhance beneficial bacteria, decrease sulfate‐reducing bacteria, modulate inflammatory intestinal microenvironment and exert cancer preventive effects in ApcMin/+ mice. Oncotarget, 7, 31226–31242. 10.18632/oncotarget.8886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L. , Tai, W. C. S. S. , Brar, M. S. , Leung, F. C. C. C. , & Hsiao, W. L. W. W. (2015). Tumor grafting induces changes of gut microbiota in athymic nude mice in the presence and absence of medicinal Gynostemma saponins. PLoS One, 10, e0126807. 10.1371/journal.pone.0126807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, T. , Li, B. , Li, Y. , Zhao, C. , Shen, J. , & Zhang, H. (2011). Catalytic synthesis and antitumor activities of sulfated polysaccharide from Gynostemma pentaphyllum Makino. Carbohydrate Polymers, 83, 554–560. 10.1016/j.carbpol.2010.08.024 [DOI] [Google Scholar]
- Chen, X. , Li, M. , Li, D. , Luo, T. , Xie, Y. , Gao, L. , Zhang, Y. , Chen, S. , Li, S. , Huang, G. , Li, W. , Su, J. , & Lai, X. (2020). Ethanol extract of Pycnoporus sanguineus relieves the dextran sulfate sodium‐induced experimental colitis by suppressing helper T cell‐mediated inflammation via apoptosis induction. Biomedicine & Pharmacotherapy, 127, 110212. 10.1016/j.biopha.2020.110212 [DOI] [PubMed] [Google Scholar]
- Chen, Z. , Zhao, Y. , Ma, X. , Lu, R. , & Song, J. (1991). [Introduction and cultivation of Gynostemma pentaphyllum Mark. in Beijing]. Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China Journal of Chinese Materia Medica, 16, 208–211,254. [PubMed] [Google Scholar]
- Cheng, T. C. , Lu, J. F. , Wang, J. S. , Lin, L. J. , Kuo, H. I. , & Chen, B. H. (2011). Antiproliferation effect and apoptosis mechanism of prostate cancer cell PC‐3 by flavonoids and saponins prepared from Gynostemma pentaphyllum . Journal of Agriculture and Food Chemistry, 59(20), 11319–11329. 10.1021/jf2018758 [DOI] [PubMed] [Google Scholar]
- Chi, A. , Tang, L. , Zhang, J. , & Zhang, K. (2012). Chemical composition of three polysaccharides from Gynostemma pentaphyllum and their antioxidant activity in skeletal muscle of exercised mice. International Journal of Sport Nutrition and Exercise Metabolism, 22(6), 479–485. 10.1123/ijsnem.22.6.479 [DOI] [PubMed] [Google Scholar]
- Choi, H. S. , Zhao, T. T. , Shin, K. S. , Kim, S. H. , Hwang, B. Y. , Lee, C. K. , & Lee, M. K. (2013). Anxiolytic effects of herbal ethanol extract from Gynostemma pentaphyllum in mice after exposure to chronic stress. Molecules, 18, 4342–4356. 10.3390/molecules18044342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Circosta, C. , De Pasquale, R. , & Occhiuto, F. (2005). Cardiovascular effects of the aqueous extract of Gynostemma pentaphyllum Makino. Phytomedicine, 12, 638–643. 10.1016/j.phymed.2004.06.023 [DOI] [PubMed] [Google Scholar]
- Devillé, C. , Gharbi, M. , Dandrifosse, G. , & Peulen, O. (2007). Study on the effects of laminarin, a polysaccharide from seaweed, on gut characteristics. Journal of the Science of Food and Agriculture, 87, 1717–1725. 10.1002/jsfa.2901 [DOI] [Google Scholar]
- Ding, Y. J. , Tang, K. J. , Li, F. L. , & Hu, Q. L. (2010). Effects of gypenosides from Gynostemma pentaphyllum supplementation on exercise‐induced fatigue in mice. African Journal of Agricultural Research, 5, 707–711. 10.5897/AJAR10.002 [DOI] [Google Scholar]
- Du, X. , Hou, Y. , Tan, H. , Han, Y. , & Zhang, Y. (2011). Hypoglycemic activity of polysaccharide from Gynostemma pentaphyllum on type 2 diabetic rats and its mechanism. Science, Technology and Engineering, 11, 5754–5758. [Google Scholar]
- Gan, L. , Zhang, S. H. , Yang, X. L. , & Xu, H. B. (2004). Immunomodulation and antitumor activity by a polysaccharide‐protein complex from Lycium barbarum . International Immunopharmacology, 4, 563–569. 10.1016/j.intimp.2004.01.023 [DOI] [PubMed] [Google Scholar]
- Gao, D. , Zhao, M. , Qi, X. , Liu, Y. , Li, N. , Liu, Z. , & Bian, Y. (2016). Hypoglycemic effect of Gynostemma pentaphyllum saponins by enhancing the Nrf2 signaling pathway in STZ‐inducing diabetic rats. Archives of Pharmacal Research, 39, 221–230. 10.1007/s12272-014-0441-2 [DOI] [PubMed] [Google Scholar]
- Gauhar, R. , Hwang, S.‐L. , Jeong, S.‐S. , Kim, J.‐E. , Song, H. , Park, D. C. , Song, K.‐S. , Kim, T. Y. , Oh, W. K. , & Huh, T.‐L. (2012). Heat‐processed Gynostemma pentaphyllum extract improves obesity in ob/ob mice by activating AMP‐activated protein kinase. Biotechnology Letters, 34, 1607–1616. 10.1007/s10529-012-0944-1 [DOI] [PubMed] [Google Scholar]
- Gou, S.‐H. , Huang, H.‐F. , Chen, X.‐Y. , Liu, J. , He, M. , Ma, Y.‐Y. , Zhao, X.‐N. , Zhang, Y. , & Ni, J.‐M. (2016). Lipid‐lowering, hepatoprotective, and atheroprotective effects of the mixture Hong‐Qu and gypenosides in hyperlipidemia with NAFLD rats. Journal of the Chinese Medical Association, 79, 111–121. 10.1016/j.jcma.2015.09.002 [DOI] [PubMed] [Google Scholar]
- He, Q. , Li, J. K. , Li, F. , Li, R. G. , Zhan, G. Q. , Li, G. , Du, W. X. , & Tan, H. B. (2015). Mechanism of action of gypenosides on type 2 diabetes and non‐alcoholic fatty liver disease in rats. World Journal of Gastroenterology, 21, 2058–2066. 10.3748/wjg.v21.i7.2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, X. , Wang, Z. , Xiao, Y. , Zhou, L. , Ruan, Z. , Chen, X. , Hu, M. , Ma, F. , Zheng, M. , Su, X. , & Deng, X. (2020). Gynostemma pentaphyllum polysaccharide prevents the growth of h22 ascites tumour by enhancing immunity rather than cytotoxicity in mice. Food and Agricultural Immunology, 31, 367–378. 10.1080/09540105.2020.1730770 [DOI] [Google Scholar]
- Hong, M. , Cai, Z. , Song, L. , Liu, Y. , Wang, Q. , & Feng, X. (2018). Gynostemma pentaphyllum attenuates the progression of nonalcoholic fatty liver disease in mice: A biomedical investigation integrated with in silico assay. Evidence‐Based Complementary and Alternative Medicine, 2018, 1–13. 10.1155/2018/8384631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou, J. , Liu, S. , Ma, Z. , Lang, X. , Wang, J. , Wang, J. , & Liang, Z. (1991). Effects of Gynostemma pentaphyllum Makino on the immunological function of cancer patients. Journal of Traditional Chinese Medicine = Chung I Tsa Chih Ying Wen Pan, 11, 47–52. [PubMed] [Google Scholar]
- Huang, G. , Khan, I. , Li, X. , Chen, L. , Leong, W. , Ho, L. T. , & Hsiao, W. L. W. (2017). Ginsenosides Rb3 and Rd reduce polyps formation while reinstate the dysbiotic gut microbiota and the intestinal microenvironment in ApcMin/+ mice. Scientific Reports, 7, 12552. 10.1038/s41598-017-12644-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, J. , Lin, B. , Xu, Q. , Guan, X. , Qian, L. , & Wang, L. (2018). Effect of Gynostemma pentaphyllum tea on lipid metabolism and gut microbiota in hyperlipidemic rats. Journal of Chinese Institute of Food Science and Technology, 1, 47–52. 10.16429/j.1009-7848.2018.06.004 [DOI] [Google Scholar]
- Huang, X. , Chen, W. , Yan, C. , Yang, R. , Chen, Q. , Xu, H. , & Huang, Y. (2019). Gypenosides improve the intestinal microbiota of non‐alcoholic fatty liver in mice and alleviate its progression. Biomedicine & Pharmacotherapy, 118, 109258. 10.1016/j.biopha.2019.109258 [DOI] [PubMed] [Google Scholar]
- Hussain, S. S. , Zhang, F. , Zhang, Y. , Thakur, K. , Naudhani, M. , Cespedes‐Acuña, C. L. , & Wei, Z. (2020). Stevenleaf from Gynostemma Pentaphyllum inhibits human hepatoma cell (HepG2) through cell cycle arrest and apoptotic induction. Food Science and Human Wellness, 9, 295–303. 10.1016/j.fshw.2020.04.011 [DOI] [Google Scholar]
- Institute for Genome Sciences (2017). NIH human microbiome project. University of Maryland School of Medicine. 10.3348/kjr.2012.13.6.776 [DOI] [Google Scholar]
- Jang, H. , Lee, J. W. , Lee, C. , Jin, Q. , Lee, M. K. , Lee, C. K. , Lee, M. K. , & Hwang, B. Y. (2016). Flavonol glycosides from the aerial parts of Gynostemma pentaphyllum and their antioxidant activity. Archives of Pharmacal Research, 39(9), 1232–1236. 10.1007/s12272-016-0793-x [DOI] [PubMed] [Google Scholar]
- Ji, X. , Shen, Y. , & Guo, X. (2018). Isolation, structures, and bioactivities of the polysaccharides from Gynostemma pentaphyllum (Thunb.) Makino: A review. BioMed Research International, 2018, 1–14. 10.1155/2018/6285134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia, D. , Rao, C. , Xue, S. , & Lei, J. (2015). Purification, characterization and neuroprotective effects of a polysaccharide from Gynostemma pentaphyllum . Carbohydrate Polymers, 122, 93–100. 10.1016/j.carbpol.2014.12.032 [DOI] [PubMed] [Google Scholar]
- Khan, I. , Huang, G. , Li, X.‐A. , Liao, W. , Leong, W. K. , Xia, W. , Bian, X. , Wu, J. , & Hsiao, W. L. W. (2019). Mushroom polysaccharides and jiaogulan saponins exert cancer preventive effects by shaping the gut microbiota and microenvironment in Apc(Min/+) mice. Pharmacological Research, 148, 104448. 10.1016/j.phrs.2019.104448 [DOI] [PubMed] [Google Scholar]
- Knight, R. , Callewaert, C. , Marotz, C. , Hyde, E. R. , Debelius, J. W. , McDonald, D. , & Sogin, M. L. (2017). The microbiome and human biology. Annual Review of Genomics and Human Genetics, 18, 65–86. 10.1146/annurev-genom-083115-022438 [DOI] [PubMed] [Google Scholar]
- Lee, H. S. , Lim, S.‐M. , Jung, J. I. , Kim, S. M. , Lee, J. K. , Kim, Y. H. , Cha, K. M. , Oh, T. K. , Moon, J. M. , Kim, T. Y. , & Kim, E. J. (2019). Gynostemma pentaphyllum extract ameliorates high‐fat diet‐induced obesity in C57BL/6N mice by upregulating SIRT1. Nutrients, 11, 2475. 10.3390/nu11102475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, B. , Zhang, X. , Wang, M. , & Jiao, L. (2015). Characterization and antioxidant activities of acidic polysaccharides from Gynostemma pentaphyllum (Thunb.) Markino. Carbohydrate Polymers, 127, 209–214. 10.1016/j.carbpol.2015.03.069 [DOI] [PubMed] [Google Scholar]
- Li, K. , Ma, C. , Li, H. , Dev, S. , He, J. , & Qu, X. (2019). Medicinal value and potential therapeutic mechanisms of Gynostemma pentaphyllum (Thunb.) Makino and its derivatives: An overview. Current Topics in Medicinal Chemistry, 19, 2855–2867. 10.2174/1568026619666191114104718 [DOI] [PubMed] [Google Scholar]
- Li, Y. , Lin, W. , Huang, J. , Xie, Y. , & Ma, W. (2016). Anti‐cancer effects of Gynostemma pentaphyllum (Thunb.) Makino (Jiaogulan). Chinese Medicine, 11, 1–16. 10.1186/s13020-016-0114-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao, W. , Khan, I. , Huang, G. , Chen, S. , Liu, L. , Leong, W. K. , Li, X. A. , Wu, J. , & Wendy Hsiao, W. L. (2020). Bifidobacterium animalis: The missing link for the cancer‐preventive effect of Gynostemma pentaphyllum . Gut Microbes, 13, 1–14. 10.1080/19490976.2020.1847629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, C. C. , Huang, P. C. , & Lin, J. M. (2000). Antioxidant and hepatoprotective effects of Anoectochilus formosanus and Gynostemma pentaphyllum . American Journal of Chinese Medicine, 28, 87–96. [DOI] [PubMed] [Google Scholar]
- Lin, M. , Wang, Y. , Zhai, X. , Xing, S. , & Piao, X. (2019). Protective effects of flavonoids from Gynostemma pentaphyllum on oxidative damage in LLC‐PK1 cells. Zhongguo Zhongyao Zazhi, 44(6), 1193–1200. 10.19540/j.cnki.cjcmm.20181226.019 [DOI] [PubMed] [Google Scholar]
- Lin, S. (2011). Research advances on the saponins of Gynostemma pentaphyllum . Drug Evaluation Research, 34, 456–464. [Google Scholar]
- Liu, J. , Li, Y. , Yang, P. , Wan, J. , Chang, Q. , Wang, T. T. Y. , Lu, W. , Zhang, Y. , Wang, Q. , & Yu, L. L. (2017). Gypenosides reduced the risk of overweight and insulin resistance in C57BL/6J mice through modulating adipose thermogenesis and gut microbiota. Journal of Agriculture and Food Chemistry, 65, 9237–9246. 10.1021/acs.jafc.7b03382 [DOI] [PubMed] [Google Scholar]
- Liu, J. , Zhang, L. , Ren, Y. , Gao, Y. , Kang, L. , & Qiao, Q. (2014). Anticancer and immunoregulatory activity of Gynostemma pentaphyllum polysaccharides in H22 tumor‐bearing mice. International Journal of Biological Macromolecules, 69, 1–4. 10.1016/j.ijbiomac.2014.05.014 [DOI] [PubMed] [Google Scholar]
- Lu, K.‐W. , Chen, J.‐C. , Lai, T.‐Y. , Yang, J.‐S. , Weng, S.‐W. , Ma, Y.‐S. , Tang, N.‐Y. , Lu, P.‐J. , Weng, J.‐R. , & Chung, J.‐G. (2010). Gypenosides causes DNA damage and inhibits expression of DNA repair genes of human oral cancer SAS cells. In Vivo, 24, 287–291. [PubMed] [Google Scholar]
- Lundqvist, L. C. E. , Rattigan, D. , Ehtesham, E. , Demmou, C. , Östenson, C.‐G. , & Sandström, C. (2019). Profiling and activity screening of Dammarane‐type triterpen saponins from Gynostemma pentaphyllum with glucose‐dependent insulin secretory activity. Scientific Reports, 9, 627. 10.1038/s41598-018-37517-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makki, K. , Deehan, E. C. , Walter, J. , & Backhed, F. (2018). The impact of dietary fiber on gut microbiota in host health and disease. Cell Host & Microbe, 23, 705–715. 10.1016/j.chom.2018.05.012 [DOI] [PubMed] [Google Scholar]
- Navarro del Hierro, J. , Herrera, T. , Fornari, T. , Reglero, G. , & Martin, D. (2018). The gastrointestinal behavior of saponins and its significance for their bioavailability and bioactivities. Journal of Functional Foods, 40, 484–497. 10.1016/j.jff.2017.11.032 [DOI] [Google Scholar]
- Nguyen, N.‐H. , Ha, T. K. Q. , Yang, J.‐L. , Pham, H. T. T. , & Oh, W. K. (2021). Triterpenoids from the genus Gynostemma: Chemistry and pharmacological activities. Journal of Ethnopharmacology, 268, 113574. 10.1016/j.jep.2020.113574 [DOI] [PubMed] [Google Scholar]
- Nguyen, P. H. , Gauhar, R. , Hwang, S. L. , Dao, T. T. , Park, D. C. , Kim, J. E. , Song, H. , Huh, T. L. , & Oh, W. K. (2011). New dammarane‐type glucosides as potential activators of AMP‐activated protein kinase (AMPK) from Gynostemma pentaphyllum . Bioorganic & Medicinal Chemistry, 19, 6254–6260. 10.1016/j.bmc.2011.09.013 [DOI] [PubMed] [Google Scholar]
- Norberg, Å. , Hoa, N. K. , Liepinsh, E. , Van Phan, D. , Thuan, N. D. , Jörnvall, H. , Sillard, R. , & Östenson, C.‐G. (2004). A novel insulin‐releasing substance, phanoside, from the plant Gynostemma pentaphyllum . Journal of Biological Chemistry, 279, 41361–41367. 10.1074/jbc.M403435200 [DOI] [PubMed] [Google Scholar]
- Qin, R. , Zhang, J. , Li, C. , Zhang, X. , Xiong, A. , Huang, F. , Yin, Z. , Li, K. , Qin, W. , Chen, M. , Zhang, S. , Liang, L. , Zhang, H. , Nie, H. , & Ye, W. (2012). Protective effects of gypenosides against fatty liver disease induced by high fat and cholesterol diet and alcohol in rats. Archives of Pharmacal Research, 35, 1241–1250. 10.1007/s12272-012-0715-5 [DOI] [PubMed] [Google Scholar]
- Quan, Y. , & Qian, M. (2010). [Effect and mechanism of gypenoside on the inflammatory molecular expression in high‐fat induced atherosclerosis rats]. Zhongguo Zhong Xi Yi Jie He Za Zhi Zhongguo Zhongxiyi Jiehe Zazhi = Chinese Journal of Integrated Traditional and Western Medicine, 30, 403–406. [PubMed] [Google Scholar]
- Razmovski‐Naumovski, V. , Huang, T. H. W. , Tran, V. H. , Li, G. Q. , Duke, C. C. , & Roufogalis, B. D. (2005). Chemistry and pharmacology of Gynostemma pentaphyllum . Phytochemistry Reviews, 4(2‐3), 197–219. 10.1007/s11101-005-3754-4 [DOI] [Google Scholar]
- Ren, D. , Zhao, Y. , Zheng, Q. , Alim, A. , & Yang, X. (2019). Immunomodulatory effects of an acidic polysaccharide fraction from herbal: Gynostemma pentaphyllum tea in RAW264.7 cells. Food and Function, 10, 2186–2197. 10.1039/c9fo00219g [DOI] [PubMed] [Google Scholar]
- Shang, X. , Chao, Y. , Zhang, Y. , Lu, C. , Xu, C. , & Niu, W. (2016). Immunomodulatory and antioxidant effects of polysaccharides from Gynostemma pentaphyllum Makino in immunosuppressed mice. Molecules, 21(8), 1085. 10.3390/molecules21081085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, S. , Wang, K. , Zhi, Y. , Shen, W. , & Huang, L. (2020). Gypenosides improves nonalcoholic fatty liver disease induced by high‐fat diet induced through regulating LPS/TLR4 signaling pathway. Cell Cycle, 19, 3042–3053. 10.1080/15384101.2020.1829800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, S.‐H. , Zhong, T.‐Y. , Peng, C. , Fang, J. , & Lv, B. (2020). Structural modulation of gut microbiota during alleviation of non‐alcoholic fatty liver disease with Gynostemma pentaphyllum in rats. BMC Complementary Medicine and Therapies, 20, 34. 10.1186/s12906-020-2835-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, S. L. , Xiao, Z. P. , Liang, H. , Wang, Y. S. , & Ji, A. G. (2013). Protective effects of Gynostemma pentaphyllum Makino polysaccharide on alcoholic hepatic injuries. Advanced Materials Research, 781‐784, 668–673. 10.4028/www.scientific.net/AMR.781-784.668 [DOI] [Google Scholar]
- Su, J. , Wang, H. , Ma, C. , Liu, C. , Rahman, M. T. , Gao, C. , & Nie, R. (2016). Hypolipidemic mechanism of gypenosides via inhibition of pancreatic lipase and reduction in cholesterol micellar solubility. European Food Research and Technology, 242, 305–312. 10.1007/s00217-015-2540-9 [DOI] [Google Scholar]
- Tai, W.‐C.‐S. , Wong, W.‐Y. , Lee, M.‐M.‐L. , Chan, B. D. , Lu, C. , & Hsiao, W.‐L.‐W. (2016). Mechanistic study of the anti‐cancer effect of Gynostemma pentaphyllum saponins in the Apc Min/+ mouse model. Proteomics, 16, 1557–1569. 10.1002/pmic.201500293 [DOI] [PubMed] [Google Scholar]
- Tsang, T.‐F. , Chan, B. , Tai, W.‐C.‐S. , Huang, G. , Wang, J. , Li, X. , Jiang, Z. H. , & Hsiao, W. L. W. (2019). Gynostemma pentaphyllum saponins induce melanogenesis and activate cAMP/PKA and Wnt/β‐catenin signaling pathways. Phytomedicine, 60, 153008. 10.1016/j.phymed.2019.153008 [DOI] [PubMed] [Google Scholar]
- Tsui, K. C. , Chiang, T. H. , Wang, J. S. , Lin, L. J. , Chao, W. C. , Chen, B. H. , & Lu, J. F. (2014). Flavonoids from Gynostemma pentaphyllum exhibit differential induction of cell cycle arrest in H460 and A549 cancer cells. Molecules, 19(11), 17663–17681. 10.3390/molecules191117663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Ha, T. K. Q. , Shi, Y.‐P. , Oh, W. K. , & Yang, J.‐L. (2018). Hypoglycemic triterpenes from Gynostemma pentaphyllum . Phytochemistry, 155, 171–181. 10.1016/j.phytochem.2018.08.008 [DOI] [PubMed] [Google Scholar]
- Wang, Y. R. , Yang, K. , Cui, W. Y. , & Piao, X. L. (2018). Effects of flavonoids from Gynostemma pentaphyllum on A549 cells damaged by hydrogen peroxide. Zhongguo Zhongyao Zazhi, 41(8), 760–773. 10.19540/j.cnki.cjcmm.2018.0034 [DOI] [PubMed] [Google Scholar]
- Wang, Z. , Wang, Z. , Huang, W. , Suo, J. , Chen, X. , Ding, K. , Sun, Q. , & Zhang, H. (2020). Antioxidant and anti‐inflammatory activities of an anti‐diabetic polysaccharide extracted from Gynostemma pentaphyllum herb. International Journal of Biological Macromolecules, 145, 484–491. 10.1016/j.ijbiomac.2019.12.213 [DOI] [PubMed] [Google Scholar]
- Xu, Z. , Chen, X. , Zhong, Z. , Chen, L. , & Wang, Y. (2011). Ganoderma lucidum polysaccharides: Immunomodulation and potential anti‐tumor activities. American Journal of Chinese Medicine, 39, 15–27. 10.1142/S0192415X11008610 [DOI] [PubMed] [Google Scholar]
- Yan, H. , Wang, X. , Niu, J. , Wang, Y. , Wang, P. , & Liu, Q. (2014). Anti‐cancer effect and the underlying mechanisms of gypenosides on human colorectal cancer SW‐480 cells. PLoS One, 9, e95609. 10.1371/journal.pone.0095609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, H. , Wang, X. , Wang, Y. , Wang, P. , & Xiao, Y. (2014). Antiproliferation and anti‐migration induced by gypenosides in human colon cancer SW620 and esophageal cancer Eca‐109 cells. Human and Experimental Toxicology, 33, 522–533. 10.1177/0960327113497771 [DOI] [PubMed] [Google Scholar]
- Yang, F. , Shi, H. , Zhang, X. , Yang, H. , Zhou, Q. , & Yu, L. L. (2013). Two new saponins from tetraploid jiaogulan (Gynostemma pentaphyllum), and their anti‐inflammatory and α‐glucosidase inhibitory activities. Food Chemistry, 141, 3606–3613. 10.1016/j.foodchem.2013.06.015 [DOI] [PubMed] [Google Scholar]
- Yoo, B. C. , Kim, K.‐H. , Woo, S. M. , & Myung, J. K. (2018). Clinical multi‐omics strategies for the effective cancer management. Journal of Proteomics, 188, 97–106. 10.1016/j.jprot.2017.08.010 [DOI] [PubMed] [Google Scholar]
- Yu, S. , Yu, J. , Dong, X. , Li, S. , & Liu, A. (2020). Structural characteristics and anti‐tumor/‐oxidant activity in vitro of an acidic polysaccharide from Gynostemma pentaphyllum . International Journal of Biological Macromolecules, 161, 721–728. 10.1016/j.ijbiomac.2020.05.274 [DOI] [PubMed] [Google Scholar]
- Zaporozhets, T. S. , Besednova, N. N. , Kuznetsova, T. A. , Zvyagintseva, T. N. , Makarenkova, I. D. , Kryzhanovsky, S. P. , & Melnikov, V. G. (2014). The prebiotic potential of polysaccharides and extracts of seaweeds. Russian Journal of Marine Biology, 40, 1–9. 10.1134/S1063074014010106 [DOI] [Google Scholar]
- Zhang, C. (2013). Protective effect of Gynostemma pentaphyllum polysaccharide on liver injury by carbon tetrachloride in rats. Chinese Journal of Experimental Traditional Medical Formulae, 19, 244–247. [Google Scholar]
- Zhao, H. , Luo, Y. , Lu, C. , Lin, N. , Xiao, C. , Guan, S. , Guo, D. A. , Liu, Z. , Ju, D. , He, X. , & Lu, A. (2010). Enteric mucosal immune response might trigger the immunomodulation activity of Ganoderma lucidum polysaccharide in mice. Planta Medica, 76, 223–227. 10.1055/s-0029-1186055 [DOI] [PubMed] [Google Scholar]
- Zhao, J. , Ming, Y. , Wan, Q. , Ye, S. , Xie, S. , Zhu, Y. , Wang, Y. , Zhong, Z. , Li, L. , & Ye, Q. (2014). Gypenoside attenuates hepatic ischemia/reperfusion injury in mice via anti‐oxidative and anti‐apoptotic bioactivities. Experimental and Therapeutic Medicine, 7, 1388–1392. 10.3892/etm.2014.1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article because no new data were created in this study.


