Abstract
A 35-year-old Japanese woman with no history of hypertension developed hypertension 5 days after normal delivery. Endocrinological and radiological examinations indicated primary aldosteronism (PA) and a 1.4-cm left adrenal tumor. The patient underwent laparoscopic adrenalectomy, and a diagnosis of aldosterone-producing adenoma was confirmed immunohistochemically. Her plasma aldosterone concentration and blood pressure normalized. Cases of PA presenting with hypertension in the postpartum period have been reported. This case suggests that PA should be considered in women with postpartum hypertension, especially in those with blood pressure that suddenly increases shortly after delivery, even if they were normotensive before and throughout pregnancy.
Keywords: adrenalectomy, aldosterone-producing adenoma, blood pressure, hypertension, immunohistochemistry, postpartum
Introduction
Primary aldosteronism (PA) is an endocrine disorder characterized by autonomous hypersecretion of the mineralocorticoid aldosterone from adrenocortical lesions (1,2). Major causes of PA are bilateral adrenal hyperplasia and unilateral aldosterone-producing adenoma (APA). Clinical manifestations of PA include sodium and fluid retention and hypertension, with or without hypokalemia, associated with increased cardiovascular morbidity and mortality.
PA is one of the most common causes of secondary hypertension and can develop at any age in both sexes. Cases of pregnant women suffering from PA have been reported (3-7); many developed PA with hypertension before pregnancy, and others developed hypertension during pregnancy. In addition, several cases of PA that presented with postpartum hypertension have been reported (8-12).
We herein report an unusual case of a woman who developed hypertension five days after delivery and was diagnosed with PA. In addition, we review previously reported cases of women without a history of hypertension who developed hypertension during the postpartum period and were diagnosed with PA.
Case Report
A 35-year-old Japanese woman, gravida 2, para 2, presented with a 7-month duration of hypertension that developed five days after normal delivery. The patient had a family history of essential hypertension in her father. The patient's medical history was unremarkable. She had no history of obesity or hypertension. The patient had never smoked cigarettes and did not drink alcohol. She was normotensive and normokalemic during and after her first pregnancy in 2010 (at 29 years old). She became pregnant for the second time in July 2015. Her blood pressure (BP) measured at the regular pregnancy checkup was normal (approximately 124/70 mmHg). Blood tests showed normal serum levels of sodium, potassium, and chloride. At 35 years old, the patient had a full-term spontaneous vaginal delivery (39 weeks and 2 days of gestation) at the Department of Obstetrics and Gynecology of Uonuma Kikan Hospital in May 2016 and gave birth to a 3,186 g healthy girl with 1- and 5-min Apgar scores of 8 and 9, respectively. The patient experienced no labor or delivery complications, such as placental abnormality or excessive bleeding.
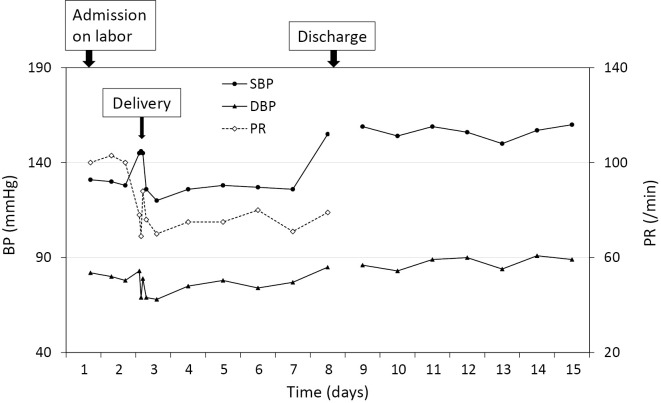
The patient's postpartum course was uneventful until 5 days after delivery, when her BP levels suddenly rose to 155/85 mmHg (Fig. 1). Because the patient had no symptoms of high BP such as headache or nausea, she was instructed to measure her BP at home every day and was discharged the same day.
Figure 1.
Serial changes in the blood pressure and pulse rate during the peripartum period (May 2016). During hospitalization (days 1 through 8), the systolic blood pressure (SBP), diastolic blood pressure (DBP), and pulse rate (PR) were measured in the morning with the patient in the supine position by nursing staff using an upper-arm-cuff device with the cuff-oscillometric method (ES-H55; Terumo, Tokyo, Japan). After discharge, the SBP and DBP were self-measured at home in the morning in a seated position using an upper-arm-cuff device with the cuff-oscillometric method (HEM-7121; Omron, Kyoto, Japan).
The patient's BP levels self-measured at home were high (approximately 155/87 mmHg; Fig. 1). Her BP levels measured at the doctor's office in June 2016 were also high (168/96 mmHg). She was diagnosed with hypertension (13) and started antihypertensive medication with oral nifedipine (20 mg/day) the same month.
To examine the possibility of secondary hypertension, a blood test was performed in November 2016 (Table 1), which showed a slightly low creatinine (0.44 mg/dL), normal electrolytes (sodium, 142 mEq/L; potassium, 4.0 mEq/L; chloride 103 mEq/L), and normal levels of adrenocorticotropic hormone (27.3 pg/mL) and cortisol (13.7 μg/dL). However, the plasma aldosterone concentration (PAC) was high (19.0 ng/dL), and the plasma renin activity (PRA) was low (<0.2 ng/mL/h), with a high PAC-to-PRA ratio (1,2). She was suspected of having PA and referred to the Department of Endocrinology and Metabolism of the same hospital in December 2016 for a further investigation and treatment.
Table 1.
Laboratory Findings (November 2016).
| Hematology | |||
| Red blood cells | 468×104 | /μL | |
| Hemoglobin | 13.8 | g/dL | (11.6-14.8) |
| Hematocrit | 42.0 | % | (35.1-44.4) |
| White blood cells | 6,100 | /μL | (3,300-8,600) |
| Platelets | 28.4×104 | /μL | (15.8-34.8) |
| Blood chemistry | |||
| Urea nitrogen | 13.2 | mg/dL | (8.0-18.4) |
| Creatinine | 0.44 | mg/dL | (0.46-0.79) |
| Sodium | 142 | mEq/L | (138-145) |
| Potassium | 4.0 | mEq/L | (3.6-4.8) |
| Chloride | 103 | mEq/L | (101-108) |
| Casual plasma glucose | 84 | mg/dL | (70-139) |
| Brain natriuretic hormone | 16.2 | pg/mL | (0-18.4) |
| Thyroid-stimulating hormone | 1.23 | μIU/mL | (0.50-5.00) |
| Free thyroxine | 1.18 | ng/dL | (0.90-1.70) |
| Free triiodothyronine | 3.33 | pg/mL | (2.30-4.00) |
| Adrenocorticotropic hormone | 27.3 | pg/mL | (7.2-63.3) |
| Cortisol | 13.7 | μg/dL | (4.0-18.3) |
| Dehydroepiandrosterone sulfate | 2,363 | ng/mL | (230-2,660) |
| Plasma renin activity | <0.2 | ng/mL/h | (0.2-2.3) |
| Aldosterone | 19.0 | ng/dL | (3.0-15.9) |
| Noradrenalin | 0.27 | ng/mL | (0.10-0.50) |
| Adrenalin | 0.08 | ng/mL | (0-0.10) |
| Dopamine | 0.01 | ng/mL | (0-0.03) |
| Urinalysis | |||
| Specific gravity | 1.017 | (1.005-1.020) | |
| Glucose | Negative | ||
| Protein | Negative | ||
| Occult blood | Negative |
Blood samples were taken in the morning with the patient in the supine position. The reference range for each parameter is shown in parentheses.
A physical examination at the time of referral showed that the patient's height, body weight, body temperature, BP, and pulse rate were 158 cm, 42.8 kg (body mass index, 17.1 kg/m2), 36.3°C, 159/86 mmHg, and 96 beats per minute, respectively. She did not complain of snoring, daytime sleeping, or muscle weakness. There were no chest rales, heart murmurs, abdominal tenderness or vascular bruits, or peripheral edema. The dorsalis pedis artery pulses were clearly palpable. No cushingoid features, such as a round face and thin skin, were found.
The patient had a positive captopril-challenge test (2); her PAC and PRA 90 minutes after the administration of oral captopril (50 mg) were 13.5 ng/dL and <0.2 ng/mL/h, respectively. She also had a positive saline-loading test (2); her PAC measured after an intravenous infusion of 0.9% saline (2 L) over 4 hours was 12.3 ng/dL. Based on these findings, the patient was diagnosed with PA.
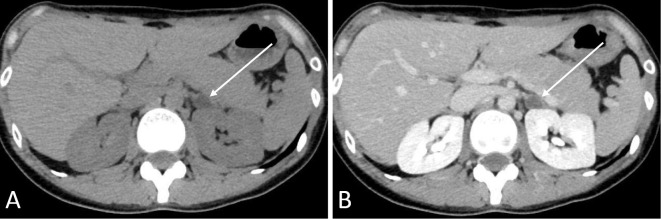
Abdominal computed tomography performed in December 2016 revealed a 1.4-cm, low-density, left adrenal tumor (Fig. 2).
Figure 2.
Abdominal computed tomography (December 2016). (A) Plane computed tomography showing a 1.4-cm, homogenous low-density left adrenal tumor with a Hounsfield Unit value of -10 (arrow). (B) Contrast-enhanced computed tomography showed less enhancement in the left adrenal tumor (arrow).
Adrenal vein sampling was performed in March 2017 (Supplementary material) to determine whether the sites of aldosterone hypersecretion were bilateral or unilateral (1,2). Although the selective index for the right adrenal vein was low, the lateralization index and contralateral suppression index were significantly high (6.1) and low (0.4), respectively. These findings suggested unilateral aldosterone hypersecretion from the left adrenal gland (14-17). The patient was informed of the test results; of the available options including repeating the adrenal vein sampling, surgery, and medical treatment, she chose adrenal surgery.
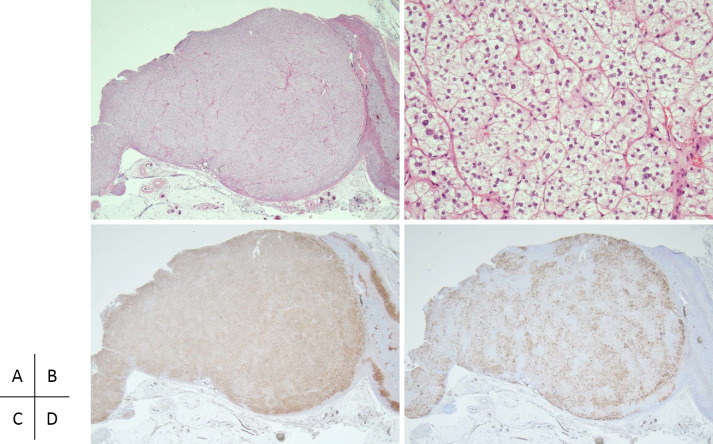
The patient underwent laparoscopic left adrenalectomy in December 2017. A microscopic examination showed that the tumor was mostly composed of clear cells laden with lipids (Fig. 3). The zona reticularis of the adjacent non-tumoral adrenal tissue was not atrophic. Immunohistochemistry revealed that the tumor exhibited positive immunoreactivity for 3β-hydroxysteroid dehydrogenase type 2 and CYP11B2, whereas most areas of the tumor were negative for 17α-hydroxylase/17,20-lyase (P450c17) immunostaining. These findings were consistent with APA (18) and ruled out the possibility of concomitant hypersecretion of cortisol (19).
Figure 3.
Histopathological findings of the resected left adrenal gland (December 2017). (A, B) Hematoxylin and Eosin staining. (A) The tumor is mostly composed of clear cells with a clear margin. (B) Extended image (high-power field) shows cord-like proliferation of clear cells in the tumor. The score of the Weiss criteria for the tumor was 0 points. (C, D) Immunohistochemistry. (C) The 3β-hydroxysteroid dehydrogenase type 2 expression is homogeneously immunolocalized in most areas of the tumor. (D) The CYP11B2 expression is heterogeneously immunolocalized in some areas of the tumor, and other areas show negative immunostaining for CYP11B2.
Blood chemistry performed in July 2018 showed normal PAC (9.6 ng/dL) and PRA (0.6 ng/mL/h). The patient's hypertension gradually improved, and she discontinued antihypertensive medication in September 2018.
The patient's subsequent clinical course has been uneventful for over 2.5 years.
Discussion
A woman with no history of hypertension before and throughout pregnancy unexpectedly developed hypertension five days after normal delivery and was diagnosed with PA. After removal of the left APA, both the PAC and BP levels normalized. To our knowledge, this is the first reported case of PA in which regular daily measurement of BP captured the exact moment when secondary hypertension developed in the postpartum period (Fig. 1).
Table 2 summarizes the characteristics of reported cases of women with no history of hypertension who developed hypertension during the postpartum period and were diagnosed with PA. All patients exhibited unilateral APA and underwent adrenalectomy without complications. In previously reported cases, although the timing or manner of the onset of high BP was undetermined, hypertension was found weeks or months after delivery. In this case, regular daily BP measurements revealed the sudden onset of hypertension within one week after delivery. These cases highlight the importance of closely monitoring BP for the early detection of postpartum hypertensive disorders including PA, even if patients were normotensive before and throughout pregnancy.
Table 2.
Summary of Reported Cases of Women with No History of Hypertension Who Developed Hypertension during the Postpartum Period and were Diagnosed with PA.
| Ref. | Age (years) | Labor or delivery complications | Time between delivery and detection of hypertension | Blood pressure (mmHg) at detection of hypertension | Time between delivery and PA diagnosis | PAC (ng/dL) | PRA (ng/mL/h) | Hypokalemia | Cause of PA | Treatment for PA | Comorbid conditions |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (8) | 23 | [-] | 2 months | 184/112 | 17 months | 19.0 | 0.02 | [+] | Right APA |
Adrenalectomy | Malignant hypertension |
| (10) | 32 | [-] | 1 month | 150/110 | 13 months | 33.5 | Low | [+] | Right APA |
Adrenalectomy | Mild kidney dysfunction |
| (10) | 30 | [-] | 2.5 weeks | 180/110 | 5 months | 21.0 | Low | [+] | Left APA |
Adrenalectomy | None |
| This case | 35 | [-] | 5 days | 155/85 | 7 months | 19.0 | <0.2 | [-] | Left APA |
Adrenalectomy | None |
APA: aldosterone-producing adenoma, PA: primary aldosteronism, PAC: plasma aldosterone concentration, PRA: plasma renin activity
The most remarkable finding in this case report was that regular daily measurement of BP successfully revealed the sudden onset of hypertension secondary to PA shortly after delivery. Normally, pregnant women exhibit a marked activation of the renin-angiotensin-aldosterone system (4-7,20). The increase in aldosterone levels is due to increased renin and angiotensin levels associated with an increased secretion of placental estrogen and progesterone. Progesterone is a competitive inhibitor of aldosterone, so the physiological effects of increased aldosterone are attenuated. Despite the significant expansion of the blood volume, pregnant women remain normotensive due to many mechanisms, including the antagonizing effects of progesterone on mineralocorticoid receptors and the peripheral vasodilation due to increased vasodilator substances, such as prostaglandins. When women with PA become pregnant, their hypertension usually persists or worsens (4-7). However, there are also reports of women with PA who had hypertension that improved during pregnancy but deteriorated after delivery (3,9,21,22). A possible explanation for the different clinical presentations of PA during pregnancy is PA severity (3,22). Pregnant women with severe or moderate PA may exhibit hypertension despite various antihypertensive mechanisms because their aldosterone levels exceed the normal pregnancy range. However, pregnant women with mild PA may be normotensive if their aldosterone concentrations are within the normal pregnancy range and excessive mineralocorticoid action is counteracted by increased progesterone. A case of a woman with PA who had hypertension that resolved after becoming pregnant but reappeared four days after delivery has been reported, wherein a drastic decrease in blood levels of progesterone and a persistent high PAC were documented (23). In our case, the patient was normotensive before and throughout pregnancy and developed hypertension five days after delivery. The severity of the patient's PA was thought to be mild based on a relatively mild elevation of PAC (24) and the absence of hypokalemia (Table 1). The immunohistochemical findings of a relatively heterogenous immunolocalization of CYP11B2 expression compared to homogeneous immunolocalization of 3β-hydroxysteroid dehydrogenase type 2 expression (Fig. 3C, D) also suggested a relatively mild overproduction of aldosterone in the APA (25,26). Thus, our patient likely developed PA without hypertension at some point during pregnancy, and then secondary hypertension developed five days after delivery with disappearance of the pregnancy-related tolerance to high BP. Physicians should be aware of possible PA in women with postpartum hypertension, especially in those with BP that suddenly increases shortly after delivery, even if they were normotensive before and throughout pregnancy.
Another possible mechanism underlying the appearance of hypertension secondary to PA shortly after delivery in our patient is an increased prolactin level. Prolactin is a pituitary hormone that plays a role in promoting lactation. Secretion of prolactin physiologically increases markedly from the first trimester to the postpartum period (27). In a recent study, increased prolactin levels were suggested to be involved in the development of APA in patients with prolactinoma, regardless of age or sex (28). Thus, an increased prolactin level associated with pregnancy was likely involved in the development of APA in our case.
PA is an important hypertensive disorder not only because of its prevalence but also because patients with PA have a higher cardiovascular morbidity and mortality than those with essential hypertension. Although PA can be cured by appropriate treatment, a delay in the diagnosis may lead to the progression of irreversible organ damage, so an early diagnosis and treatment are needed to prevent organ damage. The measurement of PAC and PRA (PAC-to-PRA ratio) is currently the most reliable means available to screen for PA. From a cost-effectiveness perspective, the screening test is recommended for high-risk patients in particular, including those with severe hypertension, resistant hypertension, hypokalemia, incidentaloma, and obstructive sleep apnea (2). Against the recommendation for selective screening, one must weigh the risk of missing or at least delaying the diagnosis of PA in some hypertensive patients; thus, the possibility of PA should be considered in all hypertensive patients (1). In our case, although the patient was not in a high-risk group for PA, the relatively young age for the onset of hypertension and sudden increase in BP after delivery were considered atypical findings for essential hypertension and raised the possibility of secondary hypertension (29). The positive screening test result (a high PAC with a high PAC-to-PRA ratio) led to the early diagnosis and successful treatment of her PA.
In conclusion, we describe a woman who unexpectedly developed hypertension five days after normal delivery and was diagnosed with PA. This case suggests that PA should be considered in women with postpartum hypertension, especially in those with BP that suddenly increases shortly after delivery.
The authors state that they have no Conflict of Interest (COI).
Supplementary Material
The selective index, calculated by dividing cortisol concentration in the adrenal vein by that in the inferior vena cava, after administration of synthetic adrenocorticotropic hormone (tetracosactide, 0.25 mg) in the elbow vein was 1.1 for the right side and 7.2 for the left side. The lateralization index, calculated by dividing the aldosterone-to-cortisol concentration ratio (ACR) in the left adrenal vein by that in the right adrenal vein, after administration of tetracosactide was 6.1. The contralateral suppression index, calculated by dividing the ACR in the right adrenal vein by that in the inferior vena cava, after administration of tetracosactide was 0.4.
Acknowledgements
The authors thank the clinical laboratory technicians of Uonuma Kikan Hospital for their technical support.
References
- 1. Nishikawa T, Omura M, Satoh F, et al. ; Task Force Committee on Primary Aldosteronism; The Japan Endocrine Society. Guidelines for the diagnosis and treatment of primary aldosteronism--the Japan Endocrine Society 2009. Endocr J 58: 711-721, 2011. [DOI] [PubMed] [Google Scholar]
- 2.Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 101: 1889-1916, 2016. [DOI] [PubMed] [Google Scholar]
- 3.Biglieri EG, Slaton PE Jr. Pregnancy and primary aldosteronism. J Clin Endocrinol Metab 27: 1628-1632, 1967. [DOI] [PubMed] [Google Scholar]
- 4.Kamoun M, Mnif MF, Charfi N, et al. Adrenal diseases during pregnancy: pathophysiology, diagnosis and management strategies. Am J Med Sci 347: 64-73, 2014. [DOI] [PubMed] [Google Scholar]
- 5.Morton A. Primary aldosteronism and pregnancy. Pregnancy Hypertens 5: 259-262, 2015. [DOI] [PubMed] [Google Scholar]
- 6.Malha L, August P. Secondary hypertension in pregnancy. Curr Hypertens Rep 17: 53, 2015. [DOI] [PubMed] [Google Scholar]
- 7.Landau E, Amar L. Primary aldosteronism and pregnancy. Ann Endocrinol (Paris) 77: 148-160, 2016. [DOI] [PubMed] [Google Scholar]
- 8.Aloia JF, Beutow G. Malignant hypertension with aldosteronoma producing adenoma. Am J Med Sci 268: 241-245, 1974. [DOI] [PubMed] [Google Scholar]
- 9.Gordon RD, Tunny TJ. Aldosterone-producing-adenoma (A-P-A): effect of pregnancy. Clin Exp Hypertens A 4: 1685-1693, 1982. [DOI] [PubMed] [Google Scholar]
- 10.Nezu M, Miura Y, Noshiro T, Inoue M. Primary aldosteronism as a cause of severe postpartum hypertension in two women. Am J Obstet Gynecol 182: 745-746, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Bretherton I, Pattison D, Pattison S, Varadarajan S. An endocrine cause of acute post-partum hypertension. Obstet Med 6: 30-32, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kilmartin C, Opu T, Podymow T, Dayan N. Primary hyperaldosteronism presenting as persistent postpartum hypertension: illustrative case and systematic review. Obstet Med 12: 190-195, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Umemura S, Arima H, Arima S, et al. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2019). Hypertens Res 42: 1235-1481, 2019. [DOI] [PubMed] [Google Scholar]
- 14.Kline GA, Chin A, So B, Harvey A, Pasieka JL. Defining contralateral adrenal suppression in primary aldosteronism: implications for diagnosis and outcome. Clin Endocrinol (Oxf) 83: 20-27, 2015. [DOI] [PubMed] [Google Scholar]
- 15.Pasternak JD, Epelboym I, Seiser N, et al. Diagnostic utility of data from adrenal venous sampling for primary aldosteronism despite failed cannulation of the right adrenal vein. Surgery 159: 267-273, 2016. [DOI] [PubMed] [Google Scholar]
- 16.Strajina V, Al-Hilli Z, Andrews JC, et al. Primary aldosteronism: making sense of partial data sets from failed adrenal venous sampling-suppression of adrenal aldosterone production can be used in clinical decision making. Surgery 163: 801-806, 2018. [DOI] [PubMed] [Google Scholar]
- 17.Lee J, Kang B, Ha J, et al. Clinical outcomes of primary aldosteronism based on lateralization index and contralateral suppression index after adrenal venous sampling in real-world practice: a retrospective cohort study. BMC Endocr Disord 20: 114, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams TA, Gomez-Sanchez CE, Rainey WE, et al. International histopathology consensus for unilateral primary aldosteronism. J Clin Endocrinol Metab 106: 42-54, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hiraishi K, Yoshimoto T, Tsuchiya K, et al. Clinicopathological features of primary aldosteronism associated with subclinical Cushing's syndrome. Endocr J 58: 543-551, 2011. [DOI] [PubMed] [Google Scholar]
- 20.Wilson M, Morganti AA, Zervoudakis I, et al. Blood pressure, the renin-aldosterone system and sex steroids throughout normal pregnancy. Am J Med 68: 97-104, 1980. [DOI] [PubMed] [Google Scholar]
- 21.Aoi W, Doi Y, Tasaki S, Mitsuoka T, Suzuki S, Hashiba K. Primary aldosteronism aggravated during peripartum period. Jpn Heart J 19: 946-953, 1978. [DOI] [PubMed] [Google Scholar]
- 22.Murakami T, Watanabe Ogura E, Tanaka Y, Yamamoto M. High blood pressure lowered by pregnancy. Lancet 356: 1980, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Ronconi V, Turchi F, Zennaro MC, Boscaro M, Giacchetti G. Progesterone increase counteracts aldosterone action in a pregnant woman with primary aldosteronism. Clin Endocrinol (Oxf) 74: 278-279, 2011. [DOI] [PubMed] [Google Scholar]
- 24.Heinrich DA, Adolf C, Rump LC, et al. Primary aldosteronism: key characteristics at diagnosis: a trend toward milder forms. Eur J Endocrinol 178: 605-611, 2018. [DOI] [PubMed] [Google Scholar]
- 25.Nanba K, Tsuiki M, Sawai K, et al. Histopathological diagnosis of primary aldosteronism using CYP11B2 immunohistochemistry. J Clin Endocrinol Metab 98: 1567-1574, 2013. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura Y, Yamazaki Y, Tezuka Y, Satoh F, Sasano H. Expression of CYP11B2 in aldosterone-producing adrenocortical adenoma: regulatory mechanisms and clinical significance. Tohoku J Exp Med 240: 183-190, 2016. [DOI] [PubMed] [Google Scholar]
- 27.Hu Y, Ding Y, Yang M, Xiang Z. Serum prolactin levels across pregnancy and the establishment of reference intervals. Clin Chem Lab Med 56: 803-807, 2018. [DOI] [PubMed] [Google Scholar]
- 28.Williams TA, Dietz AS, Theodoropoulou M, et al. Coexisting prolactinoma and primary aldosteronism: is there a pathophysiological link? J Clin Endocrinol Metab 100: E1262-E1269, 2015. [DOI] [PubMed] [Google Scholar]
- 29.Rimoldi SF, Scherrer U, Messerli FH. Secondary arterial hypertension: when, who, and how to screen? Eur Heart J 35: 1245-1254, 2014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The selective index, calculated by dividing cortisol concentration in the adrenal vein by that in the inferior vena cava, after administration of synthetic adrenocorticotropic hormone (tetracosactide, 0.25 mg) in the elbow vein was 1.1 for the right side and 7.2 for the left side. The lateralization index, calculated by dividing the aldosterone-to-cortisol concentration ratio (ACR) in the left adrenal vein by that in the right adrenal vein, after administration of tetracosactide was 6.1. The contralateral suppression index, calculated by dividing the ACR in the right adrenal vein by that in the inferior vena cava, after administration of tetracosactide was 0.4.