Abstract
Acute fibrinous and organizing pneumonia (AFOP) is rare in patients with systemic lupus erythematosus (SLE). We herein report a case of AFOP with SLE and hemophagocytic syndrome. Early-phase high-resolution computed tomography showed a fine granular lung pattern. A pathological examination revealed AFOP. An immunohistological examination revealed numerous CD163+ and fewer CD68+ macrophages present in the lung tissue and in alveolar spaces as well, including fibrin balls, the interstitium, and bronchial walls. Pneumonia and thrombocytopenia worsened during high-dose steroid therapy, plasma exchange, and intravenous immunoglobulin administration. The addition of intravenous cyclophosphamide successfully ameliorated the symptoms and radiographic lesions. Therefore, this therapy may be useful for treating severe AFOP.
Keywords: acute fibrinous and organizing pneumonia, autoimmune-associated hemophagocytic syndrome, systemic lupus erythematosus, rheumatic disease, CD163 positive macrophages, intravenous cyclophosphamide
Introduction
Acute fibrinous and organizing pneumonia (AFOP) is a relatively newly recognized histopathological manifestation presenting with fibrin “balls” within the alveolar spaces and organizing pneumonia, featuring fibrin-associated, loose, intraluminal connective tissue within the alveolar ducts and bronchioles. AFOP does not meet the classic histological criteria for diffuse alveolar damage, bronchiolitis obliterans with organizing pneumonia, or eosinophilic pneumonia (1). AFOP may be idiopathic or develop secondary to a variety of lung injuries (2) and other conditions, including drug-taking, environmental insults, infection, transplantation, and autoimmune diseases (3).
Several cases of AFOP associated with rheumatic diseases have been reported (1-11), but only one has been linked to systemic lupus erythematosus (SLE) (11); although this case was treated successfully (11), the prognosis of AFOP associated with rheumatic diseases is not always good (1,4,5), and the optimal treatment remains unclear.
We herein report a case of AFOP associated with SLE and autoimmune-associated hemophagocytic syndrome (AAHS) that worsened during initial high-dose prednisolone (PSL) therapy, including intravenous methylprednisolone (IV MP), plasma exchange, and massive intravenous immunoglobulin (IVIG) administration. However, intravascular pulsed cyclophosphamide (IV CYC) therapy was successful. Our case also indicated that CD163+ cells, previously indicated to be M2 anti-inflammatory macrophages (12), are provably involved in AFOP with SLE.
Case Report
A 25-year-old woman was admitted to Shimane University Hospital with a fever, cough, and erythema of the face and extremities. Five years earlier, she had been diagnosed with histiocytic necrotizing lymphadenitis and been initially treated with PSL at 30 mg/day. The dose was gradually reduced and maintained at 3 mg/day for several years. She had presented with a productive cough in the past two months and facial erythema after her visit to Thailand in the past month before her hospital admission.
A laboratory examination revealed proteinuria. She was first examined at the Department of Respiratory Medicine and then consulted the Department of Rheumatology. On admission, her body temperature was 36.8°C. Her heart and breathing rates were normal. Her percutaneous oxygen saturation [as revealed by pulse oximetry (SpO2)] on room air was 97%. She presented with erythema on her face and extremities, oral ulcers, and cervical and inguinal lymphadenopathy. Auscultation revealed a few right-side-dominant coarse crackles.
A urinalysis revealed proteinuria, hematuria, hyaline, and cellular casts. The urinary protein excretion level was 7.1 g/day. Blood tests revealed progressive pancytopenia from the day of admission to day 4, the day of transfer to the Department of Rheumatology with the following findings: white blood cells: 4,140 to 2,320 /μL of blood, hemoglobin (Hgb): 9.2 to 8.6 g/dL, and platelets: 166,000 to 75,000 /μL of blood. Hypoalbuminemia and elevated levels of lactate dehydrogenase (445 IU/L, normal 100-215 IU/L), ferritin (796 ng/mL, normal 5-120 ng/mL), soluble interleukin 2 receptor (1,332 U/mL, normal 144-518 U/mL), and KL-6 (591 U/mL, normal <500 U/mL) were detected. The total bilirubin levels and renal function were within normal ranges. Complements CH50, C3, and C4 were decreased to 10.5 U/mL, 26 mg/dL, and 6.9 mg/dL respectively; the levels of C1q-circulating immune complexes were elevated (5.3 μg/mL, normal <2.9 μg/mL). According to an immunofluorescence test, her antinuclear antibody status was found to be positive (1:320; homogeneous and speckled type). Antibodies to dsDNA (96.2 IU/mL, normal ≤12.0 IU/mL), Sm (11.7, normal ≤10), U1RNP (12.7, normal ≤10), SS-A (≥500, normal ≤10) and PAIgG (180 ng/107 platelets, normal ≤25 ng/107 platelets) were also evident, but direct Coombs test, lupus anticoagulants, anti-cardiolipin IgG, and anti-cardiolipin-beta2 glycoprotein I complex, anti-SS-B, and anti-glomerular basement antibodies were not detected. She was diagnosed with SLE because she met both the 1997 revised American College of Rheumatology criteria and the 2012 Systemic Lupus Erythematosus International Collaborating Clinics (SLICC) classification criteria.
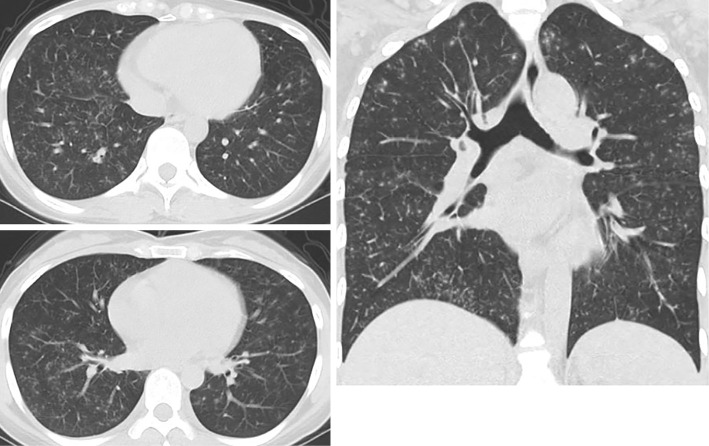
A blood gas analysis (BGA) on room air revealed a pH of 7.415, an arterial carbon dioxide partial pressure (PaCO2) of 38.5 mmHg, an arterial oxygen partial pressure (PaO2) of 81.8 mmHg, an HCO3- level of 24.1 mEq/L, and a base excess of 0.1 mmol/L. Chest computed tomography (CT) revealed diffuse, finely granular shadows in both lungs (Fig. 1). An infection assessment was extensively conducted because of her travel history and excluded by negative results on the interferon-γ release assay for tuberculosis, the virus separating test, other blood or urinary infection markers (such as beta-D-glucan, fungal antigens, antibodies against viruses, chlamydiae, and mycoplasma), cultures of sputum and gastric juice, and polymerase chain reaction. The angiotensin-converting enzyme levels were within the normal limits.
Figure 1.
A chest CT scan obtained on admission and before treatment. Diffuse finely granular shadows are evident in both lungs.
Bronchofiberscopy and bronchoalveolar lavage (BAL) performed on the day of admission revealed lymphocytosis with a reduced CD4/8 ratio and small amounts of bleeding but findings that were inconsistent with diffuse alveolar hemorrhage. A transbronchial lung biopsy revealed no specific findings.
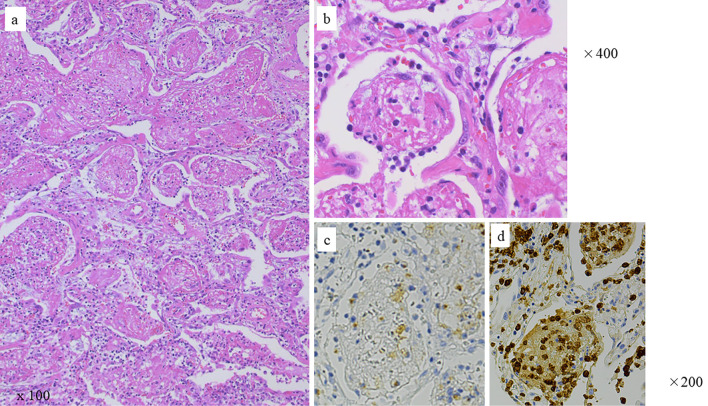
A videothoracoscopic lung biopsy was also performed because the patient had traveled abroad, and the possibility of microorganisms had to be excluded. The biopsy specimens obtained on day 3 revealed intra-alveolar fibrin balls in the alveolar spaces, an air space filling pattern with neutrophils, foam cells, an inflammatory exudate, and organizing pneumonia (Fig. 2). No alveolar hemorrhage or infectious disease was observed. The patient was diagnosed with AFOP associated with SLE.
Figure 2.
Videothoracoscopically derived lung biopsy specimens revealed fibrin balls within the alveolar spaces, foamy cells, leucocytes, an inflammatory exudate, and organizing pneumonia. Hematoxylin and Eosin staining: (a) 100× and (b) 400×. Immunohistochemistry with (c) anti-CD68 or (d) anti-CD163 mouse monoclonal antibody, followed by biotinylated anti-mouse IgG, avidinbiotin-peroxidase complex, and color developed with diaminobenzidine tetrahydrochloride (brown). Nuclei were counterstained with hematoxylin (blue). Control mouse IgG stained exudate weakly, but not cells (not shown).
An immunohistochemical examination later revealed increased numbers of macrophage linear cells; numerous CD163+ cells and a smaller number of CD 68+ cells were observed everywhere in her lung tissue and in alveolar spaces, including fibrin balls, the interstitium, and bronchial walls (Fig. 2c, d). Antibodies against IgG, C1q, or C3 were used for immunostaining, but these deposits were not detected, except for a small granular deposit of IgG in one vessel.
The patient's body temperature increased to 40 °C on day 3 accompanied by worsening pancytopenia, elevated ferritin levels, and worsening liver function tests. A bone marrow examination performed on the same day revealed the presence of hemophagocytic cells. The patient was also diagnosed with autoimmune-associated hemophagocytic syndrome (AAHS). We initiated IV MP 1,000 mg/day for 3 days followed by 1.5 mg/kg/day of PSL. Despite high-dose steroidal therapy, anemia (Hgb 7.9 g/dL), the thrombocytopenia (37,000 /μL), hyperferritinemia, and liver function worsened.
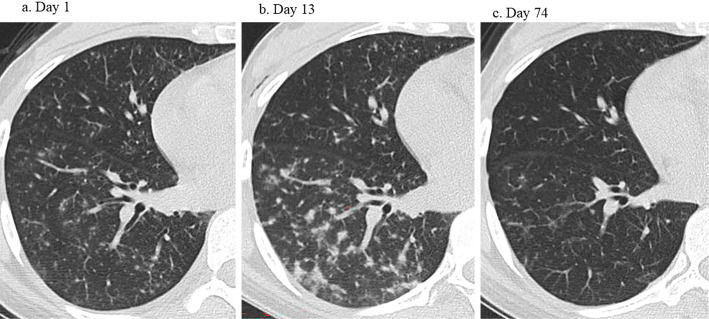
We performed plasma exchange on days 7 and 8 for the temporary reduction of cytokines causing AAHS, followed by 400 mg/kg/day IVIG for 5 days, because immune-related thrombocytopenia (17,000 /μL) might be involved under conditions of hemoptysis. However, while the platelet count improved by more than 50,000 /μL, it decreased again, and platelet transfusion did not increase the platelet count as expected. Her serum LDH also remained high, which may have been caused by AAHS. Furthermore, she required supplemental oxygen to treat hypoxia, and the lung lesions evident on CT worsened on day 13 (Fig. 3b). We administered IV CYC (750 mg) to treat respiratory symptoms and uncontrolled AAHS. Her respiratory and other symptoms and laboratory data improved.
Figure 3.
CT changes on hospital days (a) 1, (b) 13, and (c) 74. (b) The pulmonary manifestations worsened on day 13 despite high-dose prednisolone therapy, including intravenous pulsing, plasma exchange, and massive intravenous immunoglobulin administration. (c) Only slight granular shadows remained on day 74.
A renal biopsy examined after the platelet counts improved on day 65 revealed lupus nephritis type III-S (A/C) +V (International Society of Nephrology/Renal Pathology Society 2003 classification) with granular deposits of IgG, C1q, and C3.
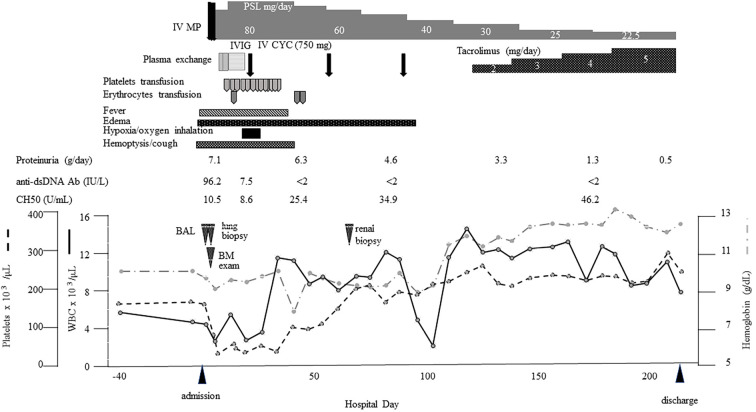
CYC was changed to tacrolimus after the third course due to concerns about the patient's fertility and neutropenia caused by CYC. We also investigated the effect of tacrolimus on improving proteinuria caused by type V lupus nephritis. The dose of tacrolimus was increased by measuring the blood concentrations, although the approved dosage for lupus nephritis is below 3 mg/day in Japan. Only slight granular shadows were evident on CT on day 74 (Fig. 3c), and her proteinuria improved. The patient's clinical course is shown in Fig. 4.
Figure 4.
The clinical course. anti-ds DNA Ab: anti-double-stranded DNA antibody, BAL: bronchoalveolar lavage, BM: bone marrow, IV CYC: intravascular cyclophosphamide pulse, IVIG: massive intravenous immunoglobulin administration, IV MP: intravenous methylprednisolone pulse, PSL: prednisolone
Discussion
AFOP was first reported as a histological pattern by Beasley et al. and was evident in the present clinical setting. AFOP does not meet the classic histological criteria for diffuse alveolar damage, bronchiolitis obliterans with organizing pneumonia, or eosinophilic pneumonia (1). The predominant histological features of AFOP are as follows: (i) organizing intra-alveolar fibrin, (ii) organizing pneumonia, and (iii) a patchy distribution. The minor features are as follows: (i) interstitial changes with acute and/or chronic inflammation, type 2 pneumocyte hyperplasia, and/or alveolar septal expansion with myxoid connective tissue, (ii) interstitial inflammation and (typically mild-to-moderate) expansion, and/or (iii) interstitial changes primarily confined to areas adjacent to the intra-alveolar fibrin balls (the intervening lung tissue exhibits only minimal changes). Pertinent negative observations are as follows: (i) hyaline membranes are not apparent, (ii) eosinophils are inconspicuous or absent, (iii) extensive bronchopneumonia and/or abscess formation is absent, and (iv) granulomatous inflammation is absent (1).
AFOP associated with rheumatic diseases has been reported (Table). No specific autoantibodies related to AFOP were detected. Anti-SSA/Ro antibody was observed in three cases (#7, #8, #12); however, the proportion of the antibody may be observed usualy in rheumatic diseases. Cases #9 and #10 possessed anti-EJ antisynthetase antibody (9,10). Therefore, this rare antibody may be associated with AFOP in some patients with anti-EJ antibody-positive antisynthetase syndrome; however, other pathologic patterns may be more frequent (12 nonspecific interstitial pneumonia and 3 unclassifiable interstitial pneumonia have been reported) (10,13). No autoantibody associated with AFOP has been found in other rheumatic diseases.
Table.
Acute Fibrinous and Organizing Pneumonia Associated with Rheumatic Diseases.
| Rheumatic diseases | Age Sex |
Symptoms | Autoantibodies | Radiographic findings | Therapy | Outcome | References | |
|---|---|---|---|---|---|---|---|---|
| 1 | Polymyositis | 78 F |
Dyspnea | Not described | Bilateral reticulonodular | CS | Death unrelated cause | 1 |
| 2 | Ankylosing spondylitis | 55 M |
Cough, dyspnea, hemoptysis | Not described | Bilateral perihilar air-space | Antibiotics | Improved | |
| 3 | Possible fibromyalgia | 58 F |
Fever, arthralgia | Not described | Consistent with atypical pneumonia | Antibiotics | Improved | |
| 4 | Dermatomyositis | 14 F |
Dyspnea | ANA | Extensive parenchymal process with patchy densities prominent in both lower lobes | Antibiotics, CS, cyclosporine, IV CYC, ventilation | Death respiratoly failure | 2 |
| 5 | Undifferentiated connective tissue disease | 39 F |
Cough, fever, dyspnea Raynaud’s phenomenon, sicca syndrome (normal salivary gland biopsy), boot and gloves neuropathic pain | p-ANCA, anti-TPO, anti-TBG | Diffuse GGA, with foci of parenchymal densification | CS, IV CYC, later IV MP ventilation | Death respiratoly failure, pulmonary hemorrhage | 5 |
| 6 | Collagen vascular disease-like condition | 47 M |
Joint pain and stiffness, myalgia, dry cough, and dyspnea | ANA | Diffuse reticular abnormalities in both lower lobes, associated with a GGA | CS, AZA, ventilation | Improved | 6 |
| 7 | Primary Sjögren's syndrome | 75 F |
Dyspnea, wheezing, productive cough dry skin, arthritis, rash and pulmonary hypertension | anti-SS-A anti-SS-B |
Bilateral multi-lobar areas of consolidation | CS | Improved | 7 |
| 8 | Primary Sjögren's syndrome | 60 F |
Fever, dyspnea, dry cough | ANA anti-SS-A | Bilateral diffuse multiple solitary nodules admixed with patchy areas of GGA | CS | Improved | 8 |
| 9 | Antisynthetase syndrome | 34 M |
Dry cough, dyspnea on exertion necrotizing myopathy | anti-EJ | Bilateral patchy infiltrates predominantly in the lower lobes | CS, IVIG, MMF | Improved | 9 |
| 10 | Antisynthetase syndrome | 66 F |
Pruritic rash, myalgia, productive cough, | Atypical pANCA, anti-EJ | Pathy peripheral airspase consolidation including foci of central lucency | CS (only initial use of MMF and AZA by intolerance) | Improved | 10 |
| 11 | Systemic lupus erythematosus, anti-phospholipid syndrome | 47 M |
Dyspnea, productive cough, hemoptysis | ANA, anti-dsDNA, anti-Sm anti-phospholipid | Peripheral and peribronchiolar areas of consolidation, maximal in the superior segments of the lower lobes with subpleural sparing in several areas | CS, oral CYC | Improved | 11 |
| 12 | Systemic lupus erythematosus | 25 F |
Fever, cough, erythema, nephritis, pancytopenia hemophagocytosis | ANA, anti-dsDNA, anti-Sm anti-U1RNP, anti-SS-A, PAIgG | Diffuse granular shadows | IV MP, CS, PE, IVIG, IV CYC | Improved | Present case |
ANA: anti-nuclear antibody, AZA: azathioprine, CS: corticosteroid, CYC: cyclophosphamide, GGA: ground-glass attenuation, IV CYC: intravenous cyclophosphamide, IVIG: intravenous immunoglobulin, IV MP; intravenous methyl-prednisolone pulse, MMF: mycophenolate mofetil, PAIgG: platelet-associated immunoglobulin-G, PE: plasma exchange, TGB: thyroxin binding globulin, TPO: thyroid peroxidase
The radiographic CT patterns also varied, exhibiting multifocal patchy opacities, diffuse multiple (but solitary) nodules, peribronchiolar areas of consolidation, nodular shadows, or fine granular shadows (such as those in our case). The lung locations also varied from diffuse to lower lobe dominance. A pathological examination is thus essential.
Pulmonary parenchymal manifestations of SLE include alveolar hemorrhage, acute lupus pneumonitis, and chronic interstitial pneumonitis, but these conditions, especially the acute forms, are relatively rare (14,15). In the present case, chest CT revealed diffuse granular shadows, causing us to suspect acute lupus pneumonitis, but intense respiratory distress did not develop. The findings of BAL differed from those of alveolar hemorrhage. Owing to the history of the patient's travel abroad, we intensively checked for microorganisms but found none. The patient was ultimately diagnosed based on the histopathological findings of the lung.
The etiology and mechanisms of AFOP in SLE or other rheumatic diseases have not yet been proven. In the patient, deposition of immune complexes of IgG, C3, and C1q in glomeruli with hypocomplinemia, elevated immune complexes, and anti-dsDNA was observed, which were not detected in lung specimens. It is possible that the cytokines involved in AAHS also cause AFOP. Hematoxylin-eosin staining of lung specimens revealed neutrophils and foam cells, which are activated macrophages laden with lipids, in air spaces. Immunohistochemical examinations with antibodies against CD68 and CD163 were performed to detect macrophages; the former is expressed on pro-inflammatory M1 macrophages, while the latter is expressed on anti-inflammatory M2 macrophages (16). CD163+ cell numbers were significantly increased, and CD68+ cells were observed in relatively smaller numbers but increased in CD68+ cells in the normal lung reported elsewhere, where these cells were scattered in the alveolar spaces (17). Both macrophages were observed everywhere in her lung tissue, in alveolar spaces, including fibrin balls, the interstitium, and bronchial walls. A higher ratio of density of CD163+ to CD68+ macrophages has also been reported in nonspecific interstitial pneumonia and cryptogenic organizing pneumonia (17). In the AFOP case we reported, the increase in these cells seemed larger and the distribution wider than the interstitial pneumonia reported previously (17). The CD163+ M2 macrophage-dominant increase has been previously demonstrated in AAHS (15). Systemic macrophage activation and related cytokines may reflect both hemophagocytosis and AFOP in our case. The patient's laboratory data and symptoms reflecting AAHS activity in the present case paralleled pulmonary symptoms. M2 macrophages are also known as producers of TGF-β, which may cause fibrin balls as well as fibrosis. Thus, macrophage activation in the lung or other tissues may be related to AFOP, although its involvement was not clear in other cases reported previously. Further careful observation of AFOP in rheumatic cases is needed to determine its etiology.
No optimal treatment for AFOP associated with SLE or other rheumatic diseases has yet been established. Cases #2 and #3 of Table did not require immunosuppressive therapies but improved with antibiotics, suggesting that an infection may have been in play. Of the other patients, 4 of 10 improved when corticosteroids alone were administered (#1, #7, #8, #10), and the other 6 received corticosteroids and immunosuppressants. Seven of 10 patients improved, and the other 3 died. Case #4 received intensive mPSL-pulsing and IVIG (2 g/kg) therapies followed by IV CYC but died 2 weeks after admission (4). Case #5 with undifferentiated connective tissue disease developed respiratory failure caused by pulmonary hemorrhaging, despite treatment with a high dose of corticosteroid (IV MP) and IV CYC and required ventilatory support in the intensive-care unit (6). Only one case of AFOP with SLE (#11) has been reported. Hariri et al. described a 47-year-old man with AFOP associated with SLE who was successfully treated with prednisone 60 mg daily for 4-6 weeks, CYC 100 mg daily, and anticoagulation therapy (11). In our patient, only the second case of SLE associated with AFOP, the addition of IV CYC to prior IV MP, plasma exchange, or IVIG was effective. The addition of IV CYC might affect AAHS, which may indirectly affect AFOP. High-dose steroid, IV MP, or plasma exchange may be effective for AFOP; however, it was difficult for our patient to wait for the effects on both respiratory and blood conditions. Unfortunately, cases #4 and #5 died despite treatment with CYC. The discrepancy between these cases and CYC-effective patients (#10 and the present case) is not clear. The differences may depend on underlying autoimmune diseases, timing of CYC introduction, or disease severity in each patient. Identifying which case requires CYC may be important. More documentation of cases of AFOP with rheumatic diseases is needed.
Ours is the second reported case of SLE with AFOP. The AFOP radiographic patterns vary, as described in this manuscript, and differ from those in a previous report (11). A lung biopsy is required to diagnose AFOP. Further research on more patients is required to establish safe and effective therapies for AFOP associated with rheumatic diseases.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors would like to thank the patient for consenting to this case report. The authors also thank Prof. Takeshi Isobe, Respiratory Medicine & Medical Oncology, Faculty of Medicine, Shimane University, for his valuable advice and Dr. Mitsuhiro Tada, who was part of the same department, for conducting the bronchoscopic examinations.
References
- 1.Beasley MB, Franks TJ, Galvin JR, Gochuico B, Travis WD. Acute fibrinous and organizing pneumonia: a histological pattern of lung injury and possible variant of diffuse alveolar damage. Arch Pathol Lab Med 126: 1064-1070, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Vasu TS, Cavallazzi R, Hirani A, Marik PE. A 64-year-old male with fever and persistent lung infiltrate. Respir Care 54: 1263-1265, 2009. [PubMed] [Google Scholar]
- 3.Arnaud D, Surani Z, Vakil A, Varon J, Surani S. Acute fibrinous and organizing pneumonia: a case report and review of the literature. Am J Case Rep 18: 1242-1246, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prahalad S, Bohnsack JF, Maloney CG, Leslie KO. Fatal acute fibrinous and organizing pneumonia in a child with juvenile dermatomyositis. J Pediatr 146: 289-292, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Valim V, Rocha RH, Couto RB, Paixão TS, Serrano EV. Acute fibrinous and organizing pneumonia and undifferentiated connective tissue disease: a case report. Case Report Rheumatol 2012: 549298, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balduin R, Giacometti C, Saccarola L, et al. Acute fibrinous and organizing pneumonia in a patient with collagen vascular disease“stigma”. Sarcoidosis Vasc Diffuse Lung Dis 24: 78-80, 2007. [PubMed] [Google Scholar]
- 7.Fasanya A, Gandhi V, DiCarlo C, Thirumala R. Acute fibrinous and organizing pneumonia in a patient with Sjögren's syndrome. Respir Med Case Rep 20: 28-30, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Zhao S, Du G, et al. Acute fibrinous and organizing pneumonia as initial presentation of primary Sjögren's syndrome: a case report and literature review. Clin Rheumatol 37: 2001-2005, 2018. [DOI] [PubMed] [Google Scholar]
- 9.Kashif M, Arya D, Niazi M, Khaja M. A rare case of necrotizing myopathy and fibrinous and organizing pneumonia with anti-EJ antisynthetase syndrome and SSA antibodies. Am J Case Rep 18: 448-453, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sauter JL, Butnor KJ. Expanding the spectrum of pulmonary histopathological manifestations of anti-synthetase syndrome: anti-EJ-associated acute fibrinous and organizing pneumonia. Histopathology 65: 581-582, 2014. [DOI] [PubMed] [Google Scholar]
- 11.Hariri LP, Unizony S, Stone J, et al. Acute fibrinous and organizing pneumonia in systemic lupus erythematosus: a case report and review of the literature. Pathol Int 60: 755-759, 2010. [DOI] [PubMed] [Google Scholar]
- 12.Do T, Tan R, Bennett M, et al. MicroRNA networks associated with active systemic juvenile idiopathic arthritis regulate CD163 expression and anti-inflammatory functions in macrophages through two distinct mechanisms. J Leuk Biol 103: 71-85, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasano H, Hagiwara E, Kitamura H, et al. Long-term clinical course of anti-glycyl tRNA synthetase (anti-EJ) antibody-related interstitial lung disease pathologically proven by surgical lung biopsy. BMC Pul Med 16: 168, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keane MP, Lynch JP 3rd. Pleuropulmonary manifestations of systemic lupus erythematosus. Thorax 55: 159-166, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murakawa Y, Taira M. Pulmonary manifestations of systemic lupus erythematosus. Riumachi-ka (Rheumatology). Forthcoming(in Japanese). [Google Scholar]
- 16.Crayne CB, Albeituni S, Nichols KE, Cron RQ. Immunology of macrophage activation syndrome. Front Immunol 10: 119, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamashita M, Saito R, Yasuhira S, et al. Distinct profiles of CD163-positive macrophages in idiopathic interstitial pneumonias. J Immunol Res 4: 2018:1436236, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]