Abstract
Hemolytic anemia and pure red cell aplasia are rare hematological complications of hepatitis B virus infection. We herein report a 24-year-old man who was diagnosed with hemolytic anemia and possible transient pure red cell anemia eight weeks after a severe episode of acute hepatitis B virus infection. Rapid recovery was observed with conservative management. Hemoglobin returned to baseline within three months. As the clinical features of hemolytic anemia associated with hepatitis B virus have not yet been elucidated, we conducted a systematic review and present an analysis of the 20 reported cases, including our present case.
Keywords: hepatitis B virus, liver failure, hemolytic anemia, pure red cell aplasia, systematic review
Introduction
Hepatitis B virus (HBV) infection affects about 400 million individuals worldwide (1,2). HBV is a partially double-stranded DNA hepadnavirus that can be transmitted by blood or other bodily fluids (3). In addition to liver diseases, such as hepatitis, cirrhosis, and hepatocellular carcinoma, HBV infection can lead to extrahepatic manifestations, including polyarthritis, polyarteritis nodosa, glomerulonephritis, and hematological disorders, in 20% of affected individuals (4-7).
Anemia is a well-known hematological complication of viral infections, including hepatitis viruses (8). Hemolytic anemia is a type of anemia characterized by the destruction of red blood cells before their normal 120-day life span, which results in jaundice and hemoglobinuria derived from free hemoglobin (9). Hemolytic anemia as an extrahepatic manifestation of acute hepatitis is rare, particularly after HBV infection (10). HBV-related pure red cell aplasia (PRCA), another cause of anemia that results from a sharp reduction in erythroid precursors in the bone marrow, is exceedingly rare (11).
We herein report a case of severe hemolytic anemia after HBV infection that was successfully treated conservatively. The extremely low reticulocyte count prompted us to suspect co-existing transient PRCA. We also present a systematic review of hemolytic anemia after HBV infection.
Case Report
A 24-year-old man was admitted for gradual-onset dyspnea and weakness. His medical history was only significant for childhood asthma. He was a social drinker but had no history of smoking. He denied any history of blood transfusions, overseas travel, intravenous drug use, tattoos, ingestion of raw meats, or family history of hepatitis. However, he admitted to sexual intercourse with a commercial sex worker without protection one month earlier.
On admission, the patient was afebrile and vital signs were normal. No altered mental status was noted. A physical examination was only remarkable for severe jaundice. Initial laboratory values were significant for total bilirubin (TB) of 13 mg/dL, direct bilirubin (DB) of 9.9 mg/dL, alanine transaminase (ALT) of 4,191 IU/L, aspartate aminotransferase of 1,795 IU/L, alkaline phosphatase of 465 IU/L, lactate dehydrogenase (LDH) of 564 IU/L, prothrombin time (PT) of 16.6 seconds (57%), and an international normalized ratio (INR) of 1.45. A complete blood count showed no abnormalities, with a hemoglobin level of 15.3 g/dL, mean corpuscular volume of 89.6 fL, white blood cell count of 3,900/μL, and platelet count of 166,000/μL. HBV surface and envelope antigens and antibodies of the immunoglobulin M (IgM) class against the hepatitis B core antigen (anti-HBc IgM) were positive. The virus was genotype B, which is prevalent in Asia, and the viral load was 5.6 log copies/mL. Human immunodeficiency virus antibody was negative. The patient was diagnosed with acute HBV infection, most likely incurred via sexual transmission. Clinical deterioration led to the diagnosis of non-comatose acute liver failure nine days after admission. The transplantation team was consulted, and entecavir (0.5 mg/day) was started. However, his laboratory values improved, and no liver transplant was necessary. The patient was discharged 24 days after admission.
The patient returned five weeks later due to new-onset cough, dyspnea, and malaise that had started two weeks earlier. The patient denied any new medications or supplements, alcohol consumption, contact with sick individuals, travel, or sexual contact after his discharge. His hemoglobin level had decreased markedly, from 13.9 (at discharge) to 5.9 g/dL. Curiously, the reticulocyte count had also decreased sharply, to 5,200/μL (0.25%). White blood cells and platelets were within the normal range. Bilirubin remained elevated (TB of 31.3 mg/dL and DB of 24.5 mg/dL), while transaminases and INR had improved. LDH had increased to 756 U/L, with an increase in LDH isozyme 1. Haptoglobin was undetectable (<10 mg/dL). Direct and indirect Coombs tests were negative. Parvovirus B19 serologies were consistent with past infection. Red blood cell-associated IgG was within the normal range, making Coombs-negative autoimmune hemolytic anemia (AIHA) unlikely. Antinuclear and anti-SSA/Ro antibodies were negative. Hepatitis envelope antigen seroconversion was confirmed. A urinalysis was positive for hemoglobin and urobilinogen. Small amounts of schistocytes were observed in the peripheral blood smear. Contrast-enhanced computed tomography showed no significant interval change, notable only for mild splenomegaly. Esophagogastroduodenoscopy and colonoscopy showed no signs of gastrointestinal bleeding. The patient was diagnosed with hemolytic anemia. Concomitant PRCA was also suspected based on the sharp decrease in reticulocytes despite severe normocytic normochromic anemia.
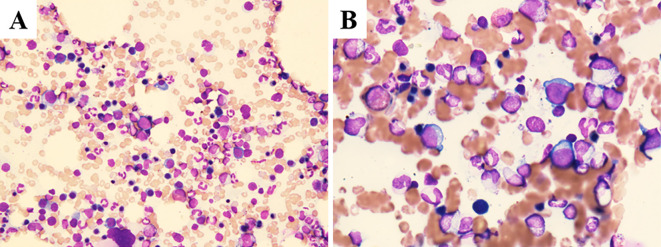
The reticulocyte count had recovered markedly to 269,500/μL (9.26%) by two days later, when bone marrow aspiration was conducted and revealed slight hypercellularity with 272,500/μL nucleated cells. Erythroid hyperplasia was confirmed with an increase in CD71+ erythroid series (Fig. 1). The results of a chromosomal analysis were unremarkable. While the erythroid hyperplasia could be attributed to a response to hemolytic anemia, hematologists believed it also reflected the recovery phase from transient PRCA.
Figure 1.
Wright-Giemsa staining of aspirated bone marrow at (A) low-power (200×) and (B) high-power (600×) magnification revealed slight hypercellularity with an increase in macroblasts and erythroblastic mitosis.
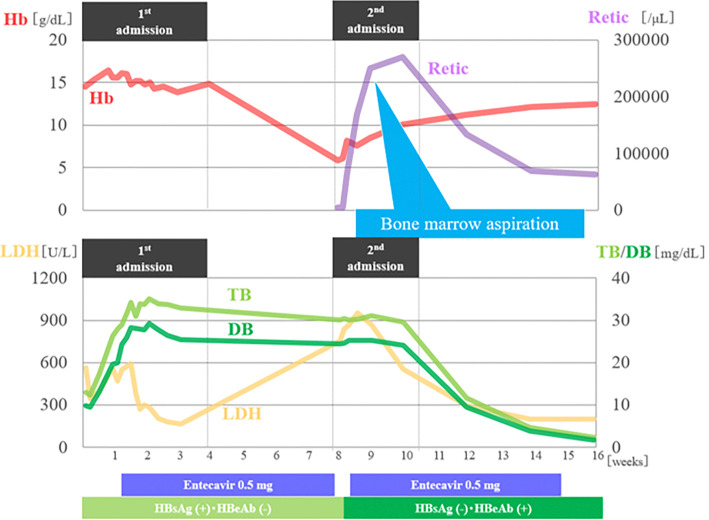
The patient recovered rapidly with conservative management, including transfusion of four units of packed red blood cells. His hemoglobin level improved to 11.2 g/dL over four weeks (Fig. 2). No recurrence of liver failure or anemia was observed during 24 months of follow-up.
Figure 2.
Clinical course timeline. Hb: hemoglobin, Retic: reticulocytes, TB: total bilirubin, LDH: lactate dehydrogenase, HBeAg: hepatitis B envelope antigen, HBeAb: hepatitis B envelope antibody
Discussion
Hemolytic anemia and HBV infection
Anemia after viral hepatitis has a relatively broad differential diagnosis, including gastrointestinal bleeding, splenomegaly due to portal hypertension, and vitamin B12 and folate deficiencies associated with alcohol consumption and nutritional deficits (8). Aplastic anemia (12), thrombocytopenia (7), leukopenia (13), hemolytic anemia (14), and PRCA (11) have all been reported in association with HBV.
Hemolytic anemia may be suspected based on clinical findings, such as anemia and jaundice, and diagnosed based on normocytic normochromic anemia, marked reticulocytosis, elevated indirect bilirubin, increased urinary urobilin, low urine urobilinogen, schistocytes on peripheral blood smear, and erythroid hyperplasia on bone marrow aspiration (9). Since the first report in 1976 (15), HBV infection complicated with hemolytic anemia remains extremely rare. Hemolytic anemia is associated more frequently with hepatitis A virus (HAV), reported in up to 2.5% of affected patients (16,17), and while rare, it has also been reported in the setting of hepatitis C virus (HCV) (18). Four mechanisms have been proposed for hemolytic anemia after viral hepatitis: weakened erythrocyte resistance due to direct viral action, rapid splenomegaly, erythrocyte fragility due to abnormal globulins, and increased hemolytic toxicants by decreased hepatic clearance (19). Hemolytic anemia may therefore occur immediately after HBV infection or during or after an episode of acute hepatitis.
There is some support for the use of antiviral agents in acute HBV infection, although data are limited (20). Entecavir may improve the clinical course of HBV-associated fulminant hepatitis and can reduce the risk of reinfection after liver transplantation (21,22). In the present case, entecavir was started when the patient first met the criteria for fulminant hepatitis and the transplantation team was consulted.
While entecavir was briefly discontinued when severe anemia was first noted, recovery from hemolytic anemia and PRCA started the day after stopping entecavir. Recovery was too rapid for entecavir to have been the cause of hemolytic anemia and PRCA. We also found no reports of hemolytic anemia or PRCA associated with entecavir. Continued improvement was observed after carefully restarting the drug, ruling out entecavir-induced hemolytic anemia and PRCA.
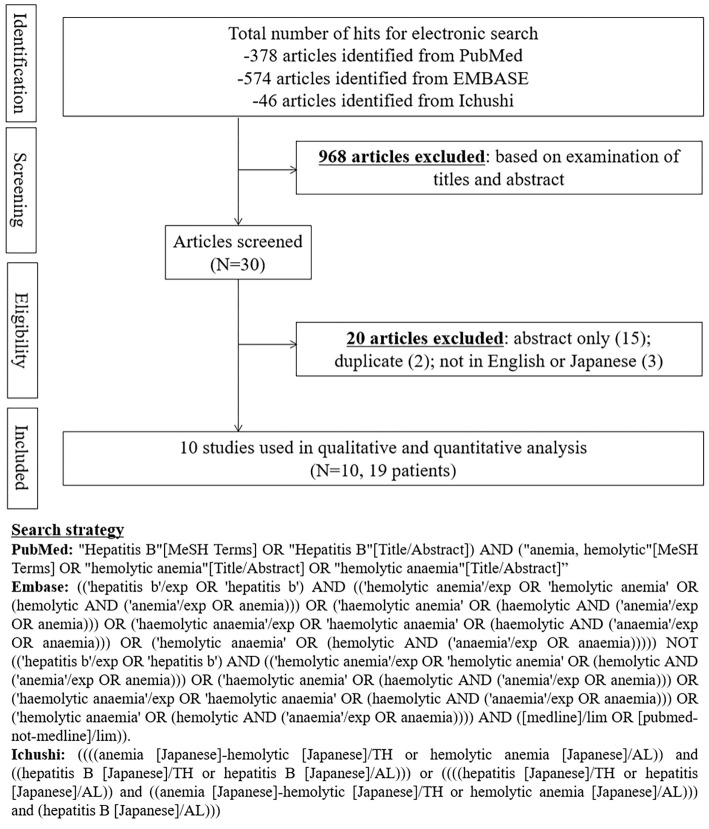
We conducted a systematic review using MeSH terms and pre-defined criteria, in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses Statement (Fig. 3). Two researchers independently searched PubMed, EMBASE, and Ichushi from their inception to September 30, 2020, to review and extract data from relevant cases. Acute HBV was diagnosed based on the detection of hepatitis B surface antigen with elevated transaminases. Hemolytic anemia was diagnosed based on the presence of anemia, hemolysis evidenced by high LDH, high bilirubin, low haptoglobin, and/or increased reticulocyte count after the exclusion of other causes of anemia. Patients were only included if hemolytic anemia occurred concurrently with or within 10 years after the onset of HBV infection.
Figure 3.
Flow diagram and search strategy for a systematic review of hemolytic anemia in the setting of hepatitis B virus infection.
Nineteen cases from 10 studies were included (14,15,23-30). The clinical characteristics of the 20 cases including our case are summarized in Table. The median age was 28 years old, and 40% were men. Most cases were reported from Asia, with 75% originating in Japan. The average time between the HBV diagnosis and hemolytic anemia was 24.5 (range: 5-61) days. A fever was present at the onset of hemolytic anemia in 65% of cases. Eighty percent had acute HBV infection, and the remaining 20% had chronic infection; 2 cases in each group were treated with antiviral agents. Three out of five acute cases with INR data had acute liver failure, defined as INR ≥1.5 with no pre-existing cirrhosis or anticoagulant therapy, with or without encephalopathy. Both peak ALT (n=6, median: 2,112 U/L, range: 780-4,191 U/L) and peak TB levels (n=5, median: 29.0 mg/dL, range: 11.6-35.1 mg/dL) tended to be very high in acute HBV cases with a delayed onset of hemolytic anemia. Erythroid hyperplasia was the sole finding in all seven cases for which bone marrow findings were reported. Steroid therapy was introduced in 53% of cases. Anemia improved within two months in 87.5% of cases, regardless of treatment. No mortality was reported.
Table.
Reports of Hemolytic Anemia in Patients with Hepatitis B Virus Infection.
| Reference | n | Age | Sex | Symptoms | Days from symptoms to admission | Days from HBV to anemia | Peak ALT | Peak TB | Laboratory values at onset of anemia | Bone marrow aspiration | Coombs test | Type of HBV infection | Acute liver failure | Treatment for HBV | Treatment for anemia | Outcome | Days to recovery from anemia | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hb (g/dL) |
ALT (U/L) |
LDH (U/L) |
TB mg/dL) |
Reticulocytes | Hapto globin (mg/dL) |
|||||||||||||||||
| 15 | 1 | 17 | F | jaundice, fever, chills, nausea, vomiting | 21 | 9 | 780 | 29 | 9.9 | 160 | NA | 29 | 27% | 0 | Erythroid hyperplasia | - | Acute | NA | NA | Transfusion | Cure | 18 |
| 20 | 1 | 2 | M | jaundice, pallor | 21 | 0 | NA | NA | 7.6 | 43 | 360 | 0.3 | 6% | 0 | NA | + (direct) | Chronic | Observation | Observation | Cure | 21 | |
| 21 | 1 | 33 | M | fever, abdominal pain | 14 | 27 | 2,615 | NA | 6.2 | 143 | 1,704 | 11.7 | 66% | ≤5 | Erythroid hyperplasia | - | Acute | No | Supportive | Steroids | Cure | Several |
| 22 | 1 | 25 | F | fever, nausea, jaundice | 21 | 48 | 1,608 | 25.7 | 5 | 120 | 3,350 | 25.7 | 16% | ≤20 | Erythroid hyperplasia | - | Acute | No | NA | Steroids | Cure | NA |
| 23 | 1 | 19 | F | fever, malaise, jaundice, abdominal pain | 14 | 37 | 1,035 | 32 | 4.9 | NA | NA | 32 | 172% | ≤10 | Erythroid hyperplasia | - | Acute | Yes | Supportive | NA | Cure | 58 |
| 14 | 10 | Mean 29.6 (16-46) | M:F 4:6 | fever (8 of 8) | NA | Mean 18.6 (5-37) | NA | NA | NA | NA | NA | NA | NA | NA | NA | - (7 of 7) | Acute | NA | NA | Steroids (5), Observation (3), Transfusion (1) | NA | NA |
| 24 | 1 | 3 | M | pallor, petechiae, ecchymoses | NA | 0 | NA | NA | 5.3 | 144 | NA | NA | 14% | NA | NA | + (direct) | Chronic | Antiviral | Steroids | Cure | NA | |
| 25 | 1 | 10 | F | pallor, malaise | NA | 0 | NA | NA | 2.7 | 121 | 975 | 3.8 | 41% | NA | Erythroid hyperplasia | + (direct) | Chronic | NA | Steroids Transfusion | Cure | 37 | |
| 26 | 1 | 66 | F | jaundice, loss of appetite, dizziness, palpitations, fatigue | 28 | 0 | NA | 11.6 | 5.3 | NA | 528 | 6.62 | 19% | 0.3 | Erythroid hyperplasia | + (direct, indirect) | Chronic | Antiviral | Steroids, rituximab | Cure | 33 | |
| 27 | 1 | 41 | F | nausea, jaundice, abdominal pain | 7 | 0 | 2,889 | 24.1 | 6.2 | 2,889 | 1,163 | 22.9 | NA | <0.24 | NA | - | Acute | Yes | Antiviral | Supportive | Cure | <120 |
| Our case | 1 | 24 | M | jaundice, fever, cough, dyspnea | 5 | 61 | 4,191 | 35.1 | 5.9 | 62 | 756 | 31.3 | 0.25% | ≤10 | Erythroid hyperplasia | - | Acute | Yes | Antiviral | Transfusion | Cure | 28 |
ALT: alanine transaminase, TB: total bilirubin, Hb: hemoglobin, LDH: lactate dehydrogenase, HBV: hepatitis B virus, NA: not available
The liver function had generally improved at the onset of the anemia, making an immunological mechanism more likely than direct hemolysis due to viral infection or liver damage. Reports of hemolytic anemia in chronic HBV infection also support this hypothesis (23,28,31).
The Coombs test positivity was low (23.5%), possibly due to Coombs-negative AIHA. AIHA is diagnosed based on evidence of a shortened erythrocyte survival in the setting of anti-red blood cell autoantibodies (32). Typical patients exhibit a positive direct antiglobulin test, but false-negatives occur in 5-10% of cases (33). Such false-negatives can occur in severe hemolysis, causing rapid erythrocyte loss (34). In the present case, Coombs-negative AIHA was essentially ruled out using red blood cell-associated IgG. The association between hemolysis and specific HBV genotypes could not be investigated in our study, as none of the case reports mentioned genotypes.
The expected increase in indirect bilirubin at the onset of hemolytic anemia was not clear in our case. We believe this is due to the extremely high TB level, which lingered after the acute HBV episode and may have masked the increase in indirect bilirubin resulting from hemolytic anemia. Thus, total and direct bilirubin trends may not be reliable indicators of hemolytic anemia in patients with severe persistent hyperbilirubinemia.
While no risk factors for HBV-induced hemolytic anemia could be identified, the prognosis appears favorable. HBV-associated hemolytic anemia should be suspected when anemia develops approximately one month after the HBV onset.
PRCA and HBV infection
PRCA is characterized by severe normocytic anemia, reticulocytopenia, and the absence of erythroblasts in otherwise normal bone marrow (35). Most acute cases are acquired, induced by either viruses or medications (35,36). Parvovirus is the most common viral cause (37). Approximately 30 cases of hepatitis-related PRCA have been reported, mostly in connection with HAV (38-51) but also with HBV (52), HCV (53), and hepatitis E virus (54). HCV cases are generally iatrogenic, resulting from treatment with interferon, ribavirin, mycophenolate mofetil, and epoetin alfa (55-58). Multiple pathological mechanisms of drug-induced PRCA have been suggested, including direct toxic effects on DNA synthesis by erythroid cells and indirect effects on accessory cells required for erythroid progenitor cells to differentiate into mature erythroblasts (59,60). Reports of HBV-related PRCA (11,52,61,62) suggest possible mechanisms, such as direct viral effects on hematopoietic cells, production of toxic substances by injured hepatocytes, an inability to inactivate erythropoietic toxins due to an impaired liver function, and autoimmune suppression of red blood cell production. However, the precise mechanism remains unknown. As many cases recover rapidly, the disease process may be transient and self-limiting in nature, possibly resulting from the reversible cell-mediated suppression of erythroid colony formation. Interestingly, the time interval of four to nine weeks from hepatitis to PRCA in published cases is similar to that of aplastic anemia, pancytopenia, and hemolytic anemia after HBV (11,49,63-66). Similar mechanisms may be involved in the viral inhibition of bone marrow hematopoiesis and selective targeting of immature bone marrow cells (67). As the mechanism underlying hepatitis-induced PRCA is not well understood, previous reports support the use of immunosuppressive agents in severe cases.
Although the delay in the bone marrow aspiration precluded a definite diagnosis of PRCA, transient HBV-associated PRCA was suspected in the present case. The time interval and transient, self-limiting nature were both consistent with previous reports.
Hemolytic anemia and PRCA
Hemolytic anemia and PRCA can occur in the same patient, either synchronously or metachronously (68). Synchronous autoimmune hemolytic anemia and PRCA have been reported in systemic lupus erythematosus (SLE), Sjögren's syndrome, HAV, human parvovirus B19, and Epstein-Barr virus (35,69-72).
There have been four reports of synchronous hemolytic anemia and PRCA associated with hepatitis viruses, all in the setting of HAV (38,42,50,71). The average time from the diagnosis of hepatitis to the onset of anemia was about three weeks. All cases responded well to treatment with steroids (1-1.5 mg/kg/day) and blood transfusions. There have been no reports of both occurring after HBV infection.
The simultaneous development of hemolytic anemia and PRCA could only be explained by direct damage to erythroid progenitor cells and the presence of peripheral destruction of mature red blood cells (73). Given the reported association between hepatitis viruses and myelosuppression (74) and the presence of antibody-related collagen diseases, such as SLE and Sjögren's syndrome, the possibility of antibody-induced hemolysis and myelosuppression should be considered (69,70). As anemia resulting from autoantibodies against the erythroid system and effective control with immunosuppressants have been reported, steroids should be considered if the clinical course is not favorable (75,76).
In our case, the marked decrease in the reticulocyte count could not be explained by hemolytic anemia alone, which usually triggers an increase in reticulocytes. The two disease processes may have both contributed to the rapid progression of anemia in our patient.
In conclusion, we herein report a case of acute HBV infection complicated by hemolytic anemia and possible PRCA that improved with conservative treatment. Further research should be conducted to determine the risk factors for hematological complications after acute viral hepatitis.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors would like to thank Dr. Ryosuke Koyamada and Dr. Takakazu Higuchi for their input on the hematological aspects of this case.
References
- 1.Lee WM. Hepatitis B virus infection. N Engl J Med 337: 1733-1745, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Lai CL, Ratziu V, Yuen MF, Poynard T. Viral hepatitis B. Lancet 362: 2089-2094, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Liang TJ. Hepatitis B: the virus and disease. Hepatology 49: S13-S21, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guillevin L, Mahr A, Callard P, et al. Hepatitis B virus-associated polyarteritis nodosa: clinical characteristics, outcome, and impact of treatment in 115 patients. Medicine (Baltimore) 84: 313-322, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Alpert E, Isselbacher KJ, Schur PH. The pathogenesis of arthritis associated with viral hepatitis. Complement-component studies. N Engl J Med 285: 185-189, 1971. [DOI] [PubMed] [Google Scholar]
- 6.Combes B, Shorey J, Barrera A, et al. Glomerulonephritis with deposition of Australia antigen-antibody complexes in glomerular basement membrane. Lancet 2: 234-237, 1971. [DOI] [PubMed] [Google Scholar]
- 7.Han SH. Extrahepatic manifestations of chronic hepatitis B. Clin Liver Dis 8: 403-418, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Casas R, Jones EA, Moreno-Otero R. Spectrum of anemia associated with chronic liver disease. World J Gastroenterol 15: 4653-4658, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhaliwal G, Cornett PA, Tierney LM Jr. Hemolytic anemia. Am Fam Physician 69: 2599-2606, 2004. [PubMed] [Google Scholar]
- 10.Phillips J, Henderson AC. Hemolytic anemia: evaluation and differential diagnosis. Am Fam Physician 98: 354-361, 2018. [PubMed] [Google Scholar]
- 11.Ide T, Sata M, Nouno R, Yamashita F, Nakano H, Tanikawa K. Clinical evaluation of four cases of acute viral hepatitis complicated by pure red cell aplasia. Am J Gastroenterol 89: 257-262, 1994. [PubMed] [Google Scholar]
- 12.Maya R, Gershwin ME, Shoenfeld Y. Hepatitis B virus (HBV) and autoimmune disease. Clin Rev Allergy Immunol 34: 85-102, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Karakus S, Arin BE, Arat Z, Karakayali H, Haberal M. Impact of hepatitis serology on development of leukopenia after solid organ transplantation. Transplant Proc 40: 199-201, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Kanematsu T, Nomura T, Higashi K, Ito M. [Hemolytic anemia in association with viral hepatitis]. Nihon Rinsho (Jpn J Clin Med) 54: 2539-2544, 1996. [PubMed] [Google Scholar]
- 15.Vanderhoof JA, Ament ME. Severe Coombs negative hemolytic anemia in hepatitis B. West J Med 125: 228-230, 1976. [PMC free article] [PubMed] [Google Scholar]
- 16.Jeong SH, Lee HS. Hepatitis A: clinical manifestations and management. Intervirology 53: 15-19, 2010. [DOI] [PubMed] [Google Scholar]
- 17.Cuthbert JA. Hepatitis A: old and new. Clin Microbiol Rev 14: 38-58, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elhajj II, Sharara AI, Taher AT. Chronic hepatitis C associated with Coombs-positive hemolytic anemia. Hematol J 5: 364-366, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Raffensperger EC. Acute acquired hemolytic anemia in association with acute viral hepatitis. Ann Intern Med 48: 1243-1253, 1958. [DOI] [PubMed] [Google Scholar]
- 20.Jochum C, Gieseler RK, Gawlista I, Fiedler A, Manka P, Saner FH. Hepatitis B-associated acute liver failure: immediate treatment with entecavir inhibits hepatitis B virus replication and potentially its sequelae. Digestion 80: 235-240, 2009. [DOI] [PubMed] [Google Scholar]
- 21.European Association for the Study of the Liver. EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J Hepatol 66: 1047-1081, 2017. [DOI] [PubMed] [Google Scholar]
- 22.Lee WM, Stravitz RT, Larson AM. Introduction to the revised American Association for the Study of Liver Diseases position paper on acute liver failure 2011. Hepatology 55: 965-967, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshioka K, Miyata H. Autoimmune haemolytic anaemia in an asymptomatic carrier of hepatitis B virus. Arch Dis Child 55: 233-234, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kazuhiro O, Hiroyuki K, Naoki AI, Hiroyuki K, Yoshihiro F. [A case of acute hepatitis type B complicated with severe hemolytic anemia]. Kanzou (Acta Hepatol Jpn) 25: 246-251, 1984. [Google Scholar]
- 25.Ishiyama T, Abe S, Horie S, Sugaya N, Wakabayashi Y, Hirose S. [Acute hepatitis type B complicated by hemolytic anemia]. Rinsho Ketsueki (Jpn J Clin Hematol) 28: 573-577, 1987. [PubMed] [Google Scholar]
- 26.Nakayama K, Kitada T, Uchida Y, Takenaka K, Sakaguchi S, Okumura M. A case of acute hepatitis B associated with hemolytic anemia. Kanzou (Acta Hepatol Jpn) 30: 673-677, 1989. [Google Scholar]
- 27.Kalayci AG, Dagdemir A, Dilber C, Albayrak D. Evans syndrome related to hepatitis B virus infection: a case that responded only to lamivudine therapy. J Pediatr Gastroenterol Nutr 32: 493-495, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Khawaja S, Abdul Muqtadir K, Taj Y. Warm autoimmune haemolytic anaemia and autoimmune hepatitis in an asymptomatic carrier of hepatitis B virus. J Pak Med Assoc 61: 512-515, 2011. [PubMed] [Google Scholar]
- 29.Zhang QL, Jia LJ, Zhang JB, Li WM, Bo YK, Li J. HBV and HCV coinfection associated with warm-type autoimmune hemolytic anemia: a case report. Turk J Haematol 31: 328-331, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furtado I, Valadares D, Nery FG. Acute hepatitis B virus infection and severe non-immune haemolytic anaemia: a rare relationship. BMJ Case Rep 2017: bcr2017221763, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gil Grande R, Gómez Pellico C, González García R, et al. [Autoimmune hemolytic anemia in a patient with chronic hepatitis B virus infection]. Rev Clin Esp 159: 217-220, 1980. [PubMed] [Google Scholar]
- 32.Packman CH. Hemolytic anemia due to warm autoantibodies. Blood Rev 22: 17-31, 2008. [DOI] [PubMed] [Google Scholar]
- 33.Garratty G. Immune hemolytic anemia associated with negative routine serology. Semin Hematol 42: 156-164, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Parker V, Tormey CA. The direct antiglobulin test: indications, interpretation, and pitfalls. Arch Pathol Lab Med 141: 305-310, 2017. [DOI] [PubMed] [Google Scholar]
- 35.Hirokawa M. [Diagnosis and management of pure red cell aplasia]. Rinsho Ketsueki (Jpn J Clin Hematol) 56: 1922-1931, 2015. [DOI] [PubMed] [Google Scholar]
- 36.Means RT, Jr. Pure red cell aplasia. Blood 128: 2504-2509, 2016. [DOI] [PubMed] [Google Scholar]
- 37.Frickhofen N, Chen ZJ, Young NS, Cohen BJ, Heimpel H, Abkowitz JL. Parvovirus B19 as a cause of acquired chronic pure red cell aplasia. Br J Haematol 87: 818-824, 1994. [DOI] [PubMed] [Google Scholar]
- 38.Chang HJ, Sinn DH, Cho SG, et al. Pure red-cell aplasia and autoimmune hemolytic anemia in a patient with acute hepatitis A. Clin Mol Hepatol 20: 204-207, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim PS, Kim IH, Kim SH, Lee SO, Kim SW. A case of severe acute hepatitis a complicated with pure red cell aplasia. Korean J Gastroenterol 60: 177-181, 2012. [DOI] [PubMed] [Google Scholar]
- 40.Yoon YK, Park DW, Sohn JW, Kim MJ, Kim I, Nam MH. Corticosteroid treatment of pure red cell aplasia in a patient with hepatitis A. Acta Haematol 128: 60-63, 2012. [DOI] [PubMed] [Google Scholar]
- 41.Lee TH, Oh SJ, Hong S, Lee KB, Park H, Woo HY. Pure red cell aplasia caused by acute hepatitis A. Chonnam Med J 47: 51-53, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koiso H, Kobayashi S, Ueki K, et al. [Pure red cell aplasia accompanied by autoimmune hemolytic anemia in a patient with type A viral hepatitis]. Rinsho Ketsueki (Jpn J Clin Hematol) 50: 424-429, 2009. [PubMed] [Google Scholar]
- 43.Della Loggia P, Cremonini L. [Acute hepatitis-associated pure red cell aplasia: a case report]. Infez Med 10: 236-238, 2002. [PubMed] [Google Scholar]
- 44.Kusaba N, Yoshida H, Ohkubo F, Shimokawa Y, Sata M. [Autoimmune thrombocytopenia and erythroid hypoplasia associated with hepatitis A]. Rinsho Ketsueki (Jpn J Clin Hematol) 41: 739-744, 2000. [PubMed] [Google Scholar]
- 45.Simmons J, Stein L, Kaufman A. Pure red cell aplasia and hepatitis A. South Med J 86: 1274-1276, 1993. [DOI] [PubMed] [Google Scholar]
- 46.Abe K, Uto H, Yamamoto Y, et al. [A case of acute hepatitis type A showing simultaneous occurrence of pure red cell aplasia and hemolytic anemia]. Nihon Shokakibyo Gakkai Zasshi (Jpn J Gastroenterol) 88: 1500-1503, 1991. [PubMed] [Google Scholar]
- 47.Tomonari A, Hirai K, Aoki H, et al. [Pure red cell aplasia and pseudothrombocytopenia associated with hepatitis A]. Rinsho Ketsueki (Jpn J Clin Hematol) 32: 147-151, 1991. [PubMed] [Google Scholar]
- 48.Tanri R, Nishimura H, Nakamura K, et al. [A case of acute hepatitis A complicated with acute pure red cell aplasia]. Nihon Naika Gakkai Zasshi (J Jpn Soc Intern Med) 79: 947-948, 1990. [PubMed] [Google Scholar]
- 49.Darchis I, Colombel JF, Cortot A, et al. Pure red cell aplasia during the course of virus A hepatitis. Gastroenterol Clin Biol 14: 1027, 1990. [PubMed] [Google Scholar]
- 50.Gundersen SG, Bjoerneklett A, Bruun JN. Severe erythroblastopenia and hemolytic anemia during a hepatitis A infection. Scand J Infect Dis 21: 225-228, 1989. [DOI] [PubMed] [Google Scholar]
- 51.Tomiyama J, Kudou H, Adachi Y, Kinugasa K, Hanada T. [A case of pure red cell aplasia following hepatitis A]. Rinsho Ketsueki (Jpn J Clin Hematol) 28: 2187-2192, 1987. [PubMed] [Google Scholar]
- 52.Sears DA, George JN, Gold MS. Transient red blood cell aplasia in association with viral hepatitis. Occurrence four years apart in siblings. Arch Intern Med 135: 1585-1589, 1975. [PubMed] [Google Scholar]
- 53.Sinha AK, Agarwal A, Lakhey M, Ansari J, Rani S. Pure red cell aplasia - report of 11 cases from eastern Nepal. Indian J Pathol Microbiol 46: 405-408, 2003. [PubMed] [Google Scholar]
- 54.Li C, Wang HF. Hepatitis E virus-related acute liver failure associated with pure red cell aplasia. Hepatobiliary Pancreat Dis Int 10: 557-558, 2011. [DOI] [PubMed] [Google Scholar]
- 55.Skabelund AJ, Hauser TR, Goist KJ. Pure red cell aplasia caused by ribavirin and interferon treatment. Clin J Gastroenterol 4: 313-317, 2011. [DOI] [PubMed] [Google Scholar]
- 56.Kato M, Ryu T, Miura H, Yamada H, Saito T. [Peginterferon alfa-2b and ribavirin-induced pure red cell aplasia during treatment of chronic hepatitis C treated by cyclosporin A with good response]. Nihon Naika Gakkai Zasshi (J Jpn Soc Intern Med) 97: 1678-1680, 2008. [DOI] [PubMed] [Google Scholar]
- 57.Hodo Y, Tsuji K, Mizukoshi E, et al. Pure red cell aplasia associated with concomitant use of mycophenolate mofetil and ribavirin in post-transplant recurrent hepatitis C. Transpl Int 19: 170-171, 2006. [DOI] [PubMed] [Google Scholar]
- 58.Stravitz RT, Chung H, Sterling RK, et al. Antibody-mediated pure red cell aplasia due to epoetin alfa during antiviral therapy of chronic hepatitis C. Am J Gastroenterol 100: 1415-1419, 2005. [DOI] [PubMed] [Google Scholar]
- 59.Yunis AA, Arimura GK, Lutcher CL, Blasquez J, Halloran M. Biochemical lesion in Dilantin-induced erythroid aplasia. Blood 30: 587-600, 1967. [PubMed] [Google Scholar]
- 60.Dessypris EN, Redline S, Harris JW, Krantz SB. Diphenylhydantoin-induced pure red cell aplasia. Blood 65: 789-794, 1985. [PubMed] [Google Scholar]
- 61.al-Awami Y, Sears DA, Carrum G, Udden MM, Alter BP, Conlon CL. Pure red cell aplasia associated with hepatitis C infection. Am J Med Sci 314: 113-117, 1997. [DOI] [PubMed] [Google Scholar]
- 62.Sreedharanunni S, Sachdeva M, Prakash G, Das R. Persistent γδ T large granular lymphocytosis in a patient with refractory pure red cell aplasia, celiac disease, and chronic hepatitis B infection. J Postgrad Med 62: 40-43, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brown KE, Tisdale J, Barrett AJ, Dunbar CE, Young NS. Hepatitis-associated aplastic anemia. N Engl J Med 336: 1059-1064, 1997. [DOI] [PubMed] [Google Scholar]
- 64.Safadi R, Or R, Ilan Y, et al. Lack of known hepatitis virus in hepatitis-associated aplastic anemia and outcome after bone marrow transplantation. Bone Marrow Transplant 27: 183-190, 2001. [DOI] [PubMed] [Google Scholar]
- 65.Foon KA, Mitsuyasu RT, Schroff RW, McIntyre RE, Champlin R, Gale RP. Immunologic defects in young male patients with hepatitis-associated aplastic anemia. Ann Intern Med 100: 657-662, 1984. [DOI] [PubMed] [Google Scholar]
- 66.Kojima S, Matsuyama K, Kodera Y, Okada J. Circulating activated suppressor T lymphocytes in hepatitis-associated aplastic anaemia. Br J Haematol 71: 147-151, 1989. [DOI] [PubMed] [Google Scholar]
- 67.Sing GK, Prior S, Fernan A, Cooksley G. Hepatitis B virus differentially suppresses myelopoiesis and displays tropism for immature hematopoietic cells. J Virol 67: 3454-3460, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sekiguchi Y, Shimada A, Imai H, et al. A case of recurrent autoimmune hemolytic anemia during remission associated with acute pure red cell aplasia and hemophagocytic syndrome due to human parvovirus B19 infection successfully treated by steroid pulse therapy with a review of the literature. Int J Clin Exp Pathol 7: 2624-2635, 2014. [PMC free article] [PubMed] [Google Scholar]
- 69.Hara A, Wada T, Kitajima S, et al. Combined pure red cell aplasia and autoimmune hemolytic anemia in systemic lupus erythematosus with anti-erythropoietin autoantibodies. Am J Hematol 83: 750-752, 2008. [DOI] [PubMed] [Google Scholar]
- 70.Komaru Y, Higuchi T, Koyamada R, et al. Primary Sjögren syndrome presenting with hemolytic anemia and pure red cell aplasia following delivery due to Coombs-negative autoimmune hemolytic anemia and hemophagocytosis. Intern Med 52: 2343-2346, 2013. [DOI] [PubMed] [Google Scholar]
- 71.Chehal A, Sharara AI, Haidar HA, Haidar J, Bazarbachi A. Acute viral hepatitis A and parvovirus B19 infections complicated by pure red cell aplasia and autoimmune hemolytic anemia. J Hepatol 37: 163-165, 2002. [DOI] [PubMed] [Google Scholar]
- 72.Katayama H, Takeuchi M, Yoshino T, et al. Epstein-Barr virus associated diffuse large B-cell lymphoma complicated by autoimmune hemolytic anemia and pure red cell aplasia. Leuk Lymphoma 42: 539-542, 2001. [DOI] [PubMed] [Google Scholar]
- 73.Saha M, Ray S, Kundu S, Chakrabarti P. Pure red cell aplasia following autoimmune hemolytic anemia: an enigma. J Postgrad Med 59: 51-53, 2013. [DOI] [PubMed] [Google Scholar]
- 74.Zeldis JB, Mugishima H, Steinberg HN, Nir E, Gale RP. In vitro hepatitis B virus infection of human bone marrow cells. J Clin Invest 78: 411-417, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mangan KF, Besa EC, Shadduck RK, Tedrow H, Ray PK. Demonstration of two distinct antibodies in autoimmune hemolytic anemia with reticulocytopenia and red cell aplasia. Exp Hematol 12: 788-793, 1984. [PubMed] [Google Scholar]
- 76.Taniguchi S, Shibuya T, Morioka E, et al. Demonstration of three distinct immunological disorders on erythropoiesis in a patient with pure red cell aplasia and autoimmune haemolytic anaemia associated with thymoma. Br J Haematol 68: 473-477, 1988. [DOI] [PubMed] [Google Scholar]