Abstract
We herein report the long-term changes in chest computed tomography (CT) findings from early sarcoidosis lesions to pleuroparenchymal fibroelastosis (PPFE)-like lesions in a 30-year-old man with granulomas on a transbronchial lung biopsy. Multiple bilateral micronodular and nodular opacities around the bronchovascular bundle in the upper lobes detected by chest CT in 2004 disappeared, but paradoxically, peripheral consolidations continued to grow at the periphery of the original lesions. Chest CT in 2017 confirmed the progression of bilateral shrinkage of the upper lobe, spread of peripheral consolidations and wedge-shaped opacities below the first rib, and bronchiectatic air bronchograms, confirming PPFE-like lesions.
Keywords: sarcoidosis, pleuroparenchymal fibroelastosis, non-caseating epithelioid granuloma, pleural thickening, subpleural consolidation
Introduction
The mechanism underlying this progression of the disease state in the chronic stage of sarcoidosis remains unclear, mainly because this disease is relatively rare, and decades of observation are frequently required before the development of pulmonary fibrosis can be seen. We previously examined computed tomography (CT) images of patients with sarcoidosis who developed chronic respiratory failure and identified characteristic findings, namely central and peripheral consolidations, a central-peripheral (C-P) band linking them, traction bronchiectasis clusters, cysts/bullae, and shrinkage of the upper lobe (SUL) (1). In particular, increases in peripheral consolidations were observed in 7 of 10 patients (1).
The term “peripheral consolidation” was defined by our group to describe the region of discrete consolidations with well-defined margins from the sixth bronchus to the area right under the pleura predominantly in the upper lobe, which were observed in the chronic phase of sarcoidosis (1). Peripheral consolidations often become more obvious during the progression of pulmonary sarcoidosis (1). However, this consolidation has scarcely been investigated and has been called “pleural thickening” in a few studies (2-4). It should be noted that this lesion is continuous from the lung field to the pleura; thus, changes in the lung field in particular need to be examined (5,6). Granulomas formed in the pleura cannot be distinguished by CT from those formed in the alveolar septa next to the pleura (5).
Similarities in radiographic findings between pulmonary sarcoidosis and pleuroparenchymal fibroelastosis (PPFE) have rarely been discussed. There have been no reports on similarities between peripheral consolidations and characteristic PPFE findings (i.e. subpleural infiltrative and/or wedge-shaped opacities) (7,8). We performed long-term observation of lesions in the lung field from the early stage in a patient with granulomas confirmed by a transbronchial lung biopsy (TBLB). We describe the disappearance of the original lesions, and at their periphery, coinciding gradual increases in peripheral consolidations and manifestation of PPFE-like lesions.
Case Report
In October 2004, a 30-year-old man with cough visited the respiratory department of a regional hospital because of abnormal shadows on chest X-ray. He had a smoking history of 10.5 pack-years. Findings on a laboratory examination were as follows: angiotensin-converting enzyme (ACE) 21.3 U/L (normal ≤21.4), soluble interleukin 2 receptor (sIL-2R) 1,060 U/mL (normal ≤613), and Krebs von den Lungen-6 glycoprotein (KL-6) 573 U/mL (normal ≤500). Chest X-ray showed diffuse micronodular, reticulated, and infiltrative opacities predominantly in the bilateral upper and middle lung fields, in addition to bilateral hilar and mediastinal lymphadenopathy (Fig. 1a). In addition, chest CT found characteristics of lung field lesions commonly seen at the early stage of sarcoidosis (i.e. multiple micronodular and infiltrative opacities predominantly around bronchovascular bundle in both upper lobes) and bilateral hilar and mediastinal lymphadenopathy (Fig. 2a). Bronchoscopy revealed reticular telangiectasis on the tracheal and bronchial walls. Bronchoalveolar lavage (BAL) results were a total cell count of 2.2×105/mL, lymphocytes 17%, and CD4/CD8 ratio 3.6. A random TBLB revealed non-caseating epithelioid granulomas and fibrotic thickening of the alveolar septa in specimens from the right lung.
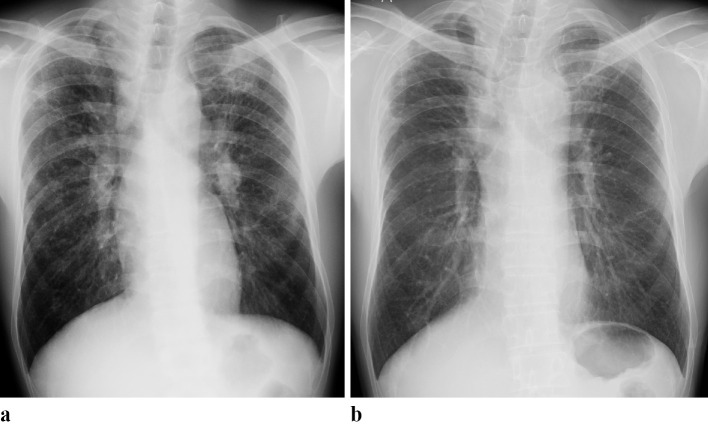
Figure 1.
Chest X-ray images. (a) In 2004, diffuse micronodular, reticulated, and infiltrative opacities predominantly in the bilateral upper and middle lung fields, and bilateral hilar and mediastinal lymphadenopathy was observed. (b) In 2017, bilateral shrinkage of the upper lung had worsened. Bilateral subpleural consolidations at the apex regions appeared, while the opacities in the lung field, observed in 2004, were decreased.
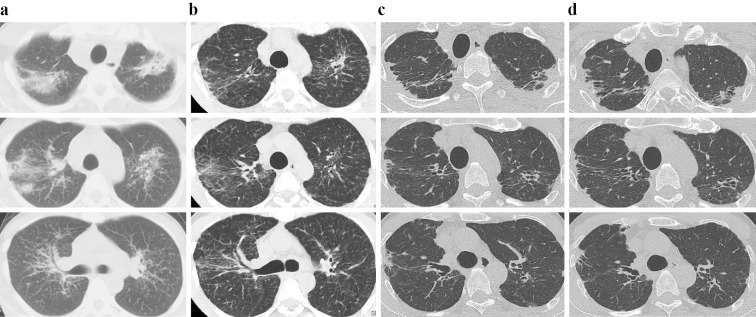
Figure 2.
Chest CT images. (a) In 2004, typical early sarcoidosis lesions in the lung field (i.e. multiple micronodular and infiltrative opacities around the bronchovascular bundle in both upper lobes) and bilateral hilar and mediastinal lymphadenopathy were observed. (b) In 2006, the micronodular and infiltrative opacities had decreased bilaterally in the lung field. (c) In 2017, the progression of bilateral shrinkage of the upper lobe was confirmed. Subpleural consolidations and wedge-shaped opacities were enhanced and spread below the anterior part of the first rib at the periphery of the infiltrative and micronodular opacification that had disappeared. Bronchiectatic air bronchograms were found within the consolidations, and PPFE-like lesions were confirmed. (d) In 2021, there were no marked changes in the consolidations or wedge-shaped opacities compared with the findings in 2017.
Pulmonary function test results were vital capacity (VC) 3.79 L (%VC 90.2%), forced expiratory volume in 1 second (FEV1) 3.63 L, FEV1% (G) 96.5%, and diffusing capacity of lung for carbon monoxide (DLCO) 18.71 mL/min/mmHg [%DLCO/alveolar volume (AV) 88.8%]. The patient had uveitis and scar infiltration on his left knee, and local treatment effectively controlled them. Taken together, these findings led to a diagnosis of sarcoidosis.
He was followed up without the use of immunosuppressive agents, including steroids. Chest CT in 2006 revealed decreases in micronodular and infiltrative opacities bilaterally in the lung field (Fig. 2b). Chest X-ray in 2017 showed progression of bilateral SUL and the formation of bilateral peripheral consolidations in the apex regions, while the opacities in the lung field observed in 2004 had disappeared almost completely (Fig. 1b). Chest CT in 2017 further confirmed progression of bilateral SUL (Fig. 2c). Peripheral consolidations and wedge-shaped opacities were enhanced and spread below the anterior part of the first rib at the periphery of the infiltrative and micronodular opacities that had disappeared. Bronchiectatic air bronchograms were found within the consolidations, thus confirming PPFE-like lesions. Pulmonary function test results in November 2017 were VC 3.55 L (%VC 89.9%), and FEV1% (G) 93.9%.
The patient was attending regular outpatient visits as of January 2021 with no obvious decreases in VC and FEV1% (G), although he complained of shortness of breath, probably due to impairment of the inspiratory muscle. Chest CT confirmed no progression in the subpleural consolidations or wedge-shaped opacities compared with 2017 (Fig. 2d). The levels of ACE and sIL-2R decreased to 12.0 U/L and 373 U/mL, respectively.
Discussion
This is a valuable report demonstrating the long-term changes in chest CT findings, from early sarcoidosis lesions to PPFE-like lesions in a patient with granulomas confirmed by TBLB. PPFE-like lesions have not yet been reported in pulmonary sarcoidosis. Two important observations were made in this case. First, our long-term observation confirmed that the early sarcoidosis lesions (i.e., bilateral multiple micronodular and nodular opacities around bronchovascular bundle in the upper lobes) detected by chest CT in 2004 had disappeared, but paradoxically, peripheral consolidations formed and continued to grow at the periphery of the original lesions. Second, chest X-ray and CT performed in 2017 confirmed the progression of bilateral SUL, spread of peripheral consolidations and wedge-shaped opacities below the first rib, and bronchiectatic air bronchograms, confirming PPFE-like lesions.
SUL and increases in peripheral consolidations have often been observed in the fibrotic process in pulmonary sarcoidosis (1). In addition, SUL and subpleural consolidations with bronchiectatic air bronchograms are significant findings in the radiographic diagnosis of PPFE (7,8), although concrete criteria have not yet been established. However, similarities in radiographic findings between pulmonary sarcoidosis and PPFE have been the subject of little attention, so revealing these similarities is expected to provide insight into the fibrotic process in pulmonary sarcoidosis.
Concerning the first observation, to our knowledge, this is the first study to demonstrate the coinciding formation of subpleural consolidations at the periphery of micronodular opacities around the bronchovascular bundle as the opacities disappeared. These consolidations were different from airspace consolidations, which are characterized by a gradual transition in intensity with poorly defined margins; instead, this case had discrete consolidations with clearly defined margins that are indicative of fibrotic lesions (9,10). Subpleural consolidations appearing in the chronic stage of sarcoidosis have been described as “pleural thickening” (2-4), but they were not continuous with the lung field, and no relationship was noted between the pleural thickening and micronodular opacities around the bronchovascular bundle. The relationship can be explained by the anatomical knowledge that lymph around the bronchovascular bundle mainly flows toward the hilum and partly toward the pleural surface (11). In other words, it is likely that macrophages phagocytosed antigens that invaded via the respiratory tract (12) in the bronchovascular bundles and pulmonary lobules and entered the lymph flow to travel to the hilum and pleura, which were observed as central and peripheral consolidations, respectively.
Concerning the second observation, in addition to the present case of sarcoidosis, we have encountered patients with pulmonary sarcoidosis who eventually developed PPFE-like lesions and developed a restrictive ventilatory defect (data not shown). Although the histological diagnosis of these PPFE-like lesions was not obtained, we did not detect mycobacterial or fungal infection nor any other pulmonary diseases during the frequent medical examinations in this patient, and we regarded these lesions as a consequence of the long clinical course of sarcoidosis rather than as secondary PPFE due to pulmonary mycobacteriosis or chronic hypersensitivity pneumonitis. The PPFE-like lesions appeared to develop when peripheral consolidations due to sarcoidosis were limited to the apex regions of the upper lobes, suggesting possible similarities in the predominance and pathology of lesion formation between PPFE and chronic sarcoidosis. One of the pathological characteristics of PPFE is lymphatic proliferation, which may be a compensatory event secondary to impaired lymphatic drainage (13). It is necessary to investigate whether or not subpleural consolidations on chest CT in the present case were associated with particular pathological findings, such as fibrosis and the confluence of granulomas; increases in elastic fibers, proliferation of podoplanin-positive myofibroblasts (14), and lymphatic proliferation (13) as seen in PPFE cases; and increases in smooth muscle and collagenous fibers as seen in idiopathic pulmonary fibrosis/usual interstitial pneumonia cases.
In conclusion, this study reported for the first time the course of development of PPFE-like lesions in pulmonary sarcoidosis, suggesting that previously diagnosed cases of PPFE may include cases of chronic sarcoidosis. Similarities in imaging findings between pulmonary sarcoidosis and PPFE may also reflect similarities in pathology. The accumulation of evidence, including pathological findings, from a greater number of cases is necessary to understand the progression of fibrosis in sarcoidosis.
Written informed consent was obtained from the patient for the publication of this case report and accompanying images.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Sawahata M, Johkoh T, Kawanobe T, et al. Computed tomography images of fibrotic pulmonary sarcoidosis leading to chronic respiratory failure. J Clin Med 9: 142, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patterson KC, Strek ME. Pulmonary fibrosis in sarcoidosis. Clinical features and outcomes. Ann Am Thorac Soc 10: 362-370, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Szwarcberg JB, Glajchen N, Teirstein AS. Pleural involvement in chronic sarcoidosis detected by thoracic CT scanning. Sarcoidosis Vasc Diffuse Lung Dis 58-62, 2005. [PubMed] [Google Scholar]
- 4.Soskel NT, Sharma OP. Pleural involvement in sarcoidosis. Curr Opin Pulm Med 6: 455-468, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Nishimura K, Itoh H, Kitaichi M, Nagai S, Izumi T. CT and pathological correlation of pulmonary sarcoidosis. Semin Ultrasound CT MR 16: 361-370, 1995. [DOI] [PubMed] [Google Scholar]
- 6.Criado E, Sánchez M, Ramírez J, et al. Pulmonary sarcoidosis: typical and atypical manifestations at high-resolution CT with pathologic correlation. Radiographics 30: 1567-1586, 2010. [DOI] [PubMed] [Google Scholar]
- 7.Sumikawa H, Johkoh T, Egashira R, et al. Pleuroparenchymal fibroelastosis-like lesions in patients with interstitial pneumonia diagnosed by multidisciplinary discussion with surgical lung biopsy. Eur J Radiol Open 7: 100298, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sumikawa H, Johkoh T, Iwasawa T, Nakanishi K, Tomiyama N. Pleuroparenchymal fibroelastosis-like lesions on chest computed tomography in routine clinical practice. Jpn J Radiol 230-236, 2019. [DOI] [PubMed] [Google Scholar]
- 9.Lähde S, Jartti A, Broas M, et al. HRCT findings in the lungs of primary care patients with lower respiratory tract infection. Acta Radiol 43: 159-163, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Aoki T, Nagata Y, Negoro Y, et al. Evaluation of lung injury after three-dimensional conformal stereotactic radiation therapy for solitary lung tumors: CT appearance. Radiology 230: 101-108, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Gurney JW. Cross-sectional physiology of the lung. Radiology 178: 1-10, 1991. [DOI] [PubMed] [Google Scholar]
- 12.Grunewald J, Grutters JC, Arkema EV, Saketkoo LA, Moller DR, Müller-Quernheim J. Sarcoidosis. Nat Rev Dis Primers 5: 45, 2019. [DOI] [PubMed] [Google Scholar]
- 13.Kinoshita Y, Watanabe K, Ishii H, Kushima H, Fujita M, Nabeshima K. Significant increases in the density and number of lymphatic vessels in pleuroparenchymal fibroelastosis. Histopathology 73: 417-427, 2018. [DOI] [PubMed] [Google Scholar]
- 14.Enomoto Y, Matsushita S, Meguro S, et al. Podoplanin-positive myofibroblasts: a pathologic hallmark of pleuroparenchymal fibroelastosis. Histopathology 72: 1209-1215, 2018. [DOI] [PubMed] [Google Scholar]