Summary
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has caused a global pandemic since late 2019 that resulted in more than 360 million population infection. Among them, less than 7% of infected individuals develop severe or critical illness. Mass vaccination has been carried out, but reinfection and vaccine breakthrough cases still occur. Besides supportive and antiviral medications, much attention has been paid in immunotherapies that aim at reducing pathological changes in the lungs. Mesenchymal stem cells (MSCs) is used as an option because of their immunomodulatory, anti-inflammatory, and regenerative properties. As of January 16, 2022, when ClinicalTrials.gov was searched for “Mesenchymal stem cells and COVID-19,” over 80 clinical trials were registered. MSC therapy was found to be safe and some effective in preclinical and clinical studies. Here, we summarize the major pathological characteristics of COVID-19 and provide scientific and rational evidence for the safety and possible effectiveness of MSCs in COVID-19 treatment.
Keywords: COVID-19, Mesenchymal stem cell, Immune therapy, Lung injury
Introduction
Coronavirus disease 2019 (COVID-19), caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has developed into a global pandemic that began in late 2019. As of January 16, 2022, a total of 5,551,970 deaths have occurred out of a total of 328,675,785 COVID-19 cases.1 Owing to the emergence of novel viral variants, including omicron, the risks of new infections, reinfections, and vaccine breakthrough infections has increased considerably worldwide, leading to a situation where the current epidemic is not yet well controlled. Similar to SARS-CoV-1 and MERS-CoV, SARS-CoV-2 belongs to the beta coronavirus family.2 Individuals infected by the virus are frequently classified based on the clinical severity of their cases and designated as asymptomatic, mild, moderate, severe, or critical.3 Severe and critical patients, particularly older adults and those with co-existing illnesses, have a high risk of mortality4 and characterizied by over-activated immune disorders. These include inflammatory hyper-responses and cytokine storms, which contribute to pulmonary tissue damage, air exchange dysfunction, acute respiratory distress syndrome (ARDS), multiple organ failure, and even death. To resolve the immune dysfunction arising from COVID-19, besides symptomatic treatment and supplemental oxygen therapy, certain immunotherapy-related clinical trials have been conducted, including treatments based on the use of glucocorticoids,5 interleukin (IL)-1 family blockers,6,7 anti-IL-6 antibodies,8 convalescent plasma,9 and antiviral specific neutralizing antibodies.10 Published data have demonstrated that immune-modulation regimens are well tolerated and show some effectiveness in clinical trial; however, further investigation is needed to confirm their efficacy in large-cohort randomized, controlled studies.
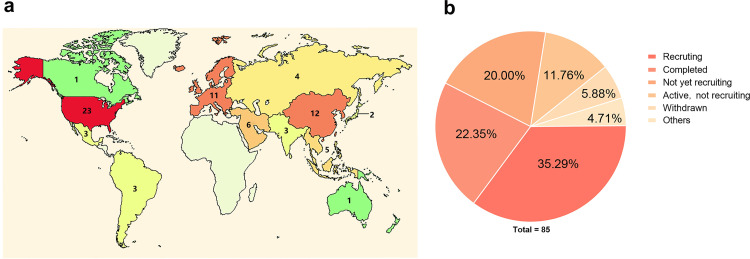
Mesenchymal stem cells (MSCs) possess immunomodulatory, anti-inflammatory, and regenerative properties.11 The safety and some effectiveness of MSCs have been investigated in various clinical trials for the treatment of several disorders, including graft-versus-host disease, inflammatory bowel disease, osteoarthritis, rheumatoid arthritis, and multiple sclerosis.12 The therapeutic roles of MSCs in preclinical models and clinical trials of acute lung injury, ARDS, and lung fibrosis suggest the feasibility of MSC treatment for COVID-19. For these reasons, MSC therapy can be considered as an optional interventional method for improving clinical outcomes in severe and critical patients. By January 16, 2022, when ClinicalTrials.gov was searched using the terms “Mesenchymal stem cells and COVID-19,” there were over 80 clinical trials registered (Figure 1). This review summarizes the pathological features of COVID-19 patients, along with the current progress and challenges of using MSCs to treat COVID-19, and also establishes the rationality of using MSCs in the treatment of the disease.
Figure 1.
A summary of clinical trials registered in ClinicalTrials.gov by searching “Mesenchymal stem cells and COVID-19”. (a) A map for all registered clinical trials. (b) The chart for the recruitment status of clinical trials.
Pathological features of COVID-19
During acute infection with SARS-CoV-2, viral replication and attack may directly result in injury to the respiratory tract and pulmonary tissues, which can trigger a series of over-active immune responses that not only play an antiviral role but also induce tissue inflammation and damage. Autopsies of dead patients with critical illness showed that the histological characteristics of lesions in the lungs were virus-induced and led to diffuse alveolar epithelial damage and inflammatory cell infiltration. This resulted in alveolar destruction with alveolar lining cell necrosis, protein-rich exudates, hyaline membrane formation, and endothelial cell membrane destruction. All of these eventually led to an increase in alveolar-capillary membrane permeability and loss of aerated lung tissue. Moreover, certain patients had fibrous deposition, mural fibrosis, and microcystic honeycombing in the lungs.13, 14, 15, 16 In contrast to other types of pneumonia, SARS-CoV-2 appears more likely to cause severe thrombotic disorders and widespread thrombosis with microangiopathy.14,17,18 In mild and moderate cases, innate and adaptive immune responses are well coordinated, which contributes to viral symptom resolution and convalesence.19 However, in severe cases, over-activation and massive infiltration of immunocytes such as macrophages, natural killer cells, and CD4+ and CD8+ T cells into the lungs result in high levels of cytokine and chemokine production.20 Uncontrolled pro-inflammatory feedback triggers a cytokine storm and accelerates local tissue damage and systemic injuries.21 Severe patients showed a significant increase in plasma levels of cytokines, including IL-2, IL-6, IL-7, IL-10, granulocyte-colony stimulating factor, interferon-inducible protein 10, monocyte chemoattractant protein-1, macrophage inflammatory protein (MIP)-1α, and tumor necrosis factor (TNF)-α.22, 23, 24, 25 The antiviral immune-related interferon (IFN) type I response was also highly impaired in the majority of patients with severe symptoms. Furthermore, the total numbers of peripheral CD4+ T cells and CD8+ T cells were significantly lower in severe and critical cases,26,27 particularly in patients with lymphocytopenia, impaired type I IFN production, and excessive activation of Th1 and inflammatory monocytes.28 A high viral load may further aggravate the ongoing inflammatory cascades.29
Generally, pulmonary inflammatory lesions were primarily resolved in the convalescence stage, while > 80% of severe/critical patients and 60% of mild/moderate patients still exhibited residual computed tomography (CT) abnormalities three months after discharge. These predominantly included ground-glass opacity followed by strip-like fibrosis.30 A recent result of a one-year follow-up study showed that 25% of individuals discharged from hospitalization still had chest CT abnormalities, including residual linear opacities and multifocal reticular/cystic lesions, and older patients with severe pneumonia, ARDS, and lymphopenia were more likely to develop CT abnormalities for over a year.31 Pulmonary fibrosis is seen as an important factor affecting long-term clinical outcomes.32,33 In an extended study of SARS-CoV-1-infected patients, lung fibrosis persisted for over seven years, suggesting that lung fibrosis could be a persistent sequela of pulmonary inflammatory injury.34 The increased expression of angiotensin-converting enzyme 2, transforming growth factor beta, connective tissue growth factor, and fibronectin35 and endothelial/epithelial-to-mesenchymal transition have all been believed to account for pulmonary fibrosis and vascular destruction post-COVID-19.36
Extrapulmonary manifestations, including thrombotic complications, myocardial dysfunction and arrhythmia, acute coronary syndromes, hepatocellular injury, gastrointestinal symptoms, acute kidney injury, and hyperglycemia, may further complicate the natural course of COVID-19 in severe and critical cases.37, 38, 39 A broad distribution of SARS-CoV-2 in the respiratory, cardiovascular, genitourinary, digestive, and immune systems and in endo/exocrine glands and the skin was identified in autopsy specimens.38,40 Renal autopsy revealed coronavirus-like particles in the cytoplasm of the renal proximal tubular epithelium, accompanied by prominent acute renal tubular injury.41 Human islet autopsy showed selective SARS-CoV-2 infection in β cells, followed by attenuated pancreatic insulin secretion and increased β cell apoptosis.42 In the heart, both compositional and gene expression changes were recorded during acute SARS-CoV-2 infection.43
Rationality of MSC treatment for COVID-19
MSCs may exert immunomodulation via autocrine, paracrine, and endocrine pathways (Figure 2).44, 45, 46 MSCs can modulate host innate and adaptive immune responses, thus reducing proinflammatory cytokine production.45,46 Given the previously described pathological characteristics of COVID-19,47,48 MSCs are considered an optional candidate for the treatment of COVID-19. In preclinical models of acute lung injury, the benefit of MSCs was associated with the alleviation of local and systemic inflammation,49 the amelioration of immune cell activation,50 and mitigated lung injury.45 MSCs have been shown to reduce the infiltration of macrophages, neutrophils, and DCs into the lungs and to decrease the levels of MIP-2, TNF-α, IL-6, IL-1β, and IL-12p70 in bronchoalveolar lavage fluid.45,46,49,51,52 Stanniocalcin-2 was found to mediate the effect of MSCs through modulating macrophage polarization from the M1 to M2 phenotype.53 In addition, MSCs induce mature DCs to become tolerogenic DCs, maintain immune tolerance, and negatively regulate the immune response.54 Furthermore, MSCs can attenuate pulmonary inflammation by regulating different T cell subsets; for example, MSCs can inhibit the activation and proliferation of T cells and induce the polarization of CCR2+CD4+ T cell subsets enriched with forkhead box P3 and IL-10.55 They can also inhibit the production of proinflammatory IL-17 and IFN-γ in T cells49 and alleviate the infiltration of proinflammatory CD8+ T cells56 and B cells expressing chemokines for neutrophil recruitment and immunoglobulin production.57 Additionally, MSC-derived extracellular vesicles could also participate in immunoregulation by decreasing the absolute count of neutrophils and TNF-α levels in an ex vivo lung injury model58 and by transferring functional mitochondria to macrophages to reduce proinflammatory cytokine production and increase their phagocytic capacity.59
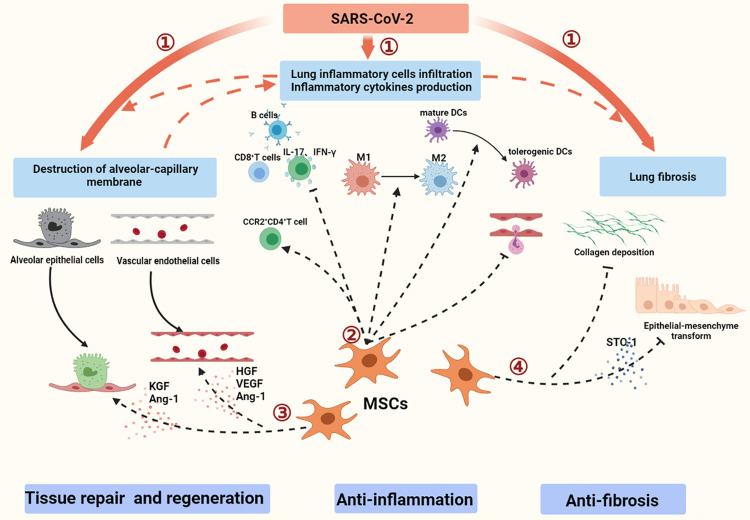
Figure 2.
A schematic overview of the pathological characteristics and potential of MSCs for treating COVID-19. (1) During the acute phase, SARS-CoV-2 infection induces an inflammatory hyper-response and pulmonary tissue damage, which may lead to the partial fibrosis of lung tissues. (2) Immunomodulatory effects of MSCs contribute to reducing inflammation. (3) MSCs promote proliferation of alveolar epithelial cells and vascular endothelial cells and reduce their apoptosis. (4) MSCs contribute to decreasing collagen deposition and inhibiting epithelial–mesenchymal transformation.
MSCs possess regenerative and differentiation properties that contribute to the repair of injured tissues. MSCs have been shown to improve alveolar fluid clearance and lung function by secreting paracrine factors that regulate membrane transport, restore the alveolar epithelial and pulmonary microvascular endothelial lining, reduce pulmonary edema, and ultimately promote the restoration of lung structure. Different cytokines are released through paracrine pathways, including keratinocyte growth factor (KGF), angiopoietin (Ang)-1, hepatocyte growth factor (HGF), and vascular endothelial growth factor (VEGF).60, 61, 62 KGF produced by MSCs may rapidly and specifically contribute to the proliferation and differentiation of alveolar epithelial type II cells.60 KGF is also related to surfactant production,61 alveolar epithelial cell apoptosis relief,63 and sodium-dependent alveolar fluid transport recovery.64 Furthermore, HGF and VEGF may have synergistic effects on the restoration of endothelial intercellular junctions, reduction of caveolin-1 protein expression, and endothelial apoptosis.65 Additionally, Ang-1 produced by MSCs has the capacity to increase endothelial cell proliferation during vascular remodeling, promote vascular stabilization during inflammation, and improve human alveolar epithelial type II cell permeability through directly affecting cytoskeletal remodeling.62,66,67
MSCs have the capacity to reshape the lung cell microenvironment by reducing the levels of profibrogenic factors. MSC infusion contributes to lung structure recovery by restoring lung epithelial cell proliferation and decreasing collagen deposition in a bleomycin-induced idiopathic pulmonary fibrosis model.68,69 The efficacy of MSCs is also associated with the correction of inappropriate epithelial–mesenchymal transformation.70
Clinical trials of MSCs for COVID-19 treatment
The safety of MSC treatment for patients with ARDS has been demonstrated in the studies listed in Table 1.71, 72, 73, 74, 75, 76 In addition, MSCs simultaneously promote the restoration of pathological lung tissues.77 Therefore, MSC therapy is considered an alternative option for patients with severe COVID-19.
Table 1.
Completed clinical studies of MSCs in the treatment of patients with ARDS.
| Numbers | Trial ID | Sources of MSCs | Phase | Numbers of patients | Doses and administration routes | Primary outcomes | Refs. |
|---|---|---|---|---|---|---|---|
| 1 | NCT01902082 | AD-MSCs | 1 | 12 | A single dose of 1 × 106 cells/ kg; IV | No adverse events or serious adverse events. The clinical effect at this dose is weak. | 71 |
| 2 | Not reported | BM-MSCs | Case report | 2 | A single dose of 2 × 106 cells/ kg; IV | Oxygenation and pulmonary compliance were improved. Levels of inflammation markers were reduced. | 72 |
| 3 | Not reported | UC-MSCs | 1 | 9 | A single dose of 1.0 × 106 cells/kg or 5.0 × 106 cells/kg or 1.0 × 107 cells/kg;IV | Tolerated without serious adverse events. | 73 |
| 4 | NCT01775774 | BM-MSCs | 1 | 9 | A single dose of 1 × 106 cells/kg or 5 × 106 cells/kg or 10 × 106 cells/kg;IV | Well tolerated and safe. | 74 |
| 5 | NCT02097641 | BM-MSCs | 2a | 60 | A single dose of 10 × 106 cells/kg; IV | No MSC-related predefined hemodynamic or respiratory adverse events. | 75 |
| 6 | NCT03042143. | UC-MSCs | 1 | 9 | A single dose of 1 or 2 or 4 × 108; IV | Well tolerated. Adverse events included apyrexia, non-sustained ventricular tachycardia, and deranged liver function. | 76 |
MSCs, mesenchymal stem cells; AD-MSCs, adipose-derived MSCs; BM-MSCs, bone marrow autologous MSCs; UC-MSCs, umbilical cord-derived MSCs; IV, intravenous injection.
To date, several clinical trials have utilized different sources of MSCs (Table 2).78 Compared with conventional treatment, MSC add-on therapy may improve clinical symptoms, which are characterized by the relief of dyspnea and a shorter recovery time.79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90 CT images of the lungs also showed notable improvements in pulmonary lesions,81, 82, 83,86,91 including a decrease in pathological lobes, ground-glass opacity, and consolidations.83 Other studies have shown that MSC add-on therapy enhanced the survival of severe/critical patients82,92 and even those who developed ARDS.86,93, 94, 95 The choice of a time window for MSC-based therapies was considered crucial; MSC transfusion for severe patients prior to intubation could reduce the risk of disease progression and 28-day mortality.83,96 Furthermore, early initiation of MSC treatment might contribute to a higher extubation rate in patients who received mechanical ventilation.97
Table 2.
Completed clinical studies of MSCs in the treatment of patients with COVID-19.
| Numbers | Trial ID | Source of MSCs | Phase | Numbers of patients | Doses and administration routes | Primary outcomes | Refs. |
|---|---|---|---|---|---|---|---|
| 1 | NCT04252118 | UC-MSCs | 1 | 18 | Three doses of 3 ×107 cells; IV | Safe and well tolerated. | 79 |
| 2 | ChiCTR2000029990 | MSC | 1 | 10 | A single dose of 1 ×106 cells/ kg; IV | No adverse effects. Pulmonary function and symptoms significantly improved. | 80 |
| 3 | Not reported | MB-MSCs | Case report | 2 | Three doses of 1 ×106 cells/ kg; IV | Values of immune indicators increased, and levels of inflammation indicators decreased. Lung exudate lesions were absorbed. | 81 |
| 4 | ChiCTR2000029606 | MB-MSCs | 1 | 44 | Three doses of 3 ×107 cells; IV | Safe and well tolerated. SpO2 was significantly improved, and chest imaging results were improved. | 82 |
| 5 | ChiCTR2000031494 | UC-MSCs | 1 | 41 | A single dose of 2 ×106 cells/ kg; IV | Clinical symptoms were relieved and alleviated in a shorter time. Levels of inflammatory factors rapidly decreased, and lymphocyte count returned to normal levels in less time. | 83 |
| 6 | Not reported | UC-MSCs | 1 | 31 | 1 × 106 cells/ kg were suspended in 100 ml normal saline. The volume of infused UC-MSCs was 100–300 mL; IV | No adverse events were observed. Laboratory parameters tended to improve. | 84 |
| 7 | Not reported | Convalescent plasma combination with UC-MSCs | Case report | 1 | Three doses of 1 × 106 cells/kg; IV | Absolute lymphocyte count increased, bilateral infiltrates were absorbed, and pulmonary function was significantly improved. | 85 |
| 8 | Not reported | UC-MSCs or PL-MSCs | Case series | 11 | Three doses of 2 × 108 cells; IV | No serious adverse events. Levels of inflammatory biomarkers significantly decreased. | 86 |
| 9 | Not reported | hESC-IMRCs | 1 | 27 | One to three doses of 3 × 106 cells/ kg; IV | No adverse events or abnormal responses related to cell therapy occurred. The area of lung fibrosis lesions decreased. | 87 |
| 10 | NCT04269525 | UC-MSCs | A pilot study | 16 | Four doses of 1 × 108 cells; IV | No infusion-related or allergic reactions. Oxygenation index was improved, and CT scan improved. Restoration of lymphocyte count and subsets. | 88 |
| 11 | NCT04416139 | UC-MSCs | 1 | 5 | A single dose of 1 × 106 cells /kg; IV | PaO2/FiO2 was improved. | 89 |
| 12 | Not reported | hESC-IMRCs | Case report | 1 | Two doses of 3 × 10 6 cells/ kg; IV | Levels of proinflammatory cytokines were decreased. SpO2 improved. | 90 |
| 13 | NCT04288102 | UC-MSCs | 2 | 100 | Three doses of 4 × 107 cells; IV | Safe and potentially effective. Lung lesion volume, especially solid component lesion volume, was reduced. The 6-minute walk test result improved. | 91 |
| 14 | NCT04457609 | UC-MSCs | 1 | 40 | A single dose of 1 × 106 cells/ kg; IV | Safe and well tolerated. Survival rate increased. | 92 |
| 15 | NCT04392778 | WJ-MSCs | 1 | 30 | Three doses of 3 × 106 cells/kg; IV | All the indicators of anti-inflammation, anti-fibrosis signs in the lungs, and levels of immunodulatory markers were dramatically improved. | 93 |
| 16 | NCT04355728 | UC-MSCs | 1/2a | 24 | Two doses of 100 ± 20 × 106 cells; IV | No serious adverse events were observed. Mortality and time to recovery decreased significantly. | 94 |
| 17 | Not reported | MSCs | 1 | 23 | Two or three doses of 1 × 106 cells/ kg; IV | Safe and well tolerated. Pulmonary function and overall outcomes were improved. | 95 |
| 18 | Not reported | UC-MSCs | 1 | 210 | A single dose of 1-2 × 106 cells/kg;IV | No adverse effects. SaO2 improved. | 96 |
| 19 | Not reported | AD-MSCs | 1 | 13 | One to three doses of 0.98 × 106 cells/kg; IV | No adverse events. Clinical symptoms and values of inflammatory parameters improved. | 97 |
| 20 | NCT04339660 | UC-MSCs | 2 | 58 | A single dose of 1 × 106 cells /kg; IV | MSC-treated patients had fewer adverse events. Clinical symptoms, values of inflammatory parameters, and CT scan improved. MSCs promoted the production of SARS-CoV-2-specific antibodies. | 98 |
| 21 | IRCT20190717044241N2 | WJ-MSCs | 1 | 5 | Three doses of 150 × 106 cells; IV | Safe and well tolerated. Levels of inflammatory biomarkers improved. | 99 |
| 22 | Not reported | UC-MSCs | Case report |
1 | Three doses of 5 × 107 cells/ kg; IV | No obvious side effects. Values of clinical indices and clinical symptoms were improved. | 100 |
| 23 | Not reported | UC-MSCs | Case report |
1 | Five doses of 1.5 × 106 cells/ kg; IV | The number of lymphocytes was increased. Respiratory and renal functions were improved. |
101 |
| 24 | Not reported | WJ-MSCs | Case report |
1 | A single dose of 1 × 106 cells /kg; IV | The percentage and counts of T cells were increased. CT scan was improved. | 102 |
| 25 | Not reported | MSCs | A retrospective study | 25 | One to three doses of 1 × 106 cells/ kg; IV | Three cases experienced treatment-related side effects including liver dysfunction, heart failure, and allergic rash. | 103 |
| 26 | Not reported | ExoFlo | 1 | 27 | 15 ml of ExoFlo was added to 100 mL of normal saline; IV | No adverse events. Clinical status, oxygenation, and laboratory values improved, levels of acute phase reactants declined. | 105 |
MSCs, mesenchymal stem cells; MB-MSCs, menstrual blood-derived MSCs; PL-MSCs, placenta-derived MSCs; hESC-IMRCs, human embryonic stem cell–derived immunity‐ and matrix‐regulatory cells; WJ-MSCs, Wharton's jelly-derived MSCs; ExoFlo, exosomes derived from allogeneic bone marrow MSCs; SaO2, arterial oxygen saturation; SpO2, oxygen saturation; CT, computed tomography.
The underlying mechanisms of MSC-based remedies for the maintenance of homeostasis, immune reconstitution, and tissue repair have been investigated in detail, and the effect of MSCs on hematopoiesis was found to correlate with the activation of a novel subpopulation of VNN2+ hematopoietic stem/progenitor like (HSPC-like) cells, which were mobilized following MSC infusion.98 Notably, the changes in inflammatory indices following MSC treatment were inconsistent. Partial data from clinical trials showed that MSCs exerted anti-inflammatory and immunoregulatory effects through reducing the levels of inflammatory biomarkers (C-reactive protein, IL-6, IL-2, IL-12, IL-8, TNF-α, and IFN-γ) and increasing the level of anti-inflammatory IL-10 and T cell counts.80,81,83,84,94,95,98, 99, 100, 101, 102 In other trials, significant changes in inflammatory factor levels were not observed.82,91,103 Therefore, the effect of MSC treatment on inflammation should be further studied in large-cohort randomized controlled trials.
In our double-blind, placebo-controlled phase 2 trial, MSC administration significantly reduced solid component lesion volume within 28 days in patients with severe COVID-19.91 During the subsequent one-year follow-up period, the patients who received MSC treatment showed reduced solid component lesion volume and improved pulmonary function, indicating that MSC therapy might have long-term benefits.104 Bone marrow MSC-derived exosomes have also been reported to be safe and effective in reducing acute phase reactant and neutrophil counts, increasing T cell counts, and improving oxygenation in severe cases.105 These data support the therapeutic potential of MSCs for severe cases with progressive lung damage.
Although most clinical trials showed that MSC treatment is generally safe and well tolerated, some MSC-related adverse events have still been recorded. The major clinical symptoms included facial flushing, fever,79 shivering,86 headache,99 liver dysfunction, heart failure, and allergic rash.95 However, adverse events were recorded in both MSC-treated patients and controls, indicating that the side effects might be infusion-related.94 Furthermore, the speed of transfusion and the baseline status of patients with co-existing illnesses may be associated with the occurrence of adverse events.
Challenges and perspective
Large-scale vaccination has provided essential protection by establishing herd immunity, which will further contribute to the control of the SARS-CoV-2 pandemic. However, there are still re-infection and post-vaccination breakthrough cases in COVID-19. Many infected individuals are asymptomatic or show mild/moderate symptoms; however, it is difficult to ensure the full recovery of severe and critical patients using current medications, including symptomatic treatment, antiviral therapy, and respiratory support treatment. MSCs may serve as an alternative immunotherapeutic option for severely affected individuals and contribute to the improvement of COVID-19 outcomes. Notably, clinical application protocols for MSCs are diverse, and optimized standard protocols will be required to maximize their therapeutic effect. Moreover, the choice of a time window for treatment, the identification of COVID-19 patients at suitable phases, and the regimen (including dosage, interval time, and round) all need to be comprehensively optimized.
Most importantly, adverse reactions related to MSC treatment and infusion also require caution. Low-grade fever, facial flushing, headache, and allergic rash after MSC infusion were reported, although these side effects were transient and generally disappeared within 24 h without any treatment. However, treatment of these symptoms may be required in severe cases. In addition, the risks of thromboembolic events associated with intravenous infusions of high doses of MSCs should also be noted. To improve safety, it is best to monitor the electrocardiogram and percutaneous oxygen saturation during MSC infusion, and to test for laboratory parameters, including liver and kidney function, blood routine, CRP, inflammatory cytokines, coagulation, procalcitonin, blood gases, and heart function, before MSC infusion.106
Follow-up studies extending over a year in length have reported that severe COVID-19 patients still experienced symptoms and persistent physiological and radiographic abnormalities.31 These data indicate that some severe COVID-19 patients will still require suitable interventions to improve their long-term prognoses. MSCs have been suggested for post-COVID-19 treatment for their antifibrotic and regeneration/differentiation properties. To determine the fate of the infused cells and to interpret the interactions between the colonized MSCs and immunocytes, MSC tracking in vivo is required. Furthermore, specific airspace and plasma biomarkers are required to predict and verify the efficacy of MSCs against COVID-19.
Both fresh and frozen MSCs with proven efficacy in clinical applications are readily available, and it is noteworthy that dead or apoptotic MSCs found in MSC preparations exert the same immunomodulatory properties as the "living MSCs" by releasing phosphatidylserine.107 In addition, MSCs derived from different donors and tissues lead to individual heterogeneity, which complicates the progress of consistent and standardized stem cell production.108,109 Therefore, it is necessary to screen qualified MSCs to avoid uncertain effects.
In summary, MSC therapy appear to serve as an alternative candidate for ameliorating inflammation, repairing lung tissue injury, and preventing long-term pulmonary disability in patients with COVID-19. However, the different groups of enrolled patients, therapeutic regimen and the time window for treatment of MSCs may lead to the disparities of data in the previous clinical trials. Prospective, multicenter, randomized and controlled trials with large sample size are still necessary to further confirm therapeutic efficacy of MSCs for the patients with severe and critical COVID-19.
Outstanding questions
Although available data from preliminary clinical trials have proved the safety and some efficiency of MSC treatment for COVID-19 patients, there are certain disparities caused by the inconsistency of inclusion and exclusion criteria. Therefore, determining the characteristics of patients who may gain more clinical benefits from MSC treatment will require more multicenter, randomized, controlled trials and long-term follow-up studies. Moreover, concomitant treatments may exert a synergistic or antagonistic role with MSCs, and treatment regimens need to be optimized. Further mechanistic studies are also needed to verify the efficacy of MSCs in COVID-19 treatment.
Search strategy and selection criteria
Data for this review were identified by searches of PubMed and Google Scholar and references from relevant articles using the search terms “SARS-CoV-2,” “COVID-19,” “immunotherapy,” “Mesenchymal stem cells,” “ARDS,” “Clinical trial,” “Lung injury,” “pulmonary fibrosis,” “Cytokine storm,” “immune response,” “clinical features,” “follow-up,” and related terms. Only articles published in English between 1994 and 2021 were included in the study. Articles were selected based on their relevance and the authors’ best judgments.
Contributors
Ruonan Xu and Fu-Sheng Wang contributed to the conception and design of the review. The draft was written by Ruonan Xu and Zhiqian Feng. Fu-Sheng Wang, Ruonan Xu critically and systemically revised the manuscript. All authors contributed to the article and approved the submitted version.
Declaration of interests
The authors declare no competing interests.
Acknowledgments
This study was supported by the funds from Innovative Research Group Project of the National Natural Science Foundation of China (81721002), the National Key Research and Development Program of China (2017YFA0105700). We also declare that the funders had no role in paper design, data collection, data analysis, interpretation and writing of the paper. Figure 1 was created using BioRender software.
Contributor Information
Ruonan Xu, Email: xuruonan2004@aliyun.com.
Fu-Sheng Wang, Email: fswang302@163.com.
References
- 1.WHO Coronavirus (COVID-19) Dashboard: https://covid19.who.int /January 16, 2022.
- 2.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Institutes of Health . National Institutes of Health (US); Bethesda (MD): 2021. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. [PubMed] [Google Scholar]
- 4.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sterne J., Murthy S., Diaz J.V., et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324(13):1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavalli G., Dagna L. The right place for IL-1 inhibition in COVID-19. Lancet Respir Med. 2021;9(3):223–224. doi: 10.1016/S2213-2600(21)00035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavalli G., De Luca G., Campochiaro C., et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2(6):e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michot J.M., Albiges L., Chaput N., et al. Tocilizumab, an anti-IL-6 receptor antibody, to treat COVID-19-related respiratory failure: a case report. Ann Oncol. 2020;31(7):961–964. doi: 10.1016/j.annonc.2020.03.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duan K., Liu B., Li C., et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020;117(17):9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta A., Gonzalez-Rojas Y., Juarez E., et al. Early treatment for COVID-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385(21):1941–1950. doi: 10.1056/NEJMoa2107934. [DOI] [PubMed] [Google Scholar]
- 11.Lopes-Pacheco M., Robba C., Rocco P., Pelosi P. Current understanding of the therapeutic benefits of mesenchymal stem cells in acute respiratory distress syndrome. Cell Biol Toxicol. 2020;36(1):83–102. doi: 10.1007/s10565-019-09493-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L.T., Ting C.H., Yen M.L., et al. Human mesenchymal stem cells (MSCs) for treatment towards immune- and inflammation-mediated diseases: review of current clinical trials. J Biomed Sci. 2016;23(1):76. doi: 10.1186/s12929-016-0289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edler C., Schröder A.S., Aepfelbacher M., et al. Dying with SARS-CoV-2 infection-an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int J Legal Med. 2020;134(4):1275–1284. doi: 10.1007/s00414-020-02317-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Z., Shi L., Wang Y., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carsana L., Sonzogni A., Nasr A., et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis. 2020;20(10):1135–1140. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magro C., Mulvey J.J., Berlin D., et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varga Z., Flammer A.J., Steiger P., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184(4):861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szabo P.A., Dogra P., Gray J.I., et al. Longitudinal profiling of respiratory and systemic immune responses reveals myeloid cell-driven lung inflammation in severe COVID-19. Immunity. 2021;54(4):797–814. doi: 10.1016/j.immuni.2021.03.005. .e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tay M.Z., Poh C.M., Rénia L., Macary P.A., Ng L. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song J.W., Zhang C., Fan X., et al. Immunological and inflammatory profiles in mild and severe cases of COVID-19. Nat Commun. 2020;11(1):3410. doi: 10.1038/s41467-020-17240-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen G., Wu D., Guo W., et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qin C., Zhou L., Hu Z., et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun Y., Dong Y., Wang L., et al. Characteristics and prognostic factors of disease severity in patients with COVID-19: the Beijing experience. J Autoimmun. 2020;112 doi: 10.1016/j.jaut.2020.102473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen G., Wu D., Guo W., et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Y., Fu B., Zheng X., et al. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl Sci Rev. 2020;7(6):998–1002. doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hadjadj J., Yatim N., Barnabei L., et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369(6504):718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou M., Xu J., Liao T., et al. Comparison of residual pulmonary abnormalities 3 months after discharge in patients who recovered from COVID-19 of different severity. Front Med. 2021;8 doi: 10.3389/fmed.2021.682087. (Lausanne) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan F., Yang L., Liang B., et al. Chest CT patterns from diagnosis to 1 year of follow-up in COVID-19. Radiology. 2021 doi: 10.1148/radiol.2021211199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spagnolo P., Balestro E., Aliberti S., et al. Pulmonary fibrosis secondary to COVID-19: a call to arms? Lancet Respir Med. 2020;8(8):750–752. doi: 10.1016/S2213-2600(20)30222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grillo F., Barisione E., Ball L., Mastracci L., Fiocca R. Lung fibrosis: an undervalued finding in COVID-19 pathological series. Lancet Infect Dis. 2021;21(4):e72. doi: 10.1016/S1473-3099(20)30582-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu X., Dong D., Ma D. Thin-Section computed tomography manifestations during convalescence and long-term follow-up of patients with severe acute respiratory syndrome (SARS) Med Sci Monit. 2016;22:2793–2799. doi: 10.12659/msm.896985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu J., Xu X., Jiang L., Dua K., Hansbro P.M., Liu G. SARS-CoV-2 induces transcriptional signatures in human lung epithelial cells that promote lung fibrosis. Respir Res. 2020;21(1):182. doi: 10.1186/s12931-020-01445-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eapen M.S., Lu W., Gaikwad A.V., et al. Endothelial to mesenchymal transition: a precursor to post-COVID-19 interstitial pulmonary fibrosis and vascular obliteration? Eur Respir J. 2020;56(4) doi: 10.1183/13993003.03167-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta A., Madhavan M.V., Sehgal K., et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao X.H., Luo T., Shi Y., et al. A cohort autopsy study defines COVID-19 systemic pathogenesis. Cell Res. 2021;31(8):836–846. doi: 10.1038/s41422-021-00523-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopes-Pacheco M., Silva P.L., Cruz F.F., et al. Pathogenesis of multiple organ injury in COVID-19 and potential therapeutic strategies. Front Physiol. 2021;12 doi: 10.3389/fphys.2021.593223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puelles V.G., Lütgehetmann M., Lindenmeyer M.T., et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383(6):590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Su H., Yang M., Wan C., et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98(1):219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu C.T., Lidsky P.V., Xiao Y., et al. SARS-CoV-2 infects human pancreatic β cells and elicits β cell impairment. Cell Metab. 2021;33(8):1565–1576. doi: 10.1016/j.cmet.2021.05.013. .e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delorey T.M., Ziegler C., Heimberg G., et al. COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets. Nature. 2021;595(7865):107–113. doi: 10.1038/s41586-021-03570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y., Chen X., Cao W., Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15(11):1009–1016. doi: 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- 45.Ionescu L., Byrne R.N., van Haaften T., et al. Stem cell conditioned medium improves acute lung injury in mice: in vivo evidence for stem cell paracrine action. Am J Physiol Lung Cell Mol Physiol. 2012;303(11):L967–L977. doi: 10.1152/ajplung.00144.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gupta N., Su X., Popov B., Lee J.W., Serikov V., Matthay M.A. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179(3):1855–1863. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- 47.Da S.K., Gobatto A., Costa-Ferro Z., et al. Is there a place for mesenchymal stromal cell-based therapies in the therapeutic armamentarium against COVID-19? Stem Cell Res Ther. 2021;12(1):425. doi: 10.1186/s13287-021-02502-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khoury M., Cuenca J., Cruz F.F., Figueroa F.E., Rocco P., Weiss D.J. Current status of cell-based therapies for respiratory virus infections: applicability to COVID-19. Eur Respir J. 2020;55(6) doi: 10.1183/13993003.00858-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hackstein H., Lippitsch A., Krug P., et al. Prospectively defined murine mesenchymal stem cells inhibit Klebsiella pneumoniae-induced acute lung injury and improve pneumonia survival. Respir Res. 2015;16:123. doi: 10.1186/s12931-015-0288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Phinney D.G., Di Giuseppe M., Njah J., et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun. 2015;6:8472. doi: 10.1038/ncomms9472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hayes M., Curley G.F., Masterson C., Devaney J., O'Toole D., Laffey J.G. Mesenchymal stromal cells are more effective than the MSC secretome in diminishing injury and enhancing recovery following ventilator-induced lung injury. Intensive Care Med Exp. 2015;3(1):29. doi: 10.1186/s40635-015-0065-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Curley G.F., Ansari B., Hayes M., et al. Effects of intratracheal mesenchymal stromal cell therapy during recovery and resolution after ventilator-induced lung injury. Anesthesiology. 2013;118(4):924–932. doi: 10.1097/ALN.0b013e318287ba08. [DOI] [PubMed] [Google Scholar]
- 53.Lv H., Liu Q., Sun Y., et al. Mesenchymal stromal cells ameliorate acute lung injury induced by LPS mainly through stanniocalcin-2 mediating macrophage polarization. Ann Transl Med. 2020;8(6):334. doi: 10.21037/atm.2020.02.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu Z., Chang W., Meng S., et al. Mesenchymal stem cells induce dendritic cell immune tolerance via paracrine hepatocyte growth factor to alleviate acute lung injury. Stem Cell Res Ther. 2019;10(1):372. doi: 10.1186/s13287-019-1488-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cao M., Liu H., Dong Y., et al. Mesenchymal stem cells alleviate idiopathic pneumonia syndrome by modulating T cell function through CCR2-CCL2 axis. Stem Cell Res Ther. 2021;12(1):378. doi: 10.1186/s13287-021-02459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu J., Feng B., Xu Y., et al. Mesenchymal stem cells alleviate LPS-induced acute lung injury by inhibiting the proinflammatory function of Ly6C(+) CD8(+) T cells. Cell Death Dis. 2020;11(10):829. doi: 10.1038/s41419-020-03036-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feng B., Zhu J., Xu Y., et al. Immunosuppressive effects of mesenchymal stem cells on lung B cell gene expression in LPS-induced acute lung injury. Stem Cell Res Ther. 2020;11(1):418. doi: 10.1186/s13287-020-01934-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park J., Kim S., Lim H., et al. Therapeutic effects of human mesenchymal stem cell microvesicles in an ex vivo perfused human lung injured with severe E. Coli pneumonia. Thorax. 2019;74(1):43–50. doi: 10.1136/thoraxjnl-2018-211576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morrison T.J., Jackson M.V., Cunningham E.K., et al. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am J Respir Crit Care Med. 2017;196(10):1275–1286. doi: 10.1164/rccm.201701-0170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ulich T.R., Yi E.S., Longmuir K., et al. Keratinocyte growth factor is a growth factor for type II pneumocytes in vivo. J Clin Invest. 1994;93(3):1298–1306. doi: 10.1172/JCI117086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yano T., Mason R.J., Pan T., Deterding R.R., Nielsen L.D., Shannon J.M. KGF regulates pulmonary epithelial proliferation and surfactant protein gene expression in adult rat lung. Am J Physiol Lung Cell Mol Physiol. 2000;279(6):L1146–L1158. doi: 10.1152/ajplung.2000.279.6.L1146. [DOI] [PubMed] [Google Scholar]
- 62.Fang X., Neyrinck A.P., Matthay M.A., Lee J.W. Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin-1. J Biol Chem. 2010;285(34):26211–26222. doi: 10.1074/jbc.M110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oswari J., Matthay M.A., Margulies S.S. Keratinocyte growth factor reduces alveolar epithelial susceptibility to in vitro mechanical deformation. Am J Physiol Lung Cell Mol Physiol. 2001;281(5):L1068–L1077. doi: 10.1152/ajplung.2001.281.5.L1068. [DOI] [PubMed] [Google Scholar]
- 64.Guery B.P., Mason C.M., Dobard E.P., Beaucaire G., Summer W.R., Nelson S. Keratinocyte growth factor increases transalveolar sodium reabsorption in normal and injured rat lungs. Am J Respir Crit Care Med. 1997;155(5):1777–1784. doi: 10.1164/ajrccm.155.5.9154891. [DOI] [PubMed] [Google Scholar]
- 65.Yang Y., Chen Q.H., Liu A.R., Xu X.P., Han J.B., Qiu H.B. Synergism of MSC-secreted HGF and VEGF in stabilising endothelial barrier function upon lipopolysaccharide stimulation via the Rac1 pathway. Stem Cell Res Ther. 2015;6:250. doi: 10.1186/s13287-015-0257-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cho C.H., Kim K.E., Byun J., et al. Long-term and sustained COMP-Ang1 induces long-lasting vascular enlargement and enhanced blood flow. Circ Res. 2005;97(1):86–94. doi: 10.1161/01.RES.0000174093.64855.a6. [DOI] [PubMed] [Google Scholar]
- 67.Korhonen E.A., Lampinen A., Giri H., et al. Tie1 controls angiopoietin function in vascular remodeling and inflammation. J Clin Invest. 2016;126(9):3495–3510. doi: 10.1172/JCI84923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zakaria D.M., Zahran N.M., Arafa S., Mehanna R.A., Abdel-Moneim R.A. Histological and physiological studies of the effect of bone marrow-derived mesenchymal stem cells on bleomycin induced lung fibrosis in adult albino rats. Tissue Eng Regen Med. 2021;18(1):127–141. doi: 10.1007/s13770-020-00294-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ortiz L.A., Gambelli F., Mcbride C., et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A. 2003;100(14):8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ono M., Ohkouchi S., Kanehira M., et al. Mesenchymal stem cells correct inappropriate epithelial-mesenchyme relation in pulmonary fibrosis using stanniocalcin-1. Mol Ther. 2015;23(3):549–560. doi: 10.1038/mt.2014.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zheng G., Huang L., Tong H., et al. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respir Res. 2014;15(1):39. doi: 10.1186/1465-9921-15-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Simonson O.E., Mougiakakos D., Heldring N., et al. In vivo effects of mesenchymal stromal cells in two patients with severe acute respiratory distress syndrome. Stem Cells Transl Med. 2015;4(10):1199–1213. doi: 10.5966/sctm.2015-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yip H.K., Fang W.F., Li Y.C., et al. Human umbilical cord-derived mesenchymal stem cells for acute respiratory distress syndrome. Crit Care Med. 2020;48(5):e391–e399. doi: 10.1097/CCM.0000000000004285. [DOI] [PubMed] [Google Scholar]
- 74.Wilson J.G., Liu K.D., Zhuo H., et al. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med. 2015;3(1):24–32. doi: 10.1016/S2213-2600(14)70291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Matthay M.A., Calfee C.S., Zhuo H., et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med. 2019;7(2):154–162. doi: 10.1016/S2213-2600(18)30418-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gorman E., Shankar-Hari M., Hopkins P., et al. Repair of acute respiratory distress syndrome by stromal cell administration (REALIST) trial: a phase 1 trial. EClinicalMedicine. 2021;41 doi: 10.1016/j.eclinm.2021.101167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wick K.D., Leligdowicz A., Zhuo H., Ware L.B., Matthay M.A. Mesenchymal stromal cells reduce evidence of lung injury in patients with ARDS. JCI Insight. 2021;6(12) doi: 10.1172/jci.insight.148983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zanirati G., Provenzi L., Libermann L.L., et al. Stem cell-based therapy for COVID-19 and ARDS: a systematic review. NPJ Regen Med. 2021;6(1):73. doi: 10.1038/s41536-021-00181-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meng F., Xu R., Wang S., et al. Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: a phase 1 clinical trial. Signal Transduct Target Ther. 2020;5(1):172. doi: 10.1038/s41392-020-00286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leng Z., Zhu R., Hou W., et al. Transplantation of ACE2(-) mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11(2):216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tang L., Jiang Y., Zhu M., et al. Clinical study using mesenchymal stem cells for the treatment of patients with severe COVID-19. Front Med. 2020;14(5):664–673. doi: 10.1007/s11684-020-0810-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu X., Jiang W., Chen L., et al. Evaluation of the safety and efficacy of using human menstrual blood-derived mesenchymal stromal cells in treating severe and critically ill COVID-19 patients: an exploratory clinical trial. Clin Transl Med. 2021;11(2):e297. doi: 10.1002/ctm2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shu L., Niu C., Li R., et al. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2020;11(1):361. doi: 10.1186/s13287-020-01875-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guo Z., Chen Y., Luo X., He X., Zhang Y., Wang J. Administration of umbilical cord mesenchymal stem cells in patients with severe COVID-19 pneumonia. Crit Care. 2020;24(1):420. doi: 10.1186/s13054-020-03142-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Peng H., Gong T., Huang X., et al. A synergistic role of convalescent plasma and mesenchymal stem cells in the treatment of severely ill COVID-19 patients: a clinical case report. Stem Cell Res Ther. 2020;11(1):291. doi: 10.1186/s13287-020-01802-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hashemian S.R., Aliannejad R., Zarrabi M., et al. Mesenchymal stem cells derived from perinatal tissues for treatment of critically ill COVID-19-induced ARDS patients: a case series. Stem Cell Res Ther. 2021;12(1):91. doi: 10.1186/s13287-021-02165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu J., Zhou X., Tan Y., et al. Phase 1 trial for treatment of COVID-19 patients with pulmonary fibrosis using hESC-IMRCs. Cell Prolif. 2020;53(12):e12944. doi: 10.1111/cpr.12944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Feng Y., Huang J., Wu J., et al. Safety and feasibility of umbilical cord mesenchymal stem cells in patients with COVID-19 pneumonia: a pilot study. Cell Prolif. 2020;53(12):e12947. doi: 10.1111/cpr.12947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Iglesias M., Butrón P., Torre-Villalvazo I., et al. Mesenchymal stem cells for the compassionate treatment of severe acute respiratory distress syndrome due to COVID 19. Aging Dis. 2021;12(2):360–370. doi: 10.14336/AD.2020.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu J., Hu Z., Wang L., et al. First case of COVID-19 infused with hESC derived immunity- and matrix-regulatory cells. Cell Prolif. 2020;53(12):e12943. doi: 10.1111/cpr.12943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shi L., Huang H., Lu X., et al. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: a randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduct Target Ther. 2021;6(1):58. doi: 10.1038/s41392-021-00488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dilogo I.H., Aditianingsih D., Sugiarto A., et al. Umbilical cord mesenchymal stromal cells as critical COVID-19 adjuvant therapy: a randomized controlled trial. Stem Cells Transl Med. 2021;10(9):1279–1287. doi: 10.1002/sctm.21-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Adas G., Cukurova Z., Yasar K.K., et al. The systematic effect of mesenchymal stem cell therapy in critical COVID-19 patients: a prospective double controlled trial. Cell Transplant. 2021;30 doi: 10.1177/09636897211024942. 9636897211024942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lanzoni G., Linetsky E., Correa D., et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: a double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl Med. 2021;10(5):660–673. doi: 10.1002/sctm.20-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Häberle H., Magunia H., Lang P., et al. Mesenchymal stem cell therapy for severe COVID-19 ARDS. J Intensive Care Med. 2021;36(6):681–688. doi: 10.1177/0885066621997365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.EN O., Pekkoc-Uyanik K.C., Alpaydin N., Gulay G.R., Simsek M. Clinical experience on umbilical cord mesenchymal stem cell treatment in 210 severe and critical COVID-19 cases in Turkey. Stem Cell Rev Rep. 2021;17(5):1917–1925. doi: 10.1007/s12015-021-10214-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sánchez-Guijo F., García-Arranz M., López-Parra M., et al. Adipose-derived mesenchymal stromal cells for the treatment of patients with severe SARS-CoV-2 pneumonia requiring mechanical ventilation. A proof of concept study. EClinicalMedicine. 2020;25 doi: 10.1016/j.eclinm.2020.100454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhu R., Yan T., Feng Y., et al. Mesenchymal stem cell treatment improves outcome of COVID-19 patients via multiple immunomodulatory mechanisms. Cell Res. 2021:1–19. doi: 10.1038/s41422-021-00573-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Saleh M., Vaezi A.A., Aliannejad R., et al. Cell therapy in patients with COVID-19 using Wharton's jelly mesenchymal stem cells: a phase 1 clinical trial. Stem Cell Res Ther. 2021;12(1):410. doi: 10.1186/s13287-021-02483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liang B., Chen J., Li T., et al. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells: a case report. Medicine. 2020;99(31):e21429. doi: 10.1097/MD.0000000000021429. (Baltimore) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tao J., Nie Y., Wu H., et al. Umbilical cord blood-derived mesenchymal stem cells in treating a critically ill COVID-19 patient. J Infect Dev Ctries. 2020;14(10):1138–1145. doi: 10.3855/jidc.13081. [DOI] [PubMed] [Google Scholar]
- 102.Zhang Y., Ding J., Ren S., et al. Intravenous infusion of human umbilical cord Wharton's jelly-derived mesenchymal stem cells as a potential treatment for patients with COVID-19 pneumonia. Stem Cell Res Ther. 2020;11(1):207. doi: 10.1186/s13287-020-01725-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen X., Shan Y., Wen Y., Sun J., Du H. Mesenchymal stem cell therapy in severe COVID-19: a retrospective study of short-term treatment efficacy and side effects. J Infect. 2020;81(4):647–679. doi: 10.1016/j.jinf.2020.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shi L., Yuan X., Yao W., et al. Human mesenchymal stem cells treatment for severe COVID-19: 1-Year follow-up results of a randomized, double-blind, placebo-controlled trial. Ebiomedicine. 2021;75 doi: 10.1016/j.ebiom.2021.103789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sengupta V., Sengupta S., Lazo A., Woods P., Nolan A., Bremer N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev. 2020;29(12):747–754. doi: 10.1089/scd.2020.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xu R., Shi L., Xie W., et al. Diagnosis and treatment guidelines for mesenchymal stem cell therapy for coronavirus disease 2019 (Beijing, 2021) Infect Dis Immun. 2021;1(2) [Google Scholar]
- 107.He X., Hong W., Yang J., et al. Spontaneous apoptosis of cells in therapeutic stem cell preparation exert immunomodulatory effects through release of phosphatidylserine. Signal Transduct Target Ther. 2021;6(1):270. doi: 10.1038/s41392-021-00688-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xie Y., Liu S., Wang L., et al. Individual heterogeneity screened umbilical cord-derived mesenchymal stromal cells with high Treg promotion demonstrate improved recovery of mouse liver fibrosis. Stem Cell Res Ther. 2021;12(1):359. doi: 10.1186/s13287-021-02430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tai C., Wang L., Xie Y., Gao T., Huang F., Wang B. Analysis of key distinct biological characteristics of human Placenta-Derived mesenchymal stromal cells and individual heterogeneity attributing to donors. Cells Tissues Organs. 2021;210(1):45–57. doi: 10.1159/000513038. [DOI] [PubMed] [Google Scholar]