SUMMARY
The sphenoid bone development occurs in both prenatal and postnatal periods. Sphenoid bone openings are used as surgical landmarks and are of great importance for neurosurgeons in everyday practice. The aim of this study was to identify morphological characteristics, postnatal development and remodeling, as well as clinical aspect of the sphenoid bone openings and to investigate their relationship and difference in size. The macerated sphenoid bones analyzed in this study were scanned by micro-computed tomography. Areas and distance in-between foramen ovale and foramen rotundum were measured. In addition, different shapes of foramen ovale were described. The most common shape of foramen ovale on both sides was oval, followed by the round, almond and elongated shapes. Modest to strong positive correlations between all foramina and age for the whole sample and both subsamples were presented, except for the right foramen rotundum area in the male subsample, which did not show significant correlation with age. Our study revealed changes in postnatal development and anatomy of foramen ovale and foramen rotundum, primarily in the aspects of size and shape, and should contribute to reducing the risk of damage to neurovascular structures during surgical procedures.
Key words: Foramen ovale, Foramen rotundum, Micro-computed tomography, Sphenoid bone
Introduction
Development of the human skull has been the subject of many studies that aimed to understand embryology and anatomy of human skull and thus enable new surgical approaches to intracranial structures (1, 2). However, data available on the sphenoid bone development during postnatal period are scarce (3, 4).
Sphenoid bone is an irregular unpaired bone at the base of the skull, consisting of a body and three pairs of processes, i.e. greater and lesser wings, and pterygoid processes. It is formed by fusion of different primordial with different embryonic origins, developing from both intramembranous and endochondral ossification with the pre-sphenoid and post-sphenoid centers (5-8). The sphenoid bone openings appear around and after the 8th gestational week, as skull base ossification occurs in the vicinity of preexisting neurovascular bundle, i.e. vessels and cranial nerves (5, 6, 9). Topographic anatomy of the sphenoid bone is of great clinical importance due to the vicinity of middle cerebral artery and trigeminal nerve. Foramen rotundum (FR), for transmission of maxillary nerve, is situated at the anterior and medial part of the sphenoid bone, while foramen ovale (FO), for transmission of mandibular nerve, accessory meningeal artery, venous plexus, and sometimes the lesser petrosal nerve is placed at the posterior border of the greater wing, behind and lateral to FR.
The sphenoid bone, as well as its openings change their shape and size during postnatal development (2, 4, 5). So far, anatomic variations in size, primarily diameters, i.e. length and width, and shape of FO and FR, as well as other sphenoid bone openings have been widely described. Most of the studies report on the results obtained on adult skull collections (10-19), while only very few studies describe results on fetal, neonatal and postnatal skulls (2, 4, 5). In addition, studies have been conducted either on dry skull collections or cadavers (10-13, 15, 17-19), or x-ray scans (13), computed tomography (CT) scans (14, 16), or even magnetic resonance imaging (MRI) scans (20), using different divider measurement devices such as Vernier, digital or other caliper (10-12, 15, 17-19), or sophisticated software (13, 14, 16).
A number of studies were conducted on Asian (4, 10, 12-15, 17, 18), South American (16, 17) and European (5, 11) populations. The variability of sphenoid bone openings, their size and shape across different world regions has been explained by population variation.
Foramen ovale was widely used as surgical landmark of high importance in neurosurgery as it enables access to the trigeminal nerve. Therefore, it is crucial to understand the exact development and topography of these structures. Approaches through FO are used for surgical treatment of trigeminal neuralgia, such as thermocoagulation of the trigeminal ganglion by radiofrequency, needle aspiration techniques, and various diagnostic procedures in different kinds of tumors (13, 17, 21-25).
The aim of this study was to identify and describe morphological characteristics, postnatal development and remodeling, as well as clinical aspect of the sphenoid bone openings, by analyzing development of FO and FR of the sphenoid bone utilizing micro-computed tomography (microCT) scanning.
Materials and Methods
Skulls
We examined 39 macerated and isolated sphenoid bones randomly selected from the Mini Skull Collection at Department of Anatomy, School of Medicine, University of Zagreb, Zagreb, Croatia, as previously described by Dumic-Cule et al. and Štoković et al. (26, 27). Skulls underwent the standard procedure of maceration whereby a clean skeleton was obtained and divided into individual bones. Damaged, fractured, or in any kind deformed isolated bones were excluded from the study. Specimens were aged from two months to seven years (including those aged seven years, i.e. before fusion of ossification centers). Appropriate ethical approval was obtained from the Research Ethics Committee of the University of Zagreb.
MicroCT
The microCT 1076 and analyzing software used in this experiment were procured from SkyScan™ (Kontich, Belgium). The SkyScan™ 1076 is a high-performance in vivo microCT scanner for preclinical research. MicroCT uses a similar technique as x-ray tomography systems used in medicine but with increased resolution. Multiple 2D x-ray images were stored while the source/detector pair was rotated. Internal structures were reconstructed as a series of 2D cross sections which were then used to analyze the two- and three-dimensional morphological parameters of the object.
The sphenoid bones were scanned, and areas and distance in-between FO and FR were measured (Fig. 1). In addition, the shape of FO was defined based on the already published classifications, as illustrated in Figure 2.
Fig. 1.

MicroCT of isolated sphenoid bone.
Fig. 2.
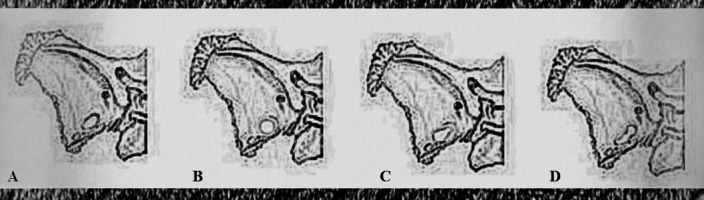
Classification of different shapes of foramen ovale: (A) oval; (B) round; (C) almond; and (D) elongated.
Statistical analysis
Statistical analysis was performed using the SPSS Statistics software package (IBM Corp., Armonk, NY, USA). Data from microCT analysis were expressed as mean ± standard deviation. The normality of data was assessed using Shapiro-Wilk test. Analyses of sex differences in FO and FR areas were calculated using Mann-Whitney U test. Correlation between age and measured areas of foramina for all subjects, as well as for the sex subsamples, was calculated using Spearman’s rho correlation coefficients. The prevalence of different FO shapes was expressed in percentage and then objectively compared between sides. Further on, differences between the areas of foramina on the left and right side were analyzed using Wilcoxon signed rank test. The values of p<0.05 were recorded separately and assumed as significant.
Results
The shapes of FO on both the right and left sides of the sphenoid bone were detected according to the classification proposed (Table 1, Fig. 2). Oval shape was most common (56.4%), followed by round (33.3%), almond (7.7%) and elongated (2.6%) shapes. Oval shaped FO was more often found on the left side, while right FO was more often round shaped (Table 1). Left FO was most commonly oval shaped in both male and female specimens, while right FO was more often round shaped in female, but equally oval and round shape in male specimens (Table 2). Descriptive statistics for all measured variables are presented in Table 3.
Table 1. Variations in the shape of foramen ovale.
| Left | Right | Total | ||||
|---|---|---|---|---|---|---|
| Frequency | Percent | Frequency | Percent | Frequency | Percent | |
| Oval | 28 | 71.8 | 16 | 41.0 | 44 | 56.4 |
| Almond | 3 | 7.7 | 3 | 7.7 | 6 | 7.7 |
| Round | 7 | 17.9 | 19 | 48.7 | 26 | 33.3 |
| Elongated | 1 | 26 | 1 | 2.6 | 2 | 2.6 |
| Total | 39 | 100.0 | 39 | 100.0 | 78 | 100.0 |
Table 2. Descriptive statistics for measured parameters in different shapes of foramen ovale.
| Oval | Almond | Round | Elongated | |||
|---|---|---|---|---|---|---|
| Left FO | Gender (n, %) |
Male | 11 57.9% |
3 15.8% |
5 26.3% |
0 0.0% |
| Female | 17 85.0% |
0 0.0% |
2 10.0% |
1 5.0% |
||
| Age (years) | M (SD) | 2.02 (1.81) |
0.78 (0.39) |
1.33 (2.09) |
6.0 (-) |
|
| Area (mm2) | M (SD) | 10.80 (4.46) | 7.96 (1.03) |
9.17 (4.51) |
8.59 (-) |
|
| Right FO | Gender (n, %) | Male | 9 47.4% |
1 5.3% |
9 47.4% |
0 0.0% |
| Female | 7 35.0% |
2 10.0% |
10 50.0% |
1 5.0% |
||
| Age (years) | M (SD) | 1.19 (0.94) |
5.00 (1.00) |
1.79 (1.97) |
6.0 (-) |
|
| Area (mm2) | M (SD) | 7.61 (3.29) |
14.88 (4.09) |
9.51 (3.65) |
8.72 (-) |
|
FO = foramen ovale; n = number; M = arithmetic mean; SD = standard deviation
Table 3. Descriptive statistics for age (years) and areas (mm2) of sphenoid bone foramina for the whole sample.
| Age | N | Min | Max | Median | Mean | SD | Shapiro-Wilk | p |
|---|---|---|---|---|---|---|---|---|
| 39 | 0.17 | 6.00 | 1.00 | 1.90 | 1.91 | 0.80 | 0.001 | |
| Left FO area | 39 | 3.12 | 19.12 | 8.95 | 10.22 | 4.27 | 0.95 | 0.10 |
| Right FO area | 39 | 2.03 | 19.60 | 8.72 | 9.13 | 3.89 | 0.98 | 0.67 |
| Left FR area | 39 | 1.30 | 6.50 | 2.95 | 3.24 | 1.31 | 0.90 | 0.002 |
| Right FR area | 39 | 1.67 | 7.27 | 3.43 | 3.54 | 1.33 | 0.95 | 0.08 |
| FO-FR distance (left) | 39 | 6.52 | 13.82 | 10.34 | 10.31 | 1.76 | 0.98 | 0.56 |
| FO-FR distance (right) | 39 | 7.33 | 13.71 | 10.30 | 10.55 | 1.73 | 0.97 | 0.45 |
FO = foramen ovale; FR = foramen rotundum; SD = standard deviation
Although age correlated with the areas of foramina (Table 4), no statistically significant age difference was found between the gender subsamples (U=155, p=0.323). Analyzing the possible sex differences in FO and FR areas, the areas of the left and right FO, as well as the right FR were not significantly different between males and females, while the area of the left FR was greater in the female subsample (U=109, p=0.037; median (males) 2.75 mm2, median (females) 3.29 mm2).
Table 4. Spearman’s rho correlation coefficients between age (years) and areas (mm2) of sphenoid bone foramina for the whole sample and sex subsamples.
| Right FO | Left FO | Right FR | Left FR | |||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| Total sample (n=39) | 0.629 | 0.001 | 0.734 | 0.001 | 0.529 | 0.001 | 0.522 | 0.001 |
| All M (n=19) | 0.570 | 0.011 | 0.729 | 0.001 | 0.391 | 0.098 | 0.488 | 0.034 |
| All F (n=20) | 0.701 | 0.001 | 0.666 | 0.002 | 0.494 | 0.027 | 0.467 | 0.044 |
FO = foramen ovale; FR = foramen rotundum; M = male; F = female
Differences between the areas of foramina on the left and right side showed significant difference between the size of the right and left FO (Z=-2.299, p=0.022), with the left one having greater size on average. There was no statistically significant difference between the areas of the left and right FR (p>0.05). The same analysis was conducted on sex based subsamples; significant difference was recorded in the male subsample for FO (Z=-2.254, p=0.024), with the left one being bigger on average (median (left) 8.76 mm2, median (right) 7.84 mm2). There was no significant difference in the female subsample between the left and right FO, and in the FR area in either male or female subsample.
Correlations between age and measured areas of foramina for the whole sample and for the subsamples based on gender were calculated. Modest to strong positive correlations were recorded between foramina area and age for the whole sample and both subsamples, with the exception of the right FR in the male subsample (Table 4, Fig. 3).
Fig. 3.

Scatter plots showing left and right foramen ovale and foramen rotundum area (mm3) correlation with age separately for males and females.
Additionally, positive correlation of age with distance between FO and FR was found for both the left (r=0.838, p<0.001) and right (r=0.744, p<0.001) size of sphenoid bone.
Discussion
Since sphenoid bone changes shape and size during development in the postnatal period, there was a change in the morphology of the bone apertures, especially pronounced in the period before ossification (1, 2). Anatomic variations in shape and dimensions of sphenoid bone openings are common, therefore, detailed developmental and anatomic analysis is of great importance for safe neurosurgical therapeutic guidelines (13, 17, 21-25).
Numerous anatomic morphometric studies of sphenoid bone openings were performed. So far, anatomic studies focused primarily on diameters, i.e. the length, width and shape of FO, FR, and other sphenoid bone openings (10-19).
Very few quantitative morphometric studies included fetal and postnatal skull bones (4, 5), while most of morphometric data were reported on adult sphenoid bone openings (10-19). Postnatal measurements were presented by Lang in 1984; the newborn FR length was about 2.5 mm, while the FO length was about 3.85 mm (5). Neither width nor surface was measured, and the author did not report any statistical differences between the right and left side morphometry (5). As mentioned previously, most morphometric data on FO and FR have been acquired in studies including adult skulls, cadavers, or patient scans (10-19). In the literature, the value of FO length and width varies at around 7-9 mm and 3-5 mm, respectively. Additionally, measurement of surface area was approximately 30 mm2. Moreover, in the majority of case studies, the measured values did not show significant difference between the left and right side for length, width, or area (4, 5, 10-19). Due to the lack of measurements on postnatal species in the literature, it is not possible to compare our results with the currently available literature. However, data from our study showed that the left FO area had a significantly larger surface when comparing to the right one, while the right FR surface was larger than the left one. Such a phenomenon could be explained by uneven ossification of the skull bones, which can take several years (2, 5, 6, 9). Although gender subsamples revealed no significant difference in FR between the left and right side in either male or female subsample, significant difference between the left and right FO was recorded in the male subsample, with the left one being bigger on average. Furthermore, FO and FR areas seemed consistent between the left and right side in the female subsample over years, whereas in the male subsample difference in size seemed to diverge with an increasing trend over years. This observation could be explained by several reasons such as hormonal and metabolic status, uneven ossification, etc. (2, 5, 6, 9). Unlike the mentioned studies, our results indicated modest to strong positive correlations between the foramina area observed and age for the whole sample and gender subsamples, with the exception of the right FR in the male subsample.
According to the literature, there is great variability between the shapes of the skull base openings (4, 5, 10). Perfect ring-shaped formation of FR was observed in all cases of fetuses after the 4th fetal month; in the prenatal period, it was mostly oval-shaped and became round-shaped after birth (4). The earliest perfect ring-shaped formation of FO was observed in the 7th fetal month and the latest at three years after birth. The bony overgrowth during its developmental process is often evidenced by the appearance of tubercle, bony spur, and bony plate surrounding the FO (19).
In various studies, FO was mainly shown to be oval-shaped (4, 5, 10-12, 19). Besides oval, FO shapes were described as almond, round, kidney, pear, strip, elongated, and irregular shape (12, 14, 19). In the majority of available studies, oval shape was described in more than 60% of specimens, followed by round and almond shapes, and in few percents, elongated or irregular shape (12, 14). In our study, oval shaped FO was presented in 56% of specimens in total, 71% on the left and 41% on the right side, followed by the round shape, almond shape, and elongated shape. The left FO was mostly oval shaped in both subsamples, while the right FO was more often round in the female subsample, and equally often round and oval in the male subsample.
Variation in the size and shape of FO could be developmentally explained since sphenoid bone develops from both intramembranous and endochondral ossification, as mentioned previously (5-8). When observing sphenoid bone openings, namely FO, most of them are formed as one main FO and accessory foramina, or as one FO divided by thin osseous trabecula. The presence of an accessory FO, as a 2.3 mm long canal, beginning with an opening in front and medial to FO and leading to near to the root of the pterygoid process, and transmitting probably some of the separate rootlets for chewing muscles has been previously described (28). Thus, the occasional presence of an accessory foramen besides the FO is probably due to the interaction of different parts during intramembranous ossification and the venous plexus from the middle meningeal veins to pterygoid venous plexus (15). In our specimens, accessory FO was found on the left side on two isolated sphenoid bones.
In clinical practice, the sphenoid bone and its openings are of great importance according to different studies; therefore, any variation in their size or shape can severely complicate both diagnostic and therapeutic procedures (13, 17, 21-25, 29, 30). Identification of mechanical compression points and the relationship between trigeminal nerve and the surrounding soft and bony structures was previously examined (24). FO is an important target in various interventions. Percutaneous micro balloon compression for treating trigeminal neuralgia via FO is a well-established technique, as well as percutaneous approach to trigeminal cave (21, 23). The FO cannulation has been used for electroencephalographic temporal lobe analysis in patients undergoing selective amygdalohippocampectomy (31). Additionally, if imaging is not enough to ascertain an accurate pathologic diagnosis of parasellar lesions, percutaneous biopsy using the transjugular-transoval route approach should be performed (25).
Our study contributed to the understanding of postnatal development and remodeling of the sphenoid bone openings in order to minimize the possible risk of damaging important anatomic structures in the mentioned endoscopic and surgical procedures and treatments.
References
- 1.Rhoton AL, Jr. The anterior and middle cranial base. Neurosurgery. 2002;51(4) Suppl:S273–302. 10.1097/00006123-200210001-00007 [DOI] [PubMed] [Google Scholar]
- 2.Azab WA. Normal development of the skull and brain. In: Greenfield J, Long C, eds. Common Neurosurgical Conditions in the Pediatric Practice. New York: Springer-Verlag, 2017. 10.1007/978-1-4939-3807-0. 10.1007/978-1-4939-3807-0 [DOI] [Google Scholar]
- 3.Lang J, Maier R, Schafhauser O. Postnatal enlargement of the foramina rotundum, ovale et spinosuum and their topographical changes. Anat Anz. 1984;156(5):351–87. [PubMed] [Google Scholar]
- 4.Yanagi S. Developmental studies on the foramen rotundum, foramen ovale and foramen spinosum of the human sphenoid bone. Hokkaido Igaku Zasshi. 1987;62(3):485–96. [PubMed] [Google Scholar]
- 5.Lang J. Clinical Anatomy of the Head, Neurocranium, Orbit and Craniocervical Region. Berlin Heidelberg: Springer-Verlag, 1983. 10.1007/978-3-642-68242-1. 10.1007/978-3-642-68242-1 [DOI] [Google Scholar]
- 6.O’Rahilly R, Muller F. Human Embryology & Teratology.3rd edition. New York: Wiley-Liss, 2001. [Google Scholar]
- 7.Catala M. Embryology of the sphenoid bone. J Neuroradiol. 2003;30(4):196–200. [in French] [PubMed] [Google Scholar]
- 8.Mérida Velasco JR, Rodriguez Vazquez JF, De la Cuadro Blanco C, Campos Lopez R, Sanchez M, Merida Velasco JA. Development of the mandibular condylar cartilage in human specimens of 10-15 weeks’ gestation. J Anat. 2009;214(1):56–64. 10.1111/j.1469-7580.2008.01009.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sperber GH, Tobias PV. Craniofacial Embryology (Dental Practical Handbooks). 4th Revised Edition. Oxford: Butterworth-Heinemann Ltd., 1989. [Google Scholar]
- 10.Ray B, Gupta N, Ghose S. Anatomic variations of foramen ovale. Kathmandu Univ Med J (KUMJ). 2005;3(1):64–8. [PubMed] [Google Scholar]
- 11.Reymond J, Charuta A, Wysocki J. The morphology and morphometry of the foramina of the greater wing of the human sphenoid bone. Folia Morphol (Warsz). 2005;64(3):188–93. [PubMed] [Google Scholar]
- 12.Somesh MS, Sridevi HB, Prabhu LV, Swamy MS, Krishnamurthy A, Murlimanju BV, et al. A morphometric study of foramen ovale. Turk Neurosurg. 2011;21(3):378–83. 10.5137/1019-5149.JTN.3927-10.2 [DOI] [PubMed] [Google Scholar]
- 13.Gupta T, Gupta SK. Original landmarks for intraoperative localization of the foramen ovale: a radio-anatomical study. Surg Radiol Anat. 2012;34(8):767–72. 10.1007/s00276-011-0846-2 [DOI] [PubMed] [Google Scholar]
- 14.Pang J, Hou S, Liu M, Feng H, Zu L, Guo Y, et al. Puncture of foramen ovale cranium in computed tomography three-dimensional reconstruction. J Craniofac Surg. 2012;23(5):1457–9. 10.1097/SCS.0b013e3182543231 [DOI] [PubMed] [Google Scholar]
- 15.Patil J, Kumar N, Rao M, Ravindra SS, Somayaji SN, Nayak SB, et al. The foramen ovale morphometry of sphenoid bone in South Indian population. J Clin Diagn Res. 2013;7(12):2668–70. 10.7860/JCDR/2013/7548.3727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sepahdari AR, Mong S. Skull base CT: normative values for size and symmetry of the facial nerve canal, foramen ovale, pterygoid canal, and foramen rotundum. Surg Radiol Anat. 2013;35(1):19–24. 10.1007/s00276-012-1001-4 [DOI] [PubMed] [Google Scholar]
- 17.Zdilla MJ, Hatfield SA, McLean KA, Laslo JM, Cyrus LM, Lambert HW. Orientation of the foramen ovale: an anatomic study with neurosurgical considerations. J Craniofac Surg. 2016;27(1):234–7. 10.1097/SCS.0000000000002332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srikantaiah VC, Shetty H. Anthropometric evaluation of foramen ovale in adult dry skulls of the Mysuru-based population. J Morphol Sci. 2019;36(01):14–6. 10.1055/s-0039-1678754 [DOI] [Google Scholar]
- 19.Prakash KG, Saniya K, Honnegowda TM, Ramkishore HS, Nautiyal A. Morphometric and anatomic variations of foramen ovale in human skull and its clinical importance. Asian J Neurosurg. 2019;14(4):1134–7. 10.4103/ajns.AJNS_243_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsutsumi S, Ono H, Ishii H. The emissary veins of the foramen ovale: an anatomical study using magnetic resonance imaging. Surg Radiol Anat. 2020;42(7):771–7. 10.1007/s00276-020-02432-8 [DOI] [PubMed] [Google Scholar]
- 21.Alvernia JE, Sindou MP, Dang ND, Maley JH, Mertens P. Percutaneous approach to the foramen ovale: an anatomical study of the extracranial trajectory with the incorrect trajectories to be avoided. Acta Neurochir (Wien). 2010;152(6):1043–53. 10.1007/s00701-010-0604-y [DOI] [PubMed] [Google Scholar]
- 22.Ciporen JN, Moe KS, Ramanathan D, Lopez S, Ledesma E, Rostomily R, et al. Multiportal endoscopic approaches to the central skull base: a cadaveric study. World Neurosurg. 2010;73(6):705–12. 10.1016/j.wneu.2010.03.033 [DOI] [PubMed] [Google Scholar]
- 23.Xiaochuan H, Xiaoyun S, Junsheng L, Ning G, Wenshi G, Zhenxing Z. Percutaneous microballoon compression for trigeminal neuralgia using Dyna-CT. Interv Neuroradiol. 2013;19(3):359–64. 10.1177/159101991301900314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang L, Diao Y, Xu Q, Zhang M. Transcranial segment of the trigeminal nerve: macro-/microscopic anatomical study using sheet plastination. Acta Neurochir (Wien). 2014;156(3):605–12. 10.1007/s00701-013-1920-9 [DOI] [PubMed] [Google Scholar]
- 25.Sindou M, Messerer M, Alvernia J, Saint-Pierre G. Percutaneous biopsy through the foramen ovale for parasellar lesions: surgical anatomy, method, and indication. In: Pickard JD, et al., eds. Advances and Technical Standards in Neurosurgery, Vol. 38. Vienna: Springer, 2012, 10.1007/978-3-7091-0676-1_3. 10.1007/978-3-7091-0676-1_3 [DOI] [PubMed] [Google Scholar]
- 26.Dumic-Cule I, Eljuga D, Izadpanah A, Erjavec I, Prgomet S, Hladnik A, et al. Dynamics of optic canal and orbital cavity development revealed by microCT. Surg Radiol Anat. 2014;36(10):989–92. 10.1007/s00276-014-1296-4 [DOI] [PubMed] [Google Scholar]
- 27.Štoković N, Trkulja V, Dumić-Čule I, Čuković-Bagić I, Lauc T, Vukičević S, et al. Sphenoid sinus types, dimensions and relationship with surrounding structures. Ann Anat. 2016;203:69–76. 10.1016/j.aanat.2015.02.013 [DOI] [PubMed] [Google Scholar]
- 28.Krmpotić-Nemanić J, Vinter I, Jalšovec D. Accessory oval foramen. Ann Anat. 2001;183(3):293–5. 10.1016/S0940-9602(01)80237-5 [DOI] [PubMed] [Google Scholar]
- 29.Bašić Kes V, Zadro Matovina L. Accommodation to diagnosis of trigeminal neuralgia. Acta Clin Croat. 2017. March;56(1):157–61. 10.20471/acc.2017.56.01.21 [DOI] [PubMed] [Google Scholar]
- 30.Bičanić I, Hladnik A, Džaja D, Petanjek Z. The anatomy of orofacial innervation. Acta Clin Croat. 2019. June;58 Suppl 1:35–42. 10.20471/acc.2019.58.s1.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wieser HG, Siegel AM. Analysis of foramen ovale electrode-recorded seizures and correlation with outcome following amygdalohippocampectomy. Epilepsia. 1991;32(6):838–50. 10.1111/j.1528-1157.1991.tb05540.x [DOI] [PubMed] [Google Scholar]


