Abstract
Background/Objective
TiCu/TiCuN is a multilayer composite coating comprising TiN and Cu, which provides excellent wear resistance and antibacterial properties. However, its applicability as a functional coating has not been widely realised, and several aspects pertaining to its properties must still be explored.
Methods
This study uses arc ion-plating technology to apply a TiCu/TiCuN coating on the surface of carbon fibre-reinforced (CFR) polyetheretherketone (PEEK) material.The safety and osteogenic activity of TiCu/TiCuN-coated CFR-PEEK materials were explored through cell experiments and animal experiments, and the molecules behind them were verified.
Results
The new material exhibits improved mechanical compatibility (mechanical strength and elastic modulus) and superior light transmittance (elimination of metal artifacts and ray refraction during radiology and radiotherapy). The proposed implant delivers excellent biocompatibility for mesenchymal stem cells and human umbilical vein endothelial cells (HUVECs), and it exhibits excellent osteogenic activity both in vitro and in vivo. Additionally, it was determined that the applied TiCu/TiCuN coating aids in upregulating the expression of angiogenesis-related signals (i.e., cluster-of-differentiation 31, α-smooth muscle actin, vascular endothelial growth factor receptor, and hypoxia-inducible factor-1α) to promote neovascularisation, which is significant for characterising the mechanism of the coating in promoting bone regeneration.
Conclusion
The current results reveal that the TiCu/TiCuN-coated CFR-PEEK implants may emerge as an advanced generation of orthopaedic implants.
Translational potential statement
The results of this study indicate that TiCu/TiCuN coating-modified CFR-PEEK materials can promote bone repair through angiogenesis and have broad clinical translation prospects.
Keywords: Bone regeneration, Carbon fibre-reinforced polyetheretherketone, Orthopaedic implant, TiCu/TiCuN, Vascularisation
1. Introduction
In recent years, the number of bone defects caused by trauma [1], bone disease [2], and bone tumour [3] has increased. Hence, the treatment of bone defects has emerged as a pressing problem for clinicians. For bone defects caused by trauma and bone diseases, an excellent reconstruction effect can be achieved via bone–graft composite metal prostheses [4,5]. However, reconstruction after bone–tumour resection is a very complex issue. In addition to considering the mechanical stability and adaptability of the reconstructed material, such patients are required to undergo frequent follow-ups, and certain patients are required to undergo radiotherapy. In such cases, medical Ti alloys and CoCrMo materials currently used in the field of bone tumour surgery exhibit the following deficiencies. Metal prostheses often generate adverse metal artifacts during radiological examinations, which affect the clinical analysis of X-ray or computed tomography (CT) data and increase the probability of missing early small tumour recurrences. The density of the medical Ti alloy is 4.43 g/cm3 and that of CoCrMo is 8.24 g/cm3. Thus, prostheses comprising these materials are extremely heavy, which may accelerate the loss of bone around the prosthesis after implantation and even cause fractures surrounding the prosthesis [6]. Moreover, the dense metal causes radiation scattering, and consequently, accurate estimation of the radiation dose is challenging for doctors. These scattered rays also damage the surrounding normal tissues [7]. The elastic modulus of normal human cortical bone is 20 GPa, whereas those of Ti–6Al–4V and CoCrMo alloys are approximately 155 and 210 GPa, respectively. This excessive difference in the elastic modulus produces a stress shielding effect, accelerates bone loss, and causes prosthesis failure [8]. Ti–6Al–4V and CoCrMo alloys are both inert metals that can provide only mechanical support; they are unable to promote bone regeneration. They exhibit inferior performance in reconstructing large, complex bone defects in patients. Patients develop serious complications, such as bedsores, pneumonia, and deep vein thromboses, owing to a long recovery time [8]. Therefore, the development of alternative materials is urgently required to effectively surpass the limitations to satisfy growing clinical needs.
Carbon fibre-reinforced (CFR) polyetheretherketone (PEEK) has been extensively for bone tumour surgery and to prepare trauma orthopaedic implant devices with appropriate clinical effects [[9], [10], [11]]. It is formed by adding carbon fibre components of various lengths and weight fractions to PEEK. Consequently, CFR-PEEK exhibits a higher mechanical strength and wear resistance than traditional PEEK materials [12]. Additionally, its mechanical parameters vary with the length and thickness, in addition to arrangement of the carbon fibre. The currently available implant-grade materials primarily include CFR-PEEK materials containing 60 or 30 wt% carbon fibres, wherein the former contains long carbon fibres. Although the tensile strength often exceeds 2000 MPa, the elastic deformation ability is sacrificed, and completing intraoperative shaping is challenging. This material can be used to prepare steel plates and intramedullary nails. Spine rod systems can provide excellent mechanical strength [[13], [14], [15]]. By contrast, the 30 wt% material is short carbon fibre composite PEEK, wherein most short carbon fibres are less than 0.4 mm in diameter. This type of material possesses both rigidity and toughness. According to the parameters, the elastic modulus of CFR-PEEK materials can be controlled within the range of 3.5–58.5 GPa, which is proximate to the elastic modulus of human bones and poses excellent mechanical adaptability. Moreover, the density of the CFR-PEEK material is 1.3 g/cm3. Under the premise of ensuring the mechanical strength needed to satisfy clinical applications, the weight of the prosthesis is reduced, which is conducive to immediate stability after the operation and reduces the risk of residual bone wear and fracture. In addition to the mechanical advantages, the light transmittance of CFR-PEEK is unmatched by most metal materials, which allows radiation to pass through during radiology inspections and treatments without affecting the results. However, CFR-PEEK materials pose certain inherent limitations, such as the lack of biological activity. In certain cases of large-scale bone defects, the same problems can occur for metal prostheses. Therefore, the development of a feasible material modification method to produce a CFR-PEEK material that promotes bone regeneration has emerged as a problem requiring further research consideration.
As an essential trace element in the human body, Cu can promote bone regeneration and antibacterial activity [[16], [17], [18]]; thus, it is an ideal material for prosthesis modification. However, a large release of Cu ions can cause tissue damage [19]. Thus, to optimise the use of the osteogenic activity of Cu ions within the scope of biological safety, our research team developed a new type of TiCu/TiCuN coating that forms TiN and Cu elements. In vivo and in vitro experimental studies have demonstrated that the coating exhibits excellent antiwear, corrosion resistance, anti-infection, and osteogenic properties [20,21]. In an experiment to promote osteogenesis, we preliminarily confirmed that the TiCu/TiCuN coating can activate the upstream signal of stromal cell derived factor-1α/C-X-C chemokine receptor type 4, which accelerates the recruitment of mesenchymal stem cells and promotes the repair of bone defects. The occurrences of accelerating bone regeneration in the downstream of the signal axis have limited exploration up to this date.
Therefore, this study raises three new questions:
-
1.
Can the TiCu/TiCuN coating be stably combined with non-metallic CFR-PEEK materials to satisfy the standards for use as implantable devices?
-
2.
Will the mechanical properties, biological behaviour, and safety of the material change if TiCu/TiCuN is combined with CFR-PEEK?
-
3.
How exactly can the TiCu/TiCuN coating increase the speed of bone regeneration?
This study explores the rationality of the clinical application of CFR-PEEK modified by TiCu/TiCuN coating based on three aspects: mechanical adaptability, biological safety, and osteogenic activity. First, the stability of the modified CFR-PEEK coating and the release characteristics of Cu2+, biological safety, and osteogenic activity are studied from a macroscopic perspective. Thereafter, the molecular mechanism triggering osteogenic activity was studied from a microscopic perspective. Therefore, this study aims to obtain a comprehensive, multilevel explanation of the aforementioned questions and provide a theoretical basis for the clinical transformation of TiCu/TiCuN-modified CFR-PEEK materials.
2. Experimental section
2.1. Preparation of materials
A disc with a diameter of 10 mm and a height of 2 mm was used in the in vitro experiment. The samples were segregated into three groups: CoCrMo, CFR-PEEK, and TiCu/TiCuN-modified CFR-PEEK groups (hereinafter referred to as “TiCu/TiCuN group”). The CoCrMo materials were obtained from Beijing Lidakang Technology Co., Ltd., and the CFR-PEEK materials were obtained from Changzhou Junhua Special Plastic Co., Ltd.
The TiCu/TiCuN-coated CFR-PEEK discs were prepared at the Institute of Metal Research, Chinese Academy of Sciences, and the preparation steps are stated as follows (Fig. 2A):
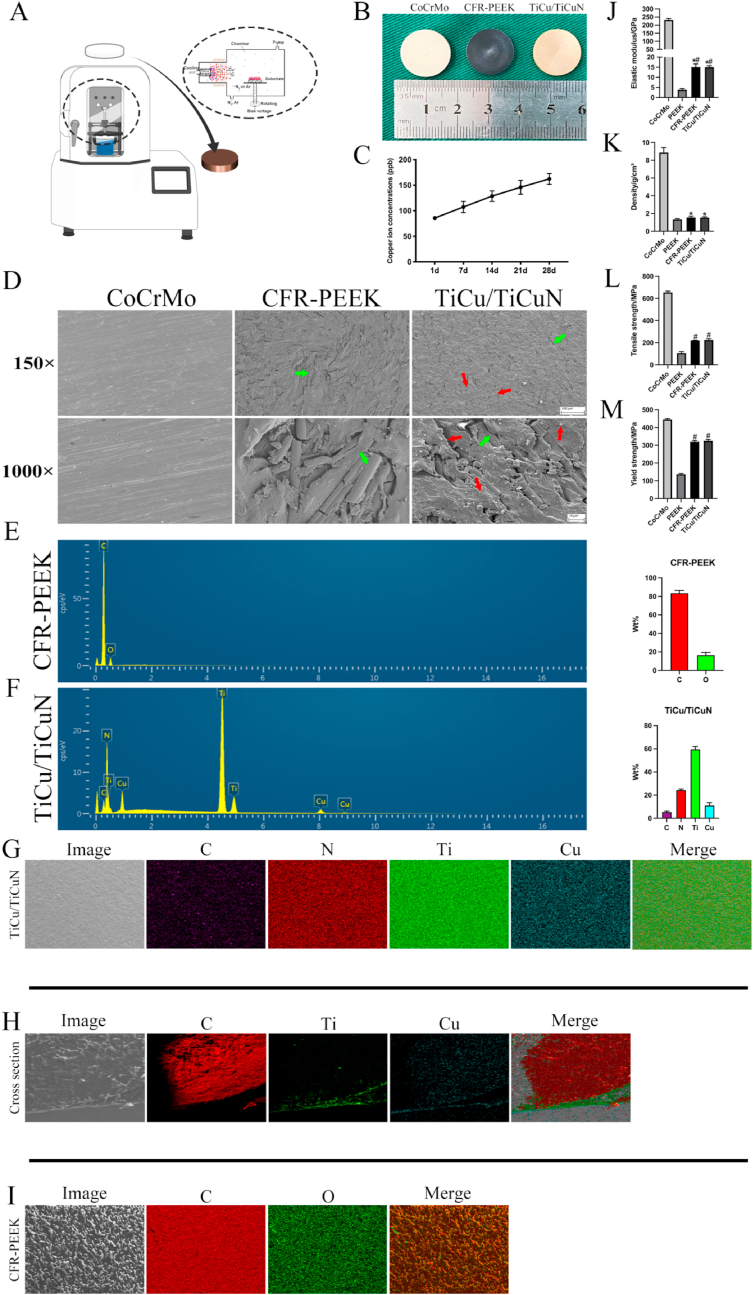
Fig. 2.
Material preparation, characterization, and mechanical testing (A) Schematic of coating preparation (B) Three groups of materials used in experiment, uniform preparation of coating on CFR-PEEK material surface (C) Cu2+ release curve (D) SEM scans of material surface microstructure (Green arrow: carbon fibre, Red arrow: TiCu/TiCuN coating) (E,F) EDS detects material composition (G,H,I) Mapping analysis (J) Modulus of elasticity (K) Density (L) Tensile strength; and (M) Yield stress.
Domestic PVD7590 arc ion-plating equipment was used for coating the target, which was a Ti90Cu10 target alloy (target purity = 99.9%). The CFR-PEEK sample was ultrasonically cleaned with absolute ethanol, dried in hot air, and placed on an equipment platform. Prior to coating, a vacuum of 5.0 × 10−3 Pa was drawn, the vacuum chamber was heated to 150 °C, and Ar gas was introduced into the chamber. Additionally, arc-enhanced glow discharge technology was used to clean the surface of the sample for 60 min at a substrate pulse bias of −150 V × 80%. Subsequently, N2 gas was introduced into the vacuum chamber, and the air pressure was maintained at 2.0 Pa. The TiCu target arc current was 70 A, the substrate pulse negative bias voltage was −50 V × 60%, the target substrate distance was approximately 150 mm, the deposition temperature was 100–150 °C, and the coating period was 35 min.
Please refer to the supplementary materials for material characterization and biological experiments.
3. Results
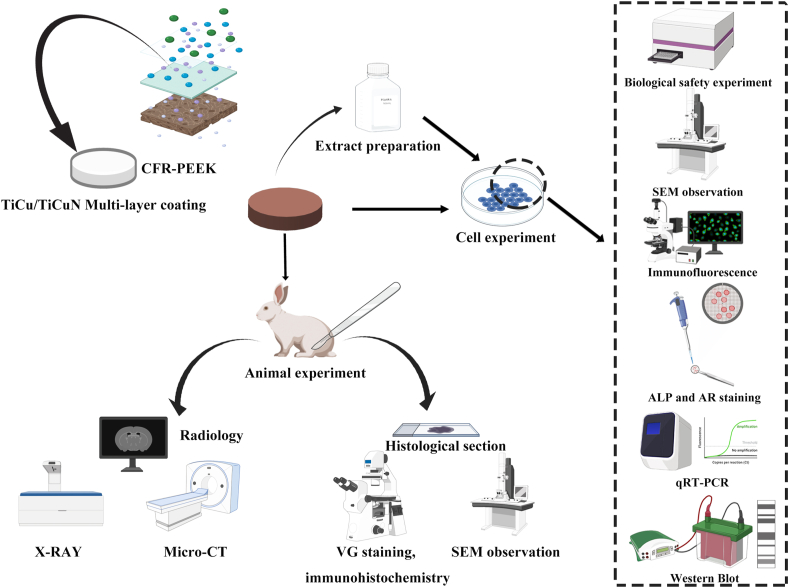
The overall design of the experiment is presented in Fig. 1.
Fig. 1.
Experimental design and implementation plan.
3.1. Implant characterisation
The general images of the materials revealed that the surfaces of the three materials were regular, and the surface of the modified CFR-PEEK material exhibited a uniform distribution of golden yellow TiCu/TiCuN (Fig. 2B).
The Cu2+ release curve exhibited a constant slope, indicating that Cu2+ was released at a uniform rate, and the release amount was within a safe range (Fig. 2C).
The material surface microstructure observed through SEM revealed that the CoCrMo caused scratches on the discs caused by the post-polishing treatment. As the CFR-PEEK material was directly cut from the extruded rods, carbon fibres with uniform distribution were scattered in various directions within the PEEK matrix and observed in the cross-section. Moreover, the TiCu/TiCuN coating was granular and uniformly distributed on the surface of the entire CFR-PEEK disc (Fig. 2D). The EDS results demonstrated that the CFR-PEEK and TiCu/TiCuN did not mix with the extraneous elements during the preparation process (Fig. 2E and F). The mapped (planar and cross-sectional) images of the TiCu/TiCuN group revealed that the material surface was uniformly covered. The designed coating thickness before spraying is 1 μm, and the actual measured coating thickness is 2 μm (Fig. 2G and H). The mapping of the CFR-PEEK material revealed that the material surface was uniform and free of impurities (Fig. 2I). Furthermore, the mechanical tests confirmed that the CFR-PEEK material exhibited excellent mechanical compatibility, and the modulus of elasticity was like that of the normal bone (Fig. 2J). Additionally, the density was considerably lower than that of the CoCrMo alloy (Fig. 2K). The tensile strength and yield stress satisfied the requirements for clinical implantation (Fig. 2L, M).
3.1.1. BMSC adhesion, proliferation, and biological safety experiment
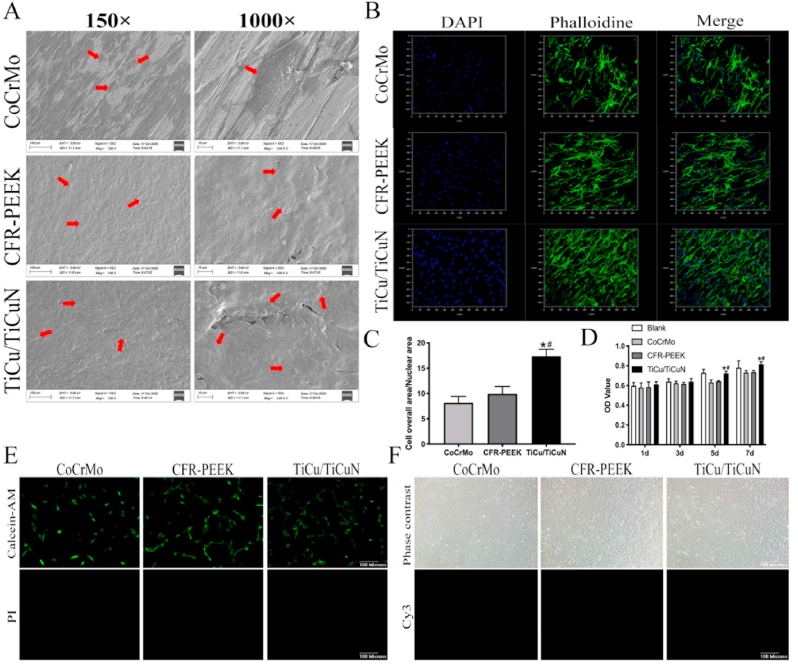
The FE-SEM results suggested that the BMSCs adhered to the surfaces of the three groups of materials after seven days of co-cultivation. The number of adhesions in the CoCrMo group was fewer than that of the other two groups (Fig. 3A).
Fig. 3.
Cell adhesion and biological safety testing (A) SEM scans of BMSCs adhering to material surface (B) Laser confocal microscope observation of number and morphology of adherent cells on material surface (C) Ratio of nucleus to cytoplasm indirectly reflects the spread of cells (D) CCK-8 experiment measures cell proliferation (E) Live/Dead staining to determine cytotoxicity (F) TUNEL staining to determine cell apoptosis.
The confocal microscopy indicated that the cell morphology adhering to the CoCrMo surface was not as significant as that of the BMSCs in the CFR-PEEK and TiCu/TiCuN groups (Fig. 3B). The TiCu/TiCuN group exhibited the largest number of cell adhesions and the optimal cell spreading and morphology. The semi-quantitative analysis of the cell adhesion resulted from the nuclear-to-mass ratio, demonstrating that the TiCu/TiCuN group possessed a superior spreading state (Fig. 3C).
The results of the CCK-8 assay on the first and third days disclosed that no significant variations were present in the cell proliferation on the surface of the three groups. By contrast, the cell proliferation rate of the TiCu/TiCuN group was significantly higher than that of the other two groups from the fifth day onward (Fig. 3D). The results of live–dead staining indicated that none of the three groups of materials exhibited demonstrable cytotoxicity to the BMSCs (Fig. 3E). Moreover, TUNEL staining indicated that apoptosis was not induced by the materials (Fig. 3F).
3.2. Osteogenic differentiation test
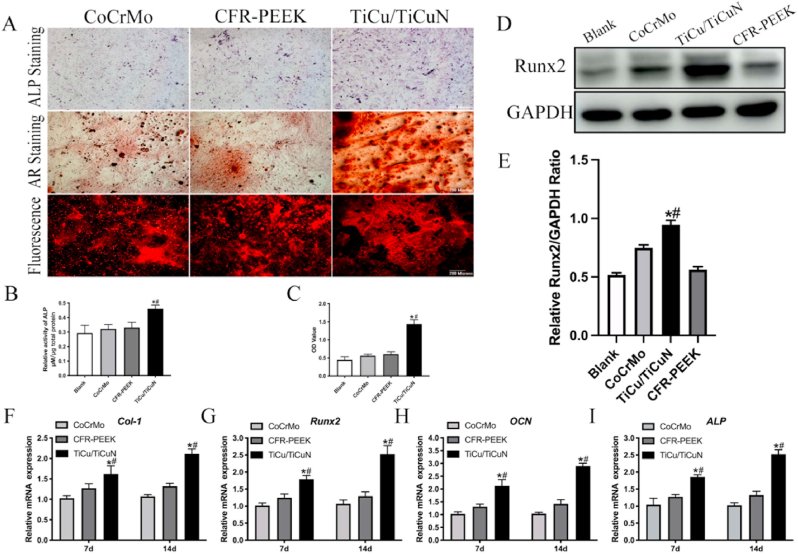
The alkaline phosphatase (ALP) live and alizarin red (AR) staining results indicated that the TiCu/TiCuN coating significantly promoted bone regeneration (Fig. 4A). The ALP semi-quantitative experiment results revealed that the TiCu/TiCuN coating promoted early bone formation (Fig. 4B). The AR semi-quantitative analysis results implied that the production of Ca nodules was enhanced under the influence of the TiCu/TiCuN coating (Fig. 4C). Western blotting revealed that the TiCu/TiCuN promoted the expression of runt-related transcription factor (Runx)-2, an important osteogenic protein (Fig. 4D and E). The PCR results confirmed that the expression of osteogenic genes, such as Runx2, collagen (Col) type 1, osteocalcin (OCN), and ALP, was upregulated by the TiCu/TiCuN coating (Fig. 4F–I).
Fig. 4.
Osteogenesis experiment (A) ALP and AR staining (B) Semi-quantitative analysis of ALP (C) AR semi-quantitative analysis (D) Western blotting analysis detects Runx2 expression (E) Semi-quantitative analysis of Runx2 expression; and (F–I) PCR analysis of expression of osteogenic genes.
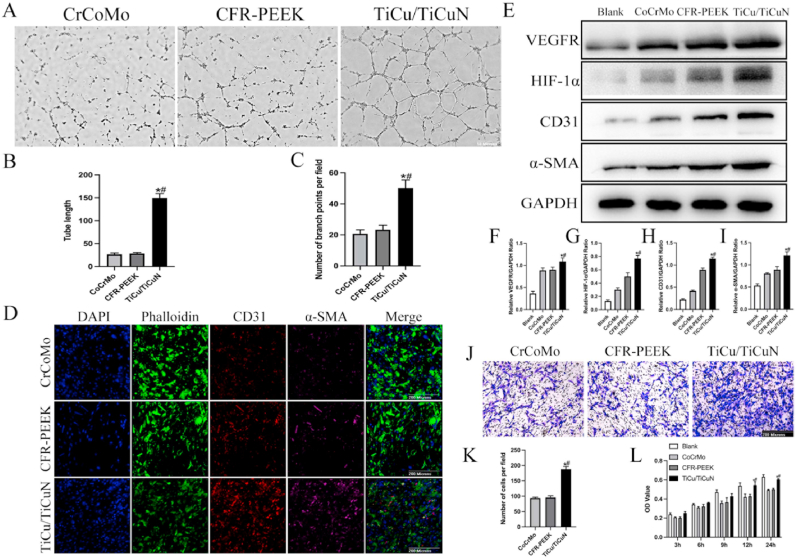
3.3. Angiogenesis experiment
The results of the tubular formation experiments disclosed that the TiCu/TiCuN coating promoted blood vessel formation by HUVECs (Fig. 5A). Furthermore, the semi-quantitative analysis of the tube length, including the number of nodes, was performed using imaging software to verify the ability of the TiCu/TiCuN coating to improve the neovascularisation quality (Fig. 5B and C).
Fig. 5.
Angiogenesis experiment (A) Tubule formation experiment (B) Semi-quantitative analysis of tube length (C) Semi-quantitative analysis of branch points (D) Immunofluorescence CD31 and α-SMA (E) Western blotting analysis detects expression of vascular-related proteins (F–I) Semi-quantitative analysis of angiogenesis-related proteins (J) Transwell experiment detects migration of HUVECs (K) Semi-quantitative analysis of Transwell experiment; and (L) CCK-8 experiment indirectly reflected early adhesion of HUVECs.
The cluster-of-differentiation (CD) 31 and α-SMA immunofluorescence staining results revealed that the expression of these two proteins was upregulated for the TiCu/TiCuN coating group (Fig. 5D). Furthermore, the western blot analysis of angiogenesis-related proteins (i.e., VEGFR, HIF-1α, CD31, and α-SMA) revealed that the developed coating significantly upregulated the protein expression (Fig. 5E–I).
Moreover, the Transwell experimental results signified that the TiCu/TiCuN coatings could promote the migration of vascular endothelial cells.
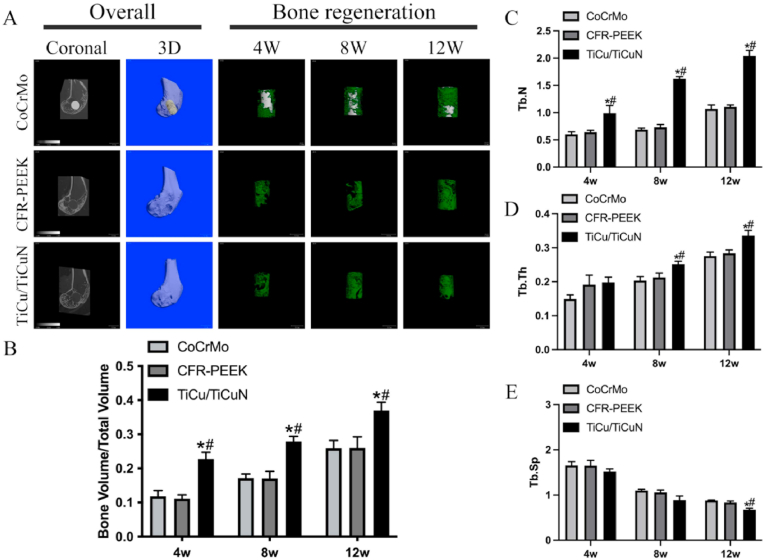
3.4. Radiology analysis
The micro-CT scan results suggested that the implant was stable without displacement after operation, and the TiCu/TiCuN coating promoted bone regeneration proximate to the bone defect (Fig. 6A and B). The analysis results of the scan data demonstrated that the regenerated bone trabecula of the TiCu/TiCuN group possessed enhanced cell population, thickness, and density (Fig. 6C–E).
Fig. 6.
Radiology analysis (A) Micro-CT scan (B) BV/TV statistics (C) Number of trabecular bones (D) Trabecular bone thickness; and (E) Trabecular bone density.
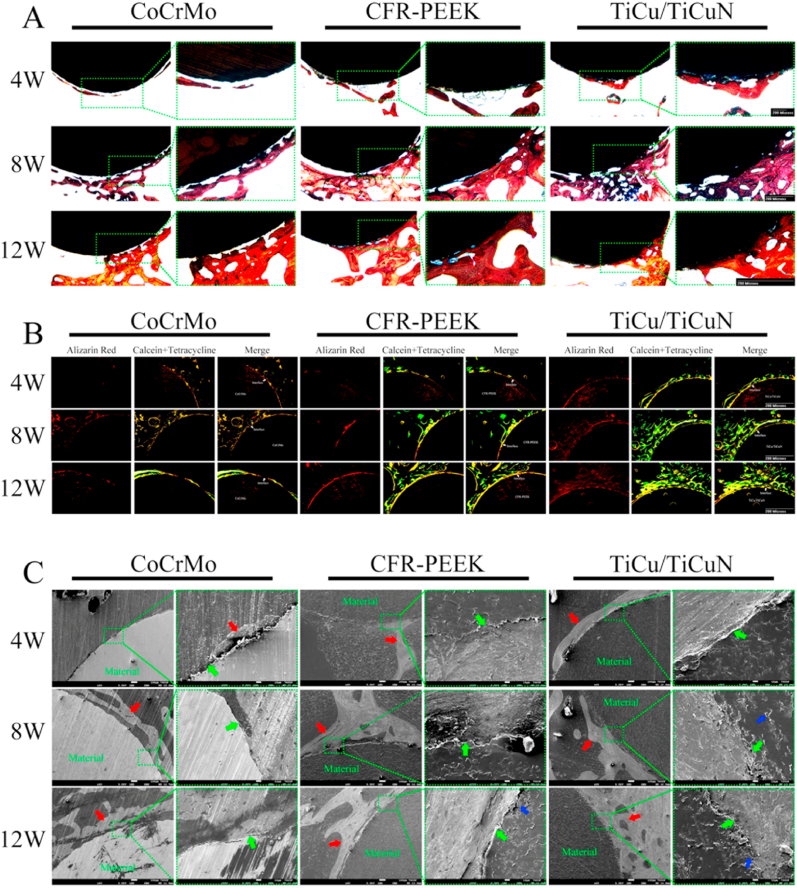
3.5. Histological analysis
The VG staining results suggested that the material and bone tissue in the TiCu/TiCuN group started to exhibit significantly strong osseointegration after 4 weeks. Similarly, the material and bone tissue in the CFR-PEEK group exhibited partial osseointegration after 8 weeks, and the osseointegration between the material and bone tissue in the CoCrMo group did not appear until after 12 weeks. However, a negligible amount of bone was integrated onto the prosthesis, and the observed amount of regenerated bone included a greater amount of regenerated bone in the TiCu/TiCuN group after 8 weeks than that in the other two groups. Nonetheless, no significant differences were observed in the amount of regenerated bone between the three groups after 12 weeks (Fig. 7A). The fluorescence triple-labelling results confirmed that the TiCu/TiCuN group displayed the fastest bone regeneration rate among the three groups, whereas that of CFR-PEEK was slower, and that of the CoCrMo group was the slowest (Fig. 7B). Moreover, the FE-SEM scans of the tissue specimens confirm that the TiCu/TiCuN coating promoted the process of osseointegration and bone regeneration, and the CFR-PEEK material possessed considerable biocompatibility, which is more conducive to bone tissue adhesion than metal (Fig. 7C).
Fig. 7.
Histological analysis (A) VG staining (B) Observation of bone regeneration speed with three fluorescent markers (C) SEM scan of tissue section to observe bone and material microstructure (Green arrow: osseointegration interface. Red arrow: bone. Blue arrow: carbon fibre).
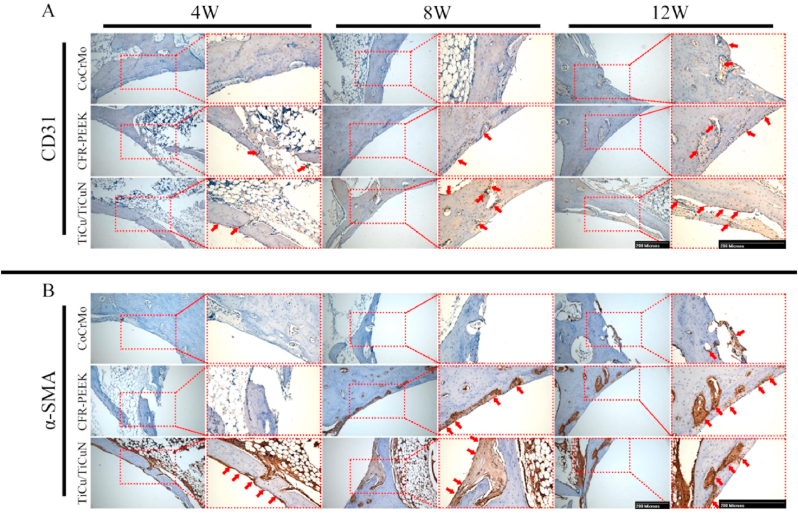
The immunohistochemistry results revealed that the vascular indices, CD31 and α-SMA, were affected by TiCu/TiCuN coating, and their expression was significantly upregulated (Fig. 8A and B) (The mechanism hypothesis is presented in Fig. 9).
Fig. 8.
Immunohistochemical analysis of (A) CD31 and (B) α-SMA (red arrow: positive performance).
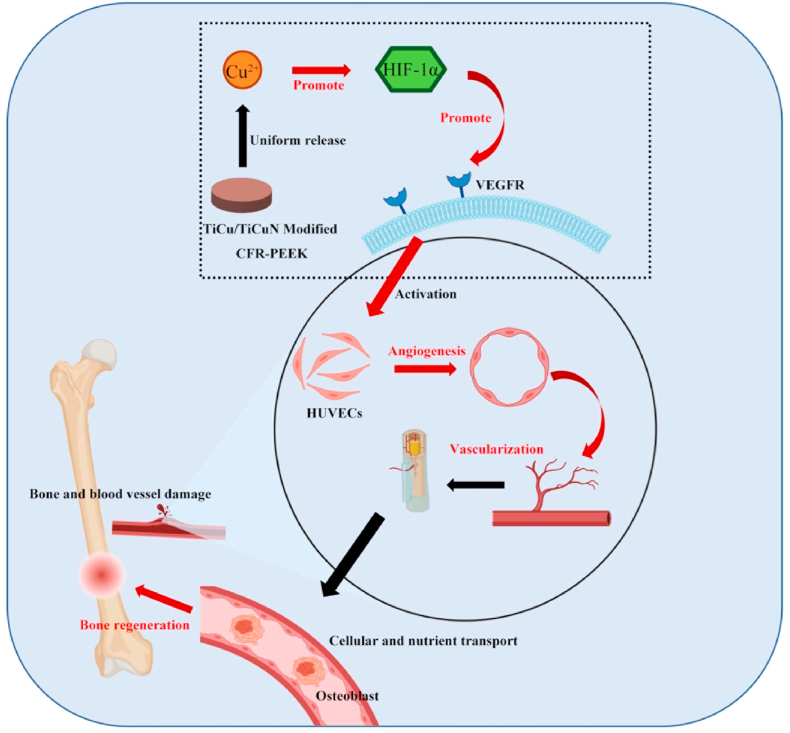
Fig. 9.
Hypothesized mechanisms of TiCu/TiCuN to promote bone defect repair by promoting angiogenesis.
As the HE staining of important organs revealed no evident damage to the heart, liver, spleen, lungs, and kidneys, the three groups of materials exhibited significant biological safety (Figure S1).
4. Discussion
Bone tumour surgery is a unique orthopaedic treatment, and its treatment plan is more complicated than those of trauma orthopaedics, spine surgery, or joint surgery, as it often involves radiotherapy and frequent follow-up. Therefore, metal artifacts and ray scattering caused by metal prostheses adversely affect quality of treatment for patients with bone tumours.
Based on these clinical issues, researchers in collaboration with specialised plastic manufacturers developed the 30 wt% CFR-PEEK material used in this study. The experimental results confirmed that the material exhibited enhanced mechanical strength and adaptability, light transmittance, and biological safety and satisfied the requirements of clinical implantation. Concurrently, we reviewed and analysed the disadvantages of CFR-PEEK materials resulting from the dearth of osteogenic activity. In particular, the CFR-PEEK material loaded with TiCu/TiCuN coating was prepared, and the mechanical properties, biological safety, and osteogenic activity of this composite material were verified alongside the rationality of its clinical application.
In this study, the selection of CFR-PEEK as the substrate-supporting TiCu/TiCuN coating was primarily based on the following considerations:
Medical-grade PEEK and PEEK-based modified materials (e.g., carbon fibre or glass fibre) have been increasingly recognised by researchers. Specifically, PEEK and carbon fibre intervertebral fusion products have yielded satisfactory results after extensive utilisation [22,23]. The manufacture of prostheses for weight-bearing components has been hindered, owing to the low tensile strength and yield strength of the pure PEEK material; the tensile strength of implanted PEEK tested in this study was 106.67 ± 11.83 MPa, and the yield strength was 137.01 ± 4.72 MPa. However, the addition of carbon fibres ameliorated the material strength. Although the excellent mechanical strength of the CFR-PEEK (60 wt%) enables successful implementation in the manufacture of fixation plates and spinal rod products, it exhibited poor elastic deformation ability and was unsuitable for use in joint prostheses. Thus, 30 wt% carbon fibre content was used for the CFR-PEEK matrix to obtain a material that can effectively replace the metal joint prosthesis.
The CFR-PEEK material satisfied the mechanical properties for clinical requirements and possessed excellent light transmittance. Additionally, the tissue morphology around the prosthesis could be prominently observed during the radiological examination, which aids in identifying the recurrence of tiny tumours. As the rays were not refracted during radiotherapy, the dose of radiotherapy could be conveniently assessed. Prior research has successfully applied TiCu/TiCuN coatings on planar metals and three-dimensional (3D) printed porous metal surfaces with complex structures. However, there is a dearth of relevant research on the preparation and biological performance of metal coatings on the surface of polymer materials. Furthermore, the relevant research should address concerns, such as the uniform preparation and release of the coating, including variations in mechanical and biological properties of the material after preparation.
The carbon fibre length of the CFR-PEEK material used in this study was 50 μm with a diameter of 7.5 μm. Additionally, the rods prepared by extrusion were cut to obtain the wafers used in the experiment, which consequently caused uneven structures of the disc surface, as observed from the SEM images. Many studies have demonstrated that a rough structure is advantageous for cell adhesion and proliferation [[24], [25], [26]]. The SEM scanning results demonstrated that the TiCu/TiCuN coating was distributed in a granular form on the surface of the CFR-PEEK substrate, uniformly covering the PEEK matrix and the carbon fibre part. The wear test results show that the TiCu/TiCuN coating improves the wear resistance of the material on both the CFR-PEEK substrate and the Ti6Al4V substrate. The wear test results also reflect the firmness of the bonding between TiCu/TiCuN and the two substrates.Thus, the experimental results address Question 1 posited in the Introduction: “Can the TiCu/TiCuN coating be stably combined with non-metallic CFR-PEEK materials to satisfy the standards for use as implantable devices?”
The uniform distribution of the coating is a prerequisite for uniform release, and the release test confirmed the uniform release characteristics of the TiCu/TiCuN coating. As discussed earlier, 30 wt% carbon fibre content was used for the CFR-PEEK material, which displayed a greater elastic deformation ability than that of the 60 wt% CFR-PEEK reported in clinical practice to date. However, certain compromises occurred in terms of mechanical strength, thereby necessitating mechanical testing. According to the mechanical test results, the yield and tensile strengths of the CFR-PEEK material were significantly lower than those of the CoCrMo material; however, the CoCrMo material displayed superior mechanical parameters compared with those of the implanted PEEK materials currently approved for medical use. Overall, the mechanical parameters of the CFR-PEEK material used in this experiment satisfied the mechanical requirements of the clinical polymer implantable devices mandated by the Chinese Medical Device Guidelines.Therefore, the experimental results demonstrate that the short carbon fiber CFR-PEEK material used in this study can meet the needs of clinical use.
After demonstrating the possibility of clinical use of materials based on mechanical adaptability, the biocompatibility and safety of materials are important concerns to be addressed [27]. First, the adhesion of cells on the surfaces of the CoCrMo, CFR-PEEK, and TiCu/TiCuN-modified CFR-PEEK groups was studied using SEM and confocal microscopy to preliminarily assess the biocompatibility of each group of materials. The fundamental observations consider the following aspects: 1) number of cells adhered onto the material surface, and 2) morphology of cell adhesion on the material surface. Owing to the influence of the TiCu/TiCuN functional coating, a greater number of BMSCs adhered to the coating group than the other two groups, and these cells were completely extended with a distinct morphology. These findings are more conducive to the differentiation and autocrine function of BMSCs [28]. However, an interesting phenomenon was observed: the cell morphology and spreading of the CFR-PEEK material surface without coating modification were improved over those of the CoCrMo group. Li et al. reported that a material surface with a spatial structure is conducive to cell adhesion and proliferation [29]. As the carbon fibre was mixed with the PEEK matrix to form rods via continuous extrusion, the experimental discs were obtained by machining the rods. Therefore, the material performance depicted an omnidirectional distribution of the carbon fibre. Its microstructure was not as smooth as that of metal materials, and uneven structures appeared on the surface, which may be the reason for CFR-PEEK to be more conducive toward the adhesion and proliferation of BMSCs.
In this experiment, the CFR-PEEK group was set as the control group to clarify whether the cell proliferation and adhesion are influenced by the surface structure or the biological function of the coating, which adds rationale to the experimental conclusions. Moreover, the biological safety of implant-grade materials was affected by the preparation process and varied after industrial processing. In addition to the material itself, if the Cu element in the TiCu/TiCuN coating exceeded the limit of 3 mg Cu/L, it causes gastrointestinal reactions [30]. Thus, this experiment did not directly apply a Cu coating; instead, it used TiN crystals to bind the Cu element. As observed from the release test results, the Cu element in the TiCu/TiCuN coating was released at a uniform rate, and the cumulative concentration at 28 days was within the safe range. Furthermore, Barceloux's research reported that the metabolism period of the Cu element in the body is 13–33 days [30]; hence, the experiment to observe the cumulative concentration of Cu2+ in 28 days can be reasonably designed for assessing safety. The experimental results indicated that the TiCu/TiCuN coating did not release a large amount of Cu2+ and caused toxic reactions. In this study, the CCK-8 experiments were designed, and the live–dead staining with TUNEL staining repeatedly demonstrated the safety of the materials toward BMSCs. Furthermore, the results showed that the TiCu/TiCuN coating and CFR-PEEK materials were neither directly toxic toward BMSCs nor did they induce BMSC apoptosis. During the initial design of the biosafety experiment, we determined the inconsistency between the results of CCK-8, live–dead staining, and TUNEL staining experiments for the rigor of the experiment. Therefore, more in-depth research needs to be conducted on the mechanism of cell death (i.e., direct death, induced apoptosis). However, owing to the high consistency of the experimental results, this segment of the experiment was not necessary.
We endowed the CFR-PEEK material with osteogenic activity via coating modification. However, before discussing the osteogenic activity of CFR-PEEK materials modified by the TiCu/TiCuN coating, we must understand that bone regeneration is a complex process involving a variety of biological processes that alter and influence each other [31]. More importantly, the regeneration and repair of blood vessels is an important biological process [[32], [33], [34]], and bone defects are often accompanied by the destruction of nutrient vessels. Owing to the destruction of the blood vessels, the nutrients in the blood lack an important channel to reach the injured site [35]. Therefore, the reconstruction of the blood supply to the bone injury site is extremely beneficial for bone repair.Lin et al. combined magnesium particles with bone cement to further enhance bone repair by promoting angiogenesis [36]. The study by Cheng and Liu also illustrates the importance of angiogenesis for bone repair [37,38]. Xie et al. determined that Cu2+ is transported by superoxide dismutase, and it can promote HIF-1α expression [ [39]], which is an important upstream signalling molecule involved in angiogenesis. Therefore, it can be speculated that the osteogenic activity of TiCu/TiCuN is based on its ability to promote vascular regeneration. Hence, rigorous osteogenesis and vascularisation experiments were designed for demonstration. First, we analysed the effect of TiCu/TiCuN on the osteogenesis process following two perspectives: early and late osteogenesis. Early osteogenesis is primarily achieved via the detection of early osteogenesis indicators, such as Runx2, ALP, Col-1, and OCN; late osteogenesis pertains to the observation of the formation of calcium nodules. The experimental design covers the entire process of osteogenesis, and the results revealed that the TiCu/TiCuN is essential for the entire process of osteogenesis. From the osteogenic differentiation experiment in Fig. 4, we can see that the semi-quantitative results of ALP and Alizarin red of CFR-PEEK material without TiCu/TiCuN coating modification are close to those of CoCrMo group. There was no statistical difference between the two groups. PCR results showed that the expression of osteogenesis-related genes in the CFR-PEEK group was higher than that in the CoCrMo group. This phenomenon may be related to the fact that the scattered carbon fibers on the surface of CFR-PEEK make the CFR-PEEK material have a certain spatial structure. On the 14th day, the expression of Runx2 protein in the CFR-PEEK group was lower than that in the CoCrMo group. After comparing this result with the PCR results of the Runx2 gene on the 14th day, we believe that the Runx2 gene in the CFR-PEEK group is in a relatively active state at this stage, but the protein translation process is in a relatively lagging state compared to the CoCrMo group. In combination with the results of cell adhesion experiments, the results of ALP and AR staining revealed that the surface-roughened materials were more conducive toward cell adhesion, but they did not necessarily promote osteogenesis. This research finding provides practical insights for clinical prosthesis treatment. Presently, during the preparation of clinical implants, implant manufacturers tend to use sandblasting and titanium spraying techniques to roughen the surface of the prosthesis [40,41]. This treatment can facilitate the adhesion of more BMSCs to the surface of the prosthesis. However, an additional condition is required to induce osteogenic differentiation of BMSCs for better promotion of bone regeneration and osseointegration. Therefore, functional coating modifications are necessary in this aspect.
A large amount of nutrients is required in the bone regeneration process. In this context, blood vessels are the “highway” for cells and nutrients to reach the injured site [[42]]; therefore, they are essential in the process of bone and tissue repair and reconstruction [43]. Consequently, this study further focused on the process of angiogenesis at the bone injury site to explore the effect of TiCu/TiCuN coating on this process. Only a few studies have explored the promotion of vascular regeneration using only Cu. Lin and Liu et al. reported that superphosphate-Cu and Mg–Cu materials promote vascular regeneration [44,45]. However, Ca phosphate and Mg exhibit appropriate biological functions as well; hence, the element enabling angiogenesis cannot be distinctly identified. In this study, we initially determined that the TiCu/TiCuN coating promotes the migration of HUVECs, and we observed that the TiCu/TiCuN coating promoted vascular regeneration via tube-forming experiments. Subsequent studies have reported that the TiCu/TiCuN activates HIF-1α signals and affects important angiogenic signals, such as VEGFR, α-SMA, and CD31, which is a further improvement over the study of the molecular mechanism, following which Cu2+ promotes bone regeneration. Since TiCu/TiCuN contains both Ti and Cu metal elements. In order to exclude the influence of Ti element on HIF-1α. We designed further experiments to verify. We co-cultured Ti6Al4V, medical stainless steel (317L), and copper-containing medical stainless steel (317-Cu) with HUVECs for 3 days to detect the expression of HIF-α gene. The results showed that there was no difference in HIF-1α expression between Ti6Al4V and 317L groups. The expression of HIF-1α was significantly up-regulated after adding Cu element (Figure S2).Therefore, this segment of the experiment answers Question 3 posited in the Introduction: “3. How exactly can the TiCu/TiCuN coating increase the speed of bone regeneration?”
5. Conclusion
In this study, a TiCu/TiCuN coating was applied to the surface of the CFR-PEEK material via the arc ion-plating technology to achieve excellent antiwear, antibacterial, and osteogenic activities and to construct a superior orthopaedic implant to traditional metal prostheses. The carbon fibre contained in the CFR-PEEK material used in this study is short carbon fibre, and its content is 30 wt%. The carbon fibres were distributed in a universal direction in a PEEK matrix. Compared with the long carbon fibre CFR-PEEK material, this material possesses both rigidity and toughness. This is the first instance, in which our team has successfully prepared a TiCu/TiCuN multilayer coating on the surface of a polymer material. Related experiments have reported that the stability of the coating was not less than that prepared on a metal surface, and Cu2+ can achieve a stable, uniform rate with safe release. Additionally, we conducted biological experiments to demonstrate that the TiCu/TiCuN-modified CFR-PEEK exhibited appropriate biocompatibility and bone regeneration activity. Furthermore, we used molecular biology methods to demonstrate that the TiCu/TiCuN coating is vital to promoting bone regeneration based on its ability to promote vascular regeneration. This ability is related to TiCu/TiCuN activating HIF-1α, which consequently affects the downstream VEGFR, α-SMA, CD31, and other signals. In summary, a safe and stable TiCu/TiCuN biocoating on the surface of CFR-PEEK was successfully prepared and the mechanism causing the osteogenic activity of the TiCu/TiCuN coating was clarified. This study provides a solid theoretical foundation for the clinical transformation of TiCu/TiCuN-modified CFR-PEEK materials.
Funding
This work was jointly supported by the Capital Health Development Scientific Research Special Project (grant number 2151000026); the Beijing Science and Technology Planning Project (grant number 2144000054); and National Natural Science Foundation of China (grant number 82072970).
Author contributions
Yu Guo: Experimental design, experiment implementation, data Formal analysis, manuscript writing; Chenglong Chen: experiment implementation, data Formal analysis, manuscript writing; Shuyuan Zhang: Experimental implementation, data Formal analysis; Ling Ren: Experimental design, article supervision, and funding; Yanhui Zhao: Coating preparation, article supervision, funding; Wei Guo: Experimental design, experimental supervision, article supervision, funding, and manuscript writing. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Declaration of competing interest
The author(s) have no conflicts of interest relevant to this article.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jot.2022.02.008.
Contributor Information
Ling Ren, Email: lren@imr.ac.cn.
Yanhui Zhao, Email: yhzhao@imr.ac.cn.
Wei Guo, Email: bonetumor@163.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Baldwin P., Li D.J., Auston D.A., Mir H.S., Yoon R.S., Koval K.J. Autograft, allograft, and bone graft substitutes: clinical evidence and indications for use in the setting of orthopaedic trauma surgery. J Orthop Trauma. 2019;33(4):203–213. doi: 10.1097/BOT.0000000000001420. [DOI] [PubMed] [Google Scholar]
- 2.Lerbs T., Cui L., Muscat C., Saleem A., van Neste C., Domizi P., et al. Expansion of bone precursors through jun as a novel treatment for osteoporosis-associated fractures. Stem Cell Rep. 2020;14(4):603–613. doi: 10.1016/j.stemcr.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zekry K.M., Yamamoto N., Hayashi K., Takeuchi A., Alkhooly A.Z.A., Abd-Elfattah A.S., et al. Reconstruction of intercalary bone defect after resection of malignant bone tumor. J Orthop Surg (Hong Kong) 2019;27(1) doi: 10.1177/2309499019832970. 2309499019832970. [DOI] [PubMed] [Google Scholar]
- 4.Hofmann A., Gorbulev S., Guehring T., Schulz A.P., Schupfner R., Raschke M., et al. CERTiFy Study Group Autologous iliac bone graft compared with biphasic hydroxyapatite and calcium sulfate cement for the treatment of bone defects in tibial plateau fractures: a prospective, randomized, open-label, multicenter study. J Bone Joint Surg Am. 2020;102(3):179–193. doi: 10.2106/JBJS.19.00680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prins J., Donders J.C.E., Helfet D.L., Wellman D.S., Klinger C.E., Redko M., et al. Periprosthetic femoral nonunions treated with internal fixation and bone grafting. Injury. 2018;49(12):2295–2301. doi: 10.1016/j.injury.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 6.Guo Y., Xie K., Jiang W., Wang L., Li G., Zhao S., et al. In vitro and in vivo study of 3D-printed porous tantalum scaffolds for repairing bone defects. ACS Biomater Sci Eng. 2019;5(2):1123–1133. doi: 10.1021/acsbiomaterials.8b01094. [DOI] [PubMed] [Google Scholar]
- 7.Shen F.H., Gasbarrini A., Lui D.F., Reynolds J., Capua J., Boriani S. Integrated custom composite polyetheretherketone/carbon fiber (PEEK/CF) vertebral body replacement (VBR) in the treatment of bone tumors of the spine: a preliminary report from a multicenter study. Spine. 2021 doi: 10.1097/BRS.0000000000004177. [DOI] [PubMed] [Google Scholar]
- 8.Guo Y., Wu J., Xie K., Tan J., Yang Y., Zhao S., et al. Study of bone regeneration and osteointegration effect of a novel selective laser-melted titanium-tantalum–niobium–zirconium alloy scaffold. ACS Biomater Sci Eng. 2019;5(12):6463–6473. doi: 10.1021/acsbiomaterials.9b00909. [DOI] [PubMed] [Google Scholar]
- 9.Ziran B.H., O'Pry E.K., Harris R.M. Carbon fiber-reinforced PEEK versus titanium tibial intramedullary nailing: a preliminary analysis and results. J Orthop Trauma. 2020;34(8):429–433. doi: 10.1097/BOT.0000000000001756. [DOI] [PubMed] [Google Scholar]
- 10.Cofano F., Di Perna G., Monticelli M., Marengo N., Ajello M., Mammi M., et al. Carbon fiber reinforced vs titanium implants for fixation in spinal metastases: a comparative clinical study about safety and effectiveness of the new “carbon-strategy. J Clin Neurosci. 2020;75:106–111. doi: 10.1016/j.jocn.2020.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Boriani S., Tedesco G., Ming L., Ghermandi R., Amichetti M., Fossati P., et al. Carbon-fiber-reinforced PEEK fixation system in the treatment of spine tumors: a preliminary report. Eur Spine J. 2018;27(4):874–881. doi: 10.1007/s00586-017-5258-5. [DOI] [PubMed] [Google Scholar]
- 12.Scholes S.C., Unsworth A. The wear properties of CFR-PEEK-Optima articulating against ceramic assessed on a multidirectional pin-on-plate machine. Proc Inst Mech Eng H. 2007;221(3):281–289. doi: 10.1243/09544119JEIM224. [DOI] [PubMed] [Google Scholar]
- 13.Mugnai R., Tarallo L., Capra F., Catani F. Biomechanical comparison between stainless steel, titanium and carbon-fiber reinforced polyetheretherketone volar locking plates for distal radius fractures. Orthop Traumatol Surg Res. 2018;104(6):877–882. doi: 10.1016/j.otsr.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Steinberg E.L., Rath E., Shlaifer A., Chechik O., Maman E., Salai M. Carbon fiber reinforced PEEK optima—a composite material biomechanical properties and wear/debris characteristics of CF-PEEK composites for orthopedic trauma implants. J Mech Behav Biomed Mater. 2013;17:221–228. doi: 10.1016/j.jmbbm.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Tedesco G., Gasbarrini A., Bandiera S., Ghermandi R., Boriani S. Composite PEEK/carbon fiber implants can increase the effectiveness of radiotherapy in the management of spine tumors. J Spine Surg. 2017;3(3):323–329. doi: 10.21037/jss.2017.06.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beyene B.B., Wassie G.A. Antibacterial activity of Cu(II) and Co(II) porphyrins: role of ligand modification. B.M.C. Chem. 2020;14(1):51. doi: 10.1186/s13065-020-00701-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L., Li G., Ren L., Kong X., Wang Y., Han X., et al. Nano-copper-bearing stainless steel promotes fracture healing by accelerating the callus evolution process. Int J Nanomed. 2017;12:8443–8457. doi: 10.2147/IJN.S146866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L., Ren L., Tang T., Dai K., Yang K., Hao Y. A novel nano-copper-bearing stainless steel with reduced Cu2+ release only inducing transient foreign body reaction via affecting the activity of NF-κB and caspase 3. Int J Nanomed. 2015;10:6725–6739. doi: 10.2147/IJN.S90249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan X., Huang J., Xu C., Bao M., Xia W., Zhu C. Differential expression of microRNAs in human endometrium after implantation of an intrauterine contraceptive device containing Copper. Mol Hum Reprod. 2021;27(9) doi: 10.1093/molehr/gaab052. gaab052. [DOI] [PubMed] [Google Scholar]
- 20.Peng C., Zhao Y., Jin S., Wang J., Liu R., Liu H., et al. Antibacterial TiCu/TiCuN multilayer films with good corrosion resistance deposited by axial magnetic field-enhanced arc ion plating. ACS Appl Mater Interfaces. 2019;11(1):125–136. doi: 10.1021/acsami.8b14038. [DOI] [PubMed] [Google Scholar]
- 21.Guo Y., Ren L., Xie K., Wang L., Yu B., Jiang W., et al. Functionalized TiCu/Ti-Cu-N-coated 3D-printed porous Ti6Al4V scaffold promotes bone regeneration through BMSC recruitment. Adv Mater Interfac. 2020;7(6) doi: 10.1002/admi.201901632. [DOI] [Google Scholar]
- 22.Seaman S., Kerezoudis P., Bydon M., Torner J.C., Hitchon P.W. Titanium vs. polyetheretherketone (PEEK) interbody fusion: meta-analysis and review of the literature. J Clin Neurosci. 2017;44:23–29. doi: 10.1016/j.jocn.2017.06.062. [DOI] [PubMed] [Google Scholar]
- 23.Heary R.F., Kheterpal A., Mammis A., Kumar S. Stackable carbon fiber cages for thoracolumbar interbody fusion after corpectomy: long-term outcome analysis. Neurosurgery. 2011;68(3):810–818. doi: 10.1227/NEU.0b013e3182077a9f. discussion 818. [DOI] [PubMed] [Google Scholar]
- 24.Xie K., Guo Y., Zhao S., Wang L., Wu J., Tan J., et al. Partially melted Ti6Al4V particles increase bacterial adhesion and inhibit osteogenic activity on 3D-printed implants: an in vitro study. Clin Orthop Relat Res. 2019;477(12):2772–2782. doi: 10.1097/CORR.0000000000000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao S., Xie K., Guo Y., Tan J., Wu J., Yang Y., et al. Fabrication and biological activity of 3D-printed polycaprolactone/magnesium porous scaffolds for critical size bone defect repair. ACS Biomater Sci Eng. 2020;6(9):5120–5131. doi: 10.1021/acsbiomaterials.9b01911. [DOI] [PubMed] [Google Scholar]
- 26.Xie K., Zhou Z., Guo Y., Wang L., Li G., Zhao S., et al. Long-term prevention of bacterial infection and enhanced osteoinductivity of a hybrid coating with selective silver toxicity. Adv Healthc Mater. 2019;8(5) doi: 10.1002/adhm.201801465. [DOI] [PubMed] [Google Scholar]
- 27.Li G., Zhang L., Wang L., Yuan G., Dai K., Pei J., et al. Dual modulation of bone formation and resorption with zoledronic acid-loaded biodegradable magnesium alloy implants improves osteoporotic fracture healing: an in vitro and in vivo study. Acta Biomater. 2018;65:486–500. doi: 10.1016/j.actbio.2017.10.033. [DOI] [PubMed] [Google Scholar]
- 28.Xie K., Wang N., Guo Y., Zhao S., Tan J., Wang L., et al. Additively manufactured biodegradable porous magnesium implants for elimination of implant-related infections: an in vitro and in vivo study. Bioact Mater. 2022;8:140–152. doi: 10.1016/j.bioactmat.2021.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li G., Wang L., Pan W., Yang F., Jiang W., Wu X., et al. In vitro and in vivo study of additive manufactured porous Ti6Al4V scaffolds for repairing bone defects. Sci rep. 2016;6:34072. doi: 10.1038/srep34072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barceloux D.G. Copper. J Toxicol Clin Toxicol. 1999;37(2):217–230. doi: 10.1081/clt-100102421. [DOI] [PubMed] [Google Scholar]
- 31.Walmsley G.G., Ransom R.C., Zielins E.R., Leavitt T., Flacco J.S., Hu M.S., et al. Stem cells in bone regeneration. Stem Cell Rev Rep. 2016;12(5):524–529. doi: 10.1007/s12015-016-9665-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qiu P., Li M., Chen K., Fang B., Chen P., Tang Z., et al. Periosteal matrix-derived hydrogel promotes bone repair through an early immune regulation coupled with enhanced Angio- and osteogenesis. Biomaterials. 2020;227:119552. doi: 10.1016/j.biomaterials.2019.119552. [DOI] [PubMed] [Google Scholar]
- 33.Wu L., Gu Y., Liu L., Tang J., Mao J., Xi K., et al. Hierarchical micro/nanofibrous membranes of sustained releasing VEGF for periosteal regeneration. Biomaterials. 2020;227:119555. doi: 10.1016/j.biomaterials.2019.119555. [DOI] [PubMed] [Google Scholar]
- 34.Lin S., Yang G., Jiang F., Zhou M., Yin S., Tang Y., et al. A magnesium-enriched 3D culture system that mimics the bone development microenvironment for vascularized bone regeneration. Adv Sci (Weinh) 2019;6(12):1900209. doi: 10.1002/advs.201900209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eelen G., Treps L., Li X., Carmeliet P. Basic and therapeutic aspects of angiogenesis updated. Circ Res. 2020;127(2):310–329. doi: 10.1161/CIRCRESAHA.120.316851. [DOI] [PubMed] [Google Scholar]
- 36.Lin X., Ge J., Wei D., Liu C., Tan L., Yang H., et al. Surface degradation–enabled osseointegrative, angiogenic and antiinfective properties of magnesium-modified acrylic bone cement. J Orthop Translat. 2019;9(17):121–132. doi: 10.1016/j.jot.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng W., Liu Y., Meng X., Zheng Z., Li L., Ke L., et al. PLGA/β-TCP composite scaffold incorporating cucurbitacin B promotes bone regeneration by inducing angiogenesis. J Orthop Translat. 2021;1(31):41–51. doi: 10.1016/j.jot.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y., Fang J., Zhang Q., Zhang X., Cao Y., Chen W., et al. Wnt10b-overexpressing umbilical cord mesenchymal stem cells promote critical size rat calvarial defect healing by enhanced osteogenesis and VEGF-mediated angiogenesis. J Orthop Translat. 2020;28(23):29–37. doi: 10.1016/j.jot.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie H., Kang Y.J. Role of Copper in angiogenesis and its medicinal implications. Curr Med Chem. 2009;16(10):1304–1314. doi: 10.2174/092986709787846622. [DOI] [PubMed] [Google Scholar]
- 40.Baleani M., Viceconti M., Toni A. The effect of sandblasting treatment on endurance properties of titanium alloy hip prostheses. Artif Organs. 2000;24(4):296–299. doi: 10.1046/j.1525-1594.2000.06486.x. [DOI] [PubMed] [Google Scholar]
- 41.Lindgren V., Galea V.P., Nebergall A., Greene M.E., Rolfson O., Malchau H., Multicenter Writing Committee Radiographic and clinical outcomes of porous titanium-coated and plasma-sprayed acetabular shells: a five-year prospective multicenter study. J Bone Joint Surg Am. 2018;100(19):1673–1681. doi: 10.2106/JBJS.17.00729. [DOI] [PubMed] [Google Scholar]
- 42.Chandrasekhar K.S., Zhou H., Zeng P., Alge D., Li W., Finney B.A., et al. Blood vessel wall-derived endothelial colony-forming cells enhance fracture repair and bone regeneration. Calcif Tissue Int. 2011;89(5):347–357. doi: 10.1007/s00223-011-9524-y. [DOI] [PubMed] [Google Scholar]
- 43.Farré-Guasch E., Bravenboer N., Helder M.N., Schulten E.A.J.M., Ten Bruggenkate C.M., Klein-Nulend J. Blood vessel formation and bone regeneration potential of the stromal vascular fraction seeded on a calcium phosphate scaffold in the human maxillary sinus floor elevation model. Materials (Basel) 2018;11(1):161. doi: 10.3390/ma11010161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin Y., Xiao W., Bal B.S., Rahaman M.N. Effect of copper-doped silicate 13–93 bioactive glass scaffolds on the response of MC3T3-E1 cells in vitro and on bone regeneration and angiogenesis in rat calvarial defects in vivo. Mater Sci Eng C Mater Biol Appl. 2016;67:440–452. doi: 10.1016/j.msec.2016.05.073. [DOI] [PubMed] [Google Scholar]
- 45.Liu C., Fu X., Pan H., Wan P., Wang L., Tan L., et al. Biodegradable Mg-Cu alloys with enhanced osteogenesis, angiogenesis, and long-lasting antibacterial effects. Sci rep. 2016;6:27374. doi: 10.1038/srep27374. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.