Author's summary
The availability of smart mobile devices (SMD) has increased continuously in recent years and affects all areas of life including medical applications. In parallel, there has been significant progress in the development of SMD applications that measure vital signs, esp. blood pressure using photoplethysmographic signals (PPG). This has enormous potential for screening and monitoring arterial hypertension. Some of these applications are already marketed, but they do not yet meet the usual criteria of validation and are not yet recommended by professional societies. The present work gives an overview of challenges and pitfalls in the use of these PPG applications.
Keywords: Blood pressure measurement, Smart devices, Photoplethysmographic blood pressure measurement, Cuffless blood pressure measurement, m-Health
Abstract
Blood pressure measurement (BPM) is an essential part of medical examination, and therefore accuracy of BPM devices is crucial. Over the past few years, there has been a rise in new BPM techniques using photoplethysmographic (PPG) signals and complex algorithms for blood pressure estimation. Especially the combination of a mobile device or a smartphone with a camera using PPG may potentially revolutionize BPM in the future. The first-ever BPM application to be approved as a medical device was one by the Korean Ministry of Food and Drug Safety in 2020, despite the lack of robust scientific evidence proving its validity. While the prospect of using these novel BPM devices is an opportunity, there are also some critical issues around calibration and utility in different patient groups that need to be resolved before they can be incorporated into daily clinical practice.
INTRODUCTION
Blood pressure (BP) measurement (BPM) is an essential part of medical examination, and undisputedly, the accuracy of BPM devices is crucial.1) The technique of BPM remained mostly unchanged during the last decades. Despite technical progress in automated cuff-based BPM devices, BPM is still based on a cuff and sphygmomanometer’s principles described by Riva Rocci and Korotkoff.2)
There is no doubt that cuff-based BPM has several problems, including the requirement for specific devices and limitations for 24-hour BP monitoring, including patients’ discomfort, which influence BP acutely.2),3) Therefore, extensive efforts have been made to develop cuffless BPM devices using pulse-transit-time or photoplethysmographic (PPG) signals. The advance of information and communication technology and the widespread mobile smartphone devices and smartwatches have brought new BPM possibilities using PPG signals. In April 2020, the BPM application (App) based on PPG signals available for the Samsung Galaxy Watch 2 (GW2) was the first one ever to be approved as a medical device by a government (Korean Ministry of Drug and Food Safety).4),5) A second device sold as a clinically validated BPM device is the Aktiia bracelet promising 24/7 BP monitoring. As a result, physicians are likely to be confronted with this new technology. Therefore, it will be of paramount importance for clinicians how the data patients retrieved by PPG devices should inform our clinical decision-making while keeping a patient-centered approach and yet evidence-based medicine.
VALIDATED PPG APPS AND DEVICES?
A prerequisite of BPM is the accuracy of the measuring device. According to current hypertension guidelines, clinicians and patients should only use BPM devices validated according to standardized protocols.3) However, even standard cuff-based BPM devices have often not been validated correctly, as reported by STRIDE BP, an international initiative for accurate BPM.1) As example in a recent study in Australia non-validated home BPM devices dominated the online marketplace resulting in roughly 18% of upper arm cuff devices (51 out of 278), 8% of wrist cuff devices (13 out of 162) and 0% of wristband wearable devices (0 out of 532) proven accurate according to international validation standards.6)
Therefore, examining the PPG Apps and devices available to date, none currently comply with the above-mentioned validation requirements. Additionally, there are presently no cuffless PPG devices officially recommended by STRIDE BP for home BPM, which is essential for hypertension diagnosis and management. Nonetheless, recent studies have shown improved accuracy by using ubiquitous or linear polynomial equations.7),8) For example, a proof-of-concept study applying an iPhone camera sensor for BPM showed bias and precision errors of −4.0 and 11.4 mmHg for systolic blood pressure and −9.4 and 9.7 mmHg for diastolic blood pressure.9) Furthermore, a smartphone-based BPM algorithm using transdermal optical imaging technology in normotensive adults falls within 5±8 mmHg of reference measurements, which fulfills a critical accuracy threshold bias and standard deviation (SD) according to the Association for the Advancement of Medical Instrumentation (AAMI) validation standard.10) The Samsung GW2 is reported by the manufacturer and the Korean Ministry of Drug and Food Safety to meet performance standards for automatic cuff-based electronic BPM devices with an average difference of less than ±5 mmHg and a maximum SD of 8 mmHg. However, there is a lack of detailed information peer-reviewed or published regarding the study population, methods, and spread of the BP data, including the measurement process during the study.4) The new Aktiia bracelet is sold as an easy 24/7 BP monitoring device which consists of a bracelet with an optical measurement unit. It can be connected to a smartphone App using Bluetooth to retrieve the BPM values. The bracelet device comes with an upper arm cuff-based automated BP device to perform a calibration measurement on the contralateral arm. In a comparative study using an adapted ISO81060-2:2013 protocol including 86 participants, the device showed promising results resulting in mean (and SD) differences between the bracelet and the reference of 0.46±7.75 mmHg for systolic and 0.39±6.86 mmHg for diastolic BP values. Both validation criteria of the ISO81060-2:2013 protocol have been fulfilled, with the caveat that the ISO81060-2 protocol was established for non-invasive sphygmomanometers and not for cuffless PPG BPM. Furthermore, there have been several protocol deviations within the study—including underrepresentation of systolic blood pressure >140 mmHg, which are of special interest in terms of diagnosis and treatment of arterial hypertension.11),12)
However, numerous Apps did not pass clinical validation. The most prominent example being the Instant BP Smartphone App, one of the top 50 best-selling iPhone Apps in 2014/2015. After an independent study, it had to be withdrawn from the market, showing a considerable overestimation of low BP values and underestimation of high BP values compared to standard BPM.13) This pattern was repeatedly seen in studies comparing PPG Apps to standard BPM. Therefore, it is crucial to know about the BP distribution and the graphical presentation in a validation study.13),14),15)
ARE PRESENT VALIDATION PROTOCOLS ADEQUATE FOR PPG DEVICES?
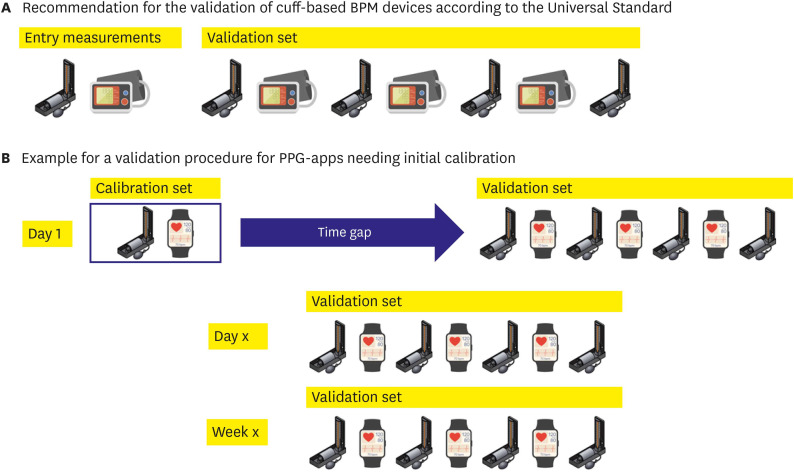
Over the last decades, several academic societies and industry standards have used various validation protocols until the proposal for a universal standard for the validation of BPM devices was finally adopted by the Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) in 2019.16) This is a highly standardized and reproducible validation protocol based on decades of experience in validating cuff-based BPM devices.16) In brief, at least 85 participants were tested under static conditions, comparing same arm, sequential measurements of a test device to the reference method using a mercury sphygmomanometer and auscultation by 2 observers. It starts with 2 initial measurements (reference and test) and 7 alternating validation measurements starting with the reference BP (Figure 1). Participants with BP difference above 12 mmHg systolic or 8 mmHg diastolic between any 2 reference BPs need to be excluded, resulting in very stable BP condition during the time needed for measurements. Next, results were analyzed according to specific validation criteria.16) However, for PPG Apps or alternative continuous cuff-less devices,16) there is no generally accepted validation protocol to date. This is highlighted in the consensus document by the AAMI/ESH/ISO, and task groups have been established to explore the methodology of such protocols.16),17) The Institute of Electrical and Electronics Engineers (IEEE) published a standard in 2014 (IEEE1708-2014), but this was criticized for having the major limitation that the reference measurement method was not described detailed enough to ensure standardization—usually a key feature of validation protocols.18),19) Additionally, there is an ISO 81060-3 standard for the clinical investigation of continuous non-invasive automated BPM devices under development. Still, as long as it is not completed and fully implemented, new devices will come up with different adaptions of validation protocols like the Aktiia device.11)
Figure 1. Measurement sequences for the validation of BPM devices. (A) Measurement sequence according to the Universal AAMI/ESH/ISO Standard for the validation16) of cuff-based BP measurement devices, started by entry measurements with the reference method and the test device, directly followed by the validation measurement set. (B) The proposal for a validation procedure for PPG Apps needs an initial calibration to the reference method. After a first calibration set, validations are set at different time points covering one calibration’s recommended time frame.
Source of illustrations: Shutterstock.com (Zern Liew; marina_ua; Victor Metelskiy).
AAMI/ESH/ISO = Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization; App = application; BP = blood pressure; BPM = blood pressure measurement; PPG = photoplethysmographic.
From a clinical point of view, there are 2 major types of PPG Apps. One type is Apps which measure BP directly, e.g., the Instant BP Smartphone App. In these cases, a validation protocol like the 2019 AAMI/ESH/ISO Standard may be suitable for validation as 2 independent measurements are compared. But the other type is Apps which need calibration to a cuff-based BPM before use, like the Samsung GW2 or the Aktiia bracelet. Afterward, BP values are estimated based on this calibration. This may lead to bias and raise critical questions about the methodology of traditional validation protocols. When the first App measurement is calibrated to the first reference measurement, the validation measurement set is directly following this calibration. Then, how can we extrapolate that this App can truly monitor dynamic changes in BP? It would be sufficient for passing the validation if the App covers a small range of BP changes around the calibration measurement. For the Samsung GW2 and the Aktiia bracelet, recalibration is recommended every 4 weeks, but how can we rely on BP values over 4 weeks after the initial validation study covering 20 minutes after calibration? At least the study team had to use several time points when investigating the device’s accuracy.11)
Given these limitations, a unique validation protocol has to be tailored, covering the first set of validation measurements and repeated measurements allowing for BP changes after different periods of time. The last set of measurements then would be mandatory before recalibration is recommended by the manufacturer. An example of such a protocol in comparison to a traditional validation protocol is given in the Figure 1.
Additionally, before applying a new technology, different patient groups that represent the general population have to be studied, e.g., subjects with large visit-to-visit BP variability or taking different drugs influencing vascular tone to challenge the new techniques in a clinical context. Moreover, there is a need for validation in special populations like the elderly, patients with cardiovascular disease, children, adolescents, or pregnancy.
PITFALLS FOR DAILY CLINICAL PRACTICE USE
There are several questions regarding the implementation into clinical practice. Correct BPM is not an easy task. BPM should be done according to standard operating procedures recommended by professional societies, including resting time, sitting position, quiet surroundings, validation and regular recalibration of devices.3) For PPG Apps needing calibration, accurate calibration will be mandatory for the accuracy. Errors during the calibration process may translate into deviations afterward. So, who will execute this calibration? Patients at home with their own devices, as we know that the majority of BP monitors on the market are not clinically validated? Or doctors in a clinical setting which might not be feasible for the younger population, current main users of PPG Apps?1) Given the enormous importance of accurate calibration, this question is essential.
The next question is how to interpret the large amount of BP values collected by the patients. The devices allow BPM at 7 days per week and even over 24 hours or collect BPM according to device-specific timings for some devices. We can assume that people will track their BP on a 24/7 basis (as the PPG App is included in a smartwatch that they wear) and bring their results into the clinics. This might be an enormous advantage, e.g., for symptom-BP correlation in case of dizziness or evaluation of nighttime BP or BP fluctuation. But actually, there is a need for studies comparing these values to our traditional cut-off BP values for home-based BPMs, which usually follow a specific protocol of a given number of BPMs in the morning and evening hours.3),20) Therefore, we will need more experience and comparative studies in the areas intended for their use, e.g., in comparison to traditional home and ambulatory BP monitoring. As an example to illustrate this challenge, the Somnotouch Non-invasive BP Measurement device is a cuff-less beat-to-beat BPM device developed explicitly for ambulatory BP monitoring. The device uses changes in the pulse-transit-time after an initial cuff-based calibration to estimate changes in BP. It has passed a validation study according to the European Society of Hypertension International Protocol revision 2010 for the validation of BP monitoring devices.21) Still, it leads to significantly higher mean systolic and diastolic BP values when compared to standard cuff-based devices over 24 hours.22),23) Reasons for that phenomenon remain unclear and include a systematic error in the algorithm, possible deviations due to a larger number of BPM, more values during physical activity by the cuffless device, and an inability to reflect pronounced BP changes, postural changes, changes in vascular tone, or hemodynamics over time.22) The technique used was not PPG, but the example illustrates that a positive validation study under laboratory conditions doesn’t predict necessarily that BP values obtained by the device are interchangeable with traditional BP measurements in the designated area of use—which is 24 hour BP measurement for the Somnotouch device. From this example we can learn, that there has to be a thorough evaluation of PPG measurements under different physiological conditions, too.
However, BPM by PPG Apps opens a new field of dynamic BP evaluation in a real-life setting. Although BP values in ‘resting condition’ are still the most critical factors determining prognosis, BP variability has been proven in many studies to be an independent and robust indicator of cardiovascular events.24) Nevertheless, conventional BPM, including ambulatory BP monitoring, mainly focused on the static BP values at resting status. PPG App-based BPM will report BP changes in daily living and during physical/emotional stress, thus opening new chapters of BP research regarding dynamic BP evaluation, e.g., response to intrinsic and extrinsic environmental factors like stress at work or seasonal climate changes. Current PPG Apps cannot measure BP in actual dynamic conditions but require a resting status for 60 seconds to get valid signals. In the user manual of the Aktiaa bracelet, e.g., the manufacturer of the device states that the BPM is done during periods of rest. However, the rapid progress of PPG technology will result in beat-to-beat signal processing.
Last but not least, one additional and important advantage has to be addressed. This technology may have the additional benefit of increasing BP awareness in the young. Currently, target customers for BPM Apps are the younger rather than the elderly. Although hypertension prevalence is low in those in the 3rd to 4th decade of life, they showed a low rate of awareness in hypertension.25),26) With a PPG App in their smartwatch, people need to measure BP using standard devices only every 4 weeks for re-calibration. For the remainder of the time, the smartwatch will track and report BP values automatically.
In conclusion, the possibilities of PPG Apps for BPM are vast and fascinating and can hopefully be integrated into clinical and research practice soon. It will open up new screening methods and monitoring possibilities in different environments. There is the possibility that BPM will be widely and readily available to people, especially for tracking over extended periods. Nonetheless, there is still no validated BPM App that is recommended by professional societies to date. Critical questions remain in PPG Apps that need calibration to a standard BPM. This includes developing specific validation protocols, execution, and publication of validation studies adhering to these protocols, and evaluation of the devices in different patient groups.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest: Hae-Young Lee, outside the submitted work, received lecture fees from Servier, Pfizer, Takeda, Menarini, MSD, Boehringer Ingelheim, Novartis, and Sanofi. Thilo Burkard has conducted validation studies as investigator-initiated trials with diverse devices, including research funding by “Centre Suisse d’Electronique et de Microtechnique” (CSEM). Outside the submitted work, Thilo Burkard received lecture fees from Servier, Amgen, Takeda, Menarini, MSD, Novartis, and personal expenses and research sponsoring from Sanofi.
Data Sharing Statement: The data generated in this study is available from the corresponding author(s) upon reasonable request.
- Conceptualization: Lee HY, Burkard T.
- Writing - original draft: Burkard T.
- Writing - review & editing: Lee HY, Burkard T.
References
- 1.Stergiou GS, O’Brien E, Myers M, et al. STRIDE BP international initiative for accurate blood pressure measurement: Systematic review of published validation studies of blood pressure measuring devices. J Clin Hypertens (Greenwich) 2019;21:1616–1622. doi: 10.1111/jch.13710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vischer AS, Burkard T. Principles of blood pressure measurement - Current techniques, office vs ambulatory blood pressure measurement. Adv Exp Med Biol. 2017;956:85–96. doi: 10.1007/5584_2016_49. [DOI] [PubMed] [Google Scholar]
- 3.Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 4.Vaidyanathan S, Jermany J, Yeh C, Bizot MN, Camisasca R. Aliskiren, a novel orally effective renin inhibitor, exhibits similar pharmacokinetics and pharmacodynamics in Japanese and Caucasian subjects. Br J Clin Pharmacol. 2006;62:690–698. doi: 10.1111/j.1365-2125.2006.02696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samsung Electronics. Samsung announces blood pressure monitoring application for Galaxy Watch devices [Internet] Suwon: Samsung; 2020. [cited 2020 September 16]. Available from: https://news.samsung.com/global/samsung-announces-blood-pressure-monitoring-application-for-galaxy-watch-devices?utm_source=ext_newsletter&utm_medium=email. [Google Scholar]
- 6.Picone DS, Deshpande RA, Schultz MG, et al. Nonvalidated home blood pressure devices dominate the online marketplace in Australia: major implications for cardiovascular risk management. Hypertension. 2020;75:1593–1599. doi: 10.1161/HYPERTENSIONAHA.120.14719. [DOI] [PubMed] [Google Scholar]
- 7.Dey J, Gaurav A, Tiwari VN. InstaBP: cuff-less blood pressure monitoring on smartphone using single PPG sensor. Annu Int Conf IEEE Eng Med Biol Soc. 2018;2018:5002–5005. doi: 10.1109/EMBC.2018.8513189. [DOI] [PubMed] [Google Scholar]
- 8.Matsumura K, Rolfe P, Toda S, Yamakoshi T. Cuffless blood pressure estimation using only a smartphone. Sci Rep. 2018;8:7298. doi: 10.1038/s41598-018-25681-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chandrasekhar A, Natarajan K, Yavarimanesh M, Mukkamala R. An iPhone application for blood pressure monitoring via the oscillometric finger pressing method. Sci Rep. 2018;8:13136. doi: 10.1038/s41598-018-31632-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo H, Yang D, Barszczyk A, et al. Smartphone-based blood pressure measurement using transdermal optical imaging technology. Circ Cardiovasc Imaging. 2019;12:e008857. doi: 10.1161/CIRCIMAGING.119.008857. [DOI] [PubMed] [Google Scholar]
- 11.Vybornova A, Polychronopoulou E, Wurzner-Ghajarzadeh A, Fallet S, Sola J, Wuerzner G. Blood pressure from the optical Aktiia Bracelet: a 1-month validation study using an extended ISO81060-2 protocol adapted for a cuffless wrist device. Blood Press Monit. 2021;26:305–311. doi: 10.1097/MBP.0000000000000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Association for the Advancement of Medical Instrumentation (US); American National Standards Institute; International Organization for Standardization. AAMI/ANSI/ISO 81060-2:2013, Non-invasive Sphygmomanometers - Part 2: Clinical Investigation of Automated Measurement Type. Arlington (VA): Association for the Advancement of Medical Instrumentation; [Google Scholar]
- 13.Plante TB, Urrea B, MacFarlane ZT, et al. Validation of the instant blood pressure smartphone App. JAMA Intern Med. 2016;176:700–702. doi: 10.1001/jamainternmed.2016.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raichle CJ, Eckstein J, Lapaire O, et al. Performance of a blood pressure smartphone App in pregnant women: the iPARR trial (iPhone App compared with standard RR measurement) Hypertension. 2018;71:1164–1169. doi: 10.1161/HYPERTENSIONAHA.117.10647. [DOI] [PubMed] [Google Scholar]
- 15.Xing X, Ma Z, Zhang M, Zhou Y, Dong W, Song M. An unobtrusive and calibration-free blood pressure estimation method using photoplethysmography and biometrics. Sci Rep. 2019;9:8611. doi: 10.1038/s41598-019-45175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stergiou GS, Palatini P, Asmar R, et al. Recommendations and practical guidance for performing and reporting validation studies according to the universal standard for the validation of blood pressure measuring devices by the Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) J Hypertens. 2019;37:459–466. doi: 10.1097/HJH.0000000000002039. [DOI] [PubMed] [Google Scholar]
- 17.Stergiou GS, Alpert B, Mieke S, et al. A universal standard for the validation of blood pressure measuring devices: Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) Collaboration Statement. J Hypertens. 2018;36:472–478. doi: 10.1097/HJH.0000000000001634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Padwal R. Cuffless blood pressure measurement: How did accuracy become an afterthought? Am J Hypertens. 2019;32:807–809. doi: 10.1093/ajh/hpz070. [DOI] [PubMed] [Google Scholar]
- 19.Institute of Electrical and Electronics Engineers (IEEE) Engineering in Medicine and Biology Society. IEEE Std 1708-2014. IEEE Standard for Wearable, Cuffless Blood Pressure Measuring Devices. New York (NY): Institute of Electrical and Electronics Engineers, Inc.; 2014. [Google Scholar]
- 20.Parati G, Stergiou GS, Asmar R, et al. European Society of Hypertension guidelines for blood pressure monitoring at home: a summary report of the Second International Consensus Conference on Home Blood Pressure Monitoring. J Hypertens. 2008;26:1505–1526. doi: 10.1097/HJH.0b013e328308da66. [DOI] [PubMed] [Google Scholar]
- 21.Bilo G, Zorzi C, Ochoa Munera JE, Torlasco C, Giuli V, Parati G. Validation of the Somnotouch-NIBP noninvasive continuous blood pressure monitor according to the European Society of Hypertension International Protocol revision 2010. Blood Press Monit. 2015;20:291–294. doi: 10.1097/MBP.0000000000000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krisai P, Vischer AS, Kilian L, Meienberg A, Mayr M, Burkard T. Accuracy of 24-hour ambulatory blood pressure monitoring by a novel cuffless device in clinical practice. Heart. 2019;105:399–405. doi: 10.1136/heartjnl-2018-313592. [DOI] [PubMed] [Google Scholar]
- 23.Socrates T, Krisai P, Vischer AS, Meienberg A, Mayr M, Burkard T. Improved agreement and diagnostic accuracy of a cuffless 24-h blood pressure measurement device in clinical practice. Sci Rep. 2021;11:1143. doi: 10.1038/s41598-020-80905-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diaz KM, Tanner RM, Falzon L, et al. Visit-to-visit variability of blood pressure and cardiovascular disease and all-cause mortality: a systematic review and meta-analysis. Hypertension. 2014;64:965–982. doi: 10.1161/HYPERTENSIONAHA.114.03903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeon YW, Kim HC. Factors associated with awareness, treatment, and control rate of hypertension among Korean young adults aged 30–49 years. Korean Circ J. 2020;50:1077–1091. doi: 10.4070/kcj.2020.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee HY, Oh GC, Sohn IS, et al. Suboptimal management status of younger hypertensive population in Korea. Korean Circ J. 2021;51:598–606. doi: 10.4070/kcj.2020.0542. [DOI] [PMC free article] [PubMed] [Google Scholar]