Abstract
Collagen XII is a regulator of corneal stroma structure and function. The current study examined the role of collagen XII in regulating corneal stromal transforming growth factor (TGF)-β activation and latency. Specifically, with the use of conventional collagen XII null mouse model, the role of collagen XII in the regulation of TGF-β latency and activity in vivo was investigated. Functional quantification of latent TGF-β in stromal matrix was performed by using transformed mink lung reporter cells that produce luciferase as a function of active TGF-β. Col12a1 knockdown with shRNA was used to test the role of collagen XII in TGF-β activation. Col12a1–/– hypertrophic stromata were observed with keratocyte hyperplasia. Increased collagen fibril forward signal was found by second harmonic generation microscopy in the absence of collagen XII. Collagen XII regulated mRNA synthesis of Serpine1, Col1a1, and Col5a1 and deposition of collagens in the extracellular matrix. A functional plasminogen activator inhibitor luciferase assay showed that collagen XII is necessary for latent TGF-β storage in the extracellular matrix and that collagen XII down-regulates active TGF-β. Collagen XII dictates stromal structure and function by regulating TGF-β activity. A hypertrophic phenotype in Col12a1–/– corneal tissue can be explained by abnormal up-regulation of TGF-β activation and decreased latent storage.
The corneal stroma has intrinsic properties that are essential for transparency, biomechanics, curvature, and avascularity. These intrinsic stromal properties are dependent on a highly organized and corneal-specific extracellular matrix and a unique population of well-interconnected cranial neural crest–derived cells unique to the stroma and known as keratocytes.1, 2, 3, 4, 5, 6
A component of this corneal-specific extracellular matrix is fibril-associated collagens with interrupted triple helices that interact with collagen fibrils, as well as basement membranes, and regulate cell-cell communication and matrix organization.2,7 Fibril-associated collagens with interrupted triple helices members, collagens XII and XIV, are known components of the stromal extracellular matrix.3,7, 8, 9 Collagen XII is a homotrimer composed of three collagen α1(XII) chains. It is a large protein with two major alternatively spliced variants, and the large variant has a glycosaminoglycan attachment site.9
The function of collagen XII is poorly understood, but it is implicated in the regulation of tissue organization and function in different tissues.10, 11, 12, 13 Deficiency in collagen XII has been associated with musculoskeletal defects, biomechanical tissue alterations, and impaired wound healing in patients with distal myopathy and a specific type of Ehlers-Danlos syndrome.14, 15, 16 In the corneal stroma, collagen XII regulates fibril density, lamellar organization, and tissue biomechanics. This is further supported by a link between decreased collagen XII deposition and keratoconus corneas, a pathologic condition characterized by a weak, ectatic cornea with abnormal distribution and orientation of collagen lamellae.17,18 The controlled expression and deposition of collagen XII during development emphasizes its important function in regulation and maintenance of tissue matrix architecture and cell behavior11,19,20 Up-regulation of collagen XII is also detected during wound healing, suggestive of a role in regulating matrix stiffness and organization of newly synthesized matrix.9,21,22 Our Col12a1–/– model, deficient for all collagen isoforms, shows increased fibril density and disorganized stromal lamellae. These changes in matrix organization modify and regulate stromal function (eg, Col12a1–/– stromata have significant structural and mechanical abnormalities, including significant resistance to compression).23
The transforming growth factor (TGF)-β system is a major regulator of multiple cell functions such as cell fate, proliferation, movement, polarity, adhesion, cytokine production, terminal differentiation, and cell death.24,25 Cells secrete TGF-β, a 25-kDa homodimeric protein, but they are not the only regulators of its biological function. TGF-β function is also regulated by its storage and release from the extracellular matrix.26, 27, 28, 29 The large latent complex, a complex of TGF-β, latency-associated protein, and latent TGF-β binding protein (LTBP), plays a critical role in modulating the action of TGF-β1 by controlling its release from the extracellular matrix.26, 27, 28, 29 Tissue fibrosis is characterized by excessive deposition of extracellular matrix proteins, like collagen, which compromises normal tissue structure and function.30 TGF-β is considered to be one of the most potent factors accelerating the progression of tissue fibrosis.31, 32, 33
Considering that collagen XII influences the extracellular matrix organization and its potential role during homeostasis and wound healing, the current study was designed to determine whether collagen XII regulates the bioavailability of TGF-β, which is closely associated with hypertrophy, in the corneal stroma. The results demonstrate that collagen XII plays a significant role in regulating TGF-β activity in the corneal stroma and that such regulatory activity has significant effects on stromal structure. The considerable therapeutic potential of this novel regulatory activity of TGF-β signaling in an extracellular context is of major importance and is likely to contribute to corneal wound healing and related fibrotic disorders.
Materials and Methods
Animals
Wild-type (WT) and collagen XII–null mice (Col12a1–/–) on C57BL/6 and 129/SvJ mixed backgrounds were used, as previously published.7,23 Corneas from mice at age 30 days old, preadult, and ≥60 days old, considered adult, were included in this study. All experiments conformed to the Use of Laboratory Animals and Association for Research in Vision and Ophthalmology statement for the use of animals in ophthalmic and vision research and were approved by the Institutional Animal Care and Use Committee of the University of South Florida College of Medicine. All mice were housed and treated in accordance with the NIH Guide for the Care and Use of Laboratory Animals.34
Corneal Stroma Cell Density
Fresh eyes were harvested from C57BL/6 mice at preadult and adult age. They were then embedded in OCT compound and frozen with isopentane (Sigma Aldrich, St. Louis, MO) on dry ice. Corneal sections (5 to 7 μm thick) were blocked using 10% donkey serum (Sigma Aldrich). DAPI Fluoromount-G clear mounting solution (SouthernBiotech, Birmingham, AL) with DAPI was used as a nuclear marker. The number of nuclei in the central cornea were counted in a ×20 magnification field. At least three different animals per age and condition were used.
Optical Coherence Tomography and Corneal Thickness Estimation
Whole eyes from euthanized mice were enucleated, and measurements were immediately obtained. Each enucleated eye was placed in a custom-made holder and placed in the Spectral Dominium Cirrus HDT Optical Coherence Tomography (Zeiss, San Francisco, CA) device for corneal thickness measurements.7 Five measurements were obtained on the vertical plane, and five measurements were obtained on the horizontal plane of the central cornea. All experiments were performed at least three times in adult corneas, where total endothelial maturation and function was achieved.
Total Collagen Assay
The measurement of total collagen deposited in the mouse cornea was performed, as previously described.35 Basically, the total collagen was quantified indirectly by a colorimetric hydroxyproline assay that generates a chromophore from hydroxyproline via reaction with p-dimethylaminobenzaldehyde (alias Ehrlich's reagent). Corneas were dissected from WT and Col12a1–/– adult mice. Samples were hydrolyzed in 6 N HCl at 100°C for 24 hours, and vacuum dried through NaOH trap, then resuspended with 0.5 mL 1 mmol/L HCl. Hydroxyproline amino acids in 0.2-mL diluted samples were converted to pyrolle-2-carboxylate by oxidation via addition of 0.1 mL of 0.06 mol/L chloramine-T in a buffer containing 70% v/v H2O, 30% v/v 2-propanol, and acetate-citrate buffer (pH 6.0) by incubation at room temperature for 20 minutes. Finally, 1.3 mL of 6.25% w/v p-dimethylaminobenzaldehyde in 2-propanol plus perchlorate acid (alias Ehrlich's solution) was added to each sample, mixed well, incubated at 55°C for 20 minutes, and cooled, and then absorbance was determined at 550 nm. The hydroxyproline concentration was determined from a standard curve (stock solution of hydroxyproline: 1 mg/mL in 1 mmol/L HCl). The mean collagen content from each cornea was calculated by using a conversion ratio of 0.125:1.0 to convert micrograms of hydroxyproline to total collagen.
SHG Microscopy of Corneal Stroma
Freshly enucleated eyes were immediately placed on Optisol media on a custom-made glass slide and imaged without any tissue manipulation or further tissue dissection within 5 minutes of enucleation. Adult corneas, age postnatal day 60, were dissected from the globe and placed as a flat mount for en face imaging. Corneal cross-sections were imaged using an Olympus MPE-RS microscope using a 25× (0.95 numerical aperture) water-immersion objective (Olympus Corp., Tokyo, Japan). Two-photon second harmonic generation (SHG) signals were generated using a mode-locked titanium/sapphire laser at 960 nm. The SHG forward-scattered signals passing through the corneal sections were collected using a 0.8 numerical aperture condenser lens with a narrow band-pass filter (465 to 485 nm). Backward-scattered SHG signals were detected with a band-pass filter (460 to 500 nm). All samples were scanned using a 2-μm z-axis step size from the back to the front of the section. All of the experimental settings and conditions were kept constant throughout the experiment.
Isolation and Culture of Corneal Fibroblasts
After euthanasia, the eyes of adult WT mice were copiously washed with betadine ophthalmic solution, and then incubated in Dulbecco’s modified Eagle’s medium (DMEM) containing 15 mg/mL dispase II (Roche Applied Science, Penzberg, Germany) at 4°C for 18 hours. The entire corneal epithelium sheet loosened by this treatment was removed by vigorous shaking. Under a dissecting microscope, the corneal stroma was separated from the sclera at the corneoscleral limbus by pressing down the limbus with a 27-gauge needle. Isolated corneal stromata were incubated overnight at 37°C in DMEM containing 1.25 mg/mL collagenase A (Roche Applied Science) and 25 μg/mL gentamicin. A keratocyte-containing cell suspension was then seeded on T25 flasks (Thermo Fisher Scientific, Waltham, MA) in DMEM containing ITS (5 μg/mL insulin, 5 μg/mL transferrin, and 5 ng/mL sodium selenite), and 25 μg/mL gentamicin supplemented with 5% fetal bovine serum (FBS). The suspension of keratocytes prepared from 12 mouse corneal buttons was seeded into each flask.
RNA Isolation and Quantification of mRNA
Whole corneas were dissected from preadult WT mice and Col12a1–/– mice, cut into small pieces, and total RNA was extracted using QIAzol Lysis Reagent (Qiagen, Hilden, Germany) and the Qiagen RNeasy MinElute Cleanup Kit (Qiagen, Venio, the Netherlands). Reverse transcription and quantitative real-time PCR analyses were performed, as previously described.23 The primer sequences used are listed in Table 1. Each sample was run in a duplicate PCR, and statistical analysis was performed on six or seven corneas from different mice.
Table 1.
Primers Used in This Article
| Gene | Forward primer | Reverse primer |
|---|---|---|
| Serpine1 | 5′-GTCTTTCCGACCAAGAGCAG-3′ | 5′-GACAAAGGCTGTGGAGGAAG-3′ |
| Col1a1 | 5′-TTCTCCTGGCAAAGACGGACTCAA-3′ | 5′-AGGAAGCTGAAGTCATAACCGCCA-3′ |
| Col5a1 | 5′-AAGCGTGGGAAACTGCTCTCCTAT-3′ | 5′-AGCAGTTGTAGGTGACGTTCTGGT-3′ |
| Col12a1 | 5′-CCCTACAACAGATGGGCCTAC-3′ | 5′-TCTTCTCCCCTGGCTTTGTA-3′ |
| Lox | 5′-CAGAGGAGAGTGGCTGAAGG-3′ | 5′-CTCAATCCCTGTGTGTGTGC-3′ |
| Actb | 5′-AGATGACCCAGATCATGTTTGAGA-3′ | 5′-CACAGCCTGGATGGCTACGT-3′ |
Lentivirus shRNA Production and Transduction
Constitutive lentiviral miR-E expression vector36 featuring puromycin drug selection and green fluorescent protein fluorescent marker was a gift from Dr. Florian Karreth at Moffitt Cancer Center and Research Institute (Tampa, FL). De novo 97-mer oligo was designed by using SplashRNA shRNA prediction tool (http://splashrna.mskcc.org, last accessed March 29, 2021).37 Three oligonucleotides (1833, 5′-TGCTGTTGACAGTGAGCGAAGAGTTGAAGATATAATCAAATAGT-GAAGCCACAGATGTATTTGATTATATCTTCAACTCT-GTGCCTACTGCCTCGGA-3′; 5121, 5′-TGCTGTTGACAGTGAGCGAAAGTACATTGTTAGATACAAATAGT-GAAGCCACAGATGTATTTGTATCTAACAATGTACT-TGTGCCTACTGCCTCGGA-3′; and 902, 5′-TGCTGTTG-ACAGTGAGCGACAGGACTGAATTTAACTTAAATA-GTGAAGCCACAGATGTATTTAAGTTAAATTCAGT-CCTGGTGCCTACTGCCTCGGA-3′; Sigma, St. Louis, MO) coding for Col12a1 shRNAs were PCR amplified using the primers miRE-Xho-fw (5′-TGAACTCGAGAAGGTATATTGCTGTTGACAGTGAGCG-3′) and miRE-EcoOligo-rev (5′-TCTCGAATTCTAGCCCCTTGAAGTCCGAGGCAGTAGGC-3′), 0.05 ng oligonucleotide template, and the PfuUltra HF kit (Agilent Technologies, Santa Clara, CA), and cloned into XhoI/EcoRI sites of miR-E recipient vectors. An oligonucleotide-targeting Renilla luciferase (Ren.713) was used as a negative control.
Plasmid DNA was amplified and purified using a HiSpeed Plasmid Midi kit (Qiagen, Hilden, Germany) and then transfected along with packaging plasmid using JetPrime (Polyplus Transfection, New York, NY) into HEK293T cells to generate lentiviruses. The cells were refed with 1.5 mL DMEM supplemented with 10% FBS and 1× antibiotic-antimycotic (Thermo Fisher, Waltham, MA) 24 and 48 hours after transfection. The culture supernatant was harvested 72 hours after transfection. The supernatant-containing lentivirus was filtered and used to infect the mouse corneal fibroblast cells. The corneal fibroblast cells were cultured with DMEM supplemented with 10% FBS and 1× antibiotic-antimycotic, as well as 2 μg/mL puromycin, to select target cells. After selection for 7 days, cells were analyzed for real-time PCR (see primer sequence in Table 1) and protein immunoblotting analysis, using rabbit anti–type XII collagen antibody (1:1000 dilution; KR33) and mouse anti–β-actin (1:1000 dilution; Millipore, Burlington, MA).
Luciferase Assay
Transformed mink lung cells, transfected with luciferase cDNA driven by plasminogen activator inhibitor (PAI-1) promoter, were a generous gift of Dr. Daniel Rifkin (New York University, New York, NY).38 Cells were cultured in DMEM supplemented with 10% FBS (Thermo Fisher). The amount of latent TGF-β in corneas isolated from Col12a1–/– and WT corneas was calculated on the basis of a comparison to a standard curve generated using different concentrations of human recombinant TGF-β1 (Sigma). For analysis and quantification of latent TGF-β, corneal stromata were minced into 10 to 12 little pieces with a blade and heated at 80°C for 10 minutes to release latent TGF-β. Equal amounts of protein were added to transformed mink lung cells for incubation for 16 hours under serum-free conditions. For analysis and quantification of active TGF-β, a co-culture system was performed, transformed mink lung cells were seeded and allowed to attach for 4 hours, and then corneal fibroblasts were seeded in serum-free DMEM and incubated for 16 hours. TGF-β signaling was inhibited by the addition of the TGF-β type I receptor/activin receptor-like kinase 5 (ALK5) inhibitor, SB431542 (Tocris Bioscience, Minneapolis, MN) to 10 μmol/L final concentration. A neutralization antibody against all three TGF-β isoforms (clone 1D11; R&D System, Minneapolis, MN) was also used. Luciferase assay was performed by using Promega's Luciferase Assay System (Promega, Madison, WI), and luminance was measured with Synergy HT plate reader (BIO-TEK, Winooski, VT).
ELISA
Total TGF-β1 antigen in mouse fibroblast culture medium was evaluated using a commercial kit, Quantikine Mouse TGF-β1 Immunoassay (R&D Systems; catalog number MB100B), according to the manufacturer's instructions. This enzyme-linked immunosorbent assay (ELISA) is used to measure active plus acid-activatable latent TGF-β1 in the cell culture supernatant. To activate the latent TGF-β1 form, each sample was acidified by 1 N HCL for 10 minutes, which was followed by neutralization by 1.2 N NaOH/0.5 mol/L HEPES. Because samples have been diluted in the activation step, the concentration read from the standard curve was multiplied by the dilution factor 1.4. All standards and samples were tested in duplicate, and the mean values were used for calculation. Same number of WT and Col12a1−/− keratocytes cells were seeded in a 6-well plate, with 3 wells for each genotype. Cells were cultured in DMEM supplemented with 1% FBS and ITS (1.0 mg/mL recombinant human insulin, 0.55 mg/mL human transferrin, and 0.5 μg/mL sodium selenite at the 100× concentration; Sigma) serum-free medium and 50 μg/mL ascorbic acid for 5 days. One day before the ELISA assay, cells were maintained in serum-free DMEM medium 24 hours before collecting medium.
Statistical Analysis
Data are presented as mean ± SD. t-Test was used to draw statistical inferences when comparing the mean of continuous dependent variables. P < 0.05 was considered statistically significant. Graph Pad Prism software version 9.1.2 (San Diego, CA) was used for statistical calculations.
Results
Collagen XII Regulates Keratocyte Density and Stromal Thickness
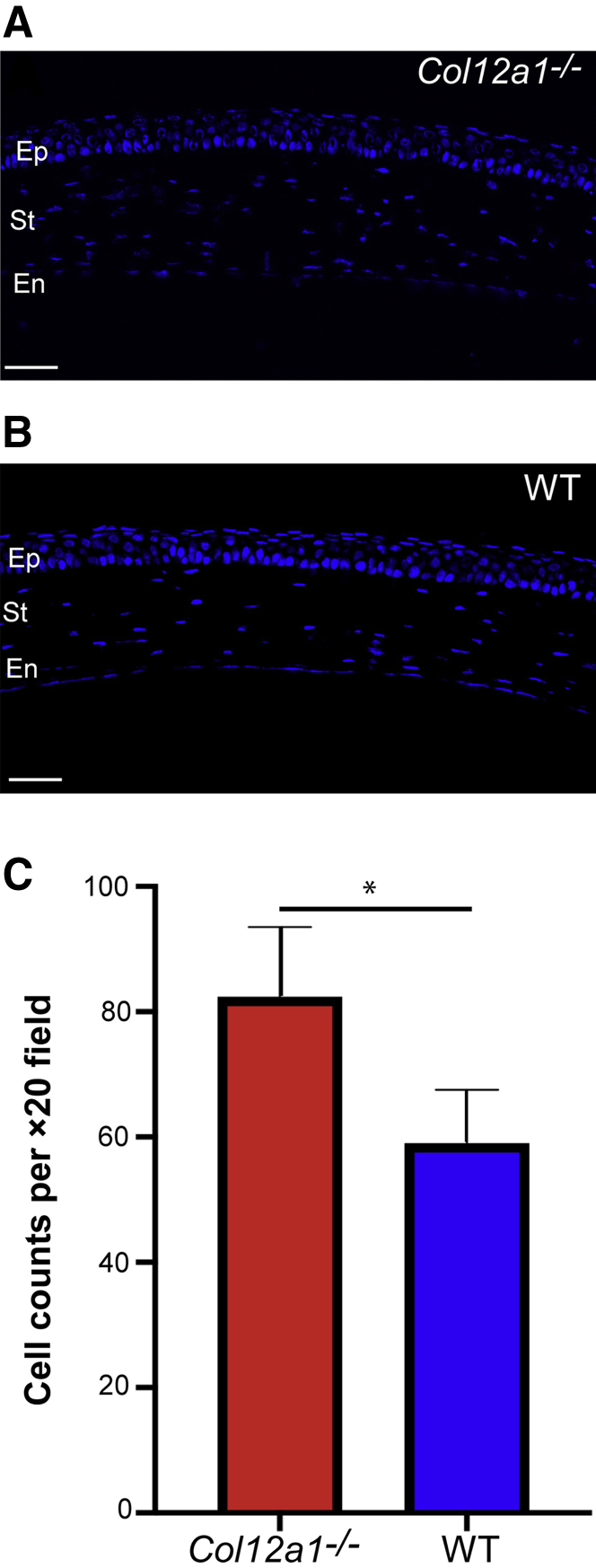
Histologic observation of stromal morphology in Col12a1−/− and WT mice at different ages suggested that collagen XII influences the number of keratocytes, as well as the thickness of stromal tissue (Figure 1, A and B). Cell density and central corneal thickness were analyzed. Keratocyte density was evaluated by counting keratocytes in corneal cross-sections at different ages in the WT and Col12a1−/− stromata. A hyperplastic number of keratocytes was found in the Col12a1−/− stromata in the preadult age. Keratocytes per ×20 field were 82.4 ± 11.1 in Col12a1−/− versus 59 ± 8.5 in WT stromata. A statistically significant difference was found between Col12a1−/− and WT (unpaired t-test; P = 0.02) (Figure 1C). At adult age, Col12a1−/− stromata continued to have a higher keratocyte density, but it was not statistically significant (71.3 ± 14.7 versus 57.6 ± 8.3; P = 0.24).
Figure 1.
Collagen XII regulates keratocyte density in the stroma (St). A: Normal keratocyte density in the wild-type (WT) stroma. B: Increased stromal cellularity is evident in the absence of collagen XII. C: A statistically significant difference in cell density is noted between both groups. ∗P < 0.05. Scale bar = 50 μm (A and B). En, endothelium; Ep, epithelium.
To quantify whether corneas were not only more populated but had deposited more extracellular matrix during development, corneal thickness was measured in the adult, wherein the influence of an immature corneal endothelial monolayer is minimal (Figure 2, A and B).7 A statistically significant difference was found between Col12a1−/− and WT adult corneal thickness. Mean corneal thickness in Col12a1−/− was 120.6 ± 5.0 μm; and in WT corneas, thickness was 100.5 ± 5.6 μm. A statistically significant difference was determined via unpaired t-test (P < 0.001) (Figure 2C). Taken together, these data demonstrate that collagen XII is a regulator of keratocyte density, stromal structure, and hierarchical organization.
Figure 2.
Collagen XII regulates corneal thickness. A and B: Optical coherence tomography imaging of a normal cornea shows in vivo corneal structure and thickness in wild-type (WT) and Col12a1−/− corneas, respectively. C: A statistically significant thicker cornea is noted in the absence of collagen XII. ∗∗∗P < 0.001.
Collagen XII Regulates the Synthesis and Deposition of Extracellular Matrix Collagens
Upon establishing that collagen XII regulated the number of keratocytes and thickness of the corneal stroma, whether the extracellular matrix macrostructure was regulated by collagen XII was investigated next. Increased collagen fibril packing in Col12a1−/− stromata has been demonstrated by electron microscopy.23 An indirect way to objectively examine the stromal hierarchical organization of collagen fibrils is by analyzing forward and backward scattered SHG signaling at different stages of stromal development (Figure 3). To assess the influence of COL12A1 on the hierarchical organization of fibrillar collagen, without tissue manipulation or chemical fixation, adult Col12a1−/− stromata were analyzed and compared with WT stromata. Cross-section SHG imaging of corneas at distinct developmental stages (postnatal days 4, 30, and 90), and en face imaging of the stroma of adult corneas from both groups was performed immediately after enucleation. Increased forward scattered and backward scattered signaling found in the tissue without collagen XII suggests fibril disorganization, increased fibril density, and abnormalities in the hierarchical organization in Col12a1−/− corneas compared with WT. These findings suggest that a matrix without COL12A1 appears with irregularities in collagen fibril morphology and structure and overall stromal hierarchical organization. To evaluate collagen levels in the stroma, an assay for total collagen content in stromata at these same ages was performed. A statistically significant increase in total collagen content was found between Col12a1−/− and WT corneas (Figure 4). Taken together, these data demonstrate that collagen XII is a regulator of collagen synthesis, packing, and deposition in the corneal stroma.
Figure 3.
Collagen XII regulates collagen fibril structure and organization in vivo. A: Second harmonic generation imaging shows higher forward scattering signal in the absence of collagen XII at all stages of development in corneal tangential images. B: Similarly, increased signaling is noted in the Col12a1−/− stroma during en face imaging of the adult postnatal day (P) 60. Scale bars: 25 μm (A); 50 μm (B). En, endothelium; Ep, epithelium; St, stroma; WT, wild type.
Figure 4.

Total collagen quantification shows regulation by collagen XII during development and in adult age. Total collagen in wild-type (WT) and Col12a1−/− corneas from different mice was analyzed in preadult postnatal day (P) 30 (A) and adult P90 (B) in WT and Col12a−/− male mice. P30 (WT n = 6, Col12a1−/− n = 10); P90 (both groups n = 5). ∗P < 0.05, ∗∗∗∗P < 0.0001.
Collagen XII Regulates Stromal TGF-β Signaling
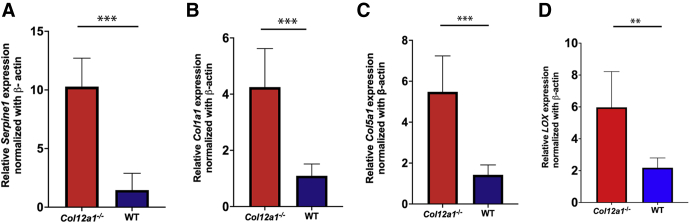
After confirmation by different techniques and assays with the observation that keratocytes and extracellular matrix are up-regulated by collagen XII, the role of TGF-β was explored to determine the underlying mechanisms. TGF-β is a well-known cytokine with significant influence on cell and matrix structure and function, and an excellent candidate to explain these preliminary observations. It was hypothesized that collagen XII is a regulator of TGF-β signaling in the stroma; and to test this, keratocyte synthetic activity in vivo at preadult age was studied by quantitative real-time PCR. Day 30 was chosen because stromal cells are known to be quiescent mitotically, but collagen synthesis is believed to be active while cornea is still maturing. The synthesis of a well-known indicator of TGF-β activity, PAI-1, whose promoter is activated by TGF-β1 or TGF-β2, was quantified.38 A statistically significant difference was found in Serpine1 mRNA expression, a gene that encodes for PAI-1, between Col12a1−/− and WT corneas with intact epithelium or after removal of epithelium layer with a combination of topical 20% alcohol and debridement (P < 0.01) (data not shown). The synthesis of collagens I and V, which are the most abundant in the corneal stroma and more relevant to fibril formation, both of which are known to be regulated by TGF-β, was additionally examined. A statistically significant difference in the mRNA expression in vivo was found in these collagens. Similarly, lysyl oxidase, an enzyme regulated by TGF-β, was increased in the absence of collagen XII (P = 0.01) (Figure 5). Together, these data suggest increased TGF-β1 signaling, as demonstrated by increased transcription of genes regulated by TGF-β in vivo.
Figure 5.
Collagen XII regulates transforming growth factor-β responsive genes in the corneal stroma in vivo in corneas with epithelium. Serpine1 (A), Col1a1 (B), Col5a1 (C), and LOX (D) mRNA expression was down-regulated by collagen XII. ∗∗P < 0.01, ∗∗∗P < 0.001. WT, wild type.
Collagen XII Regulates Latent TGF-β Storage in the Matrix
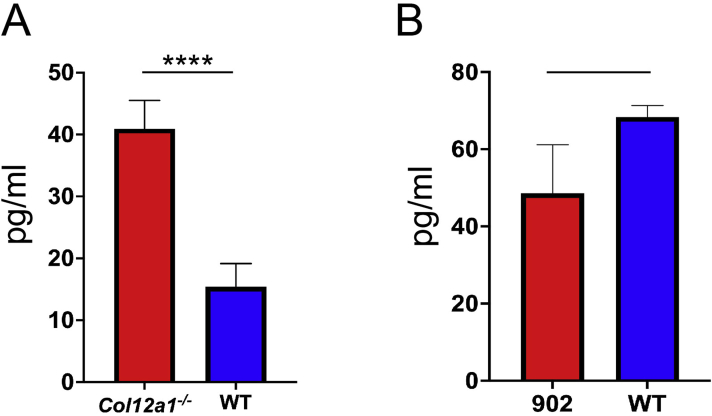
To explore how collagen XII might regulate the function of TGF-β in the cornea, a sensitive and functional assay based on the up-regulation of PAI-1 by TGF-β was utilized.38 The amount of latent TGF-β was assessed by activating all TGF-β stored in corneal tissue by heating similar quantities of corneal tissue extracts from WT and Col12a1−/− corneas for 10 minutes at 80°C. The function of TGF-β was then measured using transformed mink lung reporter cells, which produce luciferase as a function of active TGF-β.38 The first step was to make sure that PAI-1 promoter was activated by TGF-β and no other growth factors. To this end, luciferase synthesis was tested after the use of SB-431542, a specific TGF-β inhibitor, and an antibody against all three TGF-β isoforms. SB-431542 significantly abolished luciferase synthesis in the reporter cells (Figure 6A). An antibody against all three TGF-β isoforms also significantly decreased luciferase synthesis (data not shown). A standard curve indicated that latent TGF-β deposits were significantly higher in the WT matrix compared with those in Col12a1−/− matrices (t-test; P < 0.01) (Figure 6B). These findings demonstrate that collagen XII regulates the levels of latent TGF-β in the stroma.
Figure 6.
Collagen XII maintains transforming growth factor (TGF)-β latency in stromal tissue in vivo. A luciferase assay was used to functionally quantify TGF-β availability in tissue freshly harvested. A: A pilot study to demonstrate that reporter cells were responding to TGF-β signaling and no other cytokines was performed using a TGF-β–specific blocker, SB431542. B: Collagen XII allows TGF-β latency in the stromal extracellular matrix. ∗∗P < 0.01, ∗∗∗∗P < 0.0001. DMEM, Dulbecco’s modified Eagle’s medium; RLU, relative light unit; WT, wild type.
Collagen XII Regulates TGF-β Activation
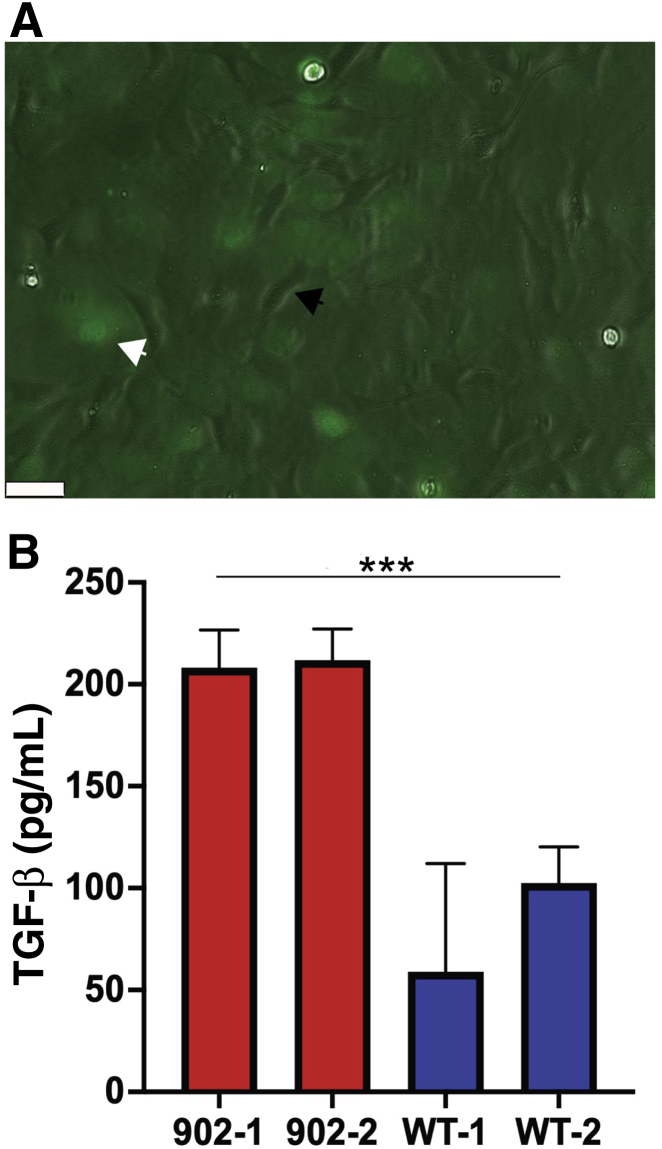
To explore whether collagen XII regulates the levels of biologically active TGF-β in the stroma, ELISA and an in vitro co-culture functional assay were utilized.39 shRNA was used to manipulate the content of collagen XII in a corneal fibroblast culture system and to study the regulation of TGF-β activation by collagen XII. Figure 7 demonstrates three different constructs used for Col12a1 silencing with shRNA. Knockdown efficiency was determined by comparing mRNA expression between target and control shRNA samples. Lentivirus construct 902 was selected for these experiments because of its significant knockdown efficiency and construct 902-infected fibroblasts were used for no more than four passages. First, total TGF-β1 was measured in the culture medium obtained from WT and Col12a1−/− corneal fibroblasts after expanding primary culture corneal fibroblasts in vitro until confluency and maintained in serum-free medium for 24 hours. The medium was collected and heated up to 80°C for 10 minutes to activate total TGF-β1 following manufacturer instructions. Statistically significant (P < 0.0001; t-test, unpaired) higher levels of total TGF-β1 were observed by ELISA in the Col12a1−/− system compared with those in WT. Cells were allowed to deposit collagen XII in the culture dish by expanding WT corneal fibroblasts and maintaining them for 5 days in serum containing medium supplemented with ascorbic acid to build matrix. After 5 days, Col12a1 expression was shut down using shRNA gene silencing to keep culture conditions equal. A group of cells transfected with construct 902 to silence Col12a1. Medium was collected after cells were maintained in serum-free medium for 24 hours. Levels of total TGF-β1 in the conditioned medium determined by ELISA were not statistically significant as compared to those in control. Finally, a co-culture system using corneal fibroblasts was transfected with construct 902 and transformed mink lung reporter cells in a 1:1 ratio. Luciferace activity was measured after 16 hours of co-culture in serum-free DMEM. Active TGF-β level was significantly (P < 0.001) higher in fibroblasts in which Col12a1 expression was silenced (Figure 8B). These data show that in vitro, collagen XII regulates active TGF-β levels and that a state of high active TGF-β occurs in the absence of collagen XII.
Figure 7.
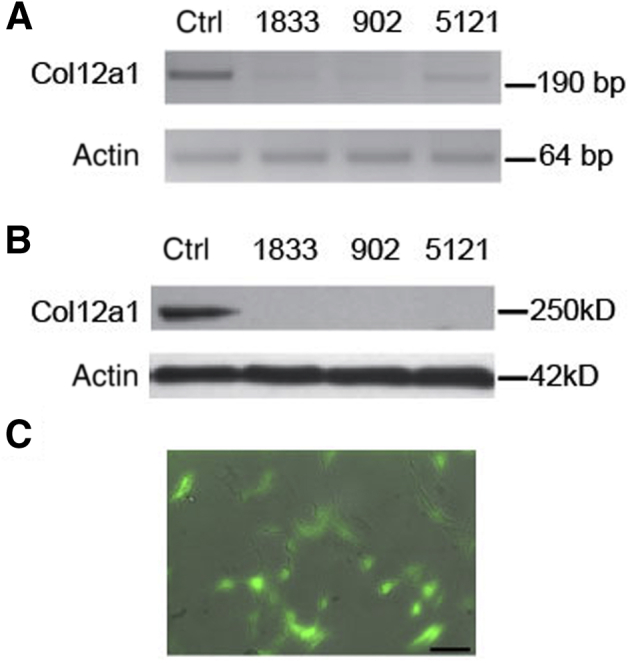
shRNA silencing of collagen XII expression in stromal fibroblast primary cultures. A: Real-time PCR of fibroblast cell culture transfected with three different constructs shows significant silencing of Col12a1 expression. B: Protein blots confirm significant silencing of Col12a1 expression after puromycin selection in primary cultures. C: Cells in primary cultures 48 hours after lentivirus transduction. Scale bar = 40 μm (C). Ctrl, control.
Figure 8.
Collagen XII regulates active transforming growth factor (TGF)-β in a co-culture system. A: Phase contrast and fluorescence microscopy shows co-culture in approximately 1:1 ratio of transformed mink lung cells, not fluorescent and black arrowhead, and fresh stromal fibroblasts, green fluorescent and white arrowhead, cells from primary culture. B: Two different clones from construct 902, each clone with at least three samples, were used. Collagen XII in the system down-regulates TGF-β activity. ∗∗∗P < 0.001. Scale bar = 50 μm (A). WT, wild type.
Discussion
Understanding the mechanisms that regulate stromal structure and function is essential for designing translational approaches to treat corneal diseases and attempting to manipulate wound healing. Collagen XII was recently shown to regulate structural and functional properties of the corneal extracellular matrix.23 The current study shows that collagen XII controls matrix deposition in the stroma during development and homeostasis, and that one of the underlying mechanisms by which collagen XII regulates the intrinsic properties of the stroma is by modulating TGF-β activity. It also demonstrates that collagen XII modulates TGF-β activation and latency.
The hypertrophic characteristics of the stroma in our Coll12a1−/− model were further explored. An increase in corneal thickness, increased collagen content, and unusual SHG signaling was observed in the cornea of this mouse model compared with WT with an abnormally high deposition of collagen in the matrix and the formation of a hypertrophic matrix. We thus hypothesized that collagen XII must have a role in regulating keratocyte synthetic activity to explain the hypertrophic stroma phenotype seen in our Coll12a1−/− knockout model.
With the observation that corneal stromata were hypertrophic in our Coll12a1−/− knockout model, subsequent explorations into unraveling the underlying mechanisms were focused on two well-characterized signaling pathways known to cause hypertrophy and hyperplasia: Yes-associated protein 1/transcriptional coactivator with PDZ-binding motif (YAP/TAZ) and TGF-β. Because exploratory research on YAP/TAZ did not yield significant findings, the potential regulation of TGF-β by collagen XII was studied. Interestingly, a recent study has shown decreased sequestration of TGF-β by the matrix in the skin in the absence of collagen XII, and at the same time, increased active TGF-β1.10 The in vitro data from the current study show similar findings and suggest that collagen XII is essential to regulate TGF-β activation and latency in the stromal matrix, and therefore is a functional regulator of TGF-β activity in the matrix.
How collagen XII regulates TGF-β latency in the matrix needs further exploration and is a complex subject. We propose two different hypotheses to explain why a matrix without collagen XII does not properly store latent TGF-β (Figure 9). The first hypothesis is that collagen XII, a large protein with multiple domains, binds latent TGF-β per se or regulates other matrix components known to bind latent TGF-β (eg, LTBP-1, fibronectin, or fibrillin) to the matrix. Therefore, in the absence of collagen XII, latent TGF-β storage is dysfunctional. The activity of TGF-β, once secreted, is elevated in the extracellular matrix, because it cannot become latent. The mRNA quantification for LTBP-1, fibrillin, and fibronectin demonstrated a statistically significant higher expression of these matrix proteins in the absence of collagen XII (data not shown). The presence of LTBPs in the cornea has not been explored in the past, and the presence of elastic fibers and elastin, as well as fibrillin, is currently being studied.40, 41, 42 Our laboratory has preliminary data suggesting that collagen XII binds LTBP-1, and studies to better define these interactions are underway. The known function of collagen XII as a matrix organizer could also play a role in regulating TGF-β latency. A disorganized matrix cannot efficiently bind or sequester TGF-β in the matrix. This hypothesis would explain the findings shown in Figure 8 and Supplemental Figure S1, where there is increased TGF-β activity in the absence of collagen XII deposition on the culture dish.
Figure 9.
Illustration summarizing the mechanism of collagen XII regulation of latent transforming growth factor (TGF)-β activation. In vivo, wild type (WT; A) and Col12a1−/− (B). In vitro, wild type (C) and Col12a1−/− (D). A minimal part of this illustration was generated with biorender.com (Toronto, ON, Canada). LTBP-1, TGF-β binding protein 1.
A second hypothesis is that collagen XII regulates TGF-β activation by regulating matrix mechanics. Collagen XII confers the corneal stroma with normal tissue mechanics.23 Interestingly, collagen XII down-regulated the expression of lysyl oxidase, suggesting another venue for matrix and tissue stiffness regulation by collagen XII. The expression of bone morphogenetic protein 1 as a regulator of lysyl oxidase synthesis was explored in the absence of significant corneal matrix expression or regulation by collagen XII. Normal matrix mechanics directs the function and differentiation of cells.43 In various tissues, fibroblasts, and myofibroblasts, cells driving fibrosis and scar contraction are gradually activated from their precursors by cues provided by the matrix.44 In different tissues, matrix stiffness and exposure to growth factors like TGF-β are among the cues provided by the matrix.32,33,45,46 Understanding the roles of collagen XII in regulating matrix-related mechanical and chemical cues (eg, latent TGF-β in the stroma) is therefore essential. Latent TGF-β storage occurs in a matrix of normal mechanics. However, a stiffer matrix or a matrix with dysfunctional mechanics, as in the case of the Col12a1−/− model,23 might be a facilitator of latent TGF-β activation. This could, at the very least, lead to the activation of TGF-β1, with continuous release into surrounding keratocytes, keeping latent TGF-β stores depleted.
Quantification of active TGF-β in tissues, or even in vitro, is complex and technically challenging because of the fast degradation of this cytokine.47 It is known that TGF-β1 in its active form lasts only 2 minutes.48,49 To study the effects of collagen XII on TGF-β activation, this study used a combination of ELISA and a co-culture system using transformed mink lung cells and corneal fibroblasts in which shRNA abolished collagen XII expression. Unfortunately, this co-culture system has intrinsic problems, including the effects on corneal fibroblasts of serum exposure, the use of a rigid nonphysiological plastic matrix, and the need of cell passage and manipulation to obtain cell clones with decreased collagen XII expression. There was variability in the luciferase readings with cell passage, but active TGF-β was increased in the fibroblast clones with early passages (less than four passages), in which collagen XII was silenced (Figure 8). The findings in this study, together with decreased latent TGF-β levels, are highly suggestive that collagen XII plays a regulatory role in TGF-β activation and keeping TGF-β latent.
A better understanding of tissue biomechanics is of the outmost importance for corneal translational research. The current findings are suggestive of the important regulatory function of collagen XII and open further research questions regarding the function of collagen XII in tissue mechanics and its effects in other aspects, including regulation of corneal thickness during development and modulation of wound healing. In summary, collagen XII, by regulating TGF-β activation and latency, is a regulator of matrix deposition during development and homeostasis. Collagen XII is needed to down-regulate active TGF-β. In the absence of collagen XII, cells are dysregulated and overstimulated to produce and deposit more collagen fibrils and matrix. This is shown in our Col12a1−/− model, where keratocytes increased collagen and matrix deposition in vivo and where SHG data clearly reflected irregular collagen organization reminiscent of collagen accumulation and matrix fibrosis. Therefore, collagen XII is not just a simple structural collagen, but a protein that greatly influences matrix properties and cell function and contributes to stromal uniqueness. Further studies are underway to evaluate the effects of collagen XII manipulation during different stages of wound healing with the purpose of improving corneal function after injury.
Acknowledgment
We thank Dr. Daniel Rifkin (New York University) for providing the luciferase reporter cells.
Footnotes
Supported by NIH/National Eye Institute grant EY029395 (E.M.E.). M.K. was supported by a grant from the Deutsche Forschungsgemeinschaft to the Research Unit FOR2722.
Disclosures: None declared.
Supplemental material for this article can be found at http://doi.org/10.1016/j.ajpath.2021.10.014.
Supplemental Data
Supplemental Figure S1.
Levels of transforming growth factor (TGF)-β1 measured by enzyme-linked immunosorbent assay in two different culture conditions. A: Total TGF-β1 in culture medium in a matrix with and without collagen XII shows increased total levels of TGF-β1 in the absence of collagen XII. B: Total TGF-β1 in culture medium in a matrix where collagen XII synthesis is blocked after a matrix containing collagen XII is formed to simulate a rescue experiment shows no significant difference in TGF-β1 expression. We ascribe the differences in TGF-β levels to different culture time and cell manipulation needed to induce Col12a1 silencing. n = 3 (A). ∗∗∗∗P < 0.0001. WT, wild type.
References
- 1.Poole C.A., Brookes N.H., Clover G.M. Keratocyte networks visualised in the living cornea using vital dyes. J Cell Sci. 1993;106(Pt 2):685–691. doi: 10.1242/jcs.106.2.685. [DOI] [PubMed] [Google Scholar]
- 2.Chen S., Mienaltowski M.J., Birk D.E. Regulation of corneal stroma extracellular matrix assembly. Exp Eye Res. 2015;133:69–80. doi: 10.1016/j.exer.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Espana E.M., Birk D.E. Composition, structure and function of the corneal stroma. Exp Eye Res. 2020;198:108137. doi: 10.1016/j.exer.2020.108137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meek K.M., Knupp C. Corneal structure and transparency. Prog Retin Eye Res. 2015;49:1–16. doi: 10.1016/j.preteyeres.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jester J.V., Moller-Pedersen T., Huang J., Sax C.M., Kays W.T., Cavangh H.D., Petroll W.M., Piatigorsky J. The cellular basis of corneal transparency: evidence for “corneal crystallins”. J Cell Sci. 1999;112(Pt 5):613–622. doi: 10.1242/jcs.112.5.613. [DOI] [PubMed] [Google Scholar]
- 6.Quantock A.J., Young R.D. Development of the corneal stroma, and the collagen-proteoglycan associations that help define its structure and function. Dev Dyn. 2008;237:2607–2621. doi: 10.1002/dvdy.21579. [DOI] [PubMed] [Google Scholar]
- 7.Hemmavanh C., Koch M., Birk D.E., Espana E.M. Abnormal corneal endothelial maturation in collagen XII and XIV null mice. Invest Ophthalmol Vis Sci. 2013;54:3297–3308. doi: 10.1167/iovs.12-11456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young B.B., Zhang G., Koch M., Birk D.E. The roles of types XII and XIV collagen in fibrillogenesis and matrix assembly in the developing cornea. J Cell Biochem. 2002;87:208–220. doi: 10.1002/jcb.10290. [DOI] [PubMed] [Google Scholar]
- 9.Massoudi D., Malecaze F., Soler V., Butterworth J., Erraud A., Fournie P., Koch M., Galiacy S.D. NC1 long and NC3 short splice variants of type XII collagen are overexpressed during corneal scarring. Invest Ophthalmol Vis Sci. 2012;53:7246–7256. doi: 10.1167/iovs.11-8592. [DOI] [PubMed] [Google Scholar]
- 10.Schonborn K., Willenborg S., Schulz J.N., Imhof T., Eming S.A., Quondamatteo F., Brinckmann J., Niehoff A., Paulsson M., Koch M., Eckes B., Krieg T. Role of collagen XII in skin homeostasis and repair. Matrix Biol. 2020;94:57–76. doi: 10.1016/j.matbio.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Izu Y., Adams S.M., Connizzo B.K., Beason D.P., Soslowsky L.J., Koch M., Birk D.E. Collagen XII mediated cellular and extracellular mechanisms regulate establishment of tendon structure and function. Matrix Biol. 2021;95:52–67. doi: 10.1016/j.matbio.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiquet M., Birk D.E., Bonnemann C.G., Koch M. Collagen XII: protecting bone and muscle integrity by organizing collagen fibrils. Int J Biochem Cell Biol. 2014;53:51–54. doi: 10.1016/j.biocel.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Izu Y., Sun M., Zwolanek D., Veit G., Williams V., Cha B., Jepsen K.J., Koch M., Birk D.E. Type XII collagen regulates osteoblast polarity and communication during bone formation. J Cell Biol. 2011;193:1115–1130. doi: 10.1083/jcb.201010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohassel P., Liewluck T., Hu Y., Ezzo D., Ogata T., Saade D., Neuhaus S., Bolduc V., Zou Y., Donkervoort S., Medne L., Sumner C.J., Dyck P.J.B., Wierenga K.J., Tennekoon G., Finkel R.S., Chen J., Winder T.L., Staff N.P., Foley A.R., Koch M., Bonnemann C.G. Dominant collagen XII mutations cause a distal myopathy. Ann Clin Transl Neurol. 2019;6:1980–1988. doi: 10.1002/acn3.50882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hicks D., Farsani G.T., Laval S., Collins J., Sarkozy A., Martoni E., Shah A., Zou Y., Koch M., Bonnemann C.G., Roberts M., Lochmuller H., Bushby K., Straub V. Mutations in the collagen XII gene define a new form of extracellular matrix-related myopathy. Hum Mol Genet. 2014;23:2353–2363. doi: 10.1093/hmg/ddt637. [DOI] [PubMed] [Google Scholar]
- 16.Delbaere S., Dhooge T., Syx D., Petit F., Goemans N., Destree A., Vanakker O., De Rycke R., Symoens S., Malfait F. Novel defects in collagen XII and VI expand the mixed myopathy/Ehlers-Danlos syndrome spectrum and lead to variant-specific alterations in the extracellular matrix. Genet Med. 2020;22:112–123. doi: 10.1038/s41436-019-0599-6. [DOI] [PubMed] [Google Scholar]
- 17.Meek K.M., Tuft S.J., Huang Y., Gill P.S., Hayes S., Newton R.H., Bron A.J. Changes in collagen orientation and distribution in keratoconus corneas. Invest Ophthalmol Vis Sci. 2005;46:1948–1956. doi: 10.1167/iovs.04-1253. [DOI] [PubMed] [Google Scholar]
- 18.Akhtar S., Bron A.J., Salvi S.M., Hawksworth N.R., Tuft S.J., Meek K.M. Ultrastructural analysis of collagen fibrils and proteoglycans in keratoconus. Acta Ophthalmol. 2008;86:764–772. doi: 10.1111/j.1755-3768.2007.01142.x. [DOI] [PubMed] [Google Scholar]
- 19.Gordon M.K., Foley J.W., Lisenmayer T.F., Fitch J.M. Temporal expression of types XII and XIV collagen mRNA and protein during avian corneal development. Dev Dyn. 1996;206:49–58. doi: 10.1002/(SICI)1097-0177(199605)206:1<49::AID-AJA5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 20.Gregory K.E., Keene D.R., Tufa S.F., Lunstrum G.P., Morris N.P. Developmental distribution of collagen type XII in cartilage: association with articular cartilage and the growth plate. J Bone Miner Res. 2001;16:2005–2016. doi: 10.1359/jbmr.2001.16.11.2005. [DOI] [PubMed] [Google Scholar]
- 21.Tzortzaki E.G., Tischfield J.A., Sahota A., Siafakas N.M., Gordon M.K., Gerecke D.R. Expression of FACIT collagens XII and XIV during bleomycin-induced pulmonary fibrosis in mice. Anat Rec A Discov Mol Cell Evol Biol. 2003;275:1073–1080. doi: 10.1002/ar.a.10120. [DOI] [PubMed] [Google Scholar]
- 22.Chaerkady R., Shao H., Scott S.G., Pandey A., Jun A.S., Chakravarti S. The keratoconus corneal proteome: loss of epithelial integrity and stromal degeneration. J Proteomics. 2013;87:122–131. doi: 10.1016/j.jprot.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun M., Zafrullah N., Devaux F., Hemmavanh C., Adams S., Ziebarth N.M., Koch M., Birk D.E., Espana E.M. Collagen XII is a regulator of corneal stroma structure and function. Invest Ophthalmol Vis Sci. 2020;61:61. doi: 10.1167/iovs.61.5.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Massague J. TGF-beta signaling in development and disease. FEBS Lett. 2012;586:1833. doi: 10.1016/j.febslet.2012.05.030. [DOI] [PubMed] [Google Scholar]
- 25.David C.J., Massague J. Contextual determinants of TGFbeta action in development, immunity and cancer. Nat Rev Mol Cell Biol. 2018;19:419–435. doi: 10.1038/s41580-018-0007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robertson I.B., Horiguchi M., Zilberberg L., Dabovic B., Hadjiolova K., Rifkin D.B. Latent TGF-beta-binding proteins. Matrix Biol. 2015;47:44–53. doi: 10.1016/j.matbio.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rifkin D.B., Rifkin W.J., Zilberberg L. LTBPs in biology and medicine: LTBP diseases. Matrix Biol. 2018;71-72:90–99. doi: 10.1016/j.matbio.2017.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holm T.M., Habashi J.P., Doyle J.J., Bedja D., Chen Y., van Erp C., Lindsay M.E., Kim D., Schoenhoff F., Cohn R.D., Loeys B.L., Thomas C.J., Patnaik S., Marugan J.J., Judge D.P., Dietz H.C. Noncanonical TGFbeta signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science. 2011;332:358–361. doi: 10.1126/science.1192149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robertson I.B., Rifkin D.B. Regulation of the bioavailability of TGF-beta and TGF-beta-related proteins. Cold Spring Harb Perspect Biol. 2016;8:a021907. doi: 10.1101/cshperspect.a021907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rockey D.C., Bell P.D., Hill J.A. Fibrosis--a common pathway to organ injury and failure. N Engl J Med. 2015;372:1138–1149. doi: 10.1056/NEJMra1300575. [DOI] [PubMed] [Google Scholar]
- 31.Kitamura H., Cambier S., Somanath S., Barker T., Minagawa S., Markovics J., Goodsell A., Publicover J., Reichardt L., Jablons D., Wolters P., Hill A., Marks J.D., Lou J., Pittet J.F., Gauldie J., Baron J.L., Nishimura S.L. Mouse and human lung fibroblasts regulate dendritic cell trafficking, airway inflammation, and fibrosis through integrin alphavbeta8-mediated activation of TGF-beta. J Clin Invest. 2011;121:2863–2875. doi: 10.1172/JCI45589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tandon A., Tovey J.C., Sharma A., Gupta R., Mohan R.R. Role of transforming growth factor beta in corneal function, biology and pathology. Curr Mol Med. 2010;10:565–578. doi: 10.2174/1566524011009060565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hinz B. The extracellular matrix and transforming growth factor-beta1: tale of a strained relationship. Matrix Biol. 2015;47:54–65. doi: 10.1016/j.matbio.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 34.Committee for the Update of the Guide for the Care and Use of Laboratory Animals; National Research Council . National Academies Press; Washington, DC: 2011. Guide for the Care and Use of Laboratory Animals: Eighth Edition. [Google Scholar]
- 35.Segev F., Heon E., Cole W.G., Wenstrup R.J., Young F., Slomovic A.R., Rootman D.S., Whitaker-Menezes D., Chervoneva I., Birk D.E. Structural abnormalities of the cornea and lid resulting from collagen V mutations. Invest Ophthalmol Vis Sci. 2006;47:565–573. doi: 10.1167/iovs.05-0771. [DOI] [PubMed] [Google Scholar]
- 36.Fellmann C., Hoffmann T., Sridhar V., Hopfgartner B., Muhar M., Roth M., Lai D.Y., Barbosa I.A., Kwon J.S., Guan Y., Sinha N., Zuber J. An optimized microRNA backbone for effective single-copy RNAi. Cell Rep. 2013;5:1704–1713. doi: 10.1016/j.celrep.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 37.Pelossof R., Fairchild L., Huang C.H., Widmer C., Sreedharan V.T., Sinha N., Lai D.Y., Guan Y., Premsrirut P.K., Tschaharganeh D.F., Hoffmann T., Thapar V., Xiang Q., Garippa R.J., Ratsch G., Zuber J., Lowe S.W., Leslie C.S., Fellmann C. Prediction of potent shRNAs with a sequential classification algorithm. Nat Biotechnol. 2017;35:350–353. doi: 10.1038/nbt.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abe M., Harpel J.G., Metz C.N., Nunes I., Loskutoff D.J., Rifkin D.B. An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem. 1994;216:276–284. doi: 10.1006/abio.1994.1042. [DOI] [PubMed] [Google Scholar]
- 39.Urban Z., Hucthagowder V., Schurmann N., Todorovic V., Zilberberg L., Choi J., Sens C., Brown C.W., Clark R.D., Holland K.E., Marble M., Sakai L.Y., Dabovic B., Rifkin D.B., Davis E.C. Mutations in LTBP4 cause a syndrome of impaired pulmonary, gastrointestinal, genitourinary, musculoskeletal, and dermal development. Am J Hum Genet. 2009;85:593–605. doi: 10.1016/j.ajhg.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feneck E.M., Lewis P.N., Ralphs J., Meek K.M. A comparative study of the elastic fibre system within the mouse and human cornea. Exp Eye Res. 2018;177:35–44. doi: 10.1016/j.exer.2018.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanlon S.D., Behzad A.R., Sakai L.Y., Burns A.R. Corneal stroma microfibrils. Exp Eye Res. 2015;132:198–207. doi: 10.1016/j.exer.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feneck E.M., Souza R.B., Lewis P.N., Hayes S., Pereira L.V., Meek K.M. Developmental abnormalities in the cornea of a mouse model for Marfan syndrome. Exp Eye Res. 2020;194:108001. doi: 10.1016/j.exer.2020.108001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bonnans C., Chou J., Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Humphrey J.D., Dufresne E.R., Schwartz M.A. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol. 2014;15:802–812. doi: 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hinz B. Myofibroblasts. Exp Eye Res. 2016;142:56–70. doi: 10.1016/j.exer.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 46.Hinz B., Lagares D. Evasion of apoptosis by myofibroblasts: a hallmark of fibrotic diseases. Nat Rev Rheumatol. 2020;16:11–31. doi: 10.1038/s41584-019-0324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Annes J.P., Munger J.S., Rifkin D.B. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 48.Saito T., Kinoshita A., Yoshiura K., Makita Y., Wakui K., Honke K., Niikawa N., Taniguchi N. Domain-specific mutations of a transforming growth factor (TGF)-beta 1 latency-associated peptide cause Camurati-Engelmann disease because of the formation of a constitutively active form of TGF-beta 1. J Biol Chem. 2001;276:11469–11472. doi: 10.1074/jbc.C000859200. [DOI] [PubMed] [Google Scholar]
- 49.Wakefield L.M., Winokur T.S., Hollands R.S., Christopherson K., Levinson A.D., Sporn M.B. Recombinant latent transforming growth factor beta 1 has a longer plasma half-life in rats than active transforming growth factor beta 1, and a different tissue distribution. J Clin Invest. 1990;86:1976–1984. doi: 10.1172/JCI114932. [DOI] [PMC free article] [PubMed] [Google Scholar]