Abstract
Metallosis is a rare and poorly understood long-term complication of instrumented surgery that can result in an inflammatory pseudotumor termed metalloma. We describe a particularly unique case and compare it to 6 analogous cases identified by PubMed and/or Medline search through July 2020. A 79-year-old male with multiple prior spinal lumbar fusion procedures presented with progressive weakness and pain. Imaging revealed a large mass surrounding the right-sided paraspinal rod with extension into the spinal canal, neural foramina, extraforaminal spaces, psoas muscle, marrow spaces, and right sided pedicles. The case presented is a unique example of a unilateral metalloma with mixed-metal instrumentation that created a progressive neurologic deficit without infection, pseudoarthrosis, or hardware failure. This case highlights the lack of understanding regarding the pathophysiology of metallosis and metalloma in spinal instrumentation. We highlight the imaging findings of metalloma to encourage early identification for removal and decompression.
Keywords: Spine, Spinal, Metalloma, Metallosis, Instrumentation
Introduction
Metallosis is a rare and poorly understood phenomenon in the surgical literature. Metallosis is defined as the deposition of metal ions in tissue [1], [2], [3], [4]. It may trigger an inflammatory response, which can result in various types of metal induced pathologies. This spectrum of disease is broadly, and poorly described in the literature as an adverse and/or allergic reaction to metal debris (AMRD), if systemic, or an adverse local tissue reaction (ALTR), if localized [1,5]. If an ALTR results in an inflammatory pseudotumor, then it is defined as a metalloma [1,2].
When observed, ARMD and/or ALTR are commonly associated with contact degradation in joint replacement surgery or in the setting of postoperative orthopedic infection [1,[5], [6], [7], [8], [9], [10]]. Due to this, it has been widely described in the orthopedic joint arthroplasty literature, but the exact pathophysiology has not been fully elucidated. Possible etiologies of metallosis are corrosion and fretting from metal-on-metal and bone-on-metal junctions resulting in the deposition of metal ions in tissue. These metal deposits may trigger physiologic reactions [11], [12], [13].
The resultant deposition of ions has been discovered in patients following instrumentation through observation of increased levels of serum metal ions [14], [15], [16]. This increase in serum metal concentrations is not correlated as always resulting in an adverse effect, and, if an adverse effect is present, it does not correlate to severity [9]. This increase in metal concentrations has also been observed in tissue samples during necroscopy [17]. Jacobs, et al. described metallosis occurring with multiple metal types including stainless steel, cobalt, and titanium implants [18]. Senaran, et al. evaluated tissue surrounding explanted spinal hardware for late post-operative pain with light microscopy, transmission electron microscopy, and scanning electron microscopy [19]. They found particulate metallic debris consisting of iron and chromium with the highest concentration around transverse rod connectors. The highest concentration of macrophages was around pedicle screws.
It is postulated that metallosis resulting in AMRD involves the breakdown of metal products by macrophages, which may trigger a cytokine induced immune response with resulting activation of T lymphocytes producing a type IV hypersensitivity reaction [20]. Towers and Kurtom reported a patient with a nickel allergy that experienced anorexia and fatigue after spinal instrumentation [21]. She lost so much weight there was concern that her hardware would erode through the skin. The hardware was removed with improved stamina and weight gain. Kim, et al. described a patient with generalized itching that resolved after hardware explant [22]. The tissues near the hardware were notable for having a lymphocytic predominance. Similarly, Shi, et al. described a patient that developed tingling and itching in the throat, dysphagia, and rash after anterior cervical discectomy and fusion [23]. Symptoms resolved following explant. Shang, et al. presented a patient with metal allergies that developed progressive back pain after spinal instrumentation that resolved following explant [24]. Curley, et al. discussed a patient that developed back pain, abdominal pain, and loss of appetite after an anterior lumbar interbody fusion (ALIF) [25]. She was found to have a presacral fluid collection and a hypersensitivity to nickel. She underwent explant with replacement of a PEEK implant with no nickel.
A feared outcome of metallosis is the formation of an inflammatory mass referred to as a metalloma. Metalloma is a result of ALTR where the immune system creates a localized granulomatous and fibrotic scar. This resultant soft tissue mass or pseudotumor is referred to as a metalloma. An increasing number are being reported in spinal surgeries, likely due to motion preserving operations like cervical disc arthroplasty, and facet replacement systems that have similarities with orthopedic joint replacements [26], [27], [28], [29], [30], [31], [32]. Rare reports exist in spinal fixation surgeries for fusion.
There are few documented cases of a metalloma creating a neurologic deficit secondary to mass effect, especially with no evidence of infection or pseudoarthrosis [2,3,[33], [34], [35], [36]]. Here, we conduct a spinal instrumentation literature review, and describe a unique case of unilateral mixed-metal metalloma causing a neurologic deficit with no evidence of infecton or pseudoarthrosis while describing the radiographic findings to aid in early diagnosis.
Case report
A 76-year-old male initially presented to an outside neurosurgeon with worsening back pain and bilateral radicular pain (right leg worse than left leg). The majority of his pain was radicular in the legs that worsened with walking. He was only able to ambulate approximately 50 feet before requiring rest. He had documented weakness in the left leg ranging from 4- to 4+ with 5 of 5 in plantarflexion. Reflexes were 2+ except for the left patellar 1+ with absent Hoffmann and Babinski signs.
Spine magnetic resonance imaging (MRI) revealed diffuse spondylosis with severe stenosis at L2-5. Spine radiographs showed spondylolisthesis at L2-3 and L3-4. He was treated urgently with a L2-5 lateral lumbar interbody fusion (LLIF) (NuVasive, San Diego, CA), L2-5 laminectomies, and L2-5 bilateral posterior instrumented fusion (PIF) with cobalt chrome rods and titanium screws (Medtronic, Minneapolis, MN) (Supplementary Table 1).
Postoperatively, he continued to have bilateral back pain with worsening radiation into the right lower extremity. The patient self-referred to our care approximately 2 years after his lumbar decompression and fusion. Now 78-years-old, he subjectively described worsening weakness in his right leg with similar left leg weakness. He was found to have a right foot drop rated 4 of 5 and hip flexion 4 of 5 with diminished reflexes, 0 at patella and 1+ at Achilles. Updated spine MRI revealed adjacent segment disease both above and below the construct with moderate stenosis at L1-2 but likely symptomatic severe right foraminal stenosis at L5-S1. Spine X-rays showed spondylolisthesis at L5-S1.
He was indicated for L5-S1 anterior lumbar interbody fusion (ALIF), L5 laminectomy, and L4-S1 PIF. The ALIF was performed with a hyperlorditic PEEK spacer and titanium screws and plates (NuVasive, San Diego, CA). Posteriorly, the cobalt chrome rods were cut bilaterally between the L4, and L5 pedicle screws. Titanium lateral connectors were placed on the remaining segment of L4 rods. Titanium pedicle screws were placed at S1 and cobalt chrome rods were passed from the L4 lateral connectors through the prior L5 and new S1 pedicle screws bilaterally (Medtronic, Minneapolis, MN) (Supplementary Table 1). Postoperatively, the patient had improved leg and back pain with residual low back pain with radiation into bilateral anterior thighs. Strength was 4+/5 dorsiflexion on right. His walking distance improved from one-quarter mile to 2 miles. Routine spine X-rays revealed mild L4-5 lateral connector loosening on the left. As he already had computed tomography (CT) proven radiographic fusion at L4-5 this was attributed as incidental and technical in nature. He was provided with a bone growth stimulator. There was evidence of fusion on subsequent X-ray at L5-S1 and stable arthrodesis L2-L5.
Approximately 1 year later at 79-years-old, the patient developed worsening falls, and ataxia following a right hip arthroplasty performed at an outside institution. His right hip arthroplasty contained cobalt chrome, ceramic, and titanium implants. He had pain in his back and down his right leg with a history consistent with pseudoclaudication. His right leg was rated 3 of 5 at the hip, 4-/5 at the knee, and 4 of 5 at the ankle with hypo-active bilateral lower extremity reflexes. He rapidly progressed to needing a cane and then a walker. Spine X-rays revealed a solid fusion mass of L2-S1 with no abnormal findings. Spine MRI and CT revealed a T1 and T2 hypointense non–enhancing mass surrounding the right-sided paraspinal rod with extension into the spinal canal, neural foramina, extraforaminal spaces, right psoas muscle and marrow spaces of the right iliac bone, sacrum, and right sided pedicles (Fig. 1, Fig. 2). The left and anterior spinal hardware had no such reaction. Routine laboratory tests were unremarkable. Inflammatory markers revealed an erythrocyte sedimentation rate (ESR) that was mildly elevated at 9.0 and white blood cell count (WBC) was normal at 8.2. He was indicated for surgical exploration for biopsy, resection, and decompression. He underwent open L4-S1 biopsy and subtotal resection of the paraspinal mass with removal of hardware at L2-S1 roughly 16 months after his extension of fusion surgery (Supplementary Table 1).
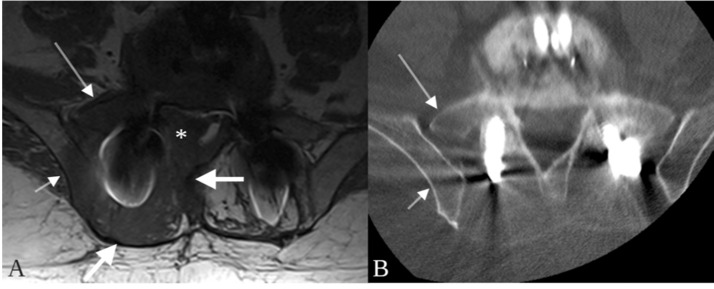
Fig. 1.
Metalloma surrounding a paraspinal rod at L4. The ovoid lesion (thick arrows) is nearly isointense to muscle on T1 (A) and T2-weighted imaging (D), and isodense to muscle on non–contrast CT (C). The mass does not enhance on post-contrast T1-weighted images (B) although a narrow margin of enhancement (B, thin arrow) is present in the right psoas muscle adjacent to the mass. The mass extends into the neural foramen and right extraforaminal space at L4 (D, asterisk).
Fig. 2.
Trans-spatial involvement of a paraspinal metalloma. On the T1-weighted image, a mass (A, thick arrows) is present in the paraspinal soft tissues surrounding a paraspinal rod. The T1 hypointense signal abnormality extends into the marrow spaces of the adjacent iliac bone (A, short thin arrow) and sacrum (A, long thin arrow) and the epidural space (A, asterisk). No lytic or sclerotic changes are present on the corresponding CT performed 3 days later (B, arrows).
Upon opening the dorsal thoracolumbar fascia, dense fibrotic tissue was encountered. The texture was “rubbery” in nature and peeled away in onion-like layers (Fig. 3A). There was a plane around the abnormal tissue that could be separated, but the tissues were firm, and difficult to mobilize. Cobbs, rongeurs, and the Sonopet (Stryker, Kalamazoo, MO) were used to aid in tissue removal. Upon reaching the hardware, a pocket of black fluid lined the rods and tulips (Fig. 3B). Both the tulips of the screws and the rods had black granular material present diffusely on them. The adjacent soft tissues and the lumbar bone were stained black. The hardware was removed, and the black filament was noted to extended into the bone (Fig. 3C). After removal of the hardware, the fusion was explored, and found to be solid with no evidence of movement. The stained tissue and stained bone were removed with use of the Sonopet. Pieces of tissue were sent for frozen and permanent pathology, fluid aspirate was sent for cytology, and multiple culture and/or gram stain swabs were sent from tissue, hardware, and fluid. Intraoperative gram stains revealed no bacteria and 1 of 3 samples had white blood cells. The frozen tissue sample was described as acute and chronic inflammatory changes with no evidence of malignancy. Pulse lavage was used and the wound was closed with a drain in the cavity.
Fig. 3.
Intraoperative photographs depicting unusual findings. (A) Fibrotic tissue encasing instrumentation with black pigment staining. (B) Black pigmented fluid collection around the instrumentation. (C) Post removal spinal instrumentation with notable black pigment staining.
Postoperatively, the patient's right lower extremity strength was improved with 4-/5 at the hip, 4+/5 at the knee, and 5 of 5 at the ankle. Final pathology revealed extensive necrosis with surrounding inflammation and fibrosis with focal deposition of black pigment of exogenous origin (metallic vs carbonaceous) (Fig. 4). Rare areas showed lymphohistiocytic reaction with giant cell formation, these were not immediately adjacent to the foreign material. Cytology results were negative for malignancy with heavy deposition of black granular pigment. Gram stain showed no organisms and none of the bacterial, fungal, or mycobacteria cultures produced a positive result. Immediate postoperative MRI revealed removal of unilateral hardware with wide resection of the mass. A follow-up spine MRI was obtained 3 months after the resection that revealed postoperative seroma at the resection site and improved mass effect (Fig. 5). His left side lumbar hardware remains intact without any evidence of any metallosis reaction.
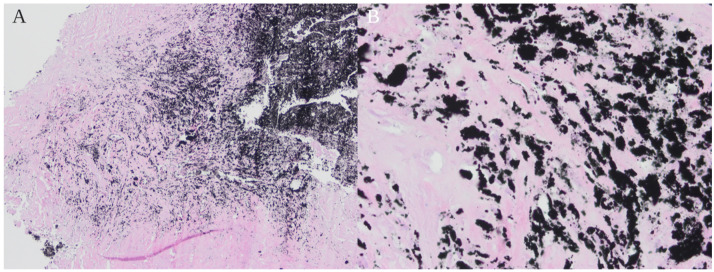
Fig. 4.
Hematoxylin and eosin-stained slides from resection of paraspinal area soft tissue mass. (A) Dense foreign black pigment material and adjacent fibrous tissue which comprised most of the specimen (40x magnification). Rare areas showed lymphohistiocytic reaction with giant cell formation, these were not immediately adjacent to the foreign material. (B) Dense foreign pigment material and adjacent fibrosis (400x magnification).
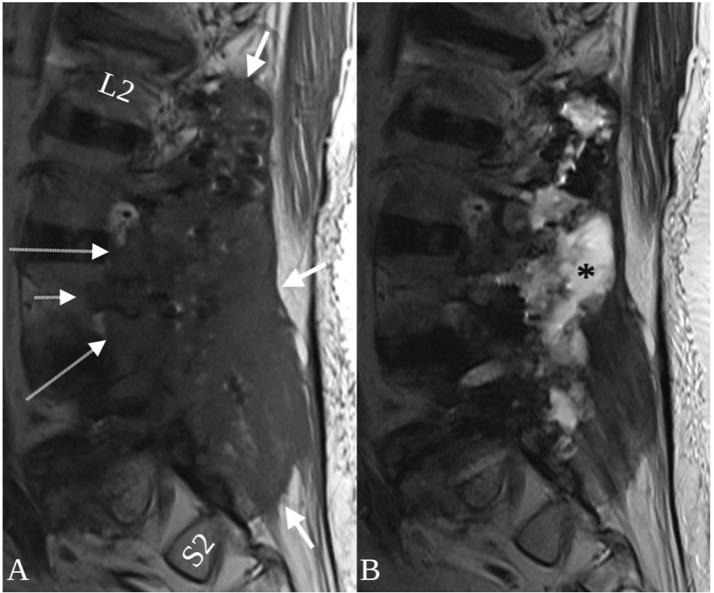
Fig. 5.
Sagittal T1 (A) and T2-weighted MRI (B) following resection of the metalloma and hardware explantation. The lesion is longitudinally extensive, spanning the paraspinal space of L2-S2 (A, thick arrows) with extension into neural foramina (A, long arrows) and a pedicle (A, short arrow). A T2 hyperintense post-operative fluid collection occupies the resection cavity (B, asterisk) and pedicle screw removal sites.
At telemedicine follow-up 3 months after surgery, the patient was subjectively “feeling better.” He was able to wean off his walker and was ambulating with a cane. He reported his dysesthesias were improved, though he continued to endorse leg weakness, worst on the right. Telemedicine follow-up and physical therapy notes 7 months from surgery reported continued improvements in leg strength and ambulation. Spine X-rays at this time revealed the remaining left sided construct with no abnormal findings. Future follow up with continued surveillance imaging is planned.
Literature review
A systematic PubMed and/or Medline literature search was performed using the algorithm (“spine” OR “spinal” AND “metalloma” OR “metallosis” AND “instrumentation”) through July 2020. We reviewed the search results for spinal instrumentation cases resulting in neurologic deficit from metalloma. Six manuscripts were identified that detailed 7 cases (Table 1) [2,3,[33], [34], [35], [36]]. We recorded patient age and sex, levels instrumented, material of instrumentation, type of instrumentation construct, and time to develop metalloma. We excluded cases that were associated with infection due to infection being a confounding factor of reoperation, pseudoarthrosis, and mass effect.
Table 1.
Case reports of spinal instrumentation associated metallomas creating neurologic deficits.
| Author; year | Age (years), Sex | Location | Metal | Instrumentation | Time to Develop |
|---|---|---|---|---|---|
| Takahashi, et al.; 200134 | 58, F | T10-L3 | Stainless steel | Sublaminar hooks | 11 mo |
| Takahashi, et al.; 200134 | 54, F | T12-L4 | Stainless steel | Sublaminar hooks | 4 y |
| Tezer, et al.; 200535 | 57, M | Unspecified thoracic | Stainless steel | Pedicle screw-hook combination | 3 y |
| Fernandez-Baillo, et al.; 20123 | 46, M | L4-5 | Titanium | Threaded cylindrical cages | 4 y |
| Li, et al.; 201636 | 58, M | L4-5 | Titanium | Pedicle screw | 2 y |
| Goldenberg, et al.; 20162 | 75, M | L4-5 | Titanium | Pedicle screw | 18 mo |
| Richman, et al.; 201733 | 19, M | T4-L1 | Stainless steel | Pedicle screw | 4 y |
| Mazur-Hart, et al.; 2021 | 79, M | L2-S1 | Titanium and cobalt chrome | Pedicle screw | 7 mo |
Discussion
Metallomas associated with spinal instrumentation are rare. As seen in our case, they can be especially harmful due to the risk of mass effect resulting in a neurologic deficit. Early identification for the removal of the offending hardware was paramount for a successful outcome. A systematic review of the literature identified 7 similar cases.
Takahashi, et al. described 2 cases of metallosis involving stainless steel instrumentation [34] Both patients had stainless steel instrumentation for thoracolumbar degenerative scoliosis with the use of sublaminar hooks. Subsequently, both patients presented with recurrent radiculopathy, and were found intraoperatively to have associated loosening of hardware. Neither patient had an MRI. Both patients underwent resection of their metallomas with extension of fusion, and ultimately had improvements in their radiculopathies.
A case report by Fernandez-Baillo, et al. reported one case of metalloma associated with titanium instrumentation [3]. Two threaded interbody cages were placed at L4-5 without posterior instrumentation. The patient re-presented with severe claudication and was found to have severe central stenosis. They provided a sagittal T2 weighted image with a hypointense mass near the operative site. Upon surgical exploration, a mass was excised, and the cages were found to be loose. The cages were removed and a circumferential fusion was performed with resolution of symptoms.
Tezer, et al. described a case of a metalloma associated with stainless steel hardware [35]. The case involved a pedicle screw-hook combination system for a traumatic T8-9 fracture. The patient presented with progressive paraparesis approximately 3 years later. Spine X-rays identified migration of a pedicle hook. Spine CT myelogram identified a focal compressive mass. MRI was not obtained. On surgical exploration, osseous fusion had been attained and the hardware was removed completely and the metalloma mass excised.
In the review by Goldenberg, et al. a metalloma case was reported with no evidence of hardware failure[2]. This case report was the second to document formation of a metalloma with a metal other than stainless steel. The patient had a metalloma associated with titanium pedicle screws and rods. The case was unique in that there was no hardware migration, loosening, or failure found on intraoperative exploration of the construct. Again, unfortunately, no MRI was able to be obtained. The patient underwent debulking of the mass with improvements in back pain and radiculopathy.
Richman, et al. has described another metalloma case without hardware failure resulting in neurologic deficit [33]. This patient presented after stainless steel pedicle screw and rod instrumentation with progressive back pain and lower extremity paresthesias. Spine imaging revealed a mass around 1 pedicle screw with cavitation of the bone. Again, no MRI was performed. During operative exploration, there was no evidence of screw loosening or hardware failure. The mass was debulked with the offending screw removed. The patient developed flaccid paralysis over the next 48 hours, which resulted in a return to the operating room for further decompression and complete hardware explant. The authors reported improvements in patient strength before discharge and a full recovery of strength at later follow up [33].
Li, et al. described a patient that returned after L4-5 fusion with recurrence of preoperative symptoms (back pain, sciatica, and neurogenic claudication) [36]. MRI revaled adjacent segment stenosis and extradural mass for which he was taken for extension of fusion. Intraoperatively, a small soft tissue metalloma mass was found at the adjacent level following instrumentation, and decompression. The patient was discharged after 5 days.
We add to the literature a case of a patient with a similar progressive back and leg pain, claudication, and lower extremity weakness. Spine imaging revealed a unilateral large paraspinal mass intimately associated with the hardware, specifically the right side of the construct with the mass centered around the rod with associated extension into the canal, neural foramen, and pelvis. The hardware used was both cobalt chrome and titanium. Upon surgical exploration, there was no evidence of screw loosening, hardware failure, or pseudoarthrosis. The hardware involved was completely removed on the right side and the metalloma mass debulked. Postoperatively the patient's pain and weakness both improved. Interestingly, the same titanium and cobalt materials on the left side, his hip arthroplasty containing titanium and cobalt, and his anterior L5-S1 fusion have shown no such reaction.
These 8 cases describe the current extent of the reported literature on spinal metallomas resulting in a neurologic deficit (Table 1). None of the cases involved the cervical spine. None were found before 6 months post-operatively. Three of the cases were associated with pseudoarthrosis, four had solid fusion contructs, and one was unreported. Four cases were stainless steel constructs, 2 were titanium, one was a mix of titanium and cobalt chrome, and one was unreported. No MRI was completed in 5 of the 8 cases and a single mid-sagittal view of an MRI is provided in 2 cases.
Descriptions in the literature of imaging findings of spinal fusion-related metallosis and pseudotumors and/or metallomas are scant. This is likely due to rarity and due to hardware-related artifact that often obscures the region of interest on CT and MR images. Fortunately, in the case presented here, the large metalloma size allowed accurate characterization of the lesion on MR imaging. The metalloma in this case was a unique mix of both benign and aggressive imaging features. Trans-spatial involvement is a feature most commonly observed in aggressive processes such as malignancy and infection. In the featured case, the lesion was centered around the fusion construct in the paraspinal space and the associated T1 hypointense signal abnormality extended into adjacent bones (pedicles, iliac bone, sacrum) and soft tissues (epidural space, extraforaminal space, retroperitoneal space and/or psoas muscle), with seemingly no hinderance posed by the fascial planes that normally separate theses spaces (Fig. 1, Fig. 2). Mass effect is another feature concerning for an aggressive process that was seen in this case. However, the lesion appeared completely non–enhancing on post-contrast T1-weighted imaging – a feature more commonly associated with non–aggressive processes (Fig. 1). The combination of trans-spatial involvement and lack of contrast-enhancement may be a finding unique to metallomas. Additional imaging clues to the diagnosis in this case are the location of the lesion, which surrounded the fusion construct, and the T1 and T2 isointensity to skeletal muscle, which has been described with metallosis and pseudotumors involving metal-on-metal hip arthroplasties and may be related to T2 gradient recalled echo (T2 GRE or T2*-weighted imaging) effects of small metal particles [37].
Early detection and intervention are important with removal of the offending instrumentation before further metalloma growth occurs. The growth rate for metallomas remains unknown. No cases have shown progression following surgical intervention and removal of the offending hardware. Previous theories have considered abnormal movement and/or micro-instability to be a trigger for the body's immune response and subsequent metalloma formation, though this has not been examined in detail. A systemic immune response would be unlikely to cause a unilateral metallosis reaction. Our review of the literature identified the majority of previously reported cases have been associated with stainless steel, but more recent reports have shown titanium can also be a culprit metal [2,3,[33], [34], [35], [36]].
The case presented here is the first reported metalloma from mixed-metal spinal instrumentation and is the first reported case with implanted cobalt chrome producing a neurologic deficit. Cobalt chrome spinal instrumentation has been reported in metallosis, but not for a metalloma creating neurologic deficit [4]. Of note, most previously reported cases have been associated with some type of hardware failure or failed fusion. Here, we report only the fourth case of a stable fusion construct with formation of a metalloma causing neurologic deficit. Additionally, we are reporting the second unilateral metalloma reaction from posterior spinal instrumentation. Finally, we are providing a thorough radiographic description of metalloma to aid in future diagnosis. Continued reporting of these rare cases is necessary to obtain a better understanding of the clinical presentation, radiographic findings, and clinical management including the surgical removal of the offending hardware.
Limitations
Limitations include those inherently associated with case reports, which includes a single case with associated bias, and retrospective design. Additional limitations include a short term follow up of 7 months.
Conclusions
The overall pathogenesis of metallosis and metalloma remains poorly understood. Hopefully, as more cases arise and are reported, more robust material science and immunologic studies will be conducted to identify an inflammatory molecule, a particular at-risk patient profile, and/or a particular metal coating antigen that would reduce or eliminate the morbidity related to metalloma formation. We add to the body of literature on spinal metallomas with a detailed radiographic description to aid in diagnosis of this rare disease.
Funding
Not Applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Ethics approval
Institutional Review Board approval is no required for less than 3 cases.
Consent to participate
Patient provided appropriate standard of care surgical consent.
Consent for publication
Patient signed an Authorization to Use and Disclose Protected Health Information.
Patient consent
The patient signed an institutional authorization to use and disclose protected health information. This can be made available upon request. Ethics approval Institutional Review Board approval is no required for less than 3 cases. Consent to participate Patient provided appropriate standard of care surgical consent for publication Patient signed an Authorization to Use and Disclose Protected Health Information.
Footnotes
Acknowledgments: The authors thank Shirley McCartney, PhD, (Oregon Health & Science University) for editorial assistance.
Competing Interests: Not Applicable.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.radcr.2022.01.041.
Appendix. Supplementary materials
References
- 1.Rony L, Lancigu R, Hubert L. Intraosseous metal implants in orthopedics: a review. Morphologie. 2018;102(339):231–242. doi: 10.1016/j.morpho.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Goldenberg Y, Tee JW, Salinas-La Rosa CM, Murphy M. Spinal metallosis: a systematic review. Eur Spine J. 2016;25(5):1467–1473. doi: 10.1007/s00586-015-4347-6. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez-Baillo N, Sanchez Marquez JM, Conde Gallego E, Martin Esteban A. Intraspinal metalloma causing lumbar stenosis after interbody fusion with cylindrical titanium cages. Acta Orthop Belg. 2012;78(6):811–814. [PubMed] [Google Scholar]
- 4.Ayers R, Miller M, Schowinsky J, Burger E, Patel V, Kleck C. Three cases of metallosis associated with spine instrumentation. J Mater Sci Mater Med. 2017;29(1):1–9. doi: 10.1007/s10856-017-6011-7. [DOI] [PubMed] [Google Scholar]
- 5.D'Angelo F, Tanas D, Gallazzi E, Zagra L. Adverse reaction to metal debris after small-head diameter metal-on-metal total hip arthroplasty: an increasing concern. Hip Int. 2018;28(2_suppl):35–42. doi: 10.1177/1120700018812993. [DOI] [PubMed] [Google Scholar]
- 6.Cipriano CA, Issack PS, Beksac B, Della Valle AG, Sculco TP, Salvati EA. Metallosis after metal-on-polyethylene total hip arthroplasty. Am J Orthop (Belle Mead NJ) 2008;37(2):E18–E25. [PubMed] [Google Scholar]
- 7.Nunez FA, Jr., Wright L, Kilpatrick SE, Seitz WH., Jr. Revision total wrist arthroplasty due to polyethylene wear, metallosis-induced carpal tunnel syndrome, distal ulnar impingement, and fourth carpometacarpal joint pain: case report and pitfalls to avoid. Hand. 2020;15(1):np1–np6. doi: 10.1177/1558944718810863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salem KH, Lindner N, Tingart M, Elmoghazy AD. Severe metallosis-related osteolysis as a cause of failure after total knee replacement. J Clin Orthop Trauma. 2020;11(1):165–170. doi: 10.1016/j.jcot.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sebecić B, Japjec M, Dojcinović B, Zgaljardić I, Staresinić M. Aggressive granulomatosis after cementless total hip arthroplasty as a result of inflammatory reaction to metal debris: case report. Acta Clin Croat. 2013;52(4):492–496. [PubMed] [Google Scholar]
- 10.Korovessis P, Petsinis G, Repanti M, Repantis T. Metallosis after contemporary metal-on-metal total hip arthroplasty. Five to nine-year follow-up. J Bone Joint Surg Am. 2006;88(6):1183–1191. doi: 10.2106/JBJS.D.02916. [DOI] [PubMed] [Google Scholar]
- 11.Hallab NJ, Jacobs JJ. Biologic effects of implant debris. Bull NYU Hosp Jt Dis. 2009;67(2):182–188. [PubMed] [Google Scholar]
- 12.Lee H, Phillips JB, Hall RM, Tipper JL. Neural cell responses to wear debris from metal-on-metal total disc replacements. Eur Spine J. 2020;29(11):2701–2712. doi: 10.1007/s00586-019-06177-w. [DOI] [PubMed] [Google Scholar]
- 13.Magone K, Luckenbill D, Goswami T. Metal ions as inflammatory initiators of osteolysis. Arch Orthop Trauma Surg. 2015;135(5):683–695. doi: 10.1007/s00402-015-2196-8. [DOI] [PubMed] [Google Scholar]
- 14.Polyzois I, Nikolopoulos D, Michos I, Patsouris E, Theocharis S. Local and systemic toxicity of nanoscale debris particles in total hip arthroplasty. J Appl Toxicol. 2012;32(4):255–269. doi: 10.1002/jat.2729. [DOI] [PubMed] [Google Scholar]
- 15.del Rio J, Beguiristain J, Duart J. Metal levels in corrosion of spinal implants. Eur Spine J. 2007;16(7):1055–1061. doi: 10.1007/s00586-007-0311-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim YJ, Kassab F, Berven SH, Zurakowski D, Hresko MT, Emans JB, et al. Serum levels of nickel and chromium after instrumented posterior spinal arthrodesis. Spine (Phila Pa 1976) 2005;30(8):923–926. doi: 10.1097/01.brs.0000158872.42802.be. [DOI] [PubMed] [Google Scholar]
- 17.Arnholt CM, White JB, Lowell JA, Perkins MR, Mihalko WM, Kurtz SM. Postmortem retrieval analysis of metallosis and periprosthetic tissue metal concentrations in total knee arthroplasty. J Arthroplasty. 2020;35(2):569–578. doi: 10.1016/j.arth.2019.08.038. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs JJ, Gilbert JL, Urban RM. Corrosion of metal orthopaedic implants. J Bone Joint Surg. 1998;80(2):268–282. doi: 10.2106/00004623-199802000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Senaran H, Atilla P, Kaymaz F, Acaroglu E, Surat A. Ultrastructural analysis of metallic debris and tissue reaction around spinal implants in patients with late operative site pain. Spine (Phila Pa 1976) 2004;29(15):1618–1623. doi: 10.1097/01.brs.0000133646.40087.8b. [DOI] [PubMed] [Google Scholar]
- 20.Gristina AG. Implant failure and the immuno-incompetent fibro-inflammatory zone. Clin Orthop Relat Res. 1994;298:106–118. PMID: 8118964. [PubMed] [Google Scholar]
- 21.Towers WS, Kurtom K. Rare systemic response to titanium spinal fusion implant: case report. Cureus. 2020;12(2):e7109. doi: 10.7759/cureus.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J. A rare case of delayed hypersensitivity reaction to metal ions secondary to a remnant pedicle screw fragment after spinal arthrodesis. Acta Orthop Traumatol Turc. 2020;54(4):461–464. doi: 10.5152/j.aott.2020.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi C, Xi Y, Sun B, He H, Wen J, Ruan Y, et al. Suspected allergy to titanium after anterior cervical discectomy and fusion using a Zero-P device: a case report. Br J Neurosurg. 2020:1–5. doi: 10.1080/02688697.2020.1718605. PMID: 32003246. [DOI] [PubMed] [Google Scholar]
- 24.Shang X, Wang L, Kou D, Jia X, Yang X, Zhang M, et al. Metal hypersensitivity in patient with posterior lumbar spine fusion: a case report and its literature review. BMC Musculoskelet Disord. 2014;15:314. doi: 10.1186/1471-2474-15-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curley KL, Krishna C, Maiti TK, McClendon J, Jr., Bendok BR. Metal hypersensitivity after spinal instrumentation: when to suspect and how to treat. World Neurosurg. 2020;139:471–477. doi: 10.1016/j.wneu.2020.04.093. [DOI] [PubMed] [Google Scholar]
- 26.Golish SR, Anderson PA. Bearing surfaces for total disc arthroplasty: metal-on-metal versus metal-on-polyethylene and other biomaterials. Spine J. 2012;12(8):693–701. doi: 10.1016/j.spinee.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 27.Harris L, Dyson E, Elliot M, Peterson D, Ulbricht C, Casey A. Delayed periprosthetic collection after cervical disc arthroplasty. J Neurosurg Spine. 2019;32(4):584–591. doi: 10.3171/2019.9.SPINE19900. [DOI] [PubMed] [Google Scholar]
- 28.Zairi F, Remacle JM, Allaoui M, Assaker R. Delayed hypersensitivity reaction caused by metal-on-metal total disc replacement. J Neurosurg Spine. 2013;19(3):389–391. doi: 10.3171/2013.6.SPINE121010. [DOI] [PubMed] [Google Scholar]
- 29.Guyer RD, Shellock J, MacLennan B, Hanscom D, Knight RQ, McCombe P, et al. Early failure of metal-on-metal artificial disc prostheses associated with lymphocytic reaction: diagnosis and treatment experience in four cases. Spine (Phila Pa 1976) 2011;36(7):E492–E497. doi: 10.1097/BRS.0b013e31820ea9a2. [DOI] [PubMed] [Google Scholar]
- 30.Goodwin ML, Spiker WR, Brodke DS, Lawrence BD. Failure of facet replacement system with metal-on-metal bearing surface and subsequent discovery of cobalt allergy: report of 2 cases. J Neurosurg Spine. 2018;29(1):81–84. doi: 10.3171/2017.10.SPINE17862. [DOI] [PubMed] [Google Scholar]
- 31.Veruva SY, Lanman TH, Isaza JE, Freeman TA, Kurtz SM, Steinbeck MJ. Periprosthetic UHMWPE wear debris induces inflammation, vascularization, and innervation after total disc replacement in the lumbar spine. Clin Orthop Relat Res. 2017;475(5):1369–1381. doi: 10.1007/s11999-016-4996-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang CC, Tang CL, Tzeng CY, Tsou HK. Metallosis after traumatic loosening of Bryan cervical disc arthroplasty: a case report and literature review. Eur Spine J. 2018;27(Suppl 3):415–420. doi: 10.1007/s00586-017-5397-8. [DOI] [PubMed] [Google Scholar]
- 33.Richman SH, Razzano AJ, Morscher MA, Riley PM. Metallosis presenting as a progressive neurologic deficit four years after a posterior spinal fusion for adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2017;42(1):E56–EE9. doi: 10.1097/BRS.0000000000001685. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi S, Delécrin J, Passuti N. Intraspinal metallosis causing delayed neurologic symptoms after spinal instrumentation surgery. Spine (Phila Pa 1976) 2001;26(13):1495–1498. doi: 10.1097/00007632-200107010-00024. [DOI] [PubMed] [Google Scholar]
- 35.Tezer M, Kuzgun U, Hamzaoglu A, Ozturk C, Kabukcuoglu F, Sirvanci M. Intraspinal metalloma resulting in late paraparesis. Arch Orthop Trauma Surg. 2005;125(6):417–421. doi: 10.1007/s00402-005-0802-x. [DOI] [PubMed] [Google Scholar]
- 36.Li YC, Yang SC, Hsu CT, Tu YK. Capsulated metallic debris tumor mass mimicking adjacent segment disease: a case report. Clin Spine Surg. 2016;29(10):E532–E5e5. doi: 10.1097/BSD.0b013e318292e685. [DOI] [PubMed] [Google Scholar]
- 37.Davis DL, Morrison JJ. Hip arthroplasty pseudotumors: pathogenesis, imaging, and clinical decision making. J Clin Imaging Sci. 2016;6(2):17. doi: 10.4103/2156-7514.181493. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.