Abstract
As per the classical view of the coagulation system, it functions solely in plasma to maintain hemostasis. An experimental approach modeling vascular reconstitution was used to show that vascular endothelial cells (ECs) endogenously synthesize coagulation factors during angiogenesis. Intracellular thrombin generated from this synthesis promotes the mitotic function of vascular endothelial cell growth factor A (VEGF-A). The thrombin concurrently cleaves C5a from EC-synthesized complement component C5 and unmasks the tethered ligand for EC-expressed protease-activated receptor 4 (PAR4). The two ligands jointly trigger EC C5a receptor-1 (C5ar1) and PAR4 signaling, which together promote VEGF receptor 2 growth signaling. C5ar1 is functionally associated with PAR4, enabling C5a or thrombin to elicit Gαi and/or Gαq signaling. EC coagulation factor and EC complement component synthesis concurrently down-regulate with contact inhibition. The connection of these processes with VEGF receptor 2 signaling provides new insights into mechanisms underlying angiogenesis. Knowledge of endogenous coagulation factor/complement component synthesis and joint PAR4/C5ar1 signaling could be applied to other cell types.
The complement and coagulation systems have been envisioned as residing exclusively in the plasma, deriving solely from the liver, and functioning independently of each other in innate immunity and in hemostasis, respectively. Because of this, although effects of complement activation fragments on the coagulation cascade and vice versa have been reported, they have been attributed to cross talk and regarded as being modulatory rather than integral to one pathway or the other.1 For example, although the immediate response to trauma includes both proteolysis of fibrinogen to form fibrin and complement activation, the two processes have been regarded as being unconnected and serving unrelated purposes.
Both coagulation and complement system products exert effects on vascular endothelial cells (ECs). Thrombin is connected to EC tissue factor production and procoagulant surface protein expression during coagulation.2, 3, 4, 5 Complement activation fragments C3a and C5a are linked to EC integrin expression and proinflammatory cytokine production during inflammation.5 Thrombin confers its modulatory effects on ECs via protease-activated receptors (PARs). Its cleavage of the N-termini of these G-protein–coupled receptors (GPCRs) unmasks their tethered ligands, thereby inducing their signaling. Four PAR family members (PAR1 to PAR4) exist, and PAR1 and PAR4, which frequently function cooperatively, are expressed on ECs.2,3,6,7 In addition to their expression on ECs, PARs are widely expressed on other cell types and implicated in many cell functions.5 Although C5a receptor-1 (C5ar1) was long thought to be strictly limited to myeloid phagocytic cells,8, 9, 10 it now is known to function in adaptive immune cells11, 12, 13, 14 as well as other cell types.15 Recent studies16 have shown that EC C5 synthesis and C5a production are interconnected with vascular endothelial cell growth factor A (VEGF-A) function. Thrombin, however, has been regarded as functioning solely in the context of coagulation in plasma.
Thrombin is generated from prothrombin in a complex termed prothrombinase that is produced by factor Va binding to factor Xa and factor II (FII) on the EC surface. Factor Xa generation, leading to the assembly of prothrombinase, is induced by the actions of factor IXa and factor VIIIa or factor VIIa and tissue factor. These processes are tightly regulated by homeostasis. The same is true for assembly of the canonical C5 convertases (C4b2a3b and C3bBbC3b) that gives rise to C5a. A previous study17 linked plasma C5a generation with thrombin in a mouse model of immune complex–mediated lung injury. Although thrombin cleaved purified complement component C5 to C5a in vitro, the authors proposed that the process constituted a compensatory mechanism for C5 cleavage connected with increased thrombin generation in plasma during disease. A role for this process in a nonhemostatic physiological process was not proposed.
C5a was recently linked with physiological VEGF-A growth induction. However, based on prior immune cell activation studies, it was theorized to be generated by the conventional alternative pathway C5 convertase C3bBbC3b. These studies demonstrated that interacting dendritic cell–CD4+ cell partners each generate C5a (and C3a) from complement components that are endogenously synthesized by both cell types,12, 14 and that the GPCR signaling plays an integral role in shaping the T-cell response. Analogous to the findings in immune cells, the studies of C5a production by ECs showed that autocrine C5ar1 signaling plays a requisite role in vascular endothelial cell growth factor receptor 2 (VEGFR2) mitotic signaling. It promotes VEGFR2 autophosphorylation, downstream growth signaling via the canonical VEGFR2 cascades, and EC transition through the cell cycle.
The findings of local complement production in ECs16 together with the observation17 that thrombin can cleave C5a from C5 in vitro prompted the hypothesis that ECs might endogenously produce coagulation factors in addition to complement components, and that EC produced thrombin might participate in EC C5a generation. Therefore, this study investigated whether endogenous thrombin generation, if locally produced within ECs, might be mechanistically linked with endogenous C5a generation in ECs, and whether these linkages might participate in EC proliferation and angiogenesis.
Materials and Methods
Cells, Antibodies, and Reagents
The sources of cells and major reagents used in each assay are given with each assay method. All cells and reagents used are listed together in detail in the major resource table (Table 1).
Table 1.
Major Resources
| Animals (in vivo studies) | |||
|---|---|---|---|
| Species | Vendor or source | Background strain | Sex |
| Mouse | Jackson Laboratories, Bar Harbor, ME | C57BL/6 | M/F |
| Mouse | C. Gerard, Children's Hospital, Boston, MA | C57BL/6, C5ar1−/− | M/F |
| Mouse | Jackson Laboratories | PAR1−/− | M/F |
| Mouse | Mutant Mouse Resource & Research Centers, University of California, Davis, CA | PAR3−/−PAR4−/− | M/F |
| Animal breeding | ||||
|---|---|---|---|---|
| Parent | Species | Vendor or source | Background strain | Other information |
| M | Mouse | Jackson Laboratories | C57BL/6 | |
| F | Mouse | Jackson Laboratories | C57BL/6 | |
| M | Mouse | C. Gerard | BALB/c, C5ar1−/− | Crossbred for 12 generations |
| F | Mouse | C. Gerard | C57BL/6 | Crossbred for 12 generations |
| M | Mouse | C. Gerard | C57BL/6, C5ar1−/− | |
| F | Mouse | C. Gerard | C57BL/6, C5ar1−/− | |
| M | Mouse | Mutant Mouse Resource & Research Centers | C57BL/6, PAR1−/− | |
| F | Mouse | Mutant Mouse Resource & Research Centers | C57BL/6, PAR1−/− | |
| M | Mouse | Mutant Mouse Resource & Research Centers | C57BL/6, PAR3−/−,PAR4−/− | |
| F | Mouse | Mutant Mouse Resource & Research Centers | C57BL/6, PAR3−/−,PAR4−/− | |
| Antibodies | ||||
|---|---|---|---|---|
| Target antigen | Vendor or source | Catalog no. | Working concentration | Lot no. (preferred but not required) |
| Human/mouse FII | ThermoFisher, Carlsbad, CA | PA1-43040 | 1 μmol/mL | SI2444095 |
| Mouse FV | Bioss, Woburn, MA | Bs-1040R | 5 μg/mL | AD121701 |
| Mouse FVII | Bioss | Bs-4846R | 5 μg/mL | YE1022W |
| Mouse FX | Bioss | Bs-9501R | 5 μg/mL | 9B26M7 |
| Human/mouse C5aR | Santa Cruz Biotechnologies, Dallas, TX | SC-53795 PE | 2 μg/mL | C1810 |
| Mouse C5a | BD Biosciences, Franklin Lakes, NJ | 558027 | 5 μg/mL | |
| Human C5a | R&D Systems, Minneapolis, MN | AF2037 | 5 μg/mL | |
| Human thrombin | ThermoFisher | HYB 109-04-02 | 5 μg/mL | UI2843443 |
| Mouse/human PAR1 | BD Biosciences | 611522 | 5 μg/mL | 6013888 |
| Mouse/human PAR4 | Abcam, Cambridge, UK | Ab5787 | 5 μg/mL | GR267332-5 |
| Human/mouse VEGFR2 | Cell Signalling Technologies, Danvers, MA | 2479 | 0.1 μg/mL | 18 |
| Reagents | ||||
|---|---|---|---|---|
| Reagent | Vendor or source | Catalog no. | Working concentration | Lot no. (preferred but not required) |
| C5a receptor antagonist, W-54011 | Calbiochem, Torrey Pines, CA | 234415 | 10 ng/mL | |
| Vorapaxar (PAR1 antagonist) | Axon Medchem, Reston, VA | 1755 | Batch no. 2 | |
| TFLLRN (PAR1 agonist) | Tocris Biosciences, Minneapolis, MN | 1464 | 0.01–100 ng/mL | |
| AY-NH2 (PAR4 agonist) | Tocris Biosciences | 1487 | 0.001–10 ng/mL | 10A |
| Bivalirudin trifluoroacetate salt | Sigma, St. Louis, MO | SML1051 | 25 μg/mL | 064M4731V |
| AS252424 (PI-3Kγ inhibitor) | Tocris Biosciences | 3671 | 10 μmol/mL | 2A/153705 |
| SU5416 (VEGFR2 antagonist) | Tocris Biosciences | 3037 | 10 μmol/mL | |
| Human plasma | |
|---|---|
| Plasma | Source |
| Plasma deficient in FII | George King Biomedical, Overland Park, KS |
| Plasma deficient in FV | George King Biomedical |
| Plasma deficient in FVII | George King Biomedical |
| Plasma deficient in FX | George King Biomedical |
| siRNA | ||||
|---|---|---|---|---|
| Target | Vendor or source | Catalog no. | ID | Working concentration, nmol/L |
| Human PAR1 | ThermoFisher | AM16708 | 146259 | 30 |
| Human PAR4 | ThermoFisher | 4392420 | s194972 | 30 |
| Cultured cells | ||
|---|---|---|
| Name | Vendor or source | Sex (F, M, or unknown) |
| bEnd.3 | ATCC, Manassas, VA | Unknown |
| Primary aortic endothelial cells; normal, human (HAECs) | ATCC | Unknown |
| HUV-EC-C (HUVECs) | ATCC | Unknown |
| 293 (HEK-293) | ATCC | Unknown |
F, female; M, male; C5ar1, C5a receptor-1; FII, factor II; FV, factor V; FVII, factor VII; FX, factor X; HAEC, human aortic endothelial cell; HUVEC, human umbilical vein endothelial cell; ID, identifier; PAR, protease-activated receptor; VEGFR2, vascular endothelial cell growth factor receptor 2.
Growth Assays and Real-Time Quantitative PCR
Murine bEnd 3 and NIH-3T3 cell lines were maintained in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum. Human umbilical vein endothelial cells (HUVECs) and human aortic endothelial cells (HAECs) were maintained in EBM-2 media containing BulletKit (Lonza, Basel, Switzerland) without added vitamin K. Except where indicated, cells were seeded at 2.5 × 104 per well in 24-well plates in complete media, after which they were transferred to media containing 0.5% serum and treated as described in the figure legends. The concentrations of C5a, C5ar1-A,12,14,16,18 thrombin,19, 20, 21 and PAR1 and PAR4 agonists and antagonists22,23 were chosen on the basis of previous studies and the manufacturer's directions. Cell numbers were counted manually. Real-time quantitative PCR was performed as described.12 Data are given as fold increases relative to basal levels corrected for actin or glyceraldehyde-3-phosphate dehydrogenase.
Coagulation Assays
A total of 100 μL of culture supernatant was incubated at 37°C for 5 minutes with 100 μL of plasma deficient in FII, factor V (FV), factor VII (FVII), or factor X (FX; George King Biomedical, Overland Park, KS). A total of 100 μL of Phospoplastin RL (R2 Diagnostics, South Bend, IN) was then added, and time to coagulation was measured while continuously mixing. Percentage clotting activity corresponds to the dilution of pooled human plasma (George King Biomedical), yielding the same clotting time.
Enzyme-Linked Immunosorbent Assays and Flow Cytometry
Enzyme-linked immunosorbent assays for C5a and flow cytometry for phosphorylated STAT3 were performed as described.12 Intracellular staining was performed following permeabilization, as in past studies,24 and the intracellular signal was calculated following subtraction of the extracellular signal.
GSK-3β Assay
The assay was performed using the glycogen synthase kinase (GSK)-3β Activity Assay kit (Sigma-Aldrich, St. Louis, MO; catalog number CS0990), per the manufacturer's protocol.
Sprouting Assay
Thoracic aortae were obtained from 8- to 14-week–old mice following perfusion with Ringer’s solution. Following removal of fibroadipose tissue, approximately 1-mm segments were suspended in a 6-mm petri dish containing complete EGM-2 (Lonza) with reduced growth factor Matrigel (Corning, Tewskbury, MA). Aortic ring sprouts were imaged on days 6 to 8, and sprout areas were determined by morphometric analysis using MetaMorph (Molecular Devices, San Jose, CA) and a Leica DMI6000 microscope (Wetzlar, Germany).
Matrigel Plug Angiogenesis Assay
Reduced growth factor Matrigel (as used in sprouting assays; 500 μL) supplemented with heparin (60 U/mL) and VEGF-A (300 ng/mL) was injected subcutaneously into the lower abdominal flank of anesthetized 8- to 14-week–old wild-type (WT) C57BL/6 mice. Ten days after injection, recovered Matrigel plugs were fixed in formalin, embedded in OCT compound, and sectioned. Sections were stained for different coagulation factors [anti-FII (Aviva Systems Biology Corp., San Diego, CA; ARP41757-P050; lot number QC12431), anti-FV (Bioss, Woburn, MA; bs-1040R; lot number AD121701), anti-FVII (Bioss; bs-4846R; lot number YE1022W), or anti-FX (Bioss; bs-9501R; lot number 9B26M7)] followed by AF546-labeled anti-rabbit. After coagulation protein staining, cells were counterstained with AF488-labeled anti-CD31 (clone 390; BD Biosciences, East Rutherford, NJ), mounted in ProLong Gold anitfade reagent with DAPI (Molecular Probes, Eugene, OR; catalog number P36935), and visualized by confocal microscopy. Images were collected using a 63× (1.4 numerical aperture) oil immersion objective on a Leica HyVolution SP8 inverted confocal microscope. Resulting images were subjected to deconvolution using Hyugens Professional (Hilversum, The Netherlands).
Confocal Microscopy
For C5ar1 and PAR4 colocalization assays, bEnd.3 cells were grown overnight on ibiTreat 8 chamber μ-Slides (ibidi, Martinsried, Germany). The slides were washed three times with 1× phosphate-buffered saline and fixed with 2% formaldehyde. Cells were stained with a cocktail containing 2 μg/mL of each of anti–C5ar1-AF488 (Abd Serotec, Oxford, UK) and anti–Par4-AF546 (Abcam, Cambridge, UK) for 1 hour. For Matrigel plug assays, plugs were implanted into the inner thighs of WT mice for 10 days. Plugs were harvested, embedded in OCT compound, and sectioned. Sections were stained with anti-FII, anti-FV, anti-FVII, or anti-FX, followed by AF546-labeled anti-rabbit secondary antibody (Ab). Cells were counterstained with anti-CD31 (green). Nuclei were visualized with DAPI (blue). For coagulation factor staining in confluent versus nonconfluent cells, HUVECs were grown to 70% and 100% confluence, fixed with 2% formaldehyde, and blocked with 5% horse serum in phosphate-buffered saline. Cells were stained with anti-FII followed by AF546-labeled anti-rabbit secondary Ab.
All images were collected using a 63× (1.4 numerical aperture) oil immersion objective on a Leica HyVolution SP8 inverted confocal microscope. Resulting images were subjected to deconvolution using Hyugens Professional.
Co-Immunoprecipitation Assays
Cells were lysed in 1× Cell Lysis Buffer (10×; Cell Signaling, Danvers, MA); catalog number 9803) supplemented with 1 mmol/L phenylmethylsulfonyl fluoride and 1 Complete Mini protease inhibitor tablet (Roche, Boston, MA; catalog number 11836153001) for 10 minutes on ice (1 mmol/L Na orthovanadate was used to inhibit phosphatase activity when lysates were to be assayed for kinase activity). Lysates were then sonicated three times for 2 minutes to break apart nucleic acids, after which the cells were centrifuged for 10 minutes at 12,000 × g. Clean supernatants were transferred to new tubes and incubated with Protein-A/G beads (Santa Cruz Biotechnology, Dallas, TX) to preclear the lysates and prevent nonspecific co-immunoprecipitation. Abs against PAR4, C5ar1, GSK-β, and CD59 were added, and samples were incubated overnight at 4°C. Protein-A/G beads were used to pull down Ab, and immunoprecipitations were assayed by Western blot analysis, enzyme-linked immunosorbent assay, or protein activity via kits, as described.
BRET Data
Bioluminescence resonance energy transfer (BRET) analyses were performed as described.25 Briefly, HEK293 cells (1 × 105) were transfected with 0.5 μg of Par4-Luc donor plasmid and increasing amounts (0 to 5 μg) of C5ar1–green fluorescent protein (GFP) acceptor plasmid or C5ar1-Luc donor plasmid and increasing amounts (0 to 5 μg) of C5ar1-GFP or Par4-GFP acceptor plasmids. Emission was detected using a PerkinElmer Life Sciences (Waltham, MA) Victor 3 plate reader equipped with the appropriate BRET2 filter set (410 nm with 80-nm bandpass and 515 nm with 30-nm bandpass; PerkinElmer Life Sciences). Emission at 410 and 515 nm was collected immediately after the addition of 5 μmol/L luciferase substrate (coelenterazine 400a; Biotium Inc., Hayward, CA). BRET signal was calculated by the ratio of emission at 515 nm to emission at 410 nm minus the BRET in the absence of GFP. In BRET studies, specific interactions are detected by a hyperbolic increase in the BRET signal (ratio of emission at 510/410 nm) as the ratio of GFP-receptor/RLuc-receptor increased, whereas nonspecific interactions increased linearly. Data were analyzed with Prism 6 (GraphPad, San Diego, CA) using best model (hyperbolic versus linear) for each experiment. GFP expression was determined by excitation at 495 nm and emission at 515 nm. Luciferase expression was determined by adding 5 μm coelenterazine H (Invitrogen, Carlsbad, CA) and reading total light emission without a filter. Each BRET assay was performed in three independent experiments.
RNA Interference
siRNAs targeting human PAR1 (identifier: 146259) and PAR4 (identifier: s194972) were purchased from ThermoFisher (Carlsbad, CA). RNA interference was performed using Lipofectamine RNAiMAX Reagent (ThermoFisher) following the manufacturer’s recommended protocol. Briefly, cells were treated with 30 nmol/L siRNA and transfection reagent at a 1:2 ratio under reduced serum conditions for 48 hours. Cells were returned to complete media, and knockdown was assessed using flow cytometry at 3 and 7 days. Silencer-Cy3 siRNA (ThermoFisher) was used as a nonspecific control.
Statistical Analysis
Power calculations and animal numbers were determined using the information provided by http://statpages.org and the therein linked Russ Lenth's power and sample-size calculator obtainable through http://www.stat.uiowa.edu/∼rlenth/Power/index.html#Download_to_run_locally (both last accessed September 12, 2021). To achieve a true difference between means of 0.5 and a power of 0.2 testing the difference between two means via an unpaired, two-tailed t-test, 10 mice were required in each group and there were 10 experimental groups. Statistical significance for all experimental data was determined by t-test (unpaired, two tailed) performed in Microsoft Excel (Seattle, WA), SigmaPlot (Systat Software, Chicago, IL), or GraphPad Prism 6, with a significance threshold value of P < 0.05. Except where indicated, all experiments were repeated at least two times in separate samples. Data are presented as mean values with SD.
Results
ECs Synthesize Coagulation Factors, and the Synthesis Is Up-Regulated by VEGF-A
To test whether ECs endogenously synthesize coagulation factors and, if so, whether VEGF-A stimulation affects their synthesis, a culture system with noncontact inhibited ECs was used to model EC growth as occurs in blood vessel reconstitution. Initial cell numbers were plated such that cells would remain <70% confluent in the presence of VEGF-A at the longest incubation time. In initial studies, murine bEnd.3 ECs incubated for 1 hour were used in the absence or presence of added VEGF-A. Immediately after harvesting the ECs, the washed cells were assayed for coagulation factor mRNA transcripts. The cells incubated with VEGF-A showed 350% to 500% increased amounts of FV, FX, FVII, and FII (prothrombin) mRNAs adjusted to fold increases over media alone (Figure 1A). Whether the mRNA synthesis translates to protein synthesis by measuring coagulation factors in culture supernatants was assessed next. Supernatants of the cells incubated in media alone shortened the clotting times of plasma samples selectively deficient in FII, FV, FVII, and FX (Figure 1B), whereas those of VEGF-A-treated cells more profoundly shortened the clotting times of each (Figure 1B). Primary aortic endothelial cells (HAECs) were used to document that the findings apply to nontransformed primary cells and human ECs. Incubation of HAECs without or with human VEGF-A showed comparable coagulation factor mRNA production (Figure 1C). These data point to endogenous production of functional FV, FX, FVII, and FII by ECs tonically and augmented production of each in response to VEGF-A stimulation.
Figure 1.
Vascular endothelial cell growth factor A (VEGF-A) induces intracellular synthesis of the components of prothrombinase. All experiments were typically done three times but in some cases at least twice. A: bEnd.3 cells were incubated for 1 hour at 37°C without or with VEGF-A (30 ng/mL) and mRNA expression of prothrombinase components [coagulation factors II (FII), V (FV), X (FX), and VII (FVII)] assayed by real-time quantitative PCR (qPCR). mRNA was normalized to β-actin. B: bEnd.3 cells were incubated for 72 hours at 37°C with media alone (0.05% fetal bovine serum) or with VEGF-A (30 ng/mL), after which their culture supernatants were assayed for their abilities to accelerate the clotting times of human plasmas deficient in FII, FV, FVII, or FX (media control without cells was <0.01 U/mL). C: Human aortic endothelial cell (HAECs) were incubated for 72 hours at 37°C without or with VEGF-A (30 ng/mL), and coagulation assays were performed as in B (media control was <0.01 U/mL). D: RNA from thoroughly perfused aortae of wild-type (WT) mice was assayed for tissue factor, FII, FV, and FX mRNA levels by qPCR. E: Matrigel plugs were implanted into the flanks of WT mice for 10 days. Plugs were harvested, embedded in OCT compound, and sectioned. Sections were stained with anti-FII, anti-FV, anti-FVII, or anti-FX (each red) and counterstained with anti-CD31 (green). Nuclei were visualized with DAPI (blue). Background signaling was minimal and suppressed. The data are representative of six separate images. ∗P < 0.05.
As a first test of whether the EC coagulation factor synthesis occurs in vivo, FV, FX, FVII, and FII production was examined in thoroughly perfused aortae from WT mice. Real-time quantitative PCR of dispase extracts were used to documente FII, FV, and FX as well as tissue factor mRNA expression (Figure 1D). Because aortae contain other cell types (eg, smooth muscle cells), and aortic ECs in situ do not proliferate (under homeostatic conditions), Matrigel plugs were implanted into the flanks of WT mice, plugs were removed 10 days later, and blood vessel growth was examined26 for intracellular coagulation factors. Confocal microscopy showed abundant FV, FX, FVII, and FII in EC cell bodies that colocalized with the EC marker CD31 (Figure 1E). These studies support the concept that the EC coagulation protein synthesis operates in vivo in proliferating ECs.
Thrombin Generation of C5a in ECs Is Integral to VEGF-A Mitotic Function
Whether EC coagulation factor production generates EC thrombin and, if so, whether the endogenously produced thrombin is mechanistically interconnected with VEGF-A mitotic function was tested next. Bivalirudin (Angiomax) or antithrombin (two different thrombin-specific inhibitors) was added to (nonconfluent) growing cultures of bEnd.3 cells in the absence or presence of added VEGF-A and EC proliferation was assessed over 72 hours. Consistent with the EC coagulation factor production being mechanistically tied to EC growth, both inhibitors completely abolished VEGF-A's induction of EC proliferation (Figure 2A). The thrombin active site inhibitors PPACK and FM19 had a similar abrogating effect (data not shown), supporting an essential role of EC coagulation factor synthesis in VEGF-A function. Taken together with the recent findings16 that EC-produced C5a plays a requisite role in EC growth, these data point to VEGF-A mitotic function, depending on activation of both EC-produced complement components and coagulation factors.
Figure 2.
Vascular endothelial cell growth factor A (VEGF-A) induces intracellular generation of C5a, C5a receptor-1 (C5ar1), and thrombin. A: bEnd.3 cells were incubated for 72 hours at 37°C without or with VEGF-A (30 ng/mL) alone, VEGF-A (30 ng/mL) + bivalirudin (Angiomax; 25 μg/mL), or VEGF-A + antithrombin (1 μmol/mL), and cell numbers were counted at 24, 48, and 72 hours. The 0.05% fetal bovine serum (FBS) was used to exclude effects of other growth factors or mediators in 10% FBS. Antithrombin can inhibit VEGF-A binding to VEGF receptor 2 but only after its cleavage, resulting from its inactivation of thrombin. B: bEnd.3 cells were incubated for 72 hours at 37°C without or with VEGF-A (30 ng/mL), or VEGF-A + antithrombin (1 μmol/mL), after which C5a in culture supernatants was assayed by enzyme-linked immunosorbent assay. C and D: bEnd.3 cells (C) and human aortic endothelial cells (HAECs; D) were incubated with VEGF-A (30 ng/mL) for 24 hours, after which intracellular and extracellular C5a and C5ar1 were measured by flow cytometry. E: bEnd.3 cells were incubated for 48 hours at 37°C with VEGF-A (30 ng/mL), after which cells were assayed for anti-total factor II (FII) reactivity extracellularly and intracellularly via flow cytometry. F: bEnd.3 cells were incubated for 48 hours at 37°C with VEGF-A (30 ng/mL) ± C3ar1-A/C5ar1-A (10 ng/mL each) (RA = combined C3ar1 and C5ar1 antagonists), after which cells were assayed for anti-total FII reactivity extracellularly and intracellularly via flow cytometry. G: HAECs were pre-incubated for 2 days in serum-free media, after which the cells were assayed for specific anti-thrombin reactivity intracellularly via flow cytometry. ∗P < 0.05, ∗∗∗P < 0.001. MFI, mean fluorescence intensity; N.D., not detectable.
The earlier studies of the neo-intimal response to femoral artery wounding27 implicated C5ar1 signaling in the proliferation of ECs into wounds but presumed that the C5a activating ligand was derived from plasma C5. On the basis of these earlier findings, the above thrombin blockade data herein, and the earlier report that thrombin can cleave C5a from purified C5 in vitro,17 we hypothesized that C5a in the setting of EC growth would derive from cleavage of EC produced C5 by EC produced thrombin. To test this idea, proliferating (nonconfluent) bEnd.3 cells were cultured with VEGF-A with or without antithrombin and the cells and their culture supernatants were assayed for C5a. Abundant C5a was generated in cultures with VEGF-A alone, whereas little, if any, was generated in the presence of thrombin blockade by antithrombin (Figure 2B). To directly determine whether VEGF-A augments thrombin-induced C5a production and determine whether the process is localized within ECs, unstimulated and VEGF-A–stimulated bEnd.3 cells were assayed for C5a without or with permeabilization of the cells. VEGF-A stimulation augmented the production of C5a as well as its C5ar1 receptor; and EC permeabilization showed that both augmentations occurred predominantly intracellularly within the ECs (Figure 2C). To verify that the findings apply to primary cells and human ECs, studies were repeated with HAECs. Comparable results were obtained (Figure 2D). Similar to that in the EC-produced C5a deriving from thrombin cleavage of EC-produced C5, added VEGF-A concomitantly augmented a prothrombin/thrombin signal intracellularly in the ECs (Figure 2E). The elicited prothrombin/thrombin signal was blunted by pharmaceutical blockade of C5ar1 (Figure 2F) interconnecting EC thrombin generation and EC C5a production. These results are consistent with the previous studies connecting VEGF-A induction of EC growth with up-regulation of autocrine C5ar1 signaling occurring intracellularly in endosomes.16
To exclude the possibility that the thrombin-induced cleavage of C5a in ECs resulted from prothrombin contained in the bovine serum-supplemented medium and document that the intracellular prothrombin/thrombin signal induced by VEGF-A contained thrombin, the primary HAECs were cultured in serum-free medium for 2 days. Intracellular fluorescence-activated cell sorting of the serum-free cells with a thrombin-specific monoclonal Ab (mAb; that does recognize prothrombin) showed a homogeneously positive anti-thrombin signal (Figure 2G). These data linking VEGF-A with intracellular generation of both thrombin and C5a taken together with the finding that antithrombin blocked the EC C5a production (Figure 1G) pointed to C5a being generated by EC produced thrombin, an issue that will be further examined below.
Locally Produced Thrombin Induces Cooperative C5ar1 and PAR Signaling That Promotes EC Proliferation
The generation of both thrombin and C5a intracellularly in proliferating ECs raised the question of whether VEGF-A induction of EC growth is dependent on the heightened generation of both. Adding C5a to primary cultures of HAEC (or to murine bEnd.3 cells) induced cell growth similar to adding VEGF-A (Figure 3A and Supplemental Figure S1A, E, and F). On the other hand, VEGF-A failed to induce growth when thrombin generation was blocked with antithrombin, even if C5a was included in the cultures (Figure 3B and Supplemental Figure S1B). Adding thrombin, like adding C5a, similarly induced the growth of both primary HAEC and bEnd.3 cells with efficiency comparable to adding VEGF-A (Figure 2C), whereas thrombin failed to do so if C5ar1 signaling was blocked by C5ar1 pharmaceutical antagonism (RA:C3ar1/C5ar1 antagonists) (Figure 3C and Supplemental Figure S1C). The findings that C5a and thrombin induced EC proliferation only in the presence of each other pointed to a mechanism in which both are jointly involved in EC growth. Neither by itself was sufficient for optimal VEGF-A induction of EC growth.
Figure 3.
Combinatorial C5a receptor-1 (C5ar1) and protease-activated receptor signaling is required for endothelial cell (EC) growth. All experiments were performed two or more times. A: Human aortic ECs (HAECs) were incubated for 72 hours at 37°C without or with vascular endothelial cell growth factor A (VEGF-A; 30 ng/mL) or C5a (30 ng/mL), and cell numbers were counted at 0, 24, 48, and 72 hours. B: HAECs were incubated for 72 hours at 37°C without or with VEGF-A (30 ng/mL) alone, VEGF-A + antithrombin (1 μmol/mL), or VEGF-A + antithrombin + C5a (10 ng/mL), and cell numbers were counted at 0, 24, 48, and 72 hours. C: HAECs were incubated at 37°C without or with VEGF-A (30 ng/mL) alone, VEGF-A + C3ar1-A/C5ar1-A (10 ng/mL each), thrombin (1 nmol/mL), or thrombin + C3ar1-A/C5ar1-A, and cell numbers were counted over 3 days. D: HAECs were incubated at 37°C with VEGF-A (30 ng/mL), VEGF-A (30 ng/mL) + vorapaxar (10 ng/mL), or VEGF-A (30 ng/mL) + vorapaxar (10 ng/mL) + C5a (10 ng/mL), and cell numbers were counted as in D. E: HAECs were incubated for 72 hours at 37°C without or with TP508 (10 μg/mL) or TP508 (10 μg/mL) plus C5a (10 ng/mL), and cell numbers were counted at 0, 24, 48, and 72 hours. F: The experiment in E was repeated, substituting TFLLRN (5 μg/mL) for TP508.
The failure of VEGF-A to induce EC growth in the presence of thrombin blockade, despite supplying its C5a product, raised the question of whether thrombin additionally provides for VEGF-A mitotic signaling via signaling through EC expressed PAR(s).28 As a first test of this idea, bEnd.3 cells (which express PAR1 and PAR4) were incubated with VEGF-A with or without vorapaxar, a drug known to inhibit human PAR1 signaling.29,30 The added vorapaxar abolished VEGF-A induction of (murine) bEnd.3 cell growth (Supplemental Figure S1D), as well as primary (human) HAEC growth (Figure 3D), irrespective of supplementing the cultures with excess C5a.
Seemingly inconsistent with the above findings, a prior study31 using confluent HUVECs found that thrombin induction of PAR1 signaling suppresses rather than promotes EC proliferation in response to epidermal growth factor and does so by disabling AKT phosphorylation. On the basis of the current data, we hypothesized that in proliferating ECs, in contrast to confluent HUVECs, which are contact inhibited, PAR signaling confers different signals. To test this concept, bEnd.3 cells and primary HAECs were cultured with TP508 (a 23–amino acid peptide from thrombin; ie, a portion of the PAR1 receptor-binding domain of thrombin) or with TFLLRN (an agonist peptide corresponding to the peptide sequence of the tethered PAR1 ligand). Each agonist was added individually, or in combination with C5a, to primary HAEC or bEnd.3 cells and EC proliferation was assessed. Although each PAR1 agonist alone induced primary HAEC (Figure 3, E and F) and bEnd.3 cell growth (Supplemental Figure S1, G and H), the inclusion of C5a had an additive effect. These findings argue that in growing ECs, PAR1 signaling augments rather than suppresses growth and that it coordinates with C5ar1 signaling in promoting EC growth. Additional studies comparing growing and confluent HAECs are discussed below.
Vorapaxar not only inhibits PAR1 signaling but also can impact PAR4 signaling as it makes heterodimers with PAR4 that coordinate in platelet activation32 in a time-dependent manner.6 siRNA studies were performed to determine whether thrombin's induction of EC growth involves PAR4 as well as PAR1 signaling and establish whether the activation of both is required for VEGF-A mitotic function. To exclude thrombin in serum, serum-free conditions were used. HAECs were treated with siRNAs targeting PAR1 or PAR4, or with Silencer (non-specific) siRNA control. Flow cytometry verified that the specific siRNAs fully abolished PAR1 and PAR4 cell surface expression (Supplemental Figure S2). The alternatively treated HAECs were first pre-incubated in serum-free medium for 16 hours, and then VEGF-A induced growth was examined over an additional 72 hours, maintaining the HAECs in serum-free medium. In contrast to the Silencer siRNA treated HAECs, which robustly grew in response to either VEGF-A or C5a (Figure 4A), the HAECs silenced in either PAR1 or PAR4 showed no growth when incubated with either agonist (Figure 4A).33
Figure 4.
Autocrine protease-activated receptor (PAR) 1/4 signaling is required for vascular endothelial cell growth factor A (VEGF-A) mitotic function, and endothelial cell (EC) prothrombinase and complement production depend on each other. A: Human aortic ECs (HAECs) were treated with siRNA targeting either PAR1 or PAR4 (or nonspecific control, Silencer) for 7 days. Following confirmation of knockdown, cells were pre-incubated for 16 hours in serum-free medium and then treated with VEGF-A (30 ng/mL) or C5a (30 ng/mL) for 3 days (in serum-free media), and growth was measured. As our studies16,33 have shown that VEGF receptor 2 (VEGFR2) signaling depends on PAR1 and PAR4 signaling (Figure 3), the loss of the VEGF-A response likely involved reduced VEGFR2 expression. B: HAECs were incubated for 1 hour at 37°C without or with C5a (10 ng/mL) or C5a + plus antithrombin (1 μmol/mL), and cells were assayed for factor II (FII), factor V (FV), factor VII (FVII), or factor X (FX) mRNAs by real-time quantitative PCR (qPCR). mRNA was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The anti-C5a monoclonal antibody (mAb) recognizes a neoepitope not present in the parental C5 protein. C: HAECs were incubated for 1 hour at 37°C without and with VEGF-A (30 ng/mL), or VEGF-A + anti-C5a mAb (1 μg/mL), and cells were assayed for FII, FV, FVII, and FX mRNAs by qPCR. mRNA was normalized to GAPDH. D: HAECs were incubated for 48 hours with VEGF-A (30 ng/mL) ± C3ar1-A/C5ar1-A (10 ng/mL each), after which cells were assayed for anti-total FII reactivity extracellularly and intracellularly by flow cytometry. E: HAECs were incubated for 1 hour at 37°C without or with thrombin (1 nmol/mL), and cells were assayed for C3, C5, C3ar1, and C5ar1 mRNAs by qPCR. mRNA was normalized to GAPDH. ∗P < 0.05.
EC-Produced Thrombin Modulates EC C5a Production and Vice Versa
The codependence of VEGF-A mitotic function on both endogenous thrombin and C5a prompted studies of whether augmented complement component and coagulation factor production induced by VEGF-A regulate each other. Adding C5a to cultures of HAEC or of bEnd.3 cells up-regulated mRNA expression of all of the EC produced coagulation proteins (Figure 4B and Supplemental Figure S1I), and antithrombin inhibited all of the up-regulations (Figure 4B and Supplemental Figure S1I). Conversely, disrupting C5ar1 signaling with a (blocking) anti-C5a mAb prevented VEGF-A's up-regulation of coagulation factor mRNAs (Figure 4C and Supplemental Figure S1J). Consistent with this, the C5ar1 blockade repressed VEGF-A's induction of intracellular prothrombin/thrombin generation (Figure 4D and Supplemental Figure S1K) (the intracellular ordinate axis is 10-fold higher than that of the extracellular ordinate axis). These data indicate that coagulation factor synthesis depends on EC C5ar1 signaling. In accordance with the mRNA expression interdependence occurring reciprocally, added thrombin up-regulated EC complement component mRNA expression (Figure 4E and Supplemental Figure S1K).
These findings point to VEGF-A's induction of autocrine PAR signaling and autocrine C5ar1 signaling occurring coordinately in ECs. The findings also point to the two systems regulating each other by virtue of thrombin being needed for generation of C5a, and C5ar1 signaling being needed for coagulation protein synthesis (Figure 4, C and D). Taken together, these data indicate that endogenous complement production in ECs up-regulates endogenous prothrombinase production and vice versa and that both up-regulations play requisite roles in VEGF-A mitotic signaling.
C5ar1 and PAR4 Are Associated, and Their Joint Signaling Is Interconnected in ECs
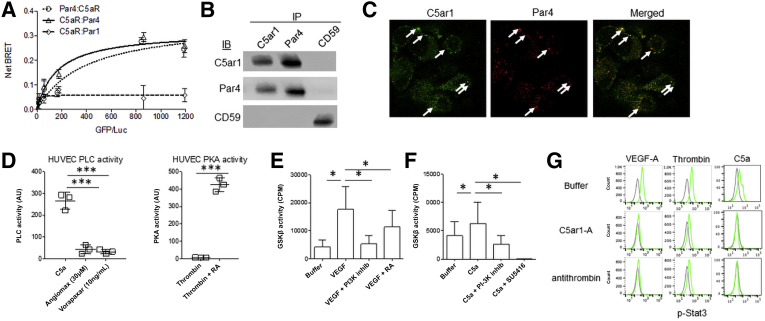
Because locally produced thrombin in ECs elicited joint C5ar1 and PAR signaling, we hypothesized that PAR1, PAR4, or both3 and C5ar1 might be in proximate contact. To test this idea, BRET analyses were done first. HEK293 (human kidney epithelial cells) cells were cotransfected with C5ar1-GFP together with PAR1-luciferase or with PAR4-luciferase. There was a dose-dependent energy transfer in the cells containing C5ar1 plus PAR4-luciferase (Figure 5A), but not PAR1-luciferase (Figure 5A). A reverse analysis with C5ar1-luciferase and PAR4-GFP yielded the same positive result (Figure 5A). These data indicate that C5ar1 and PAR4 are within 10 nm of each other in membranes. Co-immunoprecipitation assays were performed next. In support of the BRET data, anti-C5ar1 co-immunoprecipitated PAR4 and vice versa (Figure 5B), but PAR1 was not brought down in either immunoprecipitation (Figure 5B). Finally, confocal imaging with GFP-labeled anti-C5ar1 mAb and Red Fluorescent Protein (RFP)-labeled anti-PAR4 mAb showed colocalization of the two receptors (Figure 5C). These results pointed to a functionally interactive complex between PAR4 and C5ar1, an arrangement that could facilitate thrombin's ability to concurrently produce the C5ar1 and PAR4 ligands and coordinately elicit signaling34 by the two receptors.
Figure 5.
C5a receptor-1 C5ar1) and protease-activated receptors (PARs) closely interact, and phospholipase C (PLC), AKT–glycogen synthase kinase (GSK)-3β, and STAT3 activation depends on their joint signaling. All experiments were performed two or more times. A: Human 293 cells cotransfected with C5ar1–green fluorescent protein (GFP) + PAR4-luciferase (Luc), PAR4-GFP + C5ar1-Luc, or alternatively with C5ar1-GFP + PAR1-Luc, or PAR1-GFP + C5ar1-Luc. Cells also were transfected with PAR4-GFP + PAR4-Luc as a positive control. Bioluminescence resonance energy transfer (BRET) in each combination was measured after addition of Luc substrate. B: Anti-PAR4 immunoprecipitations (IPs), anti-C5ar1 IPs, and anti-CD59 IPs were prepared from bEnd.3 cell extracts, and immunoblots of the immunoprecipitated proteins were probed for associated C5ar1, PAR4, or CD59. C: bEnd.3 cells in complete media were incubated with vascular endothelial cell growth factor A (VEGF-A; 30 ng/mL) for 1 hour, after which the fixed and permeabilized cells were stained with anti–C5ar1-GFP and anti–PAR4-RFP and examined by confocal microscopy. Background signaling was minimal and suppressed. The data are representative of six separate images. Arrows represent sites of colocalization. D: Left panel: Human umbilical vein endothelial cells (HUVECs) were stimulated with C5a or C5a plus bivalirudin (Angiomax) or vorapaxar for 5 minutes, after which cells were lysed and assayed for PLC activity. Right panel: HUVECs were stimulated with thrombin or thrombin plus C3ar1-A/C5ar1-A (RA) for 5 minutes, after which cells were lysed and assayed for protein kinase A (PKA) activity. E: Serum-starved bEnd.3 cells were incubated for 72 hours at 37°C without or with VEGF-A (30 ng/mL), VEGF-A + the PI-3Kγ inhibitor AS252424 (10 μmol/mL), or VEGF-A + C3ar1-A/C5ar1-A (RA; 10 ng/mL), after which cell extracts were assayed for GSK-3β activity. F: The protocol in E was used substituting C5a (10 ng/mL) for VEGF-A and including C5a + the PI-3Kγ inhibitor AS252424 (10 μg/mL) and C5a + the VEGFR2 inhibitor SU5416 (10 μmol/mL). G: bEnd.3 cells were incubated with VEGF-A (30 ng/mL), thrombin (1 μmol/mL), or C5a (10 ng/mL) in the absence or presence of C5ar1-A (RA; 10 ng/mL) or antithrombin (1 μmol/mL), after which the cells were assayed for phosphorylated STAT3 (p-STAT3) by intracellular flow cytometry. ∗P < 0.05, ∗∗∗P < 0.001. AU, arbitrary unit; CPM, counts per minute; IB, immunoblot.
Because C5ar1 and PAR4 are both GPCRs, their association raised the possibility of the existence of additional signaling interconnections. C5ar1 [as indicated (Introduction)], is a Gi coupled GPCR such that the α subunit of its G protein represses activation of adenylyl cyclase. PAR4 is (predominantly) a Gq coupled GPCR such that its G protein α subunit activates phospholipase C (PLC). Some past studies have reported that C5ar1 can couple to Gαq as well as Gαi.35, 36, 37, 38 Others have reported that PAR4 can couple to Gαi as well as Gαq.3,5,7 To determine whether combinatorial C5ar1-PAR signaling might reconcile these observations, HUVECs were incubated with C5a alone, together with Angiomax (which blocks thrombin), or together with vorapaxar (which blocks PAR1/PAR4 signaling) (Figure 3D) and PLC activation was assayed. C5a activation of the Gαq target PLC occurred when C5a was added alone (Figure 5D), but the activation was reversed by Angiomax that blocks thrombin needed for PAR as well as C5ar1 signaling. Notably, vorapaxar (which blocks PAR1-dependent PAR4 signaling) (Figure 3D) had the same effect (Figure 5D). In the case of the Gαi signaling of PAR4, there have been reports that in platelets PAR4 can couple to the purinergic Gαi GPCR P2Y12.34 Nevertheless, although thrombin suppressed protein kinase A activation (a result of suppressed generation of the Gαi target adenylyl cyclase) in primary HAECs, it did not do so in the presence of C3ar1/C5ar1 pharmaceutical antagonists (RA) implicating C5ar1 signaling (Figure 5D). These data provide evidence that PLC can be activated by C5ar1 signaling and adenylyl cyclase can be repressed by PAR4 signaling. They argue that the cross-activation can occur by virtue of C5ar1 and PAR4 being functionally associated with each other in primary HAECs and the agonist of either GPCR being able to activate the other GPCR.
Disruption of C5ar1 or PAR Transduction Blocks AKT–GSK-β–STAT3 and AKT-mammalian target of rapamycin 2 (mTOR) Signaling
VEGF-A induction of EC proliferation is dependent on activated STAT3.39 Previous studies in immune cells14 and recent studies in ECs16 showed that C5ar1 signaling promotes STAT3 activation. To determine whether, and how, combinatorial EC C5ar1 and PAR1/4 signaling participates in STAT3 activation, VEGF-A induction of AKT–GSK-β–STAT3 signaling in ECs was examined. Addition of C5a, like that of VEGF-A (Figure 3A), to cultures of bEnd.3 cells evoked S473 phosphorylation of AKT 14, activation of GSK-β (Figure 5, E and F), and Y705 phosphorylation of STAT3 (Figure 5G). The activation of GSK-β by VEGF-A was suppressed by C5ar1 blockade (Figure 5E) and that by C5a was reversed by VEGFR2 blockade (Figure 5F). STAT3 activation by VEGF-A was suppressed by either C5ar1 or thrombin blockade, and that by thrombin was suppressed by C5ar1 blockade (Figure 5G), consistent with both PAR4 and C5ar1 signaling being essential. GSK-β activation by C5a was abolished by the VEGFR2 inhibitor SU5416 (Figure 5F). GSK-β activation by VEGF-A was abolished by the AS252424 inhibitor of phosphatidylinositol 3-kinase gamma (PI-3Kγ) (Figure 5, E and F) (ie, downstream of both C5ar114 and PAR4 transduction40,41 and possibly related to either thrombin or C5a being able to activate both C5ar1 and PAR4). Collectively, these data argue that thrombin as well as C5ar1 signaling are needed for VEGFR2-associated induction of GSK-β and STAT3 activation. The data further argue that AKT–GSK-β–STAT3 signaling is dependent on PI-3Kɣ activation.
Both C5ar1 and PAR4 Signaling Promote VEGFR2 Signaling in ECs
How joint C5ar1 and PAR1/4 signaling in ECs affects VEGF-A induction of the PI-3K–AKT–mTOR cascade was investigated next. Prior studies in CD4+ T cells12,14 showed that C5ar1 activation of PI-3Kɣ is essential for augmented PI-3K–AKT–mTOR signaling. In distinction to this linkage, early studies of CD4+ cell activation42 had linked costimulatory CD28-dependent activation of PI-3Kα with AKT-mTOR signaling. Taken together, the two results suggest that both activated PI-3K isoforms [that jointly promote phosphatidylinositol 3,4,5 trisphosphate (PIP3) assembly] may be needed for AKT-mTOR signaling in ECs as in T cells.
To characterize the respective roles of C5ar1 and of PAR4 signaling in PI-3Kɣ and of PI-3Kα activation in the context of EC proliferation, bEnd.3 ECs were first incubated with thrombin and the activation of each PI-3K isoform was assayed. The added thrombin (which jointly induces both PAR4 and C5ar1 signaling) activated both PI-3K isoforms with high efficiency (Figure 6, A and B). The activation of both isoforms occurred in <1 minute and was sustained for >180 minutes (Figure 6, A and B). The activation of both isoforms was abolished by C5ar1 antagonism, highlighting C5ar1 signaling as an obligate process in thrombin-induced activation of the two PI-3K isoforms (Figure 6, A and B). To distinguish the roles of PAR1 and PAR4 in activating each PI-3K isoform, PAR agonists and antagonists were used. Titrations of agonists specific for PAR1 and PAR4 into cultures of (nonactivated) bEnd.3 cells showed that signaling by either PAR activated PI-3Kɣ but did not activate PI-3Kα (Figure 6C). This contrasted with titrations of C5a into the culture, which activated both PI-3Kɣ and PI-3Kα, localizing the PI-3Kα activating effect of thrombin (which jointly induces PAR and C5ar1 signaling) to C5ar1 signaling (Figure 6D). Dose-response curves of either thrombin or C5a addition to bEnd.3 cells indicated that activation of PI-3Kɣ preceded that of PI-3Kα. This timing would be consistent with PI-3Kɣ functioning upstream to repress phosphatase and tensin homolog (PTEN) restraint of PIP3-dependent AKT activation, and PI-3Kα (known to be VEGFR2-activated) being mechanistically involved in augmenting PIP3 generation.
Figure 6.
Role of C3ar1, protease-activated receptors (PAR) 1/PAR4, and vascular endothelial cell growth factor receptor 2 (VEGFR2) signaling in activation of PI-3Kɣ and PI-3Kα. A and B: bEnd.3 cells were incubated for increasing times at 37°C with thrombin (1 μmol/mL) without or with C3ar1-A/C5ar1-A (10 ng/mL each), after which anti–PI-3Kγ (A) and anti–PI-3Kα (B) immunoprecipitations (IPs) of the cell extracts were assayed for PI-3K enzymatic activity. C: TFLLRN (PAR1 agonist) or ay-NH2 (PAR4 agonist) was titrated into bEnd.3 cells for 9 minutes at 37°C, after which PI-3K isoform activity was assayed, as in A and B above. D: Thrombin (1 μmol/mL; left panel) or C5a (10 ng/mL; right panel) was titrated into serum-starved bEnd.3 cells for 9 minutes, after which PI-3K isoform activity was assayed as above. E: Vascular endothelial cell growth factor A (VEGF-A), TP508, ay-NH2, and C5a were incubated with bEnd.3 cells for 9 minutes, cells were lysed, and anti-VEGFR2 IPs were prepared. The VEGFR2 IPs were eluted using alkaline solution, and the eluents were immunoprecipitated with PI-3Kα or PI-3Kγ Abs, after which PI-3K activity in the IPs was assayed as above.
Because PI-3Kɣ activation occurs on membranes nearby GPCRs in contrast to PI-3Kα activation, which typically occurs on receptor tyrosine kinases43 like VEGRF2, VEGFR2 immunoprecipitations were examined for associated PI-3Kα following EC activation with the PAR1, PAR4, and C5ar1 agonists. bEnd.3 cells were incubated with TP508 (PAR1 agonist), ay-NH2 (PAR4 agonist), C5a, or VEGF-A, after which extracts of the activated cells were incubated with anti-VEGFR2 mAb. Twenty-four hours later, VEGFR2 was immunoprecipitated from each incubation mixture and immunoprecipitated proteins were eluted from the alternatively activated bEnd.3 cells. Secondary anti–PI-3Kɣ or PI-3Kα immunoprecipitations of the eluents of the anti-VEGFR2 immunoprecipitations were prepared from each incubation culture and the secondary anti–PI-3Kɣ immunoprecipitations and anti–PI-3Kα immunoprecipitations were tested for PI-3K enzymatic activity. The anti–PI-3Kɣ immunoprecipitations from cells activated with each of the agonists uniformly lacked VEGFR2-associated PI-3Kɣ activity (Figure 6E). This would be in accordance with PI-3Kɣ (due to its membrane localization near PIP3) not being immunoprecipitated by anti-VEGFR2 mAb. In contrast, the anti–PI-3Kα immunoprecipitations from cells treated with all agonists uniformly contained PI-3Kα activity, which in all cases was abolished by the VEGFR2 antagonist SU5416. These data indicate that, in contrast to the short-term incubations, PAR1 or PAR4 signaling in ECs incubated overnight induced VEGFR2 signaling and its activation of PI-3Kα. In support of this interpretation, PI-3Kα activation by all agonists was knocked down in cells pretreated with VEGFR2 siRNA (data not shown). More studies in long-term cultures will be needed to determine whether PAR1/4 signaling transactivates directly to VEGFR2, as the recent studies have shown for C5ar1 signaling,16 whether it transactivates indirectly via the PAR4-C5ar1 complex characterized above, or whether another mechanism is involved.
Collectively, these data argue that PAR1, PAR4, and C5ar1 signaling all participate in VEGF-A's induction of EC growth. Although further studies are needed to directly validate the functional relationships and kinetics, the data are consistent with the following hypothetical formulation. They would be consistent with C5ar1 and PAR1 (because of its higher affinity than PAR4 for thrombin) initially collaborating in activating PI-3Kɣ to repress PIP3 dephosphorylation. They support prior findings that C5ar1 directly transactivates to VEGFR2 to promote its signaling.16 They further support the concept that C5ar1 Gαi signaling additionally functions to enable AKT and STAT3 activation and joint PAR4 Gαq signaling functions to enable PLC activation. The anti-VEGFR2 immunoprecipitation data following the addition to ECs of individual ligands of each receptor argue that all coordinately participate in VEGFR2-associated activation of PI-3Kα. According to the data presented herein, C5a and the PAR ligands could each induce combinatorial C5ar1 and PAR4 signaling. These results are in accordance with the vorapaxar blocking studies (Figure 3), C5ar1-PAR4 association studies (Figure 5), and the assays with PAR1 and PAR4 siRNAs (Figure 4), which show that PAR1, PAR4, and C5ar1 all collaborate in enabling VEGF-A mitotic function.
Down-Regulated C5ar1 and PAR Signaling Is Connected with Contact Inhibition
The experimental system using subconfluent ECs was chosen to model proliferating ECs (eg, ECs in the context of neo-angiogenesis or reconstitution of vasculature following blood vessel severance). In this system, VEGF-A induces joint C5ar1 and PAR1/4 signaling which is integrally interconnected with VEGF-A mitotic function. Because the current studies of subconfluent ECs differ from most studies of ECs that use confluent HUVECs to model ECs in intact blood vessels, thrombin generation and C5a production in subconfluent growing HUVECs was compared to that in confluent HUVECs. Although subconfluent HUVECs expressed coagulation factor and complement component mRNAs, the mRNA expression of both systems down-regulated at confluence (Figure 7A). In accordance with this result, the production of thrombin and of C5a was robust in growing HUVECs, whereas both were repressed in confluent (contact-inhibited) HUVECs (Figure 7, B and C). These findings indicate that PAR-C5ar1 signaling operates in growing ECs and is repressed with contact inhibition.
Figure 7.
Combinatorial C5a receptor-1 (C5ar1) and thrombin agonism drives angiogenesis. The experiments were performed two or more times. A: Human aortic endothelial cell (HAECs) grown at 70% and 100% confluence were assayed for factor II (FII), factor V (FV), factor VII (FVII), factor X (FX), C3, and C5 mRNAs by real-time quantitative PCR. mRNA was normalized to glyceraldehyde-3-phosphate dehydrogenase. B: Human umbilical vein endothelial cells (HUVECs) grown at 70% or 100% confluence were stained with antibodies against FII (red), counterstained with DAPI (blue) and CD31 (data not shown), and imaged by confocal microscopy. Three randomly selected fields of view under each condition are presented. C: HUVECs grown at 70% or 100% confluence were stimulated with vascular endothelial cell growth factor A (VEGF-A; 30 ng/mL) or media control for 24 hours, after which C5a in the culture supernatant was assayed by enzyme-linked immunosorbent assay. D: Representative sections of aortic segments from wild-type (WT) mice were incubated for 7 days in Matrigel with VEGF-A (30 ng/mL), VEGF-A + C3ar1-A/C5ar1-A (RA; 10 ng/mL each), or VEGF-A + antithrombin (1 μmol/mL). E: Quantitation of sprout areas from WT aortic segments grown without or with VEGF-A, VEGF-A + C3ar1-A/C5ar1-A, or VEGF-A + antithrombin (each point represents growth from one aortic ring; three rings were isolated per mouse). These analyses were performed four times. F: Identical studies were done for aortic segments from WT mice treated with VEGF-A (30 ng/mL) ± vorapaxar (10 ng/mL), aortic segments from C5ar1–/– mice treated with VEGF-A (30 ng/mL), and aortic segments from Par4–/–Par3–/– and Par1–/– mice treated with VEGF-A (30 ng/mL). These studies were performed three times. G: Diagram of C5ar1–protease-activated receptor (PAR) 4 interaction at confluence versus when cells are growing. H: Diagram of PAR and C5ar1 Gβγ activation of PI-3Kγ and C5ar1 PI-3Kα via transactivation to VEGF receptor 2 (VEGFR2). PAR Gαq activation of phospholipase C (PLC) and C5ar1 Gαi repression of protein kinase A (PKA) also are depicted. The proposed activation sequence is as follows: 1, extracellular stimulus (hypoxia/thrombin); 2, thrombin cleavage of endothelial cell C5 and PAR1/4; 3, PAR1/4/C5ar1 Gβɣ activation of PI-3Kɣ and phosphorylated AKT; 4, C5ar1 transactivation to VEGFR216, 5, VEGFR2 activation of PI-3Kα; 6, C5ar1/PAR4 induced ↑ PLC and ↓ PKA; 7, PI3K-AKT-mTOR, diacylglycerol/IP3, and STAT3 signaling, and 8, amplified C5ar1/PAR4/VEGFR2 signaling.16n = 2 (E). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
VEGF-A Induction of EC Sprouting Depends on Joint C5ar1 and PAR1-PAR4 Signaling
To validate that joint C5ar1 and PAR signaling is required for VEGF-A induction of EC growth physiologically, we performed sprouting assays. Aortic segments from WT mice incubated in Matrigel with VEGF-A showed robust sprouting compared with those from segments treated with media alone (1.07 versus 0.42 mm2; P < 0.05) (Figure 7D). Addition of C5ar1-A (RA) or antithrombin to the VEGF-A treated WT segments markedly attenuated the sprouting (1.07 mm2) versus 0.26 and 0.28 mm2 (P < 0.05) (Figure 7D). The results of multiple repeated assays are summarized in Figure 7E. The inclusion of vorapaxar with VEGF-A had a similar blunting effect (Figure 7F). Consistent with these results, aortic segments from C5ar1–/– mice, double PAR3–/–PAR4–/– mice, and single PAR1–/– mice (Figure 7F) showed some sprouting in response to VEGF-A, but less than that of VEGF-A-treated WT segments. These ex vivo data are consistent with the confocal analyses in Figure 1E of in vivo neo-angiogenesis in implanted Matrigel plugs showing FII, FV, FVII, and FX colocalizing in CD31+ cells. They are consistent with the multiple in vitro studies linking joint PAR1/4 and C5ar1 signaling with VEGF-A mitotic function detailed above. The data further argue that both PAR and C5ar1 signaling jointly regulate EC growth physiologically.
A diagram depicting the linkage of endogenously produced thrombin and C5a with VEGF-A mitotic function in proliferating versus contact-inhibited ECs is shown in Figure 7G. A second diagram depicting the linkage of combinatorial Gαi-Gαq signaling by the interactive PAR4-C5ar1 functional complex with joint PKC activation/protein kinase A inactivation and PI-3Kɣ/PI-3Kα signaling associated with VEGFR2 activation is shown in Figure 7H.
Discussion
Thrombin functions to repair injured blood vessels by generating fibrin and activating platelets that together seal separated vascular segments.44 In conjunction with this, it triggers EC proliferation that enables vascular reconstitution.45 The current view is that thrombin is solely generated in the intravascular compartment from prothrombinase components that are produced by the liver.46 Although a previous study found47 that thrombin can cleave C5a from complement component C5, the observation was connected with heightened plasma thrombin in disease. The studies herein provide the insight that this process operates endogenously in ECs to produce C5a and plays a requisite role in VEGF-A function.
The data show that in proliferating ECs: i) both coagulation factors, specifically FX, FV, FVII, and FII (prothrombin), which give rise to thrombin, and complement components, specifically C5 that gives rise to C5a, are jointly synthesized by ECs; and ii) thrombin (ie, endogenously produced by ECs) jointly cleaves C5a from EC synthesized C5 and unmasks the tethered ligands of EC expressed PAR1 and/or PAR4, that together induce combinatorial C5ar1 and PAR4 signaling. They show that: iii) the PAR4 and C5ar1 GPCRs are directly or indirectly complexed with each other in ECs and that iv) cooperative C5ar1 and PAR signaling in ECs is integrally involved in VEGF-A mitotic function. The experiments additionally provide evidence that: v) the associated PAR4-C5ar1 complex promotes combinatorial Gαi and Gαq signaling that induces PI-3K–AKT–mTOR and STAT3 activation as well as PLC activation. Finally, they provide evidence that: vi) C5ar1, PAR4, and PAR1 signaling all induce PI-3Kɣ activation in short-term cultures, whereas C5ar1 signaling additionally induces PI-3Kα activation. All, however, promote PI-3Kα activation in long-term cultures. The proximity of C5ar1 and PAR4, in principle, could promote efficient induction of joint C5ar1 and PAR4 signaling by EC produced thrombin, dual processes needed for VEGF-A mitotic function. Taken together, the findings support a requisite role of endogenous coagulation factor synthesis in EC growth. Mechanistically, they document an interconnection of the EC synthesized coagulation factors with EC synthesized complement via EC produced thrombin and show that thrombin-induced signaling through associated C5ar1 and PAR4 plays an integral role in VEGF-A mitotic function.
As emphasized in Results, it is important to highlight the difference between the studies investigating proliferating ECs herein and those by many others examining homeostatic ECs. Most in vitro studies of ECs have used HUVEC31 grown to confluence, such that the ECs are contact inhibited. That system is intended to model conditions that pertain in intact blood vessels. Conditions that model the reconstitution of severed vessels were achieved by titrating cell numbers such that the cultures always remained nonconfluent in the presence of added VEGF-A, helping to study effects on EC proliferation in the absence of effects of contact inhibition. The comparisons of growing versus confluent HUVEC showed that although C5ar1 and PAR signaling are robust in proliferating ECs, both are repressed in confluent ECs, raising the possibility that down-regulation of this joint signaling participates in the processes that halt cell growth in contact inhibition.
Although the synthesis/presentation of tissue factor by ECs has been documented in many past studies,48,49 its function uniformly has been connected solely with intravascular coagulation. A contemporaneous study by the Moake group50 detected FVII, FIX, and FX as well as FII in confluent cultures of HUVECs and other ECs subjected to the serum-free conditions and noted that EC-produced FX could be activated without adding plasma factors. The findings in that system likewise were interpreted solely in the context of coagulation. Other prior studies51, 52, 53 have reported EC production of factor B and factor H and coagulation factors FX and FVII, and similarly interpreted the findings in the context of intravascular complement activation and coagulation. The findings herein differ from these past findings in that they document the endogenous production by ECs of the full array of prothrombinase components, leading to the intracellular production of thrombin in ECs. This process is mechanistically interconnected with VEGF-A mitotic function rather than with coagulation.
The finding herein that thrombin is produced intracellularly is supported by the intracellular fluorescence-activated cell sorting analyses of thrombin protein, and the confocal localization of FII in the bodies of ECs growing in vivo in Matrigel. It addition, it is supported by the ability of VEGF-A stimulated (primary) HAECs to support autocrine PAR1 and PAR4 signaling in serum-free cultures, the abrogation of EC growth in serum-starved cultures by PAR inhibitors, and the findings that ECs produce all prothrombinase components that generate thrombin. Disruption of EC growth by siRNA knockdowns of PAR1 and PAR4 in serum-free media provided direct confirmation of the involvement of this signaling in EC growth. Our findings that the locally produced thrombin triggers joint C5ar1 and PAR signaling and that this cooperative signaling is needed for VEGF-A function thus tie EC produced coagulation proteins to an intracellular signaling system that plays an important role in EC growth. Although this process is distinct from coagulation per se, EC proliferation is an essential process in vascular repair. Because PAR1, PAR4,54,55 C5ar1,56,57 and VEGFR2 undergo recycling between the cell surface and endosomes intracellularly, more studies will be needed to determine precisely how Angiomax and antithrombin exert their blocking effects on VEGFR2 function. More studies also will be needed to characterize the thrombin-mediated C5 cleavage process and the joint C5ar1 and PAR4 signaling processes spatially and structurally.
BRET, co-immunoprecipitation analyses, and confocal microscopy all indicated that PAR4 and C5ar1 are intimately associated with each other (within 10 nm). The BRET studies did not detect proximity of PAR1 with C5ar1. However, the current findings that PAR1 antagonism (vorapaxar) inhibited EC proliferation, and that PAR1 agonists (TFLLRN or TP508) augmented EC proliferation, all implicated PAR1 signaling in VEGF-A function. The findings that PAR1 knockdown abolished VEGF-A induction of EC growth in serum-free media and that PAR1 genetic deficiency blunted sprouting further support this. Although the data for TP508 are controversial because a previous study indicated that TP508 activates ECs via binding to αvβ3 integrin,58 the combined data herein implicate TP508 in impacting PAR1 signaling. One possibility is that PAR1, which has high affinity for thrombin, is close to the PAR4-C5ar1 complex, allowing the tethered ligand of PAR1 to deliver thrombin to PAR4, similar to the reported cooperativity of murine PAR3 delivering thrombin to PAR4.4 Although possible, it is unlikely that the tethered ligand of PAR1 activates PAR4 because the sequences are different. A second possibility is that PAR1 and PAR4-C5ar1 signaling converge downstream. A precedent exists for the cooperativity of PAR1 signaling with PAR4 signaling in several systems.2 Because of differences in the extracellular regions of PAR1 and PAR4 and the higher affinity of PAR1 for thrombin, the tethered ligand of PAR4 is much less efficiently cleaved by thrombin.3 However, cooperation of PAR1 and PAR4 improves thrombin activation of PAR4.4 Functionally important, thrombin characteristically elicits a short burst of PAR1 signaling that is followed by a more prolonged expanse of PAR4 signaling.59
The finding that C5ar1 and PAR4 are associated with each other could account for past reports that C5ar1 GPCR signaling, although usually being Gi coupled (pertussis toxin sensitive) and adenylyl cyclase repressive, can also activate PLC. Some studies have attributed the PLC activation to the βɣ subunit of the C5ar135,60 G protein, whereas other studies37 have attributed it to alternative Gαq coupling. In the same vein, some studies have reported that PAR4, although generally being Gαq coupled, sometimes can be Gαi coupled.61 Studies in platelets61 have found that PAR4 can couple the purinergic receptor P2Y12 that conveys Gαi signaling. The current findings that C5ar1 is associated with PAR4 in ECs and that C5ar1 antagonism blocks thrombin suppression of protein kinase A, activation of AKT, and VEGFR2 signaling support the concept that in ECs the C5ar1-PAR4 complex could account for Gαq coupling of C5ar1 and Gαi coupling of PAR4. More studies will be needed to definitively characterize the interactions in ECs versus platelets of PAR4 with C5ar1 or with P2Y12.
Whether EC thrombin generation and intravascular thrombin generation are entirely separate processes or whether they function together remains to be determined. A corollary of this distinction is whether EC produced thrombin contributes to intravascular coagulation and intravascularly produced thrombin contributes to VEGF-A induction of EC growth. Our prior studies of immune cell activation using bone marrow chimeras consisting of wild-type bone marrow in complement-deficient recipients and vice versa62,63 showed that immune cell produced and (liver-produced) systemic complement function independently of each other in separate compartments. These results underline the functional importance of distinguishing between autocrine and paracrine receptor tyrosine kinase, PAR, and C5ar1 signaling loops operating to promote EC growth. The current data will provide a framework for more definitively unraveling the respective involvement of systemic versus EC produced thrombin in EC growth.
In conclusion, the data herein shed light on several past observations. First and foremost, endothelium has the means to synthesize endogenous complement and coagulation proteins that function to promote cell growth and angiogenesis. Recognizing this has important consequences is essential because blockade of both thrombin and C5ar1 is needed to reduce inflammation and brain edema following intracerebral hemorrhage.64 Also, the PAR and C5ar1 receptor pathways are both involved in the mobilization of hematopoietic stem/progenitor cells,65 the inflammatory response that follows traumatic lung injury,66 and the expression of P-selectin by ECs.67 Findings by others that disrupting PAR signaling in ECs induces apoptosis68 are consistent with our findings in immune cells that disrupting C3ar1/C5ar1 signaling similarly is pro-apoptotic.14 More studies will be needed to determine whether the PAR1, C5ar1, and PAR4 autocrine signaling loops in ECs described herein regulate growth in other cell types.
Acknowledgments
We thank Micah Harland (data), Patricia Conrad (confocal), Richard Lee (imaging), Melissa DiBlasi and Victoria Turnbill (graphics), Dawn Smith (cell culture), Kathryn Franke (mice), Maria de la Fuente (bioluminescence resonance energy transfer), Alona Merkulova (angiogenesis), and I. Pikuleva (the Ophthalmology Core) for their help.
Footnotes
Supported by NIHR01 HL109561 (M.E.M.), R01 AR067182 (M.E.M.), R01 HL052779 (A.H.S.), HL126634 (A.H.S.), R01 HL098217 (M.N.), and EY11373 (Ophthalmology Core; Principal Investigator: Irina Pikuleva).
D.Ca., M.G.S., and D.Co. contributed equally to this work.
Disclosures: None declared.
Supplemental material for this article can be found at http://doi.org/10.1016/j.ajpath.2021.09.011.
Author Contributions
D.C., M.G.S., D.C., S.S., F.A., and W.H. performed the experiments; M.G.S. analyzed the data; A.H.S. guided coagulation time and sprouting studies; M.N. guided bioluminescence resonance energy transfer analyses and protease-activated receptor studies; and M.E.M. designed and conceived the project. M.E.M. wrote the manuscript, and M.G.S., M.N., and A.H.S. edited all versions of the manuscript.
Supplemental Data
Supplemental Figure S1.
Combinatorial C5a receptor-1 (C5ar1) and protease-activated receptor (PAR) 1/PAR4 signaling is required for endothelial cell (EC) growth. A: bEnd.3 cells were incubated for 72 hours at 37°C without or with vascular EC growth factor A (VEGF-A; 30 ng/mL) or C5a (30 ng/mL), after which cell numbers were counted at 0, 24, 48, and 72 hours. B: bEnd.3 cells were incubated for 72 hours at 37°C without or with VEGF-A (30 ng/mL), VEGF-A + antithrombin (1 μmol/mL), or VEGF-A + antithrombin + C5a (10 ng/mL), after which cell numbers were counted at 0, 24, 48, and 72 hours. C: bEnd.3 cells were incubated without or with VEGF-A (30 ng/mL), VEGF-A + C3ar1-A/C5ar1-A (10 ng/mL each), thrombin (1 nmol/mL), or thrombin (1 μmol/mL) + C3ar1-A/C5ar1-A, after which cell numbers were counted over 3 days. D: bEnd.3 cells were incubated for 72 hours at 37°C without or with VEGF-A (30 ng/mL) or VEGF-A (30 ng/mL) + vorapaxar (10 ng/mL), and cell numbers were counted at 0, 24, 48, and 72 hours. E: bEnd.3 cells were incubated as in A with a fourth condition: C5a (10 ng/mL) included VEGF-A and vorapaxar. F: bEnd.3 cells were incubated for 72 hours at 37°C without or with VEGF-A (30 ng/mL) or C5a (10 ng/mL), and cell numbers were counted at 0, 24, 48, and 72 hours. G: bEnd.3 cells were incubated for 72 hours at 37°C without or with TP508 (10 μg/mL) or TP508 (10 μg/mL) plus C5a (10 ng/mL), after which cell numbers were counted at 0, 24, 48, and 72 hours. H: The experiment in G was repeated, substituting TFLLRN (5 μg/mL) for TP508. I: bEnd.3 cells were incubated for 1 hour at 37°C without or with C5a (10 ng/mL) or C5a + plus antithrombin (1 μmol/mL), after which cells were assayed for factor II (FII), factor V (FV), factor VII (FVII), and factor X (FX) mRNAs by real-time quantitative PCR (qPCR). J: bEnd.3 cells were incubated for 1 hour at 37°C without and with VEGF-A (30 ng/mL), or VEGF-A + anti-C5a mAb (1 μg/mL), after which cells were assayed for FII, FV, FVII, and FX mRNAs by qPCR. K: bEnd.3 cells were incubated for 1 hour at 37°C without or with thrombin (1 μmol/mL), and cells were assayed for C3, C5, C3ar1, and C5ar1 mRNAs by qPCR. ∗P < 0.05.
Supplemental Figure 2.
Knockdown of protease-activated receptor (PAR) 1 and PAR4 expression. Human aortic endothelial cells were treated with siRNAs that target either PAR1 or PAR4 (or nonspecific control, Silencer) for 7 days, and PAR1 and PAR4 expression levels, respectively, were measured by flow cytometry as well as real-time quantitative PCR (data not shown). ∗∗P < 0.001 and ∗∗∗P < 0.01. MFI, mean fluorescence intensity.
References
- 1.Leibundgut G., Lee J.H., Strauss B.H., Segev A., Tsimikas S. Acute and long-term effect of percutaneous coronary intervention on serially-measured oxidative, inflammatory, and coagulation biomarkers in patients with stable angina. J Thromb Thrombolysis. 2016;41:569–580. doi: 10.1007/s11239-016-1351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arachiche A., Mumaw M.M., de la Fuente M., Nieman M.T. Protease-activated receptor 1 (PAR1) and PAR4 heterodimers are required for PAR1-enhanced cleavage of PAR4 by alpha-thrombin. J Biol Chem. 2013;288:32553–32562. doi: 10.1074/jbc.M113.472373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin H., Liu A.P., Smith T.H., Trejo J. Cofactoring and dimerization of proteinase-activated receptors. Pharmacol Rev. 2013;65:1198–1213. doi: 10.1124/pr.111.004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakanishi-Matsui M., Zheng Y.W., Sulciner D.J., Weiss E.J., Ludeman M.J., Coughlin S.R. PAR3 is a cofactor for PAR4 activation by thrombin. Nature. 2000;404:609–613. doi: 10.1038/35007085. [DOI] [PubMed] [Google Scholar]
- 5.Rezaie A.R. Protease-activated receptor signalling by coagulation proteases in endothelial cells. Thromb Haemostasis. 2014;112:876–882. doi: 10.1160/TH14-02-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duvernay M.T., Temple K.J., Maeng J.G., Blobaum A.L., Stauffer S.R., Lindsley C.W., Hamm H.E. Contributions of protease-activated receptors PAR1 and PAR4 to thrombin-induced GPIIbIIIa activation in human platelets. Mol Pharmacol. 2017;91:39–47. doi: 10.1124/mol.116.106666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao P., Metcalf M., Bunnett N.W. Biased signaling of protease-activated receptors. Front Endocrinol. 2014;5:67. doi: 10.3389/fendo.2014.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jagels M.A., Travis J., Potempa J., Pike R., Hugli T.E. Proteolytic inactivation of the leukocyte C5a receptor by proteinases derived from Porphyromonas gingivalis. Infect Immunity. 1996;64:1984–1991. doi: 10.1128/iai.64.6.1984-1991.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Proctor L.M., Arumugam T.V., Shiels I., Reid R.C., Fairlie D.P., Taylor S.M. Comparative anti-inflammatory activities of antagonists to C3a and C5a receptors in a rat model of intestinal ischaemia/reperfusion injury. Br J Pharmacol. 2004;142:756–764. doi: 10.1038/sj.bjp.0705819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerard C., Gerard N.P. C5A anaphylatoxin and its seven transmembrane-segment receptor. Annu Rev Immunol. 1994;12:775–808. doi: 10.1146/annurev.iy.12.040194.004015. [DOI] [PubMed] [Google Scholar]
- 11.Paiano J., Harland M., Strainic M.G., Nedrud J., Hussain W., Medof M.E. Follicular B2 cell activation and class switch recombination depend on autocrine C3ar1/C5ar1 signaling in B2 cells. J Immunol. 2019;203:379–388. doi: 10.4049/jimmunol.1900276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strainic M.G., Shevach E.M., An F., Lin F., Medof M.E. Absence of signaling into CD4(+) cells via C3aR and C5aR enables autoinductive TGF-beta1 signaling and induction of Foxp3(+) regulatory T cells. Nat Immunol. 2013;14:162–171. doi: 10.1038/ni.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lalli P.N., Strainic M.G., Yang M., Lin F., Medof M.E., Heeger P.S. Locally produced C5a binds to T cell-expressed C5aR to enhance effector T-cell expansion by limiting antigen-induced apoptosis. Blood. 2008;112:1759–1766. doi: 10.1182/blood-2008-04-151068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strainic M.G., Liu J., Huang D., An F., Lalli P.N., Muqim N., Shapiro V.S., Dubyak G.R., Heeger P.S., Medof M.E. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity. 2008;28:425–435. doi: 10.1016/j.immuni.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Barr S.A., Caguioa J., Gruol D., Perkins G., Ember J.A., Hugli T., Cooper N.R. Neuronal expression of a functional receptor for the C5a complement activation fragment. J Immunol. 2001;166:4154–4162. doi: 10.4049/jimmunol.166.6.4154. [DOI] [PubMed] [Google Scholar]
- 16.Hwang M.S., Strainic M.G., Pohlmann E., Kim H., Pluskota E., Ramirez-Bergeron D.L., Plow E.F., Medof M.E. VEGFR2 survival and mitotic signaling depends on joint activation of associated C3ar1/C5ar1 and IL-6R-gp130. J Cell Sci. 2019;132:jcs219352. doi: 10.1242/jcs.219352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huber-Lang M., Sarma J.V., Zetoune F.S., Rittirsch D., Neff T.A., McGuire S.R., Lambris J.D., Warner R.L., Flierl M.A., Hoesel L.M., Gebhard F., Younger J.G., Drouin S.M., Wetsel R.A., Ward P.A. Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med. 2006;12:682–687. doi: 10.1038/nm1419. [DOI] [PubMed] [Google Scholar]
- 18.Strainic M.G., Liu J., An F., Bailey E., Esposito A., Hamann J., Heeger P.S., Medof M.E. CD55 is essential for CD103(+) dendritic cell tolerogenic responses that protect against autoimmunity. Am J Pathol. 2019;189:1386–1401. doi: 10.1016/j.ajpath.2019.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forbes G.L., Merkulova A., Pinheiro A., Lee J., Zeng P., Abdalian S., Walker A.Y., Wnek G.E., Schmaier A.H. Poly (acrylic acid) (PAA) is a contact system activator with properties to stop hemorrhage. Thromb Res. 2020;193:142–145. doi: 10.1016/j.thromres.2020.06.011. [DOI] [PubMed] [Google Scholar]
- 20.Schmaier A.H. A novel antithrombotic mechanism mediated by the receptors of the kallikrein/kinin and renin-angiotensin systems. Front Med (Lausanne) 2016;3:61. doi: 10.3389/fmed.2016.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stavrou E.X., Fang C., Merkulova A., Alhalabi O., Grobe N., Antoniak S., Mackman N., Schmaier A.H. Reduced thrombosis in Klkb1-/- mice is mediated by increased Mas receptor, prostacyclin, Sirt1, and KLF4 and decreased tissue factor. Blood. 2015;125:710–719. doi: 10.1182/blood-2014-01-550285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta S., Konradt C., Corken A., Ware J., Nieswandt B., Di Paola J., Yu M., Wang D., Nieman M.T., Whiteheart S.W., Brass L.F. Hemostasis vs. homeostasis: platelets are essential for preserving vascular barrier function in the absence of injury or inflammation. Proc Natl Acad Sci U S A. 2020;117:24316–24325. doi: 10.1073/pnas.2007642117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nieman M.T. RAPid signaling in platelets. Blood. 2018;132:1864–1865. doi: 10.1182/blood-2018-09-872093. [DOI] [PubMed] [Google Scholar]
- 24.Amthauer R., Kodukula K., Brink L., Udenfriend S. Phosphatidylinositol-glycan (PI-G)-anchored membrane proteins: requirement of ATP and GTP for translation-independent COOH-terminal processing. Proc Natl Acad Sci U S A. 1992;89:6124–6128. doi: 10.1073/pnas.89.13.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de la Fuente M., Noble D.N., Verma S., Nieman M.T. Mapping human protease-activated receptor 4 (PAR4) homodimer interface to transmembrane helix 4. J Biol Chem. 2012;287:10414–10423. doi: 10.1074/jbc.M112.341438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larusch G.A., Merkulova A., Mahdi F., Shariat-Madar Z., Sitrin R.G., Cines D.B., Schmaier A.H. Domain 2 of uPAR regulates single-chain urokinase-mediated angiogenesis through beta1-integrin and VEGFR2. Am J Physiol Heart Circ Physiol. 2013;305:H305–H320. doi: 10.1152/ajpheart.00110.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakuma M., Morooka T., Wang Y., Shi C., Croce K., Gao H., Strainic M., Medof M.E., Simon D.I. The intrinsic complement regulator decay-accelerating factor modulates the biological response to vascular injury. Arterioscler Thromb Vasc Biol. 2010;30:1196–1202. doi: 10.1161/ATVBAHA.110.205559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Darmoul D., Gratio V., Devaud H., Lehy T., Laburthe M. Aberrant expression and activation of the thrombin receptor protease-activated receptor-1 induces cell proliferation and motility in human colon cancer cells. Am J Pathol. 2003;162:1503–1513. doi: 10.1016/S0002-9440(10)64283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moon J.Y., Franchi F., Rollini F., Angiolillo D.J. Role for thrombin receptor antagonism with vorapaxar in secondary prevention of atherothrombotic events: from bench to bedside. J Cardiovasc Pharmacol Ther. 2018;23:23–37. doi: 10.1177/1074248417708617. [DOI] [PubMed] [Google Scholar]
- 30.Yang J., Xu K., Seiffert D. Challenges and promises of developing thrombin receptor antagonists. Recent Patents Cardiovasc Drug Discov. 2010;5:162–170. doi: 10.2174/157489010793351980. [DOI] [PubMed] [Google Scholar]
- 31.Waitkus M.S., Chandrasekharan U.M., Willard B., Haque S.J., DiCorleto P.E. STAT3-mediated coincidence detection regulates noncanonical immediate early gene induction. J Biol Chem. 2013;288:11988–12003. doi: 10.1074/jbc.M112.428516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moschonas I.C., Kellici T.F., Mavromoustakos T., Stathopoulos P., Tsikaris V., Magafa V., Tzakos A.G., Tselepis A.D. Molecular requirements involving the human platelet protease-activated receptor-4 mechanism of activation by peptide analogues of its tethered-ligand. Platelets. 2017;28:812–821. doi: 10.1080/09537104.2017.1282607. [DOI] [PubMed] [Google Scholar]
- 33.Strainic M.G., Pohlmann E., Valley C.C., Sammeta A., Hussain W., Lidke D.S., Medof M.E. RTK signaling requires C3ar1/C5ar1 and IL-6R joint signaling to repress dominant PTEN, SOCS1/3 and PHLPP restraint. FASEB J. 2020;34:2105–2125. doi: 10.1096/fj.201900677R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khan A., Li D., Ibrahim S., Smyth E., Woulfe D.S. The physical association of the P2Y12 receptor with PAR4 regulates arrestin-mediated Akt activation. Mol Pharmacol. 2014;86:1–11. doi: 10.1124/mol.114.091595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang H., Kuang Y., Wu Y., Smrcka A., Simon M.I., Wu D. Pertussis toxin-sensitive activation of phospholipase C by the C5a and fMet-Leu-Phe receptors. J Biol Chem. 1996;271:13430–13434. doi: 10.1074/jbc.271.23.13430. [DOI] [PubMed] [Google Scholar]
- 36.Lee C.H., Katz A., Simon M.I. Multiple regions of G alpha 16 contribute to the specificity of activation by the C5a receptor. Mol Pharmacol. 1995;47:218–223. [PubMed] [Google Scholar]
- 37.Buhl A.M., Eisfelder B.J., Worthen G.S., Johnson G.L., Russell M. Selective coupling of the human anaphylatoxin C5a receptor and alpha 16 in human kidney 293 cells. FEBS Lett. 1993;323:132–134. doi: 10.1016/0014-5793(93)81464-b. [DOI] [PubMed] [Google Scholar]
- 38.Amatruda T.T., 3rd, Gerard N.P., Gerard C., Simon M.I. Specific interactions of chemoattractant factor receptors with G-proteins. J Biol Chem. 1993;268:10139–10144. [PubMed] [Google Scholar]
- 39.Simons M., Gordon E., Claesson-Welsh L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol. 2016;17:611–625. doi: 10.1038/nrm.2016.87. [DOI] [PubMed] [Google Scholar]
- 40.Rahman A., True A.L., Anwar K.N., Ye R.D., Voyno-Yasenetskaya T.A., Malik A.B. Galpha(q) and Gbetagamma regulate PAR-1 signaling of thrombin-induced NF-kappaB activation and ICAM-1 transcription in endothelial cells. Circ Res. 2002;91:398–405. doi: 10.1161/01.res.0000033520.95242.a2. [DOI] [PubMed] [Google Scholar]
- 41.Xie Y., Abel P.W., Kirui J.K., Deng C., Sharma P., Wolff D.W., Toews M.L., Tu Y. Identification of upregulated phosphoinositide 3-kinase gamma as a target to suppress breast cancer cell migration and invasion. Biochem Pharmacol. 2013;85:1454–1462. doi: 10.1016/j.bcp.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stein P.H., Fraser J.D., Weiss A. The cytoplasmic domain of CD28 is both necessary and sufficient for costimulation of interleukin-2 secretion and association with phosphatidylinositol 3'-kinase. Mol Cell Biol. 1994;14:3392–3402. doi: 10.1128/mcb.14.5.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alberts B., Johnson A., Lewis J., Morgan D., Raff M.C., Roberts K., Walter P., Wilson J., Hunt T. ed 6. Garland Science; New York, NY: 2014. Molecular Biology of the Cell. [Google Scholar]
- 44.Brummel K.E., Paradis S.G., Butenas S., Mann K.G. Thrombin functions during tissue factor-induced blood coagulation. Blood. 2002;100:148–152. doi: 10.1182/blood.v100.1.148. [DOI] [PubMed] [Google Scholar]
- 45.Dupuy E., Habib A., Lebret M., Yang R., Levy-Toledano S., Tobelem G. Thrombin induces angiogenesis and vascular endothelial growth factor expression in human endothelial cells: possible relevance to HIF-1alpha. J Thromb Haemost. 2003;1:1096–1102. doi: 10.1046/j.1538-7836.2003.00208.x. [DOI] [PubMed] [Google Scholar]
- 46.Sullivan B.P., Kopec A.K., Joshi N., Cline H., Brown J.A., Bishop S.C., Kassel K.M., Rockwell C., Mackman N., Luyendyk J.P. Hepatocyte tissue factor activates the coagulation cascade in mice. Blood. 2013;121:1868–1874. doi: 10.1182/blood-2012-09-455436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oikonomopoulou K., Ricklin D., Ward P.A., Lambris J.D. Interactions between coagulation and complement--their role in inflammation. Semin Immunopath. 2012;34:151–165. doi: 10.1007/s00281-011-0280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Graf C., Ruf W. Tissue factor as a mediator of coagulation and signaling in cancer and chronic inflammation. Thromb Res. 2018;164(Suppl 1):S143–S147. doi: 10.1016/j.thromres.2018.01.023. [DOI] [PubMed] [Google Scholar]
- 49.Yu Y., Boing A.N., Hau C.M., Hajji N., Ruf W., Sturk A., Nieuwland R. Tissue factor coagulant activity is regulated by the plasma membrane microenvironment. Thromb Haemost. 2018;118:990–1000. doi: 10.1055/s-0038-1642031. [DOI] [PubMed] [Google Scholar]
- 50.Cohen C.T., Turner N.A., Moake J.L. Production and control of coagulation proteins for factor X activation in human endothelial cells and fibroblasts. Sci Rep. 2020;10:2005. doi: 10.1038/s41598-020-59058-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ku S.K., Bae J.S. Antithrombotic activities of wogonin and wogonoside via inhibiting platelet aggregation. Fitoterapia. 2014;98:27–35. doi: 10.1016/j.fitote.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 52.Lee W., Ku S.K., Bae J.S. Factor Xa inhibits HMGB1-induced septic responses in human umbilical vein endothelial cells and in mice. Thromb Haemost. 2014;112:757–769. doi: 10.1160/TH14-03-0233. [DOI] [PubMed] [Google Scholar]
- 53.Dauchel H., Julen N., Lemercier C., Daveau M., Ozanne D., Fontaine M., Ripoche J. Expression of complement alternative pathway proteins by endothelial cells: differential regulation by interleukin 1 and glucocorticoids. Eur J Immunol. 1990;20:1669–1675. doi: 10.1002/eji.1830200808. [DOI] [PubMed] [Google Scholar]
- 54.Smith T.H., Coronel L.J., Li J.G., Dores M.R., Nieman M.T., Trejo J. Protease-activated receptor-4 signaling and trafficking is regulated by the clathrin adaptor protein complex-2 independent of beta-arrestins. J Biol Chem. 2016;291:18453–18464. doi: 10.1074/jbc.M116.729285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grimsey N.J., Coronel L.J., Cordova I.C., Trejo J. Recycling and endosomal sorting of protease-activated receptor-1 is distinctly regulated by Rab11A and Rab11B proteins. J Biol Chem. 2016;291:2223–2236. doi: 10.1074/jbc.M115.702993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arbore G., West E.E., Spolski R., Robertson A.A.B., Klos A., Rheinheimer C., Dutow P., Woodruff T.M., Yu Z.X., O'Neill L.A., Coll R.C., Sher A., Leonard W.J., Kohl J., Monk P., Cooper M.A., Arno M., Afzali B., Lachmann H.J., Cope A.P., Mayer-Barber K.D., Kemper C. T helper 1 immunity requires complement-driven NLRP3 inflammasome activity in CD4(+) T cells. Science. 2016;352:aad1210. doi: 10.1126/science.aad1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liszewski M.K., Kolev M., Le Friec G., Leung M., Bertram P.G., Fara A.F., Subias M., Pickering M.C., Drouet C., Meri S., Arstila T.P., Pekkarinen P.T., Ma M., Cope A., Reinheckel T., Rodriguez de Cordoba S., Afzali B., Atkinson J.P., Kemper C. Intracellular complement activation sustains T cell homeostasis and mediates effector differentiation. Immunity. 2013;39:1143–1157. doi: 10.1016/j.immuni.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsopanoglou N.E., Papaconstantinou M.E., Flordellis C.S., Maragoudakis M.E. On the mode of action of thrombin-induced angiogenesis: thrombin peptide, TP508, mediates effects in endothelial cells via alphavbeta3 integrin. Thromb Haemostasis. 2004;92:846–857. doi: 10.1160/TH04-04-0208. [DOI] [PubMed] [Google Scholar]
- 59.Canto I., Soh U.J., Trejo J. Allosteric modulation of protease-activated receptor signaling. Mini Rev Med Chem. 2012;12:804–811. doi: 10.2174/138955712800959116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roach T.I., Rebres R.A., Fraser I.D., Decamp D.L., Lin K.M., Sternweis P.C., Simon M.I., Seaman W.E. Signaling and cross-talk by C5a and UDP in macrophages selectively use PLCbeta3 to regulate intracellular free calcium. J Biol Chem. 2008;283:17351–17361. doi: 10.1074/jbc.M800907200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thibeault P.E., LeSarge J.C., Arends D., Fernandes M., Chidiac P., Stathopulos P.B., Luyt L.G., Ramachandran R. Molecular basis for activation and biased signaling at the thrombin-activated GPCR proteinase activated receptor-4 (PAR4) J Biol Chem. 2020;295:2520–2540. doi: 10.1074/jbc.RA119.011461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sheen J.H., Strainic M.G., Liu J., Zhang W., Yi Z., Medof M.E., Heeger P.S. TLR-induced murine dendritic cell (DC) activation requires DC-intrinsic complement. J Immunol. 2017;199:278–291. doi: 10.4049/jimmunol.1700339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kwan W.H., Hashimoto D., Paz-Artal E., Ostrow K., Greter M., Raedler H., Medof M.E., Merad M., Heeger P.S. Antigen-presenting cell-derived complement modulates graft-versus-host disease. J Clin Invest. 2012;122:2234–2238. doi: 10.1172/JCI61019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li G., Fan R.M., Chen J.L., Wang C.M., Zeng Y.C., Han C., Jiao S., Xia X.P., Chen W., Yao S.T. Neuroprotective effects of argatroban and C5a receptor antagonist (PMX53) following intracerebral haemorrhage. Clin Exp Immunol. 2014;175:285–295. doi: 10.1111/cei.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hoth J.J., Wells J.D., Jones S.E., Yoza B.K., McCall C.E. Complement mediates a primed inflammatory response after traumatic lung injury. J Trauma Acute Care Surg. 2014;76:601–608. doi: 10.1097/TA.0000000000000129. discussion 608-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Borkowska S., Suszynska M., Mierzejewska K., Ismail A., Budkowska M., Salata D., Dolegowska B., Kucia M., Ratajczak J., Ratajczak M.Z. Novel evidence that crosstalk between the complement, coagulation and fibrinolysis proteolytic cascades is involved in mobilization of hematopoietic stem/progenitor cells (HSPCs) Leukemia. 2014;28:2148–2154. doi: 10.1038/leu.2014.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Foreman K.E., Vaporciyan A.A., Bonish B.K., Jones M.L., Johnson K.J., Glovsky M.M., Eddy S.M., Ward P.A. C5a-induced expression of P-selectin in endothelial cells. J Clin Invest. 1994;94:1147–1155. doi: 10.1172/JCI117430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu Z., Reiser G. PAR-1 activation rescues astrocytes through the PI3K/Akt signaling pathway from chemically induced apoptosis that is exacerbated by gene silencing of beta-arrestin 1. Neurochem Int. 2014;67:46–56. doi: 10.1016/j.neuint.2013.12.007. [DOI] [PubMed] [Google Scholar]