Abstract
Objective: To systematically review in vitro studies that evaluated the effects of plant extracts on dentin bonding strength.
Materials and Methods: Six electronic databases (PubMed, Embase, VIP, CNKI, Wanfang and The Cochrane Library) were searched from inception to September 2021 in accordance with the Preferred Reporting Items for Systematic Reviews (PRISMA). In vitro studies that compared the performance of dental adhesives with and without the plant extracts participation were included. The reference lists of the included studies were manually searched. Two researchers carried out study screening, data extraction and risk of bias assessment, independently and in duplicate. Meta-analysis was conducted using Review Manager 5.3.
Results: A total of 62 studies were selected for full-text analysis. 25 articles used the plant extracts as primers, while five added the plant extracts into adhesives. The meta-analysis included 14 articles of in vitro studies investigating the effects of different plant extract primers on dentin bonding strength of etch-and-rinse and self-etch adhesives, respectively. The global analysis showed statistically significant difference between dental adhesives with and without plant extract primers. It showed that the immediate bond strength of dental adhesives was improved with the application of plant extract primers.
Conclusion: The application of proanthocyanidin (PA) primers have positive effect on the in vitro immediate bonding strength of dental adhesives irrespective of etch-and-rinse or self-etch modes.
Keywords: dentin, bonding, plant extracts, natural crosslinkers, adhesives, primers
Introduction
Dentin bonding is the foundation of esthetic restoration (Drummond, 2008). Nowadays, manufacturers claim that dental adhesive system has already developed to the eighth generation (Taneja et al., 2017). However, irrespective of acceptable immediate bonds, the long-term bonding strength of these adhesives is inadequate (Deligeorgi et al., 2001; Hass et al., 2016a). As a result, nearly half of esthetic restorations cannot serve for more than 10 years, and dentists have to spend 60% of working hours to replace them (Mjor et al., 2000; Deligeorgi et al., 2001). Thus, the improvement of long-term bond strength is still a puzzle that needs to be solved.
Unsatisfactory long-term dentin bonds are usually attributed to two reasons: The degradation of dentin collagen within the hybrid layer; and the emergence of secondary caries at the interface (Brackett et al., 2011). A reasonable strategy to solve these problems is to modify contemporary dental adhesives with different additives, such as chlorhexidine, nano-silver, carbon nanotube and amorphous calcium phosphate (Carrilho et al., 2007; Borges et al., 2013; Zhang et al., 2013; Alkatheeri et al., 2015). Amongst these additives, plant extracts attracted great attention due to their biological safety and functional versatility (Gotti et al., 2015; Yang et al., 2017; Yu et al., 2017). Many articles have reported the advantages of natural plant extracts, including their capability to stabilize dentin collagen (La et al., 2009), and to inhibit MMPs (Du et al., 2012; Yang et al., 2016) and microbes (Kaul et al., 1985; Rigano et al., 2007). Therefore, many researchers have been attempting to dope plant extracts into adhesives or provide a separate plant extract primer to achieve high antibiotic property and improved long-term bond strength (La et al., 2009; Borges et al., 2013; Gotti et al., 2015; Yang et al., 2017).
However, the combination of different adhesives with different plant extracts may produce unpredictable results, and different concentration of plant extract primer may have different bonding performance (Macedo et al., 2009; Islam et al., 2014). Previous studies have tested a limited amount of plant extracts, using different experimental designs, with contradictory conclusions. Thus, a comprehensive overview summarizing the effect of all existing plant extracts on dental adhesives will be helpful for dental clinicians and relevant researchers.
The objectives of this study are to systematically review the in vitro studies that evaluated adhesive-dentin bond strength with or without plant extracts participation and to compare different plant extracts in terms of bond strength. The hypotheses are: no difference exists in the bond strengths when modifying the adhesives with plant extracts; no difference exists in the bond strengths when plant extract primers are used; no difference exists in the bond strength when using different concentrations of plant extracts.
Materials and Methods
Criteria for Considering Studies for This Review
Inclusion Criteria
• Studies that added plant extracts to dental adhesives or used plant extract as primers.
• Studies that compared the performance of dental adhesives with and without the participation of plant extracts.
• In vitro studies that evaluated the bond strength of dental adhesives.
Exclusion criteria
Reviews, clinical trials or case reports.
Search Strategy
A systematic electronic search was conducted by two independent reviewers (SZ and HY) using nine databases (PubMed, Embase, Web of Science, Cochrane Library, VIP, CNKI, Wanfang, OpenGrey literature and ProQuest Dissertation Abstracts) from inception to September 2021 to identify articles related to plant extracts and dental bonding. The search terms were a combination of subject terms and free-text terms (Appendix Table A1).
When multiple publications about the same intervention were identified, the most informative and relevant article was selected for inclusion.
Data Collection and Analysis
Selection of Studies
Article titles and abstracts were independently screened by two authors (SZ and HY). The authors conducted a second review when the inclusion criteria were met. The abstracts were examined by two review authors (SZ and HY) independently using the same inclusion criteria. If there were disagreements, the abstract would be assessed by the third author (FH). Then full text of all potentially relevant studies were retrieved and independently assessed in duplicate by two review authors (SZ and HY). Any disagreement regarding the eligibility of the included studies was resolved through discussion with the third reviewer.
Data Extraction and Management
Data extraction was performed independently by two authors (SZ and HY). The demographic data, plant extracts used, plant extract concentration, bonding systems, as well as outcomes were recorded (Table 1). If any information was missing, we contacted the corresponding authors via email.
TABLE 1.
Characteristics of the included studies.
| First author | Year | Country | Publication | Plant extracts | Action modes | Plant extracts concentration | Dental adhesives | Outome |
|---|---|---|---|---|---|---|---|---|
| Albuquerque N | 2019 | Brazil | Oper Dent | EGCG | Adhesive | 0.1% w/v | Single Bond 2 (3M ESPE, St. Paul, MN, United States) | MTBS |
| Yang H | 2017 | China | SCI REP | Quercetin | Adhesive | 100, 500 and 1,000 μg/ml | Single Bond 2 | MTBS |
| Yu HH | 2017 | China | Materials (Basel) | EGCG, EGCG-3Me | Adhesive | 200, 400, and 600 μg/ml | Single Bond 2 | MTBS |
| Gotti VB | 2015 | Brazil | J Adhes Dent | Quercetin | Adhesive | 5 wt% | Single Bond 2; Clearfil SE Bond (Kurary Noritake Dental; Tokyo, Japan); Easy Bond (3M ESPE, St. Paul, MN, United States) | MTBS |
| Du X | 2012 | China | J Dent | EGCG | Adhesive | 100, 200, and 300 μg/ml | Single Bond 2 | MTBS |
| Peng W | 2020 | China | Materials Science and Engineering C | Resveratrol | Primer | 1, 10, and 20 μg/ml | Single Bond Universal | MTBS |
| Zhang Z | 2020 | China | Dental Materials | EGCG | Primer | 0.01%, 0.1%, 1% | Single Bond Universal | MTBS |
| Landmayer K | 2020 | Brazil | J Prosthet Dent | EGCG; Proanthocyanidin (PA) | Primer | EGCG at 400 μM; 10% PA | Single Bond 2 | MTBS |
| Dávila-Sánchez A | 2020 | Chile | Dent Mater | Quercetin; Hesperidin; Rutin; Naringin; Proanthocyanidin | Primer | 0.065 | Single Bond Universal | MTBS |
| de Siqueira FSF | 2020 | Brazil | Clin Oral Investig | Proanthocyanidin | Primer | 0.065 | Prime and Bond Elect (Dentsply Sirona, Milford, DE, United States); Single Bond Universal; Tetric n-Bond Universal (Ivoclar Vivadent AG, Schaan, Liechtenstein) | MTBS |
| Yi L | 2019 | China | J Dent | Baicalein | Primer | 0.01%, 0.05%, and 1% w/v | Single Bond Universal | MTBS |
| Albuquerque N | 2019 | Brazil | Oper Dent | EGCG | Primer | 0.1% EGCG; or 1% PLGA/EGCG | Single Bond 2 | MTBS |
| Costa CAG | 2019 | Brazil | J Adhes Dent | EGCG | Primer | 0.1% EGCG; or 2% CHX | Clearfil SE Bond | MTBS |
| Fialho MPN | 2019 | Brazil | J Mech Behav Biomed Mater | EGCG | Primer | 0.02%; 0.2%; 0.5% | Single Bond 2 | MTBS |
| Li J | 2018 | China | Oper Dent | Baicalein | Primer | 0.1, 0.5, 2.5, and 5.0 μg/ml | Single Bond 2 | MTBS |
| Porto ICCM | 2018 | Brazil | Eur J Oral Sci | Quercetin; Resveratrol | Primer | 100, 250, 500, or 1,000 μg ml, a mixture of quercetin and resveratrol (3:1, 1:1, 1:3; vol:vol | Single Bond Universal | MTBS |
| Bacelar-Sá R | 2017 | Brazil | Braz Dent J | Proanthocyanidin | Primer | 0.065 | Single Bond Universal; Prime and Bond Elect; All-Bond 3 (Bisco Inc., Schaumburg, IL, United States); G-Aenial (GC Corp., Tokyo, Japan) | MTBS |
| Li K | 2017 | China | RSC Adv | Quercetin | Primer | 0.1, 0.5, and 1 wt% | Single Bond 2 | MTBS |
| Zheng P | 2017 | China | Sci Rep | Proanthocyanidin | Primer | 0.05 | Single Bond 2 | MTBS |
| Zhou J | 2016 | China | Dent Mater | Grape seed extract | Primer | 5 mass% | Single Bond 2 | MTBS |
| Hass V | 2016 | Brazil | Dent Mater | Proanthocyanidin | Primer | 6.5 wt% | Single Bond Plus; Tetric N-Bond | MTBS |
| Yang H | 2016 | United States | J Dent | EGCG | Primer | 0.02% and 0.1% | Single Bond 2 | MTBS |
| Zheng P | 2015 | China | Oper Dent | Grape seed extract | Primer | 0.0005 | OptiBond FL (Kerr, Scafati, Italy); Clearfil SE Bond | MTBS |
| Islam MS | 2014 | Japan | Dent Mater | Proanthocyanidin; Hesperidin | Primer | 0.5%, 1%, 2%, 5% of hesperidin (HPN) or 0.5% of proanthocyanidins (PA) | Clearfil SE Bond | MTBS |
| Liu RR | 2014 | China | Int J Oral Sci | Proanthocyanidin | Primer | 10% or 15% | Single Bond 2 | MTBS |
| Santiago SL | 2013 | Brazil | J Adhes Dent | EGCG | Primer | 0.02%, 0.1%, or 0.5% w/v | Single Bond 2 | MTBS |
| Broyles AC | 2013 | United States | J Prosthodont | Grape seed extract | Primer | 0.065 | RelyX Unicem (3M ESPE, St. Paul, MN, United States); G-Cem self-adhesive cements (GC America, Alsip, IL) | MTBS |
| Liu RR | 2012 | China | Zhonghua Kou Qiang Yi Xue Za Zhi | Proanthocyanidin | Primer | 0.15 | Single Bond 2 | MTBS |
| Macedo GV | 2009 | United States | J Dent Res | Grape seed extract | Primer | 0.065 | Single Bond 2; One Step Plus (Bisco, Schaumburg, IL, United States) | MTBS |
| Al-Ammar A | 2009 | United States | J Biomed Mater Res B Appl Biomater | Grape seed extract; Genipin | Primer | 6.5% GSE; 0.5% GE | One Step Plus; Single Bond Plus | MTBS |
Abbreviation: EGCG, epigallocatechin-3-gallate; EGCG-3Me, epigallocatechin-3-O-(3-O-methyl)-gallate; GSE, grape seed extract; GE, genipin.
Quality Assessment
Two reviewers (SZ and HY) independently assessed the risk of bias of the included studies with the assessment instrument used in a previous systematic review of in vitro studies (Sarkis-Onofre et al., 2014). Quality assessment parameters included randomized teeth, teeth free of caries or restoration, operation following the manufacturer’s instructions, given sample size, and the bonding procedures were performed by a single operator with or without blinding. The article would be given a “Yes” on the parameter if it was reported and performed appropriately in the article; and a “No” if it was not mentioned or not performed properly. Articles were classified into three levels of risk of bias according to the number of parameters that scored “Yes”: high (≤2 parameters), medium (3-4 parameters), and low (5-6 parameters) (Table 2).
TABLE 2.
Risk of bias of the studies considering aspects reported in the Materials and Methods section.
| Study | Year | Random | Caries | Adhesive | Sample | Operator | Blind | Risk |
|---|---|---|---|---|---|---|---|---|
| Peng W | 2020 | Y | Y | Y | Y | Y | N | Low |
| Zhang Z | 2020 | Y | N | Y | Y | Y | N | Medium |
| Landmayer K | 2020 | N | Y | Y | Y | N | N | Medium |
| Dávila-Sánchez A | 2020 | Y | Y | Y | Y | Y | N | Low |
| de Siqueira FSF | 2020 | Y | Y | Y | Y | Y | N | Low |
| Albuquerque N | 2019 | Y | Y | Y | Y | N | N | Medium |
| Yi L | 2019 | Y | Y | Y | Y | Y | N | Low |
| Albuquerque N | 2019 | N | Y | Y | Y | N | N | Medium |
| Costa CAG | 2019 | Y | Y | Y | Y | N | N | Medium |
| Fialho MPN | 2019 | N | Y | Y | Y | Y | N | Medium |
| Li J | 2018 | Y | Y | Y | Y | N | N | Medium |
| Porto ICCM | 2018 | Y | Y | Y | Y | N | N | Medium |
| Yang H | 2017 | Y | Y | Y | Y | N | N | Medium |
| Yu HH | 2017 | Y | Y | Y | Y | N | N | Medium |
| Bacelar-Sá R | 2017 | N | Y | Y | Y | N | N | Medium |
| Li K | 2017 | Y | Y | Y | Y | N | N | Medium |
| Zheng P | 2017 | N | Y | Y | Y | N | N | Medium |
| Zhou J | 2016 | N | Y | Y | Y | N | N | Medium |
| Hass V | 2016 | N | Y | Y | Y | N | N | Medium |
| Yang H | 2016 | N | Y | Y | Y | Y | N | Medium |
| Gotti VB | 2015 | Y | N | Y | Y | N | N | Medium |
| Zheng P | 2015 | Y | Y | Y | Y | N | N | Medium |
| Islam MS | 2014 | N | Y | Y | Y | N | N | Medium |
| Liu RR | 2014 | Y | Y | Y | Y | N | N | Medium |
| Santiago SL | 2013 | N | Y | Y | Y | N | N | Medium |
| Broyles AC | 2013 | Y | Y | Y | Y | N | N | Medium |
| Du X | 2012 | Y | N | Y | Y | N | N | Medium |
| Liu RR | 2012 | Y | Y | Y | Y | N | N | Medium |
| Macedo GV | 2009 | Y | Y | Y | Y | N | N | Medium |
| Al-Ammar A | 2009 | Y | Y | Y | Y | N | N | Medium |
Statistical Analysis
Meta-analysis was conducted using Review Manager 5.3. Each possible comparison of the bond strength of dental adhesives with or without plant extracts participation was undertaken. In order to minimize the heterogeneity, only in vitro studies comparing the same plant extracts with the same concentration was included in the global analysis. The mean difference with 95% confidence interval (CI) was calculated and p ≤ 0.05 was considered significant. Statistical heterogeneity was assessed using the modified chi-square test (Cochran’s Q), which indicates heterogeneity when p>0.1, and I2 test, which indicates heterogeneity when its values is greater than 50%. Random-effect model was used in the analysis. The publication bias was to be assessed if more than ten studies were included in a meta-analysis. Sensitivity analysis was also performed by sequentially excluding each study if there were sufficient studies (≥10).
Results
Search Strategy and Characteristics
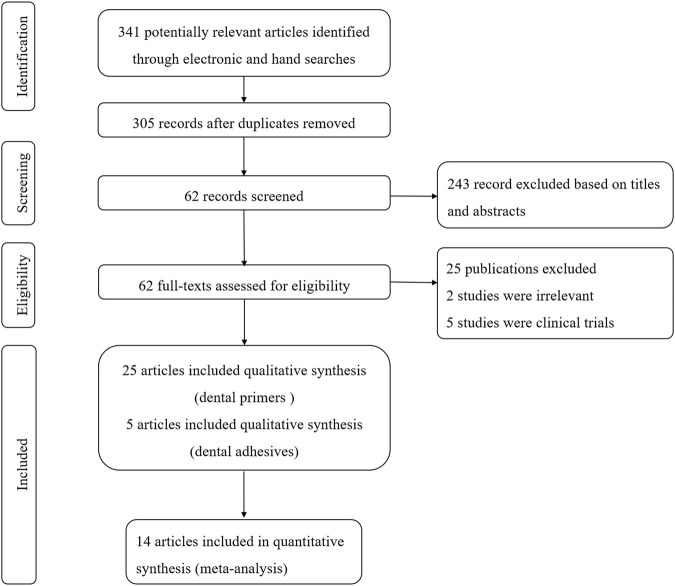
The initial search yielded 341 articles, out of which, 36 articles were eliminated after screening of titles and removal of duplicates. After abstract screening, 243 articles were excluded. A resultant sample of 62 articles was carried forward to the next stage, in which full-text copies were scrutinized. Finally, a total of 30 studies were systematically reviewed, in which 5 studies added plant extracts into adhesives and 25 studies used plant extract solution as primers (Figure 1). Twenty-nine articles were in English and 1 were in Chinese. There are nine types of plant extracts and 15 types of adhesives involved (Table 1).
FIGURE 1.
Flow chart of study selection according to PRISMA statement.
Risk of Bias
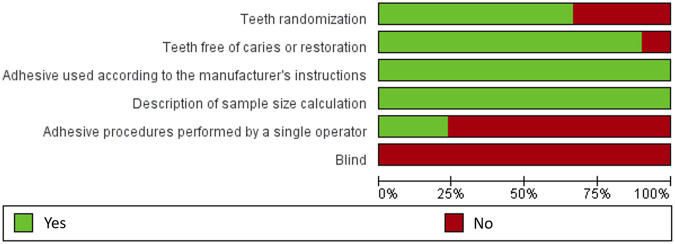
Most of the 30 studies (86.7%) exhibited a medium risk of bias, except for four (13.3%) with a low risk of bias. All of the studies used the adhesive according to the manufacturer’s instructions and described sample size calculation, but none of the studies reported blinding. A total of 20 studies (66.7%) reported random assignment of teeth, and 27 studies (90%) used teeth free of caries. Only seven studies (23.3%) reported adhesive procedure performed by a single operator. The results are described in Figure 2 and Table 2.
FIGURE 2.
Risk of bias graph judgements about each risk of bias item presented as percentages across all included studies.
Meta-Analysis
In the studies included in the meta-analysis, we only choose the data of interest. Only commercial adhesives were included, and the studies used experimental adhesives were excluded (Epasinghe et al., 2012). The effect of plant extracts on bonding strength may be related to different bonding modes such as self-etch or etch-and-rinse (Macedo et al., 2009; Bacelar-Sa et al., 2017). Hence, the disparity of the bond strength of different plant extracts in self-etch or etch-and-rinse adhesives was compared. Because aging methods were highly heterogeneous (i.e., water storage, saliva storage and PH cycling), it was not considered in the meta-analysis (Deng et al., 2014).
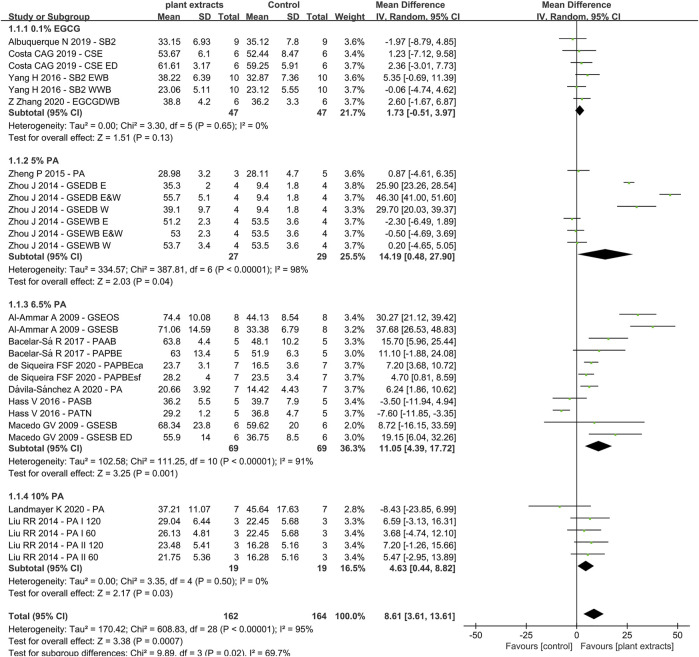
Due to the fact that different concentrations of plant extracts were used, only those with the same concentration were taken into meta-analysis. Of the 30 studies, data from 14 papers in which plant extract solution serve as primers underwent meta-analysis. The results of the meta-analysis are shown in Figures 3–5.
FIGURE 3.
Forest Plot—plant extract primers: etch-and-rinse immediate bond strength.
FIGURE 5.
Forest Plot—proanthocyanidin (PA) primers
Etch-and-Rinse Bond Strength (Plant Extract Primers)
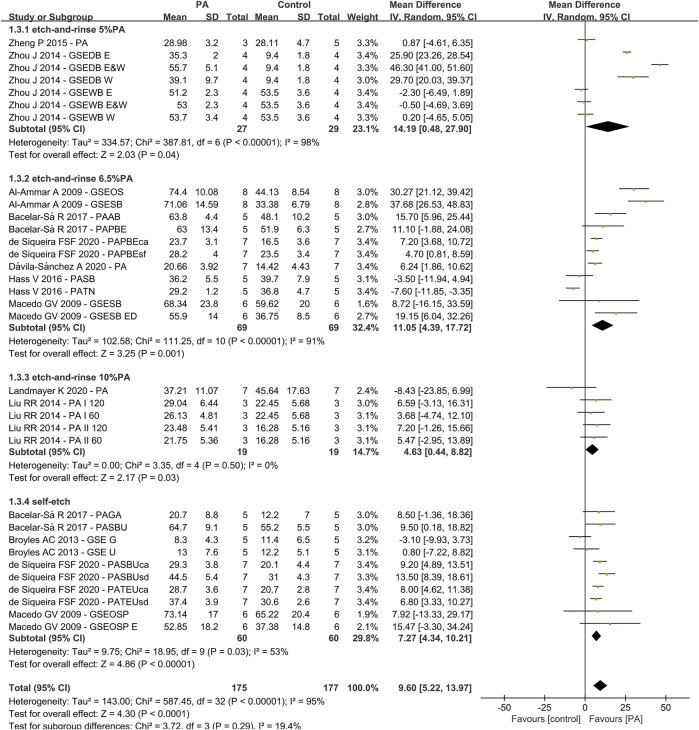
The first analysis (etch-and rinse adhesive with or without plant extract primers) was performed, and the different concentration of plant extracts were the subgroups. A total of 29 datasets were selected, while 14 studies were included (Figure 3), with the following results: Q-test p < 0.00001, I2 = 95% and overall effect p = 0.0007. Test for subgroup differences: Q-test p = 0.02 and I2 = 69.7%, which showed that the data of subgroups were consistent.
The data of subgroup using 0.1% EGCG as primer showed no statistically significant differences compared with control group (Z-test: p > 0.05). However, the result of proanthocyanidin (PA) showed that the experimental groups had significant higher bond strengths than the control groups, with overall effect p < 0.05. For primers with 5% PA and 6.5% PA, the result in the Q-test was both p < 0.01 and I2 = 98%, I2 = 91%, separately. However, the result of 10% PA in the Q-test was p > 0.05 and I2 = 0%. The results of the meta-analysis are shown in Figure 3.
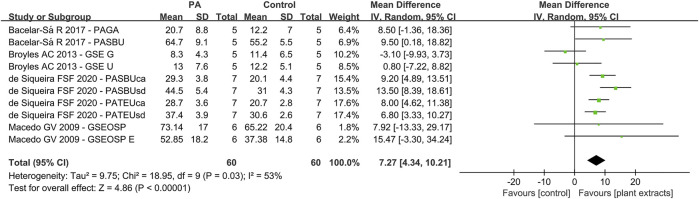
Self-Etch Bond Strength (Plant Extract Primers)
For the second analysis (self-etch adhesive with or without plant extract primers), 10 data sets were selected, with four studies included (Figure 4). The results were as followings: Q-test p < 0.05 and I2 = 53%. The global analysis showed statistically significant difference (p < 0.05).
FIGURE 4.
Forest Plot—plant extract primers: self-etch immediate bond strength.
Primers With Vs Without Proanthocyanidin
For the third analysis (primers with or without PA), 11 studies and 33 datasets were included (Figure 5). The difference between control and experimental groups were statistically significant (Q-test: p < 0.01, I2 = 95% and Z-test: p < 0.05). The differences in the test for subgroups (primers with different concentration of PA) showed the following values: chi-squared = 3.72, df = 3 (p = 0.29) and I2 = 19.4%. The meta-analysis results are shown in Figure 5.
Discussion
This systematic review is the first to verify the effects of plant extracts on dentin bonding strength from in vitro studies. Thorough database research was conducted, and data were extracted and integrated in tables. Each study was designed and performed on the basis of their own parameters (plant extract types, action modes, concentration, dental adhesives and indicators), as listed in Table 1. Nine different plant extracts were added into 15 types of adhesives or served as primers. Out of the 30 studies, the data from 14 were selected for further evaluation.
As shown in Table 1, there were different commercial adhesives used. We had undertaken several measures to avoid the discrepancy. Firstly, the details of the adhesives were listed, such as commercial name, manufacturer, and place of production. Secondly, the articles that used experimental adhesives were excluded in the present study. Thirdly, 19 of 30 included studies chose the same one commercial adhesive, Single Bond 2(3M ESPE, St. Paul, MN, United States). Furthermore, all included studies set the control group which did not add plant extracts into adhesives or serve as primers. All these strategies were helpful to eliminate the disturbance of different adhesives on research results to the utmost extent. Furthermore, the included studies all reported the manufacturers and details of plant extracts, such as resveratrol powder (Sigma–Aldrich, St. Louis, MO, United States), and the pureness of this product was listed as ≥99% (HPLC).
Since plant extract was introduced, its effectiveness in crosslinking and biocompatibility has drawn a lot of attention. Different plant extracts were investigated, listing as follows: proanthocyanidin (PA), epigallocatechin-3-gallate (EGCG), quercetin (QUE), resveratrol (RSV), baicalein (BAI), hesperidin (HES), rutin (RUT) and naringin (NAR). Firstly, despite the chemical structure differences, they all belong to plant polyphenol, which possesses antioxidant and anti-inflammatory properties. These effects are mainly derived from phenolic hydroxyl groups in polyphenols (Leopoldini et al., 2011). The highly-hydroxylated structures make them capable of forming insoluble complexes with carbohydrates and proteins (Bravo, 1998; Teixeira et al., 2002). The major force that stabilizes the plant-extract-protein complexes is hydrogen bonding between phenolic hydroxyl and peptide carbonyl (Hagerman and Butler, 1980a; Hagerman and Butler, 1980b), which is strengthened by alkyl substitution on the amide nitrogen adjacent to the carbonyl (Cannon, 1955). Therefore, the mechanical properties of collagen complex would be increased (Yang et al., 2016). Secondly, plant extracts, such as baicalein and resveratrol, can inhibit the activity of peptidases directly or indirectly by changing the catalytic domain (Mazzoni et al., 2018) or crosslinking with noncollagenous proteins regulating peptidases (Breschi et al., 2010; Cova et al., 2011). Thirdly, many plant extracts, like baicalein, are commonly used in herbal medicines to treat bacterial and viral infections. They show remarkable antimicrobial effects on different bacteria including Escherichia coli, P. cuspidatum and S.mutans (Song et al., 2006; Duan et al., 2007; Zeng et al., 2008; Chinnam et al., 2010; Jang et al., 2014). The mechanisms are not clear yet, but it might be attributed to the inhibition of the cellular growth (Paulo et al., 2010).
One of the most studied plant extracts is proanthocyanidin (PA), also known as grape seed extracts (GSE) (Al-Ammar et al., 2009; Macedo et al., 2009; Liu et al., 2012; Broyles et al., 2013; Liu et al., 2014; Islam et al., 2014; Zheng et al., 2015; Zhou et al., 2016; Hass et al., 2016b; Bacelar-Sa et al., 2017; Zheng and Chen, 2017; de Siqueira et al., 2020; Ds et al., 2020; Landmayer et al., 2020). It is a condensed tannins extracted from Vitis vinifera grapes, which has been reported to contain 79.6% polyphenols(Aguiar et al., 2014). PA is composed of flavon-3-ol subunits, catechin, epicatechin and epicatechin-3-O-gallate and linked through C4-C8 (Cavaliere et al., 2010). These components are responsible for their properties such as free-radical scavenging capacity, high affinity for protein, antioxidant potential and capacity to enhance the mechanical properties of collagen (Castellan et al., 2010; Leme-Kraus et al., 2017). Epasinghe et al. (2012) reported that incorporation of less than 3% proanthocyanidin into dental adhesive can reduce nanoleakage without comprising 24 h adhesive-dentin bond strength. The meta-analysis of the PA primer effects on bonding showed a significant positive effect compared with the control group, irrespective of the concentrations or the type of adhesive used (Al-Ammar et al., 2010; Macedo et al., 2009; Liu et al., 2014; Wiegand et al., 2015; Zhou et al., 2016; Hass et al., 2016a; Bacelar-Sa et al., 2017; Ds et al., 2020; Landmayer et al., 2020; Siqueira et al., 2020). However, the results of 5 and 6.5% PA primer revealed a heterogeneity of 98% and 91% (Figure 3). The reason might be attributed to different bonding techniques such as dry bonding and wet bonding (Zhou et al., 2016) For 10% PA primer, the bonding strength shows statistically significant elevation with no heterogeneity (Liu et al., 2014; Landmayer et al., 2020). Although the heterogeneity varies from group to group, the subgroup analysis revealed no significant differences, which also prove the effectiveness of PA primer.
Another important plant extract being intensely investigated is epigallocatechin-3-gallate (EGCG) (Du et al., 2012; Santiago et al., 2013; Yang et al., 2016; Yu et al., 2017; Albuquerque et al., 2019; Costa et al., 2019; Fialho et al., 2019; Landmayer et al., 2020; Zhang et al., 2020). It is one of the flavanols in tea, also known as catechins (Tachibana, 2011). As a representative component of green tea, it cannot be found in any plants except C. sinensis (L.) Kuntze (Tachibana, 2011). EGCG consists of a meta-5,7-dihydroxyl-substituted A ring and trihydroxy phenol structures on both the B and D rings (Peter et al., 2017). The polyphenolic structure makes EGCG good donors for hydrogen bonding (Yang et al., 2009). Thus, it has shown the ability to bring various health benefits, like anti-metastasis, anti-inflammatory and antioxidant effects (Mukhtar and Ahmad, 2000; Mereles and Hunstein, 2011; Suzuki and Isemura, 2013). The similarity of chemical structure with other flavanols like PA makes it capable of enhancing the mechanical strength of collagen. The addition of EGCG directly into adhesives has been proven to preserve the bond strength after different ageing methods (Du et al., 2012; Yu et al., 2017; Albuquerque et al., 2019). The result of EGCG primer showed no negative influence on immediate bond strength (Zhang et al., 2020). The lack of data and various ageing methods make it impossible to do meta-analysis on aged bond strength. However, plenty of articles showed EGCG primer can improve the bond stability (Landmayer et al., 2020; Zhang et al., 2020). Furthermore, Yu et al. (2017) created a derivative of EGCG, called EGCG-3Me, which can enhance the bond stability, inhibited S.mutans adhesion and hinder its growth.
There are other plant extracts included in this systematic review: quercetin (QUE) (Gotti et al., 2015; Yang et al., 2017; Ds et al., 2020), resveratrol (RSV) (Porto et al., 2018; Peng et al., 2020), baicalein (BAI) (J. Li et al., 2018; Yi et al., 2019), genipin (GEN) (Al-Ammar et al., 2009), hesperidin (HES) (Islam et al., 2014; Ds et al., 2020), rutin (RUT) (Ds et al., 2020), and naringin (NAR) (Ds et al., 2020). The molecular formula, mass and number of hydroxyphenyl radicals are listed in Table 3. The data are inadequate to perform meta-analysis.
TABLE 3.
Physical and chemical properties of the plant extracts and their possible effects on immediate bonding strength.
| Plant extracts | Molecular formula | Mol. Weight (g/mol) | Number of hydroxyphenyl radicals | Effects on immediate bonding strength |
|---|---|---|---|---|
| Proanthocyanidin | C30H26O13 | 594.5 | 7 |

|
| Epigallocatechin gallate | C22H18O11 | 458.4 | 8 |

|
| Quercetin | C15H10O7 | 302.2 | 5 |

|
| Resveratol | C14H12O3 | 228.2 | 3 |

|
| Baicalein | C15H10O5 | 270.2 | 3 |

|
| Genipin | C11H14O5 | 226.2 | 2 |

|
| Hesperidin | C28H34O15 | 610.6 | 2 |

|
| Rutin | C27H30O16 | 610.5 | 4 |

|
| Naringin | C27H32O14 | 580.5 | 2 |

|
Green = evident; Yellow = unclear; Red = not recommended.
As natural crosslinkers, there are many factors influencing the crosslinking process. For instance, 1) the molecule size; 2) the number of molecules available in the solution; 3) the solubility index of the molecule and its influence on the miscibility of the vehicle for its application in dentin; 4) the number and type of reactive sites of the molecule; 5) the characteristics of the dentin (Ds et al., 2020).
It is a paradox that the bigger molecules usually have more reactive sites that can enhance the crosslinking effect, but their ability to dissolve and diffuse would be lower than smaller ones. Moreover, the type of molecules in grape seed extracts are complex, with monomers, oligomers and polymers existing at the same time (Bravo, 1998). The size of the oligomers and polymers were larger, which makes it more difficult to diffuse into dentin tubules. According to the results of included studies, we concluded the possible effects of different plant extracts on immediate bonding strength and classified them into different colors: green means the effects on improving bonding strength were evident; yellow means more studies in need; red means probable adverse effects (Table 3).
The plant extracts are normally recognized as plant polyphenols, which encompass a wide variety of molecules that contain at least one aromatic ring with one or more hydroxyl groups (Ferrazzano et al., 2011). Although they were extracted from different plants, the similarity in their chemical structure makes it possible for them to all possess properties like antioxidation and anti-bacterium. To begin with, the highly-hydroxylated structures make them capable of forming complexes with proteins, especially proline-rich proteins in dental collagen (Bravo, 1998). This fortified crosslinking interaction helps enhance the mechanical strength of dental bonding (Yang et al., 2017; Yi et al., 2019; Peng et al., 2020). Furthermore, the polyphenolic compounds could coordinate with metal ions and compete with peptidases such as MMPs for the catalytic domain in collagen (Mazzoni et al., 2018). As a result, the enzymatic hydrolysis of hybrid layer collagen would be impeded and the adhesive-dentin interface stability would be maintained (Epasinghe et al., 2012; Yang et al., 2016; Yang et al., 2017). Besides, the plant polyphenols were considered metabolites involved in the chemical defense of plants and possess the ability to inhibit bacteria (Ferrazzano et al., 2011). There is plenty of evidence supporting the inhibition of cariogenic bacteria by phenolic compounds. The mechanisms of polyphenols against bacteria like S.mutans may include affecting cell membrane permeability, inhibiting protein synthesis, blocking ATP synthesis and inhibiting bacterial metabolism (Chinnam et al., 2010; Xie et al., 2015). Lastly, as natural crosslinkers, the plant polyphenols are non-toxic compared to synthetic compounds like chlorhexidine and glutaraldehyde. They can protect cells by inhibiting oxidative stress-induced DNA damage, lipid peroxidation and protein oxidation (Kang et al., 2012). To conclude, all these in vitro studies demonstrated that the plant extracts, consisting of polyphenols, can enhance mechanical strength of dentin collagen, maintain dentin-adhesive stability, inhibit cariogenic bacteria and resist adhesive-induced cytotoxicity.
Although plant extracts have shown plenty of advantages, there are still a large variety of aspects to be explored, such as solvent, treatment time and concentrations. First, theoretically, the effect of plant extracts would increase with the concentration. However, the solubility of the compounds were not great (Bravo, 1998). Zhang et al. (2020) reported EGCG with dimethyl sulfoxide as a solvent can exert synergistic effect on dentin-adhesive interface stability. Second, the treatment time varies from one to another. Genipin is reported to have a slow rate of cross-linking induction that the mechanical strength increased only after 40 h treatment (Bedran-Russo et al., 2007). Third, the effect of different concentration on bonding is complex. It has been shown more than 3% PA added into adhesive directly can exert adverse effect on bonding (Epasinghe et al., 2012). More studies are needed to determine the suitable solvent, treatment time and concentrations of plant extracts.
As mentioned in this review, plant extracts are actually polyphenols, which possess phenolic hydroxyl groups and aromatic rings (Ferrazzano et al., 2011). Therefore, their solubility in solvents such as ethanol are high, due to their similar chemical structure like hydroxyl groups. Furthermore, the interactions between plant extract (eg. PA) and collagen can be disrupted by detergents of hydrogen bond-weakening solvents, suggesting that PA-collagen complex formation involves primarily hydrogen bonding between the protein amide carbonyl and the phenolic hydroxyl (Hagerman and Klucher, 1986). Ethanol, on the other hand, stimulate PA and collagen interactions (Bo et al., 2010). There is no evidence that the interaction is concentration-dependent.
The present study showed the changes in dentin bond strength after adding plant extracts into adhesives or serving as primers. Although strict selection was performed to minimize heterogeneity, the data of several subgroups remained high heterogeneous. There are three reasons for heterogeneity:1. Different adhesive brands; 2. Different bonding modes (etch-and-rinse or self-etch); 3. Different dentin material (normal or eroded dentin). Also, several authors failed to report important details, such as whether the same operator performed the bonding steps of all specimens. These factors may help explain the high heterogeneity in in vitro experiments.
Conclusions
Plant extracts have positive effects on the immediate microtensile bond strength of the adhesive-dentin interface. Meta-analysis demonstrated that the use of proanthocyanidin (PA) primer, especially at the concentration of 10%, had statistically significant effect on the immediate dentin bonding strength. Considerable heterogeneity existed among the different adhesive brands, bonding modes and dentin materials used, which limited the meta-analysis approach. Further clinical research is needed to confirm the effect of plant extracts on bond strength in vivo.
Appendix
Table A1.
Search strategy used for PubMed (from inception to September 2021).
| Search terms | |
|---|---|
| #1 | Epigallocatechin gallate OR epigallocatechin-3-gallate OR epigallocatechin-3-O-gallate OR “EGCG cpd” OR epigallo-catechin gallate |
| #2 | Quercetin[MeSH] |
| #3 | Genipin |
| #4 | Proanthocyanidins[MeSH] OR “condensed tannin*” OR “anthocyanidin polymer*” OR procyanidin* |
| #5 | Naringenin |
| #6 | Hesperidin[MeSH] OR “hesperetin 7 rhamnoglucoside” OR “hesperetin 7 rutinoside” |
| #7 | “Crosslinking agent*” OR “cross link*” |
| #8 | Plant extracts[MeSH] |
| #9 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 |
| #10 | Dental cements[MeSH] OR “dental adhesive*” OR “luting agent*” OR dentistry [MeSH] |
| #11 | Bond* AND strength |
| #12 | #9 AND #10 AND #11 |
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
The conception and design of the study were performed by HY and CH. Literature retrieving and studies selection were performed by SZ and JY. Quality evaluation was carried out by CH and JY. Mathematical modeling and meta-analysis were conducted by FH and SZ. Results analysis and interpretation were undertaken by HY and SZ. The manuscript was drafted by HY and SZ. All authors read and approved the final manuscript.
Funding
This work was financially supported by National Natural Science Foundation of China (81701012), Youth Clinical Research Fund of Chinese Stomatological Association (CSA-B2018-01).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Aguiar T. R., Vidal C. M. P., Phansalkar R. S., Todorova I., Napolitano J. G., Mcalpine J. B., et al. (2014). Dentin Biomodification Potential Depends on Polyphenol Source. J. Dent Res. 93 (4), 417–422. 10.1177/0022034514523783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ammar A., Drummond J. L., Bedran-Russo A. K. (2010). The Use of Collagen Cross-Linking Agents to Enhance Dentin Bond Strength. J. Biomed. Mater. Res. B Appl. Biomater. 91 (1), 419–424. 10.1002/jbm.b.31417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ammar A., Drummond J. L., Bedran-Russo A. K. (2009). The Use of Collagen Cross-Linking Agents to Enhance Dentin Bond Strength. J. Biomed. Mater. Res. 91B (1), 419–424. 10.1002/jbm.b.31417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque N., Neri J. R., Lemos M., Yamauti M., de Sousa F., Santiago S. (2019). Effect of Polymeric Microparticles Loaded with Catechin on the Physicochemical Properties of an Adhesive System. Oper. Dent 44 (4), E202–E211. 10.2341/18-112-L [DOI] [PubMed] [Google Scholar]
- Alkatheeri M. S., Palasuk J., Eckert G. J., Platt J. A., Bottino M. C. (2015). Halloysite Nanotube Incorporation into Adhesive Systems-Effect on Bond Strength to Human Dentin. Clin. Oral Invest. 19 (8), 1905–1912. 10.1007/s00784-015-1413-8 [DOI] [PubMed] [Google Scholar]
- Bacelar-Sá R., Giannini M., Ambrosano G. M. B., Bedran-Russo A. K. (2017). Dentin Sealing and Bond Strength Evaluation of Hema-free and Multi-Mode Adhesives to Biomodified Dentin. Braz. Dent. J. 28 (6), 731–737. 10.1590/0103-6440201701522 [DOI] [PubMed] [Google Scholar]
- Bedran-Russo A. K. B., Pereira P. N. R., Duarte W. R., Drummond J. L., Yamauchi M. (2007). Application of Crosslinkers to Dentin Collagen Enhances the Ultimate Tensile Strength. J. Biomed. Mater. Res. 80B (1), 268–272. 10.1002/jbm.b.30593 [DOI] [PubMed] [Google Scholar]
- Bo H., Jaurequi J., Bao W. T., Nimni M. E. (2010). Proanthocyanidin: a Natural Crosslinking Reagent for Stabilizing Collagen Matrices. J. Biomed. Mater. Res. A 65A. [DOI] [PubMed] [Google Scholar]
- Borges B. C. D., Catelan A., Sasaki R. T., Ambrosano G. M. B., Reis A. F., Aguiar F. H. B. (2013). Effect of the Application of a Casein Phosphopeptide-Amorphous Calcium Phosphate (CPP-ACP) Paste and Adhesive Systems on Bond Durability of a Fissure Sealant. Odontology 101 (1), 52–59. 10.1007/s10266-012-0062-5 [DOI] [PubMed] [Google Scholar]
- Brackett M. G., Li N., Brackett W. W., Sword R. J., Qi Y. P., Niu L. N., et al. (2011). The Critical Barrier to Progress in Dentine Bonding with the Etch-And-Rinse Technique. J. Dentistry 39 (3), 238–248. 10.1016/j.jdent.2010.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo L. (1998). Polyphenols: Chemistry, Dietary Sources, Metabolism, and Nutritional Significance. Nutr. Rev. 56 (11), 317–333. 10.1111/j.1753-4887.1998.tb01670.x [DOI] [PubMed] [Google Scholar]
- Breschi L., Mazzoni A., Nato F., Carrilho M., Visintini E., Tjäderhane L., et al. (2010). Chlorhexidine Stabilizes the Adhesive Interface: a 2-year In Vitro Study. Dental Mater. 26 (4), 320–325. 10.1016/j.dental.2009.11.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyles A. C., Pavan S., Bedran-Russo A. K. (2013). Effect of Dentin Surface Modification on the Microtensile Bond Strength of Self-Adhesive Resin Cements. J. Prosthodont. 22 (1), 59–62. 10.1111/j.1532-849X.2012.00890.x [DOI] [PubMed] [Google Scholar]
- Cannon C. G. (1955). The Interactions and Structure of the -CONH- Group in Amides and Polyamides. Microchimica Acta 43 (2-3), 555–588. 10.1007/bf01235027 [DOI] [Google Scholar]
- Carrilho M. R. O., Carvalho R. M., de Goes M. F., di Hipólito V., Geraldeli S., Tay F. R., et al. (2007). Chlorhexidine Preserves Dentin Bond In Vitro . J. Dent Res. 86 (1), 90–94. 10.1177/154405910708600115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellan C. S., Pereira P. N., Grande R. H. M., Bedran-Russo A. K. (2010). Mechanical Characterization of Proanthocyanidin-Dentin Matrix Interaction. Dental Mater. 26 (10), 968–973. 10.1016/j.dental.2010.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaliere C., Foglia P., Gubbiotti R., Sacchetti P., Samperi R., Laganà A. (2010). Rapid-resolution Liquid Chromatography/mass Spectrometry for Determination and Quantitation of Polyphenols in Grape Berries. Rapid Commun. Mass. Spectrom. 22 (20), 3089–3099. 10.1002/rcm.3705 [DOI] [PubMed] [Google Scholar]
- Chinnam N., Dadi P. K., Sabri S. A., Ahmad M., Kabir M. A., Ahmad Z. (2010). Dietary Bioflavonoids Inhibit Escherichia coli ATP Synthase in a Differential Manner. Int. J. Biol. Macromolecules 46 (5), 478–486. 10.1016/j.ijbiomac.2010.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa C. A. G., Passos V. F., Neri J. R., Mendonça J. S., Santiago S. L. (2019). Effect of Metalloproteinase Inhibitors on Bond Strength of a Self-Etching Adhesive on Erosively Demineralized Dentin. J. Adhes. Dent 21 (4), 337–344. 10.3290/j.jad.a42930 [DOI] [PubMed] [Google Scholar]
- Cova A., Breschi L., Nato F., Ruggeri A., Jr., Carrilho M., Tjäderhane L., et al. (2011). Effect of UVA-Activated Riboflavin on Dentin Bonding. J. Dent Res. 90 (12), 1439–1445. 10.1177/0022034511423397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Siqueira F. S. F., Hilgemberg B., Araujo L. C. R., Hass V., Bandeca M. C., Gomes J. C., et al. (2020). Improving Bonding to Eroded Dentin by Using Collagen Cross-Linking Agents: 2 Years of Water Storage. Clin. Oral Invest. 24 (2), 809–822. 10.1007/s00784-019-02918-9 [DOI] [PubMed] [Google Scholar]
- Deligeorgi V., Mjör I. A., Wilson N. H. (2001). An Overview of Reasons for the Placement and Replacement of Restorations. Prim. Dental Care os8 (1), 5–11. 10.1308/135576101771799335 [DOI] [PubMed] [Google Scholar]
- Deng D., Yang H., Guo J., Chen X., Zhang W., Huang C. (2014). Effects of Different Artificial Ageing Methods on the Degradation of Adhesive-Dentine Interfaces. J. Dentistry 42 (12), 1577–1585. 10.1016/j.jdent.2014.09.010 [DOI] [PubMed] [Google Scholar]
- Drummond J. L. (2008). Degradation, Fatigue, and Failure of Resin Dental Composite Materials. J. Dent Res. 87 (8), 710–719. 10.1177/154405910808700802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ds A., Mfgb C., Jpb D., Mbd E., Bh D., Ss F., et al. (2020). Influence of Flavonoids on Long-Term Bonding Stability on Caries-Affected Dentin - ScienceDirect. Dental Mater. 36 (9), 1151–1160. 10.1016/j.dental.2020.05.007 [DOI] [PubMed] [Google Scholar]
- Du X., Huang X., Huang C., Wang Y., Zhang Y. (2012). Epigallocatechin-3-gallate (EGCG) Enhances the Therapeutic Activity of a Dental Adhesive. J. Dentistry 40 (6), 485–492. 10.1016/j.jdent.2012.02.013 [DOI] [PubMed] [Google Scholar]
- Duan C., Matsumura S., Kariya N., Nishimura M., Shimono T. (2007). In Vitro antibacterial Activities of Scutellaria Baicalensis Georgi against Cariogenic Bacterial. Pediatr. Dental J. 17 (1), 58–64. 10.1016/s0917-2394(07)70096-4 [DOI] [Google Scholar]
- Epasinghe D. J., Yiu C. K. Y., Burrow M. F., Tay F. R., King N. M. (2012). Effect of Proanthocyanidin Incorporation into Dental Adhesive Resin on Resin-Dentine Bond Strength. J. Dentistry 40 (3), 173–180. 10.1016/j.jdent.2011.11.013 [DOI] [PubMed] [Google Scholar]
- Ferrazzano G., Amato I., Ingenito A., Zarrelli A., Pinto G., Pollio A. (2011). Plant Polyphenols and Their Anti-cariogenic Properties: a Review. Molecules 16 (2), 1486–1507. 10.3390/molecules16021486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fialho M. P. N., Hass V., Nogueira R. P., França F. M. G., Turssi C. P., Basting R. T., et al. (2019). Effect of Epigallocatechin-3- Gallate Solutions on Bond Durability at the Adhesive Interface in Caries-Affected Dentin. J. Mech. Behav. Biomed. Mater. 91, 398–405. 10.1016/j.jmbbm.2018.11.022 [DOI] [PubMed] [Google Scholar]
- Gotti V. B., Feitosa V. P., Sauro S., Correr-Sobrinho L., Leal F. B., Stansbury J. W., et al. (2015). Effect of Antioxidants on the Dentin Interface Bond Stability of Adhesives Exposed to Hydrolytic Degradation. J. Adhes. Dent 17 (1), 35–44. 10.3290/j.jad.a33515 [DOI] [PubMed] [Google Scholar]
- Hagerman A. E., Klucher K. M. (1986). Tannin-protein Interactions. Prog. Clin. Biol. Res. 213 (3), 67–76. [PubMed] [Google Scholar]
- Hagerman A. E., Butler L. G. (1980a). Condensed Tannin Purification and Characterization of Tannin-Associated Proteins. J. Agric. Food Chem. 28 (5), 947–952. 10.1021/jf60231a011 [DOI] [PubMed] [Google Scholar]
- Hagerman A. E., Butler L. G. (1980b). Determination of Protein in Tannin-Protein Precipitates. J. Agric. Food Chem. 28 (5), 944–947. 10.1021/jf60231a010 [DOI] [PubMed] [Google Scholar]
- Hass V., Luque-Martinez I., Muñoz M. A., Reyes M. F., Abuna G., Sinhoreti M. A., et al. (2016a). The Effect of Proanthocyanidin-Containing 10% Phosphoric Acid on Bonding Properties and MMP Inhibition. Dent Mater. 32 (3), 468–475. 10.1016/j.dental.2015.12.007 [DOI] [PubMed] [Google Scholar]
- Hass V., Luque-Martinez I. V., Gutierrez M. F., Moreira C. G., Gotti V. B., Feitosa V. P., et al. (2016b). Collagen Cross-Linkers on Dentin Bonding: Stability of the Adhesive Interfaces, Degree of Conversion of the Adhesive, Cytotoxicity and In Situ MMP Inhibition. Dental Mater. 32 (6), 732–741. 10.1016/j.dental.2016.03.008 [DOI] [PubMed] [Google Scholar]
- Islam M. S., Hiraishi N., Nassar M., Yiu C., Otsuki M., Tagami J. (2014). Effect of Hesperidin Incorporation into a Self-Etching Primer on Durability of Dentin Bond. Dental Mater. 30 (11), 1205–1212. 10.1016/j.dental.2014.08.371 [DOI] [PubMed] [Google Scholar]
- Jang E.-J., Cha S.-M., Choi S.-M., Cha J.-D. (2014). Combination Effects of Baicalein with Antibiotics against Oral Pathogens. Arch. Oral Biol. 59 (11), 1233–1241. 10.1016/j.archoralbio.2014.07.008 [DOI] [PubMed] [Google Scholar]
- Kang K. A., Zhang R., Piao M. J., Chae S., Kim H. S., Park J. H., et al. (2012). Baicalein Inhibits Oxidative Stress-Induced Cellular Damage via Antioxidant Effects. Toxicol. Ind. Health 28 (5), 412–421. 10.1177/0748233711413799 [DOI] [PubMed] [Google Scholar]
- Kaul T. N., Middleton E., Jr., Ogra P. L. (1985). Antiviral Effect of Flavonoids on Human Viruses. J. Med. Virol. 15 (1), 71–79. 10.1002/jmv.1890150110 [DOI] [PubMed] [Google Scholar]
- La V. D., Howell A. B., Grenier D. (2009). Cranberry Proanthocyanidins Inhibit MMP Production and Activity. J. Dent Res. 88 (7), 627–632. 10.1177/0022034509339487 [DOI] [PubMed] [Google Scholar]
- Landmayer K., Liberatti G. A., Farias-Neto A. M., Wang L., Honório H. M., Francisconi-Dos-Rios L. F. (2020). Could Applying Gels Containing Chlorhexidine, Epigallocatechin-3-Gallate, or Proanthocyanidin to Control Tooth Wear Progression Improve Bond Strength to Eroded Dentin. J. Prosthet Dent 124 (6), 798–e7. 10.1016/j.prosdent.2020.05.032 [DOI] [PubMed] [Google Scholar]
- Leme-Kraus A. A., Aydin B., Vidal C. M., Phansalkar R. M., Nam J. W., Mcalpine J., et al. (2017). Biostability of the Proanthocyanidins-Dentin Complex and Adhesion Studies. J. Dent Res. 96 (4), 412–406. 10.1177/0022034516680586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopoldini M., Russo N., Toscano M. (2011). The Molecular Basis of Working Mechanism of Natural Polyphenolic Antioxidants. Food Chem. 125 (2), 288–306. 10.1016/j.foodchem.2010.08.012 [DOI] [Google Scholar]
- Li J., Chen B., Hong N., Wu S., Li Y. (2018). Effect of Baicalein on Matrix Metalloproteinases and Durability of Resin-Dentin Bonding. Oper. Dent 43 (4), 426–436. 10.2341/17-097-L [DOI] [PubMed] [Google Scholar]
- Li K., Yang H., Yan H., Sun Y., Chen X., Guo J., et al. (2017). Quercetin as a Simple but Versatile Primer in Dentin Bonding. Rsc Adv. 7, 36392–36402. 10.1039/c7ra07467k [DOI] [Google Scholar]
- Liu R.-R., Fang M., Zhang L., Tang C.-F., Dou Q., Chen J.-H. (2014). Anti-proteolytic Capacity and Bonding Durability of Proanthocyanidin-Biomodified Demineralized Dentin Matrix. Int. J. Oral Sci. 6 (3), 168–174. 10.1038/ijos.2014.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R. R., Fang M., Zhao S. J., Li F., Shen L. J., Chen J. H. (2012). The Potential Effect of Proanthocyanidins on the Stability of Resin-Dentin Bonds against thermal Cycling. Zhonghua Kou Qiang Yi Xue Za Zhi 47 (5), 268–272. 10.3760/cma.j.issn.1002-0098.2012.05.004 [DOI] [PubMed] [Google Scholar]
- Macedo G. V., Yamauchi M., Bedran-Russo A. K. (2009). Effects of Chemical Cross-Linkers on Caries-Affected Dentin Bonding. J. Dent Res. 88 (12), 1096–1100. 10.1177/0022034509351001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni A., Angeloni V., Comba A., Maravic T., Cadenaro M., Tezvergil-Mutluay A., et al. (2018). Cross-linking Effect on Dentin Bond Strength and MMPs Activity. Dental Mater. 34 (2), 288–295. 10.1016/j.dental.2017.11.009 [DOI] [PubMed] [Google Scholar]
- Mereles D., Hunstein W. (2011). Epigallocatechin-3-gallate (EGCG) for Clinical Trials: More Pitfalls Than Promises. Ijms 12 (9), 5592–5603. 10.3390/ijms12095592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mjör I. A., Moorhead J. E., Dahl J. E. (2000). Reasons for Replacement of Restorations in Permanent Teeth in General Dental Practice. Int. Dental J. 50 (6), 361–366. 10.1111/j.1875-595x.2000.tb00569.x [DOI] [PubMed] [Google Scholar]
- Mukhtar H., Ahmad N. (2000). Tea Polyphenols: Prevention of Cancer and Optimizing Health. Am. J. Clin. Nutr. 71 (6 Suppl. l), 1698S–1702S. 10.1093/ajcn/71.6.1698S [DOI] [PubMed] [Google Scholar]
- Paulo S., L., Ferreira S., Gallardo E. F. (2010). Antimicrobial Activity and Effects of Resveratrol on Human Pathogenic Bacteria. WORLD JOURNAL MICROBIOLOGY BIOTECHNOLOGY 53 (6), 716–723. 10.1007/s11274-010-0325-7 [DOI] [Google Scholar]
- Peng W., Yi L., Wang Z., Yang H., Huang C. (2020). Effects of Resveratrol/ethanol Pretreatment on Dentin Bonding Durability. Mater. Sci. Eng. C 114, 111000. 10.1016/j.msec.2020.111000 [DOI] [PubMed] [Google Scholar]
- Peter B., Bosze S., Horvath R. (2017). Biophysical Characteristics of Proteins and Living Cells Exposed to the green tea Polyphenol Epigallocatechin-3-Gallate (EGCg): Review of Recent Advances from Molecular Mechanisms to Nanomedicine and Clinical Trials. Eur. Biophys. J. 46 (1), 1–24. 10.1007/s00249-016-1141-2 [DOI] [PubMed] [Google Scholar]
- Porto I. C. C. M., Nascimento T. G., Oliveira J. M. S., Freitas P. H., Haimeur A., França R. (2018). Use of Polyphenols as a Strategy to Prevent Bond Degradation in the Dentin-Resin Interface. Eur. J. Oral Sci. 126 (2), 146–158. 10.1111/eos.12403 [DOI] [PubMed] [Google Scholar]
- Rigano D., Formisano C., Basile A., Lavitola A., Senatore F., Rosselli S., et al. (2007). Antibacterial Activity of Flavonoids and Phenylpropanoids fromMarrubium Globosumssp. Libanoticum. Phytother. Res. 21 (4), 395–397. 10.1002/ptr.2061 [DOI] [PubMed] [Google Scholar]
- Santiago S. L., Osorio R., Neri J. R., Carvalho R. M., Toledano M. (2013). Effect of the Flavonoid Epigallocatechin-3-Gallate on Resin-Dentin Bond Strength. J. Adhes. Dent 15 (6), 535–540. 10.3290/j.jad.a29532 [DOI] [PubMed] [Google Scholar]
- Sarkis-Onofre R., Skupien J., Cenci M., Moraes R., Pereira-Cenci T. (2014). The Role of Resin Cement on Bond Strength of Glass-Fiber Posts Luted into Root Canals: a Systematic Review and Meta-Analysis of In Vitro Studies. Oper. Dent 39 (1), E31–E44. 10.2341/13-070-LIT [DOI] [PubMed] [Google Scholar]
- Siqueira F., Hilgemberg B., Araujo L., Hass V., Bandeca M. C., Gomes J. C., et al. (2020). Improving Bonding to Eroded Dentin by Using Collagen Cross-Linking Agents: 2years of Water Storage. Clin. Oral Investig. 24 (2), 809–822. 10.1007/s00784-019-02918-9 [DOI] [PubMed] [Google Scholar]
- Song J.-H., Kim S.-K., Chang K.-W., Han S.-K., Yi H.-K., Jeon J.-G. (2006). In Vitro inhibitory Effects of Polygonum Cuspidatum on Bacterial Viability and Virulence Factors of Streptococcus Mutans and Streptococcus Sobrinus. Arch. Oral Biol. 51 (12), 1131–1140. 10.1016/j.archoralbio.2006.06.011 [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Isemura M. (2013). Binding Interaction between (-)-epigallocatechin Gallate Causes Impaired Spreading of Cancer Cells on Fibrinogen. Biomed. Res. 34 (6), 301–308. 10.2220/biomedres.34.301 [DOI] [PubMed] [Google Scholar]
- Tachibana H. (2011). Green tea Polyphenol Sensing. Proc. Jpn. Acad. Ser. B: Phys. Biol. Sci. 87 (3), 66–80. 10.2183/pjab.87.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneja S., Kumari M., Bansal S. (2017). Effect of Saliva and Blood Contamination on the Shear Bond Strength of Fifth-, Seventh-, and Eighth-Generation Bonding Agents: An In Vitro Study. J. Conserv Dent 20 (3), 157–160. 10.4103/0972-0707.218310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira F., Pollock M., Karim A., Jiang Y. (2002). Use of Antioxidants for the Prophylaxis of Cold-Induced Peripheral Nerve Injury. Mil. Med. 167 (9), 753–755. 10.1093/milmed/167.9.753 [DOI] [PubMed] [Google Scholar]
- Wiegand A., Zheng P., Zaruba M. T. (2015). Effect of Different Matrix Metalloproteinase Inhibitors on Microtensile Bond Strength of an Etch-And-Rinse and a Self-Etch Adhesive to Dentin. Oper. Dent. 40(1):80–86. 10.2341/13-162-L [DOI] [PubMed] [Google Scholar]
- Xie Y., Yang W., Tang F., Chen X., Ren L. (2014). Antibacterial Activities of Flavonoids: Structure-Activity Relationship and Mechanism. Cmc 22 (1), 132–149. 10.2174/0929867321666140916113443 [DOI] [PubMed] [Google Scholar]
- Yang C. S., Wang X., Lu G., Picinich S. C. (2009). Cancer Prevention by tea: Animal Studies, Molecular Mechanisms and Human Relevance. Nat. Rev. Cancer 9 (6), 429–439. 10.1038/nrc2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Guo J., Deng D., Chen Z., Huang C. (2016). Effect of Adjunctive Application of Epigallocatechin-3-Gallate and Ethanol-Wet Bonding on Adhesive-Dentin Bonds. J. Dentistry 44, 44–49. 10.1016/j.jdent.2015.12.001 [DOI] [PubMed] [Google Scholar]
- Yang H., Li K., Yan H., Liu S., Wang Y., Huang C. (2017). High-performance Therapeutic Quercetin-Doped Adhesive for Adhesive-Dentin Interfaces. Sci. Rep. 7 (1), 8189. 10.1038/s41598-017-08633-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi L., Yu J., Han L., Li T., Yang H., Huang C. (2019). Combination of Baicalein and Ethanol-Wet-Bonding Improves Dentin Bonding Durability. J. Dentistry 90, 103207. 10.1016/j.jdent.2019.103207 [DOI] [PubMed] [Google Scholar]
- Yu H.-H., Zhang L., Yu F., Li F., Liu Z.-Y., Chen J.-H. (2017). Epigallocatechin-3-gallate and Epigallocatechin-3-O-(3-O-Methyl)-Gallate Enhance the Bonding Stability of an Etch-And-Rinse Adhesive to Dentin. Materials 10 (2), 183. 10.3390/ma10020183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z., Qian L., Cao L., Tan H., Huang Y., Xue X., et al. (2008). Virtual Screening for Novel Quorum Sensing Inhibitors to Eradicate Biofilm Formation of Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 79 (1), 119–126. 10.1007/s00253-008-1406-5 [DOI] [PubMed] [Google Scholar]
- Zhang K., Li F., Imazato S., Cheng L., Liu H., Arola D. D., et al. (2013). Dual Antibacterial Agents of Nano-Silver and 12-methacryloyloxydodecylpyridinium Bromide in Dental Adhesive to Inhibit Caries. J. Biomed. Mater. Res. 101B (6), 929–938. 10.1002/jbm.b.32898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Yu J., Yao C., Yang H., Huang C. (2020). New Perspective to Improve Dentin-Adhesive Interface Stability by Using Dimethyl Sulfoxide Wet-Bonding and Epigallocatechin-3-Gallate. Dental Mater. 36 (11), 1452–1463. 10.1016/j.dental.2020.08.009 [DOI] [PubMed] [Google Scholar]
- Zheng P., Chen H. (2017). Evaluate the Effect of Different Mmps Inhibitors on Adhesive Physical Properties of Dental Adhesives, Bond Strength and Mmp Substarte Activity. Sci. Rep. 7 (1), 4975. 10.1038/s41598-017-04340-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P., Zaruba M., Attin T., Wiegand A. (2015). Effect of Different Matrix Metalloproteinase Inhibitors on Microtensile Bond Strength of an Etch-And-Rinse and a Self-Etching Adhesive to Dentin. Oper. Dent 40 (1), 80–86. 10.2341/13-162-L [DOI] [PubMed] [Google Scholar]
- Zhou J., Chiba A., Scheffel D. L. S., Hebling J., Agee K., Tagami J., et al. (2016). Cross-linked Dry Bonding: A New Etch-And-Rinse Technique. Dental Mater. 32 (9), 1124–1132. 10.1016/j.dental.2016.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.