This analysis of 2 randomized clinical trials evaluates long-term (52 weeks) efficacy and safety of upadacitinib treatment in patients with atopic dermatitis.
Key Points
Question
Is upadacitinib efficacy and safety maintained through 52 weeks of continuous treatment in patients with moderate to severe atopic dermatitis?
Findings
In this analysis of follow-up data from the large, global, replicate phase 3 Measure Up 1 and Measure Up 2 randomized clinical trials including a total of 1609 patients, once-daily upadacitinib (15 mg or 30 mg) provided durable efficacy with responses maintained through 52 weeks of treatment. No new safety risks were observed.
Meaning
This follow-up analysis of 2 randomized clinical trials found that continuous upadacitinib treatment showed a favorable longer-term benefit-risk profile in patients with moderate to severe atopic dermatitis.
Abstract
Importance
Primary results from the Measure Up 1 and Measure Up 2 studies demonstrated upadacitinib efficacy and safety through 16 weeks in patients with atopic dermatitis. Longer-term outcomes remain unknown.
Objective
To evaluate long-term (52 weeks) efficacy and safety of upadacitinib treatment in patients with atopic dermatitis.
Design, Setting, and Participants
Measure Up 1 and Measure Up 2 are ongoing double-blind, placebo-controlled, replicate phase 3 randomized clinical trials that include adults and adolescents with moderate to severe atopic dermatitis at 151 and 154 centers, respectively. Cutoffs for this analysis were December 21, 2020 (Measure Up 1), and January 15, 2021 (Measure Up 2).
Interventions
Patients were randomized 1:1:1 to receive once-daily oral upadacitinib 15 mg, 30 mg, or placebo. At week 16, patients randomized at baseline to receive upadacitinib 15 mg (273 and 260 patients in Measure Up 1 and Measure Up 2, respectively) and 30 mg (270 and 268 patients) continued assigned treatment; placebo-treated patients were rerandomized 1:1 to receive upadacitinib 15 mg (121 and 120 patients in Measure Up 1 and Measure Up 2, respectively) or 30 mg (123 and 121 patients) in a double-blinded manner.
Main Outcomes and Measures
Safety and efficacy, including 75% improvement in the Eczema Area and Severity Index and Validated Investigator Global Assessment for Atopic Dermatitis score of clear (0) or almost clear (1) with 2 or greater grades of improvement, were assessed.
Results
Measure Up 1 and Measure Up 2 included a total of 1609 patients (mean [SD] age, 33.8 [15.6] years; 727 women [45.2%]; 882 men [54.8%]). Efficacy at week 16 was maintained through week 52. At week 52, 75% improvement in the Eczema Area and Severity Index was achieved by 82.0% (95% CI, 77.0%-86.9%) and 79.1% (95% CI, 73.9%-84.4%) of patients continuing the 15-mg dose and 84.9% (95% CI, 80.3%-89.5%) and 84.3% (95% CI, 79.6%-89.0%) of patients continuing the 30-mg dose (for Measure Up 1 and Measure Up 2, respectively); Validated Investigator Global Assessment for Atopic Dermatitis score of clear (0) or almost clear (1) with 2 or greater grades of improvement was achieved by 59.2% (95% CI, 52.9%-65.5%) and 52.6% (95% CI, 46.2%-59.1%) and 62.5% (95% CI, 56.3%-68.7%) and 65.1% (95% CI, 58.9%-71.2%) of patients in the Measure Up 1 and Measure Up 2 studies, respectively. Treatment discontinuation due to adverse events was low overall but was slightly higher for the upadacitinib 30-mg dose. Both upadacitinib doses were well tolerated with no new safety signals.
Conclusions and Relevance
In this analysis of follow-up data from 2 randomized clinical trials, longer-term treatment of adolescents and adults with moderate to severe atopic dermatitis with upadacitinib demonstrated a favorable benefit-risk profile, with sustained efficacy responses through 52 weeks.
Trial Registration
ClinicalTrials.gov Identifiers: NCT03569293 (Measure Up 1) and NCT03607422 (Measure Up 2)
Introduction
Long-term oral treatment options are limited for patients with moderate to severe atopic dermatitis (AD) recalcitrant to topical therapy; most conventional oral immunosuppressive therapies are used off-label, have limited efficacy data, and are not suitable for long-term treatment owing to their safety profiles.1,2,3 Additionally, the proportion of patients achieving clear or almost-clear skin with the injectable immunotherapy dupilumab after 16 weeks is less than 50%.4 There remains an unmet need for efficacious and well-tolerated oral medications that provide rapid, sustained itch relief and skin clearance and improve quality of life for patients with moderate to severe AD.
Upadacitinib (Rinvoq; AbbVie Inc) is being investigated to treat AD in adolescents and adults.5,6,7,8,9 The week-16 results from the Measure Up 1 and Measure Up 2 studies10 demonstrated that upadacitinib was superior to placebo across assessments of disease activity, itch, skin pain, and impact of AD on quality of life and had a favorable benefit-risk profile in patients with moderate to severe AD. Clinically and statistically significant improvements in itch were observed as early as 2 days after the first dose of upadacitinib, and skin clearance (≥75% improvement in Eczema Area and Severity Index [EASI]) was observed as early as week 2.10 Both the 15-mg and 30-mg upadacitinib doses were well tolerated; no new important safety risks beyond the labeled upadacitinib safety profile were observed through week 16.10 Because AD is a chronic disease,11,12 the evaluation of the long-term benefit-risk profile of upadacitinib as monotherapy continues in the ongoing blinded extension (BE) period of Measure Up 1 and Measure Up 2. Here, we report results through 52 weeks of the BE period.
Methods
Study Design and Patients
A detailed description of the study design and patient population of the Measure Up 1 (NCT03569293) and Measure Up 2 (NCT03607422) studies was previously reported.10 Briefly, both studies are ongoing global, multicenter, double-blind, placebo-controlled, replicate phase 3 randomized clinical trials conducted in 151 and 154 centers in Measure Up 1 and Measure Up 2, respectively. Both studies consisted of a 35-day screening period, a 16-week double-blind treatment period, and a BE period up to week 260. This 52-week analysis was prespecified in the trial protocol.
Patients aged 12 to 75 years with moderate to severe AD (defined as ≥10% of body surface area affected by AD, EASI ≥16, Validated Investigator Global Assessment for AD [vIGA-AD]13 ≥3, and Worst Pruritus Numerical Rating Scale [WP-NRS] ≥4 [weekly average]) who were candidates for systemic therapy were eligible to participate. The study was conducted in accord with the International Conference for Harmonisation guidelines, applicable regulations and guidelines governing clinical study conduct, and ethical principles originating from the Declaration of Helsinki. Before patient enrollment, independent ethics committees and/or institutional review boards approved the study protocol, informed consent form(s), and recruitment materials. Patients or their parents or legal guardians provided written informed consent before screening.
Procedures
At study entry, patients were randomized 1:1:1 to receive a daily oral dose of upadacitinib 15 mg, upadacitinib 30 mg, or placebo. Randomization was stratified by baseline vIGA-AD (moderate, severe), geographic region (Measure Up 1, US/Puerto Rico/Canada, Japan, China, and other; Measure Up 2, US/Puerto Rico/Canada and other), and age group (adolescent, adult). At week 16, patients initially randomized to upadacitinib 15 mg or upadacitinib 30 mg continued treatment as originally assigned. Placebo-treated patients were rerandomized 1:1 at week 16 to upadacitinib 15 mg or 30 mg; randomization was stratified by EASI 50 response at week 16, age group, and geographic region. Study investigators, site personnel, and patients were masked to treatment assignment in the double-blind period and masked to dose in the BE period. Starting at week 16, only systemic treatments for AD were considered as rescue therapy; topical medications for AD could be used at the investigator’s discretion.
Assessments
Efficacy end points, including skin clearance, itch relief, and quality of life, were assessed through week 52 (see eMethods in Supplement 1). Safety parameters included treatment-emergent adverse events (AEs), serious AEs, AEs leading to discontinuation of study drug, deaths, AEs of special interest (AESI), and incidence of potentially clinically significant laboratory assessments.
Statistical Analysis
After all patients in the main study (adolescents and adults) completed week 52, efficacy analyses were conducted in the intention-to-treat (ITT) population, defined as all patients who were randomized at baseline in the main study. Safety analyses were performed up to the cutoff date of the week-52 analysis (Measure Up 1, December 21, 2020; Measure Up 2, January 15, 2021). The safety population included all patients in the main study who received at least 1 dose of study drug. Efficacy and safety analyses were performed for each study and for the integrated data from Measure Up 1 and Measure Up 2.
Observed case (OC) analysis during treatment with the study drug, which did not impute missing data, was the primary analysis approach used for all variables. Adverse events were summarized using exposure-adjusted event rate (EAER, events/100 patient years [PYs]), calculated as the number of AEs divided by the total exposure in 100 PYs. Laboratory assessments indicating potentially clinically significant findings were reported as the number and proportion of patients. Missing safety data were not imputed. See eMethods in Supplement 1 for additional details on statistical analysis.
Results
Patients
Demographic and baseline disease characteristics were well balanced between groups (Table 1).10 A total of 273 and 260 patients (Measure Up 1 and Measure Up 2, respectively) randomized at baseline to upadacitinib 15 mg and 270 and 268 patients to upadacitinib 30 mg continued to receive their initial treatment in the BE period and had a minimum 52 weeks of upadacitinib exposure (eFigure 1 in Supplement 1). At week 16, 485 placebo-treated patients were rerandomized (121 and 120 to upadacitinib 15 mg, 123 and 121 to upadacitinib 30 mg in Measure Up 1 and Measure Up 2, respectively); these patients had a minimum of 36 weeks’ upadacitinib exposure. During the BE period, only 1 patient permanently discontinued treatment because of COVID-19 diagnosis. This patient was treated with placebo/upadacitinib, 30 mg, in Measure Up 2. From weeks 16 to 52, concomitant topical corticosteroids/topical calcineurin inhibitor therapy was used by 79 of 281 patients (28.1%) in the upadacitinib 15-mg group and 70 of 285 patients (24.6%) in the upadacitinib 30-mg group in Measure Up 1 and 89 of 276 patients (32.2%) and 63 of 282 patients (22.3%), respectively, in Measure Up 2. Rescue medication was initiated in the BE period for 12 of 281 (4.3%), 15 of 285 (5.3%), 2 of 121 (1.7%), and 1 of 123 (0.8%) patients receiving upadacitinib 15 mg, upadacitinib 30 mg, placebo/upadacitinib 15 mg, and placebo/upadacitinib 30 mg, respectively, in Measure Up 1, and 18 of 276 (6.5%), 11 of 282 (3.9%), 3 of 120 (2.5%), and 1 of 121 (0.8%), respectively, in Measure Up 2.
Table 1. Demographic and Baseline Characteristics (Integrated Data)a.
| Characteristic | No. (%) | |||
|---|---|---|---|---|
| UPA 15 mg (n = 557) | UPA 30 mg (n = 567) | PBO/UPA 15 mg (n = 241) | PBO/UPA 30 mg (n = 244) | |
| Sex | ||||
| Female | 245 (44.0) | 250 (44.1) | 113 (46.9) | 119 (48.8) |
| Male | 312 (56.0) | 317 (55.9) | 128 (53.1) | 125 (51.2) |
| Age, mean (range), y | 33.7 (12-74) | 33.9 (12-75) | 33.3 (12-71) | 34.4 (12-75) |
| Age group, y | ||||
| <18 | 75 (13.5) | 77 (13.6) | 34 (14.1) | 35 (14.3) |
| ≥18 | 482 (86.5) | 490 (86.4) | 207 (85.9) | 209 (85.7) |
| Race and ethnicity | ||||
| American Indian/Alaska Native | 5 (0.9) | 2 (0.4) | 2 (0.8) | 5 (2.0) |
| Asian | 128 (23.0) | 133 (23.5) | 52 (21.6) | 53 (21.7) |
| Black or African American | 43 (7.7) | 25 (4.4) | 17 (7.1) | 16 (6.6) |
| Native Hawaiian or Other Pacific Islander | 3 (0.5) | 1 (0.2) | 1 (0.4) | 1 (0.4) |
| White | 366 (65.7) | 389 (68.6) | 164 (68.0) | 164 (67.2) |
| Multiple | 12 (2.2) | 17 (3.0) | 5 (2.1) | 5 (2.0) |
| Not Hispanic or Latino | 499 (89.6) | 504 (88.9) | 209 (86.7) | 218 (89.3) |
| Geographic region | ||||
| US/Puerto Rico/Canada | 234 (42.0) | 241 (42.5) | 103 (42.7) | 105 (43.0) |
| Japan | 15 (2.7) | 16 (2.8) | 5 (2.1) | 3 (1.2) |
| China | 14 (2.5) | 17 (3.0) | 5 (2.1) | 9 (3.7) |
| Other | 294 (52.8) | 293 (51.7) | 128 (53.1) | 127 (52.0) |
| BMI, mean (SD) | 25.8 (5.9) | 25.8 (5.8) | 26.8 (6.5) | 26.3 (5.5) |
| Disease duration since diagnosis, mean (SD), y | 19.7 (14.7) | 20.6 (14.3) | 21.3 (13.9) | 19.6 (14.2) |
| BSA affected, mean (SD), % | 46.8 (22.3) | 47.0 (22.6) | 47.0 (22.4) | 44.4 (22.2) |
| vIGA-AD | ||||
| Moderate (score of 3) | 280 (50.3) | 280 (49.4) | 119 (49.4) | 133 (54.5) |
| Severe (score of 4) | 277 (49.7) | 287 (50.6) | 122 (50.6) | 111 (45.5) |
| EASI, mean (SD) | 29.6 (12.3) | 29.3 (11.7) | 28.7 (12.6) | 28.4 (12.2) |
| Worst pruritus NRS, weekly average, mean (SD) | 7.2 (1.6) | 7.3 (1.5) | 7.2 (1.6) | 7.4 (1.6) |
| Worst pruritus NRS, daily average, mean (SD) | 7.3 (1.8) | 7.5 (1.7) | 7.4 (1.8) | 7.5 (1.8) |
| DLQI total score, mean (SD) | 16.6 (7.0) | 16.5 (6.9) | 16.7 (7.2) | 16.9 (6.9) |
| POEM total score, mean (SD) | 21.2 (4.9) | 21.6 (5.0) | 21.4 (5.5) | 21.9 (5.1) |
Abbreviations: BMI, body mass index, calculated as weight in kilograms divided by height in meters squared; BSA, body surface area; DLQI, Dermatology Life Quality Index; EASI, Eczema Area and Severity Index; NRS, numeric rating scale; PBO, placebo; POEM, Patient-Oriented Eczema Measure; UPA, upadacitinib; vIGA-AD, Validated Investigator Global Assessment for Atopic Dermatitis.
Data from Measure Up 1 and Measure Up 2 studies.10 Values are based on nonmissing values from randomized patients.
Efficacy
In the OC analysis in both studies, among patients who started upadacitinib treatment with the 15-mg dose on day 1, efficacy at week 52 was slightly better than at week 16 (Figures 1 and 2; eFigures 2-4 in Supplement 1); while among those who started upadacitinib treatment with the 30-mg dose on day 1, efficacy demonstrated at week 16 was maintained through week 52 (Figures 1 and 2; eFigures 2-4 in Supplement 1). At week 52, the proportions of patients who achieved EASI-75 were 82.0% (95% CI, 77.0%-86.9%) for upadacitinib 15 mg and 84.9% (95% CI, 80.3%-89.5%) for upadacitinib 30 mg in Measure Up 1 (Figure 1A, left graph), and 79.1% (95% CI, 73.9%-84.4%) and 84.3% (95% CI, 79.6%-89.0%) in Measure Up 2 (Figure 1A, middle graph). The proportion of patients who achieved vIGA-AD 0/1 at week 52 was 59.2% (95% CI, 52.9%-65.5%) for upadacitinib 15 mg and 62.5% (95% CI, 56.3%-68.7%) for upadacitinib 30 mg in Measure Up 1 (Figure 1B, left graph) and 52.6% (95% CI, 46.2%-59.1%) and 65.1% (95% CI, 58.9%-71.2%) in Measure Up 2 (Figure 1B, middle graph). At week 52, the proportion of patients who achieved WP-NRS improvement 4 or greater was 67.3% (95% CI, 61.1%-73.4%) for upadacitinib 15 mg and 67.7% (95% CI, 61.6%-73.7%) for upadacitinib 30 mg in Measure Up 1 (Figure 1C, left graph) and 62.4% (95% CI, 56.1%-68.7%) and 72.9% (95% CI, 67.1%-78.7%) in Measure Up 2 (Figure 1C, middle graph).
Figure 1. Efficacy Over Time for EASI 75, vIGA-AD 0/1, and Worst Pruritus NRSa Improvement of 4 or Greater Through Week 52 (ITT Population and Integratedb Data, OC Analysis).
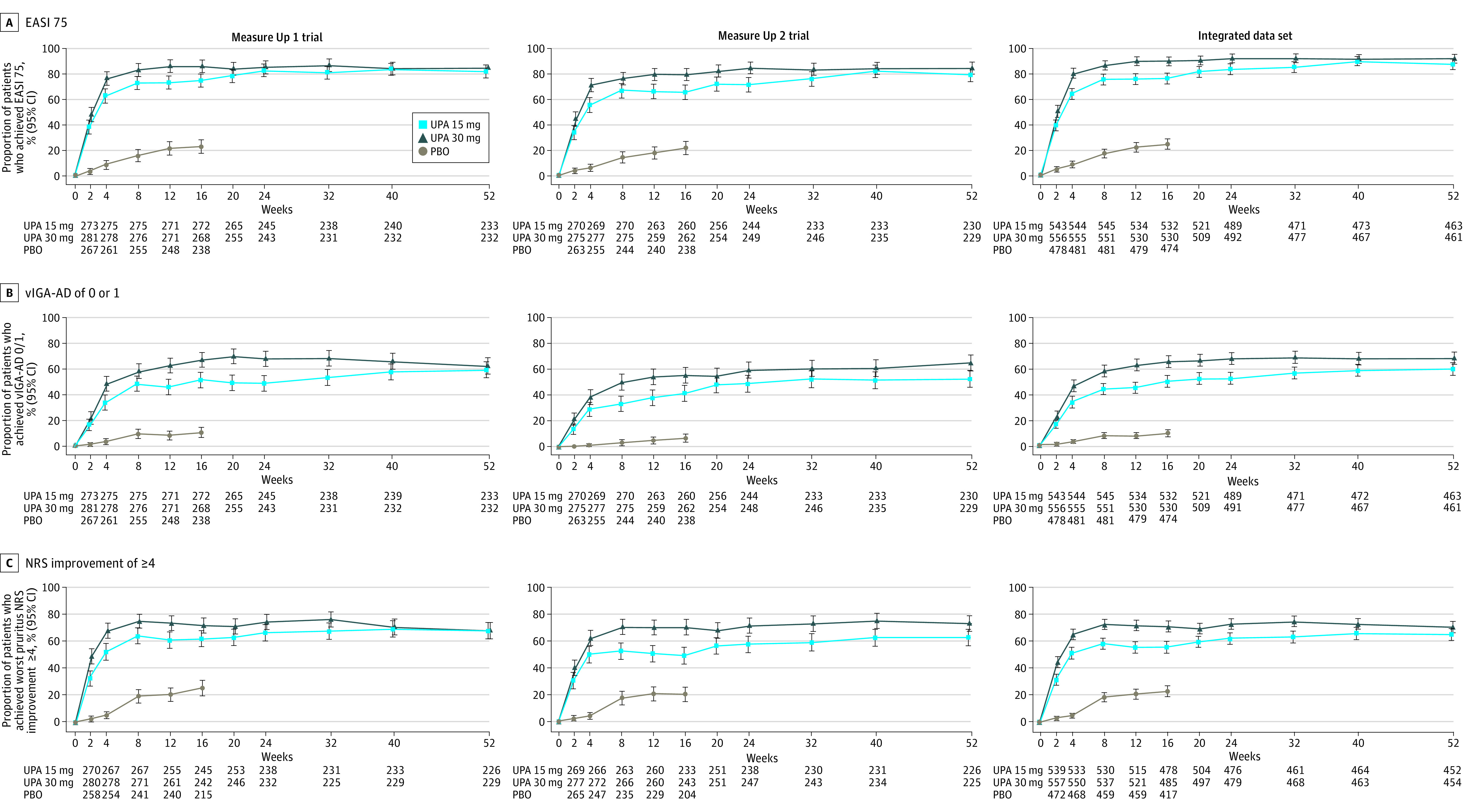
Response rates at each visit for EASI 75 (A), vIGA-AD 0/1 (B), and worst pruritus NRSa improvement of 4 or greater (C) in the Measure Up 1 trial, the Measure Up 2 trial, and the integratedb data set.
aBased on weekly average (through week 16); based on study visit (weeks 20 through 52) for patients with worst pruritus NRS of 4 or greater at baseline.
bData from Measure Up 1 and Measure Up 2 studies.10
EASI 75 indicates 75% or greater improvement in Eczema Area and Severity Index; ITT, intention to treat for the main study; NRS, numeric rating scale; OC, observed case; PBO, placebo; UPA, upadacitinib; vIGA-AD 0/1, Validated Investigator Global Assessment for Atopic Dermatitis of clear (0) or almost clear (1) with 2 or more grades of reduction.
Figure 2. Efficacy Over Time for EASI 90, EASI 100, vIGA-AD 0 With a Reduction From Baseline of 2 or Greater, and Worst Pruritus NRS 0/1a Through Week 52 (ITT Population and Integratedb Data, OC Analysis).
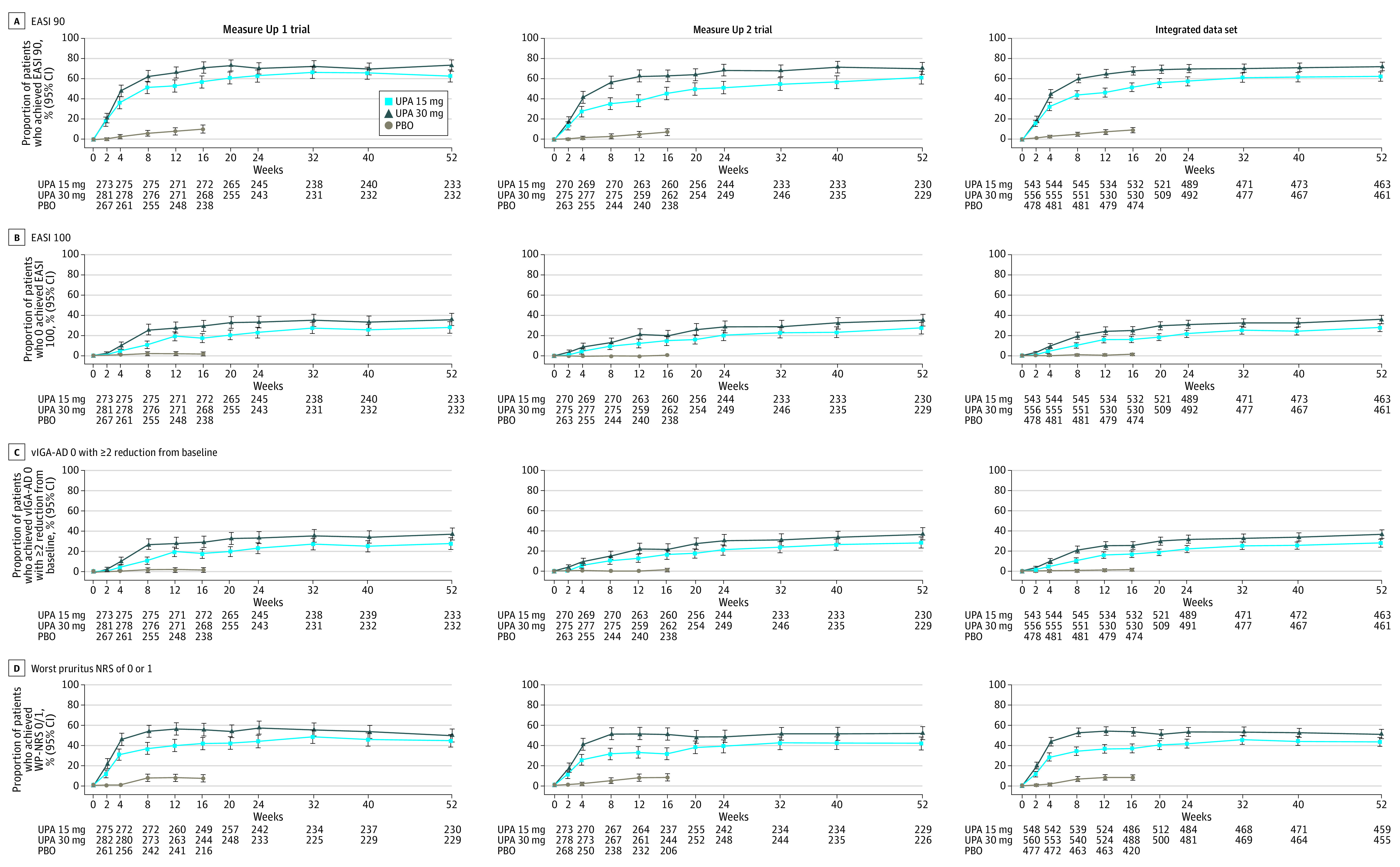
aFor patients with worst pruritus NRS greater than 1 at baseline.
bData from Measure Up 1 and Measure Up 2 studies.10
EASI 90/100 indicates 90% or greater/100% improvement in Eczema Area and Severity Index; ITT, intention to treat for the main study; NRS, numeric rating scale; OC, observed case; PBO, placebo; UPA, upadacitinib; vIGA-AD 0, Validated Investigator Global Assessment for Atopic Dermatitis of clear with 2 or greater grades of reduction.
For the more stringent end points of EASI-90, EASI-100, vIGA-AD 0, and WP-NRS 0/1, responses were maintained or improved at week 52 vs week 16 (OC analysis). At week 52, EASI-90 was achieved by 62.7% (95% CI, 56.5%-68.9%) for upadacitinib 15 mg and 73.3% (95% CI, 67.6%-79.0%) for upadacitinib 30 mg of patients in Measure Up 1 (Figure 2A, left graph) and 61.3% (95% CI, 55.0%-67.6%) and 70.3% (95% CI, 64.4%-76.2%) in Measure Up 2 (Figure 2A, middle graph); EASI-100 was achieved by 27.9% (95% CI, 22.1%-33.7%) and 35.8% (95% CI, 29.6%-41.9%) in Measure Up 1 (Figure 2B, left graph) and 27.8% (95% CI, 22.0%-33.6%) and 35.8% (95% CI, 29.6%-42.0%) in Measure Up 2 (Figure 2B, middle graph). The proportion of patients who achieved vIGA-AD 0 at week 52 was 27.9% (95% CI, 22.1%-33.7%) for upadacitinib 15 mg and 37.1% (95% CI, 30.9%-43.3%) for upadacitinib 30 mg in Measure Up 1 (Figure 2C, left graph), and 28.3% (95% CI, 22.4%-34.1%) and 37.1% (95% CI, 30.9%-43.4%) in Measure Up 2 (Figure 2C, middle graph). At week 52, WP-NRS 0/1 was achieved by 44.8% (95% CI, 38.4%-51.2%) for upadacitinib 15 mg and 50.2% (95% CI, 43.7%-56.7%) for upadacitinib 30 mg of patients in Measure Up 1 (Figure 2D, left graph) and 41.9% (95% CI, 35.5%-48.3%) and 52.2% (95% CI, 45.7%-58.7%) in Measure Up 2 (Figure 2D, middle graph). Improvements in EASI and WP-NRS occurred rapidly (within 1 week) and continued to increase, with improvements at week 16 maintained through week 52 in both studies (eFigures 2A and 2B in Supplement 1).
Meaningful improvements occurred in several patient-reported outcome assessments, including Dermatology Life Quality Index (DLQI; eFigure 3 in Supplement 1); Hospital Anxiety and Depression Scale (HADS; eFigure 4 in Supplement 1); and the sleep, emotional state, and daily activities domains of the Atopic Dermatitis Impact Scale (ADerm-IS; eFigure 5 in Supplement 1). These improvements occurred at the earliest weekly time point at which they were assessed, with improvements at week 16 maintained through week 52.
Comparable results were obtained with the multiple imputation (categorical end points) or mixed-effects model for repeated measures (continuous end points) statistical analyses (eFigures 6-8 in Supplement 1). The integrated population (Table 2) exhibited similar efficacy results at weeks 16 (eFigure 9 in Supplement 1) and 52 (Figures 1 and 2; eFigure 2 in Supplement 1), as did the adolescent population (eFigure 10 in Supplement 1). Placebo-treated patients rerandomized at week 16 to receive upadacitinib had response rates through study week 52 similar to those who received upadacitinib from day 1 (eFigures 11-13 in Supplement 1).
Table 2. Loss of Response and Achievement of EASI 75 at Week 52 by Week 16 Response Status (ITT Population).
| UPA 15 mg | UPA 30 mg | |||
|---|---|---|---|---|
| No.a | No. (%) [95% CI] | No.a | No. (%) [95% CI] | |
| Proportion of patients who experienced loss of response after wk 16 up to wk 52 among wk-16 EASI 75 and vIGA-AD 0/1 responders a , b | ||||
| Measure Up 1 | ||||
| Overallc | 133 | 4 (3.0) [0.1-5.9] | 176 | 6 (3.4) [0.7-6.1] |
| Week 20 | 131 | 0 | 170 | 2 (1.2) [0-2.8] |
| Week 24 | 120 | 0 | 166 | 1 (0.6) [0-1.8] |
| Week 32 | 126 | 4 (3.2) [0.1-6.2] | 160 | 1 (0.6) [0-1.8] |
| Week 40 | 120 | 1 (0.8) [0-2.5] | 159 | 1 (0.6) [0-1.9] |
| Week 52 | 121 | 0 | 161 | 2 (1.2) [0-3.0] |
| Measure Up 2 | ||||
| Overallc | 107 | 6 (5.6) [1.2-10.0] | 142 | 7 (4.9) [1.4-8.5] |
| Week 20 | 106 | 0 | 139 | 0 |
| Week 24 | 102 | 2 (2.0) [0-4.7] | 139 | 3 (2.2) [0-4.6] |
| Week 32 | 102 | 2 (2.0) [0-4.7] | 135 | 1 (0.7) [0-2.2] |
| Week 40 | 101 | 0 | 133 | 2 (1.5) [0-3.6] |
| Week 52 | 100 | 3 (3.0) [0-6.3] | 129 | 4 (3.1) [0.1-6.1] |
| Proportion of patients achieving EASI 75 at wk 52 among wk-16 EASI 75 responders d | ||||
| Measure Up 1 | 175 | 153 (87.4) [82.5-92.3] | 207 | 183 (88.4) [84.0-92.8] |
| Measure Up 2 | 157 | 137 (87.3) [82.0-92.5] | 189 | 172 (91.0) [86.9-95.1] |
| Integrated population | 332 | 290 (87.3) [83.8-90.9] | 396 | 355 (89.6) [86.6-92.6] |
| Proportion of patients achieving EASI 75 at wk 52 among wk-16 EASI 75 nonresponders e | ||||
| Measure Up 1 | 57 | 37 (64.9) [52.5-77.3] | 24 | 14 (58.3) [38.6-78.1] |
| Measure Up 2 | 73 | 45 (61.6) [50.5-72.8] | 35 | 16 (45.7) [29.2-62.2] |
| Integrated population | 130 | 82 (63.1) [54.8-71.4] | 59 | 30 (50.8) [38.1-63.6] |
Abbreviations: EASI 75, 75% or greater improvement in Eczema Area and Severity Index; ITT, intention to treat for the main study; UPA, upadacitinib; vIGA-AD 0/1, Validated Investigator Global Assessment for Atopic Dermatitis of clear (0) or almost clear (1) with 2 or more grades of reduction.
Loss of response was defined as loss of 50% or greater of the week-16 EASI response and vIGA-AD 2 or greater after week 16.
Responders were defined as patients who achieved EASI 75 and vIGA-AD 0/1 at week 16.
At any time between week 16 up to week 52.
Responders were defined as patients who achieved EASI 75 at week 16.
Nonresponders were defined as patients who did not achieve EASI 75 at week 16.
Few patients treated with upadacitinib 15 mg (3.0% and 5.6%, Measure Up 1 and Measure Up 2, respectively) or upadacitinib 30 mg (3.4% and 4.9%, respectively) who experienced EASI-75 and vIGA-AD 0/1 responses at week 16 lost response, defined as loss of 50% or greater of the week-16 EASI response and vIGA-AD 2 or greater after week 16 (Table 2). Most patients who achieved EASI-75 response at week 16 maintained EASI-75 response at week 52 (87.4% and 87.3% treated with upadacitinib 15 mg in Measure Up 1 and Measure Up 2, respectively, and 88.4% and 91.0% treated with upadacitinib 30 mg in Measure Up 1 and Measure Up 2, respectively; Table 2).
Safety
Safety results are presented in Table 3. In both studies, the rate of treatment discontinuations due to AEs was low overall, though higher in the upadacitinib 30-mg group vs the 15-mg group. The EAERs of AEs, serious AEs, and AESIs at week 52 were similar or lower than EAERs observed through week 16 (eTable 1 in Supplement 1). Similar safety results were observed in the adolescent population (eTable 2 in Supplement 1).
Table 3. Treatment-Emergent Adverse Events (TEAEs) During Administration of UPA Through Week 52.
| AEs | UPA 15 mg | UPA 30 mg | ||||
|---|---|---|---|---|---|---|
| Measure Up 1 (n = 401)a | Measure Up 2 (n = 396)a | Combined (n = 797)b | Measure Up 1 (n = 408)a | Measure Up 2 (n = 403)a | Combined (n = 811)b | |
| Events (events/100 PY) | ||||||
| PY = 490.9 | PY = 462.4 | PY = 953.3 | PY = 501.0 | PY = 477.2 | PY = 978.2 | |
| Any TEAE | 1288 (262.4) | 1114 (240.9) | 2402 (252.0) | 1658 (330.9) | 1293 (270.9) | 2951 (301.7) |
| Serious AEs | 32 (6.5) | 33 (7.1) | 65 (6.8) | 50 (10.0) | 33 (6.9) | 83 (8.5) |
| AEs leading to discontinuation of study drug | 22 (4.5) | 21 (4.5) | 43 (4.5) | 39 (7.8) | 31 (6.5) | 70 (7.2) |
| Deaths | 0 | 0 | 0 | 1 (0.2)c | 0 | 1 (0.1) |
| AESIs d | ||||||
| Serious infections | 10 (2.0) | 11 (2.4) | 21 (2.2) | 23 (4.6) | 12 (2.5) | 35 (3.6) |
| Opportunistic infection excluding tuberculosis and herpes zoster | 5 (1.0) | 13 (2.8) | 18 (1.9) | 15 (3.0) | 5 (1.0) | 20 (2.0) |
| Herpes zostere | 17 (3.5) | 17 (3.7) | 34 (3.6) | 28 (5.6) | 25 (5.2) | 53 (5.4) |
| Active tuberculosis | 1 (0.2) | 0 | 1 (0.1) | 0 | 1 (0.2) | 1 (0.1) |
| NMSCf | 1 (0.2) | 3 (0.6) | 4 (0.4) | 3 (0.6) | 1 (0.2) | 4 (0.4) |
| Cancer other than NMSCg | 2 (0.4) | 0 | 2 (0.2) | 2 (0.4) | 3 (0.6) | 5 (0.5) |
| Lymphoma | 0 | 0 | 0 | 0 | 1 (0.2)h | 1 (0.1) |
| Hepatic disorder | 29 (5.9) | 27 (5.8) | 56 (5.9) | 52 (10.4) | 42 (8.8) | 94 (9.6) |
| Adjudicated gastrointestinal perforation | 0 | 0 | 0 | 0 | 0 | 0 |
| Anemia | 3 (0.6) | 12 (2.6) | 15 (1.6) | 19 (3.8) | 18 (3.8) | 37 (3.8) |
| Neutropenia | 11 (2.2) | 5 (1.1) | 16 (1.7) | 23 (4.6) | 11 (2.3) | 34 (3.5) |
| Lymphopenia | 4 (0.8) | 3 (0.6) | 7 (0.7) | 7 (1.4) | 2 (0.4) | 9 (0.9) |
| CPK elevation | 36 (7.3) | 31 (6.7) | 67 (7.0) | 58 (11.6) | 51 (10.7) | 109 (11.1) |
| Kidney dysfunction | 0 | 0 | 0 | 2 (0.4) | 1 (0.2) | 3 (0.3) |
| Adjudicated MACE | 1 (0.2) | 0 | 1 (0.1) | 0 | 0 | 0 |
| Adjudicated VTE | 1 (0.2) | 0 | 1 (0.1) | 0 | 1 (0.2) | 1 (0.1) |
Abbreviations: AE, adverse event; AESI, adverse event of special interest; CPK, creatine phosphokinase; MACE, major adverse cardiovascular event; NMSC, nonmelanoma skin cancer; PY, patient year; TEAE, treatment-emergent adverse event; UPA, upadacitinib; VTE, venous thromboembolic event.
Includes all patients in the main study who received at least 1 dose of UPA.
Data from Measure Up 1 and Measure Up 2 studies.10
Myocardial infarction (COVID-19 related) occurred in a patient in their 60s 28 days after the last dose of study medication.
Prespecified AESIs were based on the known UPA safety profile6 and previous safety observations for UPA7 and other JAK inhibitors.14
Searched using a prespecified grouped term.
Four cases of NMSC were reported in the UPA 15-mg group: 1 Bowen disease (patient in their 50s on day 7), 1 basal cell carcinoma (patient in their 60s on day 22), and 2 cases of squamous cell carcinoma (1 in a patient in their 50s on day 21 and 1 in a patient in their 50s on day 113); 4 NMSCs were reported in the UPA 30-mg group: 1 basal cell carcinoma (patient in their 50s on day 610), 2 cases of squamous cell carcinoma of the skin (1 in a patient in their 60s on day 218 and 1 in a patient in their 60s on day 434), and 1 cutaneous T-cell lymphoma stage I (see footnote h).
Breast and colon cancer were diagnosed on days 520 and 550 in a patient in their 40s taking UPA 15 mg; 1 case each of endometrial adenocarcinoma (patient in their 60s on day 515), kidney cell carcinoma (patient in their 50s on day 318), anal squamous cell cancer (patient in their 60s on day 64), breast cancer (patient in their 50s on day 21), and gastric cancer (patient in their 70s on day 36) in the UPA 30-mg group.
A case of cutaneous T-cell lymphoma in a patient in their 50s taking UPA 30 mg on day 194 deemed unrelated to study drug and nonserious by the investigator; after medical review, the patient likely had cutaneous T-cell lymphoma at baseline.
One death (myocardial infarction related to COVID-19 diagnosis) occurred on study day 399 (28 days after last study drug dose) in a patient in their 60s treated with upadacitinib 30 mg in Measure Up 1 (Table 3); the patient had type 2 diabetes mellitus, hypertension, obesity, and hypercholesterolemia.
For the combined studies, the EAER of serious infection was higher with upadacitinib 30 mg compared with upadacitinib 15 mg (3.6/100 PYs vs 2.2/100 PYs), while the EAER of opportunistic infection (excluding tuberculosis and herpes zoster) was similar between treatment groups (1.9/100 PYs with upadacitinib 15 mg and 2.0/100 PYs with upadacitinib 30 mg; Table 3). The most common serious infection was pneumonia in Measure Up 1 and eczema herpeticum in Measure Up 2. All opportunistic infections (excluding tuberculosis and herpes zoster) were cases of eczema herpeticum or Kaposi varicelliform eruption, except for an esophageal candidiasis in a patient taking upadacitinib 30 mg. Across both studies, most events of eczema herpeticum were nonserious; 1 and 2 events reported in the upadacitinib 15-mg and 30-mg groups, respectively, led to treatment discontinuation. The EAERs of herpes zoster were higher with upadacitinib 30 mg compared with upadacitinib 15 mg (5.4/100 PYs vs 3.6/100 PYs, respectively; Table 3). Most herpes zoster events were nonserious, except for 3 in the upadacitinib 30-mg group and 2 in the upadacitinib 15-mg group. One herpes zoster event led to treatment discontinuation. Most herpes zoster (75 of 82 [91.5%]) events involved a single or 2 dermatomes; no central nervous system, ocular, lung, or liver involvement was reported. One case of tuberculosis was reported in a patient in their 20s taking upadacitinib 15-mg and another was reported in a patient in their 20s taking upadacitinib 30-mg; both were serious, deemed possibly related to study drug, and led to treatment withdrawal. Overall, the incidence of COVID-19 cases was 27 of 809 patients (3.3%) in Measure Up 1 and 29 of 799 patients (3.6%) in Measure Up 2 and similar between treatment groups; most events were nonserious. Serious events of COVID-19 were reported in 5 patients (0.3%); 1 led to treatment discontinuation.
One adjudicated major adverse cardiovascular event (nonfatal stroke) was reported in a patient in their 60s with uncontrolled hypertension who was taking upadacitinib 15 mg, which was classified as serious, deemed unrelated to the study drug, and resulted in discontinuation of study treatment. Two adjudicated venous thromboembolic events were reported: 1 case of deep vein thrombosis (grade 4 in severity) in a patient in their 40s taking upadacitinib 15 mg with a previous history of deep vein thrombosis, and 1 pulmonary embolism (grade 3 in severity) related to COVID-19 diagnosis in a patient in their 70s taking upadacitinib 30 mg—both events were deemed serious and unrelated to the study treatment by the investigator, and both patients discontinued study treatment.
The EAER of malignant neoplasm was 0.6/100 PYs with upadacitinib 15 mg and 0.9/100 PYs with upadacitinib 30 mg. Nonmelanoma skin cancer (NMSC) was the most reported malignant neoplasm in both treatment groups. Except for breast cancer in 2 patients, other types of cancers were reported in a single patient. Among non-NMSC cancers, 3 of 7 occurred within 3 months of study drug initiation (anal cancer, breast cancer, and gastric cancer). A case of kidney cancer had clinical findings (kidney mass and hematuria) at study entry indicating that the cancer was present at the time of randomization.
In both studies, the most frequently reported treatment-emergent AEs (≥5% in any treatment group) were acne, cough, headache, herpes zoster, oral herpes, urinary tract infection, upper respiratory tract infection, nasopharyngitis, atopic dermatitis, and elevation in blood creatine phosphokinase level (eTable 3 in Supplement 1). Acne events were nonserious, and treatment discontinuation was rare (1 of 1609 [0.1%]). Most acne events involved the face and consisted primarily of inflammatory papules, pustules, and/or comedones. Acne required no intervention in 34 of 84 (40.5%) and 61 of 131 (46.6%) patients in the upadacitinib 15-mg and 30-mg groups, respectively; most remaining cases were managed with topical therapies. The most common risk factors were history of acne and/or family history of acne.
Adverse events of hepatic disorder (most were transaminase elevations), anemia, lymphopenia, and elevations in creatine phosphokinase were reported more frequently in upadacitinib 30-mg vs upadacitinib 15-mg groups (Table 3). These laboratory-related AEs were generally transient, mild to moderate, and asymptomatic. Grade 3 to 4 anemia, lymphopenia, neutropenia, and elevated aspartate aminotransferase, alanine aminotransferase, and creatine phosphokinase levels were infrequent across all treatment groups (eTable 4 in Supplement 1); the frequency of these laboratory abnormalities was higher in the upadacitinib 30-mg group vs the 15-mg group. Changes in laboratory parameters occurred by weeks 8 to 12 and were stable thereafter (eFigure 14 in Supplement 1). Treatment discontinuations due to laboratory-related toxic effects were infrequent.
Discussion
In Measure Up 1 and Measure Up 2, both doses of upadacitinib maintained efficacy responses achieved at week 16 (primary end point)10 through week 52 for several measures of AD disease activity (including vIGA-AD 0/1, vIGA-AD 0, and EASI-75/90/100), patient-reported itch (WP-NRS improvement ≥4, WP-NRS 0/1), and patient-reported quality of life (DLQI, HADS, and ADerm-IS Sleep, Daily Activities, and Emotional State). Collectively, these results indicate that AD treatment with upadacitinib led to robust and comprehensive long-term disease control. Placebo-treated patients who were rerandomized at week 16 to upadacitinib achieved similar response trends through study week 52 to those who received upadacitinib from day 1, confirming the findings from the double-blind period. While upadacitinib 15 mg achieved and maintained high levels of efficacy, there was greater relative benefit with the 30-mg dose. Patients receiving upadacitinib 30 mg consistently reached numerically greater response rates and higher degrees of improvement than did patients receiving upadacitinib 15 mg for all outcome measures assessed through week 52. The numerically better outcomes with upadacitinib 30 mg through week 52 are more apparent when using more stringent thresholds of disease activity (vIGA 0, EASI-90/100).
No new safety risks were observed beyond those noted in the 16-week treatment period,10 and no new safety risks were identified for upadacitinib compared with the rheumatoid arthritis and psoriatic arthritis studies.6 The EAERs observed in Measure Up 1 and Measure Up 2 through 52 weeks were generally comparable to those observed at week 16 and generally similar to or lower than the rates reported following upadacitinib treatment in patients with rheumatoid arthritis.15 While there was a higher rate of safety events with upadacitinib 30 mg, these events were mostly mild to moderate in severity and infrequently led to treatment discontinuation. The risk profiles for both doses are generally comparable. A few events, however, such as serious infections, herpes zoster, and laboratory-related toxic effects were reported more frequently with upadacitinib 30 mg vs 15 mg. Adjudicated major adverse cardiovascular events, adjudicated venous thromboembolism, and deaths were infrequently reported in the AD studies. Except for NMSC, no specific type or pattern of malignant neoplasms was observed. Many of the malignant neoplasms excluding NMSC were either diagnosed within a short period of time (<3 months) from the first dose of study drug or had physical findings at baseline. The safety profile of upadacitinib was similar between adolescents and the overall population. There were no reports of malignant neoplasm, adjudicated major adverse cardiovascular events, adjudicated venous thromboembolic events, or deaths in adolescent patients.
Altogether, while longer-term treatment of moderate to severe AD with upadacitinib (both 15-mg and 30-mg doses) has a favorable benefit-risk profile, upadacitinib 30 mg may provide added benefit for patients with higher disease burdens that may require more rapid or more complete response to treatment.
Limitations
While 52-week results are reported here, these studies will follow patients through 260 weeks; longer-term data will be valuable for evaluating the efficacy and safety of upadacitinib for the chronic disease of atopic dermatitis. Because of the predominance of White patients in the study populations, additional studies in underrepresented populations may be needed. Although not a limitation of these studies, many patients use systemic treatments in combination with topical treatments rather than as monotherapy; this practice was evaluated in a separate study of upadacitinib in atopic dermatitis (the AD Up study).16
Conclusions
In this analysis of follow-up data from the ongoing phase 3 Measure Up 1 and Measure Up 2 randomized clinical trials, results through 52 weeks demonstrated the maintenance of efficacy and an acceptable longer-term safety profile of upadacitinib monotherapy in adolescents and adults with moderate to severe AD.
eMethods.
eFigure 1. Patient Disposition
eFigure 2. Efficacy Over Time: A, Percent Change From Baseline in EASI and B, Percent Change From Baseline in Worst Pruritus NRS Through Week 52 (ITT Population and Integrated Data, OC Analysis)
eFigure 3. Efficacy Over Time: A, DLQI Improvement ≥4; B, DLQI 0/1 Through Week 52 (ITT Population and Integrated Data, OC Analysis)
eFigure 4. Efficacy Over Time: A, HADS-A <8 and HADS-D <8; B, Percent Change in HADS From Baseline Through Week 52 (ITT Population and Integrated Data, OC Analysis)
eFigure 5. Efficacy Over Time: A, ADerm-IS Sleep; B, Emotional State; and C, Daily Activities Through Week 52 (ITT Population and Integrated Data, OC Analysis)
eFigure 6. Efficacy Over Time: A, EASI 75; B, vIGA-AD 0/1; and C, Worst Pruritus NRS Improvement ≥4 Through Week 52 (ITT Population and Integrated data, MI analysis)
eFigure 7. Efficacy Over Time: A, EASI 90; B, EASI 100; and C, Worst Pruritus NRS 0/1 Through Week 52 (ITT Population and Integrated Data, MI Analysis)
eFigure 8. Efficacy Over Time: A, Percent Change From Baseline in EASI; B, Percent Change From Baseline in Worst Pruritus NRS Through Week 52 (ITT Population and Integrated Data, MMRM Analysis)
eFigure 9. Efficacy Over Time: A, EASI 75; B, vIGA-AD 0/1; C, EASI 90; D, Worst Pruritus NRS Improvement ≥4; E, EASI 100; and F, Percent Change in Worst Pruritus NRS From Baseline by Visit Through Week 16 (Placebo-controlled Population, Integrated Data)
eFigure 10. Efficacy Over Time: A, EASI 75; B, vIGA-AD 0/1; and C, Worst Pruritus NRS Improvement ≥4 Through Week 52 (Adolescent Population, OC Analysis)
eFigure 11. Efficacy Over Time for Crossover Cohort: A, EASI 75; B, vIGA-AD 0/1; and C, Worst Pruritus NRS Improvement ≥4 Through Week 52 (Integrated Data, OC and MI Analysis)
eFigure 12. Efficacy Over Time for Crossover Cohort: A, EASI 90; B, EASI 100 Through Week 52 (Integrated Data, OC and MI Analyses)
eFigure 13. Efficacy Over Time for Crossover Cohort: A, Percent Change From Baseline in EASI; B, Percent Change From Baseline in Worst Pruritus NRS Through Week 52 (Integrated Data, OC and MMRM Analyses)
eFigure 14. Hematology and Chemistry Parameters Over Time Through Week 52 (ITT Population)
eTable 1. Treatment-emergent Adverse Events Through Week 16 (Safety Population)
eTable 2. Treatment-emergent Adverse Events Through Week 52 (Adolescent Population)
eTable 3. Most Frequently Reported Treatment-emergent Adverse Events (≥ 5% of Patients in Any Treatment Group) During Administration of UPA Through Week 52
eTable 4. Grades 3–4 Hematology and Chemistry Parameters Through Week 52 (All UPA Population)
Data Sharing Statement
References
- 1.Megna M, Napolitano M, Patruno C, et al. Systemic treatment of adult atopic dermatitis: a review. Dermatol Ther (Heidelb). 2017;7(1):1-23. doi: 10.1007/s13555-016-0170-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cork MJ, Danby SG, Ogg GS. Atopic dermatitis epidemiology and unmet need in the United Kingdom. J Dermatolog Treat. 2020;31(8):801-809. doi: 10.1080/09546634.2019.1655137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moyle M, Cevikbas F, Harden JL, Guttman-Yassky E. Understanding the immune landscape in atopic dermatitis: the era of biologics and emerging therapeutic approaches. Exp Dermatol. 2019;28(7):756-768. doi: 10.1111/exd.13911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simpson EL, Bieber T, Guttman-Yassky E, et al. ; SOLO 1 and SOLO 2 Investigators . Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335-2348. doi: 10.1056/NEJMoa1610020 [DOI] [PubMed] [Google Scholar]
- 5.Nader A, Stodtmann S, Friedel A, Mohamed MF, Othman AA. Pharmacokinetics of upadacitinib in healthy subjects and subjects with rheumatoid arthritis, Crohn’s disease, ulcerative colitis, or atopic dermatitis: population analyses of phase 1 and 2 clinical trials. J Clin Pharmacol. 2020;60(4):528-539. doi: 10.1002/jcph.1550 [DOI] [PubMed] [Google Scholar]
- 6.Rinvoq (updacitinib) extended-release tablets, for oral use. Prescribing information. AbbVie Inc; 2019.
- 7.Guttman-Yassky E, Thaçi D, Pangan AL, et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2020;145(3):877-884. doi: 10.1016/j.jaci.2019.11.025 [DOI] [PubMed] [Google Scholar]
- 8.Parmentier JM, Voss J, Graff C, et al. In vitro and in vivo characterization of the JAK1 selectivity of upadacitinib (ABT-494). BMC Rheumatol. 2018;2:23. doi: 10.1186/s41927-018-0031-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157(9):1047-1055. doi: 10.1001/jamadermatol.2021.3023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397(10290):2151-2168. doi: 10.1016/S0140-6736(21)00588-2 [DOI] [PubMed] [Google Scholar]
- 11.Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396(10247):345-360. doi: 10.1016/S0140-6736(20)31286-1 [DOI] [PubMed] [Google Scholar]
- 12.Weidinger S, Beck LA, Bieber T, Kabashima K, Irvine AD. Atopic dermatitis. Nat Rev Dis Primers. 2018;4(1):1. doi: 10.1038/s41572-018-0001-z [DOI] [PubMed] [Google Scholar]
- 13.Simpson E, Bissonnette R, Eichenfield LF, et al. The Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD): the development and reliability testing of a novel clinical outcome measurement instrument for the severity of atopic dermatitis. J Am Acad Dermatol. 2020;83(3):839-846. doi: 10.1016/j.jaad.2020.04.104 [DOI] [PubMed] [Google Scholar]
- 14.He H, Guttman-Yassky E. JAK inhibitors for atopic dermatitis: an update. Am J Clin Dermatol. 2019;20(2):181-192. doi: 10.1007/s40257-018-0413-2 [DOI] [PubMed] [Google Scholar]
- 15.Cohen SB, van Vollenhoven RF, Winthrop KL, et al. Safety profile of upadacitinib in rheumatoid arthritis: integrated analysis from the SELECT phase 3 clinical programme [abstract P209]. Ann Rheum Dis. 2020;59(2)(suppl):keaa111.204. doi: 10.1093/rheumatology/keaa111.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silverberg JI, de Bruin-Weller M, Bieber T, et al. Upadacitinib plus topical corticosteroids in atopic dermatitis: Week 52 AD Up study results. J Allergy Clin Immunol. Published online August 14, 2021. doi: 10.1016/j.jaci.2021.07.036 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eFigure 1. Patient Disposition
eFigure 2. Efficacy Over Time: A, Percent Change From Baseline in EASI and B, Percent Change From Baseline in Worst Pruritus NRS Through Week 52 (ITT Population and Integrated Data, OC Analysis)
eFigure 3. Efficacy Over Time: A, DLQI Improvement ≥4; B, DLQI 0/1 Through Week 52 (ITT Population and Integrated Data, OC Analysis)
eFigure 4. Efficacy Over Time: A, HADS-A <8 and HADS-D <8; B, Percent Change in HADS From Baseline Through Week 52 (ITT Population and Integrated Data, OC Analysis)
eFigure 5. Efficacy Over Time: A, ADerm-IS Sleep; B, Emotional State; and C, Daily Activities Through Week 52 (ITT Population and Integrated Data, OC Analysis)
eFigure 6. Efficacy Over Time: A, EASI 75; B, vIGA-AD 0/1; and C, Worst Pruritus NRS Improvement ≥4 Through Week 52 (ITT Population and Integrated data, MI analysis)
eFigure 7. Efficacy Over Time: A, EASI 90; B, EASI 100; and C, Worst Pruritus NRS 0/1 Through Week 52 (ITT Population and Integrated Data, MI Analysis)
eFigure 8. Efficacy Over Time: A, Percent Change From Baseline in EASI; B, Percent Change From Baseline in Worst Pruritus NRS Through Week 52 (ITT Population and Integrated Data, MMRM Analysis)
eFigure 9. Efficacy Over Time: A, EASI 75; B, vIGA-AD 0/1; C, EASI 90; D, Worst Pruritus NRS Improvement ≥4; E, EASI 100; and F, Percent Change in Worst Pruritus NRS From Baseline by Visit Through Week 16 (Placebo-controlled Population, Integrated Data)
eFigure 10. Efficacy Over Time: A, EASI 75; B, vIGA-AD 0/1; and C, Worst Pruritus NRS Improvement ≥4 Through Week 52 (Adolescent Population, OC Analysis)
eFigure 11. Efficacy Over Time for Crossover Cohort: A, EASI 75; B, vIGA-AD 0/1; and C, Worst Pruritus NRS Improvement ≥4 Through Week 52 (Integrated Data, OC and MI Analysis)
eFigure 12. Efficacy Over Time for Crossover Cohort: A, EASI 90; B, EASI 100 Through Week 52 (Integrated Data, OC and MI Analyses)
eFigure 13. Efficacy Over Time for Crossover Cohort: A, Percent Change From Baseline in EASI; B, Percent Change From Baseline in Worst Pruritus NRS Through Week 52 (Integrated Data, OC and MMRM Analyses)
eFigure 14. Hematology and Chemistry Parameters Over Time Through Week 52 (ITT Population)
eTable 1. Treatment-emergent Adverse Events Through Week 16 (Safety Population)
eTable 2. Treatment-emergent Adverse Events Through Week 52 (Adolescent Population)
eTable 3. Most Frequently Reported Treatment-emergent Adverse Events (≥ 5% of Patients in Any Treatment Group) During Administration of UPA Through Week 52
eTable 4. Grades 3–4 Hematology and Chemistry Parameters Through Week 52 (All UPA Population)
Data Sharing Statement


