Key Points
Question
What are the characteristics and outcomes of type 2 myocardial infarction (T2MI) compared with type 1 myocardial infarction (T1MI) in patients presenting to the emergency department (ED) with acute chest discomfort?
Findings
In this cohort study 6253 patients, 251 patients (4.0%) and 1027 patients (16.4%) were diagnosed with T2MI and T1MI, respectively, and had comparable all-cause and cardiovascular mortality at 2 years. Tachyarrhythmia and hypertension were responsible for more than two-thirds of patients with T2MI and had lower mortality compared with patients with hypotension, hypoxemia, or anemia.
Meaning
Improved understanding of the specifics of patients with T2MI should help improve management strategies.
This cohort study explores the characteristics and outcomes of patients with type 2 myocardial infarction compared with those with type 1 myocardial infarction.
Abstract
Importance
In contrast to type 1 myocardial infarction (T1MI) caused by atherothrombosis, characteristics and outcomes of type 2 myocardial infarction (T2MI) caused by supply-demand mismatch are incompletely understood.
Objective
To explore the characteristics and outcomes of patients with T2MI compared with those with T1MI.
Design, Setting, and Participants
In a prospective, international, multicenter cohort study including 12 emergency departments (EDs) in 5 European countries, unselected patients presenting with acute chest discomfort were enrolled from April 2006 to April 2018. Follow-up was done by telephone or in written form 3, 12, and 24 months after hospital discharge. Data were analyzed from April 2006 to April 2020.
Interventions
The final diagnoses of T2MI and T1MI were centrally adjudicated according to the Fourth Universal Definition of Myocardial Infarction by 2 independent cardiologists, including the pathophysiological trigger of T2MI.
Main Outcomes and Measures
Patient characteristics and outcomes, including 2-year all-cause and cardiovascular mortality and future T2MI and T1MI events.
Results
Of 6253 included patients, 2078 (33.2%) were women, and the median (IQR) age was 61 (48-74) years. Among 6253 patients with acute chest discomfort, the final adjudicated diagnosis was T2MI in 251 patients (4.0%), with tachyarrhythmia and hypertension responsible for two-thirds of cases, and T1MI in 1027 patients (16.4%). All-cause and cardiovascular mortality were comparable at 2 years (T2MI: adjusted hazard ratio, 1.0; 95% CI, 0.7-1.5; T1MI: adjusted hazard ratio, 0.7; 95% CI, 0.4-1.1). Patients with tachyarrhythmia or hypertension as their underlying trigger of T2MI had a lower mortality compared with patients with hypotension, hypoxemia, or anemia. Future T2MI was more likely among patients with index T2MI compared with patients with index T1MI (hazard ratio, 3.2; 95% CI, 1.4-7.5). Similarly, future T1MI was more likely to occur among patients with index T1MI (hazard ratio, 3.0; 95% CI, 1.2-7.4).
Conclusions and Relevance
Among patients with T2MI, tachyarrhythmia and hypertension were responsible for more than two-thirds of T2MI cases. While T2MI and T1MI had comparable all-cause and cardiovascular mortality at 2 years, patients with tachyarrhythmia or hypertension as their underlying trigger of T2MI had a lower mortality compared with patients with hypotension, hypoxemia, or anemia. Future T2MI occurred 3-fold more frequently among patients with T2MI vs T1MI as the index event. Improved understanding of the specifics of patients with T2MI should help improve management strategies.
Introduction
Myocardial infarction (MI) is one of the leading causes of death in Europe and in the US.1,2 Evidence-based care for MI has been established by large randomized clinical trials documenting important benefits to patients with early coronary revascularization, dual antiplatelet therapy, and high-dose statins.2,3
Recently, it has become apparent that plaque rupture and/or plaque erosion is not the underlying pathophysiology of all acute myocardial infarctions.2,3 Myocardial oxygen supply-demand mismatch caused by tachycardia, hypotension, or hypertension is the predominant mechanism in a substantial proportion of acute myocardial infarctions and is classified as type 2 MI (T2MI) according to the Fourth Universal Definition of Myocardial Infarction.1 Unfortunately, there are relevant uncertainties regarding the characteristics, management, and outcomes of T2MI compared with type 1 MI (T1MI).4,5,6,7,8 We therefore aimed to explore characteristics, management, and outcomes of patients with T2MI compared with patients with T1MI in a large prospective multicenter study enrolling unselected patients presenting to the emergency department (ED) with acute chest discomfort.
Methods
Study Design and Patient Population
The Advantageous Predictors of Acute Coronary Syndrome Evaluation (APACE) study is an ongoing prospective international multicenter study including 12 centers in 5 countries aimed at advancing early diagnosis of MI.9,10,11,12,13,14 Adult patients presenting to the ED with acute chest discomfort were enrolled. Each patient had to provide written informed consent to be included in the study. Patients with terminal kidney disease requiring regular dialysis and patients in cardiogenic shock were excluded. For this analysis, patients were also excluded if the final diagnosis was unclear after adjudication and if the measurement of cardiac troponin at ED presentation was missing. The study was carried out according to the principles of the Declaration of Helsinki and approved by the local ethics committees as well as the Ethics Commission of Northwestern and Central Switzerland (EKNZ). Data were obtained and analyzed according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline (eTable 2 in Supplement 1).15
Clinical Assessment
All patients underwent clinical assessment, including standardized and detailed medical history with assessment of 34 predefined chest pain characteristics, vital signs, physical examination, 12-lead electrocardiography (ECG), continuous ECG rhythm monitoring, pulse oximetry, standard blood testing, and chest radiography, if indicated. Levels of cardiac troponins (cTn), including high-sensitivity cTn (hs-cTn) in some centers, were measured at presentation and serially thereafter if clinically indicated. Treatment of patients was left to the discretion of the attending physician.16 Calculation of the estimated glomerular filtration rate was performed using the abbreviated Modification of Diet in Renal Disease (MDRD) formula.17
Central Adjudication of T1MI and T2MI
All cases were adjudicated by 2 independent cardiologists (T.N., J.B., L.K., O.M., D.I.K., F.J.M.-S., M.C., R.T., K.W., M.R.G., or C.M.) according to the Fourth Universal Definition of Myocardial Infarction based on detailed review of patient history, physical examination, results of laboratory testing, radiologic testing, ECG, echocardiography, cardiac exercise stress test, lesion severity, and morphology in coronary angiography and 90-day follow-up.1,2,10,11,12,13,14,16 In case of disagreement regarding the diagnosis, a third cardiologist (T.N., J.B., L.K., O.M., D.I.K., F.J.M.-S., M.C., R.T., K.W., M.R.G., or C.M.) reviewed and adjudicated. Two sets of serial cTn measurements were performed for the adjudication of the final diagnosis: serial cTn measurements obtained as part of local routine clinical care (different cTn and hs-cTn assays) and serial measurements of hs-cTn T from study blood samples performed centrally in the core laboratory (University Hospital Basel, Basel, Switzerland) to take advantage of the higher sensitivity and higher overall diagnostic accuracy offered by hs-cTn T.16,18
In addition to evidence of myocardial necrosis in a clinical setting consistent with acute myocardial ischemia, T1MI was defined as spontaneous MI related to a primary atherothrombotic coronary event, such as plaque erosion or rupture, intraluminal coronary thrombus, or distal microembolization.1 T2MI was defined as secondary to myocardial oxygen supply-demand mismatch in the context of bradyarrhythmias or tachyarrhythmias, hypoxemia, hypotension, hypertension, severe anemia, or coronary artery spasm, coronary embolism, and nonatherosclerotic dissection.1 For T2MI, underlying coronary artery disease (CAD) was possible but not mandatory.1 To qualify for T2MI, the same dynamic changes in hs-cTn T were required as for T1MI. Documentation of a clear trigger was essential for the diagnosis of T2MI as recommended.1 Coronary angiography was not mandatory for a diagnosis of T1MI to limit the effect of selection bias owing to clinical referral to coronary angiography. Different etiologies of acute cardiomyocyte injury, such as myocarditis, Takotsubo syndrome, and acute heart failure, were adjudicated as other cardiac pathologies distinct from T2MI and T1MI, as recommended.1 Blood sampling and laboratory methods have been described in detail elsewhere.10,11,12,13,14
Follow-up and Clinical End Points
Follow-up was done by telephone or in written form 3, 12, and 24 months after hospital discharge. Information about clinical end points, including cardiovascular (CV) and all-cause mortality, was obtained by reviewing all available clinical information, including contact with patients, family physicians, and national mortality registries. All documents were reviewed by experienced cardiologists. Therapies that have been shown to improve outcomes in patients with MI, such as the use of coronary revascularization, dual antiplatelet therapy, and high-dose statins,2 were assessed at hospital discharge.
Statistical Analysis
Continuous variables were expressed as medians and IQRs and categorical variables as counts and percentages. Baseline characteristics, findings, and in-hospital procedures between those with T2MI and those with T1MI were compared using the Mann-Whitney U test for continuous variables and by the Pearson χ2 test for categorical variables. Two-year all-cause mortality, according to the adjudicated diagnosis, and future MI were plotted in Kaplan-Meier curves, and the log-rank test was used for comparison of survival between the 2 groups. For 2-year CV mortality competing risk analysis using the Fine-Gray method was used. Cumulative incidence functions were plotted. For the outcome analyses, patients were included only once.
Multivariable Cox proportional hazards models with adjustments for age, sex, and comorbidities were used to evaluate the association of T2MI vs T1MI with 2-year all-cause and CV mortality. Considering the total number of patients with T2MI in our study and to avoid overfitting in the model, 2 multivariable models were built for prognostic purposes, the second having a total of 11 covariates. The covariates included in the multivariable models were selected based on clinical knowledge, regardless of the P value. Model A included age and sex. Model B included age, sex, estimated glomerular filtration rate, peak hs-cTn T, hypertension, diabetes, dyslipidemia, history of ischemic or hemorrhagic stroke, liver disease, obstructive lung disease, and malignancy. For future MI, we only adjusted for model A. Proportional hazard assumption was tested by means of the Schoenfeld residuals.
All hypothesis testing was 2-tailed, and P values less than .05 were considered to indicate statistical significance. The statistical analyses were performed using IBM SPSS Statistics for Windows version 26.0 (IBM Corp) and Stata version 16.1 (Stata Corp).
Results
Patient Characteristics
From April 2006 to April 2018, 6253 patients were eligible for analysis. The median (IQR) age was 61 (48-74) years, and 2078 patients (33.2%) were women. The final adjudicated diagnosis was T2MI in 251 patients (4.0%) and T1MI in 1027 patients (16.4%) (eFigure in Supplement 1). Most baseline characteristics were comparable between patients with T2MI and T1MI (Table 1). Differences included a higher proportion of women (T2MI, 90 of 251 [35.9%]; T1MI, 267 of 1027 [26.0%]; P = .002) and a lower prevalence of CV risk factors in T2MI. The median (IQR) heart rate was higher in the T2MI group (89 [73-119] beats per minute vs 76 [65-88] beats per minute; P < .001), while the median (IQR) systolic blood pressure was lower in patients with T2MI compared with those with T1MI (135 [117-153] mm Hg vs 144 [127-161] mm Hg; P < .001).
Table 1. Baseline Characteristics for Differentiation Between Type 2 Myocardial Infarction (T2MI) and Type 1 Myocardial Infarction (T1MI).
| Characteristic | No. (%) | P valuea | ||
|---|---|---|---|---|
| All patients (N = 6253) | T2MI (n = 251) | T1MI (n = 1027) | ||
| Age, median (IQR), y | 61 (48-74) | 71 (60-80) | 69 (57-79) | .25 |
| Sex | ||||
| Male | 4175 (67) | 161 (64) | 760 (74) | .002 |
| Female | 2078 (33) | 90 (36) | 267 (26) | |
| BMI, median (IQR)b | 26 (24-30) | 26 (24-29) | 27 (24-29) | .32 |
| Risk factors | ||||
| Hypertension | 3700 (59) | 193 (77) | 757 (74) | .30 |
| Hypercholesterolemia | 2937 (47) | 132 (53) | 629 (61) | .01 |
| Diabetes | 1062 (17) | 65 (26) | 261 (25) | .88 |
| Current smoking | 1580 (25) | 49 (20) | 272 (26) | .02 |
| History of smoking | 2312 (37) | 99 (39) | 418 (41) | .72 |
| Positive family history of CAD | 944 (15) | 23 (9) | 162 (16) | .007 |
| Medical history | ||||
| Coronary artery disease | 1966 (31) | 104 (41) | 407 (40) | .60 |
| Myocardial infarction | 1400 (22) | 67 (27) | 311 (30) | .26 |
| Myocardial revascularization | 1641 (26) | 77 (31) | 324 (32) | .79 |
| Peripheral artery disease | 326 (5.2) | 25 (10) | 103 (10) | .97 |
| Ischemic/hemorrhagic stroke | 324 (5.2) | 22 (8.8) | 80 (7.8) | .61 |
| Vital parameters, median (IQR) | ||||
| SBP, mm Hg | 140 (125-156) | 135 (117-153) | 144 (127-161) | <.001 |
| Heart rate, beats per minute | 76 (66-89) | 89 (73-119) | 76 (65-88) | <.001 |
| Oxygen saturation, % | 98 (97-100) | 98 (96-99) | 98 (96-99) | .36 |
| Biochemistry, median (IQR) | ||||
| hs-cTn T, ng/L | ||||
| Baseline | 8 (4-20) | 30 (17-59) | 63 (29-291) | <.001 |
| Absolute change within 1 h | 1 (0-2) | 5 (2-13) | 11 (4-39) | <.001 |
| Peak value within 6 h | 9 (5-24) | 40 (26-84) | 113 (45-332) | <.001 |
| Hemoglobin, g/dL | 14.3 (13.2-15.3) | 14.1 (12.5-15.3) | 14.3 (13.0-15.4) | .04 |
| BNP, pg/mLc | 142 (44-509) | 1036 (201-3463) | 558 (169-1648) | .20 |
| Creatinine clearance, mL/min/1.73 m2 | 84 (68-100) | 70 (52-90) | 76 (61-94) | .001 |
Abbreviations: BMI, body mass index; BNP, brain-type natriuretic peptide; CAD, coronary artery disease; hs-cTn, high-sensitivity cardiac troponin; SBP, systolic blood pressure.
SI conversion factor: To convert hemoglobin to g/L, multiply by 10; BNP to ng/L multiply by 1.
P values for comparison between T2MI and T1MI.
Calculated as weight in kilograms divided by height in meters squared.
Data available in 1225 patients overall, 51 patients with T2MI, and 221 patients with T1MI.
T2MI Phenotypes
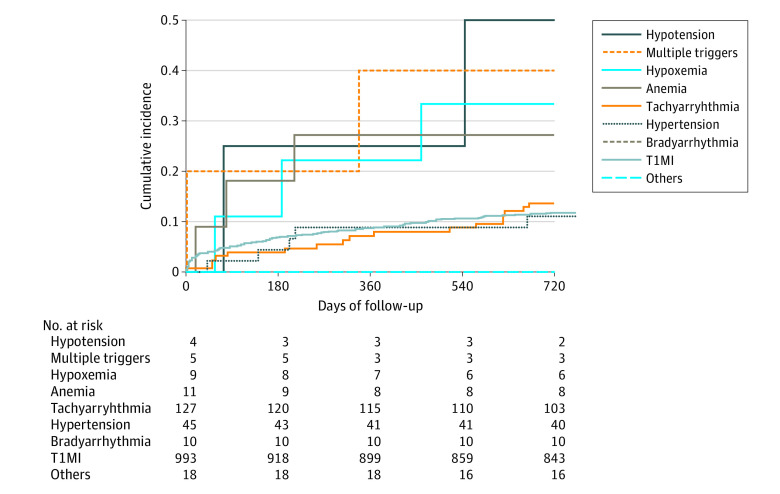
The dominant underlying pathophysiological mechanisms among 251 patients with T2MI were tachyarrhythmia (135 [53.8%]), bradyarrhythmia (10 [4.0%]), hypertension (47 [18.7%]), hypotension (4 [1.6%]), anemia (11 [4.4%]), hypoxemia (9 [3.6%]), coronary artery dissection (3 [1.2%]), coronary artery spasm (14 [5.6%]), coronary embolism (2 [0.8%]), multiple triggers (7 [2.8%]), or unknown (9 [3.6%]).
In-Hospital Procedures
Treatment markedly differed between patients with T2MI and T1MI and included percutaneous coronary intervention or coronary artery bypass grafting in 9 patient with T2MI (3.6%) and in 772 patients with T1MI (75.2%; P < .001). A total of 35 patients with T2MI (13.9%) and 784 patients with T1MI (76.3%) were discharged taking dual antiplatelet therapy (P < .001). Statin therapy at discharge was also less common in patients with T2MI compared with those with T1MI (135 [53.8%] vs 922 [89.8%]; P < .001). In contrast, oral anticoagulation was more often prescribed in those with T2MI vs T1MI (77 [30.7%] vs 139 [13.5%]; P < .001) (Table 2).
Table 2. Findings and In-Hospital Procedures.
| Finding | No. (%) | P valuea | ||
|---|---|---|---|---|
| All patients (N = 6253) | T2MI (n = 251) | T1MI (n = 1027) | ||
| In hospital procedures | ||||
| Coronary angiography | 1552 (25) | 69 (27.5) | 880 (86) | <.001 |
| 1-Vessel disease | 357 (5.7) | 12 (4.8) | 232 (23) | <.001 |
| 2-Vessel disease | 367 (5.9) | 7 (2.8) | 249 (24) | <.001 |
| 3-Vessel disease | 579 (9.3) | 10 (4.0) | 377 (37) | <.001 |
| Percutaneous coronary intervention | 921 (15) | 8 (3.2) | 684 (67) | <.001 |
| CABG | 129 (2.1) | 1 (0.4) | 88 (8.6) | <.001 |
| Ergometry, MPS, PET, or SE | 1329 (21) | 46 (18) | 178 (17) | .71 |
| Positive findings on ergometry, MPS, PET, or SE | 324 (5.2) | 14 (5.6) | 58 (5.6) | .97 |
| ECG at presentation | ||||
| Left bundle branch block | 226 (3.6) | 25 (10) | 51 (5.0) | .003 |
| ST-segment elevation | 275 (4.4) | 9 (3.6) | 169 (16) | <.001 |
| ST-segment depression | 550 (8.8) | 54 (22) | 295 (29) | .02 |
| T-wave inversion | 773 (12) | 50 (20) | 265 (26) | .05 |
| No significant ECG abnormalities | 4528 (72.4) | 127 (51) | 401 (39) | .001 |
| Medication at presentation | ||||
| Aspirin | 2152 (34) | 102 (41) | 446 (43) | .42 |
| DAPT | 515 (8.2) | 20 (8.0) | 104 (10) | .30 |
| ACE/ARB inhibitors | 2371 (38) | 133 (53) | 478 (47) | .07 |
| β-Blockers | 2054 (33) | 118 (47) | 380 (37) | .004 |
| Calcium antagonists | 931 (15) | 49 (20) | 193 (19) | .79 |
| Nitrates | 586 (9.4) | 27 (11) | 151 (15) | .11 |
| Statins | 2126 (34) | 107 (43) | 407 (40) | .39 |
| Oral anticoagulation | 610 (9.8) | 46 (18) | 85 (8.3) | <.001 |
| Medication at discharge | ||||
| Aspirin | 2920 (47) | 124 (49) | 937 (91) | <.001 |
| DAPT | 1382 (22) | 35 (14) | 784 (76) | <.001 |
| ACE/ARB inhibitors | 2924 (47) | 152 (61) | 824 (80) | <.001 |
| β-Blockers | 2771 (44) | 170 (68) | 809 (79) | <.001 |
| Calcium antagonists | 1058 (17) | 67 (27) | 202 (20) | .01 |
| Nitrates | 787 (13) | 39 (16) | 236 (23) | .01 |
| Statins | 2921 (47) | 135 (54) | 922 (90) | <.001 |
| Oral anticoagulation | 851 (14) | 77 (31) | 139 (14) | <.001 |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor antagonist; CABG, coronary artery bypass grafting; DAPT dual antiplatelet therapy; ECG, electrocardiography; MPS, myocardial perfusion scanning; PET, positron emission tomography; SE, stress echocardiography; T1MI, type 1 myocardial infarction; T2MI, type 2 myocardial infarction.
P values for comparison between T2MI and T1MI.
Long-term All-Cause and CV Mortality
The median (IQR) duration of follow-up was 821 (739-1375) days. There were 33 deaths (13.9%) in the T2MI group and 116 deaths (11.7%) in the T1MI group over 730 days. Accordingly, 2-year all-cause mortality and 2-year CV mortality were comparable between patients with T2MI and T1MI (all-cause mortality: T2MI, 33 of 238 [13.9%]; T1MI, 116 of 993 [11.7%]; log-rank P value = .39; CV mortality: T2MI, 18 of 238 [7.6%]; T1MI, 92 of 993 [9.3%]; Fine-Gray P value = .39) (Figure 1A and B). Considering competing risks, the 2-year CV mortality was also similar between patients with T2MI and T1MI (hazard ratio [HR], 0.80; 95% CI, 0.49-1.33; P = .39). After adjustment for age, sex, and baseline comorbidities, similar findings emerged (eTable 1 in Supplement 1).
Figure 1. Cumulative Incidence of Mortality at 730 Days and Future Myocardial Infarction After Index Type 2 Myocardial Infarction (T2MI) and Type 1 Myocardial Infarction (T1MI).
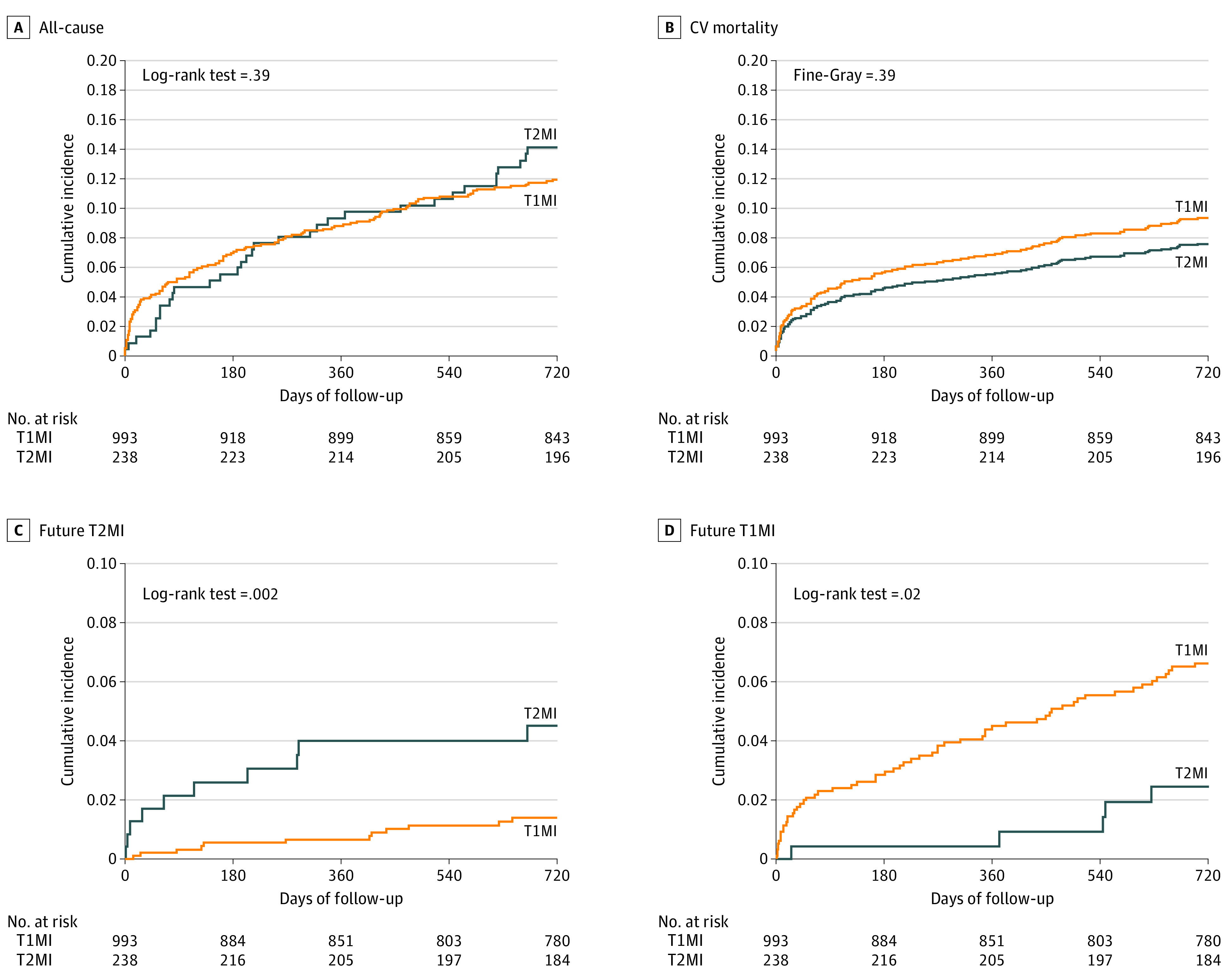
A and B, Two-year all-cause mortality and 2-year cardiovascular mortality. Adjusted rates of both were similar between patients with T2MI and T1MI. C and D, Future T2MI and T1MI. Patients who developed a future T2MI more often had an index T2MI than T1MI, whereas the reverse was seen for patients who developed a future T1MI.
Future MI
Future T2MI was more likely to occur among patients with index T2MI vs index T1MI (unadjusted HR, 3.44; 95% CI, 1.49-7.97; P = .004; adjusted HR, 3.20; 95% CI, 1.37-7.50; P = .007). Similarly, future T1MI was more likely to occur among patients with index T1MI (unadjusted HR, 2.91; 95% CI, 1.17-7.24; P = .02; adjusted HR, 2.98; 95% CI, 1.19-7.44; P = .02) (Figure 1C and D).
All-Cause Mortality Stratified by T2MI Phenotype
The cumulative incidence of 2-year all-cause mortality differed among the different mechanisms provoking T2MI. The mortality of patients with tachyarrhythmia or hypertension as their underlying trigger of T2MI was lower compared with patients with hypotension, hypoxemia, or anemia as the underlying trigger (Figure 2).
Figure 2. Cumulative Incidence of All-Cause Mortality at 730 Days of Follow-up.
The cumulative incidence is stratified by the underlying cause of type 2 myocardial infarction. Patients with bradyarrhythmia or the subgroup others had the best prognoses. The subgroups type 1 myocardial infarction (T1MI), hypertension, and tachyarrhythmia had more favorable prognoses than multiple triggers, including hypotension, hypoxemia, or anemia. Multiple triggers defined as more than 1 of the mentioned reasons; others included coronary artery spasm, coronary embolism, and coronary artery dissection.
Discussion
In this large prospective multicenter study enrolling unselected patients presenting with acute chest discomfort to the ED, we explored the characteristics, management, and outcomes of patients with T2MI compared with T1MI. We report 5 major findings. First, the prevalence of T2MI was one-fourth that of T1MI. Second, tachyarrhythmia (135 [53.8%]) and hypertension (47 [18.7%]) were by far the most common T2MI phenotypes. Third, T2MI and T1MI had comparable all-cause and CV mortality at 2 years. Fourth, all-cause mortality substantially differed among the different T2MI phenotypes, with the common triggers, such as tachyarrhythmia and hypertension, being associated with a much lower mortality compared with uncommon triggers, such as hypotension, hypoxemia, and anemia. Fifth, future T2MI occurred 3-fold as often among patients with T2MI as the index event compared with patients with T1MI as the index event. In contrast, future T1MI occurred 3-fold more frequently among patients with T1MI compared with patients with T2MI as the index event.
These findings extend and corroborate important observations made in Minnesota from 2003 to 2012.6 Using cTn T measurements irrespective of the indication and the clinical setting, including critically ill patients in the intensive care unit and the perioperative period, 5460 patients had at least 1 elevated conventional cTn T concentration. Of these, 1365 patients were classified as having index T1MI (mean age, 69 years; 37% women), and 1054 were classified as having T2MI (mean age, 74 years; 54% women). CV mortality was comparable between those with T2MI and T1MI, while all-cause mortality was higher in patients with T2MI. Subclassification of T2MI by cause demonstrated a more favorable prognosis when the principal provoking mechanism was arrhythmia compared with postoperative status, hypotension, anemia, and hypoxia. After index T2MI, as in this study, the most common MI during follow-up was a recurrent T2MI, whereas the occurrence of a new T1MI was relatively uncommon (estimated rates at 5 years, 9.7% and 1.7%, respectively).6 Similarly, in Edinburgh, among 2122 patients with an elevated conventional cTn I irrespective of the indication and setting, 55.2% had T1MI and 20.2% had T2MI. Again, CV mortality was comparable between those with T2MI and T1MI, while all-cause mortality was higher in those with T2MI.5
These findings help to address uncertainties regarding the major determinants of the prevalence of T2MI and outcomes of T2MI compared with T1MI. In contrast to previous assumptions,6 the use of hs-cTn T and I instead of less sensitive, conventional cTn T and I is only a minor confounder. This study included serial measurements of hs-cTn T for the central adjudication of patients presenting with a homogenous presenting symptom of acute chest discomfort and found T2MI to be much less frequent than T1MI. However, using all measurements performed with a less sensitive, conventional cTn T assay, irrespective of the indication and the clinical setting, when including critically ill patients in the intensive care unit and the perioperative period, T2MI was as common as or even more common than T1MI.4,8 When restricting the assessment to the perioperative period and using hs-cTn T, T2MI was the clearly dominant MI etiology.19
Beyond the clinical setting and the presence and severity of underlying cardiac and noncardiac disease, this study extended and corroborated the insights obtained in the Minnesota cohort6 that tachyarrhythmia and hypertension were associated with more favorable outcomes. These findings underscore that even within a cohort of patients with a homogenous presenting symptom, such as acute chest discomfort, and in a homogenous clinical setting, such as the ED, T2MI is not a single clinical entity but rather a heterogeneous group of phenotypic clusters with myocardial oxygen supply-demand mismatch as the underlying mechanism.
This diversity also highlights that the immediate therapeutic approaches to T2MI must be highly individualized and focused on rapid reversal of the trigger. However, long-term therapeutic consequences may be much more homogenous in targeting myocardial oxygen tolerance, particularly given the high-risk of T2MI recurrence. These therapeutic measures should include optimal blood pressure and lipid control. This concept is supported, for example, by a parallel reduction in T1MI and T2MI by more often achieving low-density lipoprotein target levels in patients with established CV disease with alirocumab vs placebo.20
Particularly when assessing the prevalence of T2MI in broader patient populations, it is important to closely follow the principles highlighted in the Fourth Universal Definition of Myocardial Infarction1—that is, mainly the fact that acute heart failure, sepsis, Takotsubo cardiomyopathy, and myocarditis are entities distinct from T2MI, that should not be misclassified as T2MI.4 This misclassification unfortunately occurred in some prior studies that had reported higher all-cause mortality owing to broadly labeled T2MI vs T1MI.8 It is also important to highlight that preexisting CAD is a major confounder of mortality among patients with T2MI.5,11 In the absence of CAD, patients with T2MI have a very low risk of death.11 This is of particular importance given the dominance of tachyarrhythmia as the trigger for T2MI and the possible psychological and medicolegal harm associated with labeling a 20-year-old patient with acute chest discomfort and ST-segment depression, both indicative of myocardial ischemia, within an episode of atrioventricular-nodal reentry tachycardia as T2MI. These insights have led experts to argue that patients with T2MI should be further subclassified according to the presence or absence of CAD.21,22
Limitations
This study has several limitations that should be considered. First, by exclusively enrolling patients presenting to the ED with acute chest discomfort, this study largely avoided bias induced by possible changes in the selection of patients for cTn and hs-cTn testing. However, we cannot comment on characteristics and outcomes of T2MI occurring in critically ill patients or in the perioperative setting. Second, we also cannot comment on characteristics and outcomes of T2MI in patients with terminal kidney disease requiring long-term hemodialysis, as they were excluded. Third, even though we used a stringent methodology for central adjudication by experienced cardiologists, including serial measurements of hs-cTn T and strict adherence to the Fourth Universal Definition of Myocardial Infarction, we still may have misclassified a small number of patients.23 Fourth, as this study required written informed consent and as cardiogenic shock was an exclusion criteria, there is likely an underrepresentation of critically ill patients presenting with acute chest discomfort.
Conclusions
The prevalence of T2MI was one-fourth that of T1MI, with tachyarrhythmia and hypertension being responsible for more than two-thirds of T2MI cases, among patients presenting to the ED with acute chest discomfort. T2MI and T1MI had comparable all-cause and CV mortality at 2 years. Patients with tachyarrhythmia or hypertension as their underlying trigger of T2MI had a lower mortality than patients with hypotension, hypoxemia, or anemia as the underlying trigger. Future T2MI occurred 3-fold more frequently among patients with T2MI compared with those with T1MI as the index event. Improved understanding of the specifics of patients with T2MI should help improve their management strategies.
eFigure. Patient flow diagram.
eTable 1. Multivariable Cox proportional hazards models with adjustments.
eTable 2. STROBE Statement—checklist of items that should be included in reports of observational studies.
eReferences.
APACE Investigators.
References
- 1.Thygesen K, Alpert JS, Jaffe AS, et al. ; ESC Scientific Document Group . Fourth Universal Definition of Myocardial Infarction (2018). Eur Heart J. 2019;40(3):237-269. doi: 10.1093/eurheartj/ehy462 [DOI] [PubMed] [Google Scholar]
- 2.Collet J-P, Thiele H, Barbato E, et al. ; ESC Scientific Document Group . 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent st-segment elevation: the Task Force for the Management of Acute Coronary Syndromes in Patients Presenting Without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2021;42(14):1289-1367. doi: 10.1093/eurheartj/ehaa575 [DOI] [PubMed] [Google Scholar]
- 3.O’Gara PT, Kushner FG, Ascheim DD, et al. ; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127(4):362-425. doi: 10.1161/CIR.0b013e3182742c84 [DOI] [PubMed] [Google Scholar]
- 4.Sandoval Y, Jaffe AS. Type 2 myocardial infarction: JACC Review Topic of the Week. J Am Coll Cardiol. 2019;73(14):1846-1860. doi: 10.1016/j.jacc.2019.02.018 [DOI] [PubMed] [Google Scholar]
- 5.Chapman AR, Shah ASV, Lee KK, et al. Long-term outcomes in patients with type 2 myocardial infarction and myocardial injury. Circulation. 2018;137(12):1236-1245. doi: 10.1161/CIRCULATIONAHA.117.031806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raphael CE, Roger VL, Sandoval Y, et al. Incidence, trends, and outcomes of type 2 myocardial infarction in a community cohort. Circulation. 2020;141(6):454-463. doi: 10.1161/CIRCULATIONAHA.119.043100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaggin HK, Liu Y, Lyass A, et al. Incident type 2 myocardial infarction in a cohort of patients undergoing coronary or peripheral arterial angiography. Circulation. 2017;135(2):116-127. doi: 10.1161/CIRCULATIONAHA.116.023052 [DOI] [PubMed] [Google Scholar]
- 8.Vargas KG, Haller PM, Jäger B, et al. Variations on classification of main types of myocardial infarction: a systematic review and outcome meta-analysis. Clin Res Cardiol. 2019;108(7):749-762. doi: 10.1007/s00392-018-1403-3 [DOI] [PubMed] [Google Scholar]
- 9.Advantageous Predictors of Acute Coronary Syndromes Evaluation (APACE) Study (APACE). ClinicalTrials.gov identifier: NCT00470587. Updated April 20, 2021. Accessed April 30, 2021. https://clinicaltrials.gov/ct2/show/NCT00470587
- 10.Troester V, Strebel I, Nestelberger T, et al. ; APACE Investigators . Association of previous myocardial infarction and time to presentation with suspected acute myocardial infarction. J Am Heart Assoc. 2021;10(1):e017829. doi: 10.1161/JAHA.120.017829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schoepfer H, Nestelberger T, Boeddinghaus J, et al. ; APACE (Advantageous Predictors of Acute Coronary Syndrome Evaluation) Investigators . Effect of a proposed modification of the type 1 and type 2 myocardial infarction definition on incidence and prognosis. Circulation. 2020;142(21):2083-2085. doi: 10.1161/CIRCULATIONAHA.120.048920 [DOI] [PubMed] [Google Scholar]
- 12.Boeddinghaus J, Nestelberger T, Koechlin L, et al. ; APACE Investigators . Early diagnosis of myocardial infarction with point-of-care high-sensitivity cardiac troponin I. J Am Coll Cardiol. 2020;75(10):1111-1124. doi: 10.1016/j.jacc.2019.12.065 [DOI] [PubMed] [Google Scholar]
- 13.Nestelberger T, Boeddinghaus J, Greenslade J, et al. ; APACE and ADAPT Investigators . Two-hour algorithm for rapid triage of suspected acute myocardial infarction using a high-sensitivity cardiac troponin I assay. Clin Chem. 2019;65(11):1437-1447. doi: 10.1373/clinchem.2019.305193 [DOI] [PubMed] [Google Scholar]
- 14.Boeddinghaus J, Twerenbold R, Nestelberger T, et al. ; APACE Investigators . Clinical use of a new high-sensitivity cardiac troponin I assay in patients with suspected myocardial infarction. Clin Chem. 2019;65(11):1426-1436. doi: 10.1373/clinchem.2019.304725 [DOI] [PubMed] [Google Scholar]
- 15.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Prev Med. 2007;45(4):247-251. doi: 10.1016/j.ypmed.2007.08.012 [DOI] [PubMed] [Google Scholar]
- 16.Nestelberger T, Boeddinghaus J, Badertscher P, et al. ; APACE Investigators . Effect of definition on incidence and prognosis of type 2 myocardial infarction. J Am Coll Cardiol. 2017;70(13):1558-1568. doi: 10.1016/j.jacc.2017.07.774 [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Coresh J, Greene T, et al. ; Chronic Kidney Disease Epidemiology Collaboration . Using standardized serum creatinine values in the Modification of Diet in Renal Disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247-254. doi: 10.7326/0003-4819-145-4-200608150-00004 [DOI] [PubMed] [Google Scholar]
- 18.Haaf P, Drexler B, Reichlin T, et al. High-sensitivity cardiac troponin in the distinction of acute myocardial infarction from acute cardiac noncoronary artery disease. Circulation. 2012;126(1):31-40. doi: 10.1161/CIRCULATIONAHA.112.100867 [DOI] [PubMed] [Google Scholar]
- 19.Puelacher C, Gualandro DM, Lurati Buse G, et al. Etiology of peri-operative myocardial infarction/injury after noncardiac surgery and associated outcome. J Am Coll Cardiol. 2020;76(16):1910-1912. doi: 10.1016/j.jacc.2020.08.043 [DOI] [PubMed] [Google Scholar]
- 20.White HD, Steg PG, Szarek M, et al. ; ODYSSEY OUTCOMES Investigators . Effects of alirocumab on types of myocardial infarction: insights from the ODYSSEY OUTCOMES trial. Eur Heart J. 2019;40(33):2801-2809. doi: 10.1093/eurheartj/ehz299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Lemos JA, Newby LK, Mills NL. A proposal for modest revision of the definition of type 1 and type 2 myocardial infarction. Circulation. 2019;140(22):1773-1775. doi: 10.1161/CIRCULATIONAHA.119.042157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thygesen K, Jaffe AS. Should myocardial infarction type 2 be regarded as two separate entities? Eur Heart J. 2019;40(33):2810-2812. doi: 10.1093/eurheartj/ehz451 [DOI] [PubMed] [Google Scholar]
- 23.Chapman AR, Adamson PD, Shah ASV, et al. ; High-STEACS Investigators . High-sensitivity cardiac troponin and the universal definition of myocardial infarction. Circulation. 2020;141(3):161-171. doi: 10.1161/CIRCULATIONAHA.119.042960 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Patient flow diagram.
eTable 1. Multivariable Cox proportional hazards models with adjustments.
eTable 2. STROBE Statement—checklist of items that should be included in reports of observational studies.
eReferences.
APACE Investigators.