Highlights
-
•
Sterilization ways affected the nutrition and flavor profile of apple juice.
-
•
Sonication was helpful to increase ascorbic acid level.
-
•
Microwave sterilization caused the highest volatile compound loss.
-
•
The soluble pectin and soluble protein in juice decreased significantly after HPS.
Keywords: Cloudy apple juice, Ultrasonic sterilization, Nutrition, Flavor
Abstract
Sterilization plays an important role in extending the shelf-life of apple juice; however, it also affects the nutritional and flavor profile of the juice. This study was initiated to evaluate the effects of several common sterilization methods (conventional pasteurization, microwave sterilization, ultrasonic sterilization, and ultra-high-pressure sterilization) on some important quality parameters of apple juice. The results showed that the content of soluble pectin and soluble protein in juice decreased significantly after ultra-high-pressure sterilization. Sonication was found to be effective in increasing the level of ascorbic acid in apple juice. The sugar:acid ratio increased significantly after pasteurization, microwave sterilization, and ultra-high-pressure sterilization, which changed the taste of juice. Microwave sterilization caused the highest volatile compound loss, while ultra-high-pressure sterilization led to a higher retention rate of volatile compounds in juice. This study could be helpful in seeking suitable sterilization methods retaining the quality of cloudy apple juice.
1. Introduction
Apples and apple juice are extensively consumed across the world, not only because of their superior storage and manufacturing capabilities, but also because they have a high nutritional value and a wide range of flavors [1], [2]. A greater understanding of the factors that influence juice quality would help commercial delivery of favorable traits.
Sterilization is a necessary technique in ensuring the safety and extending the shelf-life of apple juice. Traditional thermal sterilization of fruit juice is still the most extensively used technique for shelf-life extension and fruit juice preservation. Nevertheless, it frequently results in unfavorable alterations in the sensory properties of the juice [3]. Ultrasonic sterilization (US) is a promising non-thermal processing method and a potential alternative to traditional thermal pasteurization. The application of sonication could generate high-quality, microbiologically safe, and high-nutritional-value juices according to recent studies, such as orange juice [4] and blackberry juice [5]. Microwave sterilization (MS) provides similar benefits as conventional sterilization, but can further improve product quality and reduce the time of exposure to energy [6]; therefore, it maintains more natural organoleptic features of the juice and has a lower risk of causing the loss of crucial thermolabile nutrients [7]. Ultra-high-pressure sterilization (HPS) is a new sterilization technology that allows for the processing of different kinds of foodstuffs in continuous fluid, inactivating pathogenic and most of the spoiling microorganisms with high efficiency. One of the main advantages of HPS is that it is less damaging to the functional and nutritive properties of fruit juice, like its antioxidant capacity, polyphenol composition, and provitamin A content and vitamin C, in comparison with the normally used thermal treatments [8], [9]. The European Commission has embraced products treated by high pressure in the category of novel foods regulated by Novel Foods Legislation. In short, different sterilization methods have their own characteristics, advantages, and disadvantages, and thus they have different effects on raw materials.
The main purpose of this research was to study the effects of different sterilization methods (conventional pasteurization [CP], MS, US, and HPS) on the nutrient content (soluble pectin, soluble protein, and ascorbic acid) and flavor (titratable acid content [TAC], total soluble solid [TSS], electronic nose [E-nose], and GC–MS) of cloudy apple juice. This could provide some theoretical references to seek suitable sterilization methods to improve sensory and edible qualities of cloudy apple juice.
2. Materials and methods
2.1. Raw material and treatment
Fresh ‘Ralls’ apples (Malus pumila Mill.) were got from mature fruit trees in Jinzhou, Liaoning Province, China. Apples with uniform color, uniform size and moderate maturity were chosen at random for the experiment. After cleaning and cutting into apple pieces and then pulped with ascorbic acid (1 g/kg) using a SRQ-7315 multi-functional food blender (Ainishideng Electric Appliance Co., LTD, Foshan, China). Apple juice was then filtered through a 300-mesh filter cloth to remove impurities (screen mesh diameter approximately 48 μm).
2.2. Sterilization methods
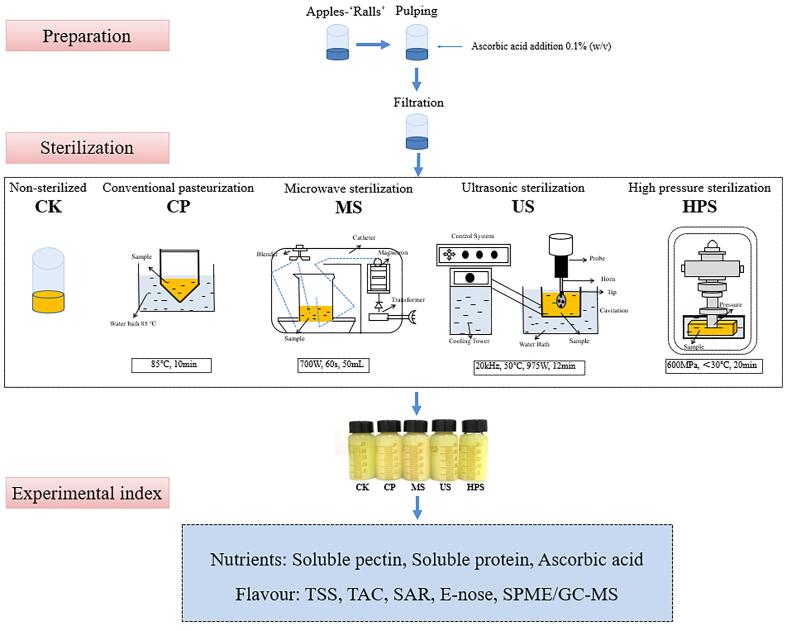
During simplified pilot-scale manufacturing, cloudy apple juice was sterilized using diverse methods, and five sets of samples were collected: the CP, MS, US, and HPS groups and the unsterilized control group (CK) (Fig. 1). Apple juice (50 mL) was sterilized in the following ways. CP: A sterile bottle with apple juice was put in a hot water bath at 85 °C for 10 min. MS: Apple juice was treated in a microwave (Midea M1-L213C, 23 L) at 700 W for 60 s [10], the juice temperature reached 62 °C after MS. US: Apple juice was ultrasound-sterilized for 12 min at 50 °C in a circulating water bath using an ultrasonic sterilizer (JRA-20CQ, Wuxi, China). The US treatment was given at a constant frequency of 20 kHz, and the power output was 975 W. HPS: Apple juice was treated at 600 MPa for 20 min using an HPS production line (HPP.L2-600/0.6, Tianjin, China) [11], [12]. After sterilization, all juices were cooled to room temperature (25 ± 0.5 °C) for subsequent study. The juices sample treatments and preparations were carried out in triplicate. All sterilization treatments (CP, MS, US, and HPS) reduced the microbial load of the cloudy apple juice to below 1 CFU/mL (data not shown).
Fig. 1.
The processing course of cloudy apple juice and the different sterilization methods. CK: non-sterilized control group, CP: conventional pasteurization, MS: microwave sterilization, US: ultrasonic sterilization, HPS: ultra-high-pressure sterilization.
2.3. Soluble pectin
The soluble pectin content was determined following the approach reported by Fraeye et al. [13] with slight modifications. Each sample (5 mL of apple juice) was mixed with 95% ethanol (25 mL). After boiling for 25 min, it was cooled to room temperature and centrifuged at 8000 × g for 20 min at 4 °C. The supernatant was discarded and 95% ethanol was added to a total volume of 30 mL. After boiling and centrifugation, the alcohol-insoluble residue was dissolved in deionized water and centrifuged at 15,000 × g for 20 min. Then the supernatants, containing the soluble pectin, were dissolved in 50 mL of deionized water. Pectin content was evaluated using a colorimetric method with a spectrophotometer (UV-2700, Shimadzu, Tokyo, Japan) at 530 nm. The pectin concentration is expressed as D-galacturonic acid equivalents.
2.4. Soluble protein
The soluble protein concentration was determined by the method of Lowry et al. [14]. Bovine serum albumin was used as a standard protein. Cloudy apple juice (2 mL) was mixed with deionized water (5 mL) and centrifuged at 12,000 × g for 20 min at 4 °C. Coomassie Brilliant Blue G-250 solution (5 mL) was mixed with supernatant (1 mL). After allowing the diluted sample to stand for 2 min, the absorbance at 595 nm was measured.
2.5. Ascorbic acid
The ascorbic acid content was measured based on the 2,6-dichlorophenol-indophenol (DCPIP) visual titration method [15]. Apple juice (10 mL) was mixed with 20 g/L oxalic acid solution in a 100 mL volumetric flask. The solution was filtered after 10 min. 10 mL of filtrate in a triangular flask was titrate with the standardized dye solution (2,6-dichloroindophenol-indophenol and sodium bicarbonate) until the color was pink (color should persist for P15 s), with oxalic acid solution as blank. The results are given in milligrams of ascorbic acid per 100 mL sample.
2.6. Total soluble solid, titratable acid content, and sugar:acid ratio
TSS was calculated as °Brix at 25 ± 1 °C using a digital refractometer (PAL-3 hand-held sugar meter, ATAGO, Japan). The TAC was determined using the acid-alkali neutralization technique. The TAC in apple juice was established using the consumption of sodium hydroxide. Malic acid has a conversion coefficient of 0.067 [16]. Sugar:acid ratio (SAR) was obtained through dividing TSS by the TAC.
2.7. Electronic nose
An olfactory machine (PEN3 E-nose system) was used to evaluate the smell characteristics of juice. Nine mental oxide sensors made up the sensor array for the experiments. The name of the sensors has the following response characteristics: R1 (aromatic compounds); R3 (ammonia and aromatic compounds); R4 (hydrogen); R5 (olefin and aromatic compounds); R6 (hydrocarbons); R7 (hydrogen sulfide); R8 (alcohols and partially aromatic compounds); R9 (aromatic compounds and organic sulfides); and R10 (alkanes (methane, etc.). After each test, a calibration technique for the sensor probe by using zero gas (activated carbon filtered gas) can be used to reduce the impact of changes in external parameters on air relative humidity, temperature changes and sensor changes over time.
The sample (15 mL) was collected into a beaker of 50 mL. The beaker was sealed with clingfilm and balanced for 30 min to ensure that the headspace volatiles are sufficient for detection [17]. The gases present in the samples were pushed at 300 mL/min through the sensor array, causing changes in the conductivity of the sensor. The measuring phase lasted for 120 s, which was long enough for the sensors to reach a stable signal value.
2.8. SPME and GC–MS analysis
For volatile extraction by solid-phase microextraction (SPME), 50/30 µm divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) fiber (Supelco, Bellefonte, USA) was used. The same extraction procedure was previously employed for the aroma analysis of grapes with some modification [18]. Apple juice (5 mL) was taken into a 20 mL glass bottle (including the diaphragm) and added 20 μL (0.473 mg/mL) cyclohexanone (internal standard solution) to avoid internal interference in the determination procedure. The 50/30 μm DVB/CAR/PDMS coated extraction needle inserted into the sample flask, and the samples were balanced at 55 °C in a magnetic stirrer for 5 min, followed by magnetic stirring for 25 min for the volatile flavor adsorption extraction process. The SPME fibre was immediately injected into a GC–MS injector at 250 °C for 5 min to desorb volatile compounds. The SPME fibre was cleaned at 250 °C for 5 min after each injection. Standards of n-alkanes (C8-C20) (Sigma-Aldrich Co.) were used for retention index (RI) conversion.
Chromatographic condition has the following setting: GC–MS (Agilent Technologies 7890A/5975C, Agilent, USA) was used with a HP-5MS capillary column (30 mm length × 0.25 mm i.d. × 0.25 µm film thickness) and splitless injection mode was adopted. High purity helium, as a carrier gas, was used at a constant flow rate of 1.2 mL/min. The inlet temperature was set at 250 °C. The temperatures were programmed as follows: 40 °C with a 5 min holding time, increase to 120 °C at 4 °C min−1, increase to 250 °C at 10 °C min−1, and held for 6 min at the final temperature. Mass spectrometry conditions included the interface temperature of GC–MS was 280 °C, the temperatures of the ion source and quadrupole were maintained at 230 °C and 150 °C, respectively, the mass canning range was from 30 to 550 amu, the scan rate is 3.8 scan/s, and mass spectra were obtained at 70 eV in the electron ionization+ (EI+) mode [19].
Moreover, the National Institute of Standards and Technology (NIST 11, Wiley 7.0) library and retention times (RTs) were used to identify the volatile compounds observed, and only compounds with greater than 70% certainty were used. Using cyclohexanone as an internal standard, semi-quantitative measurements were performed. The volatile compound content was determined by comparing the peak area of each component to that of the internal standard.
2.9. Statistical analysis
All of the nutrient content (soluble pectin, soluble protein and ascorbic acid) and flavor (TSS, TAC, E-nose and GC–MS) measurements were repeated 3 times. One-way analysis of variance (ANOVA) was conducted on different groups (CK, CP, MS, US, HPS) of cloudy apple juice using SPSS Statistics 22.0 to test significant differences. The plots curved with Origin 8.5, and values were shown as mean ± standard deviation at a significance level of P < 0.05.
3. Results and discussion
3.1. Differences in soluble pectin, soluble protein, and ascorbic acid contents
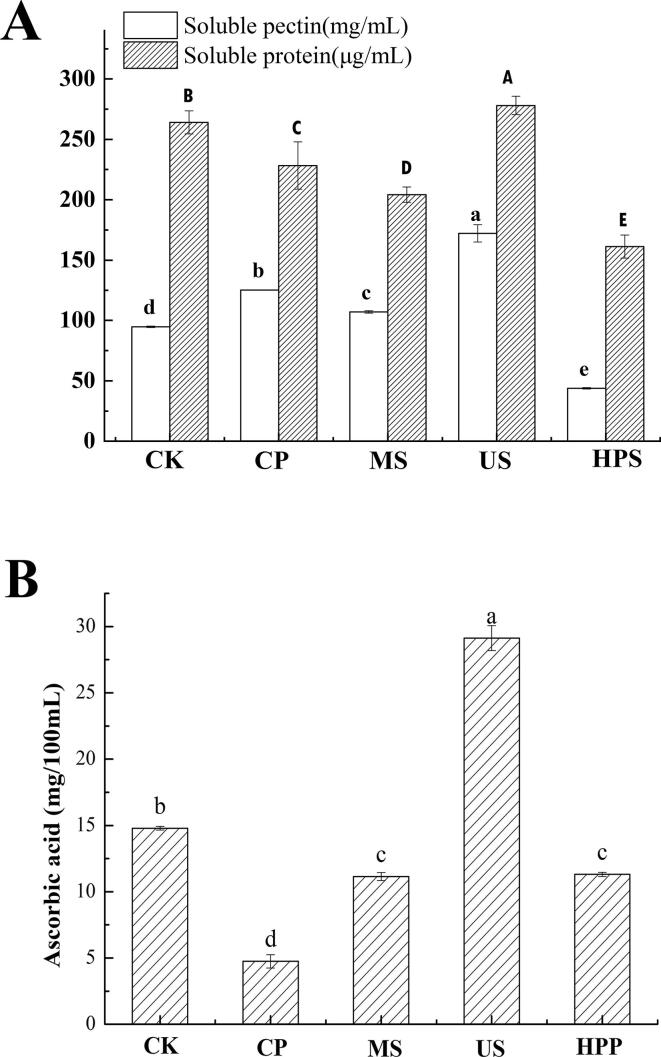
The soluble pectin and soluble protein contents of apple juice following various sterilization methods are shown in Fig. 2A, which shows a significant difference among all the samples (P < 0.05). The soluble pectin content in unsterilized apple juice was 94.76 ± 0.61 mg/mL. All sterilization methods except for HPS increased the soluble pectin content significantly (P < 0.05). The rise in soluble pectin concentration after CP (125.16 ± 0.17 mg/mL) and MS (106.98 ± 0.97 mg/mL) might mainly be due to the thermal hydrolysis and pectin methyl esterase (PME) activity. PME is a pectate methyl ester enzyme with a higher heat resistance and can act on soluble pectin present in the cloudy fruit juice [20]. It has been found that adequate heat treatment could promote the hydrolysis of pectin in the cell wall [21]. The optimal temperature range for PME activity is broad, ranging from 30 to 65 °C [22], [23]. With the pasteurization temperature increased, part of pectin was destroyed when it reached the temperature range of PME activity during heating, and then PME activity was inhibited with subsequent temperature increase. The juice temperature reached 62 °C after MS, which was close to the active temperature range of PME, making the action of PME more significant and the hydrolysis degree of pectin greater. The soluble pectin content in juice after US was the highest. This is primarily because the higher temperature and ultrasonic mechanical action. Pectin could bind non-covalently with proteins and polyphenols in cloudy apple juice [24]. Mechanical action of ultrasound destroys the pectin-protein-phenol polymers and cell wall pectin macromolecules, a prerequisite for pectin release [25]. However, the soluble pectin content in the HPS group (43.75 ± 0.63 mg/g) was significantly lower (P < 0.05), and this is perhaps due to two main reasons. One reason is that the atomic distance decreases with pressure increasing, which increases the hydrogen bonding of endogenous enzymes and rearranges the protein structure, resulting in an increase in endogenous enzyme activity [26]. The other possible reason is that the high pressure promoted the binding of PME to the substrate, which accelerated the catalytic degradation of soluble pectin [27].
Fig. 2.
Effects of sterilization methods on (A) soluble pectin and soluble protein contents and (B) ascorbic acid content of cloudy apple juice. Different letters (a–d, A–E) indicate significant differences (P < 0.05) between different sterilization methods. Experiments were performed in triplicate; vertical bars indicate ± SD (P < 0.05).
Soluble protein is one of the primary nutrients and also the main components of cloud particles in apple juice. It is essential for the stability of apple juice. The soluble protein content was significantly affected by different sterilization methods in apple juice (Fig. 2A). All sterilization methods significantly decreased the soluble protein content compared to CK, except US, which increased the soluble protein content (the soluble protein content was reduced by approximately 38.9% after HPS). This might be because high-intensity ultrasound improves protein solubility by modifying protein conformation and structure in such a way that hydrophilic regions of amino acids from inside are exposed to water [28].
Ascorbic acid is an essential vitamin in human nutrition and is primarily supplied by fruits and vegetables. In the current study, the amount of ascorbic acid changed significantly (P < 0.05) after all sterilization treatments compared to CK (Fig. 2B). Ascorbic acid is a heat-sensitive bioactive compound [3], and it is greatly degraded through the aerobic pathway. CP juice showed a serious loss of ascorbic acid because of the increased processing temperatures. However, ascorbic acid content was increased significantly after US, and earlier studies indicated higher levels of ascorbic acid content in ultrasound-treated fruit juices such as mango juice and orange juice as well as kasturi lime fruit juice [29], [30], [31]. This could probably be attributed to two reasons. First, the ultrasonic amplitude allows greater penetration of solvent and better extractability of organic acids from the matrix [32]. Second, dissolved oxygen is eliminated during cavitation. Degraded polysaccharides, which have better antioxidant properties than ordinary polysaccharides [33], were obtained by US treatment. The rise in antioxidant capacity can also be attributable to the increased polyphenol and organic acid levels in cloudy apple juice caused by cavitation [34]. Free hydroxyl radicals can be produced in phenolic compounds and degraded polysaccharides which are comparable to vitamin C [35]. The redox reaction between L-ascorbic acid and 2,6-dichloroindophenol is used to determine the concentration. The ascorbic acid content rose as the increase in the levels of reducing substances. After MS and HPS, the ascorbic acid content was reduced by about 24%.
3.2. Differences in total soluble solid, titratable acid content, and sugar:acid ratio
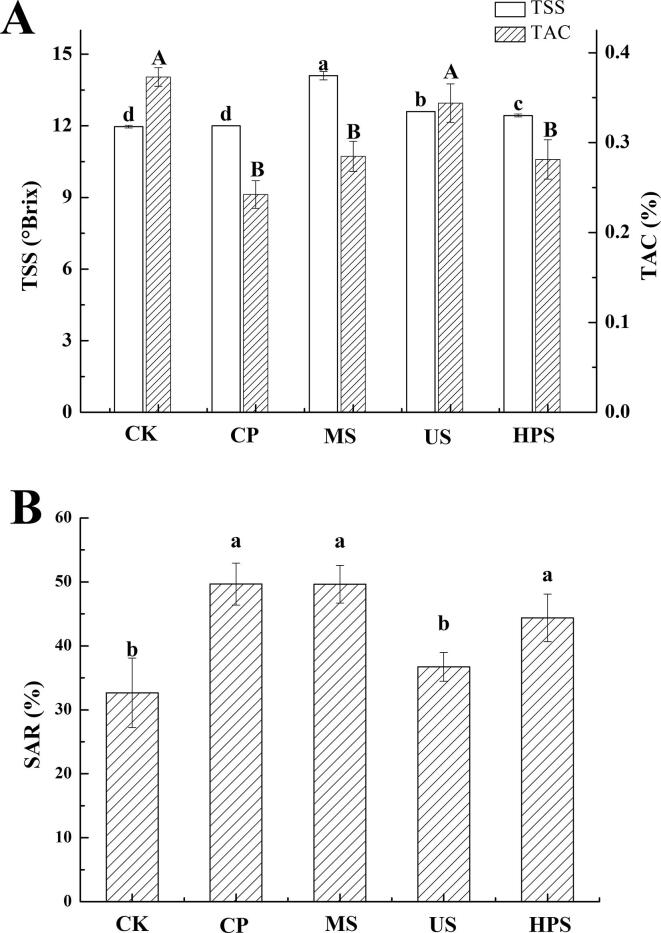
Sugars and acids of juice are responsible for the major taste features (sweetness and sourness, respectively). The TSS of apple juice (not from concentrate juice) must be at least 10 °Brix, according to EU standards (Directive 2012/12/EU) and worldwide standards (Codex Stan 48-1981). In the present work, the TSS of different apple juices, unadjusted for acidity, were all over the acceptable °Brix level. As shown in Fig. 3A, the TSS of apple juice increased significantly after MS (14.10 ± 0.17 °Brix), US (12.60 ± 0.00 °Brix), and HPS (12.43 ± 0.06 °Brix). But the TSS in juice showed no significant changes after CP (P > 0.05). Khandpur et al. drew a similar conclusion in orange juice and sweet lime juice after US [36]. MS and HPS preserve the inherent sensory characteristics of the juice and shorten the time it is exposed to energy, reducing the risk of losing critical thermolabile nutrients. The TAC showed no significant changes after US, while it decreased after CP, MS, and HPS. As a whole, the SAR (Fig. 3B) increased significantly (P < 0.05) after CP, MS, and HPS treatments, while there was no significant difference after US.
Fig. 3.
Effects of sterilization methods on (A) total soluble solid (TSS) and titratable acid content (TAC) and (B) sugar:acid ratio (SAR) of cloudy apple juice. Different letters (a–d, A–B) indicate significant differences (P < 0.05) among different sterilization methods for apple juice. Experiments were performed in triplicate; vertical bars indicate ± SD (P < 0.05).
3.3. Electronic nose analysis
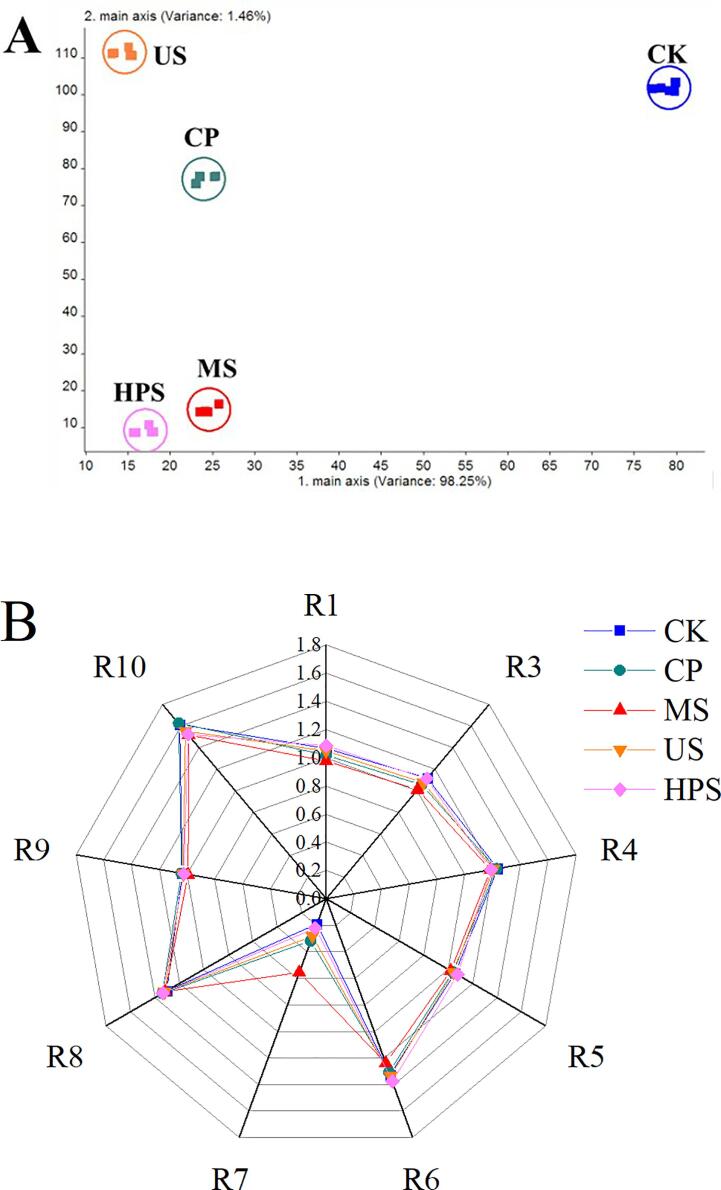
The cross-sensitive chemical sensor array in an E-nose mimics the human olfactory system in order to identify and distinguish complicated volatiles [37]. Fig. 4A shows the linear discriminant analysis (LDA) results of aroma components using the E-nose. LDA highlights the spatial distribution of aromatic components in samples as well as the distances between them. The first discriminant function (LD1) described 98.25% of the total variance, whereas the second discriminant function (LD2) explained 1.46%. The larger the dispersion between data collection points, the higher the group distinction. There were no overlaps in volatile flavor data acquisition points among different apple juices, indicating that LDA was capable to distinguishing the aroma. LD1 was the primary variance, so the distance on the horizontal coordinate axis could better represent the smell difference. It could be seen that the position of CK was far away from all sterilized groups on the horizontal coordinate axis, which indicated an obvious change of scent in apple juice after sterilization. However, little difference in odor was shown among these sterilization methods by LDA.
Fig. 4.
The E-nose analysis of cloudy apple juice. (A) Linear discriminant analysis (LDA). (B) Radar diagram of response values of E-nose sensors.
Based on the E-nose profile radar graphs of juice (Fig. 4B), the responses of the nine sensor arrays of the E-nose to the aroma volatiles are shown after different sterilization conditions. All sensor response values except those for hydrogen sulfide (R7) and alkanes (R10) were higher after HPS than after any of the other sterilization methods, indicating that fruit juice flavor is relatively rich after HPS. As a whole, from Fig. 4B, sensors R1, R3, R6, and R7 showed relatively obvious changes comparing different sterilized conditions. R1, R3, R6, and R7 are sensitive to aromatic compounds, ammonia and aromatic compounds, hydrocarbons, and hydrogen sulfide, respectively. However, the mechanisms underlying these changes need to be further researched.
3.4. Volatile compounds
The volatile compounds of apple juice were extracted by SPME and analyzed using GC–MS (Table 1). Odor-active volatile chemicals such as esters, alcohols, and aldehydes are responsible for the aroma of apple juice. About 25 kinds of volatile compounds, including 16 types of esters, five types of aldehydes, and four types of alcohol, were found in apple juice with a matching degree more than 80%. Compared to the CK group, the content of volatile compounds in apple juice almost decreased to zero after sterilization. Among these sterilization methods, the highest loss of volatile compounds was found in the MS group.
Table 1.
The levels of volatile components in apple juice after different sterilization methods.
| RIa | Volatile compound | Content (μg·kg−1) |
||||
|---|---|---|---|---|---|---|
| CK | CP | MS | US | HPS | ||
| Esters | ||||||
| 807 | Butyl acetate | 3.27 ± 0.98c | NDb | ND | ND | ND |
| 900 | Propyl butyrate | ND | 1.30 ± 0.01 | ND | ND | 1.47 ± 0.43 |
| 908 | Butyl propionate | 1.71 ± 0.42 | ND | ND | ND | 1.01 ± 0.23 |
| 930 | Methyl caproate | 1.09 ± 0.03 | 0.81 ± 0.10 | 0.28 ± 0.02 | 0.55 ± 0.12 | 0.73 ± 0.14 |
| 975 | Propyl-2-methyl butyrate | ND | 1.79 ± 0.40 | ND | ND | ND |
| 996 | Butyl butyrate | 28.74 ± 4.51 | 10.61 ± 0.01 | 2.55 ± 0.01 | 8.66 ± 1.63 | 9.95 ± 2.72 |
| 1003 | Ethyl hexanoate | 7.48 ± 1.45 | ND | ND | ND | ND |
| 1018 | Hexyl acetate | 3.95 ± 0.75 | 2.47 ± 0.93 | ND | 1.84 ± 0.30 | 1.98 ± 0.75 |
| 1073 | Butyl 2-methylbutanoate | 14.84 ± 2.18 | 4.17 ± 0.27 | 1.30 ± 0.03 | 4.80 ± 0.22 | 5.00 ± 1.49 |
| 1146 | Butyl-2-methyl butyrate | 5.41 ± 0.70 | ND | ND | 2.24 ± 0.31 | 2.62 ± 0.84 |
| 1166 | Pentyl-2-methyl butyrate | 2.01 ± 0.17 | 0.74 ± 0.26 | ND | 0.71 ± 0.03 | 0.84 ± 0.23 |
| 1201 | Octyl-2-methyl-propionate | 254.77 ± 3.64 | ND | ND | ND | 78.49 ± 7.35 |
| 1202 | Hexyl butyrate | ND | 44.12 ± 0.03 | 24.98 ± 0.36 | 59.21 ± 0.02 | ND |
| 1262 | Hexyl-2-methyl- butyrate | 194.65 ± 1.44 | 36.94 ± 3.81 | 34.05 ± 1.18 | 67.92 ± 0.77 | 75.87 ± 0.12 |
| 1884 | Hexyl caproate | 73.82 ± 2.99 | 15.55 ± 0.06 | 17.45 ± 1.34 | 29.84 ± 2.15 | 32.79 ± 1.50 |
| 1934 | Methyl hexadecanoate | ND | 3.65 ± 0.32 | ND | ND | ND |
| Sum | 591.75 ± 9.46 | 122.14 ± 14.26 | 80.60 ± 2.94 | 175.77 ± 9.27 | 208.27 ± 15.14 | |
| Aldehydes | ||||||
| <800 | Hexanal | 86.5 ± 2.13 | ND | ND | 24.81 ± 4.98 | ND |
| 877 | (E)-2-Hexenal, | 114.76 ± 8.69 | 74.68 ± 1.79 | 21.02 ± 0.67 | 81.32 ± 2.09 | 116.47 ± 0.74 |
| 900 | Heptanal | 11.60 ± 2.42 | ND | 2.24 ± 0.12 | ND | 2.19 ± 0.42 |
| 1002 | Octanal | ND | ND | 1.84 ± 0.16 | 2.43 ± 0.30 | 2.88 ± 0.57 |
| 1207 | Decanal | 7.73 ± 0.53 | 2.17 ± 0.55 | 1.79 ± 0.60 | 2.57 ± 0.08 | ND |
| Sum | 220.62 ± 13.76 | 76.85 ± 2.34 | 26.88 ± 1.55 | 111.13 ± 7.45 | 121.55 ± 1.74 | |
| Alcohols | ||||||
| 1002 | 1-Hexanol | 85.08 ± 1.38 | 26.89 ± 0.43 | 13.43 ± 0.99 | 33.62 ± 7.55 | 38.00 ± 0.10 |
| 1028 | 1-Heptanol | 2.19 ± 0.06 | ND | ND | ND | ND |
| 1047 | 2-Ethyl-1-hexanol | 4.15 ± 0.87 | 4.12 ± 0.24 | 4.14 ± 0.14 | 4.34 ± 0.15 | 4.72 ± 0.98 |
| 1299 | 1-Octanol | 16.45 ± 1.27 | 5.78 ± 1.49 | 1.83 ± 0.09 | 5.76 ± 0.97 | 8.06 ± 0.02 |
| Sum | 107.88 ± 3.57 | 36.79 ± 2.17 | 19.40 ± 1.22 | 43.72 ± 8.67 | 50.79 ± 1.10 | |
RI (retention index): Standards of n-alkanes (C8–C20) were used for RI conversion.
ND: Not detected.
Values are presented as mean ± standard deviation (P < 0.05).
The most noticeable contributors to the aroma profile of intact apples and apple juices are volatile esters, which are linked with the “fruity” qualities of fruit flavor [38]. Unsterilized apple juice contained a total of 12 ester compounds, and the total content of ester compounds was 591.75 ± 9.46 μg/kg. After sterilization, the total ester content was severely reduced. Several chemical changes occurred during the processes of sterilization, and some new compounds (which are not present in the CK group) appeared, such as propyl-2-methyl butyrate, hexyl butyrate, and methyl hexadecanoate. The increase in the levels of these flavor components may be the consequence of flavor precursors being activated by heat or flavor compounds being released from cell membranes. Moreover, this could also be due to heat treatment enhanced the hydrophobic effects between aroma compounds and macromolecules [39]. The total ester compound content of US apple juice was 175.77 ± 9.27 μg/kg, and the decrease in the levels of volatile compounds in US apple juice may be due to the extreme physical conditions inside the bubbles during cavitation collapse at the microlevel [40] and several chemical reactions that occur at the same time or separately [41]. The highest reduction rate of ester compounds in apple juice was found in HPS juice, indicating that HPS treatment preserved the flavor more than other sterilization methods.
Aldehydes are also thought to be crucial in the aroma of fruit juice since they have positive log odor units [42]. The oxidative degradation of unsaturated fatty acids is primarily responsible for the formation of aldehydes [43]. Five types of aldehydes were identified in apple juice, with (E)-2-hexenal being the most abundant one. (E)-2-hexenal imparts a green leafy and grassy sensory quality to apple flavor [44]. After HPS treatment the concentration of (E)-2-hexenal was the highest, close to that of the unsterilized group, and (E)-2-hexenal levels were severely reduced after MS. Compared to CK, octanal appeared in MS, US, and CP juices, suggesting that auto-oxidation and enzymatic oxidation may be involved [45].
Apple juice included four different types of alcohol. Alcohols have a low odorant activity and can be found in low concentrations in the form of glycoside binding in fruits [46]. 1–1-Hexanol is the most abundant alcohol in apple juice and is generally lost after sterilization treatment. 1-Heptanol was no longer detected after all sterilization treatments. The content of 2-Ethyl-1-hexanol in the five groups was not significantly different, indicating that 2-Ethyl-1-hexanol has a high tolerance to temperature, pressure, and chemical reactions.
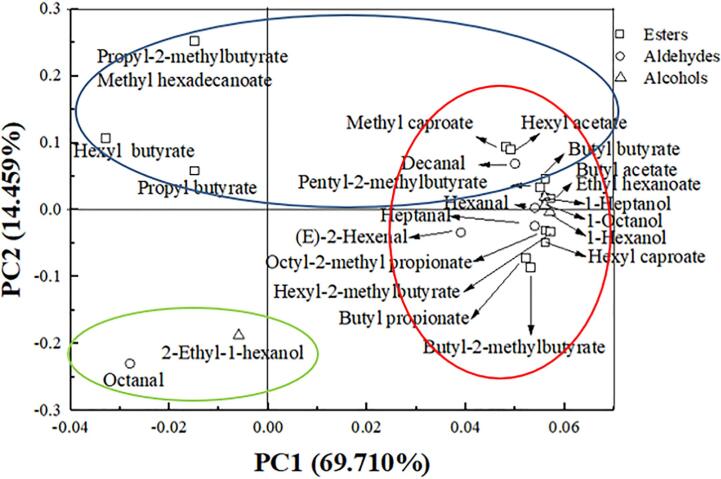
3.5. Principal component analysis
The complex data matrix generated by principal component analysis (PCA) is shown in Fig. 5, showing the loading plots for apple juice volatile compounds (Table 1) by the first two principal components (PC1 and PC2), which contributed 69.71% and 14.45%, respectively, to the total variation. In the first quadrant, there were six kinds of esters, two kinds of alcohol, and two types of aldehydes, which may have a greater impact on apple flavor. The GC–MS study revealed that the three flavor compounds butyl acetate, ethyl hexanoate, and 1-heptanol were only found in non-sterilized juice (Table 1), and they all located in the first quadrant by PCA (Fig. 5). So butyl acetate, ethyl hexanoate, and 1-heptanol might be the most important volatile compounds influenced by sterilization processes. 2-Ethyl-1-hexanol and octanal were located in the third quadrant, indicating that they might be less affected by sterilization methods of apple juice, and this was consistent with the results of GC–MS study.
Fig. 5.
Principal component analysis (PCA) of volatile compounds of apple juice.
4. Conclusion
The four sterilization methods had different influences on the nutritional value and flavor of cloudy apple juice. Among them, CP and MS increased the SAR and soluble protein content, whereas they reduced the TAC, soluble pectin content, and ascorbic acid content of apple juice. ultrasonic sterilization significantly improved the soluble protein, ascorbic acid, and soluble pectin contents and TSS of apple juice. HPS treatment significantly reduced the soluble pectin and soluble protein contents in juice; however, it showed a higher retention rate of volatile compounds in apple juice. Compared with other sterilization methods, MS showed a serious loss of juice flavor compounds. Therefore, ultrasonic sterilization is the better sterilization method in terms of flavor and nutritional composition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (No. 31972031), and the Scientific Research Fund Project of Liaoning Province, China (No. LJKZ1016). We are grateful to Drs. Danshi Zhu and Dr. He Liu (Bohai University, CN) for their critical review of this manuscript.
Contributor Information
Danshi Zhu, Email: zhudanshi1978@163.com.
Yueyi Zhang, Email: zhangyueyi0309@163.com.
Chengcheng Kou, Email: kcc542030659@163.com.
Pushun Xi, Email: 3427450040@qq.com.
He Liu, Email: liuhe2069@163.com.
References
- 1.Lee B., Seo J.D., Rhee J.K., Kim C.Y. Heated apple juice supplemented with onion has greatly improved nutritional quality and browning index. Food Chem. 2016;201:315–319. doi: 10.1016/j.foodchem.2016.01.092. [DOI] [PubMed] [Google Scholar]
- 2.Persic M., Mikulic-Petkovsek M., Slatnar A., Veberic R. Chemical composition of apple fruit, juice and pomace and the correlation between phenolic content, enzymatic activity and browning, LWT-Food. Sci. Technol. 2017;82:23–31. doi: 10.1016/j.lwt.2017.04.017. [DOI] [Google Scholar]
- 3.Odriozola-Serrano I., Soliva-Fortuny R., Martín-Belloso O. Changes of health-related compounds throughout cold storage of tomato juice stabilized by thermal or high intensity pulsed electric field treatments. Innov. Food Sci. Emerg. Technol. 2008;9(3):272–279. doi: 10.1016/j.ifset.2007.07.009. [DOI] [Google Scholar]
- 4.Valero M., Recrosio N., Saura D., Muñoz N., Martí N., Lizama V. Effects of ultrasonic treatments in orange juice processing. J. Food Eng. 2007;80(2):509–516. doi: 10.1016/j.jfoodeng.2006.06.009. [DOI] [Google Scholar]
- 5.Tiwari B.K., O’Donnell C.P., Cullen P.J. Effect of sonication on retention of anthocyanins in blackberry juice. J. Food Eng. 2009;93(2):166–171. doi: 10.1016/j.jfoodeng.2009.01.027. [DOI] [Google Scholar]
- 6.Cañumir J.A., Celis J.E., de Bruijn J., Vidal L.V. Pasteurisation of apple juice by using microwaves, LWT-Food. Sci. Technol. 2002;35(5):389–392. doi: 10.1006/fstl.2001.0865. [DOI] [Google Scholar]
- 7.Igual M., García-Martínez E., Camacho M.M., Martínez-Navarrete N. Effect of thermal treatment and storage on the stability of organic acids and the functional value of grapefruit juice. Food Chem. 2010;118(2):291–299. doi: 10.1016/j.foodchem.2009.04.118. [DOI] [Google Scholar]
- 8.Suárez-Jacobo Á., Rüfer C.E., Gervilla R., Guamis B., Roig-Sagués A.X., Saldo J. Influence of ultra-high pressure homogenisation on antioxidant capacity, polyphenol and vitamin content of clear apple juice. Food Chem. 2011;127(2):447–454. doi: 10.1016/j.foodchem.2010.12.152. [DOI] [PubMed] [Google Scholar]
- 9.Velazquez-Estrada R.M., Hernandez-Herrero M.M., Rufer C.E., Guamis-Lopez B., Roig-Sagues A.X. Influence of ultra high pressure homogenization processing on bioactive compounds and antioxidant activity of orange juice. Innov. Food Sci. Emerg. Technol. 2013;18:89–94. doi: 10.1016/j.ifset.2013.02.005. [DOI] [Google Scholar]
- 10.Jiang B.o., Mantri N., Hu Y.a., Lu J., Jiang W.u., Lu H. Evaluation of bioactive compounds of black mulberry juice after thermal, microwave, ultrasonic processing, and storage at different temperatures. Food Sci. Technol. Int. 2015;21(5):392–399. doi: 10.1177/1082013214539153. [DOI] [PubMed] [Google Scholar]
- 11.Apichartsrangkoon A., Wongfhun P., Gordon M.H. Flavor Characterization of Sugar-Added Pennywort (Centella asiatica L.) Juices Treated with Ultra-High Pressure and Thermal Processes. J. Food Sci. 2009;74:C643–C646. doi: 10.1111/j.1750-3841.2009.01358.x. [DOI] [PubMed] [Google Scholar]
- 12.Dogan C., Erkmen O. High pressure inactivation kinetics of Listeria monocytogenes inactivation in broth, milk, and peach and orange juices. J. Food Eng. 2004;62(1):47–52. doi: 10.1016/S0260-8774(03)00170-5. [DOI] [Google Scholar]
- 13.Fraeye I., Duvetter T., Verlent I., Ndaka Sila D., Hendrickx M., Van Loey A. Comparison of enzymatic de-esterification of strawberry and apple pectin at elevated pressure by fungal pectinmethylesterase. Innov. Food Sci. Emerg. Technol. 2007;8(1):93–101. doi: 10.1016/j.ifset.2006.07.004. [DOI] [Google Scholar]
- 14.Lowry OliverH., Rosebrough NiraJ., Farr A.L., Randall RoseJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193(1):265–275. doi: 10.1016/S0021-9258(19)52451-6. [DOI] [PubMed] [Google Scholar]
- 15.Varming C., Petersen M.A., Toldam-Andersen T.B. Ascorbic acid contents in Danish apple cultivars and commercial apple juices, LWT-Food. Sci. Technol. 2013;54(2):597–599. doi: 10.1016/j.lwt.2013.06.024. [DOI] [Google Scholar]
- 16.Zhu D., Kou C., Shen Y., Xi P., Cao X., Liu H.e., Li J. Effects of different processing steps on the flavor and colloidal properties of cloudy apple juice. J. Sci. Food Agric. 2021;101(9):3819–3826. doi: 10.1002/jsfa.v101.910.1002/jsfa.11016. [DOI] [PubMed] [Google Scholar]
- 17.Zhu D., Shen Y., Wei L., Xu L., Cao X., Liu H.e., Li J. Effect of particle size on the stability and flavor of cloudy apple juice. Food Chem. 2020;328:126967. doi: 10.1016/j.foodchem.2020.126967. [DOI] [PubMed] [Google Scholar]
- 18.Zhu D., Li J., Xu Y., Liu H., Meng X. Changes of Volatile Compounds in Table Grapes During Postharvest Storage by SPME Coupled with GC-MS. Asian J. Chem. 2013;25:9647–9652. doi: 10.14233/ajchem.2013.15113. [DOI] [Google Scholar]
- 19.Zhu D., Ren X., Wei L., Cao X., Ge Y., Liu H.e., Li J. Collaborative analysis on difference of apple fruits flavour using electronic nose and electronic tongue. Sci. Hortic. 2020;260:108879. doi: 10.1016/j.scienta.2019.108879. [DOI] [Google Scholar]
- 20.CASTALDO D., LOVOI A., QUAGLIUOLO L., SERVILLO L., BALESTRIERI C., GIOVANE A. Orange Juices and Concentrates Stabilization by a Proteic Inhibitor of Pectin Methylesterase. J. Food Sci. 1991;56(6):1632–1634. doi: 10.1111/jfds.1991.56.issue-610.1111/j.1365-2621.1991.tb08658.x. [DOI] [Google Scholar]
- 21.Shen Y., Zhu D., Xi P., Cai T., Cao X., Liu H.e., Li J. Effects of temperature-controlled ultrasound treatment on sensory properties, physical characteristics and antioxidant activity of cloudy apple juice. LWT- Food Science and Technology. 2021;142:111030. doi: 10.1016/j.lwt.2021.111030. [DOI] [Google Scholar]
- 22.Castro S.M., Van Loey A., Saraiva J.A., Smout C., Hendrickx M. Activity and process stability of purified green pepper (Capsicum annuum) pectin methylesterase, Journal of Agricultural and Food. Chemistry. 2004;52(18):5724–5729. doi: 10.1021/jf0352071. [DOI] [PubMed] [Google Scholar]
- 23.Salas-Tovar J.A., Flores-Gallegos A.C., Contreras-Esquivel J.C., Escobedo-Garcia S., Gonzalez-Herrera S.M., Morales-Castro J., Rodriguez-Herrera R. Ultrasound-vacuum infusion effect on jalapeno pepper (Capsicum annuum L.) blanching and thermal behavior of its pectin methylesterase, LWT-Food. Sci. Technol. 2018;95:150–156. doi: 10.1016/j.lwt.2018.04.081. [DOI] [Google Scholar]
- 24.Pinelo M., Zeuner B., Meyer A.S. Juice clarification by protease and pectinase treatments indicates new roles of pectin and protein in cherry juice turbidity. Food Bioprod. Process. 2010;88(2-3):259–265. doi: 10.1016/j.fbp.2009.03.005. [DOI] [Google Scholar]
- 25.Wellala C., Bi J., Liu X., Liu J., Lyu J., Wu X. Juice related water-soluble pectin characteristics and bioaccessibility of bioactive compounds in oil and emulsion incorporated mixed juice processed by high pressure homogenization. Food Hydrocolloids. 2019;93:56–67. doi: 10.1016/j.foodhyd.2019.02.011. [DOI] [Google Scholar]
- 26.B. Krebbers, A.M. Matser, S.W. Hoogerwerf, R. Moezelaar, M.M.M. Tomassen, R.W.v.d. Berg, Combined high-pressure and thermal treatments for processing of tomato puree: evaluation of microbial inactivation and quality parameters, Innovative Food Science & Emerging Technologies. 4 (2003) 377-385. https://doi.org/10.1016/S1466-8564(03)00045-6.
- 27.DEROECK A, SILA D, DUVETTER T, VANLOEY A, HENDRICKX M. Effect of high pressure/high temperature processing on cell wall pectic substances in relation to firmness of carrot tissue. Food Chem. 2008;107(3):1225–1235. doi: 10.1016/j.foodchem.2007.09.076. [DOI] [Google Scholar]
- 28.Krešić Greta, Lelas Vesna, Jambrak Anet Režek, Herceg Zoran, Brnčić Suzana Rimac. Influence of novel food processing technologies on the rheological and thermophysical properties of whey proteins. J. Food Eng. 2008;87(1):64–73. doi: 10.1016/j.jfoodeng.2007.10.024. [DOI] [Google Scholar]
- 29.Guerrouj K., Sanchez-Rubio M., Taboada-Rodriguez A., Cava-Rolla R.M., Marin-Iniesta F. Sonication at mild temperatures enhances bioactive compounds and microbiological quality of orange juice. Food Bioprod. Process. 2016;99:20–28. doi: 10.1016/j.fbp.2016.03.007. [DOI] [Google Scholar]
- 30.Bhat Rajeev, Kamaruddin Nor Shuaidda Bt Che, Min-Tze Liong, Karim A.A. Sonication improves kasturi lime (Citrus microcarpa) juice quality. Ultrason. Sonochem. 2011;18(6):1295–1300. doi: 10.1016/j.ultsonch.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Wang Jingyi, Xie Bijun, Sun Zhida. Quality parameters and bioactive compound bioaccessibility changes in probiotics fermented mango juice using ultraviolet-assisted ultrasonic pre-treatment during cold storage, LWT-Food. Sci. Technol. 2021;137:110438. doi: 10.1016/j.lwt.2020.110438. [DOI] [Google Scholar]
- 32.Bhat S., Sharma H.K. Combined effect of blanching and sonication on quality parameters of bottle gourd (Lagenaria siceraria) juice. Ultrason. Sonochem. 2016;33:182–189. doi: 10.1016/j.ultsonch.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 33.Yan S.L., Pan C., Yang X.Q., Chen S.J., Qi B., Huang H. Degradation of Codium cylindricum polysaccharides by H2O2-Vc-ultrasonic and H2O2-Fe2+-ultrasonic treatment: Structural characterization and antioxidant activity. Int. J. Biol. Macromol. 2021;182:129–135. doi: 10.1016/j.ijbiomac.2021.03.193. [DOI] [PubMed] [Google Scholar]
- 34.Aadil Rana Muhammad, Zeng Xin-An, Han Zhong, Sun Da-Wen. Effects of ultrasound treatments on quality of grapefruit juice. Food Chem. 2013;141(3):3201–3206. doi: 10.1016/j.foodchem.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Gu Jinyan, Zhang Haihui, Wen Chaoting, Zhang Jixian, He Yuanqing, Ma Haile, Duan Yuqing. Purification, characterization, antioxidant and immunological activity of polysaccharide from Sagittaria sagittifolia L. Food Res. Int. 2020;136:109345. doi: 10.1016/j.foodres.2020.109345. [DOI] [PubMed] [Google Scholar]
- 36.Khandpur P., Gogate P.R. Evaluation of ultrasound based sterilization approaches in terms of shelf life and quality parameters of fruit and vegetable juices. Ultrason. Sonochem. 2016;29:337–353. doi: 10.1016/j.ultsonch.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 37.Casale M., Oliveri P., Armanino C., Lanteri S., Forina M. NIR and UV-vis spectroscopy, artificial nose and tongue: Comparison of four fingerprinting techniques for the characterisation of Italian red wines. Anal. Chim. Acta. 2010;668(2):143–148. doi: 10.1016/j.aca.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 38.I. Lara, A. Ortiz, G. Echeverría, M.L. López, J. Graell, Development of aroma-synthesising capacity throughout fruit maturation of 'Mondial Gala' apples, J. Hortic Sci. Biotech. 83 (2008) 253-259. https://doi.org/10.1080/14620316.2008.11512377.
- 39.Jouquand Céline, Ducruet Violette, Giampaoli Pierre. Partition coefficients of aroma compounds in polysaccharide solutions by the phase ratio variation method. Food Chem. 2004;85(3):467–474. doi: 10.1016/j.foodchem.2003.07.023. [DOI] [Google Scholar]
- 40.Mason T.J., Joyce E., Phull S.S., Lorimer J.P. Potential uses of ultrasound in the biological decontamination of water. Ultrason. Sonochem. 2003;10(6):319–323. doi: 10.1016/S1350-4177(03)00102-0. [DOI] [PubMed] [Google Scholar]
- 41.Rodríguez Óscar, Eim Valeria, Rosselló Carmen, Femenia Antoni, Cárcel Juan A, Simal Susana. Application of power ultrasound on the convective drying of fruits and vegetables: effects on quality. J. Sci. Food Agric. 2018;98(5):1660–1673. doi: 10.1002/jsfa.2018.98.issue-510.1002/jsfa.8673. [DOI] [PubMed] [Google Scholar]
- 42.Wang D., Cai J., Zhu B.Q., Wu G.F., Duan C.Q., Chen G., Shi Y. Study of free and glycosidically bound volatile compounds in air-dried raisins from three seedless grape varieties using HS-SPME with GC-MS. Food Chem. 2015;177:346–353. doi: 10.1016/j.foodchem.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 43.Chen Q., Song J., Bi J., Meng X., Wu X. Characterization of volatile profile from ten different varieties of Chinese jujubes by HS-SPME/GC-MS coupled with E-nose. Food Res. Int. 2018;105:605–615. doi: 10.1016/j.foodres.2017.11.054. [DOI] [PubMed] [Google Scholar]
- 44.Amaro Ana L., Beaulieu John C., Grimm Casey C., Stein Rebecca E., Almeida Domingos P.F. Effect of oxygen on aroma volatiles and quality of fresh-cut cantaloupe and honeydew melons. Food Chem. 2012;130(1):49–57. doi: 10.1016/j.foodchem.2011.06.052. [DOI] [Google Scholar]
- 45.Hashizume M., Okugawa T., Gordon M.H., Mottram D.S. Light-induced off-flavour in cloudy apple juice. Dev. Food Sci. 2006;43:273–276. doi: 10.1016/S0167-4501(06)80065-8. [DOI] [Google Scholar]
- 46.Nikfardjam Martin Pour, Maier Daniel. Development of a headspace trap HRGC/MS method for the assessment of the relevance of certain aroma compounds on the sensorial characteristics of commercial apple juice. Food Chem. 2011;126(4):1926–1933. doi: 10.1016/j.foodchem.2010.12.021. [DOI] [PubMed] [Google Scholar]