Abstract
Inflammation is a defensive reaction for external stimuli to the human body and generally accompanied by immune responses, which is associated with multiple diseases such as atherosclerosis, type 2 diabetes, Alzheimer’s disease, psoriasis, asthma, chronic lung diseases, inflammatory bowel disease, and multiple virus-associated diseases. Epigenetic mechanisms have been demonstrated to play a key role in the regulation of inflammation. Common epigenetic regulations are DNA methylation, histone modifications, and non-coding RNA expression; among these, histone modifications embrace various post-modifications including acetylation, methylation, phosphorylation, ubiquitination, and ADP ribosylation. This review focuses on the significant role of histone modifications in the progression of inflammatory diseases, providing the potential target for clinical therapy of inflammation-associated diseases.
Keywords: inflammation, immune, epigenetic, inflammatory diseases, histone modifications
Introduction
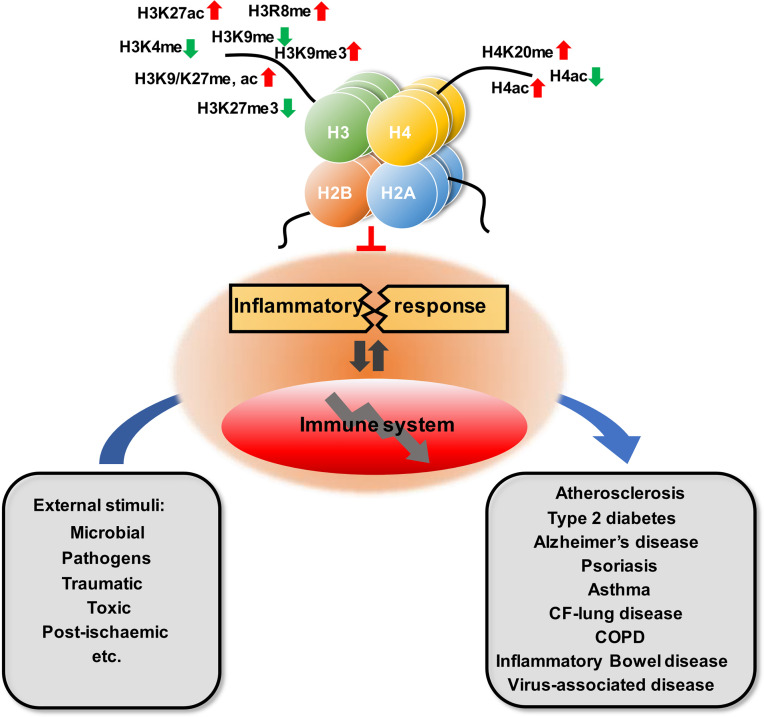
Inflammation is a response of the immune system to harmful stimuli including but not limited to microbial stimuli, pathogens, traumatic stimuli, toxic stimuli, or post-ischemic stimuli (1). It is a frequent occurrence to protect organisms from the spread of infection and recover the affected compartments to a normal state, which is called “resolution” (2). However, if the inflammation fails to subside, it will turn to a trouble itself and contribute to the pathogenesis of a great sort of inflammatory diseases (3).
The study of epigenetics, especially histone modifications, and their involvement in inflammation disease is still an emerging research filed, but it is becoming to attract more and more attention and growing at a fast pace. As the basic proteins in both eukaryotic and prokaryotic cells, histones combined with DNA constitute the nucleosome structure (4). Post-translational modifications (PTMs) of proteins are rising as a pivotal means by which chromatin could be altered to regulate gene expression; in other words, intracellular metabolites can modulate immunity. PTMs of histones on both the histone core and N-terminal tails affect a variety of biological processes such as transcription, replication, and chromosome maintenance (5).
Related studies have shown that the modifications of histones are closely associated with the occurrence of inflammatory diseases, including atherosclerosis, type 2 diabetes, Alzheimer’s disease, psoriasis, asthma, multiple chronic lung diseases, and inflammatory bowel disease ( Table 1 and Figure 1 ). This article offers an illustrative landscape of histone modifications as significant regulators for chronic or virus-related inflammatory diseases, providing a perspective on the therapy of inflammatory diseases.
Table 1.
Histone modifications in inflammatory diseases.
| Diseases | Target | Modification | Regulators | Reference |
|---|---|---|---|---|
| Atherosclerosis | HDAC1 | Deacetylation | KLF5, miR-224-3p | (6, 7) |
| HDAC3 | Deacetylation | miR-19b | (8) | |
| HDAC4 | Deacetylation | miR-200b-3p | (9) | |
| HDAC6, H3K9 | Deacetylation | NORAD | (10) | |
| SIRT6 | Deacetylation | (11) | ||
| H3K9, H3K27 | Methylation↓ | (12) | ||
| H3K27 | Trimethylation↓ | EZH2 | (13) | |
| Type 2 diabetes | H3K9, H2AK119, | Dimethylation, Acetylation, Ubiquitination↑ | DBP | (14) |
| H2BK120 | Trimethylation↑ | GLUT4 | (15) | |
| Alzheimer’s disease | H3K9 | Deacetylation | Keap1 | (16) |
| HDAC3 | Methylation, Acetylation↑ | amyloid-β42 | (17) | |
| H3K9, H3K27 | ||||
| SIRT1, 3, 6 | Deacetylation↓ | (18) | ||
| H3K4, H3K9 | Trimethylation | (19) | ||
| Psoriasis | H3, H4 | Acetylation↓ | (20) | |
| H3K4 | Methylation↑ | |||
| H3K27 | Trimethylation↑ | EZH2, JMJD3 | (21, 22) | |
| H3K27 | Acetylation↑ | (23) | ||
| Asthma | H3K9, K14, K18, K23, K27, K36, H2B1KK120, B2BK20, BK16, BK20, BK108ac, BK116ac, BK120ac | Acetylation↑ | (24) | |
| H2BK5, H2BK11 | Acetylation↓ | |||
| H3 | Citrullination↑ | (25) | ||
| HDAC4 | Deacetylation↑ | Slug, CXCL12 | (26) | |
| Chronic obstructive pulmonary disease | H3K4, H3K27 | Methylation, Acetylation↑ | IL6-AS1 | (27) |
| H3K9 | Trimethylation↓ | (28) | ||
| HDAC2 | Deacetylation↓ | (29) | ||
| SIRT1 | Deacetylation↓ | (30) | ||
| Cystic fibrosis lung disease | HDAC6 | Deacetylation | (31) | |
| HDAC7 | Deacetylation | (32) | ||
| Inflammatory bowel disease | H3K27 | Trimethylation | EZH2 | (33) |
| H3K4 | Trimethylation↑ | (34) | ||
| H4K20 | Monomethylation | SETD8 | (35) | |
| H3R8 | Methylation↑ | PRMT2 | (36) | |
| H3K27 | Acetylation | (37) | ||
| H1, H3 | Citrullination | (38) | ||
| SIRT1 | Deacetylation↓ | (39) | ||
| Virus-associated disease | ||||
| SV40 | H3/H4 | Hyperacetylate | P300/CBP | (40, 41) |
| H3K9/H4K20 | Methylation | (42) | ||
| Merkel cell polymavirus | H3K27 | Trimethylation↓ | (43) | |
| H3K27 | Acetylation | P300/CBP | (44) | |
| HPV | H3 | Acetylation↓ | E6 and p300/CBP, TIP60, HDAC1, HDAC2 | (45, 46) |
| H3 | Acetylation↑ | E7 and p300/CBP | (44) | |
| H3 | Methylation ↑ | E6/E7 and EZH2 | (47) | |
| H3 | Methylation↓ | E6/E7 and KDM6A/KDM6B | (48) | |
| HBV | H3/H4 | Acetylation | HBx and p300/CBP, HDAC1, SIRT1 | (49, 50) |
| H3K4/H3K9 | Methylation | HBx and SETDB1, EZH2, SMYD3, | (51, 52) | |
| HDV | H3 | Acetylation | (53) | |
| HCV | H3/H4 | Acetylation | H2AX | (54) |
| H3 | Methylation | KDM5B, LSD1, G9a, EZH2 | (55, 56) | |
| HIV | H3 | Acetylation | CTIP2, HDAC1/2, BRD4 | (57, 58) |
| H3 | Methylation | LSD1, SET1, EZH2 | (57, 59) | |
| SARS-CoV-2 | H3 | Acetylation | NSP5 and HDAC2, SIRT1 | (60, 61) |
| H3K9 | Methylation | |||
| H3 | Citrullination↑ | PAD4 | (62–64) | |
Symbol ↑ represents upregulated,symbol ↓ represents downregulated.
Figure 1.
Overview of the role of histone modification in inflammatory disease.
Immunity and Inflammation to Inflammatory Disease
Inflammation is a coordinated immune response to tissue injury and pathogenic or non-pathogenic infections. The process involves a variety of cell responses, including immune cell migration and cytokine release (65). The function of inflammation is to repair the damage or resist the infection and restore the balanced state through redeploying the immune system (66). In the acute phase of inflammation, immune cells migrate to the injury site to contain the infection and repair damaged tissue, and then the lesion begins to heal. Effective, rapid, and targeted resolution is the ideal inflammatory response. Persistent inflammatory response can give rise to serious consequences, such as tissue damage, immune dysfunction, organ lesion, cancer, and even death, finally resulting in chronic diseases (67–70). The inflammatory process involves complex regulations of target genes through mass of signalings or epigenetic mechanisms.
Histone Post-Translational Modifications
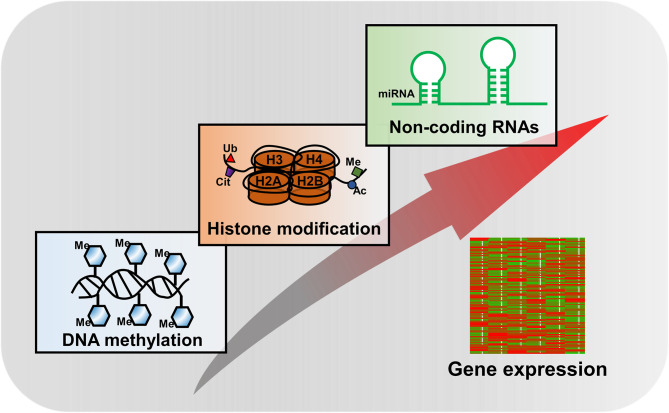
Histone modifications are one of the key components of epigenetic mechanisms, producing heritable changes in gene expression without alterations in the DNA sequence ( Figure 2 ). Over the past two decades, explosive discoveries of epigenetics have been laid down to unravel the mysteries of various biological processes.
Figure 2.
Major epigenetic mechanisms in regulation of gene expression.
The nucleosome core is formed by a histone octamer consisting of two copies of H2A, H2B, H3, and H4 and surrounded by ~150 base pairs of DNA, which is the basic functional unit of chromatin (71). PTMs of histones mostly present on the amino-terminal tail domains of the histones, but yet new data showed it also happened on the core of histones which could alter chromatin architecture by directly mediating the protein–DNA interaction (72). Besides the long-studied PTMs such as acetylation, methylation, ubiquitination, phosphorylation, citrullination, glycosylation, formylation, deamination, ADP ribosylation, proline isomerization, and sumoylation (4), a series of novel PTMs were identified recently, including crotonylation (73), propionylation, butyrylation (74), and lactylation (75). Among these, histone acetylation and methylation are the best understood PTMs.
Histone acetylation is mostly related to active transcription, while histone methylation is a kind of complex which depends on the specific methylated sites to regulate transcriptional states. The coexistence of mono-methylated H3K4 (H3K4me1) and acetylated H3K27 (H3K27ac) is the hallmark of active enhancers (76, 77). Histone methylation is commonly mediated by histone methyltransferases (HMTs), which specifically occurs on histone H3 and H4 at distinct lysine or arginine residues (78). Moreover, the enzyme family of the Complex of Proteins Associated with Set1 (COMPASS) is indispensable for H3K4 methylation (79, 80). Normally, histone methylation is considered to be a stable epigenetic mark because it sustains a stable overall charge of the histone tails, while with increasing level of methylation, it will lead to the increase of basicity, hydrophobicity, and affinity for DNA and then alter chromatin and regulate gene transcription (78).
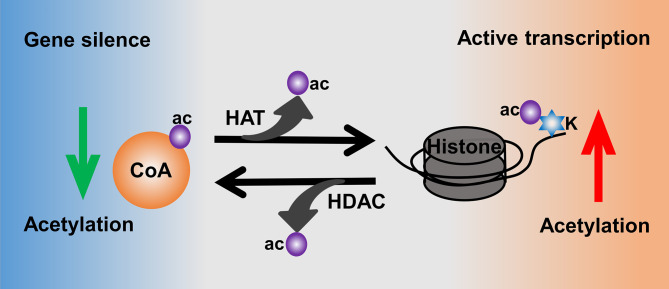
Moreover, histone acetylation is highly mediated by the activities of histone acetyltransferases (HATs) and histone deacetylases (HDACs) (81) ( Figure 3 ). HATs catalyze the transfer of acetyl from donor-acetyl coenzyme A to lysine residues of the histone peptide, which could increase the level of histone acetylation to make the chromatin sustain an active transcription (78). In contrast, HDACs are responsible for catalyzing the removal of acetyl from ϵ-amino groups of conserved lysine residues at the histone amino terminal tail, thus leading to a low level of acetylation as well as heterochromatin and gene silence (82, 83). There are 18 human HDACs identified and categorized into four classes: class I are Rpd3-like proteins and composed of HDAC1, HDAC2, HDAC3, and HDAC8; class II are Hda1-like proteins consisting of HDAC4~HDAC7 and HDAC9~10; class III are Sir2-like proteins including SIRT1~7; and class IV only contains HDAC11 (84).
Figure 3.
Histone acetylation mediated by the activities of HATs and HDACs.
Otherwise, the polycomb group consists of PRC1 and PRC2 protein complexes maintaining catalytic properties for distinct histone modifications (85). As an E3 ubiquitin ligase, PRC1 mediated histone H2A mono-ubiquitylation for gene silencing (86, 87), whereas PRC2 compacts chromatin and catalyzes the methylation of histone H3K27 via its catalytic subunit EZH1/2 (88). Furthermore, additional enzymes related to histone modifications include the phosphorylation of specific serine groups by histone kinases (HKs) (89), catalyzing the conversion of butyryl-CoA to crotonyl-CoA by acyl-CoA dehydrogenase, ACADS, acyl-CoA oxidase, and ACOX3 (90); the attachment of ubiquitin (Ub) by PRC1 (91); the ADP-ribosylation of histones by poly(ADP-ribose) (PAR) units (92); and sumoylation by small ubiquitin-like modifiers (SUMOs) (93). Although plenty of histone modifications has been identified, the hidden mechanisms for epigenetic regulation still have a long way to go.
Histone Modifications in Inflammatory Diseases
Atherosclerosis
Atherosclerosis (AS) is characterized by large and medium arteries, which is caused by chronic inflammatory disorder of the arterial vessel wall, and it is commonly considered as a major contributor of cardiovascular diseases (CVDs) including stroke and myocardial infarction (94, 95). Monocyte-derived macrophages mainly contribute to the development of AS (96, 97). Accumulating research showed the significant effects of histone modifications in the progression of AS. Therein, HDACs play a non-negligible role (98). HDAC1 was targeted by microRNA (miR)-410 to increase the level of IKBα through suppression of NF-κB by KLF5, thus preventing the development of AS (6). Meanwhile, overexpression of HDAC1 suggested to promote the anti-AS effects of miR-224-3p-mediated FOSL2 inhibition via HIF1α deacetylation (7). The defect of myeloid HDAC2 in high-calorie diet-fed (HFD) LDLR-/- male mice leads to a significant AS reduction without affecting plasma lipid and lipoprotein profiles. The inhibition of HDAC2 prevents monocytes and macrophages from nutrient stress-induced dysfunction and reprogramming which could transform into atherosclerosis (99). Excessive HDAC3 prevents inflammation to restrain atherosclerosis progression through inactivating NF-κB/p65 via upregulation of PPARγ, and it was mediated by miR-19b (8). Epicardial adipose tissue (EAT) may target HDAC4 and cause endothelial cell damage induced by miR-200b-3p, promoting oxidative stress (9). LncRNA non-coding RNA activated by DNA damage (NORAD) is a novel long non-coding RNA (lncRNA); it could recruit HDAC6 to enhance H3K9 deacetylation and consequently suppress the transcription of the VFGF gene which was supposed to enhance vascular endothelial cell injury and atherosclerosis (10). Endothelial–mesenchymal transition (EndMT) is associated with atherosclerosis and plaque instability. Both in vivo and ex vivo knockout of Hdac9 could prevent EndMT through sustained endothelial protein expression and alleviate the increase in mesenchymal proteins, thus slowing the development of atherosclerosis (100). HDAC9 is also a regulator of atherosclerosis plaque stability and IKK activation to drive inflammatory responses in both endothelial and macrophages cells (101). A lower expression of Sirtuin 1 (SIRT1) leads to ovariectomy (OVX)-induced arterial senescence and atherosclerosis in apolipoprotein E-knockout (ApoE-KO) mice (102). SIRT3 has been reviewed to play multiple roles in the development and regression of atherosclerosis (103). The expression of SIRT6 is reduced in both human and mouse plaque of vascular smooth muscle cells (VSMCs), and it is indicated to regulate telomere maintenance and VSMC lifespan as well as inhibit atherosclerosis depending on its deacetylase activity (11).
In addition to histone acetylation, histone methylation and citrullination also play a decisive role in atherosclerosis progression. Methylation of H3K9 and H3K27 was decreased in atherosclerosis plaques in smooth muscle cells (SMCs), and H3K4 methylation showed a significant association with the severity of atherosclerosis (12). Besides, histone H3K27 trimethylation could be catalyzed by PRC2 with EZH2, which is deemed to increase macrophage inflammatory responses (13, 104). A recent study showed that Ezh2-deficient mice reduced the levels of H3K27me3 and decreased H3K27 methyltransferase activity and also showed a significant reduction of lesion size suggesting the improvement of atherosclerosis (105). Citrullinated histones (Cit-histones) are associated with neutrophil extracellular trap (NET) release and are involved in different AS events in vitro. Cit-histones were pro-atherogenic mediators which can accelerate low-density lipoprotein aggregation when it is released at the lesion, thus slowing down atherosclerosis progression (106).
Type 2 Diabetes
Over the years, type 2 diabetes (T2D) becomes a worldwide disease with a leading prevalence of incidence. Unhealthy diets, physical inactivity, and aging are the major risks of T2D, which can lead to impaired insulin action and secretion (107). It has long been uncovered that chronic inflammatory processes and epigenetic mechanisms were underlying in the pathogenesis of T2D (108–110). Recently, increasing data have focused on the role of histone modifications in T2D progression. Increasing levels of histone modifications such as H3K9me2, H3K9Ac, H2AK119Ub, and H2BK120Ub in the heart of T2D rats can be attenuated by telmisartan, which could ameliorate T2D cardiomyopathy (14). Besides, exendin-4 induced a reversal of pancreatic histone H3K9 and H3K23 acetylation, and H3K4 mono-methylation and H3K9 di-methylation might improve T2D progression (111). Increased H3K9me3 in the Slc2a4 promoter could reduce the expression of GLUT4, encoded by Slc2a4, which contributes to glycemic impairment in T2D (15). Acetylation of H3K9 at the promoter region of clock gene Dbp and DBP mRNA expression in omental adipose tissue, a compartment related to the mechanism of T2D, was significantly lower in T2D patients (112).
Inhibition of HDACs has been frequently reported to attenuate T2D progression. Reduction of pancreatic β cell mass is a hallmark of T2D which can alter insulin signaling. Moreover, reducing the expression of HDAC6 in pancreatic β cells tends to downregulate insulin signaling (113). Selective inhibition of HDAC3 by RGFP966 enhanced insulin secretion and synthesis which might retard the development of T2D (114). In addition, inhibition of HDAC3 accelerates vascular endothelial proliferation during vascular impairment caused by T2D through activating Nrf2 signaling by inhibiting Keap1 synthesis and Nrf2–Nox4 association, which indicated the potential of HDAC3 as an epigenetic regulator in T2D-related vascular complications (16). Elevated levels of HDAC7 lead to β cell dysfunction and the defects of T2D (115). SIRT4 has been reported to deactivate AMPK signaling, which leads to insulin resistance responses including inflammation and oxidative stress and inhibits insulin secretion directly, ultimately producing T2B (116). Defect of SIRT6 was supposed to increase H3K9 and H3K56 acetylation and TXNIP expression, which is important in maintaining β cell function and viability (117).
Alzheimer’s Disease
Alzheimer’s disease (AD) is a typical age-related neurodegenerative disease, which is induced by chronic neuroinflammation with increased microglia and astrocyte activation, leading to cognitive impairment and dementia (118–122). Accumulation of amyloid-β plaques and tau tangles are two representative pathological characterizations of AD. Recent research has shed light on the critical role of epigenetic regulations especially histone modifications in AD progression (123–125). Using genome-wide RNA-interference-based screening, Yuan et al. identified 59 genes that might regulate age-related behavioral deterioration including cognitive decline in aging Caenorhabditis elegans (126, 127). Moreover, a neuronal histone 3 lysine 9 methyltransferase is one of the 59 genes identified; it was found to increase with age in the frontal cortex and correlate positively with AD progression. Meanwhile, multi-omics integrated by transcriptomic, proteomic, and epigenomic analyses of the postmortem human brain with AD revealed that the histone acetyltransferases for H3K27ac and H3K9ac were upregulated at the mRNA level and enriched specific to AD tissues at the protein level (17). In the same study with a fly model of AD, increasing levels of H3K27ac and H3K9ac in genome-wide aggravated amyloid-β42-driven neurodegeneration are observed. In parallel, however, another epigenome-wide association study employing the H3K9ac mark in 669 human prefrontal cortices discerned tau protein burden but not amyloid-β affecting 5,990 out of 26,384 H3K9ac domains, which showed a greater effect on the AD-related brain epigenome (128). Sirtuins have long been discussed to exert multiple functions in brain aging and neurodegenerative diseases such as AD (129–131). The expressions of SIRT1, SIRT3, and SIRT6 in the hippocampus and saliva were 1.5- to 4.9-fold reduced in elderly AD patients compared to healthy individuals of corresponding ages (18). Several other studies also indicated that SIRT1, SIRT3, and SIRT6 were reduced in AD patients in both mRNA and protein levels (132–134).
HDAC inhibitors were deemed to be innovative agents for AD therapy as its involvement in neurodevelopment, memory formation, and cognitive processes (135, 136). HDAC6 has been indicated to regulate tau acetylation reversibly, and a HDAC6-dependent surveillance mechanism that inhibits toxic tau accumulation has been put forward (137). Choi et al. found a HDAC6 inhibitor, CKD-504, which could dramatically change the tau interactome to degrade pathological tau in amyloid plaques and neurofibrillary tangles of AD model mouse brains through the proteasomal pathway, in the end rescuing cognitive decline in AD model mice (138). Another HDAC6 inhibitor, MPToG211, can significantly reduce tau phosphorylation and aggregation, which cause ubiquitination of phosphorylated tau proteins through reducing the binding of Hsp90 and HDAC6. This inhibitory activity ameliorates learning and memory impairment in AD animal models (139).
Despite genome-wide changes of histone methylations being displayed in aging and cognitive functioning, the impact of the diverse arrays of histone methylation has not been deciphered even in AD (140, 141). H3K4me3 has been targeted to recover prefrontal cortex synaptic function and memory-related behaviors by compound WDR5-0103, a newly identified H3K4me3 inhibitor (142). Likewise, H3K9me3 highly occupied epigenomes involved in synaptic transmission, neuronal differentiation, and cell motility, to remodel heterochromatin condensation, leading to a downregulation of the synaptic pathology of sporadic AD (19).
Psoriasis
Psoriasis is a recurrent and chronic inflammatory skin disease with multiple pathological features such as vascular hyperplasia, abnormal keratinocyte proliferation, and infiltration of inflammatory cells into the dermis and epidermis (143). Multiple factors including genetic susceptibility, environmental factors, and innate and adaptive immune responses were involved in psoriasis, making it a complex disease. However, the exact etiology remains largely unknown. In recent years, accumulating studies have linked epigenetic network imbalances with psoriasis, considering it as one of the major causative elements for the disease (144, 145). In this regard, we focus on the effect of histone modifications on psoriasis. An analysis of peripheral blood mononuclear cells isolated from both psoriasis patients before and after therapeutic drug administration and healthy individuals shows that the levels of acetylated H3 and H4 were reduced while the level of methylated H3K4 was increased (20). The expression of H3K27me3 and its trimethylation mediator, EZH2, was also increased in the epidermis of psoriatic lesional skin compared to the normal one. Moreover, knockdown of EZH2 caused an abnormal proliferation of keratinocytes which could be reversed by its target gene Kallikrein-8 (KLK8) (21). Another study identified grainyhead-like 2 (GRHL2) binding at the promoter region of target gene EDC, which might inhibit the recruitment of histone demethylase Jmjd3 to the EDC promoters and increase the level of H3K27me3 leading to the inhibition of keratinocyte differentiation (22). ChIP-seq with anti-H3K27Ac in psoriatic and healthy skin identified an overexpressed enrichment of H3K27Ac in psoriasis (23). HDAC1 was overexpressed in psoriasis patients while SIRT1 was decreased in the basal layer of psoriasis patients compared to healthy controls (146). Activation of SIRT1 by resveratrol induced human keratinocyte damage through blocking the Akt pathway (147). The evidence linked histone modifications with psoriasis progression providing a therapeutic target for psoriasis.
Asthma
Asthma is a common chronic inflammatory respiratory disease characterized by coughing, breath shortness, chest tightness, and wheezing, usually triggered by noxious agents or aeroallergens (148). Airway inflammation, hyperresponsiveness, and remodeling are the major contributors to the development of asthma (149). The role of epigenetic mechanisms in the pathophysiological process of asthma is progressively identified and confirmed (150–152). Using proteomics analysis of asthmatic lung tissues, Ren et al. identified 15 differentially modified acetylation sites, among which thirteen sites were upregulated including H3K9ac, H3K14ac, H3K18ac, H3K23ac, H3K27ac, H3K36ac, H2B1KK120ac, H2B2BK20ac, H2BK16ac, H2BK20ac, H2BK108ac, H2BK116ac, and H2BK120ac, while two sites were downregulated including H2BK5ac and H2BK11ac. These are potential acetylation sites related to asthma pathogenesis (24). The histone acetylation of orosomucoid 1 like protein 3 (ORMDL3) was mediated by histone acetylase p300 using a dual-luciferase reporter assay. p300 increased the mRNA levels of endogenous ORMDL3 by activating transcription from the ORMDL3 promoter. ORMDL3 expression and HAT activity were increased in the lung tissues of asthmatic mice. When p300 expression and HAT activity as well as aceH3 levels were impeded by C646, the expression of ORMDL3 would be reduced and relieve airway hyperreactivity, which improves airway inflammation and remodeling in asthma (153). With a genome-wide profiling of the enhancer-associated histone modification H3K27ac in bronchial epithelial cells (BECs) from asthma patients, 4,321 (FDR < 0.05) regions were identified to exhibit differential H3K27ac enrichment between individuals with or without asthma (154). Inhibiting H3K27me3 demethylation by a selective inhibitor GSK-J4 could improve the typical hallmarks of asthma, including airway inflammation, hyperresponsiveness, and remodeling, then alleviate the development of asthmatic disease (155). A study showed an elevated circulating H3cit level in stable asthmatics which is related to the enhanced lung extracellular traps (ETs) (25).
The activity of HDAC is distinctive in asthma progression (156). HAT and HDAC activities were associated inversely in blood monocytes isolated from healthy individuals and patients with asthma, and HAT activity was increased while HDAC activity was reduced during neutrophilic airway inflammation (157). HDAC1 protein expression was inhibited by intranasal curcumin to retard asthma severity in an allergic asthmatic mouse model (158). HDAC2 protein expression could be reduced by cigarette smoke exposure via enhancing AKT signaling, and it can be reversed by roxithromycin (RMX) treatment to increase HDAC2 expression and reduce airway inflammation (159). The expression of HDAC4 was upregulated in lung tissues of asthmatic mice, and it could deacetylate Kruppel-like factor 5 (KLF5) to upregulate Slug and CXC chemokine ligand-12 (CXCL12), thus triggering airway remodeling and promoting progression of asthma (26). Overexpression of SIRT6 reduced cell migration and proliferation, suppressed the activation of Smad3 phosphorylation induced by TGF-β1 treatment, and at the same time decreased the H3K9 acetylation level and the transcriptional activity of the c-Jun promoter (160). Moreover, SIRT1 restrained the inflammatory cytokine expression in primary bone marrow-derived macrophages (BMDMs) through the ERK/p38 MAPK pathways (161). The modulations suggested that upregulation of the expression of SIRT6 might improve airway remodeling in asthma.
Chronic Obstructive Pulmonary Disease
Chronic obstructive pulmonary disease (COPD) is also a chronic inflammatory airway disease similar to asthma, involving an obstruction in airflow, which is reversible in asthma while being progressive and irreversible in COPD (162, 163). Aberrant neutrophilic inflammation is characterized by COPD, which contributes to airway damage and leads to alveoli loss, mucus production increase, and mucociliary dysfunction (164, 165). The underlying pathogenesis of COPD is still largely unknown. Existing research indicated that epigenetic mechanisms are associated with the disease progression in COPD (166). A novel lncRNA, interleukin 6 antisense RNA 1 (IL6-AS1) supposed to recruit early B-cell factor 1 to the IL-6 promoter to increase the H3K4 methylation and H3K27 acetylation, therein, increases airway inflammation in COPD (27). SUV39H1 is a histone methyltransferase; the levels of SUV39H1 and H3K9me3 were reduced in COPD patients. Reduction of SUV39H1 by administration of its specific inhibitor, chaetocin, or genetic knockdown, leads to a loss of H3K9me3 and enhances inflammatory responses in COPD (28). A proteome study of COPD and histone lysine crotonylation (Kcr) found 190 proteins upregulated and 151 proteins downregulated, among which 90 proteins were regulated by differentially expressed crotonylation sites and expressed differentially in COPD (167).
Targeting HDACs as a therapy approach for COPD has drawn more and more attention within recent scientific research (168). It has been shown that HDAC2 was downregulated in skeletal muscle of COPD patients (29). In addition, the HDAC2 protein level was decreased upon PM2.5 exposure, and myeloid-specific deficiency of HDAC2 enhanced PM2.5-induced M2 alveolar macrophage polarization which resulted in the progressiveness of COPD (169). Similarly, knockout of HDAC2 enhanced cigarette smoke (CS)-induced DNA damage, inflammatory response, and cellular senescence in mouse models, indicating that HDAC2 is the key player in CS-associated COPD disease (170). SIRT1 was shown to maintain a lower expression in CD28nullCD8 + T and NKT-like cells than in CD28+ cells from COPD patients and healthy controls, which was related to increased IFNγ and TNFα production, steroid resistance, and disease progression. Increasing expression of SIRT1 by treatment of multiple specific drugs such as prednisolone can reverse these activities as to reduce systemic inflammation in COPD (30).
CF-Lung Disease (Cystic Fibrosis)
Cystic fibrosis (CF) lung disease is another life-threatening chronic inflammatory lung disease with various mutations in the cystic fibrosis transmembrane conductance regulator (CFTR), which assists in the regulation and clearance of mucus (171, 172). As a familial autosomal recessive disease, the related epigenetic mechanisms were gradually revealed (173). Increasing evidence showed that HDAC inhibitors could largely assist in correcting protein-misfolding diseases such as CF-related diseases (174). The most notable binding partner of HDAC6 is alpha-tubulin, which is reduced in CF cells. Inhibiting the expression of HDAC6 enables the restoration of tubulin acetylation to normal levels in CF cells (175) and reversal of the growth defects such as height and weight caused by CF (176). Depletion of HDAC6 in CF mouse models can also regain the growth and responsive activity to bacterial challenge and inflammatory phenotypes of wild-type mice (31, 177). Moreover, in HEK293 cells, inhibition of HDAC6 by suberoylanilide hydroxamic acid (SAHA) regulated both innate and adaptive immune responses of CF-lung disease-associated pathogenesis and progression (178). The majority of CF patients have a deletion of Phe 508 (△F508), which induces an efficient degradation of CFTR and then leads to premature lung failure (171). △F508 CFTR interacted with at least 638 proteins, which forms a △F508 CFTR interactome, and remodeling the interactome could promote the rescue of cystic fibrosis development (179). Hutt et al. revealed that HDAC7 was beneficial for restoring △F508 function through a SAHA-sensitive mechanism (32).
Inflammatory Bowel Disease
Inflammatory bowel disease (IBD) is a chronic inflammatory disease with distinct gastrointestinal disorders, which maintains two common clinical forms including ulcerative colitis (UC) and Crohn’s disease (180). Not only genetic predisposition but also environmental factors and imbalance of intestinal bacterial flora tend to induce IBD incidence (181–183). As a multifactorial disease, the exact etiology of IBD still needs to be unraveled, but recent studies have linked the pathogeny of IBD with epigenetic mechanisms (184–186). H3K27me3 can be regulated by extracellular vesicles to control the differentiation of Th17 cells in ulcerative colitis, which plays a distinct role in the pathogenesis of IBD (187). Inhibiting the activity of EZH2 on H3K27me3 promoted the development of functional myeloid-derived suppressor cells (MDSCs), which was beneficial to promoting the anti-inflammatory effect to treat IBD (33). A large proportion of genes enriched in newly diagnosed pediatric IBD patients maintained a significant level of H3K4me3, which was related to the severity of intestinal inflammation during the progression of IBD (34). SETD8 is a typical histone H4K20 methyltransferase in which silence of SETD8 could significantly decrease the enrichment of H4K20me1 in the p62 promoter to regulate the expression of p62 and inhibit the inflammatory response in colitis (35). The level of H3K9 acetylation was decreased upon dextran sulfate sodium (DSS) treatment, which was reported to induce colitis by lessening the macrophage amount and the secreted inflammatory cytokines (188). Protein arginine methyltransferase 2 (PRMT2) presents a high expression in IBD patients; it could increase the asymmetric methylation of H3R8 at the promoter of the suppressor of cytokine signaling3 (SOCS3) to mediate colitis progression (36). A genome-wide profiling of H3K27ac of colon tissues from DSS-induced chronic colitis mouse model identified 56 candidate genes that are potentially involved in H3K27ac change, among which special typical enhancers were upregulated by H3K27ac that might be associated with the development of intestinal inflammation (37). Citrullination of histone H1 and H3 forms NETs to activate fibroblasts into myofibroblasts in triggering fibrosis, thus potentially alleviating IBD (38).
HDAC inhibitors (HDACi) were shown to play a positive role in the course of IBD (189–191). HDAC2, HDAC3, HDAC6, HDAC9, and HDAC10 have been reported to be especially associated with IBD (192). The family member IL-35 (EBI3/IL-12p35) was indicated to induce anti-inflammatory activity in UC (193), and the activity could be upregulated by histone acetylation via HDACi administration (194). SIRT1 displayed a lower activity in various IBD models, which is important for the generation of oxidative stress and the production of pro-inflammatory cytokines (39).
Virus-Associated Diseases
Virus infections are closely correlated with many diseases, such as cancer and pneumonia. Inflammation induced by virus is the main driving force in disease development (195). Both virus life cycle and host cell viability are regulated by epigenetic modifications. After infection, the virus DNA was transported to the nucleus and then integrated into chromatin to form the virus genome nucleosomes (41). Although the composition of virus genome nucleosomes is simple, the epigenetic regulation especially histone modifications plays an important role in the expression of appropriate genes. Meanwhile, the infection also changes the epigenetic modifications in host cells, even resulting in the transformation of a normal cell to a cancer cell (41, 69, 196). Clarifying the changes in epigenetic modifications is increasingly needed in the therapy of virus-related diseases.
Polyomavirus
Polyomavirus infection can lead to chronic inflammation-associated diseases, such as cancer and nephropathy (197, 198). SV40 is a polyomavirus with a small double strand and circular DNA, which has been used as a model system to study basic aspects of histone modifications after virus infection. The viral DNA and histone were organized into a minichromosome, presented in virions and infected cells.
In the SV40 virions, H3 and H4 were hyperacetylated, along with the methylation of H3 on lysine9 (H3K9me1, H3K9me2, H3K9me3) and H4 on lysine 20 (H4K20me) (40, 199). Each methylated form of H4 was involved in different biological functions in cellular chromatin. H4K20me1 appears to associate with transcriptionally activation, while H4K20me2 is especially associated with DNA damage and repair (42, 200). It is consensus that H4K20me3 is concentrated in heterochromatin (201, 202). In the SV40 minichromosome, H4K20me1 appears to associate with transcription and may be necessary to compact minichromosome into the virion (40). The T-antigen in SV40 interacted with HAT and HDAC family members, such as p300, CREB-binding protein (CBP), HDAC1, and HDAC3 in host cells, resulting in the dysregulation of the genome (41). The T-antigen was associated with the inflammation responses (197). For the other polyomavirus, Merkel cell polyomavirus (MCPyV), the H3K27 trimethylation (H3K27me3) was repressed and the p300/CBP was upregulated (43, 203). The MCPyV sT was reported to interact with PP4 and the interaction interferes with the NF-kB pathway which was involved in the inflammation response (198).
Papillomavirus
HPV is a human papillomavirus with a circular, double-stranded DNA (204). High-risk HPV is the etiological factor of cervical carcinoma which is the fourth most common malignancy in women worldwide. HPV proteins are involved in the development of chronic inflammation which is the pivotal causal factor in the development of HPV carcinogenesis (205). It has been known that during HPV infection, certain epigenetic alterations occurred in HPV and host cellular genomes (206).
The main oncoproteins E5, E6, and E7 in HPV play important roles in viral life cycle and cancer development (207). The E5, E6, and E7 oncoproteins can activate the NF-κB pathway which is related to the progression of cervical carcinoma (208). NF-κB is the transcription factor to connect the immune system activation, chronic inflammatory responses, and carcinogenesis (209). Recent research suggested that the expression of E6/E7 was increased in cervical inflammation (205). Therefore, regulation of the translation and post-translation modification of these oncoproteins is essential to controlling the progression of inflammation. E6 and E7 mainly participated in the epigenetic regulations in host cells. E6 inhibited the activity of p300/CBP to affect the H3 acetylation, while E7 binds to p300/CBP to stimulate their activity (44, 210). The interactions of E6 and E7 with HATs downregulated the expression of the chemotactic interleukin 8 (IL-8) in immune cells, thereby affecting the inflammatory response (47, 211). E6 can also target HAT TIP60 to reduce the acetylation of histone H4 (45). The expression levels of HDAC1 and HDAC2 were increased, but the mechanism was not clear, which may be associated with the E6/E7-dependent elevation of SIRT1 expression (46).
Besides the acetylation, HPV infection can affect histone methylation distinctly. Changing the repressive mark H3K27me is a common histone modification in many cancers. HPV E6 and E7 stimulated the expression of FOXM1 and E2F1, respectively; both of them could bind the EZH2 promoter to enhance transcription (212). Otherwise, E6 also enhanced EZH2 transcription through inhibiting repression protein p53 (47). PRC1 bound to H3K27me-marked chromatin to silence gene expression through monoubiquitinating lysine 119 of histone H2A (213). However, researchers found that the H3K27me3 level was decreased in E6/E7-expressing cells. One reason is that E6/E7 upregulated the expression of KDM6A and KDM6B, which are demethylases targeting H3K27me (214). Controlling the balance of H3K27me may be a therapeutic intervention for HPV-associated malignancy.
Hepatitis Virus
HBV is hepatitis B virus with a single strand of DNA, 3.2 kb in size, the smallest among human DNA viruses. In the nucleus of infected cells, HBV formed a covalently closed circular DNA (cccDNA) minichromosome with host histone and non-histone proteins (196). HBV infection can cause acute, chronic, or occult hepatitis. With the infection, many people were at the risk of hepatocellular carcinoma (HCC). The main reason of treatment failure is the inability to eliminate the cccDNA (51). Strong evidence suggested that epigenetic regulations on both cccDNA and host genome were essential for viral life and pathogenesis.
HBx, bound to cccDNA, is the only regulatory protein encoded by HBV, playing an important role in the viral replication (215). HBx recruited p300/CBP acetyltransferase to cccDNA resulting in acetylation of H3 and H4, consequently activating transcription (49). HBx can directly interact with HADC1 and SIRT1 protein associated with the low HBV replication (50). In HBx mutant cells, the cccDNA-bound histones were hypoacetylated, resulting in low transcription (51). As mentioned above, HBx participated in the regulation of gene expression and viral replication through mediating histone acetylation.
Apart from histone acetylation, HBx also mediated methylation through affecting the level of H3K4me and H3K9me. In the absence of HBx, H3ac and H3K4me3 were decreased, and H3 dimethylation and tri-methylation (H3K9me) were increased with the concomitant transcriptional silencing and chromatin condensing. HBx affects histone methylation by several pathways. HBx stimulated the expression of SETDB1, the histone lysine 9-specific methyltransferase (51). HBx upregulated EZH2 expression and increased the half-life of EZH2. Furthermore, HBx increased the expression of the H3K4-specific methyltransferase, Set and MYND-domain containing 3 (SMYD3) (52). Histone methylation was correlated with the condensation of chromatin. In other words, HBx could regulate chromatin activation through mediating histone methylation (216).
HDV is hepatitis delta virus with a subviral satellite RNA virus that replicates only when it is surrounded by the helper protein-HBV surface antigen (217). Although the role of HDV in the development of hepatocellular carcinoma has not been well investigated, many epidemiological studies favored that it enhanced the development of liver cirrhosis and increased the risk of HCC with HBV superinfection. With the expression of HDV antigen HDAg, the transcription of CLU increased, which was associated with increased acetylation of histone H3 (53).
HCV is hepatitis C virus with a single-stranded RNA virus and is a unique human oncovirus that replicates in the cytoplasm exclusively (218). HCV infection induced the inhibition of histone H4 methylation/acetylation and histone H2AX phosphorylation through overexpression of phosphatase A catalytic subunit alpha (PP2Ac) with a significant expression change in genes important for hepatocarcinogenesis (54). Otherwise, histone H3 acetylation on lysine9/27, H3 acetylation on lysine14, and histone H2A acetylation on lysine5 also changed in HCV-infected cells (219).
For the role of histone methylation in HCV-infected cells, it has been reported that the expressions of KDM5B/JARID1B and LSD1, members of histone demethylase, were increased. Overexpression of KDM5B and LSD1 resulted in poor prognosis in HCC. Further studies may demonstrate KDM5B/LSD1 as a therapeutic target (55). The histone lysine methylase, G9a for lysine 9 of histone 3 (H3K9), is also associated with the progression of HCC prognosis (56). In China, overexpression of EZH2 was considered as a promising biomarker for HCC patients (220).
Human Immunodeficiency Virus
HIV-1 is human immunodeficiency virus type 1, with a single-stranded positive-sense RNA that replicated in CD4+ human immune cells. HIV-1 was integrated into the host cell genome after infection, establishing a stable latency which is the major obstacle for HIV cure (221). The proviral HIV genome was regulated by host epigenetic modification machinery, while HIV proteins affect the gene expression of host cells. The cellular epigenetic regulator mainly affected the viral promoter located in the 5′ long terminal repeat (LTR) sequence (222).
Histone deacetylases (HDAC1/2) were recruited on HIV LTRs; as a consequence, the transcription was suppressed (223). Histone acetylation was generally considered to promote gene expression, and the methylation on histones produced a complex scenario to control transcription. In microglial cells, CTIP2/BCL11B recruited many types of enzyme-chromatin-modifying complexes to establish the heterochromatic environment to repress HIV-1 gene expression. CTIP2 and LSD1 bound to the Sp1 site in LTR. CTIP2 sequentially recruited HDAC1/2 to acetylate H3 and HMT SUV39H1 to catalyze H3K9me3 which was recognized by HP1. In parallel, LSD1 recruited the COMPASS complex containing the histone methyltransferase SET1 to stimulate H3K4me3 (57, 224, 225). The bromodomain (BD) and extra-terminal domain (ET) protein, BRD4, consists of two conserved BDs that selectively bind to acetyl-lysine residues of histones. BRD4 was recruited to the HIV promoter to suppress gene expression, resulting in promotion of latency (58). EZH2 together with the EZH2-mediated H3K27me3 showed a higher level at the LTR of silenced HIV proviruses. Another methyltransferase G9a for histone H3 lysine 9 (H3K9) was responsible for transcriptional repression through promoting repressive demethylation at H3K9 (59). The relationship between PTM of histones and HIV-1 viral latency provides the potential therapeutic strategy. A successful HIV curative strategy needs to reverse HIV latency to purge hidden viral reservoirs or enhance HIV latency to silence HIV transcription permanently. Some epigenetic modifying agents have been suggested for transcription control of HIV-1 latency, such as histone deacetylase inhibitors (HDACi), histone methyltransferase inhibitors (HMTi), and histone demethylase inhibitors (226).
COVID-19
The coronavirus disease 2019 (COVID-19) pandemic was caused by acute respiratory syndrome coronavirus 2. The virus infection resulted in dysregulated immune responses and mass of acute inflammation. However, there is no specific drug. The virus life cycle and the host immune response to infection were associated with various epigenetic regulations, especially histone modifications which can be the therapeutic target. In a recent protein interactome analysis, hundreds of human proteins were identified to interact with SARS-CoV-2 proteins. Among these, eight proteins were associated with epigenetic regulations. HADC2 was identified to interact with NSP5 (non-structural protein 5) which participates in the formation of the replicase–transcriptase complex (60). HDAC2 can suppress inflammatory gene expression while the activity and expression of HDAC2 were inhibited in peripheral lung and alveolar macrophages with pulmonary diseases, such as COPD (227). The detection indicated that NSP5 was likely to inhibit HADC2, which then influenced HADC2-based inflammation responses. Another evidence was that ACE2 (angiotensin-converting enzyme 2) which was regulated by some histone modification proteins, such as HAT1 and HDAC2, was highly expressed in a number of severe COVID-19 patients (61, 228). SIRT1, another ACE2 epigenetic regulator, was also upregulated in several COVID-19 patients. Furthermore, ACE2 was especially regulated by histone methylation (H3K4mel and H3K4me3) and histone acetylation (H3K27ac) (62, 228).
The complication risks of COVID-19 are highly age-dependent. The age-dependent epigenetic regulation may be the foundation of age-associated severity of COVID-19 symptoms (62, 229). Histone modifications and the levels of histone proteins changed during aging, which dramatically influence chromatin compaction and gene expression. Solid evidence suggested that the acetylation level of H4 was reduced during aging. In kidney and liver tissue with age, the level of H4K20me3, a marker of constitutive heterochromatin, was increased. In some studies in vitro, the level of H3K9me3 was decreased (230). It is necessary to elucidate whether the epigenetic change during aging affects the severity of COVID-19 directly.
NETs were elevated in COVID-19 patients due to the higher level of 3 markers, cell-free DNA, myeloperoxidase-DNA, and citrullinated histone H3 (Cit-H3) (62, 63). Cit-H3 was one of the histone modifications, with the conversion of arginine to citrulline which is targeted by peptidylarginine deiminase 4 (PAD4) (64). Higher arginine contents change the charge distribution then affect the interaction with DNA, leading to chromatin decondensation and transcription activation (230). Meanwhile, Cit-H3 is an important epigenetic modification for stem cell pluripotency. Therefore, the modification of Cit-H3 might be crucial for epigenetic therapy of COVID-19.
Concluding Remarks
Over the past two decades, the understanding of histone modifications in the pathogenesis of inflammatory diseases has been largely extended. In this review, we present an illustrative but not comprehensive overview of the key role of histone modifications in regulating the progression of inflammatory diseases. In this regard, we showed involvements of histone PTMs in AS, T2D, AD, psoriasis, asthma, COPD, CF-lung disease, inflammatory bowel disease, and virus-associated diseases, but it is not limited to the diseases mentioned here; there are still more inflammatory diseases related to histone modifications, such as periodontitis, spondyloarthritis, many types of cardiometabolic diseases, chronic kidney disease, colorectal cancer, and most common neurodegenerative diseases as well as demyelinating disorders. Numerous studies focusing on histone PTM-related molecular mechanisms using multi-omics methods combined with cell-based systems and experimental animal models have profoundly contributed to the current knowledge on this topic.
Accumulating data have shown that distinct histone modifications are enriched in inflammatory organisms suffering from chronic inflammatory diseases or virus-associated inflammatory diseases. The imbalance of activating and repressing histone modifications stimulates the development of a wide range of diseases ranging from autoimmunity, cardiovascular pathology, viscera injury, neurodegenerative disorder to cancer.
On the basis of salient cognition of these mechanisms, therapeutic applications of the histone modifications targeting for inflammatory diseases are expected to expand: HDAC inhibitors as clinical drugs; new generations of vaccines that alter histone PTMs levels; enzymes modulating histone PTMs; and development of inducers of distinct histone proteins for the treatment of inflammation paralysis in inflammatory diseases. In fact, epigenetic drugs nowadays attract particular interest to the clinic owing to their characteristic reversible and transient effects. Only continuous research on the mechanisms of histone modifications will be able to achieve these aims and fulfill the potentials through the comprehending of the role of histones post-translational modifications in inflammatory diseases.
Author Contributions
YL, TQ, and XC conceived of the review and participated in its design. YL, TQ, GW, YQ, WW, YK, and TX summarized all the data and information acquired for the review. YL, TQ, TX and XC wrote the manuscript and prepared the table and figures. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (32100620, 32000363) and The Natural Science Foundation of Zhejiang Province (LQ21C060006).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Nathan C. Points of Control in Inflammation. Nature (2002) 420(6917):846–52. doi: 10.1038/nature01320b [DOI] [PubMed] [Google Scholar]
- 2. Feehan KT, Gilroy DW. Is Resolution the End of Inflammation? Trends Mol Med (2019) 25(3):198–214. doi: 10.1016/j.molmed.2019.01.006 [DOI] [PubMed] [Google Scholar]
- 3. Nathan C, Ding A. Nonresolving Inflammation. Cell (2010) 140(6):871–82. doi: 10.1016/j.cell.2010.02.029 [DOI] [PubMed] [Google Scholar]
- 4. Kouzarides T. Chromatin Modifications and Their Function. Cell (2007) 128(4):693–705. doi: 10.1016/j.cell.2007.02.005 [DOI] [PubMed] [Google Scholar]
- 5. Black JC, Van Rechem C, Whetstine JR. Histone Lysine Methylation Dynamics: Establishment, Regulation, and Biological Impact. Mol Cell (2012) 48(4):491–507. doi: 10.1016/j.molcel.2012.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nan S, Wang Y, Xu C, Wang H. Interfering microRNA-410 Attenuates Atherosclerosis via the HDAC1/KLF5/Ikbα/NF-κb Axis. Mol Ther Nucleic Acids (2021) 24:646–57. doi: 10.1016/j.omtn.2021.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang H, Sugimoto K, Lu H, Yang WY, Liu JY, Yang HY, et al. HDAC1-Mediated Deacetylation of HIF1α Prevents Atherosclerosis Progression by Promoting miR-224-3p-Mediated Inhibition of FOSL2. Mol Ther Nucleic Acids (2021) 23:577–91. doi: 10.1016/j.omtn.2020.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang J, Xu X, Li P, Zhang B, Zhang J. HDAC3 Protects Against Atherosclerosis Through Inhibition of Inflammation via the microRNA-19b/Pparγ/NF-κb Axis. Atherosclerosis (2021) 323:1–12. doi: 10.1016/j.atherosclerosis.2021.02.013 [DOI] [PubMed] [Google Scholar]
- 9. Zhang F, Cheng N, Du J, Zhang H, Zhang C. MicroRNA-200b-3p Promotes Endothelial Cell Apoptosis by Targeting HDAC4 in Atherosclerosis. BMC Cardiovasc Disord (2021) 21(1):172. doi: 10.1186/s12872-021-01980-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kai H, Wu Q, Yin R, Tang X, Shi H, Wang T, et al. LncRNA NORAD Promotes Vascular Endothelial Cell Injury and Atherosclerosis Through Suppressing VEGF Gene Transcription via Enhancing H3K9 Deacetylation by Recruiting Hdac6. Front Cell Dev Biol (2021) 9:701628. doi: 10.3389/fcell.2021.701628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grootaert MOJ, Finigan A, Figg NL, Uryga AK, Bennett MR. SIRT6 Protects Smooth Muscle Cells From Senescence and Reduces Atherosclerosis. Circ Res (2021) 128(4):474–91. doi: 10.1161/circresaha.120.318353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Greißel A, Culmes M, Burgkart R, Zimmermann A, Eckstein HH, Zernecke A, et al. Histone Acetylation and Methylation Significantly Change With Severity of Atherosclerosis in Human Carotid Plaques. Cardiovasc Pathol (2016) 25(2):79–86. doi: 10.1016/j.carpath.2015.11.001 [DOI] [PubMed] [Google Scholar]
- 13. Zhang X, Wang Y, Yuan J, Li N, Pei S, Xu J, et al. Macrophage/microglial Ezh2 Facilitates Autoimmune Inflammation Through Inhibition of Socs3. J Exp Med (2018) 215(5):1365–82. doi: 10.1084/jem.20171417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kadakol A, Malek V, Goru SK, Pandey A, Gaikwad AB. Telmisartan and Esculetin Combination Ameliorates Type 2 Diabetic Cardiomyopathy by Reversal of H3, H2A, and H2B Histone Modifications. Indian J Pharmacol (2017) 49(5):348–56. doi: 10.4103/ijp.IJP_710_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yonamine CY, Alves-Wagner AB, Esteves JV, Okamoto MM, Correa-Giannella ML, Giannella-Neto D, et al. Diabetes Induces Tri-Methylation at Lysine 9 of Histone 3 at Slc2a4 Gene in Skeletal Muscle: A New Target to Improve Glycemic Control. Mol Cell Endocrinol (2019) 481:26–34. doi: 10.1016/j.mce.2018.11.006 [DOI] [PubMed] [Google Scholar]
- 16. Huang S, Chen G, Sun J, Chen Y, Wang N, Dong Y, et al. Histone Deacetylase 3 Inhibition Alleviates Type 2 Diabetes Mellitus-Induced Endothelial Dysfunction via Nrf2. Cell Commun Signal (2021) 19(1):35. doi: 10.1186/s12964-020-00681-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nativio R, Lan Y, Donahue G, Sidoli S, Berson A, Srinivasan AR, et al. An Integrated Multi-Omics Approach Identifies Epigenetic Alterations Associated With Alzheimer’s Disease. Nat Genet (2020) 52(10):1024–35. doi: 10.1038/s41588-020-0696-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pukhalskaia AE, Dyatlova AS, Linkova NS, Kozlov KL, Kvetnaia TV, Koroleva MV, et al. Sirtuins as Possible Predictors of Aging and Alzheimer’s Disease Development: Verification in the Hippocampus and Saliva. Bull Exp Biol Med (2020) 169(6):821–4. doi: 10.1007/s10517-020-04986-4 [DOI] [PubMed] [Google Scholar]
- 19. Lee MY, Lee J, Hyeon SJ, Cho H, Hwang YJ, Shin JY, et al. Epigenome Signatures Landscaped by Histone H3K9me3 Are Associated With the Synaptic Dysfunction in Alzheimer’s Disease. Aging Cell (2020) 19(6):e13153. doi: 10.1111/acel.13153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ovejero-Benito MC, Reolid A, Sánchez-Jiménez P, Saiz-Rodríguez M, Muñoz-Aceituno E, Llamas-Velasco M, et al. Histone Modifications Associated With Biological Drug Response in Moderate-to-Severe Psoriasis. Exp Dermatol (2018) 27(12):1361–71. doi: 10.1111/exd.13790 [DOI] [PubMed] [Google Scholar]
- 21. Zhang T, Yang L, Ke Y, Lei J, Shen S, Shao S, et al. EZH2-Dependent Epigenetic Modulation of Histone H3 Lysine-27 Contributes to Psoriasis by Promoting Keratinocyte Proliferation. Cell Death Dis (2020) 11(10):826. doi: 10.1038/s41419-020-03028-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen W, Xiao Liu Z, Oh JE, Shin KH, Kim RH, Jiang M, et al. Grainyhead-Like 2 (GRHL2) Inhibits Keratinocyte Differentiation Through Epigenetic Mechanism. Cell Death Dis (2012) 3(12):e450. doi: 10.1038/cddis.2012.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Masalha M, Ben-Dov IZ, Ram O, Meningher T, Jacob-Hirsch J, Kassem R, et al. H3K27Ac Modification and Gene Expression in Psoriasis. J Dermatol Sci (2021) 103(2):93–100. doi: 10.1016/j.jdermsci.2021.07.003 [DOI] [PubMed] [Google Scholar]
- 24. Ren Y, Li M, Bai S, Kong L, Su X. Identification of Histone Acetylation in a Murine Model of Allergic Asthma by Proteomic Analysis. Exp Biol Med (Maywood) (2021) 246(8):929–39. doi: 10.1177/1535370220980345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kuczia P, Zuk J, Iwaniec T, Soja J, Dropinski J, Malesa-Wlodzik M, et al. Citrullinated Histone H3, a Marker of Extracellular Trap Formation, Is Increased in Blood of Stable Asthma Patients. Clin Transl Allergy (2020) 10:31. doi: 10.1186/s13601-020-00337-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wei W, Chen W, He N. HDAC4 Induces the Development of Asthma by Increasing Slug-Upregulated CXCL12 Expression Through KLF5 Deacetylation. J Transl Med (2021) 19:258. doi: 10.1186/s12967-021-02812-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yi E, Zhang J, Zheng M, Zhang Y, Liang C, Hao B, et al. Long Noncoding RNA IL6-AS1 is Highly Expressed in Chronic Obstructive Pulmonary Disease and Is Associated With Interleukin 6 by Targeting miR-149-5p and Early B-Cell Factor 1. Clin Transl Med (2021) 11(7):e479. doi: 10.1002/ctm2.479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen TT, Wu SM, Ho SC, Chuang HC, Liu CY, Chan YF, et al. SUV39H1 Reduction Is Implicated in Abnormal Inflammation in COPD. Sci Rep (2017) 7:46667. doi: 10.1038/srep46667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. To M, Swallow EB, Akashi K, Haruki K, Natanek SA, Polkey MI, et al. Reduced HDAC2 in Skeletal Muscle of COPD Patients. Respir Res (2017) 18(1):99. doi: 10.1186/s12931-017-0588-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hodge G, Tran HB, Reynolds PN, Jersmann H, Hodge S. Lymphocyte Senescence in COPD Is Associated With Decreased Sirtuin 1 Expression in Steroid Resistant Pro-Inflammatory Lymphocytes. Ther Adv Respir Dis (2020) 14:1753466620905280. doi: 10.1177/1753466620905280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Corey DA, Rymut SM, Kelley TJ. Alleviation of Depression-Like Behavior in a Cystic Fibrosis Mouse Model by Hdac6 Depletion. Sci Rep (2020) 10(1):16278. doi: 10.1038/s41598-020-73298-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hutt DM, Herman D, Rodrigues AP, Noel S, Pilewski JM, Matteson J, et al. Reduced Histone Deacetylase 7 Activity Restores Function to Misfolded CFTR in Cystic Fibrosis. Nat Chem Biol (2010) 6(1):25–33. doi: 10.1038/nchembio.275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhou J, Huang S, Wang Z, Huang J, Xu L, Tang X, et al. Targeting EZH2 Histone Methyltransferase Activity Alleviates Experimental Intestinal Inflammation. Nat Commun (2019) 10(1):2427. doi: 10.1038/s41467-019-10176-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kelly D, Kotliar M, Woo V, Jagannathan S, Whitt J, Moncivaiz J, et al. Microbiota-Sensitive Epigenetic Signature Predicts Inflammation in Crohn’s Disease. JCI Insight (2018) 3(18):e122104. doi: 10.1172/jci.insight.122104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen P, Zhu H, Mao Y, Zhuo M, Yu Y, Chen M, et al. SETD8 Involved in the Progression of Inflammatory Bowel Disease via Epigenetically Regulating P62 Expression. J Gastroenterol Hepatol (2021) 36(10):2850–63. doi: 10.1111/jgh.15550 [DOI] [PubMed] [Google Scholar]
- 36. Li J, Pan X, Ren Z, Li B, Liu H, Wu C, et al. Protein Arginine Methyltransferase 2 (PRMT2) Promotes Dextran Sulfate Sodium-Induced Colitis by Inhibiting the SOCS3 Promoter via Histone H3R8 Asymmetric Dimethylation. Br J Pharmacol (2022) 179(1):141–58. doi: 10.1111/bph.15695 [DOI] [PubMed] [Google Scholar]
- 37. Chen M, Li Q, Cao N, Deng Y, Li L, Zhao Q, et al. Profiling of Histone 3 Lysine 27 Acetylation Reveals its Role in a Chronic DSS-Induced Colitis Mouse Model. Mol Omics (2019) 15(4):296–307. doi: 10.1039/c9mo00070d [DOI] [PubMed] [Google Scholar]
- 38. Dragoni G, De Hertogh G, Vermeire S. The Role of Citrullination in Inflammatory Bowel Disease: A Neglected Player in Triggering Inflammation and Fibrosis? Inflammation Bowel Dis (2021) 27(1):134–44. doi: 10.1093/ibd/izaa095 [DOI] [PubMed] [Google Scholar]
- 39. Devi K, Singh N, Jaggi AS. Dual Role of Sirtuin 1 in Inflammatory Bowel Disease. Immunopharmacol Immunotoxicol (2020) 42(5):385–91. doi: 10.1080/08923973.2020.1790595 [DOI] [PubMed] [Google Scholar]
- 40. Balakrishnan L, Gefroh A, Milavetz B. Histone H4 Lysine 20 Mono- and Tri-Methylation Define Distinct Biological Processes in SV40 Minichromosomes. Cell Cycle (2010) 9(7):1320–32. doi: 10.4161/cc.9.7.11123 [DOI] [PubMed] [Google Scholar]
- 41. Milavetz BI, Balakrishnan L. Viral Epigenetics. Methods Mol Biol (2015) 1238:569–96. doi: 10.1007/978-1-4939-1804-1_30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pesavento JJ, Yang H, Kelleher NL, Mizzen CA. Certain and Progressive Methylation of Histone H4 at Lysine 20 During the Cell Cycle. Mol Cell Biol (2008) 28(1):468–86. doi: 10.1128/MCB.01517-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Busam KJ, Pulitzer MP, Coit DC, Arcila M, Leng D, Jungbluth AA, et al. Reduced H3K27me3 Expression in Merkel Cell Polyoma Virus-Positive Tumors. Mod Pathol (2017) 30(6):877–83. doi: 10.1038/modpathol.2017.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bernat A, Avvakumov N, Mymryk JS, Banks L. Interaction Between the HPV E7 Oncoprotein and the Transcriptional Coactivator P300. Oncogene (2003) 22(39):7871–81. doi: 10.1038/sj.onc.1206896 [DOI] [PubMed] [Google Scholar]
- 45. Jha S, Vande Pol S, Banerjee NS, Dutta AB, Chow LT, Dutta A. Destabilization of TIP60 by Human Papillomavirus E6 Results in Attenuation of TIP60-Dependent Transcriptional Regulation and Apoptotic Pathway. Mol Cell (2010) 38(5):700–11. doi: 10.1016/j.molcel.2010.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Langsfeld ES, Bodily JM, Laimins LA. The Deacetylase Sirtuin 1 Regulates Human Papillomavirus Replication by Modulating Histone Acetylation and Recruitment of DNA Damage Factors NBS1 and Rad51 to Viral Genomes. PloS Pathog (2015) 11(9):e1005181. doi: 10.1371/journal.ppat.1005181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Scarth JA, Patterson MR, Morgan EL, Macdonald A. The Human Papillomavirus Oncoproteins: A Review of the Host Pathways Targeted on the Road to Transformation. J Gen Virol (2021) 102(3):001540. doi: 10.1099/jgv.0.001540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hyland PL, McDade SS, McCloskey R, Dickson GJ, Arthur K, McCance DJ, et al. Evidence for Alteration of EZH2, BMI1, and KDM6A and Epigenetic Reprogramming in Human Papillomavirus Type 16 E6/E7-Expressing Keratinocytes. J Virol (2011) 85(21):10999–1006. doi: 10.1128/JVI.00160-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pollicino T, Belloni L, Raffa G, Pediconi N, Squadrito G, Raimondo G, et al. Hepatitis B Virus Replication Is Regulated by the Acetylation Status of Hepatitis B Virus cccDNA-Bound H3 and H4 Histones. Gastroenterology (2006) 130(3):823–37. doi: 10.1053/j.gastro.2006.01.001 [DOI] [PubMed] [Google Scholar]
- 50. Deng JJ, Kong KE, Gao WW, Tang HV, Chaudhary V, Cheng Y, et al. Interplay Between SIRT1 and Hepatitis B Virus X Protein in the Activation of Viral Transcription. Biochim Biophys Acta Gene Regul Mech (2017) 1860(4):491–501. doi: 10.1016/j.bbagrm.2017.02.007 [DOI] [PubMed] [Google Scholar]
- 51. Riviere L, Gerossier L, Ducroux A, Dion S, Deng Q, Michel ML, et al. HBx Relieves Chromatin-Mediated Transcriptional Repression of Hepatitis B Viral cccDNA Involving SETDB1 Histone Methyltransferase. J Hepatol (2015) 63(5):1093–102. doi: 10.1016/j.jhep.2015.06.023 [DOI] [PubMed] [Google Scholar]
- 52. Yang L, He J, Chen L, Wang G. Hepatitis B Virus X Protein Upregulates Expression of SMYD3 and C-MYC in HepG2 Cells. Med Oncol (2009) 26(4):445–51. doi: 10.1007/s12032-008-9144-1 [DOI] [PubMed] [Google Scholar]
- 53. Abbas Z, Abbas M, Abbas S, Shazi L. Hepatitis D and Hepatocellular Carcinoma. World J Hepatol (2015) 7(5):777–86. doi: 10.4254/wjh.v7.i5.777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Duong FH, Christen V, Lin S, Heim MH. Hepatitis C Virus-Induced Up-Regulation of Protein Phosphatase 2A Inhibits Histone Modification and DNA Damage Repair. Hepatology (2010) 51(3):741–51. doi: 10.1002/hep.23388 [DOI] [PubMed] [Google Scholar]
- 55. Shigek Y, Hayami S, Ueno M, Miyamoto A. Overexpression of KDM5B Jarid1b Is Associated With Poor Prognosis in Hepatocellular Carcinoma. Oncotarget (2018) 9:34320–35. doi: 10.18632/oncotarget.26144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Testoni B, Durantel D, Lebosse F, Fresquet J, Helle F, Negro F, et al. Ribavirin Restores IFNalpha Responsiveness in HCV-Infected Livers by Epigenetic Remodelling at Interferon Stimulated Genes. Gut (2016) 65(4):672–82. doi: 10.1136/gutjnl-2014-309011 [DOI] [PubMed] [Google Scholar]
- 57. Lange UC, Verdikt R, Ait-Ammar A, Van Lint C. Epigenetic Crosstalk in Chronic Infection With HIV-1. Semin Immunopathol (2020) 42(2):187–200. doi: 10.1007/s00281-020-00783-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Alamer E, Zhong C, Hajnik R, Soong L, Hu H. Modulation of BRD4 in HIV Epigenetic Regulation: Implications for Finding an HIV Cure. Retrovirology (2021) 18(1):3. doi: 10.1186/s12977-020-00547-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Imai K, Togami H, Okamoto T. Involvement of Histone H3 Lysine 9 (H3K9) Methyltransferase G9a in the Maintenance of HIV-1 Latency and its Reactivation by BIX01294. J Biol Chem (2010) 285(22):16538–45. doi: 10.1074/jbc.M110.103531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, White KM, et al. A SARS-CoV-2 Protein Interaction Map Reveals Targets for Drug Repurposing. Nature (2020) 583(7816):459–68. doi: 10.1038/s41586-020-2286-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sen R, Garbati M, Bryant K, Lu Y. Epigenetic Mechanisms Influencing COVID-19. Genome (2021) 64(4):372–85. doi: 10.1139/gen-2020-0135 [DOI] [PubMed] [Google Scholar]
- 62. Chlamydas S, Papavassiliou AG, Piperi C. Epigenetic Mechanisms Regulating COVID-19 Infection. Epigenetics (2021) 16(3):263–70. doi: 10.1080/15592294.2020.1796896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA. Neutrophil Extracellular Traps in COVID-19. JCI Insight (2020) 5(11):e138999. doi: 10.1172/jci.insight.138999 10.1172/jci.insight.138999DS1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Vossenaar ER, Zendman AJ, van Venrooij WJ, Pruijn GJ. PAD, a Growing Family Of Citrullinating Enzymes: Genes, Features and Involvement In Disease. Bioessays (2003) 25(11):1106–18. doi: 10.1002/bies.10357 [DOI] [PubMed] [Google Scholar]
- 65. Barton GM. A Calculated Response: Control of Inflammation by the Innate Immune System. J Clin Invest (2008) 118(2):413–20. doi: 10.1172/JCI34431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nathan C. Points of Control in Inflammatin. Nature (2002) 420:846–52. doi: 10.1038/nature01320 [DOI] [PubMed] [Google Scholar]
- 67. Broom LJ, Kogut MH. Inflammation: Friend or Foe for Animal Production? Poult Sci (2018) 97(2):510–4. doi: 10.3382/ps/pex314 [DOI] [PubMed] [Google Scholar]
- 68. Germolec DR, Shipkowski KA, Frawley RP, Evans E. Markers of Inflammation. Methods Mol Biol (2018) 1803:57–79. doi: 10.1007/978-1-4939-8549-4_5 [DOI] [PubMed] [Google Scholar]
- 69. Minarovits J, Demcsak A, Banati F, Niller HH. Epigenetic Dysregulation in Virus-Associated Neoplasms. Adv Exp Med Biol (2016) 879:71–90. doi: 10.1007/978-3-319-24738-0_4 [DOI] [PubMed] [Google Scholar]
- 70. Sun Y, Chen P, Zhai B, Zhang M, Xiang Y, Fang J, et al. The Emerging Role of Ferroptosis in Inflammation. BioMed Pharmacother (2020) 127:110108. doi: 10.1016/j.biopha.2020.110108 [DOI] [PubMed] [Google Scholar]
- 71. Bentley GA, Lewit-Bentley A, Finch JT, Podjarny AD, Roth M. Crystal Structure of the Nucleosome Core Particle at 16 A Resolution. J Mol Biol (1984) 176(1):55–75. doi: 10.1016/0022-2836(84)90382-6 [DOI] [PubMed] [Google Scholar]
- 72. Tessarz P, Kouzarides T. Histone Core Modifications Regulating Nucleosome Structure and Dynamics. Nat Rev Mol Cell Biol (2014) 15(11):703–8. doi: 10.1038/nrm3890 [DOI] [PubMed] [Google Scholar]
- 73. Tan M, Luo H, Lee S, Jin F, Yang JS, Montellier E, et al. Identification of 67 Histone Marks and Histone Lysine Crotonylation as a New Type of Histone Modification. Cell (2011) 146(6):1016–28. doi: 10.1016/j.cell.2011.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chen Y, Sprung R, Tang Y, Ball H, Sangras B, Kim SC, et al. Lysine Propionylation and Butyrylation Are Novel Post-Translational Modifications in Histones. Mol Cell Proteomics (2007) 6(5):812–9. doi: 10.1074/mcp.M700021-MCP200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhang D, Tang Z, Huang H, Zhou G, Cui C, Weng Y, et al. Metabolic Regulation of Gene Expression by Histone Lactylation. Nature (2019) 574(7779):575–80. doi: 10.1038/s41586-019-1678-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Creyghton MP, Cheng AW, Welstead GG, Kooistra T, Carey BW, Steine EJ, et al. Histone H3K27ac Separates Active From Poised Enhancers and Predicts Developmental State. Proc Natl Acad Sci USA (2010) 107(50):21931–6. doi: 10.1073/pnas.1016071107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Heintzman ND, Hon GC, Hawkins RD, Kheradpour P, Stark A, Harp LF, et al. Histone Modifications at Human Enhancers Reflect Global Cell-Type-Specific Gene Expression. Nature (2009) 459(7243):108–12. doi: 10.1038/nature07829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Biel M, Wascholowski V, Giannis A. Epigenetics–an Epicenter of Gene Regulation: Histones and Histone-Modifying Enzymes. Angew Chem Int Ed Engl (2005) 44(21):3186–216. doi: 10.1002/anie.200461346 [DOI] [PubMed] [Google Scholar]
- 79. Shilatifard A. The COMPASS Family of Histone H3K4 Methylases: Mechanisms of Regulation in Development and Disease Pathogenesis. Annu Rev Biochem (2012) 81:65–95. doi: 10.1146/annurev-biochem-051710-134100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Schneider J, Wood A, Lee JS, Schuster R, Dueker J, Maguire C, et al. Molecular Regulation of Histone H3 Trimethylation by COMPASS and the Regulation of Gene Expression. Mol Cell (2005) 19(6):849–56. doi: 10.1016/j.molcel.2005.07.024 [DOI] [PubMed] [Google Scholar]
- 81. Kurdistani SK, Grunstein M. Histone Acetylation and Deacetylation in Yeast. Nat Rev Mol Cell Biol (2003) 4(4):276–84. doi: 10.1038/nrm1075 [DOI] [PubMed] [Google Scholar]
- 82. Steunou AL, Rossetto D, Côté J. Regulating Chromatin by Histone Acetylation. New York: Springer; (2014) 147–221. [Google Scholar]
- 83. Gao Y, Zhang H, Lirussi F, Garrido C, Ye XY, Xie T. Dual Inhibitors of Histone Deacetylases and Other Cancer-Related Targets: A Pharmacological Perspective. Biochem Pharmacol (2020) 182:114224. doi: 10.1016/j.bcp.2020.114224 [DOI] [PubMed] [Google Scholar]
- 84. Seto E, Yoshida M. Erasers of Histone Acetylation: The Histone Deacetylase Enzymes. Cold Spring Harb Perspect Biol (2014) 6(4):a018713. doi: 10.1101/cshperspect.a018713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kassis JA, Kennison JA, Tamkun JW. Polycomb and Trithorax Group Genes in Drosophila. Genetics (2017) 206(4):1699–725. doi: 10.1534/genetics.115.185116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wang H, Wang L, Erdjument-Bromage H, Vidal M, Tempst P, Jones RS, et al. Role of Histone H2A Ubiquitination in Polycomb Silencing. Nature (2004) 431(7010):873–8. doi: 10.1038/nature02985 [DOI] [PubMed] [Google Scholar]
- 87. de Napoles M, Mermoud JE, Wakao R, Tang YA, Endoh M, Appanah R, et al. Polycomb Group Proteins Ring1A/B Link Ubiquitylation of Histone H2A to Heritable Gene Silencing and X Inactivation. Dev Cell (2004) 7(5):663–76. doi: 10.1016/j.devcel.2004.10.005 [DOI] [PubMed] [Google Scholar]
- 88. Margueron R, Reinberg D. The Polycomb Complex PRC2 and Its Mark in Life. Nature (2011) 469(7330):343–9. doi: 10.1038/nature09784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Nowak SJ, Corces VG. Phosphorylation of Histone H3: A Balancing Act Between Chromosome Condensation and Transcriptional Activation. Trends Genet (2004) 20(4):214–20. doi: 10.1016/j.tig.2004.02.007 [DOI] [PubMed] [Google Scholar]
- 90. Fang Y, Xu X, Ding J, Yang L, Doan MT, Karmaus PWF, et al. Histone Crotonylation Promotes Mesoendodermal Commitment of Human Embryonic Stem Cells. Cell Stem Cell (2021) 28(4):748–63.e7. doi: 10.1016/j.stem.2020.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Morgan MAJ, Shilatifard A. Reevaluating the Roles of Histone-Modifying Enzymes and Their Associated Chromatin Modifications in Transcriptional Regulation. Nat Genet (2020) 52(12):1271–81. doi: 10.1038/s41588-020-00736-4 [DOI] [PubMed] [Google Scholar]
- 92. Chen Q, Bian C, Wang X, Liu X, Ahmad Kassab M, Yu Y, et al. ADP-Ribosylation of Histone Variant H2AX Promotes Base Excision Repair. EMBO J (2021) 40(2):e104542. doi: 10.15252/embj.2020104542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ryu HY, Zhao D, Li J, Su D, Hochstrasser M. Histone Sumoylation Promotes Set3 Histone-Deacetylase Complex-Mediated Transcriptional Regulation. Nucleic Acids Res (2020) 48(21):12151–68. doi: 10.1093/nar/gkaa1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, et al. Atherosclerosis. Nat Rev Dis Primers (2019) 5(1):56. doi: 10.1038/s41572-019-0106-z [DOI] [PubMed] [Google Scholar]
- 95. Wei X, Ying M, Dehaini D, Su Y, Kroll AV, Zhou J, et al. Nanoparticle Functionalization With Platelet Membrane Enables Multifactored Biological Targeting and Detection of Atherosclerosis. ACS Nano (2018) 12(1):109–16. doi: 10.1021/acsnano.7b07720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. De Paoli F, Staels B, Chinetti-Gbaguidi G. Macrophage Phenotypes and Their Modulation in Atherosclerosis. Circ J (2014) 78(8):1775–81. doi: 10.1253/circj.cj-14-0621 [DOI] [PubMed] [Google Scholar]
- 97. Groh L, Keating ST, Joosten LAB, Netea MG, Riksen NP. Monocyte and Macrophage Immunometabolism in Atherosclerosis. Semin Immunopathol (2018) 40(2):203–14. doi: 10.1007/s00281-017-0656-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zhao TC, Wang Z, Zhao TY. The Important Role of Histone Deacetylases in Modulating Vascular Physiology and Arteriosclerosis. Atherosclerosis (2020) 303:36–42. doi: 10.1016/j.atherosclerosis.2020.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Wang L, Ahn YJ, Asmis R. Inhibition of Myeloid HDAC2 Upregulates Glutaredoxin 1 Expression, Improves Protein Thiol Redox State and Protects Against High-Calorie Diet-Induced Monocyte Dysfunction and Atherosclerosis. Atherosclerosis (2021) 328:23–32. doi: 10.1016/j.atherosclerosis.2021.05.002 [DOI] [PubMed] [Google Scholar]
- 100. Lecce L, Xu Y, V’Gangula B, Chandel N, Pothula V, Caudrillier A, et al. Histone Deacetylase 9 Promotes Endothelial-Mesenchymal Transition and an Unfavorable Atherosclerotic Plaque Phenotype. J Clin Invest (2021) 131(15):e131178. doi: 10.1172/jci131178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Asare Y, Campbell-James TA, Bokov Y, Yu LL, Prestel M, El Bounkari O, et al. Histone Deacetylase 9 Activates IKK to Regulate Atherosclerotic Plaque Vulnerability. Circ Res (2020) 127(6):811–23. doi: 10.1161/circresaha.120.316743 [DOI] [PubMed] [Google Scholar]
- 102. Sasaki Y, Ikeda Y, Miyauchi T, Uchikado Y, Akasaki Y, Ohishi M. Estrogen-SIRT1 Axis Plays a Pivotal Role in Protecting Arteries Against Menopause-Induced Senescence and Atherosclerosis. J Atheroscler Thromb (2020) 27(1):47–59. doi: 10.5551/jat.47993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Liu Y, Shen X, Pang M, Sun Z, Qian Y, Xue W, et al. Role of Histone Deacetylase Sirt3 in the Development and Regression of Atherosclerosis. Life Sci (2021) 272:119178. doi: 10.1016/j.lfs.2021.119178 [DOI] [PubMed] [Google Scholar]
- 104. Neele AE, de Winther MPJ. Repressing the Repressor: Ezh2 Mediates Macrophage Activation. J Exp Med (2018) 215(5):1269–71. doi: 10.1084/jem.20180479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Neele AE, Chen HJ, Gijbels MJJ, van der Velden S, Hoeksema MA, Boshuizen MCS, et al. Myeloid Ezh2 Deficiency Limits Atherosclerosis Development. Front Immunol (2020) 11:594603. doi: 10.3389/fimmu.2020.594603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Haritha VH, George A, Shaji BV, Anie Y. NET-Associated Citrullinated Histones Promote LDL Aggregation and Foam Cell Formation In vitro . Exp Cell Res (2020) 396(2):112320. doi: 10.1016/j.yexcr.2020.112320 [DOI] [PubMed] [Google Scholar]
- 107. Chatterjee S, Khunti K, Davies MJ. Type 2 Diabetes. Lancet (2017) 389(10085):2239–51. doi: 10.1016/s0140-6736(17)30058-2 [DOI] [PubMed] [Google Scholar]
- 108. Mushtaq G, Khan JA, Kumosani TA, Kamal MA. Alzheimer’s Disease and Type 2 Diabetes via Chronic Inflammatory Mechanisms. Saudi J Biol Sci (2015) 22(1):4–13. doi: 10.1016/j.sjbs.2014.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Sommese L, Benincasa G, Lanza M, Sorriento A, Schiano C, Lucchese R, et al. Novel Epigenetic-Sensitive Clinical Challenges Both in Type 1 and Type 2 Diabetes. J Diabetes Complicat (2018) 32(11):1076–84. doi: 10.1016/j.jdiacomp.2018.08.012 [DOI] [PubMed] [Google Scholar]
- 110. Ling C, Rönn T. Epigenetics in Human Obesity and Type 2 Diabetes. Cell Metab (2019) 29(5):1028–44. doi: 10.1016/j.cmet.2019.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Tu P, Huang B, Li M, Zhang Y, Bao S, Tu N, et al. Exendin-4 may Improve Type 2 Diabetes by Modulating the Epigenetic Modifications of Pancreatic Histone H3 in STZ-Induced Diabetic C57BL/6 J Mice. J Physiol Biochem (2021) 77(4):1–9. doi: 10.1007/s13105-021-00835-8 [DOI] [PubMed] [Google Scholar]
- 112. Ushijima K, Suzuki C, Kitamura H, Shimada K, Kawata H, Tanaka A, et al. Expression of Clock Gene Dbp in Omental and Mesenteric Adipose Tissue in Patients With Type 2 Diabetes. BMJ Open Diabetes Res Care (2020) 8(1):e001465. doi: 10.1136/bmjdrc-2020-001465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Inoue H, Asahara SI, Sugiura Y, Kawada Y, Imai A, Hara C, et al. Histone Deacetylase 6 Regulates Insulin Signaling in Pancreatic β Cells. Biochem Biophys Res Commun (2021) 534:896–901. doi: 10.1016/j.bbrc.2020.10.078 [DOI] [PubMed] [Google Scholar]
- 114. Lei L, Bai G, Wang X, Liu S, Xia J, Wu S, et al. Histone Deacetylase 3-Selective Inhibitor RGFP966 Ameliorates Impaired Glucose Tolerance Through β-Cell Protection. Toxicol Appl Pharmacol (2020) 406:115189. doi: 10.1016/j.taap.2020.115189 [DOI] [PubMed] [Google Scholar]
- 115. Daneshpajooh M, Bacos K, Bysani M, Bagge A, Ottosson Laakso E, Vikman P, et al. HDAC7 Is Overexpressed in Human Diabetic Islets and Impairs Insulin Secretion in Rat Islets and Clonal Beta Cells. Diabetologia (2017) 60(1):116–25. doi: 10.1007/s00125-016-4113-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Choubey SK, Prabhu D, Nachiappan M, Biswal J, Jeyakanthan J. Molecular Modeling, Dynamics Studies and Density Functional Theory Approaches to Identify Potential Inhibitors of SIRT4 Protein From Homo Sapiens : A Novel Target for the Treatment of Type 2 Diabetes. J Biomol Struct Dyn (2017) 35(15):3316–29. doi: 10.1080/07391102.2016.1254117 [DOI] [PubMed] [Google Scholar]
- 117. Qin K, Zhang N, Zhang Z, Nipper M, Zhu Z, Leighton J, et al. SIRT6-Mediated Transcriptional Suppression of Txnip Is Critical for Pancreatic Beta Cell Function and Survival in Mice. Diabetologia (2018) 61(4):906–18. doi: 10.1007/s00125-017-4542-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Breunig JJ, Guillot-Sestier MV, Town T. Brain Injury, Neuroinflammation and Alzheimer’s Disease. Front Aging Neurosci (2013) 5:26. doi: 10.3389/fnagi.2013.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Gambino CM, Sasso BL, Bivona G, Agnello L, Ciaccio M. Aging and Neuroinflammatory Disorders: New Biomarkers and Therapeutic Targets. Curr Pharm Des (2019) 25(39):4168–74. doi: 10.2174/1381612825666191112093034 [DOI] [PubMed] [Google Scholar]
- 120. Mishra A, Brinton RD. Inflammation: Bridging Age, Menopause and Apoeϵ4 Genotype to Alzheimer’s Disease. Front Aging Neurosci (2018) 10:312. doi: 10.3389/fnagi.2018.00312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Sawikr Y, Yarla NS, Peluso I, Kamal MA, Aliev G, Bishayee A. Neuroinflammation in Alzheimer’s Disease: The Preventive and Therapeutic Potential of Polyphenolic Nutraceuticals. Adv Protein Chem Struct Biol (2017) 108:33–57. doi: 10.1016/bs.apcsb.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 122. Stylianou E. Epigenetics of Chronic Inflammatory Diseases. J Inflammation Res (2019) 12:1–14. doi: 10.2147/jir.S129027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Berson A, Nativio R, Berger SL, Bonini NM. Epigenetic Regulation in Neurodegenerative Diseases. Trends Neurosci (2018) 9:587–98. doi: 10.1016/j.tins.2018.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Narayan P, Dragunow M. Alzheimer’s Disease and Histone Code Alterations. Adv Exp Med Biol (2017) 978:321–36. doi: 10.1007/978-3-319-53889-1_17 [DOI] [PubMed] [Google Scholar]
- 125. Stoccoro A, Coppedè F. Role of Epigenetics in Alzheimer’s Disease Pathogenesis. Neurodegener Dis Manag (2018) 8(3):181–93. doi: 10.2217/nmt-2018-0004 [DOI] [PubMed] [Google Scholar]
- 126. Yuan J, Chang SY, Yin SG, Liu ZY, Cheng X, Liu XJ, et al. Two Conserved Epigenetic Regulators Prevent Healthy Ageing. Nature (2020) 579(7797):118–22. doi: 10.1038/s41586-020-2037-y [DOI] [PubMed] [Google Scholar]
- 127. Han ZJ, Xue WW, Tao L, Zhu F. Identification of Novel Immune-Relevant Drug Target Genes for Alzheimer’s Disease by Combining Ontology Inference With Network Analysis. CNS Neurosci Ther (2018) 24(12):1253–63. doi: 10.1111/cns.13051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Klein HU, McCabe C, Gjoneska E, Sullivan SE, Kaskow BJ, Tang A, et al. Epigenome-Wide Study Uncovers Large-Scale Changes in Histone Acetylation Driven by Tau Pathology in Aging and Alzheimer’s Human Brains. Nat Neurosci (2019) 22(1):37–46. doi: 10.1038/s41593-018-0291-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Jęśko H, Wencel P, Strosznajder RP, Strosznajder JB. Sirtuins and Their Roles in Brain Aging and Neurodegenerative Disorders. Neurochem Res (2017) 42(3):876–90. doi: 10.1007/s11064-016-2110-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Khan H, Tiwari P, Kaur A, Singh TG. Sirtuin Acetylation and Deacetylation: A Complex Paradigm in Neurodegenerative Disease. Mol Neurobiol (2021) 58(8):3903–17. doi: 10.1007/s12035-021-02387-w [DOI] [PubMed] [Google Scholar]
- 131. Yeong KY, Berdigaliyev N, Chang Y. Sirtuins and Their Implications in Neurodegenerative Diseases From a Drug Discovery Perspective. ACS Chem Neurosci (2020) 11(24):4073–91. doi: 10.1021/acschemneuro.0c00696 [DOI] [PubMed] [Google Scholar]
- 132. Kaluski S, Portillo M, Besnard A, Stein D, Einav M, Zhong L, et al. Neuroprotective Functions for the Histone Deacetylase Sirt6. Cell Rep (2017) 18(13):3052–62. doi: 10.1016/j.celrep.2017.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Lu Y, Tan L, Wang X. Circular HDAC9/microRNA-138/Sirtuin-1 Pathway Mediates Synaptic and Amyloid Precursor Protein Processing Deficits in Alzheimer’s Disease. Neurosci Bull (2019) 35(5):877–88. doi: 10.1007/s12264-019-00361-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Song S, Li B, Jia Z, Guo L. Sirtuin 3 mRNA Expression is Downregulated in the Brain Tissues of Alzheimer’s Disease Patients: A Bioinformatic and Data Mining Approach. Med Sci Monit (2020) 26:e923547. doi: 10.12659/msm.923547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. De Simone A, Milelli A. Histone Deacetylase Inhibitors as Multitarget Ligands: New Players in Alzheimer’s Disease Drug Discovery? ChemMedChem (2019) 14(11):1067–73. doi: 10.1002/cmdc.201900174 [DOI] [PubMed] [Google Scholar]
- 136. Thomas EA, D’Mello SR. Complex Neuroprotective and Neurotoxic Effects of Histone Deacetylases. J Neurochem (2018) 145(2):96–110. doi: 10.1111/jnc.14309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Trzeciakiewicz H, Ajit D, Tseng JH, Chen Y, Ajit A, Tabassum Z, et al. An HDAC6-Dependent Surveillance Mechanism Suppresses Tau-Mediated Neurodegeneration and Cognitive Decline. Nat Commun (2020) 11(1):5522. doi: 10.1038/s41467-020-19317-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Choi H, Kim HJ, Yang J, Chae S, Lee W, Chung S, et al. Acetylation Changes Tau Interactome to Degrade Tau in Alzheimer’s Disease Animal and Organoid Models. Aging Cell (2020) 19(1):e13081. doi: 10.1111/acel.13081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Fan SJ, Huang FI, Liou JP, Yang CR. The Novel Histone De Acetylase 6 Inhibitor, MPT0G211, Ameliorates Tau Phosphorylation and Cognitive Deficits in an Alzheimer’s Disease Model. Cell Death Dis (2018) 9(6):655. doi: 10.1038/s41419-018-0688-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Basavarajappa BS, Subbanna S. Histone Methylation Regulation in Neurodegenerative Disorders. Int J Mol Sci (2021) 22(9):4654. doi: 10.3390/ijms22094654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Lardenoije R, Iatrou A, Kenis G, Kompotis K, Steinbusch HW, Mastroeni D, et al. The Epigenetics of Aging and Neurodegeneration. Prog Neurobiol (2015) 131:21–64. doi: 10.1016/j.pneurobio.2015.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Cao Q, Wang W, Williams JB, Yang F, Wang ZJ, Yan Z. Targeting Histone K4 Trimethylation for Treatment of Cognitive and Synaptic Deficits in Mouse Models of Alzheimer’s Disease. Sci Adv (2020) 6(50):eabc8096. doi: 10.1126/sciadv.abc8096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med (2009) 361(5):496–509. doi: 10.1056/NEJMra0804595 [DOI] [PubMed] [Google Scholar]
- 144. Chandra A, Ray A, Senapati S, Chatterjee R. Genetic and Epigenetic Basis of Psoriasis Pathogenesis. Mol Immunol (2015) 64(2):313–23. doi: 10.1016/j.molimm.2014.12.014 [DOI] [PubMed] [Google Scholar]
- 145. Shao S, Gudjonsson JE. Epigenetics of Psoriasis. Adv Exp Med Biol (2020) 1253:209–21. doi: 10.1007/978-981-15-3449-2_8 [DOI] [PubMed] [Google Scholar]
- 146. Hwang YJ, Na JI, Byun SY, Kwon SH, Yang SH, Lee HS, et al. Histone Deacetylase 1 and Sirtuin 1 Expression in Psoriatic Skin: A Comparison Between Guttate and Plaque Psoriasis. Life (Basel) (2020) 10(9):157. doi: 10.3390/life10090157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Lee JH, Kim JS, Park SY, Lee YJ. Resveratrol Induces Human Keratinocyte Damage via the Activation of Class III Histone Deacetylase, Sirt1. Oncol Rep (2016) 35(1):524–9. doi: 10.3892/or.2015.4332 [DOI] [PubMed] [Google Scholar]
- 148. Fanta CH. Asthma. N Engl J Med (2009) 360(10):1002–14. doi: 10.1056/NEJMra0804579 [DOI] [PubMed] [Google Scholar]
- 149. Wang J, Shang YX, Cai XX, Liu LY. Vasoactive Intestinal Peptide Inhibits Airway Smooth Muscle Cell Proliferation in a Mouse Model of Asthma via the ERK1/2 Signaling Pathway. Exp Cell Res (2018) 364(2):168–74. doi: 10.1016/j.yexcr.2018.01.042 [DOI] [PubMed] [Google Scholar]
- 150. Alashkar Alhamwe B, Alhamdan F, Ruhl A, Potaczek DP, Renz H. The Role of Epigenetics in Allergy and Asthma Development. Curr Opin Allergy Clin Immunol (2020) 20(1):48–55. doi: 10.1097/aci.0000000000000598 [DOI] [PubMed] [Google Scholar]
- 151. Gomez JL. Epigenetics in Asthma. Curr Allergy Asthma Rep (2019) 19(12):56. doi: 10.1007/s11882-019-0886-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Long A, Bunning B, Sampath V, DeKruyff RH, Nadeau KC. Epigenetics and the Environment in Airway Disease: Asthma and Allergic Rhinitis. Adv Exp Med Biol (2020) 1253:153–81. doi: 10.1007/978-981-15-3449-2_6 [DOI] [PubMed] [Google Scholar]
- 153. Cheng Q, Shang Y, Huang W, Zhang Q, Li X, Zhou Q. P300 Mediates the Histone Acetylation of ORMDL3 to Affect Airway Inflammation and Remodeling in Asthma. Int Immunopharmacol (2019) 76:105885. doi: 10.1016/j.intimp.2019.105885 [DOI] [PubMed] [Google Scholar]
- 154. McErlean P, Kelly A, Dhariwal J, Kirtland M, Watson J, Ranz I, et al. Profiling of H3K27Ac Reveals the Influence of Asthma on the Epigenome of the Airway Epithelium. Front Genet (2020) 11:585746. doi: 10.3389/fgene.2020.585746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Yu Q, Yu X, Zhao W, Zhu M, Wang Z, Zhang J, et al. Inhibition of H3K27me3 Demethylases Attenuates Asthma by Reversing the Shift in Airway Smooth Muscle Phenotype. Clin Exp Allergy (2018) 48(48):1439–52. doi: 10.1111/cea.13244 [DOI] [PubMed] [Google Scholar]
- 156. Royce SG, Karagiannis TC. Histone Deacetylases and Their Role in Asthma. J Asthma (2012) 49(2):121–8. doi: 10.3109/02770903.2011.648298 [DOI] [PubMed] [Google Scholar]
- 157. Gunawardhana LP, Gibson PG, Simpson JL, Powell H, Baines KJ. Activity and Expression of Histone Acetylases and Deacetylases in Inflammatory Phenotypes of Asthma. Clin Exp Allergy (2014) 44(1):47–57. doi: 10.1111/cea.12168 [DOI] [PubMed] [Google Scholar]
- 158. Islam R, Dash D, Singh R. Intranasal Curcumin and Sodium Butyrate Modulates Airway Inflammation and Fibrosis via HDAC Inhibition in Allergic Asthma. Cytokine (2022) 149:155720. doi: 10.1016/j.cyto.2021.155720 [DOI] [PubMed] [Google Scholar]
- 159. Xia M, Xu H, Dai W, Zhu C, Wu L, Yan S, et al. The Role of HDAC2 in Cigarette Smoke-Induced Airway Inflammation in a Murine Model of Asthma and the Effect of Intervention With Roxithromycin. J Asthma (2018) 55(4):337–44. doi: 10.1080/02770903.2017.1337788 [DOI] [PubMed] [Google Scholar]
- 160. Liu F, Shang YX. Sirtuin 6 Attenuates Epithelial-Mesenchymal Transition by Suppressing the TGF-β1/Smad3 Pathway and C-Jun in Asthma Models. Int Immunopharmacol (2020) 82:106333. doi: 10.1016/j.intimp.2020.106333 [DOI] [PubMed] [Google Scholar]
- 161. Lai T, Su G, Wu D, Chen Z, Chen Y, Yi H, et al. Myeloid-Specific SIRT1 Deletion Exacerbates Airway Inflammatory Response in a Mouse Model of Allergic Asthma. Aging (Albany NY) (2021) 13(11):15479–90. doi: 10.18632/aging.203104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Simpson JL, Scott R, Boyle MJ, Gibson PG. Inflammatory Subtypes in Asthma: Assessment and Identification Using Induced Sputum. Respirology (2006) 11(1):54–61. doi: 10.1111/j.1440-1843.2006.00784.x [DOI] [PubMed] [Google Scholar]
- 163. Speizer FE, Ware JH. Exploring Different Phenotypes of COPD. N Engl J Med (2015) 373(2):185–6. doi: 10.1056/NEJMe1503888 [DOI] [PubMed] [Google Scholar]
- 164. Hoenderdos K, Condliffe A. The Neutrophil in Chronic Obstructive Pulmonary Disease. Am J Respir Cell Mol Biol (2013) 48(5):531–9. doi: 10.1165/rcmb.2012-0492TR [DOI] [PubMed] [Google Scholar]
- 165. Liu T, Wang FP, Wang G, Mao H. Role of Neutrophil Extracellular Traps in Asthma and Chronic Obstructive Pulmonary Disease. Chin Med J (Engl) (2017) 130(6):730–6. doi: 10.4103/0366-6999.201608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Zhang L, Valizadeh H, Alipourfard I, Bidares R, Aghebati-Maleki L, Ahmadi M. Epigenetic Modifications and Therapy in Chronic Obstructive Pulmonary Disease (COPD): An Update Review. Copd (2020) 17(3):333–42. doi: 10.1080/15412555.2020.1780576 [DOI] [PubMed] [Google Scholar]
- 167. Gan Q, Tang D, Yan Q, Chen J, Xu Y, Xue W, et al. Differential Expression Study of Lysine Crotonylation and Proteome for Chronic Obstructive Pulmonary Disease Combined With Type II Respiratory Failure. Can Respir J (2021) 2021:6652297. doi: 10.1155/2021/6652297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Shanmugam G, Rakshit S, Sarkar K. HDAC Inhibitors: Targets for Tumor Therapy, Immune Modulation and Lung Diseases. Transl Oncol (2021) 16:101312. doi: 10.1016/j.tranon.2021.101312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Jiang Y, Zhao Y, Wang Q, Chen H, Zhou X. Fine Particulate Matter Exposure Promotes M2 Macrophage Polarization Through Inhibiting Histone Deacetylase 2 in the Pathogenesis of Chronic Obstructive Pulmonary Disease. Ann Transl Med (2020) 8(20):1303. doi: 10.21037/atm-20-6653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Sundar IK, Rashid K, Gerloff J, Rangel-Moreno J, Li D, Rahman I. Genetic Ablation of Histone Deacetylase 2 Leads to Lung Cellular Senescence and Lymphoid Follicle Formation in COPD/Emphysema. FASEB J (2018) 32(9):4955–71. doi: 10.1096/fj.201701518R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Riordan JR. CFTR Function and Prospects for Therapy. Annu Rev Biochem (2008) 77:701–26. doi: 10.1146/annurev.biochem.75.103004.142532 [DOI] [PubMed] [Google Scholar]
- 172. Fahy JV, Dickey BF. Airway Mucus Function and Dysfunction. N Engl J Med (2010) 363(23):2233–47. doi: 10.1056/NEJMra0910061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Sirinupong N, Yang Z. Epigenetics in Cystic Fibrosis: Epigenetic Targeting of a Genetic Disease. Curr Drug Targets (2015) 16(9):976–87. doi: 10.2174/1389450116666150416114514 [DOI] [PubMed] [Google Scholar]
- 174. Anglès F, Hutt DM, Balch WE. HDAC Inhibitors Rescue Multiple Disease-Causing CFTR Variants. Hum Mol Genet (2019) 28(12):1982–2000. doi: 10.1093/hmg/ddz026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Rymut SM, Harker A, Corey DA, Burgess JD, Sun H, Clancy JP, et al. Reduced Microtubule Acetylation in Cystic Fibrosis Epithelial Cells. Am J Physiol Lung Cell Mol Physiol (2013) 305(6):L419–31. doi: 10.1152/ajplung.00411.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Rymut SM, Corey DA, Valerio DM, Erokwu BO, Flask CA, Kelley TJ, et al. Improved Growth Patterns in Cystic Fibrosis Mice After Loss of Histone Deacetylase 6. Sci Rep (2017) 7(1):3676. doi: 10.1038/s41598-017-03931-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Rosenjack J, Hodges CA, Darrah RJ, Kelley TJ. HDAC6 Depletion Improves Cystic Fibrosis Mouse Airway Responses to Bacterial Challenge. Sci Rep (2019) 9(1):10282. doi: 10.1038/s41598-019-46555-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Bodas M, Mazur S, Min T, Vij N. Inhibition of Histone-Deacetylase Activity Rescues Inflammatory Cystic Fibrosis Lung Disease by Modulating Innate and Adaptive Immune Responses. Respir Res (2018) 19(1):2. doi: 10.1186/s12931-017-0705-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Pankow S, Bamberger C, Calzolari D, Martínez-Bartolomé S, Lavallée-Adam M, Balch WE, et al. ΔF508 CFTR Interactome Remodelling Promotes Rescue of Cystic Fibrosis. Nature (2015) 528(7583):510–6. doi: 10.1038/nature15729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Kaplan GG. The Global Burden of IBD: From 2015 to 2025. Nat Rev Gastroenterol Hepatol (2015) 12(12):720–7. doi: 10.1038/nrgastro.2015.150 [DOI] [PubMed] [Google Scholar]
- 181. Ananthakrishnan AN. Environmental Risk Factors for Inflammatory Bowel Diseases: A Review. Dig Dis Sci (2015) 60(2):290–8. doi: 10.1007/s10620-014-3350-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Cleynen I, Boucher G, Jostins L, Schumm LP, Zeissig S, Ahmad T, et al. Inherited Determinants of Crohn’s Disease and Ulcerative Colitis Phenotypes: A Genetic Association Study. Lancet (2016) 387(10014):156–67. doi: 10.1016/s0140-6736(15)00465-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Rapozo DC, Bernardazzi C, de Souza HS. Diet and Microbiota in Inflammatory Bowel Disease: The Gut in Disharmony. World J Gastroenterol (2017) 23(12):2124–40. doi: 10.3748/wjg.v23.i12.2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Ray G, Longworth MS. Epigenetics, DNA Organization, and Inflammatory Bowel Disease. Inflammation Bowel Dis (2019) 25(2):235–47. doi: 10.1093/ibd/izy330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Wawrzyniak M, Scharl M. Genetics and Epigenetics of Inflammatory Bowel Disease. Swiss Med Wkly (2018) 148:w14671. doi: 10.4414/smw.2018.14671 [DOI] [PubMed] [Google Scholar]
- 186. Hornschuh M, Wirthgen E, Wolfien M, Singh KP, Wolkenhauer O, Däbritz J. The Role of Epigenetic Modifications for the Pathogenesis of Crohn’s Disease. Clin Epigenet (2021) 13(1):108. doi: 10.1186/s13148-021-01089-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Chen Q, Duan X, Xu M, Fan H, Dong Y, Wu H, et al. BMSC-EVs Regulate Th17 Cell Differentiation in UC via H3k27me3. Mol Immunol (2020) 118:191–200. doi: 10.1016/j.molimm.2019.12.019 [DOI] [PubMed] [Google Scholar]
- 188. Liu M, Ding J, Zhang H, Shen J, Hao Y, Zhang X, et al. Lactobacillus Casei LH23 Modulates the Immune Response and Ameliorates DSS-Induced Colitis via Suppressing JNK/p-38 Signal Pathways and Enhancing Histone H3K9 Acetylation. Food Funct (2020) 11(6):5473–85. doi: 10.1039/d0fo00546k [DOI] [PubMed] [Google Scholar]
- 189. Ali MN, Choijookhuu N, Takagi H, Srisowanna N, Nguyen Nhat Huynh M, Yamaguchi Y, et al. The HDAC Inhibitor, SAHA, Prevents Colonic Inflammation by Suppressing Pro-Inflammatory Cytokines and Chemokines in DSS-Induced Colitis. Acta Histochem Cytochem (2018) 51(1):33–40. doi: 10.1267/ahc.17033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Friedrich M, Gerbeth L, Gerling M, Rosenthal R, Steiger K, Weidinger C, et al. HDAC Inhibitors Promote Intestinal Epithelial Regeneration via Autocrine Tgfβ1 Signalling in Inflammation. Mucosal Immunol (2019) 12(3):656–67. doi: 10.1038/s41385-019-0135-7 [DOI] [PubMed] [Google Scholar]
- 191. Gerbeth L, Glauben R. Histone Deacetylases in the Inflamed Intestinal Epithelium-Promises of New Therapeutic Strategies. Front Med (Lausanne) (2021) 8:655956. doi: 10.3389/fmed.2021.655956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Felice C, Lewis A, Armuzzi A, Lindsay JO, Silver A. Review Article: Selective Histone Deacetylase Isoforms as Potential Therapeutic Targets in Inflammatory Bowel Diseases. Aliment Pharmacol Ther (2015) 41(1):26–38. doi: 10.1111/apt.13008 [DOI] [PubMed] [Google Scholar]
- 193. Fonseca-Camarillo G, Furuzawa-Carballeda J, Yamamoto-Furusho JK. Interleukin 35 (IL-35) and IL-37: Intestinal and Peripheral Expression by T and B Regulatory Cells in Patients With Inflammatory Bowel Disease. Cytokine (2015) 75(2):389–402. doi: 10.1016/j.cyto.2015.04.009 [DOI] [PubMed] [Google Scholar]
- 194. Wetzel A, Scholtka B, Gerecke C, Kleuser B. Epigenetic Histone Modulation Contributes to Improvements in Inflammatory Bowel Disease via EBI3. Cell Mol Life Sci (2020) 77(23):5017–30. doi: 10.1007/s00018-020-03451-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Woller N, Kuhnel F. Virus Infection, Inflammation and Prevention of Cancer. Recent Results Cancer Res (2014) 193:33–58. doi: 10.1007/978-3-642-38965-8_3 [DOI] [PubMed] [Google Scholar]
- 196. Balakrishnan L, Milavetz B. Epigenetic Regulation of Viral Biological Processes. Viruses (2017) 9(11):346. doi: 10.3390/v9110346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Costa C, Cavallo R. Polyomavirus-Associated Nephropathy. World J Transplant (2012) 2(6):84–94. doi: 10.5500/wjt.v2.i6.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Pietropaolo V, Prezioso C, Moens U. Merkel Cell Polyomavirus and Merkel Cell Carcinoma. Cancers (Basel) (2020) 12(7):1774. doi: 10.3390/cancers12071774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Balakrishnan L, Milavetz B. Histone Hyperacetylation in the Coding Region of Chromatin Undergoing Transcription in SV40 Minichromosomes Is a Dynamic Process Regulated Directly by the Presence of RNA Polymerase II. J Mol Biol (2007) 365(1):18–30. doi: 10.1016/j.jmb.2006.09.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Talasz H, Lindner HH, Sarg B, Helliger W. Histone H4-Lysine 20 Monomethylation Is Increased in Promoter and Coding Regions of Active Genes and Correlates With Hyperacetylation. J Biol Chem (2005) 280(46):38814–22. doi: 10.1074/jbc.M505563200 [DOI] [PubMed] [Google Scholar]
- 201. Kourmouli N, Jeppesen P, Mahadevhaiah S, Burgoyne P, Wu R, Gilbert DM, et al. Heterochromatin and Tri-Methylated Lysine 20 of Histone H4 in Animals. J Cell Sci (2004) 117(Pt 12):2491–501. doi: 10.1242/jcs.01238 [DOI] [PubMed] [Google Scholar]
- 202. Sarg B, Helliger W, Talasz H, Koutzamani E, Lindner HH. Histone H4 Hyperacetylation Precludes Histone H4 Lysine 20 Trimethylation. J Biol Chem (2004) 279(51):53458–64. doi: 10.1074/jbc.M409099200 [DOI] [PubMed] [Google Scholar]
- 203. Pietropaolo V, Prezioso C, Moens U. Role of Virus-Induced Host Cell Epigenetic Changes in Cancer. Int J Mol Sci (2021) 22(15):8346. doi: 10.3390/ijms22158346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Bravo IG, Félez-Sánchez M. Papillomaviruses: Viral Evolution, Cancer and Evolutionary Medicine. Evol Med Public Health (2015) 2015(1):32–51. doi: 10.1093/emph/eov003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Sadri Nahand J, Moghoofei M, Salmaninejad A, Bahmanpour Z, Karimzadeh M, Nasiri M, et al. Pathogenic Role of Exosomes and microRNAs in HPV-Mediated Inflammation and Cervical Cancer: A Review. Int J Cancer (2020) 146(2):305–20. doi: 10.1002/ijc.32688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Burley M, Roberts S, Parish JL. Epigenetic Regulation of Human Papillomavirus Transcription in the Productive Virus Life Cycle. Semin Immunopathol (2020) 42(2):159–71. doi: 10.1007/s00281-019-00773-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Nguyen HP, Ramirez-Fort MK, Rady PL. The Biology of Human Papillomaviruses. Curr Probl Dermatol (2014) 45:19–32. doi: 10.1159/000355959 [DOI] [PubMed] [Google Scholar]
- 208. Kim SH, Oh JM, No JH, Bang YJ, Juhnn YS, Song YS. Involvement of NF-kappaB and AP-1 in COX-2 Upregulation by Human Papillomavirus 16 E5 Oncoprotein. Carcinogenesis (2009) 30(5):753–7. doi: 10.1093/carcin/bgp066 [DOI] [PubMed] [Google Scholar]
- 209. Akira S, Uematsu S, Takeuchi O. Pathogen Recognition and Innate Immunity. Cell (2006) 124(4):783–801. doi: 10.1016/j.cell.2006.02.015 [DOI] [PubMed] [Google Scholar]
- 210. Patel D, Huang S-M, Baglia LA, McCance DJ. The E6 Protein of Human Papillomavirus Type16 Binds to an Inhibites Co-Acitvatin by Cbp and P300. EMBO J (1999) 18:5061–72. doi: 10.1093/emboj/18.18.5061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Kany S, Vollrath JT, Relja B. Cytokines in Inflammatory Disease. Int J Mol Sci (2019) 20(23):6008. doi: 10.3390/ijms20236008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Chen PM, Cheng YW, Wang YC, Wu TC, Chen CY, Lee H. Up-Regulation of FOXM1 by E6 Oncoprotein Through the MZF1/NKX2-1 Axis Is Required for Human Papillomavirus-Associated Tumorigenesis. Neoplasia (2014) 16(11):961–71. doi: 10.1016/j.neo.2014.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Nichol JN, Dupere-Richer D, Ezponda T, Licht JD, Miller WH., Jr. H3K27 Methylation: A Focal Point of Epigenetic Deregulation in Cancer. Adv Cancer Res (2016) 131:59–95. doi: 10.1016/bs.acr.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Hyland PL, McDade SS, McCloskey R, Dickson GJ, Arthur K, McCance DJ, et al. Human Papillomavirus E7 Oncoprotein Induces KDM6A and KDM6B Histone Demethylase Expression and Causes Epigenetic Reprogramming. J Virol (2011) 85(21):10999–1006. doi: 10.1128/JVI.00160-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Belloni L, Pollicino T, De Nicola F, Guerrieri F, Raffa G, Fanciulli M, et al. Nuclear HBx Binds the HBV Minichromosome and Modifies the Epigenetic Regulation of cccDNA Function. Proc Natl Acad Sci USA (2009) 106(47):19975–9. doi: 10.1073/pnas.0908365106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Dandri M. Epigenetic Modulation in Chronic Hepatitis B Virus Infection. Semin Immunopathol (2020) 42(2):173–85. doi: 10.1007/s00281-020-00780-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Huang CR, Lo SJ. Hepatitis D Virus Infection, Replication and Cross-Talk With the Hepatitis B Virus. World J Gastroenterol (2014) 20(40):14589–97. doi: 10.3748/wjg.v20.i40.14589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Jeong SW, Jang JY, Chung RT. Hepatitis C Virus and Hepatocarcinogenesis. Clin Mol Hepatol (2012) 18(4):347–56. doi: 10.3350/cmh.2012.18.4.347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Zhou P, Xia J, Zhou YJ, Wan J, Li L, Bao J, et al. Proportions of Acetyl-Histone-Positive Hepatocytes Indicate the Functional Status and Prognosis of Cirrhotic Patients. World J Gastroenterol (2015) 21(21):6665–74. doi: 10.3748/wjg.v21.i21.6665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Cai MY, Tong ZT, Zheng F, Liao YJ, Wang Y, Rao HL, et al. EZH2 Protein: A Promising Immunomarker for the Detection of Hepatocellular Carcinomas in Liver Needle Biopsies. Gut (2011) 60(7):967–76. doi: 10.1136/gut.2010.231993 [DOI] [PubMed] [Google Scholar]
- 221. Deeks SG, Lewin SR, Ross AL, Ananworanich J, Benkirane M, Cannon P, et al. International AIDS Society Global Scientific Strategy: Towards an HIV Cure 2016. Nat Med (2016) 22(8):839–50. doi: 10.1038/nm.4108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Abdel-Hameed EA, Ji H, Shata MT. HIV-Induced Epigenetic Alterations in Host Cells. Adv Exp Med Biol (2016) 879:27–38. doi: 10.1007/978-3-319-24738-0_2 [DOI] [PubMed] [Google Scholar]
- 223. Williams SA, Chen L-F, Kwon H, Ruiz-Jarabo CM, Verdin E, Greene WC. NFkB P50 Promotes HIV Latency Through HDAC Recruitment and Repression of Transcriptional Initiation. EMBO J (2005) 25:139–49. doi: 10.1038/sj.emboj.7600900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Marban C, Suzanne S, Dequiedt F, de Walque S, Redel L, Van Lint C, et al. Recruitment of Chromatin-Modifying Enzymes by CTIP2 Promotes HIV-1 Transcriptional Silencing. EMBO J (2007) 26(2):412–23. doi: 10.1038/sj.emboj.7601516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Rohr O, Marban C, Aunis D, Schaeffer E. Regulation of HIV-1 Gene Transcription: From Lymphocytes to Microglial Cells. J Leukoc Biol (2003) 74(5):736–49. doi: 10.1189/jlb.0403180 [DOI] [PubMed] [Google Scholar]
- 226. Spivak AM, Planelles V. Novel Latency Reversal Agents for HIV-1 Cure. Annu Rev Med (2018) 69:421–36. doi: 10.1146/annurev-med-052716031710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Barnes PJ. Role of HDAC2 in the Pathophysiology of COPD. Annu Rev Physiol (2009) 71:451–64. doi: 10.1146/annurev.physiol.010908.163257 [DOI] [PubMed] [Google Scholar]
- 228. Pinto BGG, Oliveira AER, Singh Y, Jimenez L, Goncalves ANA, Ogava RLT, et al. ACE2 Expression Is Increased in the Lungs of Patients With Comorbidities Associated With Severe COVID-19. J Infect Dis (2020) 222(4):556–63. doi: 10.1093/infdis/jiaa332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Promislow DEL. A Geroscience Perspective on COVID-19 Mortality. J Gerontol A Biol Sci Med Sci (2020) 75(9):e30–3. doi: 10.1093/gerona/glaa094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Feser J, Tyler J. Chromatin Structure as a Mediator of Aging. FEBS Lett (2011) 585(13):2041–8. doi: 10.1016/j.febslet.2010.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]