Abstract
The values of the pharmacokinetic parameters of the nucleoside antiretroviral agent β-d-2′,3′-didehydro-2′,3′-dideoxy-5-fluorocytidine (D-D4FC) in rhesus monkeys were determined with a two-compartment model after the administration of a single dose. The average values for the terminal half-life, renal clearance, and total systemic clearance for the intravenous administration route were 3.6 h and 0.31 and 0.43 liter · kg−1 · h−1, respectively. The oral bioavailability of D-D4FC averaged 41%. For the intravenous administration route, 76% of the compound was recovered intact in the urine within 8 h, indicating that D-D4FC was eliminated mainly by renal excretion. D-D4FC was detected in the cerebrospinal fluid (CSF) at similar concentrations after administration by both the intravenous and oral routes. D-D4FC levels in plasma and CSF were higher than the median effective concentration for human immunodeficiency virus type 1 in vitro.
Advances in the therapy of human immunodeficiency virus type 1 (HIV-1) and hepatitis B virus (HBV) have produced numerous compounds that are selective and effective in vitro, in animal models, and in humans. Pharmacokinetic studies with relevant animal models prior to administration to humans are critical in determining the pharmacological characteristics necessary for the treatment of chronic infections such as those with HIV-1 and HBV, which necessitate prolonged treatment. Favorable characteristics for anti-HIV agents developed for clinical use include a relatively long half-life, good oral bioavailability, metabolic stability, and penetration into the central nervous system.
β-d-2′,3′-Didehydro-2′,3′-dideoxy-5-fluorocytidine (D-D4FC) has potent antiviral activity in vitro and in animal models (5, 11, 12, 15). Of significance is the finding that D-D4FC does not demonstrate significant cross-resistance to any of the licensed antiretroviral agents (12). D-D4FC was previously studied in woodchucks and was found to have a favorable pharmacokinetic profile with a long half-life (4.71 and 10.75 h after administration by the intravenous [i.v.] and oral [p.o.] routes, respectively) and good oral bioavailability (F), averaging 34.1% (8). The objectives of this study were to determine the values of the pharmacokinetic parameters of D-D4FC in rhesus monkeys after the i.v. and p.o. administration of a single dose. Rhesus monkeys were selected since nucleosides generally behave similarly in rhesus monkeys and humans (1, 10).
The synthesis and chemical characterization of D-D4FC will be reported elsewhere (13). The purity of this compound was ≥99.99% as determined by high-pressure liquid chromatography (HPLC). The internal standard, 2′,3′-didehydro-3′-deoxythymidine (D-D4T), was synthesized as reported previously (2). For i.v. and p.o. administration, D-D4FC was dissolved in phosphate-buffered saline (PBS) and water, respectively. HPLC-grade acetonitrile and all other chemicals (analytical grade) were obtained from Fisher Scientific (Fair Lawn, N.J.).
Three 3-year-old male rhesus monkeys (Macaca mulatta) weighing 4.5 to 5 kg were used for the pharmacokinetic studies, and one monkey served as an untreated control animal. The animals were maintained at the Yerkes Regional Primate Research Center of Emory University, which is fully accredited by the American Association for Accreditation of Laboratory Animal Care, in accordance with guidelines established by the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (9a). For comparison with previously reported studies by our group, monkeys were administered 33.3 mg of D-D4FC per kg of body weight i.v. (in 10 ml of PBS). After a 3-week washout period, immediately after p.o. administration of 10 ml of 1 M NaHCO3, they received 33.3 mg/kg p.o. in a total volume of 10 ml of water by gastric intubation followed by 3 ml of a water flush. A control animal was administered PBS or water without drug. Animals were maintained under anesthesia for 4 h after drug administration with a mixture of ketamine HCl (60 mg) and tiletamine HCl plus zolazepam HCl (Telazol; 20 mg) administered intramuscularly. Animals were monitored for alertness and were given additional anesthesia (30 to 60 mg of ketamine HCl) as necessary. Animals were maintained most of the time on their sides on a warm heating pad and were covered with a blanket. Blood samples were taken prior to and at 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, and 24 h after drug administration through the femoral vein while the animals were lying on their backs. Cerebrospinal fluid (CSF) samples were taken from all treated monkeys at 0.5, 1, 2, and 3 h after drug administration by cisternal or lumbar tap with a 22-gauge needle. The monkeys were catheterized for urine collection. Urine samples were collected at 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, and 8 h after dosing. Plasma, CSF, and urine samples were frozen at −70°C until analysis.
D-D4FC concentrations in biological fluids were measured by HPLC. Five hundred microliters of acetonitrile was added to a 50-μl plasma sample in a microcentrifuge tube, and 50 μl of D-D4T (20 μg/ml) was added as an internal standard before being thoroughly vortexed and centrifuged at 11,200 × g for 5 min. The supernatant was evaporated to dryness in a Speed Vac model SC 110 concentrator (Forma Scientific, Inc., Marietta, Ohio). Samples were reconstituted with 200 μl of 2% acetonitrile in sodium phosphate buffer (pH 7), and 50 μl was injected onto the HPLC column. For CSF samples, 50 μl of CSF sample was added to 50 μl of internal standard (D-D4T; 1 μg/ml), and 50 μl was injected onto a reverse-phase high-pressure liquid chromatograph for analysis. For urine samples, 10 μl of urine was added to 50 μl of internal standard (D-D4T; 200 μg/ml) and 940 μl of 2% acetonitrile sodium phosphate buffer (pH 7). A 50-μl sample was injected onto the HPLC column.
HPLC was performed with a Waters HPLC system (Millipore Corporation, Milford, Mass.) equipped with a model 600 controller, a model 996 photodiode array detector, and a model 717plus autosampler. Millennium 2010 software (Millipore Corporation) was used for system control, data acquisition, and processing. Chromatography was performed on a Whatman PartiSphere C18 column (4.6 by 250 mm; particle diameter, 5 μm; Whatman Inc., Clifton, N.J.). The mobile phase was a linear gradient from 100% A (2% acetonitrile in water) to 85% A and 15% B (100% acetonitrile) in 12 min; this phase was then held for 3 min before it was changed to 100% mobile phase B. The flow rate was maintained at 1.5 ml/min. The compounds were detected at a UV wavelength of 280 nm. The D-D4FC concentrations in the samples were determined from the slope of the standard curve of the ratio of the peak area for D-D4FC to the peak area for D-D4T versus the standard D-D4FC concentration. The range of linearity for D-D4FC was 0.02 to 100 μg/ml. The limits of quantitation of the analytical method for D-D4FC were 0.02, 0.02, and 1 μg/ml for CSF, plasma, and urine samples, respectively. The accuracies of the assay methods were greater than 96%. The intra- and interday relative standard deviations at low, medium, and high concentrations were less than 8%.
The stability of D-D4FC was determined under various pH conditions. After 4 h of incubation in pH 4 buffer at room temperature, the peak area of D-D4FC decreased by 49%, with a new peak detected at 3.2 min. This peak corresponded to fluorocytosine (5-FC) when it was compared to the retention time and UV spectrum of standard 5-FC. The half-life for D-D4FC at pH 4 was 3.8 h. When D-D4FC was incubated with PBS buffer (pH 7.4) at room temperature, only 2% was converted to 5-FC after 1 week. The half-life for D-D4FC at pH 7.4 was 253 days. D-D4FC is completely stable in basic solution for at least 3 h, with no detectable breakdown to 5-FC. D-D4FC was also found to be stable in human whole blood when it was incubated in human whole blood at 37°C for 16 h. D-D4FC is unstable in acid, because under these conditions the formation of the base is thermodynamically favored due to the more stable oxonium ion, and 5-FC is released. This instability has been observed in animals and humans treated with the chemically related, clinically approved compound D-D4T (stavudine) (3, 14). Therefore, in clinical practice, oral D-D4FC should be given with an antacid agent or in a buffered solution or the compound should be formulated to protect it from gastric acids. No protection from acid is necessary if the compound is administered intravenously since D-D4FC is stable in human blood.
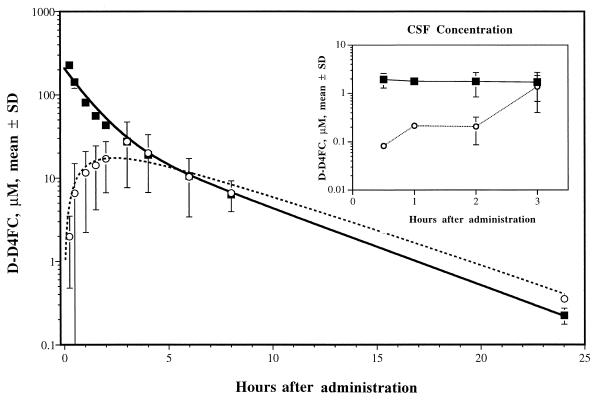
The data for the three monkeys after i.v. and p.o. administration were fit simultaneously to a two-compartment open mammillary pharmacokinetic model (6) with a nonlinear least-squares curve-fitting program (WinNonlin, version 01.5A, 1997; Scientific Consulting, Inc., Apex, N.C.). Initial estimates for model parameters were obtained by resolving the curves for individual animals into sums of exponentials by the method of residuals (7). A weighting factor of 1/(predicted value)2 was used for the fitting. This method permits the first-order oral absorption rate constant (ka) and F to be estimated simultaneously with the two-compartment parameters. The equation used for oral absorption allowed for a lag time (tlag) between the time of administration and the onset of absorption. The resulting model parameters, together with the corresponding model-independent parameters, are contained in Table 1. Model fit to averaged data and the concentrations observed in rhesus monkeys for a single dose of 33.3 mg of D-D4FC per kg given both i.v. and p.o. is shown in Fig. 1. The average values for the distribution and elimination half-lives (t1/2α and t1/2β, respectively) were 0.7 and 3.6 h, respectively. The fraction of compound absorbed (F) by the oral route ranged from 0.24 to 0.49 (average, 0.41), indicating a varied F, with close to half the p.o. dose of D-D4FC reaching the systemic circulation. The maximum concentration of drug in serum (Cmax) and the time to Cmax (Tmax) ranged from 21.1 to 47.5 μM and 1 to 4 h, respectively, with no inverse correlation between these two parameters. The highest Cmax (47.5 μM) corresponded to a Tmax of 3 h, while the lowest Cmax (21.1 μM) corresponded to a Tmax of 1 h. Absorption rates (ka) ranged from 0.50 to 0.86 h−1 (average, 0.6 h−1), and mean absorption times (MATs) were between 2.7 and 3.4 h (average, 3.1 h). Variations in the calculated MATs for the data for the p.o. route of administration but not the data for the i.v. route of administration (range, 2.9 to 3.1 h), together with the need for a tlag to model two of the three sets of data for the p.o. route of administration, suggests that differences in gastric emptying times may be partially responsible for the variance in the concentrations in plasma achieved in these animals after p.o. dosing. However, other gastrointestinal tract factors have not been ruled out.
TABLE 1.
Values of pharmacokinetic parameters of D-D4FC derived from monkeys given 33.3 mg/kg by i.v. or p.o. route
| Route and model parametera | Mean (n = 3) | Minimum | Maximum | Fit to averaged data |
|---|---|---|---|---|
| i.v. route | ||||
| t1/2α (h) | 0.69 | 0.61 | 0.83 | 0.77 |
| t1/2β (h) | 3.57 | 3.46 | 3.75 | 3.26 |
| % AUC from α phase | 53.5 | 46.3 | 57.8 | 53.2 |
| % AUC from β phase | 46.5 | 42.2 | 53.7 | 46.8 |
| k10 (h−1) | 0.63 | 0.57 | 0.69 | 0.58 |
| k12 (h−1) | 0.27 | 0.17 | 0.34 | 0.20 |
| k21 (h−1) | 0.31 | 0.29 | 0.36 | 0.33 |
| Vcentral (liter · kg−1) | 0.68 | 0.58 | 0.79 | 0.70 |
| VSS (liter · kg−1) | 1.24 | 1.16 | 1.32 | 1.13 |
| CLsys (liter · kg−1 · h−1) | 0.43 | 0.40 | 0.45 | 0.40 |
| AUC (μM · h) | 345.5 | 327.4 | 363.9 | 363.5 |
| AUMC (μM · h−2) | 1,014.1 | 917.1 | 1,075.7 | 1,017.3 |
| MRTi.v. (h) | 2.93 | 2.80 | 3.12 | 2.80 |
| r (n) | 0.973 (10) | 0.937 (10) | 0.994 (10) | 0.970 |
| CLR (liter · kg−1 · h−1) | 0.31 | 0.27 | 0.35 | |
| p.o. route | ||||
| ka (h−1) | 0.64 | 0.50 | 0.86 | 0.26 |
| tlag (h) | 0.88 | 0.00 | 1.83 | 0.00 |
| F | 0.41 | 0.24 | 0.49 | 0.41 |
| r | 0.94 | 0.90 | 0.99 | 0.90 |
| Cmax (μM) | 33.4 | 21.1 | 47.5 | |
| Tmax | 2.67 | 1.00 | 4.00 | |
| AUC (μM · h) | 172.0 | 104.6 | 211.7 | |
| AUMC (μM · h2) | 1,029.8 | 669.7 | 1,242.1 | |
| MRT p.o. (h) | 6.06 | 5.56 | 6.40 | |
| MAT (h) | 3.13 | 2.68 | 3.42 |
The values of the model parameters were determined by simultaneously fitting the data for plasma following administration by the i.v. and oral routes to a two-compartment model. The model parameters included t1/2α and t1/2β, the respective disposition half-lives; k10 is the elimination rate constant from the central compartment; k12 and k21 are the disposition rate constants between the central and peripheral compartment; Vcentral is the distribution volume of the central compartment; VSS is the distribution volume once the compound has distributed throughout animal; CLR and CLsys are the renal and systemic clearances, respectively; Cmax is the observed maximum concentration in plasma, while Tmax is the time to Cmax. F is the fraction of the oral dose of compound absorbed. CLR was calculated for the i.v. dose as the ratio of compound recovered in the urine/AUC. AUC and AUMC values are the area under zero and first moment plasma concentration-versus-time curves, respectively; MRT is the mean residence time; r is the correlation coefficient, and n is the number of observation points. ka is the first-order oral absorption rate constant. The mean absorption time (MAT) was calculated as the difference between each animal’s own MRT after p.o. administration and the corresponding MRT after i.v. administration (4). CLR was calculated for the i.v. dose as the ratio of compound recovered in the urine to the corresponding AUC. CLR was not calculated for the oral dose, since renal excretion had not yet plateaued at the end of the urine collection interval.
FIG. 1.
Model fits to averaged data and observed concentrations in rhesus monkey plasma after the administration of a single dose of 33.3 mg of D-D4FC per kg by the i.v. (squares) and p.o. (circles) routes. The corresponding concentrations in CSF are depicted in the inset.
Drug concentrations in urine were measured from 0.25 to 8 h after i.v. and p.o. administration of 33.3 mg/kg. In contrast to D-D4T, only about half of the dose of which was recovered in the urine of monkeys (3), 76% of the original dose of D-D4FC was recovered in the urine within 8 h after i.v. administration. Average values for renal clearance (CLR) and for total systemic clearance (CLsys) were 0.31 and 0.43 liter · kg−1 · h−1, respectively. The fraction of the compound recovered in the urine was not calculated for monkeys receiving the p.o. dose, since the amount accumulated in the urine had not plateaued within the 8 h of collection, although by 8 h 25% unchanged D-D4FC was recovered. The high fraction of drug recovered in the urine indicates that D-D4FC was eliminated mainly by renal excretion after it was administered by the i.v. route. Since the kidneys are well-perfused organs and are likely to be in equilibrium with the central compartment, the assumption that elimination is from the central compartment appears to be valid. Similarities in the plasma concentration-versus-time profiles indicated similar disposition rate constants and mean residence times (Table 1), suggesting that drug disposition did not vary significantly between monkeys.
Previous studies with rhesus monkeys treated with 3′-azido-3′-deoxythymidine (AZT) (1), 3′-fluoro-3′-deoxythymidine (FLT), and D-D4T (10) indicated that glucuronidation of AZT and FLT was the primary mechanism of clearance of the drugs in monkeys, and the concentration of D4T glucuronide metabolite in urine was highly variable, with no glucuronide detected in one-half of the urine samples. Cretton et al. (3) also reported that no glucuronidation of D-D4T was found in rhesus monkeys. Determination of whether 5′-glucuronide formation can occur involves hydrolysis of the glucuronide to the nucleoside with β-glucuronidase under strong acidic conditions (9), which may degrade antiviral agents such as D-D4FC and D4T. Due to the confirmed instability of D-D4FC under acidic conditions, we are unable to determine if D-D4FC is excreted in the urine as a glucuronide metabolite. However, no major polar peak was detected by HPLC analysis of urine samples from any of the monkeys, suggesting that D-D4FC is not glucuronidated to a significant level.
Penetration of antiretroviral nucleosides into the central nervous system is essential, since this compartment is an important sanctuary for HIV-1. The CSF D-D4FC concentration-versus-time profiles after i.v. and p.o. administration of 33.3 mg of D-D4FC per kg to the three monkeys (Fig. 1, inset) indicate that D-D4FC could be detected in the CSF of all three monkeys at 0.5 h after i.v. administration. The D-D4FC concentration in CSF did not decline noticeably for up to 3 h after administration. However, following p.o. administration, D-D4FC could not be detected in CSF samples from two-thirds of the monkeys until 2 h after dosing. At 3 h, the D-D4FC concentration in CSF reached the same level as that 3 h after i.v. administration, consistent with a slower absorption of the compound after p.o. administration. The apparent Cmaxs in CSF were 1.7 and 1.4 μM at 3 h after administration by the i.v. and p.o. routes, respectively. Since the median effective concentrations (EC50) of D-D4FC against HIV-1 in acutely infected human lymphocytes is 0.07 μM (12), it appears that high and sustained antiviral levels are attained in this compartment.
This study characterized the single-dose pharmacokinetics of D-D4FC in rhesus monkeys administered 33.3 mg/kg. Irrespective of the route of administration, D-D4FC plasma and CSF concentrations were above the EC50 for HIV-1 for a prolonged period of time. Absorption of the nucleoside was variable after p.o. administration; the average oral bioavailability of D-D4FC was 41%. D-D4FC was eliminated intact mainly by renal excretion and was detected in CSF at similar concentrations after administration by both the i.v. and p.o. routes. Studies with animal models of chronic HIV-1 infection are ongoing and are leading to advanced toxicological evaluations with mammals.
Acknowledgments
This work was supported by NIH grants AI-41980 (to R.F.S.), AI-28731 (to D.C.L.), and RR00165 (to H.M.M.), the Emory Center for AIDS Research (to R.F.S.), the U.S. Department of Veterans Affairs (to R.F.S.), and the Georgia Veterans Affairs Research Center for AIDS and HIV Infections (to R.F.S.).
REFERENCES
- 1.Boudinot F D, Schinazi R F, Gallo J M, McClure H M, Anderson D C, Doshi K J, Kambhampathi P C, Chu C K. 3′-Azido-2′,3′-dideoxyuridine (AzddU): comparative pharmacokinetics with 3′-azido-3′-deoxythymidine (AZT) in monkeys. AIDS Res Hum Retroviruses. 1990;6:219–228. doi: 10.1089/aid.1990.6.219. [DOI] [PubMed] [Google Scholar]
- 2.Cosford N D P, Schinazi R F. Selenium nucleophiles for the preparation of antiviral nucleosides. J Org Chem. 1991;56:2161–2165. [Google Scholar]
- 3.Cretton E M, Zhou Z, Kidd L B, McClure H M, Kaul S, Hitchcock M J M, Sommadossi J-P. In vitro and in vivo disposition and metabolism of 3′-deoxy-2′,3′-didehydrothymidine. Antimicrob Agents Chemother. 1993;37:1816–1825. doi: 10.1128/aac.37.9.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cutler D J. Theory of mean absorption time, an adjunct to conventional bioavailability studies. J Pharm Pharmacol. 1978;30:476–478. doi: 10.1111/j.2042-7158.1978.tb13296.x. [DOI] [PubMed] [Google Scholar]
- 5.Faraj A, Schinazi R F, Juodawlkis A, Lesnikowski Z, McMillan A, Morrow C D, Sommadossi J-P. Effects of β-d-2′,3′-didehydro-2′,3′-dideoxy-5-fluorocytidine 5′-triphosphate (β-D-D4FC-TP) and its β-l-enantiomer 5′-triphosphate (β-l-D4FC-TP) on viral DNA polymerases. Antivir Res. 1997;34:A66. (abstr. 86). [Google Scholar]
- 6.Gabrielsson J, Weiner D, editors. Pharmacokinetic and pharmacodynamic data analysis. Concepts and applications. Stockholm, Sweden: Swedish Pharmaceutical Press; 1994. pp. 175–184. [Google Scholar]
- 7.Gibaldi M, Perrier D, editors. Pharmacokinetics. 2nd ed. New York, N.Y: Marcel Dekker, Inc.; 1982. pp. 433–444. [Google Scholar]
- 8.Lam S S, Boudinot F D, Ascenzi M A, Tennant B C, Liotta D C, Shi J, Schinazi R F. Preclinical pharmacokinetics of β-d-2′,3′-didehydro-2′,3′-dideoxy-5-fluorocytidine (D-D4FC) in woodchucks. Boston, Mass: American Association of Pharmaceutical Scientists; 1997. [Google Scholar]
- 9.Marsh C A. Chemistry of d-glucuronic acid and its glycosides. In: Dutton G J, editor. Glucuronic acid, free and combined. New York, N.Y: Academic Press, Inc.; 1986. pp. 4–136. [Google Scholar]
- 9a.National Institutes of Health. Guide for the care and use of laboratory animals. Bethesda, Md: National Institutes of Health; 1988. [Google Scholar]
- 10.Schinazi R F, Boudinot F D, Doshi K J, McClure H M. Pharmacokinetics of 3′-fluoro-3′-deoxythymidine and 3′-deoxy-2′,3′-didehydrothymidine in rhesus monkeys. Antimicrob Agents Chemother. 1990;34:1214–1219. doi: 10.1128/aac.34.6.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schinazi, R. F., B. E. Korba, and B. C. Tennant. Unpublished data.
- 12.Schinazi R F, Ma L, Shi J, Hurwitz S J, Schlueter Wirtz S, Kupfer S, Juodawlkis A, McAtee J, Liotta D C, McClure H M, Faraj A, Sommadossi J-P. Fifth Conference on Retrovirus and Opportunistic Infections. 1998. Nucleosides with dual anti-HIV and HBV activity, abstr. 629; p. 198. [Google Scholar]
- 13.Shi, J., J. J. McAtee, S. Schlueter-Wirtz, P. Tharnish, A. Juodawlkis, D. C. Liotta, and R. F. Schinazi. Synthesis and biological evaluation of 2′,3′-didehydro-2′,3′-dideoxy-5-fluorocytidine (D4FC) analogs: discovery of carbocyclic nucleoside triphosphates with potent inhibitory activity against HIV-1 reverse transcriptase. J. Med. Chem. [DOI] [PubMed]
- 14.Sommadossi, J.-P. 1995. Comparison of metabolism and in vitro antiviral activity of stavudine versus other 2′,3′-dideoxynucleoside analogues. J. Infect. Dis. 171(Suppl. 2):S88–S92. [DOI] [PubMed]
- 15.Ussery M A, Wood O L, Kunder S C, Bacho M A, Broud D D, Papermaster S F, Hall B E, Ciavarella A, Nielsen C J, Schinazi R F. Anti-HIV activity in the HuPBMC SCID mouse model of six novel nucleoside analogs: (−)-FTC, (±)-FTC, D-DAPD, D-D4FC, CS-92 and CS-87. Antivir Res. 1998;37:A49. (abstr. 33). [Google Scholar]