Abstract
Background:
We aimed to evaluate the efficacy and safety of biologic agents targeting three main cytokines, that is, nerve growth factor (NGF), interleukin-1 (IL-1), and tumor necrosis factor-α (TNF-α), for osteoarthritis (OA) treatment.
Methods:
Databases (PubMed, Embase, and Cochrane Library) and ClinicalTrials.gov were systematically searched for randomized placebo-controlled trials (RCTs) of biologic agents from inception to November 15, 2020. The outcomes were the mean change in pain, function scores, and the risk of adverse effects (AEs).
Results:
Out of the 28 studies with 29 RCTs (8555 individuals) included, biologic agents were superior to placebo in pain relief (standardized mean difference [SMD] = 0.28, 95% confidence interval [CI] = 0.17–0.38, p < 0.001) and function improvement (SMD = 0.30, 95% CI = 0.18–0.43, p < 0.001). The incidence of any AEs (risk ratio [RR] = 1.09, 95% CI = 1.05–1.14, p < 0.001) and discontinuations due to AEs (RR = 1.39, 95% CI = 1.05–1.83, p = 0.021) were higher following treatment with biologic agents while no significant difference was found in serious AEs. Subgroup analyses showed that NGF inhibitors provided superior pain relief (SMD = 0.36, 95% CI = 0.26–0.47, p < 0.001) and function improvement (SMD = 0.41, 95% CI = 0.30–0.51, p < 0.001), whereas IL-1 inhibitors and TNF-α inhibitors did not. Meanwhile, NGF inhibitors increased the incidence of any AEs (RR = 1.12, 95% CI = 1.07–1.17, p < 0.001) and discontinuations due to AEs (RR = 1.48, 95% CI = 1.07–2.06, p = 0.018). IL-1 inhibitors and TNF-α inhibitors showed no difference in safety compared with placebo.
Conclusions:
The efficacy and safety of biologic agents vary by mechanism of action. NGF inhibitors can relieve OA-related pain and improve function but involve safety concerns. IL-1 inhibitors and TNF-α inhibitors are relatively safe options but with limited efficacy.
Keywords: biologic agents, IL-1, meta-analysis, NGF, osteoarthritis, TNF-α
Introduction
Osteoarthritis (OA), the leading cause of pain and disability globally, is characterized as a whole joint disease involving cartilage, subchondral bone, and synovium.1,2 OA is a complex chronic disorder that most commonly affects the knee, followed by the hand and hip.1,2 With the combined impact of population aging and rise of obesity,2,3 the number of patients with OA worldwide has risen by 48% from 1990 to 2019 3 and is expected to increase continuously over the coming years, which seriously reduces patients’ quality of life and intensifies socioeconomic costs.4–6 The current pharmaceutical treatments for patients with OA, such as nonsteroidal anti-inflammatory drugs (NSAIDs), acetaminophen, duloxetine, and intra-articular glucocorticoid injection, can be effective but also have substantial limitations.7,8 For example, NSAIDs exhibit a small effect size in some patients and involve great safety concerns in prolonged use including NSAID-induced gastrointestinal toxic effects, especially in elderly individuals.9–11 Therefore, it is urgent to identify a novel therapeutic medication to treat this debilitating condition.
Biologic agents have achieved distinct effects in the treatment of rheumatic disorders such as rheumatoid arthritis (RA).12–14 This successful treatment approach has greatly encouraged the conduct of randomized controlled trials (RCTs) on biologic agents in OA. Biotherapeutic strategy as such has been used to treat OA by modulating or inhibiting the effects of major cytokines, which is of a similar mechanism to the successful strategy for the treatment of RA. 15 There were three main types of cytokine blockers used in OA, targeting the nerve growth factor (NGF), interleukin-1 (IL-1), and the tumor necrosis factor-α (TNF-α), respectively. These cytokines are all involved in pain pathways of OA. Specifically, TNF-α, IL-1, and NGF can modulate pain via nociceptor sensitization.16,17 In particular, the expression of NGF can be induced by the upregulation of IL-1 and TNF-α in the case of OA.18,19 The understanding of the cytokine network associated with the pathogenesis of OA has enhanced the rationale of studies exploring whether this biotherapeutic approach has an effect on symptom improvement.
Controversy concerning the efficacy and safety of biologic agents in OA remains in the existing literature, which presents mixed outcomes of success20–22 and failure.23–25 Several meta-analyses indicated that NGF inhibitors had shown effects of pain relief and function improvement relative to placebo in OA but with inconsistent safety performance.26–29 Contrary to the results of NGF inhibitors, two meta-analyses evaluated the efficacy of IL-1 inhibitors and TNF-α inhibitors, and ended up with conclusions of ineffectiveness for OA.30,31 Nevertheless, despite the increase of relevant clinical trials, no comprehensive meta-analysis has been undertaken to date to evaluate the efficacy and safety of these three main cytokine blockers in the treatment of OA. Therefore, the present meta-analysis was intended to examine the efficacy and safety of biologic agents based on the current RCTs investigating NGF inhibitors, IL-1 inhibitors, and TNF-α inhibitors.
Methods
Search strategy
We systematically searched the databases PubMed, Embase, and Cochrane Library for RCTs involving biologic agents in the treatment of OA from inception to November 15, 2020. As three main types of cytokine blockers, these biologic agents target NGF, IL-1, or TNF-α. Additional relevant trials were retrieved through ClinicalTrials.gov. There were no restrictions on language and publication date. The details of the search strategy are available in Supplementary Table 1. The references of the identified articles and previous review articles were manually searched to avoid omitting other related studies.
Selection criteria
The inclusion criteria were as follows: (1) population: adult patients diagnosed with OA of knee, hip, or hand; (2) intervention: intravenous, subcutaneous, or intra-articular administration of biologic agents; (3) comparator: only placebo acting as a control group; (4) outcomes: mean change from baseline in pain and function scores, incidence of any adverse events (AEs) and serious AEs, and incidence of discontinuations due to AEs; (5) study design: either full texts or abstracts of RCTs containing available data.
Exclusion criteria included case reports, letters, editorials, reviews, conference abstracts with unavailable indicators and other unrelated studies. Trials without placebo controls were excluded. In addition, if the intervention or control group of a trial was in combination with NSAIDs or other analgesics, the trial would be excluded. When there were several articles from the same study, only the most recent, complete, and relevant study was included to avoid duplication.
Data extraction
F.M. and H.L. independently screened each record in strict accordance with the inclusion and exclusion criteria using NoteExpress 3.3.0 software. F.M. and H.L. extracted data from eligible studies independently, including study name, author, year, publication type, study design, participant characteristics, the studied pain condition, intervention details, duration of follow-up, and outcome measures. In case of disagreement, a third reviewer would be consulted until reaching consensus. The data of mean change, standard error, standard deviation, and 95% confidence interval (CI) in tables and texts were obtained directly from the literature. For articles showing graphic results, the GetData Graph Digitizer software version 2.22 was used to extract the data. For crossover trials, data were only extracted from the first period. If multiple intervention doses were present in a trial, subgroups would be combined into one group for analysis. The scale with the highest sensitivity to change would be used in case of multiple pain scales reported in a study. 32 The function subscale of Western Ontario and McMaster Universities Arthritis Index (WOMAC) was used for the assessment of functional improvement. If a study did not measure or report WOMAC function, the function subscale of Australian/Canadian Osteoarthritis Hand Index score (AUSCAN) or one of the other functional measurement scales was used instead.
Quality assessment
F.M. and H.F. independently assessed the risk of bias of the included trials using Cochrane’s risk of bias tool, covering sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting and other sources of bias. 33 Each source of bias was rated as low risk of bias, high risk of bias or unclear risk of bias.
Statistical analysis
This study was registered on PROSPERO (CRD42021246922). Stata 12.0 software (Stata Corporation, College Station, TX, USA) was used for statistical analysis. In meta-analysis, continuous variables were represented by standardized mean difference (SMD), and binary data was expressed by risk ratio (RR). Both SMD and RR were reported along with 95% CI. The heterogeneity was reported using the Cochrane Q test 34 and the inconsistency index value (I²). 35 According to the size of heterogeneity, the pooled effects and their respective 95% CIs were calculated using fixed-effects or random-effects models. When I² value was less than 50%, we used a fixed-effects model. On the contrary, a random-effects model would be used. Funnel plots and Egger’s regression tests were used to detect publication bias. If funnel plot indicated asymmetry by Egger’s regression test, a trim and fill analysis would be conducted. A p value less than 0.05 was considered statistically significant.
Subgroup analyses were conducted in terms of the target of action (NGF, IL-1, or TNF-α). A sensitivity analysis was performed to evaluate the impacts of any single study on the pooled outcomes.
Results
Study selection
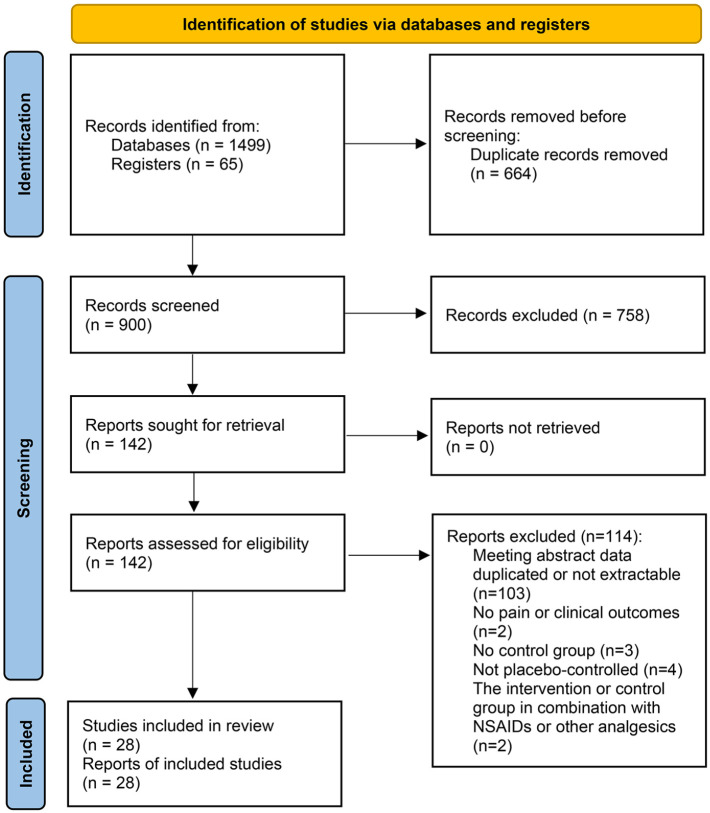
After an exhaustive literature search, a total of 1499 articles from databases and 65 studies from registers were preliminarily identified (Figure 1), from which 664 studies were removed due to duplication and 758 articles were excluded by reviewing titles and abstracts. After reading the full texts of the remaining articles, another 114 articles were excluded for the reasons of (1) conference abstract data duplicated or not extractable (n = 103); (2) no pain or clinical outcomes (n = 2); (3) no control group (n = 3); 4) control group that is not a placebo (n = 4); or (5) the intervention or control group in combination with NSAIDs or other analgesics (n = 2). Ultimately, 28 eligible studies comparing biologic agents with placebo in patients with OA were included in this meta-analysis.
Figure 1.
Flow chart of the literature search and study selection.
Study characteristics
The baseline characteristics of the included studies are summarized in Table 1. Twenty-eight eligible studies (26 full texts and 2 abstracts) included 29 trials (one study 36 contained two trials), which were all randomized, double-blinded, and placebo-controlled. Except for one crossover trial, 37 the others were all parallel trials. For the two abstracts, data were obtained through ClinicalTrials.gov. Eventually, a total of 8555 individuals clinically or radiographically diagnosed with OA were included in this meta-analysis. All the included articles were published in English, between 2009 and 2020.
Table 1.
Baseline characteristics of studies included in the meta-analysis.
| Studies | Type | Group | Target | Intervention | Treatment period | N | Female (%) | Age (y) | Joint | Duration since diagnosis (y) |
|---|---|---|---|---|---|---|---|---|---|---|
| Chevalier et al. 38 | RCT | Anakinra 50 mg | IL-1 | IA; once | 1 day | 34 | 50.0 | 63.3 | Knee | 8.1 |
| Anakinra 150 mg | 67 | 68.7 | 62.6 | 5.2 | ||||||
| Placebo | 69 | 63.8 | 62.2 | 6.0 | ||||||
| Lane et al. 21 | RCT | Tanezumab 10 µg/kg | NGF | IV q8 wk; twice | 16 weeks | 74 | 66.2 | 58.3 | Knee | NA |
| Tanezumab 25 µg/kg | 74 | 67.6 | 59.9 | NA | ||||||
| Tanezumab 50 µg/kg | 74 | 50.0 | 60.4 | NA | ||||||
| Tanezumab 100 µg/kg | 74 | 59.5 | 57.1 | NA | ||||||
| Tanezumab 200 µg/kg | 74 | 54.1 | 58.4 | NA | ||||||
| Placebo | 74 | 56.8 | 58.1 | NA | ||||||
| Nagashima et al. 39 | RCT | Tanezumab 10 µg/kg | NGF | IV; once | 1 day | 15 | 66.7 | 59.3 | Knee | 4.5 |
| Tanezumab 25 µg/kg | 15 | 53.3 | 57.3 | 7.3 | ||||||
| Tanezumab 50 µg/kg | 15 | 73.3 | 60.7 | 4.2 | ||||||
| Tanezumab 100 µg/kg | 16 | 75.0 | 58.1 | 3.8 | ||||||
| Tanezumab 200 µg/kg | 6 | 83.3 | 60.0 | 5.4 | ||||||
| Placebo | 16 | 68.8 | 59.4 | 7.9 | ||||||
| Cohen(A) et al. 23 | RCT | AMG108 100 mg IV | IL-1 | IV or SC q4 wk; | 12 weeks | 12 | 91.7 | 61.1 | Knee | 6.9 |
| AMG108 300 mg IV | 3 times | 12 | 58.3 | 62.8 | 10.2 | |||||
| AMG108 300 mg SC | 12 | 41.7 | 59.6 | 6.6 | ||||||
| AMG108 75 mg SC | 12 | 75.0 | 62.3 | 10.0 | ||||||
| Placebo | 16 | 62.5 | 60.8 | 9.6 | ||||||
| Cohen(B) et al. 23 | RCT | AMG108 300 mg | IL-1 | SC q4 wk; 3 times | 12 weeks | 80 | 67.5 | 61.3 | Knee | 6.1 |
| Placebo | 80 | 67.5 | 60.1 | 6.1 | ||||||
| Brown et al. 22 | RCT | Tanezumab 2.5 mg | NGF | IV q8 wk; 3 times | 24 weeks | 172 | 54.7 | 60.8 | Knee | 7.3 |
| Tanezumab 5 mg | 172 | 58.7 | 62.1 | 7.5 | ||||||
| Tanezumab 10 mg | 174 | 60.9 | 61.4 | 9.5 | ||||||
| Placebo | 172 | 69.2 | 62.2 | 8.2 | ||||||
| Verbruggen et al. 24 | RCT | Adalimumab 40 mg | TNF-α | SC q2 wk; 26 times | 52 weeks | 30 | 86.7 | 61.9 | Hand | 9.6 |
| Placebo | 30 | 83.3 | 60.7 | 14.4 | ||||||
| NCT01160822(A) et al. 40 | RCT | Canakinumab 150 mg | IL-1 | IA; once | 1 day | 6 | 50.0 | 58.3 | Knee | NA |
| Canakinumab 300 mg | 7 | 57.1 | 61.0 | NA | ||||||
| Canakinumab 600 mg | 6 | 33.3 | 64.2 | NA | ||||||
| Placebo | 5 | 40.0 | 57.8 | NA | ||||||
| NCT01160822(B) et al. 40 | RCT | Canakinumab 600 mg | IL-1 | IA; once | 1 day | 45 | 68.9 | 61.4 | Knee | NA |
| Naproxen 500 mg | 53 | 64.2 | 62.2 | NA | ||||||
| Placebo | 47 | 66.0 | 60.3 | NA | ||||||
| Spierings et al. 41 | RCT | Tanezumab 5 mg | NGF | IV q8 wk; twice | 16 weeks | 161 | 59.6 | 57.8 | Knee | 7.6 |
| Tanezumab 10 mg | 150 | 62.7 | 57.0 | or | 7.5 | |||||
| Oxycodone 10–40 mg | 158 | 62.7 | 57.6 | Hip | 6.2 | |||||
| Placebo | 141 | 65.2 | 57.2 | 7.4 | ||||||
| Brown et al. 42 | RCT | Tanezumab 2.5 mg | NGF | IV q8 wk; 3 times | 24 weeks | 155 | 65.2 | 62.4 | Hip | 6.0 |
| Tanezumab 5 mg | 154 | 59.7 | 61.8 | 6.3 | ||||||
| Tanezumab 10 mg | 157 | 56.1 | 63.3 | 5.6 | ||||||
| Placebo | 155 | 66.5 | 61.9 | 5.6 | ||||||
| Sanga et al. 43 | RCT | Fulranumab 1 mg q4 wk | NGF | SC q4 wk or q8 wk; 4 times or 2 times | 12 weeks | 77 | 58.4 | 61.2 | Knee | NA |
| Fulranumab 3 mg q4 wk | 79 | 58.2 | 60.8 | or | NA | |||||
| Fulranumab 3 mg q8 wk | 76 | 59.2 | 60.5 | Hip | NA | |||||
| Fulranumab 6 mg q8 wk | 78 | 60.3 | 60.7 | NA | ||||||
| Fulranumab 10 mg q8 wk | 78 | 53.8 | 61.4 | NA | ||||||
| Placebo | 78 | 55.1 | 61.3 | NA | ||||||
| Ekman(A) et al. 36 | RCT | Tanezumab 5 mg | NGF | IV q8 wk; twice | 16 weeks | 206 | 59.2 | 61.1 | Knee | 7.9 |
| Tanezumab 10 mg | 208 | 61.5 | 61.1 | 8.5 | ||||||
| Naproxen 500 mg | 206 | 62.6 | 61.4 | 7.2 | ||||||
| Placebo | 208 | 57.7 | 60.9 | 9.0 | ||||||
| Ekman(B) et al. 36 | RCT | Tanezumab 5 mg | NGF | IV q8 wk; twice | 16 weeks | 211 | 65.1 | 60.1 | Knee | 6.4 |
| Tanezumab 10 mg | 209 | 63.5 | 59.8 | or | 6.8 | |||||
| Naproxen 500 mg | 211 | 61.2 | 59.2 | Hip | 7.7 | |||||
| Placebo | 209 | 64.5 | 60.3 | 6.3 | ||||||
| Brown et al. 44 | RCT | Tanezumab 5 mg | NGF | IV q8 wk; 3 times | 24 weeks | 73 | 60.3 | 57.8 | Knee | NA |
| Tanezumab 10 mg | 74 | 63.5 | 58.0 | or | NA | |||||
| Placebo | 72 | 54.2 | 56.3 | Hip | NA | |||||
| Tiseo et al. 45 | RCT | Fasinumab 0.03 mg/kg | NGF | IV q8 wk; twice | 16 weeks | 53 | 60.4 | 59.0 | Knee | NA |
| Fasinumab 0.1 mg/kg | 53 | 67.9 | 60.3 | NA | ||||||
| Fasinumab 0.3 mg/kg | 54 | 68.5 | 58.8 | NA | ||||||
| Placebo | 55 | 78.2 | 59.1 | NA | ||||||
| Chevalier et al. 46 | RCT | Adalimumab 40 mg | TNF-α | SC q15 d; twice | 1 month | 41 | 87.8 | 62.8 | Hand | 13.5 |
| Placebo | 42 | 83.3 | 62.2 | 13.5 | ||||||
| Gow et al. 47 | RCT | AMG403 3 mg | NGF | SC q4 wk; 4 times | 16 weeks | 6 | 33.3 | 53.0 | Knee | NA |
| AMG403 10 mg | 6 | 50.0 | 48.7 | NA | ||||||
| AMG403 20 mg | 6 | 50.0 | 52.7 | NA | ||||||
| Placebo | 6 | 83.3 | 54.7 | NA | ||||||
| Mayorga et al. 48 | RCT | Fulranumab 3 mg | NGF | SC q4 wk; 4 times | 16 weeks | 48 | 62.5 | 59.2 | Knee | NA |
| Fulranumab 9 mg | 50 | 60.0 | 58.8 | NA | ||||||
| Oxycodone 20–50 mg | 50 | 50.0 | 58.6 | NA | ||||||
| Placebo | 48 | 52.1 | 60.9 | NA | ||||||
| Wang et al. 49 | RCT | ABT981 0.3 mg/kg q2 wk | IL-1 | SC q2 wk or q4 wk; | 8 weeks or | 7 | 71.4 | 61.3 | Knee | NA |
| ABT981 1 mg/kg q2 wk | 4 times or 3 times | 12 weeks | 7 | 71.4 | 62.6 | NA | ||||
| ABT981 3 mg/kg q2 wk | 7 | 100 | 61.4 | NA | ||||||
| Placebo q2 wk | 6 | 83.3 | 60.0 | NA | ||||||
| ABT981 3 mg/kg q4 wk | 7 | 100 | 60.0 | NA | ||||||
| Placebo q4 wk | 2 | 100 | 55.0 | NA | ||||||
| Birbara et al. 26 | RCT | Tanezumab 2.5 mg SC | NGF | SC or IV q8 wk; | 16 weeks | 74 | 64.9 | 61.0 | Knee | 7.3 |
| Tanezumab 5 mg SC | twice | 63 | 57.1 | 60.3 | 9.1 | |||||
| Tanezumab 10 mg SC | 86 | 62.8 | 58.2 | 8.7 | ||||||
| Tanezumab 10 mg IV | 84 | 57.1 | 59.6 | 8.2 | ||||||
| Placebo | 72 | 65.3 | 61.3 | 9.6 | ||||||
| Walicke et al. 50 | RCT | Tanezumab 3 µg/kg | NGF | IV; once | 1 day | 4 | 100.0 | 47.3 | Knee | NA |
| Tanezumab 10 µg/kg | 4 | 75.0 | 52.8 | NA | ||||||
| Tanezumab 30 µg/kg | 4 | 50.0 | 51.5 | NA | ||||||
| Tanezumab 100 µg/kg | 6 | 33.3 | 51.8 | NA | ||||||
| Tanezumab 300 µg/kg | 6 | 33.3 | 53.7 | NA | ||||||
| Tanezumab 1000 µg/kg | 6 | 66.7 | 52.8 | NA | ||||||
| Placebo | 12 | 75.0 | 49.8 | NA | ||||||
| Aitken et al. 37 | RCT | Adalimumab 40 mg | TNF-α | SC q2 wk; 6 times | 12 weeks | 18 | 83.3 | 63.1 | Hand | NA |
| Placebo | 25 | 72.0 | 61.2 | NA | ||||||
| Kloppenburg et al. 51 | RCT | Etanercept 50/25mg | TNF-α | SC q1 wk; 52 times | 1 year | 45 | 82.2 | 59.4 | Hand | 6.2 |
| Placebo | 45 | 80.0 | 60.1 | 7.3 | ||||||
| NCT01144143 et al. 52 | RCT | Infliximab 10 mg | TNF-α | IA; once | 1 day | 8 | 62.5 | NA | Knee | NA |
| MP 80 mg | 4 | 100 | NA | NA | ||||||
| Placebo | 4 | 100 | NA | NA | ||||||
| Schnitzer et al. 53 | RCT | Tanezumab 2.5 mg | NGF | SC q8 wk; twice | 16 weeks | 231 | 62.8 | 60.9 | Knee | 6.4 |
| Tanezumab 2.5/5 mg | 233 | 64.8 | 61.2 | or | 7.2 | |||||
| Placebo | 232 | 67.7 | 60.4 | Hip | 6.9 | |||||
| Kelly et al. 54 | RCT | Fulranumab 1 mg | NGF | SC q4 wk; 4 times | 16 weeks | 81 | 69.1 | 62.0 | Knee | NA |
| Fulranumab 3 mg | 83 | 51.8 | 63.0 | or | NA | |||||
| Placebo | 81 | 65.4 | 64.4 | Hip | NA | |||||
| Dakin et al. 55 | RCT | Fasinumab 1 mg | NGF | SC q4 wk; 4 times | 16 weeks | 85 | 69.4 | 60.7 | Knee | NA |
| Fasinumab 3 mg | 84 | 64.3 | 60.7 | or | NA | |||||
| Fasinumab 6 mg | 85 | 60.0 | 60.1 | Hip | NA | |||||
| Fasinumab 9 mg | 84 | 64.3 | 61.5 | NA | ||||||
| Placebo | 83 | 65.1 | 60.1 | NA | ||||||
| Kloppenburg et al. 56 | RCT | Lutikizumab 200 mg | IL-1 | SC q2 wk; 12 times | 24 weeks | 64 | 82.8 | 66.0 | Hand | 11.0 |
| Placebo | 67 | 86.6 | 66.0 | 11.0 | ||||||
| Fleischmann et al. 57 | RCT | Lutikizumab 25 mg | IL-1 | SC q2 wk; 26 times | 52 weeks | 89 | 70.8 | 61.6 | Knee | 7.6 |
| Lutikizumab 100 mg | 85 | 62.4 | 60.2 | 7.9 | ||||||
| Lutikizumab 200 mg | 88 | 64.8 | 59.1 | 8.7 | ||||||
| Placebo | 85 | 61.2 | 59.5 | 7.9 | ||||||
| Berenbaum et al. 58 | RCT | Tanezumab 2.5 mg | TNF-α | SC q8 wk; 3 times | 24 weeks | 283 | 70.0 | 65.2 | Knee | 6.0 |
| Tanezumab 5 mg | 284 | 68.0 | 65.2 | or | 6.7 | |||||
| Placebo | 282 | 69.5 | 64.2 | Hip | 7.4 |
IA, intra-articular; IL-1, interleukin-1; IV, intravenous; MP, methylprednisolone; NA, data not available; NGF, nerve growth factor; RCT, randomized controlled trial; SC, subcutaneous; TNF-α, tumor necrosis factor-α.
Risk of bias assessment of included trials
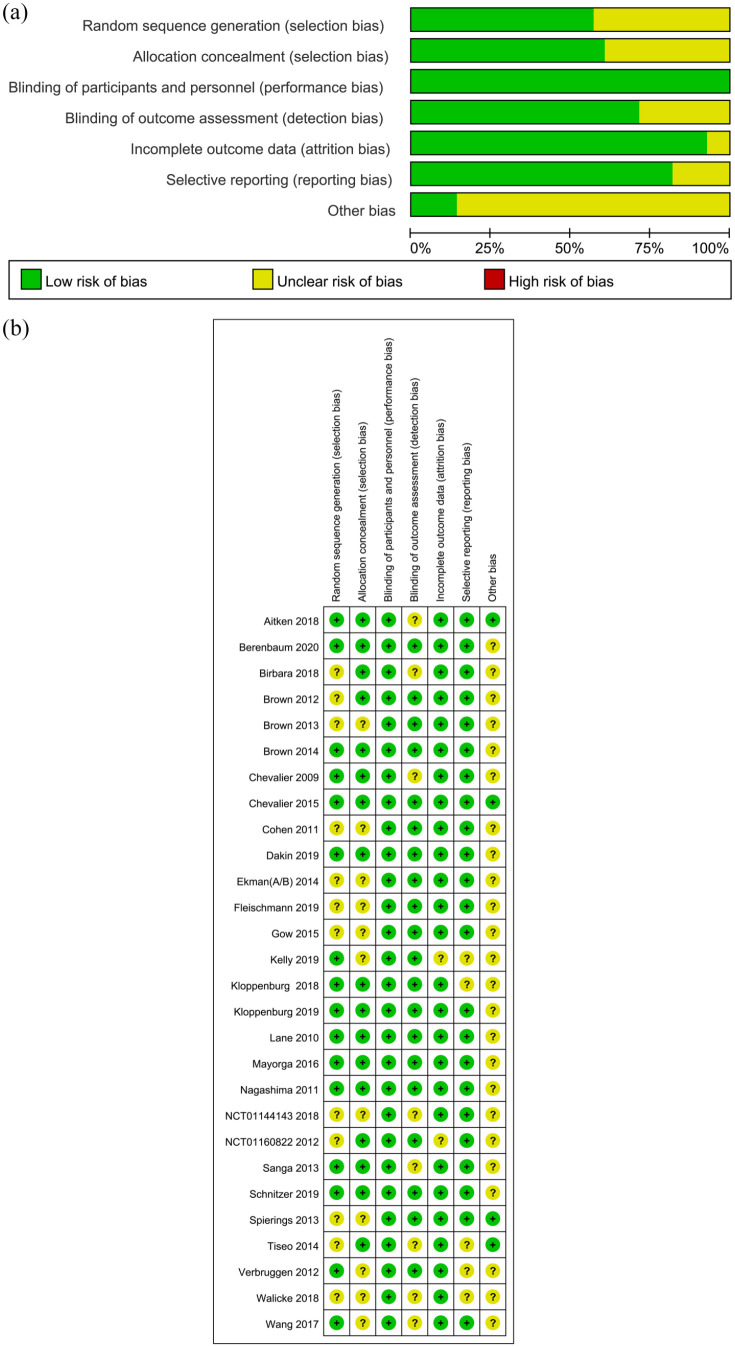
According to the Cochrane Handbook for Systematic Reviews of Interventions (version 5.1.0), we assessed the risk of bias of each included study, and the details are shown in Figure 2. As for random sequence generation, 16 studies were rated as low risk of bias. The allocation concealment in 11 studies was not illustrated in detail. As for blinding of participants and personnel, all of the studies were rated as low risk of bias. The details of blinding of outcome assessment could not be adequately obtained from eight studies, which were therefore rated as unclear risk of bias. As for attrition bias and reporting bias, two and five studies were considered unclear risk, respectively. Other sources of bias were unclear in most of included studies (24/28).
Figure 2.
Risk of bias assessment of included studies.
Efficacy of biologic agents in OA
Pain
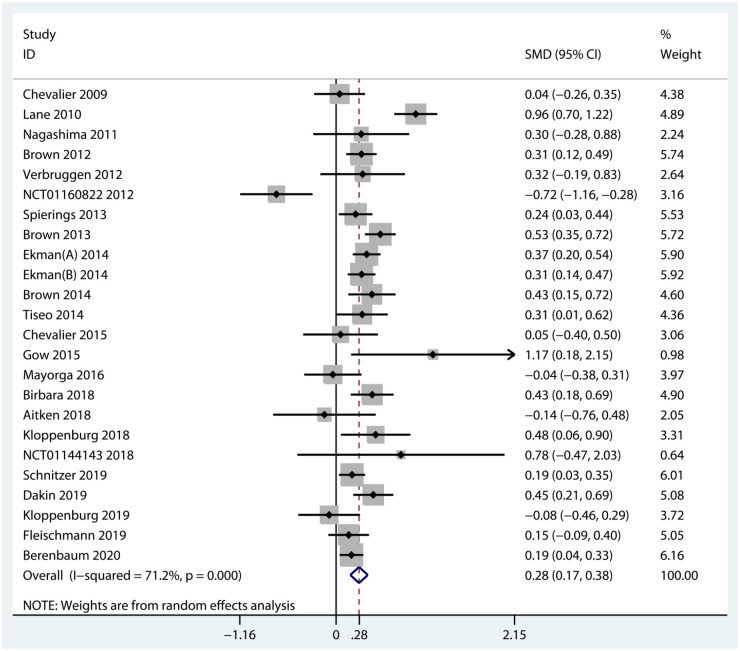
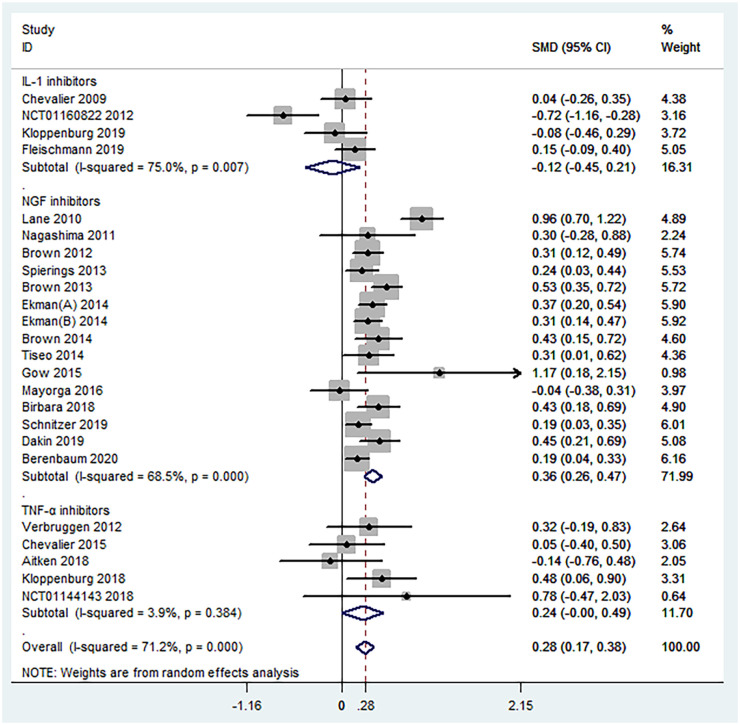
A total of 24 RCTs including 7383 participants diagnosed with knee, hip, or hand OA reported the mean change from baseline in pain scores. Overall, biologic agents appeared to be statistically superior to placebo with regard to pain relief (SMD = 0.28, 95% CI = 0.17–0.38, p < 0.001, I2 = 71.2%) (Figure 3). Subgroup analyses against the mechanism of action demonstrated that NGF inhibitors (SMD = 0.36, 95% CI = 0.26–0.47, p < 0.001, I2 = 68.5%) were significantly superior to placebo in pain relief. On the contrary, both IL-1 inhibitors (SMD = −0.12, 95% CI = −0.45 to 0.21, p = 0.481, I2 = 75.0%) and TNF-α inhibitors (SMD = 0.24, 95% CI = −0.00 to 0.49, p = 0.050, I2 = 3.9%) were found no statistical significance in pain relief. Details of subgroup analyses are presented in Table 2 and Figure 4.
Figure 3.
Forest plot for pain improvement of biologic agents compared with placebo in OA.
Table 2.
Results of overall and subgroup meta-analysis for the efficacy and safety of biologic agents in OA.
| Analysis | No. of trials | ES (95% CI) | p value | I2 (p value) |
|---|---|---|---|---|
| Pain | ||||
| Overall | 24 | 0.28 (0.17 to 0.38) | <0.001 | 71.2% (<0.001) |
| Subgroup analysis | ||||
| Mechanism of action | ||||
| NGF inhibitors | 15 | 0.36 (0.26 to 0.47) | <0.001 | 68.5% (<0.001) |
| IL-1 inhibitors | 4 | −0.12 (−0.45 to 0.21) | 0.481 | 75.0% (0.007) |
| TNF-α inhibitors | 5 | 0.24 (−0.00 to 0.49) | 0.050 | 3.9% (0.384) |
| Function | ||||
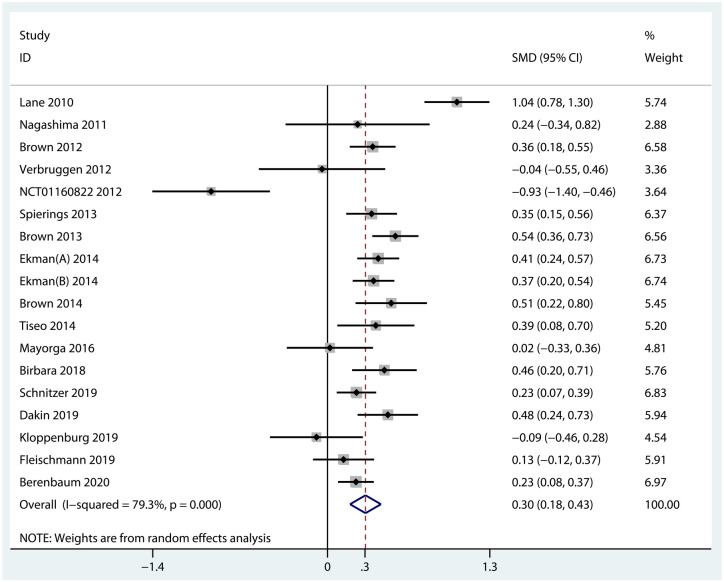
| Overall | 18 | 0.30 (0.18 to 0.43) | <0.001 | 79.3% (<0.001) |
| Subgroup analysis | ||||
| Mechanism of action | ||||
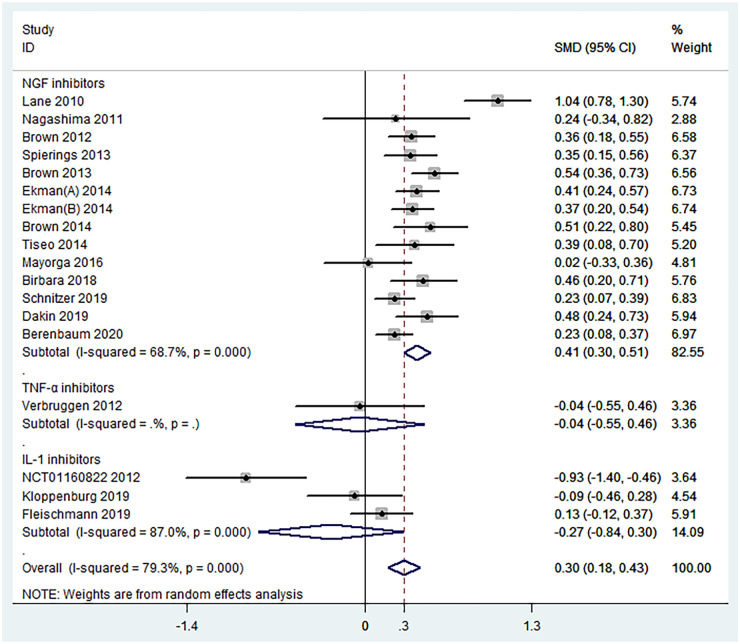
| NGF inhibitors | 14 | 0.41 (0.30 to 0.51) | <0.001 | 68.7% (<0.001) |
| IL-1 inhibitors | 3 | −0.27 (−0.84 to 0.30) | 0.354 | 87.0% (<0.001) |
| TNF-α inhibitors | 1 | −0.04 (−0.55 to 0.46) | 0.865 | – |
| Any AEs | ||||
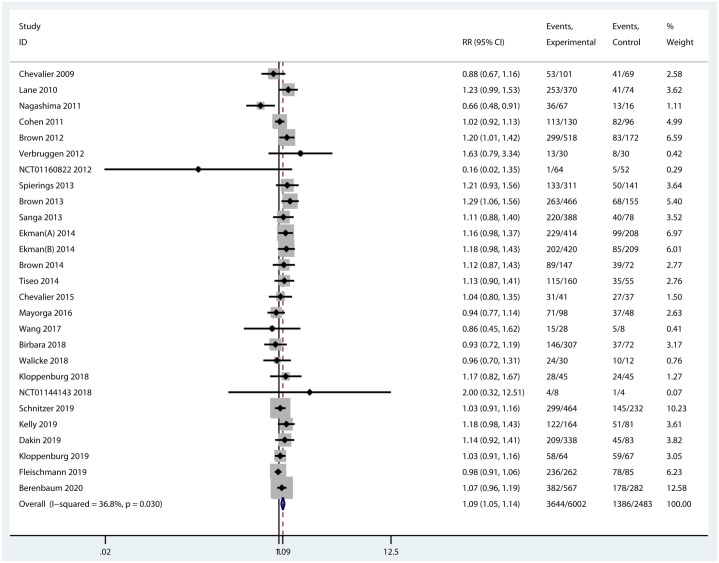
| Overall | 27 | 1.09 (1.05 to 1.14) | <0.001 | 36.8% (0.030) |
| Subgroup analysis | ||||
| Mechanism of action | ||||
| NGF inhibitors | 17 | 1.12 (1.07 to 1.17) | <0.001 | 31.1% (0.108) |
| IL-1 inhibitors | 6 | 0.97 (0.91 to 1.03) | 0.328 | 4.8% (0.386) |
| TNF-α inhibitors | 4 | 1.18 (0.96 to 1.47) | 0.123 | 0.0% (0.561) |
| Serious AEs | ||||
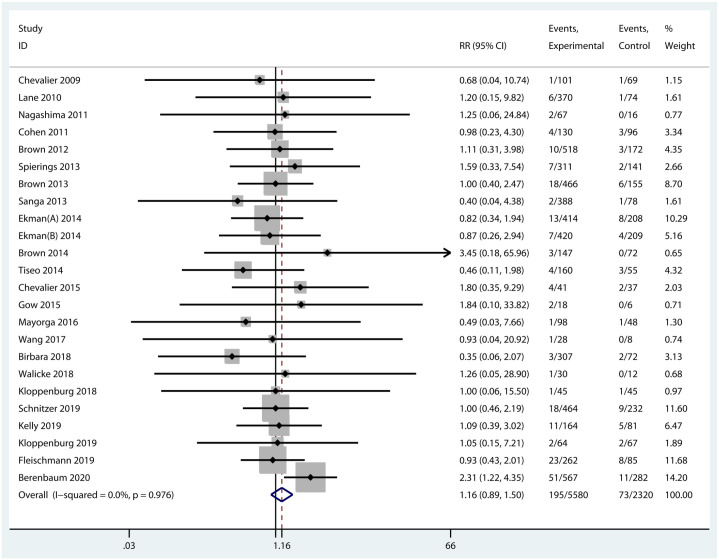
| Overall | 24 | 1.16 (0.89 to 1.50) | 0.265 | 0.0% (0.976) |
| Subgroup analysis | ||||
| Mechanism of action | ||||
| NGF inhibitors | 17 | 1.20 (0.89 to 1.61) | 0.228 | 0.0% (0.815) |
| IL-1 inhibitors | 5 | 0.94 (0.51 to 1.73) | 0.838 | 0.0% (0.999) |
| TNF-α inhibitors | 2 | 1.55 (0.38 to 6.23) | 0.541 | 0.0% (0.717) |
| Discontinuations due to AEs | ||||
| Overall | 23 | 1.39 (1.05 to 1.83) | 0.021 | 0.0% (0.636) |
| Subgroup analysis | ||||
| Mechanism of action | ||||
| NGF inhibitors | 16 | 1.48 (1.07 to 2.06) | 0.018 | 0.0% (0.637) |
| IL-1 inhibitors | 4 | 0.94 (0.52 to 1.68) | 0.828 | 0.0% (0.530) |
| TNF-α inhibitors | 3 | 2.15 (0.62 to 7.51) | 0.229 | 21.8% (0.279) |
AEs, adverse events; CI, confidence interval; ES, effect size; I2, inconsistency index value; IL-1, interleukin-1; NGF, nerve growth factor; No., number; TNF-α, tumor necrosis factor-α.
Figure 4.
Forest plot for subgroup analyses in the improvement of pain conducted in accordance with mechanism of action. NGF = nerve growth factor; IL-1 = interleukin-1; TNF-α = tumor necrosis factor-α.
Function. Data from 18 RCTs were pooled to evaluate the efficacy of biologic agents in function improvement. The results indicated that when compared with placebo, biologic agents achieved a significant improvement in terms of function scores (SMD = 0.30, 95% CI = 0.18–0.43, p < 0.001, I2 = 79.3%) (Figure 5). Specifically, NGF inhibitors were statistically superior to placebo (SMD = 0.41, 95% CI = 0.30–0.51, p < 0.001, I2 = 68.7%), but TNF-α inhibitors (SMD = −0.04, 95% CI =−0.55 to 0.46, p = 0.865) and IL-1 inhibitors (SMD = −0.27, 95% CI = −0.84 to 0.30, p = 0.354, I2 = 87.0%) were ineffective (Figure 6). The results of overall and subgroup meta-analyses for the function improvement of biologic agents in OA are shown in Table 2.
Figure 5.
Forest plot for function improvement of biologic agents compared with placebo in OA.
Figure 6.
Forest plot for subgroup analyses in the improvement of function conducted in accordance with mechanism of action. NGF = nerve growth factor; IL-1 = interleukin-1; TNF-α = tumor necrosis factor-α.
Safety of biologic agents in OA
Any AEs
All the included trials provided data on the incidence of any AEs. Nevertheless, one of them was a crossover randomized trial and was excluded on account of the data combining both treatment periods. Among any AEs, abnormal peripheral sensation, musculoskeletal and connective tissue disorders, gastrointestinal disorders, and infections were commonly observed in patients treated with anti-NGFs. Injection site reactions, neutropenia, and infections were more frequent with OA patients treated with IL-1 inhibitors. More subjects in the treatment of TNF-α inhibitors had injection site reactions and infections (Supplementary Table 2). Intravenous and subcutaneous administrations of biologic agents were generally similar in the incidence of AEs. The most common AE was musculoskeletal and connective tissue disorders such as arthralgia in patients treated with intra-articular IL-1 and TNF-α inhibitors injection. Overall, the incidence of any AEs was significantly different between biologic agents and placebo (RR = 1.09, 95% CI = 1.05–1.14, p < 0.001, I2 = 36.8%) (Figure 7). Subgroup analyses revealed that the incidence of any AEs of NGF inhibitors was significantly higher than that of placebo (RR = 1.12, 95% CI = 1.07–1.17, p < 0.001, I2 = 31.1%). On the contrary, there was no significant difference in any AEs compared TNF-α inhibitors (RR = 1.18, 95% CI = 0.96–1.47, p = 0.123, I2 = 0.0%) and IL-1 inhibitors (RR = 0.97, 95% CI = 0.91–1.03, p = 0.328, I2 = 4.8%) with placebo (Supplementary Figure 1).
Figure 7.
Forest plot for any AEs of biologic agents compared with placebo in OA.
Serious AEs
An AE, which was life-threatening, disabling, leading to hospitalization or death, or leading to a birth defect or congenital anomaly, was classified as a serious AE. Musculoskeletal and connective tissue disorders, infections, and gastrointestinal disorders were the most common serious AEs in subjects treated with NGF blockers. Serious infections were observed in RCTs of agents targeting IL-1 and few serious complications occurred in TNF antagonist therapy. Notably, no significant difference was found between biologic agents and placebo in terms of the incidence of serious AEs (RR = 1.16, 95% CI = 0.89–1.50, p = 0.265, I2 = 0.0%) (Figure 8). Compared with placebo, all the three types of cytokine blockers were not associated with any significantly increased incidence of serious AEs (Supplementary Figure 1). Moreover, no obvious difference was observed in serious AEs of biologic agents in different routes of administration.
Figure 8.
Forest plot for serious AEs of biologic agents compared with placebo in OA.
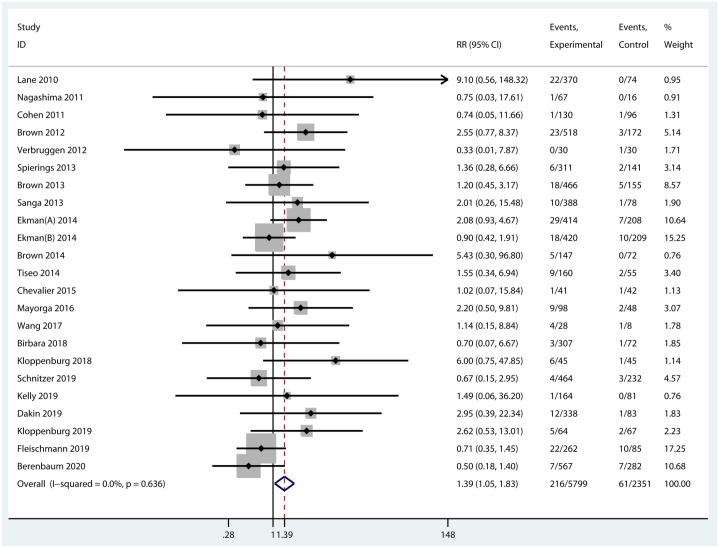
Discontinuations due to AEs
The number of discontinued patients due to AEs was extracted from 23 RCTs with the data available. The incidence of discontinuations due to AEs was statistically higher in experimental groups than that in the control group (RR = 1.39, 95% CI = 1.05–1.83, p = 0.021, I2 = 0.0%) (Figure 9). Specifically, the incidence of discontinuations of NGF inhibitors was significantly increased compared with placebo (RR = 1.48, 95% CI = 1.07–2.06, p = 0.018, I2 = 0.0%). Nevertheless, differences in discontinuations due to AEs were not significant between the other two inhibitors with placebo (Supplementary Figure 1).
Figure 9.
Forest plot for discontinuations of biologic agents due to AEs compared with placebo in OA.
Sensitivity analyses and publication bias
Sensitivity analyses were conducted to examine the influence of a single study on the pooled effects. After removing each individual study, the overall effect of each main outcome did not change statistically. All funnel plots showed no asymmetry by Egger’s regression tests (Supplementary Figure 2).
Discussion
This meta-analysis comprehensively investigated the efficacy and safety of biologic agents including NGF inhibitors, IL-1 inhibitors, and TNF-α inhibitors in patients with OA. The pooled results indicated that biologic agents were significantly superior to placebo in pain relief and function improvement with a higher incidence of any AEs and discontinuations due to AEs. Besides, NGF inhibitors had significant effects in pain relief and function improvement and were associated with higher risk of any AEs and withdrawals due to AEs. On the contrary, both IL-1 inhibitors and TNF-α inhibitors showed no difference compared with placebo in terms of efficacy and safety.
Comparisons with other studies
Several meta-analyses have been reported to evaluate the efficacy and safety of NGF inhibitors in OA.27–29,59–63 However, half of them only focused on tanezumab.28,29,60,61 Tanezumab demonstrated superiority in pain relief and function improvement compared with placebo, which was consistent with the results of this meta-analysis. The discovery of safety varied according to tanezumab dose explored. Yu et al. 28 evaluated the safety of low-dose tanezumab and no significant difference was found in terms of withdrawal due to AEs. Fan et al. 29 explored the safety of tanezumab administered as a fixed dosing regimen and there was significant difference in serious AEs compared with placebo. The results of safety for tanezumab in the other two studies were the same as this meta-analysis.60,61 In addition, there were four meta-analyses including all three NGF inhibitors, tanezumab, fulranumab, and fasinumab.27,59,62,63 Like the studies of tanezumab above, the efficacy results of anti-NGFs were consistent with this meta-analysis, but the safety findings were not absolutely the same. Among them, the article conducted by Yang et al. 27 indicated that pooled differences of AEs rates between experimental and control groups were not significant. However, it only included six OA trials and omitted several trials eligible for their inclusion criteria. 64 Schnitzer et al. published a meta-analysis in 2015 and found that safety, determined by odds ratios of withdrawals due to AEs, at the lower doses was better than higher doses and appeared similar to placebo. This study included 13 RCTs to evaluate the efficacy and safety of NGF inhibitors in the treatment of hip and knee OA. 59 Ever since Schnitzer et al.’s work, a certain number of high-quality RCTs investigating monoclonal NGF antibodies in the treatment of OA have been published.26,44,47,50,53-55,58 The network meta-analysis conducted by Cao et al. 62 compared the efficacy and safety of the anti-NGF antibody with NSAIDs and opioids in the treatment of OA and found that anti-NGFs were not associated with higher withdrawal rates related to AEs. But only nine RCTs of NGF inhibitors were included due to particular exclusion criteria which was dose-escalation studies of a single drug. Similarly, another meta-analysis conducted by Seah et al. 63 evaluated the effectiveness of NGF inhibitors in the treatment of hip and knee OA, and only included 13 eligible studies. This study found that anti-NGFs were not associated with higher incidence of serious AEs but were associated with significant increase of discontinuation due to AEs. As for IL-1 inhibitors and TNF-α inhibitors, there were two published studies evaluating the efficacy and safety of these two in OA.30,31 Persson et al. 30 reported that the efficacy of biologic disease-modifying anti-rheumatic drugs, IL-1 inhibitors and TNF-α inhibitors, was not superior to placebo in the treatment of OA. It is noteworthy that this meta-analysis included six related RCTs and contained four kinds of IL-1 inhibitors and TNF-α inhibitors (adalimumab, etanercept, anakinra, and infliximab) without inclusion of the other three inhibitors (i.e. lutikizumab, AMG 108, and canakinumab). Another meta-analysis conducted by Cao et al. 31 only evaluated the efficacy and safety of lutikizumab, an anti–IL-1α/β dual-variable domain immunoglobulin, and only included two RCTs of lutikizumab. Lutikizumab showed no improvement either in pain or function, but was of fine tolerance for patients with OA. Both of the two meta-analyses above did not include clinical trials investigating any kinds of IL-1 inhibitors and TNF-α inhibitors and only involved several blockers.
To update the evidence of biologic agents in the treatment of OA, we included 29 RCTs covering tanezumab (12 trials), fulranumab (4 trials), fasinumab (2 trials), adalimumab (3 trials), etanercept (1 trial), infliximab (1 trial), anakinra (1 trial), lutikizumab (3 trials), AMG 108 (1 trial), and canakinumab (1 trial) in our meta-analysis. Compared with previous studies which were either narrative review or meta-analysis only involving a few biological agents, the present work comprehensively evaluated the efficacy and safety of three main biologic agents targeting IL-1, TNF-α, and NGF. Meanwhile, considering that all the included trials are double-blinded randomized placebo-controlled trials, our subgroup analyses on the mechanism of action could provide indirect comparisons for these three main biologic agents.
Possible explanations
Although the pooled results indicated that biologic agents provided statistically significant effects in pain relief and function improvement, subgroup analyses on the mechanism of action showed that, except for NGF inhibitors, IL-1 inhibitors, and TNF-α inhibitors were found no statistical significance. These promising results of NGF inhibitors suggested that NGF played a significant role in pain pathways which had been confirmed by several experimental studies.16,65–67 Different from NGF inhibitors addressing the mechanisms of pain in a nonspecific way, IL-1 and TNF-α blockers target pro-inflammatory cytokines directly involved in the catabolic and anabolic of cartilage.68,69 However, taking into account the rapid clearance and short half-life of cytokine blockers, such disappointing results may be attributed to the insufficient drug exposure in the affected joint. 38 In consideration of the location of disease, hand OA often affects multiple joints compared with OA of the weight-bearing joints such as the knees and hips. We specially conducted a subgroup analysis for the treatment of hand OA with IL-1 and TNF-α blockers, and the results indicated that there was no significant difference in the efficacy and safety of the two blockers compared with placebo (Supplementary Figures 3 and 4). However, in several studies of patients with erosive hand OA, daily subcutaneous injections of 100 mg of anakinra improved pain after 3 months of treatment, 70 and subcutaneous treatment with 40 mg of adalimumab every 2 weeks for 1 year significantly decreased the number of new erosions in the patient subset with clinical interphalangeal joint swelling at baseline. 24 These promising studies implied that the efficacy of IL-1 and TNF inhibitors may depend on the inflammatory phenotype of OA. 15 In a trial of IL-1 inhibitors conducted by Schieker et al., 71 canakinumab can reduce the consequences of large joint OA such as total hip or knee replacement and were with less OA-related AEs. Such promising findings from the exploratory analysis of this RCT encouraged investigation of IL-1 blockers in OA treatment. Furthermore, the ratio of endogenous IL-1 receptor antagonist to IL-1β was high in the synovial fluid so that the effect of exogenous IL-1 inhibitors was limited. 72 The interaction between IL-1 and TNF-α in OA suggested that inhibitors targeting both of them simultaneously might be needed to effectively reduce the expression of matrix metalloproteinases and aggrecanases. 73 Nevertheless, serious adverse reactions including infection forced researchers to discreetly consider this strategy. 74
With respect to the safety of biologic agents, further subgroup analyses showed that NGF inhibitors had a higher incidence of any AEs and discontinuations due to AEs compared with the placebo group, whereas the other two blockers did not. Although NGF inhibitors have distinct effects in OA-related pain relief and function improvement, the US Food and Drug Administration (FDA) suspended all related trials until 2012 due to rapid progressive OA which happened in patients using NGF inhibitors and was in relation to dose and the combination of NSAIDs.75,76 The safety of subcutaneous tanezumab injection in patients with OA pain was generally similar to intravenous administration in terms of the incidence of rapid progressive OA.21,26,42,53 The tanezumab Adjudication Committee reviewed joint-related AEs in 249 of 386 patients and 68 of which were classified as rapid progressive OA in total 9810 patients treated with tanezumab monotherapy or tanezumab combined with NSAIDs. 77 Furthermore, a total of 18 of 88 patients reported as joint-related AEs were classified as rapid progressive OA among 1353 participants treated in the nine phase I and II studies of fulranumab. 78 Recently, the marketing application of tanezumab, an NGF antibody, for OA had been rejected by FDA in consideration of safety. 79 Therefore, the safety of NGF inhibitors reveals a demand for further investigations and great cautiousness in the upcoming trials. Contrary to NGF inhibitors, IL-1 inhibitors and TNF-α inhibitors were of favorable tolerance in the treatment of OA, but attention should still be paid to the risk of infection, even if there are no safety concerns.
Limitations
There were several limitations in this study. First, this study could not provide direct comparisons among the three targeted therapies, and a network meta-analysis was failed to be conducted due to lack of direct comparisons. However, as mentioned above, we retrieved all available data from double-blinded randomized placebo-controlled trials and most of the included studies were of high quality, which could provide indirect comparisons for their efficacy and safety. Second, in consideration of complete evidence capture, conference abstracts that were lack of stricter peer review were included as full texts in this study. Unfortunately, thorough examination of the method and critical assessment of the risk of bias were not allowed in conference abstracts. Third, as one of the factors affecting the efficacy and safety of biological agents, the potential effect of dose of injection was not examined, due to different divisions of dose subgroups in RCTs. A meta-analysis of tanezumab indicated that low-dose tanezumab (10 or 25 μg/kg and 2.5 mg) had similar effects in pain relief and function improvement and caused a lower incidence of AEs in OA. 61 Fourth, maintaining a steady drug concentration is important for the effectiveness of the medications and hence the lack of analysis for the effect of treatment duration on the efficacy of biologic agents was another limitation. Finally, all the 14 trials of tanezumab were sponsored by a pharmaceutical company (Pfizer Inc., New York, NY, USA), which might lead to overestimation of the effect of biologic agents.
Implication for research
Although previous studies and this meta-analysis supported the distinct efficacy of NGF blockers, controversy still exists based on the current evidence in the treatment of OA using anti-NGFs. The high risk of rapid progressive OA has led to marketing application failure. Considering that such AEs have not been observed in trials of other chronic diseases, such as low back pain, 80 whether characteristic and selection of patients in the trials of NGF inhibitors play a role in the incidence of rapid progressive OA deserves further clinical and preclinical studies, and identifying the phenotype of patients who showed significant efficacy without exhibiting a safety concern would be helpful for OA treatments. 81 Moreover, on account of rapid clearance and short half-life of IL-1 inhibitors and TNF-α inhibitors,15,38 it is necessary to build a relatively stable system to allow these two cytokine blockers to stay in the affected joints for a longer time. Nevertheless, due to the lack of efficacy of blockers inhibiting IL-1 and TNF-α, it should also be considered that IL-1 and TNF may not be the right targets for OA treatment.82,83 Deeper understanding of the molecular mechanism of pain in OA is urgently needed in the upcoming studies to develop more effective therapeutic medications. It is worth noting that more and more other cytokines such as IL-6, a cytokine in the synovial fluid, become attractive and promising targets,84–86 and maybe inhibitors targeting them can be novel treatment of OA in the future.
Conclusion
The results of combining all biologic agents showed statistically significant pain relief and function improvement. Specifically, NGF inhibitors provide significant pain relief and function improvement but with nonignorable safety concerns. Although with favorable tolerance, neither IL-1 inhibitors nor TNF-α inhibitors reduce OA-related pain and improve function. The results imply that the efficacy and safety of biologic agents vary by mechanism of action.
Supplemental Material
Supplemental material, sj-docx-1-tab-10.1177_1759720X221080377 for Efficacy and safety of biologic agents for the treatment of osteoarthritis: a meta-analysis of randomized placebo-controlled trials by Fanqiang Meng, Hui Li, Haoran Feng, Huizhong Long, Zidan Yang, Jiatian Li, Yuqing Wang and Dongxing Xie in Therapeutic Advances in Musculoskeletal Disease
Acknowledgments
Everyone who contributed significantly to this study has been listed.
Footnotes
Author contributions: Fanqiang Meng: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Hui Li: Conceptualization; Data curation; Formal analysis; Methodology; Writing – original draft; Writing – review & editing.
Haoran Feng: Data curation; Formal analysis; Investigation.
Huizhong Long: Data curation; Formal analysis; Investigation.
Zidan Yang: Methodology; Supervision; Validation; Visualization.
Jiatian Li: Formal analysis; Methodology.
Yuqing Wang: Formal analysis; Methodology; Software.
Dongxing Xie: Conceptualization; Methodology; Writing – original draft; Writing – review & editing.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (81930071, 81772413), the Key Research and Development Program of Hunan Province (2018SK2072, 2018SK2074), the Hunan Provincial Innovation Key Foundation for Postgraduate (CX20200389), the Central South University’s Innovation Key Foundation for Postgraduate (1053320192237), and the Postgraduate Independent Exploration and Innovation Project of Central South University (2018zzts256).
ORCID iDs: Fanqiang Meng  https://orcid.org/0000-0003-2200-1859
https://orcid.org/0000-0003-2200-1859
Dongxing Xie  https://orcid.org/0000-0003-2672-4544
https://orcid.org/0000-0003-2672-4544
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Fanqiang Meng, Department of Orthopaedics, Xiangya Hospital, Central South University, Changsha, China.
Hui Li, Department of Orthopaedics, Xiangya Hospital, Central South University, Changsha, China; Hunan Key Laboratory of Joint Degeneration and Injury, Xiangya Hospital, Central South University, Changsha, China; Hunan Engineering Research Center for Osteoarthritis, Changsha, China; National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, China.
Haoran Feng, Department of Orthopaedics, Xiangya Hospital, Central South University, Changsha, China.
Huizhong Long, Department of Orthopaedics, Xiangya Hospital, Central South University, Changsha, China.
Zidan Yang, Hunan Key Laboratory of Joint Degeneration and Injury, Xiangya Hospital, Central South University, Changsha, China.
Jiatian Li, Department of Orthopaedics, Xiangya Hospital, Central South University, Changsha, China.
Yuqing Wang, Department of Orthopaedics, Xiangya Hospital, Central South University, Changsha, China.
Dongxing Xie, Department of Orthopaedics, Xiangya Hospital, Central South University, 87 Xiangya Road, Changsha, Hunan 410008, China; Hunan Key Laboratory of Joint Degeneration and Injury, Xiangya Hospital, Central South University, Changsha, China; Hunan Engineering Research Center for Osteoarthritis, Changsha, China; National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, China.
References
- 1. Prieto-Alhambra D, Judge A, Javaid MK, et al. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: influences of age, gender and osteoarthritis affecting other joints. Ann Rheum Dis 2014; 73: 1659–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet 2019; 393: 1745–1759. [DOI] [PubMed] [Google Scholar]
- 3. Hunter DJ, March L, Chew M. Osteoarthritis in 2020 and beyond: a Lancet Commission. Lancet 2020; 396: 1711–1712. [DOI] [PubMed] [Google Scholar]
- 4. Hunter DJ, Schofield D, Callander E. The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol 2014; 10: 437–441. [DOI] [PubMed] [Google Scholar]
- 5. Losina E, Paltiel AD, Weinstein AM, et al. Lifetime medical costs of knee osteoarthritis management in the United States: impact of extending indications for total knee arthroplasty. Arthritis Care Res (Hoboken) 2015; 67: 203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ackerman IN, Pratt C, Gorelik A, et al. Projected burden of osteoarthritis and rheumatoid arthritis in Australia: a population-level analysis. Arthritis Care Res (Hoboken) 2018; 70: 877–883. [DOI] [PubMed] [Google Scholar]
- 7. Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage 2019; 27: 1578–1589. [DOI] [PubMed] [Google Scholar]
- 8. Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol 2020; 72: 220–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Atiquzzaman M, Karim ME, Kopec J, et al. Role of nonsteroidal antiinflammatory drugs in the association between osteoarthritis and cardiovascular diseases: a longitudinal study. Arthritis Rheumatol 2019; 71: 1835–1843. [DOI] [PubMed] [Google Scholar]
- 10. Gregori D, Giacovelli G, Minto C, et al. Association of pharmacological treatments with long-term pain control in patients with knee osteoarthritis: a systematic review and meta-analysis. JAMA 2018; 320: 2564–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O’Neil CK, Hanlon JT, et al. Adverse effects of analgesics commonly used by older adults with osteoarthritis: focus on non-opioid and opioid analgesics. Am J Geriatr Pharmacother 2012; 10: 331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bui VL, Brahn E. Cytokine targeting in rheumatoid arthritis. Clin Immunol 2019; 206: 3–8. [DOI] [PubMed] [Google Scholar]
- 13. Abramson SB, Yazici Y. Biologics in development for rheumatoid arthritis: relevance to osteoarthritis. Adv Drug Deliv Rev 2006; 58: 212–225. [DOI] [PubMed] [Google Scholar]
- 14. Burmester GR, Feist E, Dorner T. Emerging cell and cytokine targets in rheumatoid arthritis. Nat Rev Rheumatol 2014; 10: 77–88. [DOI] [PubMed] [Google Scholar]
- 15. Chevalier X, Eymard F, Richette P. Biologic agents in osteoarthritis: hopes and disappointments. Nat Rev Rheumatol 2013; 9: 400–410. [DOI] [PubMed] [Google Scholar]
- 16. Lin CL, Heron P, Hamann SR, et al. Functional distinction between NGF-mediated plasticity and regeneration of nociceptive axons within the spinal cord. Neuroscience 2014; 272: 76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sachs D, Cunha FQ, Poole S, et al. Tumour necrosis factor-alpha, interleukin-1beta and interleukin-8 induce persistent mechanical nociceptor hypersensitivity. Pain 2002; 96: 89–97. [DOI] [PubMed] [Google Scholar]
- 18. Eibl JK, Strasser BC, Ross GM. Structural, biological, and pharmacological strategies for the inhibition of nerve growth factor. Neurochem Int 2012; 61: 1266–1275. [DOI] [PubMed] [Google Scholar]
- 19. Raychaudhuri SP, Raychaudhuri SK, Atkuri KR, et al. Nerve growth factor: a key local regulator in the pathogenesis of inflammatory arthritis. Arthritis Rheum 2011; 63: 3243–3252. [DOI] [PubMed] [Google Scholar]
- 20. Maksymowych WP, Russell AS, Chiu P, et al. Targeting tumour necrosis factor alleviates signs and symptoms of inflammatory osteoarthritis of the knee. Arthritis Res Ther 2012; 14: R206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lane NE, Schnitzer TJ, Birbara CA, et al. Tanezumab for the treatment of pain from osteoarthritis of the knee. N Engl J Med 2010; 363: 1521–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brown MT, Murphy FT, Radin DM, et al. Tanezumab reduces osteoarthritic knee pain: results of a randomized, double-blind, placebo-controlled phase III trial. J Pain 2012; 13: 790–798. [DOI] [PubMed] [Google Scholar]
- 23. Cohen SB, Proudman S, Kivitz AJ, et al. A randomized, double-blind study of AMG 108 (a fully human monoclonal antibody to IL-1R1) in patients with osteoarthritis of the knee. Arthritis Res Ther 2011; 13: R125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Verbruggen G, Wittoek R, Vander Cruyssen B, et al. Tumour necrosis factor blockade for the treatment of erosive osteoarthritis of the interphalangeal finger joints: a double blind, randomised trial on structure modification. Ann Rheum Dis 2012; 71: 891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Richette P, Ravaud P, Maheu E, et al. A randomized, multicentre, double blind, placebo controlled study of anti TNF ALPHA (ADALIMUMAB) in refractory hand osteoarthritis. Ann Rheum Dis 2013; 72: A54. [DOI] [PubMed] [Google Scholar]
- 26. Birbara C, Dabezies EJ, Jr, Burr AM, et al. Safety and efficacy of subcutaneous tanezumab in patients with knee or hip osteoarthritis. J Pain Res 2018; 11: 151–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang S, Huang Y, Ye Z, et al. The efficacy of nerve growth factor antibody for the treatment of osteoarthritis pain and chronic low-back pain: a meta-analysis. Front Pharmacol 2020; 11: 817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yu Y, Lu ST, Sun JP, et al. Safety of low-dose tanezumab in the treatment of hip or knee osteoarthritis: a systemic review and meta-analysis of randomized phase III clinical trials. Pain Med 2021; 22: 585–595. [DOI] [PubMed] [Google Scholar]
- 29. Fan ZR, Ma JX, Wang Y, et al. Efficacy and safety of tanezumab administered as a fixed dosing regimen in patients with knee or hip osteoarthritis: a meta-analysis of randomized controlled phase III trials. Clin Rheumatol 2021; 40: 2155–2165. [DOI] [PubMed] [Google Scholar]
- 30. Persson MSM, Sarmanova A, Doherty M, et al. Conventional and biologic disease-modifying anti-rheumatic drugs for osteoarthritis: a meta-analysis of randomized controlled trials. Rheumatology (Oxford) 2018; 57: 1830–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cao Z, Li Y, Wang W, et al. Is lutikizumab, an anti-interleukin-1α/β dual variable domain immunoglobulin, efficacious for osteoarthritis? Results from a bayesian network meta-analysis. Biomed Res Int 2020; 2020: 9013283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jüni P, Reichenbach S, Dieppe P. Osteoarthritis: rational approach to treating the individual. Best Pract Res Clin Rheumatol 2006; 20: 721–740. [DOI] [PubMed] [Google Scholar]
- 33. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med 1997; 127: 820–826. [DOI] [PubMed] [Google Scholar]
- 35. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ekman EF, Gimbel JS, Bello AE, et al. Efficacy and safety of intravenous tanezumab for the symptomatic treatment of osteoarthritis: 2 randomized controlled trials versus naproxen. J Rheumatol 2014; 41: 2249–2259. [DOI] [PubMed] [Google Scholar]
- 37. Aitken D, Laslett LL, Pan F, et al. A randomised double-blind placebo-controlled crossover trial of HUMira (adalimumab) for erosive hand OsteoaRthritis – the HUMOR trial. Osteoarthritis Cartilage 2018; 26: 880–887. [DOI] [PubMed] [Google Scholar]
- 38. Chevalier X, Goupille P, Beaulieu AD, et al. Intraarticular injection of anakinra in osteoarthritis of the knee: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum 2009; 61: 344–352. [DOI] [PubMed] [Google Scholar]
- 39. Nagashima H, Suzuki M, Araki S, et al. Preliminary assessment of the safety and efficacy of tanezumab in Japanese patients with moderate to severe osteoarthritis of the knee: a randomized, double-blind, dose-escalation, placebo-controlled study. Osteoarthritis Cartilage 2011; 19: 1405–1412. [DOI] [PubMed] [Google Scholar]
- 40. ClinicalTrials.gov. To Determine the Safety, Tolerability, Pharmacokinetics and Effect on Pain of a Single Intra-articular Administration of Canakinumab in Patients With Osteoarthritis in the Knee, https://clinicaltrials.gov/ct2/show/NCT01160822 (2012, accessed 14 February 2021).
- 41. Spierings ELH, Fidelholtz J, Wolfram G, et al. A phase III placebo- and oxycodone-controlled study of tanezumab in adults with osteoarthritis pain of the hip or knee. Pain 2013; 154: 1603–1612. [DOI] [PubMed] [Google Scholar]
- 42. Brown MT, Murphy FT, Radin DM, et al. Tanezumab reduces osteoarthritic hip pain: results of a randomized, double-blind, placebo-controlled phase III trial. Arthritis Rheum 2013; 65: 1795–1803. [DOI] [PubMed] [Google Scholar]
- 43. Sanga P, Katz N, Polverejan E, et al. Efficacy, safety, and tolerability of fulranumab, an anti-nerve growth factor antibody, in the treatment of patients with moderate to severe osteoarthritis pain. Pain 2013; 154: 1910–1919. [DOI] [PubMed] [Google Scholar]
- 44. Brown MT, Herrmann DN, Goldstein M, et al. Nerve safety of tanezumab, a nerve growth factor inhibitor for pain treatment. J Neurol Sci 2014; 345: 139–147. [DOI] [PubMed] [Google Scholar]
- 45. Tiseo PJ, Kivitz AJ, Ervin JE, et al. Fasinumab (REGN475), an antibody against nerve growth factor for the treatment of pain: results from a double-blind, placebo-controlled exploratory study in osteoarthritis of the knee. Pain 2014; 155: 1245–1252. [DOI] [PubMed] [Google Scholar]
- 46. Chevalier X, Ravaud P, Maheu E, et al. Adalimumab in patients with hand osteoarthritis refractory to analgesics and NSAIDs: a randomised, multicentre, double-blind, placebo-controlled trial. Ann Rheum Dis 2015; 74: 1697–1705. [DOI] [PubMed] [Google Scholar]
- 47. Gow JM, Tsuji WH, Williams GJ, et al. Safety, tolerability, pharmacokinetics, and efficacy of AMG 403, a human anti-nerve growth factor monoclonal antibody, in two phase I studies with healthy volunteers and knee osteoarthritis subjects. Arthritis Res Ther 2015; 17: 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mayorga AJ, Wang S, Kelly KM, et al. Efficacy and safety of fulranumab as monotherapy in patients with moderate to severe, chronic knee pain of primary osteoarthritis: a randomised, placebo- and active-controlled trial. Int J Clin Pract 2016; 70: 493–505. [DOI] [PubMed] [Google Scholar]
- 49. Wang SX, Abramson SB, Attur M, et al. Safety, tolerability, and pharmacodynamics of an anti-interleukin-1α/β dual variable domain immunoglobulin in patients with osteoarthritis of the knee: a randomized phase 1 study. Osteoarthritis Cartilage 2017; 25: 1952–1961. [DOI] [PubMed] [Google Scholar]
- 50. Walicke PA, Hefti F, Bales R, et al. First-in-human randomized clinical trials of the safety and efficacy of tanezumab for treatment of chronic knee osteoarthritis pain or acute bunionectomy pain. Pain Rep 2018; 3: e653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kloppenburg M, Ramonda R, Bobacz K, et al. Etanercept in patients with inflammatory hand osteoarthritis (EHOA): a multicentre, randomised, double-blind, placebo-controlled trial. Ann Rheum Dis 2018; 77: 1757–1764. [DOI] [PubMed] [Google Scholar]
- 52. ClinicalTrials.gov. Treatment Of Knee Osteoarthritis With Intra-Articular Infliximab, https://clinicaltrials.gov/ct2/show/NCT01144143 (2018, accessed 14 February 2021).
- 53. Schnitzer TJ, Easton R, Pang S, et al. Effect of Tanezumab on Joint Pain, Physical Function, and Patient Global Assessment of Osteoarthritis Among Patients With Osteoarthritis of the Hip or Knee: A Randomized Clinical Trial. JAMA 2019; 322: 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kelly KM, Sanga P, Zaki N, et al. Safety and efficacy of fulranumab in osteoarthritis of the hip and knee: results from four early terminated phase III randomized studies. Curr Med Res Opin 2019; 35: 2117–2127. [DOI] [PubMed] [Google Scholar]
- 55. Dakin P, DiMartino SJ, Gao H, et al. The Efficacy, Tolerability, and Joint Safety of Fasinumab in Osteoarthritis Pain: A Phase IIb/III Double-Blind, Placebo-Controlled, Randomized Clinical Trial. Arthritis Rheumatol 2019; 71: 1824–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kloppenburg M, Peterfy C, Haugen IK, et al. Phase IIa, placebo-controlled, randomised study of lutikizumab, an anti-interleukin-1α and anti-interleukin-1β dual variable domain immunoglobulin, in patients with erosive hand osteoarthritis. Ann Rheum Dis 2019; 78: 413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fleischmann RM, Bliddal H, Blanco FJ, et al. A Phase II Trial of Lutikizumab, an Anti-Interleukin-1α/β Dual Variable Domain Immunoglobulin, in Knee Osteoarthritis Patients With Synovitis. Arthritis Rheumatol 2019; 71: 1056–1069. [DOI] [PubMed] [Google Scholar]
- 58. Berenbaum F, Blanco FJ, Guermazi A, et al. Subcutaneous tanezumab for osteoarthritis of the hip or knee: efficacy and safety results from a 24-week randomised phase III study with a 24-week follow-up period. Ann Rheum Dis 2020; 79: 800–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schnitzer TJ, Marks JA. A systematic review of the efficacy and general safety of antibodies to NGF in the treatment of OA of the hip or knee. Osteoarthritis Cartilage 2015; 23 Suppl 1: S8–S17. [DOI] [PubMed] [Google Scholar]
- 60. Kan SL, Li Y, Ning GZ, et al. Tanezumab for patients with osteoarthritis of the knee: a meta-analysis. PLoS ONE 2016; 11: e0157105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chen J, Li J, Li R, et al. Efficacy and safety of tanezumab on osteoarthritis knee and hip pains: a meta-analysis of randomized controlled trials. Pain Med 2017; 18: 374–385. [DOI] [PubMed] [Google Scholar]
- 62. Cao Z, Zhou J, Long Z, et al. Targeting nerve growth factor, a new option for treatment of osteoarthritis: a network meta-analysis of comparative efficacy and safety with traditional drugs. Aging (Albany NY) 2020; 13: 1051–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Seah KTM, Rammanohar J, Sutton J, et al. The effectiveness of anti-nerve growth factor monoclonal antibodies in the management of pain in osteoarthritis of the hip and knee: a PRISMA systematic review and meta-analysis. Pain Med 2021; 22: 1185–1204. [DOI] [PubMed] [Google Scholar]
- 64. Rizzo RRN, Wewege MA, Leake HB, et al. Commentary: the efficacy of nerve growth factor antibody for the treatment of osteoarthritis pain and chronic low-back pain: a meta-analysis. Front Pharmacol 2021; 12: 619344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ghilardi JR, Freeman KT, Jimenez-Andrade JM, et al. Neuroplasticity of sensory and sympathetic nerve fibers in a mouse model of a painful arthritic joint. Arthritis Rheum 2012; 64: 2223–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhao L, Huang J, Fan Y, et al. Exploration of CRISPR/Cas9-based gene editing as therapy for osteoarthritis. Ann Rheum Dis 2019; 78: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. LaBranche TP, Bendele AM, Omura BC, et al. Nerve growth factor inhibition with tanezumab influences weight-bearing and subsequent cartilage damage in the rat medial meniscal tear model. Ann Rheum Dis 2017; 76: 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum 2001; 44: 1237–1247. [DOI] [PubMed] [Google Scholar]
- 69. Goldring SR, Goldring MB. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin Orthop Relat Res 2004; 427 Suppl: S27–S36. [DOI] [PubMed] [Google Scholar]
- 70. Bacconnier L, Jorgensen C, Fabre S. Erosive osteoarthritis of the hand: clinical experience with anakinra. Ann Rheum Dis 2009; 68: 1078–1079. [DOI] [PubMed] [Google Scholar]
- 71. Schieker M, Conaghan PG, Mindeholm L, et al. Effects of interleukin-1beta inhibition on incident hip and knee replacement: exploratory analyses from a randomized, double-blind, placebo-controlled trial. Ann Intern Med 2020; 173: 509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Richette P, François M, Vicaut E, et al. A high interleukin 1 receptor antagonist/IL-1beta ratio occurs naturally in knee osteoarthritis. J Rheumatol 2008; 35: 1650–1654. [PubMed] [Google Scholar]
- 73. Bondeson J, Blom AB, Wainwright S, et al. The role of synovial macrophages and macrophage-produced mediators in driving inflammatory and destructive responses in osteoarthritis. Arthritis Rheum 2010; 62: 647–657. [DOI] [PubMed] [Google Scholar]
- 74. Pelletier JP, Martel-Pelletier J. DMOAD developments: present and future. Bull NYU Hosp Jt Dis 2007; 65: 242–248. [PubMed] [Google Scholar]
- 75. Halvorson KG, Kubota K, Sevcik MA, et al. A blocking antibody to nerve growth factor attenuates skeletal pain induced by prostate tumor cells growing in bone. Cancer Res 2005; 65: 9426–9435. [DOI] [PubMed] [Google Scholar]
- 76. Seidel MF, Lane NE. Control of arthritis pain with anti-nerve-growth factor: risk and benefit. Curr Rheumatol Rep 2012; 14: 583–588. [DOI] [PubMed] [Google Scholar]
- 77. Hochberg MC, Tive LA, Abramson SB, et al. When is osteonecrosis not osteonecrosis?: adjudication of reported serious adverse joint events in the tanezumab clinical development program. Arthritis Rheumatol 2016; 68: 382–391. [DOI] [PubMed] [Google Scholar]
- 78. Hochberg MC. Serious joint-related adverse events in randomized controlled trials of anti-nerve growth factor monoclonal antibodies. Osteoarthritis Cartilage 2015; 23 Suppl 1: S18–S21. [DOI] [PubMed] [Google Scholar]
- 79. Business Wire. Joint FDA advisory committee votes on application for tanezumab for the treatment of osteoarthritis pain. www.businesswire.com/news/home/20210325005905/en/ (2021, accessed 26 May 2021).
- 80. Kivitz AJ, Gimbel JS, Bramson C, et al. Efficacy and safety of tanezumab versus naproxen in the treatment of chronic low back pain. Pain 2013; 154: 1009–1021. [DOI] [PubMed] [Google Scholar]
- 81. Wise BL, Seidel MF, Lane NE. The evolution of nerve growth factor inhibition in clinical medicine. Nat Rev Rheumatol 2021; 17: 34–46. [DOI] [PubMed] [Google Scholar]
- 82. Bondeson J. Are we moving in the right direction with osteoarthritis drug discovery. Expert Opin Ther Targets 2011; 15: 1355–1368. [DOI] [PubMed] [Google Scholar]
- 83. Bougault C, Gosset M, Houard X, et al. Stress-induced cartilage degradation does not depend on the NLRP3 inflammasome in human osteoarthritis and mouse models. Arthritis Rheum 2012; 64: 3972–3981. [DOI] [PubMed] [Google Scholar]
- 84. Rose-John S, Waetzig GH, Scheller J, et al. The IL-6/sIL-6R complex as a novel target for therapeutic approaches. Expert Opin Ther Targets 2007; 11: 613–624. [DOI] [PubMed] [Google Scholar]
- 85. Latourte A, Cherifi C, Maillet J, et al. Systemic inhibition of IL-6/Stat3 signalling protects against experimental osteoarthritis. Ann Rheum Dis 2017; 76: 748–755. [DOI] [PubMed] [Google Scholar]
- 86. Richette P. Efficacy of tocilizumab in patients with hand osteoarthritis: double blind, randomised, placebo-controlled, multicentre trial. Ann Rheum Dis. Epub ahead of print 14 October 2020. DOI: 10.1136/annrheumdis-2020-218547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tab-10.1177_1759720X221080377 for Efficacy and safety of biologic agents for the treatment of osteoarthritis: a meta-analysis of randomized placebo-controlled trials by Fanqiang Meng, Hui Li, Haoran Feng, Huizhong Long, Zidan Yang, Jiatian Li, Yuqing Wang and Dongxing Xie in Therapeutic Advances in Musculoskeletal Disease