Abstract
Background:
The choice between infliximab (IFX) and vedolizumab (VED) as a first-line biological agent in moderate-to-severe ulcerative colitis (UC) can be difficult. Second-line vedolizumab (VED) efficacy may decline following prior infliximab (IFX) treatment failure in UC patients. However, it is not known whether second-line IFX efficacy declines after failure of first-line VED.
Aims:
We aimed to compare first-line and second-line persistence of IFX and VED, in particular whether second-line IFX persistence declines after failure of first-line VED.
Methods:
Persistence of IFX and VED was analysed from the Australian Pharmaceutical Benefits Scheme registry data as either first- or second-line treatment in UC. Propensity score matching (1:1) was conducted in the comparison of first-line treatments. Cox proportional hazard regression analysis was used to identify significant predictors and expressed as a hazard ratio (HR and 95% CI).
Results:
There were 420 subjects with moderate-to-severe UC who received either first-line IFX (n = 251) or VED (n = 169), with 774 patient-years of follow-up. First-line VED had significantly longer persistence than first-line IFX (>50.2 versus 22.2 months, p = 0.001). Fifty-three subjects failed first-line IFX and swapped to second-line VED (IFX→VED group). Twenty-two subjects failed first-line VED group and swapped to second-line IFX (VED→IFX group). First-line VED persistence was significantly longer than second-line VED (>50.2 versus 32.0 months, p = 0.03), but first-line IFX persistence was not statistically significantly different to second-line IFX (27.6 months versus > 38.6 months, p = 0.30). Immunomodulator co-therapy was significantly associated with a lower risk of nonpersistence of first-line VED (HR: 0.55, 95% CI: 0.33–0.89, p = 0.02) and IFX (HR: 0.63,95%CI: 0.33–0.92, p = 0.02).
Conclusion:
VED had a significantly longer persistence than IFX as first-line biological agent but does not disadvantage second-line IFX use in moderate-to-severe UC. VED after IFX is associated with significantly poorer persistence. VED, therefore, should be considered as the first-line biological agent of choice in UC.
Keywords: infliximab, sequencing, ulcerative colitis, vedolizumab
Introduction
Ulcerative colitis (UC) is an increasingly prevalent chronic inflammatory bowel disease (IBD) with 15% of patients having an aggressive disease course.1,2 The cumulative risk of relapse is 70–80% at 10 years 2 and as such, many patients require maintenance treatment with an advanced therapy. 3 Recent guidelines recommend the use of infliximab (IFX) or vedolizumab (VED) as a first-line biological agent for patients with moderate-to-severe UC who are naïve to biological therapy. 4 However, 10–40% of patients have a primary nonresponse to IFX, while 23–46% of patients have a secondary loss of response to IFX at 12 months after induction. 5 Furthermore, 35% of patients lose response to VED at 12 months. 6 Therefore, many patients will switch to a second-line biological agent. VED may be less effective in those who have failed prior anti-tumour necrosis factor-alpha (anti-TNFα) therapy including IFX.7,8 The effectiveness of second-line IFX after failure of VED, however, is less well-known. The optimal sequencing of these agents as first- and second-line agents is a key knowledge gap that requires addressing to reduce disease burden. 9
Treatment persistence measures continuing medication prescription based on ongoing therapeutic efficacy in the absence of significant adverse effects. 10 Persistence reflects willingness by patient and their physician to continue a medication in the absence of therapeutic failure or a better treatment alternative. 11 Importantly, it reflects real-world practice that allows for treatment optimisation including dose-adjustment, addition of temporary induction treatments and other strategies. The rate of failure and duration of persistence for various drugs are different and provide important comparative efficacy data given the paucity of head-to-head trials of advanced therapies in UC.10,12
Persistence data, in some respects, may be even more informative than head-to-head trials. Population-based persistence data are cost-efficient and might include sufficient statistical power to identify clear clinical differences through long-term follow-up. Persistence data may be similar to clinical drug trials in that both have strict inclusion and exclusion eligibility criteria, collect data prospectively, include data on prior or ongoing use of conventional and biological agents, and are multicentre and generalizable. The Persistence in Australian National IBD Cohort (PANIC) is a large real-world national population-based registry with 8219 patient-years of follow-up, in which biological agents were fully funded without stipulated hierarchical prescribing order. 13 Hence, this database allows for an objective comparison of persistence between biological agents.
The primary aim of this population-based prospective study was to compare the persistence of IFX and VED as first- and second-line therapies, including second-line IFX following VED failure versus first-line IFX use. Factors affecting the persistence of these biological agents were examined.
Methods
Study population
Consecutive adult subjects (aged ⩾18 years) prescribed IFX or VED for the treatment of moderate-to-severe UC were included. The diagnosis of moderate-to-severe UC had to be definite using the Copenhagen diagnostic criteria 14 and we excluded biological agents prescribed to treat acute severe UC, Crohn’s disease or rheumatological/dermatological indications.
Study design and data source
The Persistence Australian National IBD Cohort (PANIC) study used prospective script data, including co-therapy with immunomodulators, collected from the Australian Pharmaceutical Benefits Scheme (PBS) database from December 2014 to September 2019. The PBS subsidises treatment with biological agents under the National Health Act 1953. Patients with UC and a partial Mayo score of ⩾6 are provided biological agents at no cost if they fail to achieve adequate treatment response following a 3-month course of mesalazine and either 3 months of thiopurine (or shorter due to toxicity) or at least a 6-week course of a corticosteroid. 15 Following induction therapy, demonstration of response to PBS subsidised treatment must be conducted 6-monthly in order to continuing maintenance treatment. The PBS does not enforce hierarchical prescribing order so there is no need to prescribe anti-TNFα agents or biosimilars prior to VED. Script data were linked to the patients’ unique Medicare number. Data were analysed using a 10% random sample, which is a standardised accepted strategy. 13 PBS data were acquired and collated by an independent provider (Model Solutions, Australia) and validated through a second provider to ensure robustness of data. All analyses, however, were performed by the researchers.
Study definitions
Persistence was defined as the duration of time from initiation to discontinuation of biological therapy for moderate-to-severe UC. 11 Nonpersistence was defined as discontinuation of that agent and represented objectively as a switch in or out of biological class or complete cessation. Persistence rate was the proportion of patients who continued on a biological agent at a particular time point. Immunomodulator co-therapy was defined as the concurrent use of a thiopurine or methotrexate during the period of biological agent use.
Sample size calculation
Sample size calculation was based on results from a retrospective real-world study that found a persistence rate of 78% for VED patients and 64% for IFX patients with IBD at 24 months. 16 Assuming a two-sided alpha of 0.05 and a power of 80%, a total of 249 IFX and 166 VED patients would be required at a ratio of three IFX patients to every two VED patients. The 3:2 ratio of IFX:VED was based on PBS biological agent prescription use for UC in Australia. 17
Study outcomes, propensity score matching and statistical analysis
Baseline characteristics of continuous variables were described as medians with interquartile ranges (IQR). Categorical variables were described as percentages. Outcomes comparing the persistence of first-line and second-line IFX and VED were analysed using unadjusted Kaplan–Meier survival curves and statistically significant differences in persistence were identified using the log-rank test. Cox proportional hazard regression was used to identify factors affecting persistence, adjusting for covariates and described as hazard ratios (HR) and 95% confidence intervals (CI). Secondary outcomes included comparing the persistence rates between each biological agent at 12, 24, 36 and 48 months using chi-square testing. We also analysed the persistence of first-line IFX and VED stratified by immunomodulator co-therapy using unadjusted Kaplan–Meier survival curves and log-rank testing. Propensity score matching was also performed in first-line therapy comparisons to control for age and gender as possible confounders of medication persistence. Using the propensity scores, first-line VED patients were matched with first-line IFX patients using a 1:1 matching algorithm, with a proximity calliper of 0.1. A p value of <0.05 was considered statistically significant. Bonferroni correction was applied in the setting of multiple comparisons during analysis of persistence rates. Statistical analysis was performed using IBM SPSS software version 27.0 (SPSS Inc., Chicago, IL, USA).
Ethics approval
The study was approved by the Services Australia External Request Evaluation Ethics Committee with approval number RMS1531. The reporting of this study conforms to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. 18
Results
Study population and baseline characteristics
In total, 420 patients were prescribed either IFX (251 patients) or VED (169 patients) as a first-line biological agent for moderate-to-severe UC, equating to 774 person-years of follow-up. Males comprised 54.2% (136/251) of the IFX group and 49.7% (84/169) of the VED group (p = 0.38). The median age of the VED group (44.0 years, IQR: 33.0–56.0) was older than the IFX group (37.0 years, IQR: 28.0–54.0, p = 0.003). The baseline characteristics of the study population are shown in Table 1.
Table 1.
Baseline characteristics of moderate-to-severe UC patients using first-line and second-line infliximab or vedolizumab.
| Biological agent | Infliximab n = 251 |
Vedolizumab n = 169 |
p-value |
|---|---|---|---|
| Male, n (%) | 136 (54.2%) | 84 (49.7%) | 0.38 |
| Median age, years (IQR) | 37.0 (28.0–54.0) | 44.0 (33.0–56.0) | 0.003 |
| Immunomodulator co-therapy, n (%) | 160 (63.7%) | 80 (47.3%) | 0.001 |
| Thiopurine, n (%) | 145 (57.8%) | 75 (44.4%) | 0.01 |
| Methotrexate, n (%) | 21 (8.4%) | 7 (4.1%) | 0.09 |
| Time on combination therapy between biological agent and immunomodulator, months (IQR) | 17.3 (5.1–27.6) | 13.3 (3.6–17.5) | 0.03 |
IQR, interquartile range; UC, ulcerative colitis.
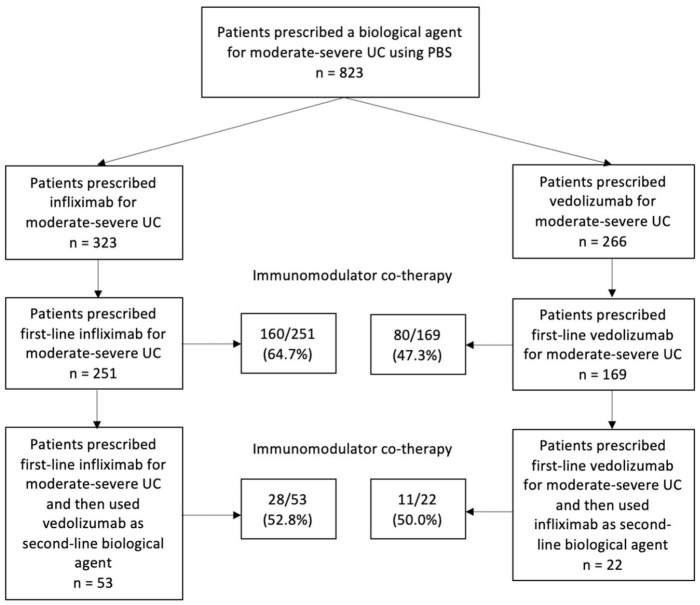
Figure 1 illustrates the PBS prescription of IFX and VED as first-line and second-line biological agents. From the 251 patients who used IFX as a first-line biological agent, 53 used VED as a second-line biological agent (IFX→VED group). From the 169 patients who used VED as a first-line biological agent, 22 used IFX as a second-line biological agent (VED→IFX group). The baseline characteristics of the IFX→VED and VED→IFX groups are shown in Table 2. The median ages of the IFX→VED and VED→IFX groups were 38.0 years (IQR: 28.5–55.0) and 41.5 years (IQR: 29.5–58.8), respectively (p = 0.30). About 67.9% (36/53) of the IFX→VED group were treated with immunomodulator co-therapy during first-line biological agent use, compared with 36.4% (8/22) of the VED→IFX group (p = 0.01). About 52.8% (28/53) of the IFX→VED group were treated with immunomodulator co-therapy during second-line biological agent use, compared with 50.0% (11/22) of the VED→IFX group (p = 0.82).
Figure 1.
Flow diagram of infliximab and vedolizumab choice in moderate-to-severe UC from PBS.
Table 2.
Baseline characteristics of moderate-to-severe UC patients using second-line vedolizumab or infliximab (IFX→VED and VED→IFX groups, respectively).
| Biological agent | IFX→VED n = 53 |
VED→IFX n = 22 |
p-value |
|---|---|---|---|
| Male, n (%) | 32 (60.4%) | 14 (63.6%) | 0.79 |
| Median age, years (IQR) | 38.0 (28.5 – 55.0) | 41.5 (29.5 – 58.8) | 0.30 |
| First-line immunomodulator co-therapy | 36 (67.9%) | 8 (36.4%) | 0.01 |
| Second-line immunomodulator co-therapy | 28 (52.8%) | 11 (50.0%) | 0.82 |
IFX, infliximab; IQR, interquartile range; UC, ulcerative colitis; VED, vedolizumab.
Persistence of infliximab and vedolizumab as first-line biological agents in moderate-to-severe UC
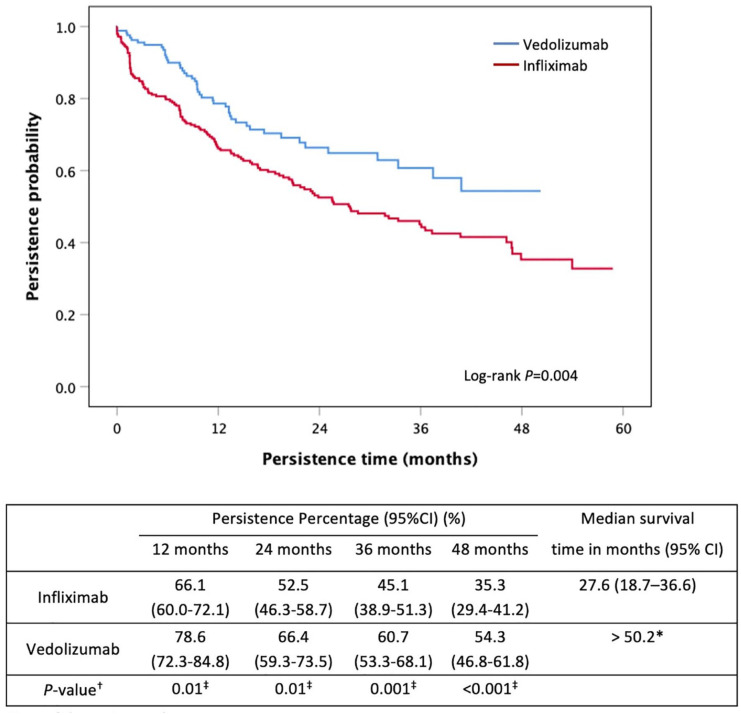
VED had a significantly longer persistence than IFX when used as a first-line biological agent in moderate-to-severe UC [>50.2 months versus 27.6 months (95%CI: 18.7–36.6), p = 0.004] (Figure 2). VED use was associated with a 43% lower risk of nonpersistence than IFX (HR: 0.57, 95% CI: 0.41–0.80, p = 0.001) (Table 3). Persistence rates and their 95% CIs at 12, 24, 36 and 48 months are reported in Figure 2. Immunomodulator co-therapy use was higher in the IFX group (63.7%, 160/251) than the VED group (47.3%, 80/169; p = 0.001) and was associated with a 44% lower risk of nonpersistence of first-line biological therapy (HR: 0.56, 95% CI: 0.42–0.77, p < 0.001).
Figure 2.
Kaplan–Meier curve for persistence of first-line infliximab and vedolizumab in moderate-to-severe UC.
Table 3.
Predictors of medication nonpersistence.
| HR (95% CI) | p-value | |
|---|---|---|
| First-line biological therapy of vedolizumab and infliximab | ||
| Female versus male gender | 1.03 (0.76–1.39) | 0.84 |
| Age | 1.00 (0.99–1.01) | 0.67 |
| VED versus IFX | 0.57 (0.41–0.80) | 0.001 |
| Immunomodulator co-therapy | 0.56 (0.42–0.77) | <0.001 |
| Second-line biological therapy of infliximab (VED→IFX group) and vedolizumab (IFX→VED) group | ||
| Female versus male gender | 0.61 (0.25–1.49) | 0.28 |
| Age | 0.98 (0.96–1.01) | 0.34 |
| VED versus IFX | 1.66 (0.63–4.40) | 0.31 |
| Immunomodulator co-therapy | 0.66 (0.30–1.47) | 0.31 |
| Vedolizumab use as a first-line or second-line biological therapy | ||
| Female versus male gender | 0.85 (0.52–1.39) | 0.51 |
| Age | 1.00 (0.98–1.01) | 0.59 |
| First-line VED versus second-line VED | 0.56 (0.34–0.92) | 0.02 |
| Immunomodulator co-therapy | 0.55 (0.33–0.89) | 0.02 |
| Infliximab use as a first-line or second-line biological therapy | ||
| Female versus male gender | 0.86 (0.59–1.25) | 0.43 |
| Age | 1.00 (0.99–1.01) | 0.90 |
| First-line IFX versus second-line IFX | 1.49 (0.61–3.67) | 0.39 |
| Immunomodulator co-therapy | 0.63 (0.44–0.92) | 0.02 |
CI, confidence interval; HR, hazard ratio; IFX, infliximab; VED, vedolizumab.
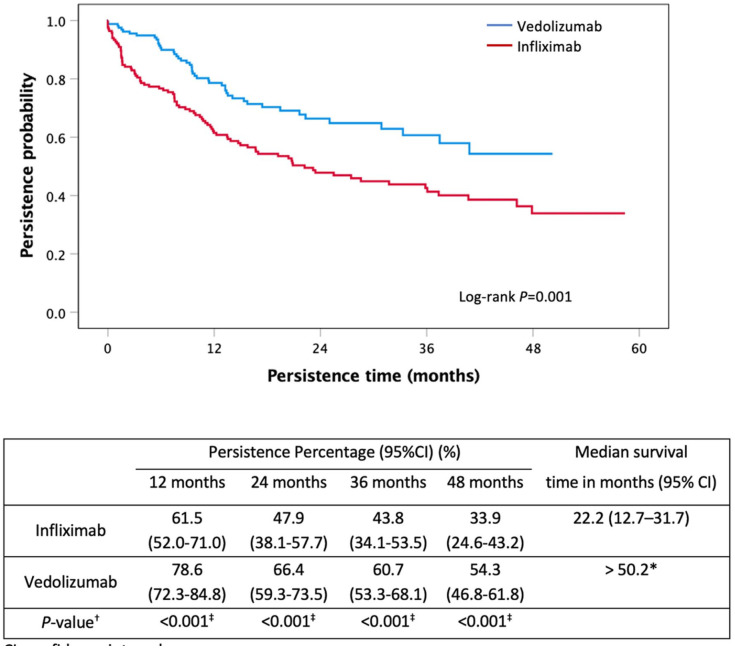
Using propensity score matching, 169 IFX patients were matched with 169 VED patients using age and gender as covariates. VED had a significant longer persistence than IFX as a first-line biological agent (>50.2 months versus 22.2 months, p = 0.001) (Figure 3).
Figure 3.
Kaplan–Meier curve for persistence of first-line infliximab and vedolizumab in moderate-to-severe UC using propensity score matching.
Persistence of infliximab and vedolizumab as second-line biological agents in moderate-to-severe UC
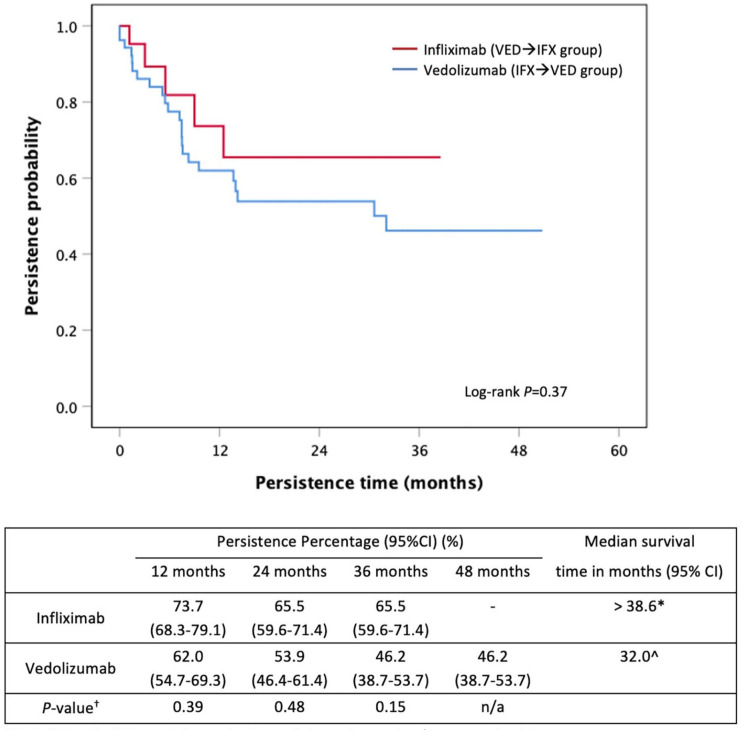
There was no significant difference between the persistence of second-line IFX in the VED→IFX group versus second-line VED use in the IFX→VED group (>38.6 months versus 32.0 months, p = 0.37, Figure 4). However, there were numerically higher persistence rates with second-line IFX in the VED→IFX group compared with second-line VED in the IFX→VED group at 12 and 24 months (Figure 4). No significant predictors of nonpersistence were identified (Table 3).
Figure 4.
Kaplan–Meier curve for persistence of second-line infliximab and vedolizumab in VED → IFX and IFX → VED groups, respectively.
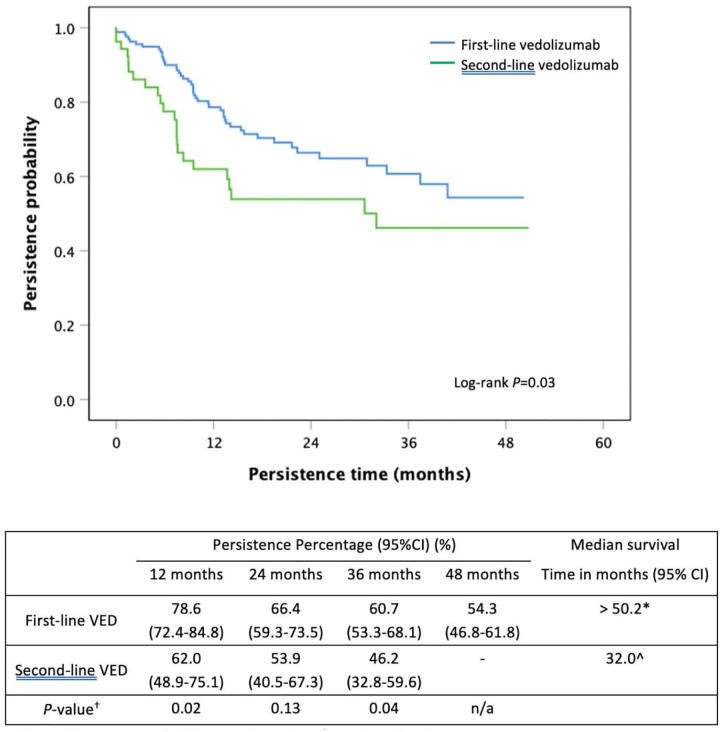
Persistence comparison between first-line vedolizumab and second-line vedolizumab
First-line VED use had a significantly longer persistence than when used as a second-line biological agent (>50.2 months versus 32.0 months, p = 0.03, Figure 5). First-line VED use was associated with a 44% lower risk of nonpersistence compared with second-line VED use (HR: 0.56, 95% CI: 0.34–0.92, p = 0.02). Persistence rates at 12, 24 and 36 months between first-line VED and second-line VED are shown in Figure 5. Immunomodulator co-therapy occurred in 47.3% (80/169) of the first-line VED group versus 52.8% (28/53, p = 0.49) of the second-line VED group, and was associated with a 45% lower risk of nonpersistence of VED therapy (HR: 0.55, 95% CI: 0.33–0.89, p = 0.02).
Figure 5.
Kaplan–Meier curve for persistence of first-line vedolizumab compared with second-line vedolizumab in moderate-to-severe UC.
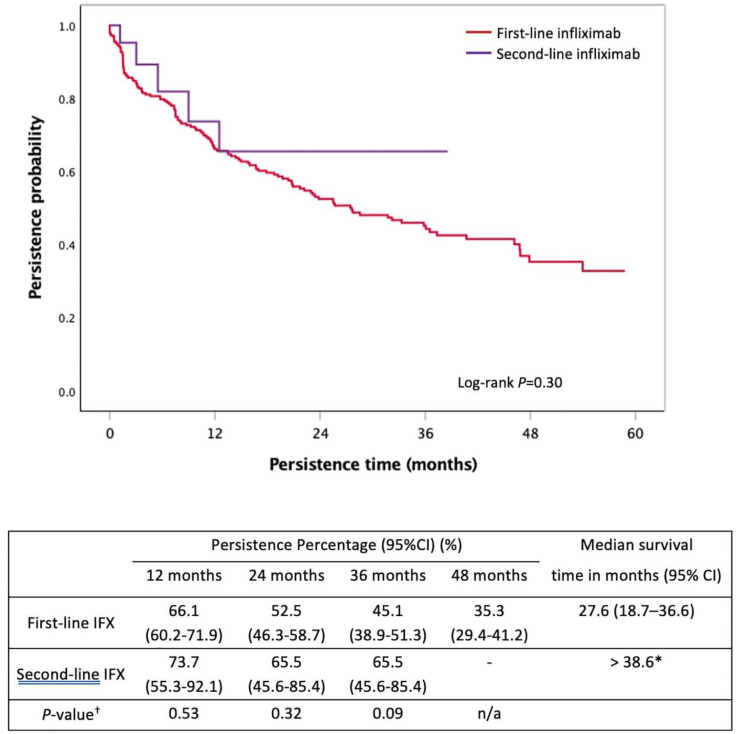
Persistence comparison between first-line infliximab and second-line infliximab
There was no significant difference between first-line IFX and second-line IFX persistence [27.6 months (95% CI: 18.7–36.6) versus > 38.6 months, p = 0.30, Figure 6]. The use of IFX as a first- versus second-line agent did not significantly predict for nonpersistence (HR: 1.49, 95%CI 0.61–3.67, p = 0.39). Immunomodulator co-therapy occurred in 64.7% (160/251) of the first-line IFX group versus 50.0% (11/22, p = 0.20) of the second-line IFX group and was associated with a 37% lower risk of nonpersistence of IFX therapy (HR: 0.63, 95% CI: 0.44–0.92, p = 0.02).
Figure 6.
Kaplan–Meier curve for persistence of first-line infliximab compared with second-line infliximab in moderate-to-severe UC.
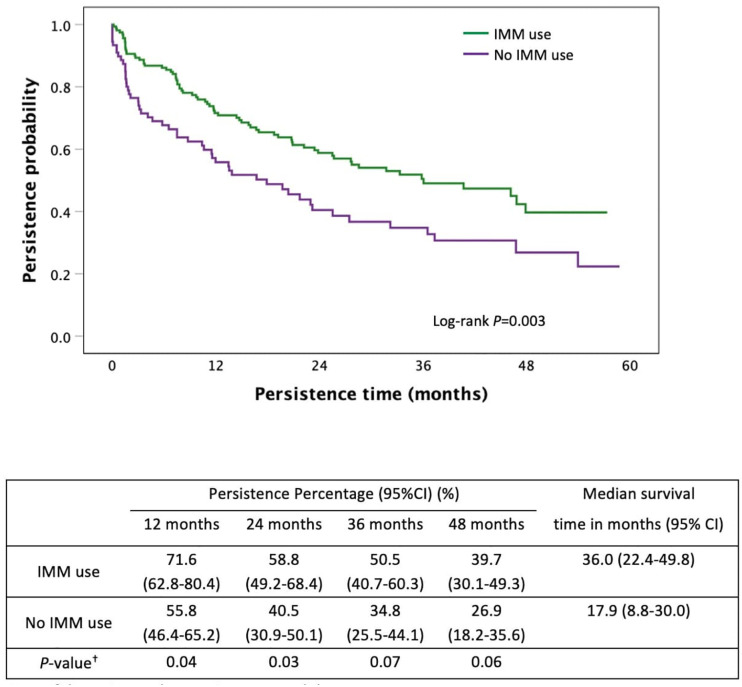
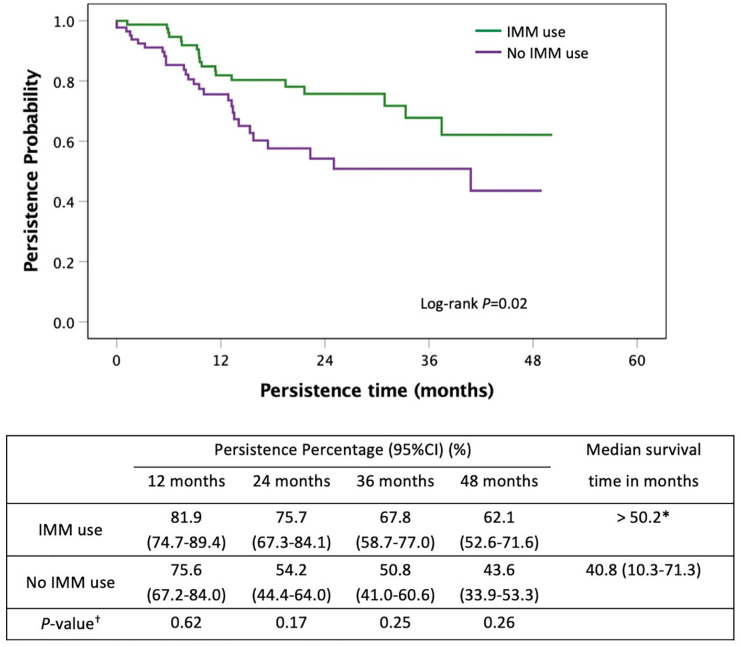
Persistence of infliximab and vedolizumab stratified by immunomodulator use
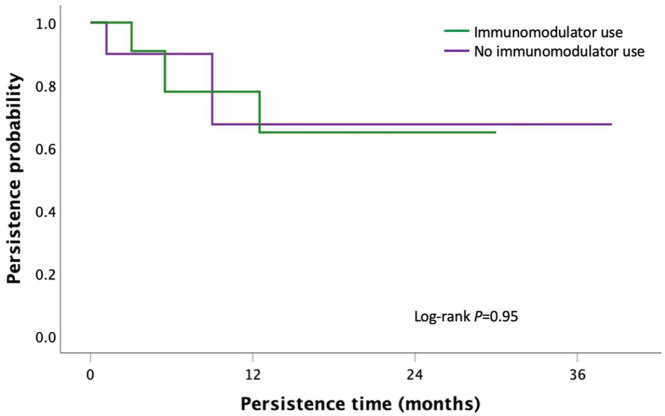
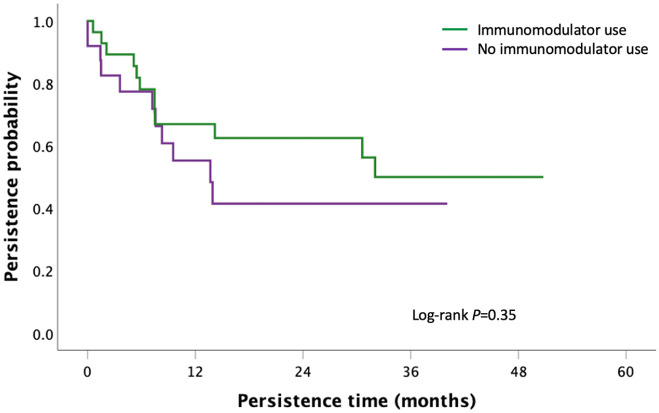
First-line IFX and first-line VED had a significantly longer persistence when used in combination with an immunomodulator compared with monotherapy [36.0 months (95% CI: 22.4–49.8) versus 17.9 months (95% CI: 8.8–26.9), p = 0.003 for IFX group; > 50.2 months versus 40.8 months (95% CI: 10.3–71.3), p = 0.02 for VED group, Figures 7 and 8]. There was no significant difference in persistence between second-line IFX (p = 0.95) and VED (p = 0.30) when stratified by immunomodulator co-therapy (Figures 9 and 10).
Figure 7.
Kaplan–Meier curve for persistence of first-line infliximab stratified by immunomodulator co-therapy in moderate-to-severe UC.
Figure 8.
Kaplan–Meier curve for persistence of first-line vedolizumab stratified by immunomodulator co-therapy in moderate-to-severe UC.
Figure 9.
Kaplan–Meier curve for persistence of second-line infliximab stratified by immunomodulator co-therapy in moderate-to-severe UC.
Figure 10.
Kaplan–Meier curve for persistence of second-line vedolizumab stratified by immunomodulator co-therapy in moderate-to-severe UC.
Discussion
In this large population-based prospective registry providing 774 patient-years of real-world follow-up, the persistence of VED was significantly longer than IFX as a first-line biological agent in moderate-to-severe UC. Persistence of second-line IFX after VED exposure was not significantly impaired when compared against second-line VED after IFX exposure. First-line VED persistence had significantly longer persistence than second-line VED. There was no significant difference in persistence between first-line IFX and second-line IFX. These data support the use of VED as first-line biological treatment in moderate-to-severe UC given the longer persistence when used first-line and the decline in persistence when used second-line. Conversely, IFX persistence when used as a second-line agent after switch from VED was not impaired.
There is a lack of data on the long-term effectiveness of biological agents in UC, and persistence data provide an indirect approach for assessing the long-term therapeutic benefit and safety of therapies. 19 The VARSITY (An Efficacy and Safety Study of Vedolizumab Intravenous [IV] Compared to Adalimumab Subcutaneous [SC] in Participants With Ulcerative Colitis) study recently demonstrated that VED was superior to adalimumab in achieving clinical remission and endoscopic improvement; however, there are no direct comparisons between IFX and VED. 12 In the absence of head-to-head comparisons between IFX and VED, real-world persistence data act as a surrogate marker for treatment efficacy, safety and acceptability by patients and physicians. 19 Our results demonstrate significantly greater persistence of first-line VED over first-line IFX in both short term (12 months) and long term (24, 36 and 48 months) (Figure 2). The results from this PANIC study are supported by a retrospective study which also demonstrated that VED had higher persistence rates than IFX in biological-naïve IBD patients. 16 The addition of propensity score matching was relevant due to the significant increased age of VED subjects compared with IFX in the PANIC registry. We previously demonstrated the increasing age of IBD subjects in developed countries 1 and for age to be a significant determinant in the deciding a biological agent in IBD. 20 Therefore, propensity score matching was performed to reduce the selection bias associated with older age and the risk of adverse effects. 21
Consistent with other studies,7,8 we found VED had significantly longer persistence when used as a first-line agent versus second-line use following IFX failure. To our knowledge, however, there are no data comparing second-line biological agents in IFX→VED with VED→IFX sequences. There is a need to explore the correct positioning of biological agents, as less than half of all UC patients continue their first-line biological agent after 1 year. 19 The persistence of second-line IFX (after VED exposure) of 65.5% (95% CI: 59.6–71.4%) compared against second-line VED of 46.2% (95% CI: 38.7–53.7%) at 36 months (p = 0.15, Figure 3) suggests a trend of IFX being a better second-line agent than VED. There have been no studies that evaluated persistence up to 3 years previously with others limited to only 6 months of follow-up.22,23 The persistence of IFX was not dissimilar when used first-line or second-line following VED.
First-line VED use did not impair the effectiveness of second-line IFX use, as supported by the EVOLVE study which also found that anti-TNF effectiveness may not be compromised by prior VED exposure. 24 However, our study found that IFX therapy impaired subsequent VED persistence.7,8 A possible explanation is a pharmacokinetic impact of prior IFX exposure on VED trough levels, with one study noting lower VED trough levels at week 6 compared with biological-naïve patients (22.5 versus 36.0 µg/ml, p = 0.03). 25 Previous research has also demonstrated that MAdCAM-1 is downregulated by TNFα blockade, so prior IFX-exposed patients may need higher doses of vedolizumab to bind a higher target burden. 26 These detectable biological changes, therefore, support VED to be used as first-line biological agent in moderate-to-severe UC, followed by IFX as a second-line agent.
Immunomodulator use improved the persistence of first-line IFX and first-line VED. Immunomodulator co-therapy is known to reduce immunogenicity and improve the persistence and effectiveness of IFX. 13 However, the benefit of combination immunotherapy with VED is less clear. This is the first study to demonstrate the increased persistence of first-line VED when combined with immunomodulators through the use of prospectively collected data for immunomodulators in our PANIC registry. We found larger differences in persistence rates between combination VED and immunomodulator co-therapy versus VED monotherapy after 12 months of VED use (Figure 8), suggesting a greater benefit with long-term follow-up. Other studies suggest a lack of advantage of combination therapy versus monotherapy but were limited to 6–12 months of data and may not have verified ongoing immunomodulator use. 27 The smaller sample size of the EVOLVE study (22 patients in the combination group) identified numerically higher persistence in the combination group at 18 and 24 months but might be underpowered to demonstrate statistical significance. 28 It is possible that newer monoclonal antibodies, although less immunogenic than older anti-TNFα agents, are still complex glycoproteins that may result in the eventual development of antibodies. Additive anti-inflammatory effects of immunomodulators with VED are also possible. A prospective study primarily focusing on the benefits of combination therapy with VED is recommended.
This study has limitations. First, this study was not a randomised controlled trial controlling for patient characteristics and disease severity. However, there is no proposed randomised controlled trial of IFX versus VED currently planned, and it would not be possible to include a sufficient sample size and follow-up duration to be able to follow-up those that require switching to a second-line treatment. Real-world evidence with sufficient patient-years of follow-up may address these data gaps. Second, the clinical, biochemical, and endoscopic outcomes are not known. Although patients with acute severe UC were excluded, it is possible that patients were prescribed IFX as they had a more severe phenotype, hence biasing the results. However, as only experienced gastroenterologists prescribe these agents in Australia, it is expected that relevant clinical information would be incorporated into any decision to continue or discontinue treatment. The population-based database also means data are not limited to specialised centres so that data can be generalised. Third, data on therapeutic drug monitoring and corticosteroid use are unknown. However, current conventional practice allows for treatment escalation of IFX and VED to be provided under compassionate access at no cost to the hospitals or subjects so it is likely that dose optimisation is performed prior to treatment switching. Whereas clinical trials might deem such dose escalations as treatment failure on intention-to-treat, persistence data allow these subjects to be included and provide a more realistic comparison of treatment efficacy. Ideally, correcting for clinical characteristics beyond age in propensity score matching might further reduce bias in comparing subjects on VED versus IFX. However, age was the most important factor identified previously 18 and subjected to propensity score matching. Also, there was a relatively small number of patients in the second-line groups which may impact on the results. However, there is limited available data on second-line use (particularly IFX) so our results will be beneficial. Finally, only a randomly selected 10% of the population database could be used for analysis due to national ethical criteria for data accessibility. However, this was a random sampling without selection bias and similar publications were deemed robust given the large patient-years of follow-up. 29
Strengths include the use of a large, national, population-based study where biological agents are prescribed without a hierarchical prescribing order and are fully reimbursed indefinitely. All subjects had verified Mayo scores of ⩾6 which indicates consistency of disease severity. All data were collected prospectively with clinical remission being an absolute requirement for ongoing treatment. Whether treatment escalation was performed or concurrent immunomodulator was used was decided between the clinician and patients consistent with real-world practice. Furthermore, individual switches to other biological agents and immunomodulator co-therapy are prospectively recorded and long-term follow-up data beyond 4 years is an advantage over clinical trials that have relatively short durations of follow-up. The population-based data also mean the results are more generalizable. In the absence of large head-to-head studies with long-term follow-up, population-based persistence data can effectively compare the efficacy of IFX versus VED.
Conclusion
In summary, the PANIC cohort with real-world data set of nonhierarchical prescribing of biological agents supports the use of VED over IFX as a first-line biological agent in moderate-to-severe UC. Following failure of VED, there is no impact on IFX persistence when used second-line. Immunomodulator treatment significantly increases the persistence of VED, but further prospective data are required to confirm this observation. Our findings will assist in the positioning of biological therapies in moderate-to-severe UC.
Supplemental Material
Supplemental material, sj-docx-1-tag-10.1177_17562848221080793 for Vedolizumab has longer persistence than infliximab as a first-line biological agent but not as a second-line biological agent in moderate-to-severe ulcerative colitis: real-world registry data from the Persistence Australian National IBD Cohort (PANIC) study by Aviv Pudipeddi, Yanna Ko, Sudarshan Paramsothy and Rupert W. Leong in Therapeutic Advances in Gastroenterology
Acknowledgments
We acknowledge Services Australia for providing the Pharmaceutical Benefits Scheme data for this research project.
Footnotes
Author contributions: Aviv Pudipeddi: Data curation; Formal analysis; Methodology; Resources; Software; Writing – original draft; Writing – review & editing.
Yanna Ko: Formal analysis; Writing – review & editing.
Sudarshan Paramsothy: Conceptualisation; Writing – review & editing.
Rupert Leong: Conceptualisation; Data curation; Formal analysis; Resources; Supervision; Visualisation; Writing – review & editing.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Aviv Pudipeddi has received speaker fees from Takeda. Yanna Ko reports no conflicts. Sudarshan Paramsothy is a consultant for Finch Therapeutics and received speaker fees from Ferring, Janssen and Takeda. Rupert Leong reports advisory board fees from AbbVie, Aspen, Celgene, Ferring, Gilead, Hospira, Janssen, MSD, Pfizer and Takeda; research fees from Gastrointestinal Society of Australia (GESA), Endochoice, Janssen, National Health and Medical Research Council of Australia, Shire and Takeda; and speaker fees from Emerge Health, Ferring, Janssen, Shire and Takeda.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Rupert W. Leong  https://orcid.org/0000-0001-5944-3488
https://orcid.org/0000-0001-5944-3488
The data underlying this article was provided by Services Australia by permission. Deidentified individual participant data will be shared on request to the corresponding author with permission from Services Australia.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Aviv Pudipeddi, Gastroenterology and Liver Services, Concord Repatriation General Hospital, Sydney, NSW, Australia; Faculty of Medicine and Health, Concord Clinical School, The University of Sydney, Sydney, NSW, Australia.
Yanna Ko, Gastroenterology and Liver Services, Concord Repatriation General Hospital, Sydney, NSW, Australia; Faculty of Medicine and Health, Concord Clinical School, The University of Sydney, Sydney, NSW, Australia.
Sudarshan Paramsothy, Gastroenterology and Liver Services, Concord Repatriation General Hospital, Sydney, NSW, Australia; Faculty of Medicine and Health, Concord Clinical School, The University of Sydney, Sydney, NSW, Australia.
Rupert W. Leong, Gastroenterology and Liver Services, Concord Repatriation General Hospital, Hospital Road, Concord, Sydney, NSW 2139, Australia; Faculty of Medicine and Health, Concord Clinical School, The University of Sydney, Sydney, NSW, Australia.
References
- 1. Pudipeddi A, Liu J, Kariyawasam V, et al. High prevalence of Crohn disease and ulcerative colitis among older people in Sydney. Med J Aust 2021; 214: 365–370. [DOI] [PubMed] [Google Scholar]
- 2. Fumery M, Singh S, Dulai PS, et al. Natural history of adult ulcerative colitis in population-based cohorts: a systematic review. Clin Gastroenterol Hepatol 2018; 16: 343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jeong DY, Kim S, Son MJ, et al. Induction and maintenance treatment of inflammatory bowel disease: a comprehensive review. Autoimmun Rev 2019; 18: 439–454. [DOI] [PubMed] [Google Scholar]
- 4. Feuerstein JD, Isaacs KL, Schneider Y, et al. AGA clinical practice guidelines on the management of moderate to severe ulcerative colitis. Gastroenterology 2020; 158: 1450–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ben-Horin S, Kopylov U, Chowers Y. Optimizing anti-TNF treatments in inflammatory bowel disease. Autoimmun Rev 2014; 13: 24–30. [DOI] [PubMed] [Google Scholar]
- 6. Schmidt E, Kochhar G, Hartke J, et al. Predictors and management of loss of response to vedolizumab in inflammatory bowel disease. Inflamm Bowel Dis 2018; 24: 2461–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Feagan BG, Rubin DT, Danese S, et al. Efficacy of vedolizumab induction and maintenance therapy in patients with ulcerative colitis, regardless of prior exposure to tumor necrosis factor antagonists. Clin Gastroenterol Hepatol 2017; 15: 229–239. [DOI] [PubMed] [Google Scholar]
- 8. Narula N, Peerani F, Meserve J, et al. Vedolizumab for ulcerative colitis: treatment outcomes from the VICTORY Consortium. Am J Gastroenterol 2018; 113: 1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Danese S, Fiorino G, Peyrin-Biroulet L. Positioning therapies in ulcerative colitis. Clin Gastroenterol Hepatol 2020; 18: 1280–1290. [DOI] [PubMed] [Google Scholar]
- 10. Patel H, Lissoos T, Rubin DT. Indicators of suboptimal biologic therapy over time in patients with ulcerative colitis and Crohn’s disease in the United States. PLoS One 2017; 12: e0175099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health 2008; 11: 44–47. [DOI] [PubMed] [Google Scholar]
- 12. Sands BE, Peyrin-Biroulet L, Loftus EV, Jr, et al. Vedolizumab versus adalimumab for moderate-to-severe ulcerative colitis. N Engl J Med 2019; 381: 1215–1226. [DOI] [PubMed] [Google Scholar]
- 13. Ko Y, Paramsothy S, Yau Y, et al. Superior treatment persistence with ustekinumab in Crohn’s disease and vedolizumab in ulcerative colitis compared with anti-TNF biological agents: real-world registry data from the Persistence Australian National IBD Cohort (PANIC) study. Aliment Pharmacol Ther 2021; 54: 292–301. [DOI] [PubMed] [Google Scholar]
- 14. Langholz E. Ulcerative colitis. An epidemiological study based on a regional inception cohort, with special reference to disease course and prognosis. Dan Med Bull 1999; 46: 400–415. [PubMed] [Google Scholar]
- 15. Australian Government, Services Australia. Ulcerative colitis – adult patient, 2020, https://www.servicesaustralia.gov.au/organisations/health-professionals/forms/pb127 (accessed 20 March 2020).
- 16. Patel H, Latremouille-Viau D, Burne R, et al. Comparison of real-world treatment outcomes with vedolizumab versus infliximab in biologic-naïve patients with inflammatory bowel disease. Crohns Colitis 360 2019; 1: otz022. [Google Scholar]
- 17. Pharmaceutical Benefits Scheme. Analysis of medicines used to treat ulcerative colitis: drug utilisation sub-committee (DUSC), 2017, https://www.pbs.gov.au/industry/listing/participants/public-release-docs/2017-06/ulcerative-colitis-dusc-prd-2017-06.pdf (accessed 5 November 2020).
- 18. Von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007; 335: 806–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen C, Hartzema AG, Xiao H, et al. Real-world pattern of biologic use in patients with inflammatory bowel disease: treatment persistence, switching, and importance of concurrent immunosuppressive therapy. Inflamm Bowel Dis 2019; 25: 1417–1427. [DOI] [PubMed] [Google Scholar]
- 20. Chan W, Kariyawasam V, Kim S, et al. Gastroenterologists’ preference and risk perception on the use of immunomodulators and biological therapies in elderly patients with ulcerative colitis: an international survey. Eur J Gastroenterol Hepatol 2020; 32: 976–983. [DOI] [PubMed] [Google Scholar]
- 21. Pudipeddi A, Kariyawasam V, Haifer C, et al. Safety of drugs used for the treatment of Crohn’s disease. Expert Opin Drug Saf 2019; 18: 357–367. [DOI] [PubMed] [Google Scholar]
- 22. Ritter T, Fourment C, Kuten SA, et al. Second-line biologic therapy after vedolizumab. Am J Gastroenterol 2019; 114(Suppl.): S429–S430. [Google Scholar]
- 23. Bressler B, Yarur A, Kopylov U, et al. Clinical effectiveness of first-line anti-TNF therapies and second-line anti-TNF therapy post-discontinuation in patients with ulcerative colitis or Crohnʼs disease. Am J Gastroenterol 2019; 114(Suppl.): S373. [Google Scholar]
- 24. Bressler B, Yarur A, Silverberg M, et al. Vedolizumab and anti-TNFα real-world outcomes in biologic-naïve inflammatory bowel disease patients: results from the EVOLVE study. J Crohns Colitis 2021; 15: 1694–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liefferinckx C, Minsart C, Cremer A, et al. Early vedolizumab trough levels at induction in inflammatory bowel disease patients with treatment failure during maintenance. Eur J Gastroenterol Hepatol 2019; 31: 478–485. [DOI] [PubMed] [Google Scholar]
- 26. Biancheri P, Di Sabatino A, Rovedatti L, et al. Effect of tumor necrosis factor-α blockade on mucosal addressin cell-adhesion molecule-1 in Crohn’s disease. Inflamm Bowel Dis 2013; 19: 259–264. [DOI] [PubMed] [Google Scholar]
- 27. Yzet C, Diouf M, Singh S, et al. No benefit of concomitant immunomodulator therapy on efficacy of biologics that are not tumor necrosis factor antagonists in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2020; 19: 668–679. [DOI] [PubMed] [Google Scholar]
- 28. Bressler B, Yarur A, Kopylov U, et al. P419 Clinical effectiveness and safety of first-line biologic vedolizumab as a monotherapy or combination therapy in ulcerative colitis and Crohn’s disease patients: results from the EVOLVE study. J Crohns Colitis 2020; 14(Suppl. 1): S381–S382. [Google Scholar]
- 29. Simons LA, Ortiz M, Calcino G. Persistence with a single pill versus two pills of amlodipine and atorvastatin: the Australian experience, 2006-2010. Med J Aust 2011; 195: 134–137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tag-10.1177_17562848221080793 for Vedolizumab has longer persistence than infliximab as a first-line biological agent but not as a second-line biological agent in moderate-to-severe ulcerative colitis: real-world registry data from the Persistence Australian National IBD Cohort (PANIC) study by Aviv Pudipeddi, Yanna Ko, Sudarshan Paramsothy and Rupert W. Leong in Therapeutic Advances in Gastroenterology