Abstract
Advances in 3D cell culture, microscale fluidic control, and cellular analysis have enabled the development of more physiologically-relevant engineered models of human organs with precise control of the cellular microenvironment. Engineered models have been used successfully to answer fundamental biological questions and to screen therapeutics, but these often neglect key elements of the immune system. There are immune elements in every tissue that contribute to healthy and diseased states. Including immune function will be essential for effective preclinical testing of therapeutics for inflammatory and immune-modulated diseases. In this review, we first discuss the key components to consider in designing engineered immune-competent models in terms of physical, chemical, and biological cues. Next, we review recent applications of models of immunity for screening therapeutics for cancer, preclinical evaluation of engineered T cells, modeling autoimmunity, and screening vaccine efficacy. Future work is needed to further recapitulate immune responses in engineered models for the most informative therapeutic screening and evaluation.
Keywords: Tumor, drug screening, autoimmunity, vaccine screening, lymphatics, organ-on-chip, preclinical, engineered T cells
Graphical Abstract
1. Introduction
Engineered tissues have reached an advanced level of complexity such that precise control of the microenvironment is possible, thus enabling accurate mimicry of particular elements of organ structure and function in vivo [1]. By combining tunable hydrogels, specific cell-types, and soluble factors, numerous engineered tissues have been developed, including brain [2], lungs [3], gut [4], skin [5], kidney [6], regions of the lymph node [7], and tumors [8], to cite a few recent examples. Engineered tissues can be combined with microphysiological systems (MPSs) to generate “tissues on-chip” with precise control of fluid flow, drug delivery, and patterning. Engineered tissues provide direct experimental access to human biology instead of relying solely on animal models, as well as the potential for personalized medicine with patient-specific cell sourcing. One of the first applications of such models has been as a platform for in vitro testing and screening of therapeutics, first to test pharmacokinetics and toxicity in models of healthy tissue, and more recently to test the efficacy of the treatment in models of disease [9–12].
Now, the combination of fundamental immunological concepts with tools and concepts from engineering has begun to produce so-called “immuno-competent” models of specific organs and tissues for drug testing and screening, though this field is still in its early days. A multitude of strategies have been developed to model primary immune tissues and peripheral tissues with an immune component, including ex vivo slices, microfluidics, and engineered tissues, summarized in a previous review [13]. In this review, we first examine traditional drug screening methodology, followed by an analysis of the key components to an engineered model that that must be considered to accurately mimic the immune response. We then examine recent efforts involving MPSs and engineered tissues that integrate immune components, with a focus on models developed to screen drug efficacy, drug delivery, cancer therapeutics, vaccines, and the efficacy of engineered T cells. We also discuss and provide perspective on the potential for engineered models to be translated clinically and commercially for widespread use of screening therapeutics.
2. Therapeutic Screening Methodology
Traditional drug screening has relied on 2D cell cultures of tissue-specific cells to look at efficacy and toxicity via viability, activation, proliferation, protein production, and migration measurements of the target cell types. For immune-specific screening, current methods largely focus on confirming effects on immune cell function, such as natural killer (NK) cell or CD8+ T cell cytolytic activity, ability of phagocyte to kill microorganisms, and T cell proliferation [14], or on measuring drug toxicity on immune cells [15]. Evaluation of cytolytic activity is typically performed via labeling target cells with dyes and taking a colorimetric measurement to determine plasma membrane damage [16,17]. Phagocytic activity has been evaluated by a number of methods including colony counting of bacteria post-phagocyte incubation, imaging of fluorescently labelled bacteria after phagocytosis, and cytokine gene expression via reverse transcriptase polymerase chain reaction (RT-PCR), as reviewed by Fugetta et al [14]. T cell proliferation has been identified as a key response for evaluation of immunogenicity of drugs, often quantified via radiolabeling or dilution of cytoplasmic fluorescent dyes and reported as a stimulation index. In vitro mixed cultures, e.g. of murine splenocytes or human white blood cells, are well suited for in-depth analysis of immune cell responses to drug concentrations at varied concentrations, with analysis of the population via flow cytometry [15]. However, while the aforementioned protocols provide high throughput and informative methods for initial studies of the immunogenicity of drugs, traditional screening methods often do not predict drug performance in vivo [18]. Better in vitro drug screening protocols are needed to improve the accuracy of predictions.
One potential strategy to improve the accuracy of drug screening is to move to a 3D microenvironment to better recapitulate the effects of the tissue microenvironment, including structural features of the extracellular matrix, cellular organization, biophysical cues, and binding sites. A 3D microenvironment can be provided by encapsulation in biomaterials or using organoid technology. Organoids, also called spheroids, are self-assembled cell aggregates that can encompass more than one cell type to mimic densely packed tissues. Organoids have been used to screen drugs in most organ systems in the body. Taking cancer as an example, it has been shown that tumor cells in 3D have different drug sensitivity and drug delivery profiles than in 2D monolayers [19,20]. In a study by Souza et al, a magnetic 3D bioprinting system was used to create spheroids of human prostate cancer cells to compare to 2D cell cultures [19]. The spheroids showed lower proliferation rates than the 2D cultures, but these rates are suspected to be closer to proliferation rates in tumors found in vivo [21]. Additionally, the spheroids were more resistant to chemotherapeutics than the 2D cultures, most notably at lower concentrations [19]. These differences may be mediated by a combination of biological effects, e.g. if cells enter a different state when in the 3D environment, and transport-limitation effects, i.e. restriction of access of drugs to the interior of the spheroid compared to in a 2D monolayer.
In a comparative study by Imamura et al, 2D breast cancer cultures were compared to 3D spheroids for drug screening. To test drug sensitivity, breast cancer cells were plated directly on standard tissue culture plates or Nanoculture 3D plates that encourage spheroid formation. On day 3, chemotherapy drugs were added, and on day 6, viability was quantified. Results showed that the breast cancer cell lines that formed dense spheroids (T-47D, BT-474 and BT-549) had increased drug resistance compared to 2D cultures. The presence of hypoxia was quantified using a hypoxia probe, LOX-1, which is quenched by oxygen. Ultimately, the spheroid cultures better recapitulated known tumor attributes, such as hypoxia in the core of the 3D culture and drug resistance [22]. The ability of such models to recapitulate human tissues and immune functions in 3D may provide more predictive information compared to animal models, although they are of course a reductionist system.
In addition to drug screening, measurement of drug delivery is another area where engineered immune competent models have the potential to make a significant impact. In particular, the pharmacodynamics (PD) and pharmacokinetics (PK) of drug candidates are key considerations toward drug efficacy, and are currently measured in vivo in animal models or in vitro in 2D culture to gain regulatory approval. Mouse models of PD and PK as they relate to the immune system are in use [23,24], as well as combination in vitro / mouse models [25,26]. Interestingly, in vitro systems are well used and reviewed for PK [27,28], but are typically suspension culture based. Engineered immune-competent models could better mimic the tissue environment for prediction of PD and PK [29]. Recent work by Tsamandouras et al shows that immune competent microphysiological systems can be used for studying PK, allowing for quantification of key parameters such as metabolic clearance and permeability, and how they are impacted by inter-organ crosstalk [30]. Engineered models of immunity are well positioned to answer key PK and PD questions during preclinical screening.
While it has been shown that 3D models can better simulate a physiologically relevant environment for drug screening, the inclusion of immune elements in that 3D microenvironment is only recently beginning. For example, the immune response to foreign pathogens such as bacteria, fungi, and viruses have been modelled in engineered tissues in a variety of organs but not yet used for drug screening. Models featuring tissue-resident phagocytic cells have been used to study Salmonella in the intestine [31], Aspergillus fungus in the lung [32], and Zika and Dengue viral infection in the brain [33]. Similarly, organoid and spheroid models have been developed for a number of human organs that have the potential to be used for drug screening, but still need to have immune function incorporated to make them useful for testing of immunotherapies. For example, 3D cultured organoids representing the lung [34–38], kidney [39], intestines [40–42], liver [43,44], pancreas [43], and brain [45,46] all have been applied to study how the novel SARS-CoV-2 virus infects and impacts organ function throughout the body. These organoids allow for efficient screening of therapeutic agents and provide models of tissues throughout the body for a comprehensive understanding of infection but would benefit from the inclusion of immune elements. The first step is often to incorporate a single tissue-resident cell type of the innate immune response, such as neutrophils or macrophages. For example, a human bronchial airway-on-chip was recently developed and applied to studying the SARS-CoV-2 virus by Si et al that incorporated neutrophil responses to examine how different antiviral drugs can be used to fight infection [47]. These models are poised to be applied to drug screening to examine immune response to potential therapeutics.
3. Key considerations when designing a model of tissue-based immunity
We argue that immune-competent models may provide superior insight into drug efficacy prior to in vivo testing, but creating such models is not trivial. The complexities of the immune system are difficult to recreate in entirety in vitro, and special care should be paid to parameters that can affect immune system function and outcomes.
Most engineered systems consist of cells embedded in some sort of biomaterial, either natural or synthetic, with the goal of recapitulating a physiological state. The specific components that exist within these systems vary significantly from application to application, but the design parameters are common when selecting specific elements for a model. In particular, appropriate biological, physical and chemical cues must be incorporated (Figure 1), as cell behavior can be impacted, for example, by chemical makeup of a matrix, the stiffness, porosity, and biodegradability [48]. Thus, the selection of the biomaterial backbone is a major element of an engineered model, with most of these systems consisting of hydrogels. The hydrogel material also impacts immunomodulation, with the ability to enhance or reduce immune cell activation [49,50]. Many reviews exist to summarize critical hydrogel properties [48,51–54]; here we will summarize three important design aspects of engineered models for drug screening in the context of immunity.
Fig 1.

Key components to consider when designing an immuno-competent 3D culture model. An engineered model can incorporate specific physical, chemical, and biological cues that have implications on outcomes and physiological relevance. Physical cues include stiffness and porosity of the matrix, surface topography, and fluid flow rates and shear stress. Chemical cues include drug release, the content of the cell culture medium, cytokines and growth factors used as supplements or secreted by the cells themselves, and binding moieties in the matrix or on the culture surface. Biological cues typically represent the cells included in the model, specifically which cell types should be included, in what activation states, as well as cell sourcing (e.g. primary or stem cell-derived; patient-specific or sex-matched). All three types of cues can have potential impacts on immune cells, for example, on their activation state, motility, and receptor expression.
3.1. Physical Cues: Stiffness, Fluid Flow, and Porosity
Physical tissue properties such as the extracellular matrix (ECM) stiffness, permeability, and interstitial flow depend strongly on physiological state and have significant impacts on immune cell activation, proliferation, and function [55,56]. Within the body, the stiffness of tissues varies greatly, from soft tissues such as brain, around 0.1–3 kPa, to stiff tissues such as bone, up to 4 GPa (Table 1). Therefore, hydrogels should be engineered at a stiffness that best mimics the tissue of interest. Stiffness can be tested via shear rheometry or dynamic mechanical analysis and is typically reported in measures of Pascals [53].
Table 1.
Physical parameters including stiffness, interstitial fluid flow, and porosity for various tissues in vivo.
| Tissue | Stiffness (kPa) | Interstitial Flow (µm/s) | Porosity (%) | Pore size (µm) | Primary ECM components | Refs |
|---|---|---|---|---|---|---|
| Brain | 0.1 – 3.3 | 0.175 | ~20 | 20–60 | Hyaluronic Acid, versican, aggrecan, GHAP | [57–60] |
| Bone | 2 – 4 ×106 | 6–9 ×105 (cortical channels); 0.4–2 ×105 (bulk ECM) | 15 (Compact); 70 (Trabecular) | 50 (vascular), 0.10 (Lacunar-canalicular), <0.001 (collagen-apatite) | Collagen type I, hydroxyapatate | [61–65] |
| Skeletal muscle | 12 | 0.12 | 8–9 (rat) | 24–48 (rabbit) | Collagen I and III, proteglycans | [61,66–69] |
| Lymph node | 0.12–9.5 | 150–700 | Collagen III, fibronectin, collagen IV | [70–74] | ||
| Skin | 0.8–7 ×103 | 9.7 | 60–85 | 40–80 | Collagen I, III, IV, elastin | [75–81] |
| Intestine | 0.5–1.25 | 15–16 (rat) | 6–10×10−4 | collagen types I, III, IV, and VI, laminin, fibronectin | [82–84] | |
| Gliomas | 0.07–13.5 | 2 | 35–43 | 0.012 (bulk - rat); 0.1–0.7 (vasculature – rat and human) | Hyaluronic Acid, versican, BEHAB, Tenascin C | [85–92] |
| Breast and connective tissue stroma | 0.400 | 0.1–2 | 20 | 1–20 | Collagens I and III, fibronectin | [93–98] |
Many studies have demonstrated the effects of stiffness and other mechanical cues on immune cells. For example, Hickey et al engineered an artificial T-cell stimulating matrix that preserved T cell phenotype and function ex vivo with stiffnesses ranging from 0.2 kPa to 3 kPa. Tuning the stiffness of the engineered matrix had effects on T cells, with higher proliferation and stimulation in softer gels [99]. Saitakis et al tested stiffnesses of 0.5, 6.4, and 100 kPa and found that altering stiffness modulated T lymphocyte migration, morphology, and cytokine secretion. Only the highest stiffness caused changes to T cell metabolic activity and cell cycle. T cell migration velocity and distance as well as cell spreading increased with increasing stiffness [100]. Therefore, T cells recognize a wide range of stiffnesses in the body and alter their behavior accordingly; we refer the interested reader to a recent review on T cell mechanobiology [101]. Stiffness also alters dendritic cell phenotype, antigen presentation, and metabolism [102,103]. Dendritic cells showed higher proliferation, activation, and cytokine production when cultured at a stiffness (50 kPa) higher than physiological values (2 kPa) [103]. Mennens et al found that mid-range stiffness of 12 kPa caused inhibition of dendritic cell differentiation and maturation [102]. Additionally, macrophages responded to increasing stiffness by polarizing toward an inflammatory phenotype [104–106] and B cells were more efficiently activated by antigens when on stiffer surfaces and stiffness further impacts B cell class switching and proliferation [107]. We note that stiffness can be both a chemical and physical cue, translating to the presentation of binding sites on matrices.
Similarly, fluid flow also has impacts on immune cells, inducing proliferation, migration with or against the direction of flow, and immune cell polarization [13]. Antigen specific binding of T cells and dendritic cells is increasingly disrupted as shear stress is increased (0.01 to 12.0 Dyn cm−2), and significantly disrupted at high shear stress typically found in the arteries (120 Dyn cm−2) [108]. Flow also regulates stromal cell organization [70,109]. Low levels of fluid flow (0.1–1 µm/sec) increase lymphatic permeability and enhance dendritic cell migration through lymphatics [110]. In addition, tumor lymphatics have enhanced fluid flow, which increases communication between tumor and lymph node [111,112]. In the body, flow can vary from 0.1 µm/s in the stroma, brain, and muscle to 10 µm/s in skin lymphatics (Table 1). In vivo, it seems that much of the biological response to flow depends on linear velocity (length/time) and/or shear rate (1/s). While experimental fluid flow through hydrogels is often reported in volumetric flow rates (volume per time), consideration of the linear flow rates may be important for cellular function.
Flow through a matrix is directly related to porosity. Hydrogels used for 3D culture are typically highly porous, which facilitates transport of oxygen, nutrients, and waste by flow and diffusion. Porosity is defined as the amount of pore space relative to the scaffold. Porosity can be measured through a multitude of methods, some of which include gravimetric methods, mercury porosimetry, liquid displacement, and scanning electron microscopy [52]. Typically, pore size is measured via imaging, such as SEM or microcomputed tomography. In the body, porosity ranges from 8% (muscle) to 85% (skin) and pore size ranges from 6 angstroms (gut) to 80 microns (skin) (Table 1). Porosity and pore size can affect cell proliferation, differentiation, and angiogenesis [52].
The ability of immune cells to deform and migrate through small pores is also dependent on porosity. Non-adherent immune cells typically utilize amoeboid migration, characterized by fast migration speeds, rounded cell shapes, and no adherence. Rather than relying on degrading the extracellular matrix, T-lymphocytes migrate along the matrix and squeeze through any existing gaps. This ability is seen for gaps approximately 2 µm and above with migration velocities up to 30 µm per minute [113]. The maximum migration velocity varies greatly among immune cells, with lower speeds for B cells (15 µm/min) and dendritic cells (10 µm/min), and the slowest migration observed in monocytes (5 µm/min) [114]. Mandeville et al showed that neutrophils were unable to migrate through 0.8 µm pores, but migrated faster along those pores than a non-porous material [115]. Tylek et al found that with decreasing pore size, with a minimum of 40 µm examined, macrophages were more polarized to the M2 anti-inflammatory phenotype [116]. Ford et al found that macrophages were unable to migrate in dense networks, but when co-cultured with fibroblasts, the fibroblasts degraded the ECM to provide tunnel networks for macrophage migration [117]. Porous biomaterials themselves with controlled release properties of cytokines and therapeutics can also be ideal for immune modulation, as discussed in a review by Jeong et al [118]. Therefore, the porosity of a designed system must allow for immune cell migration or provide additional cell types to aid immune cells in highly dense networks.
3.2. Chemical Cues: Drug release and mass transport
Hydrogels are utilized both as a platform for drug delivery and as a substrate for engineered tissue models. Desired properties vary greatly based on application and specific tissue properties. For drug delivery applications, controlling a hydrogel’s release properties is essential. A hydrogel’s release mechanism can be diffusion-controlled, swelling-controlled, or chemically-controlled [54]. The language used to describe drug release profiles varies greatly within the literature. For some hydrogels, release profiles are stable and linear [119,120]. Other hydrogels have differing release profiles based on drug polarity. In poly-ethylene glycol(PEG)-polylactic-co-glycolic acid(PLGA)-PEG hydrogels, hydrophilic drugs release over a short period of time (2 weeks) with a first-order release profile while hydrophobic drugs take a longer period of time (2 months) and follow an S-shaped release profile [121]. In Elastin-Like Polypeptide-Collagen Hydrogels, the release profiles differed significantly between drugs, based on their chemistry. The drug rhBMP-2 had a linear release profile while doxycycline had a bi-phasic release profile with an initial burst and then gradual release, even though both drugs are hydrophilic [122]. With such high variation of release profiles between hydrogels and drugs, each system must be characterized individually.
In an in vitro model, just as in vivo, mass transport of applied drugs, nutrients, and secreted signals has a significant impact on the observed efficacy. Diffusion and flow through a 3D matrix are directly related to porosity, as discussed above. In addition to these physical parameters, diffusion is also controlled by concentration gradients. In immune-competent models in particular, diffusion of cytokines is a critical aspect to consider. Indeed, a local cytokine niche may form around cytokine producing cells, whose niche size is controlled by the competition between the rates of secretion, diffusion, and consumption of the cytokine [123]. Understanding the mechanisms of cytokine diffusion are essential toward immune outcomes, where cytokines can guide migration, control inflammation, and regulate differentiation and apoptosis. The physicochemical properties of a 3D matrix can be tailored to match in vivo tissues for maximum physiological relevance. However, it is difficult to know the appropriate concentrations throughout a tissue, especially at a local level. Ideally, we would need to know the distribution of cytokines within a tissue and the locally available gradients surrounding tissues and cells. To do this, better detection methods are needed in situ [124].
3.3. Biological Cues: Cell selection, sourcing, and matching
Fundamental to developing a model is choosing the required cell types and desired outcomes. As it is neither possible nor desirable to include every single cell type that is present in vivo, the cell types should be selected based on the desired functional output of the model (Fig 2). To screen innate immune functions, tissue-resident cells such as macrophages, natural killer cells, neutrophils, or dendritic cells may be required. For adaptive immunity, T cells and/or B cells are needed, as well as dendritic cells if antigen presentation is required. For example, if screening the effect of an immunotherapy for breast cancer on the tumor-killing activity of T cells, minimally the model must contain both tumor cells and a specific set of T cells. Supportive cells such as stroma, macrophages, or antigen-presenting cells may also be needed, depending on the mechanism of the immunotherapy. Models of tumor growth and invasion may require inclusion of macrophages, which can either inhibit tumor progression [125–128] or promote it through immune suppression [129,130], enhancement of tumor cell migration [131–134], and stimulation of angiogenesis [135–137]. Macrophages may also contribute to tumor chemoresistance, making them an essential part of therapeutic screening [138–140]. In another example, a model may be desired for pre-clinical screening of vaccines. Modeling an entire vaccine response from injection to protection would be immense and unfeasible with current technology, so it may be sensible to model steps of the vaccine response separately. For example, a model for testing antigen uptake might include epithelial skin (or muscle) cells and antigen presenting cells, whereas a model for B cell class-switching in response to an activated CD4+ T cell should include B cells and CD4+ T cells at a minimum. In some cases, it may also be useful to include cells that are responsible for immune surveillance, e.g. the lymphatic endothelial cells (LECs) that make up the lymphatic drainage system, or the fibroblastic reticular cells that form the backbone and traffic control system of the lymph node.
Fig 2.

The immune system is made up of a diversity of cells. The innate system is the first line of defense for the host and includes cells such as macrophages, natural killer cells, and a number of additional cells with cytolytic or cytotoxic activity. Adaptive immunity begins in the lymph node and moves to peripheral tissues for an antigen-specific immune response. Immune surveillance occurs throughout the body, with a lymphatic system delivering lymph fluid to lymph nodes for surveillance.
Once the cellular components are chosen, it is necessary to identify a cell culture medium that supports the viability and function of all the cells in combination. Known as the “common media” problem, this is an ongoing challenge for any co-culture model. Progress toward common media has been made in the multi-organ-chip field. For example, a mixture of testis organoid media and endothelial growth media was used to culture liver, cardiac, lung, blood vessel, testis, and brain organoids together [141]; a mixture of hepatocyte culture media and cardiomyocyte maintenance media was used to culture lung, liver, and cardiac organoids together [142]; and a growth factor deprived induced pluripotent stem cell (iPSC) media was used to culture intestine, liver, and neuronal models together [143]. However, these models have not yet incorporated immune elements, which may provide additional challenges toward developing a universal media.
Like media choice, the sourcing of immune cells has great implications on in vitro models and can present unique challenges and opportunities. Donated human blood proves a readily available source of circulating white blood cells. Large quantities of these cells can also be obtained from leuko-reduction collars, which are discard products after platelet donation via apheresis [144]. During the COVID-19 pandemic, however, donations have been down (go donate blood!) and it has been more difficult to source donated blood or leuko-reduction collars in significant quantities, but hopefully this situation will resolve in the next year. Donated white blood cells contain a mixture of cell types (e.g. T cells, B cells, granulocytes, monocytes) in a mixture of activation states (e.g. naive, effector, and memory T cells) [145,146], a limitation that must be acknowledged or else accounted for by purification of the cells prior to use in the model system. Furthermore, unlike cell lines or even primary fibroblasts, naive lymphocytes, and many other immune cells, do not proliferate in culture. Thus, they must be obtained fresh every few days or must be treated with a mitogen to induce proliferation and activation. When working with primary human cells, it is not uncommon to observe large (e.g. 10-fold) variations in response to stimulation from donor to donor, which means that each experiment must be conducted with internal (same-donor) controls or else powered with a large number of replicates to detect differences between treatment conditions.
As an alternative to donor-derived cells, some cells of the immune system may be derived from embryonic or induced pluripotent stem cells. Embryonic stem cells (ESCs) can have immunogenic effects after transplantation, and strategies such as parthenogenesis [147] and somatic cell nuclear transfer (SCNT) [148,149] have been developed to address this issue [150]. Parthenogenesis uses activated oocytes that have not yet been fertilized, while SCNT removes the nucleus of an oocyte and replaces it with the nucleus of a donor’s somatic cells. After pathogenesis or SCNT is performed, the oocytes proceed to the blastocyst stage before collecting the produced ESCs [150]. Alternatively iPSCs are generated by using cells taken from an adult donor and induced back into a pluripotent stage, avoiding the potential ethical issues of ESCs. Progenitor hematopoietic stem cells [151–153] and a variety of immune cells, including macrophages [154,155], microglia [156,157], natural killer cells [158], and T cells [159,160] have been derived from iPSCs [161]. For example, human microglia are challenging to source and cannot be readily replaced by murine microglia due to differences in cytokine production and drug responses, iPSC-derived microglia provide a readily available source of human microglia that can be easily expanded in vitro [161]. iPSC-derived macrophages have been used to study infectious diseases such as Zika and Dengue virus, macrophage related diseases such as liver fibrosis, and for suppression of gastric and peritoneal cancers in mouse models [162]. iPSC-differentiation protocols continue to be a hot area of research, towards the dream of producing all of the required cell types for engineered models and organs-on-chip from a single patient’s cells [163].
Despite the promise of iPSC-derived cell sourcing, a major challenge in utilizing iPSC-derived immune cells comes from ensuring they have tissue-specific phenotypes, as they are often immature. On the other hand, commonly utilized macrophage lines such as murine RAW 264.7 and human THP cell lines also have a lack of tissue-specific phenotypes. A second challenge is that immune cells derived from iPSCs are rarely used for engineered tissue models. iPSC-derived T cells have been produced for immunotherapy, but by design these have a single antigen-specificity rather than with the whole repertoire of antigen specificities seen in vivo [164,165]. The restricted antigen-specificity may limit their usefulness in engineered models of immunity where recognition of more than one antigen is required.
Beyond immune cells, when screening potential therapeutics, the type and source of other cell types, such as tumor cells or stromal cells, also impacts results. For example, patient-derived tumor cells provide the most informative model in the clinic, and typically have desired outcomes of tumor cell death [166–168], reduction of tumor cell proliferation, and reduction of invasion [169].
Finally, sex and HLA-matching are interesting variables to consider, whose effects on 3D models of immunity are not yet well understood. The potential for sex-matching in vitro models is a growing aspiration in the field. As reviewed by Klein and Flanagan, sex-differences in immune system function have been observed pre- and post- puberty, including differences in autoimmune disorder occurrence, infectious disease susceptibility, and responses to vaccination [170]. Sex hormones regulate innate immune cells and affect susceptibility to viral infection among other impacts. Human females have higher antigen-presenting cell efficiency, higher macrophage activation, higher T cell proliferation, greater numbers of B cells, and higher antibody production. Human males have higher pro-inflammatory cytokine production, larger thymuses, and greater numbers of natural killer cells [170]. Thus, it may be possible to detect sex differences in the immune response of 3D models if all cells are sourced from donors of the same sex. Furthermore, clinical studies have demonstrated that hematopoietic stem cell grafts that are not sex-matched from donor to recipient have an increased rate of rejection and failure [171]. In this context, it is possible that non-sex-matched models may experience immune rejection in long-term models of adaptive immunity, but this has not been tested yet. In principle, HLA-matching should also be considered in long-term culture models, to avoid activation of T cells from one donor against tissue-specific cells from another donor, as occurs in graft-vs-host disease [172]. Similar to sex matching, HLA-matching has not been explored yet in engineered models of disease, possibly because most engineered models of immune function have been short term or restricted to cells of the innate immune system.
4. Applications of engineered models for drug screening
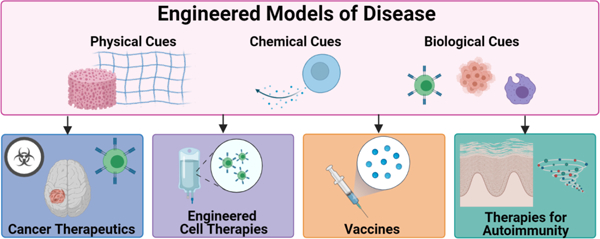
The need for models to screen therapeutics for both immune modulation and for immune-related pathologies is high. This area of development is nascent compared to other types of therapeutic screening platforms, yet the potential is high. There are several malignancies that have leveraged current tissue-engineered platforms to test and incorporate immune elements for better patient specificity and predicting outcomes (Figure 3). These include cancer and autoimmune diseases. Platforms have also been developed for testing of immuno-modulatory therapies, including vaccine development and engineered immune cells. These engineered systems will be discussed here.
Fig 3.

Applications of Engineered Models of Disease. Immunocompetent models that recapitulate specific tissue features can be used to screen cancer therapeutics, engineered cell therapies, vaccines, and therapies for autoimmunity.
4.1. Applied Models Toward Screening of Cancer Therapeutics
Modeling solid tumors in vitro is of great interest to the broader scientific community. Treating solid tumors comes with unique challenges, such as the enhanced permeability and retention effect (EPR), whereby solid tumors exhibit leaky vasculature, compressed lymphatics, dense ECM, and high interstitial fluid pressure (IFP) [173]. These phenomena alter the delivery of drug particles to the tumor and has been modeled mathematically [174], but remains to be well studied in vitro. Further, the tumor microenvironment (TME) contributes greatly to drug efficacy (or inefficacy) and cancer progression [175]. The TME includes blood and lymphatic vasculature, aberrant extracellular matrix deposition, stromal cells, and immune cells, and each component is typically hijacked by the tumor to further its progression rather than aid the host.
An important application of in vitro tumor models is the screening and evaluation of therapeutics. Glioblastoma, for example, is well studied in vitro in terms of therapeutic screening [176,177], but inclusion of immune elements has lagged behind. There are multiple potential reasons for this, including the misconception of the brain as an immune-privileged organ; glioblastoma, the most common of brain tumors, being largely immune-cold; and the difficulty in understanding and analyzing what the immune makeup of tumors in the brain is. Luckily, there has been recent progress on all of these fronts, and with the advent of immunotherapies for brain tumors, renewed interest in better simulating the immune niche [178–182]. Many engineered brain tissue models have focused on inflammatory response from microglia, but do not investigate this response during cancer [13]. A lot of progress has been made toward recapitulating the TME in various cancer types [166–169]. The field has taken steps toward the inclusion of immune elements, such as the lymphatic system and macrophages.
An exciting new development is the generation of in vitro models that replication some aspect of the spatially organization of the tumor microenvironment. Previously, most models have consisted of homogenous cells in hydrogel systems or organically grown organoids for tumor modeling, but these systems can lack the spatial organization of complex tissues. Bioprinting allows enhanced spatial control of extruded biomaterials and cells, and thus is useful in modeling the complex heterogeneous tumor microenvironment. However, the inclusion of immune cells in 3D bioprinted tumor models has lagged behind. Grolman et al established a simple hydrogel extrusion method for fabricating differentially organized 3D macrophage-breast cancer cultures, depending on flow rate (Fig 4A) [183]. The inner channel is hollow and contains macrophages (RAW 264.7), while the outer shell consists of breast adenocarcinoma (MDA-MB-231) cells in peptide-conjugated alginate hydrogel. The peptide-conjugated alginate fibers provide physical binding moieties for the cancer cells. The interactions between MDA-MB-231 cells and RAW 264.7 cells are well studied, and thus the authors chose them for co-culture. In designing this model, physical, chemical, and biological cues were considered. The macrophages were shown to migrate into the alginate hydrogel and interact with tumor cells over time under normal conditions, but remained in the hollow inner channel following the inhibition of migration by Gefitinib, zoledronic acid, and a Rac1 inhibitor. The authors propose this platform could be used to optimize drug compounds that disrupt tumor cell - macrophage interactions through the use of spatially-organized 3D hydrogels [183].
Fig 4.

Engineered cancer models with immune elements. (A) To examine macrophage invasion into tumors, a rapid 3D extrusion technique was used to fabricate spatially organized models of the tumor bulk (tumor cells in red) containing a hollow vessel-like structure (outlined in grey dashes). By day 4, immortalized macrophages (green) invaded from the vessel into the tumor cell-laden hydrogel readily, but this invasion was inhibited by Gefitinib (GEF), Rac1 inhibitor (RA), and zoledronic acid (ZA), pharmacological agents that inhibit migration. Scale bars are 400 microns. Adapted with permission from Grolman et al [183]. (B, C) A 3D bioprinted glioblastoma model was used to investigate macrophage contributions to tumor mass. (B) Glioma stem cells (GSCs, green) were cultured with or without anti-inflammatory M2 macrophages (red), and astrocytes and neural stem cells (NSCs) were bioprinted around the tumor bulk (pink). (C) Fluorescent images of bioprinted glioblastoma, with 3264 GSCs in green and macrophages in red. Scale bars are 1 mm. Adapted with permission from Tang et al [185]. (D-K) To examine blood and lymphatic capillary contribution to melanoma progression, a 3D-cultured skin melanoma model was developed. (D, E) In a skin model without melanoma, composite immunofluorescence images show that human blood endothelial cells (CD31, green) and lymphatic endothelial cells (podoplanin, red) formed separate blood and lymphatic capillary networks. Scale bars: 50 µm (D) and 150 µm (E). (F-K) Melanoma spheroids (red) incorporated into the model integrated with the microenvironment, shown by immunostaining (green) of epidermal markers filaggrin (F, G) and involucrin (H, I); nuclei stained with Hoechst (blue). Scale bars: 100 µm. Boxed regions magnified in G and I. (J, K) Hematoxylin and eosin staining of the melanoma model (boxed region magnified in K). Scale bar: 200 µm in J, 50 µm in K. Adapted with permission from Bourland et al [200].
Further, spatially organized models can recapitulate key features of the TME. As an example, the microenvironment of glioblastoma greatly impacts its behavior, with aberrant vessels preventing drug delivery and pro-tumor immune cells supporting tumor progression [175]. Two-dimensional co-cultures have shown that glioblastoma cells can steer macrophages toward an anti-inflammatory polarization that is considered pro-tumor [184]. In a bioprinted glioblastoma model, the contributions of macrophages toward glioblastoma progression in systems with and without the brain microenvironment were examined. Gelatin methacrylate (GelMA) and glycidyl methacrylate-hyaluronic acid (GMHA) hydrogels were used to develop an ECM similar to that of glioblastoma in vivo, with GelMA providing mechanical stability and the GMHA providing HA, a key ECM component of the brain. These biomaterials came together to provide desirable mechanical properties, with the model’s tumor core reaching a stiffness around 3 kPa and the peripheral brain tissue region reaching around 1 kPa. The hydrogel overall has a porosity of 53% and pore size of 85 µm, as reported by Tang et al. This bioprinted model therefore demonstrates precise and tunable control of mechanical properties and binding moieties that recapitulate in vivo tissue. Further, the model incorporates key biological cues in the form of chosen cell types. The tumor core was made up of patient-derived glioblastoma stem cells (GSCs) with or without macrophages (THP-1 polarized to M2), while the peripheral brain tissue was made up of astrocytes and neural progenitor cells. Ultimately, the presence of macrophages enhanced glioblastoma invasion and progression, and when the macrophage-glioblastoma interactions were disrupted, the glioblastoma cells showed enhanced sensitivity to chemotherapy and slower disease progression (Fig 4B-C) [185]. 3D bioprinted glioblastoma models therefore provide a platform to model disease progression and physiologically relevant interactions between cell types.
Interstitial fluid flow, integral to immune responses, also enhances the invasion of tumor cells in the context of the brain both in engineered microenvironments and in vivo, thus indicating that it is a vital force in tumor response [186,187]. In a microfluidic device developed by Lee et al, macrophages and pancreatic ductal adenocarcinoma tumor cells were embedded in 2.5 mg/mL collagen hydrogels and subjected to interstitial fluid flow (IFF) at a rate of approximately 3 µm/s. The authors designed the microfluidic device so that tumor cells and macrophages were in adjacent channels, but not directly co-cultured, to simulate tumor-originating IFF. Chemical cues, such as tumor secreted factors IL-8 and CCL2, were also precisely controlled in the model. Collagen was chosen as a matrix that is tumor-related and commonly used. The microfluidic device allows precise control of IFF as a physical cue and organizes the spatial distribution of the cell types to create physiologically relevant biological cues. They found that macrophages had increased migration speeds in response to tumor cell secreted factors and IFF alone, but no synergistic effect above the observed increased migration when tumor cell secreted factors and IFF were combined [188].
Vasculature is a key component of the immune microenvironment and is directly related to fluid flow. When vasculature is surrounding and within the tumor stroma, it can greatly impact tumor metastasis and chemoresistance. Immune cells can traffic through the vasculature for delivery to a tissue. While some immune cells are tissue resident, most are delivered via the vasculature when an immune response is warranted, and therefore, vasculature is essential to consider when developing in vitro models for therapeutic screening. Many models of the vasculature have been developed; current strategies for developing vascularized tissues in vitro often rely on microfluidic platforms and bioprinting [189,190]. In one tumor-relevant example, Hachey et al developed a vascularized micro-tumor on chip device of colorectal cancer and was able to recapitulate known patient-specific responses [191].
While many models have focused on recapitulating the blood vasculature, models of lymphatic vessels have lagged behind, but some progress has been made recently [192,193]. Lymphatic vasculature regulates interstitial fluid and serves as a network for immune cell trafficking. Solid tumors are known to metastasize through the lymphatic system, and once in the lymphatics, can travel to the other organs in the body. Localizing chemotherapy agents to the lymphatics is challenging without altering typical drug delivery. Intravenous or oral drugs do not accumulate at sufficient concentrations in the lymphatics, leading to the necessity of innovations such as nanoparticle carrier systems and alternate delivery routes, such as subcutaneous or intramuscular drug administration [194]. Further, tumors can co-opt the lymphatic system to support tumor progression and metastasis and suppress the immune response [195]. In a bioprinted breast cancer model on a chip, Cao et al designed a GelMA hydrogel with PEG diacrylate (PEGDA) to recapitulate the permeability of lymphatic vessels and GelMA with 8-arm polyethylene glycol-octaacrylate (PEGOA) to recapitulate the permeability of blood vessels. The model was designed around these key physical cues to replace biological cues provided by these vessels in vivo. MCF-7 breast cancer cells showed higher viability after exposure to a chemotherapeutic, DOX, when both blood and lymphatic channels were included, compared to a blood vessel channel only. The authors speculate this could be due to lymphatic drainage of the drug. Ultimately, the model demonstrates that the combination and number of blood and lymphatic vessels can drastically vary drug diffusion profiles [196].
Lymphatic vessels play an important role in the progression and metastasis of melanoma, and thus a variety of in vitro methods have been developed to study melanoma that are highly focused on lymph node/lymphatic interactions. 3D culture matters here, as antigen specific T lymphocytes have impaired ability to recognize melanoma cells in 3D compared to 2D tissue culture plastic systems [197–199]. Beyond 3D structure alone, Bourland et al incorporated melanoma spheroids into an engineered skin model, stacking cell sheets of fibroblasts, spheroids, keratinocytes, and human microvascular ECs, providing a highly physiologically relevant model of melanoma (Fig 4D-K) [200]. The model showed the formation of both blood and lymphatic capillaries, supported by VEGF-A and VEGF-C secretion, as well as a stratified epidermis, as identified by involucrin and filaggrin immunostaining. The melanoma spheroids expanded during culture and invaded into other layers of the model. The model was then treated with vemurafenib, a cancer drug commonly used to treat melanoma. The melanoma spheroids responded to vemurafenib by decreasing proliferation 4-fold [200]. However, some tumor cells, often located near fibroblasts, continued to proliferate, even after 12 days of treatment, aligning with previous studies showing that cancer-associated fibroblasts (CAFs) enhance tumor progression. When vemurafenib was applied to spheroids alone, it resulted in a greater decrease in proliferation compared to spheroids in the 3D skin model, showing the importance and contribution of the microenvironment to cancer progression and drug response [200]. Votanopoulos et al fabricated patient-matched organoids by combining cells from lymph node and melanoma biopsies that were able to activate naive T cells to promote tumor cell death. The response to anticancer drugs differed from patient to patient, and in certain cases was enhanced by the inclusion of lymph node cells [201]. Inclusion of the lymphatics is necessary toward understanding the efficacy of therapeutics in vivo, as the lymphatics can alter drug delivery profiles, enhance or reduce drug efficacy, and contribute to cancer progression.
Recently, an MPS model of the lymph node subcapsular sinus was used to mimic the entry path of cancer cells into the lymph node [71]. Interestingly, monocytes lining the lymphatics enhanced cancer cell adhesion in a flow-dependent manner. The authors intend to add pulsatile flow to the model to further match lymphatic flow properties. MPS systems such as these provide models that can reproduce great complexity on a small, controllable scale to better understand tumor/lymphatic dynamics in the body [202].
Overall, engineered tissues that recapitulate the 3D tumor microenvironment and aspects of immunity have proven to enhance the clinical relevance of in vitro studies. Patient-specific engineered models are feasible and could be implemented as a point of care system for personalized medicine. Current patient-specific screening requires biopsy of the necessary tissue and challenges may occur in obtaining high counts of the desired cell types. In the future, induced pluripotent stem cell technology may reduce the need for invasive biopsies, once appropriate derivation protocols are established. Future work in the field should strengthen the inclusion of immune elements in in vitro models, especially adaptive immune responses, to better screen potential therapeutic agents.
4.2. In Vitro Platforms for Preclinical Evaluation of Engineered T-Cells
Engineered T-cells have the potential to transform the way cancer is treated as higher tumor specificity is achieved [203,204]. With genetically engineered antigen specific receptors, engineered T cells can precisely target tumor cells to allow the immune system to clear the malignancy. Tumor cells are capable of evading T cell receptor (TCR) engineered T cells due to the reliance on major histocompatibility complex (MHC) proteins, but chimeric antigen receptor (CAR) T cells are MHC-independent, and thus found to be more potent against tumor cells [205]. In vitro platforms are essential to testing the safety and efficacy of engineered T cells, providing higher throughput, greater flexibility, patient specificity, and precise control of the microenvironment compared to in vivo animal studies. Engineered T cells are already in use clinically to treat hematological cancers with great success [206]. Many preclinical models for hematological cancers focus on mouse models and 2D cell culture [207–209]. Meanwhile, solid tumors provide a different challenge due to the stiff and dense microenvironment. The efficacy of engineered T cells is drastically reduced against solid tumors due to insufficient migration into the tumor bulk, poor amplification, and poor persistence [210]. Developing engineered tissue models of solid tumors for engineered T-cell screening is essential for translating in vitro work to the clinic.
T cell activity in solid tumors have been modeled in vitro via numerous methods, including spheroids and 3D hydrogel cultures. Jacob et al developed a protocol to generate patient-derived glioblastoma organoids that can be cultured in suspension with engineered T cells for the evaluation of engineered T cell efficacy [211]. Wallstabe et al utilized decellularized porcine jejunum (BioVaSc) as a scaffold for A549 lung and MDA-MB-231 breast cancer cells to examine engineered T cell effectiveness under static and dynamic conditions (Fig 5A). The BioVaSc scaffold provides an intact basement membrane for physiologically relevant epithelial tumor adherence. A bioreactor is used to expose the T cells to shear stresses of approximately 0.0004 dyn/cm2, which is considered below physiological values. However, with dynamic flow, the T cells were effective for 5 days, compared to only 3 in static culture, and had enhanced invasion into the tumor bulk [212]. These results can directly correlate to understanding the conditions necessary for T cell efficacy in solid tumors.
Fig 5.

In vitro platforms used for screening engineered T cells. (A) In 3D lung (A549) and breast (MDA-MB-231) tumor models, cytotoxicity and migration of CAR T cells were examined. Nuclei are labeled with DAPI (blue) and control T cells and CAR T cells are labeled in green. CAR T cells showed enhanced migration into the tumor bulk compared to normal T cells. Scale bar is 100 µm. Adapted with permission from Wallstabe et al [212] (B) 3D microdevices were used to investigate cytotoxicity of engineered T cells under hypoxia. HepG2-Env target cells (red) were cultured with mRNA T cell receptor engineered T cells (green) overnight. T cells were more migratory in normoxia. Adapted with permission from Pavesi et al [213]. (C). A 3D tumor model was used to investigate CAR T cell killing efficacy under hypoxia. SKOV3 Human epithelial ovarian cancer cells were incubated with normal type (NT)-T cells or CAR-T cells at a 20:1 ratio for 24, 48, and 72 hours and labeled with viability dyes (live, green; dead, red). Scale bar is 1000 µm. Adapted with permission from Ando et al [214].
Microfluidic platforms provide a unique opportunity for precise control of a 3D microenvironment, especially oxygenation and perfusion, while testing T cell function in tumor models. For example, Pavesi et al developed a perfusable 3D microfluidic model of hepatic cancer (HepG2-Env) in collagen gel for the preclinical evaluation of engineered T cells (Fig 5B). Two different methods of engineered T cell production were examined: retroviral-transduced T cells and activated mRNA-electroporated engineered T cells. The influence of oxygen levels and inflammatory cytokines on the effectiveness of the engineered T cells was evaluated in the microfluidic device and a 2D culture system. In general, the engineered T cells circulating in the microfluidic device were more effective at higher oxygen levels (20% compared to 2%) and in the presence of inflammation. The responses of the engineered T cells in 2D culture did not match that of the microfluidic device, demonstrating the necessity of more complex 3D systems for the evaluation of engineered T cells [213]. Ando et al developed a microfluidic device utilizing GelMA hydrogel that contained an oxygen gradient to model the hypoxic tumor core (Fig 5C). GelMA was chosen for its mechanical tunability and optimized to provide 6 kPa of stiffness and a pore size of 8.47 ± 3.02 µm. Enhanced killing of SKOV3 human epithelial ovarian cancer cells by CAR T cells was observed at the edges of the hypoxic model (1% oxygen), where the oxygen content was physiologically relevant to healthy tissue. Under ambient air conditions (21% oxygen), no enhancement of efficacy was seen [214]. In an engineered breast cancer on-chip model, Aung et al photo-patterned endothelial cells, monocytes, and cancer cells in a gelatin-based hydrogel, and subsequently perfused the model with T cells. Under hypoxic conditions, more T cells were recruited than in conditions with dispersed cancer cells. The presence of monocytes further enhanced T cell recruitment [215]. This platform could further be used to assess engineered T cell recruitment. Interestingly, these results show a potential dichotomy between T cell recruitment and function under hypoxia.
These in vitro models have provided invaluable insight into the dynamics of engineered T cells in regards to treating solid tumors. It is shown that engineered T cells behave differently in 2D compared to 3D [213], that dynamic culture impacts engineered T cell efficacy [212], and that engineered T cells are not as effective in hypoxic conditions [213,214]. With many established in vitro models for evaluating engineered T cells, future work could include examining potential mechanisms and therapeutics to boost engineered T cell efficacy for solid tumors as well as optimizing treatment conditions. Future work could also focus on vascularizing these platforms to look at how engineered T cells traffic through vasculature into the tumor bulk.
4.3. Engineered models of allergy and autoimmunity, with case study of Inflammatory Bowel Disease
Generating microfluidic models that properly represent allergic responses and autoimmune diseases will be a crucial step towards developing biomimetic platforms that can be used to test drug therapies. So far, with a few notable exceptions described below, the majority of models that incorporate immunity into organs associated with autoimmune diseases have focused primarily on immune cell interactions with healthy tissue rather than modeling a particular disease or conducting drug testing [13,216].
While they don’t directly cause tissue destruction, asthma and allergic responses are similar to autoimmune diseases because they both result from overactive immune responses, with vastly different results. There are existing microfluidic models that aim to recapitulate the inflammation found in an asthmatic lung through the use of patient-derived tissues [217] or stimulation with inflammatory cytokines [218], but these devices lacked an immune cell component needed to fully represent asthmatic conditions as seen in vivo. In a 2016 study, Ramadan and Ting utilized a skin-on-a-chip cultured with dendritic cells to model allergic contact dermatitis using chemical (LPS) and physical (UV light) stimulation [219]. To generate a skin model on-chip, epidermal cells were cultured on a membrane above suspended dendritic cells, and Transepithelial Electrical Resistance (TEER) was used to determine barrier integrity. By stimulating the cells with LPS, there was less cytokine expression in a co-culture of epidermal cells and dendritic cells, as opposed to dendritic cells alone, showing that the epidermal layer formed a protective barrier against LPS inflammation [219]. Further, an immunocompetent lung model was developed that incorporates fibroblasts, epithelial cells, and dendritic cells into an electrospun scaffold to model the upper airway epithelium and has been tested with an allergen as proof of concept [220]. By including antigen presentation, such a model could form the basis for future studies of allergies.
In addition to allergic responses, psoriasis stands out as an autoimmune disease that has been modeled in vitro [221–223]. A hallmark of psoriasis is chronic inflammation where skin cells, immune cells, and environmental factors contribute to skin inflammation and epidermal thickening, but the disease pathogenesis and etiology is still widely unknown [221]. A model for this autoimmune disease is tissue engineered human skin equivalents, which have reached the commercial market and are used by clinicians for skin grafts. Initially, these engineered human skin models incorporated keratinocytes without the presence of an immune element to study psoriasis [221,222], but in a 2014 study from van den Bogaard et al., immune cells were incorporated to better mimic hallmarks of the disease. Activated T cells and their associated cytokines were co-cultured with the established human skin equivalent comprised of keratinocytes suspended in a 3D matrix [223]. After a period of 2 days, immune cell migration into the dermis was observed, with inflammation similar to psoriasis after a 4 day period [223].
Like psoriasis, Inflammatory Bowel Disease (IBD) has been one of the first autoimmune diseases to be addressed in vitro using engineered models. The level of complexity, of course, depends on the purpose of the model and what it is trying to reproduce. Building on a strong foundation of gut-on-a-chip models, the strategy often has been to adapt existing models to integrate immunity and inflammation. A simple model of the gut was developed by Beaurivage et al., where human intestinal epithelial cells were cultured within a hydrogel channel in the OrganoPlate microfluidic device (Fig 6A) [224]. To introduce inflammation into the system, the epithelial cells were co-cultured with monocyte-derived macrophages. Eventually, the authors plan to use this device for drug development or for personalized medicine [224]. In the same device, Gjorevski et al. cultured intestinal epithelial cells with immune cells in hydrogel within a central channel with two adjacent channels for media and stimulant perfusion [225]. Once pro-inflammatory mediators were introduced, the macrophages were activated and the barrier stability decreased. When neutrophils were introduced in one of the perfusion channels, they successfully infiltrated the hydrogel intestinal model, further increasing the inflammation-based damage [225].
Fig 6.

Microfluidic and engineered models of IBD. (A) By co-culturing macrophages next to an intestinal epithelial cell (IEC) lined channel, an inflammatory condition were induced in a gut-on-chip model, which resulted in an increase in cytokine secretion. Adapted with permission from Beaurivage et al. [224] (B) Epithelial cells and macrophages were co-cultured in separate layers within a 3D bi-layer silk scaffold. The macrophages were distributed evenly throughout the outer scaffold, while the epithelial cells formed a monolayer on the inner ring of the scaffold. Adapted with permission from Roh et al. [226] (C) A Transwell co-culture model contained an intestinal epithelia cell later (i) on top of a collagen layer (vii) containing dendritic cells (ii) and macrophages (vi). The apical compartment (v) contained a pro-inflammatory compound (iv). The insert was separated from the basolateral compartment (viii) via a filter membrane (iii). When stained with antibodies specific for the tight junction protein, ZO-1 (red), there is a decrease in tight junctions in the inflamed model. Adapted with permission from Leonard et al. [157] (D) A microfluidic device was designed to incorporate full-thickness intestinal tissue with two separate flow regions both above and below the explanted tissue. Tissue from patients with IBD showed inflammation before on-chip culture (i) and after on-chip culture for 72 hrs (ii). Adapted with permission from Dawson et al. [232].
Tissue engineering-based models have also been used to mimic inflammation in IBD. For example, an engineered model from Roh et al. used spongy silk materials to form a cylindrical intestine model complete with a lumen, microvilli, and mucus (Fig 6B) [226]. With human colon organoids in the inner layer to form an epithelium and monocyte-derived macrophages in the outer layer, the engineered model was able to upregulate the secretion of inflammatory cytokines associated with IBD when exposed to inflammatory stimuli [226]. In addition to mimicking IBD-related inflammation, models of the gut have also been used for drug screening in the inflamed intestinal microenvironment. A 3D co-culture system from Leonard et al. integrated macrophages, dendritic cells, and epithelial cells to test the response to various nanomaterials, bacteria, and drugs (Fig 6C) [227–229].
In IBD, the chronic inflammation in the gut can lead to mucosal injury and damage to villi. A device developed by Kim et al. integrated elements from the gut microbiome, epithelial deformation, and immune cells to generate a gut-on-a-chip platform that was used to study the inflammation and villus damage present in a diseased state [230]. Fluid flow was used on the microfluidic device to encourage villi formation in response to luminal flow, and peripheral blood mononuclear cells were co-cultured with the endothelial barrier and villus. In response to challenge with endotoxin, this model recapitulated the secretion of pro-inflammatory cytokines, damage to villi, and loss of barrier integrity seen in vivo [230]. In a study from Shin and Kim, the same device was used to study intestine-immune cell cross-talk during the initiation of inflammation in the intestine [231]. To induce inflammation, the device was dosed with dextran sodium sulfate (DSS), resulting in epithelial barrier damage and impairment of both the villous microstructures and mucous production. Studying cell-cell interactions in this model revealed that close contact of DSS-sensitized epithelial cells with immune cells elevated the oxidative stress levels, leading to inflammation in the gut model [231].
Finally, to better integrate the complexity seen within the diseased state, Dawson et al. incorporated intestinal tissue explants into a perfusion platform to study IBD patient-specific tissues (Fig 6D) [232]. The device holds a section of full thickness human intestinal tissue while independently perfusing media on the luminal and serosal sides of the tissue [232]. Inflamed tissue was biopsied from patients suffering from IBD and cultured on-chip for 72 hrs. Since an inflamed state was maintained for at least 72 hrs, this platform will be useful for testing patient-specific drug responses going forward.
4.4. Screening Vaccine Efficacy and Safety in Vitro
Vaccines generally fall into one of five categories: live attenuated (avirulent), inactivated (killed), purified subunits, viral vectors, and DNA vaccines [233,234]. Live attenuated vaccines and inactivated vaccines contain a non-pathogenic strain or killed strain, respectively, that is able to cause both humoral and cell-mediated immune responses. Purified subunit, viral vector, and DNA vaccines are the predominant vaccines in development today as they only contain a part (protein or gene) of the pathogenic microorganism and thus carry no risk of infection. Whereas in attenuated and inactivated vaccines, the pathogen provides both the antigen and the pathogen-associated molecular patterns (PAMP), subunit vaccines require an adjuvant to trigger pattern recognition receptors on antigen presenting cells.
Currently, vaccines are screened in vitro in traditional 2D cultures and in vivo in animal models. During preclinical trials, live attenuated vaccine potency is evaluated in vitro by evaluating transfection efficiency and viral infectivity in 2D culture assays [234]. 2D culture assays are also used to measure vaccine efficacy through antibody production and cytokine secretion following re-exposure of an antigen. For subunit vaccines, preclinical potency tests require animal studies, often involving mice and, eventually, Rhesus monkeys [235,236]. The animal models are essential for assessing safety, determining the start and length of the induced immune response, and determining the best route of exposure (i.e. nasal spray versus intramuscular injection) [237]. These metrics depend on communication and cell migration between multiple organ systems, which is currently only possible to model in living animals. Given that the cost of an epidemic infectious disease vaccine can cost from $31–68 million [238] to pass from preclinical trials and through clinical trials with a 94% chance of failure [239], there is a significant need for more accurate, human-relevant in vitro models for evaluating vaccine efficacy. This need is especially clear amid the coronavirus pandemic. Currently available subunit (mRNA) COVID vaccines were developed and received emergency FDA approval in just under 12 months, though with a substantial price tag of at least $10 billion from the U.S. government [240]. The efficiency of future pandemic responses depends on better models.
The lymph nodes (LN) are an organizing center of the adaptive immune response, where activated T cells interact with B cells at the lymph node follicle to produce antibodies [233,241]. The LN plays an essential role in a vaccine response, so modeling the LN with tissue engineering is a promising front for improving vaccine testing. As reviewed in [13], numerous models of the LN have emerged recently; here we briefly discuss models that are potentially relevant for drug or vaccine screening.
One approach to modeling drug or vaccine responses in the lymph node has used modular cultures, e.g. in bioreactors or transwells. For example, a bioreactor system termed “human artificial lymph node” (HuALN) has been constructed of polysulfone and consists of 2 culture chambers separated by an oxygen permeable membrane [242]. The device debuted in 2010 having human B and T cells in 3D co-culture, and has since been demonstrated as a platform for testing vaccines and unwanted immune responses to protein therapeutics. B and T cells cluster in the bioreactor, then form micro-organoids following stimulation by a drug or antigen. Inflammation status and T cell response has been monitored by collecting effluent from the outlet of the bioreactor and analyzing for an array of cytokines. HuALN has been commercialized (owned by ProGenBio) and is advertised as a vaccine testing platform. In another example, the “Modular IMmune In vitro Construct” (MIMIC®) is a transwell-based platform [243] by the U.S. company VaxDesign (now owned by Sanofi Pasteur). MIMIC® was used to measure cytokine and antibody production by B and T cells from donors who had received a tetanus vaccine or influenza vaccines. While the exact make-up of this system is proprietary, available literature illustrates that the transwell can be arranged in a variety of configurations depending on the model. To model the response to tetanus vaccine, authors collected purified B- and CD4+ T-cells from donors before and after they received a tetanus vaccine. When purified cells were cultured in the MIMIC® well with autologous antigen-pulsed dendritic cells, the cells from vaccinated individuals produced tetanus-specific antibodies [244]. Analogous tests were performed to test various influenza vaccines, and the expected age-dependent responses were reported [245]. So far, these tests demonstrate that the MIMIC® system predicts antigen-challenge experiments when working with cells from vaccinated donors. De novo prediction of the immune response to ex vivo vaccination of cells from previously unvaccinated donors is not yet possible in these or other systems, so animal models or human donors are still essential to test vaccine efficacy.
An alternative approach to vaccine testing and lymph node modeling is to form organoids. A foundational study by Purwada et al. demonstrated that culturing naïve primary B cells and an engineered fibroblast line in a hydrogel matrix with stiffness similar to a lymph node results in organoids forming that are roughly 200 um in diameter [7]. These clustered B cells could be differentiated into germinal center B cells more efficiently than cells in 2D culture and were shown to undergo antibody class-switching, further supporting the idea that considering the microscale organization of the lymph node is crucial for building a vaccine or drug testing platform [246]. Future work with organoid-based lymph node models may aim to include additional biomimetic features such as surrounding T cells or a lymph node sinus, for example. Of course, implementing such features requires more development and validation, which is why this approach is not yet being used for vaccine or drug testing.
In general, the current state of vaccine testing platforms favors those that are easy to construct, such as transwell-based platforms, and have few cell types. While these platforms do allow for monitoring a vaccine response in a more biomimetic setting than traditional 2D culture, their simplicity may result in the same pit-falls of pre-clinical 2D culture assays; that unexpected side effects or low efficacy could be observed in clinical trials. For example, without stromal cells and micro-scale spatial biomimicry (e.g., the lymph node sinus and germinal center) key aspects of antigen presentation, B and T cell activation, and antibody production may not be occurring as they do in vivo. Testing platforms that can retain or reproduce the microarchitecture of the lymph node are currently still in development. For example, Ball et al. and Belanger et al. have shown that lymph node slices, which retain the spatial organization and lymph node-resident cell populations, are useful to compare vaccine adjuvants via the response to ex vivo antigen challenge after in vivo vaccination [247,248]. While retaining most of the biological information is promising, there remain challenges with slice-to-slice variation and access to tissue for pre-clinical translation. Furthermore, due to lymphocyte egress, lymph node slices are currently best used for short-term experiments, e.g. antigen recall, not the multi-week cultures needed to study germinal center development. In preliminary work, Shanti et al. developed microfluidic devices that allow patterning of an immune cell-laden hydrogel in a biomimetic orientation, but complex immune function has not yet been demonstrated in this system [249]. Nevertheless, the promise of recreating the spatial organization of the lymph node using microfluidics, hydrogels, and human immune cells may open the door to more accurate vaccine testing in the future.
5. Engineered models in translation (clinical and commercial perspective)
The use of animal models and 2D culture systems for evaluating drug efficacy is known to be imperfect. Approximately 90% of compounds that pass pre-clinical drug screening fail during clinical trials [250]. Microphysiological systems and engineered tissues have emerged as a potential solution to enhance the predictability of pre-clinical models. These complex microscale in vitro systems can recapitulate key elements of human physiology, including spatial organization, fluid flow, extracellular matrix, and key cell types. In the future, connecting microphysiological systems of different organs could translate toward a “human on chip” that encompasses a number organ systems [29,163,251,252]. However, the commercial use of complex in vitro models is limited due to a lack of validation, low throughput rates, and a lack of compatibility with standard assessment techniques [253]. A recent review by Zhang et al examines the potential of current in vitro microphysiological models for improving pre-clinical models and identifies that for commercialization, a focus on high throughput for each stage of the model is essential [253]. Dittrich and Manz point out that major challenges with implementing microfluidics include standardization of materials, interfaces, and geometries as well as refining the ease of handling and robustness of these systems [254]. A review by Dehne et al points out that microphysiological systems are a young research field, but that nearly all major pharmaceutical companies are involved in MPS feasibility studies. The review further summarizes examples of specific companies and funded research within the field of microphysiological systems and discusses the challenges of meeting legislative requirements to replace traditional in vitro methods with microphysiological systems [255].
Moving forward, the field is taking steps to implement microphysiological systems as the gold standard of drug screening. The inclusion of immune elements into engineered models faces many barriers, such as challenges with sourcing and co-culturing primary immune cells, the challenges of designing biomaterials that allow for physiologically relevant immune cell migration, and the difficulty of recapitulating the complexity of robust immune responses. However, while the immune response is still largely omitted from commercialized model systems, its integration has been a high priority in government funding for years, e.g. with funding from the National Institutes of Health, DARPA, BARDA, and other defense-related agencies. These efforts will likely bear fruit over the next few years with the first round of immune-related models ready for commercialization and translation. As discussed above, a number of models of various aspects of immunity have been established, but are not widely applied toward drug screening. Immunogenicity screening is essential toward achieving clinical success. At the Children’s Mercy Hospital in Kansas City, up to 65% of patients are undergoing treatment for IBD-developed antidrug antibodies [256]. Current strategies for evaluating immunogenicity include ligand-binding immuno-assays that rely on positive controls created in non-human species [257]. The techniques used to evaluate immunogenicity vary widely and results are often misleading, leading to a need for better screening via microphysiological platforms or computational modeling, and clearer outputs and data presentation [257,258]. Therefore, there is a need for the field to focus more closely on including immune elements in future microphysiological systems and engineered tissue models. Current work toward optimizing universal media, demonstrating the major contributions of the immune system toward drug delivery and efficacy, and developing immune-competent models will serve to advance the utilization of immune-competent models in preclinical drug screening.
6. Challenges and Opportunities
As discussed above, the integration of immunity into engineered tissue models for screening therapeutics and drugs is a necessary step for the field to enhance predictive power of therapeutic efficacy and safety. With the challenges of achieving regulatory approval of these models for preclinical assessment, including additional levels of complexity via immune elements will provide additional challenges toward attaining approval. Reliable and reproducible cell sourcing, always a challenge for engineered models of human tissues, is especially challenging for immune-related cells. Patient-to-patient variability must be accounted for when using primary cells for applications other than personalized medicine, while iPSC-derivation protocols are not yet available to produce polyclonal populations of lymphocytes or well-validated antigen-presenting cells. Current work looking at deriving decellularized ECM matrices and induced pluripotent stem cells from biopsies could provide patient-specific biomaterials and cells [259]. As the organ-on-chip community moves toward incorporating the major organ systems into larger interconnected devices [252], the benefits of adding immune organs such as lymph nodes could be an opportunity to create an immunocompetent “human on chip”. Additionally, as immune-competent engineered models progress, more consideration can be given to reproducing the time scales of immune responses and recapitulating robust adaptive immune responses. Modeling adaptive immune responses will require consideration of the low frequency of antigen-specific T cells and B cells in the circulation, the extended timescale (10’s of days) compared to innate immune responses (minutes – hours), and the compartmentalization of adaptive immune responses into initiation at the site of insult, drainage to the lymph node or spleen, dynamics in the lymph node and spleen themselves, and finally a return to the site of insult for immunity. In the past, a major barrier has been engaging immunologists, engineers, and physical scientists to talk with one another, but this is changing in recent years with the boom in “immune engineering” (the field of controlling or analyzing immunity via principles from engineering and physical science) and a suite of new conferences and symposia to build up the community. Inclusion of immunity in engineered tissues and MPSs provides an exciting opportunity to have predictive preclinical screening that is comparable to patient responses in the clinic.
Conclusion
Engineered models provide a powerful tool toward in vitro screening of drugs, therapies, and vaccines. With the ability to manipulate physical and biochemical cues, models can be designed that mimic important tissue parameters. Traditional 2D drug screening does not provide this precise control or recapitulate in vivo conditions. Models have been developed for the study of IBD, autoimmunity, and cancer, but the inclusion of immune elements often lags behind. Engineered models of tumors that include immune elements show enhanced chemoresistance and offer the potential for more accurate drug screening than traditional 2D models. Such engineered tumor models provide a physiologically relevant platform for preclinical assessment of engineered T cell efficacy. Meanwhile, a variety of engineered models for screening vaccines in vitro are in development. Emerging models are remarkably biomimetic, and more validation may lead to their translation to pre-clinical vaccine testing. In summary, many models utilized for drug screening fail to include immune elements even though they can drastically alter drug delivery and drug efficacy, and therefore should be considered a vital part of drug screening.
Highlights.
Immunity impacts all tissues and should be considered in models for drug screening
Immune-competent models are impacted by physical, chemical, and biological cues
Recent tumor-immune models feature spatial organization, flow, and vasculature
Tumor models can be used to test the efficacy of engineered T-cell therapies
Models of autoimmunity and vaccination are early stage but promising
Sourcing of immune cells is a significant challenge with opportunity for innovation
Acknowledgments
This publication was supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB) under award number U01EB029127 through the National Institutes of Health (NIH), with co-funding from the National Center for Advancing Translational Sciences (NCATS). Additional support was provided by the National Cancer Institute under award number R37CA222563 to JMM and by the National Institute of Allergy and Infectious Diseases under award number R01AI131723 to RRP. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. JH was supported by the Institute for Critical Technology and Applied Science Scholarship through Virginia Tech. JMZ was supported by the Graduate Research Fellowship Program through the National Science Foundation (NSF). Graphical abstract, Figure 1, Figure 2, and Figure 3 were created with Biorender.com.
Abbreviations
- MPSs
microphysiological systems
- NK
natural killer
- HCQ
hydroxychloroquine
- ECM
extracellular matrix
- ESCs
Embryonic stem cells
- SCNT
somatic cell nuclear transfer
- iPSCs
induced pluripotent stem cells
- EPR
enhanced permeability and retention effect
- IFP
interstitial fluid pressure
- TME
tumor microenvironment
- TEER
Transepithelial Electrical Resistance
- IBD
Inflammatory Bowel Disease
- DSS
dextran sodium sulfate
- PAMP
pathogen-associated molecular patterns
- LN
Lymph node
- HuALN
human artificial lymph node
- MIMIC®
Modular IMmune In vitro Construct
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Gomes ME, Rodrigues MT, Domingues RMA, Reis RL, Tissue Engineering and Regenerative Medicine: New Trends and Directions—A Year in Review, Tissue Engineering Part B: Reviews 23 (2017) 211–224. 10.1089/ten.teb.2017.0081. [DOI] [PubMed] [Google Scholar]
- [2].Park J, Lee BK, Jeong GS, Hyun JK, Lee CJ, Lee S-H, Three-dimensional brain-on-a-chip with an interstitial level of flow and its application as an in vitro model of Alzheimer’s disease, Lab Chip 15 (2015) 141–150. 10.1039/C4LC00962B. [DOI] [PubMed] [Google Scholar]
- [3].Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE, Reconstituting Organ-Level Lung Functions on a Chip, Science 328 (2010) 1662–1668. 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].de Haan P, Ianovska MA, Mathwig K, van Lieshout GAA, Triantis V, Bouwmeester H, Verpoorte E, Digestion-on-a-chip: a continuous-flow modular microsystem recreating enzymatic digestion in the gastrointestinal tract, Lab Chip 19 (2019) 1599–1609. 10.1039/C8LC01080C. [DOI] [PubMed] [Google Scholar]
- [5].Wufuer M, Lee G, Hur W, Jeon B, Kim BJ, Choi TH, Lee S, Skin-on-a-chip model simulating inflammation, edema and drug-based treatment, Sci Rep 6 (2016) 37471. 10.1038/srep37471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wilmer MJ, Ng CP, Lanz HL, Vulto P, Suter-Dick L, Masereeuw R, Kidney-on-a-Chip Technology for Drug-Induced Nephrotoxicity Screening, Trends in Biotechnology 34 (2016) 156–170. 10.1016/j.tibtech.2015.11.001. [DOI] [PubMed] [Google Scholar]
- [7].Purwada A, Jaiswal MK, Ahn H, Nojima T, Kitamura D, Gaharwar AK, Cerchietti L, Singh A, Ex vivo engineered immune organoids for controlled germinal center reactions, Biomaterials 63 (2015) 24–34. 10.1016/j.biomaterials.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shirure VS, Bi Y, Curtis MB, Lezia A, Goedegebuure MM, Goedegebuure SP, Aft R, Fields RC, George SC, Tumor-on-a-chip platform to investigate progression and drug sensitivity in cell lines and patient-derived organoids, Lab Chip 18 (2018) 3687–3702. 10.1039/C8LC00596F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nam K-H, Smith AST, Lone S, Kwon S, Kim D-H, Biomimetic 3D Tissue Models for Advanced High-Throughput Drug Screening, J Lab Autom 20 (2015) 201–215. 10.1177/2211068214557813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Carvalho MR, Lima D, Reis RL, Oliveira JM, Correlo VM, Anti-Cancer Drug Validation: the Contribution of Tissue Engineered Models, Stem Cell Rev and Rep 13 (2017) 347–363. 10.1007/s12015-017-9720-x. [DOI] [PubMed] [Google Scholar]
- [11].Vanderburgh J, Sterling JA, Guelcher SA, 3D Printing of Tissue Engineered Constructs for In Vitro Modeling of Disease Progression and Drug Screening, Ann Biomed Eng 45 (2017) 164–179. 10.1007/s10439-016-1640-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Caddeo S, Boffito M, Sartori S, Tissue Engineering Approaches in the Design of Healthy and Pathological In Vitro Tissue Models, Front. Bioeng. Biotechnol 5 (2017) 40. 10.3389/fbioe.2017.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hammel JH, Cook SR, Belanger MC, Munson JM, Pompano RR, Modeling Immunity In Vitro: Slices, Chips, and Engineered Tissues, Annu. Rev. Biomed. Eng 23 (2021) annurev-bioeng-082420–124920. 10.1146/annurev-bioeng-082420-124920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fuggetta MP, Lanzilli G, Fioretti D, Rinaldi M, In vitro end points for the assessment of cellular immune response-modulating drugs, Expert Opinion on Drug Discovery 4 (2009) 473–493. 10.1517/17460440902821632. [DOI] [PubMed] [Google Scholar]
- [15].Frick A, Fedoriw Y, Richards K, Damania B, Parks B, Suzuki O, Benton C, Chan E, Thomas R, Wiltshire T, Immune cell-based screening assay for response to anticancer agents: applications in pharmacogenomics, PGPM (2015) 81. 10.2147/PGPM.S73312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Neri S, Mariani E, Meneghetti A, Cattini L, Facchini A, Calcein-Acetyoxymethyl Cytotoxicity Assay: Standardization of a Method Allowing Additional Analyses on Recovered Effector Cells and Supernatants, Clin Diagn Lab Immunol 8 (2001) 1131–1135. 10.1128/CDLI.8.6.1131-1135.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kolber MA, Quinones RR, Gress RE, Henkert PA, Measurement of cytotoxicity by target cell release and retention of the fluorescent dye bis-carboxyethyl-carboxyfluorescein (BCECF), Journal of Immunological Methods 108 (1988) 255–264. 10.1016/0022-1759(88)90427-9. [DOI] [PubMed] [Google Scholar]
- [18].Astashkina A, Mann B, Grainger DW, A critical evaluation of in vitro cell culture models for high-throughput drug screening and toxicity, Pharmacology & Therapeutics 134 (2012) 82–106. 10.1016/j.pharmthera.2012.01.001. [DOI] [PubMed] [Google Scholar]
- [19].Souza AG, Silva IBB, Campos-Fernandez E, Barcelos LS, Souza JB, Marangoni K, Goulart LR, Alonso-Goulart V, Comparative Assay of 2D and 3D Cell Culture Models: Proliferation, Gene Expression and Anticancer Drug Response, CPD 24 (2018) 1689–1694. 10.2174/1381612824666180404152304. [DOI] [PubMed] [Google Scholar]
- [20].Van Zundert I, Fortuni B, Rocha S, From 2D to 3D Cancer Cell Models—The Enigmas of Drug Delivery Research, Nanomaterials 10 (2020) 2236. 10.3390/nano10112236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chignola R, Schenetti A, Andrighetto G, Chiesa E, Foroni R, Sartoris S, Tridente G, Liberati D, Forecasting the growth of multicell tumour spheroids: implications for the dynamic growth of solid tumours: Forecasting spheroid growth, Cell Proliferation 33 (2000) 219–229. 10.1046/j.1365-2184.2000.00174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Imamura Y, Mukohara T, Shimono Y, Funakoshi Y, Chayahara N, Toyoda M, Kiyota N, Takao S, Kono S, Nakatsura T, Minami H, Comparison of 2D- and 3D-culture models as drug-testing platforms in breast cancer, Oncology Reports 33 (2015) 1837–1843. 10.3892/or.2015.3767. [DOI] [PubMed] [Google Scholar]
- [23].Banks WA, Niehoff ML, Ponzio NM, Erickson MA, Zalcman SS, Pharmacokinetics and modeling of immune cell trafficking: quantifying differential influences of target tissues versus lymphocytes in SJL and lipopolysaccharide-treated mice, J Neuroinflammation 9 (2012) 714. 10.1186/1742-2094-9-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Texler B, Zollner A, Reinstadler V, Reider SJ, Macheiner S, Jelusic B, Pfister A, Watschinger C, Przysiecki N, Tilg H, Oberacher H, Moschen AR, Tofacitinib-Induced Modulation of Intestinal Adaptive and Innate Immunity and Factors Driving Cellular and Systemic Pharmacokinetics, Cellular and Molecular Gastroenterology and Hepatology (2021) S2352345X21001922. 10.1016/j.jcmgh.2021.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang H, Rayburn ER, Wang W, Kandimalla ER, Agrawal S, Zhang R, Immunomodulatory oligonucleotides as novel therapy for breast cancer: pharmacokinetics, in vitro and in vivo anticancer activity, and potentiation of antibody therapy, Mol Cancer Ther 5 (2006) 2106–2114. 10.1158/1535-7163.MCT-06-0158. [DOI] [PubMed] [Google Scholar]
- [26].Morris EC, Rebello P, Thomson KJ, Peggs KS, Kyriakou C, Goldstone AH, Mackinnon S, Hale G, Pharmacokinetics of alemtuzumab used for in vivo and in vitro T-cell depletion in allogeneic transplantations: relevance for early adoptive immunotherapy and infectious complications, Blood 102 (2003) 404–406. 10.1182/blood-2002-09-2687. [DOI] [PubMed] [Google Scholar]
- [27].De Buck SS, Mackie CE, Physiologically based approaches towards the prediction of pharmacokinetics: in vitro–in vivo extrapolation, Expert Opinion on Drug Metabolism & Toxicology 3 (2007) 865–878. 10.1517/17425255.3.6.865. [DOI] [PubMed] [Google Scholar]
- [28].Houston J, Galetin A, Methods for Predicting In Vivo Pharmacokinetics Using Data from In Vitro Assays, CDM 9 (2008) 940–951. 10.2174/138920008786485164. [DOI] [PubMed] [Google Scholar]
- [29].Sasserath T, Rumsey JW, McAleer CW, Bridges LR, Long CJ, Elbrecht D, Schuler F, Roth A, Bertinetti‐LaPatki C, Shuler ML, Hickman JJ, Differential Monocyte Actuation in a Three‐Organ Functional Innate Immune System‐on‐a‐Chip, Adv. Sci 7 (2020) 2000323. 10.1002/advs.202000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tsamandouras N, Chen WLK, Edington CD, Stokes CL, Griffith LG, Cirit M, Integrated Gut and Liver Microphysiological Systems for Quantitative In Vitro Pharmacokinetic Studies, AAPS J 19 (2017) 1499–1512. 10.1208/s12248-017-0122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Barrila J, Yang J, Crabbé A, Sarker SF, Liu Y, Ott CM, Nelman-Gonzalez MA, Clemett SJ, Nydam SD, Forsyth RJ, Davis RR, Crucian BE, Quiriarte H, Roland KL, Brenneman K, Sams C, Loscher C, Nickerson CA, Three-dimensional organotypic co-culture model of intestinal epithelial cells and macrophages to study Salmonella enterica colonization patterns, Npj Microgravity 3 (2017) 10. 10.1038/s41526-017-0011-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chandorkar P, Posch W, Zaderer V, Blatzer M, Steger M, Ammann CG, Binder U, Hermann M, Hörtnagl P, Lass-Flörl C, Wilflingseder D, Fast-track development of an in vitro 3D lung/immune cell model to study Aspergillus infections, Sci Rep 7 (2017) 11644. 10.1038/s41598-017-11271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Abreu CM, Gama L, Krasemann S, Chesnut M, Odwin-Dacosta S, Hogberg HT, Hartung T, Pamies D, Microglia Increase Inflammatory Responses in iPSC-Derived Human BrainSpheres, Front. Microbiol 9 (2018) 2766. 10.3389/fmicb.2018.02766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Han Y, Yang L, Duan X, Duan F, Nilsson-Payant BE, Yaron TM, Wang P, Tang X, Zhang T, Zhao Z, Bram Y, Redmond D, Houghton S, Nguyen D, Xu D, Wang X, Uhl S, Huang Y, Johnson JL, Xiang J, Wang H, Pan FC, Cantley LC, tenOever BR, Ho DD, Evans T, Schwartz RE, Chen HJ, Chen S, Identification of Candidate COVID-19 Therapeutics using hPSC-derived Lung Organoids, Microbiology, 2020. 10.1101/2020.05.05.079095. [DOI] [Google Scholar]
- [35].Suzuki T, Itoh Y, Sakai Y, Saito A, Okuzaki D, Motooka D, Minami S, Kobayashi T, Yamamoto T, Okamoto T, Takayama K, Generation of human bronchial organoids for SARS-CoV-2 research, Cell Biology, 2020. 10.1101/2020.05.25.115600. [DOI] [Google Scholar]
- [36].Lamers MM, Vaart J, Knoops K, Riesebosch S, Breugem TI, Mykytyn AZ, Beumer J, Schipper D, Bezstarosti K, Koopman CD, Groen N, Ravelli RBG, Duimel HQ, Demmers JAA, Verjans GMGM, Koopmans MPG, Muraro MJ, Peters PJ, Clevers H, Haagmans BL, An organoid‐derived bronchioalveolar model for SARS‐CoV‐2 infection of human alveolar type II‐like cells, EMBO J 40 (2021). 10.15252/embj.2020105912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Han Y, Duan X, Yang L, Nilsson-Payant BE, Wang P, Duan F, Tang X, Yaron TM, Zhang T, Uhl S, Bram Y, Richardson C, Zhu J, Zhao Z, Redmond D, Houghton S, Nguyen D-HT, Xu D, Wang X, Jessurun J, Borczuk A, Huang Y, Johnson JL, Liu Y, Xiang J, Wang H, Cantley LC, tenOever BR, Ho DD, Pan FC, Evans T, Chen HJ, Schwartz RE, Chen S, Identification of SARS-CoV-2 inhibitors using lung and colonic organoids, Nature 589 (2021) 270–275. 10.1038/s41586-020-2901-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Salahudeen AA, Choi SS, Rustagi A, Zhu J, van Unen V, de la O SM, Flynn RA, Margalef-Català M, Santos AJM, Ju J, Batish A, Usui T, Zheng GXY, Edwards CE, Wagar LE, Luca V, Anchang B, Nagendran M, Nguyen K, Hart DJ, Terry JM, Belgrader P, Ziraldo SB, Mikkelsen TS, Harbury PB, Glenn JS, Garcia KC, Davis MM, Baric RS, Sabatti C, Amieva MR, Blish CA, Desai TJ, Kuo CJ, Progenitor identification and SARS-CoV-2 infection in human distal lung organoids, Nature 588 (2020) 670–675. 10.1038/s41586-020-3014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Monteil V, Kwon H, Prado P, Hagelkrüys A, Wimmer RA, Stahl M, Leopoldi A, Garreta E, Hurtado del Pozo C, Prosper F, Romero JP, Wirnsberger G, Zhang H, Slutsky AS, Conder R, Montserrat N, Mirazimi A, Penninger JM, Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2, Cell 181 (2020) 905–913.e7. 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zang R, Gomez Castro MF, McCune BT, Zeng Q, Rothlauf PW, Sonnek NM, Liu Z, Brulois KF, Wang X, Greenberg HB, Diamond MS, Ciorba MA, Whelan SPJ, Ding S, TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes, Sci. Immunol 5 (2020) eabc3582. 10.1126/sciimmunol.abc3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhou J, Li C, Liu X, Chiu MC, Zhao X, Wang D, Wei Y, Lee A, Zhang AJ, Chu H, Cai J-P, Yip CC-Y, Chan IH-Y, Wong KK-Y, Tsang OT-Y, Chan K-H, Chan JF-W, To KK-W, Chen H, Yuen KY, Infection of bat and human intestinal organoids by SARS-CoV-2, Nat Med (2020). 10.1038/s41591-020-0912-6. [DOI] [PubMed] [Google Scholar]
- [42].Lamers MM, Beumer J, van der Vaart J, Knoops K, Puschhof J, Breugem TI, Ravelli RBG, Paul van Schayck J, Mykytyn AZ, Duimel HQ, van Donselaar E, Riesebosch S, Kuijpers HJH, Schippers D, van de Wetering WJ, de Graaf M, Koopmans M, Cuppen E, Peters PJ, Haagmans BL, Clevers H, SARS-CoV-2 productively infects human gut enterocytes, Science (2020) eabc1669. 10.1126/science.abc1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Yang L, Han Y, Nilsson-Payant BE, Gupta V, Wang P, Duan X, Tang X, Zhu J, Zhao Z, Jaffré F, Zhang T, Kim TW, Harschnitz O, Redmond D, Houghton S, Liu C, Naji A, Ciceri G, Guttikonda S, Bram Y, Nguyen D-HT, Cioffi M, Chandar V, Hoagland DA, Huang Y, Xiang J, Wang H, Lyden D, Borczuk A, Chen HJ, Studer L, Pan FC, Ho DD, tenOever BR, Evans T, Schwartz RE, Chen S, A Human Pluripotent Stem Cell-based Platform to Study SARS-CoV-2 Tropism and Model Virus Infection in Human Cells and Organoids, Cell Stem Cell 27 (2020) 125–136.e7. 10.1016/j.stem.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhao B, Ni C, Gao R, Wang Y, Yang L, Wei J, Lv T, Liang J, Zhang Q, Xu W, Xie Y, Wang X, Yuan Z, Liang J, Zhang R, Lin X, Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids, Protein Cell 11 (2020) 771–775. 10.1007/s13238-020-00718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Song E, Zhang C, Israelow B, Lu P, Weizman O-E, Liu F, Dai Y, Szigeti-Buck K, Yasumoto Y, Wang G, Castaldi C, Heltke J, Ng E, Wheeler J, Alfajaro MM, Fontes B, Ravindra NG, Van Dijk D, Mane S, Gunel M, Ring A, Wilen CB, Horvath TL, Louvi A, Farhadian SF, Bilguvar K, Iwasaki A, Neuroinvasive potential of SARS-CoV-2 revealed in a human brain organoid model, Microbiology, 2020. 10.1101/2020.06.25.169946. [DOI] [Google Scholar]
- [46].Ramani A, Müller L, Ostermann PN, Gabriel E, Abida‐Islam P, Müller‐Schiffmann A, Mariappan A, Goureau O, Gruell H, Walker A, Andrée M, Hauka S, Houwaart T, Dilthey A, Wohlgemuth K, Omran H, Klein F, Wieczorek D, Adams O, Timm J, Korth C, Schaal H, Gopalakrishnan J, SARS ‐CoV‐2 targets neurons of 3D human brain organoids, EMBO J 39 (2020). 10.15252/embj.2020106230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Si L, Bai H, Rodas M, Cao W, Oh CY, Jiang A, Moller R, Hoagland D, Oishi K, Horiuchi S, Uhl S, Blanco-Melo D, Albrecht RA, Liu W-C, Jordan T, Nilsson-Payant BE, Golynker I, Frere J, Logue J, Haupt R, McGrath M, Weston S, Zhang T, Plebani R, Soong M, Nurani A, Kim SM, Zhu DY, Benam KH, Goyal G, Gilpin SE, Prantil-Baun R, Gygi SP, Powers RK, Carlson KE, Frieman M, tenOever BR, Ingber DE, A human-airway-on-a-chip for the rapid identification of candidate antiviral therapeutics and prophylactics, Nat Biomed Eng 5 (2021) 815–829. 10.1038/s41551-021-00718-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Shoichet MS, Polymer Scaffolds for Biomaterials Applications, Macromolecules 43 (2010) 581–591. 10.1021/ma901530r. [DOI] [Google Scholar]
- [49].Singh A, Peppas NA, Hydrogels and Scaffolds for Immunomodulation, Adv. Mater 26 (2014) 6530–6541. 10.1002/adma.201402105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sadtler K, Wolf MT, Ganguly S, Moad CA, Chung L, Majumdar S, Housseau F, Pardoll DM, Elisseeff JH, Divergent immune responses to synthetic and biological scaffolds, Biomaterials 192 (2019) 405–415. 10.1016/j.biomaterials.2018.11.002. [DOI] [PubMed] [Google Scholar]
- [51].Caccavo D, Cascone S, Lamberti G, Barba AA, Hydrogels: experimental characterization and mathematical modelling of their mechanical and diffusive behaviour, Chem. Soc. Rev 47 (2018) 2357–2373. 10.1039/C7CS00638A. [DOI] [PubMed] [Google Scholar]
- [52].Loh QL, Choong C, Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size, Tissue Engineering Part B: Reviews 19 (2013) 485–502. 10.1089/ten.teb.2012.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Oyen ML, Mechanical characterisation of hydrogel materials, International Materials Reviews 59 (2014) 44–59. 10.1179/1743280413Y.0000000022. [DOI] [Google Scholar]
- [54].Ghasemiyeh P, Mohammadi-Samani S, Hydrogels as Drug Delivery Systems; Pros and Cons, Trends in Pharmaceutical Sciences 5 (2019). 10.30476/tips.2019.81604.1002. [DOI] [Google Scholar]
- [55].Huse M, Mechanical forces in the immune system, Nat Rev Immunol 17 (2017) 679–690. 10.1038/nri.2017.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Moreau HD, Piel M, Voituriez R, Lennon-Duménil A-M, Integrating Physical and Molecular Insights on Immune Cell Migration, Trends in Immunology 39 (2018) 632–643. 10.1016/j.it.2018.04.007. [DOI] [PubMed] [Google Scholar]
- [57].Syková E, Nicholson C, Diffusion in Brain Extracellular Space, Physiological Reviews 88 (2008) 1277–1340. 10.1152/physrev.00027.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Rauti R, Renous N, Maoz BM, Mimicking the Brain Extracellular Matrix in Vitro : A Review of Current Methodologies and Challenges, Isr. J. Chem 60 (2020) 1141–1151. 10.1002/ijch.201900052. [DOI] [Google Scholar]
- [59].Nicholson C, Hrabětová S, Brain Extracellular Space: The Final Frontier of Neuroscience, Biophysical Journal 113 (2017) 2133–2142. 10.1016/j.bpj.2017.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kamali-Zare P, Nicholson C, Brain extracellular space: geometry, matrix and physiological importance, Basic Clin Neurosci 4 (2013) 282–286. [PMC free article] [PubMed] [Google Scholar]
- [61].Butcher DT, Alliston T, Weaver VM, A tense situation: forcing tumour progression, Nat Rev Cancer 9 (2009) 108–122. 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Lin X, Patil S, Gao Y-G, Qian A, The Bone Extracellular Matrix in Bone Formation and Regeneration, Front. Pharmacol 11 (2020) 757. 10.3389/fphar.2020.00757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Schaffler MB, Burr DB, Stiffness of compact bone: Effects of porosity and density, Journal of Biomechanics 21 (1988) 13–16. 10.1016/0021-9290(88)90186-8. [DOI] [PubMed] [Google Scholar]
- [64].Dillaman RM, Roer RD, Gay DM, Fluid movement in bone: Theoretical and empirical, Journal of Biomechanics 24 (1991) 163–177. 10.1016/0021-9290(91)90386-2. [DOI] [PubMed] [Google Scholar]
- [65].Cardoso L, Fritton SP, Gailani G, Benalla M, Cowin SC, Advances in assessment of bone porosity, permeability and interstitial fluid flow, Journal of Biomechanics 46 (2013) 253–265. 10.1016/j.jbiomech.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Gillies AR, Lieber RL, Structure and function of the skeletal muscle extracellular matrix: Skeletal Muscle ECM, Muscle Nerve 44 (2011) 318–331. 10.1002/mus.22094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Wang Q, Pei S, Lu XL, Wang L, Wu Q, On the characterization of interstitial fluid flow in the skeletal muscle endomysium, Journal of the Mechanical Behavior of Biomedical Materials 102 (2020) 103504. 10.1016/j.jmbbm.2019.103504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Ling GN, Kromash MH, The Extracellular Space of Voluntary Muscle Tissues, Journal of General Physiology 50 (1967) 677–694. 10.1085/jgp.50.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Shapiro L, Elsangeedy E, Lee H, Atala A, Yoo JJ, Lee SJ, Ju YM, In vitro evaluation of functionalized decellularized muscle scaffold for in situ skeletal muscle regeneration, Biomed. Mater 14 (2019) 045015. 10.1088/1748-605X/ab229d. [DOI] [PubMed] [Google Scholar]
- [70].Tomei AA, Siegert S, Britschgi MR, Luther SA, Swartz MA, Fluid Flow Regulates Stromal Cell Organization and CCL21 Expression in a Tissue-Engineered Lymph Node Microenvironment, J Immunol 183 (2009) 4273–4283. 10.4049/jimmunol.0900835. [DOI] [PubMed] [Google Scholar]
- [71].Birmingham KG, O’Melia MJ, Bordy S, Reyes Aguilar D, El-Reyas B, Lesinski G, Thomas SN, Lymph Node Subcapsular Sinus Microenvironment-On-A-Chip Modeling Shear Flow Relevant to Lymphatic Metastasis and Immune Cell Homing, IScience 23 (2020) 101751. 10.1016/j.isci.2020.101751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Hirsch S, Guo J, Reiter R, Papazoglou S, Kroencke T, Braun J, Sack I, MR Elastography of the Liver and the Spleen Using a Piezoelectric Driver, Single-Shot Wave-Field Acquisition, and Multifrequency Dual Parameter Reconstruction: Multi-frequency MR Elastography of the Liver and the Spleen, Magn. Reson. Med 71 (2014) 267–277. 10.1002/mrm.24674. [DOI] [PubMed] [Google Scholar]
- [73].Miyaji K, Furuse A, Nakajima J, Kohno T, Ohtsuka T, Yagyu K, Oka T, Omata S, The stiffness of lymph nodes containing lung carcinoma metastases: A new diagnostic parameter measured by a tactile sensor, Cancer 80 (1997) 1920–1925. . [DOI] [PubMed] [Google Scholar]
- [74].Arda K, Ciledag N, Aktas E, Arıbas BK, Köse K, Quantitative Assessment of Normal Soft-Tissue Elasticity Using Shear-Wave Ultrasound Elastography, American Journal of Roentgenology 197 (2011) 532–536. 10.2214/AJR.10.5449. [DOI] [PubMed] [Google Scholar]
- [75].Cizauskaite U, Bernatoniene J, Innovative Natural Ingredients-Based Multiple Emulsions: The Effect on Human Skin Moisture, Sebum Content, Pore Size and Pigmentation, Molecules 23 (2018) 1428. 10.3390/molecules23061428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Karimi A, Navidbakhsh M, Haghighatnama M, Haghi AM, Determination of the axial and circumferential mechanical properties of the skin tissue using experimental testing and constitutive modeling, Computer Methods in Biomechanics and Biomedical Engineering 18 (2015) 1768–1774. 10.1080/10255842.2014.961441. [DOI] [PubMed] [Google Scholar]
- [77].Fischer M, Franzeck UK, Herrig I, Costanzo U, Wen S, Schiesser M, Hoffmann U, Bollinger A, Flow velocity of single lymphatic capillaries in human skin, American Journal of Physiology-Heart and Circulatory Physiology 270 (1996) H358–H363. 10.1152/ajpheart.1996.270.1.H358. [DOI] [PubMed] [Google Scholar]
- [78].Rossitto G, Mary S, Chen JY, Boder P, Chew KS, Neves KB, Alves RL, Montezano AC, Welsh P, Petrie MC, Graham D, Touyz RM, Delles C, Tissue sodium excess is not hypertonic and reflects extracellular volume expansion, Nat Commun 11 (2020) 4222. 10.1038/s41467-020-17820-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Rossitto G, Touyz RM, Petrie MC, Delles C, Much Ado about N…atrium: modelling tissue sodium as a highly sensitive marker of subclinical and localized oedema, Clinical Science 132 (2018) 2609–2613. 10.1042/CS20180575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Weber L, Kirsch E, Müller P, Krieg T, Collagen Type Distribution and Macromolecular Organization of Connective Tissue in Different Layers of Human Skin, Journal of Investigative Dermatology 82 (1984) 156–160. 10.1111/1523-1747.ep12259720. [DOI] [PubMed] [Google Scholar]
- [81].Linden IH, Laden E, Erickson JO, Armen D, Electron Microscopic Study of Normal Skin Collagen and Elastic Fibers1, Journal of Investigative Dermatology 24 (1955) 83–88. 10.1038/jid.1955.13. [DOI] [PubMed] [Google Scholar]
- [82].Linnankoski J, Mäkelä J, Palmgren J, Mauriala T, Vedin C, Ungell A, Lazorova L, Artursson P, Urtti A, Yliperttula M, Paracellular Porosity and Pore Size of the Human Intestinal Epithelium in Tissue and Cell Culture Models, Journal of Pharmaceutical Sciences 99 (2010) 2166–2175. 10.1002/jps.21961. [DOI] [PubMed] [Google Scholar]
- [83].Stewart DC, Berrie D, Li J, Liu X, Rickerson C, Mkoji D, Iqbal A, Tan S, Doty AL, Glover SC, Simmons CS, Quantitative assessment of intestinal stiffness and associations with fibrosis in human inflammatory bowel disease, PLoS ONE 13 (2018) e0200377. 10.1371/journal.pone.0200377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Fine KD, Santa Ana CA, Porter JL, Fordtran JS, Effect of changing intestinal flow rate on a measurement of intestinal permeability, Gastroenterology 108 (1995) 983–989. 10.1016/0016-5085(95)90193-0. [DOI] [PubMed] [Google Scholar]
- [85].Streitberger K-J, Reiss-Zimmermann M, Freimann FB, Bayerl S, Guo J, Arlt F, Wuerfel J, Braun J, Hoffmann K-T, Sack I, High-Resolution Mechanical Imaging of Glioblastoma by Multifrequency Magnetic Resonance Elastography, PLoS ONE 9 (2014) e110588. 10.1371/journal.pone.0110588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Pepin KM, McGee KP, Arani A, Lake DS, Glaser KJ, Manduca A, Parney IF, Ehman RL, Huston J, MR Elastography Analysis of Glioma Stiffness and IDH1 -Mutation Status, AJNR Am J Neuroradiol 39 (2018) 31–36. 10.3174/ajnr.A5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Sakai N, Takehara Y, Yamashita S, Ohishi N, Kawaji H, Sameshima T, Baba S, Sakahara H, Namba H, Shear Stiffness of 4 Common Intracranial Tumors Measured Using MR Elastography: Comparison with Intraoperative Consistency Grading, AJNR Am J Neuroradiol 37 (2016) 1851–1859. 10.3174/ajnr.A4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Kingsmore KM, Logsdon DK, Floyd DH, Peirce SM, Purow BW, Munson JM, Interstitial flow differentially increases patient-derived glioblastoma stem cell invasion via CXCR4, CXCL12, and CD44-mediated mechanisms, Integrative Biology 8 (2016) 1246–1260. 10.1039/c6ib00167j. [DOI] [PubMed] [Google Scholar]
- [89].Vargová L, Homola A, Zámečník J, Tichý M, Beneš V, Syková E, Diffusion parameters of the extracellular space in human gliomas: Diffusion in Human Gliomas, Glia 42 (2003) 77–88. 10.1002/glia.10204. [DOI] [PubMed] [Google Scholar]
- [90].Bellail AC, Hunter SB, Brat DJ, Tan C, Van Meir EG, Microregional extracellular matrix heterogeneity in brain modulates glioma cell invasion, The International Journal of Biochemistry & Cell Biology 36 (2004) 1046–1069. 10.1016/j.biocel.2004.01.013. [DOI] [PubMed] [Google Scholar]
- [91].Miroshnikova YA, Mouw JK, Barnes JM, Pickup MW, Lakins JN, Kim Y, Lobo K, Persson AI, Reis GF, McKnight TR, Holland EC, Phillips JJ, Weaver VM, Tissue mechanics promote IDH1-dependent HIF1α–tenascin C feedback to regulate glioblastoma aggression, Nat Cell Biol 18 (2016) 1336–1345. 10.1038/ncb3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Sarin H, Kanevsky AS, Wu H, Sousa AA, Wilson CM, Aronova MA, Griffiths GL, Leapman RD, Vo HQ, Physiologic upper limit of pore size in the blood-tumor barrier of malignant solid tumors, J Transl Med 7 (2009) 51. 10.1186/1479-5876-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Acerbi I, Cassereau L, Dean I, Shi Q, Au A, Park C, Chen YY, Liphardt J, Hwang ES, Weaver VM, Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration, Integrative Biology 7 (2015) 1120–1134. 10.1039/c5ib00040h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Chary SR, Jain RK, Direct measurement of interstitial convection and diffusion of albumin in normal and neoplastic tissues by fluorescence photobleaching., Proceedings of the National Academy of Sciences 86 (1989) 5385–5389. 10.1073/pnas.86.14.5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Shieh AC, Swartz MA, Regulation of tumor invasion by interstitial fluid flow, Phys. Biol 8 (2011) 015012. 10.1088/1478-3975/8/1/015012. [DOI] [PubMed] [Google Scholar]
- [96].Wolf K, Alexander S, Schacht V, Coussens LM, von Andrian UH, van Rheenen J, Deryugina E, Friedl P, Collagen-based cell migration models in vitro and in vivo, Seminars in Cell & Developmental Biology 20 (2009) 931–941. 10.1016/j.semcdb.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Ianniello C, Moy L, Fogarty J, Schnabel F, Adams S, Axelrod D, Axel L, Brown R, Madelin G, Multinuclear MRI to disentangle intracellular sodium concentration and extracellular volume fraction in breast cancer, Sci Rep 11 (2021) 5156. 10.1038/s41598-021-84616-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Yao W, Li Y, Ding G, Interstitial Fluid Flow: The Mechanical Environment of Cells and Foundation of Meridians, Evidence-Based Complementary and Alternative Medicine 2012 (2012) 1–9. 10.1155/2012/853516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Hickey JW, Dong Y, Chung JW, Salathe SF, Pruitt HC, Li X, Chang C, Fraser AK, Bessell CA, Ewald AJ, Gerecht S, Mao H, Schneck JP, Engineering an Artificial T‐Cell Stimulating Matrix for Immunotherapy, Adv. Mater 31 (2019) 1807359. 10.1002/adma.201807359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Saitakis M, Dogniaux S, Goudot C, Bufi N, Asnacios S, Maurin M, Randriamampita C, Asnacios A, Hivroz C, Different TCR-induced T lymphocyte responses are potentiated by stiffness with variable sensitivity, ELife 6 (2017) e23190. 10.7554/eLife.23190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].de la Zerda A, Kratochvil MJ, Suhar NA, Heilshorn SC, Review: Bioengineering strategies to probe T cell mechanobiology, APL Bioengineering 2 (2018) 021501. 10.1063/1.5006599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Mennens SFB, Bolomini-Vittori M, Weiden J, Joosten B, Cambi A, van den Dries K, Substrate stiffness influences phenotype and function of human antigen-presenting dendritic cells, Sci Rep 7 (2017) 17511. 10.1038/s41598-017-17787-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Chakraborty M, Chu K, Shrestha A, Revelo XS, Zhang X, Gold MJ, Khan S, Lee M, Huang C, Akbari M, Barrow F, Chan YT, Lei H, Kotoulas NK, Jovel J, Pastrello C, Kotlyar M, Goh C, Michelakis E, Clemente-Casares X, Ohashi PS, Engleman EG, Winer S, Jurisica I, Tsai S, Winer DA, Mechanical Stiffness Controls Dendritic Cell Metabolism and Function, Cell Reports 34 (2021) 108609. 10.1016/j.celrep.2020.108609. [DOI] [PubMed] [Google Scholar]
- [104].Sridharan R, Cavanagh B, Cameron AR, Kelly DJ, O’Brien FJ, Material stiffness influences the polarization state, function and migration mode of macrophages, Acta Biomaterialia 89 (2019) 47–59. 10.1016/j.actbio.2019.02.048. [DOI] [PubMed] [Google Scholar]
- [105].Friedemann M, Kalbitzer L, Franz S, Moeller S, Schnabelrauch M, Simon J-C, Pompe T, Franke K, Instructing Human Macrophage Polarization by Stiffness and Glycosaminoglycan Functionalization in 3D Collagen Networks, Adv. Healthcare Mater 6 (2017) 1600967. 10.1002/adhm.201600967. [DOI] [PubMed] [Google Scholar]
- [106].Blakney AK, Swartzlander MD, Bryant SJ, The effects of substrate stiffness on the in vitro activation of macrophages and in vivo host response to poly(ethylene glycol)-based hydrogels, J Biomed Mater Res A 100 (2012) 1375–1386. 10.1002/jbm.a.34104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Zeng Y, Yi J, Wan Z, Liu K, Song P, Chau A, Wang F, Chang Z, Han W, Zheng W, Chen Y-H, Xiong C, Liu W, Substrate stiffness regulates B-cell activation, proliferation, class switch, and T-cell-independent antibody responses in vivo: Cellular immune response, Eur. J. Immunol 45 (2015) 1621–1634. 10.1002/eji.201444777. [DOI] [PubMed] [Google Scholar]
- [108].Moura Rosa P, Gopalakrishnan N, Ibrahim H, Haug M, Halaas Ø, The intercell dynamics of T cells and dendritic cells in a lymph node-on-a-chip flow device, Lab Chip 16 (2016) 3728–3740. 10.1039/C6LC00702C. [DOI] [PubMed] [Google Scholar]
- [109].Curbishley SM, Eksteen B, Gladue RP, Lalor P, Adams DH, CXCR3 Activation Promotes Lymphocyte Transendothelial Migration across Human Hepatic Endothelium under Fluid Flow, The American Journal of Pathology 167 (2005) 887–899. 10.1016/S0002-9440(10)62060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Miteva DO, Rutkowski JM, Dixon JB, Kilarski W, Shields JD, Swartz MA, Transmural Flow Modulates Cell and Fluid Transport Functions of Lymphatic Endothelium, Circulation Research 106 (2010) 920–931. 10.1161/CIRCRESAHA.109.207274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Harrell MI, Iritani BM, Ruddell A, Tumor-Induced Sentinel Lymph Node Lymphangiogenesis and Increased Lymph Flow Precede Melanoma Metastasis, The American Journal of Pathology 170 (2007) 774–786. 10.2353/ajpath.2007.060761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Pathak AP, Artemov D, Neeman M, Bhujwalla ZM, Lymph Node Metastasis in Breast Cancer Xenografts Is Associated with Increased Regions of Extravascular Drain, Lymphatic Vessel Area, and Invasive Phenotype, Cancer Res 66 (2006) 5151–5158. 10.1158/0008-5472.CAN-05-1788. [DOI] [PubMed] [Google Scholar]
- [113].Wolf K, Müller R, Borgmann S, Bröcker Eva.-B., Friedl P, Amoeboid shape change and contact guidance: T-lymphocyte crawling through fibrillar collagen is independent of matrix remodeling by MMPs and other proteases, Blood 102 (2003) 3262–3269. 10.1182/blood-2002-12-3791. [DOI] [PubMed] [Google Scholar]
- [114].Lämmermann T, Bader BL, Monkley SJ, Worbs T, Wedlich-Söldner R, Hirsch K, Keller M, Förster R, Critchley DR, Fässler R, Sixt M, Rapid leukocyte migration by integrin-independent flowing and squeezing, Nature 453 (2008) 51–55. 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- [115].Mandeville JTH, Lawson MA, Maxfield FR, Dynamic imaging of neutrophil migration in three dimensions: mechanical interactions between cells and matrix, J Leukoc Biol 61 (1997) 188–200. 10.1002/jlb.61.2.188. [DOI] [PubMed] [Google Scholar]
- [116].Tylek T, Blum C, Hrynevich A, Schlegelmilch K, Schilling T, Dalton PD, Groll J, Precisely defined fiber scaffolds with 40 μ m porosity induce elongation driven M2-like polarization of human macrophages, Biofabrication 12 (2020) 025007. 10.1088/1758-5090/ab5f4e. [DOI] [PubMed] [Google Scholar]
- [117].Ford AJ, Orbach SM, Rajagopalan P, Fibroblasts stimulate macrophage migration in interconnected extracellular matrices through tunnel formation and fiber alignment, Biomaterials 209 (2019) 88–102. 10.1016/j.biomaterials.2019.03.044. [DOI] [PubMed] [Google Scholar]
- [118].Jeong M, Kim H, Park J-H, Porous Materials for Immune Modulation, Open Material Sciences 4 (2018) 1–14. 10.1515/oms-2018-0001. [DOI] [Google Scholar]
- [119].Thongchai K, Chuysinuan P, Thanyacharoen T, Techasakul S, Ummartyotin S, Characterization, release, and antioxidant activity of caffeic acid-loaded collagen and chitosan hydrogel composites, Journal of Materials Research and Technology 9 (2020) 6512–6520. 10.1016/j.jmrt.2020.04.036. [DOI] [Google Scholar]
- [120].Jeyanthi R, Rao KP, Controlled release of anticancer drugs from collagen-poly(HEMA) hydrogel matrices, Journal of Controlled Release 13 (1990) 91–98. 10.1016/0168-3659(90)90078-8. [DOI] [Google Scholar]
- [121].Jeong B, Bae YH, Kim SW, Drug release from biodegradable injectable thermosensitive hydrogel of PEG–PLGA–PEG triblock copolymers, Journal of Controlled Release 63 (2000) 155–163. 10.1016/S0168-3659(99)00194-7. [DOI] [PubMed] [Google Scholar]
- [122].Pal P, Nguyen QC, Benton AH, Marquart ME, Janorkar AV, Drug‐Loaded Elastin‐Like Polypeptide–Collagen Hydrogels with High Modulus for Bone Tissue Engineering, Macromol. Biosci 19 (2019) 1900142. 10.1002/mabi.201900142. [DOI] [PubMed] [Google Scholar]
- [123].Oyler-Yaniv A, Oyler-Yaniv J, Whitlock BM, Liu Z, Germain RN, Huse M, Altan-Bonnet G, Krichevsky O, A Tunable Diffusion-Consumption Mechanism of Cytokine Propagation Enables Plasticity in Cell-to-Cell Communication in the Immune System, Immunity 46 (2017) 609–620. 10.1016/j.immuni.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Belanger MC, Anbaei P, Dunn AF, Kinman AWL, Pompano RR, Spatially Resolved Analytical Chemistry in Intact, Living Tissues, Anal. Chem 92 (2020) 15255–15262. 10.1021/acs.analchem.0c03625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Wang P, Wang H, Huang Q, Peng C, Yao L, Chen H, Qiu Z, Wu Y, Wang L, Chen W, Exosomes from M1-Polarized Macrophages Enhance Paclitaxel Antitumor Activity by Activating Macrophages-Mediated Inflammation, Theranostics 9 (2019) 1714–1727. 10.7150/thno.30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Chen D, Xie J, Fiskesund R, Dong W, Liang X, Lv J, Jin X, Liu J, Mo S, Zhang T, Cheng F, Zhou Y, Zhang H, Tang K, Ma J, Liu Y, Huang B, Chloroquine modulates antitumor immune response by resetting tumor-associated macrophages toward M1 phenotype, Nat Commun 9 (2018) 873. 10.1038/s41467-018-03225-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Hussain SF, Yang D, Suki D, Aldape K, Grimm E, Heimberger AB, The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses1, Neuro-Oncology 8 (2006) 261–279. 10.1215/15228517-2006-008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Wang Y-C, He F, Feng F, Liu X-W, Dong G-Y, Qin H-Y, Hu X-B, Zheng M-H, Liang L, Feng L, Liang Y-M, Han H, Notch Signaling Determines the M1 versus M2 Polarization of Macrophages in Antitumor Immune Responses, Cancer Res 70 (2010) 4840–4849. 10.1158/0008-5472.CAN-10-0269. [DOI] [PubMed] [Google Scholar]
- [129].Doedens AL, Stockmann C, Rubinstein MP, Liao D, Zhang N, DeNardo DG, Coussens LM, Karin M, Goldrath AW, Johnson RS, Macrophage Expression of Hypoxia-Inducible Factor-1α Suppresses T-Cell Function and Promotes Tumor Progression, Cancer Res 70 (2010) 7465–7475. 10.1158/0008-5472.CAN-10-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Jia X, Feng G, Wang Z, Du Y, Shen C, Hui H, Peng D, Li Z, Kong D, Tian J, Activation of mesenchymal stem cells by macrophages promotes tumor progression through immune suppressive effects, Oncotarget 7 (2016) 20934–20944. 10.18632/oncotarget.8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Maolake A, Izumi K, Shigehara K, Natsagdorj A, Iwamoto H, Kadomoto S, Takezawa Y, Machioka K, Narimoto K, Namiki M, Lin W-J, Wufuer G, Mizokami A, Tumor-associated macrophages promote prostate cancer migration through activation of the CCL22-CCR4 axis, Oncotarget 8 (2017) 9739–9751. 10.18632/oncotarget.14185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Bohrer LR, Schwertfeger KL, Macrophages Promote Fibroblast Growth Factor Receptor-Driven Tumor Cell Migration and Invasion in a Cxcr2-Dependent Manner, Mol Cancer Res 10 (2012) 1294–1305. 10.1158/1541-7786.MCR-12-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Steitz AM, Steffes A, Finkernagel F, Unger A, Sommerfeld L, Jansen JM, Wagner U, Graumann J, Müller R, Reinartz S, Tumor-associated macrophages promote ovarian cancer cell migration by secreting transforming growth factor beta induced (TGFBI) and tenascin C, Cell Death Dis 11 (2020) 249. 10.1038/s41419-020-2438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Zhang S, Che D, Yang F, Chi C, Meng H, Shen J, Qi L, Liu F, Lv L, Li Y, Meng Q, Liu J, Shang L, Yu Y, Tumor-associated macrophages promote tumor metastasis via the TGF-β/SOX9 axis in non-small cell lung cancer, Oncotarget 8 (2017) 99801–99815. 10.18632/oncotarget.21068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Bingle L, Lewis CE, Corke KP, Reed MWR, Brown NJ, Macrophages promote angiogenesis in human breast tumour spheroids in vivo, Br J Cancer 94 (2006) 101–107. 10.1038/sj.bjc.6602901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Chen P, Huang Y, Bong R, Ding Y, Song N, Wang X, Song X, Luo Y, Tumor-Associated Macrophages Promote Angiogenesis and Melanoma Growth via Adrenomedullin in a Paracrine and Autocrine Manner, Clin Cancer Res 17 (2011) 7230–239. 10.1158/1078-0432.CCR-11-1354. [DOI] [PubMed] [Google Scholar]
- [137].Ehling J, Bartneck M, Wei X, Gremse F, Fech V, Möckel D, Baeck C, Hittatiya K, Eulberg D, Luedde T, Kiessling F, Trautwein C, Lammers T, Tacke F, CCL2-dependent infiltrating macrophages promote angiogenesis in progressive liver fibrosis, Gut 63 (2014) 1960–1971. 10.1136/gutjnl-2013-306294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Kuwada K, Kagawa S, Yoshida R, Sakamoto S, Ito A, Watanabe M, Ieda T, Kuroda S, Kikuchi S, Tazawa H, Fujiwara T, The epithelial-to-mesenchymal transition induced by tumor-associated macrophages confers chemoresistance in peritoneally disseminated pancreatic cancer, J Exp Clin Cancer Res 37 (2018) 307. 10.1186/s13046-018-0981-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Melcher V, Graf M, Interlandi M, Moreno N, de Faria FW, Kim SN, Kastrati D, Korbanka S, Alfert A, Gerß J, Meyer zu Hörste G, Hartmann W, Frühwald MC, Dugas M, Schüller U, Hasselblatt M, Albert TK, Kerl K, Macrophage-tumor cell interaction promotes ATRT progression and chemoresistance, Acta Neuropathol 139 (2020) 913–936. 10.1007/s00401-019-02116-7. [DOI] [PubMed] [Google Scholar]
- [140].Baghdadi M, Wada H, Nakanishi S, Abe H, Han N, Putra WE, Endo D, Watari H, Sakuragi N, Hida Y, Kaga K, Miyagi Y, Yokose T, Takano A, Daigo Y, Seino K, Chemotherapy-Induced IL34 Enhances Immunosuppression by Tumor-Associated Macrophages and Mediates Survival of Chemoresistant Lung Cancer Cells, Cancer Res 76 (2016) 6030–6042. 10.1158/0008-5472.CAN-16-1170. [DOI] [PubMed] [Google Scholar]
- [141].Rajan SAP, Aleman J, Wan M, Pourhabibi Zarandi N, Nzou G, Murphy S, Bishop CE, Sadri-Ardekani H, Shupe T, Atala A, Hall AR, Skardal A, Probing prodrug metabolism and reciprocal toxicity with an integrated and humanized multi-tissue organ-on-a-chip platform, Acta Biomaterialia 106 (2020) 124–135. 10.1016/j.actbio.2020.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Skardal A, Murphy SV, Devarasetty M, Mead I, Kang H-W, Seol Y-J, Shrike Zhang Y, Shin S-R, Zhao L, Aleman J, Hall AR, Shupe TD, Kleensang A, Dokmeci MR, Jin Lee S, Jackson JD, Yoo JJ, Hartung T, Khademhosseini A, Soker S, Bishop CE, Atala A, Multi-tissue interactions in an integrated three-tissue organ-on-a-chip platform, Sci Rep 7 (2017) 8837. 10.1038/s41598-017-08879-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Ramme AP, Koenig L, Hasenberg T, Schwenk C, Magauer C, Faust D, Lorenz AK, Krebs A-C, Drewell C, Schirrmann K, Vladetic A, Lin G-C, Pabinger S, Neuhaus W, Bois F, Lauster R, Marx U, Dehne E-M, Autologous induced pluripotent stem cell-derived four-organ-chip, Future Science OA 5 (2019) FSO413. 10.2144/fsoa-2019-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Farh KK-H, Marson A, Zhu J, Kleinewietfeld M, Housley WJ, Beik S, Shoresh N, Whitton H, Ryan RJH, Shishkin AA, Hatan M, Carrasco-Alfonso MJ, Mayer D, Luckey CJ, Patsopoulos NA, De Jager PL, Kuchroo VK, Epstein CB, Daly MJ, Hafler DA, Bernstein BE, Genetic and epigenetic fine mapping of causal autoimmune disease variants, Nature 518 (2015) 337–343. 10.1038/nature13835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Blumenreich MS, The White Blood Cell and Differential Count, in: Walker HK, Hall WD, Hurst JW (Eds.), Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed., Butterworths, Boston, 1990. http://www.ncbi.nlm.nih.gov/books/NBK261/ (accessed May 14, 2021). [Google Scholar]
- [146].Kleiveland CR, Peripheral Blood Mononuclear Cells, in: Verhoeckx K, Cotter P, López-Expósito I, Kleiveland C, Lea T, Mackie A, Requena T, Swiatecka D, Wichers H (Eds.), The Impact of Food Bioactives on Health, Springer International Publishing, Cham, 2015: pp. 161–167. 10.1007/978-3-319-16104-4_15. [DOI] [Google Scholar]
- [147].Revazova ES, Turovets NA, Kochetkova OD, Kindarova LB, Kuzmichev LN, Janus JD, Pryzhkova MV, Patient-Specific Stem Cell Lines Derived from Human Parthenogenetic Blastocysts, Cloning and Stem Cells 9 (2007) 432–449. 10.1089/clo.2007.0033. [DOI] [PubMed] [Google Scholar]
- [148].Byrne JA, Pedersen DA, Clepper LL, Nelson M, Sanger WG, Gokhale S, Wolf DP, Mitalipov SM, Producing primate embryonic stem cells by somatic cell nuclear transfer, Nature 450 (2007) 497–502. 10.1038/nature06357. [DOI] [PubMed] [Google Scholar]
- [149].Brambrink T, Hochedlinger K, Bell G, Jaenisch R, ES cells derived from cloned and fertilized blastocysts are transcriptionally and functionally indistinguishable, Proceedings of the National Academy of Sciences 103 (2006) 933–938. 10.1073/pnas.0510485103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Klimanskaya I, Rosenthal N, Lanza R, Derive and conquer: sourcing and differentiating stem cells for therapeutic applications, Nat Rev Drug Discov 7 (2008) 131–142. 10.1038/nrd2403. [DOI] [PubMed] [Google Scholar]
- [151].Lu Y-F, Cahan P, Ross S, Sahalie J, Sousa PM, Hadland BK, Cai W, Serrao E, Engelman AN, Bernstein ID, Daley GQ, Engineered Murine HSCs Reconstitute Multilineage Hematopoiesis and Adaptive Immunity, Cell Reports 17 (2016) 3178–3192. 10.1016/j.celrep.2016.11.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Rahman M, Brauer P, Zúñiga-Pflücker J, Zandstra P, Controlled generation of hematopoietic progenitor cells from pluripotent stem cells using microenvironmental cues, Experimental Hematology 41 (2013) S28. 10.1016/j.exphem.2013.05.113. [DOI] [Google Scholar]
- [153].Kennedy M, Awong G, Sturgeon CM, Ditadi A, LaMotte-Mohs R, Zúñiga-Pflücker JC, Keller G, T Lymphocyte Potential Marks the Emergence of Definitive Hematopoietic Progenitors in Human Pluripotent Stem Cell Differentiation Cultures, Cell Reports 2 (2012) 1722–1735. 10.1016/j.celrep.2012.11.003. [DOI] [PubMed] [Google Scholar]
- [154].Ackermann M, Kempf H, Hetzel M, Hesse C, Hashtchin AR, Brinkert K, Schott JW, Haake K, Kühnel MP, Glage S, Figueiredo C, Jonigk D, Sewald K, Schambach A, Wronski S, Moritz T, Martin U, Zweigerdt R, Munder A, Lachmann N, Bioreactor-based mass production of human iPSC-derived macrophages enables immunotherapies against bacterial airway infections, Nat Commun 9 (2018) 5088. 10.1038/s41467-018-07570-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Zhang H, Xue C, Shah R, Bermingham K, Hinkle CC, Li W, Rodrigues A, Tabita-Martinez J, Millar JS, Cuchel M, Pashos EE, Liu Y, Yan R, Yang W, Gosai SJ, VanDorn D, Chou ST, Gregory BD, Morrisey EE, Li M, Rader DJ, Reilly MP, Functional Analysis and Transcriptomic Profiling of iPSC-Derived Macrophages and Their Application in Modeling Mendelian Disease, Circ Res 117 (2015) 17–28. 10.1161/CIRCRESAHA.117.305860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Pandya H, Shen MJ, Ichikawa DM, Sedlock AB, Choi Y, Johnson KR, Kim G, Brown MA, Elkahloun AG, Maric D, Sweeney CL, Gossa S, Malech HL, McGavern DB, Park JK, Differentiation of human and murine induced pluripotent stem cells to microglia-like cells, Nat Neurosci 20 (2017) 753–759. 10.1038/nn.4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Douvaras P, Sun B, Wang M, Kruglikov I, Lallos G, Zimmer M, Terrenoire C, Zhang B, Gandy S, Schadt E, Freytes DO, Noggle S, Fossati V, Directed Differentiation of Human Pluripotent Stem Cells to Microglia, Stem Cell Reports 8 (2017) 1516–1524. 10.1016/j.stemcr.2017.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Zeng J, Tang SY, Toh LL, Wang S, Generation of “Off-the-Shelf” Natural Killer Cells from Peripheral Blood Cell-Derived Induced Pluripotent Stem Cells, Stem Cell Reports 9 (2017) 1796–1812. 10.1016/j.stemcr.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Suwanpitak S, Promnakhon N, Netsrithong R, Wattanapanitch M, Efficient Generation of iPSC-Derived Hematoendothelial Progenitors and Specification Toward T cell Lineage, in: Springer US, New York, NY, 2021. 10.1007/7651_2021_355. [DOI] [PubMed] [Google Scholar]
- [160].Iriguchi S, Yasui Y, Kawai Y, Arima S, Kunitomo M, Sato T, Ueda T, Minagawa A, Mishima Y, Yanagawa N, Baba Y, Miyake Y, Nakayama K, Takiguchi M, Shinohara T, Nakatsura T, Yasukawa M, Kassai Y, Hayashi A, Kaneko S, A clinically applicable and scalable method to regenerate T-cells from iPSCs for off-the-shelf T-cell immunotherapy, Nat Commun 12 (2021) 430. 10.1038/s41467-020-20658-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Hasselmann J, Blurton‐Jones M, Human iPSC‐derived microglia: A growing toolset to study the brain’s innate immune cells, Glia 68 (2020) 721–739. 10.1002/glia.23781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Bernareggi D, Pouyanfard S, Kaufman DS, Development of innate immune cells from human pluripotent stem cells, Experimental Hematology 71 (2019) 13–23. 10.1016/j.exphem.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Low LA, Mummery C, Berridge BR, Austin CP, Tagle DA, Organs-on-chips: into the next decade, Nat Rev Drug Discov 20 (2021) 345–361. 10.1038/s41573-020-0079-3. [DOI] [PubMed] [Google Scholar]
- [164].Kawamoto H, Masuda K, Nagano S, Maeda T, Cloning and expansion of antigen-specific T cells using iPS cell technology: development of “off-the-shelf” T cells for the use in allogeneic transfusion settings, Int J Hematol 107 (2018) 271–277. 10.1007/s12185-018-2399-1. [DOI] [PubMed] [Google Scholar]
- [165].Nishimura T, Nakauchi H, Generation of Antigen-Specific T Cells from Human Induced Pluripotent Stem Cells, in: Boyd AS (Ed.), Immunological Tolerance, Springer New York, New York, NY, 2019: pp. 25–40. 10.1007/978-1-4939-8938-6_3. [DOI] [PubMed] [Google Scholar]
- [166].Zanoni M, Piccinini F, Arienti C, Zamagni A, Santi S, Polico R, Bevilacqua A, Tesei A, 3D tumor spheroid models for in vitro therapeutic screening: a systematic approach to enhance the biological relevance of data obtained, Sci Rep 6 (2016) 19103. 10.1038/srep19103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Kim M, Mun H, Sung CO, Cho EJ, Jeon H-J, Chun S-M, Jung DJ, Shin TH, Jeong GS, Kim DK, Choi EK, Jeong S-Y, Taylor AM, Jain S, Meyerson M, Jang SJ, Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening, Nat Commun 10 (2019) 3991. 10.1038/s41467-019-11867-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Mazzocchi AR, Rajan SAP, Votanopoulos KI, Hall AR, Skardal A, In vitro patient-derived 3D mesothelioma tumor organoids facilitate patient-centric therapeutic screening, Sci Rep 8 (2018) 2886. 10.1038/s41598-018-21200-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Benton G, DeGray G, Kleinman HK, George J, Arnaoutova I, In Vitro Microtumors Provide a Physiologically Predictive Tool for Breast Cancer Therapeutic Screening, PLoS ONE 10 (2015) e0123312. 10.1371/journal.pone.0123312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Klein SL, Flanagan KL, Sex differences in immune responses, Nat Rev Immunol 16 (2016) 626–638. 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- [171].Stern M, Passweg JR, Locasciulli A, Socié G, Schrezenmeier H, Békássy AN, Fuehrer M, Hows J, Korthof ET, McCann S, Tichelli A, Zoumbos NC, Marsh JCW, Bacigalupo A, Gratwohl A, Influence of Donor/Recipient Sex Matching on Outcome of Allogeneic Hematopoietic Stem Cell Transplantation for Aplastic Anemia, Transplantation 82 (2006) 218–226. 10.1097/01.tp.0000226156.99206.d1. [DOI] [PubMed] [Google Scholar]
- [172].Ferrara JLM, Levine JE, Reddy P, Holler E, Graft-versus-host disease, Lancet 373 (2009) 1550–1561. 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Ozcelikkale A, Moon H, Linnes M, Han B, In vitro microfluidic models of tumor microenvironment to screen transport of drugs and nanoparticles: In vitro microfluidic models of tumor microenvironment, WIREs Nanomed Nanobiotechnol 9 (2017) e1460. 10.1002/wnan.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Stapleton S, Milosevic M, Allen C, Zheng J, Dunne M, Yeung I, Jaffray DA, A Mathematical Model of the Enhanced Permeability and Retention Effect for Liposome Transport in Solid Tumors, PLoS ONE 8 (2013) e81157. 10.1371/journal.pone.0081157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Roberts LM, Munson J, Modulating Microenvironments for Treating Glioblastoma, Curr. Tissue Microenviron. Rep 1 (2020) 99–111. 10.1007/s43152-020-00010-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Tate KM, Munson JM, Assessing drug response in engineered brain microenvironments, Brain Research Bulletin 150 (2019) 21–34. 10.1016/j.brainresbull.2019.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Cornelison RC, Munson JM, Perspective on Translating Biomaterials Into Glioma Therapy: Lessons From in Vitro Models, Front. Mater 5 (2018) 27. 10.3389/fmats.2018.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Turk OM, Woodall RC, Gutova M, Brown CE, Rockne RC, Munson JM, Delivery strategies for cell-based therapies in the brain: overcoming multiple barriers, Drug Deliv. and Transl. Res 11 (2021) 2448–2467. 10.1007/s13346-021-01079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Wu S, Yang W, Zhang H, Ren Y, Fang Z, Yuan C, Yao Z, The Prognostic Landscape of Tumor-Infiltrating Immune Cells and Immune Checkpoints in Glioblastoma, Technol Cancer Res Treat 18 (2019) 153303381986994. 10.1177/1533033819869949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Schmitt MJ, Company C, Dramaretska Y, Barozzi I, Göhrig A, Kertalli S, Großmann M, Naumann H, Sanchez-Bailon MP, Hulsman D, Glass R, Squatrito M, Serresi M, Gargiulo G, Phenotypic Mapping of Pathologic Cross-Talk between Glioblastoma and Innate Immune Cells by Synthetic Genetic Tracing, Cancer Discov 11 (2021) 754–777. 10.1158/2159-8290.CD-20-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Klemm F, Maas RR, Bowman RL, Kornete M, Soukup K, Nassiri S, Brouland J-P, Iacobuzio-Donahue CA, Brennan C, Tabar V, Gutin PH, Daniel RT, Hegi ME, Joyce JA, Interrogation of the Microenvironmental Landscape in Brain Tumors Reveals Disease-Specific Alterations of Immune Cells, Cell 181 (2020) 1643–1660.e17. 10.1016/j.cell.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Martinez-Lage M, Lynch TM, Bi Y, Cocito C, Way GP, Pal S, Haller J, Yan RE, Ziober A, Nguyen A, Kandpal M, O’Rourke DM, Greenfield JP, Greene CS, Davuluri RV, Dahmane N, Immune landscapes associated with different glioblastoma molecular subtypes, Acta Neuropathol Commun 7 (2019) 203. 10.1186/s40478-019-0803-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Grolman JM, Zhang D, Smith AM, Moore JS, Kilian KA, Rapid 3D Extrusion of Synthetic Tumor Microenvironments, Adv. Mater 27 (2015) 5512–5517. 10.1002/adma.201501729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Cui X, Morales R-TT, Qian W, Wang H, Gagner J-P, Dolgalev I, Placantonakis D, Zagzag D, Cimmino L, Snuderl M, Lam RHW, Chen W, Hacking macrophage-associated immunosuppression for regulating glioblastoma angiogenesis, Biomaterials 161 (2018) 164–178. 10.1016/j.biomaterials.2018.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Tang M, Xie Q, Gimple RC, Zhong Z, Tam T, Tian J, Kidwell RL, Wu Q, Prager BC, Qiu Z, Yu A, Zhu Z, Mesci P, Jing H, Schimelman J, Wang P, Lee D, Lorenzini MH, Dixit D, Zhao L, Bhargava S, Miller TE, Wan X, Tang J, Sun B, Cravatt BF, Muotri AR, Chen S, Rich JN, Three-dimensional bioprinted glioblastoma microenvironments model cellular dependencies and immune interactions, Cell Res (2020). 10.1038/s41422-020-0338-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Hermida MA, Kumar JD, Schwarz D, Laverty KG, Di Bartolo A, Ardron M, Bogomolnijs M, Clavreul A, Brennan PM, Wiegand UK, Melchels FPW, Shu W, Leslie NR, Three dimensional in vitro models of cancer: Bioprinting multilineage glioblastoma models, Advances in Biological Regulation 75 (2020) 100658. 10.1016/j.jbior.2019.100658. [DOI] [PubMed] [Google Scholar]
- [187].Heinrich MA, Bansal R, Lammers T, Zhang YS, Michel Schiffelers R, Prakash J, 3D‐Bioprinted Mini‐Brain: A Glioblastoma Model to Study Cellular Interactions and Therapeutics, Adv. Mater 31 (2019) 1806590. 10.1002/adma.201806590. [DOI] [PubMed] [Google Scholar]
- [188].Lee SWL, Seager RJ, Litvak F, Spill F, Sieow JL, Leong PH, Kumar D, Tan ASM, Wong SC, Adriani G, Zaman MH, and Kamm RD, Integrated in silico and 3D in vitro model of macrophage migration in response to physical and chemical factors in the tumor microenvironment, Integrative Biology 12 (2020) 90–108. 10.1093/intbio/zyaa007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Osaki T, Sivathanu V, Kamm RD, Vascularized microfluidic organ-chips for drug screening, disease models and tissue engineering, Current Opinion in Biotechnology 52 (2018) 116–123. 10.1016/j.copbio.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Massa S, Sakr MA, Seo J, Bandaru P, Arneri A, Bersini S, Zare-Eelanjegh E, Jalilian E, Cha B-H, Antona S, Enrico A, Gao Y, Hassan S, Acevedo JP, Dokmeci MR, Zhang YS, Khademhosseini A, Shin SR, Bioprinted 3D vascularized tissue model for drug toxicity analysis, Biomicrofluidics 11 (2017) 044109. 10.1063/1.4994708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Hachey SJ, Movsesyan S, Nguyen QH, Burton-Sojo G, Tankazyan A, Wu J, Hoang T, Zhao D, Wang S, Hatch MM, Celaya E, Gomez S, Chen GT, Davis RT, Nee K, Pervolarakis N, Lawson DA, Kessenbrock K, Lee AP, Lowengrub J, Waterman ML, Hughes CCW, An in vitro vascularized micro-tumor model of human colorectal cancer recapitulates in vivo responses to standard-of-care therapy, Lab Chip 21 (2021) 1333–1351. 10.1039/D0LC01216E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Henderson AR, Ilan IS, Lee E, A bioengineered lymphatic vessel model for studying lymphatic endothelial cell‐cell junction and barrier function, Microcirculation (2021). 10.1111/micc.12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Gong MM, Lugo-Cintron KM, White BR, Kerr SC, Harari PM, Beebe DJ, Human organotypic lymphatic vessel model elucidates microenvironment-dependent signaling and barrier function, Biomaterials 214 (2019) 119225. 10.1016/j.biomaterials.2019.119225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Cote B, Rao D, Alany RG, Kwon GS, Alani AWG, Lymphatic changes in cancer and drug delivery to the lymphatics in solid tumors, Advanced Drug Delivery Reviews 144 (2019) 16–34. 10.1016/j.addr.2019.08.009. [DOI] [PubMed] [Google Scholar]
- [195].Schaaf MB, Garg AD, Agostinis P, Defining the role of the tumor vasculature in antitumor immunity and immunotherapy, Cell Death Dis 9 (2018) 115. 10.1038/s41419-017-0061-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Cao X, Ashfaq R, Cheng F, Maharjan S, Li J, Ying G, Hassan S, Xiao H, Yue K, Zhang YS, A Tumor‐on‐a‐Chip System with Bioprinted Blood and Lymphatic Vessel Pair, Adv. Funct. Mater 29 (2019) 1807173. 10.1002/adfm.201807173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Ghosh S, Rosenthal R, Zajac P, Weber WP, Oertli D, Heberer M, Martin I, Spagnoli GC, Reschner A, Culture of Melanoma Cells in 3-Dimensional Architectures Results in Impaired Immunorecognition by Cytotoxic T Lymphocytes Specific for Melan-A/MART-1 Tumor-Associated Antigen:, Annals of Surgery 242 (2005) 851–858. 10.1097/01.sla.0000189571.84213.b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Feder-Mengus C, Ghosh S, Weber WP, Wyler S, Zajac P, Terracciano L, Oertli D, Heberer M, Martin I, Spagnoli GC, Reschner A, Multiple mechanisms underlie defective recognition of melanoma cells cultured in three-dimensional architectures by antigen-specific cytotoxic T lymphocytes, Br J Cancer 96 (2007) 1072–1082. 10.1038/sj.bjc.6603664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Ramgolam K, Lauriol J, Lalou C, Lauden L, Michel L, de la Grange P, Khatib A-M, Aoudjit F, Charron D, Alcaide-Loridan C, Al-Daccak R, Melanoma Spheroids Grown Under Neural Crest Cell Conditions Are Highly Plastic Migratory/Invasive Tumor Cells Endowed with Immunomodulator Function, PLoS ONE 6 (2011) e18784. 10.1371/journal.pone.0018784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Bourland J, Fradette J, Auger FA, Tissue-engineered 3D melanoma model with blood and lymphatic capillaries for drug development, Sci Rep 8 (2018) 13191. 10.1038/s41598-018-31502-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Votanopoulos KI, Forsythe S, Sivakumar H, Mazzocchi A, Aleman J, Miller L, Levine E, Triozzi P, Skardal A, Model of Patient-Specific Immune-Enhanced Organoids for Immunotherapy Screening: Feasibility Study, Ann Surg Oncol 27 (2020) 1956–1967. 10.1245/s10434-019-08143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].McClatchey PM, Hannen E, Thomas SN, Microfluidic Platforms for the Interrogation of Intravascular Cellular Trafficking Mechanisms Influenced by Hemodynamic Forces, in: Singh A, Gaharwar AK (Eds.), Microscale Technologies for Cell Engineering, Springer International Publishing, Cham, 2016: pp. 197–218. 10.1007/978-3-319-20726-1_9. [DOI] [Google Scholar]
- [203].Sukumaran S, Watanabe N, Bajgain P, Raja K, Mohammed S, Fisher WE, Brenner MK, Leen AM, Vera JF, Enhancing the Potency and Specificity of Engineered T Cells for Cancer Treatment, Cancer Discov 8 (2018) 972–987. 10.1158/2159-8290.CD-17-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Turtle CJ, Hudecek M, Jensen MC, Riddell SR, Engineered T cells for anti-cancer therapy, Current Opinion in Immunology 24 (2012) 633–639. 10.1016/j.coi.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Labanieh L, Majzner RG, Mackall CL, Programming CAR-T cells to kill cancer, Nat Biomed Eng 2 (2018) 377–391. 10.1038/s41551-018-0235-9. [DOI] [PubMed] [Google Scholar]
- [206].June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC, CAR T cell immunotherapy for human cancer, Science 359 (2018) 1361–1365. 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- [207].Lin L, Cho S-F, Xing L, Wen K, Li Y, Yu T, Hsieh PA, Chen H, Kurtoglu M, Zhang Y, Andrew Stewart C, Munshi N, Anderson KC, Tai Y-T, Preclinical evaluation of CD8+ anti-BCMA mRNA CAR T cells for treatment of multiple myeloma, Leukemia 35 (2021) 752–763. 10.1038/s41375-020-0951-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Sommer C, Boldajipour B, Kuo TC, Bentley T, Sutton J, Chen A, Geng T, Dong H, Galetto R, Valton J, Pertel T, Juillerat A, Gariboldi A, Pascua E, Brown C, Chin SM, Sai T, Ni Y, Duchateau P, Smith J, Rajpal A, Van Blarcom T, Chaparro-Riggers J, Sasu BJ, Preclinical Evaluation of Allogeneic CAR T Cells Targeting BCMA for the Treatment of Multiple Myeloma, Molecular Therapy 27 (2019) 1126–1138. 10.1016/j.ymthe.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Siegler EL, Wang P, Preclinical Models in Chimeric Antigen Receptor–Engineered T-Cell Therapy, Human Gene Therapy 29 (2018) 534–546. 10.1089/hum.2017.243. [DOI] [PubMed] [Google Scholar]
- [210].Wang X, Wu Z, Qiu W, Chen P, Xu X, Han W, Programming CAR T cells to enhance anti-tumor efficacy through remodeling of the immune system, Front. Med 14 (2020) 726–745. 10.1007/s11684-020-0746-0. [DOI] [PubMed] [Google Scholar]
- [211].Jacob F, Ming G, Song H, Generation and biobanking of patient-derived glioblastoma organoids and their application in CAR T cell testing, Nat Protoc 15 (2020) 4000–4033. 10.1038/s41596-020-0402-9. [DOI] [PubMed] [Google Scholar]
- [212].Wallstabe L, Göttlich C, Nelke LC, Kühnemundt J, Schwarz T, Nerreter T, Einsele H, Walles H, Dandekar G, Nietzer SL, Hudecek M, ROR1-CAR T cells are effective against lung and breast cancer in advanced microphysiologic 3D tumor models, JCI Insight 4 (2019) e126345. 10.1172/jci.insight.126345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Pavesi A, Tan AT, Koh S, Chia A, Colombo M, Antonecchia E, Miccolis C, Ceccarello E, Adriani G, Raimondi MT, Kamm RD, Bertoletti A, A 3D microfluidic model for preclinical evaluation of TCR-engineered T cells against solid tumors, JCI Insight 2 (2017) e89762. 10.1172/jci.insight.89762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Ando Y, Siegler EL, Ta HP, Cinay GE, Zhou H, Gorrell KA, Au H, Jarvis BM, Wang P, Shen K, Evaluating CAR-T Cell Therapy in a Hypoxic 3D Tumor Model, Adv. Healthcare Mater 8 (2019) 1900001. 10.1002/adhm.201900001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Aung A, Kumar V, Theprungsirikul J, Davey SK, Varghese S, An Engineered Tumor-on-a-Chip Device with Breast Cancer–Immune Cell Interactions for Assessing T-cell Recruitment, Cancer Res 80 (2020) 263–275. 10.1158/0008-5472.CAN-19-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Irimia D, Wang X, Inflammation-on-a-Chip: Probing the Immune System Ex Vivo, Trends in Biotechnology 36 (2018) 923–937. 10.1016/j.tibtech.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Benam KH, Villenave R, Lucchesi C, Varone A, Hubeau C, Lee H-H, Alves SE, Salmon M, Ferrante TC, Weaver JC, Bahinski A, Hamilton GA, Ingber DE, Small airway-on-a-chip enables analysis of human lung inflammation and drug responses in vitro, Nat Methods 13 (2016) 151–157. 10.1038/nmeth.3697. [DOI] [PubMed] [Google Scholar]
- [218].Nawroth JC, Lucchesi C, Cheng D, Shukla A, Ngyuen J, Shroff T, Varone A, Karalis K, Lee H-H, Alves S, Hamilton GA, Salmon M, Villenave R, A Microengineered Airway Lung Chip Models Key Features of Viral-induced Exacerbation of Asthma, Am J Respir Cell Mol Biol 63 (2020) 591–600. 10.1165/rcmb.2020-0010MA. [DOI] [PubMed] [Google Scholar]
- [219].Ramadan Q, Ting FCW, In vitro micro-physiological immune-competent model of the human skin, Lab Chip 16 (2016) 1899–1908. 10.1039/C6LC00229C. [DOI] [PubMed] [Google Scholar]
- [220].Harrington H, Cato P, Salazar F, Wilkinson M, Knox A, Haycock JW, Rose F, Aylott JW, Ghaemmaghami AM, Immunocompetent 3D Model of Human Upper Airway for Disease Modeling and In Vitro Drug Evaluation, Mol. Pharmaceutics 11 (2014) 2082–2091. 10.1021/mp5000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Niehues H, van den Bogaard EH, Past, present and future of in vitro 3D reconstructed inflammatory skin models to study psoriasis, Exp Dermatol 27 (2018) 512–519. 10.1111/exd.13525. [DOI] [PubMed] [Google Scholar]
- [222].Chamcheu JC, Esnault S, Adhami VM, Noll AL, Banang-Mbeumi S, Roy T, Singh SS, Huang S, Kousoulas KG, Mukhtar H, Fisetin, a 3,7,3′,4′-Tetrahydroxyflavone Inhibits the PI3K/Akt/mTOR and MAPK Pathways and Ameliorates Psoriasis Pathology in 2D and 3D Organotypic Human Inflammatory Skin Models, Cells 8 (2019) 1089. 10.3390/cells8091089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].van den Bogaard EH, Tjabringa GS, Joosten I, Vonk-Bergers M, van Rijssen E, Tijssen HJ, Erkens M, Schalkwijk J, Koenen HJPM, Crosstalk between Keratinocytes and T Cells in a 3D Microenvironment: A Model to Study Inflammatory Skin Diseases, Journal of Investigative Dermatology 134 (2014) 719–727. 10.1038/jid.2013.417. [DOI] [PubMed] [Google Scholar]
- [224].Beaurivage C, Naumovska E, Chang Y, Elstak E, Nicolas A, Wouters H, van Moolenbroek G, Lanz H, Trietsch S, Joore J, Vulto P, Janssen R, Erdmann K, Stallen J, Kurek D, Development of a Gut-on-a-Chip Model for High Throughput Disease Modeling and Drug Discovery, IJMS 20 (2019) 5661. 10.3390/ijms20225661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Gjorevski N, Avignon B, Gérard R, Cabon L, Roth AB, Bscheider M, Moisan A, Neutrophilic infiltration in organ-on-a-chip model of tissue inflammation, Lab Chip 20 (2020) 3365–3374. 10.1039/D0LC00417K. [DOI] [PubMed] [Google Scholar]
- [226].Roh TT, Chen Y, Paul HT, Guo C, Kaplan DL, 3D bioengineered tissue model of the large intestine to study inflammatory bowel disease, Biomaterials 225 (2019) 119517. 10.1016/j.biomaterials.2019.119517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Leonard F, Screening of budesonide nanoformulations for treatment of inflammatory bowel disease in an inflamed 3D cell-culture model, ALTEX 29 (2012) 275–285. 10.14573/altex.2012.3.275. [DOI] [PubMed] [Google Scholar]
- [228].Leonard F, Collnot E-M, Lehr C-M, A Three-Dimensional Coculture of Enterocytes, Monocytes and Dendritic Cells To Model Inflamed Intestinal Mucosa in Vitro, Mol. Pharmaceutics 7 (2010) 2103–2119. 10.1021/mp1000795. [DOI] [PubMed] [Google Scholar]
- [229].Susewind J, de Souza Carvalho-Wodarz C, Repnik U, Collnot E-M, Schneider-Daum N, Griffiths GW, Lehr C-M, A 3D co-culture of three human cell lines to model the inflamed intestinal mucosa for safety testing of nanomaterials, Nanotoxicology (2015) 1–10. 10.3109/17435390.2015.1008065. [DOI] [PubMed] [Google Scholar]
- [230].Kim HJ, Li H, Collins JJ, Ingber DE, Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip, Proc Natl Acad Sci USA 113 (2016) E7–E15. 10.1073/pnas.1522193112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Shin W, Kim HJ, Intestinal barrier dysfunction orchestrates the onset of inflammatory host–microbiome cross-talk in a human gut inflammation-on-a-chip, Proc Natl Acad Sci USA 115 (2018) E10539–E10547. 10.1073/pnas.1810819115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Dawson A, Dyer C, Macfie J, Davies J, Karsai L, Greenman J, Jacobsen M, A microfluidic chip based model for the study of full thickness human intestinal tissue using dual flow, Biomicrofluidics 10 (2016) 064101. 10.1063/1.4964813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Murphy K, Weaver C, Weaver C, Janeway’s Immunobiology, W.W. Norton & Company, 2016. 10.1201/9781315533247. [DOI] [Google Scholar]
- [234].Artaud C, Kara L, Launay O, Vaccine Development: From Preclinical Studies to Phase 1/2 Clinical Trials, in: Ariey F, Gay F, Ménard R (Eds.), Malaria Control and Elimination, Springer, New York, NY, 2019: pp. 165–176. 10.1007/978-1-4939-9550-9_12. [DOI] [PubMed] [Google Scholar]
- [235].Kennedy RC, Shearer MH, Hildebrand W, Nonhuman primate models to evaluate vaccine safety and immunogenicity, Vaccine 15 (1997) 903–908. 10.1016/S0264-410X(96)00277-0. [DOI] [PubMed] [Google Scholar]
- [236].Govindarajan D, Meschino S, Guan L, Clements DE, ter Meulen JH, Casimiro DR, Coller B-AG, Bett AJ, Preclinical development of a dengue tetravalent recombinant subunit vaccine: Immunogenicity and protective efficacy in nonhuman primates, Vaccine 33 (2015) 4105–4116. 10.1016/j.vaccine.2015.06.067. [DOI] [PubMed] [Google Scholar]
- [237].Gerdts V, Wilson HL, Meurens F, van Drunen Littel - van den Hurk S, Wilson D, Walker S, Wheler C, Townsend H, Potter AA, Large Animal Models for Vaccine Development and Testing, ILAR Journal 56 (2015) 53–62. 10.1093/ilar/ilv009. [DOI] [PubMed] [Google Scholar]
- [238].Gouglas D, Thanh Le T, Henderson K, Kaloudis A, Danielsen T, Hammersland NC, Robinson JM, Heaton PM, Røttingen J-A, Estimating the cost of vaccine development against epidemic infectious diseases: a cost minimisation study, The Lancet Global Health 6 (2018) e1386–e1396. 10.1016/S2214-109X(18)30346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Pronker ES, Weenen TC, Commandeur H, Claassen EHJHM, Osterhaus ADME, Risk in Vaccine Research and Development Quantified, PLOS ONE 8 (2013) e57755. 10.1371/journal.pone.0057755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].CLEVE M, What the Lightning-Fast Quest for COVID Vaccines Means for Other Diseases, Nature 589 (2021). [DOI] [PubMed] [Google Scholar]
- [241].Iwasaki A, Omer SB, Why and How Vaccines Work, Cell 183 (2020) 290–295. 10.1016/j.cell.2020.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Giese C, Lubitz A, Demmler CD, Reuschel J, Bergner K, Marx U, Immunological substance testing on human lymphatic micro-organoids in vitro, Journal of Biotechnology 148 (2010) 38–45. 10.1016/j.jbiotec.2010.03.001. [DOI] [PubMed] [Google Scholar]
- [243].Higbee RG, Byers AM, Dhir V, Drake D, Fahlenkamp HG, Gangur J, Kachurin A, Kachurina O, Leistritz D, Ma Y, Mehta R, Mishkin E, Moser J, Mosquera L, Nguyen M, Parkhill R, Pawar S, Poisson L, Sanchez-Schmitz G, Schanen B, Singh I, Song H, Tapia T, Warren W, Wittman V, An Immunologic Model for Rapid Vaccine Assessment — A Clinical Trial in a Test Tube, Alternatives to Laboratory Animals 37 (2009) 19–27. 10.1177/026119290903701S05. [DOI] [PubMed] [Google Scholar]
- [244].Byers AM, Tapia TM, Sassano ER, Wittman V, In vitro antibody response to tetanus in the MIMICTM system is a representative measure of vaccine immunogenicity, Biologicals 37 (2009) 148–151. 10.1016/j.biologicals.2009.02.018. [DOI] [PubMed] [Google Scholar]
- [245].Dauner A, Agrawal P, Salvatico J, Tapia T, Dhir V, Shaik SF, Drake DR, Byers AM, The in vitro MIMIC® platform reflects age-associated changes in immunological responses after influenza vaccination, Vaccine 35 (2017) 5487–5494. 10.1016/j.vaccine.2017.03.099. [DOI] [PubMed] [Google Scholar]
- [246].Purwada A, Singh A, Immuno-engineered organoids for regulating the kinetics of B-cell development and antibody production, Nature Protocols 12 (2017) 168–182. 10.1038/nprot.2016.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Ball AG, Belanger MC, Pompano RR, Detergent wash improves vaccinated lymph node handling ex vivo, Journal of Immunological Methods 489 (2021) 112943. 10.1016/j.jim.2020.112943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Belanger MC, Ball AG, Catterton MA, Kinman AWL, Anbaei P, Groff BD, Melchor SJ, Lukens JR, Ross AE, Pompano RR, Acute Lymph Node Slices Are a Functional Model System to Study Immunity Ex Vivo, ACS Pharmacol. Transl. Sci 4 (2021) 128–142. 10.1021/acsptsci.0c00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Shanti A, Samara B, Abdullah A, Hallfors N, Accoto D, Sapudom J, Alatoom A, Teo J, Danti S, Stefanini C, Multi-compartment 3D-cultured organ-on-a-chip: Towards a biomimetic lymph node for drug development, Pharmaceutics 12 (2020) 464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Kola I, Landis J, Can the pharmaceutical industry reduce attrition rates?, Nat Rev Drug Discov 3 (2004) 711–716. 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- [251].Oleaga C, Bernabini C, Smith AST, Srinivasan B, Jackson M, McLamb W, Platt V, Bridges R, Cai Y, Santhanam N, Berry B, Najjar S, Akanda N, Guo X, Martin C, Ekman G, Esch MB, Langer J, Ouedraogo G, Cotovio J, Breton L, Shuler ML, Hickman JJ, Multi-Organ toxicity demonstration in a functional human in vitro system composed of four organs, Sci Rep 6 (2016) 20030. 10.1038/srep20030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Novak R, Ingram M, Marquez S, Das D, Delahanty A, Herland A, Maoz BM, Jeanty SSF, Somayaji MR, Burt M, Calamari E, Chalkiadaki A, Cho A, Choe Y, Chou DB, Cronce M, Dauth S, Divic T, Fernandez-Alcon J, Ferrante T, Ferrier J, FitzGerald EA, Fleming R, Jalili-Firoozinezhad S, Grevesse T, Goss JA, Hamkins-Indik T, Henry O, Hinojosa C, Huffstater T, Jang K-J, Kujala V, Leng L, Mannix R, Milton Y, Nawroth J, Nestor BA, Ng CF, O’Connor B, Park T-E, Sanchez H, Sliz J, Sontheimer-Phelps A, Swenor B, Thompson G, Touloumes GJ, Tranchemontagne Z, Wen N, Yadid M, Bahinski A, Hamilton GA, Levner D, Levy O, Przekwas A, Prantil-Baun R, Parker KK, Ingber DE, Robotic fluidic coupling and interrogation of multiple vascularized organ chips, Nat Biomed Eng 4 (2020) 407–420. 10.1038/s41551-019-0497-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Zhang H, Whalley RD, Ferreira AM, Dalgarno K, High throughput physiological micro-models for in vitro pre-clinical drug testing: a review of engineering systems approaches, Prog. Biomed. Eng 2 (2020) 022001. 10.1088/2516-1091/ab7cc4. [DOI] [Google Scholar]
- [254].Dittrich PS, Manz A, Lab-on-a-chip: microfluidics in drug discovery, Nat Rev Drug Discov 5 (2006) 210–218. 10.1038/nrd1985. [DOI] [PubMed] [Google Scholar]
- [255].Dehne E-M, Hasenberg T, Marx U, The ascendance of microphysiological systems to solve the drug testing dilemma, Future Science OA 3 (2017) FSO0185. 10.4155/fsoa-2017-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Vermeire S, Gils A, Accossato P, Lula S, Marren A, Immunogenicity of biologics in inflammatory bowel disease, Therap Adv Gastroenterol 11 (2018) 1756283X1775035. 10.1177/1756283X17750355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Shakhnovich V, Meibohm B, Rosenberg A, Kierzek AM, Hasenkamp R, Funk RS, Thalhauser CJ, Graaf PH, Wang YC, Hamuro L, Immunogenicity in Clinical Practice and Drug Development: When is it Significant?, Clin Transl Sci 13 (2020) 219–223. 10.1111/cts.12717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Gunn GR, Sealey DCF, Jamali F, Meibohm B, Ghosh S, Shankar G, From the bench to clinical practice: understanding the challenges and uncertainties in immunogenicity testing for biopharmaceuticals: Challenges of Immunogenicity Testing, Clin Exp Immunol 184 (2016) 137–146. 10.1111/cei.12742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Edri R, Gal I, Noor N, Harel T, Fleischer S, Adadi N, Green O, Shabat D, Heller L, Shapira A, Gat-Viks I, Peer D, Dvir T, Personalized Hydrogels for Engineering Diverse Fully Autologous Tissue Implants, Adv. Mater 31 (2019) 1803895. 10.1002/adma.201803895. [DOI] [PubMed] [Google Scholar]


