Abstract
Data regarding the efficacy and safety of smoking-cessation pharmacotherapy after stroke are lacking. We systematically reviewed data on this topic by searching Medline, Cochrane, and Clinicaltrials.gov to identify randomized clinical trials (RCT) and observational studies that assessed the efficacy and safety of nicotine replacement therapy (NRT), varenicline, and bupropion in patients with stroke and TIA. We included studies that reported rates of smoking cessation, worsening or recurrent cerebrovascular disease, seizures, or neuropsychiatric events. We identified 2 RCTs and 6 observational studies; 3 included ischemic stroke and TIA, 2 subarachnoid hemorrhage (SAH), and 3 did not specify. Four studies assessed efficacy; cessation rates ranged from 33% to 66% with pharmacological therapy combined with behavioral interventions versus 15% to 46% without, but no individual study demonstrated a statistically significant benefit. Safety data for varenicline and buopropion in ischemic stroke were scarce. Patients with SAH who received NRT had more seizures (9% vs 2%; P=0.024) and delirium (19% vs 7%; P=0.006) in one study, but less frequent vasospasm in 3 studies. In conclusion, combined with behavioral interventions, smoking-cessation therapies resulted in numerically higher cessation rates. Limited safety data may prompt caution regarding seizures and delirium in patients with subarachnoid hemorrhage.
Keywords: Cerebrovascular Disease, Stroke, Transient Ischemic Attack (TIA), Secondary Prevention, Smoking Cessation, Pharmacology
Introduction
Eighteen percent of patients hospitalized with stroke nationwide are current smokers, and the proportion exceeds 40% among young patients with stroke [1, 2]. Smoking is associated with worse outcomes, including recurrent stroke and mortality, after ischemic stroke, transient ischemic attack (TIA), and subarachnoid hemorrhage (SAH) [3–6] However, smoking cessation within 6 months of ischemic stroke and transient ischemic attack (TIA) has been associated with a markedly reduced long-term risk of vascular events and death [7].
The efficacy of nicotine replacement therapy (NRT), bupropion, and varenicline for smoking cessation in the general population is established [8]. This is not the case for patients with cerebrovascular disease. The American Heart Association/American Stroke Association (AHA/ASA) secondary prevention guidelines assert that smoking-cessation pharmacotherapy is effective in general, while acknowledging that data from patients with stroke and TIA are unavailable [9]. The AHA/ASA Guidelines for the Early Management of Patients with Acute Ischemic Stroke were recently updated to include recommendations regarding the use of select pharmacotherapies;[10] however, the cited data are sparse and largely drawn from studies of the general population. In contrast, abundant data specific to patients with cardiovascular disease informed the recent American College of Cardiology endorsement of smoking-cessation pharmacotherapy for patients hospitalized with cardiovascular disease[11].
Efficacy and safety findings from patients with cardiovascular disease[12–14] may not be generalizable to stroke and TIA. Patients with stroke may have higher rates of spontaneous, un-aided cessation from stroke-related attenuation of nicotine dependence and stays in smoke-free rehabilitation environments[15–20]. Further, these patients pose unique safety considerations related to vulnerability to cerebral ischemia,[21–23] seizures,[24, 25] and neuropsychiatric symptoms[26, 27]. In light of these considerations, we performed a systematic review to evaluate the evidence for efficacy and safety of smoking-cessation pharmacotherapy in patients with stroke and TIA.
Methods
We conducted a systematic review of randomized clinical trials and observational studies that investigated the efficacy and safety of smoking-cessation pharmacotherapy for patients with stroke and TIA. We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standards[28]. We reviewed only previously published data; institutional review board approval and individual patient informed consent were not required.
Search Strategy
We queried the Medline, Cochrane Library, and clinicaltrials.gov databases last on October 23, 2019. We adapted a published search strategy [12] to retrieve studies regarding cerebrovascular disease (Supplemental Material). We additionally reviewed references of included articles and relevant reviews. Search results were exported into a web-based systematic review software (Covidence, Veritas Health Innovation, Melbourne, Australia).
Study Selection
Two independent reviewers (NSP, SSO) screened titles and abstracts. Articles selected by at least one reviewer were selected for full-text review by both reviewers; consensus and a tie-breaker (JW) were used to select the final articles. Study selection criteria were established a priori; restrictive criteria were not used given the suspected paucity of data on this topic. We included both randomized trials and observational studies that compared pharmacological intervention(s) (NRT, varenicline, and bupropion) to a non-pharmacological or non-interventional control, in addition to single-arm observational studies. Studies that used behavioral co-interventions alongside pharmacological therapy were included. We limited studies to those that included only, or separately reported adequate subgroup data for, adult patients with stroke and TIA. We included all stroke types to maximize scope. Studies that reported either the efficacy outcome of smoking cessation or at least one safety outcome were included. Pre-specified safety outcomes were: recurrent or worsening cerebrovascular disease, death, clinical outcomes, seizures, and neuropsychiatric events. We excluded single case reports and unpublished abstracts. Only articles published in English were included.
Data Abstraction
After a standardized data form was created and iteratively revised, two reviewers (NSP, SSO) independently abstracted data. After collation, discrepancies were resolved by consensus and through discussion with an additional reviewer (JW). Study characteristics were country, design, setting, publication year, study population characteristics, number of patients, and duration of follow-up. Patient characteristics were demographics, type of cerebrovascular disease, and smoking duration and frequency. We recorded the nature, dose, duration, and behavioral co-interventions for pharmacological interventions. We recorded the number of patients with self-reported or biochemically-validated smoking cessation, with preference given to biochemically-validated cessation rates. We additionally abstracted the number of patients experiencing each adverse outcome.
Study Quality Assessment
Two reviewers (NSP, SSO) independently rated the overall quality of evidence by outcome of interest using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach[29]. This approach allows joint consideration of data from heterogeneous study designs (randomized clinical trials and observational studies) for each individual outcome while simultaneously considering overall design quality, consistency of findings, directness, precision, strength of association, and bias in individual studies. The overall quality is described as “very low”, “low”, “moderate”, and “high”.
Synthesis of Results
We calculated pooled frequencies where possible for the outcomes of smoking cessation and individual adverse events. However, after completion of study selection, we decided not to perform statistical between-group comparisons or a formal, quantitative meta-analysis given the marked heterogeneity in study designs, populations, and nature of interventions and outcomes in the available studies.
Data Availability
Our detailed search strategy is available in Table I and Table II of the Supplemental Materials. All data included in this review are available in Tables 1 and 2.
Table 1.
Summary of Included Studies
| 1st author | Year | Design | Population/Setting | Pharmacotherapy | Co-intervention | Number of patients (n)* | Efficacy Outcome(s) | Safety Outcome(s) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Rx. | Control | ||||||||
| Frandsen | 2012 | Randomized trial | IS, TIA In-patient | Free NRT | Intensive behavioral intervention | 94 | 49 | 45 | 6-mo. cessation, self-reported and exhaled CO | None |
| Papadakis | 2011 | Randomized trial† | IS, TIA Clinic | Free NRT, bupropion, varenicline | Behavioral intervention | 28 | 15 | 13 | 6-mo. continuous and 7-day point abstinence (exhaled CO) | None |
| Lee | 2005 | Observational | IS In-patient | NRT, bupropion, varenicline | Intensive behavioral intervention | 157 | 86 | 71 | 1-year sustained and 1, 3, 6, 9, 12 mo. point abstinence | None |
| Çelik | 2015 | Observational | Stroke Community | Bupropion, varenicline | Support program | 141 | 141 | N/A | 1-year smoking status | None |
| Carandang | 2011 | Observational | SAH ICU | NRT patch | None | 258 | 87 | 171 | None | Vasospasm‡, DCI, seizures, delirium, 3-month mortality |
| Seder | 2011 | Observational | SAH ICU | NRT patch | None | 234 | 128 | 106 | None | Vasospasm‡, in-hospital mortality, Glasgow Outcome Scale |
| Panos | 2010 | Observational§ | SAH ICU | NRT patch | None | 88 | 56 | 32 | None | Vasospasm |
| Hubbard | 2005 | Observational‖ | Stroke Community | NRT | N/A | 506 | 506 | N/A | None | Recurrent stroke |
Abbreviations: Rx, pharmacotherapy; IS, ischemic stroke; TIA, transient ischemic attack; NRT, nicotine replacement therapy; CO, carbon monoxide; SAH, subarachnoid hemorrhage; ICU, intensive care unit; DCI, delayed cerebral ischemia.
For studies that included patients with and without cerebrovascular disease, the numbers reported here are only for subgroups with cerebrovascular disease.
Randomized patients to a cost-free pharmacotherapy or provision of a prescription for pharmacotherapy. More patients in the cost-free group initiated and complied with pharmacotherapy.
Rates of angiographic and clinical vasospasm were reported.
This study included patients admitted to a neurosurgical ICU. Only data for patients with SAH were included for this systematic review. We assumed that all of the reported occurrences of vasospasm occurred in patients with SAH.
This study evaluated the risk of stroke after NRT prescription; in a secondary analysis, the outcome was recurrent stroke among patients with prior stroke. Only data pertaining to the latter analysis were included here.
Table 2.
GRADE Quality Assessment of Included Studies and Summary of Findings, By Outcome of Interest
| Studies | Design Quality | Study Quality | Consistency | Directness | Other Factors* | Overall Quality† | Summary of Findings |
|---|---|---|---|---|---|---|---|
| Efficacy – Smoking Cessation | |||||||
| Frandsen Papadakis Lee Çelik | Low quality – pilot RCTs and observational data | No additional serious limitations | No important inconsistency | Uncertainty: co-interventions and non-distinct control group | Sparse data and high risk of bias in non-blinded randomized trials | Very low | Numerically increased cessation in individual studies with interventions including pharmacotherapy |
| Safety – Recurrent or Worsening Cerebrovascular Disease | |||||||
| Seder Carandang Panos Hubbard | Low quality – observational | No additional serious limitations | No important inconsistency | No uncertainty about directness | No other major limitations, and +1 level for strength of associations | Moderate | Less vasospasm with NRT, after adjusting for Fisher grade. DCI rates similar. Recurrent stroke not increased with NRT in one study. |
| Safety – Mortality | |||||||
| Seder Carandang | Low quality – observational | No additional serious limitations | No important inconsistency | No uncertainty about directness | Sparse data | Very low | Less in-hospital mortality and 3-month mortality (after adjusting for age, cerebral edema, grade) with NRT. |
| Safety – Clinical Outcomes | |||||||
| Carandang | Low quality – observational | No additional serious limitations | Data too sparse to assess consistency | Uncertainty: outcome measure | Sparse data | Very low | More good functional outcomes at 3 months with NRT, after adjusting for severity and grade. |
| Safety – Seizures | |||||||
| Seder | Low quality – observational | No additional serious limitations | Data too sparse to assess consistency | Uncertainty: outcome measure | No other major limitations | Low | More seizures with NRT in single observational study. |
| Safety – Neuropsychiatric Events (Delirium) | |||||||
| Seder | Low quality – observational | No additional serious limitations | Data too sparse to assess consistency | Uncertainty: outcome measure | No other major limitations | Low | More delirium with NRT in single observational study. |
Abbreviations: GRADE, Grading of Recommendations, Assessment, Development, and Evaluation; RCT, randomized clinical trial; NRT, nicotine replacement therapy; DCI, delayed cerebral ischemia.
Other factors are: imprecision/sparse data, strength of association, bias, dose-response gradient, possibility of residual confounding.
Overall quality determined after iteratively up- or down-grading quality based on study quality, consistency, directness, and other factors, after assigning an initial quality rating based on design quality.
Results
The search identified 452 potentially relevant publications, of which 8 met inclusion criteria (Figure). We identified 2 randomized trials and 6 observational studies (Table 1). Of the 8 studies, 3 included patients with ischemic stroke and TIA, 3 included SAH, and 2 did not specify. A total of 1,506 patients with cerebrovascular disease were included: 279 ischemic stroke or TIA, 580 SAH, and 647 stroke not specified. Study settings included intensive care units (3), inpatient wards (2), outpatient clinics (2), and unspecified (1). Readiness to quit was assessed in 3 studies. Pharmacological interventions were NRT in 4 studies, a choice of varenicline or bupropion in 1 study, and a choice of NRT, varenicline, or bupropion in 1 study. A total of 1,068 (71%) patients were randomized to or received pharmacotherapy-containing cessation interventions. In 4 studies, a behavioral co-intervention of variable intensity was provided, and 4 studies were observational without a specified control intervention. Rates of smoking cessation were reported in 4 studies; 2 studies reported biochemically validated rates. Mortality was reported in 2 studies, clinical outcomes in 1 study, recurrent or worsening cerebrovascular disease in 4 studies, seizures in 1 study, and neuropsychiatric events in 1 study. The duration of follow-up ranged from the index hospitalization only to up to 1 year.
Figure.
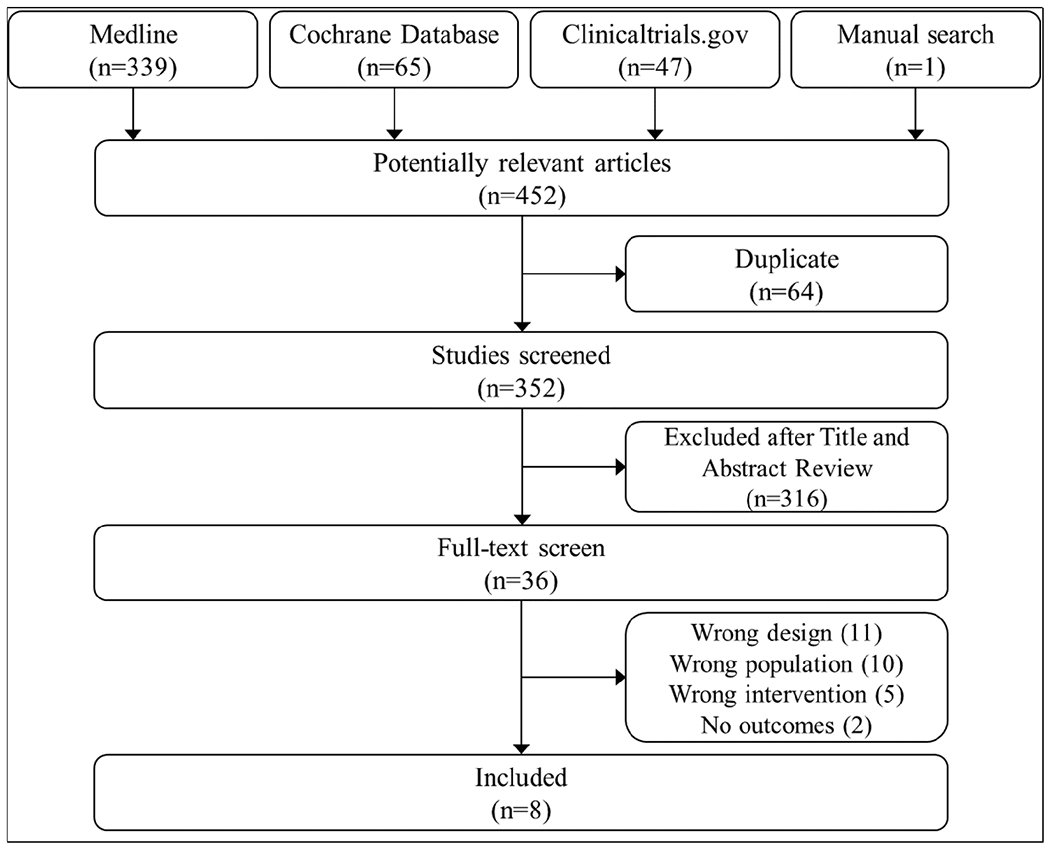
Study selection flow diagram.
Two reviewers independently screened titles and abstracts and then full-text manuscripts prior to including 8 manuscripts in this systematic review.
Efficacy
We did not identify any randomized, placebo-controlled, double-blinded trials that evaluated the efficacy of a pharmacological intervention in patients with cerebrovascular disease. Two pilot studies randomized patients with ischemic stroke or TIA to interventions that included cost-free pharmacological therapy[30, 31]. In one study, 28 patients received counselling and were randomized to cost-free pharmacotherapy or a standard prescription for pharmacotherapy; a nominally higher proportion of patients given cost-free pharmacotherapy used and was compliant with medication, and these patients had a non-significantly higher odds of abstinence[30]. In the second study, 94 patients were randomized to a 30-minute individual counseling session or an intensive intervention comprised of a 5-session program and free NRT[31]. Again, patients randomized to receive the intensive intervention had a non-significantly higher rate of cessation. In both, the lack of blinding conferred a high risk of bias.
We also identified an observational study of 157 Korean men with ischemic stroke that assessed smoking cessation in a before-after analysis of an intensive behavioral intervention that included consideration for pharmacotherapy (varenicline, bupropion, or NRT)[32]. Patients in the intervention period had three times the odds of smoking cessation compared to those treated in the standard of care period; however, they did not find that use of pharmacotherapy was itself associated with smoking cessation. In these pilot randomized trials and one observational study, cessation rates ranged from 33% to 66% with interventions including pharmacotherapy or cost-free pharmacotherapy versus 15% to 46% without, without any statistically significant differences between approaches in any individual study. In aggregate, of the 150 patients who received interventions including pharmacotherapy or who were randomized to interventions that included cost-free pharmacotherapy, 83 (55%) quit smoking. Of the 129 patients in control groups, 52 (40%) quit smoking. Excluding the trial comparing cost-free versus standard of care prescriptions,[30] 78 (58%) of 135 patients in pharmacotherapy groups versus 50 (43%) of 116 patients in non-pharmacotherapy groups quit smoking. For comparison, in an analysis of a nationwide smoking-cessation program from Turkey that reported rates of 1-year cessation among patients with a prior stroke not further specified, 34% of patients taking a cessation aide quit smoking[33]. Overall, the quality of evidence for smoking cessation efficacy was “very low” by the GRADE criteria (Table 2).
Safety
Studies reporting smoking-cessation rates did not report adverse events, and no safety studies for patients with ischemic stroke and TIA were identified. A registry-based, self-controlled study of NRT reported that the risk of first stroke, not further specified, was not increased after prescription of an NRT[34]. With regards to the safety endpoint of recurrent or worsening cerebrovascular disease, they performed a secondary analysis of recurrent stroke and reported that the risk of recurrent stroke was not increased after prescription of an NRT, but they did not provide measures of effect. Two single-center, observational studies of NRT in patients with SAH reported rates of vasospasm[35, 36]. An additional study included patients with SAH alongside other patients admitted to a neurosurgical intensive care unit and adjusted for presence of SAH when examining the association between NRT and vasospasm[37]. Rates of angiographic vasospasm were similar in patients with SAH receiving and not receiving NRT in individual studies. In aggregate, vasospasm was seen in 72 (27%) of 263 patients receiving NRT and 117 (38%) of 304 patients not receiving NRT. In two studies of patients with SAH reporting clinical vasospasm rates,[35, 36] vasospasm was observed in 49 (23%) of 215 patients receiving NRT and 80 (29%) of 277 patients not receiving NRT. One study adjusted for Fisher grade and found NRT was associated with less clinical vasospasm (odds ratio [OR], 0.45; 95% confidence interval [CI], 0.23-0.88)[35]. Last, one study reported that rates of delayed cerebral ischemia did not differ between patients receiving (25%) and not receiving (23%) NRT (P=0.65)[36]. Overall, we deemed evidence regarding recurrent or worsening cerebrovascular disease to be moderate in quality by the GRADE criteria (Table 2).
Data regarding mortality, clinical outcomes, seizures, and neuropsychiatric adverse events were scarce. In one study of patients with SAH, NRT was associated with a lower odds of 3-month mortality after adjusting for age, cerebral edema, and SAH grade (OR, 0.12; 95% CI, 0.04-0.39)[36]. A separate study of patients with SAH reported a nominally lower rate of in-hospital mortality among those who received NRT (2% versus 7%)[35]. This study also reported that NRT-treated patients were more likely to have a good functional outcome at discharge (Glasgow Outcome Scale score <4) after adjusting for clinical severity and grade (OR, 2.17; 95% CI, 1.19-3.97). A single study of patients with SAH reported both higher rates of seizures (9% vs 2%; P=0.024) and delirium (19% vs 7%; P=0.006) among patients receiving NRT[36]. The quality of data for each of these outcomes was “very low” by the GRADE criteria (Table 2).
Discussion
Data supporting the efficacy and safety of smoking-cessation pharmacotherapy in patients with acute cerebrovascular diseases are limited and of generally low quality. Numerically higher rates of smoking cessation were observed among patients given interventions that included pharmacotherapy, albeit in combination with intensive behavioral therapy. Safety data were scarce, except for data suggesting no increase, and potentially a decrease, in rates of vasospasm among patients with SAH treated with NRT. A single study raised the possibility of higher rates of seizure and delirium in these patients.
Prior reviews on this topic differed in scope by including studies of behavioral, non-pharmacological interventions and not reporting safety data[38] or restricting inclusion to studies of NRT in patients with SAH[39]. In this systematic review, we comprehensively evaluated efficacy and safety data for smoking-cessation pharmacotherapy in patients with stroke and TIA in a framework informed by considerations specific to this population. Whereas randomized, placebo-controlled trials have assessed the efficacy and safety of bupropion and varenicline for patients with cardiovascular diseases including acute coronary syndrome,[14, 40–43] no such data exist for patients with stroke and TIA. A registered randomized study of NRT in patients with SAH (NCT02350335) has not yet been published and will not be informative regarding other therapies or for other forms of stroke. In contrast to patients with cardiovascular diseases, patients with stroke and TIA uniquely experience reductions in nicotine dependence from insular and basal ganglia injury[17, 18, 44]. Whether NRT or varenicline, which is a partial nicotine receptor agonist, provide additional reductions in dependence and smoking urges in patients with mesolimbic pathway disruption is unknown. Further, patients with stroke often have prolonged admissions in smoke-free environments, such as inpatient rehabilitation centers, and studies of patients with cardiovascular disease have found that longer length of stay and participation in cardiac rehabilitation facilitate smoking cessation[20, 45]. These biological and care-related factors may result in spontaneous cessation rates that are higher than in the general or cardiovascular disease population, such that the benefit of pharmacotherapy may be reduced. Demonstrating the efficacy of cessation pharmacotherapy for patients with stroke and TIA is necessary to establish effective, standardized approaches for secondary prevention both in the acute and chronic settings.
With the exception of data regarding vasospasm in patients with SAH, which had previously been noted,[39] safety data in patients with stroke or TIA are limited. There are several important safety concerns that remain unaddressed by the current literature. First, bupropion increases the risk of seizures[25] and is formally contraindicated in patients at risk of seizures. The package insert for varenicline was also recently updated to reflect post-marketing observations of seizures[46]. Stroke, particularly hemorrhagic stroke, is associated with an increased risk of seizures[24]. Whether bupropion and varenicline are associated with an excess risk of seizures in patients with recent or any prior stroke is unknown. Second, although the Evaluating Adverse Events in a Global Smoking Cessation Study (EAGLES) demonstrated that varenicline was safe in patients with a prior history of chronic psychiatric comorbidities,[47] it is unclear whether these data are applicable to patients with stroke, who face an increased risk of suicide[26, 27]. Third, a randomized clinical trial suggested that varenicline was safe for use in patients with acute coronary syndrome[14]. However, this study was not powered for safety outcomes, and a large observational study found varenicline use to be associated with an increased risk of cardiovascular events[48]. The American College of Cardiology cites these data in tempering their recommendations regarding in-hospital initiation of varenicline after acute coronary syndromes[43]. Whether varenicline can safely be initiated during hospitalization for stroke and TIA, to ideally achieve therapeutic levels by the time of discharge, is unknown. Last, animal data suggest that nicotine may increase infarct size and cerebral edema after stroke,[21–23] which raises concerns regarding in-hospital initiation of NRT after ischemic stroke. Determining the cerebrovascular safety profile of cessation pharmacotherapy is necessary before making strong recommendations, especially with regards to in-hospital initiation after acute ischemic stroke and TIA.
The strengths of this systematic review include a priori specification of safety outcomes of interest, a robust search strategy, and broad inclusion criteria. This approach resulted in substantial heterogeneity, precluding a formal quantitative meta-analysis, but permitted a comprehensive overview of cessation pharmacotherapy efficacy and safety data. The results remain hypothesis-generating. However, we have outlined areas of interest that deserve further pharmacoepidemiological or randomized study.
Conclusions
There were insufficient high-quality data to conclusively assess the efficacy and safety of smoking-cessation pharmacotherapy in patients with stroke and TIA. More data specific to this patient population are ideally needed in order to make strong recommendations regarding the use of smoking-cessation pharmacotherapy.
Supplementary Material
Sources of Support:
NIH/NINDS (T32-NS07153; PI: Elkind, trainee: Parikh).
Disclosures:
Dr. Elkind serves as the Chairman of the Advisory Committee to the American Stroke Association and on the National, Founders Affiliate, and New York City boards of the American Heart Association. He receives royalties for chapters on stroke from UpToDate. Dr. Parikh, Dr. Salehi Omran, Dr. Kamel, and Dr. Willey: none.
References
- [1].Otite FO, Liaw N, Khandelwal P, Malik AM, Romano JG, Rundek T, et al. Increasing prevalence of vascular risk factors in patients with stroke: A call to action. Neurology. 2017;89:1985–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].George MG, Tong X, Bowman BA. Prevalence of Cardiovascular Risk Factors and Strokes in Younger Adults. JAMA Neurol. 2017;74:695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kaplan RC, Tirschwell DL, Longstreth WT, Manolio TA, Heckbert SR, Lefkowitz D, et al. Vascular events, mortality, and preventive therapy following ischemic stroke in the elderly. Neurology. 2005;65:835–42. [DOI] [PubMed] [Google Scholar]
- [4].Kim J, Gall SL, Dewey HM, Macdonell RA, Sturm JW, Thrift AG. Baseline smoking status and the long-term risk of death or nonfatal vascular event in people with stroke: a 10-year survival analysis. Stroke. 2012;43:3173–8. [DOI] [PubMed] [Google Scholar]
- [5].Ovbiagele B, Weir CJ, Saver JL, Muir KW, Lees KR. Effect of smoking status on outcome after acute ischemic stroke. Cerebrovasc Dis. 2006;21:260–5. [DOI] [PubMed] [Google Scholar]
- [6].Wermer MJ, Greebe P, Algra A, Rinkel GJ. Incidence of recurrent subarachnoid hemorrhage after clipping for ruptured intracranial aneurysms. Stroke. 2005;36:2394–9. [DOI] [PubMed] [Google Scholar]
- [7].Epstein KA, Viscoli CM, Spence JD, Young LH, Inzucchi SE, Gorman M, et al. Smoking cessation and outcome after ischemic stroke or TIA. Neurology. 2017;89:1723–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013:CD009329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–236. [DOI] [PubMed] [Google Scholar]
- [10].Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019:STR0000000000000211. [Google Scholar]
- [11].Barua RS, Rigotti NA, Benowitz NL, Cummings KM, Jazayeri MA, Morris PB, et al. 2018 ACC Expert Consensus Decision Pathway on Tobacco Cessation Treatment: A Report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2018. [DOI] [PubMed] [Google Scholar]
- [12].Suissa K, Larivière J, Eisenberg MJ, Eberg M, Gore GC, Grad R, et al. Efficacy and Safety of Smoking Cessation Interventions in Patients With Cardiovascular Disease: A Network Meta-Analysis of Randomized Controlled Trials. Circ Cardiovasc Qual Outcomes. 2017;10. [DOI] [PubMed] [Google Scholar]
- [13].Benowitz NL, Pipe A, West R, Hays JT, Tonstad S, McRae T, et al. Cardiovascular Safety of Varenicline, Bupropion, and Nicotine Patch in Smokers: A Randomized Clinical Trial. JAMA Intern Med. 2018;178:622–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Eisenberg MJ, Windle SB, Roy N, Old W, Grondin FR, Bata I, et al. Varenicline for Smoking Cessation in Hospitalized Patients With Acute Coronary Syndrome. Circulation. 2016;133:21–30. [DOI] [PubMed] [Google Scholar]
- [15].McCarthy MJ, Huguet N, Newsom JT, Kaplan MS, McFarland BH. Predictors of smoking patterns after first stroke. Soc Work Health Care. 2013;52:467–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sienkiewicz-Jarosz H, Zatorski P, Ryglewicz D, Bienkowski P. Reasons for quitting smoking in patients with first-ever ischemic stroke. Eur Addict Res. 2012;18:275–8. [DOI] [PubMed] [Google Scholar]
- [17].Abdolahi A, Williams GC, Benesch CG, Wang HZ, Spitzer EM, Scott BE, et al. Immediate and Sustained Decrease in Smoking Urges After Acute Insular Cortex Damage. Nicotine Tob Res. 2017;19:756–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gaznick N, Tranel D, McNutt A, Bechara A. Basal ganglia plus insula damage yields stronger disruption of smoking addiction than basal ganglia damage alone. Nicotine Tob Res. 2014;16:445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Twardella D, Loew M, Rothenbacher D, Stegmaier C, Ziegler H, Brenner H. The diagnosis of a smoking-related disease is a prominent trigger for smoking cessation in a retrospective cohort study. J Clin Epidemiol. 2006;59:82–9. [DOI] [PubMed] [Google Scholar]
- [20].Katz DA, Buchanan DM, Weg MWV, Faseru B, Horwitz PA, Jones PG, et al. Does outpatient cardiac rehabilitation help patients with acute myocardial infarction quit smoking? Prev Med. 2019;118:51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bradford ST, Stamatovic SM, Dondeti RS, Keep RF, Andjelkovic AV. Nicotine aggravates the brain postischemic inflammatory response. Am J Physiol Heart Circ Physiol. 2011;300:H1518–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Paulson JR, Yang T, Selvaraj PK, Mdzinarishvili A, Van der Schyf CJ, Klein J, et al. Nicotine exacerbates brain edema during in vitro and in vivo focal ischemic conditions. J Pharmacol Exp Ther. 2010;332:371–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wang L, Kittaka M, Sun N, Schreiber SS, Zlokovic BV. Chronic nicotine treatment enhances focal ischemic brain injury and depletes free pool of brain microvascular tissue plasminogen activator in rats. J Cereb Blood Flow Metab. 1997;17:136–46. [DOI] [PubMed] [Google Scholar]
- [24].Merkler AE, Gialdini G, Lerario MP, Parikh NS, Morris NA, Kummer B, et al. Population-Based Assessment of the Long-Term Risk of Seizures in Survivors of Stroke. Stroke. 2018;49:1319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Beyens MN, Guy C, Mounier G, Laporte S, Ollagnier M. Serious adverse reactions of bupropion for smoking cessation: analysis of the French Pharmacovigilance Database from 2001 to 2004. Drug Saf. 2008;31:1017–26. [DOI] [PubMed] [Google Scholar]
- [26].Eriksson M, Glader EL, Norrving B, Asplund K. Poststroke suicide attempts and completed suicides: a socioeconomic and nationwide perspective. Neurology. 2015;84:1732–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Harnod T, Lin CL, Kao CH. Risk of Suicide Attempt in Poststroke Patients: A Population-Based Cohort Study. J Am Heart Assoc. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9, W64. [DOI] [PubMed] [Google Scholar]
- [29].Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Papadakis S, Aitken D, Gocan S, Riley D, Laplante MA, Bhatnagar-Bost A, et al. A randomised controlled pilot study of standardised counselling and cost-free pharmacotherapy for smoking cessation among stroke and TIA patients. BMJ open. 2011;1:e000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Brunner Frandsen N, Sørensen M, Hyldahl TK, Henriksen RM, Bak S. Smoking cessation intervention after ischemic stroke or transient ischemic attack. A randomized controlled pilot trial. Nicotine Tob Res. 2012;14:443–7. [DOI] [PubMed] [Google Scholar]
- [32].Lee MJ, Park E, Kim HC, Lee HS, Cha MJ, Kim YD, et al. Timely Interventions can Increase Smoking Cessation Rate in Men with Ischemic Stroke. J Korean Acad Nurs. 2016;46:610–7. [DOI] [PubMed] [Google Scholar]
- [33].Çelik İ, Yüce D, Hayran M, Erman M, Kılıçkap S, Buzgan T, et al. Nationwide Smoking Cessation Treatment Support Program--Turkey project. Health Policy. 2015;119:50–6. [DOI] [PubMed] [Google Scholar]
- [34].Hubbard R, Lewis S, Smith C, Godfrey C, Smeeth L, Farrington P, et al. Use of nicotine replacement therapy and the risk of acute myocardial infarction, stroke, and death. Tob Control. 2005;14:416–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Carandang RA, Barton B, Rordorf GA, Ogilvy CS, Sims JR. Nicotine replacement therapy after subarachnoid hemorrhage is not associated with increased vasospasm. Stroke. 2011;42:3080–6. [DOI] [PubMed] [Google Scholar]
- [36].Seder DB, Schmidt JM, Badjatia N, Fernandez L, Rincon F, Claassen J, et al. Transdermal nicotine replacement therapy in cigarette smokers with acute subarachnoid hemorrhage. Neurocrit Care. 2011;14:77–83. [DOI] [PubMed] [Google Scholar]
- [37].Panos NG, Tesoro EP, Kim KS, Mucksavage JJ. Outcomes associated with transdermal nicotine replacement therapy in a neurosurgery intensive care unit. Am J Health Syst Pharm. 2010;67:1357–61. [DOI] [PubMed] [Google Scholar]
- [38].Edjoc RK, Reid RD, Sharma M. The effectiveness of smoking cessation interventions in smokers with cerebrovascular disease: a systematic review. BMJ Open. 2012;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Turgeon RD, Chang SJ, Dandurand C, Gooderham PA, Hunt C. Nicotine replacement therapy in patients with aneurysmal subarachnoid hemorrhage: Systematic review of the literature, and survey of Canadian practice. J Clin Neurosci. 2017;42:48–53. [DOI] [PubMed] [Google Scholar]
- [40].Rigotti NA, Pipe AL, Benowitz NL, Arteaga C, Garza D, Tonstad S. Efficacy and safety of varenicline for smoking cessation in patients with cardiovascular disease: a randomized trial. Circulation. 2010;121:221–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Windle SB, Dehghani P, Roy N, Old W, Grondin FR, Bata I, et al. Smoking abstinence 1 year after acute coronary syndrome: follow-up from a randomized controlled trial of varenicline in patients admitted to hospital. CMAJ. 2018;190:E347–E54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Tonstad S, Farsang C, Klaene G, Lewis K, Manolis A, Perruchoud AP, et al. Bupropion SR for smoking cessation in smokers with cardiovascular disease: a multicentre, randomised study. Eur Heart J. 2003;24:946–55. [DOI] [PubMed] [Google Scholar]
- [43].Barua RS, Rigotti NA, Benowitz NL, Cummings KM, Jazayeri MA, Morris PB, et al. 2018 ACC Expert Consensus Decision Pathway on Tobacco Cessation Treatment: A Report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2018. [DOI] [PubMed] [Google Scholar]
- [44].Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Perez GH, Nicolau JC, Romano BW, Laranjeira R. Depression: a predictor of smoking relapse in a 6-month follow-up after hospitalization for acute coronary syndrome. Eur J Cardiovasc Prev Rehabil. 2008;15:89–94. [DOI] [PubMed] [Google Scholar]
- [46].Labs P. CHANTIX (varenicline) [package insert]. February 2019.
- [47].Anthenelli RM, Benowitz NL, West R, St Aubin L, McRae T, Lawrence D, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387:2507–20. [DOI] [PubMed] [Google Scholar]
- [48].Gershon AS, Campitelli MA, Hawken S, Victor C, Sproule BA, Kurdyak P, et al. Cardiovascular and Neuropsychiatric Events after Varenicline Use for Smoking Cessation. Am J Respir Crit Care Med. 2018;197:913–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Our detailed search strategy is available in Table I and Table II of the Supplemental Materials. All data included in this review are available in Tables 1 and 2.
Table 1.
Summary of Included Studies
| 1st author | Year | Design | Population/Setting | Pharmacotherapy | Co-intervention | Number of patients (n)* | Efficacy Outcome(s) | Safety Outcome(s) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Rx. | Control | ||||||||
| Frandsen | 2012 | Randomized trial | IS, TIA In-patient | Free NRT | Intensive behavioral intervention | 94 | 49 | 45 | 6-mo. cessation, self-reported and exhaled CO | None |
| Papadakis | 2011 | Randomized trial† | IS, TIA Clinic | Free NRT, bupropion, varenicline | Behavioral intervention | 28 | 15 | 13 | 6-mo. continuous and 7-day point abstinence (exhaled CO) | None |
| Lee | 2005 | Observational | IS In-patient | NRT, bupropion, varenicline | Intensive behavioral intervention | 157 | 86 | 71 | 1-year sustained and 1, 3, 6, 9, 12 mo. point abstinence | None |
| Çelik | 2015 | Observational | Stroke Community | Bupropion, varenicline | Support program | 141 | 141 | N/A | 1-year smoking status | None |
| Carandang | 2011 | Observational | SAH ICU | NRT patch | None | 258 | 87 | 171 | None | Vasospasm‡, DCI, seizures, delirium, 3-month mortality |
| Seder | 2011 | Observational | SAH ICU | NRT patch | None | 234 | 128 | 106 | None | Vasospasm‡, in-hospital mortality, Glasgow Outcome Scale |
| Panos | 2010 | Observational§ | SAH ICU | NRT patch | None | 88 | 56 | 32 | None | Vasospasm |
| Hubbard | 2005 | Observational‖ | Stroke Community | NRT | N/A | 506 | 506 | N/A | None | Recurrent stroke |
Abbreviations: Rx, pharmacotherapy; IS, ischemic stroke; TIA, transient ischemic attack; NRT, nicotine replacement therapy; CO, carbon monoxide; SAH, subarachnoid hemorrhage; ICU, intensive care unit; DCI, delayed cerebral ischemia.
For studies that included patients with and without cerebrovascular disease, the numbers reported here are only for subgroups with cerebrovascular disease.
Randomized patients to a cost-free pharmacotherapy or provision of a prescription for pharmacotherapy. More patients in the cost-free group initiated and complied with pharmacotherapy.
Rates of angiographic and clinical vasospasm were reported.
This study included patients admitted to a neurosurgical ICU. Only data for patients with SAH were included for this systematic review. We assumed that all of the reported occurrences of vasospasm occurred in patients with SAH.
This study evaluated the risk of stroke after NRT prescription; in a secondary analysis, the outcome was recurrent stroke among patients with prior stroke. Only data pertaining to the latter analysis were included here.
Table 2.
GRADE Quality Assessment of Included Studies and Summary of Findings, By Outcome of Interest
| Studies | Design Quality | Study Quality | Consistency | Directness | Other Factors* | Overall Quality† | Summary of Findings |
|---|---|---|---|---|---|---|---|
| Efficacy – Smoking Cessation | |||||||
| Frandsen Papadakis Lee Çelik | Low quality – pilot RCTs and observational data | No additional serious limitations | No important inconsistency | Uncertainty: co-interventions and non-distinct control group | Sparse data and high risk of bias in non-blinded randomized trials | Very low | Numerically increased cessation in individual studies with interventions including pharmacotherapy |
| Safety – Recurrent or Worsening Cerebrovascular Disease | |||||||
| Seder Carandang Panos Hubbard | Low quality – observational | No additional serious limitations | No important inconsistency | No uncertainty about directness | No other major limitations, and +1 level for strength of associations | Moderate | Less vasospasm with NRT, after adjusting for Fisher grade. DCI rates similar. Recurrent stroke not increased with NRT in one study. |
| Safety – Mortality | |||||||
| Seder Carandang | Low quality – observational | No additional serious limitations | No important inconsistency | No uncertainty about directness | Sparse data | Very low | Less in-hospital mortality and 3-month mortality (after adjusting for age, cerebral edema, grade) with NRT. |
| Safety – Clinical Outcomes | |||||||
| Carandang | Low quality – observational | No additional serious limitations | Data too sparse to assess consistency | Uncertainty: outcome measure | Sparse data | Very low | More good functional outcomes at 3 months with NRT, after adjusting for severity and grade. |
| Safety – Seizures | |||||||
| Seder | Low quality – observational | No additional serious limitations | Data too sparse to assess consistency | Uncertainty: outcome measure | No other major limitations | Low | More seizures with NRT in single observational study. |
| Safety – Neuropsychiatric Events (Delirium) | |||||||
| Seder | Low quality – observational | No additional serious limitations | Data too sparse to assess consistency | Uncertainty: outcome measure | No other major limitations | Low | More delirium with NRT in single observational study. |
Abbreviations: GRADE, Grading of Recommendations, Assessment, Development, and Evaluation; RCT, randomized clinical trial; NRT, nicotine replacement therapy; DCI, delayed cerebral ischemia.
Other factors are: imprecision/sparse data, strength of association, bias, dose-response gradient, possibility of residual confounding.
Overall quality determined after iteratively up- or down-grading quality based on study quality, consistency, directness, and other factors, after assigning an initial quality rating based on design quality.


