Abstract
Background
According to clinical guidelines, dopamine agonists are the first‐line treatment of restless legs syndrome (RLS).
Objectives
To evaluate efficacy and safety of dopamine agonists for RLS.
Search methods
We searched the Cochrane Central Register of Controlled Trials (The Cochrane Library 2008, Issue 4), MEDLINE, EMBASE, PsycINFO and CINAHL, from January 1985 to December 2008, plus reference lists of articles. We contacted pharmaceutical companies.
Selection criteria
We included double‐blind randomised controlled trials (RCTs) of dopamine agonist treatment versus placebo or other treatment for a period of at least seven days in patients with RLS (≥ 18 years). Outcomes included the International RLS Severity Rating Scale (IRLS), Clinical Global Impressions (CGI‐I), polysomnography and self rated sleep quality, quality of life, daytime functioning, and safety parameters.
Data collection and analysis
Two reviewers extracted data separately; assessed risk of bias; and contacted pharmaceutical companies and authors for additional information. We collected dropout rates due to adverse events and experience of adverse events.
Main results
We included 35 placebo controlled and three active controlled RCTs (N = 7365). The mean reduction on the IRLS was −5.7 points lower in dopamine agonist treatment compared to placebo (95% confidence interval (CI) −6.7 to −4.7). Periodic limb movements in sleep per hour of sleep (PLMS‐Index; PLMSI) were −22.4/h lower than in placebo (95% CI −27.8 to −16.9). Self rated quality of sleep and disease specific quality of life were improved by a standardised mean difference (SMD) of 0.40 (95% CI 0.33 to 0.47) and 0.34 (95% CI 0.23 to 0.44), respectively. Patients were more likely to drop out (odds ratio (OR) 1.82, 95% CI 1.35 to 2.45) and experienced more adverse events under dopamine agonist treatment than with placebo (OR 1.82, 95% CI 1.59 to 2.08). Visual inspection of forest plots showed the highest efficacy in three studies investigating cabergoline and pergolide (N = 3). Active controlled trials investigated effects of cabergoline, pergolide, and pramipexole in a number of outcomes. The IRLS score was lower with cabergoline and pramipexole compared to levodopa (MD −5.3, 95% CI −8.4 to −2.1). Only four studies investigated treatment efficacy up to seven months. The most severe side effect, augmentation, was not assessed reliably.
Authors' conclusions
The meta‐analyses show the superiority of dopamine agonists over placebo in RCTs up to seven months. Cabergoline and pramipexole showed larger efficacy compared to levodopa in some but not all outcomes.
Keywords: Humans, Benzothiazoles, Benzothiazoles/therapeutic use, Cabergoline, Dopamine Agonists, Dopamine Agonists/therapeutic use, Ergolines, Ergolines/therapeutic use, Levodopa, Levodopa/therapeutic use, Pergolide, Pergolide/therapeutic use, Pramipexole, Randomized Controlled Trials as Topic, Restless Legs Syndrome, Restless Legs Syndrome/drug therapy, Severity of Illness Index
Plain language summary
Dopamine agonists for restless legs syndrome
Restless legs syndrome (RLS) is a sensorimotor disorder characterised by an urge to move the limbs which is usually associated with unpleasant sensations. Symptoms are worse during rest, in the evening, and at night and improve by movement. The course of the disorder is usually chronic. Dopamine agonists are recommended as first‐line treatment for RLS.
We could include 38 trials in the meta‐analyses which investigated the efficacy and safety of dopamine agonist treatment compared to placebo or to other treatments for RLS. The studies were performed mostly in European and Northern American countries. Treatment durations varied from one week to seven months, but most treatments had durations of one to 12 weeks. Patients suffered from moderate to severe RLS and were treated with the dopamine agonists cabergoline, lisuride, pergolide, pramipexole, ropinirole, rotigotine, and sumanirole.
Dopamine agonists lead to a larger improvement on the International RLS Severity Rating Scale (IRLS) compared to placebo. Clinicians rated RLS symptoms as more improved with dopamine agonists compared to placebo (CGI‐I). Also periodic limb movements in sleep were significantly reduced by dopamine agonists compared to placebo. Sleep efficiency was also slightly improved. Patients rated their quality of sleep and quality of life as markedly improved. Patients were, however, more likely to discontinue dopamine agonist treatment and experienced more adverse events when treated with dopamine agonists compared to placebo. All dopamine agonists were superior to placebo except sumanirole. Indirect descriptive comparisons revealed the highest efficacy for the ergoline dopamine agonists cabergoline and pergolide, which has to be weighed against potentially serious side effects such as cardiac valve fibrosis. The non‐ergoline dopamine agonists lisuride, pramipexole, rotigotine, and ropinirole showed adequate efficacy.
Augmentation, a serious adverse event in dopaminergic treatment, has not been sufficiently assessed. Future studies need to investigate long‐term efficacy of dopamine agonists against placebo or other active treatment and the frequency and the impact of augmentation on treatment outcome during dopaminergic treatment.
Summary of findings
Summary of findings for the main comparison. Summary of findings: dopamine agonists versus placebo.
| Dopamine agonists compared with placebo for restless legs syndrome | ||||||
|
Patient or population: patients with restless legs syndrome according to IRLSSG, 18 years or older Settings: outpatient settings in Europe, North America and Japan. Intervention: treatment with dopamine agonists for at least seven days Comparison: placebo treatment for at least seven days | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Dopamine agonists | |||||
|
1 IRLS range: 0 to 40 (= severe) |
The mean IRLS change from baseline ranged across control groups from ‐1.8 to ‐13.4. | The mean IRLS change from baseline in the intervention groups was ‐5.74 larger (95% CI ‐6.74 to ‐4.74). | 6380 (30 studies) |
+++O moderate |
Inconsistent results (I² = 75%) which can be explained partly by medication subgroups and possibility of publication bias. | |
|
2 Periodic limb movements in sleep (PLMS Index) PLMS per hour of sleep (8 trials) or time in bed (7 trials) |
The mean PLMS Index change ranged across control groups from 21 to ‐16.6. | The mean PLMS Index change in the intervention groups was ‐22.86 larger (95%CI ‐28.3 to ‐17.41). | 1141 (15 studies) |
+++O moderate |
Inconsistent results (I² = 73%) which can be explained partly by medication subgroups and possibility of publication bias. | |
|
3 Sleep efficiency Percentage of total sleep time per time in bed |
The mean sleep efficiency change ranged across control groups from 0.6 to 6.1 percent. | The mean sleep efficiency change in the intervention groups was 4.61 percent larger (95% CI 2.14 to 7.04). | 677 (11 studies) |
++++ high |
||
|
4 Number of dropouts due to adverse events |
38 per 1000 | 66 per 1000 (50 to 86) | OR 1.82 (1.35 to 2.45) | 7054 (34 studies) |
++++ high |
|
|
5 Clinical Global Impressions ‐ Improvement of condition (CGI‐I) Rating of 1 = very much improved to 7 = very much worse |
50 per 100 | 72 per 100 (67 to 77) |
RR 1.44 (1.34 to 1.54) | 6338 (27 studies) |
++++ high |
|
|
6 Subjective quality of sleep SMD on questionnaires MOS, RLS‐6, PSQI, VAS |
No comparable data can be given for placebo group as SMDs of different questionnaires were calculated. | The mean subjective quality of sleep in the intervention groups had a SMD of 0.40 (95% CI 0.33 to 0.47). | 4592 (22 studies) |
++++ high |
||
|
7 Quality of life SMD on 2 RLS‐QoL questionnaires |
The mean subjective quality of life in the intervention groups had a SMD of 0.34 (95% CI 0.23 to 0.44). | 4312 (17 studies) |
+++O moderate |
Inconsistent results (I² = 61%) which can be explained partly by medication subgroups. No comparable data can be given for placebo group as SMDs of different questionnaires were calculated. |
||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds Ratio; RR: Risk Ratio; SMD: standardised mean difference | ||||||
GRADE Working Group grades of evidence High quality (++++): Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality (+++O): Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality (++OO): Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality (+OOO): We are very uncertain about the estimate.
Summary of findings 2. Summary of findings: dopamine agonists versus levodopa.
| Dopamine agonists compared with levodopa for restless legs syndrome | ||||||
|
Patient or population: patients with restless legs syndrome Settings: outpatient settings in Europe Intervention: treatment with dopamine agonists cabergoline, pergolide, pramipexole for at least seven days Comparison: treatment with levodopa for at least seven days | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Levodopa | Other dopamine agonists | |||||
|
1 IRLS range: 0 to 40 (= severe) |
The mean IRLS change ranged across levodopa groups from ‐4.4 to ‐9.55. | The mean IRLS change in the intervention groups was ‐5.25 larger (95% CI ‐8.40 to ‐2.10). | 383 (2 studies) | +++O moderate |
Estimated effect based on only two studies. | |
|
2 Periodic limb movements per hour of time in bed |
The mean PLMI was ‐7.7 in the levodopa group. | The mean PLMI in the pramipexole was ‐3.80 larger (95% CI ‐9.08 to 1.48, P = 0.16). | 39 (1 study) | ++OO low |
Methods of the study were not sufficiently reported. Estimated effect based on only one study. Treatment difference was not significant. |
|
|
3 Number of drop outs due to adverse events |
2 per 100 | 3 per 100 (2 to 5) | OR 1.70 (95% CI 0.96 to 3.01, P = 0.07) | 504 (3 studies) |
+++O moderate | Treatment difference was not significant and the result shows no to a significant effect. |
|
4 Clinical Global Impressions ‐ Improvement of condition (CGI‐I) Rating of 1 = very much improved to 7 = very much worse |
58 per 100 | 72 per 100 (58 to 72) | RR 1.19 (95% CI 0.91 to 1.56) | 422 (2 studies) | +++O moderate | Treatment difference was not significant and the result shows no to a significant effect. |
|
5 Subjective quality of sleep RLS‐6; scale satisfaction with sleep: 0 to 10 (= low satisfaction) |
The mean change in satisfaction with sleep was ‐2.8 in levodopa. | The mean change in satisfaction with sleep in cabergoline was ‐0.63 larger (95% CI ‐1.35 to 0.09, P = 0.09). | 344 (1 study) | ++OO low |
Estimated effect based on only one study. Treatment difference was not significant. |
|
|
6 Quality of life RLS‐QoL: 0 to 60 (= severe impairment) |
The mean change in quality of life was ‐10.38 in levodopa. | The mean difference in RLS‐QoL in the intervention group was ‐5.54 larger (95% CI ‐8.43 to ‐2.65). | 314 (1 study) | +++O moderate | Estimated effect based on only one study. | |
|
7 Number of patients experiencing adverse events |
54 per 100 | 72 per 100 (63 to 80) | OR 2.87 (95% CI 0.43 to 19.00) | 461 (3 studies) | +++O moderate | Treatment difference was not significant and the result shows no to a significant effect. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds Ratio; RR: Risk Ratio | ||||||
GRADE Working Group grades of evidence High quality (++++): Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality (+++O): Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality (++OO): Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality (+OOO): We are very uncertain about the estimate.
Summary of findings 3. Summary of findings: subgroups of dopamine agonists on IRLS.
| Subgroups of dopamine agonists compared with placebo for restless legs syndrome | ||||||
|
Patient or population: patients with restless legs syndrome Settings: outpatient settings in Europe, North America, Australia and Japan Intervention: treatment with dopamine agonists for at least seven days Comparison: treatment with placebo for at least seven days | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Dopamine agonists | |||||
|
IRLS all dopamine agonists range: 0 to 40 (= severe) |
The mean IRLS ranged across control groups from ‐1.8 to ‐13.4. | The mean IRLS in the intervention groups was ‐5.74 larger (95% CI ‐6.74 to ‐4.74). | 6380 (30 studies) | +++O moderate |
Inconsistent results (I² = 75%) which can be explained partly by medication subgroups and possibility of publication bias. | |
|
1 IRLS cabergoline |
The mean IRLS ranged across control groups from ‐3.3 to ‐7.9. | The mean IRLS in the intervention groups was ‐11.49 larger (95% CI ‐15.14 to ‐7.84). | 127 (2 studies) | ++++ high |
||
|
3 IRLS lisuride |
The mean IRLS ranged across control groups from ‐6.9 to ‐8.2. | The mean IRLS in the intervention groups was ‐8.00 larger (95% CI ‐10.28 to ‐5.72). | 378 (2 studies) | ++++ high |
||
|
2 IRLS pergolide |
The post mean IRLS was 23.2 in the control group. | The mean IRLS in the intervention group was ‐11.70 larger (95% CI ‐14.8 to ‐8.6). | 97 (1 study) | ++++ high |
||
|
4 IRLS pramipexole |
The mean IRLS ranged across control groups from ‐5.7 to ‐12.2. | The mean IRLS in the intervention groups was ‐5.16 larger (95% CI ‐6.87 to ‐3.45). | 2256 (8 studies) | +++O moderate |
Inconsistent results (76%) in pramipexole trials. | |
|
5 IRLS ropinirole |
The mean IRLS ranged across control groups from ‐0.3 to ‐13.4. | The mean IRLS in the intervention groups was ‐4.19 larger (95% CI ‐5.4 to ‐2.97). | 2301 (11 studies) | +++O moderate |
Inconsistent results (58%) in ropinirole trials. | |
|
6 IRLS rotigotine |
The mean IRLS ranged across control groups from ‐8.0 to ‐9.9. | The mean IRLS in the intervention groups was ‐6.98 larger (95% CI ‐8.99 to ‐4.96). | 958 (5 studies) | ++++ high |
||
|
7 IRLS sumanirole |
The mean IRLS was ‐10.1 in the control group. | The mean IRLS in the intervention group was ‐1.83 larger (95% CI ‐4.71 to 1.05). | 263 (1 study) | ++++ high |
||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; I²: Inconsistency | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Background
Description of the condition
Restless legs syndrome (RLS) ‐ previously called 'the most common disorder you never heard of' ‐ is a frequent, though often under‐diagnosed, disorder with a high impact on sleep. The syndrome was first described in detail by Ekbom 1945. Obligatory diagnostic criteria were established half a century later by the International Restless Legs Syndrome Study Group (IRLSSG, Walters 1995). These criteria were revised at a consensus conference held at the National Institute of Health (Allen 2003). The essential criteria, supportive criteria, and associated features of the disease are summarised in Table 4.
1. Diagnosis criteria of restless legs syndrome.
| Essential criteria |
| 1. An urge to move the legs, usually accompanied or caused by uncomfortable and unpleasant sensations in the legs (sometimes the urge to move is present without the uncomfortable sensations and sometimes the arms or other body parts are involved in addition to the legs). 2. The urge to move or unpleasant sensations begin or worsen during periods of rest or inactivity such as lying or sitting. 3. The urge to move or unpleasant sensations are partially or totally relieved by movement, such as walking or stretching, at least as long as the activity continues. 4. The urge to move or unpleasant sensations are worse in the evening or night than during the day or only occur in the evening or night (when symptoms are very severe, the worsening at night may not be noticeable but must have been present previously). |
| Supportive criteria and associated features of RLS |
|
Table 1: Diagnosis criteria of restless legs syndrome
| Essential criteria |
| 1. An urge to move the legs, usually accompanied or caused by uncomfortable and unpleasant sensations in the legs (sometimes the urge to move is present without the uncomfortable sensations and sometimes the arms or other body parts are involved in addition to the legs). 2. The urge to move or unpleasant sensations begin or worsen during periods of rest or inactivity such as lying or sitting. 3. The urge to move or unpleasant sensations are partially or totally relieved by movement, such as walking or stretching, at least as long as the activity continues. 4. The urge to move or unpleasant sensations are worse in the evening or night than during the day or only occur in the evening or night (when symptoms are very severe, the worsening at night may not be noticeable but must have been present previously). |
| Supportive criteria and associated features of RLS |
|
Epidemiological surveys in Western Europe and in the USA indicate that up to 10% of the population are afflicted with RLS. In females the prevalence is twice as high as in males and increases with age (Berger 2004; Berger 2007; Högl 2003; Phillips 2000; Rothdach 2000; Ulfberg 2001). According to recent surveys, one third of the people reporting RLS symptoms (i.e. 2% to 3% of the population) are impaired by the symptoms and their sequelae and may be in need of medical treatment (Hening 2004a; Tison 2005).
Periodic limb movements while awake (PLMW) and during sleep (PLMS) are supporting features of the syndrome. PLMS are motor phenomena monitored during polysomnography and occur in approximately 80% of RLS patients (Montplaisir 1997). PLMS monitoring is routinely performed in polysomnography where a bilateral surface electromyogram of the anterior tibial muscles is recorded. Scoring of PLMS is carried out according to standard criteria (Bonnet 1993; Iber 2007; Zucconi 2006). PLMS also occur frequently in several other sleep disorders and may be present in subjects who do not complain of sleep disturbance. PLMS are also common in the elderly, but are seen more frequently in patients with RLS (Allen 2003; for an overview see Hornyak 2004). Although the presence of PLMS is not specific to RLS, an elevated (> 15/h) PLMS index (number of PLMS per hour of sleep, PLMSI; American Academy of Sleep Medicine 2005) is supportive of the diagnosis of RLS (Allen 2003). In 40% to 60% of cases there is a family history of the disorder, which suggests a genetic predisposition for RLS (Stefansson 2007; Winkelmann 2007). Generally, patients with positive family history experience an earlier onset of symptoms (before the age of 30 to 45 years) than patients without afflicted relatives. A positive response to levodopa also supports the diagnosis of RLS, with almost 90% of patients showing a 50% relief of symptoms when treated with this agent (Stiasny‐Kolster 2006).
Sleep disturbances are commonly associated with RLS and are usually the reason why patients seek medical advice (Hening 2004a). Sleep disturbances are also considered to be a feature of the full expression of the disorder. However, due to the frequent occurrence of sleep problems in other disorders and their limited occurrence in patients with milder RLS, they are not considered to be necessary for, or supportive of the diagnosis of RLS (Allen 2003). The natural course of the disorder varies greatly for those with milder RLS. For patients whose symptoms start early in adult life and who eventually seek treatment, typically, the severity and frequency of symptoms increase over time. Thus, the disorder is generally considered to be a chronic condition. Physical examinations usually do not result in pathological findings for patients with idiopathic (primary) RLS (i.e. of unknown cause). However, it is important for clinicians to look for factors that may exacerbate or trigger symptoms (secondary RLS). Beside the established causes of secondary RLS (e.g. end‐stage renal disease, pregnancy, and iron deficiency), an increasing number of conditions including several neurological diseases such as multiple sclerosis, polyneuropathy, and cerebellar ataxias seem to be associated with the disorder (Allen 2007; Connor 2008; Manconi 2004; Schöls 1998; Walters 2007).
Previous and current treatments aim for symptomatic relief of both the unpleasant sensations and the urge to move and thereby aim to improve sleep disturbance. Restless legs syndrome‐associated curtailment of sleep may result in daytime problems such as fatigue, tiredness, and impaired functioning, as well as impaired quality of life (Kushida 2007; Talati 2009).
Description of the intervention
Since the 1980s, therapy has focused on levodopa and dopamine agonists (Stiasny‐Kolster 2009; Trenkwalder 2008). In the past few years, several studies examining a variety of dopaminergic substances have been published. However, no controlled studies have yet investigated long‐term treatment effects (i.e. for more than a year). A few meta‐analyses have recently been undertaken and examined effects of dopaminergic medication such as levodopa and dopamine agonists on RLS (Baker 2008; Conti 2007; Hansen 2009; Quilici 2008; Talati 2009; Zintzaras 2010). Second‐line treatment options include antiepileptic drugs such as gabapentin, gabapentin enacarbil, and valproic acid as well as pregabalin and opioids (Eisensehr 2004; Garcia‐Borreguero 2002; Kushida 2009; Walters 1993; for overview see Conti 2008; Silber 2004). Although some of these agents are often used in the treatment of RLS (e.g. opioids), the number of studies investigating substances other than dopaminergic drugs is still limited.
How the intervention might work
The aetiology of the disorder is not sufficiently understood, but comprises a complex network system reflected in the many different topographical, genetic, and biochemical causes of RLS, either in isolation or in combination (Trenkwalder 2010). It is generally accepted that a dysfunction of the central nervous dopaminergic system may play a major role in those phenotypes with response to dopaminergic agents (Hening 2004b; Trenkwalder 2004). Dopaminergic neurotransmission can modulate neuronal interactions at very low doses, contributing to cortical plasticity. Neuromodulators such as norepinephrine, dopamine, and 5‐hydroxytryptamine (5‐HT) can also modulate spinal motor neuron excitability at least fivefold (Heckman 2009) interacting with both the motor and sensory system causing RLS symptoms. Differential responses of early and late flexor reflexes to dopaminergic agents and opioids combined with plasticity changes might explain why dopaminergic‐induced hyperexcitability can occur during augmentation in RLS (Paulus 2006). Brain iron storage may be involved in many phenotypes of RLS and also may interact with augmentation induced by dopaminergic therapy. Currently, it seems, that supplying iron is both a symptomatic and in some cases, i.e. pregnancy and iron deficiency anaemia, a curative way of treating RLS, although the mechanism of low brain iron in RLS is not yet understood. Other curative treatments for idiopathic RLS are not known.
Why it is important to do this review
Recent reviews have described the efficacy and safety of dopaminergic treatment for RLS. However, these meta‐analyses investigated either a selection of dopaminergic drugs or only a limited number of outcome parameters (Baker 2008; Conti 2007; Hansen 2009; Quilici 2008; Zintzaras 2010).
We undertook the present evaluation in order to systematically assess the therapeutic efficacy of all dopamine agonists investigated in RLS. Therefore, we used a pre‐reviewed study protocol which included searching several databases; assessing quality of all included studies; and the evaluation of a wide range of clinically relevant aspects of treatment effects.
Compared to previous reviews we included a higher number of studies and additional clinically relevant outcome parameters. We investigated the effects on symptom severity using the IRLS (International RLS Severity Rating Scale) and the CGI (Clinical Global Impressions). Furthermore, we thoroughly analysed effects of dopamine agonist treatment on polysomnography parameters. To assess treatment effects we evaluated changes in the PLMS index (PLMSI, see above) as well as sleep efficiency. Further comprehensive analyses included questionnaires on quality of sleep, daytime functioning, quality of life, and the patients’ global impression of change of the disorder (for description of the questionnaires see Table 5). Safety parameters such as dropout rates and major adverse events were recorded.
2. Summary of questionnaires in trials and their scoring.
| Questionnaire | Description | Measurement |
| IRLS | Symptom severity scale with a total score of 0 to 40 for 10 questions (rating 0 to 4; Walters 2003). | Mild RLS: 0 to 10 Moderate RLS: 11 to 20 Severe RLS: 21 to 30 Very severe RLS: 31 to 40 |
| Clinical Global Impressions ‐ Improvement Scale | Evaluation by clinician regarding improvement of condition (National Institute of Mental Health 1976). | Rating 1 (very much better) to 7 (very much worse) |
| SF‐A | Subscore of questions regarding subjective quality of sleep (Goertelmeyer 1985). | Rating 1 to 5 → transformed into SMD |
| PSQI | Index score of 18 questions regarding sleep of the past 4 weeks with a cut‐off score of ≥ 5 for "bad" sleepers (Buysse 1989). | 0 to 21 points → transformed into SMD |
| MOS Sleep Problems Index II | Index score of 6 questions regarding severity of sleep problems (Hays 2005). | Rating 0 (lowest) to 100 (highest) → transformed into SMD |
| MOS somnolence | Score of 3 questions regarding severity of sleep problems (Hays 2005). | Rating 0 (lowest) to 100 (highest) → transformed into SMD |
| RLS‐6 satisfaction with sleep | Question regarding satisfaction with sleep in the past 7 days (Kohnen 2004). | Rating 0 (totally satisfied) to 10 (totally unsatisfied) → transformed into SMD |
| RLS‐6 daytime tiredness | Question regarding daytime somnolence in the past 7 days (Kohnen 2004). | Rating 0 (not at all) to 10 (very tired) → transformed into SMD |
| John Hopkins RLS QoL | Total score of 10 questions investigating health related quality of life in RLS patients (Abetz 2005). | 0 (lowest) to 100 (highest) → transformed into SMD |
| QoL‐RLS | Total score of 12 questions investigating health related quality of life in RLS patients (6‐point Likert scale; Kohnen 2002). | 0 to 60 (high impairment) → transformed into SMD |
IRLS: International RLS Severity Rating Scale; SF‐A: Schlaffragebogen‐A; PSQI: Pittsburgh Sleep Quality Inventory; MOS: Medical Outcomes Study 12‐item Sleep Scale; John Hopkins RLS QoL: Restless Legs Syndrome Quality of Life questionnaire; QoL‐RLS: Restless Legs Syndrome Quality of Life questionnaire.
Objectives
To evaluate the efficacy and safety of dopamine agonists for the treatment of RLS compared to placebo and other active treatments.
Methods
Criteria for considering studies for this review
Types of studies
We included all double‐blind and randomised controlled trials (RCTs) investigating the treatment of RLS with a dopamine agonist versus placebo or another drug, enclosing trial designs with parallel groups as well as cross‐over trials.
Types of participants
Adult patients (18 years or older) had to have a diagnosis of primary or secondary RLS according to diagnostic criteria defined by the IRLSSG (Allen 2003; Walters 1995).
Types of interventions
The experimental intervention consisted of any dose or regimen of a dopamine agonist (DA) by any route (oral, intravenous, or transdermal) for a minimum of seven days. In the control intervention, either placebo or other comparative drugs were used.
Types of outcome measures
Endpoints had to be validated instruments. Divided into primary and secondary endpoints, studies had to present at least one of the following endpoints:
Primary outcomes
International RLS Severity Rating Scale (IRLS).
PLMSI (number of PLM per total sleep time or time in bed).
Sleep efficiency (total sleep time during time in bed).
Number of dropouts due to adverse events (safety parameter).
Secondary outcomes
Clinical Global Impressions ‐ Improvement (CGI‐I).
Self rated quality of sleep (description of the included questionnaires see below).
Disease‐specific quality of life (description of the included questionnaires see below).
Additional outcomes which were expected to be useful for explaining effects:
Patient Global Impressions (PGI).
Number of patients experiencing adverse events (safety parameter).
Number of patients with augmentation (according to the definition of Allen 2003; safety parameter).
Daytime tiredness (description of the included questionnaires see below).
Search methods for identification of studies
The following resources were used for identification of relevant studies in any language.
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library 2008, Issue 4), MEDLINE (January 1985 to December 2008), EMBASE (January 1985 to December 2008), PsycINFO (January 1985 to December 2008), and CINAHL (January 1985 to December 2008) to obtain all possibly relevant trials. As specified in the protocol, we excluded studies not using recently accepted diagnosis criteria from the meta‐analyses. We did not search the Cochrane Movement Disorders Group's Trials Register as this database had not been updated by the Cochrane Movement Disorders Group. The respective search strategies are displayed in the Appendices.
Searching other resources
We searched online databases for additional unpublished studies. We accessed the Internet sites www.clinicaltrials.gov, www.clinicalstudyresults.org, and those trial registers that were offered online by relevant pharmaceutical companies. These were the trial sites of Boehringer Ingelheim, GlaxoSmithKline, and Lilly. Table 6 lists numbers of studies retrieved in these searches. We checked recent reviews and the reference lists of all included studies for additional publications in any language. We contacted the first authors of the following trials: Earley 1998; Montplaisir 1999; Pieta 1998; Staedt 1997. We also contacted the following pharmaceutical companies for information regarding additional data and not yet published trials: Boehringer Ingelheim, GlaxoSmithKline, Hoffmann La‐Roche, Lilly, Pfizer, Axxonis, and Schwarz Pharma (UCB Group). We received information on studies until November 2009 and closed the database in December 2009.
3. Number of retrieved trials/ additional information for published trials in online searches.
| Online resource | Retrieved additional trials | Additional information for published trials |
| clinical trials | 8 | 9 |
| GlaxoSmithKline | 2 | 5 |
| Boehringer Ingelheim | 0 | 5 |
| Lilly | 0 | 0 |
| clinical study results | 0 | 0 |
Internet sites:
www.clinicaltrials.gov
www.gsk‐clinicalstudyregister.com
http://trials.boehringer‐ingelheim.com
www.lillytrials.com
www.clinicalstudyresults.org
Data collection and analysis
Selection of studies
Two reviewers (HS and MH, the latter with support of CL, see acknowledgments) reviewed independently all obtained references to assess their potential relevance. Subsequently, the selected studies were assessed for inclusion from the full text. Authorship and results were not blinded. In both steps, any disagreements were resolved by discussion.
Data extraction and management
Two reviewers (HS and MH, the latter with support of CL) independently extracted data using a prepared form, and afterwards they cross‐checked resulting data files to resolve any disagreements and errors.
Extracted data included diagnosis criteria, study type, numbers of patients in treatment groups, doses given and process of titration, age, gender, ethnicity, country of trial, duration of symptoms, duration of treatment, occurrence of adverse events, and dropouts due to adverse events.
All questionnaires which were used in any of the included studies are presented in Table 5.
In the majority of studies, RLS severity was assessed by the International RLS Severity Rating Scale (IRLS; Walters 2003), which is a validated severity rating scale with 10 items rated from 0 to 4 and a total score of 0 to 40. Scores of 1 to 10 represent mild, 11 to 20 moderate, 21 to 30 severe, and 31 to 40 points indicate very severe symptoms. Symptom improvement was furthermore investigated by assessing responder rates of the Patient Global Impressions scale (PGI; National Institute of Mental Health 1976) and responder rates of the Clinical Global Impressions – Improvement scale (CGI‐I; National Institute of Mental Health 1976). Self rated quality of sleep was assessed by the Sleep Problems Index II of the Medical Study Outcomes Sleep Questionnaire (MOS; Hays 2005), the scale “satisfaction with sleep” of the RLS‐6 Scales (Kohnen 2004), the scale “sleep quality” of the questionnaire Schlaffragebogen‐A (SF‐A; Goertelmeyer 1985), the Pittsburgh Sleep Quality Index (PSQI; Buysse 1989), and Visual Analogue Scales (VAS) assessing sleep quality. In a few studies, self rated quality of sleep was assessed by two questionnaires. We extracted data of the more frequently used scale. Polysomnography data assessing PLMSI and sleep efficiency were also evaluated. When the PLMSI was not assessed, number of periodic limb movements (PLM), including PLM during sleep and during wake, divided per hour of time in bed were extracted (PLMI). We extracted data from restless legs‐specific instruments assessing disease‐specific quality of life (Hopkins RLS‐QoL by Abetz 2005 and QoL‐RLS by Kohnen 2002). We assessed daytime tiredness based on the scales “somnolence” of the MOS, “daytime tiredness” of the RLS‐6 scales, and item 5 of the IRLS (tiredness or sleepiness related to RLS symptoms). Safety parameters such as dropout rates due to adverse events and number of patients experiencing adverse events were extracted.
Dichotomous data comprised the endpoints CGI‐I and PGI, dropout rates due to adverse events and number of patients experiencing adverse events. Continuous (interval‐scaled) data included baseline as well as end‐of‐treatment data and change from baseline means if available, together with respective standard deviations or standard errors.
Assessment of risk of bias in included studies
Two reviewers (HS, MH, the latter with support of CL) independently performed assessment of methodological quality using the Cochrane Collaboration's tool for assessing bias (Reviewer's Handbook, chapter 8). Resulting disagreements were resolved by discussion. Criteria such as randomisation, allocation concealment, and blinding were classified in each trial. Low to high risk of bias was assigned (Reviewer's Handbook). The results of each trial are displayed in the Characteristics of included studies section and are also presented together in Figure 1 and Figure 2. By consensus, CT, RK, MH, and HS decided upon the quality of the evidence of each outcome (see Table 1; Table 2; Table 3 for results).
1.
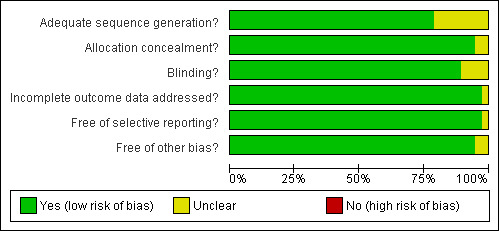
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
2.
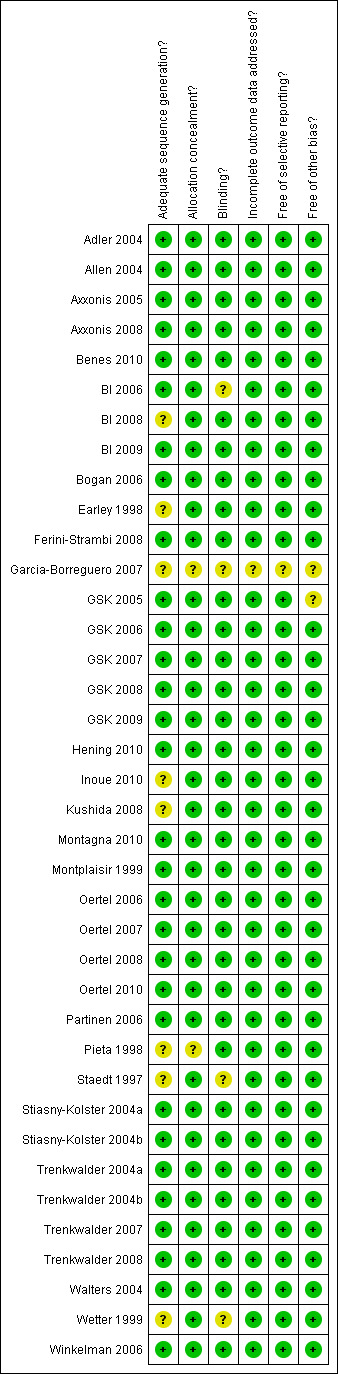
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Measures of treatment effect
Dichotomous data
We converted responder rates on the PGI and CGI‐I scales into risk ratios with 95% confidence intervals. Risk ratios above 1 indicate a better response with treatment than placebo or other active drug.
We analysed safety parameters such as the number of dropouts due to adverse events and the number of patients experiencing adverse events using odds ratios (OR) with 95% confidence intervals. Odds ratios above 1 indicate more frequent negative events in the treatment group compared to negative events in the placebo or other active group.
Continuous data
We analysed continuous (interval‐scaled) data of questionnaires using mean differences with 95% confidence intervals for the primary outcomes IRLS score, PLMSI, and sleep efficiency. Negative mean differences indicate a better response in the treatment group for IRLS and PLMSI, positive mean differences indicate a better response in the treatment group for sleep efficiency. In trials with multiple treatment arms, we pooled results of all treatment arms in order to compare the overall treatment effect to the placebo effect.
We computed standard errors of mean differences from reported analysis (paired t‐tests) in the cross‐over trials when feasible (in trials Adler 2004; BI 2006; Pieta 1998; Wetter 1999).
The secondary outcomes quality of sleep, quality of life, and daytime tiredness were assessed with different questionnaires in the included trials. Therefore, we calculated standardised mean differences (SMD) for these outcomes, i.e. Hedges' adjusted g, with a confidence interval of 95%. Standardised mean differences include values of 0.2 representing a small effect, 0.5 representing a moderate effect, and 0.8 indicating a large effect (Cohen 1988). Positive values for quality of sleep and quality of life and negative values for daytime tiredness indicate superiority of treatment over placebo.
Unit of analysis issues
Six of the included studies were double‐blind randomised cross‐over trials. Nine of the included studies used also minimum treatment doses, i.e. doses that fell out of the range of usually administered doses in routine care. Minimum doses were excluded from meta‐analyses and only recommended doses were pooled. The minimum doses were defined by consensus (CT, MH, RK, HS) for lisuride (2.5 mg/24 h), pramipexole (0.125 mg), and rotigotine (0.5 mg/24 h and 1.0 mg/24 h). In three studies, the dopamine agonists cabergoline, pergolide, and pramipexole were compared to levodopa treatment, respectively.
Dealing with missing data
When available, we extracted data from intention‐to‐treat analysis including the last observation carried forward (LOCF). In seven, mainly cross‐over trials, we had to revert to the reported per protocol data.
We aimed to obtain additional information for 37 of 38 included trials. Therefore, we contacted pharmaceutical companies (Axxonis, Boehringer Ingelheim, GlaxoSmithKline, Lilly, Pfizer, and Schwarz Pharma) and authors of the following trials: Adler 2004; Earley 1998; Montplaisir 1999; Pieta 1998; Staedt 1997.
Assessment of heterogeneity
We used Chi² tests to measure statistical heterogeneity of study results. The implemented I² statistics give an estimate of the degree of this heterogeneity. Values of 0% to 40% represent low heterogeneity; values of 30% to 60% represent moderate heterogeneity, whereas values of 50% to 90% may indicate substantial, and 75% to 100% considerable heterogeneity according to the Cochrane Handbook for Systematic Reviews of Interventions (Reviewer's Handbook, chapter 9).
Assessment of reporting biases
We examined funnel plots and investigated asymmetry coefficients to identify possible publication bias (Egger 1997).
Data synthesis
We chose random‐effects models to pool the data, as we could not expect any common underlying effect due to the diversity of study populations and medications. As analyses of covariance were used in most trials, we applied the generic inverse variance method for continuous outcomes. As we could obtain means and standard deviations for the calculation of SMDs, we used the inverse variance method for SMDs. For dichotomous outcomes, we applied odds ratios with the Mantel‐Haenszel method for safety parameters and risk ratios with the Mantel‐Haenszel method for the two responder outcomes.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses with each dopamine agonist in order to investigate efficacy and safety of each dopamine agonist treatment separately, and to identify sources of heterogeneity of treatment effects. We visually inspected treatment effects and their confidence intervals in indirect comparisons in order to observe this heterogeneity between the dopamine agonist subgroups. In order to investigate methodological heterogeneity, we also assessed the influence of study type on treatment effects by visual inspection (cross‐over trial versus parallel group trial). Separate meta‐analyses were performed for trials with active comparator treatments.
We assessed the influence of study quality on the heterogeneity of IRLS treatment effects by comparing treatment effects of studies with low risk of bias to those with unclear risk of bias (bias category ‘randomisation’, see above). The comparison was only made for this bias category, as in other bias categories, possible risk of bias was present in very few studies.
We exploratively investigated the influence of treatment duration on the IRLS treatment effect in a meta‐regression analysis, since dopamine agonist treatment is a symptomatic and long‐term treatment for RLS. To analyse the possible reasons for heterogeneous treatment effects, we performed an additional meta‐regression to investigate the effect of number of study sites on IRLS treatment effect. We performed this explorative analysis as we were under the impression that small studies may show higher treatment effects. We hypothesized that with increasing number of study sites, patient populations become more heterogeneous. Lastly, we assessed the influence of the mean baseline IRLS score on IRLS treatment effect. To this end we performed univariable meta‐regressions with each possible predictor separately as well as a multivariable meta‐regression including all three predictors (treatment duration, number of study sites, and mean baseline IRLS).
In order to investigate heterogeneous IRLS treatment effects, an additional explorative subgroup analysis was performed. As over half of the included studies were performed predominantly in European countries, the influence of predominantly European versus other study origin on IRLS treatment effect was evaluated.
Results
Description of studies
Results of the search
Overall, we obtained 501 English and non‐English publications searching the electronic databases. Sixty‐four of these publications were potentially eligible after screening of titles and abstracts. Twenty‐three fulfilled eligibility criteria after inspection of full texts and were included. The search in additional online databases yielded eight additional and unpublished trials and five trials which were listed in online databases and presented at scientific meetings. Two more trials were retrieved by checking reference lists. Thus, 38 trials were identified with 23 fully published trials, seven trials published as abstracts and partly online, and eight trials presented online. Information regarding additional trials and data was provided by Axxonis, Boehringer Ingelheim, GlaxoSmithKline, Pfizer, and Schwarz Pharma.
Included studies
Design and sample sizes
Overall, 38 randomised and double‐blind studies were included for this review comprising 7365 participants (ranging from eight to 402 per study). Thirty‐two of these studies were randomised parallel group trials (Allen 2004; Axxonis 2005; Axxonis 2008; Benes 2010; BI 2008; BI 2009; Bogan 2006; Earley 1998; Ferini‐Strambi 2008; Garcia‐Borreguero 2007; GSK 2005; GSK 2006; GSK 2007; GSK 2008; GSK 2009; Hening 2010; Inoue 2010; Kushida 2008; Montagna 2010; Oertel 2006; Oertel 2007; Oertel 2008; Oertel 2010; Partinen 2006; Stiasny‐Kolster 2004a; Stiasny‐Kolster 2004b; Trenkwalder 2004a; Trenkwalder 2004b; Trenkwalder 2007; Trenkwalder 2008; Walters 2004; Winkelman 2006). The other six trials were randomised cross‐over trials (Adler 2004; BI 2006; Montplaisir 1999; Pieta 1998; Staedt 1997; Wetter 1999). Twenty‐seven of the studies were published in peer reviewed journals; the remaining 11 studies were obtained from conference abstracts and partly from online registers. In the present review, these are referred to by the pharmaceutical company who initiated the study (Axxonis: Axxonis 2005; Axxonis 2008; Boehringer Ingelheim: BI 2006; BI 2008; BI 2009; GlaxoSmithKline: GSK 2005; GSK 2006; GSK 2007; GSK 2008; GSK 2009).
Patients received cabergoline in three studies, lisuride in two, and pergolide in five studies. Pramipexole was used in 10 trials, ropinirole in 12, and rotigotine in five trials. One trial investigated sumanirole, a dopamine agonist which has not been licensed. One of the lisuride trials compared treatment with lisuride to treatment with ropinirole and placebo.
Three of the 38 trials investigated dopamine agonist treatment against levodopa (BI 2006; Staedt 1997; Trenkwalder 2007).
Methods, patients, interventions, and relevant outcomes of all included trials are described in the Characteristics of included studies section.
Setting and location
Five studies (Adler 2004; Earley 1998; Montplaisir 1999; Partinen 2006; Staedt 1997) were conducted in one centre; all other studies were multi‐centre studies. Patients were recruited from outpatient clinic settings and private practices.
Studies were conducted in the USA (N = 11), Germany (N = 6), Canada (N = 1), Finland (N = 1), Japan (N = 1), Switzerland (N = 1), and the United Kingdom (N = 1). Multinational studies were conducted in European countries (N = 10), Northern American and European countries (N = 1) and Northern American countries (N = 1). A further four studies were conducted in Europe with additional study sites in Australia (N = 3) and Korea (N = 1).
Treatment duration in cabergoline studies varied from five to eight weeks. Both lisuride trials were conducted over a period of 12 weeks. Pergolide studies ranged from 10 days to six weeks and pramipexole studies from three to 12 weeks with one further trial lasting 26 weeks. Ropinirole trials had treatment durations of 12 weeks, with the exception of two studies with durations of four and 26 weeks, respectively. Rotigotine studies had treatment durations of seven days to seven weeks with two studies having durations of seven months. In the sumanirole trial, patients were treated for eight weeks.
Participants
Diagnosis of RLS was made according to the criteria defined by the International Restless Legs Study Group (IRLSSG; Allen 2003; Walters 1995) with exception of one study, in which diagnosis was not explicitly made according to valid criteria but used acceptable diagnostic criteria (Staedt 1997).
All studies but one (Pieta 1998, N = 8 uremic patients) included almost entirely patients with primary RLS. Symptom severity at baseline was moderate to very severe (mean baseline IRLS score ranging from a score of 21 (BI 2006) to 31.5 (Oertel 2006)).
Patients were 55.1 years old (mean), ranging from a mean of 42.5 years (Pieta 1998) to 60.5 years (Axxonis 2005). A mean percentage of 64.4 female patients participated in the trials, ranging from 45.5% (Staedt 1997) to 84% (GSK 2008).
Interventions
Study drugs were given orally with the exception of lisuride and rotigotine, which were applied transdermally using skin patches.
Flexible up‐titration to optimised dose was used in 21 trials. Forced up‐titration was performed either to one fixed level (Oertel 2006; Pieta 1998; Trenkwalder 2007), to the highest tolerated level (Adler 2004; GSK 2005; Inoue 2010; Montplaisir 1999), or to multiple doses investigated in multiple study arms (Axxonis 2005; BI 2008; Garcia‐Borreguero 2007; Oertel 2008; Partinen 2006; Stiasny‐Kolster 2004a; Stiasny‐Kolster 2004b; Trenkwalder 2008; Hening 2010; Winkelman 2006).
Maximum doses included 2.0 mg cabergoline in two studies. In the active controlled cabergoline study a maximum dose of 3.0 mg was used. Lisuride studies implemented 7.5 and 10.0 mg/48 h at the maximum. In pergolide studies a maximum dose of 0.25 mg was used in two studies, 0.65 mg in one, and 0.75 mg in two studies. In pramipexole trials, 0.25 mg was used in one study, 0.75 mg in eight, and 1.5 mg in one study at the maximum. In ropinirole studies, the maximum dose was 2.0 mg in one study, 4.0 mg in seven studies, and 6.0 mg in four studies. In rotigotine studies, maximum doses ranged from 2.0 mg/24 h (one study) over 3.0 mg/24 h (three studies) to 4.0 mg/24 h (one study). In the sumanirole study, 4.0 mg was used as the maximum dose. We excluded doses which are not recommended in practice, but which were included in dose‐finding studies using multiple treatment arms (see also methods).
Doses of the comparator drug levodopa in the three active controlled studies were 300 mg/75 mg levodopa/benserazide (Trenkwalder 2007), 300 mg/75 mg levodopa/benserazide dual release formulation (BI 2006), and 400 mg/100 mg levodopa/carbidopa (Staedt 1997).
Outcomes
The IRLS was widely used to assess severity of symptoms and therapeutic effect. Other scales were used for symptom assessment and evaluation of secondary outcomes as well as safety parameters. Polysomnography parameters included the PLMSI and sleep efficiency.
Excluded studies
We excluded 41 publications while checking the full text copies for eligibility. Nineteen reports were of trials which were published in full elsewhere. Four trials had a withdrawal design. Thirteen studies did not investigate patients in a randomised controlled design, four other publications were overviews on RLS and one study was not completed. Characteristics of excluded studies and reasons for exclusion are presented separately.
Risk of bias in included studies
We primarily report on quality of assessment of the primary outcomes IRLS, PLMSI, sleep efficiency, and dropout rates due to adverse events. All other outcomes included questionnaires and ratings and were, therefore, similar to the IRLS regarding the method of assessment.
Allocation
Randomisation included computer‐generated randomisation lists in 26 trials as stated in the study protocol or confirmed on request. Allocation of treatment was mostly performed with numbered packages and was partly based on an Interactive Voice Response System. Nine studies investigating PLMSI reported on randomisation procedure whereas six studies did not report sufficiently on that issue. In the majority of studies, allocation concealment was performed by dispensing numbered packages as described above. Adequate performance of randomisation could be assured in seven of 12 trials investigating sleep efficiency with reports of numbered packages in the majority of trials. The majority of studies contributing safety data reported adequately on randomisation and allocation concealment.
Blinding
Blinding of participants, investigators, and data analysts was sufficiently described in most trial reports for the IRLS and for dropout analysis. Blinded polysomnography rating was performed in 13 studies with insufficient information of blinding of polysomnography scoring in two more trials.
Incomplete outcome data
In most studies, the method of the last observation carried forward (LOCF) was used for inclusion of incomplete outcome data. Seven of the polysomnography studies reported per protocol data. In the majority of studies, the dropout rate from placebo and dopamine agonist treatment was not different. As all studies with high dropout rates (i.e. 20% to 25%) used the LOCF method, the likelihood of bias due to high numbers of dropouts can be considered low.
Selective reporting
The majority of studies investigated a wide range of questionnaire assessments regarding symptom severity, quality of sleep, daytime functioning, and disease‐specific quality of life as well as polysomnography parameters. In a few trials, not all implemented measures were reported in the final study report although they had been mentioned either in the protocol or in result reports accessible online.
Other potential sources of bias
Seven studies were supported by governmental and pharmaceutical grants, 30 studies were fully sponsored by pharmaceutical companies, and one study did not report on funding. We could not ascertain any other major source of bias.
Effects of interventions
See: Table 1; Table 2; Table 3
Comparison I: Dopamine agonists versus placebo
1a) Change on the severity scale IRLS
Thirty trials assessed change from baseline as measured by the IRLS. The mean difference (MD) was −5.74 points in favour of dopamine agonist treatment compared to placebo treatment (95% confidence interval (CI) −6.74 to −4.74). This comparison showed considerable heterogeneity (I² = 75%). Results of the only cross‐over trial overlapped with the overall mean difference (see comparison 1.1 and Figure 3).
3.
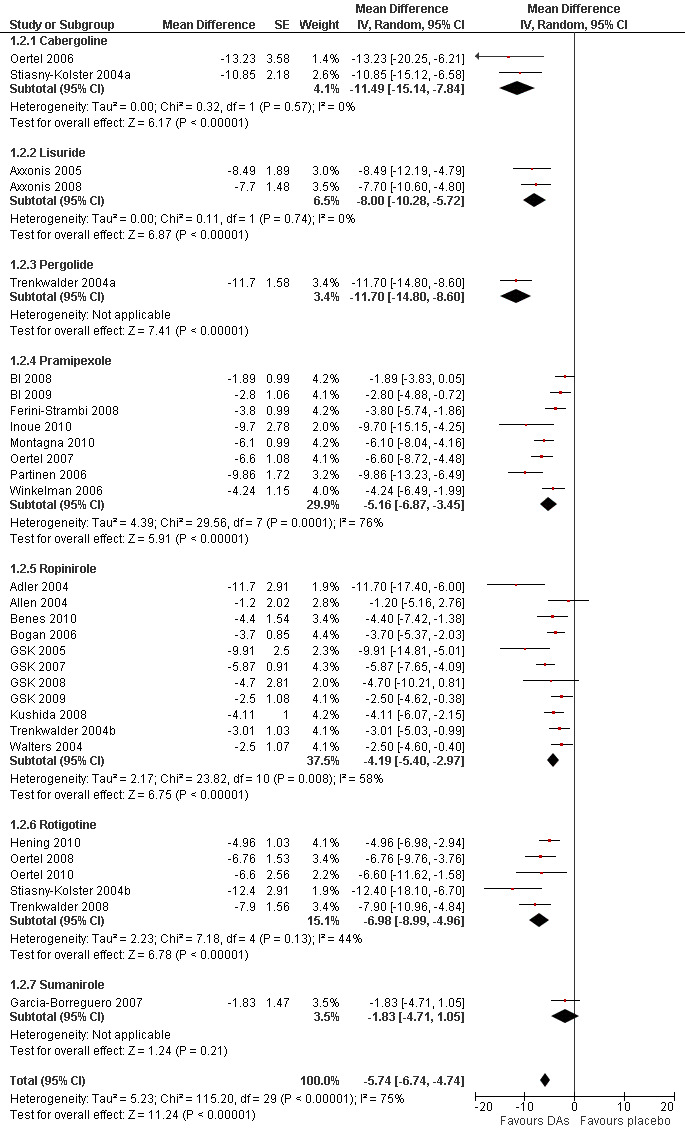
Forest plot of comparison: 1 Dopamine agonists versus placebo, outcome: 1.2 Medication subgroups: change on IRLS.
1b) Medication subgroup analysis of change on IRLS
The analysis of change from baseline on the IRLS revealed the following results in the medication subgroups (see also Figure 3 for all medication subgroups and Table 3): One pergolide and two cabergoline trials showed similarly high treatment differences between treatment and placebo with a MD of −11.70 for one pergolide trial (95% CI −14.8 to −8.6) and −11.49 points for the cabergoline trials (95% CI −15.14 to −7.84, I² = 0%). Trials using transdermal systems such as the lisuride (MD −8.0, 95% CI −10.28 to −5.72, I² = 0) and rotigotine trials also showed high treatment effects (MD −6.98, 95% CI −8.99 to −4.96, I² = 44%). Somewhat lower effects were found in pramipexole studies (MD −5.16, 95% CI −6.88 to −3.43, I² = 76%), followed by ropinirole studies (MD −4.19, 95% CI −5.4 to −2.97, I² = 58%). The sumanirole study showed a treatment difference of −1.83 points (95% CI −4.71 to 1.05).
Visual inspection of confidence intervals revealed that those of the subgroups cabergoline and pergolide showed the highest effects, those of pramipexole and ropinirole showed lower effects. No significant treatment effect was observed with sumanirole. The confidence interval of lisuride overlapped with those of cabergoline, pergolide, pramipexole, and rotigotine and was higher than that of ropinirole.
2a) Change in periodic limb movements in sleep index (PLMSI)
Data from 15 trials were analysed regarding the treatment effect of dopamine agonists on the PLMSI. Data of eight trials contributing PLM data per hour of total sleep time and seven trials including PLM data per hour of time in bed were pooled. Mean difference in reductions of PLMSI was −22.38 per hour of sleep (or hour in bed) when compared to placebo (95% CI −27.82 to −16.94, I² = 73%). Cross‐over trials were analysed separately and showed higher treatment effects over placebo than parallel group trials but confidence intervals overlapped slightly (see comparison 1.3 and Figure 4).
4.
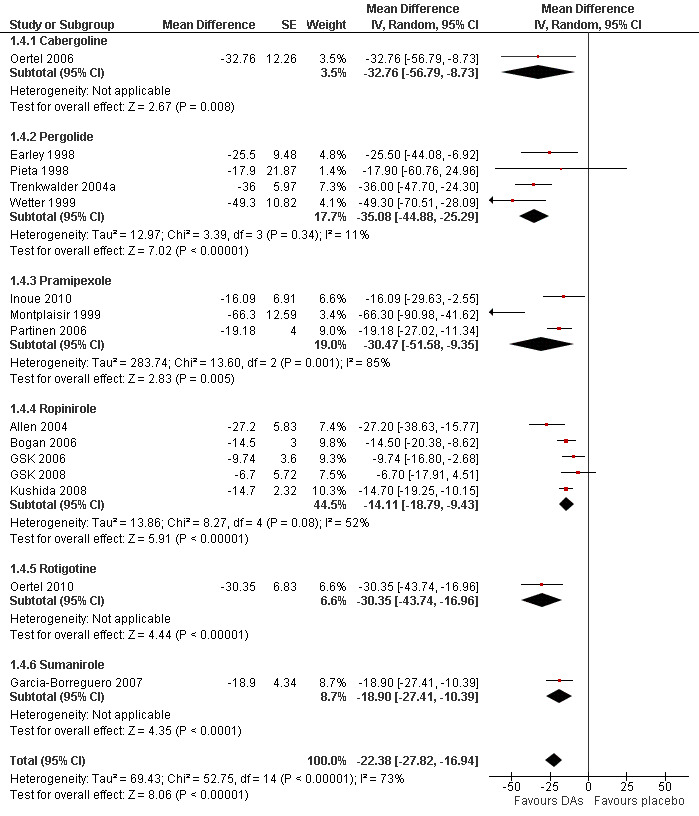
Forest plot of comparison: 1 Dopamine agonists versus placebo, outcome: 1.4 Medication subgroups: change in periodic limb movements in sleep.
2b) Medication subgroup analysis of change in periodic limb movements in sleep index (PLMSI)
Treatment difference was high with a wide confidence interval in one cabergoline trial (MD −32.76/h, 95% CI −56.79 to −8.73). In the other medication subgroups, larger effects resulted in pergolide (MD −35.08/h, 95% CI −44.88 to −25.29, I² = 11%), pramipexole (MD −30.47/h, 95% CI −51.58 to −9.35, I² = 85%), and in one rotigotine trial (MD −30.35/h, 95% CI −43.74 to −16.96). Somewhat lower effects resulted in the sumanirole trial (MD −18.90/h, 95% CI −27.41 to −10.39) and in ropinirole trials (MD −14.11/h, 95% CI −18.79 to −9.43, I² = 52%; see Figure 4 for all subgroups).
3a) Change in sleep efficiency
Eleven trials investigated sleep efficiency assessed in polysomnography. Mean difference of improvement in sleep efficiency was 4.53% favouring dopamine agonists (95% CI 2.00 to 7.06, I² = 48%) including heterogeneous effects in cross‐over trials (see comparison 1.5 and Figure 5).
5.
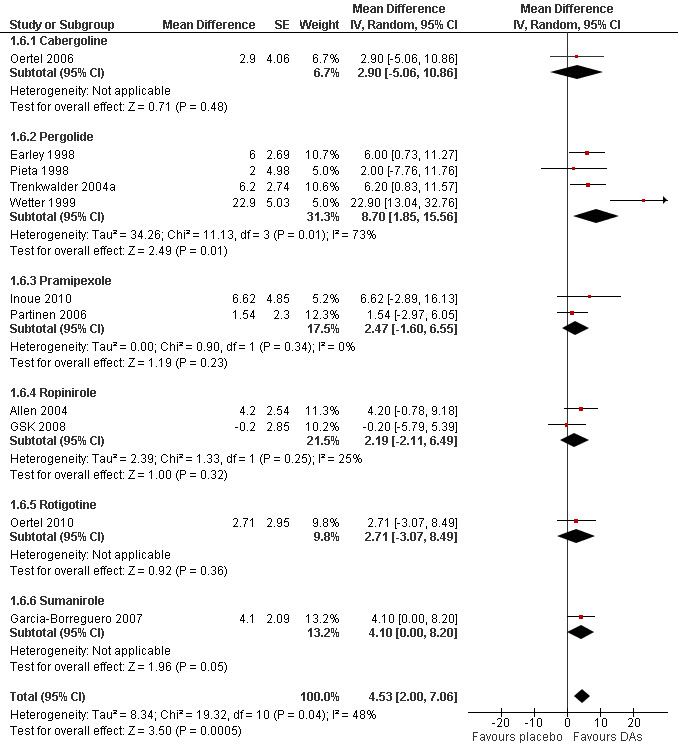
Forest plot of comparison: 1 Dopamine agonists versus placebo, outcome: 1.6 Medication subgroups: change in sleep efficiency.
3b) Medication subgroup analysis of change in sleep efficiency
Pergolide trials were heterogeneous (I² = 73%) with a mean difference of 8.7% (95% CI 1.85 to 15.86). Effects did not differ from placebo in the following medication subgroups: sumanirole (MD 4.1%), cabergoline (MD 2.9%), rotigotine (MD 2.71%), pramipexole (MD 2.47%), and ropinirole (MD 2.19%; see Figure 5).
4a) Number of dropouts due to adverse events
Thirty‐four trials assessed dropout rates due to adverse events. Patients were more likely to drop out of dopamine agonist treatment compared to placebo treatment ( OR 1.82, 95% CI 1.35 to 2.45, I² = 41%, see Figure 6). The assumed control group risk (median of dropouts in placebo treatments) of 38 dropouts in 1000 patients increases to 66 of 1000 patients dropping out of treatment when treated with dopamine agonists. This corresponds with a risk difference of 28 out of 1000 patients who would drop out of treatment due to adverse events when treated with a dopamine agonist compared to placebo treatment.
6.
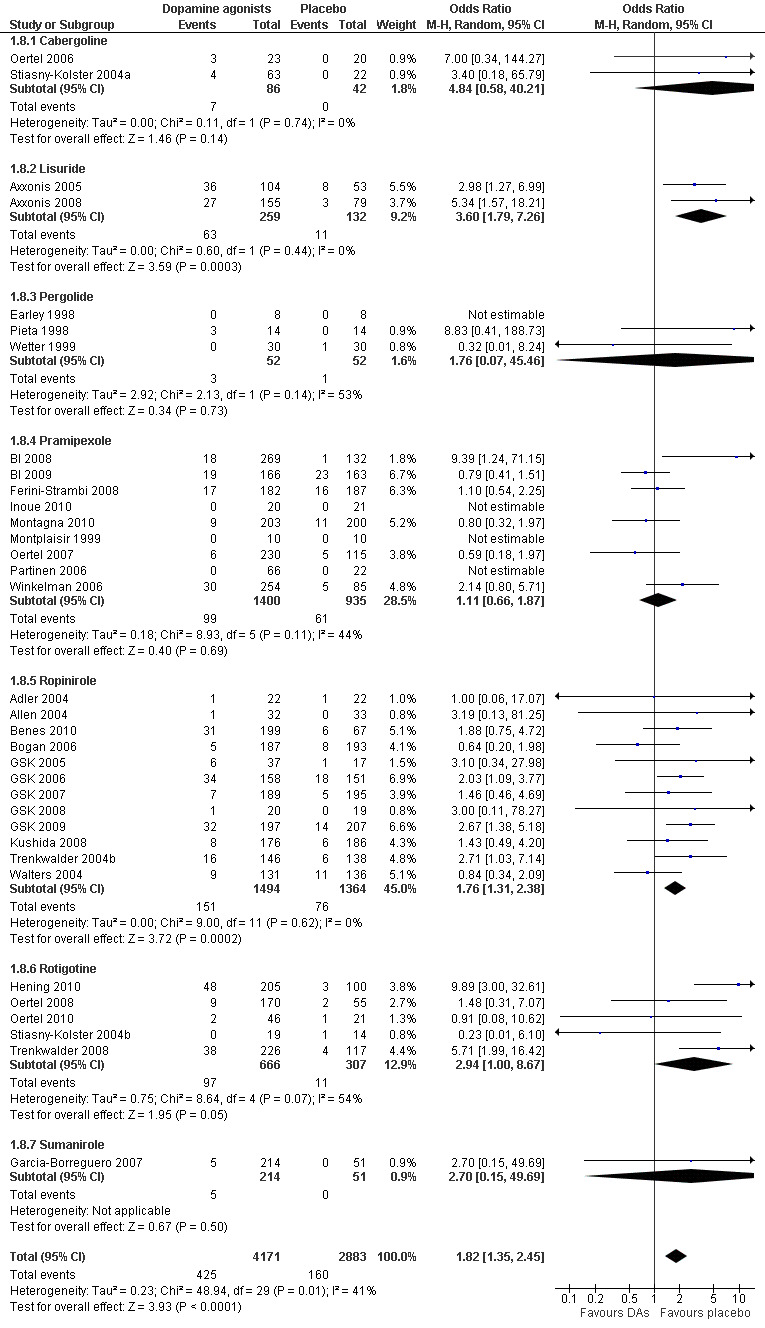
Forest plot of comparison: 1 Dopamine agonists versus placebo, outcome: 1.8 Medication subgroups: number of dropouts due to adverse events.
4b) Medication subgroup analysis of dropouts due to adverse events
In the medication subgroups cabergoline, pergolide, pramipexole, rotigotine, and the only sumanirole trial, there were no differences regarding dropout rates compared to placebo (see comparison 1.8 and Figure 6). Differences resulted in lisuride (OR 3.6, 95% CI 1.79 to 7.26) and ropinirole trials (OR 1.76, 95% CI 1.31 to 2.38) indicating elevated dropouts due to adverse events in active treatment compared to placebo treatment.
5a) Responder rates on CGI‐I
Twenty‐seven trials reported on CGI‐I. Patients treated with dopamine agonists responded to a greater extent on the CGI‐I than those on placebo treatment (Risk Ratio (RR) 1.44, 95% CI 1.34 to 1.54, I² = 49%, see Figure 7).
7.
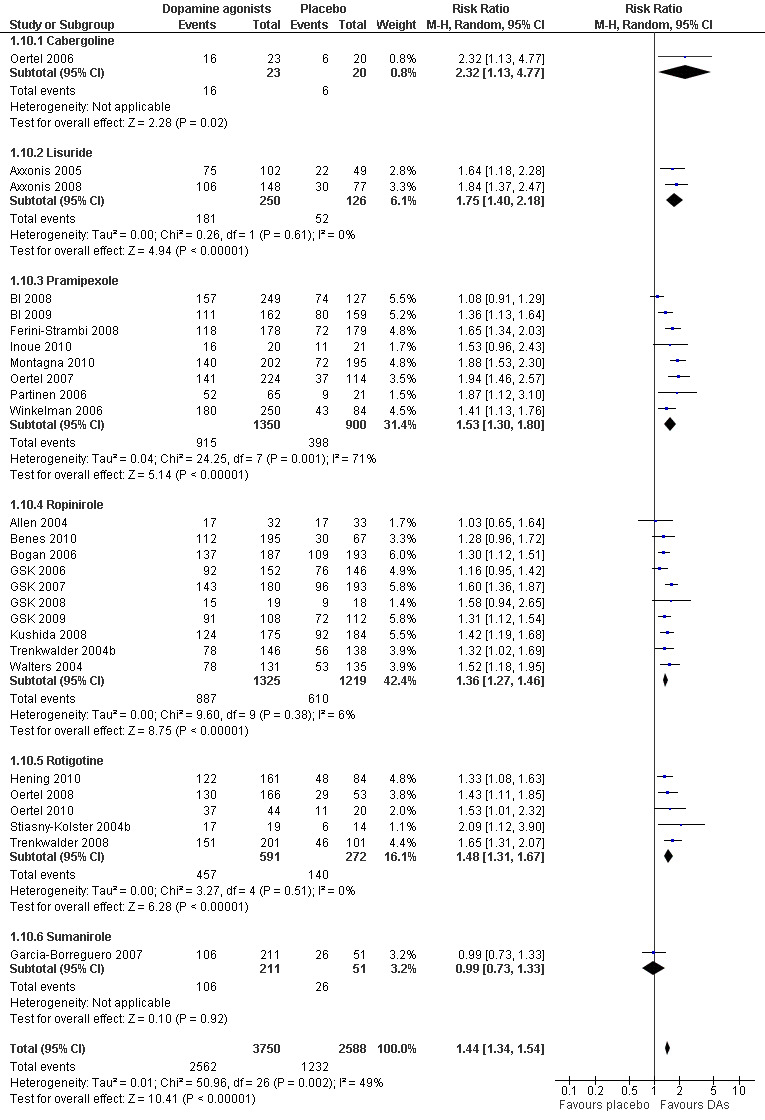
Forest plot of comparison: 1 Dopamine agonists versus placebo, outcome: 1.10 Medication subgroups: responder rates on CGI‐I.
5b) Medication subgroup analysis of responder rates on CGI‐I
Risk ratios of the subgroups ropinirole (RR 1.36), rotigotine (RR 1.48), pramipexole (RR 1.53, I² = 71%), and lisuride (RR 1.75) differed only slightly with the exception of one cabergoline trial showing a higher RR of 2.32 (95% CI 1.13 to 4.77) and the sumanirole trial showing no effect (see comparison 1.10 and Figure 7 for details).
6a) Change in self rated quality of sleep
Quality of sleep was investigated in 22 trials and improved more with dopamine agonists compared to placebo (standardised mean difference (SMD) 0.40, 95% CI 0.33 to 0.47, I² = 16%, see Figure 8).
8.
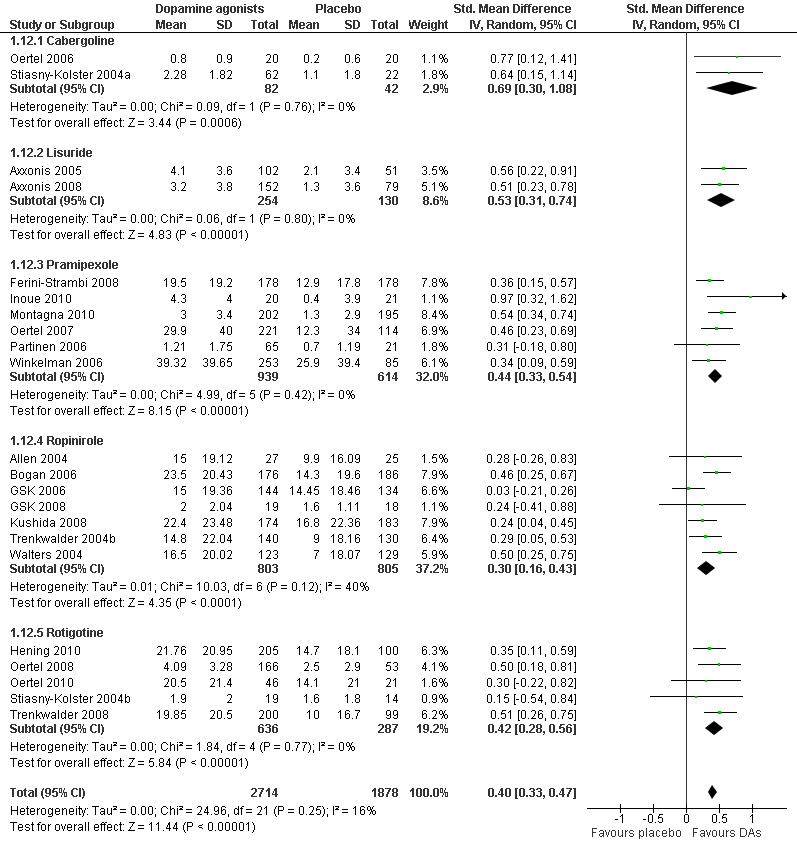
Forest plot of comparison: 1 Dopamine agonists versus placebo, outcome: 1.12 Medication subgroups: change in self‐rated quality of sleep.
6b) Medication subgroup analysis of change in self rated quality of sleep
When looking at medication subgroups, cabergoline, lisuride, pramipexole, rotigotine, and ropinirole trials showed larger treatment effects compared to placebo in descending order (see comparison 1.12, see Figure 8).
7a) Change in disease‐specific quality of life
Disease‐specific quality of life (QoL) was assessed in 17 trials by the QoL‐RLS (Kohnen 2002; N = 7) and the Hopkins RLS‐QoL (Abetz 2005; N = 10). The results showed a small effect of dopamine agonists over placebo (SMD 0.34, 95% CI 0.23 to 0.44, I² = 61%, see Figure 9).
9.
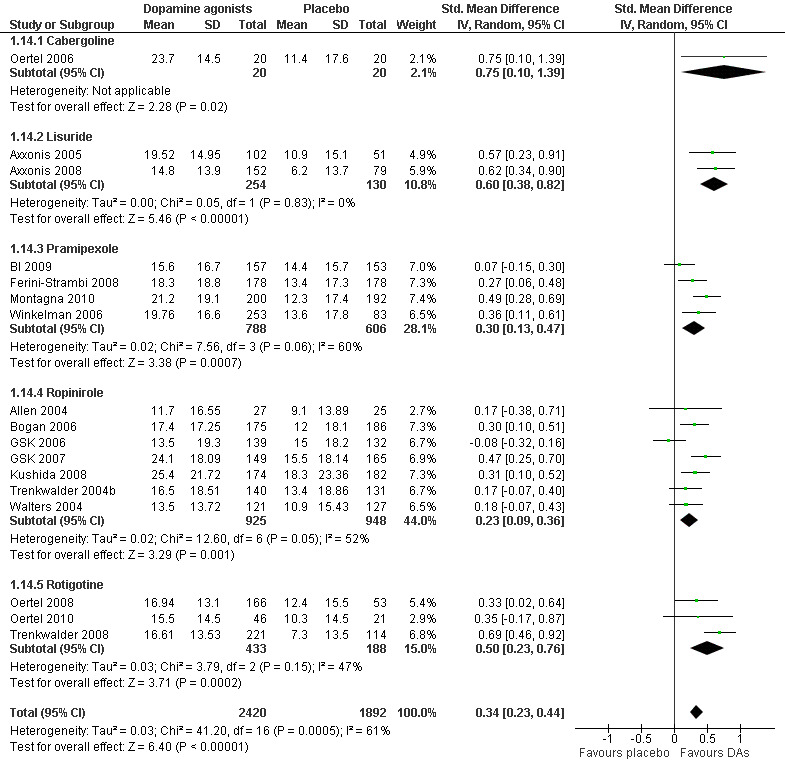
Forest plot of comparison: 1 Dopamine agonists versus placebo, outcome: 1.14 Medication subgroups: change in quality of life.
7b) Medication subgroup analysis of disease‐specific change in quality of life
One cabergoline study investigating QoL revealed a large treatment effect over placebo (SMD 0.75, 95% CI 0.10 to 1.39). Moderate effects resulted in two lisuride trials (SMD 0.60, 95% CI 0.38 to 0.82) and in rotigotine trials (SMD 0.50, 95% CI 0.23 to 0.76, I² = 47%). Pramipexole and ropinirole studies showed smaller effects on QoL with SMDs of 0.30 and 0.23 with substantial to moderate heterogeneity (I² = 60% and 52%, respectively; see comparison 1.14 and Figure 9).
8a) Responder rates on PGI
In 13 trials investigating PGI, patients were more likely to respond when treated with dopamine agonists (RR 1.53, 95% CI 1.34 to 1.75) but study results showed substantial heterogeneity (I² = 73%).
8b) Medication subgroup analysis of responder rates on PGI
One pergolide trial with PGI assessment obtained the highest RR (4.51) followed by a lower RR for pramipexole (1.57; I² = 74%), ropinirole (1.35; I² = 73%), and rotigotine trials (1.34; I² = 0%; see comparison 1.16). Divergent confidence intervals indicate markedly lower effects in pramipexole, ropinirole, and rotigotine compared to pergolide.
9a) Number of patients experiencing adverse events
In 33 trials reporting on this safety parameter, significantly more patients experienced adverse events when treated with dopamine agonists compared to placebo‐treated patients (OR 1.82, 95% CI 1.59 to 2.08, I² = 24%, see comparison 1.17).
9b) Medication subgroup analysis of number of patients experiencing adverse events
Study data of patients with adverse events showed significantly higher odds ratios in rotigotine (OR 2.41, I² = 2%), ropinirole (OR 2.07, I² = 12%), and pramipexole trials (OR 1.48, I² = 0%) than in placebo trials. The effects of lisuride, pergolide, and cabergoline did not significantly differ from those of placebo treatment. Cabergoline trials showed considerable heterogeneity (I² = 79%). In the only sumanirole trial, the numbers of patients with adverse events did not differ between treatment and placebo (see comparison 1.18).
10a) Change in daytime tiredness
In 21 trials assessing daytime tiredness, dopamine agonist treatment reduced daytime tiredness compared to placebo treatment (SMD −0.24, 95% CI −0.31 to −0.17, I² = 29%) representing a small effect (see comparison 1.19).
10b) Medication subgroup analysis of change in daytime tiredness
Largest treatment differences in daytime tiredness were shown in lisuride trials (SMD −0.47; I² = 36%). Lower treatment differences were seen in one cabergoline trial (SMD −0.31), pramipexole (SMD −0.25; I² = 75%), rotigotine (SMD −0.24, I² = 0%), and ropinirole trials (SMD −0.19; I² = 10%, see comparison 1.20).
10a) Augmentation
Augmentation was not reliably and comparably assessed in the included trials; therefore, we could not perform a meta‐analysis on this outcome.
Visual inspection of funnel plots and asymmetry coefficients showed a possibility of publication bias or small study effects for the outcomes IRLS and PLMSI (IRLS coefficient = −3.31, P = 0.001 and PLMSI coefficient = −2.48, P = 0.009; see Figure 10 for IRLS). When examining the two funnel plots of IRLS and PLMSI effects, medication subgroups differed in treatment effects and precision (presented as the inverse of the standard error) with, for example, cabergoline and lisuride showing larger effects with larger standard errors, whereas other medication subgroups showed treatment effects closer to the mean treatment effect and smaller standard errors.
10.
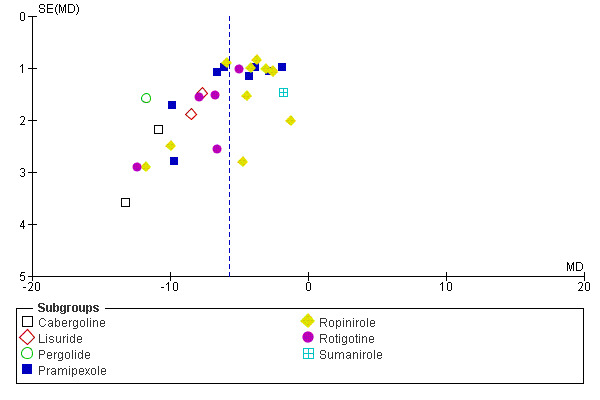
Funnel plot of comparison: 1 Dopamine agonists versus placebo, outcome: 1.2 Medication subgroups: change on IRLS.
11) Additional subgroup analyses
Subgroup analysis of effect of randomisation on IRLS treatment effect
A descriptive subgroup analysis of 30 studies was performed to investigate study quality as a source of heterogeneity of IRLS treatment effects. To this aim, studies were divided into those with low and those with unclear risk of bias regarding randomisation. Noteworthy, studies with unclear risk of bias regarding randomisation were those studies which did not report adequately on the randomisation procedure. The IRLS treatment difference was −3.54 in studies with unclear risk of randomisation bias (95% CI −5.83 to −1.24, I² = 66%) and −6.07 in studies with low risk of bias (95% CI −7.14 to −5.00, I² = 74%, see comparison 1.21). In conclusion, study quality was no source of heterogeneity of the overall effect as both subgroups showed substantial heterogeneity and an overlap of the confidence intervals by inspection.
Explorative analyses of possible predictors for treatment effects
We performed explorative univariable and multiple meta‐regressions with the three predictors: baseline severity of RLS, treatment duration, and number of investigating sites. Mean treatment difference of the IRLS was regressed on these predictors.
Results of the univariable meta‐regression: The number of investigating sites within a trial was negatively associated with the size of treatment difference (P = 0.001). Studies with longer duration showed smaller treatment effects than studies with shorter duration (P = 0.027). Baseline scores of the IRLS had no effect on IRLS treatment effects (P = 0.12; see Appendix 6).
Result of the multiple meta‐regression: When analysing the association between each of the three factors and treatment effects on the IRLS, we found that the number of study sites within a trial was still associated with the magnitude of IRLS mean differences (P = 0.001). Treatment duration was no longer predictive (P = 0.43) whereas higher baseline IRLS scores tended to lead to larger treatment differences on the IRLS (P = 0.09, see Appendix 7).
A further explorative subgroup analysis investigating the effect of the location of study sites on IRLS treatment effect was performed. This was due to the fact that more than half of the studies were conducted predominantly in Europe (N = 18 European sites versus N = 12 other study sites assessing IRLS). Indirect comparisons of the two subgroups showed that IRLS treatment differences were larger in studies conducted in European countries (MD −6.88, 95% CI −8.31 to −5.45, I² = 77%) compared to studies conducted predominantly in other countries (MD −4.13, 95% CI −5.31 to −2.95, I² = 61%). Noteworthy, results of both subgroups showed substantial to considerable heterogeneity (see comparison 1.22).
Comparison II: Dopamine agonists versus levodopa
In three trials, the dopamine agonists cabergoline, pergolide, and pramipexole were compared to levodopa.
1) Change on the severity scale IRLS
In two trials (cabergoline or pramipexole versus levodopa), the change from baseline on the IRLS was larger with dopamine agonists compared to levodopa (MD −5.25 points, 95% CI −8.40 to −2.10). Results showed a moderate heterogeneity (I² = 55%).
2) Change in periodic limb movements index (PLMI)
One trial investigated the index of periodic limb movements per time in bed (PLMI) when treated with pramipexole in comparison to levodopa. Pramipexole and levodopa effects were not different on the PLMI (MD −3.80/h, 95% CI −9.08 to 1.48; P = 0.16).
3) Number of dropouts due to adverse events
Numbers of patients dropping out of treatment due to adverse events were slightly, but not significantly, larger when treated with dopamine agonists compared to levodopa (OR 1.70, 95% CI 0.96 to 3.01; P = 0.07). The effect of one trial investigating pergolide was not estimable as no patients dropped out of the study.
4) Responder rates on CGI‐I
On the CGI‐I, patients were more likely to respond to treatment when treated with cabergoline compared to levodopa, whereas response to pramipexole treatment was similar to response to levodopa treatment (see comparison 2.4).
5) Change in self rated quality of sleep
One trial investigated change in self rated quality of sleep during treatment with cabergoline versus treatment with levodopa. Quality of sleep showed a tendency to improve after treatment for six weeks with cabergoline, but this change was not statistically significant (P = 0.09; see comparison 2.5).
6) Change in disease‐specific quality of life
One trial investigating change in disease‐specific quality of life in cabergoline versus levodopa found a larger improvement with cabergoline treatment (MD −5.54 points, 95% CI −8.43 to −2.65).
7) Number of patients experiencing adverse events
The experience of adverse events was investigated in all of the three trials. The number of patients with adverse events was slightly lower in pramipexole treatment compared to levodopa treatment (OR 0.32), but higher in cabergoline (OR 2.06) and pergolide (OR 26.67) when comparing these to levodopa. The pooled effect of dopamine agonists was not different from levodopa and substantial heterogeneity was seen (I² = 63%, see comparison 2.7).
8) Change in daytime tiredness
Treatment effects did not differ between cabergoline and levodopa in one trial investigating daytime tiredness (see comparison 2.8).
No active controlled trial investigated the endpoints sleep efficiency and Patient Global Impressions. Therefore, we cannot draw any conclusions regarding these endpoints in actively controlled trials.
Comparison III: Lisuride versus ropinirole
One trial compared treatment with lisuride to ropinirole directly and to placebo . Reductions on the IRLS after treatment were larger with lisuride compared to ropinirole with a mean difference of −3.00 points (95% CI −5.70 to −0.30). Quality of life was more improved with lisuride treatment than with ropinirole (MD −4.50, 95% CI −8.12 to −0.88) whereas response to both treatments and daytime tiredness did not differ between treatments (comparisons 3.3 and 3.7). Patients dropped out of treatments and experienced adverse events at a similar rate (comparisons 3.2 and 3.6).
Discussion
Summary of main results
Dopamine agonist treatment showed superiority over placebo treatment regarding all investigated efficacy endpoints. Marked effects were seen on the IRLS and PLMSI as well as on PGI and CGI‐I. The mean treatment effect of the IRLS was close to six points which is regarded as a difference of clinical relevance (Trenkwalder 2007). Small to moderate effects were shown in outcomes such as sleep efficiency, self rated quality of sleep, quality of life, and daytime tiredness as well as the safety parameters number of dropouts due to adverse events and number of patients experiencing adverse events (see Table 1; Table 3).
All medication subgroups indicated efficacy on the IRLS above the non‐inferiority margin of three points and four of seven medication subgroups (cabergoline, pergolide, lisuride, rotigotine) showed a treatment difference above six points which indicates a clinically relevant improvement (Trenkwalder 2007). Visual inspection of confidence intervals revealed that those of the subgroups cabergoline and pergolide showed highest effects, those of pramipexole and ropinirole showed lower effects. No significant effect was observed in sumanirole. The confidence interval of lisuride overlapped with those of cabergoline, pergolide, pramipexole, and rotigotine and was higher than that of ropinirole.
Sleep efficiency was overall slightly, but significantly, improved. When looking at improvement in each dopamine agonist, a favourable effect was only present with pergolide.
Patients rated their daytime tiredness as improved with dopamine agonist treatment. The only exception was patients treated with cabergoline in one trial, who rated their daytime tiredness as similarly improved as with placebo (comparison 1.21).
Dropouts due to adverse events occurred more often in patients receiving lisuride and ropinirole compared to placebo, whereas there were no differences in the treatments with cabergoline, pergolide, pramipexole, rotigotine, and sumanirole. Patients receiving pramipexole, ropinirole, or rotigotine experienced more adverse events compared to placebo, whereas treatments with cabergoline, lisuride, and pergolide showed a similar risk of adverse events compared with placebo.
When looking at symptom improvement on the outcomes IRLS, PLMSI, quality of life, and PGI, treatment effects were evident in all but the sumanirole medication subgroups. However, substantial heterogeneity remained in the pramipexole and ropinirole trials.
Sumanirole was the only dopamine agonist with low or no efficacy in RLS treatment. The dopamine agonist differed from placebo only when measuring PLMSI. Treatment effects on the IRLS were larger in treatment groups with higher doses.
Univariable meta‐regression showed a negative effect of higher number of study sites and longer treatment duration on IRLS treatment effect. In a multiple meta‐regression, effects of number of study sites remained significant, and a tendency towards higher treatment effects in the more severely affected patients was seen.
Three trials comparing a dopamine agonist (cabergoline, pergolide, pramipexole) to levodopa showed superiority of dopamine agonists on the IRLS (cabergoline, pramipexole) and with regard to quality of life (cabergoline). The number of patients experiencing adverse events was higher during treatment with cabergoline and pergolide compared to levodopa. No treatment difference between dopamine agonists and levodopa was seen on the following outcomes: PLMSI, dropout rates due to adverse events, CGI‐I, self rated quality of sleep, and daytime tiredness (see Table 2). Only one study compared two dopamine agonists (and placebo). In this study, lisuride was superior in reduction of IRLS score and improvement of quality of life compared to ropinirole.
In summary, dopamine agonist treatment shows a greater efficacy in RLS treatment than placebo. In some aspects, dopamine agonists are even more effective than levodopa. However, treatment effects have to be weighed against an increase in the dropout rate due to adverse events and in a higher number of patients experiencing adverse events.
Overall completeness and applicability of evidence
Patients were recruited from outpatient settings. They suffered from moderate to very severe RLS and represent the patient population requiring treatment.
Treatment durations varied from one week to 30 weeks with durations of 12 weeks in many studies. Efficacy of drug treatment can be investigated reliably in these time periods. However, there is a general lack of controlled evidence for long‐term efficacy and safety of dopamine agonists in RLS, although RLS is a chronic disorder and treatment with dopaminergic agents is symptomatic, i.e. a long‐term medication. An indication regarding long‐term efficacy is provided by univariable meta‐regression. The results of this analysis indicated a slight decrease of efficacy on the IRLS with increased treatment duration.
Earlier reviews including meta‐analyses reported greater effects in pramipexole treatment compared to ropinirole treatment acknowledging that pramipexole studies were of shorter duration (Baker 2008; Quilici 2008). The presented meta‐analyses included a further number of studies with longer treatment durations. In these meta‐analyses, we were not able to replicate findings indicating differences in treatment efficacy between pramipexole and ropinirole (Kohnen 2008).
In summary, dopamine agonist treatment showed superiority over placebo. All relevant aspects of symptom severity and wellbeing‐related improvements were assessed. The only three active controlled studies showed greater effects with dopamine agonists compared to levodopa regarding symptom severity and quality of life improvement and overall similar numbers of patients experiencing adverse events.
Quality of the evidence
We searched all relevant databases and public trial registers provided by the pharmaceutical companies and by government (see Table 6) and included unpublished trials, which were only presented in online trial registers. We furthermore contacted pharmaceutical companies. Therefore, we presume that there are not many additional, relevant RCTs, if any.
Due to only one obtained trial investigating a small number of exclusively uremic (secondary) RLS patients (N = 8), we cannot draw any conclusions regarding uremic RLS. Evidence for treatment of uremic or any other form of secondary RLS is clearly missing.
The majority of the included studies were adequately planned and performed according to requirements for RCTs. Most study publications adequately described the methods for randomisation, allocation concealment, and blinding during the study either in study reports or on request. The majority of studies were initiated by pharmaceutical companies. In those studies, standard study procedures were applied. In a few sponsored studies, we could not obtain satisfactory information on randomisation and blinding, which led to some restriction in the quality rating of methodological appropriateness. We did not obtain sufficient information regarding study procedures and performance of a few investigator‐initiated trials. Therefore, we had to rate their likelihood for bias as “unclear”, even though we are aware that an insufficient study report does not necessarily represent inadequate performance of the study. In summary, methodological standards were adequately reported in the majority of studies.
When investigating parameters with marked heterogeneity (I² > 50%) of overall treatment effects (IRLS, PLMSI, quality of life, PGI), medication subgroup analyses showed homogeneous effects, with the exception of pramipexole and ropinirole. Both pramipexole and ropinirole showed heterogeneous treatment results on the IRLS, PLMSI, and quality of life (I² = 52% to 85%). Additionally, treatment differences on the PGI were heterogeneous in pramipexole (I² = 74%). One pramipexole (BI 2008) and one ropinirole trial (GSK 2005) contributed predominantly to the heterogeneous treatment effects on the IRLS. Both studies were unpublished and retrieved from online registers. In the pramipexole study, the medication dose was relatively low (0.25 mg) compared to the other included pramipexole studies (mean dose of 0.46 mg). The ropinirole study used an escalating dose regimen (GSK 2005). Heterogeneity of studies contributing to the treatment effect on PLMSI could not be traced back to methodological factors. The lower treatment effect of the only middle‐term, 26‐week pramipexole study (BI 2009) contributed considerably to the heterogeneity in pramipexole studies on the disease‐specific QoL (I² = 60%). Heterogeneity of PGI in the pramipexole subgroup was increased markedly by the lower treatment effect of the pramipexole study (BI 2008). Furthermore, a general reason for heterogeneity might be the wide range of RLS severity at baseline due to inclusion of patients with an IRLS score of ≥ 15 in most studies. Although baseline severity might not substantially have influenced treatment results, we found a non‐significant tendency towards larger treatment effects in patients with more severe RLS symptoms.
In active controlled trials, heterogeneity of treatment effect was present on the IRLS, as well as in the number of patients with adverse events in the studies investigating cabergoline and pramipexole. These differences in treatment effects can be traced back to the different medications.
Only four studies investigated the effects of dopamine agonists against other dopaminergic agents. Therefore, a conclusive comparison of dopaminergic medications regarding their efficacy in RLS cannot be made. In the meta‐analyses of dopamine agonists against placebo, treatment effects in medication subgroups did not differ markedly and confidence intervals of the medication subgroups overlapped in most endpoints. Marked treatment differences only resulted pertaining to the IRLS treatment effect when indirectly comparing medication subgroups of non‐ergoline substances to subgroups of ergoline treatments by visually inspecting forest plots. IRLS treatment effects of the ergoline treatments with cabergoline and pergolide were higher compared with non‐ergoline treatments such as pramipexole and ropinirole. Confidence intervals of the ergoline lisuride overlapped with those of cabergoline and pergolide and were superior to ropinirole. This finding was supported by a larger effect of pergolide on the PGI compared to pramipexole, ropinirole, and rotigotine. It has to be mentioned that the highest treatment differences were found in medication subgroups with few trials investigating a relatively low number of patients (cabergoline, pergolide), whereas studies with lower treatment effects such as in the medication subgroups ropinirole and pramipexole investigated a large number of patients and therefore are more likely to be representative. Notably, ergoline dopamine agonists, which were investigated in the earlier studies showing higher efficacy results, are considered today as third‐line treatment because of the risk of cardiac valvular fibrosis. Only one study (Axxonis 2008) compared dopamine agonist substances directly. All other comparisons of medication subgroups are indirect and descriptive. The results show low to substantial heterogeneity both considering overall treatment effects and the medication subgroups. Thus, differences in treatment effects between medication subgroups observed by visual inspection of the forest plots should be considered as hypothesis generating and should be treated with caution. In future, direct head‐to‐head trials of dopamine agonists are needed.
Results on all endpoints showed small to moderate treatment effects which were precise in every endpoint.
Asymmetry in funnel plots of the IRLS (see Figure 10) and PLMSI might be an indication of selective publication of studies with positive results. A closer inspection of the studies, however, shows that a number of small studies were conducted in “expert” study centres. These centres focus on clinical and experimental research on RLS and might, therefore, investigate a more severely affected patient population which shows the higher treatment effects. Varying effects between the medication subgroups, i.e. larger effects in cabergoline and lisuride with larger standard errors, also contribute to the skewed funnel plots. These factors put the seemingly skewed distribution of effects into perspective.
Potential biases in the review process
We made extensive efforts to prevent bias in the search for relevant trials and respective data as well as meta‐analyses of included data; therefore, we assume that the potential biases in the review process are limited. We searched all relevant databases without language restrictions. The reviewers decided separately upon eligibility, collected data independently, and checked resulting data files as well as data directly contributing to meta‐analyses, resolving disagreements and errors. When data had been insufficiently presented, we asked pharmaceutical companies and authors for more information in order to include all assessed questionnaires and instruments and information relevant to bias.
The presented work is independent from sponsoring of pharmaceutical companies, as it was supported by the German Ministry for Education and Research (Bundesministerium für Bildung und Forschung ‐ BMBF, Project number DLR 01KG0723).
Agreements and disagreements with other studies or reviews
A recent meta‐analysis included a considerable number of dopamine agonist studies and investigated effects on the IRLS and CGI‐I (Zintzaras 2010). Two other meta‐analyses especially compared pramipexole to ropinirole on the IRLS and CGI‐I (Baker 2008; Quilici 2008). The meta‐analysis results overlap with our results. Contrary to reported superiority of pramipexole over ropinirole, we could not find a marked difference between pramipexole and ropinirole. Confidence intervals overlapped to a great extent in our analysis (pramipexole: 95% CI −6.87 to −3.45 versus ropinirole: 95% CI −5.4 to −2.97). Possibly, the differences between effects become smaller due to the inclusion of a higher number of studies and of studies with longer duration.
One meta‐analysis pooled results of individual patient data assessed in six ropinirole studies investigating sleep related data and found moderate changes regarding subjective sleep data, including daytime somnolence in favour of ropinirole compared to placebo (Hansen 2009). These data are supported by our data, indicating modest changes in daytime somnolence represented by small effect sizes.
The presented results on quality of life instruments are similar to previous results of a meta‐analysis evaluating pramipexole, ropinirole, and rotigotine (Talati 2009). Our findings, which include more detailed data provided by pharmaceutical companies and data on additional dopamine agonists, however, indicate slightly more pronounced effects compared to previous results (SMD of 0.34, 95% CI 0.23 to 0.44 versus SMD of 0.2, 95% CI 0.10 to 0.30; Talati 2009).
Authors' conclusions
Implications for practice.
This meta‐analysis according to Cochrane criteria has been the first to comprehensively assess efficacy and safety of dopamine agonist treatment in an extended number of clinically relevant outcome parameters. Meta‐analysis results show that all dopamine agonists except sumanirole are superior to placebo in the treatment of RLS. Therefore, the use of dopamine agonists can be recommended for the treatment of RLS. In the three studies with the active comparator levodopa, the dopamine agonists cabergoline, pergolide, and pramipexole showed superiority over levodopa in some, but not all outcomes.
The efficacy of three cabergoline and pergolide studies was the highest but has to be weighed against the known side effects such as fibrosis, especially cardiac valve fibrosis, which need to be monitored regularly. The non‐ergoline dopamine agonists pramipexole, rotigotine, and ropinirole showed adequate efficacy. By inspecting the forest plots, the number of dropouts due to adverse events and the presence of adverse events were slightly higher in non‐ergoline dopamine agonists.
Implications for research.
Additional studies are needed in order to enhance the robustness of findings especially for those dopamine agonists (including cabergoline, pergolide, lisuride, and sumanirole) which have been investigated in only a few studies. Furthermore, there is a lack of large scale studies comparing efficacious dopamine agonist treatments directly against other dopamine agonists. Additionally, dopamine agonist treatments need to be compared to other treatment options like anticonvulsants and opioids. A major limitation of the majority of previous studies is the lack of identification of augmentation. Generally, long‐term studies are needed in order to evaluate sustained efficacy as well as safety, especially regarding the development of augmentation over time.
What's new
| Date | Event | Description |
|---|---|---|
| 13 November 2008 | Amended | Converted to new format |
Acknowledgements
The presented work was supported by a grant from the German Federal Ministry for Education and Research (Bundesministerium für Bildung und Forschung ‐ BMBF, grant number DLR 01KG0723) to MH.
We also thank Carolin Laux for supporting the project during data collection and data management.
We are grateful to Bruce Grant for language correction of the review.
Appendices
Appendix 1. Central (OVID) search strategy
AF dopamin* agonist*
AF cabergolin*
AF pergolid*
AF bromocriptin*
AF Alpha‐Dihydroergocryptin*
AF pramipexol*
AF ropinirol*
AF rotigotin*
AF apomorphin*
AF piribedil*
AF lisurid*
AF talipexol*
or/1‐12
AF “rls”
AF “restless leg*“
14 or 15
13 and 16
Appendix 2. CINAHL (EBSCO) search strategy
TX dopamin* agonist*
TX cabergolin*
TX pergolid*
TX bromocriptin*
TX Alpha‐Dihydroergocryptin*
TX pramipexol*
TX ropinirol*
TX rotigotin*
TX apomorphin*
TX piribedil*
TX lisurid*
TX talipexol*
or/1‐12
AF “rls”
AF “restless leg*“
14 or 15
13 and 16
Appendix 3. EMBASE search strategy
(random* or factorial* or crossover* or "cross over*" or placebo* or (doubl* adj blind*) or (singl* adj blind*) or assign* or allocat* or volunteer*).mp
Randomized Controlled Trial/
(l‐dopa or levodopa* or ldopa or "l dopa").mp
Levodopa/
(rls or "restless leg*").mp
Restless Legs Syndrome/
(1 or 2) and (3 or 4) and (5 or 6)
Appendix 4. MEDLINE (OVID) search strategy
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
or/ 1‐8
humans.sh.
9 and 10
AF dopamin* agonist*
AF cabergolin*
AF pergolid*
AF bromocriptin*
AF Alpha‐Dihydroergocryptin*
AF pramipexol*
AF ropinirol*
AF rotigotin*
AF apomorphin*
AF piribedil*
AF lisurid*
AF talipexol*
or/12‐23
AF rls
AF restless leg*
25 or 26
11 and 24 and 27
Appendix 5. PsycINFO (EBSCO) search strategy
TX dopamin* agonist*
TX cabergolin*
TX pergolid*
TX bromocriptin*
TX Alpha‐Dihydroergocryptin*
TX pramipexol*
TX ropinirol*
TX rotigotin*
TX apomorphin*
TX piribedil*
TX lisurid*
TX talipexol*
or/1‐12
TX “rls”
TX “restless leg*“
14 or 15
13 and 16
Appendix 6. Univariable meta‐regressions
| Explanatory variable | Coefficient (B) | 95% confidence interval | Statistical significance | Proportion of heterogeneity explained (R²) | Interpretation |
| Number of sites | 0.097 | 0.05 to 0.15 | 0.001 | 0.42 | Studies with more investigating sites result in lower IRLS treatment effects than studies with less investigating sites. |
| Duration of treatment | 0.023 | 0.003 to 0.042 | 0.027 | 0.15 | Studies with longer treatment duration show lower IRLS treatment effects than studies with shorter treatment duration. |
| Baseline severity on the IRLS | ‐0.42 | ‐0.96 to 0.11 | 0.12 | 0.06 | Baseline severity has no influence on treatment effect. |
Appendix 7. Mutivariable meta‐regression
| Explanatory variable | Coefficient (B) | 95% confidence interval | Statistical significance | Proportion of heterogeneity explained (R²) | Interpretation |
| Number of sites | 0.097 | 0.04 to 0.15 | 0.001 | Studies with more investigating sites result in lower IRLS treatment effects than studies with less investigating sites. | |
| Duration of treatment | 0.006 | ‐0.01 to 0.024 | 0.429 | Studies with differing treatment duration have no differential influence on IRLS treatment effects. | |
| Baseline severity on the IRLS | ‐0.346 | ‐0.75 to 0.059 | 0.091 | 0.56 | More severe baseline severity scores on the IRLS in tendency lead to larger treatment effects on the IRLS. |
Data and analyses
Comparison 1. Dopamine agonists versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change on IRLS | 30 | Mean Difference (Random, 95% CI) | ‐5.74 [‐6.74, ‐4.74] | |
| 1.1 Cross‐over trial | 1 | Mean Difference (Random, 95% CI) | ‐11.7 [‐17.40, ‐4.00] | |
| 1.2 Parallel group trials | 29 | Mean Difference (Random, 95% CI) | ‐5.61 [‐6.60, ‐4.62] | |
| 2 Medication subgroups: change on IRLS | 30 | Mean Difference (Random, 95% CI) | ‐5.74 [‐6.74, ‐4.74] | |
| 2.1 Cabergoline | 2 | Mean Difference (Random, 95% CI) | ‐11.49 [‐15.14, ‐7.84] | |
| 2.2 Lisuride | 2 | Mean Difference (Random, 95% CI) | ‐8.00 [‐10.28, ‐5.72] | |
| 2.3 Pergolide | 1 | Mean Difference (Random, 95% CI) | ‐11.7 [‐14.80, ‐8.60] | |
| 2.4 Pramipexole | 8 | Mean Difference (Random, 95% CI) | ‐5.16 [‐6.87, ‐3.45] | |
| 2.5 Ropinirole | 11 | Mean Difference (Random, 95% CI) | ‐4.19 [‐5.40, ‐2.97] | |
| 2.6 Rotigotine | 5 | Mean Difference (Random, 95% CI) | ‐6.98 [‐8.99, ‐4.96] | |
| 2.7 Sumanirole | 1 | Mean Difference (Random, 95% CI) | ‐1.83 [‐4.71, 1.05] | |
| 3 Change in periodic limb movements in sleep index | 15 | Mean Difference (Random, 95% CI) | ‐22.38 [‐27.82, ‐16.94] | |
| 3.1 Cross‐over trials | 3 | Mean Difference (Random, 95% CI) | ‐49.64 [‐71.71, ‐27.58] | |
| 3.2 Parallel group trials | 12 | Mean Difference (Random, 95% CI) | ‐19.00 [‐23.46, ‐14.54] | |
| 4 Medication subgroups: change in periodic limb movements in sleep index | 15 | Mean Difference (Random, 95% CI) | ‐22.38 [‐27.82, ‐16.94] | |
| 4.1 Cabergoline | 1 | Mean Difference (Random, 95% CI) | ‐32.76 [‐56.79, ‐8.73] | |
| 4.2 Pergolide | 4 | Mean Difference (Random, 95% CI) | ‐35.08 [‐44.88, ‐25.29] | |
| 4.3 Pramipexole | 3 | Mean Difference (Random, 95% CI) | ‐30.47 [‐51.58, ‐9.35] | |
| 4.4 Ropinirole | 5 | Mean Difference (Random, 95% CI) | ‐14.11 [‐18.79, ‐9.43] | |
| 4.5 Rotigotine | 1 | Mean Difference (Random, 95% CI) | ‐30.35 [‐43.74, ‐16.96] | |
| 4.6 Sumanirole | 1 | Mean Difference (Random, 95% CI) | ‐18.9 [‐27.41, ‐10.39] | |
| 5 Change in sleep efficiency | 11 | Mean Difference (Random, 95% CI) | 4.53 [2.00, 7.06] | |
| 5.1 Cross‐over trials | 2 | Mean Difference (Random, 95% CI) | 12.44 [‐8.04, 32.92] | |
| 5.2 Parallel group trials | 9 | Mean Difference (Random, 95% CI) | 3.61 [1.81, 5.41] | |
| 6 Medication subgroups: change in sleep efficiency | 11 | Mean Difference (Random, 95% CI) | 4.53 [2.00, 7.06] | |
| 6.1 Cabergoline | 1 | Mean Difference (Random, 95% CI) | 2.9 [‐5.06, 10.86] | |
| 6.2 Pergolide | 4 | Mean Difference (Random, 95% CI) | 8.70 [1.85, 15.56] | |
| 6.3 Pramipexole | 2 | Mean Difference (Random, 95% CI) | 2.47 [‐1.60, 6.55] | |
| 6.4 Ropinirole | 2 | Mean Difference (Random, 95% CI) | 2.19 [‐2.11, 6.49] | |
| 6.5 Rotigotine | 1 | Mean Difference (Random, 95% CI) | 2.71 [‐3.07, 8.49] | |
| 6.6 Sumanirole | 1 | Mean Difference (Random, 95% CI) | 4.1 [0.00, 8.20] | |
| 7 Number of dropouts due to adverse events | 34 | 7054 | Odds Ratio (M‐H, Random, 95% CI) | 1.82 [1.35, 2.45] |
| 8 Medication subgroups: number of dropouts due to adverse events | 34 | 7054 | Odds Ratio (M‐H, Random, 95% CI) | 1.82 [1.35, 2.45] |
| 8.1 Cabergoline | 2 | 128 | Odds Ratio (M‐H, Random, 95% CI) | 4.84 [0.58, 40.21] |
| 8.2 Lisuride | 2 | 391 | Odds Ratio (M‐H, Random, 95% CI) | 3.60 [1.79, 7.26] |
| 8.3 Pergolide | 3 | 104 | Odds Ratio (M‐H, Random, 95% CI) | 1.76 [0.07, 45.46] |
| 8.4 Pramipexole | 9 | 2335 | Odds Ratio (M‐H, Random, 95% CI) | 1.11 [0.66, 1.87] |
| 8.5 Ropinirole | 12 | 2858 | Odds Ratio (M‐H, Random, 95% CI) | 1.76 [1.31, 2.38] |
| 8.6 Rotigotine | 5 | 973 | Odds Ratio (M‐H, Random, 95% CI) | 2.94 [1.00, 8.67] |
| 8.7 Sumanirole | 1 | 265 | Odds Ratio (M‐H, Random, 95% CI) | 2.70 [0.15, 49.69] |
| 9 Responder rates on CGI‐I | 27 | 6338 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [1.34, 1.54] |
| 10 Medication subgroups: responder rates on CGI‐I | 27 | 6338 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [1.34, 1.54] |
| 10.1 Cabergoline | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 2.32 [1.13, 4.77] |
| 10.2 Lisuride | 2 | 376 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [1.40, 2.18] |
| 10.3 Pramipexole | 8 | 2250 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [1.30, 1.80] |
| 10.4 Ropinirole | 10 | 2544 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [1.27, 1.46] |
| 10.5 Rotigotine | 5 | 863 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [1.31, 1.67] |
| 10.6 Sumanirole | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.73, 1.33] |
| 11 Change in self rated quality of sleep | 22 | 4592 | Std. Mean Difference (IV, Random, 95% CI) | 0.40 [0.33, 0.47] |
| 12 Medication subgroups: change in self rated quality of sleep | 22 | 4592 | Std. Mean Difference (IV, Random, 95% CI) | 0.40 [0.33, 0.47] |
| 12.1 Cabergoline | 2 | 124 | Std. Mean Difference (IV, Random, 95% CI) | 0.69 [0.30, 1.08] |
| 12.2 Lisuride | 2 | 384 | Std. Mean Difference (IV, Random, 95% CI) | 0.53 [0.31, 0.74] |
| 12.3 Pramipexole | 6 | 1553 | Std. Mean Difference (IV, Random, 95% CI) | 0.44 [0.33, 0.54] |
| 12.4 Ropinirole | 7 | 1608 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [0.16, 0.43] |
| 12.5 Rotigotine | 5 | 923 | Std. Mean Difference (IV, Random, 95% CI) | 0.42 [0.28, 0.56] |
| 13 Change in disease specific quality of life | 17 | 4312 | Std. Mean Difference (IV, Random, 95% CI) | 0.34 [0.23, 0.44] |
| 14 Medication subgroups: change in disease specific quality of life | 17 | 4312 | Std. Mean Difference (IV, Random, 95% CI) | 0.34 [0.23, 0.44] |
| 14.1 Cabergoline | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 0.75 [0.10, 1.39] |
| 14.2 Lisuride | 2 | 384 | Std. Mean Difference (IV, Random, 95% CI) | 0.60 [0.38, 0.82] |
| 14.3 Pramipexole | 4 | 1394 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [0.13, 0.47] |
| 14.4 Ropinirole | 7 | 1873 | Std. Mean Difference (IV, Random, 95% CI) | 0.23 [0.09, 0.36] |
| 14.5 Rotigotine | 3 | 621 | Std. Mean Difference (IV, Random, 95% CI) | 0.50 [0.23, 0.76] |
| 15 Responder rates on PGI | 13 | 3321 | Risk Ratio (IV, Random, 95% CI) | 1.53 [1.34, 1.75] |
| 16 Medication subgroups: responder rates on PGI | 13 | 3321 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [1.34, 1.75] |
| 16.1 Pergolide | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 4.51 [2.31, 8.80] |
| 16.2 Pramipexole | 8 | 2260 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [1.30, 1.89] |
| 16.3 Ropinirole | 2 | 655 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [1.06, 1.73] |
| 16.4 Rotigotine | 2 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [1.14, 1.57] |
| 17 Number of patients experiencing adverse events | 33 | 7049 | Odds Ratio (M‐H, Random, 95% CI) | 1.82 [1.59, 2.08] |
| 18 Medication subgroups: number of patients experiencing adverse events | 33 | 7049 | Odds Ratio (M‐H, Random, 95% CI) | 1.82 [1.59, 2.08] |
| 18.1 Cabergoline | 2 | 128 | Odds Ratio (M‐H, Random, 95% CI) | 2.66 [0.32, 21.99] |
| 18.2 Lisuride | 2 | 391 | Odds Ratio (M‐H, Random, 95% CI) | 1.72 [0.90, 3.27] |
| 18.3 Pergolide | 3 | 131 | Odds Ratio (M‐H, Random, 95% CI) | 1.97 [0.94, 4.15] |
| 18.4 Pramipexole | 8 | 2320 | Odds Ratio (M‐H, Random, 95% CI) | 1.48 [1.24, 1.77] |
| 18.5 Ropinirole | 12 | 2841 | Odds Ratio (M‐H, Random, 95% CI) | 2.07 [1.71, 2.50] |
| 18.6 Rotigotine | 5 | 973 | Odds Ratio (M‐H, Random, 95% CI) | 2.41 [1.75, 3.31] |
| 18.7 Sumanirole | 1 | 265 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.49, 1.92] |
| 19 Change in daytime tiredness | 21 | 4965 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.31, ‐0.17] |
| 20 Medication subgroups: change in daytime tiredness | 21 | 4965 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.31, ‐0.17] |
| 20.1 Cabergoline | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.93, 0.32] |
| 20.2 Lisuride | 2 | 384 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.74, ‐0.19] |
| 20.3 Pramipexole | 4 | 1411 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.47, ‐0.04] |
| 20.4 Ropinirole | 9 | 2186 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.28, ‐0.10] |
| 20.5 Rotigotine | 5 | 944 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.38, ‐0.10] |
| 21 Subgroup analysis: Effect of randomisation on change on IRLS | 30 | Mean Difference (Random, 95% CI) | ‐5.74 [‐6.74, ‐4.74] | |
| 21.1 Randomisation unclear | 4 | Mean Difference (Random, 95% CI) | ‐3.54 [‐5.83, ‐1.24] | |
| 21.2 Randomisation clear | 26 | Mean Difference (Random, 95% CI) | ‐6.07 [‐7.14, ‐3.00] | |
| 22 Subgroup analysis: Effect of study origin on change on IRLS | 30 | Mean Difference (Random, 95% CI) | ‐5.74 [‐6.74, ‐4.74] | |
| 22.1 Europe | 18 | Mean Difference (Random, 95% CI) | ‐6.88 [‐8.31, ‐5.45] | |
| 22.2 Other origin | 12 | Mean Difference (Random, 95% CI) | ‐4.13 [‐5.31, ‐2.95] |
1.1. Analysis.
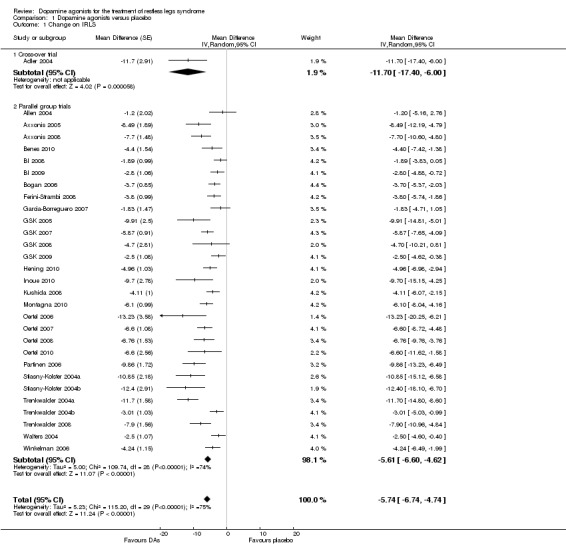
Comparison 1 Dopamine agonists versus placebo, Outcome 1 Change on IRLS.
1.2. Analysis.
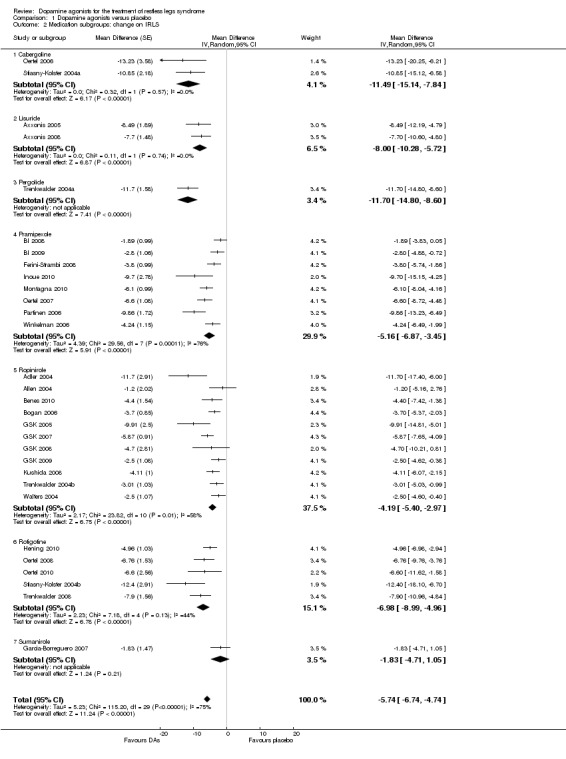
Comparison 1 Dopamine agonists versus placebo, Outcome 2 Medication subgroups: change on IRLS.
1.3. Analysis.
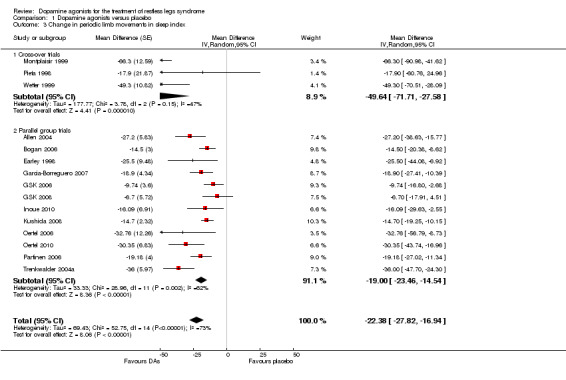
Comparison 1 Dopamine agonists versus placebo, Outcome 3 Change in periodic limb movements in sleep index.
1.4. Analysis.
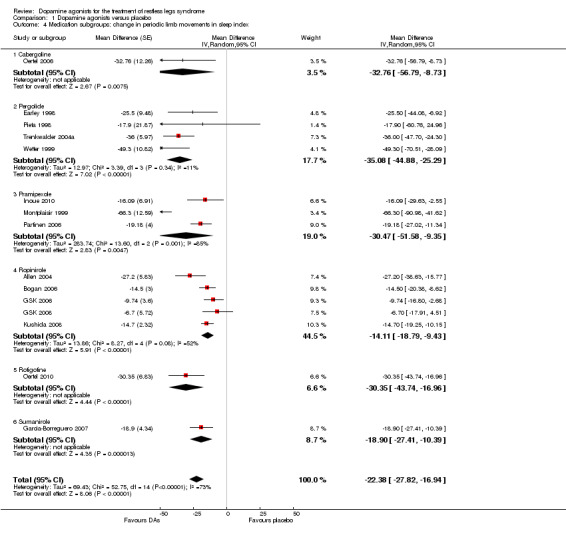
Comparison 1 Dopamine agonists versus placebo, Outcome 4 Medication subgroups: change in periodic limb movements in sleep index.
1.5. Analysis.
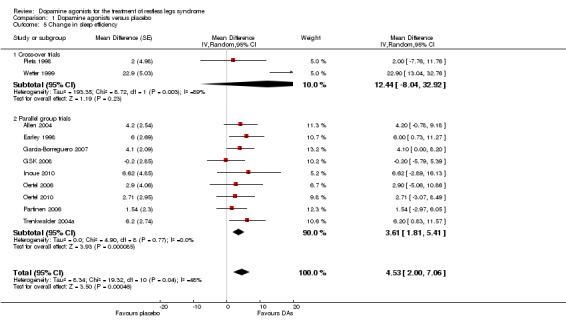
Comparison 1 Dopamine agonists versus placebo, Outcome 5 Change in sleep efficiency.
1.6. Analysis.
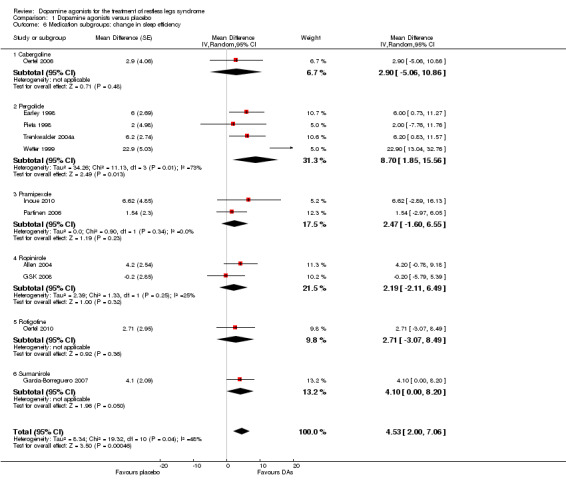
Comparison 1 Dopamine agonists versus placebo, Outcome 6 Medication subgroups: change in sleep efficiency.
1.7. Analysis.
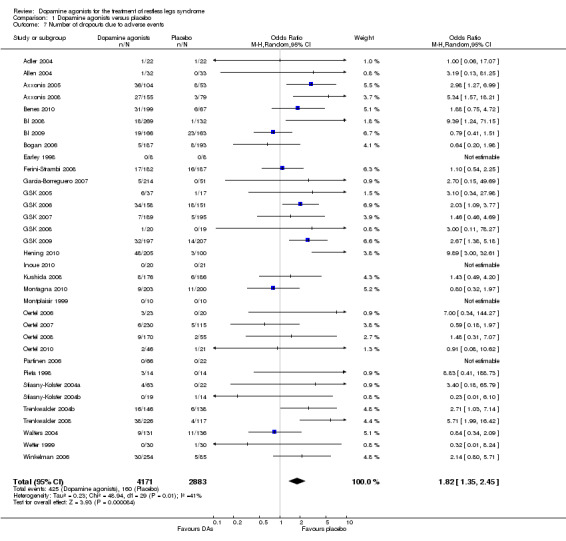
Comparison 1 Dopamine agonists versus placebo, Outcome 7 Number of dropouts due to adverse events.
1.8. Analysis.
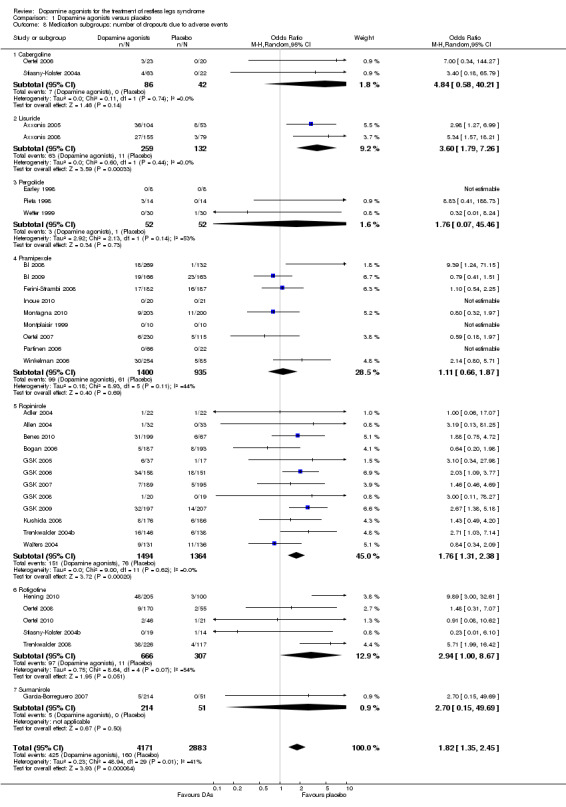
Comparison 1 Dopamine agonists versus placebo, Outcome 8 Medication subgroups: number of dropouts due to adverse events.
1.9. Analysis.
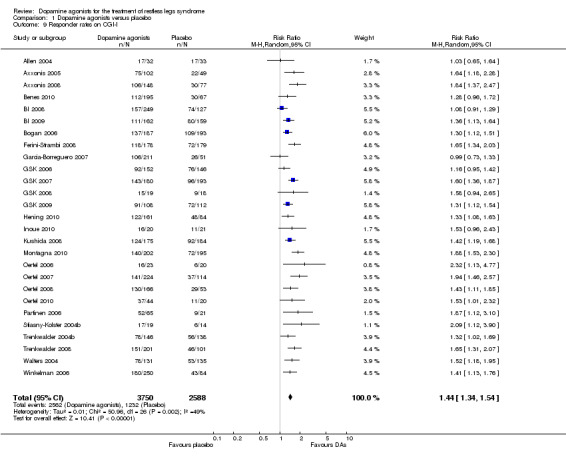
Comparison 1 Dopamine agonists versus placebo, Outcome 9 Responder rates on CGI‐I.
1.10. Analysis.
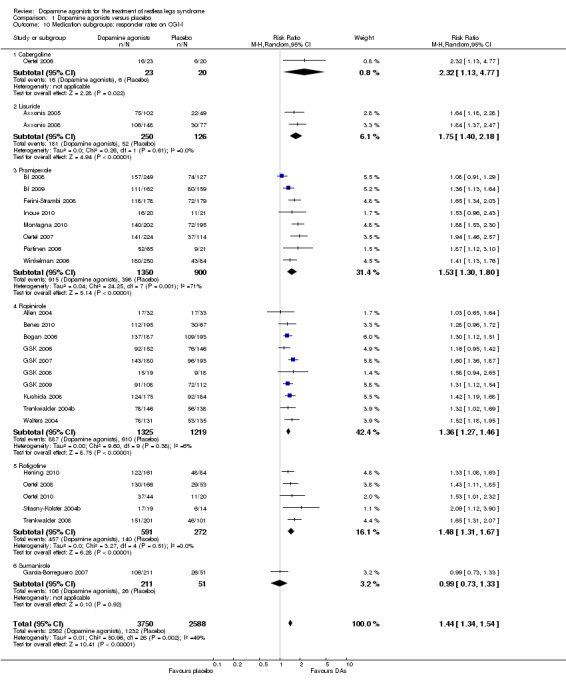
Comparison 1 Dopamine agonists versus placebo, Outcome 10 Medication subgroups: responder rates on CGI‐I.
1.11. Analysis.
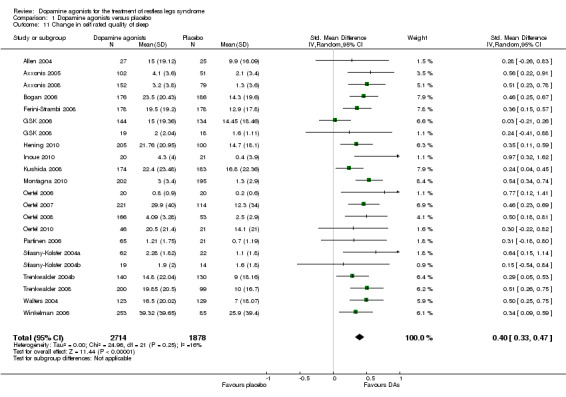
Comparison 1 Dopamine agonists versus placebo, Outcome 11 Change in self rated quality of sleep.
1.12. Analysis.
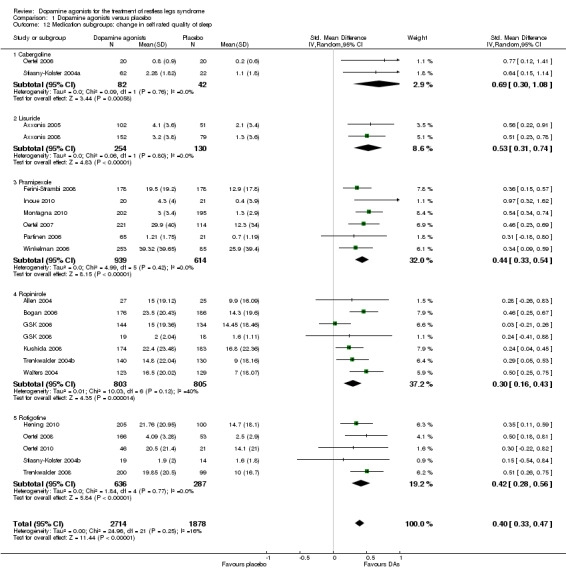
Comparison 1 Dopamine agonists versus placebo, Outcome 12 Medication subgroups: change in self rated quality of sleep.
1.13. Analysis.
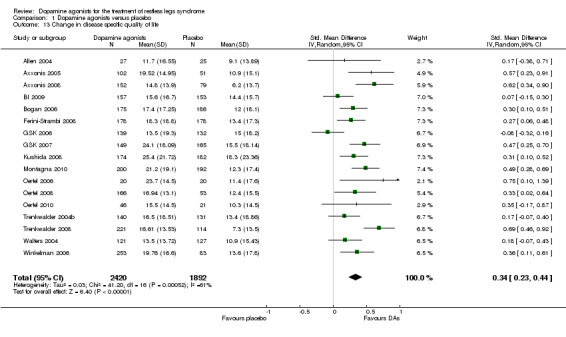
Comparison 1 Dopamine agonists versus placebo, Outcome 13 Change in disease specific quality of life.
1.14. Analysis.
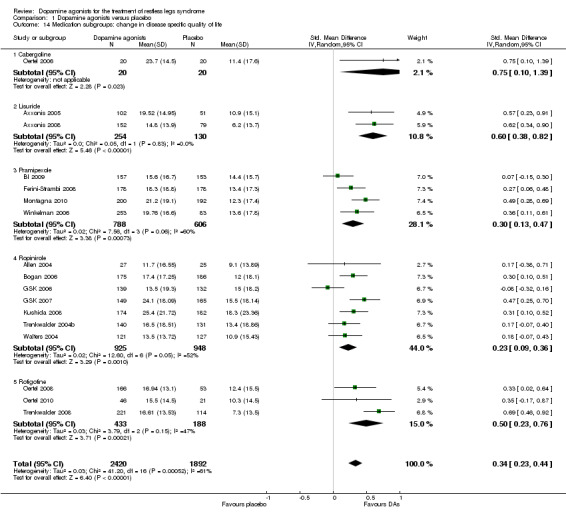
Comparison 1 Dopamine agonists versus placebo, Outcome 14 Medication subgroups: change in disease specific quality of life.
1.15. Analysis.
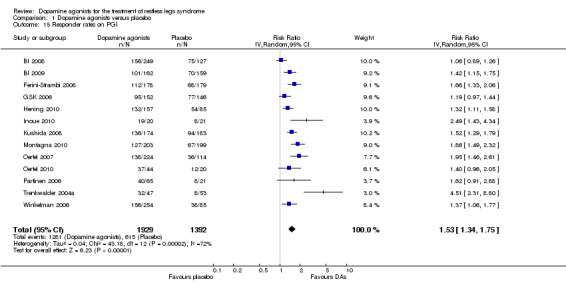
Comparison 1 Dopamine agonists versus placebo, Outcome 15 Responder rates on PGI.
1.16. Analysis.
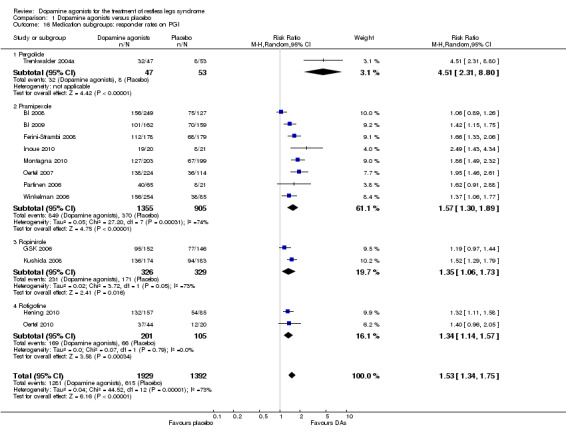
Comparison 1 Dopamine agonists versus placebo, Outcome 16 Medication subgroups: responder rates on PGI.
1.17. Analysis.
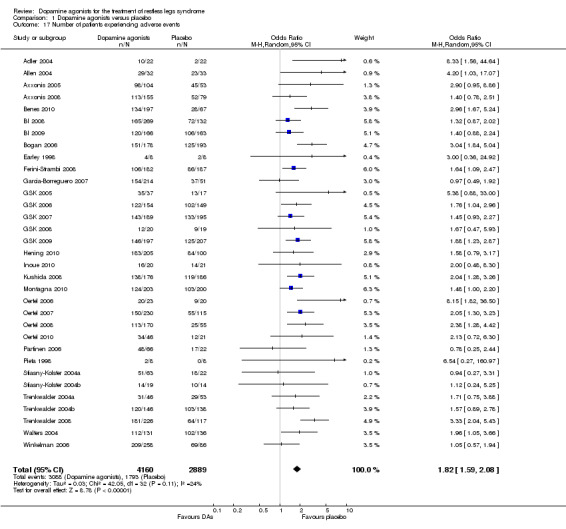
Comparison 1 Dopamine agonists versus placebo, Outcome 17 Number of patients experiencing adverse events.
1.18. Analysis.
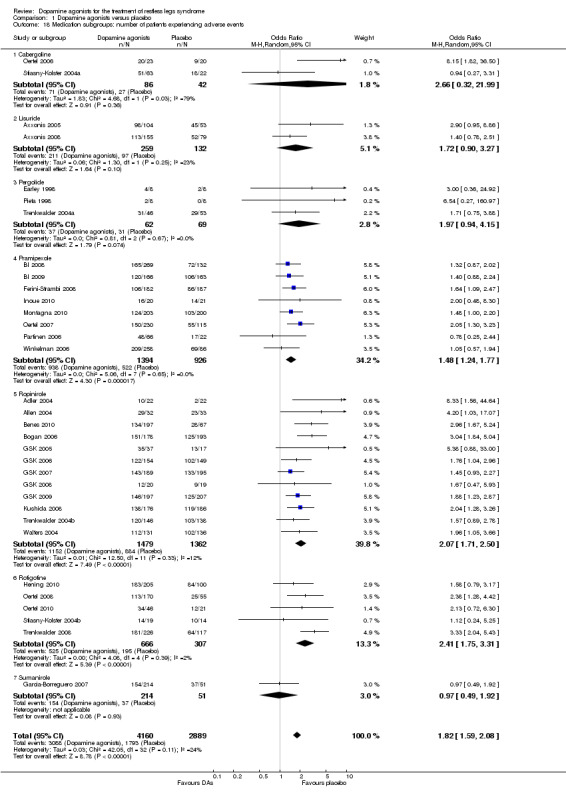
Comparison 1 Dopamine agonists versus placebo, Outcome 18 Medication subgroups: number of patients experiencing adverse events.
1.19. Analysis.
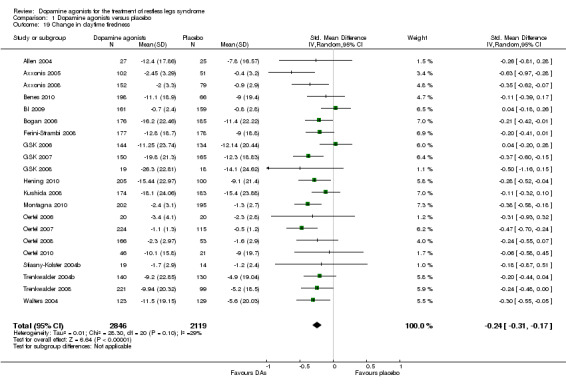
Comparison 1 Dopamine agonists versus placebo, Outcome 19 Change in daytime tiredness.
1.20. Analysis.
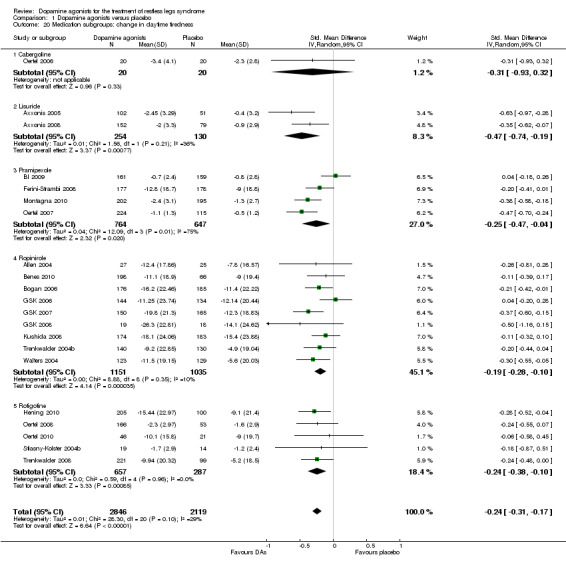
Comparison 1 Dopamine agonists versus placebo, Outcome 20 Medication subgroups: change in daytime tiredness.
1.21. Analysis.
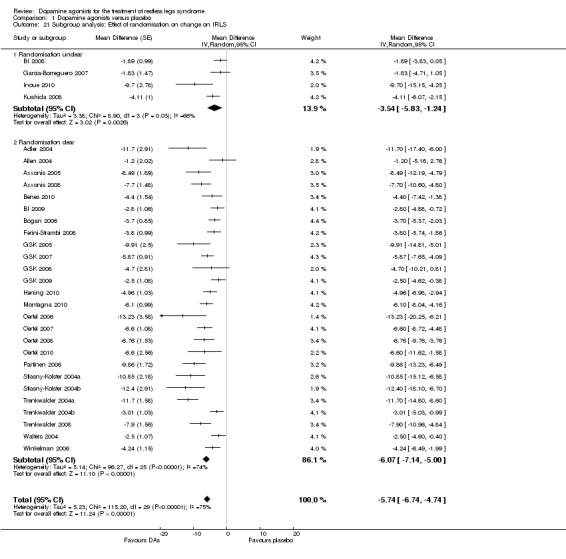
Comparison 1 Dopamine agonists versus placebo, Outcome 21 Subgroup analysis: Effect of randomisation on change on IRLS.
1.22. Analysis.
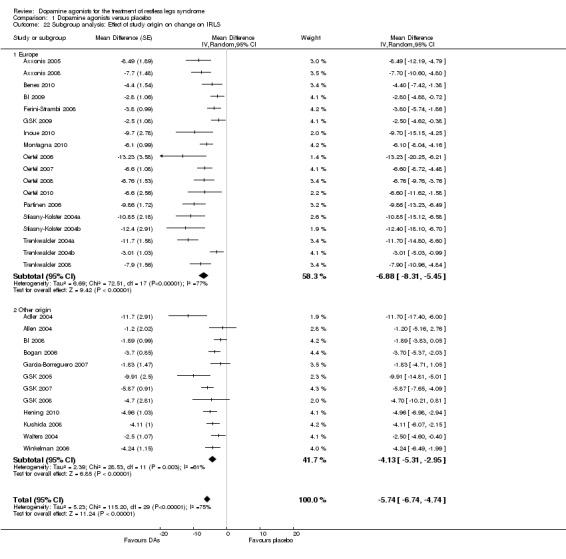
Comparison 1 Dopamine agonists versus placebo, Outcome 22 Subgroup analysis: Effect of study origin on change on IRLS.
Comparison 2. Active trials: dopamine agonists versus levodopa.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change on IRLS | 2 | 422 | Mean Difference (IV, Random, 95% CI) | ‐5.25 [‐8.40, ‐2.10] |
| 2 Change in periodic limb movements in sleep | 1 | 78 | Mean Difference (IV, Random, 95% CI) | ‐3.80 [‐9.08, 1.48] |
| 3 Number of dropouts due to adverse events | 3 | 504 | Odds Ratio (M‐H, Random, 95% CI) | 1.70 [0.96, 3.01] |
| 4 Responder rates on CGI‐I | 2 | 422 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.91, 1.56] |
| 5 Change in self rated quality of sleep | 1 | 344 | Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐1.35, 0.09] |
| 6 Change in quality of life | 1 | 314 | Mean Difference (IV, Random, 95% CI) | ‐5.54 [‐8.43, ‐2.65] |
| 7 Number of patients experiencing adverse events | 3 | 461 | Odds Ratio (M‐H, Random, 95% CI) | 2.87 [0.43, 19.00] |
| 8 Change in daytime tiredness | 1 | 344 | Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.41, 0.95] |
2.1. Analysis.
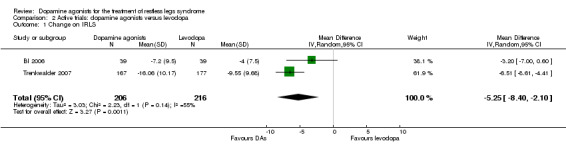
Comparison 2 Active trials: dopamine agonists versus levodopa, Outcome 1 Change on IRLS.
2.2. Analysis.
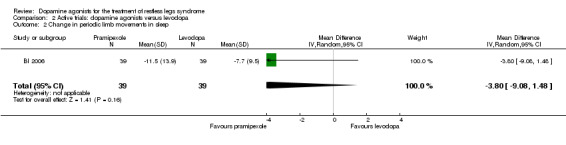
Comparison 2 Active trials: dopamine agonists versus levodopa, Outcome 2 Change in periodic limb movements in sleep.
2.3. Analysis.
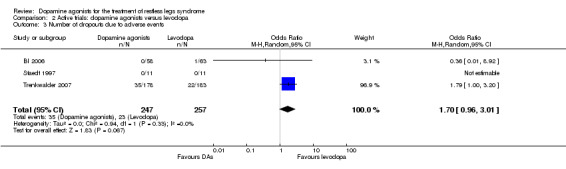
Comparison 2 Active trials: dopamine agonists versus levodopa, Outcome 3 Number of dropouts due to adverse events.
2.4. Analysis.
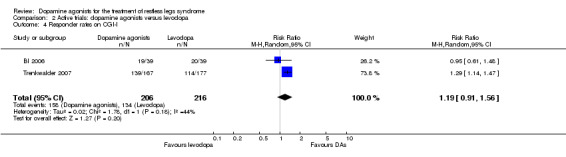
Comparison 2 Active trials: dopamine agonists versus levodopa, Outcome 4 Responder rates on CGI‐I.
2.5. Analysis.
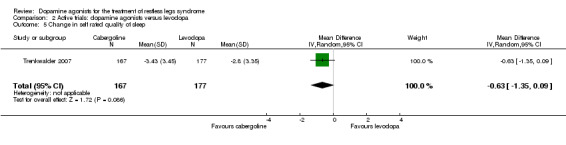
Comparison 2 Active trials: dopamine agonists versus levodopa, Outcome 5 Change in self rated quality of sleep.
2.6. Analysis.
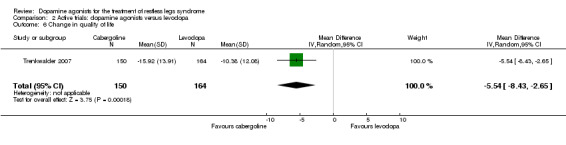
Comparison 2 Active trials: dopamine agonists versus levodopa, Outcome 6 Change in quality of life.
2.7. Analysis.
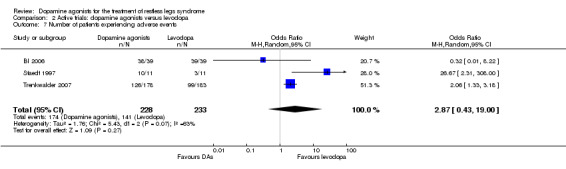
Comparison 2 Active trials: dopamine agonists versus levodopa, Outcome 7 Number of patients experiencing adverse events.
2.8. Analysis.
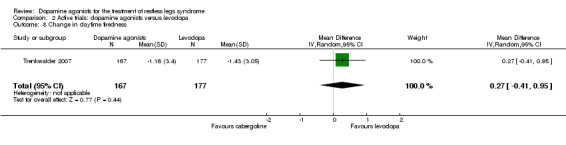
Comparison 2 Active trials: dopamine agonists versus levodopa, Outcome 8 Change in daytime tiredness.
Comparison 3. Active trials: lisuride vs. ropinirole.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change on IRLS | 1 | 230 | Mean Difference (IV, Random, 95% CI) | ‐3.0 [‐5.70, ‐0.30] |
| 2 Number of dropouts due to adverse events | 1 | 233 | Odds Ratio (M‐H, Random, 95% CI) | 2.53 [1.00, 6.42] |
| 3 Responders on CGI‐I | 1 | 223 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.82, 1.16] |
| 4 Change in self‐rated quality of sleep | 1 | 230 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.75, 1.15] |
| 5 Change in quality of life | 1 | 230 | Mean Difference (IV, Random, 95% CI) | ‐4.5 [‐8.12, ‐0.88] |
| 6 Number of patients experiencing adverse events | 1 | 233 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.43, 1.52] |
| 7 Change in daytime tiredness | 1 | 230 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐1.38, 0.38] |
3.1. Analysis.
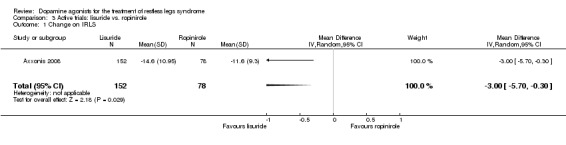
Comparison 3 Active trials: lisuride vs. ropinirole, Outcome 1 Change on IRLS.
3.2. Analysis.
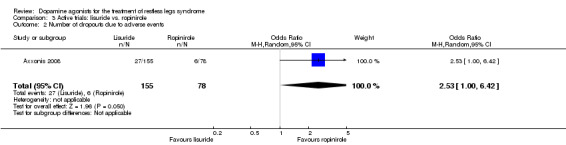
Comparison 3 Active trials: lisuride vs. ropinirole, Outcome 2 Number of dropouts due to adverse events.
3.3. Analysis.
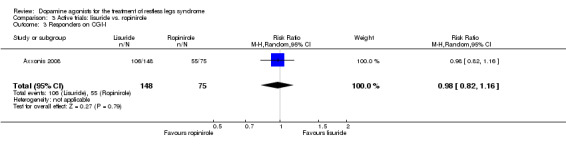
Comparison 3 Active trials: lisuride vs. ropinirole, Outcome 3 Responders on CGI‐I.
3.4. Analysis.
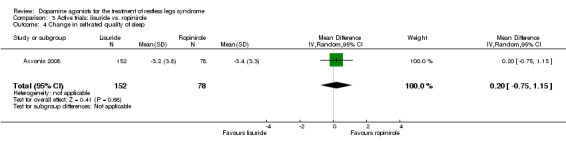
Comparison 3 Active trials: lisuride vs. ropinirole, Outcome 4 Change in self‐rated quality of sleep.
3.5. Analysis.
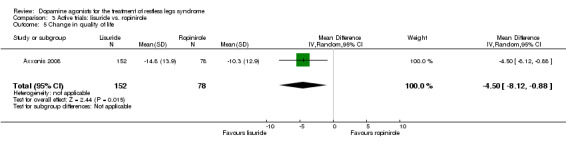
Comparison 3 Active trials: lisuride vs. ropinirole, Outcome 5 Change in quality of life.
3.6. Analysis.
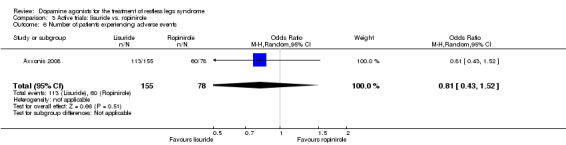
Comparison 3 Active trials: lisuride vs. ropinirole, Outcome 6 Number of patients experiencing adverse events.
3.7. Analysis.
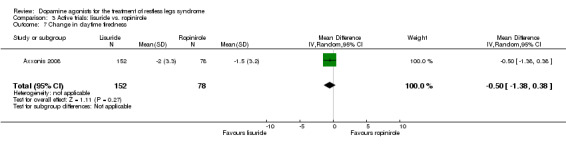
Comparison 3 Active trials: lisuride vs. ropinirole, Outcome 7 Change in daytime tiredness.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adler 2004.
| Methods | Randomised controlled cross‐over trial of ropinirole versus placebo Drop outs/withdrawals: ITT analysis of 22 patients (6 men), premature discontinuations in ropinirole (one in each period) and placebo (one in period 1) |
|
| Participants | Included/analysed: 22/22 Demographics: 6 male, age 60 years Diagnosis: RLS according to IRLSSG criteria and IRLS > 10 Setting: 1 site in the USA Baseline: IRLS score of 25.0 |
|
| Interventions | Intervention: forced up‐titration of ropinirole (twice daily) from 0.5 mg to 6 mg per day in 3 weeks (lower doses if up‐titration not tolerated), maintenance for 1 week Control: placebo for 4 weeks Washout between phases of 1 week |
|
| Outcomes | Observation period: 2 weeks washout and 4 weeks each treatment period Change of symptoms: IRLS Safety: number of withdrawals due to AEs, number of patients with AEs |
|
| funding source | Two authors (CH Adler and MD Sethi) received compensation in excess of $10,000. | |
| Notes | ITT: intention‐to‐treat; IRLS: International Restless Legs Study Group Rating Scale; AE: adverse event | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | The project statistician created the randomised treatment allocation schedule by using a computer random number generator. |
| Allocation concealment? | Low risk | Study drug was packaged for each patient by the Mayo Clinic Pharmacy. |
| Blinding? All outcomes | Low risk | Placebo tablets of the same size, shape, colour, and taste as the ropinirole tablets were provided by GlaxoSmithKline. The investigators, clinical staff, and patients did not know the treatment assignments until after the data were analysed. |
| Incomplete outcome data addressed? All outcomes | Low risk | Subjects who discontinued study drug prematurely had outcomes measured at the time of discontinuation and were included in the analysis. |
| Free of selective reporting? | Low risk | All results reported as prespecified. |
| Free of other bias? | Low risk | Low indication of other bias. |
Allen 2004.
| Methods | Randomised controlled parallel group trial of ropinirole versus placebo Dropouts/withdrawals: ITT (29/30), premature discontinuations of 4 in ropinirole group, 6 in placebo group |
|
| Participants | Included/analysed: 32 (ropinirole) and 33 (placebo)/ 29 (ropinirole) and 30 (placebo) Demographics: 26 male, age 55.4 (ropinirole) and 53.3 (placebo) years Diagnosis: RLS according to IRLSSG criteria and IRLS ≥ 15, symptoms ≥ 15 nights/last month and PLMS ≥ 5/H Setting: 15 sites in USA Baseline: PLMS‐Index of 48.5 (ropinirole) and 35.7 (placebo) |
|
| Interventions | Intervention: flexible up‐titration of ropinirole (once daily) from 0.25 to 4 mg in 8 weeks, maintenance for 4 weeks Control: placebo for 12 weeks |
|
| Outcomes | Observation period: ≥ 7 days washout, 12 weeks treatment Change of symptoms: IRLS, CGI responders Objective quality of sleep: SE, PLM‐Index (TIB, using PSG) Subjective quality of sleep: MOS sleep problems index II Daytime tiredness: MOS somnolence Quality of life: RLS‐QoL Safety: number of withdrawals due to AEs, number of patients with AEs |
|
| funding source | The study was supported by GlaxoSmithKline Research and Development. | |
| Notes | CGI: Clinical Global Impressions; SE: sleep efficiency; PLMS‐I: Periodic Limb Movements in Sleep Index per hour of time in bed (TIB); MOS: Medical Outcomes Study Sleep Scale with scales sleep problems index II and somnolence; RLS‐QoL: RLS Quality of Life Questionnaire | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | The allocation schedule was generated in blocks using the Sponsor's Coding Memo System. |
| Allocation concealment? | Low risk | RAMOS assigned a randomisation number and one container number. |
| Blinding? All outcomes | Low risk | Tablets were identical in appearance and packaged to be indistinguishable irrespective of treatment. Blinded status of study team, site monitors, investigators and patients was maintained. |
| Incomplete outcome data addressed? All outcomes | Low risk | Dropouts and LOCF method reported. |
| Free of selective reporting? | Low risk | All results reported as prespecified. |
| Free of other bias? | Low risk | Low indication of other bias. |
Axxonis 2005.
| Methods | Randomised controlled parallel group trial of lisuride patches versus placebo Dropouts/withdrawals: ITT (210) |
|
| Participants | Included/analysed: 157 (lisuride) and 53 (placebo)/ 154 (lisuride) and 51 (placebo) Demographics: 31.9% male, age 60.5 years Diagnosis: RLS according to IRLSSG criteria and RLS Diagnostic Clinical Interview ≥ 10, IRLS total score ≥ 15, RLS severity during rest ≥ 3 Setting: 25 sites in Germany and Austria Baseline: IRLS score of 28.8 (lisuride) and 29 (placebo) |
|
| Interventions | Intervention: fixed doses of lisuride patches (every 48 hours) in 3 arms: 2.5 mg, 5.0 mg, 10.0 mg, maintenance for 12 weeks Control: fixed dose of placebo patches for 12 weeks |
|
| Outcomes | Observation period: ≥ 7 days washout, 12 weeks treatment Change of symptoms: IRLS, CGI responders Subjective quality of sleep: RLS‐6 satisfaction with sleep Daytime tiredness: RLS‐6 daytime tiredness Quality of life: RLS‐QoL Safety: number of withdrawals due to AEs, number of patients with AEs |
|
| funding source | The study was supported by Axxonis Pharma AG. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | The computer‐based randomised treatment period was double‐blind. |
| Allocation concealment? | Low risk | Each patient was provided with the respective patient kit according to his/her randomisation number. |
| Blinding? All outcomes | Low risk | The placebo and lisuride patches were identical in composition, size and colour with the exception that placebo patches did not contain lisuride. Every patient received an identical number of active patches or matched placebo patches of identical size. [...] statistical Analysis Plan which was to be finalized prior to unblinding of the randomisation code. |
| Incomplete outcome data addressed? All outcomes | Low risk | LOCF method used for incomplete outcome data. |
| Free of selective reporting? | Low risk | All review specific outcomes reported after request. |
| Free of other bias? | Low risk | Low indication of other bias. |
Axxonis 2008.
| Methods | Randomised controlled parallel group trial of lisuride patches versus ropinirole and placebo Dropouts/withdrawals: ITT (309) |
|
| Participants | Included/analysed: both 152 (lisuride), 78 (ropinirole) and 79 (placebo) Demographics: 26.2% male, age 58.6 years Diagnosis: RLS according to IRLSSG criteria and RLS Diagnostic Clinical Interview > 10, IRLS total score ≥ 15, RLS severity during rest ≥ 3 Setting: 37 sites in Germany and Austria Baseline: IRLS score of 28.8 (lisuride) and 29 (placebo) |
|
| Interventions | Intervention: fixed doses of lisuride patches (every 48 hours) in 3 arms: 2.5 mg, 5.0 mg, 10.0 mg, maintenance for 12 weeks Control: fixed dose of placebo patches for 12 weeks |
|
| Outcomes | Observation period: ≥ 7 days washout, 12 weeks treatment Change of symptoms: IRLS, CGI responders Subjective quality of sleep: RLS‐6 Quality of life: RLS‐QoL Safety: number of withdrawals due to AEs, number of patients with AEs |
|
| funding source | The study was supported by Axxonis Pharma AG. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | The computer‐based randomised treatment period was double‐blind. |
| Allocation concealment? | Low risk | A patient received the lowest available randomisation number and was treated with the corresponding batch of study medication throughout the double‐blind treatment period. |
| Blinding? All outcomes | Low risk | Active drug and placebo, i.e. lisuride patches and placebo patches as well as ropinirole capsules and placebo capsules, were identical in shape, size, and colour with the exception that placebo patches did not contain lisuride and placebo capsules did not contain ropinirole. (2) Active drug and placebo and their accompanying packaging and labelling were identical in appearance. A detailed description of the planned analysis was fixed in a Statistical Analysis Plan which was to be finalized prior to unblinding of the randomisation code. |
| Incomplete outcome data addressed? All outcomes | Low risk | LOCF method used for incomplete outcome data. |
| Free of selective reporting? | Low risk | All review specific outcomes reported after request. |
| Free of other bias? | Low risk | Low indication of other bias. |
Benes 2010.
| Methods | Randomised controlled parallel‐group trial of ropinirole versus placebo Dropouts/withdrawals: ITT (171/60) with premature discontinuations of 54 (ropinirole) and 29 (placebo) |
|
| Participants | Included/ analysed: 199 (ropinirole) and 67 (placebo)/ 171 (ropinirole) and 60 (placebo) Demographics: 27.3% (ropinirole) and 32.8% male, age 58.2 (ropinirole) and 59.5 (placebo) years Setting: 62 centres in Germany Baseline: IRLS of 28.5 (ropinirole) and 29.0 (placebo) |
|
| Interventions | Intervention: flexible up‐titration of ropinirole (once daily) from 0.25 mg to 0.5 (1 week) to 4 mg in 12 weeks Control: placebo for 12 weeks |
|
| Outcomes | Observation period: 12 weeks treatment Change of symptoms: IRLS, CGI responders Daytime tiredness: MOS somnolence Safety: number of withdrawals due to AEs, number of patients with AEs |
|
| funding source | The study was supported by GlaxoSmithKline. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | The allocation schedule was generated by the sponsor´s (GlaxoSmithKline Germany) coding system. Investigators were not aware of the block size used. |
| Allocation concealment? | Low risk | Numbered boxes were distributed in ascending order. |
| Blinding? All outcomes | Low risk | Identical tablets for active and placebo treatment; neither investigator nor patient knew if treatment was active or placebo. |
| Incomplete outcome data addressed? All outcomes | Low risk | Dropouts and reasons reported. |
| Free of selective reporting? | Low risk | Outcomes changed in planning phase of study, investigated and reported as in lastly updated protocol. |
| Free of other bias? | Low risk | Low indication of other bias. |
BI 2006.
| Methods | Randomised controlled cross‐over trial of pramipexole versus levodopa Dropouts/withdrawals: PP (39), with 6 (levodopa) and 3 (ppx) premature discontinuations |
|
| Participants | Included/analysed: 67/ 39 Demographics: 16 male, age: 56.9 years Diagnosis: RLS according to IRLSSG criteria, symptom presence almost every day, PLMI > 5 Setting: 6 Swiss centres including Basel, Bern, Lugano, Luzern, Zürich, Zurzach Baseline: IRLS score of 21.1 (levodopa) and 20.8 (ppx), PLMI (actigraphy) of 21.1 (levodopa) and 21.5 (ppx) |
|
| Interventions | Intervention 1: flexible up‐titration of pramipexole (once daily) from 0.25 to 0.75 mg in 2 weeks, maintenance for 2 weeks Intervention 2: flexible up‐titration of levodopa‐dual‐release (once daily) from 100/25 mg to 300/75 mg for 2 weeks, maintenance for 2 weeks Washout between phases of 2 weeks |
|
| Outcomes | Observation period: 2 weeks run‐in, 4 weeks each treatment period Change of symptoms: IRLS, CGI responders Objective quality of sleep: PLM‐Index (using actigraphy) Safety: number of withdrawals due to AEs, number of patients with AEs |
|
| funding source | The study was supported by Boehringer Ingelheim, Inc. | |
| Notes | ppx: pramipexole | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomization schedule was provided by BI Pharma GmbH & Co KG. |
| Allocation concealment? | Low risk | Medication package with the lowest available number was allocated to the patient. |
| Blinding? All outcomes | Unclear risk | Tablets were packaged in identical hard gelatine capsules, the code was restricted to authorised personnel, such as staff involved in packaging of study medication. Blinding of data analysts not reported. |
| Incomplete outcome data addressed? All outcomes | Low risk | Dropouts and reasons reported by BI on request. |
| Free of selective reporting? | Low risk | Results reported as prespecified. |
| Free of other bias? | Low risk | Low indication of other bias. |
BI 2008.
| Methods | Randomised controlled parallel‐group trial of pramipexole versus placebo Dropouts/withdrawals: ITT (123/126/127) with premature exclusions of (18/15/11) |
|
| Participants | Included/analysed: 132 (intervention A), 137 (intervention B), 132 (placebo) / 123 (intervention A), 126 (intervention B), 127 (placebo) Demographics: 35 (A), 45 (B) and 52 (C) male, age 49.8 (A), 47.6 (B) and 49.6 (C) years Diagnosis: Idiopathic RLS according to IRLSSG criteria, IRLS > 15 Setting: 50 sites in the USA Baseline: IRLS of 25 (both treatment groups) and 24.9 (placebo) |
|
| Interventions | Intervention A: fixed dose of pramipexole 0.25 mg (once daily) for 6 weeks Intervention B: fixed up‐titration of pramipexole (once daily) from 0.125 mg (1 week) to 0.25 mg (once daily) for 5 weeks Control C: placebo for 6 weeks |
|
| Outcomes | Observation period: 6 weeks treatment Change of symptoms: IRLS, CGI responders, PGI responders Safety: number of withdrawals due to AEs, number of patients with AEs |
|
| funding source | The study was supported by Boehringer Ingelheim, Inc. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Insufficient information. |
| Allocation concealment? | Low risk | Assigning the next available medication kit by lowest kit number. |
| Blinding? All outcomes | Low risk | Each placebo tablet matched the corresponding pramipexole tablet by size and shape; both patient and investigator were blinded. |
| Incomplete outcome data addressed? All outcomes | Low risk | Dropouts and reasons reported. |
| Free of selective reporting? | Low risk | Outcomes reported by pharmaceutical company after request as prespecified. |
| Free of other bias? | Low risk | Low indication of other bias. |
BI 2009.
| Methods | Randomised controlled parallel group trial of pramipexole verus placebo Dropouts/withdrawals: Analysis with LOCF (166/163) with premature discontinuations of 35 (pramipexole) and 60 (placebo) |
|
| Participants | Included/ analysed for primary endpoint: 166 (pramipexole) and 163 (placebo)/ 162 (pramipexole) and 159 (placebo) Demographics: 38.2% (pramipexole) and 52.3% male, age 57.9 (ropinirole) and 55.8 (placebo) years Setting: 42 centres in 9 European countries Baseline: IRLS of 23.9 (pramipexole) and 23.5 (placebo) |
|
| Interventions | Intervention: flexible up‐titration of pramipexole (once daily) from 0.125 mg to 0.75 mg in 4 weeks and further 26 weeks Control: placebo for 12 weeks and further 26 weeks |
|
| Outcomes | Observation period: 26 weeks treatment Change of symptoms: IRLS, CGI responders, PGI responders Daytime tiredness: RLS‐6 daytime tiredness Quality of life: RLS‐QoL Safety: number of withdrawals due to AEs, number of patients with AEs |
|
| funding source | The study was supported by Boehringer Ingelheim GmbH. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation code provided by BI Pharma GmbH & Co. KG, the commercial program PMX CTM Release 3.3.0, Propack Data GmbH, was used to generate the code listing. |
| Allocation concealment? | Low risk | Patients were assigned the lowest available medication number available. |
| Blinding? All outcomes | Low risk | Both patients and investigators were blinded as to the treatment allocation, persons involved had no access to the treatment allocation schedule. |
| Incomplete outcome data addressed? All outcomes | Low risk | Dropouts and reasons reported. |
| Free of selective reporting? | Low risk | Outcomes reported as prespecified. |
| Free of other bias? | Low risk | Low indication of other bias. |
Bogan 2006.
| Methods | Randomised controlled parallel group trial of ropinirole versus placebo Dropouts/withdrawals: ITT (187/193) with premature discontinuations of 23 in ropinirole and 26 in placebo |
|
| Participants | Included/analysed: 187 (ropinirole) and 194 (placebo)/187 (ropinirole) and 193 (placebo) Demographics: 78 (ropinirole) and 70 (placebo) male, age 52.2 (ropinirole) and 52.4 (placebo) Diagnosis: RLS according to IRLSSG criteria and IRLS ≥ 15, symptoms ≥ 15 nights/previous month Setting: 47 sites in USA Baseline: IRLS score of 22.0 (ropinirole) and 21.6 (placebo), PLM‐I (actigraphy) of 39.8 (ropinirole) and 32.8 (placebo) |
|
| Interventions | Intervention: flexible up‐titration of ropinirole (once daily) from 0.25 to 4 mg in 10 weeks, maintenance for 2 weeks Control: placebo for 12 weeks |
|
| Outcomes | Observation period: ≥ 7 days washout, 12 weeks treatment Change of symptoms: IRLS, CGI responders Objective sleep quality: PLM‐Index (using actigraphy) Subjective sleep quality: MOS sleep problems index II Daytime tiredness: MOS somnolence Quality of life: RLS‐QoL Safety: number of withdrawals due to AEs, number of patients with AEs |
|
| funding source | The study was supported by GlaxoSmithKline Research and Development. | |
| Notes | PLM‐Index per time in bed (TIB) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomised using sponsor’s coding memo system. |
| Allocation concealment? | Low risk | Two bottles of study medication were distributed. |
| Blinding? All outcomes | Low risk | Study tablets and their packaging were identical in appearance, a blinded central reader was used for PSG. Patients, investigators and site monitors were kept blind during whole study. |
| Incomplete outcome data addressed? All outcomes | Low risk | All drop outs and reasons reported. |
| Free of selective reporting? | Low risk | All results reported as prespecified. |
| Free of other bias? | Low risk | Low indication of other bias. |
Earley 1998.
| Methods | Randomised controlled parallel group trial of pergolide versus placebo Dropouts/withdrawals: ITT (15) with missing post data of 1 patient |
|
| Participants | Included/analysed: 16/15 Demographics: 3 (pergolide) and 5 (placebo) male, age 62.5 (pergolide) and 56.5 (placebo) years Diagnosis: RLS according to IRLSSG criteria and PLMS > 15/h Setting: 1 centre in the USA Baseline: PLMS‐I (TST) 48.9 (pergolide) and 48.7 (placebo) |
|
| Interventions | Intervention: flexible up‐titration of pergolide (twice daily) from 0.1 mg to 0.65 mg per day in 2 weeks, maintenance for 5 days Control: placebo for 18 days |
|
| Outcomes | Observation period: 4 days washout, 18 days treatment Quality of sleep: SE, PLMS‐Index (using PSG) Safety: number of withdrawals due to AEs, number of patients with AEs |
|
| funding source | The study was supported in part by a gift from Athena Neurosciences, Inc. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomly generated schedule. |
| Allocation concealment? | Low risk | Two separate packets containing the maximum number of capsules. |
| Blinding? All outcomes | Low risk | Implementation of identical capsules, the investigator (R.P.A.) was blinded to any dose adjustments or reports of adverse reactions. |
| Incomplete outcome data addressed? All outcomes | Low risk | Description of invalid data of 1 patient (placebo) present. |
| Free of selective reporting? | Low risk | Results reported as prespecified, results of placebo‐group only in graph. |
| Free of other bias? | Low risk | Low indication of other bias. |
Ferini‐Strambi 2008.
| Methods | Randomised controlled parallel group trial of pramipexole (ppx) versus placebo Dropouts/withdrawals: ITT (178/179) with dropouts of 27 (ppx) and 52 (placebo) |
|
| Participants | Included/analysed:182 (ppx) and 187 (placebo)/ 178 (ppx) and 179 (placebo) Demographics: 50 (ppx) and 68 (placebo) male, age 56.3 (ppx) and 56.9 (placebo) years Diagnosis: RLS according to IRLSSG criteria and RLS symptoms present at least 2 to 3 days per week during the last 3 months prior to baseline; IRLS total score > 15 at baseline Setting: 49 sites in Europe Baseline: IRLS score of 24.2 (ppx) and 24.6 (placebo) |
|
| Interventions | Intervention: flexible up‐titration of pramipexole (once daily) from 0.125 mg to 0.75 mg in 4 weeks, maintenance for 8 weeks Control: placebo for 12 weeks |
|
| Outcomes | Observation period: 2 weeks washout, 12 weeks treatment Change of symptoms: IRLS, CGI responders, PGI responders Subjective quality of sleep: MOS sleep problems index II Daytime tiredness: MOS somnolence Quality of life: RLS‐QoL Safety: number of withdrawals due to AEs, number of patients with AEs |
|
| funding source | The study was supported by Boehringer Ingelheim GmbH. | |
| Notes | ppx: pramipexole; PGI: Patient Global Impressions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Clin‐Pro/LBL Version 5.2 release 1 software (Clinical Systems, Inc.) |
| Allocation concealment? | Low risk | Patients were assigned to the lowest medication number available. |
| Blinding? All outcomes | Low risk | Patients, investigators, and study site personnel were blinded to treatment identity without access to randomisation schedule. |
| Incomplete outcome data addressed? All outcomes | Low risk | All dropouts and reasons reported. |
| Free of selective reporting? | Low risk | All results reported as prespecified with additional report of RLS‐6 scales 4‐6 on request. |
| Free of other bias? | Low risk | Low indication of other bias. |
Garcia‐Borreguero 2007.
| Methods | Randomised controlled parallel group trial of sumanirole versus placebo Dropouts/withdrawals: ITT (56/49/59/54 for sumanirole doses of 0.5, 1.0, 2.0, 4.0 mg and 52 for placebo) with premature discontinuations of 8/9/10/7 (in sumanirole doses) and 10 (placebo) and PP analysis for IRLS (40/39/44/35 for sumanirole doses and 38 for placebo) |
|
| Participants | Included/analysed: both as ITT: 56/49/59/54 (sumanirole) and 52 (placebo); PP: 40/39/44/35 (sumanirole) and 38 (placebo) Demographics: 25/30/18/13 (sumanirole) and 22 (placebo) male, age 52.9 (placebo) to 55.4 years (4 mg sumanirole) Diagnosis: RLS according to IRLSSG criteria, IRLS ≥ 20 and RLS‐6 scale ≥4 in ≥12 weeks and PLMS ≥11/h Setting: 48 sites in the USA, Canada, Spain, Germany, Italy, UK Baseline: IRLS score of 25.4, 24.7, 26.1 and 26.5 (ascending sumanirole groups) and 25.2 (placebo), PLMS‐I of 39.9, 53.8, 39.2, 33.8 (ascending sumanirole groups) and 33.4 (placebo) |
|
| Interventions | Intervention: fixed up‐titration of sumanirole (once daily) to arms of 0.5 mg, 1 mg, 2 mg and 4 mg in 5 weeks, maintenance for 3 weeks Control: placebo for 8 weeks |
|
| Outcomes | Observation period: 8 weeks treatment Change of symptoms: IRLS (PP population), CGI responders Objective quality of sleep: SE, PLMS‐Index (using PSG) Safety: number of withdrawals due to AEs, number of patients with AEs |
|
| funding source | The study was funded by Pfizer Inc. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Insufficient information |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Insufficient information |
| Free of selective reporting? | Unclear risk | Results reported as prespecified in methods section without report of CGI‐S and ESS. Additionally, sleep efficiency and sleep latency were described. |
| Free of other bias? | Unclear risk | Insufficient information |
GSK 2005.
| Methods | Randomised controlled parallel‐group trial of ropinirole versus placebo Dropouts/withdrawals: ITT (37/17) with premature discontinuations of 8 (ropinirole) and 4 (placebo) |
|
| Participants | Included/ analysed: both 37 (ropinirole) and 17 (placebo) Demographics: 10 (ropinirole) and 4 (placebo) male, age 50.9 (ropinirole) and 56.2 (placebo) years Diagnosis: RLS according to IRLSSG criteria, IRLS total score ≥ 15, RLS symptoms ≥ 15 nights/ per month Setting: 11 centres in the USA Baseline: IRLS score of 26.0 (ropinirole) and 27.4 (placebo) |
|
| Interventions | Intervention: forced up‐titration of ropinirole (once daily) from 0.25 mg to 4 mg in 7 weeks Control: placebo for 7 weeks |
|
| Outcomes | Observation period: ≤ 7 days washout, 7 weeks treatment Change of symptoms: IRLS Safety: number of withdrawals due to AEs, number of patients with AEs |
|
| funding source | The study was supported by GlaxoSmithKline | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | A randomisation schedule was prepared prior to the start of the study by Clinical Pharmacology Statistics and Data Sciences (CPSDS), GlaxoSmithKline, Harlow, UK. Patients were assigned to study treatment in accordance with the randomisation schedule. |
| Allocation concealment? | Low risk | RAMOS assigned a randomisation number and two container numbers. |
| Blinding? All outcomes | Low risk | Active ropinirole and placebo tablets were identical in appearance and packaging to maintain the double‐blind nature of the study. The blinded status of the site monitors, investigators, and patient was thus maintained at all times. |
| Incomplete outcome data addressed? All outcomes | Low risk | All dropouts and reasons reported. |
| Free of selective reporting? | Low risk | All results reported as prespecified. |
| Free of other bias? | Unclear risk | Population size was based on feasibility. |
GSK 2006.
| Methods | Randomised controlled parallel‐group trial of ropinirole versus placebo Dropouts/withdrawals: ITT (154/149) with premature discontinuations of 48 in ropinirole and 37 in placebo |
|
| Participants | Included/analysed: 158 (ropinirole) and 151 (placebo)/ 154 (ropinirole) and 149 (placebo) Demographics: 45 (ropinirole) and 37 (placebo) male, age 56.2 (ropinirole) and 56.8 (placebo) years Diagnosis: RLS according to IRLSSG criteria, RLS symptoms ≥ 15 nights/ previous four weeks, which were considered to be moderate/severe Setting: 60 centres in the UK Baseline: IRLS score of 23.5 in both groups, PLMI (actigraphy) of 19.93 (ropinirole) and 22.67 (placebo) |
|
| Interventions | Intervention: flexible up‐titration of ropinirole (once daily) from 0.25 (2 days) to 0.5 mg (5 days) and finally to 4 mg in 11 weeks Control: placebo for 12 weeks |
|
| Outcomes | Obsevation period: 12 weeks treatment Change of symptoms: CGI responders, PGI responders Objective sleep quality: PLM‐Index ("time down during night", using actigraphy) Subjective sleep quality: MOS sleep problems index II Daytime tiredness: MOS somnolence Quality of life: RLS‐QoL Safety: number of withdrawals due to AEs, number of patients with AEs |
|
| funding source | The study was supported by GlaxoSmithKline. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | The randomisation was produced using GSK internal software. |
| Allocation concealment? | Low risk | Bottles were uniquely numbered according to the randomisation schedule. Patients were not aware which treatment these numbers applied to and neither were the physicians. |
| Blinding? All outcomes | Low risk | See above. |
| Incomplete outcome data addressed? All outcomes | Low risk | All dropouts and reasons reported. |
| Free of selective reporting? | Low risk | All results reported as prespecified. |
| Free of other bias? | Low risk | Low indication of other bias. |
GSK 2007.
| Methods | Randomised controlled parallel group trial of ropinirole versus placebo Dropouts/withdrawals: ITT (187/195) with premature discontinuations of 34 (ropinirole) and 35 (placebo) |
|
| Participants | Included/analysed: 189 (ropinirole) and 195 (placebo)/187 (ropinirole) and 195 (placebo) Demographics: 66 (ropinirole) and 63 (placebo) male, age 52.1 (ropinirole) years and 52.3 (placebo) years Diagnosis: RLS according to IRLSSG criteria and the RLS Diagnostic Clinical Interview, IRLS total score ≥ 15 Setting: 57 centres in the USA and 8 centres in Canada Baseline: IRLS score of 25.2 (ropinirole) and 25.3 (placebo) |
|
| Interventions | Intervention: flexible up‐titration of ropinirole (once daily) from 0.5 mg to 6 mg in 12 weeks Control: placebo for 12 weeks |
|
| Outcomes | Observation period: ≥ 7 days washout, 12 weeks treatment Change of symptoms: IRLS, CGI responders Daytime tiredness: MOS somnolence Quality of life: RLS‐QoL Safety: number of withdrawals due to AEs, number of patients with AEs |
|
| funding source | The study was supported by GlaxoSmithKline Research and Development. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | The randomisation was created in the GSK RANDALL system. |
| Allocation concealment? | Low risk | Investigators... obtain a randomisation number. |
| Blinding? All outcomes | Low risk | The blinded status of the study team, site monitors, investigators, and patient maintained at all times. |
| Incomplete outcome data addressed? All outcomes | Low risk | Dropouts and reasons reported. |
| Free of selective reporting? | Low risk | All results reported as prespecified. |
| Free of other bias? | Low risk | Low indication of other bias. |
GSK 2008.
| Methods | Randomised controlled parallel‐group trial of ropinirole‐CR versus placebo Dropouts/withdrawals: ITT (19/18) with premature discontinuations of 3 in ropinirole‐CR and 5 in placebo |
|
| Participants | Included/analysed: 20 (ropinirole) and 19 (placebo)/19 (ropinirole) and 18 (placebo) Demographics: 4 (ropinirole) and 2 (placebo) male, age 50.9 (ropinirole) and 49.8 (placebo) years Diagnosis: RLS according to IRLSSG criteria, PLMAI ≥ 5, sleep efficiency < 85% or latency > 20 minutes, RLS symptoms present ≥ 20 evenings or nights/ previous month, IRLS score ≥ 20 Setting: 18 study sites in the United States Baseline: IRLS score of 27.3 (ropinirole) and 26.5 (placebo), PLMI (TIB) of 50.2 (ropinirole) and 41.2 (placebo) |
|
| Interventions | Intervention: flexible up‐titration of ropinirole (once daily) from 0.5 mg to 6 mg in 12 weeks Control: placebo for 12 weeks |
|
| Outcomes | Observation period: 12 weeks treatment Change of symptoms: IRLS, CGI responders Objective quality of sleep: SE, PLM‐Index (TIB, using PSG) Subjective sleep quality: VAS (0‐10 = excellent) Daytime tiredness: MOS somnolence Safety: number of withdrawals due to AEs, number of patients with AEs |
|
| funding source | The study was supported by GlaxoSmithKline. | |
| Notes | PLMAI: Periodic Limb Movement Arousal Index per TIB | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | The randomization schedule was generated in GSK RANDALL system. |
| Allocation concealment? | Low risk | RAMOS (Registration and Medication Ordering System) was used to manage the randomisation of the patients and the ordering, dispensing and tracking of study medication. The study coordinator obtained a bottle number from RAMOS and ensured that the bottle dispensed to the subject was the correct bottle. |
| Blinding? All outcomes | Low risk | The blinded status of study was maintained at all times for the study team (including statistician), site monitors, subjects, investigators and polysomnography technologists. Once all subjects had completed the study, all data were in‐house and all queries resolved; the protocol violators were determined and the database frozen, the study was then unblinded. |
| Incomplete outcome data addressed? All outcomes | Low risk | All dropouts and reasons reported. |
| Free of selective reporting? | Low risk | All endpoints were investigated as presented in protocol. However, primary endpoints were changed from PLMAI and sleep latency and several secondary outcomes in the study protocol to the primary endpoint PLMI and several secondary outcomes in the online publication of the pharmaceutical company. |
| Free of other bias? | Low risk | The study was abandoned prematurely after 39 subjects were randomised for administrative reasons unrelated to safety or efficacy. |
GSK 2009.
| Methods | Randomised controlled parallel group trial of ropinirole versus placebo Dropouts/withdrawals: ITT (196/205) with premature discontinuations of 76 (ropinirole) and 59 (placebo) |
|
| Participants | Included/analysed: 197 (ropinirole) and 207 (placebo)/196 (ropinirole) and 205 (placebo) Demographics: 37.2% (ropinirole) and 36.6% male, age 56.5 (ropinirole) and 56.1 (placebo) years Setting: 35 centres in 9 European countries and Australia Baseline: IRLS of 27.7 (ropinirole) and 27.5 (placebo) |
|
| Interventions | Intervention: flexible up‐titration of ropinirole (once daily) from 0.25 mg to 2.0 mg in 12 weeks and further 26 weeks Control: placebo for 12 weeks and further 26 weeks |
|
| Outcomes | Observation period: 12 weeks treatment Change of symptoms: IRLS, CGI responders Safety: number of withdrawals due to AEs, number of patients with AEs |
|
| funding source | The study was supported by GlaxoSmithKline. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | The randomisation schedule was generated in GSK RANDALL system. |
| Allocation concealment? | Low risk | RAMOS was used to mange the randomisation of the patients and the ordering, dispensing and tracking of study medication. Investigators phoned into RAMOS at screening to register the subject and again at baseline to obtain a randomisation number. Each patient was assigned to a subject number and randomisation number. The study coordinator obtained a bottle number from RAMOS and ensured that the bottle dispensed to the subject was the correct bottle. |
| Blinding? All outcomes | Low risk | The blinded status of the study was maintained at all times for the study team (including statistician), site monitors, subjects, and investigators. Once all subjects had completed the study, all data were in‐house and all queries resolved; the protocol violators were determined and the database frozen, the study was then unblinded. |
| Incomplete outcome data addressed? All outcomes | Low risk | All dropouts and reasons reported. |
| Free of selective reporting? | Low risk | All results reported as prespecified. |
| Free of other bias? | Low risk | Low indication of other bias. |
Hening 2010.
| Methods | Randomised controlled parallel‐group trial of rotigotine versus placebo Dropouts/withdrawals: ITT (99/101/99/106 (rotigotine groups in ascending order) and 100 (placebo)) with dropouts of 23/47/36/46 (rotigotine) and 33 (placebo) |
|
| Participants | Included/analysed: 99/101/99/106 (rotigotine) and 100 (placebo)/99/101/99/106 (rotigotine) and 100 (placebo) Demographics: 35.5% (2 mg rotigotine) to 43% (placebo) male, age ranging from 51.3 (3 mg rotigotine) to 52.9 (0.5 mg rotigotine) years Diagnosis: RLS according to IRLSSG criteria, IRLS total score ≥ 15, CGI‐S ≥ 4 Setting: 63 sites in the USA Baseline: IRLS score of 23.5, 23.1, 23.2, 23.6 (ascending rotigotine groups) and 23.5 (placebo) |
|
| Interventions | Intervention: fixed doses of rotigotine (once daily, up‐titrated in 4 weeks) in 4 arms of 1.125 mg, 2.25 mg, 4.5 mg and 6.75 mg, maintenance for 6 months Control: placebo patches for 7 months |
|
| Outcomes | Observation period: 1 week washout, 7 months treatment Change of symptoms: IRLS, CGI responders, PGI responders Subjective quality of sleep: MOS sleep problems index II, additionally RLS‐6 satisfaction with sleep Daytime tiredness: MOS somnolence, additionally RLS‐6 daytime tiredness Safety: number of withdrawals due to AEs, number of patients with AEs |
|
| funding source | The study was sponsored by Schwarz Biosciences GmbH, UCB Group. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomization was performed in blocks by the sponsor based on a computer‐generated randomisation list. |
| Allocation concealment? | Low risk | To enrol a subject, the investigator must call the Interactive Voice Response System (IVRS). To randomise a subject, the investigator had to call the IVRS and provide brief details of the subject to be randomised. The IVRS automatically informed the investigator of the subjects randomisation number. The IVRS allocated kit numbers to the subject based on the randomisation number during the course of the trial. |
| Blinding? All outcomes | Low risk | Subjects and investigators were blinded as to trial medication and dose. Subjects were randomised to receive placebo or rotigotine. Rotigotine and placebo‐matched patches and their accompanying packaging were identical in appearance. A detailed description was described in a Statistical Analysis Plan which was finalized prior to unblinding of the randomisation code. |
| Incomplete outcome data addressed? All outcomes | Low risk | Numbers of patients in groups and dropouts were given by monitoring company. |
| Free of selective reporting? | Low risk | All relevant results reported by monitoring company after request. |
| Free of other bias? | Low risk | Low indication of other bias. |
Inoue 2010.
| Methods | Randomised controlled parallel group trial of pramipexole verus placebo Dropouts/withdrawals: ITT analysis (20/21), with premature discontinuations of 4 (placebo) |
|
| Participants | Included/analysed: 20 (ppx) and 21 (placebo) Demographics: 45% (ppx) and 52% (placebo) male, 48.7 (ppx) and 62.3 (placebo) years Diagnosis: RLS according to IRLSSG criteria, PLMI ≥ 5/hour, RLSRS > 15, symptoms ≥ once a week/ previous month Setting: Yoyogi Somnological Clinic and other 7 hospitals in Japan Baseline: IRLS of 23.4 (ppx) and 25.1 (placebo) and PLMS‐I of 25.8 (ppx) and 46.1 (placebo) |
|
| Interventions | Intervention: forced up‐titration of pramipexole (once daily) from 0.125 mg to 0.75 mg as tolerated in 3 weeks, maintenance for 3 weeks Control: placebo for 6 weeks |
|
| Outcomes | Observation period: 14 days washout, 6 weeks treatment Change of symptoms: IRLS, CGI responders, PGI responders Objective sleep quality: SE, PLMS‐Index (using PSG) Subjective quality of sleep: PSQI Safety: number of withdrawals due to AEs, number of patients with AEs |
|
| funding source | This study was supported by Boehringer Ingelheim GmbH. | |
| Notes | ppx: pramipexole; PSQI: Pittsburgh Sleep Quality Index | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomisation code present. |
| Allocation concealment? | Low risk | A randomised treatment number was allocated in ascending numerical order. Corresponding coded medication packages were provided by Nippon BI. |
| Blinding? All outcomes | Low risk | No persons who were directly involved in the planning or analysis of the trial were granted access to the randomisation schedule. |
| Incomplete outcome data addressed? All outcomes | Low risk | Dropouts and reasons reported. |
| Free of selective reporting? | Low risk | Results reported as prespecified in the study protocol. Additionally, data were reported in the online publication of the pharmaceutical company for parameters not prespecified in the study protocol such as polysomnography data, SIT, PSQI, ESS, CGI and PGI. |
| Free of other bias? | Low risk | Low indication of other bias. |
Kushida 2008.
| Methods | Randomised controlled parallel group trial of ropinirole versus placebo Dropouts/ withdrawals: ITT (175/184) with premature discontinuations of 24 (ropinirole) and 25 (placebo) |
|
| Participants | Included/analysed: 176 (ropinirole) and 187 (placebo)/175 (ropinirole) and 184 (placebo) Demographics: 67 (ropinirole) and 78 (placebo) male, age 51.4 (ropinirole) and 50.4 (placebo) years Diagnosis: RLS according to IRLSSG criteria, IRLS ≥ 20, Insomnia Severity Index ≥ 15, symptom onset after 5 pm, RLS symptoms ≥ 15 nights/ previous month Setting: 47 centres in the USA Baseline: IRLS score of 26.0 (both groups) PLMI (of "time down during night", actigraphy) |
|
| Interventions | Intervention: flexible up‐titration of ropinirole (twice daily) from 0.5 to 6.0 mg in 12 weeks Control: placebo for 12 weeks |
|
| Outcomes | Observation period: 10 days washout, 12 weeks treatment Change of symptoms: IRLS, CGI responders, PGI responders Objective quality of sleep: PLM‐Index (using actigraphy) Subjective quality of sleep: MOS sleep problems index II Daytime tiredness: MOS somnolence Quality of life: RLS‐QoL Safety: number of withdrawals due to AEs, number of patients with AEs |
|
| funding source | The study was supported by GlaxoSmithKline Research and Development. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Insufficient information |
| Allocation concealment? | Low risk | Subjects will be assigned to one of the two treatment arms in accordance with the randomisation schedule and randomisation number. |
| Blinding? All outcomes | Low risk | The blinded status of the study team, site monitors, investigators and subjects will be maintained at all times. |
| Incomplete outcome data addressed? All outcomes | Low risk | Dropouts and reasons reported. |
| Free of selective reporting? | Low risk | Results reported as prespecified with additional information for PGI, CGI and IRLS data by pharmaceutical publication and requested information of GSK. |
| Free of other bias? | Low risk | Low indication of other bias. |
Montagna 2010.
| Methods | Randomised controlled parallel group trial of pramipexole versus placebo Dropouts/withdrawals: ITT (404) and exclusion of 1 patient not having received at least 1 dose of drug |
|
| Participants | Included/analysed: 203 (ppx) and 200 (placebo) Demographics: 33% and 27% male, age 55.0 (ppx) and 56.1 (placebo) years Diagnosis: RLS according to IRLSSG criteria, IRLS > 15, rating of item 10 > 2, BDI total ≤ 28 Setting: 52 sites in 8 European countries and Korea Baseline: IRLS score of 25.9 (ppx) and 25.8 (placebo) |
|
| Interventions | Intervention: flexible up‐titration of pramipexole (once daily) from 0.125 mg to 0.75 mg in 4 weeks, maintenance for 8 weeks Control: placebo for 12 weeks |
|
| Outcomes | Observation period: 2 weeks washout, 12 weeks treatment Change of symptoms: IRLS, CGI responders, PGI responders Subjective quality of sleep: RLS‐6 satisfaction with sleep Daytime tiredness: RLS‐6 daytime tiredness Quality of life: RLS‐QoL Safety: number of withdrawals due to AEs, number of patients with AEs |
|
| funding source | The study was supported by Boehringer Ingelheim, Inc. | |
| Notes | ppx: pramipexole | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation code was provided by BI Pharmaceuticals, Inc., USA, using ClinPro/LBL Version 5.2 release 1 software. |
| Allocation concealment? | Low risk | Coded medication packages provided by BI and assigned lowest available treatment number at the site to each patient. |
| Blinding? All outcomes | Low risk | Patients, investigators, and study site personnel were blinded to treatment identity. |
| Incomplete outcome data addressed? All outcomes | Low risk | Dropouts and reasons reported. |
| Free of selective reporting? | Low risk | All results reported as prespecified with additional PGI and IRLS data. |
| Free of other bias? | Low risk | Low indication of bias. |
Montplaisir 1999.
| Methods | Randomised controlled cross‐over trial of pramipexole versus placebo Dropouts/withdrawals: PP (10) and exclusion of 1 patient due to scheduling problems |
|
| Participants | Included/analysed: 11/10 Demographics: 5 male, age 49.3 years Diagnosis: RLS according to IRLSSG criteria, disturbed sleep onset, symptoms for 3 nights/week ≥ 1 year and PLMS ≥ 10/H Setting: 1 site in Canada Baseline: PLMS‐I (TST) of 77.1 in all 10 patients |
|
| Interventions | Intervention: flexible up‐titration of pramipexole (once daily) from 0.375 mg, 0.75 mg to 1.5 mg in 2 weeks, maintenance for 2 weeks Control: placebo for 4 weeks Washout in between phases of 2 weeks |
|
| Outcomes | Observation period: 2 weeks washout, 4 weeks each treatment period Objective quality of sleep: PLMS‐Index (using PSG) Safety: number of withdrawals due to AEs |
|
| funding source | The study was supported by a grant from Pharmacia and Upjohn Inc. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Allocation generated electronically by the department responsible for packaging new investigational drugs. |
| Allocation concealment? | Low risk | Allocation concealed by coded packaging and papers. |
| Blinding? All outcomes | Low risk | Pramipexole tablets and identical looking placebo tablets, patients, investigators, sleep specialist and sponsor were all blinded to treatment sequence. |
| Incomplete outcome data addressed? All outcomes | Low risk | Dropout of one patient before treatment start reported and explained. |
| Free of selective reporting? | Low risk | Results reported as prespecified without data report for non significant sleep data. |
| Free of other bias? | Low risk | Low indication of bias. |
Oertel 2006.
| Methods | Randomised controlled parallel group trial of cabergoline versus placebo Dropouts/ withdrawals: PP (20/20) with premature discontinuations of 3 patients in the cabergoline group |
|
| Participants | Included/analysed: 43/ 40 Demographics: 6 (cabergoline) and 5 (placebo) male, age 57.3 (cabergoline) and 55.5 (placebo) years Diagnosis: RLS according to IRLSSG criteria, IRLS ≥ 10, RLS‐6 "severity at night" ≥ 4 and PLMS‐AI > 5 Setting: 7 German centres Baseline: IRLS score of 31.2 (cabergoline) and 31.8 (placebo), PLMS‐I (TST) 52.3 (cabergoline) and 61.7 (placebo) |
|
| Interventions | Intervention: fixed up‐titration of cabergoline (once daily) from 0.5 mg to 2.0 mg in 2 weeks, maintenance for 3 weeks Control: placebo for 5 weeks |
|
| Outcomes | Observation period: 5 half lives washout, 5 weeks treatment Change of symptoms: IRLS, CGI responders Objective quality of sleep: SE, PLMS‐Index (using PSG) Subjective quality of sleep: RLS‐6 satisfaction with sleep Daytime tiredness: RLS‐6 daytime tiredness Quality of life: QoL‐RLS Safety: number of withdrawals due to AEs, number of patients with AEs |
|
| funding source | The study was supported by Pfizer GmbH and by the Bundesministerium für Bildung und Forschung (BMBF, Germany). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation performed at sponsor’s statistical department before patient enrolment. |
| Allocation concealment? | Low risk | Numbered boxes, patients assigned by the investigators to one of the two treatments after the medication numbers in ascending order. |
| Blinding? All outcomes | Low risk | Visually identical placebo tablets, blinding was not broken before the total trial database had been locked. |
| Incomplete outcome data addressed? All outcomes | Low risk | 3 patients dropped out due to intolerable AEs in cabergoline group. |
| Free of selective reporting? | Low risk | All results reported as prespecified with no report of CGI‐therapeutic effect and side effects in results section as prespecified in methods section. |
| Free of other bias? | Low risk | Low indication of other bias. |
Oertel 2007.
| Methods | Randomised controlled parallel group trial of pramipexole versus placebo Dropouts/withdrawals: ITT (224/114) with premature discontinuations of 12 (ppx) and 8 (placebo) |
|
| Participants | Included/analysed: 230 (ppx) and 115 (placebo)/224 (ppx) and 114 (placebo) Demographics: 80 (ppx) and 36 (placebo) male, age 55.4 (ppx) and 55.8 (placebo) years Diagnosis: RLS according to IRLSSG criteria, IRLS > 15, symptoms for 2 to 3 days per week/ past 3 months Setting: 37 sites in 5 European countries Baseline: IRLS score of 24.7 (ppx) and 24.9 (placebo) |
|
| Interventions | Intervention: flexible up‐titration of pramipexole (once daily) from 0.125 mg, 0.25 mg, 0.5 mg to 0.75 mg in 4 weeks, maintenance for 2 weeks Control: placebo for 6 weeks |
|
| Outcomes | Observation period: 2 weeks washout, 6 weeks treatment Change of symptoms: IRLS, CGI responders, PGI responders Subjective quality of sleep: VAS (0‐10 = very dissatisfied with sleep) Daytime tiredness: IRLS item 5 Safety: number of withdrawals due to AEs, number of patients with AEs |
|
| funding source | The study was supported by Boehringer Ingelheim International. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation code provided by BI Pharma GmbH & Co KG, Medical Data Services, using the commercial programme ClinPro/LBL version 5.2 release 1. |
| Allocation concealment? | Low risk | Coded medication packages were provided by BI GmbH & Co KG, a randomised treatment number was allocated in ascending numerical order. |
| Blinding? All outcomes | Low risk | Tablets were identical in size, colour, shape and appearance. No person directly involved in the planning or analysis, neither patient nor investigator was granted access to the randomisation schedule. |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop outs and reasons reported. |
| Free of selective reporting? | Low risk | Results reported as prespecified. Additionally, SF‐36 and ESS were reported in online publication of the pharmaceutical company. |
| Free of other bias? | Low risk | Low indication of other bias. |
Oertel 2008.
| Methods | Randomised controlled parallel group trial of rotigotine versus placebo Dropouts/withdrawals: ITT (50/64/49/64/53 (rotigotine doses) and 53 (placebo)) with premature discontinuations of 5/5/1/7/5 (rotigotine doses) and 8 (placebo) patients |
|
| Participants | Included/analysed: 52/64/49/65/56 (rotigotine doses) and 55 (placebo)/ 50/64/49/64/53 (rotigotine doses) and 53 (placebo) Demographics: 26% (1.125 mg cabergoline) to 42.9% (2.25 mg rotigotine) male, age 57.3 (1 mg rotigotine) to 59.9 years (4 mg rotigotine) Diagnosis: RLS according to IRLSSG criteria, IRLS ≥ 15 Setting: 34 hospital outpatient units, sleep centres or private practices of neurologists in Austria, Germany, Spain Baseline: IRLS score of 27.4 (3 mg rotigotine) to 28.2 (4 mg rotigotine) |
|
| Interventions | Intervention: fixed doses of rotigotine patches (once daily, partly up‐titrated in up to 2 weeks) in 6 arms: 1.125 mg, 2.25 mg, 4.5 mg, 6.75 mg and 9 mg, maintenance for 4 weeks Control: fixed dose of placebo patches for 6 weeks |
|
| Outcomes | Observation period: 4 weeks washout for DAs, otherwise 1 week washout, 6 weeks treatment Change of symptoms: IRLS, CGI responders Subjective quality of sleep: RLS‐6 satisfaction with sleep Daytime tiredness: RLS‐6 daytime tiredness Quality of life: RLS‐QoL Safety: number of withdrawals due to AEs, number of patients with AEs |
|
| funding source | The study was supported by Schwarz Pharma. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomization by the sponsor’s biometrical department using the procedure “PLAN” from the SAS statistical package, Version 8.2. |
| Allocation concealment? | Low risk | Medication numbers in ascending order. |
| Blinding? All outcomes | Low risk | Placebo and rotigotine patches were identical in composition, size and colour. All placebo and active rotigotine patches and their accompanying packaging were identical in appearance. Each subject received an identical number of active patches or matched placebo patches of identical size. |
| Incomplete outcome data addressed? All outcomes | Low risk | Dropouts and reasons reported. |
| Free of selective reporting? | Low risk | All results were reported as prespecified. CGI therapeutic effect was not reported as described in methods section of the online publication of the pharmaceutical company and in the publication. |
| Free of other bias? | Low risk | Low indication of other bias. |
Oertel 2010.
| Methods | Randomised controlled parallel‐group trial of rotigotine versus placebo Dropouts/withdrawals: ITT (46/21) with dropouts of 5 (rotigotine) and 1 (placebo) |
|
| Participants | Included/analysed: both 46 (rotigotine) and 21 (placebo) Demographics: 11 (rotigotine) and 7 (placebo) male, age 60.8 (rotigotine) and 55.2 (placebo) years Diagnosis: RLS according to IRLSSG criteria, IRLS total score ≥ 15, CGI Item‐S ≥ 4, PLMI (time in bed) ≥ 15 Setting: 11 sites in 5 European countries Baseline: IRLS score of 26.3 (rotigotine) and 25.4 (placebo) |
|
| Interventions | Intervention: flexible up‐titration of rotigotine (once daily) from 2.25 mg, 4.5 mg to 6.75 mg in 3 weeks (± 3 days), maintenance for 4 weeks Control: placebo patches for 7 weeks |
|
| Outcomes | Observation period: 7 weeks treatment Change of symptoms: IRLS, CGI responders, PGI responders Objective quality of sleep: SE, PLMS‐Index (using PSG) Subjective quality of sleep: MOS sleep problems index II Daytime tiredness: MOS somnolence Quality of life: RLS‐QoL Safety: number of withdrawals due to AEs, number of patients with AEs |
|
| funding source | The study was sponsored by Schwarz Biosciences GmbH, UCB Group. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomization and patch supply were performed by the study sponsor and managed by an interactive voice response telephone system based on a computer‐generated randomisation list stratified by site in blocks of 6. |
| Allocation concealment? | Low risk | See above. |
| Blinding? All outcomes | Low risk | Rotigotine and placebo patches were identical in size and appearance. Participants, investigators and polysomnography raters were blinded to the treatment assignment. |
| Incomplete outcome data addressed? All outcomes | Low risk | Dropouts and reasons reported. |
| Free of selective reporting? | Low risk | Results reported as prespecified in the study protocol and methods section. Additionally, polysomnography data, the RLS‐6 scales, the MOS, ESS, ASRS, CGI 2 and 3, RLS‐QoL and ratings of depression and tolerability were implemented. |
| Free of other bias? | Low risk | Low indication of other bias. |
Partinen 2006.
| Methods | Randomised controlled parallel group trial of pramipexole versus placebo Dropouts/withdrawals: ITT (21/22/22/21 (ppx) and 21 (placebo)) with premature discontinuations of 1 patient in the pramipexole group and 1 patient in the placebo group |
|
| Participants | Included/analysed: 109/107 Demographics: 28 male, age 56.2 years Diagnosis: RLS according to IRLSSG criteria, IRLS ≥ 15, weekly symptoms = 3 months and PLMS ≥ 5/h Setting: 1 research centre in Finland Baseline: IRLS score of 22.4, 23.0, 23.6 and 21.7 (ascending ppx groups) and 22.9 (placebo); PLMS‐I (TST) 22.3, 29.4, 24.75, 29.68 (ppx groups) and 42.85 (placebo) |
|
| Interventions | Intervention: fixed doses of pramipexole (once daily, partly up‐titrated) in 4 arms of 0.125 mg, 0.25 mg, 0.5 mg and 0.75 mg for 3 weeks Control: placebo for 3 weeks |
|
| Outcomes | Observation period: 1 week washout of RLS medication, 3 weeks treatment Change of symptoms: IRLS, CGI responders, PGI responders Objective quality of sleep: SE, PLMS‐Index (using PSG) Subjective quality of sleep: SSQ (1‐10 = excellent sleep quality) Safety: number of withdrawals due to AEs, number of patients with AEs |
|
| funding source | The study was supported by Boehringer Ingelheim GmbH. | |
| Notes | ppx: pramipexole; SSQ: subjective sleep quality scale ‐ VAS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation code provided by BI Pharma GmbH & Co KG, Medical Data Services, using the commercial programme ClinPro/LBL version 5.2 release 1. |
| Allocation concealment? | Low risk | Coded medication packages were provided by BI GmbH & Co KG, a randomised treatment number was allocated in ascending numerical order. |
| Blinding? All outcomes | Low risk | Tablets were identical in size, colour, shape and appearance. No person directly involved in the planning or analysis, neither patient nor investigator was granted access to the randomisation schedule. |
| Incomplete outcome data addressed? All outcomes | Low risk | Dropouts and reasons reported. |
| Free of selective reporting? | Low risk | Results reported as prespecified. |
| Free of other bias? | Low risk | Low indication of other bias. |
Pieta 1998.
| Methods | Randomised controlled cross‐over trial of pergolide versus placebo Dropouts/withdrawals: PP (8) with dropouts of 6 |
|
| Participants | Included/analysed: 14/8 Demographics: 3 male, age 42.5 years Diagnosis: Patients on chronic haemodialysis, continuous peritoneal dialysis and symptoms of restless legs Setting: 2 university hospitals in Canada Baseline: none; severe secondary RLS |
|
| Interventions | Intervention: fixed up‐titration of pergolide (once daily) from 0.05 mg to 0.25 mg in 8 nights, maintenance 2 nights Control: placebo for 10 nights Washout between phases of 1 week |
|
| Outcomes | Observation period: 3 days washout before trial, 10 days each treatment period Objective quality of sleep: SE, PLMS‐Index (using PSG) Safety: number of withdrawals due to AEs, number of patients with AEs |
|
| funding source | The study was supported by the Kidney Foundation of Canada. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Insufficient information |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes | Low risk | Leg movements were counted separately by blinded scorers and scored for association with arousals. |
| Incomplete outcome data addressed? All outcomes | Low risk | All dropouts and reasons reported. |
| Free of selective reporting? | Low risk | Results described as prespecified and data partly reported. |
| Free of other bias? | Low risk | Low indication of other bias. |
Staedt 1997.
| Methods | Randomised active controlled cross‐over trial of levodopa versus pergolide Dropouts/ withdrawals: ITT (11), no premature discontinuations |
|
| Participants | Included/ analysed: 11 Demographics: 6 male, age 57.6 years Diagnosis: patients with a history of restlessness and paraesthesias at night and/or day Setting: 1 centre in Germany Baseline: PLMS‐disturbed time of sleep 164 ± 80.4 min |
|
| Interventions | Intervention 1: flexible up‐titration of single dose levodopa/benserazide from 250 mg to 500 mg in 16 days Intervention 2: flexible up‐titration of single dose pergolide from 0.125 mg to 0.25 mg in 16 days Washout of 24 hours between treatment periods |
|
| Outcomes | Safety: number of withdrawals due to AEs, number of patients with AEs | |
| funding source | No funding stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Insufficient information |
| Allocation concealment? | Low risk | Medication concealed in capsules for double‐blind administration. |
| Blinding? All outcomes | Unclear risk | Insufficient information, not mentioned if research staff was blinded. |
| Incomplete outcome data addressed? All outcomes | Low risk | No incomplete outcome data. |
| Free of selective reporting? | Low risk | All results reported as prespecified, additional sleep data reported. |
| Free of other bias? | Low risk | Low indication of other bias. |
Stiasny‐Kolster 2004a.
| Methods | Randomised controlled parallel group trial of cabergoline versus placebo Dropouts/withdrawals: ITT (84) with 6 premature discontinuations in the cabergoline group |
|
| Participants | Included/analysed: 85/ 84 Demographics: 29.4% male, age 56.1 years Diagnosis: RLS according to IRLSSG criteria, RLS‐6 "severity at night" ≥ 4 Setting: 10 German centres Baseline: IRLS score of 27.2, 25.2 and 27.7 (ascending cabergoline groups) and 26.0 (placebo) |
|
| Interventions | Intervention: fixed up‐titration of cabergoline (once daily) from 0.5 to 1.0 mg (3 days), to 1.5 mg and to 2.0 mg (4 and 7 days) in 2 weeks, 3 weeks maintenance Control: placebo for 5 weeks |
|
| Outcomes | Observation period: 1 to 2 (for levodopa) weeks washout, 5 weeks treatment Change of symptoms: IRLS Subjective quality of sleep: RLS‐6 satisfaction with sleep Safety: number of withdrawals due to AEs, number of patients with AEs |
|
| funding source | The study was supported by Pfizer GmbH and the Bundersministerium für Bildung und Forschung (BMBF, Germany). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomization was performed [...] centrally with a computer program. |
| Allocation concealment? | Low risk | Patients assigned to one of the available medication numbers in ascending order, numbered containers dispensed. |
| Blinding? All outcomes | Low risk | Visually identical placebo tablets, study blind was not broken before the total database of the trial had been frozen. |
| Incomplete outcome data addressed? All outcomes | Low risk | Dropouts before and during trial and reasons reported. |
| Free of selective reporting? | Low risk | All results reported as prespecified. |
| Free of other bias? | Low risk | Low indication of other bias. |
Stiasny‐Kolster 2004b.
| Methods | Randomised controlled parallel group trial of rotigotine versus placebo Dropouts/withdrawals: ITT (63) with one premature discontinuation in the placebo group |
|
| Participants | Included/analysed: both 17/13/19 (rotigotine) and 14 (placebo) Demographics: 36.5% male, age 58.3 years (ranging from 54.6 to 60.1 years in groups) Diagnosis: RLS according to IRLSSG criteria, IRLS ≥ 10 and RLS‐6 "severity during the day when at rest" ≥ 3 Setting: 9 sites in Germany Baseline: IRLS score of 26.1, 26.6 and 25.9 (ascending rotigotine groups) and 25.0 (placebo) |
|
| Interventions | Intervention: fixed dose of rotigotine patch (once daily) in 3 arms of 1.125 mg, 2.25 mg and 4.5 mg for 1 week Control: placebo patches for 1 week |
|
| Outcomes | Observation period: 1 to 4 (for DAs) weeks washout, 1 week treatment Change of symptoms: IRLS, CGI responders Subjective quality of sleep: RLS‐6 satisfaction with sleep Daytime tiredness: RLS‐6 daytime tiredness Safety: number of withdrawals due to AEs, number of patients with AEs |
|
| funding source | The study was supported by Schwarz Pharma AG. | |
| Notes | DA: dopamine agonist | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomization was done by the Sponsor by use of a SAS procedure. |
| Allocation concealment? | Low risk | Subject numbers were attributed to subjects in a consecutive, ascending order. If at the baseline visit the subject fulfilled all inclusion and exclusion criteria, the subject was allocated to the next free consecutive randomisation number, starting from 80001. |
| Blinding? All outcomes | Low risk | Placebo and rotigotine patches were identical in composition with the exception that the placebo patches did not contain rotigotine. All placebo and active rotigotine patches and their accompanying packaging were identical in appearance. Each subject received an identical number of active patches or matched placebo of identical size. By these measures, the subjects and all other persons involved in the trial, remained blind until the randomisation code list was opened. |
| Incomplete outcome data addressed? All outcomes | Low risk | Dropouts and reasons reported. |
| Free of selective reporting? | Low risk | Results were reported as prespecified. |
| Free of other bias? | Low risk | Low indication of other bias. |
Trenkwalder 2004a.
| Methods | Randomised controlled parallel group trial of pergolide versus placebo Dropouts/withdrawals: ITT (100) with premature discontinuations of 8 (pergolide) and 9 (placebo) |
|
| Participants | Included/analysed: both 100 Demographics: 40 male, age 56.2 years Diagnosis: RLS according to IRLSSG criteria, sleep disturbances requiring treatment for the past 3 months, PLMS‐AI > 5 and SOL > 25 or sleep efficiency ≤ 85% Setting: 17 centres in 6 European countries (Belgium, Finland, Germany, Italy, The Netherlands, Spain) and Australia Baseline: IRLS score of 23.7 (pergolide) and 25.0 (placebo), PLM‐I (TIB) of 39.6 (pergolide) and 44.2 (placebo) |
|
| Interventions | Intervention: fixed up‐titration of pergolide (once daily) from 0.05 mg to 0.25 mg in 8 days, flexible adjustment to 0.75 mg in 6 weeks total including a minimum of 10 days maintenance Control: placebo for 6 weeks |
|
| Outcomes | Observation period: single‐blind washout period of 10 to 14 days, 6 weeks treatment Change of symptoms: IRLS, PGI responders Objective quality of sleep: SE, PLM‐Index (TIB, using PSG) Safety: number of patients with AEs |
|
| funding source | The study was supported by Eli Lilly and Company. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated random number sequence. |
| Allocation concealment? | Low risk | Kit numbers were assigned. |
| Blinding? All outcomes | Low risk | Identical capsules, PSG recordings were read at an independent centre by a blinded rater. |
| Incomplete outcome data addressed? All outcomes | Low risk | Dropouts and reasons reported. |
| Free of selective reporting? | Low risk | All results reported as prespecified. |
| Free of other bias? | Low risk | Low indication of other bias. |
Trenkwalder 2004b.
| Methods | Randomised controlled parallel group trial of ropinirole versus placebo Dropouts/withdrawals: ITT (146/138) with premature discontinuations of 34 (ropinirole) and 29 (placebo) |
|
| Participants | Included/analysed: 147 (ropinirole) and 139 (placebo)/146 (ropinirole) and 138 (placebo) Demographics: 58 (ropinirole) and 47 (placebo) male, age 54.0 (ropinirole) and 56.2 (placebo) years Diagnosis: RLS according to IRLSSG criteria, IRLS ≥ 15 and symptoms ≥ 15 nights in the previous month (or same frequency before treatment) Setting: 43 hospitals, sleep centres, and neurology clinics in 10 European countries (Austria, Belgium, France, Germany, Italy, Netherlands, Norway, Spain, Sweden, United Kingdom) Baseline: IRLS score of 24.4 (ropinirole) and 25.2 (placebo) |
|
| Interventions | Intervention: flexible up‐titration of ropinirole (once daily) from 0.25 mg to 4.0 mg (2 reductions due to AEs possible) in 7 weeks, maintenance for 5 weeks Control: placebo for 12 weeks |
|
| Outcomes | Observation period: ≤ 7 days washout, 12 weeks treatment Change of symptoms: IRLS, CGI responders Subjective quality of sleep: MOS sleep problems index II Daytime tiredness: MOS somnolence Quality of life: RLS‐QoL Safety: number of withdrawals due to AEs, number of patients with AEs |
|
| funding source | The study was supported by GlaxoSmithkline Research and Development. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Sponsor’s coding memo system |
| Allocation concealment? | Low risk | Randomisation and medication ordering system (RAMOS) to register and randomise patients. |
| Blinding? All outcomes | Low risk | Identical looking tablets; investigators, patients and study monitors were blinded to the treatment status of the patients at all times. |
| Incomplete outcome data addressed? All outcomes | Low risk | Dropouts and reasons reported. |
| Free of selective reporting? | Low risk | All results reported as prespecified. |
| Free of other bias? | Low risk | Low indication of other bias. |
Trenkwalder 2007.
| Methods | Randomised active controlled parallel‐group trial with cabergoline and levodopa Dropouts/withdrawals: ITT (178 (cabergoline/183 (levodopa)) with premature discontinuations of 39 (cabergoline) and 38 (levodopa) in 6 weeks and PP (104 (cabergoline) and 100 (levodopa)) |
|
| Participants | Included/analysed: 178 (cabergoline) and 183 (levodopa)/ ITT: 178 (cabergoline), 183 (levodopa), PP: 104 (cabergoline), 100 (levodopa) Demographics of ITT: 58 (cabergoline) and 46 (levodopa), age 56.9 (cabergoline) and 58.7 (levodopa) years Diagnosis: RLS according to IRLSSG criteria, IRLS ≥ 10 and RLS‐6 "severity at night" ≥ 4 Setting: 51 centres in four European countries Baseline: IRLS score of 25.6 (cabergoline) and 25.8 (levodopa) |
|
| Interventions | Intervention 1: forced up‐titration to single dose levodopa/benserazide 250 mg (in 8 days), severe cases 375 mg (81 patients) Intervention 2: forced up‐titration to single dose cabergoline 2 mg (in 14 days), severe cases 3 mg (30 patients) for 42 (short term) and 210 days (long term) |
|
| Outcomes | Observation period:1 week single‐blind placebo run‐in, 6 weeks treatment Change of symptoms: IRLS, CGI Subjective quality of sleep: RLS‐6 satisfaction with sleep Daytime tiredness: RLS‐6 daytime tiredness Quality of life: RLS‐QoL Safety: number of withdrawals due to AEs, number of patients with AEs |
|
| funding source | The study was supported by Pfizer GmbH. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Sequentially assigned to one of the two treatments by the investigators using medication numbers in ascending order for each block of 4 which was allocated to the study site after central randomisation using the program SAS Proc. Plan Version v 8.2. |
| Allocation concealment? | Low risk | Medication numbers in ascending order. |
| Blinding? All outcomes | Low risk | Identical looking tablets. |
| Incomplete outcome data addressed? All outcomes | Low risk | Dropouts and reasons described. |
| Free of selective reporting? | Low risk | All results reported as prespecified with further report of short term data for secondary outcomes following request. |
| Free of other bias? | Low risk | Low indication of other bias. |
Trenkwalder 2008.
| Methods | Randomised controlled parallel group trial of rotigotine versus placebo Dropouts/withdrawals: ITT (112/109/112 (rotigotine groups in ascending order) and 114 (placebo)) with dropouts of 31/25/40 (rotigotine) and 49 (placebo) |
|
| Participants | Included/analysed: 115/112/114 (rotigotine) and 117 (placebo)/112/109/112 (rotigotine) and 114 (placebo) Demographics: 27% to 34% male, age ranging from 56.5 (3 mg rotigotine) to 59.7 (placebo) years Diagnosis: RLS according to IRLSSG criteria, IRLS ≥ 15 and CGI "severity of symptoms" ≥ 4 Setting: 49 centres in eight European countries (Austria, Finland, Germany, Italy, Netherlands, Spain, Sweden, UK) Baseline: IRLS score of 28.1, 28.2 and 28.0 (ascending rotigotine groups) and 28.1 (placebo) |
|
| Interventions | Intervention: fixed up‐titration of rotigotine (once daily) to 3 arms: 2.25 mg, 4.5 mg and 6.75 mg in 3 weeks, maintenance until month 6 Control: placebo patches for 6 months and 3 weeks |
|
| Outcomes | Observation period: up to 4 weeks washout, 6 months and 3 weeks treatment Change of symptoms: IRLS, CGI responders, PGI responders Subjective quality of sleep: MOS sleep problems index II Daytime tiredness: MOS somnolence Quality of Life: RLS‐QoL Safety: number of withdrawals due to AEs, number of patients with AEs |
|
| funding source | The study was sponsored by Schwarz Biosciences GmbH, UCB Group. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated randomisation list |
| Allocation concealment? | Low risk | Interactive voice response system to allocate patients |
| Blinding? All outcomes | Low risk | Identical looking tablets |
| Incomplete outcome data addressed? All outcomes | Low risk | Dropouts and reasons reported. |
| Free of selective reporting? | Low risk | All results reported as prespecified with additional results for MOS, CGI‐I responders and RLS‐QoL. |
| Free of other bias? | Low risk | Low indication of other bias. |
Walters 2004.
| Methods | Randomised controlled parallel group trial of ropinirole versus placebo Dropouts/withdrawals: ITT(131/135) with premature discontinuations of 29 (ropinirole) and 29 (placebo) |
|
| Participants | Included/analysed: 131 (ropinirole) and 136 (placebo)/131 (ropinirole) and 135 (placebo) Demographics: 42% (ropinirole) and 38.5 % (placebo) male, age 54.9 (ropinirole) and 56.0 (placebo) years Diagnosis: RLS according to IRLSSG criteria, IRLS ≥ 15 and symptoms ≥ 15 nights/ previous month Setting: 46 centres in Australia, Europe and North America Baseline: IRLS score of 23.6 (ropinirole) and 24.8 (placebo) |
|
| Interventions | Intervention: Flexible up‐titration of ropinirole (once daily) from 0.25 mg to 4.0 mg in 7 weeks, maintenance for 5 weeks Control: placebo for 12 weeks |
|
| Outcomes | Observation period: 1 week washout, 12 weeks treatment Change of symptoms: IRLS, CGI responders Subjective quality of sleep: MOS sleep problems index II Daytime tiredness: MOS somnolence Quality of life: RLS‐QoL Safety: number of withdrawals due to AEs, number of patients with AEs |
|
| funding source | The study was supported by GlaxoSmithKline Research and Development. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Centralized allocation system, the Registration and Medication Order System (RAMOS) |
| Allocation concealment? | Low risk | Allocation of container number for the medication via RAMOS |
| Blinding? All outcomes | Low risk | Identical looking tablets |
| Incomplete outcome data addressed? All outcomes | Low risk | Dropouts and reasons reported |
| Free of selective reporting? | Low risk | All results reported as prespecified without data report of MOS, SF‐36 and WPAI. |
| Free of other bias? | Low risk | Low indication of other bias. |
Wetter 1999.
| Methods | Randomised controlled cross‐over trial of pergolide versus placebo Dropouts/withdrawals: PP (28) with premature discontinuations of 1 (pergolide) and 1 (placebo) |
|
| Participants | Included/analysed: 30/28 Demographics: 12 male, age 57.2 years Diagnosis: RLS according to IRLSSG criteria, PLMS‐I > 5, SOL > 25 and/or sleep efficiency < 85% Setting: Max Planck Institute of Psychiatry in Munich and Department of Neurology at University of Marburg, Germany Baseline: CGI‐Severity of condition rating of 5.1 (rating 1‐7 = extremely ill) |
|
| Interventions | Intervention: flexible up‐titration of pergolide (once daily) from 0.05 mg to 0.75 mg in 2 weeks, maintenance for 2 weeks Control: placebo for 4 weeks Washout between phases of 1 week |
|
| Outcomes | Observation period: 2 weeks washout before trial, 4 weeks each treatment period Objective quality of sleep: SE, PLM‐Index (using PSG) Safety: number of withdrawals due to AEs |
|
| funding source | Eli Lilly and Company provided the drugs for this study. Lilly Deutschland GmbH provided a $25,000 grant for a part‐time research nurse. No pertinent financial support to the authors or their immediate families was given. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Insufficient information |
| Allocation concealment? | Low risk | Study numbers in consecutive order |
| Blinding? All outcomes | Unclear risk | No information about blinding of PSG raters or other investigators |
| Incomplete outcome data addressed? All outcomes | Low risk | Dropouts and reasons reported |
| Free of selective reporting? | Low risk | All results reported as prespecified without report of SOL and PLMS total data. |
| Free of other bias? | Low risk | Low indication of other bias. |
Winkelman 2006.
| Methods | Randomised controlled parallel group trial of pramipexole versus placebo Dropouts/withdrawals: ITT (88/79/87 (ppx) and 85 (placebo)) with dropouts of 10/19/23 (ppx) and 11 (placebo) |
|
| Participants | Included/analysed: 88/80/90 (ppx) and 86 (placebo)/88/79/87 (ppx) and 85 (placebo) Demographics: 33.3% (0.75 mg ppx) to 45.6% (0.5 mg ppx) male, age ranging from 49.6 (0.5 mg ppx) to 53.4 (0.25 mg ppx) years Diagnosis: RLS according to IRLSSG criteria, IRLS > 15, symptoms for 2‐3 days ≥ previous 3 months Setting: 43 sites in the United States Baseline: IRLS score of 23.4, 22.9 and 24.1 (ascending ppx groups) and 23.5 (placebo) |
|
| Interventions | Intervention: fixed up‐titration of pramipexole (once daily) from 0.125 mg in 1 week to 3 arms in 3 weeks: 0.25 mg, 0.5 mg, 0.75 mg, maintenance for 9 weeks Control: placebo for 12 weeks |
|
| Outcomes | Observation period: 2 weeks washout, 12 weeks treatment Change of symptoms: IRLS, CGI responders, PGI responders Subjective quality of sleep: VAS (0‐50) Quality of life: RLS‐QOL Safety: number of withdrawals due to AEs, number of patients with AEs |
|
| funding source | JW Winkelman has received honoraria during the course of this study from Boehringer Ingelheim, Inc. | |
| Notes | ppx: pramipexole | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Standard randomisation program ClinPro/LBL, Version 5.2, release 1 (Clinical Systems Inc.) |
| Allocation concealment? | Low risk | Medication numbers in ascending numerical order |
| Blinding? All outcomes | Low risk | Sub‐investigators who performed solely the CGI‐I assessment were unaware of the patients’ IRLS self‐ratings and of reported adverse events. |
| Incomplete outcome data addressed? All outcomes | Low risk | All dropouts and reasons reported. |
| Free of selective reporting? | Low risk | All results reported as prespecified, with IRLS and quality of sleep described in figures. |
| Free of other bias? | Low risk | Low indication of other bias. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abetz 2005 | Validation study of the RLS‐QoL using pooled data from TREAT RLS 1 (Trenkwalder 2004b) and TREAT RLS 2 (Walters 2004). |
| Abetz 2006 | Validation study of the IRLS using pooled data from TREAT RLS 1 and TREAT RLS (see above). |
| Allen 1998 | Results of the study were published in Earley 1998. |
| Anonymous 2006 | Overview on RLS. |
| Benes 2005 | Results of the study were published in Benes 2006. |
| Benes 2006 | Study included a first open label phase and a second withdrawal phase. |
| Benes 2006a | Publication presenting two open label studies. |
| Bingham 2002 | Planned study, data were not analysed. |
| Bliwise 2005 | Study included a first open label phase and a second withdrawal phase. |
| Ebell 1999 | The reference which had been cited in CINAHL was not detectable. |
| Freeman 2001 | Open label study. |
| Gunning 1999 | Results of the study were published in Earley 1998. |
| Kinge 2005 | No control group was implemented in the study. |
| Manconi 2003 | Open label study. |
| Miranda 2003 | Open label study. |
| Miranda 2004 | No control group was implemented in the study. |
| Montplaisir 1998 | Results of the study were published in Montplaisir 1999. |
| Montplaisir 2006 | Study included a first open label phase and a second withdrawal phase. |
| Morgan 2008 | Analysis using pooled data from TREAT RLS 1 and 2 (Trenkwalder 2004b; Walters 2004). |
| Noel 1998 | Open label study. |
| Oertel 2005 | Results of the study were published in Oertel 2008. |
| Partinen 2006a | Results of the study were published in Partinen 2006. |
| Partinen 2006b | Results of the study were published in Partinen 2006. |
| Penzel 2002 | Open label study. |
| Plowman 2005 | Overview on RLS. |
| Powell 2007 | Overview on RLS treatment results. |
| Reading 2007 | Overview on sleep problems. |
| Reuter 1999 | Inadequate design. |
| Staedt 1998 | No control group was implemented in the study. |
| Stiasny 2000 | Survey study. |
| Stiasny 2001 | Open label study. |
| Stiasny 2002 | Results of the study were published in Stiasny‐Kolster 2004a. |
| Tagaya 2002 | Results of the study were fully published in Wetter 1999. |
| Thorpy 2005 | Discussion of TREAT RLS 2 (Walters 2004). |
| Trenkwalder 2001a | Results of the study were published in Trenkwalder 2004b. |
| Trenkwalder 2001b | Results of the study were published in Trenkwalder 2001b. |
| Trenkwalder 2005 | Results of the study were published in Trenkwalder 2007. |
| Trenkwalder 2006 | Study included a first open label phase and a second withdrawal phase. |
| Wetter 1998 | Results of the study were published in Wetter 1999. |
| Winkelman 2005 | Results of the study were published in Winkelman 2006. |
| Yee 2004 | Results of the study were published in Walters 2004. |
Differences between protocol and review
Two outcomes defined in the protocol were specified in detail, three outcomes were added and one outcome parameter was dropped after discussion in the light of actual research before search for publications.
PLMS‐Index and number of patients dropping out due to adverse events were defined as primary and not as secondary outcome parameters. Sleep efficiency assessed in polysomnography was appended as primary outcome.
We added questionnaires assessing self and clinician rated general improvement (PGI and CGI‐I) in the secondary outcome section. We excluded the planned outcome patient satisfaction with treatment as the number of RLS trials investigating this parameter was very low.
We investigated not only the first phase of cross‐over trials, but included both phases of cross‐over trials and partly corrected statistically for the different design (see Reviewer's Handbook, chapter 16.4).
We included search dates from 1985 (instead of 1995) to 2009 in order to obtain all possibly relevant trials. The only relevant study published before 1995 (Walters 1988), however, could not be included in the meta‐analysis due to the predefined exclusion criteria.
We conducted additional searches in online trial registers provided by pharmaceutical companies, the Pharmaceutical Research and Manufacturers of America (PhRMA) and the U.S. National Institutes for Health.
Instead of implementing fixed‐effect models where data were homogeneous, we performed all meta‐analyses using random‐effects models as recommended by the Reviewer's Handbook.
We did not conduct subgroup analyses but separate meta‐analyses on efficacy of dopamine agonist treatment compared to other active treatments. The influence of treatment duration was assessed in additional meta‐regressions instead of subgroup analysis. Furthermore, meta‐regressions of the influence of number of investigating sites and baseline IRLS score on IRLS treatment effect were performed.
Due to the study designs of the included trials (flexible dosing regimen, mostly similar dosing), it was not possible to investigate influence of different dosages on treatment effect.
As we performed subgroup analyses by comparing studies at low risk of bias and studies at moderate risk of bias, sensitivity analyses were considered as redundant and were not performed as described in the protocol.
Contributions of authors
MH: Performing previous work that was the foundation of the current review, conceiving the review, designing the review, coordinating the review, providing general advice on the review, securing funding for the review, screening of obtained publications regarding eligibility, appraising quality of papers, data collection for the review, writing to study authors for additional information, interpretation of data, writing the review, providing a clinical perspective, providing a policy perspective, providing a consumer perspective.
HS: Undertaking searches, screening search results, organizing retrieval of papers, screening retrieved publications regarding eligibility, appraising quality of papers, extracting data from papers, writing to study authors for additional information, obtaining and screening data on unpublished studies, data management for the review, entering data into RevMan, analysis of data, interpretation of data, writing the review, providing a methodological perspective.
CT: Performing previous work that was the foundation of the current review, coordinating the review, securing funding for the review, providing general advice on the review, interpretation of data, writing the review, providing a clinical perspective, providing a policy perspective, providing a consumer perspective.
RK: Performing previous work that was the foundation of the current review, conceiving the review, designing the review, coordinating the review, securing funding for the review, providing general advice on the review, providing additional data of included trials, interpretation of data, providing a methodological perspective.
LK: Conceiving the review, designing the review, providing general advice on the review, writing the protocol, designing search strategies, analysis of data, interpretation of data.
DR: Coordinating the review, securing funding for the review, providing general advice on the review.
Sources of support
Internal sources
Department of Psychiatry and Psychotherapy, University Hospital Freiburg, Germany.
External sources
-
German Ministry for Education and Research, Germany.
(Bundesministerium für Bildung und Forschung ‐ BMBF, Project number DLR 01KG0723).
Declarations of interest
Hanna Scholz has no conflicts of interest to declare.
Claudia Trenkwalder has been an advisor for Boehringer Ingelheim, Cephalon, Mundipharm, Orion Pharma, Novartis, Solvay, Axxonis and UCB. She has received lecture honoraria from Boehringer Ingelheim, UCB, TEVA and AstraZeneca.
Ralf Kohnen received honoraria for advisory board membership from Pfizer, USA; Axxonis, Germany; Roche, Germany; UCB, Germany; Jazzpharma, USA.
Dieter Riemann received research support from DFG, BMBF, EU (public funding) and from Takeda, Sanofi‐Aventis, Organon, Actelion and Omron. He was on the speakers bureau of Sanofi‐Aventis, Takeda, Servier, Lundbeck, Boehringer Ingelheim, GSK, Cephalon and Merz Pharmaceuticals. He was also a member of advisory boards for Sanofi‐Aventis, Lundbeck, GSK, Takeda and Actelion. Dr. Riemann declares that the above mentioned activities have no influence on the content of this article.
Levente Kriston has no conflicts of interest to declare.
Magdolna Hornyak received honoraria and/or lecture fees from Boehringer Ingelheim, Germany; GlaxoSmithKline, Germany; Roche, Germany; Pfizer Inc., Germany.
Edited (no change to conclusions)
References
References to studies included in this review
Adler 2004 {published data only}
- Adler CH, Hauser RA, Sethi K, Caviness JN, Marlor L, Anderson WM, et al. Ropinirole for restless legs syndrome: a placebo‐controlled crossover trial. Neurology 2004;62(8):1405‐7. [DOI] [PubMed] [Google Scholar]
Allen 2004 {published and unpublished data}
- Allen R, Becker PM, Bogan R, Schmidt M, Kushida CA, Fry JM, et al. Ropinirole decreases periodic leg movements and improves sleep parameters in patients with restless legs syndrome. Sleep: Journal of Sleep and Sleep Disorders Research 2004;27(5):907‐14. [DOI] [PubMed] [Google Scholar]
- SKF‐101468/191. A 12 week, double‐blind, placebo‐controlled, parallel group study to assess the efficacy, safety and tolerability of ropinirole in subjects with restless legs syndrome (RLS) suffering from periodic leg movements of sleep (PLMS). http://www.gsk‐clinicalstudyregister.com/result_detail.jsp?protocolId=101468%2f191&studyId=2514&compound=Ropinirole (accessed 15 January 2009).
Axxonis 2005 {unpublished data only}
- Benes H on behalf of the TULIR study group. Transdermal lisuride in patients with idiopathic restless legs syndrome: results from a placebo‐controlled, double‐blind, randomized, multicenter, 12‐week dose‐finding study. Sleep Medicine 2005;6(S2):S156. [Google Scholar]
Axxonis 2008 {unpublished data only}
- Benes H, Kohnen R. Transdermal lisuride in patients with severe restless legs syndrome: results from a placebo‐ and ropinirole‐controlled, double‐blind, randomized, multicenter, 12‐week efficacy and tolerability study. Sleep 2008;31 Abstract Suppl:A266. [Google Scholar]
Benes 2010 {published and unpublished data}
- Benes H, Mattern W, Peglau I, Dreykluft T, Bergmann L, Hansen C, et al. on behalf of the ROAD‐RLS study group. Ropinirole improves depressive symptoms and restless legs syndrome severity in RLS patients: a multicentre, randomised, placebo‐controlled study. Journal of Neurology revision submitted. [DOI] [PubMed]
- RRL 106721. A multi‐center, randomized, placebo‐controlled, double‐blind phase IIIb study of the effects of ropinirole on mood and (sub‐clinical) depression in the therapy of patients with moderate and severe idiopathic restless legs syndrome. http://www.gsk‐clinicalstudyregister.com/files/pdf/21025.pdf (accessed 12 June 2009).
BI 2006 {published and unpublished data}
- 248.518. A double‐blind, randomised, crossover trial investigating the efficacy and safety of the dopamine agonist pramipexole (Sifrol®, 0.25‐0.75 mg per day) versus levodopa / benserazide (Madopar® DR, 125‐375 mg per day) in patients with restless legs syndrome. http://trials.boehringer‐ingelheim.com/res/trial/data/pdf/248.518_U08‐2249_upload.pdf (accessed 16 March 2009).
- Mathis J, Bassetti C. Comparison of pramipexole (PPX) versus levodopa/benserazide (L/B) in the treatment of restless legs syndrome (RLS): A double blind, randomized, Swiss multi‐centre crossover trial. Movement Disorders 2006;21 Suppl 15:S442. [Google Scholar]
BI 2008 {unpublished data only}
- 248.616. A Phase IV randomised, double‐blind, active and placebo‐controlled, 6‐week trial to investigate the efficacy and safety of a starting (and fixed) dose 0.25 mg pramipexole (Mirapex®) in patients with idiopathic Restless Legs Syndrome. http://trials.boehringer‐ingelheim.com/res/trial/data/pdf/248.616_U08‐3876_upload19194.pdf (accessed 4 March 2009).
BI 2009 {published and unpublished data}
- 248.629. A phase IV randomised, double‐blind, placebo‐controlled, dose titration trial with pramipexole (Sifrol®, Mirapexin®) 0.125‐0.75 mg/day per os to investigate the long‐term efficacy, safety and tolerability in patients with idiopathic moderate to severe Restless Legs Syndrome for 26 weeks. http://trials.boehringer‐ingelheim.com/res/trial/data/pdf/248.629_U09‐1047.pdf (accessed 4 December 2009).
- Högl B. Efficacy and safety of pramipexole for restless legs syndrome: a 26‐week, randomized, placebo‐controlled, clinical trial in the European Union. AAN Congress; 2009 May 25‐June 2, Seattle 2009.
- Högl B, Poewe W, Garcia‐Borreguero D, Ferini‐Strambi L, Trenkwalder C. 26‐week effects of pramipexole on quality of life in restless legs syndrome. Presented at The Movement Disorder Society’s13th Annual International Congress of Parkinson’s Disease and Movement Disorders; 2009 June 7–11; Paris. 2009.
Bogan 2006 {published and unpublished data}
- Bogan RK, Fry JM, Schmidt MH, Carson SW, Ritchie SY, Group TRUS. Ropinirole in the treatment of patients with restless legs syndrome: a US‐based randomized, double‐blind, placebo‐controlled clinical trial. Mayo Clinic Proceedings 2006;81(1):17‐27. [DOI] [PubMed] [Google Scholar]
- SKF‐101468/249. A 12 week, double‐blind, placebo‐controlled, parallel group study to assess the efficacy and safety of ropinirole in patients suffering from restless legs syndrome (RLS). http://www.gsk‐clinicalstudyregister.com/result_detail.jsp?protocolId=101468%2f249&studyId=19934&compound=Ropinirole (accessed 4 February 2009).
Earley 1998 {published data only}
- Earley CJ, Yaffee JB, Allen RP. Randomized, double‐blind, placebo‐controlled trial of pergolide in restless legs syndrome. Neurology 1998;51(6):1599‐602. [DOI] [PubMed] [Google Scholar]
Ferini‐Strambi 2008 {published data only (unpublished sought but not used)}
- Ferini‐Strambi L, Aarskog D, Partinen M, Chaudhuri KR, Sohr M, Verri D, et al. Effect of pramipexole on RLS symptoms and sleep: a randomized, double‐blind, placebo‐controlled trial. Sleep Medicine 2008;9(8):874‐81. [DOI] [PubMed] [Google Scholar]
Garcia‐Borreguero 2007 {published data only}
- Garcia‐Borreguero D, Winkelman J, Adams A, Ellis A, Morris M, Lamb J, et al. Efficacy and tolerability of sumanirole in restless legs syndrome: a phase II, randomized, double‐blind, placebo‐controlled, dose‐response study. Sleep Medicine 2007;8(2):119‐27. [DOI] [PubMed] [Google Scholar]
GSK 2005 {published and unpublished data}
- Kelly M, Mistry P. Tolerability of a forced‐dose escalating regimen of ropinirole in patients with RLS. Movement Disorders 2005;20 Suppl 10:S63. [Google Scholar]
- SKF‐101468/207. A double‐blind, randomized, placebo‐controlled, parallel‐group study to investigate the tolerability of a dose‐escalating regimen of ropinirole in patients suffering from Restless Legs Syndrome (RLS). http://www.gsk‐clinicalstudyregister.com/result_detail.jsp?protocolId=101468%2f207&studyId=2523&compound=Ropinirole (accessed 12 January 2009).
GSK 2006 {unpublished data only}
- ROP 101892. A 12‐week, double‐blind, placebo‐controlled, parallel‐group study to assess the effectiveness of ropinirole in patients willing to take regular medication for their restless legs syndrome in a primary care setting. http://www.gsk‐clinicalstudyregister.com/result_detail.jsp?protocolId=101892&studyId=23627&compound=Ropinirole (accessed 12 January 2009).
GSK 2007 {published and unpublished data}
- Becker P, Bogan R, Earl N. Ropinirole CR ‐ a novel extended‐release formulation ‐ demonstrates onset of symptom improvement from the first night of treatment in patients with moderate‐to‐severe primary restless legs syndrome. Sleep 2007;30(Abstract Suppl):A282. [Google Scholar]
- SKF‐101468/205. A 12‐week, double‐blind, placebo‐controlled, parallel‐group study to assess the efficacy and safety of ropinirole controlled release for RLS (CR‐RLS) in patients with restless legs syndrome. http://www.gsk‐clinicalstudyregister.com/result_detail.jsp?protocolId=101468%2f205&studyId=19930&compound=Ropinirole (accessed 4 March 2009).
GSK 2008 {unpublished data only}
- RRL 103660. A 12‐week, multi‐center, double‐blind, placebo‐controlled, parallel‐group, flexible dose polysomnography study of ropinirole controlled release for restless legs syndrome (CR‐RLS) in RLS patients with sleep disturbance and periodic limb movements (PLM) during sleep. http://www.gsk‐clinicalstudyregister.com/result_detail.jsp?protocolId=RRL103660&studyId=21024&compound=Ropinirole (accessed 4 March 2009).
GSK 2009 {unpublished data only}
- ROR 104836. A randomised, double‐blind, placebo‐controlled, parallel group study to evaluate the efficacy and safety of ropinirole for 26 weeks and to further evaluate the incidence of augmentation and rebound for a further 40 weeks open‐label extension treatment period in subjects suffering from moderate to severe restless legs syndrome. http://www.gsk‐clinicalstudyregister.com/files/pdf/21020.pdf (accessed 4 November 2009).
Hening 2010 {published and unpublished data}
- Hening WA, Allen RP, Ondo WG, Walters AS, Winkelman JW, Becker P, et al. the SP792 Study Group. Rotigotine improves restless legs syndrome: a 6‐month randomized, double‐blind, placebo‐controlled trial in the United States.. Movement Disorders 2010;25(11):1675‐83. [DOI] [PubMed] [Google Scholar]
- SP792. A multicenter, randomized, double‐blind, placebo‐controlled, 5‐arm parallel‐group trial to investigate theefficacy and safety of 4 different transdermal doses of rotigotine in subjects with idiopathic restless legs syndrome. http://www.clinicaltrials.gov/ct2/show/NCT00135993 (accessed 4 March 2009).
Inoue 2010 {published and unpublished data}
- 248.557. A randomised, double‐blind, placebo‐controlled study to evaluate the efficacy and safety of pramipexole with the dose range from 0.125 mg to 0.75 mg orally once daily for 6 weeks in patients with primary restless legs syndrome. http://trials.boehringer‐ingelheim.com/res/trial/data/pdf/248.557_U06‐3385_new.pdf (accessed 4 March 2009).
- Inoue Y, Fujita M, Shimizu T, Emura N, Kuroda K, Uchimura N. Efficacy and safety of pramipexole in Japanese patients with restless legs syndrome. Movement Disorders 2006;21 Suppl 15:S442. [Google Scholar]
- Inoue Y, Hirata K, Kuroda K, Fujita M, Shimizu T, Emura N, et al. Efficacy and safety of pramipexole in Japanese patients with primary restless legs syndrome: A polysomnographic randomized, double‐blind, placebo‐controlled study. Sleep Medicine 2010;11:11‐16. [DOI] [PubMed] [Google Scholar]
Kushida 2008 {published and unpublished data}
- Kushida CA, Geyer J, Tolson JM, Asgharian A. Patient‐ and physician‐rated measures demonstrate the effectiveness of ropinirole in the treatment of restless legs syndrome. Clinical Neuropharmacology 2008;31(5):281‐6. [DOI] [PubMed] [Google Scholar]
- RRL 100013. A 12‐week, double‐blind, placebo‐controlled, twice‐daily dosing study to assess the efficacy and safety of ropinirole in patients suffering from restless legs syndrome (RLS) requiring extended treatment coverage. http://www.gsk‐clinicalstudyregister.com/result_detail.jsp?protocolId=100013&studyId=23628&compound=Ropinirole (accessed 12 January 2009).
Montagna 2010 {published and unpublished data}
- 248.604. A phase IV randomised, double‐blind, placebo‐controlled, dose titration trial with 0.125‐0.75 mg/day pramipexole (Sifrol®, Mirapexin®) orally for 12 weeks to investigate the safety and efficacy in out‐patients with idiopathic Restless Legs Syndrome associated with mood disturbances. http://trials.boehringer‐ingelheim.com/res/trial/data/pdf/248.604_U07‐2329upload_new19148.pdf (accessed 4 March 2009).
- Hornyak M, Montagna P, Ulfberg J, Hong S, Koester J, Crespi G. Treatment of daytime RLS symptoms with pramipexole in patients with mood disturbance. Neurology 2008;70(11 Suppl 1):A293 [P05.167]. [Google Scholar]
- Montagna P, Hornyak M, Ulfberg J, Bong Hong S, Koester J, Crespi G, et al. Randomized trial of pramipexole for patients with restless legs syndrome and mood disturbance. Sleep Medicine in press. [DOI] [PubMed]
- Montagna P, Hornyak M, Ulfberg J, Hong S, Koester J, Crespi G. Pramipexole for the treatment of RLS and associated depressive symptoms. Neurology 2008;70(11 Suppl 1):A294 [P085.173]. [Google Scholar]
- Montagna P, Hornyak M, Ulfberg J, Hong S, Koester J, Crespi G. Rapid onset of action and sustained efficacy of pramipexole in RLS patients with mood disturbance. Neurology 2008;70(11 Suppl 1):A292‐3 [P05.166]. [Google Scholar]
Montplaisir 1999 {published data only}
- Montplaisir J, Nicolas A, Denesle R, Gomez‐Mancilla B. Restless legs syndrome improved by pramipexole: a double‐blind randomized trial. Neurology 1999;52(5):938‐43. [DOI] [PubMed] [Google Scholar]
Oertel 2006 {published data only (unpublished sought but not used)}
- CABAS‐0067‐033. A double‐blind, randomized, placebo‐controlled multi‐center efficacy study for the treatment of patients with Restless Legs Syndrome (RLS). http://www.clinicaltrials.gov/ct2/show/NCT00627003 (accessed 4 March 2009).
- Oertel WH, Benes H, Bodenschatz R, Peglau I, Warmuth R, Happe S, et al. Efficacy of cabergoline in restless legs syndrome: a placebo‐controlled study with polysomnography (CATOR). Neurology 2006;67(6):1040‐6. [DOI] [PubMed] [Google Scholar]
Oertel 2007 {published and unpublished data}
- 248.520. A randomised, double‐blind, placebo‐controlled dose titration trial with 0.125‐0.75 mg pramipexole (Sifrol®) orally to investigate the safety and efficacy in out‐patients with idiopathic Restless Legs Syndrome for 6 weeks followed by 46 weeks open‐label or double‐blind treatment period. http://trials.boehringer‐ingelheim.com/res/trial/data/pdf/248.520__U05‐1394‐01_new.pdf (accessed 4 March 2009).
- Oertel WH, Stiasny‐Kolster K, Bertholdt B, Hallström Y, Albo J, Leissner L, et al. for The Pramipexole RLS Study Group. Efficacy of pramipexole in restless legs syndrome: a six‐week, multicenter, randomized, double‐blind study (effect‐RLS study). Movement Disorders: Official Journal of the Movement Disorder Society 2007;22(2):213‐9. [DOI] [PubMed] [Google Scholar]
Oertel 2008 {published and unpublished data}
- Oertel WH, Benes H, Garcia‐Borreguero D, Geisler P, Högl B, Saletu B, et al. Efficacy of rotigotine transdermal system in severe restless legs syndrome: A randomized, double‐blind, placebo‐controlled, six‐week dose‐finding trial in Europe. Sleep Medicine 2008;9(3):228‐39. [DOI] [PubMed] [Google Scholar]
- SP709. Multi‐center, double‐blind, randomized, placebo‐controlled, six‐arm, parallel‐group, dose‐finding trial to determine efficacy, safety and tolerability of five different transdermal doses of rotigotine in subjects with idiopathic restless legs syndrome. http://www.clinicaltrials.gov/ct2/show/NCT00243217 (accessed 4 March 2009).
Oertel 2010 {published and unpublished data}
- Oertel W, Benes H, Garcia‐Borreguero D, Hoegl B, Poewe W, Montagna P, et al. Rotigotine transdermal patch in idiopathic restless legs syndrome. Sleep Medicine 2010;11(9):848‐56. [DOI] [PubMed] [Google Scholar]
- SP794. A multicenter, double‐blind, randomized, placebo‐controlled, two‐arm, parallel‐group, sleep lab trial to investigate the efficacy and safety of transdermal rotigotine in subjects with idiopathic restless legs syndrome. http://www.clinicaltrials.gov/ct2/show/NCT00275236 (accessed 4 March 2009).
Partinen 2006 {published data only (unpublished sought but not used)}
- 248.515. A double‐blind, placebo‐controlled dose‐finding study to investigate efficacy and safety of different doses (0.125, 0.25, 0.5, 0.75 mg) of pramipexole administered once daily orally over three weeks in patients with idiopathic restless legs syndrome (RLS), followed by a 26‐week open‐label dose‐finding study (0.125 mg – 0.75 mg) to investigate long‐term efficacy of pramipexole in patients with RLS. http://trials.boehringer‐ingelheim.com/res/trial/data/pdf/248.515_U04‐2112_new.pdf (accessed 4 March 2009).
- Partinen M, Hirvonen K, Jama L, Alakuijala A, Hublin C, Tamminen I, et al. Efficacy and safety of pramipexole in idiopathic restless legs syndrome: a polysomnographic dose‐finding study‐‐the PRELUDE study. Sleep Medicine 2006;7(5):407‐17. [DOI] [PubMed] [Google Scholar]
Pieta 1998 {published data only}
- Pieta J, Millar T, Zacharias J, Fine A, Kryger M. Effect of pergolide on restless legs and leg movements in sleep in uremic patients. Sleep 1998;21(6):617‐22. [DOI] [PubMed] [Google Scholar]
Staedt 1997 {published data only}
- Staedt J, Wassmuth F, Ziemann U, Hajak G, Ruther E, Stoppe G. Pergolide: treatment of choice in restless legs syndrome (RLS) and nocturnal myoclonus syndrome (NMS). A double‐blind randomized crossover trial of pergolide versus L‐Dopa. Journal of Neural Transmission 1997;104(4‐5):461‐8. [DOI] [PubMed] [Google Scholar]
Stiasny‐Kolster 2004a {published and unpublished data}
- Stiasny‐Kolster K, Benes H, Peglau I, Hornyak M, Holinka B, Wessel K, et al. Effective cabergoline treatment in idiopathic restless legs syndrome. Neurology 2004;63(12):2272‐9. [DOI] [PubMed] [Google Scholar]
Stiasny‐Kolster 2004b {published and unpublished data}
- Stiasny‐Kolster K, Kohnen R, Schollmayer E, Moller JC, Oertel WH, Rotigotine Sp 666 Study G. Patch application of the dopamine agonist rotigotine to patients with moderate to advanced stages of restless legs syndrome: a double‐blind, placebo‐controlled pilot study. Movement Disorders 2004;19(12):1432‐8. [DOI] [PubMed] [Google Scholar]
Trenkwalder 2004a {published data only}
- Trenkwalder C, Hundemer HP, Lledo A, Swieca J, Polo O, Wetter TC, et al. Efficacy of pergolide in treatment of restless legs syndrome: the PEARLS Study. Neurology 2004;62(8):1391‐7. [DOI] [PubMed] [Google Scholar]
Trenkwalder 2004b {published and unpublished data}
- SKF‐101468/190. A 12 week, double‐blind, placebo‐controlled, parallel group study to assess the efficacy and safety of ropinirole in patients suffering from restless legs syndrome (RLS). http://www.gsk‐clinicalstudyregister.com/result_detail.jsp?protocolId=101468%2f190&studyId=2480&compound=Ropinirole (accessed 15 January 2009).
- Trenkwalder C, Garcia‐Borreguero D, Montagna P, Lainey E, Weerd AW, Tidswell P, et al. Ropinirole in the treatment of restless legs syndrome: results from the TREAT RLS 1 study, a 12 week, randomised, placebo controlled study in 10 European countries. Journal of Neurology, Neurosurgery & Psychiatry 2004;75:92‐7. [PMC free article] [PubMed] [Google Scholar]
Trenkwalder 2007 {published and unpublished data}
- CABAS‐0067‐031. A double‐blind, randomized, active‐controlled multicenter efficacy trial for the treatment of patients with Restless Legs Syndrome (RLS). http://www.clinicaltrials.gov/ct2/show/NCT00625547 (accessed 4 March 2009).
- Trenkwalder C, Benes H, Grote L, Happe S, Hogl B, Mathis J, et al. Cabergoline compared to levodopa in the treatment of patients with severe restless legs syndrome: results from a multi‐center, randomized, active controlled trial. Movement Disorders 2007;22(85):696‐703. [DOI] [PubMed] [Google Scholar]
Trenkwalder 2008 {published and unpublished data}
- SP790. A multi‐center, randomized, double‐blind, placebo‐controlled, four‐arm parallel‐group trial to investigate the efficacy and safety of three different transdermal doses of rotigotine in subjects with idiopathic restless legs syndrome. http://www.clinicaltrials.gov/ct2/show/NCT00136045 (accessed 4 March 2009).
- Trenkwalder C, Benes H, Poewe W, Oertel WH, Garcia‐Borreguero D, Weerd AW, et al. Efficacy of rotigotine for treatment of moderate‐to‐severe restless legs syndrome: a randomised, double‐blind, placebo‐controlled trial.[see comment]. Lancet Neurology 2008;7(7):595‐604. [DOI] [PubMed] [Google Scholar]
Walters 2004 {published and unpublished data}
- SKF‐101468/194. A 12 week, double.blind, placebo‐controlled, parallel group study to assess the efficacy and safety of ropinirole in patients suffering from restless legs syndrome (RLS).. http://www.gsk‐clinicalstudyregister.com/result_detail.jsp?protocolId=101468%2f194&studyId=2363&compound=Ropinirole (accessed 15 January 2009).
- Walters AS, Ondo WG, Dreykluft T, Grunstein R, Lee D, Sethi K, et al. Ropinirole is effective in the treatment of restless legs syndrome. TREAT RLS 2: a 12‐week, double‐blind, randomized, parallel‐group, placebo‐controlled study. Movement Disorders 2004;19(12):1414‐23. [DOI] [PubMed] [Google Scholar]
Wetter 1999 {published data only}
- Wetter TC, Stiasny K, Winkelmann J, Buhlinger A, Brandenburg U, Penzel T, et al. A randomized controlled study of pergolide in patients with restless legs syndrome.[see comment]. Neurology 1999;52(5):944‐50. [DOI] [PubMed] [Google Scholar]
Winkelman 2006 {published and unpublished data}
- 248.543. A randomized, double‐blind, placebo‐controlled, parallel group clinical trial comparing fixed doses of 0.25 mg, 0.50 mg and 0.75 mg pramipexole (Mirapex®) administered orally to investigate the safety and efficacy in patients with idiopathic restless legs syndrome for 12 weeks. http://trials.boehringer‐ingelheim.com/res/trial/data/pdf/248.543_U05‐3094_new.pdf (accessed 4 March 2009).
- Winkelman JW, Sethi KD, Kushida CA, Becker PM, Koester J, Cappola JJ, et al. Efficacy and safety of pramipexole in restless legs syndrome. Neurology 2006;67(6):1034‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abetz 2005 {published data only}
- Abetz L, Arbuckle R, Allen RP, Mavraki E, Kirsch J. The reliability, validity and responsiveness of the Restless Legs Syndrome Quality of Life questionnaire (RLSQoL) in a trial population. Health & Quality of Life Outcomes 2005;3:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Abetz 2006 {published data only}
- Abetz L, Arbuckle R, Allen RP, Garcia‐Borreguero D, Hening W, Walters AS, et al. The reliability, validity and responsiveness of the International Restless Legs Syndrome Study Group rating scale and subscales in a clinical‐trial setting. Sleep Medicine 2006;7(4):340‐9. [DOI] [PubMed] [Google Scholar]
Allen 1998 {published data only}
- Allen RP, Earley CJ, Hening WA, Walters AS, Yaffee J, Wagner ML. Pergolide treatment of the restless legs syndrome: a double‐blind placebo‐controlled study with objective assessment of leg movements. Neurology 1998;50(4 Suppl 4):A68. [Google Scholar]
Anonymous 2006 {published data only}
- Anonymous. Ropinirole: new indication. Restless legs: disproportionate adverse effects. Prescrire International 2006;15(85):173‐6. [PubMed] [Google Scholar]
Benes 2005 {published data only}
- Benes H. Transdermal lisuride in patients with idiopathic restless legs syndrome: results from a placebo controlled double blind randomized multicenter 12 week dose‐finding study. Sleep Medicine 2005;6 Suppl 2:S136. [Google Scholar]
Benes 2006 {published data only}
- Benes H. Transdermal lisuride: short‐term efficacy and tolerability study in patients with severe restless legs syndrome. Sleep Medicine 2006;7(1):31‐5. [DOI] [PubMed] [Google Scholar]
Benes 2006a {published data only}
- Benes H, Deissler A, Rodenbeck A, Engfer A, Kohnen R. Lisuride treatment of restless legs syndrome: first studies with monotherapy in de novo patients and in combination with levodopa in advanced disease. Journal of Neural Transmission 2006;113(1):87‐92. [DOI] [PubMed] [Google Scholar]
Bingham 2002 {published data only}
- Bingham C. Pergolide for restless legs in uraemia. National Research Register, UK [http://www.nrr.nhs.uk/] 2002.
Bliwise 2005 {published data only}
- Bliwise DL, Freeman A, Ingram CD, Rye DB, Chakravorty S, Watts RL. Randomized, double‐blind, placebo‐controlled, short‐term trial of ropinirole in restless legs syndrome. Sleep Medicine 2005;6(2):141‐7. [DOI] [PubMed] [Google Scholar]
Ebell 1999 {published data only}
- Ebell M. Is pergolide effective in the short‐term treatment of restless leg syndrome (RLS)?. Evidence‐Based Practice 1999;2(4):5, insert 2p. [Google Scholar]
Freeman 2001 {published data only}
- Freeman A, Rye DB, Bliwise D, Chakravorty S, Krulewicz S, Watts RL. Ropinirole for Restless Legs Syndrome (RLS): An open label and double blind placebo‐controlled study. Neurology 2001;56(8 Suppl 3):A5. [Google Scholar]
Gunning 1999 {published data only}
- Gunning K, Gay C. Treatment of restless leg syndrome with pergolide. Journal of Family Practice 1999;48(4):250. [PubMed] [Google Scholar]
Kinge 2005 {published data only}
- Kinge E, Lossius M I, Mowincel P. [Ropinirole treatment of restless legs] [Danish]. Ugeskrift for Laeger 2005;167(11):1284‐6. [PubMed] [Google Scholar]
Manconi 2003 {published data only}
- Manconi M, Casetta I, Govoni V, Cesnik E, Ferini‐Strambi L, Granieri E. Pramipexole in Restless Legs syndrome. Evaluation by suggested immobilization test. Journal of Neurology 2003;250(12):1494‐5. [DOI] [PubMed] [Google Scholar]
Miranda 2003 {published data only}
- Miranda M, Fabres L, Kagi M, Aguilera L, Alvo M, Elgueta L, et al. [Treatment of restless legs syndrome in uremic patients undergoing dialysis with pramipexole: preliminary results]. [Spanish]. Revista Medica de Chile 2003;131(6):700‐1. [PubMed] [Google Scholar]
Miranda 2004 {published data only}
- Miranda M, Kagi M, Fabres L, Aguilera L, Alvo M, Elgueta L, et al. Pramipexole for the treatment of uremic restless legs in patients undergoing hemodialysis. Neurology 2004;62(5):831‐2. [DOI] [PubMed] [Google Scholar]
Montplaisir 1998 {published data only}
- Montplaisir J, Nicolas A, Denesle R, Gomez‐Mancilla B. Pramipexole alleviates sensory and motor symptoms of restless legs syndrome. Neurology 1998;51(1):311‐2. [Google Scholar]
Montplaisir 2006 {published data only}
- 101468/188. A study of the maintained efficacy and safety of ropinirole versus placebo in the long term treatment of restless legs syndrome (RLS). http://www.gsk‐clinicalstudyregister.com/result_detail.jsp?protocolId=101468%2f188&studyId=2482&compound=Ropinirole (accessed 15 January 2009).
- Montplaisir J, Karrasch J, Haan J, Volc D. Ropinirole is effective in the long‐term management of restless legs syndrome: a randomized controlled trial. Movement Disorders 2006;21(10):1627‐35. [DOI] [PubMed] [Google Scholar]
Morgan 2008 {published data only}
- Morgan JC, Ames M, Sethi KD. Response to ropinirole in restless legs syndrome is independent of baseline serum ferritin. Journal of Neurology, Neurosurgery & Psychiatry 2008;79(8):964‐5. [DOI] [PubMed] [Google Scholar]
Noel 1998 {published data only}
- Noel S, Korri H, Vanderheyden JE. Low dosage of pergolide in the treatment of restless legs syndrome. Acta Neurologica Belgica 1998;98(1):52‐3. [PubMed] [Google Scholar]
Oertel 2005 {published data only}
- Oertel WH, Benes H, Geisler P, Eisensehr I, Garcia‐Borreguero D, Clarencach P, et al. Rotigotine patch efficacy and safety in the treatment of severe idiopathic restless legs syndrome results from a multinational double blind placebo controlled multi center dose finding study. Sleep Medicine 2005;6 Suppl 2:S159. [Google Scholar]
Partinen 2006a {published data only}
- Partinen M, Hirvonen K, Jama L, Alakuijala A, Hubin C, Tamminen I, et al. Effects of pramipexole on periodic limb movements (PLMs) in restless legs syndrome (RLS) a polysomnographic study. Sleep Medicine 2006;7:S126. [DOI] [PubMed] [Google Scholar]
Partinen 2006b {published data only}
- Partinen M, Hirvonen K, Jama L, Alakuijala A, Hublin C, Tamminen I, et al. Clinician ratings and patient self‐ratings of improvement after 3 weeks of double‐blind placebo‐controlled pramipexole for restless legs syndrome (RLS). Sleep Medicine 2006;7:S126. [Google Scholar]
Penzel 2002 {published data only}
- Penzel T, Brandenburg U, Peter JH, Otto R, Hundemer HP, Lledo A, et al for the PEARLS Study Group. A new design of a polysomnography‐based multi‐center treatment study for the restless legs syndrome. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology 2002;113(4):571‐8. [DOI] [PubMed] [Google Scholar]
Plowman 2005 {published data only}
- Plowman BK, Boggie DT, Morreale AP, Schaefer MG, DeLattre ML, Chan H. Clinical consultation. Sleep attacks in patients receiving dopamine‐receptor agonists. American Journal of Health‐System Pharmacy 2005;62(5):537‐40. [DOI] [PubMed] [Google Scholar]
Powell 2007 {published data only}
- Powell D. Advance reports. Patch for Parkinson's disease improves restless legs syndrome. Geriatric Medicine 2007;37(2):58. [Google Scholar]
Reading 2007 {published data only}
- Reading P. Parasomnias: the spectrum of things that go bump in the night. Practical Neurology 2007;7(1):6‐15. [PubMed] [Google Scholar]
Reuter 1999 {published data only}
- Reuter I, Ellis CM, Chaudhuri KR. Nocturnal subcutaneous apomorphine infusion in Parkinson's disease and restless legs syndrome. Acta Neurologica Scandinavica 1999;100(3):163‐7. [DOI] [PubMed] [Google Scholar]
Staedt 1998 {published data only}
- Staedt J, Hunerjager H, Ruther E, Stoppe G. Pergolide: treatment of choice in Restless Legs Syndrome (RLS) and Nocturnal Myoclonus Syndrome (NMS). Longterm follow up on pergolide. Short communication. Journal of Neural Transmission 1998;105(2‐3):265‐8. [DOI] [PubMed] [Google Scholar]
Stiasny 2000 {published data only}
- Stiasny K, Moller JC, Oertel WH. Safety of pramipexole in patients with restless legs syndrome. Neurology 2000;55(10):1589‐90. [DOI] [PubMed] [Google Scholar]
Stiasny 2001 {published data only}
- Stiasny K, Wetter TC, Winkelmann J, Brandenburg U, Penzel T, Rubin M, et al. Long‐term effects of pergolide in the treatment of restless legs syndrome. Neurology 2001;56(10):1399‐402. [DOI] [PubMed] [Google Scholar]
Stiasny 2002 {published data only}
- Stiasny K, Uberall M, Oertel WH. Cabergoline in restless legs syndrome (RLS) ‐ a double‐blind placebo‐controlled multicenter dose‐finding trial. European Journal of Neurology 2002;9 Suppl 2:50. [Google Scholar]
Tagaya 2002 {published data only}
- Tagaya H, Wetter T, Winkelmann J, Rubin M, Hundemer H, Trenkwalder C, et al. Pergolide restores sleep maintenance but impairs sleep EEG. Sleep Medicine 2002;3:49‐54. [DOI] [PubMed] [Google Scholar]
Thorpy 2005 {published data only}
- Thorpy M. Ropinirole in the treatment of restless legs syndrome. Current Neurology and Neuroscience Reports 2005;5(2):137‐9. [DOI] [PubMed] [Google Scholar]
Trenkwalder 2001a {published data only}
- Trenkwalder C, Brandenburg U, Hundemer P, Lledo A, Quail D, Swieca J. A randomized long‐term placebo‐controlled multicenter trial of pergolide in the treatment of Restless Legs Syndrome with central evaluation of polysomnographic data. Neurology 2001;56(8 Suppl 3):A5‐6. [Google Scholar]
Trenkwalder 2001b {published data only}
- Trenkwalder C, Brandenburg U, Hundemer HP, Lledo A, Quail D, Swieca J. A long‐term controlled multicenter trial of pergolide in the treatment of restless legs syndrome with central evaluation of polysomnographic data. Journal of the Neurological Sciences 2001;187 Suppl 1:S432. [Google Scholar]
Trenkwalder 2005 {published data only}
- Trenkwalder C, Kohen R. Augmentation and lack of efficacy with dopaminergic treatment in [patients with RLS double blind comparison between cabergoline and l‐dopa in the treatment of patients with severe restless legs syndrome. Sleep Medicine 2005;6 Suppl 2:S162. [Google Scholar]
Trenkwalder 2006 {published data only}
- 245.546. A double‐blind, placebo‐controlled, randomised withdrawal study of 3 months’ duration in patients suffering from idiopathic Restless Legs Syndrome who responded to a preceding, 6‐month treatment with open‐label pramipexole including titration (0.125, 0.25, 0.5, 0.75 mg orally q.n.). http://trials.boehringer‐ingelheim.com/res/trial/data/pdf/248.546_U05‐1532_new_upload.pdf (accessed 4 March 2009).
- Trenkwalder C, Stiasny‐Kolster K, Kupsch A, Oertel WH, Koester J, Reess J. Controlled withdrawal of pramipexole after 6 months of open‐label treatment in patients with restless legs syndrome. Movement Disorders 2006;21(9):1404‐10. [DOI] [PubMed] [Google Scholar]
Wetter 1998 {published data only}
- Wetter TC, Stiasny K, Winkelmann J, Buhlinger A, Brandenburg U, Peter JH, et al. A polysomnographic, controlled study of pergolide in the treatment of restless legs syndrome. Neurology 1998;50(4 Suppl 4):A69. [Google Scholar]
Winkelman 2005 {published data only}
- Winkelman J, Sethi K, Kushida C, Becker P, Mahowald M. Pramipexole is efficacious and safe in treating RLS patients results of a 12 weeks placebo controlled fixed dose study. Sleep Medicine 2005;6 Suppl 2:S74. [Google Scholar]
Yee 2004 {published data only}
- Yee B, Dreykluft T, Walters A, Ondo W, Sethi K, Hyman N, et al. Ropinirole versus placebo in restless legs syndrome: efficacy, safety and quality of life results from a 12‐week double‐blind international study. Internal Medicine Journal 2004;34(3):A26. [Google Scholar]
Additional references
Abetz 2005
- Abetz L, Vallow SM, Kirsch J, Allen RP, Washburn T, Earley CJ. Validation of the Restless Legs Syndrome Quality of Life questionnaire. Health and quality of life outcomes 2005;8(2):157‐67. [DOI] [PubMed] [Google Scholar]
Allen 2003
- Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisir J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Medicine 2003;4:101‐19. [DOI] [PubMed] [Google Scholar]
Allen 2007
- Allen RP, Earley CJ. The role of iron in restless legs syndrome. Movement Disorders 2007;22 Suppl 18:S440‐8. [DOI] [PubMed] [Google Scholar]
American Academy of Sleep Medicine 2005
- American Academy of Sleep Medicine. Periodic limb movement disorder. In: American Academy of Sleep Medicine editor(s). The International classification of sleep disorders, 2nd edition: Diagnostic and coding manual. Westchester, Illinois: American Academy of Sleep Medicine, 2005:182‐6. [Google Scholar]
Baker 2008
- Baker WL, White CM, Coleman CI. Effect of nonergot dopamine agonists on symptoms of restless legs syndrome. Annals of Family Medicine 2008;6(3):253‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berger 2004
- Berger K, Luedemann J, Trenkwalder C, John U, Kessler C. Parity and the risk of restless legs syndrome in the general population. Archives of Internal Medicine 2004;164(2):196‐202. [DOI] [PubMed] [Google Scholar]
Berger 2007
- Berger K, Kurth T. RLS epidemiology ‐ Frequencies, risk factors and methods in population studies. Movement Disorders 2007;22:S420‐3. [DOI] [PubMed] [Google Scholar]
Bonnet 1993
- Bonnet M, Carley D, Carskadon M, Easton P, Guilleminault C, Harper R, et al. Recording and scoring leg movements. Sleep 1993;16:748‐59. [PubMed] [Google Scholar]
Buysse 1989
- Buysse D, Reynolds C, Monk T, Berman S, Kupfer D. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research 1989;28(2):193‐213. [DOI] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical power analysis in the behavioral sciences. 2nd Edition. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc, 1988. [Google Scholar]
Connor 2008
- Connor JR. Pathophysiology of restless legs syndrome: evidence for iron involvement. Current Neurology and Neuroscience Reports 2008;8:162‐6. [DOI] [PubMed] [Google Scholar]
Conti 2007
- Conti CF, Oliveira MM, Andriolo RB, Saconato H, Atallah AN, Valbuza JS, et al. Levodopa for idiopathic restless legs syndrome: evidence‐based review. Movement Disorders 2007;22(13):1943‐51. [DOI] [PubMed] [Google Scholar]
Conti 2008
- Conti CF, Oliveira MM, Valbuza JS, Prado LB, Carvalho LB, Prado GF. Anticonvulsants to treat idiopathic restless legs syndrome: systematic review. Arquivos de neuro‐psiquiatria 2008;66(2B):431‐5. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. British Medical Journal 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eisensehr 2004
- Eisensehr I, Ehrenberg BL, Rogge Solti S, Noachtar S. Treatment of idiopathic restless legs syndrome (RLS) with slow‐release valproic acid compared with slow‐release levodopa/benserazid. Journal of Neurology 2004;251:579‐83. [DOI] [PubMed] [Google Scholar]
Ekbom 1945
- Ekbom K. Restless legs: a clinical study. Acta Medica Scandinavica 1945;158:1‐123. [Google Scholar]
Garcia‐Borreguero 2002
- Garcia‐Borreguero D, Larrosa O, Llave Y, Verger K, Masramon X, Hernandez G. Treatment of restless legs syndrome with gabapentin: a double‐blind, cross‐over study. Neurology 2002;59(10):1573‐9. [DOI] [PubMed] [Google Scholar]
Goertelmeyer 1985
- Goertelmeyer R. On the development of a standardized sleep inventory for the assessment of sleep. In: Kubicki S, Herrmann W editor(s). Methods of sleep research. Stuttgart/New York: Fischer, 1985. [Google Scholar]
Hansen 2009
- Hansen RA, Song L, Moore CG, Gilsenan AW, Kim MM, Calloway MO, Murray MD. Effect of ropinirole on sleep outcomes in patients with restless legs syndrome: meta‐analysis of pooled individual patient data from randomized controlled trials. Pharmacotherapy 2009;29(3):255‐62. [DOI] [PubMed] [Google Scholar]
Hays 2005
- Hays RD, Martin SA, Sesti AM, Spritzer KL. Psychometric properties of the Medical Outcomes Study Sleep measure. Sleep Medicine 2005;6(1):41‐4. [DOI] [PubMed] [Google Scholar]
Heckman 2009
- Heckman CJ, Mottram C, Quinlan K, Theiss R, Schuster J. Motoneuron excitability: the importance of neuromodulatory inputs. Clinical Neurophysiology 2009;120(12):2040‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hening 2004a
- Hening W, Walters AS, Allen RP, Montplaisir J, Myers A, Ferini‐Strambi L. Impact, diagnosis and treatment of restless legs syndrome (RLS) in a primary care population: the REST (RLS epidemiology, symptoms, and treatment) primary care study. Sleep Medicine 2004;5(3):237‐46. [DOI] [PubMed] [Google Scholar]
Hening 2004b
- Hening W. The clinical neurophysiology of the restless legs syndrome and periodic limb movements. Part I: diagnosis, assessment, and characterization. Clinical Neurophysiology 2004;115(9):1965‐74. [DOI] [PubMed] [Google Scholar]
Hornyak 2004
- Hornyak M, Trenkwalder C. Restless legs syndrome and periodic limb movement disorder in the elderly. Journal of Psychosomatic Research 2004;56(5):543‐8. [DOI] [PubMed] [Google Scholar]
Högl 2003
- Högl B, Saletu M, Brandauer E, Frauscher B, Müller J, Seppi K, et al. Prevalence of Restless Legs Syndrome in the central European alpine region of South Tyrol. Sleep 2003;26:A344. [Google Scholar]
Iber 2007
- Iber C, Ancoli‐Israel S, Chesson A, Quan SF for the American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events: Rules, terminology and technical specifications. 1st Edition. Westchester, Illinois: American Academy of Sleep Medicine, 2007. [Google Scholar]
Kohnen 2002
- Kohnen R, Benes H, Heinrich CR, Kurella B. Development of the disease‐specific Restless Legs Syndrome Quality of Life (RLS‐QoL) questionnaire. Movement Disorders 2002;17 Suppl 5:P743. [Google Scholar]
Kohnen 2004
- Kohnen R, Oertel WH, Stiasny‐Kolster K, Benes H, Trenkwalder C. Severity rating of restless legs syndrome: validation of the RLS‐6 scales. Sleep 2004;27 Suppl 1:A304. Abstract 680. [Google Scholar]
Kohnen 2008
- Kohnen R. The promises of meta‐analyses in current RLS research: is 1+1 more than 2?. Sleep Medicine 2008;9(7):709‐11. [DOI] [PubMed] [Google Scholar]
Kushida 2007
- Kushida C, Martin M, Nikam P, Blaisdell B, Wallenstein G, Ferini‐Strambi L, et al. Burden of restless legs syndrome on health‐related quality of life. Quality of Life Research 2007;16(4):617‐24. [DOI] [PubMed] [Google Scholar]
Kushida 2009
- Kushida CA, Becker PM, Ellenbogen AL, Canafax DM, Barrett RW, XP052 Study Group. Randomized, double‐blind, placebo‐controlled study of XP13512/GSK1838262 in patients with RLS. Neurology 2009;72(5):439‐46. [DOI] [PubMed] [Google Scholar]
Manconi 2004
- Manconi M, Govoni V, Vito A, Economou NT, Cesnik E, Mollica G, et al. Pregnancy as a risk factor for restless legs syndrome. Sleep Medicine 2004;5(3):305‐8. [DOI] [PubMed] [Google Scholar]
Montplaisir 1997
- Montplaisir J, Boucher S, Poirier G, Lavigne G, Lapierre O, Lespérance P. Clinical, polysomnographic, and genetic characteristics of restless legs syndrome: a study of 133 patients diagnosed with new standard criteria. Movement Disorders 1997;12(1):61‐5. [DOI] [PubMed] [Google Scholar]
National Institute of Mental Health 1976
- National Institute of Mental Health (NIMH). Early clinical drug evaluation unit (ECDEU). Clinical Global Impressions. In: Guy W editor(s). ECDEU assessment manual for psychopharmacology. revised. Rockville MD: NIMH, Psychopharmacology Research Branch, 1976. [Google Scholar]
Paulus 2006
- Paulus W, Schomburg ED. Dopamine and the spinal cord in restless legs syndrome: does spinal cord physiology reveal a basis of augmentation?. Sleep Medicine Reviews 2006;10:185‐96. [DOI] [PubMed] [Google Scholar]
Phillips 2000
- Phillips B, Young T, Finn L, Asher K, Hening WA, Purvis C. Epidemiology of restless legs symptoms in adults. Archives of Internal medicine 2000;160:2137‐41. [DOI] [PubMed] [Google Scholar]
Quilici 2008
- Quilici S, Abrams KR, Nicolas A, Martin M, Petit C, Lleu PL, et al. Meta‐analysis of the efficacy and tolerability of pramipexole versus ropinirole in the treatment of restless legs syndrome. Sleep Medicine 2008;9(7):715‐26. [DOI] [PubMed] [Google Scholar]
Reviewer's Handbook
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008.. Available from www.cochrane‐handbook.org.
Rothdach 2000
- Rothdach AJ, Trenkwalder C, Haberstock J, Keil U, Berger K. Prevalence and risk factors of RLS in an elderly population. Neurology 2000;54:1064‐8. [DOI] [PubMed] [Google Scholar]
Schöls 1998
- Schöls L, Haan J, Riess O, Amoridis G, Przuntek H. Sleep disturbance in spinocerebellar ataxias: is the SCA3 mutation a cause of restless legs syndrome?. Neurology 1998;51:1603‐7. [DOI] [PubMed] [Google Scholar]
Silber 2004
- Silber MH, Ehrenberg BL, Allen RP, Buchfuhrer MJ, Earley CJ, Hening WA, et al. An algorithm for the management of restless legs syndrome. Mayo Clinic Proceedings 2004;79(7):916‐22. [DOI] [PubMed] [Google Scholar]
Stefansson 2007
- Stefansson H, Rye DB, Hicks A, Petursson H, Ingason A, Thorgeirsson TE. A genetic risk factor for periodic limb movements in sleep. New England Journal of Medicine 2007;357(7):639‐47. [DOI] [PubMed] [Google Scholar]
Stiasny‐Kolster 2006
- Stiasny‐Kolster K, Kohnen R, Möller JC, Trenkwalder C, Oertel WH. Validation of the "L‐DOPA test" for diagnosis of restless legs syndrome. Sleep Medicine 2006;21(9):1333‐9. [DOI] [PubMed] [Google Scholar]
Stiasny‐Kolster 2009
- Stiasny‐Kolster. Restless legs syndrome [Restless‐Legs‐Syndrom]. Somnology 2009;13(1):115‐27. [Google Scholar]
Talati 2009
- Talati R, Phung OJ, Mather J, Coleman CI. Effect of non‐ergot dopamine agonists on health‐related quality of life of patients with restless legs syndrome. The Annals of Pharmacotherapy 2009;43:813‐21. [DOI] [PubMed] [Google Scholar]
Tison 2005
- Tison F, Crochard A, Léger D, Bouée S, Lainey E, Hasnaoui A. Epidemiology of restless legs syndrome in French adults: a nationwide survey: the INSTANT Study. Neurology 2005;65(2):239‐46. [DOI] [PubMed] [Google Scholar]
Trenkwalder 2004
- Trenkwalder C, Paulus W. Why do restless legs occur at rest?‐pathophysiology of neuronal structures in RLS. Neurophysiology of RLS (part 2). Clinical Neurophysiology 2004;115(9):1975‐88. [DOI] [PubMed] [Google Scholar]
Trenkwalder 2007
- Trenkwalder C, Kohnen R, Allen RP, Benes H, Ferini‐Strambi L, Garcia‐Borreguero D, et al. Clinical trials in restless legs syndrome ‐ recommendations of the european RLS Study Group (EURLSSG). Sleep Medicine 2007;22 Suppl 18:S495‐504. [DOI] [PubMed] [Google Scholar]
Trenkwalder 2008
- Trenkwalder C, Hening WA, Montagna P, Oertel WH, Allen RP, Walters AS, et al. Treatment of restless legs syndrome: an evidence‐based review and implications for clinical practice. Movement Disorders 2008;23(16):2267‐302. [DOI] [PubMed] [Google Scholar]
Trenkwalder 2010
Ulfberg 2001
- Ulfberg J, Nyström B, Carter N, Edling C. Prevalence of restless legs syndrome among men aged 18 to 64 years: An association with somatic disease and neuropsychiatric symptoms. Movement Disorders 2001;16:1159‐63. [DOI] [PubMed] [Google Scholar]
Walters 1988
- Walters AS, Hening WA, Kavey N, Chokroverty S, Gidro‐Frank S. A double‐blind randomized crossover trial of bromocriptine and placebo in restless legs syndrome. Annals of Neurology 1988;24(3):455‐8. [DOI] [PubMed] [Google Scholar]
Walters 1993
- Walters AS, Wagner ML, Hening WA, Grasing K, Mills R, Chokroverty S, et al. Successful treatment of the idiopathic restless legs syndrome in a randomized double‐blind trial of oxycodone versus placebo. Sleep 1993;16:327‐32. [DOI] [PubMed] [Google Scholar]
Walters 1995
- Walters AS, The International Restless Legs Syndrome Study Group. Toward a better definition of restless legs syndrome. Movement Disorders 1995;10:634‐42. [DOI] [PubMed] [Google Scholar]
Walters 2003
- Walters AS, LeBrocq C, Dhar A, Hening W, Rosen R, Allen RP, et al. International Restless Legs Syndrome Study Group. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Medicine 2003;4:121‐32. [DOI] [PubMed] [Google Scholar]
Walters 2007
- Walters AS. Restless legs syndrome and periodic limb movements in sleep. Continuum: Lifelong Learning in Neurology 2007;13(3):115‐38. [Google Scholar]
Winkelmann 2007
- Winkelmann J, Schormair B, Lichtner P, Ripke S, Xiong L, Jalilzadeh S, et al. Genome‐wide association study of restless legs syndrome identifies common variants in three genomic regions. Nature Genetics 2007;39(8):1000‐6. [DOI] [PubMed] [Google Scholar]
Zintzaras 2010
- Zintzaras E, Kitsios GD, Papathanasiou AA, Konitsiotis S, Miligkos M, Rodopoulou P, et al. Randomized trials of dopamine agonists in restless legs syndrome: a systematic review, quality assessment, and meta‐analysis. Clinical Therapeutics 2010;32(2):221‐37. [DOI] [PubMed] [Google Scholar]
Zucconi 2006
- Zucconi M, Ferri R, Allen R, Baier PC, Bruni O, Chokroverty S, et al. International Restless Legs Syndrome Study Group (IRLSSG). The official World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG). Sleep Medicine 2006;7(2):175‐83. [DOI] [PubMed] [Google Scholar]


