Abstract
Anti-fog sprays and solutions are used on eyeglasses to minimize the condensation of water vapor, particularly while wearing a mask. Given their water-repellent properties, we sought to characterize per- and polyfluorinated alkyl substance (PFAS) compounds in four anti-fog spray products, five anti-fog cloth products, and two commercial fluorosurfactant formulations suspected to be used in preparing anti-fog products. Fluorotelomer alcohols (FTOHs) and fluorotelomer ethoxylates (FTEOs) were detected in all products and formulations. While 6:2 FTOH and the 6:2 FTEO polymeric series were predominant, one anti-fog cloth and one formulation contained 8:2, 10:2, 12:2, 14:2, and 16:2 FTOH and FTEO polymeric series. PFAS concentrations varied in samples and were detected at levels up to 25,000 μg/mL in anti-fog sprays and 185,000 μg (g cloth)−1 in anti-fog cloth products. The total organic fluorine (TOF) measurements of anti-fog products ranged from 190 to 20,700 μg/mL in sprays and 44,200 to 131,500 μg (g cloth)−1 in cloths. Quantified FTOHs and FTEOs accounted for 1−99% of TOF mass. In addition, all four anti-fog sprays and both commercial formulations exhibited significant cytotoxicity and adipogenic activity (either triglyceride accumulation and/or pre-adipocyte proliferation) in murine 3T3-L1 cells. Results suggest that FTEOs are a significant contributor to the adipogenic activity exhibited by the anti-fog sprays. Altogether, these results suggest that FTEOs are present in commercial products at toxicologically relevant levels, and more research is needed to fully understand the health risks from using these PFAS-containing products.
Keywords: PFAS, fluorotelomer ethoxylate (FTEOs), anti-fog, adipogenic activity, endocrine disruptors, 3T3-L1
Graphical Abstract
INTRODUCTION
Due to the COVID19 pandemic, there has been an increase in the use of protective gear, including masks and face shields, particularly among medical staff and other essential workers. The fogging of eyeglasses while wearing full protective gear can be challenging, and a variety of approaches are used to overcome this problem, including applying dish soap, hand sanitizer, iodophor (iodine complexed with a solubilizing agent), or antifogging agents to the goggles.1 Anti-fog solutions have been one of the solutions recommended by health care professionals to help prevent fogging of glasses while wearing masks.2–4 Many of these products are marketed as “safe” and “non-toxic”; however, the ingredients on these products are not fully disclosed, although the ingredients listed on some products indicate the presence of fluorinated compounds. Given that they provide a water-repellant property, it seemed likely that these products could contain per- and polyfluoroalkyl substances (PFAS).
PFAS are a large class of chemical compounds that have been used for their stain- and water-repellent properties in commercial products for decades.5–15 Due to the widespread use of PFAS, they have been detected nearly ubiquitously in environmental matrices and human serum.7,9,11,13,14,16–23 PFAS have been shown to have a number of toxicological effects in laboratory studies and have been associated with thyroid disorders, immunotoxic effects, and various cancers in epidemiology studies.20,24–28
Most research to date has focused on perfluoroalkyl acids (PFAAs), such as perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS), which are known to be more toxic than many other studied PFAS. However, there are multiple classes of PFAS. For example, fluorotelomer alcohols (FTOHs) are primarily used in the production of PFAS for commercial applications,6,29–36 although FTOHs are sometimes used directly in fast food packaging and stain- and water-repellent textiles.31,37–39 Due to these direct and indirect uses (i.e., manufacturing residual) of FTOHs in commercial products, they have been widely detected in environmental samples and human serum.6,30,40–42 FTOHs are of particular concern in the indoor environment where they are released from commercial products and are frequently a dominant class of PFAS detected in dust and indoor air.17,33,41,43–52 Previous studies have shown that FTOHs and other precursor compounds can transform to more toxic and stable ionic PFAAs via aerobic and metabolic pathways.6,9,29,41,43,53–63 This could be particularly important for exposure in the indoor environment as FTOHs measured in indoor air have been found to be significantly correlated with serum PFAAs, suggesting that metabolic transformations are occurring in the body and that exposure to FTOHs may be a source of exposure to the more toxic PFAS in the indoor environment.64,65
Fluorotelomer ethoxylates (FTEOs), in contrast, are a class of fluorinated compounds that have been infrequently studied. Frömel and Knepper (2010) studied the biodegradation of FTEOs from commercial mixtures in a wastewater treatment plant (WWTP),66 but to the best of our knowledge, FTEOs have not yet been identified in any commercial products. Several types of polyethoxylated surfactants, and some PFAS, have been shown to induce adipogenesis in vitro, implicating them as potential endocrine disruptors;67–71 however, no research has determined if fluorinated polyethoxylates could produce similar effects.
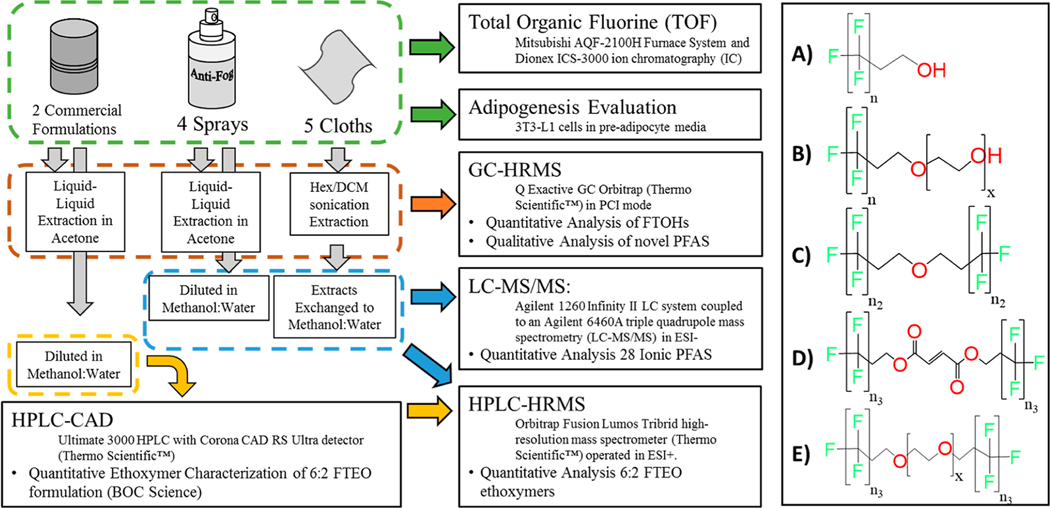
Given that these anti-fog products claim to prevent condensation of water vapor on eyeglasses, we sought to determine if these products contained a PFAS chemistry. The two main objectives of this study were to (1) identify and characterize PFAS compounds present in commercially available anti-fog sprays and cloth wipes and (2) investigate the adipogenic activity of the anti-fog sprays in a common in vitro pre-adipocyte model. More specifically, 10 non-ionic PFAS were targeted via gas chromatography (GC)–high-resolution mass spectrometry (HRMS) methods and 28 ionic PFAS were targeted via liquid chromatography–mass spectrometry (LC–MS/MS) methods. Additional analyses using high-performance liquid chromatography (HPLC)–HRMS methods were employed to quantify novel analytes (FTEOs) in anti-fog products and two PFAS commercial formulations. HPLC combined with charged aerosol detection (CAD) was employed to determine ethoxymer distribution in commercial mixtures, and an ion chromatography method was used to measure total organic fluorine (TOF) in all anti-fog samples and commercial mixtures. In vitro assays were also used to characterize the adipogenic activity in anti-fog sprays, commercial formulations, and individual analytes of interest. Figure 1 illustrates a simplified version of the sample workflow and analyses used that are presented in full in the Methods and Materials section.
Figure 1.
Analytical workflow for sample analysis (left) and chemical structures identified in anti-fog products (right). A represents FTOHs, B represents FTEOs, C represents fluorotelomer ethers, D represents fluorotelomer fumarates, and E represents FTEO ethers. Compounds were identified with n as 6, 8, 10, 12, 14, or 16; n2 as 6, 8, or 10; n3 as 6; and x ranging from 1 to 8 via GC–MS analysis and from 2 to 13 via LC-MS analysis.
METHODS AND MATERIALS
Anti-fog Consumer Products and Commercial Formulations.
Four anti-fog sprays and five anti-fog cloths were purchased from Amazon.com. The products were selected based on the highest number of positive reviews at the time of purchase (Table S1). Two commercially relevant non-ionic fluorosurfactant formulations were also analyzed. A 6:2 FTEO mixture (polyethylene oxide and mono(3,3,4,4,5,5,6,6,7,7,8,8,8)-tridecafluorooctyl ether; CAS#: 52440–44-4) was obtained from BOC Sciences, and a legacy sample of Zonyl FSN-100 (E.I. du Pont de Nemours & Company) was received as a gift of Prof. Jennifer Field (Oregon State University).
Analytical Methods for GC Analysis PFASs in Anti-fog Products.
Anti-fog sprays were diluted in a variety of analytical grade solvents (hexane, ethyl acetate, acetone, and dichloromethane) to determine which solution was optimal for GC–MS analysis. Based on the peak responses on a Q-Exactive GC-Orbitrap, acetone produced the optimal results. Serial dilutions of anti-fog sprays were created in acetone and spiked with isotopically labeled 2-perfluorohexyl-[1,2-13C2]-ethanol(6:2) and 2-perfluorooctyl-[1,2-13C2]-ethanol(8:2) (Wellington Laboratories, Guelph, Ontario). Anti-fog cloth products were analyzed by cutting and weighing out ∼0.5 g of sections of cloth and extracting via sonication in 10 mL of 1:1 hexane/dichloromethane three times. A small aliquot of the combined extract was added to a GC vial, diluted with ethyl acetate to 1 mL, and spiked with 13C 6:2 FTOH and 13C 8:2 FTOH. All samples were analyzed in triplicates.
The samples were analyzed based on previously published methods.72 Briefly, FTOHs were analyzed on a Q Exactive GC hybrid quadrupole-Orbitrap GC–MS/MS system (Thermo Scientific) operated in the full-scan positive chemical ionization (PCI) mode. Seven additional PFAS precursor compounds were screened but not found in any samples analyzed (Table S2). The GC was equipped with an Agilent J&W DB-WAX GC capillary column (30 m × 0.25 mm ID and 0.25 μm film thicknesses) with methane as the reagent gas flowing at 1.5 mL/min. The programmable temperature vaporizer inlet was operated in the splitless injection mode with a 1 μL injection. The GC oven temperature program was 50 °C for 2 min, 50−70 °C at 3 °C/ min, 70−130 °C at 10 °C/min, 130−250 at 20 °C/min, and held for 20 min. The ion source was kept at 250 °C. The samples were run with a scan range of 70−1050 m/z and quantified using the TraceFinder software. The analytes were measured with a standard targeted approach that included a five-point calibration curve and included the use of isotopically labeled standards.
LC−MS/MS Methods for Ionic PFAS Analysis.
Ionic PFAS were analyzed by an Agilent 1260 Infinity II LC system coupled to an Agilent 6460A triple quadrupole mass spectrometry (LC–MS/MS). Chromatographic separation was achieved under gradient conditions using a C18 column (Agilent Zorbax Eclipse XDB-C18, 4.6 × 50 mm and 1.8 μm particle size) preceded by a 4.6 × 5 mm XDB-C18 guard column. The mobile phases water (A) and methanol (B) were both modified with 2 mM ammonium acetate. The gradient program is as follows: initial condition 30% B, held for 1.5 min, increased to 95% B over 2 min, held for 6 min, increased to 100% B over 3 min, returned to the initial condition 30% B over 0.5 min, and held for 5.5 min. The flow rate was 0.4 mL/min, the column temperature was 45 °C, and the injection volume was 20 μL. Quantification was performed using multiple reaction monitoring transitions and run in the electrospray negative mode. Full results for ionic PFAS are presented in Table S3. Analytes were measured with a standard targeted approach that included a five-point calibration curve and included the use of isotopically labeled standards.
HPLC–CAD Methods for Characterizing Ethoxymer Distribution.
FTEOs in the 6:2 FTEO fluorosurfactant formulation were separated and quantified by HPLC with CAD using an Ultimate 3000 HPLC with a Corona CAD RS Ultra detector (Thermo Fisher Scientific). The separation was conducted as described previously for alkylphenol ethoxylate surfactants using a mixed-mode high-performance size exclusion chromatography method.73 The column (Shodex MSPak GF-310 4D, 150 × 4.6 mm, cross-linked polyvinyl alcohol phase) was held at 60 °C and operated under gradient conditions with a flow rate of 0.2 mL/min. The mobile phases were water (A) and methanol (B). From the initial conditions of 50:50 A/B, solvent B was increased to 100% in 22.7 min with a 10 min hold at 100% B. The column was returned to initial conditions in 5 min and held for post-run equilibration for 10 additional minutes. The sample injection volume was 5 μL. The charged aerosol detector was operated at a 25 °C nebulizer temperature and 10 Hz data acquisition with a digital filter setting of 3. The CAD response is proportional to the total mass (quantity) injected for nonvolatile compounds, and this response does not vary appreciably depending on the functional group or chemical structure across a wide range of molecule classes. 6:2 FTEO ethoxymers were identified by the corresponding retention time from analogous HPLC–HRMS analysis (below), and the relative quantity of each individual ethoxymer in the mixture was calculated as the % of the total peak area for the full HPLC–CAD chromatogram. Three replicate analyses were conducted. This analysis provided a high-confidence quantification of the 6:2 FTEO ethoxymers present in the 6:2 FTEO fluorosurfactant formulation acquired from BOC Sciences. Due to the complexity of the mixture (extensive co-elution of different FTEO ethoxymers), we were unable to characterize the Zonyl FSN-100 via HPLC–CAD analysis.
HPLC–HRMS Methods for FTEO Quantification in Commercial Products.
6:2 FTEO ethoxymers were quantified in anti-fog sprays and cloth extracts by HPLC–HRMS. The HPLC separation conditions were exactly as described above (HPLC-CAD methods). Detection was performed using an Orbitrap Fusion Lumos Tribrid high-resolution mass spectrometer (Thermo Fisher Scientific) operated in the positive-ion electrospray mode. The source conditions were electrospray voltage = 3300 V, sheath gas and auxiliary gas = 35 and 7 arbitrary units, respectively, ion transfer tube temperature = 350 °C, and vaporizer temperature = 275 °C. Spectra were acquired in the Orbitrap analyzer at 240,000 resolution over an m/z range of 300−1100 and an ion funnel RF amplitude of 60%. Spectral acquisition was internally calibrated using the Easy-IC reagent ion source to achieve mass accuracy typically <1 ppm (RMS). Quantitation of individual 6:2 FTEO ethoxymers was conducted from accurate mass extracted ion chromatograms (2 ppm) with external standard quantitation versus a six-point calibration curve prepared from 0.1 to 50 μg/mL Σ6:2 FTEO. Anti-fog sprays were diluted 1:1000 or 1:100 in 50:50 methanol/water, and dichloromethane extracts of anti-fog cloths were evaporated to dryness under a gentle nitrogen stream and reconstituted in an equal volume of methanol prior to dilution (1:1000) in 50:50 methanol/water. The injection volumes were 5 μL in all cases. This analysis provided a highconfidence quantification of the 6:2 FTEO ethoxymers present in the anti-fog products with the use of the 6:2 FTEO fluorosurfactant formulation characterized via HPLC–CAD as described above.
TOF Measurement.
All anti-fog products and the 6:2 FTEO commercial formulation were analyzed for TOF using previously published methods.74 TOF in this context refers to organic-bound fluorine or organofluorine. The fluorine contents in the four anti-fog sprays and the 6:2 FTEO formulation were diluted by methanol and water and then analyzed in triplicates by a Mitsubishi AQF-2100H furnace system. The fluorine atoms in all forms were mineralized into fluoride by combustion, which was then absorbed into reagent water. The formed fluoride concentration was quantified by a Dionex ICS-3000 ion chromatography (IC) to back-calculate the total fluorine (TF) concentrations in the samples. The same anti-fog spray samples were also diluted by water and analyzed for their inorganic fluoride (IF) concentrations directly using IC. The TOF was determined as the difference between the TF and IF levels in the same sample.75 In all the tested anti-fog spray samples, the TF levels were dominated by TOF, with IF contributing to 0.07−1.43% of TF levels.
The five cloth samples were analyzed in triplicate for extractable organofluorine to represent their TOF levels. Each cloth sample (0.05 g) was extracted by 1 mL of hexane:dichloromethane (1:1 v/v) mixture under sonication three times. The combined extracts were diluted by methanol, combusted in the Mitsubishi furnace system, and analyzed for fluoride after combustion.
Adipogenesis Evaluation of Anti-fog Solutions.
3T3L1 cells (Zenbio cat# SP-L1-F, lot# 3T3–062104, passage 8–12; Research Triangle Park, NC) were maintained as described in detail previously68,69,76,77 in pre-adipocyte media (Dulbecco’s modified Eagle’s medium―high glucose; DMEM-HG; Gibco cat# 11995, supplemented with 10% bovine calf serum and 1% penicillin and streptomycin). The cells were seeded into 96-well tissue culture plates (Greiner cat# 655090), grown to confluency, and allowed 48 h to undergo growth arrest and initiate clonal expansion. The medium was then replaced with controls and/or test solution dilutions in differentiation cocktail media (DMEM-HG with 10% fetal bovine serum, 1% penicillin/ streptomycin, 1.0 μg/mL human insulin, and 0.5 mM 3isobutyl-1-methylxanthine, IBMX). After 48 h of induction, the medium was replaced with fresh dilutions of all test chemicals and treatments in adipocyte maintenance media (differentiation media without IBMX), and this was refreshed every 2−3 days until the plates were assayed. 10 days after induction, the plates were assayed for triglyceride accumulation and pre-adipocyte proliferation. The medium was removed from the plates, and the cells were rinsed with Dulbecco’s phosphate-buffered saline (DPBS; Gibco cat # 14040), removed, and replaced with a 200 μL dye mixture [19 mL of DPBS, 1 drop/mL of NucBlue Live ReadyProbes Reagent (Thermo cat #R37605) and 500 μL of 40 μg/mL Nile Red (Sigma 72485–100MG)]. The plates were protected from light and incubated at room temperature for approximately 40 min, and then the fluorescence was measured using excitation at 485 nm and emission at 572 nm for Nile Red and 360 and 460 nm for NucBlue, respectively.
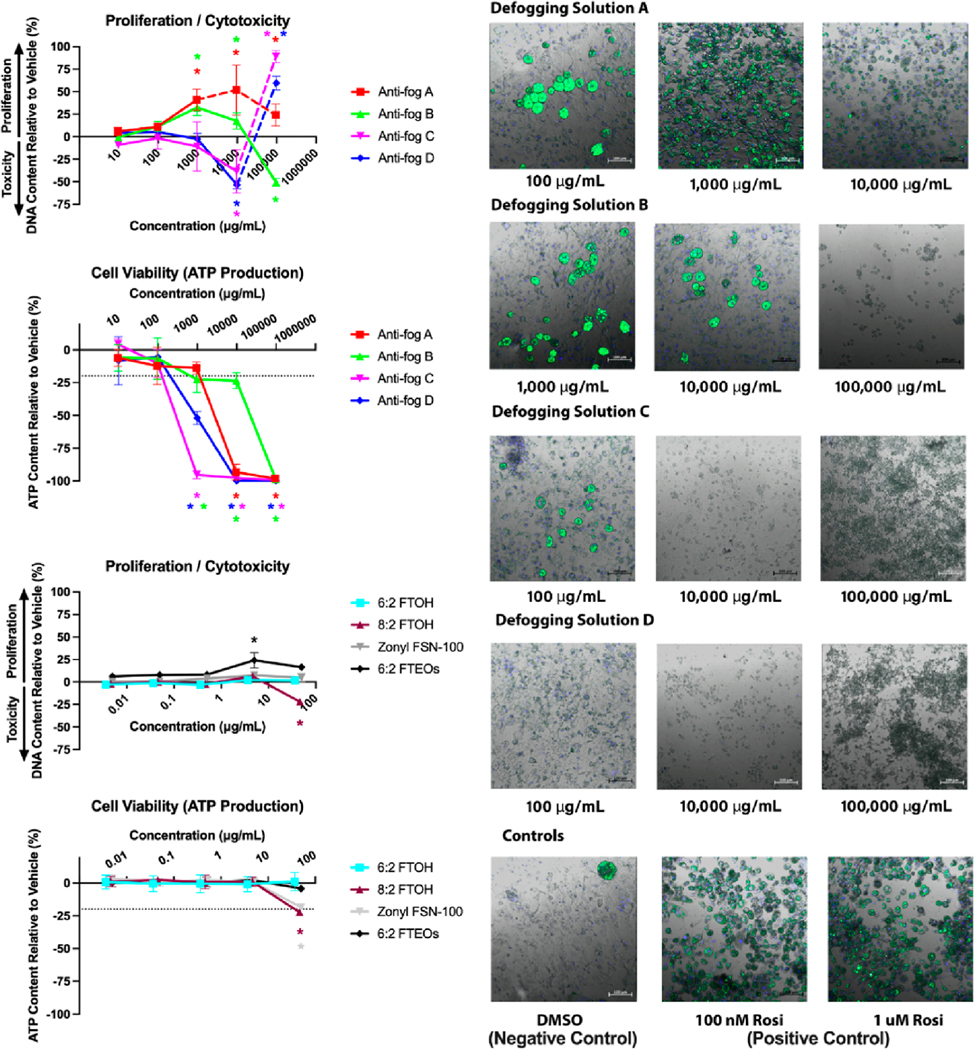
For triglyceride accumulation data, percent activities were calculated relative to the maximal rosiglitazone-induced fold induction over the intra-assay differentiated vehicle control (0.1% dimethyl sulfoxide) responses. The DNA content was calculated as percent change from differentiated vehicle control responses for each chemical at each concentration and was then used to normalize the total triglyceride values to obtain the triglyceride content per unit DNA (a proxy for triglyceride accumulation per cell). DNA content measurements in the adipogenesis assay can denote either pre-adipocyte proliferation (positive responses) and/or cytotoxicity (negative values) across a dose response. However, the DNA content assays can occasionally provide non-specific increases in this system, so two additional cell health measures were included to provide consensus determinations of toxicity. First, visual confirmation (qualitative) was performed using a Zeiss LSM 800 fluorescence confocal imaging system (Figure 2) to assess specific versus nonspecific staining and cell integrity. After fluorescence measurements and microscopy, the CellTiter-Glo 2.0 assay (Promega cat # PRG9242) was utilized to assess the metabolic activity of cells via ATP measurements. Briefly, 100 μL of media was removed from plates and replaced with 100 μL of the CellTiter-Glo reagent, mixed, incubated for 10 min, and then read on a plate reader for quantification of luminescence. All three cell health determinations were compared to determine the potential cytotoxic responses by our test chemicals. Four technical (replicates within each assay plate) and three biological replicates (separate cell passages/assays) were performed for every tested chemical and concentration for each of these assays. Given the lack of available information on commercial sprays and contaminants present, we tested these at 1:1000 dilutions from the actual product. In contrast, we performed more controlled dose response ranges for individual and defined mixtures, where we had more information available to select realistic toxicological dosing concentrations below presumed toxicity.
Figure 2.
Cytotoxicity and cell health measures for anti-fog sprays and constituent chemicals. 3T3-L1 pre-adipocytes were differentiated while exposed to sprays and constituent chemicals and then assayed for DNA content (cytotoxicity), ATP production (cell viability), and fluorescent microscopy (qualitative visual confirmation). The DNA content reported as increase (pre-adipocyte proliferation) or decrease (cytotoxicity) relative to differentiated solvent control response. ATP production reported as a decrease in ATP produced relative to differentiated solvent control response. Data presented as mean ± SEM from three independent experiments. Fluorescence microscopy used as a third confirmatory measure of toxicity for anti-fog sprays (green fluorescence measures triglyceride accumulation staining and blue fluorescence represents nuclear staining).
RESULTS AND DISCUSSION
Product Characterization.
Several different PFAS were detected in all products, and their chemical backbones are summarized in Figure 1. FTOHs and FTEOs were detected in every product. 6:2 FTOH and the 6:2 FTEO series were the predominant PFAS compounds observed and were detected in every product. FTEO ethoxymers were identified via GC–HRMS and HPLC–HRMS/MS by predicting the exact mass for each isomer up to 15 ethoxy units and monitoring for the protonated molecular ion in each sample. Identifications were additionally confirmed with fragments common to 6:2 FTOH and the series (Table S4). Ethoxymers in the 6:2 FTEO polymeric series were detected from 1 to 8 ethoxy units using GC–MS and from 2 to 13 ethoxy units using HPLC–HRMS. While the 6:2 compounds were most widely detected in samples, 8:2, 10:2, 12:2, 14:2, and 16:2 FTOH and FTEO series were detected in anti-fog cloth A. All products contained similar PFAS, some of which are novel and, to our knowledge, have not been reported in the literature. All products, except for anti-fog spray A, had a compound that included two partially fluorinated chains (6:2 fluorination pattern) connected by a single ether bond (Figure 1c). Anti-fog spray A instead was found to contain two partially fluorinated chains (6:2 fluorination pattern) with a fumarate diester bridge. Anti-fog cloth A also had similar compounds (i.e., ester-bonded fluorinated chains) with 6:2−8:2, 8:2−8:2, 8:2−10:2, and 10:2−10:2 fluorination patterns (Table S4). In our method, the 8:2−12:2 fluorotelomer ether appeared to coelute with the isomeric 10:2−10:2 fluorotelomer ether (Figure S11). Larger fluorotelomer ethers were likely present but not observed using GC–HRMS due to their high masses. It seems possible that these compounds were not intentionally produced but were instead the result of side dimerization reactions, for example, manufacturing byproducts. One anti-fog spray (A) and two anti-fog cloths (C&D) also contained compounds with two partially fluorinated chains (6:2 fluorination pattern) connected by an ethoxyl chain length ranging from 1 to 8. Chemical identifiers, including CAS number, IUPAC Name, SMILES, and INCHI-Key, for all the identified PFAS compounds are listed in the Supporting Information (Table S5). Seven additional non-ionic PFAS (including 6:2 fluorotelomer acrylate, 6:2 fluorotelomer methacrylate, and 8:2 fluorotelomer acrylate) were targeted for quantification in this study but were not detected in products (Table S2).
The concentration of 6:2 FTOH in the anti-fog sprays ranged from 3.43 to 10,600 μg/mL (Table 1). While we did screen for 4:2, 8:2, 10:2, 12:2, 14:2, and 16:2 FTOH in all products, no other FTOHs were detected in any of the anti-fog sprays. In the anti-fog cloths, the levels of 6:2 FTOH ranged from 1.29 to 38.4 μg (g cloth)−1. Both 8:2 FTOH and 10:2 FTOH were detected in cloth A at 127 and 15.5 μg (g cloth)−1, respectively. 12:2 FTOH, 14:2 FTOH, and 16:2 FTOH were also detected in cloth A but were not quantified due to the lack of authentic standards. Qualitatively, 12:2 FTOH and 14:2 FTOH were present at levels equal to or greater than 6:2 FTOH in this product based on standard-normalized instrument responses (Table S6). The median FTOH concentrations reported here in these sprays and cloths are similar to what has previously been reported in food contact paper, treated textiles, floor waxes, and stone/wood sealants.33,38,39 However, the upper limit detected in anti-fog products (10,600 μg/mL) is an order of magnitude higher than previously measured.
Table 1.
Concentrations of FTOH and 6:2 FTEOs in Anti-fog Sprays and Clothsa
| Concentration (μg/mL) | Concentration (μg/g) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Spray A | Spray B | Spray C | Spray D | Cloth A | Cloth B | Cloth C | Cloth D | Cloth E | |
| 6:2FTOH | 10,600 | 25.8 | 3.46 | 3.43 | 1.29 | 3.95 | 38.4 | 7.94 | 9.62 |
| 8:2FTOH | - | - | - | - | 127 | - | - | - | - |
| 10:2FTOH | - | - | - | - | 15.5 | - | - | - | - |
| 6:2FTEO1b | N.Q. | N.Q. | N.Q. | N.Q. | N.Q. | N.Q. | N.Q. | N.Q. | N.Q. |
| 6:2FTEO2 | 35.2 | 1.08 | 0.193 | 0.194 | 1.29 | 53.3 | 126 | 208 | 244 |
| 6:2FTEO3 | 129 | 2.55 | 0.8 | 0.804 | 3.64 | 260 | 566 | 746 | 726 |
| 6:2FTEO4 | 1010 | 16.2 | 8.84 | 8.89 | 28.9 | 2770 | 6140 | 7340 | 5780 |
| 6:2FTEO5 | 2670 | 42.8 | 33 | 32.1 | 99 | 9420 | 22,100 | 23,400 | 17,200 |
| 6:2FTEO6 | 2790 | 50.1 | 52.9 | 51.4 | 134 | 12,200 | 29,200 | 33,200 | 21,300 |
| 6:2FTEO7 | 2200 | 46.8 | 64.6 | 63 | 125 | 12,200 | 29,300 | 30,400 | 20,800 |
| 6:2FTEO8 | 1720 | 44.3 | 73.4 | 75.9 | 104 | 10,600 | 26,300 | 28,400 | 18,900 |
| 6:2FTEO9 | 1280 | 35.4 | 76 | 78.3 | 77.9 | 7540 | 21,900 | 21,500 | 15,800 |
| 6:2FTEO10 | 964 | 28 | 77.6 | 79.7 | 57.2 | 5380 | 16,500 | 17,000 | 11,800 |
| 6:2FTEO11 | 616 | 17.2 | 67.3 | 66 | 35.7 | 3280 | 11,100 | 10,900 | 7830 |
| 6:2FTEO12 | 429 | 9.53 | 39.8 | 49.2 | 25 | 1720 | 6800 | 6230 | 4450 |
| 6:2FTEO13 | 517 | 7.19 | 31.1 | 57.1 | 7.34 | 1680 | 6090 | 5930 | 3120 |
| Σionic PFASc | 1.37 | 0.062 | 0.019 | 0.037 | 2.09 | 0.156 | 1.51 | 2.68 | 0.825 |
| ΣPFAS | 25,000 | 327 | 529 | 566 | 702 | 67,100 | 176,000 | 185,000 | 128,000 |
| TOF measurement | 20,700 (508) |
221 (3) | 202 (2) | 190 (1) | 46,800 (5200) |
44,200 (2200) |
131,500 (2200) |
92,000 (2700) |
73,900 (2900) |
| % TOF explained by FTEOs and FTOHs | 60% | 57% | 88% | 99% | 1% | 55% | 48% | 72% | 62% |
TOF measurements are reported as the average of triplicate analysis, with standard deviations in parenthesis.
6:2FTEO1 was unable to be quantified via HPLC–HRMS methods as the other FTEOs were and is denoted as N.Q.
See Table S3 for the list of ionic PFAS quantified.
In general, the FTEOs were present in anti-fog products at levels greater than FTOHs (Tables 1 and S6). The Σ6:2 FTEO2−13 was present at levels up to 14,400 μg/mL in sprays and up to 185,000 μg (g cloth)−1 in the cloths. In general, the anti-fog cloths had much greater levels of FTEOs relative to FTOHs (Table S6). Intuitively, this may be explained by the fact that the volatile FTOHs are likely not retained well on the cloths for extended periods of time. The 8:2, 10:2, 12:2, 14:2, and 16:2 FTEO series were also identified via GC–HRMS in anti-fog cloth A, all from 1 to 8 ethoxy units, respectively, though not quantified due to the lack of authentic standards.
Anti-fog products were also analyzed for a suite of 28 ionic PFAS, including PFSAs, PFCAs, FTSAs, FTCAs, diPAPs, GenX, and FOSAA using LC–MS/MS (Table S3). Many ionic PFAS were detected in products at trace levels (Table S3). Σ(ionic PFAS) ranged from 19 to 1370 ng/mL in anti-fog sprays and 156 to 2680 ng (g cloth)−1 in anti-fog sprays. Perfluoroalkyl carboxylic acids were the most abundant PFAS with PFBA or PFHxA being most prevalent in anti-fog sprays and PFHpA or PFPeA being most prevalent in anti-fog cloths. While a nonnegligible amount of legacy ionic PFAS was detected in these products, they were present at levels several orders of magnitude lower than FTOHs and FTEOs.
TOF contents of the anti-fog products were also quantified (Table 1). Of the four anti-fog sprays, spray A had the highest TOF content of 20,700 μg/mL with sprays B, C, and D at 221, 202, and 190 μg/mL, respectively. For the five anti-fog cloths, the TOF contents ranged from 44,200 μg (g cloth)−1 in cloth B to 131,500 μg (g cloth)−1 in cloth C. These TOF values are higher than the extractable organic fluorine concentrations measured in cosmetics (up to 1720 μg g−1)78 and total fluorine (TF) concentrations measured up to 60 μg g−1 in fast food packaging.10 It makes an intuitive sense that anti-fog products, with the sole purpose of water repellency, would have higher fluorine content than cosmetics or food packaging, where PFASs are an additive.
6:2 FTOH and Σ6:2 FTEOs accounted for 57−99% of TOF levels in anti-fog sprays and for 1−72% of TOF levels in anti-fog cloths (Table 1). The trace levels of ionic PFAS detected in antifog products only accounted for 0.01−0.03% of TOF in the antifog sprays and an even smaller portion in anti-fog cloths. Presumably, the remaining fluorine mass was associated with the PFAS discussed above. This is especially relevant for anti-fog cloth A, where 8:2, 10:2, 12:2, 14:2, and 16:2 FTEOs were present in the product. In anti-fog cloth A, 8:2 FTEOs and 10:2 FTEOs were present at levels that appear to be over an order of magnitude higher than the 6:2 FTEOs based on instrument normalized responses (Table S6). For the other products, the unquantified PFAS, namely, 6:2 fluorotelomer fumarate, 6:2−6:2 fluorotelomer ether, 6:2 FTEO1, and ethoxymers of a chain length >13, likely account for the remaining fluorine mass quantified by the TOF measurements. For example, 6:2 fluorotelomer fumarate was the second most abundant peak (behind 6:2 FTOH) in spray A, which likely explains the lower contribution of TOF explained by our quantitative analysis. Similarly, 6:2−6:2 fluorotelomer ethers were the second most abundant peak (behind 6:2 FTOH) in spray B, and this spray was the only spray to contain FTEO ethers, which we were unable to quantify (Table S6). This again likely explains the lower contribution of TOF explained by our quantitative analysis. These results suggest that, while there may still be unidentified PFAS in these products, we have identified the most prevalent compounds.
Characterization of Non-Ionic Fluorosurfactant Formulations.
After identifying the FTEOs in these anti-fog products, we sought to determine the commercial PFAS source of these compounds and provide an estimate of their contribution in the products. We acquired an older Zonyl FSN-100 formulation from Dr. Jennifer Field (Oregon State University) and purchased a new FTEO formulation (hereafter referred to as “6:2 FTEO formulation”) from BOC Sciences for PFAS characterization. The 6:2 FTEO formulation contained 6:2 FTOH, the 6:2 FTEO series, and 6:2–6:2 fluorotelomer ether (Table S6). 6:2 FTOH was present in the 6:2 FTEO formulation at a concentration of 7310 ± 140 μg/g. The Zonyl FSN-100 formulation, in contrast, contained FTOHs and FTEO series for 6:2, 8:2, 10:2, 12:2, 14:2, and 16:2 fluorination patterns, as well as 6:2−6:2, 6:2−8:2, 8:2−8:2, 8:2−10:2, and 10:2−10:2 fluorotelomer ethers (Table S6). 6:2, 8:2, and 10:2 FTOH were present at 834 ± 120, 754 ± 26, and 482 ± 28 μg/g, respectively, in the Zonyl FSN-100 formulation. The TOF measured in the 6:2 FTEO formulation was 447.57 ± 11.55 mg g−1. Due to the limitations on the available amount, we were unable to conduct TOF analysis on the Zonyl FSN-100 mixture.
HPLC–CAD was used to quantify the ethoxymer distribution of the 6:2 FTEO formulation. The distribution peaked at six EO, with an asymmetric profile favoring shorter chains (Table S7), consistent with commercial production via ethylene oxide polymerization on an alcohol hydrophobe. Ethoxylate chain lengths from 2 to 13 accounted for 92.74% of the formulation (Table S7). Presumably, 6:2 FTOH, 6:2 FTEO1, and 6:2−6:2 fluorotelomer ether account for the remaining percentage, though it could not be confirmed via HPLC–CAD analysis. Due to the complexity of the mixture (extensive co-elution of different FTEO ethoxymers), we were unable to quantitatively characterize the Zonyl FSN-100 via HPLC–CAD analysis.
The 6:2 FTEO formulation most closely resembled the formulation present in sprays C and D and cloths B and C, while the Zonyl FSN-100 formulation most closely resembled the formulation present in cloth A. Spray B and cloths D and E all appear to be produced from the same chemical formulation, not identified in this study, which includes the 6:2 FTEO ethers. Spray A, while similar to the 6:2 FTEO formulation, included the 6:2 fluorotelomer fumarate in the place of 6:2−6:2 fluorotelomer ether, suggesting that it may stem from a different FTEO commercial mixture that was likely manufactured using a modified procedure.
Adipogenic Activity of Anti-fog Spray Products.
Since prior research observed high adipogenic activity for similar polyethoxylated surfactants,69,71 we sought to determine if these fluorinated ethoxylate products and mixtures would also elicit activity. All the four anti-fog spray solutions, both commercial formulations, and four individual component chemicals present in the sprays (6:2 FTOH, 8:2 FTOH, diethylene glycol butyl ether, and 1-butoxypropanol) were characterized for their potential toxicity using several metrics, including assessments of cytotoxicity (DNA content), cell viability (ATP production), and a qualitative microscopy evaluation. All of these were also assessed for their adipogenic activity (triglyceride accumulation and pre-adipocyte proliferation) in murine 3T3-L1 preadipocytes. Cloths were not tested.
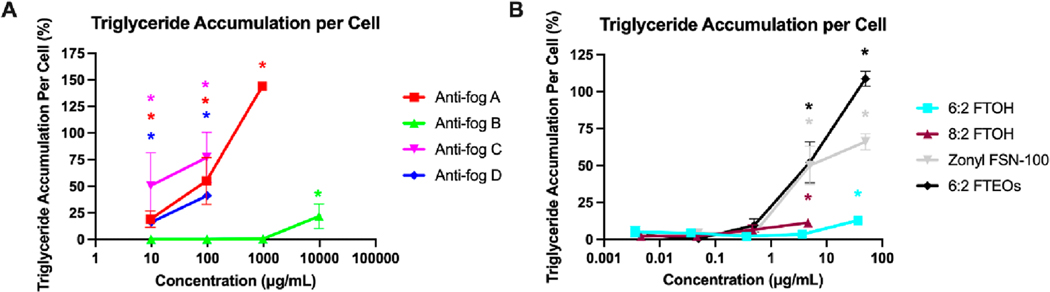
Figure 2 presents the results from the cell health/cytotoxicity testing. In general, the commercial anti-fog spray products were much more toxic than any of the individual chemicals present in the sprays (e.g., 6:2 FTOH, 8:2 FTOH, diethylene glycol butyl ether, and 1-butoxypropanol) or commercial chemical mixtures tested (i.e., 6:2 FTEO mixture and Zonyl FSN-100). Anti-fog spray A inhibited cell viability at doses of 10,000 and 100,000 μg/mL based on cell viability (ATP production) and visual confirmation via microscopy, despite DNA content measurements appearing to increase at 10,000 μg/mL. Anti-fog B marginally inhibited the cell viability at concentrations of 1000 and 10,000 μg/mL (ATP production), with normal microscopy and positive effects on DNA content at these doses. At 100,000 μg/mL, all three measures demonstrated consistent toxicity. Anti-fog sprays C and D were most toxic, with cell viability and visual confirmation suggesting cytotoxicity at doses ≥1000 μg/ mL (despite apparent increase in DNA content at 100,000 μg/ mL). Of the individual chemicals and commercial mixtures, 8:2 FTOH was cytotoxic at the highest dose (46 μg/mL and 100 μM) in both DNA and ATP content assays. The Zonyl FSN-100 formulation inhibited cell viability at the highest dose (50 μg/ mL), though there were no apparent effects on DNA content. 6:2 FTOH, the 6:2 FTEO formulation, and both nonfluorinated additives (diethylene glycol butyl ether and 1-butoxypropanol) did not demonstrate cytotoxicity at any doses tested in this study. At concentrations below the cytotoxicity thresholds discussed above, anti-fog sprays exhibited a range of adipogenic activities. Triglyceride accumulation is presented in Figure 3 and is only shown for doses that were not deemed cytotoxic based on the three measures described above. Anti-fog A exhibited the greatest degree of activity, with approximately 145% triglyceride accumulation induced at 1000 μg/mL, relative to the maximal rosiglitazone-induced (positive control) response (set at 100%). Anti-fog A also promoted pre-adipocyte proliferation, with 40% increased DNA content relative to the differentiated vehicle control at 1000 μg/mL (Figure 2). Antifog B exhibited minor adipogenic activity, with 22% triglyceride accumulation induced at 10,000 μg/mL, and 32% increased DNA content at 1000 μg/mL. Anti-fog sprays C and D exhibited 71 and 41% triglyceride accumulation at 100 μg/mL, respectively, and neither promoted significant cell proliferation (i.e., increased DNA content) at any concentration tested.
Figure 3.
Anti-fog sprays and fluorinated components induce pre-adipocyte differentiation. 3T3-L1 pre-adipocytes were differentiated while exposed to sprays and constituent chemicals and then assayed for triglyceride accumulation (marker of adipocyte differentiation) via Nile red neutral lipid droplet staining. Triglyceride accumulation is depicted for the anti-fog spray commercial products (A) and for the fluorinated component chemicals and commercial mixtures (B). Data presented as percent triglyceride accumulation per cell (normalized to DNA content) relative to the maximal intraassay response for the rosiglitazone positive control. Data presented as mean ± SEM from three independent experiments. Data for triglyceride accumulation per cell at doses that were deemed cytotoxic are not shown.
Of the individual chemicals tested, 6:2 FTOH exhibited minor adipogenic activity, with 13% triglyceride accumulation induced at 36 μg/mL (100 μM) and no effects on pre-adipocyte proliferation. Similarly, 8:2 FTOH exhibited 11% triglyceride accumulation at ∼5 μg/mL (10 μM). The two non-fluorinated additives were inactive for both triglyceride accumulation and proliferation (Figure S1). In contrast, the commercial mixtures exhibited robust adipogenic activity. The 6:2 FTEO formulation and Zonyl FSN-100 formulation exhibited 109 and 66% triglyceride accumulation at 50 μg/mL, respectively. The 6:2 FTEO formulation also promoted 24% pre-adipocyte proliferation at 10 μg/mL, though the Zonyl FSN-100 had no proliferative effects.
We have previously reported the adipogenic activities of a small number of PFAS, including 6:2 and 8:2 FTOHs, neither of which exhibited significant activity in our assay previously (at lower concentrations than we tested herein).68 However, 8:2 fluorotelomer acrylate (1H,1H,2H,2H-heptadecafluorodecyl acrylate) exhibited significant effects on triglyceride accumulation in our hands,68 and others have demonstrated adipogenic effects for other PFAS in this model.67 We have also previously reported extremely potent and efficacious triglyceride accumulation and pre-adipocyte proliferation for various alkylphenols and alcohol polyethoxylates.69 It is therefore perhaps unsurprising that the ethoxylated fluorotelomer compounds identified in this study exhibit activity in this assay. We should note that our results provide a note of caution on the interpretation of high-throughput toxicity testing. While some chemicals exhibited an apparent increase in DNA content at high doses (e.g., sprays A, C, and D), fluorescent imaging and cell viability assays confirmed cell death at these concentrations (Figure 2).
Last, we sought to estimate the potential PFAS exposure by using these sprays as indicated. We measured the density of each defogger spray (Table S8). Based on these densities, and our measurement of PFAS, we estimate that approximately 2.5% of the mass of spray A is composed of PFAS, whereas in sprays B, C, and D, PFAS ranged from 0.03 to 0.06%. Therefore, a 1000 μg/ mL dose of spray A would be ∼25 μg/mL total PFAS and would fall between the 5 and 50 μg/mL dose of the 6:2 FTEO formulation. The 135% triglyceride accumulation observed at 1000 μg/mL of spray A correlates well with the interpolated 25 μg/mL activity (∼100% triglyceride accumulation) observed at for the 6:2 FTEO commercial mixture. Since we were able to completely characterize spray A (the ingredient list was on the product bottle) and we know that the two non-fluorinated additives present in spray A were not active in our assay, we can conclude that the 6:2 FTEOs are a significant driver to the adipogenic activity exhibited by spray A in our model.
Similarly, the 10,000 μg/mL dose of spray B would be ∼3 μg/ mL total PFAS (i.e., dose of the 6:2 FTEO formulation). The 20% triglyceride accumulation observed at 10,000 μg/mL of spray B correlates well with the 3 μg/mL activity (∼30% triglyceride accumulation) observed for the 6:2 FTEO commercial mixture. While we were unable to completely characterize spray B, we can reasonably postulate that the 6:2 FTEOs are a significant driver of the adipogenic activity exhibited by spray B in our model.
Sprays C and D are more difficult to interpret within our assay due to high levels of cytotoxicity. Sprays C and D demonstrate equivalent effects on triglyceride accumulation to A at 10 μg/ mL, despite having much lower total PFAS levels in the product. This high degree of cytotoxicity and adipogenic activity cannot be predicted by the known levels of PFAS in these products. Contrary to sprays A and B, sprays C and D had several highly abundant features present in the chromatogram that we were unable to identify. These features were present at levels many orders of magnitude higher than the any fluorinated compound identified in the sprays, contrary to what was observed in sprays A and B. Therefore, contrary to the strong line of evidence we have for 6:2 FTEO driving activity in sprays A and B, we believe that the unidentified additives may be driving the activity for sprays C and D that we see in our model. Though without a true identification, we cannot be certain.
Implications.
While we only measured a small number of anti-fog products, we found that FTOHs and FTEOs were quantitatively important components in all of them. The FTEOs explained a majority of the TOF measured in the samples, demonstrating the importance of the TOF (or similar) approach, as a regular targeted method would have been insufficient to characterize the full PFAS content in these samples. The presence of PFAS compounds in these anti-fog products is unsurprising, though the quantity was unexpected. Using the measured densities of each spray, we estimate that ∼3.5 mg of PFAS is discharged to the target surface and surrounding environment with each pump of spray A. Sprays B, C, and D all fall below 100 μg of PFAS per use (Table S8). To put this in context, if only 1% of the total PFAS from each use of the spray enters the body (via inhalation/dermal absorption), the PFAS exposure would equate to 1−35 μg of PFAS. This amount of PFAS exposure is a 14−500× greater dose than one would receive if consuming 1 L of water at the U.S. EPA health advisory level for PFAS/PFOA of 70 part per trillion (ng/L). The application notes for spray A state that one application will be effective for ∼24 h, which indicates that this product has the potential to be a significant daily exposure source. In addition, the instructions on spray A recommended rubbing the product onto the eyeglass surface with your finger. Given the mobility of FTOHs in the indoor environment, these products have the potential to be an important source of PFAS precursors in the indoor environment (namely, air and dust). Previous studies have shown that FTOHs in air are correlated with serum PFAA levels, suggesting that they may be an important precursor class for PFAAs that are more toxic and have longer half-lives in the body.64,79
Significant effects on triglyceride accumulation were observed for all four anti-fog sprays and on pre-adipocyte proliferation for two of the four solutions. Even at concentrations as low as 10 μg/mL, anti-fog spray A exhibited ∼20% triglyceride accumulation or equivalent to approximately 5 nM of the positive control, rosiglitazone. Importantly, the biological activity was observed at an in vitro dose that is less than the dose applied to eyeglasses from one pump of the spray based on their densities (the 1000 μg/mL in vitro dose is equivalent to ∼190 μg of solution, with approximately 1000 times the quantity released in each spray). Sprays C and D similarly exhibited significant triglyceride accumulation at the 10 μg/mL dose, though it is unclear how much of this activity is attributable to FTEOs. Regardless of the main driver of adipogenic activity in sprays C and D, it is similarly observed at an in vitro dose that is less than the dose applied to eyeglasses from one pump of the spray, thereby warranting the concern.
While the production and use of anti-fog products is a clear potential exposure source of FTEOs, little is known about their fate in the human body or the environment. Frömel and Knepper (2010) found FTEOs biodegraded in a WWTP under aerobic conditions to FTEO carboxylates (FTEOC), with little evidence of them degrading beyond that.66 However, further research is needed to fully understand the potential pathways for FTEOs to degrade further to FTOHs and subsequently stable ionic PFAA. Given that the aforementioned study only analyzed FTEO biodegradation under a single set of conditions, there is still much unknown about the biodegradation potential of these compounds. Even less is known about the transformation of FTEOs via metabolic processes, as it has not been studied.
To the best of our knowledge, no research to date has been conducted to determine how widespread FTEOs are used in consumer products and how much of it is being produced every year. These products were manufactured in several different countries, specifically the United States, China, and Korea (Table S1), and therefore, it seems likely that there is global use of these products. This is especially pertinent given the recent EU ban on long-chain perfluorinated carboxylic acids (C9–14 PFCAs), their salts and precursors that will take effect in February 2023,80 and the progress toward more restrictive regulation in the United States.81 While a majority of the analytes detected in this study were short-chain PFAS (C6), one sample and one commercial mixture contained long-chain PFAS (C10–C16 FTOHs/FTEOs). These analytes could be classified as precursors for long-chain PFCAs and would thus be subject to the new EU regulations. More research is needed to elucidate the uses of these novel compounds in commercial products.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported in part by a grant from the Environment Protection Agency (CR-83948201–0; H.M.S.), a major research instrumentation grant from the National Science Foundation (CBET-1828257; H.M.S.), the National Institute of Environmental Health Sciences (R00 ES030405; C.D.K.), and the North Carolina Policy Collaborative through an appropriation from the North Carolina General Assembly (Y.H., V.F.P., and M.S.). We also wish to thank Michael and Annie Falk for establishing the Falk Exposomics Laboratory (H.M.S. and P.L.F.). Last, we wish to thank Duncan Hay for assistance with laboratory analysis.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.1c06990.
Additional plots for adipogenesis results, additional information for the anti-fog products, as well as chemical descriptors, structures, mass-to-charge ratios, spectra, and relative response ratios for identified fluorinated compounds; results in full for LC–MS analysis of ionic PFAS and fluorotelomer ethoxymer distribution in commercial FTEO formulation determined by HPLC–CAD analysis; and PCI spectra for all the identified PFAS in this study (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.est.1c06990
The authors declare no competing financial interest.
Contributor Information
Nicholas J. Herkert, Nicholas School of the Environment, Duke University, Durham, North Carolina 27708, United States.
Christopher D. Kassotis, Institute of Environmental Health Sciences and Department of Pharmacology, Wayne State University, Detroit, Michigan 48202, United States
Sharon Zhang, Nicholas School of the Environment, Duke University, Durham, North Carolina 27708, United States.
Yuling Han, Department of Civil and Environmental Engineering, University of North Carolina at Charlotte, Charlotte, North Carolina 28223, United States.
Vivek Francis Pulikkal, Department of Civil and Environmental Engineering, University of North Carolina at Charlotte, Charlotte, North Carolina 28223, United States.
Mei Sun, Department of Civil and Environmental Engineering, University of North Carolina at Charlotte, Charlotte, North Carolina 28223, United States.
P. Lee Ferguson, Nicholas School of the Environment and Department of Civil and Environmental Engineering, Duke University, Durham, North Carolina 27708, United States.
Heather M. Stapleton, Nicholas School of the Environment, Duke University, Durham, North Carolina 27708, United States.
REFERENCES
- (1).Hu Y. Prevention of Fogging of Protective Eyewear for Medical Staff During the COVID-19 Pandemic. J. Emerg. Nurs. 2020, 46, 564–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Hazanchuk V. How to Wear a Face Mask Without Fogging Your Glasses. EyeSmart; The American Academy of Ophthalmic, 2021. [Google Scholar]
- (3).How to Keep Your Glasses From Fogging Up While Wearing a Mask. Health Essentials; Cleveland Clinic, 2020. [Google Scholar]
- (4).Vence T. The Best Anti-Fog for Glasses and a Mask. Wirecutter; The New York Times Company, 2021. [Google Scholar]
- (5).Barzen-Hanson KA; Roberts SC; Choyke S; Oetjen K; McAlees A; Riddell N; McCrindle R; Ferguson PL; Higgins CP; Field JA Discovery of 40 Classes of Per- and Polyfluoroalkyl Substances in Historical Aqueous Film-Forming Foams (AFFFs) and AFFF-Impacted Groundwater. Environ. Sci. Technol. 2017, 51, 2047–2057. [DOI] [PubMed] [Google Scholar]
- (6).Buck RC; Franklin J; Berger U; Conder JM; Cousins IT; De Voogt P; Jensen AA; Kannan K; Mabury SA; van Leeuwen SP Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integrated Environ. Assess. Manag. 2011, 7, 513–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Houtz EF; Higgins CP; Field JA; Sedlak DL Persistence of perfluoroalkyl acid precursors in AFFF-impacted groundwater and soil. Environ. Sci. Technol. 2013, 47, 8187–8195. [DOI] [PubMed] [Google Scholar]
- (8).Houtz EF; Sutton R; Park J-S; Sedlak M. Poly-and perfluoroalkyl substances in wastewater: Significance of unknown precursors, manufacturing shifts, and likely AFFF impacts. Water Res. 2016, 95, 142–149. [DOI] [PubMed] [Google Scholar]
- (9).Prevedouros K; Cousins IT; Buck RC; Korzeniowski SH Sources, fate and transport of perfluorocarboxylates. Environ. Sci. Technol. 2006, 40, 32–44. [DOI] [PubMed] [Google Scholar]
- (10).Schaider LA; Balan SA; Blum A; Andrews DQ; Strynar MJ; Dickinson ME; Lunderberg DM; Lang JR; Peaslee GF Fluorinated compounds in US fast food packaging. Environ. Sci. Technol. Lett. 2017, 4, 105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Zareitalabad P; Siemens J; Hamer M; Amelung W. Perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) in surface waters, sediments, soils and wastewater - A review on concentrations and distribution coefficients. Chemosphere 2013, 91, 725–732. [DOI] [PubMed] [Google Scholar]
- (12).Wang Z; Cousins IT; Scheringer M; Hungerbuehler K. Hazard assessment of fluorinated alternatives to long-chain perfluoroalkyl acids (PFAAs) and their precursors: Status quo, ongoing challenges and possible solutions. Environ. Int. 2015, 75, 172–179. [DOI] [PubMed] [Google Scholar]
- (13).Hu XC; Andrews DQ; Lindstrom AB; Bruton TA; Schaider LA; Grandjean P; Lohmann R; Carignan CC; Blum A; Balan SA Detection of poly-and perfluoroalkyl substances (PFASs) in US drinking water linked to industrial sites, military fire training areas, and wastewater treatment plants. Environ. Sci. Technol. Lett. 2016, 3, 344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Lindstrom AB; Strynar MJ; Libelo EL Polyfluorinated Compounds: Past, Present, and Future. Environ. Sci. Technol. 2011, 45, 7954–7961. [DOI] [PubMed] [Google Scholar]
- (15).Wang Z; DeWitt JC; Higgins CP; Cousins IT A Never-Ending Story of Per- and Polyfluoroalkyl Substances (PFASs)? Environ. Sci. Technol. 2017, 51, 2508–2518. [DOI] [PubMed] [Google Scholar]
- (16).Björklund JA; Thuresson K; De Wit CA Perfluoroalkyl compounds (PFCs) in indoor dust: concentrations, human exposure estimates, and sources. Environ. Sci. Technol. 2009, 43, 2276–2281. [DOI] [PubMed] [Google Scholar]
- (17).Haug LS; Huber S; Schlabach M; Becher G; Thomsen C. Investigation on per-and polyfluorinated compounds in paired samples of house dust and indoor air from Norwegian homes. Environ. Sci. Technol. 2011, 45, 7991–7998. [DOI] [PubMed] [Google Scholar]
- (18).Kannan K; Corsolini S; Falandysz J; Fillmann G; Kumar KS; Loganathan BG; Mohd MA; Olivero J; Wouwe NV; Yang JH Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries. Environ. Sci. Technol. 2004, 38, 4489–4495. [DOI] [PubMed] [Google Scholar]
- (19).Kato K; Wong L-Y; Jia LT; Kuklenyik Z; Calafat AM Trends in Exposure to Polyfluoroalkyl Chemicals in the U.S. Population: 1999–2008. Environ. Sci. Technol. 2011, 45, 8037–8045. [DOI] [PubMed] [Google Scholar]
- (20).Lewis R; Johns L; Meeker J. Serum biomarkers of exposure to perfluoroalkyl substances in relation to serum testosterone and measures of thyroid function among adults and adolescents from NHANES 2011–2012. Int. J. Environ. Res. Publ. Health 2015, 12, 6098–6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Olsen GW; Burris JM; Ehresman DJ; Froehlich JW; Seacat AM; Butenhoff JL; Zobel LR Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ. Health Perspect. 2007, 115, 1298–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Olsen GW; Church TR; Miller JP; Burris JM; Hansen KJ; Lundberg JK; Armitage JB; Herron RM; Medhdizadehkashi Z; Nobiletti JB Perfluorooctanesulfonate and other fluorochemicals in the serum of American Red Cross adult blood donors. Environmental health perspectives 2003, 111, 1892–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Padilla-Sánchez JA; Papadopoulou E; Poothong S; Haug LS Investigation of the Best Approach for Assessing Human Exposure to Poly- and Perfluoroalkyl Substances through Indoor Air. Environ. Sci. Technol. 2017, 51, 12836–12843. [DOI] [PubMed] [Google Scholar]
- (24).Caron-Beaudoin É; Ayotte P; Laouan Sidi EA; Gros-Louis McHugh N; Lemire M; Lemire M. Exposure to perfluoroalkyl substances (PFAS) and associations with thyroid parameters in First Nation children and youth from Quebec. Environ. Int. 2019, 128, 13–23. [DOI] [PubMed] [Google Scholar]
- (25).Coperchini F; Awwad O; Rotondi M; Santini F; Imbriani M; Chiovato L. Thyroid disruption by perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA). J. Endocrinol. Invest. 2017, 40, 105–121. [DOI] [PubMed] [Google Scholar]
- (26).Grandjean P. Delayed discovery, dissemination, and decisions on intervention in environmental health: a case study on immunotoxicity of perfluorinated alkylate substances. Environ. Health 2018, 17, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Lau C; Anitole K; Hodes C; Lai D; Pfahles-Hutchens A; Seed J. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol. Sci. 2007, 99, 366–394. [DOI] [PubMed] [Google Scholar]
- (28).Vieira VM; Hoffman K; Shin H-M; Weinberg JM; Webster TF; Fletcher T. Perfluorooctanoic acid exposure and cancer outcomes in a contaminated community: a geographic analysis. Environ. Health Perspect. 2013, 121, 318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Dinglasan MJA; Ye Y; Edwards EA; Mabury SA Fluorotelomer alcohol biodegradation yields poly- and perfluorinated acids. Environ. Sci. Technol. 2004, 38, 2857–2864. [DOI] [PubMed] [Google Scholar]
- (30).Dinglasan-Panlilio MJA; Mabury SA Significant residual fluorinated alcohols present in various fluorinated materials. Environ. Sci. Technol. 2006, 40, 1447–1453. [DOI] [PubMed] [Google Scholar]
- (31).Herzke D; Olsson E; Posner S. Perfluoroalkyl and polyfluoroalkyl substances (PFASs) in consumer products in Norway–A pilot study. Chemosphere 2012, 88, 980–987. [DOI] [PubMed] [Google Scholar]
- (32).Kotthoff M; Müller J; Jürling H; Schlummer M; Fiedler D. Perfluoroalkyl and polyfluoroalkyl substances in consumer products. Environ. Sci. Pollut. Res. 2015, 22, 14546–14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Liu X; Guo Z; Folk EE; Roache NF Determination of fluorotelomer alcohols in selected consumer products and preliminary investigation of their fate in the indoor environment. Chemosphere 2015, 129, 81–86. [DOI] [PubMed] [Google Scholar]
- (34).Sinclair E; Kim SK; Akinleye HB; Kannan K. Quantitation of gas-phase perfluoroalkyl surfactants and fluorotelomer alcohols released from nonstick cookware and microwave popcorn bags. Environ. Sci. Technol. 2007, 41, 1180–1185. [DOI] [PubMed] [Google Scholar]
- (35).Trier X; Granby K; Christensen JH Polyfluorinated surfactants (PFS) in paper and board coatings for food packaging. Environ. Sci. Pollut. Res. 2011, 18, 1108–1120. [DOI] [PubMed] [Google Scholar]
- (36).Vestergren R; Herzke D; Wang T; Cousins IT Are imported consumer products an important diffuse source of PFASs to the Norwegian environment? Environ. Pollut. 2015, 198, 223–230. [DOI] [PubMed] [Google Scholar]
- (37).Begley TH; White K; Honigfort P; Twaroski ML; Neches R; Walker RA Perfluorochemicals: potential sources of and migration from food packaging. Food Addit. Contam 2005, 22, 1023–1031. [DOI] [PubMed] [Google Scholar]
- (38).Rice PA; Aungst J; Cooper J; Bandele O; Kabadi SV Comparative analysis of the toxicological databases for 6:2 fluorotelomer alcohol (6:2 FTOH) and perfluorohexanoic acid (PFHxA) (vol 138, pg 1, 2020). Food Chem. Toxicol. 2020, 139, 111249. [DOI] [PubMed] [Google Scholar]
- (39).Yuan G; Peng H; Huang C; Hu J. Ubiquitous occurrence of fluorotelomer alcohols in eco-friendly paper-made food-contact materials and their implication for human exposure. Environ. Sci. Technol. 2016, 50, 942–950. [DOI] [PubMed] [Google Scholar]
- (40).Beesoon S; Genuis SJ; Benskin JP; Martin JW Exceptionally high serum concentrations of perfluorohexanesulfonate in a Canadian family are linked to home carpet treatment applications. Environ. Sci. Technol. 2012, 46, 12960–12967. [DOI] [PubMed] [Google Scholar]
- (41).Ellis DA; Martin JW; De Silva AO; Mabury SA; Hurley MD; Sulbaek Andersen MP; Wallington TJ Degradation of fluorotelomer alcohols: a likely atmospheric source of perfluorinated carboxylic acids. Environ. Sci. Technol. 2004, 38, 3316–3321. [DOI] [PubMed] [Google Scholar]
- (42).Eriksson U; Kärrman A; Rotander A; Mikkelsen B; Dam M. Perfluoroalkyl substances (PFASs) in food and water from Faroe Islands. Environ. Sci. Pollut. Res. 2013, 20, 7940–7948. [DOI] [PubMed] [Google Scholar]
- (43).Fromme H; Tittlemier SA; Völkel W; Wilhelm M; Twardella D. Perfluorinated compounds - Exposure assessment for the general population in western countries. Int. J. Hyg Environ. Health 2009, 212, 239–270. [DOI] [PubMed] [Google Scholar]
- (44).Huber S; Haug LS; Schlabach M. Per-and polyfluorinated compounds in house dust and indoor air from northern Norway–A pilot study. Chemosphere 2011, 84, 1686–1693. [DOI] [PubMed] [Google Scholar]
- (45).Jian J-M; Guo Y; Zeng L; Liang-Ying L; Lu X; Wang F; Zeng EY Global distribution of perfluorochemicals (PFCs) in potential human exposure source–a review. Environ. Int. 2017, 108, 51–62. [DOI] [PubMed] [Google Scholar]
- (46).Shoeib M; Harner T; Webster G; Lee SC Indoor sources of poly-and perfluorinated compounds (PFCS) in Vancouver, Canada: implications for human exposure. Environ. Sci. Technol. 2011, 45, 7999–8005. [DOI] [PubMed] [Google Scholar]
- (47).Strynar MJ; Lindstrom AB Perfluorinated compounds in house dust from Ohio and North Carolina, USA. Environ. Sci. Technol. 2008, 42, 3751–3756. [DOI] [PubMed] [Google Scholar]
- (48).Tian Z; Kim S-K; Shoeib M; Oh J-E; Park J-E Human exposure to per-and polyfluoroalkyl substances (PFASs) via house dust in Korea: implication to exposure pathway. Sci. Total Environ. 2016, 553, 266–275. [DOI] [PubMed] [Google Scholar]
- (49).Winkens K; Giovanoulis G; Koponen J; Vestergren R; Berger U; Karvonen AM; Pekkanen J; Kiviranta H; Cousins IT Perfluoroalkyl acids and their precursors in floor dust of children’s bedrooms–Implications for indoor exposure. Environ. Int. 2018, 119, 493–502. [DOI] [PubMed] [Google Scholar]
- (50).Wu Y; Romanak K; Bruton T; Blum A; Venier M. Per- and polyfluoroalkyl substances in paired dust and carpets from childcare centers. Chemosphere 2020, 251, 126771. [DOI] [PubMed] [Google Scholar]
- (51).Xu Z; Fiedler S; Pfister G; Henkelmann B; Mosch C; Völkel W; Fromme H; Schramm K-W Human exposure to fluorotelomer alcohols, perfluorooctane sulfonate and perfluorooctanoate via house dust in Bavaria, Germany. Sci. Total Environ. 2013, 443, 485–490. [DOI] [PubMed] [Google Scholar]
- (52).Zheng G; Boor BE; Schreder E; Salamova A. Indoor exposure to per- and polyfluoroalkyl substances (PFAS) in the childcare environment. Environ. Pollut. 2020, 258, 113714. [DOI] [PubMed] [Google Scholar]
- (53).Butt CM; Muir DCG; Mabury SA Biotransformation pathways of fluorotelomer-based polyfluoroalkyl substances: A review. Environ. Toxicol. Chem. 2014, 33, 243–267. [DOI] [PubMed] [Google Scholar]
- (54).Frömel T; Knepper TP Mass spectrometry as an indispensable tool for studies of biodegradation of surfactants. Trac. Trends Anal. Chem. 2008, 27, 1091–1106. [Google Scholar]
- (55).Gebbink WA; Berger U; Cousins IT Estimating human exposure to PFOS isomers and PFCA homologues: the relative importance of direct and indirect (precursor) exposure. Environ. Int. 2015, 74, 160–169. [DOI] [PubMed] [Google Scholar]
- (56).Liu J; Lee LS; Nies LF; Nakatsu CH; Turco RF Biotransformation of 8: 2 fluorotelomer alcohol in soil and by soil bacteria isolates. Environ. Sci. Technol. 2007, 41, 8024–8030. [DOI] [PubMed] [Google Scholar]
- (57).Liu J; Wang N; Szostek B; Buck RC; Panciroli PK; Folsom PW; Sulecki LM; Bellin CA 6–2 Fluorotelomer alcohol aerobic biodegradation in soil and mixed bacterial culture. Chemosphere 2010, 78, 437–444. [DOI] [PubMed] [Google Scholar]
- (58).Martin JW; Mabury SA; O’Brien PJ Metabolic products and pathways of fluorotelomer alcohols in isolated rat hepatocytes. Chem.-Biol. Interact. 2005, 155, 165–180. [DOI] [PubMed] [Google Scholar]
- (59).Nilsson H; Kärrman A; Rotander A; van Bavel B; Lindström G; Westberg H. Inhalation exposure to fluorotelomer alcohols yield perfluorocarboxylates in human blood? Environ. Sci. Technol. 2010, 44, 7717–7722. [DOI] [PubMed] [Google Scholar]
- (60).Rand AA; Mabury SA Is there a human health risk associated with indirect exposure to perfluoroalkyl carboxylates (PFCAs)? Toxicology 2017, 375, 28–36. [DOI] [PubMed] [Google Scholar]
- (61).Wang N; Szostek B; Buck RC; Folsom PW; Sulecki LM; Gannon JT 8–2 Fluorotelomer alcohol aerobic soil biodegradation: Pathways, metabolites, and metabolite yields. Chemosphere 2009, 75, 1089–1096. [DOI] [PubMed] [Google Scholar]
- (62).Wang Z; Cousins IT; Scheringer M; Hungerbühler K. Fluorinated alternatives to long-chain perfluoroalkyl carboxylic acids (PFCAs), perfluoroalkane sulfonic acids (PFSAs) and their potential precursors. Environ. Int. 2013, 60, 242–248. [DOI] [PubMed] [Google Scholar]
- (63).Rand AA; Mabury SA Protein binding associated with exposure to fluorotelomer alcohols (FTOHs) and polyfluoroalkyl phosphate esters (PAPs) in rats. Environ. Sci. Technol. 2014, 48, 2421–9. [DOI] [PubMed] [Google Scholar]
- (64).Fraser AJ; Webster TF; Watkins DJ; Nelson JW; Stapleton HM; Calafat AM; Kato K; Shoeib M; Vieira VM; McClean MD Polyfluorinated Compounds in Serum Linked to Indoor Air in Office Environments. Environ. Sci. Technol. 2012, 46, 1209–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Makey CM; Webster TF; Martin JW; Shoeib M; Harner T; Dix-Cooper L; Webster GM Airborne Precursors Predict Maternal Serum Perfluoroalkyl Acid Concentrations. Environ. Sci. Technol. 2017, 51, 7667–7675. [DOI] [PubMed] [Google Scholar]
- (66).Frömel T; Knepper TP Fluorotelomer ethoxylates: Sources of highly fluorinated environmental contaminants part I: Biotransformation. Chemosphere 2010, 80, 1387–1392. [DOI] [PubMed] [Google Scholar]
- (67).Watkins AM; Wood CR; Lin MT; Abbott BD The effects of perfluorinated chemicals on adipocyte differentiation in vitro. Mol. Cell. Endocrinol. 2015, 400, 90–101. [DOI] [PubMed] [Google Scholar]
- (68).Kassotis CD; Hoffman K; Stapleton HM Characterization of Adipogenic Activity of Semi-volatile Indoor Contaminants and House Dust. Environ. Sci. Technol. 2017, 51, 8735–8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Kassotis CD; Kollitz EM; Ferguson PL; Stapleton HM Nonionic ethoxylated surfactants induce adipogenesis in 3T3-L1 cells. Toxicol. Sci. 2018, 162, 124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Bowers RR; Temkin AM; Guillette LJ; Baatz JE; Spyropoulos DD The commonly used nonionic surfactant Span 80 has RXRα transactivation activity, which likely increases the obesogenic potential of oil dispersants and food emulsifiers. Gen. Comp. Endocrinol. 2016, 238, 61–68. [DOI] [PubMed] [Google Scholar]
- (71).Temkin AM; Bowers RR; Magaletta ME; Holshouser S; Maggi A; Ciana P; Guillette LJ; Bowden JA; Kucklick JR; Baatz JE Effects of crude oil/dispersant mixture and dispersant components on PPAR γ activity in vitro and in vivo: identification of dioctyl sodium sulfosuccinate (DOSS; CAS# 577–11-7) as a probable obesogen. Environmental health perspectives 2016, 124, 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Shoeib M; Harner T; Lee SC; Lane D; Zhu J. Sorbentimpregnated polyurethane foam disk for passive air sampling of volatile fluorinated chemicals. Anal. Chem. 2008, 80, 675–682. [DOI] [PubMed] [Google Scholar]
- (73).Ferguson PL; Iden CR; Brownawel BJ Analysis of nonylphenol and nonylphenol ethoxylates in environmental samples by mixed-mode high-performance liquid chromatography-electrospray mass spectrometry. J. Chromatogr. A 2001, 938, 79–91. [DOI] [PubMed] [Google Scholar]
- (74).Han Y; Pulikkal VF; Sun M. Comprehensive Validation of the Adsorbable Organic Fluorine Analysis and Performance Comparison of Current Methods for Total Per- and Polyfluoroalkyl Substances in Water Samples. ACS ES&T Water 2021, 1, 1474–1482. [Google Scholar]
- (75).Dubocq F; Wang T; Yeung LWY; Sjöberg V; Kärrman A. Characterization of the Chemical Contents of Fluorinated and Fluorine-Free Firefighting Foams Using a Novel Workflow Combining Nontarget Screening and Total Fluorine Analysis. Environ. Sci. Technol. 2019, 54, 245–254. [DOI] [PubMed] [Google Scholar]
- (76).Kassotis CD; Masse L; Kim S; Schlezinger JJ; Webster TF; Stapleton HM Characterization of adipogenic chemicals in three different cell culture systems: implications for reproducibility based on cell source and handling. Sci. Rep. 2017, 7, 42104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Kassotis CD; Hoffman K; Phillips AL; Zhang S; Cooper EM; Webster TF; Stapleton HM Characterization of adipogenic, PPARgamma, and TRbeta activities in house dust extracts and their associations with organic contaminants. Sci. Total Environ. 2021, 758, 143707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Schultes L; Vestergren R; Volkova K; Westberg E; Jacobson T; Benskin JP Per- and polyfluoroalkyl substances and fluorine mass balance in cosmetic products from the Swedish market: implications for environmental emissions and human exposure. Environ. Sci.: Processes Impacts 2018, 20, 1680–1690. [DOI] [PubMed] [Google Scholar]
- (79).Fraser AJ; Webster TF; Watkins DJ; Strynar MJ; Kato K; Calafat AM; Vieira VM; McClean MD Polyfluorinated compounds in dust from homes, offices, and vehicles as predictors of concentrations in office workers’ serum. Environ. Int. 2013, 60, 128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Perfluoroalkyl chemicals (PFAS). https://echa.europa.eu/hot-topics/perfluoroalkyl-chemicals-pfas (July 12, 2021).
- (81).EPA’s Per- and Polyfluoroalkyl Substances (PFAS) Action Plan. Report 823-R-18–004; U.S. Environmental Protection Agency: Washington, DC, 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.