Abstract
The current study investigates the potential for topical delivery of a fluticasone propionate (FP) and levocetirizine dihydrochloride (CTZ)-loaded microemulsion (ME) for the management of atopic dermatitis. Various microemulsion components were chosen based on their solubility and emulsification capabilities, and the ternary phase diagram was constructed. A total of 12 microemulsion formulations were screened for various attributes like vesicle size, polydispersity index, ζ-potential, percent transmittance, density, and pH. The average globule size and ζ-potential of FP and levocetirizine-containing ME were 52.12 nm and −2.98 ζ-potential, respectively. Transmission electron microscopy confirmed the spherical nature of the globules. The developed system not only controlled the release of both drugs but also enhanced the efficacy of the drugs on a rodent model. Histopathological studies confirmed the safety of the developed system. The present findings provide evidence for a scalable and simpler approach for the management of atopic dermatitis.
1. Introduction
Atopic dermatitis (AD) is a general skin condition, characterized as an inflammatory, relapsing, chronic, and pruritic skin syndrome. The actual etiological reasons for AD development are still under exploration.1 Environmental factors coupled with genetic reasons are the generally recognized factors for the progression of AD. It is characterized by erythema coupled with edema, lichenification, eczematous lesions, and xerosis. The plasma levels of eosinophils, IgE, IL-13, IL-4, and mast cells are also reported to be elevated.2 Eventually, there are two categories of AD, known as intrinsic AD and extrinsic AD. Intrinsic AD constitutes clinical cases of around 20% of patients and may not elevate the allergic sensitization and serum IgE levels. On the other hand, extrinsic AD widely affects around 80% of the affected patients and is characterized by an involvement of IgE-mediated sensitization and elevation of serum (IgE) levels.3 Most of the atopic indicators are also associated like asthma, food allergic reactions, and allergic rhinitis and most prominently affect children as well as adults with an incidence of 15–30%. As genetic predisposition is there, when such people come in contact with the allergen or any allergic body, it occurs just before any hypersensitivity reaction.4 In the past few years, there have been reports on the role of oxidative stress (OS) in AD.5 Three phases of AD are encountered. (1) Acute AD for a short duration of time: Vesicular types of lesions are noticeable, which are filled with fluid and covered with crustlike flares. There is also an involvement of a majority of the Th2 cytokines.6 (2) Subacute AD: Wounds are highly swollen and covered with a dry red scaly skin. (3) Chronic AD for a longer duration of time: Due to excess scratching, the skin becomes thick and tough. AD is associated with certain types of reactions like IgE-independent Th1 (delayed), IgE-mediated mast cell type (immediate), and IgE-Th2 (late) but, eventually, theories related to these are still unclear.
One of the categories, which is known as topical corticosteroids (TCSs), is targeted therapy in AD.7 Also, the use of these categories brings about some adverse events like acne eruption, changes in skin (atrophic changes), and adrenal suppression.8 Usage of drugs like tacrolimus, sirolimus, and pimecrolimus is another option to corticosteroids, which belongs to the category of topical calcineurin inhibitors (TCIs).9 In the present era, steroids are well known for their anti-inflammatory activity. Glucocorticosteroids are most frequently used in the treatment of dermatological disorders possessing anti-inflammatory and antiproliferative effects.10 To minimize the toxicity of glucocorticosteroids, fluticasone propionate (FP) was developed without impairing their efficacy. Basically, it is the first synthetic carbothioate glucocorticoid classified as a potent anti-inflammatory drug.11 Today, FP is primarily recommended for anti-inflammatory activity, especially in AD. It is a medium-strength synthetic fluorinated corticosteroid (under class III) used for topical skin diseases. It is available in the market in various formulations, commonly as cream and ointment, in the range of 0.005–0.05%.12
Many research studies have proved that cetirizine is effective in skin treatment as an anti-inflammatory drug that minimizes histamine, bradykinin, and various allergic mediators.13 Various adverse events were noticed after oral administration of cetirizine like sedation, ocular dryness, and dry mouth.14 Hence, to minimize these side effects and to target the inflamed skin, we will deliver levocetirizine dihydrochloride through the topical route. Administering cetirizine through the oral route is one of the traditional therapies of administration. Recently, many researchers have reported that cetirizine possesses an effective topical therapeutic activity toward allergic dermatitis, along with the ability to minimize certain adverse effects caused by oral delivery.15 Levocetirizine dihydrochloride is an enantiomeric derivative of cetirizine possessing antihistaminic activity toward various allergic mediators.16 The pharmacological activity shows that levocetirizine is capable of modifying allergic mediators that are produced by eosinophils such as cytokines, antiproteinases, and growth factor.17 The study performed suggested that levocetirizine reduces the expression of IL-5 in CD4+ cells and IL-13 in CD4+ and CD8+ cells. Moreover, it enhances the expression of IL-10 in CD4+ cells. It is well known that IL-5 recruits and activates eosinophils, which results in degranulation and release of granule proteins, which ultimately get deposited in skin lesions in AD. The topical application of levocetirizine reduces systemic side effects like drowsiness, dry mouth, fever, and cough and provides targeted therapy by reducing flares.18 No such marketed formulations containing levocetirizine topical formulations are available in the market to date. However, factors such as solubility, providing superior drug retention and permeation, reducing systemic side effects by avoiding first-pass metabolism, providing rapid and controlled delivery, enhancing the therapeutic effect, providing specific targeting toward diseases, and providing a greater amount of active ingredients in a deeper layer of the skin are still required; hence, there is a need to develop a microformulation that can meet most of these requirements.
In the past few years, a dynamic approach came into existence, known as “nanosized drug carriers”, for formulations comprising topical management. Many nanoparticulate carriers were devised for topical management, like micelles, solid–lipid nanoparticles, nanospheres, liposomes, microemulsion, nanoemulsions, dendrimers, NLCs, gold nanoparticles, and inorganic particles like quantum dots.19,20 A microemulsion (ME) is a composition of oil, water, and surfactant and is a clear and transparent system.21 MEs have a nanodispersive droplet-like system, which is thermodynamically stable and optically isotropic in nature.22 Topically, ME provides biocompatibility and higher drug penetration through the stratum corneum. Among the various mechanisms followed by microemulsions is their composition, consisting of ex-surfactants and cosurfactants acting as absorption enhancers and providing a high wetting ability to the large interfacial area. The inner phase of the system acts as a reservoir for active pharmaceutical ingredients, which disperse on the application site from the outer phase of the system.23 Microemulsions are beneficial for both water-soluble and water-insoluble drug carriers, which have broad compatibility and can penetrate by diffusing the horny layer and increase the extent of the drug in systemic circulation. MEs are particularly interesting carriers for topical delivery, but they have some issues associated with topical application. The small viscosity of the microemulsion designed for topical use results in it dripping from the skin surface, making its application inconvenient.24 Therefore, with the help of a thickening agent, it is transformed into a complex semisolid system. Hence, hydrogels act as colloidal gels, which can enhance the viscosity and remain on the skin surface for a longer duration.
We prepared a microemulsion with levocetirizine dihydrochloride to improve the transdermal absorption of fluticasone propionate. A combination of these drugs may be a better option for treating chronic AD as both of these drugs have the potential to treat symptoms associated with AD. FP provides powerful anti-inflammatory activity by reducing scaling, erythema, and excoriation on the AD skin, whereas AD decreases IFN response and increases IL-4, IgE, and Th2 responses, among other things. Thus, we utilized levocetirizine dihydrochloride, which increases IFN levels and lowers IgE, eosinophils, and other mediators. As a result, the symptoms of AD are lessened.
Hence, the objective of this work is to design and evaluate novel microemulsion-based gels loaded with FP and levocetirizine dihydrochloride for safe and effective topical delivery in the management of atopic dermatitis.
2. Results
2.1. Solubility Studies
The solubility of FP and levocetirizine was tested in a number of solvents. Tween 80 was shown to have the maximum solubility of FP and levocetirizine for surfactants, IPM for oils, ethanol for cosurfactants, and phosphate buffer pH 5.5 for the aqueous phase. The solubility investigations revealed that FP had solubilities of 13 and 3.5 mg/mL, while levocetirizine had solubilities of 24 and 4 mg/mL for Tween 80 and isopropyl palmitate, respectively. Various components were chosen for the final formulation of the microemulsion based on the drug solubility.
2.2. Ternary Phase Diagram
Figure 1 depicts the ternary phase diagram for oil, water, and surfactants. The ternary phase diagram supports the selection of Tween 80 as a surfactant. The microemulsion containing Tween 80 was found to be clear and stable and had a better flowability, and the solubility of the drug was found to be much higher in Tween 80. Hence, Tween 80 was selected as the surfactant. The microemulsion region was identified as the one with a high surfactant content area and where a large amount of water solubilized without phase separation. The endpoint for the microemulsion titration was considered as the appearance of turbidity and the formation of a gel at the bottom. The ternary phase diagram was obtained using PCP-Triangular Excel software.
Figure 1.
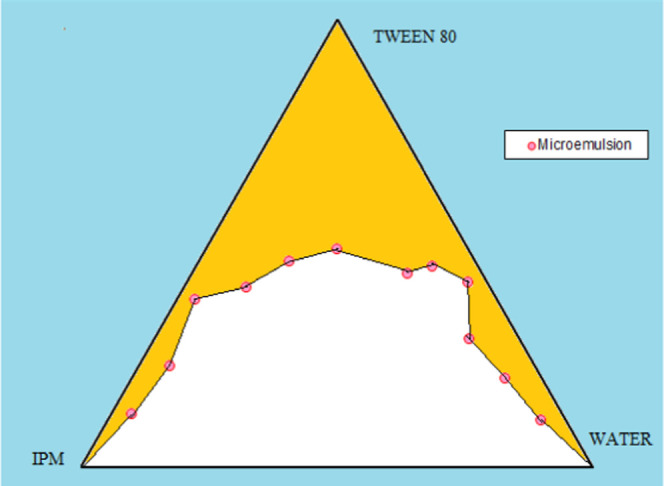
Ternary phase diagram showing the ME and the position of ME.
2.3. Microemulsion Formulation Preparation
The ME was prepared in 12 sets with different concentrations of excipients containing ethanol, phospholipid, IPM, and Tween 80, as depicted in Table 1, followed by characterization. Among them, set 10 was found to be clear and freely spreadable with a uniform particle size. The formulation is shown in Figure 2.
Table 1. Sets of Microemulsion Formulations.
| sl. no. | ethanol (g) | phospholipid (g) | Tween 80 (g) | IPM (g) | water (g) | total (g) | PDI | ζ-potential (mV) | particle size (nM) | percent transmittance | density (g/L) | pH |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 0.2 | 2.5 | 1 | 5.3 | 10 | 0.198 | –2.41 | 119.1 | 99.4 | 0.912 | 7.45 |
| 2 | 0.6 | 0.35 | 2.5 | 1 | 5.55 | 10 | 0.215 | –3.71 | 131.4 | 94.6 | 1.001 | 7.16 |
| 3 | 0.2 | 0.2 | 5 | 1 | 3.6 | 10 | 0.244 | –2.58 | 149.5 | 82.4 | 0.667 | 7.35 |
| 4 | 0.3 | 0.387 | 2.812 | 1.125 | 5.376 | 10 | 0.351 | –2.8 | 47.52 | 88.5 | 0.773 | 6.91 |
| 5 | 0.4 | 0.275 | 3.125 | 1.25 | 4.95 | 10 | 0.469 | –2.27 | 30.88 | 79.8 | 0.691 | 6.64 |
| 6 | 0.6 | 0.2 | 3.75 | 1 | 4.45 | 10 | 0.395 | –2.25 | 55.75 | 84.4 | 0.88 | 6.4 |
| 7 | 0.3 | 0.237 | 4.062 | 1.125 | 4.275 | 10 | 0.355 | –1.8 | 319.1 | 81.2 | 0.792 | 7.11 |
| 8 | 0.2 | 0.35 | 3.75 | 1 | 4.7 | 10 | 0.445 | –1.8 | 396.5 | 78.2 | 0.776 | 6.87 |
| 9 | 1 | 0.2 | 2.5 | 1 | 5.3 | 10 | 0.45 | –2.25 | 40.63 | 86.4 | 0.887 | 6.74 |
| 10 | 0.7 | 0.237 | 2.8125 | 1.125 | 5.126 | 10 | 0.514 | –1.22 | 18.79 | 82.93 | 0.72 | 6.37 |
| 11 | 0.2 | 0.2 | 2.5 | 2 | 5.1 | 10 | 0.377 | –1.4 | 47.14 | 68.4 | 0.931 | 6.85 |
| 12 | 0.3 | 0.237 | 2.812 | 1.625 | 5.023 | 10 | 0.417 | –3.42 | 21.91 | 91.4 | 0.755 | 6.9 |
Figure 2.

Pictorial presentation of the drug-loaded microemulsion.
2.4. Microemulsion Characterization
2.4.1. Micromeritics and Particle Size
The ζ-potential and PDI of the formulated microemulsion were found to be closer to zero. This is possible due to the nonionic property of surfactants.21 The low PDI indicates homogeneity, while a ζ-potential greater than −15 mV indicates stability.35 The formulated microemulsion set 10 was found to have the smallest particle size, followed by the formulation containing set 12, and the largest particle size was for set 8. The difference in particle size was due to the different concentrations of the components used in the formulation. Hence, set 10 was found to have the smallest particle size of 18.79 nm with a ζ-potential of −1.22 for the oil droplet, which can provide better emulsifying proficiency and be utilized for the final drug-loaded microemulsion containing FP and levocetirizine. The mean particle size was found to be 52.12 nm, and the ζ-potential was −2.98.
2.4.2. Drug Loading and Drug Entrapment
Drug loading and entrapment studies were performed and are depicted in Table 2. The % entrapment efficiency of the drug-loaded microemulsion was compared with that of the dual drug-loaded microemulsion. The drug loading and percent entrapment were found to be higher in the drug-loaded microemulsion. The entrapment of the drug in the carrier was due to entrapment of the drug in the lipid core. From the reported values, the percentage loading of FP was found to be 0.1%,36 the percentage entrapment was found to be 49%, and the reported percentage entrapment for levocetirizine was found to be 76.88%.14
Table 2. % Entrapment and % Drug Loading of FP and Levocetirizine Microemulsions.
| sl. no. | formulation | % entrapment | % drug Loading |
|---|---|---|---|
| 1 | FP-loaded microemulsion | 40 ± 11.4 | 0.097 |
| 3 | Levocetirizine-loaded microemulsion | 66.8 ± 14.1 | 2.226 |
| 5 | Fluticasone with the levocetirizine-loaded microemulsion | 20 ± 1.17 | 0.056 |
| 68 ± 15.43 | 2.248 |
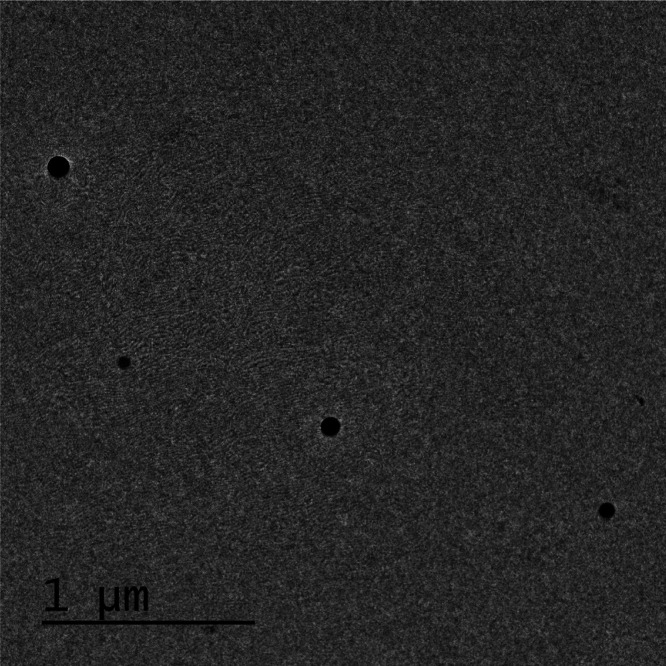
2.5. Transmission Electron Microscopy (TEM)
The TEM microphotograph is shown in Figure 3. The picture clearly depicts the presence of segregated globules with a spherical shape. The figures confirm the absence of aggregation and show the formation of a microemulsion of the desired size and morphology.
Figure 3.
TEM microphotograph of the developed ME.
2.6. Evaluation Studies
2.6.1. Drug Release and Release Kinetics
Figure 4 shows the formulation containing the drug-loaded microemulsion of FP, levocetirizine, and the dual drug-loaded microemulsion of FP with levoceterizine compared with plain FP and levocetirizine as a cumulative drug release graph versus time in a release media of PBS 5.5 pH, which resembles the normal pH of the skin. The drug release was found to be higher in the drug-loaded microemulsion compared to the plain drug due to increased solubility of the drug in the lipid matrix. On fitting of various release models, we found that the release pattern did not follow a particular model. The r2 values of various models are listed in Table 3.
Figure 4.
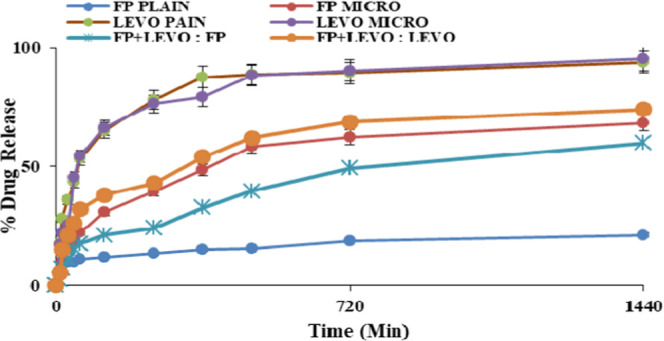
Cumulative drug release of FP and levocetirizine alone and that of the drug-loaded microemulsion at pH 5.5.
Table 3. r2 Values of Different Kinetic Models for Various Formulations at pH 5.5.
| |
r2 |
|||||
|---|---|---|---|---|---|---|
| formulations | pH | zero order | first order | Higuchi | Korsmeyer–Peppas | |
| FP with the levocetirizine-loaded microemulsion | FP | 5.5 | 0.843 | 0.933 | 0.979 | 0.860 |
| levocetirizine | 0.906 | 0.959 | 0.937 | 0.778 | ||
The best kinetic model that accurately describes drug release was found using linear regression analysis. The zero-order (mg/cm2 versus time), Higuchi (mg/cm2 versus square root of time), first-order (log of mg/cm2 versus time), and Korsmeyer–Peppas models were all tested. For the zero-order, first-order, Higuchi, and Korsmeyer–Peppas models, the correlation coefficients for FP were 0.843, 0.933, 0.979, and 0.860, respectively. For levocetirizine, the r2 values for the zero-order, first-order, Higuchi, and Korsmeyer–Peppas models were 0.906, 0.959, 0.937, and 0.778, respectively.
The FP and levocetirizine release profiles from ME followed Higuchi’s model, according to comparisons between these different correlation coefficients. This kinetic model, based on Fick’s law, depicts a linear relationship between the square root of time and the total amount of drug released, implying that drug release was regulated by a diffusion mechanism in which the drug transfer rate per unit area is proportional to the concentration gradient between the two sides of the diffusion layer.38 The drug concentration gradient that occurred between the donor and receptor compartments, as well as the drug’s water solubility and partition coefficient, influenced the diffusion of FP and levocetirizine into the receiver medium. The phenomenon of diffusion by gradient across the hydrophilic synthetic membranes reduced the drug concentration in the internal phase of the microemulsion as a result of the higher drug concentration in the vehicle, causing misalignment of the surfactant film and subsequent drug transfer to the external phase of the formulation. Thus, Higuchi’s model suggests that the microemulsion system is critical in the drug release process since diffusion through this vehicle constitutes the release’s limiting phase.
Cavalcanti and colleagues studied topical microemulsions containing pentoxyfilline (PTX). Three distinct linear regression models were tested: zero order (g/cm2 versus time), Higuchi (g/cm2 versus square root of time), and first order (log of g/cm2 versus time). For the zero-order, Higuchi, and first-order models, the correlation coefficients were 0.9567, 0.9989, and 0.8023, respectively. The PTX release profile from ME followed Higuchi’s model, according to comparisons between these different correlation coefficients.39
Victor and co-workers performed a study on Na3CaDTPA containing a microemulsion-based hydrogel for topical delivery and compared the release profiles of the active substance from conventional gel and microemulsion-gel formulations through hydrophilic and hydrophobic membranes. No statistically significant differences were found between the amounts of drug released after 6 h from the two formulations, regardless of the membrane used. Mathematical modeling of the drug release data suggests a quasi-Fickian diffusion mechanism for both conventional and microemulsion-based hydrogels.40
2.7. Ex Vivo Permeation Study
Drug permeation across the rodent skin was found to be better for the microemulsion-based gel vis-à-vis the conventional product for both drugs. Despite the maintenance of proper sink conditions, the permeation of both drugs from the conventional product was quite less in comparison to that from the microemulsion-based gel, as shown in Figure 5.
Figure 5.
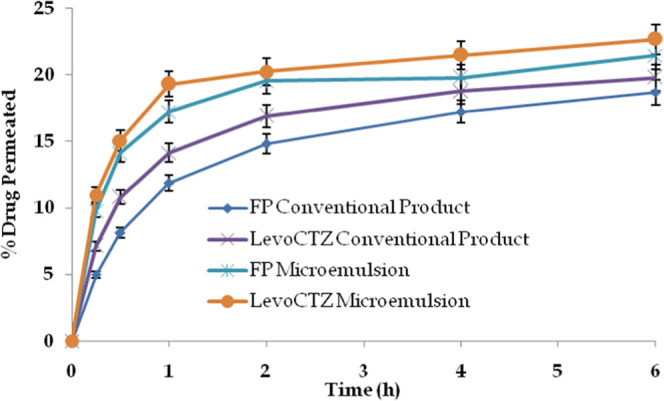
Drug permeation profile of both drugs from the studied formulations across rodent skin (n = 3).
Levoceterizine, being more water-soluble, exhibited a relatively better drug permeation profile in comparison to FP. Conventional formulations are devoid of biocompatible materials like phospholipids; hence, the permeation of both drugs was quite less compared to that of the microemulsion-based gel.41,42 On the other hand, the microemulsion contained the oil component, such as isopropyl myristate, which is known for its penetration enhancement potential, and phospholipids, one of the structural units of skin having a nanometric size, which enhanced the permeation across the skin layers.43
The percent drug retention of FP and levoceterizine in the experimental skin from the conventional product was found to be 2.31 and 1.72%, respectively. Notably, the values of percent drug retention from the microemulsion-based gel were observed to be 4.97% for FP and 3.17% for levocetirizine from the developed microemulsion-based system. The findings are promising as the drug deposited in the skin layers is the drug available for pharmacological action that is desired locally, especially in conditions like atopic dermatitis. The results provide an inference for the suitability of the developed system in conditions like atopic dermatitis.
2.8. In Vivo Study
2.8.1. Hydrogen Peroxide-Induced Atopic Dermatitis in Rats
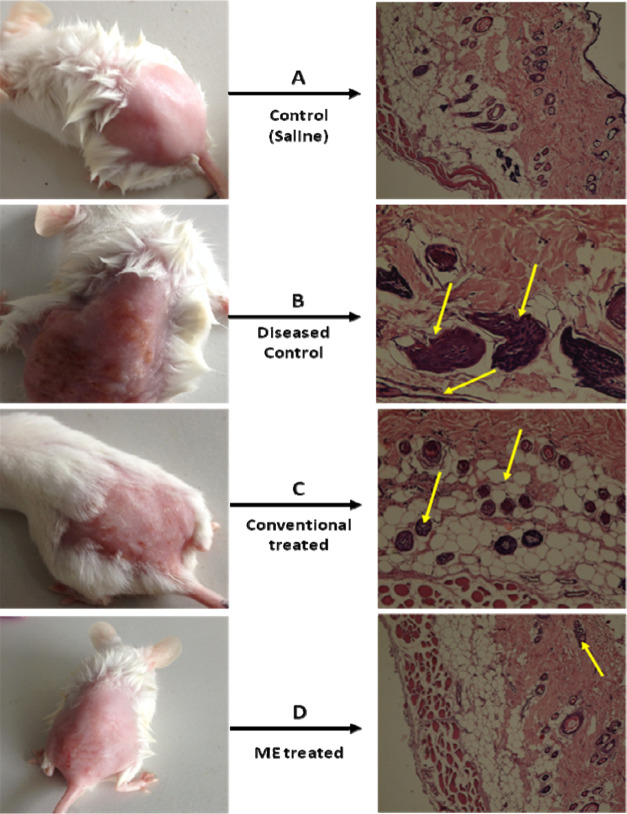
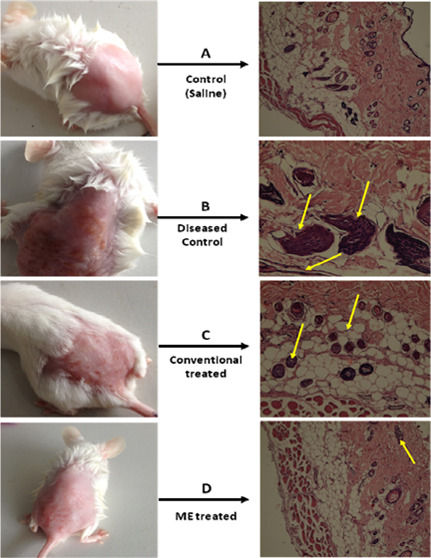
In this model, a 10% HPO solution was applied topically to the dorsal skin of Wistar rats twice daily for 7 consecutive days. To confirm the elicitation of atopic dermatitis, animals from group A (diseased control) were sacrificed on the 3rd, 5th, and 7th days.44 The degree of atopic dermatitis generated was evaluated on the basis of the histopathology of the HPO-treated skin, as shown in Figure 6.
Figure 6.
Images of the representative rodent groups receiving various treatments on the last day and the respective histopathological slides.
Histopathological examination revealed epidermal thickening with mild inflammation on the 3rd day. On the 5th day, the above-mentioned changes progressed and the border between the epidermis and dermis became irregular, with keratinocytes showing signs of pyknosis. On the 7th day, the epidermis was partially detached from the dermis, leaving a space filled with fluid between them; necrotic keratinocytes were seen scattered in the basal layer, and inflammation was extended to the deeper layers.
From a histopathological examination, the severity of dermatitis was ascertained, and treatment was started in group D animals with a microemulsion gel and in group C animals with a conventional cream. The formulations were applied twice daily for 7 days. However, treatment with formulations other than the conventional cream inhibited these atopic skin symptoms from day 5, and the microemulsion-treated gel had a better effect as compared to the conventional cream. The conventional cream did not have any effect against AD in short-term administration. Interestingly, on day 7, the microemulsion gel ameliorated the hydrogen peroxide-induced skin to a greater extent than the conventional cream, which indicates that the prepared drug-loaded microemulsion gel could decrease AD symptoms and that the concentration of the surfactants and cosurfactants did not irritate the AD-like skin. At some level, conventional creams irritate the skin, resulting in erythema, dryness, and itching in AD patients. Moreover, we observed that the ME gel completely repaired the scaling and erythema from the AD skin on day 7, while with conventional therapy, erythema, scaling, and excoriation on day 7 were still not completely gone, as shown in Table 4. The efficacy of the formulations was assessed on the 3rd, 5th, and 7th days on the basis of the parameters given below.
Table 4. Mean Inflammatory Scores Observed for the Groups Treated with (A) Control, (B) Microemulsion Gel Formulation, and (C) MKT Cream Formulation for 7 Days.
| days |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| 3rd | 5th | 7th | |||||||
| group | erythema | excoriation | scaling | erythema | excoriation | scaling | erythema | excoriation | scaling |
| control | 3 | 3 | 3 | 3 | 3 | 2 | 3 | 2 | 2 |
| microemulsion gel | 2 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 |
| conventional cream | 3 | 3 | 2 | 2 | 2 | 1 | 2 | 1 | 1 |
2.8.2. Physical Evaluation: Inflammation Scores
The severity of dermatitis on the dorsal skin was assessed by evaluating the dryness, crust, and keratinization. The total scores of the skin severity were defined as the sum of the individual scores for each of the following three signs and symptoms: erythema (hemorrhage), excoriation (erosion), and scaling (dryness), on the dorsal region.45 Inflammation scores were recorded on the 3rd, 5th, and 7th days, as shown in Table 4.
From a physical evaluation, it was concluded that the microemulsion-gel-treated skin showed signs of healing as the levels of erythema, excoriation, and scaling were reduced significantly. However, on the 7th day, the conventional-cream-treated skin was still not completely healed. % Inhibition of inflammation in the case of the microemulsion gel was found to be significantly higher than that of conventional cream formulations (Figure 7).
Figure 7.
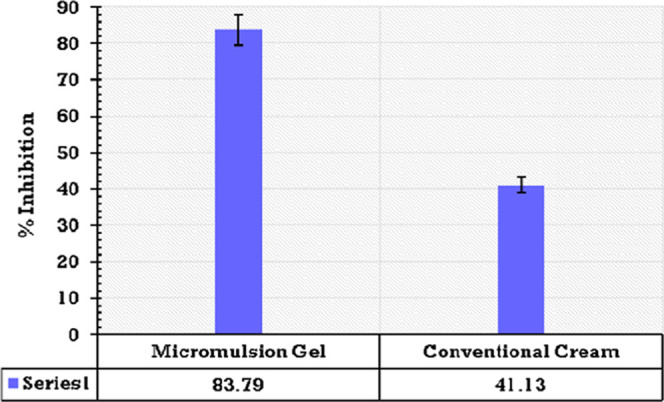
Bar graph showing % inhibition of the microemulsion gel and a conventional cream formulation.
2.8.3. Histopathological Examination
The extent of healing of the skin was confirmed by histopathological examination. On the 3rd day, the microemulsion-gel-treated skin showed signs of epidermal healing, and no fluid was observed between the epidermis and dermis although inflammation was still present. However, in the conventional cream formulation, epidermis thickening as well as fluid between the epidermis and dermis was still present. On the 5th day, healing further progressed with the microemulsion gel as the inflammation was significantly reduced and only a few necrotic keratinocytes were observed. However, in the case of the conventional cream formulation, epidermal healing was observed, but inflammation was still not mitigated. On the 7th day, the epidermis and dermis were fully recovered with no signs of inflammation and necrotic keratinocytes in the case of the microemulsion gel. However, in the case of the conventional cream formulation, the epidermis and dermis were not yet fully recovered as mild inflammation was still present along with a few necrotic keratinocytes. These results show the superiority of the microemulsion gel over the conventional cream formulation, as depicted in Figure 6. The better drug transport characteristics of the vesicular carrier can be attributed to the interaction of the biocompatible components with the skin resulting in better penetration, which is expected to deliver the molecules to the desired target site without disturbing the normal surrounding tissues.46
3. Conclusions
The goal of our study was to create a dermal delivery system for fluticasone propionate and levocetirizine dihydrochloride that could successfully deliver in the deeper layers of the skin and reduce the onset of action time for the treatment of chronic AD. The formulated topical system with optimum topical characteristics and histopathological safety was capable of permeating the skin barrier effectively. The therapeutic efficacy of FP and levocetirizine-loaded ME on hydrogen peroxide-induced atopic dermatitis was improved during in vivo pharmacodynamic activity. Between the two drugs, the microemulsion-based gel was found to have superior drug penetration across the rodent skin than the conventional product. This topical system has the potential to lower the high plasma levels of eosinophils, IgE, IL-13, IL-4, and mast cells by counteracting locally produced histamine and other allergic and inflammatory mediators by binding their particular receptor. Furthermore, the topical preparation may hydrate the skin and minimize the irritation caused by an allergic reaction. There is currently no such formulation on the market; clinical trials are needed to determine the efficacy and safety of topical formulations on human skin.
4. Methods
4.1. Materials
Fluticasone propionate was kindly provided by M/s Century Pharmaceuticals Ltd., Gujarat, India. Levocetirizine dihydrochloride was kindly provided by M/s Biogenetic Ltd., Himachal Pradesh, India. Isopropyl myristate and isopropyl palmitate were kindly provided by M/s CDH [Pvt] Ltd., New Delhi, India. Phospholipid was a gift sample from Punjab University, India. Tween 80 was purchased from Sisco Research Laboratories Pvt. Ltd., Maharashtra, India. Ethanol and methanol were purchased from Sigma-Aldrich, and double-distilled water was utilized in the entire study.
All other chemicals and reagents were of analytical or chromatographic grade.
4.2. Solubility Studies of FP and Levocetirizine Dihydrochloride in Oils, Surfactants, and Cosurfactants
Solubility studies were performed to determine the best suitable candidate of ME formulation with a high drug-loading capacity. Solubility studies were performed in various solvents, viz. aqueous solvents (distilled water, phosphate-buffered saline (PBS) of pH (PBS 6.8), (PBS 7.8) and (PBS 5.5)), surfactants (Labrafil, Labrasol, Tween 20, Tween 40, Tween 60, Tween 80, Span 20, and Span 80), cosurfactants (methanol and ethanol), and oils (castor oil, isopropyl myristate, tocopherol, oleic acid, lecithin, and isopropyl palmitate). An excess of FP and levocetirizine was allowed to dissolve in 2 mL of each solvent on a shaker bath for 24 h at 37 °C to achieve dissolution equilibrium. Lastly, the sample was allowed to centrifuge at 10 000 rpm for 10 min, and the supernatants were assessed for the amount of drug under a UV–visible spectrophotometer. All of the studies were performed in triplicate.21
4.3. Ternary Phase Diagram
The ternary phase diagram was constructed to identify the ME region by finding different concentration ranges of constituents, i.e., surfactants, oils, water, and cosurfactants. The study was carried out by the water titration method. TO study the solubility behavior of fluticasone propionate and levocetirizine dihydrochloride, isopropyl myristate (IPM) was selected as the oil phase. The ternary phase diagram was created using various Tweens (Tween 20, Tween 40, Tween 60, Tween 80). Various surfactant-to-water ratios ranging from 1:9 to 9:1 were obtained and titrated with IPM until turbidity/droplet settling/gel formation occurred. The combination was completely mixed using a vortex mixer, and the prepared sample was evaluated against a light versus dark backdrop. If the sample has an isotropic nature/clear solution (microemulsion), or if the sample appears milky or has phase separation, then it is not a microemulsion. The endpoint between a microemulsion and nonmicroemulsion was considered by measuring the midpoint between a cloudy and a clear solution. Similarly, ratios ranging from 1:9 to 9:1 between IPM and surfactants were prepared and titrated against water until the endpoints were noticed.21,25
4.4. Microemulsion Formulation Preparation
On the basis of the desired characteristic, the composition of the microemulsion was selected for the final formulation. The microemulsion was prepared by mixing an aqueous phase and an organic phase. The organic phase consists of IPM, phospholipid, and ethanol, and the aqueous phase consists of Tween 80, water, FP, and levocetirizine. Phospholipid was allowed to dissolve in ethanol, and then, IPM was added. Then, a certain amount of FP and levocetirizine was added in the system and allowed to dissolve in Tween 80 containing water as the aqueous phase. Finally, the aqueous phase was slowly poured drop by drop into the organic phase under a mild magnetic stirring condition for 5 min. The whole study was performed at ambient temperature.
4.5. Preparation of Microemulsion Gel Formulation
To make the microemulsion rheologically favorable for topical application, it was incorporated in a secondary vehicle.
4.5.1. Preparation of 10% Stock Gel
Carbopol, 1 g, was dispersed in 6.5 mL of water in a 10 mL beaker and kept overnight in a refrigerator. Next day, it was removed from the refrigerator and 1.5 g of ethanol was added, followed by the subsequent addition of 1 g of triethanolamine. The mixture was mixed properly using a glass rod.
4.5.2. Incorporation of Drug-Loaded Microemulsion into Carbopol Gel
Carbopol equivalent to 2% of the total carbopol content from a 10% stock gel was weighed and mixed properly with the remaining amount of the microemulsion, simply by levigation, which led to gelatination.26
4.6. Microemulsion Characterization
4.6.1. Micromeretics and Surface Charge
Particle size and ζ-potential are crucial factors for the physical stability of the formulations. The nonflocculation of the system was due to the smaller particle size, and the decrease in flocculation occurred due to the accurate ζ-potential.
Particle size and ζ-potential studies of drug-loaded ME were measured at 25C via dynamic light scattering at the Birla Institute of Technology and Science (BITS), Pilani, Rajasthan, India, using a Malvern Zetasizer (M/s Malvern, Worcestershire, U.K.). Determination of the polydispersity (PI) index was also done with the same equipment, which provided the width of the size distribution. The study was performed in triplicate.
4.6.2. Drug Entrapment and Drug-Loading Studies
The dialysis method was used for the identification of loading as well as entrapment efficiency. The formulation containing the drug-loaded microemulsion (FP, levocetirizine, and FP with levocetirizine) containing a drug equivalent to 1 mg was weighed and packed individually in a dialysis bag and stirred for 2 h containing 50 mL of methanol on magnetic stirrers. An aliquot of 2 mL was taken after 2 h and analyzed under a UV spectrophotometer at the respective wavelength. The amount of unentrapped drug was subtracted from the total amount of drug, and percent entrapment was calculated, whereas for drug loading, the part of the drug entrapped per 100 parts of the carrier was reported.27
4.7. Transmission Electron Microscopy
The drug-loaded microemulsion was assessed under a transmission electron microscope (JEM 100CX11, Japan) for the determination of surface morphology and shape. The microemulsion sample was allowed to adsorb on a copper grid and further stained with 2% phosphotungstic acid at ambient temperature, and the study was performed for 30 s.
4.8. Evaluation Studies
4.8.1. In Vitro Drug Release and Release Kinetics
The in vitro release study was performed using the dialysis bag method. Microemulsions loaded with FP, levocetirizine, and FP with levocetirizine were compared to plain FP and levocetirizine (equivalent to 1 mg of drug); they were placed in a dialysis bag containing 1 mL of 0.5% Tween 80 and suspended separately into 50 mL of phosphate buffer pH 5.5 containing 1% Tween 80 solution with continuous stirring. Aliquots of 2 mL each were taken at regular time intervals with proper replacement of the media to maintain the sink condition. Cumulative drug release was determined by scanning under a UV spectrophotometer.28,29 To discover the best-fitting release model, the data was fitted to multiple models, including zero-order (mg/cm2 versus time), Higuchi (mg/cm2 versus square root of time), first-order (log of mg/cm2 versus time), and Korsmeyer–Peppas models. To better explain the release profile, a linear regression analysis was performed, and the mathematical model with the highest coefficient of linear correlation (r2) was chosen.30
4.9. Ex Vivo Permeation Study
4.9.1. Preparation of Skin
The skin permeation study was carried out following approval from the Panjab University Institutional Animal Ethics Committee for the purpose of controlling and supervising animal experiments by the Indian Government (Ref letter no. PU/IAEC/S/14/69). Male mice weighing 234 g were used for the study. During the length of the experiment, the mice were appropriately confined and fed a balanced diet of food and water. The mice were fasted for one night before the experiment. The mice were sacrificed using the cervical dislocation method, and the abdomen skin of the mice was excised for the experiment after the hair on the skin was removed. Before application, the skin was cleansed twice with regular saline water and thoroughly examined.31
4.9.2. Skin Permeation Study
To identify the potential of the prepared formulation in terms of permeability, an ex vivo permeation study was performed using the Franz diffusion cell assembly, where mice skin acts as a permeable bridge.25,32 Before the permeation study, 13 × 13 mm2 skin pieces were cut and soaked in PBS buffer for 15 min for complete hydration. The skin was placed on the donor compartment facing the stratum corneum layer toward the donor. The formulation containing 1 g of drug-loaded microemulsion or 1 g of drug-loaded microemulsion gel was placed on the stratum corneum layer as the donor compartment and occluded with a parafilm to prevent evaporation of water from the system. The receptor compartment was filled with the medium under constant stirring at 600 rpm and maintaining the temperature up to 37 ± 0.2° by a circulating water bath jacket throughout the experiment. The receptor compartment contained 15 mL of saline along with PEG 400, which provided a sink condition for drug-loaded formulations. The experiment was performed for 24 h for all of the formulations, and 0.5 mL of sample was withdrawn from the receptor compartment after a specific time interval and replaced with an equal amount of fresh medium. Finally, the withdrawn sample was vortexed for 2 min using 1 mL of methanol and filtered over using 0.22 mm membrane filters. The cumulative amount of drug permeated from the skin was accessed using HPLC and quantified using a reported method.33,34
4.10. In Vivo Study
The in vivo pharmacodynamic study was performed using 16 Laca mice, weighing 15–25 g, and aged 2–3 months of either sex. This dermatitis study obtained approval from the Animal Ethics Committee of Punjab University for the purpose of control and supervision of experiments on animals by the government of India (Ref letter no. PU/IAEC/S/14/69). All of the rats were caged in a standard condition with a proper meal and diet at specific air conditioning in an animal house at 25 ± 1 °C, with a 12:12 h dark/light condition.
4.10.1. Hydrogen Peroxide-Induced Atopic Dermatitis in Rats
The mice were divided into four different groups before the experiment—1st group: control (normal saline); 2nd group: diseased control; 3rd group: conventional treated; and 4th group: ME treated. Each group consisted of four mice weighing 15–25 g. The mice were shifted to the laboratory 1 week before the experiment. The dorsal portion of the mouse skin was shaved using a depilatory cream (Veet) applied on the dorsal portion of rats for 10 min and swabbed with cotton to remove the hair in a direction from the tail to head without damaging the skin. Then, 1 mL of solution containing hydrogen peroxide (HPO) was placed on the dorsal skin of rats 24 h after the shaving for all three groups except for the control (saline) group for the development of AD. The process was continued for 7 consecutive days. During the days of treatment with HPO, the diseased control group was sacrificed on the 3rd, 5th, and 7th days for the histopathological assessment of HPO-treated skin. The treatment was started on the 3rd group with conventional therapy and on the 4th group with the drug-loaded ME gel, and dosing was done as per another study.16 The prepared formulation was applied twice daily for 7 days, and the efficacy was evaluated on the 3rd, 5th, and 7th days. The degree of inflammation and erythematic scores were evaluated and calculated.
4.10.2. Physical Evaluation: Inflammation Scores
The physical manifestation of AD appearing on the dorsal skin was identified by addressing dryness, keratinization, edema, lichenification, eczematous lesions, and xerosis on a scoring basis, i.e., score 0 indicates no inflammation, score 1 indicates mild inflammation, score 3 indicates moderate inflammation, and score 4 indicates severe inflammation. The scoring system was majorly based on three symptoms—erythema, scaling, and excoriation, which defined the cruelty of dorsal skin.
% Inhibition of inflammation was calculated using the following equation
where Sc is the total scoring of the control and St is the total scoring of the test.
4.11. Histopathological Study
The effects of the prepared formulations, i.e., ME and ME gel, on the dorsal skin were assessed for safety of the formulation. Histological assessments were conducted on an excised sample of the skin. On the 3rd, 5th, and 7th days of the week, each animal from each group was sacrificed and the dorsal skin was excised carefully. The skin specimen was fixed using the Franz diffusion cell assembly, similar to that in the drug permeation study. The dorsal skin was treated with normal saline NS (control group), ME, and ME gel for 12 h. After 12 h, skin were fixed in 10% formalin, washed with NS, and dehydrated with ethanol. Finally, the skin sample was embedded in paraffin, cut into fine slices, and treated with hematoxylin and eosin for staining before observing under a light microscope at 40X. The results were compared with those of the untreated rat skin.16,17
4.12. Stability Study
The optimized ME was kept in sealed transparent containers for identifying the stability as per ICH regulations and guidelines and stored at temperatures of 5 ± 2, 25 ± 2, 40 ± 2 °C, and 75 ± 5 RH for a duration of 3 months. The formulation was assayed at 0, 15, 30, 60, and 90 days’ time intervals in terms of pH, color change, drug content, clarity, and homogeneity.
4.13. Statistical Analysis
One-way ANOVA was accomplished to identify the differences in different groups. GraphPad Prism v6.0 software was used for all of the statistical analyses. All of the values were denoted in terms of mean ± SD, and the results were considered significant when *p < 0.05, **p < 0.01, and ***p < 0.001.
Acknowledgments
The authors acknowledge the DST-FIST facilities of the Department of Pharmacy, Central University of Rajasthan (SR/FST/LS2-654/2016). The support of Prof. O. P. Katare, University Institute of Pharmaceutical Sciences, Panjab University, Chandigarh, India, during the animal studies is highly acknowledged.
The authors declare no competing financial interest.
References
- Ghosh N.; Mitra S.; Banerjee E. R. Therapeutic Effects of Topically-Administered Guar Gum Nanoparticles in Oxazolone-Induced Atopic Dermatitis in Mice. Biomed. Res. Ther. 2018, 5, 2305–2325. 10.15419/bmrat.v5i5.444. [DOI] [Google Scholar]
- Patel N.; Feldman S. R. Management of Atopic Dermatitis. Adherence in Atopic Dermatitis. Introduction. Adv. Exp. Med. Biol. 2017, 1027, 139–159. 10.1007/978-3-319-64804-0. [DOI] [PubMed] [Google Scholar]
- Renert-Yuval Y.; Guttman-Yassky E. What’s New in Atopic Dermatitis. Dermatol. Clin. 2019, 37, 205–213. 10.1016/j.det.2018.12.007. [DOI] [PubMed] [Google Scholar]
- Dinulos J. G.; Trickett A.; Crudele C. New Science and Treatment Paradigms for Atopic Dermatitis. Curr. Opin. Pediatr. 2018, 30, 161–168. 10.1097/MOP.0000000000000560. [DOI] [PubMed] [Google Scholar]
- Cho B. S.; Kim J. O.; Ha D. H.; Yi Y. W. Exosomes Derived from Human Adipose Tissue-Derived Mesenchymal Stem Cells Alleviate Atopic Dermatitis. Stem Cell Res. Ther. 2018, 9, 187 10.1186/s13287-018-0939-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicki R.; Trzeciak M.; Wilkowska A.; Sokołowska-Wojdyło M.; Ługowska-Umer H.; Barańska-Rybak W.; Kaczmarski M.; Kowalewski C.; Kruszewski J.; Maj J.; Silny W.; Śpiewak R.; Petranyuk A. Special Paper Atopic Dermatitis: Current Treatment Guidelines. Statement of the Experts of the Dermatological Section, Polish Society of Allergology, and the Allergology Section, Polish Society of Dermatology. Postepy Dermatol. Alergol. 2015, 4, 239–249. 10.5114/pdia.2015.53319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegfried E. C.; Jaworski J. C.; Kaiser J. D.; Hebert A. A. Systematic Review of Published Trials: Long-Term Safety of Topical Corticosteroids and Topical Calcineurin Inhibitors in Pediatric Patients with Atopic Dermatitis. BMC Pediatr. 2016, 16, 75 10.1186/s12887-016-0607-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson E. L.; Lacour J. P.; Spelman L.; Galimberti R.; Eichenfield L. F.; Bissonnette R.; King B. A.; Thyssen J. P.; Silverberg J. I.; Bieber T.; Kabashima K.; Tsunemi Y.; Costanzo A.; Guttman-Yassky E.; Beck L. A.; Janes J. M.; DeLozier A. M.; Gamalo M.; Brinker D. R.; Cardillo T.; Nunes F. P.; Paller A. S.; Wollenberg A.; Reich K. Baricitinib in Patients with Moderate-to-Severe Atopic Dermatitis and Inadequate Response to Topical Corticosteroids: Results from Two Randomized Monotherapy Phase III Trials. Br. J. Dermatol. 2020, 183, 242–255. 10.1111/bjd.18898. [DOI] [PubMed] [Google Scholar]
- Silverberg J. I.; Toth D.; Bieber T.; Alexis A. F.; Elewski B. E.; Pink A. E.; Hijnen D.; Jensen T. N.; Bang B.; Olsen C. K.; Kurbasic A.; Weidinger S. Tralokinumab plus Topical Corticosteroids for the Treatment of Moderate-to-Severe Atopic Dermatitis: Results from the Double-Blind, Randomized, Multicentre, Placebo-Controlled Phase III ECZTRA 3 Trial. Br. J. Dermatol. 2021, 184, 450–463. 10.1111/bjd.19573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano I.; Safroneeva E.; Roumet M. C.; Comer G. M.; Eagle G.; Schoepfer A.; Falk G. W. Randomised Clinical Trial: The Safety and Tolerability of Fluticasone Propionate Orally Disintegrating Tablets versus Placebo for Eosinophilic Oesophagitis. Aliment. Pharmacol. Ther. 2020, 51, 750–759. 10.1111/apt.15670. [DOI] [PubMed] [Google Scholar]
- Liu L.; Ong G. A Randomized, Open-Label Study to Evaluate an Intermittent Dosing Regimen of Fluticasone Propionate 0.05% Cream in Combination with Regular Emollient Skin Care in Reducing the Risk of Relapse in Pediatric Patients with Stabilized Atopic Dermatitis. J. Dermatol. Treat. 2018, 29, 501–509. 10.1080/09546634.2017.1401211. [DOI] [PubMed] [Google Scholar]
- Rubio-Gomis E.; Martinez-Mir I.; Morales-Olivas F. J.; Martorell-Aragones A.; Palop-Larrea V.; Bernalte-Sesé A.; Cerda-Mir J. C.; Polo-Martín P.; Febrer I.; Aranda-Grau L.; Llosa-Cortes I.; Tejedor-Sanz M. J.; Julia-Benito J. C.; Alvarez-de-Laviada-Mulero T.; Planelles-Cantarino M. V.; Apolinar-Valiente E.; Loriente-Tur M.; Abella-Bazataqui A. M.; Alvarez-Gonzalez I.; Morales-Carpi C.; Burches-Greus M. E.; Ferrer-Bautista A. B.; Felix-Toledo R.; Marmaneu-Laguia D.; Garcia-Martinez V. E.; Beltran-Marques M. A.; Rodriguez-Gracia B. Fluticasone in Mild to Moderate Atopic Dermatitis Relapse: A Randomized Controlled Trial. Allergol. Immunopathol. 2018, 46, 378–384. 10.1016/j.aller.2017.12.001. [DOI] [PubMed] [Google Scholar]
- Goindi S.; Kumar G.; Kaur A. Novel Flexible Vesicles Based Topical Formulation of Levocetirizine: In Vivo Evaluation Using Oxazolone-Induced Atopic Dermatitis in Murine Model. J. Liposome Res. 2014, 24, 249–257. 10.3109/08982104.2014.899365. [DOI] [PubMed] [Google Scholar]
- Goindi S.; Kumar G.; Kumar N.; Kaur A. Development of Novel Elastic Vesicle-Based Topical Formulation of Cetirizine Dihydrochloride for Treatment of Atopic Dermatitis. AAPS PharmSciTech 2013, 14, 1284–1293. 10.1208/s12249-013-0017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He A.; Feldman S. R.; Fleischer A. B. An Assessment of the Use of Antihistamines in the Management of Atopic Dermatitis. J. Am. Acad. Dermatol. 2018, 79, 92–96. 10.1016/j.jaad.2017.12.077. [DOI] [PubMed] [Google Scholar]
- Pal R. R.; Parashar P.; Singh I.; Saraf S. A. Tamanu Oil Potentiated Novel Sericin Emulgel of Levocetirizine: Repurposing for Topical Delivery against DNCB-Induced Atopic Dermatitis, QbD Based Development and in Vivo Evaluation. J. Microencapsulation 2019, 36, 432–446. 10.1080/02652048.2019.1637474. [DOI] [PubMed] [Google Scholar]
- Pal R. R.; Maurya A. K.; Parashar P.; Saraf S. A. A Comparative Study of Levocetirizine Loaded Vesicular and Matrix Type System for Topical Application: Appraisal of Therapeutic Potential against Atopic Dermatitis. J. Pharm. Innovation 2020, 16, 469–480. 10.1007/s12247-020-09465-x. [DOI] [Google Scholar]
- Lei D.; Guo C.; Greenberger P. Iatrogenic Systemic Allergic Contact Dermatitis From Cetirizine and Levocetirizine. Ann. Allergy, Asthma Immunol. 2018, 121, S131–S132. 10.1016/j.anai.2018.09.436. [DOI] [Google Scholar]
- Kumar P.; Raza K.; Kaushik L.; Malik R.; Arora S.; Katare O. Role of Colloidal Drug Delivery Carriers in Taxane-Mediated Chemotherapy: A Review. Curr. Pharm. Des. 2016, 22, 5127–5143. 10.2174/1381612822666160524144926. [DOI] [PubMed] [Google Scholar]
- Shershakova N.; Baraboshkina E.; Andreev S.; Purgina D.; Struchkova I.; Kamyshnikov O.; Nikonova A.; Khaitov M. Anti-Inflammatory Effect of Fullerene C 60 in a Mice Model of Atopic Dermatitis. J. Nanobiotechnol. 2016, 14, 8 10.1186/s12951-016-0159-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza K.; Singh B.; Mahajan A.; Negi P.; Bhatia A.; Katare O. P. Design and Evaluation of Flexible Membrane Vesicles (FMVs) for Enhanced Topical Delivery of Capsaicin. J. Drug Target. 2011, 19, 293–302. 10.3109/1061186X.2010.499464. [DOI] [PubMed] [Google Scholar]
- Froelich A.; Osmałek T.; Snela A.; Kunstman P.; Jadach B.; Olejniczak M.; Roszak G.; Białas W. Novel Microemulsion-Based Gels for Topical Delivery of Indomethacin: Formulation, Physicochemical Properties and in Vitro Drug Release Studies. J. Colloid Interface Sci. 2017, 507, 323–336. 10.1016/j.jcis.2017.08.011. [DOI] [PubMed] [Google Scholar]
- Das S.; Lee S. H.; Chia V. D.; Chow P. S.; Macbeath C.; Liu Y.; Shlieout G. Development of Microemulsion Based Topical Ivermectin Formulations: Pre-Formulation and Formulation Studies. Colloids Surf., B 2020, 189, 110823 10.1016/j.colsurfb.2020.110823. [DOI] [PubMed] [Google Scholar]
- Praça F. G.; Viegas J. S. R.; Peh H. Y.; Garbin T. N.; Medina W. S. G.; Bentley M. V. L. B. Microemulsion Co-Delivering Vitamin A and Vitamin E as a New Platform for Topical Treatment of Acute Skin Inflammation. Mater. Sci. Eng. C 2020, 110, 110639 10.1016/j.msec.2020.110639. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Michniak-Kohn B. Investigation of Microemulsion Microstructures and Their Relationship to Transdermal Permeation of Model Drugs: Ketoprofen, Lidocaine, and Caffeine. Int. J. Pharm. 2011, 421, 34–44. 10.1016/j.ijpharm.2011.09.014. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Michniak-Kohn B. B. Investigation of Microemulsion and Microemulsion Gel Formulations for Dermal Delivery of Clotrimazole. Int. J. Pharm. 2018, 536, 345–352. 10.1016/j.ijpharm.2017.11.041. [DOI] [PubMed] [Google Scholar]
- Aggarwal N.; Goindi S.; Khurana R. Formulation, Characterization and Evaluation of an Optimized Microemulsion Formulation of Griseofulvin for Topical Application. Colloids Surf., B 2013, 105, 158–166. 10.1016/j.colsurfb.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Tunki L.; Kulhari H.; Vadithe L. N.; Kuncha M.; Bhargava S.; Pooja D.; Sistla R. Modulating the Site-Specific Oral Delivery of Sorafenib Using Sugar-Grafted Nanoparticles for Hepatocellular Carcinoma Treatment. Eur. J. Pharm. Sci. 2019, 137, 104978 10.1016/j.ejps.2019.104978. [DOI] [PubMed] [Google Scholar]
- Golmohammadzadeh S.; Farhadian N.; Biriaee A.; Dehghani F.; Khameneh B. Preparation, Characterization and in Vitro Evaluation of Microemulsion of Raloxifene Hydrochloride. Drug Dev. Ind. Pharm. 2017, 43, 1619–1625. 10.1080/03639045.2017.1328430. [DOI] [PubMed] [Google Scholar]
- Dredán J.; Antal I.; Rácz I. Evaluation of Mathematical Models Describing Drug Release from Lipophilic Matrices. Int. J. Pharm. 1996, 145, 61–64. 10.1016/S0378-5173(96)04725-4. [DOI] [Google Scholar]
- Zhao L.; Wang Y.; Zhai Y.; Wang Z.; Liu J.; Zhai G. Ropivacaine Loaded Microemulsion and Microemulsion-Based Gel for Transdermal Delivery: Preparation, Optimization, and Evaluation. Int. J. Pharm. 2014, 477, 47–56. 10.1016/j.ijpharm.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Fouad S. A.; Basalious E. B.; El-Nabarawi M. A.; Tayel S. A. Microemulsion and Poloxamer Microemulsion-Based Gel for Sustained Transdermal Delivery of Diclofenac Epolamine Using in-Skin Drug Depot: In Vitro/in Vivo Evaluation. Int. J. Pharm. 2013, 453, 569–578. 10.1016/j.ijpharm.2013.06.009. [DOI] [PubMed] [Google Scholar]
- Vitorino C.; Almeida A.; Sousa J.; Lamarche I.; Gobin P.; Marchand S.; Couet W.; Olivier J. C.; Pais A. Passive and Active Strategies for Transdermal Delivery Using Co-Encapsulating Nanostructured Lipid Carriers: In Vitro vs. in Vivo Studies. Eur. J. Pharm. Biopharm. 2014, 86, 133–144. 10.1016/j.ejpb.2013.12.004. [DOI] [PubMed] [Google Scholar]
- Badihi A.; Frušić-Zlotkin M.; Soroka Y.; Benhamron S.; Tzur T.; Nassar T.; Benita S. Topical Nano-Encapsulated Cyclosporine Formulation for Atopic Dermatitis Treatment: Topical Cyclosporine NCs for AD. Nanomed.: Nanotechnol., Biol. Med. 2020, 24, 102140 10.1016/j.nano.2019.102140. [DOI] [PubMed] [Google Scholar]
- Negi P.; Singh B.; Sharma G.; Beg S.; Raza K.; Katare O. P. Phospholipid Microemulsion-Based Hydrogel for Enhanced Topical Delivery of Lidocaine and Prilocaine: QbD-Based Development and Evaluation. Drug Delivery 2016, 23, 941–957. 10.3109/10717544.2014.923067. [DOI] [PubMed] [Google Scholar]
- Souto E. B.; Doktorovová S.; Araújo J.; Garcia M. L.; Rakovsk E. Formulating Fluticasone Propionate in Novel PEG-Containing Nanostructured Lipid Carriers (PEG-NLC). Colloids Surf., B 2010, 75, 538–542. 10.1016/j.colsurfb.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Djekic L.; Primorac M.; Filipic S.; Agbaba D. Investigation of Surfactant/Cosurfactant Synergism Impact on Ibuprofen Solubilization Capacity and Drug Release Characteristics of Nonionic Microemulsions. Int. J. Pharm. 2012, 433, 25–33. 10.1016/j.ijpharm.2012.04.070. [DOI] [PubMed] [Google Scholar]
- Cavalcanti A. L. M.; Reis M. Y. F. A.; Silva G. C. L.; Ramalho ÍM. M.; Guimarães G. P.; Silva J. A.; Saraiva K. L. A.; Damasceno B. P. G. L. Microemulsion for Topical Application of Pentoxifylline: In Vitro Release and in Vivo Evaluation. Int. J. Pharm. 2016, 506, 351–360. 10.1016/j.ijpharm.2016.04.065. [DOI] [PubMed] [Google Scholar]
- Cojocaru V.; Ranetti A. E.; Hinescu L. G.; Ionescu M.; Cosmescu C.; Poştoarcă A. G.; Cinteză L. O. Formulation and Evaluation of in Vitro Release Kinetics of Na3cadtpa Decorporation Agent Embedded in Microemulsion-Based Gel Formulation for Topical Delivery. Farmacia 2015, 63, 656–664. [Google Scholar]
- Beg S.; Raza K.; Kumar R.; Chadha R.; Katare O. P.; Singh B. Improved Intestinal Lymphatic Drug Targeting via Phospholipid Complex-Loaded Nanolipospheres of Rosuvastatin Calcium. RSC Adv. 2016, 6, 8173–8187. 10.1039/c5ra24278a. [DOI] [Google Scholar]
- Raza K.; Singh B.; Lohan S.; Sharma G.; Negi P.; Yachha Y.; Prakash O. Nano-Lipoidal Carriers of Tretinoin with Enhanced Percutaneous Absorption, Photostability, Biocompatibility and Anti-Psoriatic Activity. Int. J. Pharm. 2013, 456, 65–72. 10.1016/j.ijpharm.2013.08.019. [DOI] [PubMed] [Google Scholar]
- Katare O.; Raza K.; Singh B.; Dogra S. Novel Drug Delivery Systems in Topical Treatment of Psoriasis: Rigors and Vigors. Indian J. Dermatol. Venereol. Leprol. 2010, 76, 612–621. 10.4103/0378-6323.72451. [DOI] [PubMed] [Google Scholar]
- Nolte T.; Zadeh-Khorasani M.; Safarov O.; Rueff F.; Varga R.; Herbach N.; Wanke R.; Wollenberg A.; Mueller T.; Gropp R.; Wolf E.; Siebeck M. Induction of Oxazolone-Mediated Features of Atopic Dermatitis in NOD-Scid IL2Rγnull Mice Engrafted with Human Peripheral Blood Mononuclear Cells. Dis. Model. Mech. 2013, 6, 125–134. 10.1242/dmm.009167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatano Y.; Man M. Q.; Uchida Y.; Crumrine D.; Scharschmidt T. C.; Kim E. G.; Mauro T. M.; Feingold K. R.; Elias P. M.; Holleran W. M. Maintenance of an Acidic Stratum Corneum Prevents Emergence of Murine Atopic Dermatitis. J. Invest. Dermatol. 2009, 129, 1824–1835. 10.1038/jid.2008.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshio T.; Sasaki Y.; Funakoshi-Tago M.; Aizu-Yokota E.; Sonoda Y.; Matsuoka H.; Kasahara T. Dermatophagoides Farinae Extract Induces Severe Atopic Dermatitis in NC/Nga Mice, Which Is Effectively Suppressed by the Administration of Tacrolimus Ointment. Int. Immunopharmacol. 2009, 9, 403–411. 10.1016/j.intimp.2008.12.013. [DOI] [PubMed] [Google Scholar]