Abstract
In recent drug development efforts, particular emphasis has been devoted to the chemical interference with the NLRP3 inflammasome. A series of 12 tailored sulfonylureas was designed, prepared through convergent syntheses with a final sodium hydride-promoted reaction of isocyanates and sulfonamides, and subjected to a systematic, high-performance liquid chromatography-based survey of the chemical stability, a critical issue of sulfonylureas in terms of preparation, storage, and application. NLRP3 binding was determined by surface plasmon resonance spectroscopy. Sulfonylurea 2 was identified to be equipotent and similarly stable compared to the prototypical NLRP3 inhibitor MCC950.
Introduction
Sulfonylureas had been the first-line drugs for diabetics for almost 6 decades. Their molecular targets, sulfonylurea receptors, are subunits of the octameric ATP-sensitive potassium channels. Upon sulfonylurea binding, the potassium efflux is decreased and the voltage-gated calcium channels are activated, ultimately leading to insulin secretion.1 In 2001, an established antidiabetic drug, glibenclamide, was observed to inhibit the formation of mature interleukin-1β.2 Sulfonylureas that affect the accompanied cellular response have been designated cytokine release inhibitory drugs (CRIDs).2 The most prominent representative of this class of compounds,3,4 the diaryl sulfonylurea derivative MCC950 (1, Table 1), is also referred to as CRID3 or CP-456,773. Some of its relatives, but not MCC950 itself, were able to stimulate insulin secretion.5 The anti-inflammatory activity of MCC950 was attributed to the inhibition of the NLRP3 inflammasome.6 This finding paved the way for further studies on the specific interaction of the NLRP3 inflammasome with low-molecular-weight, drug-like compounds, which proved of great value as pharmacological tool compounds7,8 and progressed into clinical trials.9
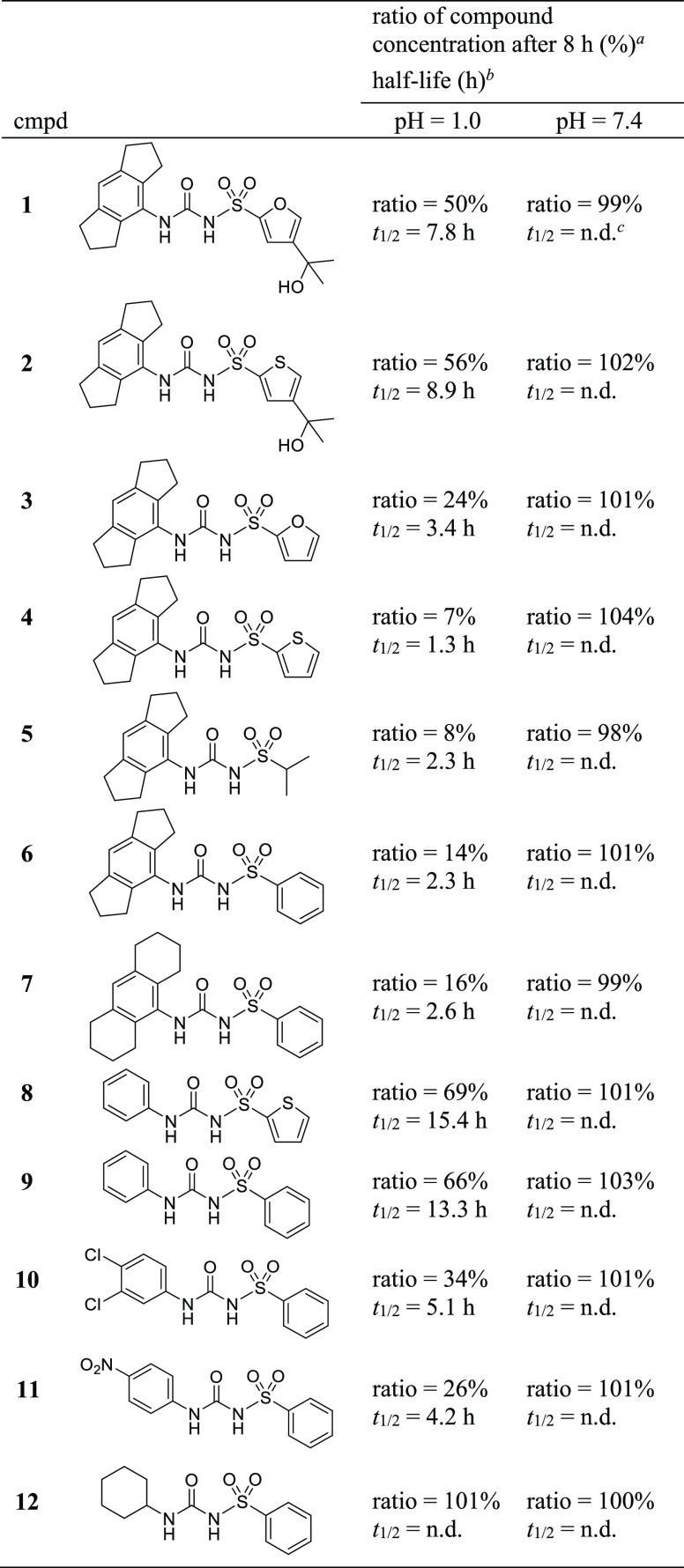
Table 1. Sulfonylureas 1–12 and Their Chemical Stability.
Areas under the curve (AUCs) of the chromatograms after 8 h of incubation at the indicated pH were determined for the test compound and the internal standard. Ratios were calculated as a measure of stability using eq S1 (Supporting Information).
Ratio values at nine different time points were plotted versus incubation time and fitted to the equation of exponential decay. Half-lives, t1/2, were calculated using eq S2 (Supporting Information).
Not detectable; no decomposition observed within 8 h.
NLRP3 is a tripartite inflammasome sensor protein.10 Its activation can be ubiquitously triggered by damage- or pathogen-associated molecular patterns, which leads to the oligomerization of NLRP3, followed by the recruitment of the adaptor protein ASC and pro-caspase-1 to an intracellular supramolecular complex. The assembly of the inflammasome platform induces the autoactivation of caspase-1. Acting as an effector protein, it cleaves pro-interleukin-1β and pro-interleukin-18 to the mature cytokines and gasdermin-D to its N- and C-terminal domains. Subsequently, the gasdermin-D N-terminal domain forms membrane pores resulting in pyroptosis and the secretion of the pro-inflammatory cytokines.11 NLPR3 has attracted great attention as a major therapeutic target and a key player in a variety of pathologies, for example, rheumatoid arthritis, osteoarthritis, Crohn’s disease, gout, coronary artery diseases, hepatic and pulmonary fibroses, as well as nonalcoholic steatohepatitis.9,12
The molecular interaction of MCC950 and NLPRP3 involves a high-affinity, noncovalent binding of the inhibitor to the Walker B ATP-hydrolysis motif of the NACHT domain,7 converting the NLRP3 “open” structure into a “closed” inactive conformation.7,13,14 Thus, NLRP3 inhibitors of this chemotype are thought to act as interdomain glues to lock the protein in an inactive conformation.14
The extraordinary potency of MCC950 with an IC50 value of 7.5 nM was demonstrated in a cell-based interleukin-1β assay.6 Pharmacokinetic studies showed the oral bioavailability and a plasma half-life of 3.27 h.6 MCC950 metabolites exhibited a strongly reduced inhibitory activity.15
Despite the resurgence of sulfonylureas as NLRP3-targeting drug candidates, a systematic study on their chemical stability has not been conducted yet. We performed a positional scanning with respect to the substituents at the sulfur and the terminal nitrogen of the central sulfonylurea core. Whereas the conventional antidiabetic sulfonylureas comprise N-alkyl, S-aryl derivatives, we paid attention to the N,S-diaryl substitution pattern of NLRP3 inflammasome inhibitors.
Results and Discussion
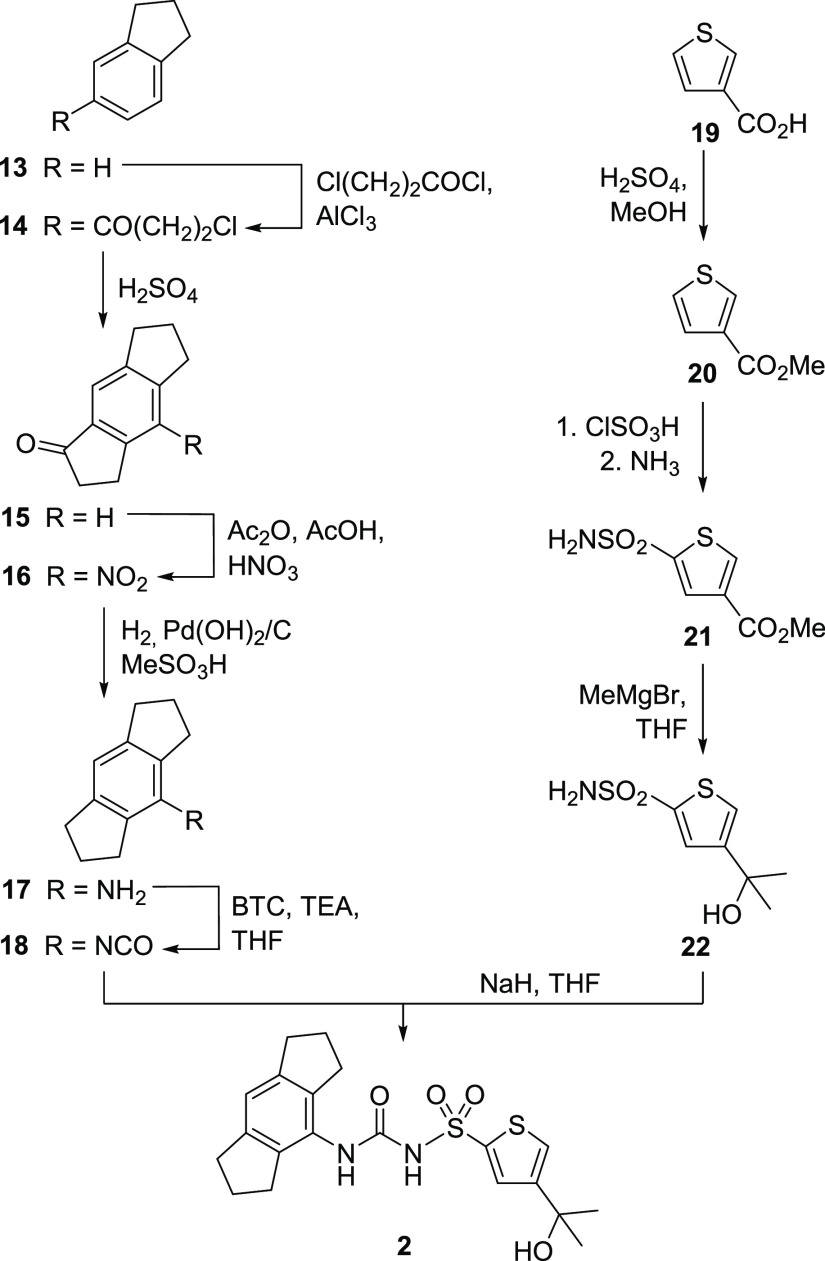
A series of 12 substituted sulfonylureas (Table 1) was conceptualized in order to stepwise modify the structure of MCC950 (1). The preparation of one representative, compound 2, is depicted in Scheme 1, whereas all other syntheses are provided in the Supporting Information. There is extensive patent literature on MCC950 and its relatives. Some of our test compounds appear in patents,16,17 which is specified in the Supporting Information. We conceived a common strategy toward these sulfonylureas. In the case of product 2, one branch of the convergent synthesis led to 1,2,3,5,6,7-hexahydro-s-indacen-4-amine (17),4 a key building block for particularly active NLRP3 inhibitors. Indane (13) was subjected to a Friedel–Crafts acylation with 3-chloropropionyl chloride in dichloromethane in the presence of aluminum chloride. The resulting ketone 14 underwent Friedel–Crafts alkylation under much harsher conditions with concd H2SO4 to afford the tricyclic intermediate 15. Deviating from the reported procedure,4 the substrate for the subsequent nitration was dissolved in a mixture of CH2Cl2, acetic anhydride, and acetic acid,18 and fuming HNO3 was slowly added at 0 °C. Both functionalities of the resulting nitro ketone 16 were reduced with Pearlman’s catalyst with additive methanesulfonic acid. We obtained the hexahydro-s-indacen-4-amine 17 after column chromatography as colorless needles.
Scheme 1. Synthesis of Sulfonylurea 2.
The second branch toward sulfonylurea 2 started with thiophene-3-carboxylic acid (19), which was esterified to 20. The following one-pot synthesis involved chlorosulfonylation and ammonolysis to produce the thiophene sulfonamide 21. In order to introduce the desired tertiary alcohol group, a double Grignard reaction with methyl magnesium bromide was employed for the synthesis of 22. Next, we performed the convergent step to the final sulfonylurea 2. For this purpose, triethylamine (TEA) was added to a suspension of bis(trichloromethyl) carbonate (BTC) in THF at 0 °C, and a solution of the hexahydro-s-indacen-4-amine 17 in THF was added slowly to avoid the formation of a symmetrical urea. The isocyanate 18 was obtained after separation of insoluble material and, without further purification, combined with a mixture of sulfonamide 22 and sodium hydride in THF to afford product 2.16
The first branch to 17 and 18 was also utilized for sulfonylureas 1,43,174,175, and 6(19) (Table 1), who share the hexahydro-s-indacene substituent with 2. The analogous route via Friedel–Crafts chemistry to the octahydroanthracene building block for the preparation of 7 was less advantageous than the catalytic hydrogenation of 9-aminoanthracene (see the Supporting Information, Scheme S1).
The furan analogue of compound 22 was in principal prepared as shown in the right branch of Scheme 1 starting from ethyl 3-furoate, but the conditions of the chlorosulfonylation and ammonolysis had to be precisely adapted (Scheme S3).20 The final step toward all synthesized sulfonylureas was performed in an analogous manner by reacting the respective isocyanates and sulfonamides.
This series of sulfonylureas was subjected to stability assays using an high-performance liquid chromatography (HPLC) method. We analyzed five different pH conditions. Data are listed in Table 1 (see also Table S1 and Figures S1–S12). By trend, all compounds with calculated pKa values in the range of 4–5.5 (https://scifinder-n.cas.org) showed a high stability at pHs 7.4 and 8.4 but underwent decomposition at pH 1 (Figure 1) and pH 4 to form the corresponding aromatic primary amine and the corresponding sulfonamide (see Figure S13). This finding clearly indicated that deprotonation at the sulfonylurea moiety stabilized the compounds. As reported for N,S-diaryl sulfonylurea herbicides,21 the neutral form was more susceptible to hydrolysis than the anionic form with the negative charge distributed throughout the sulfonylurea moiety, thus reducing the electrophilicity of the carbonyl carbon. Accordingly, liquid formulations of N-alkyl-S-aryl sulfonylureas, for example, glibenclamide, were stable,22 but not those of N,S-diaryl sulfonylurea herbicides. The latter could be stabilized by salt formulation.23 Compounds 9–12 exhibited different stability at pH 1, reflecting the electron-withdrawing (10, 11) or electron-donating (12) properties of the N-substituent. The glibenclamide fragment 12 with N-alkyl substitution was the only compound lacking decomposition at pH 1.
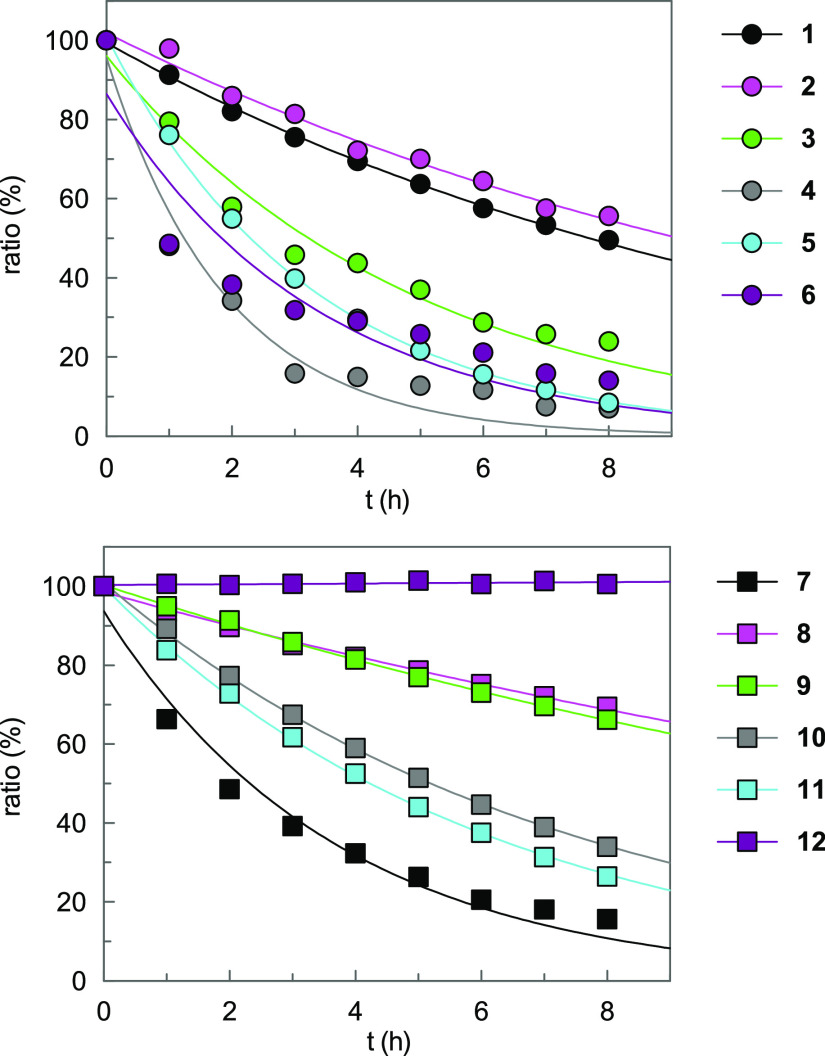
Figure 1.
Time course of decomposition of sulfonylureas 1–12. Compounds (0.1 mM) were incubated at 37 °C for 8 h at pH 1 (0.1 M HCl, 20% MeCN) in the presence of an internal standard (0.1 mM) and subjected to HPLC analysis. The ratios are plotted versus incubation time.
Unexpectedly, the introduction of two trimethylene bridges at the N-phenyl substituent decreased the stability at pH 1. The hexahydro-s-indacene containing compounds 4 and 6 showed 12-fold and 6-fold shorter half-lives than their benzene analogues 8 and 9, respectively. The hexahydro-s-indacene derivatives 3 and 5 were unstable at pH 1 as well. To ascertain a specific effect of this tricyclic ring system, we expanded the adjacent alkylidene units and investigated the octahydroanthracene derivative 7, which, however, was similarly unstable as its counterpart 6. Such a degree of instability at pH 1 is not only limiting a gastrointestinal passage of compounds when applied orally but also impedes their preparation and work-up under acidic conditions.
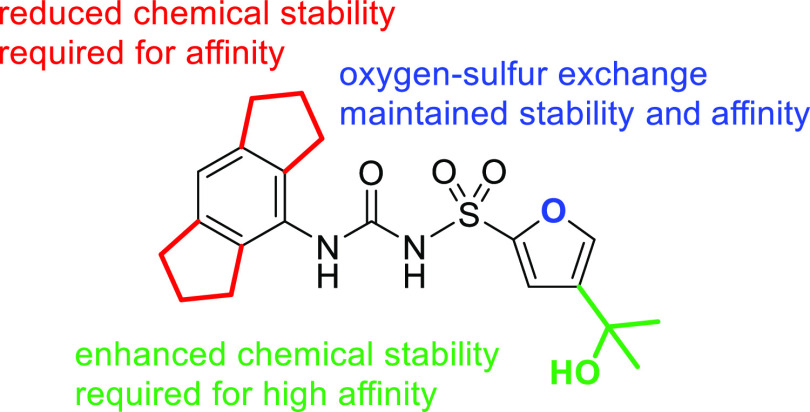
We analyzed the influence of the tertiary alcohol group on the compound’s stability and compared 1 with 3, as well as 2 with 4. An unforeseen extension of the half-lives by a factor of 2 and 7, respectively, was determined for 1 and 2, caused by the presence of the 2-hydroxy-propyl-2-yl residue at position 4 of the furan or thiophene ring. Next, the affinity of selected diaryl sulfonylureas to human NLRP3 was determined by surface plasmon resonance (SPR) spectroscopy.
The recombinant biotinylated human NLRP3-NACHT domain, derived from HEK293T cells, was immobilized on a streptavidin-functionalized sensor chip, and binding of ligands was studied in the presence of ADP. Notable NLRP3 affinity was not expected for compounds 8–12.24 Among the sulfonylureas 1–7, we selected MCC950 (1), 2, 6, and 7 for SPR analysis (Figure 2). The SPR data revealed compounds 1 and 2 to be NLRP3 ligands with high affinity (Table 2). Our finding showed that the exchange of the ring oxygen (in 1) by sulfur (in 2) was well tolerated, which offers justification for the development of thiophene-based NLRP3 ligands. This is also reflected in the patent literature.16 An unsubstituted aromatic S-substituent reduced the affinity as exemplified with compound 6, indicating that the tertiary alcohol group (in 1 and 2) contributed to the NLRP3–ligand interaction. Noteworthy, the replacement of the hexahydro-s-indacene moiety with an octahydroanthracene moiety caused the total loss of binding ability of 7.
Figure 2.
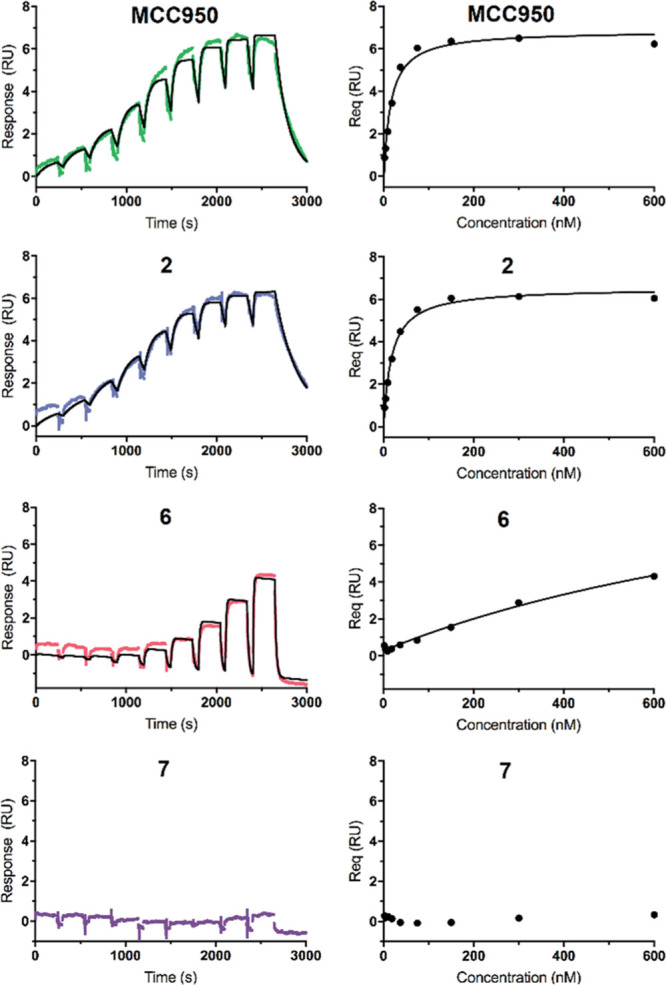
SPR sensorgrams (left) and saturation curves (right) of MCC950, 2, 6, and 7 injected on single channels. The chip surface was loaded with human NLRP3-NACHT. RU is the response unit, Req the steady-state response. The binding parameters are listed in Table 2. Data were fitted to a 1:1 binding model.
Table 2. SPR Binding Behavior Data of Selected Sulfonylureas to Human NLRP3a.
| cmpd | ka (105M–1 s–1) | kd (10–3 s–1) | KD.kin (nM) | KD,eq (nM) |
|---|---|---|---|---|
| 1b | 5.3 ± 2.5 | 6.2 ± 1.3 | 15 ± 4 | 21 ± 4 |
| 2 | 2.5 ± 0.2 | 3.9 ± 1.1 | 15 ± 4 | 19 ± 2 |
| 6 | >2.0 | >25 | n.d.c | >600d |
| 7 | n.d.e | n.d.e | n.d.e | n.d.e |
Second-order on-rate constants for association (ka), first-order off-rate constants for dissociation (kd), kinetic dissociation constants (KD,kin), and equilibrium dissociation constant (KD,eq) for selected sulfonylureas (n = 3).
A KD value of 10.6 nM obtained by SPR for binding of 1 to NLRP3ΔLRR in the presence of ATP was reported.7
Not determined due to the fast binding kinetics of compound 6.
Insufficient saturation of immobilized NLRP3 with compound 6.
Binding of 7 to NLRP3 was not detectable.
As a conclusion, our series of MCC950 analogues revealed that subtle changes in the inhibitor structure influenced chemical stability under acidic conditions. Truncation of the alkylidene bridges at the tricyclic unit caused an unanticipated increase of stability. However, the hexahydro-s-indacene moiety has commonly been established to achieve NLRP3 inhibition. Our study demonstrated that the stability of hexahydro-s-indacene-containing compounds can be regained through the introduction of the tertiary alcohol group, which additionally improved the compounds’ potency. The combination of both structural features accounts for the high suitability of MCC950 as a pharmacological tool compound. With the diaryl sulfonylurea 2, we introduce a thiophene bioisostere of MCC950 (1), which was equipotent and similarly stable. This discovery suggests further synthetic options toward NLRP3 ligands based on thiophene chemistry.
Acknowledgments
T.K. was supported by a fellowship from the Jürgen Manchot Foundation, Düsseldorf, Germany, and D.F. by a fellowship from the Studienstiftung des deutschen Volkes, Bonn, Germany. M.Ge. was funded by the Deutsche Forschungsgemeinschaft under Germany’s Excellence Strategy-EXC2151-390873048. Compound 32 was provided by Dr. Christian Steinebach.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c00125.
General information, preparation of compounds, analytical data, HPLC stability, and SPR spectroscopy measurements (PDF)
The authors declare no competing financial interest.
Dedication
This work is dedicated to Professor Dr. Kurt Eger on the occasion of his 80th birthday.
Supplementary Material
References
- Lv W.; Wang X.; Xu Q.; Lu W. Mechanisms and characteristics of sulfonylureas and glinides. Curr. Top. Med. Chem. 2020, 20, 37–56. 10.2174/1568026620666191224141617. [DOI] [PubMed] [Google Scholar]
- Perregaux D. G.; McNiff P.; Laliberte R.; Hawryluk N.; Peurano H.; Stam E.; Eggler J.; Griffiths R.; Dombroski M. A.; Gabel C. A. Identification and characterization of a novel class of interleukin-1 post-translational processing inhibitors. J. Pharmacol. Exp. Ther. 2001, 299, 187–197. [PubMed] [Google Scholar]
- Laliberte R. E.; Perregaux D. G.; Hoth L. R.; Rosner P. J.; Jordan C. K.; Peese K. M.; Eggler J. F.; Dombroski M. A.; Geoghegan K. F.; Gabel C. A. Glutathione S-transferase omega 1-1 is a target of cytokine release inhibitory drugs and may be responsible for their effect on interleukin-1 beta posttranslational processing. J. Biol. Chem. 2003, 278, 16567–16578. 10.1074/jbc.m211596200. [DOI] [PubMed] [Google Scholar]
- Urban F. J.; Jasys V. J.; Raggon J. W.; Buzon R. A.; Hill P. D.; Eggler J. F.; Weaver J. D. Novel synthesis of 1-(1,2,3,5,6,7-hexahydro-s-indacen-4-yl)-3-[4-(1-hydroxy-1-methyl-ethyl)-furan-2-sulfonyl]urea, an anti-inflammatory agent. Synth. Commun. 2003, 33, 2029–2043. 10.1081/scc-120021029. [DOI] [Google Scholar]
- Hill J. R.; Coll R. C.; Sue N.; Reid J. C.; Dou J.; Holley C. L.; Pelingon R.; Dickinson J. B.; Biden T. J.; Schroder K.; Cooper M. A.; Robertson A. A. B. Sulfonylureas as concomitant insulin secretagogues and NLRP3 inflammasome inhibitors. ChemMedChem 2017, 12, 1449–1457. 10.1002/cmdc.201700270. [DOI] [PubMed] [Google Scholar]
- Coll R. C.; Robertson A. A. B.; Chae J. J.; Higgins S. C.; Muñoz-Planillo R.; Inserra M. C.; Vetter I.; Dungan L. S.; Monks B. G.; Stutz A.; Croker D. E.; Butler M. S.; Haneklaus M.; Sutton C. E.; Núñez G.; Latz E.; Kastner D. L.; Mills K. H. G.; Masters S. L.; Schroder K.; Cooper M. A.; O’Neill L. A. J. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 2015, 21, 248–255. 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll R. C.; Hill J. R.; Day C. J.; Zamoshnikova A.; Boucher D.; Massey N. L.; Chitty J. L.; Fraser J. A.; Jennings M. P.; Robertson A. A. B.; Schroder K. MCC950 directly targets the NLRP3 ATP-hydrolysis motif for inflammasome inhibition. Nat. Chem. Biol. 2019, 15, 556–559. 10.1038/s41589-019-0277-7. [DOI] [PubMed] [Google Scholar]
- a Vande Walle L.; Stowe I. B.; Šácha P.; Lee B. L.; Demon D.; Fossoul A.; Van Hauwermeiren F.; Saavedra P. H. V.; Šimon P.; Šubrt V.; Kostka L.; Stivala C. E.; Pham V. C.; Staben S. T.; Yamazoe S.; Konvalinka J.; Kayagaki N.; Lamkanfi M. MCC950/CRID3 potently targets the NACHT domain of wild-type NLRP3 but not disease-associated mutants for inflammasome inhibition. PLoS Biol. 2019, 17, e3000354 10.1371/journal.pbio.3000354. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Hill J. R.; Shao X.; Massey N. L.; Stauff J.; Sherman P. S.; Robertson A. A. B.; Scott P. J. H. Synthesis and evaluation of NLRP3-inhibitory sulfonylurea [11C]MCC950 in healthy animals. Bioorg. Med. Chem. Lett. 2020, 30, 127186. 10.1016/j.bmcl.2020.127186. [DOI] [PubMed] [Google Scholar]; c Keuler T.; Gatterdam K.; Akbal A.; Lovotti M.; Marleaux M.; Geyer M.; Latz E.; Gütschow M. Development of fluorescent and biotin probes targeting NLRP3. Front. Chem. 2021, 9, 642273. 10.3389/fchem.2021.642273. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Kennedy C. R.; Goya Grocin A.; Kovačič T.; Singh R.; Ward J. A.; Shenoy A. R.; Tate E. W. A probe for NLRP3 inflammasome inhibitor MCC950 identifies carbonic anhydrase 2 as a novel target. ACS Chem. Biol. 2021, 16, 982–990. 10.1021/acschembio.1c00218. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Xu Y.; Xu Y.; Blevins H.; Lan Y.; Liu Y.; Yuan G.; Striar R.; Zagaroli J. S.; Tocci D. R.; Langan A. G.; Zhang C.; Zhang S.; Wang C. Discovery of carbon-11 labeled sulfonamide derivative: A PET tracer for imaging brain NLRP3 inflammasome. Bioorg. Med. Chem. Lett. 2021, 34, 127777. 10.1016/j.bmcl.2021.127777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaid A. G.; Spencer K. B. Strategies for targeting the NLRP3 inflammasome in the clinical and preclinical space. J. Med. Chem. 2021, 64, 101–122. 10.1021/acs.jmedchem.0c01307. [DOI] [PubMed] [Google Scholar]
- a Song N.; Liu Z.-S.; Xue W.; Bai Z.-F.; Wang Q.-Y.; Dai J.; Liu X.; Huang Y.-J.; Cai H.; Zhan X.-Y.; Han Q.-Y.; Wang H.; Chen Y.; Li H.-Y.; Li A.-L.; Zhang X.-M.; Zhou T.; Li T. NLRP3 phosphorylation is an essential priming event for inflammasome activation. Mol. Cell 2017, 68, 185–197. 10.1016/j.molcel.2017.08.017. [DOI] [PubMed] [Google Scholar]; b Stutz A.; Kolbe C.-C.; Stahl R.; Horvath G. L.; Franklin B. S.; van Ray O.; Brinkschulte R.; Geyer M.; Meissner F.; Latz E. NLRP3 inflammasome assembly is regulated by phosphorylation of the pyrin domain. J. Exp. Med. 2017, 214, 1725–1736. 10.1084/jem.20160933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan D.; Vande Walle L.; Lamkanfi M. Therapeutic modulation of inflammasome pathways. Immunol. Rev. 2020, 297, 123–138. 10.1111/imr.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan M. S. J.; Olhava E. J.; Roush W. R.; Seidel H. M.; Glick G. D.; Latz E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug Discovery 2018, 17, 588–606. 10.1038/nrd.2018.97. [DOI] [PubMed] [Google Scholar]
- Tapia-Abellán A.; Angosto-Bazarra D.; Martínez-Banaclocha H.; de Torre-Minguela C.; Cerón-Carrasco J. P.; Pérez-Sánchez H.; Arostegui J. I.; Pelegrin P. MCC950 closes the active conformation of NLRP3 to an inactive state. Nat. Chem. Biol. 2019, 15, 560–564. 10.1038/s41589-019-0278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker C.; Mattes H.; Wright M.; Boettcher A.; Hinniger A.; Hughes N.; Kapps-Fouthier S.; Eder J.; Erbel P.; Stiefl N.; Mackay A.; Farady C. J. Crystal structure of NLRP3 NACHT domain with an inhibitor defines mechanism of inflammasome inhibition. J. Mol. Biol. 2021, 433, 167309. 10.1016/j.jmb.2021.167309. [DOI] [PubMed] [Google Scholar]
- Salla M.; Butler M. S.; Pelingon R.; Kaeslin G.; Croker D. E.; Reid J. C.; Baek J. M.; Bernhardt P. V.; Gillam E. M. J.; Cooper M. A.; Robertson A. A. B. Identification, synthesis, and biological evaluation of the major human metabolite of NLRP3 inflammasome inhibitor MCC950. ACS Med. Chem. Lett. 2016, 7, 1034–1038. 10.1021/acsmedchemlett.6b00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littman B. H.; Woodworth T. L. G.; Dombrowski M. A.. Combination treatment with IL-1RA and diaryl sulphonyl urea compounds. WO 2001019390 A1, 2001.
- O’Neill L.; Coll R.; Cooper M.; Robertson A.; Schroder K.. Sulfonylureas and related compounds and use of same. WO 2016131098 A1, 2016.
- Tsimerman M.; Mallik D.; Matsuo T.; Otani T.; Tamao K.; Organ M. G. Sterically demanding imidazolinium salts through the activation and cyclization of formamides. Chem. Commun. 2012, 48, 10352–10354. 10.1039/c2cc36329a. [DOI] [PubMed] [Google Scholar]
- Agarwal S.; Sasane S.; Shah H. A.; Pethani J. P.; Deshmukh P.; Vyas V.; Iyer P.; Bhavsar H.; Viswanathan K.; Bandyopadhyay D.; Giri P.; Mahapatra J.; Chatterjee A.; Jain M. R.; Sharma R. Discovery of N-cyano-sulfoximineurea derivatives as potent and orally bioavailable NLRP3 inflammasome inhibitors. ACS Med. Chem. Lett. 2020, 11, 414–418. 10.1021/acsmedchemlett.9b00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salla M.; Butler M. S.; Massey N. L.; Reid J. C.; Cooper M. A.; Robertson A. A. B. Synthesis of deuterium-labelled analogues of NLRP3 inflammasome inhibitor MCC950. Bioorg. Med. Chem. Lett. 2018, 28, 793–795. 10.1016/j.bmcl.2017.12.054. [DOI] [PubMed] [Google Scholar]
- a Hay J. V. Chemistry of sulfonylurea herbicides. Pestic. Sci. 1990, 29, 247–261. 10.1002/ps.2780290303. [DOI] [Google Scholar]; b Brown H. M. Mode of action, crop selectivity, and soil relations of the sulfonylurea herbicides. Pestic. Sci. 1990, 29, 263–281. 10.1002/ps.2780290304. [DOI] [Google Scholar]; c Sarmah A. K.; Sabadie J. Hydrolysis of sulfonylurea herbicides in soils and aqueous solutions: A review. J. Agric. Food Chem. 2002, 50, 6253–6265. 10.1021/jf025575p. [DOI] [PubMed] [Google Scholar]
- Estevez P.; Boscolo O.; Quiroga E.; Fernandez Penuto R.; Buontempo F.; Tripodi V.; Lucangioli S. Development and stability study of glibenclamide oral liquid paediatric formulations for the treatment of permanent neonatal diabetes mellitus. Eur. J. Hosp. Pharm. 2016, 23, 213–218. 10.1136/ejhpharm-2015-000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. C. Chemical stabilization study for sulfonylurea herbicides. Korean J. Weed Sci. 1997, 17, 135–138. [Google Scholar]
- a Hill J. R.; Coll R. C.; Schroder K.; Robertson A. A. B. Design, synthesis and evaluation of an NLRP3 inhibitor diazirine photoaffinity probe. Tetrahedron Lett. 2020, 61, 151849. 10.1016/j.tetlet.2020.151849. [DOI] [Google Scholar]; b Glick G.; Ghosh S.; Roush W. R.. Compounds and compositions for treating conditions associated with NLRP activity. WO 2017184624 A1, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.