Abstract
Although the uterine cervix responds to the female sex hormone change, the role of progesterone in cervical cancer is poorly understood. It has been shown that medroxyprogesterone acetate (MPA) regresses cervical cancer in the transgenic mouse model expressing human papillomavirus type 16 E6 and E7 oncogenes. As MPA interacts most strongly with progesterone receptor (PR), we reasoned that PR would contribute to MPA-induced regression of cervical cancer. We also hypothesized that estrogen influences the therapeutic activity of MPA because it promotes cervical cancer growth in the same mouse model. The present study showed that the deletion of Pgr in the cervical cancer cells ablated the MPA's therapeutic effect in the human papillomavirus transgenic mouse model. Additionally, estrogen attenuated cancer regression by MPA in the same model system. These observations indicate that MPA can effectively regress cervical cancer only when cancer cells express PR and estrogen levels are low. These results suggest that, if translatable, MPA should be administered when estrogen levels are low in patients with PR-positive cervical cancer.
High-risk human papillomaviruses (HPVs) are causally associated with cervical cancer.1 It is a multistage disease starting from cervical intraepithelial neoplasia (CIN), culminating in invasive cancer. HPV vaccination rates are low among women in developing countries and women of low socioeconomic status in developed countries (most cervical cancers arise in these women) because of low awareness of the link between HPV and cervical cancer, high costs for vaccination, and social and religious issues.2, 3, 4 Cervical cancer remains the third most common cancer and the third most frequent cause of cancer death in women worldwide.5
HPV E6 and E7 oncoproteins are primary drivers of HPV-induced cancers, including cervical cancer. They promote cancers by binding and inhibiting or activating multiple cellular proteins. The most notable activities of E6 and E7 are to inactivate p53 and retinoblastoma protein (pRb) tumor suppressor proteins, respectively.6 CIN3, high-grade dysplasia, develops in only up to 40% of women who are persistently infected with high-risk HPVs.7 More than two-thirds of CIN3 cases regress spontaneously without intervention.8 E6 and E7 alone do not transform primary human keratinocytes in vitro.9 These observations support that HPV is necessary but insufficient for cervical cancer and that other cofactors are also crucial for cervical carcinogenesis.
The use of oral contraceptives for ≥5 years increases the risk of cervical cancer by fourfold in HPV-infected women.10 HPV-positive women who have seven or more full-term pregnancies are at a higher risk of cervical carcinoma than those with zero to six full-term pregnancies.11 These variables involve female sex hormones, estrogen and progesterone, that activate estrogen receptor α and progesterone receptor (PR), respectively.12 Epidemiologic studies investigating the correlation of individual hormones with cervical cancer risk need to be interpreted with caution because they have not stratified data based on the status of high-risk HPVs.13
K14E6 and K14E7 transgenic mice have been powerful tools to study the pathogenesis of cervical cancer. Like the human disease, cervical cancer in these mice develops at the cervical transformation zone through multiple stages in a cofactor-dependent manner.14,15 In addition, human and mouse cervical cancers share critical biomarkers, such as p16Ink4a and cyclin E.16 These genetically engineered mice have been helpful to dissect the mechanism of E6 and E7 and have revealed the individual role of estrogen and progestin in cervical cancer.15,17, 18, 19, 20 Treatment with exogenous estrogen for 6 to 9 months promotes cervical cancer in these mice.14,15 A progestin called medroxyprogesterone acetate (MPA) promotes the regression of cervical cancer and CIN in K14E6/K14E7 and K14E7 mice.20, 21, 22 Although MPA is synthetic progesterone, it binds not only PR but also glucocorticoid receptor and mineralocorticoid receptor.23,24 MPA inhibits cervical cancer development in K14E7/Pgr+/− mice but not in K14E7/Pgr−/− mice, demonstrating the requirement of PR for cervical cancer prevention by MPA.21 Herein, K14E7 single transgenic and conditional Pgr knockout mice were used to investigate the roles of epithelial PR and estrogen in the efficacy of MPA for cervical cancer treatment. The study shows that MPA promotes regression of PR-positive cervical cancer only, and estrogen attenuates its therapeutic effect. These results suggest that the level of estrogen is important for the efficacy of MPA in treating PR-positive cervical cancer.
Materials and Methods
Mice and Hormone Treatments
K14E7 [Tg(KRT14−HPV16E7)2304Plam], Wnt7a-Cre [Tg(Wnt7a-EGFP/cre)#Bhr], and Pgrf/f (Pgrtm4.1Lyd) mice were described previously.25, 26, 27 K14E7 mice were originally generated with the FVB/N strain, and the other two genotypes were backcrossed to FVB/N for at least 10 generations. K14E7/Wnt7a-Cre/Pgrf/f males were mated with Pgrf/f females. Wnt7a-Cre is expressed in sperm progenitor cells (diploid), and thus the Pgr gene is deleted in all mature sperms (haploids) regardless of Wnt7a-Cre genotype.28 In consequence, all offspring had the Pgrf/− genotype. All mice were genotyped by PCR using genomic DNAs isolated from tail or toe biopsies. For estrogen treatment, slow-release drug pellets were used to provide 0.05 mg of 17β-estradiol over 60 days (Innovative Research of America, Sarasota, FL; catalog number SE-121). Estrogen pellets were implanted underneath the dorsal skin at 6 to 8 weeks of age and replenished every 60 days, as described previously.21 Medroxyprogesterone acetate injectable suspension (Greenstone LLC, Peapack, NJ; product UPC 00359762453716) was diluted to 30 mg/mL in phosphate-buffered saline (PBS) and injected into mice once a month via the i.p. route (0.15 mL equivalent to 4.5 mg of MPA per injection). These hormone dosages were determined in previous studies.14,20 Some mice were intraperitoneally injected with 0.15 mL of bromodeoxyuridine (BrdU) solution (25 mg/mL) 1 hour before euthanasia. All procedures were approved by the University of Houston Institutional Animal Care and Use Committee.
Tissue Processing and Histopathologic Analysis
Female reproductive tracts were harvested at study end points, fixed in 4% paraformaldehyde at 4°C, processed in an Excelsior AS tissue processor (Thermo Fisher Scientific, Kalamazoo, MI), and paraffin embedded. Prepared tissues were serially sectioned (5 μm thick) throughout the cervix, resulting in approximately 100 sections per tissue. Every tenth slide was stained with hematoxylin and eosin and subjected to histopathologic analyses, as described previously.14 Criteria for disease grades included the thickness of basal-like epithelial layer, presence of nuclear atypia, and invasion into stroma. If a mouse had multiple grades of neoplastic diseases, the mouse was assigned with the worst disease grade. For example, cancer was assigned when a mouse had CIN3 and cancer.
Immunohistochemistry
Tissue sections were deparaffinized in xylene, rehydrated in a series of ethanol solution, and washed in PBS. Antigen was retrieved by microwaving tissue sections in 10 mmol/L sodium citrate buffer (pH 6.0) for 20 minutes. For BrdU immunohistochemistry, sections were incubated in 2N HCl for 20 minutes and washed with PBS. Processed sections were incubated with primary antibodies diluted in 5% goat serum (PR, 1:1000; K10, 1:50; BrdU, 1:50). Antibodies were purchased from Millipore Sigma, Burlington, MA [BrdU (Mobu-1); catalog number EMNA-61], Sigma-Aldrich, St. Louis, MO [PR (SP2); catalog number SAB5500165], and Thermo Scientific, Fremont, CA [K10 (DE-K10); catalog number MA5-13705]. After washes in PBS, sections were incubated with Alexa488-conjugated anti−rabbit IgG (Life Technologies, Carlsbad, CA; catalog number A11008) or anti-mouse IgG (Life Technologies; catalog number A11001). Terminal deoxynucleotidyl transferase dUTP nick end labeling assay was performed with an ApopTaq Fluorescein in situ apoptosis detection kit (Millipore-Sigma; catalog number S7110), according to the manufacturer's instructions. Nuclei were stained for 30 seconds with 10 μg/mL of Hoechst 33258 solution (Sigma-Aldrich; catalog number B2883). Slides were mounted with Gelvatol mounting medium.
Microscopy and Digital Image Analysis
Stained sections were visualized with an Eclipse Ti2 microscope (Nikon Instruments Inc., Melville, NY). Representative images were obtained with a Nikon DS-Qi2 monochrome camera or a DS-Ri2 color camera using Nikon NIS-Elements imaging software version 5.2. Cancer area was measured using Nikon NIS-Elements and tissue sections containing the largest area for each cancer. To determine proliferation and apoptosis indexes, two to four random microscopic fields per cancer were imaged with a 20× objective lens, and cells were counted using ImageJ version 1.53f (NIH, Bethesda, MD), downloaded from https://imagej.nih.gov/ij on November 2, 2020. Nuclei were pseudocolored red using Adobe Photoshop 2020 version 21 (Adobe, San Jose, CA).
Statistical Analysis
MSTAT software version 6.5.1 was used to perform Wilcoxon rank-sum test (cancer area and tumor multiplicity) and Fisher exact test (cancer incidence). It was downloaded from https://mcardle.wisc.edu/mstat on October 1, 2019. A t-test was used to analyze proliferation and apoptosis indexes. P ≤ 0.05 was considered statistically significant and denoted in figures.
Results
MPA Fails to Regress PR-Negative Cervical Cancer
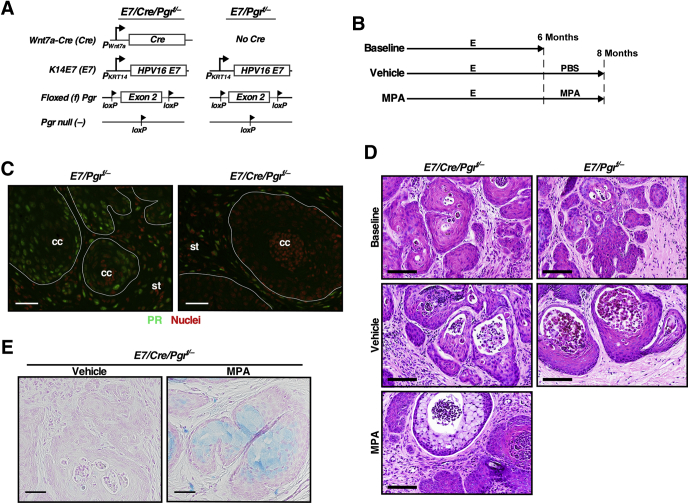
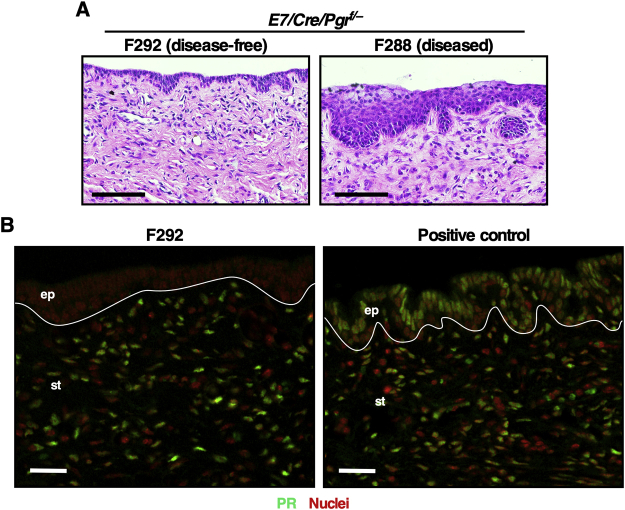
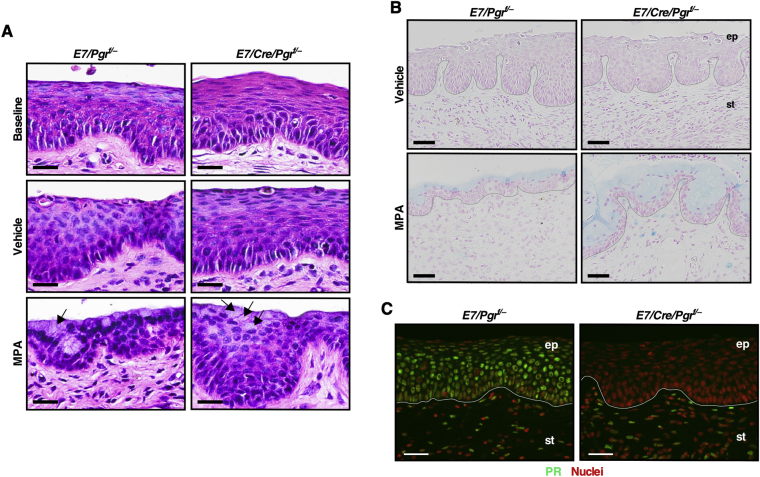
The first goal was to determine whether epithelial Pgr is necessary for MPA-mediated cervical cancer regression. The Wnt7a-Cre transgenic allele was used because it specifically ablates PR expression in the epithelium of female reproductive tracts.29 Generated genotypes were K14E7/Wnt7a-Cre/Pgrf/− and K14E7/Pgrf/−, which will be referred to hereafter as E7/Cre/Pgrf/− and E7/Pgrf/−, respectively (Figure 1A). All mice were treated with estrogen for 6 months, and each genotype was divided into three groups (Figure 1B). The baseline control group of each genotype was sacrificed immediately. Cancer incidences were 70% (n = 10) in E7/Pgrf/− and 83.3% (n = 6) in E7/Cre/Pgrf/−, which were not significantly different (Table 1). The remaining mice had high-grade CIN lesions. This was consistent with previous results showing that estrogen-induced cervical disease burden was similar between E7/Pgr+/+ and E7/Pgr−/−.20 These results indicated that most mice had cervical cancer before further treatment. The other groups of mice were further treated with PBS vehicle (vehicle group) or MPA (MPA group) for 2 additional months (Figure 1B). Cancer incidences in the vehicle group were not significantly different between E7/Pgrf/− (75%) and E7/Cre/Pgrf/− (100%) (P = 0.42) (Table 1). In the MPA group, cancer incidence in E7/Cre/Pgrf/− mice (86.7%) was significantly higher than that in E7/Pgrf/− mice (0%) (P = 1.54 × 10−6). Cancer incidences in vehicle-treated (100%) and MPA-treated E7/Cre/Pgrf/− mice (86.7%) were not significantly different (P = 1). As expected, PR was undetectable in cervical cancers in E7/Cre/Pgrf/− mice, unlike those in E7/Pgrf/− control mice (Figure 1C). These results demonstrate that PR should be expressed in cancer cells for MPA to regress cervical cancer efficiently. There was one E7/Cre/Pgrf/− mouse in the MPA group that had no cervical neoplastic diseases (Table 1). The epithelium in this mouse was hypoplastic compared with the other diseased mice in the same group (Supplemental Figure S1A). Consistent with its genotype, PR expression was absent in the epithelium of this mouse (Supplemental Figure S1B).
Figure 1.
Medroxyprogesterone acetate (MPA) fails to regress progesterone receptor (PR)–negative cervical cancer. A: Schematic show modified alleles in each genotype. B: Treatment regimens are depicted. C: PR is not expressed in cervical cancer cells in E7/Cre/Pgrf/− mice. Cancer sections from the cervical canal were stained for PR (green). Nuclei were stained with Hoechst 33258 and pseudocolored red. White lines separate cervical cancer (cc) from surrounding stroma (st). D: Shown are high-magnification images of representative hematoxylin and eosin–stained cervical cancers. Note that there were no cancers in MPA-treated E7/Pgrf/− mice. E: Cervical cancer sections were stained with Alcian blue. Nuclei were stained with nuclear fast red. Scale bars: 50 μm (C and E); 100 μm (D). E, estrogen; PBS, phosphate-buffered saline.
Table 1.
Summary of Worst Cervical Neoplastic Diseases
| Group | Genotype | Group size, n | No disease | Dysplasia only |
Cancer and dysplasia | Cancer incidence, % | ||
|---|---|---|---|---|---|---|---|---|
| CIN1 | CIN2 | CIN3 | ||||||
| Baseline | E7/Pgrf/− | 10 | 0 | 0 | 2 | 1 | 7 | 70.0 |
| E7/Cre/Pgrf/− | 6 | 0 | 0 | 1 | 0 | 5 | 83.3 | |
| Vehicle | E7/Pgrf/− | 8 | 0 | 0 | 1 | 1 | 6 | 75.0 |
| E7/Cre/Pgrf/− | 4 | 0 | 0 | 0 | 0 | 4 | 100 | |
| MPA | E7/Pgrf/− | 14 | 11 | 0 | 3 | 0 | 0 | 0∗ |
| E7/Cre/Pgrf/− | 15 | 1 | 0 | 1 | 0 | 13 | 86.7 | |
| E | E7/Pgrf/− | 8 | 0 | 0 | 0 | 0 | 8 | 100 |
| E7/Cre/Pgrf/− | 10 | 0 | 0 | 0 | 0 | 10 | 100 | |
| MPA/E | E7/Pgrf/− | 11 | 0 | 0 | 0 | 0 | 11 | 100 |
| E7/Cre/Pgrf/− | 10 | 0 | 0 | 0 | 0 | 10 | 100 | |
CIN, cervical intraepithelial neoplasia; E, estrogen; MPA, medroxyprogesterone acetate.
P < 0.001 compared with the other groups (Fisher exact test).
MPA Induces Mucinification of PR-Negative Cervical Cancer
As expected, cancers arising in baseline and vehicle groups were well differentiated and histologically similar regardless of genotypes (Figure 1D). Terminal differentiation is associated with anti-cancer activities.30 Mucinification is a form of terminal differentiation of cervical epithelial cells. Interestingly, cancers in MPA-treated E7/Cre/Pgrf/− mice had cells with clear cytoplasm and showed positive staining for Alcian blue (Figure 1, D and E), which are the hallmarks of mucinification induced by MPA.20 The epithelium in MPA-treated E7/Cre/Pgrf/− mice was dysplastic and thick but stained positive for Alcian blue (Supplemental Figure S2, A and B). PR was not expressed in epithelial cells of those mice (Supplemental Figure S2C). These results demonstrate that MPA-induced mucinification was independent of PR in cancer and epithelial cells and implicated stromal PR in this process.
Estrogen Prevents MPA-Induced Regression of Cervical Cancer
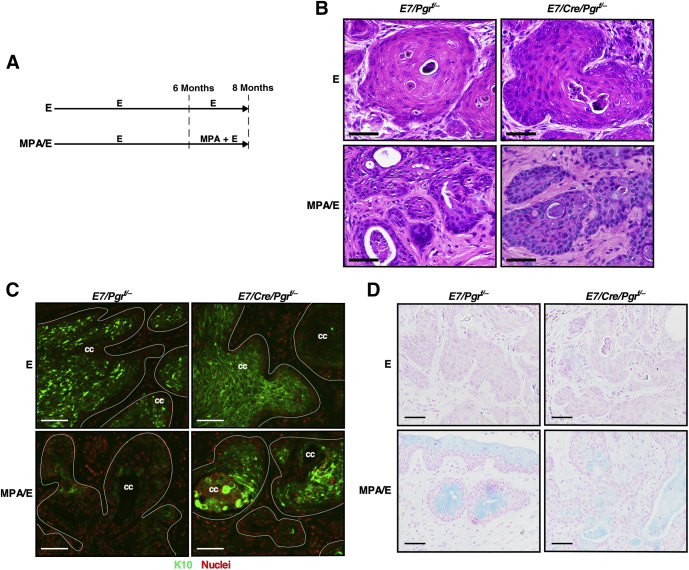
The next question was whether exogenous estrogen influences the efficacy of MPA in regressing cervical cancer. E7/Pgrf/− and E7/Cre/Pgrf/− mice were treated with estrogen for 6 months, which promoted cervical cancer at similar levels in both genotypes (Table 1). Other groups of each genotype were then further treated with MPA plus estrogen (MPA/E group) or estrogen alone (E group) for 2 additional months (Figure 2A). There was no difference in cancer incidence between E7/Pgrf/− and E7/Cre/Pgrf/− mice (100% in both genotypes) when treated with estrogen alone (E group) (Table 1). All E7/Cre/Pgrf/− mice (n = 10) treated with MPA plus estrogen had cervical cancer (MPA/E group) (Table 1). This was expected because MPA failed to regress cervical cancer in the same genotype of mice even in the absence of exogenous estrogen (MPA group) (Table 1). Cervical cancer incidence was 100% in E7/Pgrf/− mice (n = 11) treated with MPA plus estrogen (MPA/E group) (Table 1). The incidence was significantly higher than that in the same genotype of mice treated with MPA alone (MPA group; P = 2.24 × 10−7) (Table 1). These results demonstrate that exogenous estrogen suppressed the therapeutic effect of MPA.
Figure 2.
Exogenous estrogen (E) inhibits medroxyprogesterone acetate (MPA)–mediated regression of progesterone receptor–positive cervical cancer. A: The treatment regimen is shown. B: Cervical cancer sections in each group were stained with hematoxylin and eosin. C: Cervical cancer sections were stained with an anti-K10 antibody (green). Hoechst 33258–stained nuclei were pseudocolored red. White lines show the boundary of cervical cancer (cc). D: Cervical cancer sections were subjected to Alcian blue staining. Nuclei were counterstained with nuclear fast red. Scale bars = 50 μm (B–D).
MPA Deregulates Differentiation of PR+ Cervical Cancer Cells Even in the Presence of Exogenous Estrogen
Cervical cancers in E7/Pgrf/− and E7/Cre/Pgrf/− mice treated with estrogen alone (E groups) were well differentiated, as evidenced by areas of less densely distributed nuclei (Figure 2B) and strong staining for squamous differentiation marker K10 (Figure 2C). However, cancers in E7/Pgrf/− mice treated with MPA plus estrogen were poorly differentiated (Figure 2B). Also, K10 staining was dramatically reduced in these cancers (Figure 2C). K10 staining was not reduced in E7/Cre/Pgrf/− mice treated with MPA plus estrogen (MPA/E group) (Figure 2C). These results demonstrate that MPA inhibits squamous differentiation of PR-positive cervical cancer cells even in the presence of exogenous estrogen. Cervical cancers in the MPA/E group were positive for Alcian blue staining regardless of the genotype (Figure 2D), indicating that estrogen did not block MPA-induced mucinification.
MPA Inhibits Cervical Cancer Growth in the Presence of Exogenous Estrogen
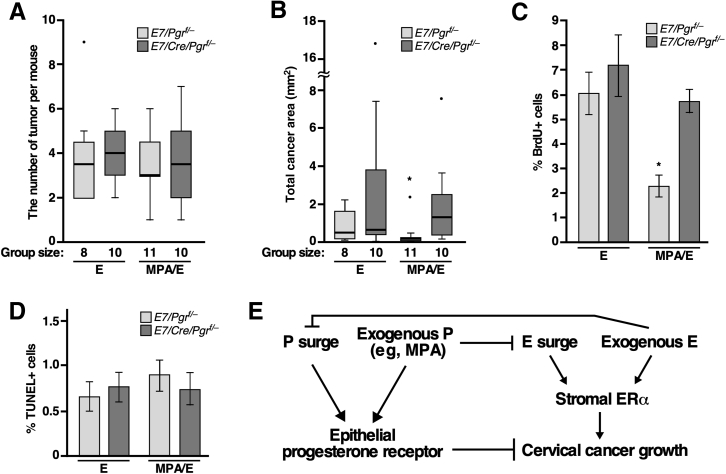
Although MPA did not reduce cancer incidence in the presence of exogenous estrogen, whether MPA had any anti-cervical cancer activity under this condition remained to be determined. Tumor multiplicity was analyzed first, and there were no differences among groups (Figure 3A). Total cancer areas were analyzed next. Cancer size was similar between E7/Pgrf/− mice (ie, PR+ cancers) and E7/Cre/Pgrf/− mice (ie, PR− cancers) in the E group (Figure 3B). It was also similar in E7/Cre/Pgrf/− mice treated with estrogen plus MPA. However, total cancer area was significantly smaller in E7/Pgrf/− mice that were treated with estrogen plus MPA compared with the other groups (Figure 3B). Cell proliferation was compared by measuring BrdU incorporation into the genomic DNA. Consistent with cancer size results, the proliferation index was significantly lower in E7/Pgrf/− mice in the MPA/E group compared with the other groups (Figure 3C). Only a small fraction (<1%) of cancer cells were positive for terminal deoxynucleotidyl transferase dUTP nick end labeling in all groups (Figure 3D), suggesting that apoptosis did not contribute to the differences in cancer size. These results support the idea that MPA blocks the growth of PR+ cervical cancer even in the presence of exogenous estrogen by inhibiting cancer cell proliferation.
Figure 3.
Medroxyprogesterone acetate (MPA) inhibits cervical cancer growth in the presence of exogenous estrogen (E). A: Tumor multipolicy in each group is shown in a box plot. There was no statistically significant difference among groups. Light and dark gray boxed areas represent E7/Pgrf/− and E7/Cre/Pgrf/− genotypes, respectively. See Figure 2A for treatment regimens for E and MPA/E groups. B: A box plot was used to show total cancer area in each group. Dots represent outliers. Note that total cancer area in E7/Pgrf/− mice in MPA/E group was significantly smaller compared with the other groups. Group sizes are indicated in the graph. C: Cervical sections were stained for bromodeoxyuridine (BrdU), and the results were quantified. More than 500 cells per cancer in average were counted. P value is compared with the other groups. D: Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay results were quantified. There was no statistically significant difference among groups. E: Model for the interplay between E and progesterone (P). See text for details. Results are shown as means ± SEM (A–D). n = 4 (C); n = 3 (D). ∗P < 0.05. ER, estrogen receptor.
Discussion
Estrogen and progesterone levels change between phases of the menstrual cycle. Estrogen levels vary nearly 10-fold among healthy women.31 In addition, there are behavioral (eg, oral contraceptive use) and pathologic (eg, obesity and polycystic ovary syndrome) conditions that affect the levels of these hormones. Furthermore, progestins, including MPA, bind not only PR but also other hormone receptors.24,32 It is important to understand the mechanism by which a progestin exerts its activity in specific tissues and whether estrogen influences the effect of progestin.
In cancer-bearing mice treated with MPA alone, cervical cancer regressed completely in E7/Pgrf/− mice, but not in E7/Cre/Pgrf/− mice (Table 1). These results demonstrated that MPA requires cancer epithelial PR to promote cervical cancer regression. It is consistent with the previously published results supporting that epithelial PR is a ligand-dependent tumor suppressor in cervical cancer.33 Intriguingly, one MPA-treated E7/Cre/Pgrf/− mouse had no disease (Table 1), and its epithelium was hypoplastic (Supplemental Figure S1A). Stromal PR did not appear to be responsible for these phenotypes because MPA promotes cervical hypoplasia in E7/Pgr−/− mice in which PR is neither expressed in epithelium nor in stroma.21 These observations raise a possibility that MPA may be effective in treating a small fraction of PR-negative cervical cancers. In this regard, a small number of PR-negative endometrial cancers have responded to MPA therapy.34,35
The current results do not rule out the possibility that PR expressed in cancer stroma is also necessary for the anti-cervical cancer activity of MPA. In this regard, MPA promoted mucinification of PR-negative cervical cancer cells in E7/Cre/Pgrf/− mice (Figure 1E) but not in in E7/Pgr−/− mice.21 PR is expressed in cancer stroma in E7/Cre/Pgrf/− mice but not in E7/Pgr−/− mice. These results suggest that stromal PR, rather than epithelial PR, mediates MPA-induced mucinification of cervical cancer cells. Differentiation of cancer cells is the major mechanism of cancer regression by retinoids.30 High levels of stromal PR are associated with better survival of cervical cancer patients.36 Although these results suggest that stromal PR contributes to MPA's therapeutic effect, it is obviously insufficient because MPA had no impact on cancer growth in E7/Cre/Pgrf/− mice (Figure 1E and Table 1). These observations further support the model that PR and estrogen receptor α function primarily in cervical cancer cells and cancer stroma, respectively.37
Exogenous estrogen inhibited the therapeutic efficacy of MPA in Pgr-intact E7/Pgrf/− mice (Table 1). However, MPA still suppressed cervical cancer growth in the presence of exogenous estrogen (Figure 3, B and C). MPA regresses CIN lesions and prevents cervical cancer development in the presence of exogenous estrogen.21 The regression of CIN occurs in almost all mice when treated with MPA for 3 months but only in 40% of mice when treated for 2 months. It is possible that cervical cancer regresses completely even in the presence of exogenous estrogen if treated with MPA for a longer period. Nonetheless, the current results indicate that exogenous estrogen attenuates, but not abolishes, the anti-cervical cancer activity of MPA. We propose that the net effect of tumor-suppressive function of progesterone and epithelial PR and protumorigenic action of estrogen and stromal estrogen receptor α determines whether cervical cancer grows or regresses (Figure 3E).18,19,33 If correct, an increased MPA dosage would promote cervical cancer regression even if estrogen levels are high. It is plausible that both PR activation and estrogen surge inhibition are necessary for MPA to block cervical cancer growth efficiently (Figure 3E). Consistently, the ablation of epithelial PR expression or the estrogen supplement inhibited MPA-induced cancer regression (Table 1).
In summary, these results demonstrate that epithelial PR is necessary for MPA's therapeutic efficacy and that exogenous estrogen attenuates it. If translatable to patients, MPA would be effective in treating PR+ cervical cancers, which account for 20% to 40% of all cases.38, 39, 40 Data from these studies suggest that MPA is more efficient during early follicular phase and late luteal phase at which estrogen levels are low. These results will help design biomarker-driven clinical trials and interpret their outcomes.
Footnotes
Supported in part by NIH grant R01 CA188646 (S.-H.C.), Cancer Prevention and Research Institute of Texas grant RP180275 (S.-H.C.), and University of Houston Large Core Equipment grant (S.-H.C.).
S.B. and F.F.M. contributed equally to this work.
Disclosures: None declared.
Current address of Y.P., Department of Molecular and Cellular Biology, Baylor College of Medicine, Houston, TX.
Supplemental material for this article can be found at http://doi.org/10.1016/j.ajpath.2021.10.008.
Author Contributions
F.F.M. and S.-H.C. conceived and designed experiments; S.B., F.F.M., E.U., and Y.P. performed experiments; S.B., F.F.M., E.U., Y.P., and S.-H.C. analyzed data and drafted and reviewed the manuscript; S.-H.C. secured funding and had full access to all the data in the study.
Supplemental Data
Supplemental Figure S1.
A: Cervical epithelial sections in E7/Cre/Pgrf/− mice were stained with hematoxylin and eosin. Note that the epithelium in the disease-free mouse is hypoplastic, unlike a diseased mouse. F288 and F292 are histology sample identifiers. B: The disease-free mouse sample shown in A was stained for progesterone receptor (green). A tissue section from E7/Cre/Pgrf/− mice was used as positive control. Nuclei were counterstained with Hoechst 33258 and pseudocolored red. White lines separate epithelium (ep) and stroma (st). Scale bars: 100 μm (A); 30 μm (B).
Supplemental Figure S2.
A: Shown are representative images of hematoxylin and eosin–stained cervical epithelia. Note that E7/Cre/Pgrf/− mice in medroxyprogesterone acetate (MPA) group had epithelial cells with clear cytoplasm similar to E7/Pgrf/− mice (black arrows). B: Cervical sections were stained with Alcian blue. Nuclei were counterstained with nuclear fast red. Black lines separate epithelium (ep) and stroma (st). C: Cancer sections from cervical canal were stained for progesterone receptor (green). Nuclei were counterstained with Hoechst 33258 and pseudocolored red. White lines separate ep and st. Scale bars: 25 μm (A); 50 μm (B and C).
References
- 1.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans Human papillomaviruses. IARC Monogr Eval Carcinog Risks Hum. 2007;90:1–636. [PMC free article] [PubMed] [Google Scholar]
- 2.Downs L.S., Smith J.S., Scarinci I., Flowers L., Parham G. The disparity of cervical cancer in diverse populations. Gynecol Oncol. 2008;109:22–30. doi: 10.1016/j.ygyno.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Larson H.J., Brocard P., Garnett G. The India HPV-vaccine suspension. Lancet. 2010;376:572–573. doi: 10.1016/S0140-6736(10)60881-1. [DOI] [PubMed] [Google Scholar]
- 4.Strohl A.E., Mendoza G., Ghant M.S., Cameron K.A., Simon M.A., Schink J.C., Marsh E.E. Barriers to prevention: knowledge of HPV, cervical cancer, and HPV vaccinations among African American women. Am J Obstet Gynecol. 2015;212:65.e1–65.e5. doi: 10.1016/j.ajog.2014.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferlay J., Colombet M., Soerjomataram I., Mathers C., Parkin D.M., Pineros M., Znaor A., Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 6.Moody C.A., Laimins L.A. Human papillomavirus oncoproteins: pathways to transformation. Nat Rev Cancer. 2010;10:550–560. doi: 10.1038/nrc2886. [DOI] [PubMed] [Google Scholar]
- 7.Castle P.E., Rodriguez A.C., Burk R.D., Herrero R., Wacholder S., Alfaro M., Morales J., Guillen D., Sherman M.E., Solomon D., Schiffman M. Short term persistence of human papillomavirus and risk of cervical precancer and cancer: population based cohort study. BMJ. 2009;339:b2569. doi: 10.1136/bmj.b2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCredie M.R., Sharples K.J., Paul C., Baranyai J., Medley G., Jones R.W., Skegg D.C. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. 2008;9:425–434. doi: 10.1016/S1470-2045(08)70103-7. [DOI] [PubMed] [Google Scholar]
- 9.DiPaolo J.A., Woodworth C.D., Popescu N.C., Notario V., Doniger J. Induction of human cervical squamous cell carcinoma by sequential transfection with human papillomavirus 16 DNA and viral Harvey ras. Oncogene. 1989;4:395–399. [PubMed] [Google Scholar]
- 10.Moreno V., Bosch F.X., Munoz N., Meijer C.J., Shah K.V., Walboomers J.M., Herrero R., Franceschi S. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: the IARC multicentric case-control study. Lancet. 2002;359:1085–1092. doi: 10.1016/S0140-6736(02)08150-3. [DOI] [PubMed] [Google Scholar]
- 11.Munoz N., Franceschi S., Bosetti C., Moreno V., Herrero R., Smith J.S., Shah K.V., Meijer C.J., Bosch F.X. Role of parity and human papillomavirus in cervical cancer: the IARC multicentric case-control study. Lancet. 2002;359:1093–1101. doi: 10.1016/S0140-6736(02)08151-5. [DOI] [PubMed] [Google Scholar]
- 12.Weikum E.R., Liu X., Ortlund E.A. The nuclear receptor superfamily: a structural perspective. Protein Sci. 2018;27:1876–1892. doi: 10.1002/pro.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung S.H. Targeting female hormone receptors as cervical cancer therapy. Trends Endocrinol Metab. 2015;26:399–401. doi: 10.1016/j.tem.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riley R.R., Duensing S., Brake T., Munger K., Lambert P.F., Arbeit J.M. Dissection of human papillomavirus E6 and E7 function in transgenic mouse models of cervical carcinogenesis. Cancer Res. 2003;63:4862–4871. [PubMed] [Google Scholar]
- 15.Shai A., Brake T., Somoza C., Lambert P.F. The human papillomavirus E6 oncogene dysregulates the cell cycle and contributes to cervical carcinogenesis through two independent activities. Cancer Res. 2007;67:1626–1635. doi: 10.1158/0008-5472.CAN-06-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brake T., Connor J.P., Petereit D.G., Lambert P.F. Comparative analysis of cervical cancer in women and in a human papillomavirus-transgenic mouse model: identification of minichromosome maintenance protein 7 as an informative biomarker for human cervical cancer. Cancer Res. 2003;63:8173–8180. [PubMed] [Google Scholar]
- 17.Balsitis S., Dick F., Dyson N., Lambert P.F. Critical roles for non-pRb targets of human papillomavirus type 16 E7 in cervical carcinogenesis. Cancer Res. 2006;66:9393–9400. doi: 10.1158/0008-5472.CAN-06-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung S.H., Shin M.K., Korach K.S., Lambert P.F. Requirement for stromal estrogen receptor alpha in cervical neoplasia. Horm Cancer. 2013;4:50–59. doi: 10.1007/s12672-012-0125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Son J., Park Y., Chung S.H. Epithelial oestrogen receptor alpha is dispensable for the development of oestrogen-induced cervical neoplastic diseases. J Pathol. 2018;245:147–152. doi: 10.1002/path.5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoo Y.A., Son J., Mehta F.F., DeMayo F.J., Lydon J.P., Chung S.H. Progesterone signaling inhibits cervical carcinogenesis in mice. Am J Pathol. 2013;183:1679–1687. doi: 10.1016/j.ajpath.2013.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baik S., Mehta F.F., Chung S.H. Prevention of cervical cancer by medroxyprogesterone acetate through progesterone receptor in an HPV transgenic mouse model. Am J Pathol. 2019;189:2463–2472. doi: 10.1016/j.ajpath.2019.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta F.F., Baik S., Chung S.H. Recurrence of cervical cancer and its resistance to progestin therapy in a mouse model. Oncotarget. 2017;8:2372–2380. doi: 10.18632/oncotarget.13676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Govender Y., Avenant C., Verhoog N.J., Ray R.M., Grantham N.J., Africander D., Hapgood J.P. The injectable-only contraceptive medroxyprogesterone acetate, unlike norethisterone acetate and progesterone, regulates inflammatory genes in endocervical cells via the glucocorticoid receptor. PLoS One. 2014;9:e96497. doi: 10.1371/journal.pone.0096497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selman P.J., Wolfswinkel J., Mol J.A. Binding specificity of medroxyprogesterone acetate and proligestone for the progesterone and glucocorticoid receptor in the dog. Steroids. 1996;61:133–137. doi: 10.1016/0039-128x(95)00216-d. [DOI] [PubMed] [Google Scholar]
- 25.Fernandez-Valdivia R., Jeong J., Mukherjee A., Soyal S.M., Li J., Ying Y., Demayo F.J., Lydon J.P. A mouse model to dissect progesterone signaling in the female reproductive tract and mammary gland. Genesis. 2010;48:106–113. doi: 10.1002/dvg.20586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song S., Pitot H.C., Lambert P.F. The human papillomavirus type 16 E6 gene alone is sufficient to induce carcinomas in transgenic animals. J Virol. 1999;73:5887–5893. doi: 10.1128/jvi.73.7.5887-5893.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winuthayanon W., Hewitt S.C., Orvis G.D., Behringer R.R., Korach K.S. Uterine epithelial estrogen receptor alpha is dispensable for proliferation but essential for complete biological and biochemical responses. Proc Natl Acad Sci U S A. 2010;107:19272–19277. doi: 10.1073/pnas.1013226107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franco H.L., Rubel C.A., Large M.J., Wetendorf M., Fernandez-Valdivia R., Jeong J.W., Spencer T.E., Behringer R.R., Lydon J.P., Demayo F.J. Epithelial progesterone receptor exhibits pleiotropic roles in uterine development and function. FASEB J. 2012;26:1218–1227. doi: 10.1096/fj.11-193334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehta F.F., Son J., Hewitt S.C., Jang E., Lydon J.P., Korach K.S., Chung S.H. Distinct functions and regulation of epithelial progesterone receptor in the mouse cervix, vagina, and uterus. Oncotarget. 2016;7:17455–17467. doi: 10.18632/oncotarget.8159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang X.H., Gudas L.J. Retinoids, retinoic acid receptors, and cancer. Annu Rev Pathol. 2011;6:345–364. doi: 10.1146/annurev-pathol-011110-130303. [DOI] [PubMed] [Google Scholar]
- 31.Schuring A.N., Kelsch R., Pierscinski G., Nofer J.R. Establishing reference intervals for sex hormones on the analytical platforms Advia Centaur and Immulite 2000XP. Ann Lab Med. 2016;36:55–59. doi: 10.3343/alm.2016.36.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beck C.A., Estes P.A., Bona B.J., Muro-Cacho C.A., Nordeen S.K., Edwards D.P. The steroid antagonist RU486 exerts different effects on the glucocorticoid and progesterone receptors. Endocrinology. 1993;133:728–740. doi: 10.1210/endo.133.2.8344212. [DOI] [PubMed] [Google Scholar]
- 33.Park Y., Baik S., Ho C., Lin C.Y., Chung S.H. Progesterone receptor is a haploinsufficient tumor-suppressor gene in cervical cancer. Mol Cancer Res. 2021;19:42–47. doi: 10.1158/1541-7786.MCR-20-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ehrlich C.E., Young P.C., Stehman F.B., Sutton G.P., Alford W.M. Steroid receptors and clinical outcome in patients with adenocarcinoma of the endometrium. Am J Obstet Gynecol. 1988;158:796–807. doi: 10.1016/0002-9378(88)90075-0. [DOI] [PubMed] [Google Scholar]
- 35.Thigpen J.T., Brady M.F., Alvarez R.D., Adelson M.D., Homesley H.D., Manetta A., Soper J.T., Given F.T. Oral medroxyprogesterone acetate in the treatment of advanced or recurrent endometrial carcinoma: a dose-response study by the Gynecologic Oncology Group. J Clin Oncol. 1999;17:1736–1744. doi: 10.1200/JCO.1999.17.6.1736. [DOI] [PubMed] [Google Scholar]
- 36.Hong M.K., Wang J.H., Su C.C., Li M.H., Hsu Y.H., Chu T.Y. Expression of estrogen and progesterone receptor in tumor stroma predicts favorable prognosis of cervical squamous cell carcinoma. Int J Gynecol Cancer. 2017;27:1247–1255. doi: 10.1097/IGC.0000000000001004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee S.A., Baik S., Chung S.H. Functional roles of female sex hormones and their nuclear receptors in cervical cancer. Essays Biochem. 2021;65:941–950. doi: 10.1042/EBC20200175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fonseca-Moutinho J.A., Cruz E., Carvalho L., Prazeres H.J., de Lacerda M.M., da Silva D.P., Mota F., de Oliveira C.F. Estrogen receptor, progesterone receptor, and bcl-2 are markers with prognostic significance in CIN III. Int J Gynecol Cancer. 2004;14:911–920. doi: 10.1111/j.1048-891X.2004.14529.x. [DOI] [PubMed] [Google Scholar]
- 39.Fujiwara H., Tortolero-Luna G., Mitchell M.F., Koulos J.P., Wright T.C., Jr. Adenocarcinoma of the cervix: expression and clinical significance of estrogen and progesterone receptors. Cancer. 1997;79:505–512. [PubMed] [Google Scholar]
- 40.Pyeon D., Newton M.A., Lambert P.F., den Boon J.A., Sengupta S., Marsit C.J., Woodworth C.D., Connor J.P., Haugen T.H., Smith E.M., Kelsey K.T., Turek L.P., Ahlquist P. Fundamental differences in cell cycle deregulation in human papillomavirus-positive and human papillomavirus-negative head/neck and cervical cancers. Cancer Res. 2007;67:4605–4619. doi: 10.1158/0008-5472.CAN-06-3619. [DOI] [PMC free article] [PubMed] [Google Scholar]