Abstract
Alterations in ocular blood flow have been identified as important risk factors for the onset and progression of numerous diseases of the eye. In particular, several population-based and longitudinal-based studies have provided compelling evidence of hemodynamic biomarkers as independent risk factors for ocular disease throughout several different geographic regions. Despite this evidence, the relative contribution of blood flow to ocular physiology and pathology in synergy with other risk factors and comorbidities (e.g., age, gender, race, diabetes and hypertension) remains uncertain. There is currently no gold standard for assessing all relevant vascular beds in the eye, and the heterogeneous vascular biomarkers derived from multiple ocular imaging technologies are non-interchangeable and difficult to interpret as a whole. As a result of these disease complexities and imaging limitations, standard statistical methods often yield inconsistent results across studies and are unable to quantify or explain a patient’s overall risk for ocular disease.
Combining mathematical modeling with artificial intelligence holds great promise for advancing data analysis in ophthalmology and enabling individualized risk assessment from diverse, multi-input clinical and demographic biomarkers. Mechanism-driven mathematical modeling makes virtual laboratories available to investigate pathogenic mechanisms, advance diagnostic ability and improve disease management. Artificial intelligence provides a novel method for utilizing a vast amount of data from a wide range of patient types to diagnose and monitor ocular disease. This article reviews the state of the art and major unanswered questions related to ocular vascular anatomy and physiology, ocular imaging techniques, clinical findings in glaucoma and other eye diseases, and mechanistic modeling predictions, while laying a path for integrating clinical observations with mathematical models and artificial intelligence. Viable alternatives for integrated data analysis are proposed that aim to overcome the limitations of standard statistical approaches and enable individually tailored precision medicine in ophthalmology.
Keywords: Ocular blood flow, Glaucoma, Mathematical models, Vascular risk factors, Artificial intelligence
1. Introduction
Alterations in ocular blood flow and vascular regulation have been associated with many pathological conditions of the eye. Hemodynamic deficits have been identified extensively in glaucomatous optic neuropathy and have been investigated in patients with age-related macular degeneration (AMD), diabetic retinopathy (DR), retinal vein occlusion (RVO), retinal artery occlusion (RAO), and non-arteritic ischemic optic neuropathy (NAION) (Weinreb and Harris, 2009; Arciero et al., 2019; Prada et al., 2019a). Impaired blood flow autoregulation, increased intraocular and mean arterial pressures, and reduced oxygen delivery to tissue are some of the mechanisms hypothesized to cause these hemo-dynamic deficits observed in the clinic. However, the extent to which vascular factors contribute to the onset or progression of the disease and the mechanisms by which these factors act must be elucidated in order to have any impact on the diagnosis and treatment strategies for these diseases.
Ocular imaging techniques have advanced dramatically in recent decades, making available a vast amount of new ocular blood flow data from across many different geographical regions for multiple eye diseases (Harris et al., 2010). However, the interpretation of these data remains very challenging for numerous reasons. First, clinical and experimental measurements are the result of complex interactions among multiple local and systemic factors, and so isolating the source for the change in one biomarker is nearly impossible. This complexity is confounded by patient-specific factors such as race, age, family history, and co-morbidities such as obesity, diabetes and systemic hypertension. Second, using animal models as surrogates for human research is highly problematic due to vastly differing size and species-dependent characteristics of ocular structures and physiology. Third, statistics, machine learning, deep learning, and artificial intelligence (AI) are data-driven techniques that identify trends and predict outcomes but do not explain the mechanisms underlying the data in order to suggest a more targeted treatment approach (Hogarty et al., 2019; Obermeyer and Emanuel, 2016; Ting et al., 2019).
Mechanism-driven mathematical modeling has emerged as an interdisciplinary tool that can account for multiple factors defining a system and unravel the effects of individual or combined components on the system. To date, mechanism-driven models have been used to study many components of the ocular circulation, including blood flow regulation mechanisms, oxygen transport, venous collapsibility, and biomechanical responses to changes in intraocular pressure and cerebrospinal fluid pressure. These models have the capability to reveal complex relationships among blood pressure (BP), hemodynamics, and oxygenation. Still, these models alone are not sufficient to account for all factors that vary among individuals (such as age, gender, race and family history) or to definitively resolve clinical questions such as the controversy of whether vascular deficits are primary or secondary to retinal ganglion cell death and tissue loss in glaucoma. Rather, combining longitudinal data collection using advanced ocular imaging techniques with mechanism-based mathematical modeling and databased methods (such as AI) is the necessary paradigm that will inspire new treatments and enable precision medicine.
The proposed paradigm calls for active collaborations across medicine, mathematics, engineering, physics and computer science. This article aims to facilitate such interdisciplinary collaborations by providing a critical review of the state of the art and major unanswered questions related to ocular vascular anatomy and physiology (section 2), ocular imaging techniques (Section 3), clinical findings in glaucoma and other eye diseases (Sections 4 and 5), and mechanistic modeling predictions (Section 6), while laying a path for integrating clinical observations with mathematical models and artificial intelligence (Section 7).
This review employs a comprehensive literature search using the OVID Medline search engine, the Web of Science database, and all available library databases capable of reference cross-matching to obtain and review for inclusion all relevant peer reviewed published articles. While not every article written on the topic(s) of ocular blood flow and glaucoma and/or ophthalmic disease can be included, the authors vetted all relevant articles examining and discussing each, reaching consensus on inclusion of those most pertinent to this review. In addition, the majority of previous Progress in Retinal Eye Research manuscripts on related topics are included in this review.
2. Anatomy and physiology of the ocular vasculature
As with any functional organ system, a healthy vasculature is required to maintain homeostasis and protect against disease in the eye. Unique to the eye, the ocular vasculature is structured in a very complex way to nourish the tissues of the eye without interfering with visual function (Prada et al., 2019b). Some components of the eye are very rich in vascular supply, such as the choroid, while other areas are completely avascular, such as the vitreous humor and lens. Advances in ocular imaging have improved the understanding of this complex vascular architecture, but there are still many areas that are not fully understood.
The vascular supply to the retina has been well-established. The central retinal artery (CRA) supplies the inner two-thirds of the retina, while the choriocapillaries, which arise from the posterior ciliary arteries, supply the deeper outer layers. The CRA branches into four major arteries, each of which supplies approximately one quadrant of the retina (Harris et al., 2010). Retinal arterioles and venules lie within the superficial retinal nerve fiber layer, while the retinal capillaries form several layers parallel to the retinal surface. The retina also exhibits a superficial network of fine capillaries in the peripapillary region, known as the radial peripapillary capillary plexus (RPCP). Three additional retinal vascular networks run through the retinal thickness and exhibit lobular configurations as opposed to those in the RPCP that run parallel to retinal nerve fiber layer axons. The most superficial network, known as the superficial vascular plexus (SVP), is supplied by the CRA and is composed of arteries, arterioles, capillaries, venules, and veins located primarily in the ganglion cell layer. The two deeper networks, the intermediate capillary plexus and deep capillary plexus (DCP), are supplied by vertical anastomoses from the SVP. Cellular functionality, regulation of retinal hemodynamics, and their involvement in ocular pathologies are outlined in detail in two previous Progress in Retinal Eye Research publications (Poumaras et al., 2008) and (Kur et al., 2012).
Recent literature has conflicted over whether the SVP, intermediate capillary plexus, and DCP run in parallel or in series. A study utilizing projection-resolved optical coherence tomography angiography demonstrated the deeper intermediate capillary plexus and DCP to be supplied by vertical anastomoses from the SVP, supporting the theory of a parallel connection between the networks (Campbell et al., 2017). In contrast, a study using high-density confocal microscopy revealed that the majority of arterial flow runs through the SVP where it splits to RPCP on one side and to the serially arranged intermediate capillary plexus and DCP on the other side (Fouquet et al., 2017). Furthermore, a recent study utilizing optical coherence tomography angiography (OCTA) found that all collateral vessels associated with retinal vein occlusion run through the DCP, suggesting that venous drainage occurs primarily through the DCP and supporting the theory of serial arrangement (Freund et al., 2018). Establishing how these three capillary networks are connected is important since it would help explain why the networks respond differently to physiological changes. Mathematical modeling could be employed to simulate the effect of different network arrangements on hemodynamic responses to healthy and pathologic conditions (Chiaravalli, 2018; Chiaravalli et al., 2019); such theoretical results could help generate more informed hypotheses regarding capillary arrangements that could be confirmed or refuted using additional imaging techniques.
Venous drainage from the eye primarily occurs through the central retinal vein (CRV) and the vortex veins. These veins merge with the superior and inferior ophthalmic veins that drain into the cavernous sinus, the pterygoid venous plexus, and the facial vein. It is not fully understood whether valves exist in these veins or not. The lack of valves has frequently been stated as a reason for the spread of infection from the mid-face to the cavernous sinus. However, evidence has demonstrated the existence of valves in the superior ophthalmic vein, its two main tributaries, and the facial vein (Zhang and Stringer, 2010). The spread of infection would therefore result from communications between the facial vein and cavernous sinus as well as the direction of blood flow rather than the absence of venous valves.
Despite the advances in imaging, several questions remain open regarding the anatomy and physiology of other ocular vascular beds. For example, it remains unclear whether the anastomotic blood exchange in the ring of Zinn Haller can compensate for an insufficiency of a single posterior ciliary artery, which helps to supply blood to the prelaminar and laminar portion of the optic nerve head (Pasquale et al., 2009). Furthermore, it is poorly understood whether there are anastomoses between the capillary or pre-capillary bed of the intra-laminar region, the pre-laminar region and the retinal nerve fiber layer region. If there are such anastomoses, it would be clinically important to identify whether they are fully or partially functional in the case of a sudden occlusion at the pre-capillary or intra-capillary level. A similar uncertainty remains regarding the anastomotic blood exchange between the choroidal vascular bed and the vascular system of the ciliary body (Pasquale et al., 2009). The description of choroidal ganglion cells has led to a focus on the physiological and pathophysiological role of these cells on the regulation of choroidal blood circulation (May et al., 2004).
One impediment to fully understanding the vascular mechanisms involved in ocular pathologies is the highly complex and intertwined heterogeneous anatomical architecture and physiology (Bharadwaj et al., 2013; Zhang., 1994). Within the retina, two separate vascular systems provide nourishment to different retinal regions: the CRA and its branches provide blood supply to the inner retina (autoregulated), while the choroid nourishes the outer retina with sympathetic innervation. The structure of the retinal vessels arising from the CRA is particularly complex, with different anatomical characteristics, such as dichotomous or sidearms type of branching, depending of the region considered. The number of layers of capillaries also varies in the different retinal regions, thus increasing the complexity of the anatomy of the ocular vasculature. Similarly, the nature of the anatomy of the choroidal vascular supply is composite, with various vessels’ arrangements, concentration and diameters in the different areas (Zhang, 1994). The complex nature of the anatomy of the retinal vascularization is reflected in the multiple characteristics of its functional aspects. In fact, the retinal vessels are lined by endothelial cells that are responsible both for the oxygenation and nourishment of the retinal tissues, and for its protection via the constitution of the blood-retinal barrier (Bharadwaj et al., 2013). A significant limitation to understanding ocular hemodynamics and eye disease stems from the inaccessibility and complexity of inner-ocular vascular tissue.
In addition to the multifaceted anatomical structure and separate, yet interwoven, physiological controls is the highly varied modalities for quantifying hemodynamic biomarkers and their significant technological limitations. Quantifying blood flow in real time and the physiological response to perturbations and disease is significantly limited in humans. As no gold standard exists for assessing all relevant vascular beds and tissues, diverse biomarkers from a vascular tissue are often presented in isolation as a surrogate for ocular blood flow. In reality, they represent only one facet of the ocular vasculature, and often are limited by technological and methodological constraints. Such limitations include the assessment of blood velocities instead of actual flow and the small and often highly isolated sampling areas. Some vascular biomarkers within the eye have been well established in relationship to ocular pathologies such as glaucoma, especially within the retina, while other vascular tissue remains largely unspecified in human health and disease. The ability to integrate these diverse biomarkers into a single map of vascular health represents a critical goal for future research. For instance, in glaucoma it is not well understood how a reduction in inner retinal flow by a certain percentage may be offset by a simultanious increase in choroidal and/or ONH circulation. The use of mathematical molding which accounts for multi-input biomarkers and artificial intelligence applications may allow for a more global assessment of vascular health and better define its involvement in ophthalmic disease.
3. Methods of observation of ocular blood flow
The ability to quantify biomarkers accurately from all relevant ocular vascular beds in ophthalmic disease remains challenging due to the technical limitations of imaging technologies. Various imaging modalities (e.g., Laser Doppler, Laser Speckle, ultrasonography) have been employed in an attempt to analyze various aspects of retinal, retrobulbar, optic nerve head (ONH), and choroidal blood flow, but there is no current gold standard for assessing all relevant vascular beds of the eye. Ocular hemodynamic biomarkers are often limited to measures of blood flow velocity (color Doppler imaging), blood flow using arbitrary units (confocal scanning laser Doppler imaging), or circulation time within certain sizes of blood vessels (Schmetterer and Garhofer, 2007; Weinreb and Harris, 2009; Wei et al., 2018). These various vascular biomarkers are often interpreted as surrogates of vascular health and/or metabolism; however, each modality outcome has its own specificity and significant limitations (SchmettererPopulation-based studies evaluating and Garhofer, 2007; Weinreb and Harris, 2009). In the following subsections, data from imaging modalities assessing retinal, retrobulbar, and ONH flow and their limitations are discussed.
3.1. Imaging of the retina
Fluorescein angiography (FA) is one of the primary modalities used to assess the retinal vasculature, which yields measurements of retinal circulation time and arteriovenous passage time. FA has the significant limitation of being invasive with severe adverse allergic reactions occurring in some patients. Studies have shown that retinal circulation time is prolonged in ischemic retinal disease and glaucoma (Bertram et al., 1991; Plange et al., 2008; Osamura et al., 2017). Importantly, Schulte et al. utilized suction cups to raise intraocular pressure (IOP) and found that arteriovenous passage time increased significantly from 1.6 ± 0.4 to 3.0 ± 0.8 s (P < 0.01). The authors suggested their results indicate an insufficiency of retinal autoregulation after an acute rise in IOP, even in healthy subjects when IOP reaches levels of 30 mmHg or greater (Schulte et al., 1996).
Other retinal hemodynamic assessment technologies include confocal scanning laser Doppler flowmetry, which yields measures of the retinal microcirculation with limitation of the analysis being measured in arbitrary units, and the retinal functional imager (RFI), which yields measures of blood velocity of medium-sized vessels (Nelson et al., 2005). Using Heidelberg confocal scanning laser flowmetry, blood flow in the neuroretinal rim was found to correspond to regional visual field defects in NTG patients, and reductions in blood flow were associated with reductions in function (Sato et al., 2006). Laser Speckle Flowgraphy (LSFG) is a technique for measuring the relative flow velocity of red blood cells in the retina and ONH (Fercher and Briers, 1981). While capable of analyzing relative changes in blood flow over time, historically LSFG has not been as effective for comparison among patients and is limited to relative flow velocity. However, more recent LSFG analysis techniques have shown usability for interindividual and intergroup comparisons (Aizawa et al., 2014). Recently, LSFG-derived tissue mean blur rate was found to correlate with OCTA-assessed circumpapillary vessel density in the outer retinal layer with significant differences between mild glaucoma and healthy controls (Kohmoto et al., 2019). LSFG has also been utilized for ONH assessment, whose outcomes are further discussed below. Retinal vessel width has been shown to be a useful indicator of vascular compromise in relation to visual field loss (Sugiyama et al., 2000). Novel advancements in integrating Doppler functionality in OCT have recently provided non-invasive and higher resolution imaging of the retinal vasculature as detailed by Leitgeb et al. (2014), with a noted limitation of fully understanding Doppler angle and its influence on device outcomes. More information on these and other modalities are available in a previous Progress in Retinal and Eye Research article (Harris et al., 1999) and their findings in glaucoma are further detailed in the World Glaucoma Association Consensus Series 6 publication (Weinreb and Harris, 2009).
3.2. Imaging of the retrobulbar blood supply
Color Doppler Imaging (CDI) uses ultrasound Doppler to quantify blood flow velocity and to calculate downstream vascular resistance within the retrobulbar blood vessels (Stalmans et al., 2011). Currently, CDI is the only imaging modality used to assess large ocular blood vessels such as the ophthalmic artery, central retinal artery and short posterior ciliary arteries. The outcomes of CDI include pulsatility index (PI), peak systolic velocity (PSV) and end diastolic velocity (EDV); these provide a means to calculate a vascular resistance index (RI), which was first described by Pourcelot (1974)(. Many associated analyses have found CDI biomarkers to be reduced in glaucoma patients compared to controls (Weinreb and Harris, 2009). For example, correlation analysis of retinal nerve fiber has been found to be in positive association with CRA EDV (r = 0.395; p < 0.05) and CRA PI (r = 0.403; p < 0.05) in OAG subjects but not in healthy controls (Januleviciene et al., 2008). Garhöfer et al. found blood flow velocities in the OA, CRA, and short posterior ciliary arteries to be significantly reduced in OAG patients compared with controls (P < 0.01 in all vessels) and further identified that the correlation between mean flow velocity and mean arterial BP in the CRA was stronger in OAG subjects than in controls (Garhöfer et al., 2010).
CDI has also been used to show that glaucoma patients exhibit faulty autoregulation of central retinal artery blood flow during posture change. When changing from the upright to supine posture, Evans et al. found normal subjects demonstrated a significant increase in OA EDV (p = 0.016) and significant decrease in OA RI (p = 0.0006) and CRA RI (p = 0.016), while glaucoma patients demonstrated similar changes in OA measures of EDV (p = 0.02) and RI (p = 0.04) but no change in CRA biomarkers (Evans et al., 1999). These findings suggest that the relationship between downstream vascular resistance in blood vessels that perfuse the retina and the larger ophthalmic branch of the internal carotid artery may be altered in certain glaucoma patients. Faulty autoregulation of retinal blood flow to baroreceptor response and/or chronic constriction of the blood supply to the anterior retina may be present in glaucoma patients. In a previous study (Sines et al., 2007) glaucoma and ocular hypertensive subjects demonstrated significantly different responses to hypercapnia. Specifically, ocular hypertensive patients demonstrated normal vasodilatory response during hypercapnia with increased volumetric blood flow to the retina while patients with OAG did not demonstrate this response. Together these data show significant differences to known perturbations of vascular reactivity in glaucoma subjects highlighting possible vasospasm at or downstream from the CRA resulting in decreased volumetric blood flow to the retina during physiological perturbation.
Dozens of other small and pilot studies have identified retrobulbar blood flow biomarkers to be lower in glaucomatous patients than nonglaucomatous subjects (Weinreb and Harris, 2009). Furthermore, computer-aided analysis of CDI waveforms has enabled the identification of novel waveform parameters that differentiate among different demographics and disease severity of OAG patients (Carichino et al., 2019). The great advantages of CDI are that it is ubiquitous in large hospitals, does not rely on clear media or fixation, and is completely non-invasive. Its main limitation is the lack of ability to quantify blood flow, as most CDI techniques do not assess blood vessel diameter. CDI also requires a skilled ultra-sonographer, since angle corrections are often made for certain retrobulbar blood vessels such as the ophthalmic artery and inter-observer variations in technique may alter outcomes (Rusia et al., 2011; Stalmans et al., 2011).
3.3. Imaging of the optic nerve head
Imaging of the ONH has historically proven to be difficult with many common misconceptions regarding the complex vasculature (Hayreh, 2001a) and the significant limitations of hemodynamic modalities to accurately image the tissues (Hayreh, 2001b). Recently, OCTA has provided a novel imaging method generating fast angiographic images of the ONH and retina (Koustenis et al., 2017; Van Melkebeke et al., 2018). Adapted from traditional OCT, OCTA uses b-scans of the same cross-section to compare differences in the backscattered OCT signal intensity or amplitude to generate a picture of blood flow at a specific point in time and map the vasculature. Segmentation of layers for analysis has become much easier with advancements in OCTA. OCTA also shows vascular details just as well as dye techniques, although it does not show slow vessel leaks visualized using dye angiography (Matsunaga et al., 2014). The main outcomes for OCTA are papillary vascular density, peripapillary vascular density, and ONH flow area (Bazvand et al., 2017). Recently in a comprehensive historical review of OCTA was made available in Progress and Retinal Eye Research which details both the history of development, current utilizations and specificity of OCTA for clinical and research paradigms (Spaide et al., 2018). In terms of OCTA data in glaucoma, several studies have found OCTA to be able to discriminate between glaucomatous and healthy eyes. Using OCTA, parapapillary choroidal microvasculature dropout has been associated with progressive retinal nerve fiber layer (RNFL) thinning in OAG (Kim et al., 2019), while Lee et al. found both progressed high-tension glaucoma and NTG patients to have an impaired vascular intake before significant disease development compared to well-controlled cases (Lee et al., 2019). Diurnal change of IOP, ocular perfusion pressure (OPP) and OCTA-assessed retinal vessel density (RVD) was also recently found to be significantly greater in OAG eyes than in healthy eyes. Specifically, at the 8:00pm time point, macular RVD of the healthy group increased to the highest level (44.12 ± 2.95%) while that of the OAG group decreased to the lowest level (40.41 ± 2.54%) (Baek et al., 2019). The ability to provide blood flow analysis as well as traditional ocular structure evaluation using a single device is a major advancement and advantage of OCTA (Koustenis et al., 2017). Tissue-specific assessment limitations and other considerations are important in understanding data from OCTA studies. Analysis of the deep layers of the retina is limited due to artefact, compared to traditional dye angiography, with artefact possibly being misinterpreted as choroidal neovascularization (de Carlo et al., 2015). Also, another important limitation of OCTA is represented by the fact that it detects areas where flow is above a certain threshold (~300 mm/s). However, when the flow is too low and below the threshold, it falsely appears as an area of undetected flow.
Older methods of assessing the ONH include the aforementioned LSFG modality which has also been shown to detect differences in ONH blood flow between NTG patients and healthy controls (Mursch-Edlmayr et al., 2018). Recently, LSFG-measured blood flow in ONH capillaries was found to be higher in glaucoma suspect eyes compared to controls but less elevated in eyes with functional loss (Gardiner et al., 2019).
3.4. Imaging techniques for oxygen saturation
Oxygenation of the ONH involves a complex interplay between the systemic vasculature (including blood pressure), IOP, localized vascular controls and cellular functionality. A previous article in Progress and Retinal Eye Research by Stefánsson et al. (2005) details these relationships and their involvement in progressive optic neuropathy. Retinal oximetry offers the potential for directly assessing blood oxygen saturation levels by measuring hemoglobin oxygen saturation (SO2) in retinal structures through photographic fundus imaging (Harris et al., 2008; Tobe et al., 2013; Stefánsson et al., 2017). Fundus photography is coupled with a beam splitter to a digital camera with the image data filtered into wavelengths, with specific wavelengths corresponding to oxygenated and deoxygenated blood. Specialized analysis software then determines the SO2 of delivered blood, based on the log relationship of brightness levels between the inside and outside of vessels at 570 nm (not sensitive to oxyhemoglobin) and 600 nm (sensitive to oxyhemoglobin) (Stefánsson et al., 2017).
Several studies have identified higher oxygen saturation in retinal veins and lower arteriovenous oxygen saturation difference in glaucoma subjects compared to controls. For example, Olafsdottir et al. found that advanced glaucoma patients had higher venous oxygen saturation levels and a lower arteriovenous difference compared to healthy controls (Olafsdottir et al., 2014). Similarly, Ramm et al. identified higher mean oxygen saturation of retinal veins (64.36 ± 7.11 vs. 59.78 ± 8.47; p = 0.01) alongside lower arteriovenous oxygen saturation difference (33.07 ± 5.24 vs. 37.53 ± 6.95, p = 0.002) in glaucomatous subjects compared to healthy controls (Ramm et al., 2014). Using automated perimetry, dual wavelength scanning laser ophthalmoscopy and Doppler optical coherence tomography, Aref et al. recently found that combined biomarkers of retinal blood flow and oxygen saturation were indicative of impaired oxygen delivery and augmented retinal oxygen extraction fraction in glaucomatous individuals (Aref et al., 2019). Recently, Shimazaki identified higher mean retinal venous oxygen saturation in NTG patients (n = 22; p = 0.007) in the worse hemifield (57.0 ± 7.5%) compared to the better hemifield (54.3 ± 7.0%) (Shimazaki et al., 2018).
Interestingly, when examining glaucoma disparity in patients of African descent, Siesky et al. found that mean oxygen saturation in retinal arteries was 89.9% ± 8.9% in OAG patients of African descent and 94.7% ± 7.9% in OAG patients of European descent (p = 0.25) (Siesky et al., 2013). Additionally, mean oxygen saturation in venules was 65.5 ± 10.7% in persons of African descent compared to 58.3 ± 20.5% in European descent (p = 0.33). The mean arteriovenous difference (A/V-diff) in oxygen saturation was also significantly lower in persons of African descent (24.4% ± 9.3%) than in those of European descent (36.4% ± 14.1%); (p = 0.03). These significant differences existed despite no significant differences in IOP, OPP, or visual field defects (Siesky et al., 2013). The reduction of A/V-diff in oxygen saturation may be explained by lower vascular density due to capillary dropout, reduced capillary function, and/or overall reduced retinal perfusion reducing functional oxygen utilization in patients of African descent. Conversely, limitations of oximetry include not accounting for differing levels of retinal pigment which may influence comparisons between persons and/or groups of persons.
Retinal oximetry has also revealed increased venous saturation levels and reduced arteriovenous differences in diabetic retinopathy patients, and that both biomarkers correlate with the severity of the disease (Hammer et al., 2009; Jorgensen et al., 2014, 2016). This imaging technique is also utilized to confirm CRV occlusion (CRVO) and measure the severity of the occlusion. A significant advantage of this modality is that it has limited invasive (dilation, bright light) concerns, and it utilizes fundus photography which is common for clinical visits. Despite the novel biomarkers that this technology has provided in several ocular diseases, a leading limitation is its measurement of oxygen saturation levels in retinal blood vessels rather than oxygenation levels in retinal tissue. Recently, a retinal oximeter has been developed that uses targeted multichannel spectrometry to provide retinal tissue oximetry measurements over a wide spectral range (Zilia, 2019).
A significant shortcoming of statistical analysis of these studies is that it cannot determine if the decreased arteriovenous difference observed in glaucoma subjects is related to lower oxygen consumption secondary to neuropathy or if it is a primary insult to retinal ganglion cells. Larger longitudinal data are required to understand more fully the importance of oximetry biomarkers in ocular disease.
4. Role of the vasculature in glaucoma
4.1. Vascular risk factors for glaucoma
Traditionally, IOP was considered to be the single most significant risk factor for the development and progression of OAG (Leske et al., 2003). This is based on the mechanical theory of glaucoma, which postulates that the increased pressure within the eye damages the retinal nerve fibers resulting in glaucomatous pathology and subsequent vision loss. However, many patients develop and experience progression of glaucoma despite their IOP remaining within normal range, while other patients exhibiting IOP elevation, or ocular hypertension (OHT), do not suffer glaucomatous damage (Weinreb and Harris, 2009; Bengtsson and Heijl, 2016). Additionally, OAG patients with well-controlled IOP have been shown to develop progressive damage to the optic nerve (Heijl et al., 2002). The search for alternative mechanisms for the onset and progression of OAG led to the proposition of the vascular theory in the early 19th century; by the latter half of the 20th century, both the mechanical and vascular theories were widely accepted as possible contributors to the disease process and may even combine synergistically (Flammer, 1985; Weinreb and Harris, 2009). Over the past several decades, ocular perfusion pressure (calculated as BP minus IOP) and ocular blood flow became mainstays in research of understanding glaucoma risk factors (Flammer, 1985; Weinreb and Harris, 2009; Costa et al., 2014). Other risk factors for glaucoma include systemic vascular dysregulation, age, race, migraine, disc hemorrhage, body mass index, diabetes mellitus, cerebrospinal fluid pressure, BP. These factors and their relationship to the ocular vasculature are discussed in the following sections.
4.1.1. Systemic vascular dysregulation
Vascular disease that includes ocular vascular insult presents with biomarkers ranging from migraine symptoms (discussed below) to cold fingertips, nailfold capillary responses, and alterations in cerebral blood flow. Primary (systemic) vascular dysregulation has been previously described as “Flammer’s syndrome” which has been defined as the presence of primary vascular dysregulation in combination with observations of atypical vascular response to stimuli in various organs and tisuses including the hands and feet (cold temperature, physical stress, etc.) (Konieczka et al., 2014). In addition to the information previously discussed, systemic vascular disease has been shown to affect the eye and brain in unison. Specifically, decreased cerebral and ocular blood flow, as well as impaired vascular autoregulation, have been identified in OAG and have been shown to correlate with visual field loss. In patients with NTG, diffuse cerebral ischemic changes have been detected through magnetic resonance imaging (Harris et al., 2013; Sugiyama et al., 2006). Middle cerebral artery (MCA) mean flow velocity, measured by transcranial Doppler, demonstrated significant correlation with electroretinogram (ERG) amplitude, mean sensitivity, mean defect, and contrast sensitivity loss. These findings revealed that reductions in cerebral blood flow are associated with decreased ERG amplitude in glaucoma patients (Harris et al., 2007). Using nailfold capillaroscopy, Philip et al. found decreased resting peripheral capillary blood flow at the nailfold of the fourth digit of the non-dominant hand in NTG (Philip et al., 2019). Similarly, reduced resting nailfold capillary blood flow was found to be present in OAG patients independent of covariates including BP and IOP (Cousins et al., 2019).
4.1.2. Age
Increasing age has been suggested as a major risk factor for the development and progression of glaucoma. Several population-based studies have examined the association between age and the risk of development of glaucoma. While some studies have shown a positive correlation (Wu and Leske, 1997), others have shown mixed results (Nomura et al., 1999). The Eye Disease Prevalence research group conducted an in-depth analysis of six population based studies and concluded that while the prevalence of glaucoma was 0.68% in the age group 40–49, the prevalence increased to 7.74% in the age group of adults over 80 years of age (Friedman et al., 2004).
With increasing age comes changes throughout the body including weakening of ocular structures and changes to the ocular vasculature. Previously, Harris et al. analyzed the effects of age on retrobulbar blood vessels (Harris et al., 2000). The authors found ophthalmic arterial enddiastolic velocity to be decreased and the Pourcelot resistance indices to be increased with advancing age (each P < 0.001). Conversely, PSV in the ophthalmic artery (OA) and CRA flow velocities were unaffected by age (Harris et al., 2000). A recent study analyzed age-related vascular changes in patients with OAG, patients suspected of glaucoma and age-matched controls. ANCOVA results showed significant interaction between age and group (p < 0.05) for five out of nine retrobulbar blood vessel parameters evaluated. Specifically, the authors concluded that while age may play a role in changes in the retrobulbar blood supply and glaucoma risk, it was not a significant determining risk factor (Asejczyk-Widlicka et al., 2015). Therefore, while age may be an independent risk factor for OAG, there is evidence to show that the vascular effects of aging provide a mechanistic explanation alongside structural weakening in the development and progression of OAG. Additionally, individual genetic susceptibility likely intersects with age-related changes, as a recent study found OAG risk alleles were associated with an earlier age at glaucoma diagnosis (Fan et al., 2019).
4.1.3. Race
Racial differences in glaucoma onset and progression have been demonstrated in various population and non-population based studies. In the black American population, OAG prevalence has been estimated to be six times higher than whites. While differences in IOP are inconsistent, the prevalence of diabetes and hypertension is higher in blacks (Sommer et al., 1991; Racette et al., 2003; Ferdinand, 2008; Huck et al., 2014). The exact mechanisms for these differences in glaucoma risk remain uncertain, but several vascular elements have been identified as affecting black OAG subjects more than their white counterparts (Guidoboni et al., 2013). Specifically, blacks have been reported to have the highest rates of obesity, diabetes, cardiovascular disease and hypertension with earlier onset, greater severity, and corresponding organ damage (Ferdinand, 2008). When considering the ocular vasculature, it is logical that a compromised systemic vascular system would equate to poor ocular circulation and potentially compromised vascular autoregulation. Indeed, low OPP and reduced directly-assessed ocular vascular biomarkers have been identified in black OAG patients (Leske et al., 2008; Huck et al., 2014; Kanakamedala et al., 2014; Siesky et al., 2015, 2016). In addition, Kaskan et al. identified lower retrobulbar blood flow biomarkers in healthy persons of African Descent compared to persons of European descent (Kaskan et al., 2016). Other racial groups have less available information on specific risk factors, although slightly elevated risk has been identified in persons of Middle Eastern descent falling in between those of Latin and African descent (Alshawa et al., 2017). It has yet to be determined if ocular manifestations of vascular dysfunction are fully linked to systemic vascular disease and/or are representative of compromised brain/eye circulation. Interestingly, a link between vascular insult in OAG and Alzheimer’s disease has been suggested with shared vascular compromise (Hutchins et al., 2018). Differences in OPP and ocular blood flow studies are detailed in the following sections.
4.1.4. Migraine
Migraine and vasospasm have been proposed as risk factors for the development of OAG since they may be indicative of systemic vascular dysfunction. Migraine attacks are known to cause a transient reduction in ocular perfusion (Guthauser et al., 1988), and several studies have shown that periodic vasoconstriction causing ischemia can eventually lead to visual field changes in glaucoma (Drance et al., 1973; Gasser et al., 1987; Flammer et al., 2013). The relationship between migraines, glaucoma and IOP level has been investigated in several studies and has produced contrasting results. The Blue Mountains Eye study found an association between migraine and glaucoma in patients aged 70–79 years, which was marginally stronger in glaucoma patients with elevated IOP (Wang et al., 1997). Meanwhile, other studies have found that migraine is significantly more frequent in patients with NTG than in high-pressure glaucoma or control subjects (Cursiefen et al., 2000). Crusifen et al. investigated the relationship between NTG and migraine in 154 glaucomatous patients (56 normal-pressure subgroup and 98 high-pressure glaucoma subgroup), 55 patients with OHT, and 75 control subjects via a standardized questionnaire based on International Headache Society criteria. In the analysis, 17% of participants suffered from migraine and 7% suffered from tension headache (episodic and chronic). Migraine was significantly more common in patients with NTG (28%) compared with healthy control (12%; P < 0.05) and patients with high-pressure glaucoma (10%; P < 0.01) (Cursiefen et al., 2000). The significance of family history and gender in the connection between glaucoma and migraine was analyzed by Gramar et al. (Gramar et al., 2015). Study results showed that glaucoma patients often described a family history of migraine, and that patients with family history of glaucoma had a significantly higher rate of occurrence of migraine than patients without a family history of glaucoma. Patients with NTG had a 63.5% increased age-corrected probability for migraine (P = 0.007) compared to patients with OAG. This could potentially indicate a polygenetic etiology of the two diseases, which both reveal a familial component (Gramer et al., 2015). The authors also postulated that the higher frequency of migraine in females could contribute to the female preponderance in NTG.
4.1.5. Disc hemorrhage
Over the last few decades, it has been established that the presence of optic disc hemorrhage (DH) is closely linked with OAG and is strongly associated with both structural and functional progression of glaucoma (Sugiyama et al., 1997; Kim and Park, 2006). The pathophysiology of disc hemorrhage has been explored more extensively in the past several years, however its underlying mechanism remains unclear. The important mechanisms regarding the pathogenesis of disc hemorrhage currently proposed suggest they are secondary to mechanical vascular disruption and/or they are secondary to increased vascular susceptibilities. Recent advances in OCTA have generated persuasive evidence of mechanical vascular disruption as a mechanism involved in the pathogenesis of DH in OAG (Kim and Park, 2017). Additionally, recent findings suggest that DH may vary in its significance according to its location, recurrence, and the associated underlying mechanism(s). Furlanetto et al. conducted an in-depth analysis of The Low-Pressure Glaucoma Treatment study group and identified several risk factors for the occurrence of a DH. These include a history of migraine (hazard ratio (HR): 5.737; p = 0.012), the use of systemic β-blockers (HR = 5.585; p = 0.036), low mean systolic BP (HR:1.06; p = 0.02), low mean arterial OPP (HR: 1.172; P = 0.007) and a narrow baseline neuro retinal rim (HR: 2.91; P = 0.048) (Furlanetto et al., 2014). Clinically, optic DH have been found to be closely coupled with RNFL defects, and in patients with NTG, the DH have been linked to the location and size of the peripapillary atrophy. Sugiyama et al. analyzed 48 eyes of 42 patients and found 64 DHs, indicating that many eyes had more than one DH. RNFL defects were seen in 47 (97.9%) of 48 eyes, and 51 DH (79.7%) coincided with the location of RNFL defects. These 51 hemorrhages were located on the border (41.2%) or adjacent to the border (58.8%) between the RNFL defect and the apparently healthy RNFL (Sugiyama et al., 1997). Functional deterioration of OAG has been observed in patients with DH, and the presence of repeated DH give an elevated risk for glaucoma progression in comparison to the presence of a single hemorrhage. Kim and Park studied 57 eyes of 54 patients and identified that 26 eyes (45.6%) revealed recurrent DH, and 31 eyes (54.4%) single DH. Optic disc changes, including RNFL thinning after DH, was found to be significantly greater in patients with recurrent DH (P = 0.004, log rank test). However, no significant differences were found between the 2 groups with respect to rate of visual field changes (P = 0.10, log rank test) (Kim and Park, 2006). Additionally, optic DH has been identified as an independent predictive factor for the development of OAG in ocular hypertensives (Budenz et al., 2017). In the Ocular Hypertensive Treatment Study, Budenz et al. followed 169 patients over a period of 13 years and identified 179 eyes with optic DH. They identified older age, higher IOP, larger cup-disc ratio, thinner central cornea and black race as risk factors for optic DH which were similar to the risk factors for the development of OAG (Budenz et al., 2017).
A conjecture has also been proposed on potential causes of disc hemorrhages, inspired by mathematical modeling. Specifically, it has been found that the combination of abrupt time variations in IOP, that occur physiologically with eye blinking or rubbing, combined with a lack of structural viscoelasticity in ocular tissues, which has been found to be associated with glaucomatous damage in monkeys (Downs et al., 2005), could lead to microstructural damage and hemorrhages due to local fluid-dynamical alterations (Banks et al., 2017; Bociu et al., 2016, 2019; Prada et al., 2016; Verri et al., 2018). Clinical or experimental data are needed in order to confirm this conjecture.
Detection of DH by clinical examination can be affected by eye movement, leading to misdiagnosis or failure to diagnose and thereby potentially delaying appropriate treatment. The advent of the use of stereo disc photography eliminates eye movement and allows clinicians more time to make a detailed examination of the fundus. However, small sized DH may be missed on a background of the orange-red retina or the red colored blood vessel, and so new improvements in imaging modalities with higher resolution have been developed. On the enhanced stereo disc photography image, it is seen that micro disc hemorrhages (less than 0.01-mm2 area) existed in a significant percentage of OAG eyes, prior to the appearance of conventional stereo disc photography images or being visible on clinical examination. Ha et al. compared visual field progression in patients with micro DH and those with macro DH and found that the visual field progression can be detected more than 1 year earlier in the micro DH group compared to the macro DH group using enhanced stereo disc photography. Thus, they concluded that micro-DH should be regarded as a clinically meaningful disease-progression predictor (Ha et al., 2019). Given the close association of DH with progressive glaucomatous damage, improved accurate assessment of the presence or absence of DH in glaucomatous eyes with an understanding of individualized risk is essential to improving OAG diagnosis and management.
4.1.6. Body mass index
Obesity has reached epidemic levels worldwide (Finkelstein et al., 2012). Multiple studies have investigated the relationship between OAG and body mass index (BMI) with conflicting results (Kim et al., 2014; Kyari et al., 2016; Lin et al., 2018; Pasquale et al., 2010; Springelkamp et al., 2017). Some studies showed an increased risk for OAG in subjects with lower BMI (Kyari et al., 2016; Lin et al., 2018; Pasquale et al., 2010; Springelkamp et al., 2017). However, a higher BMI has also been shown to be a risk factor for progression to glaucoma (Kim et al., 2014). In a recent meta-analysis of 15 studies (2,445,980 subjects), Liu et al. found a positive correlation between glaucoma and both general and abdominal adiposity (pooled relative risk ratio, RR: 1.09, 95%confidence intervals (CI) 0.87–1.37; RR: 1.28, 95%CI 1.15–1.41, respectively) (Liu et al., 2017). Indeed, the relationship between BMI in glaucomatous patients has yet to be fully understood. Data from the Indianapolis Glaucoma Progression Study (IGPS) were analyzed in order to elucidate this relationship in two studies published in 2013 (Ngo et al., 2013) and 2018 (Ng et al., 2018). In 2013, Ngo et al. investigated the relationships between BP, OPP, IOP in OAG patients with normal weight (NW; n = 38; BMI = 18.5–24.9 kg/m2), overweight (OW; n = 43; BMI = 25.0–29.9 kg/m2) and obesity (OB; n = 34; BMI ≥30 kg/m2) (Ngo et al., 2013). The authors found a different correlation between the variables analyzed in OAG patients of different BMI categories. Specifically, a significant positive correlation was demonstrated in NW patients between changes in systolic BP (SBP) and IOP (r = 0.36, p = 0.0431). In OW and OB OAG subjects, a negative correlation was shown between changes in IOP and OPP (OW: r = −0.56, p = 0.0002; OB: r = −0.38, p = 0.0499).
In 2018, Ng et al. examined the relationship between the changes in structural optic nerve head parameters and functional progression in 77 OAG patients (29 NW; 28 OW; 20 OB) over a five-year period (Ng et al., 2018). Changes in ONH morphology were found to be more predictive of functional progression in OW OAG patients than in NW OAG patients. Changes in cup area, cup volume and cup/disk area ratio were more predictive of functional progression in OW patients, compared to NW or OB patients, with a significant difference between BMI groups (p = 0.0064, 0.0024, 0.0085, respectively). These findings suggest that BMI may affect the interplay between known risk factors for glaucoma onset and progression, such as IOP, OPP and BP. Additionally, glaucoma structural and functional progression may occur differently in NW, OW, and OB OAG patients. Future studies are needed to further investigate the relationship between BMI and OAG in order to tailor treatments in OAG patients with different BMI categories.
4.1.7. Diabetes mellitus
The increasing number of obese subjects worldwide is directly related to a parallel epidemic of diabetes mellitus (DM), in particular type 2 (DMT2). In fact, obesity and DMT2 share common risk factors, such as family history, lack of physical activity, and hyper caloric and unhealthy diet (Volaco, 2018). The World Health Organization estimated that the number of people with diabetes mellitus (DM) has risen from 108 million in 1980 to 422 million in 2014, the majority being type 2 (WHO, 2019). DM is a disease characterized by hyperglycemic stress on both the macro and microvasculature. A well-understood pathophysiologic mechanism of DM describes endothelial cell damage, compromising the resilience of the vasculature supplying many structures, including the eye (Creager et al., 2003; Gerber et al., 2015). In physiologic conditions, nitric oxide released by endothelial cells is responsible for dilation of the vasculature in response to stress; however, this process is impaired by the glycemic damage of longstanding diabetes (Creager et al., 2003). Chronic hyperglycemia also causes oxidative stress and consequent cell damage induced by the reactive oxygen species (Creager et al., 2003). The result is dysfunction in the organs most prone to diminished perfusion, such as the kidney and eye.
In diabetic patients, microvascular changes in the retina are known to cause DR, a leading cause of preventable blindness in adults (Cheung et al., 2010). Diabetic patients with DR have reduced vascular reactivity and regulation (Grunwald et al., 1984a, 1996; Grunwald et al., 1995; Gilmore et al., 2008). Several studies have shown common pathophysiological mechanisms between DM and OAG. In addition to vascular dysregulation (Gerber et al., 2015), both diseases have been shown to exhibit alterations in endothelin-1 and nitric oxide expression and production (Doganay et al., 2002). Although the relationship between diabetes and glaucoma has been investigated in several studies, it is still not fully understood. Specifically, several large-scale clinical trials have evaluated the relationship between DM and OAG, and the results show that DM represents a controversial risk factor for glaucomatous optic neuropathy. In fact, while some large clinical trials have reported no association between diabetes and glaucoma (Gordon et al., 2008; de Voogd et al., 2006; Tielsch et al., 1995a), other studies reported an increased risk of OAG in diabetics (Chopra et al., 2008; Dielemans et al., 1996; Hennis et al., 2003; Klein et al., 1994; Mitchell et al., 1997). In a recent meta-analysis of 47 studies including 2,981,242 subjects in 16 countries, Zhao et al. found a significant increase in OAG risk in diabetic patients compared to non-diabetic (pooled RR: 1.48, 95%CI 1.29–1.71) (Zhao et al., 2015). In 2018, Rim et al. investigated the relationship between DM and glaucoma risk in a retrospective propensity score-matched cohort study on 166,716 Korean aged ≥ 40 years (nondiabetic: 58,358; diabetic: 58,358) (Rim et al., 2018). An increased risk of OAG was detected in patients with DM compared to those without after adjustment for gender and age over a 10-year follow-up period (HR: 1.19, 95%CI 1.09–1.30). Since both DM and OAG are chronic diseases, further longitudinal studies are needed in populations from different ethnicities in order to investigate the interplay between DM and OAG on the progression of both diseases over long periods of time.
Vascular abnormalities may be a risk factor for both OAG and diabetes, and two studies investigated ocular hemodynamics using different imaging devices in glaucomatous patients with and without DM. Lee et al. investigated the effects of changes in retinal capillary blood flow assessed via Heidelberg retinal flowmetry on the ONH morphology evaluated by Heidelberg Retinal Tomography (HRT) and OCT in 66 OAG patients (14 with DM; 52 without DM) at baseline and 3-year follow-up (Lee et al., 2014). The authors found a stronger correlation between changes in ONH parameters and retinal capillary flow in OAG patients with DM, compared to those without DM. Specifically, changes in the superior and inferior retinal avascular area strongly correlated with ONH changes in OAG diabetic patients (r ≥ 0.90; p ≤ 0.03). A strong correlation was also found between changes in the inferior mean flow and in the ONH cup area (r = 0.97; p = 0.0029), cup/disc area ratio (r = 0.96; p = 0.0070), linear cup/disc ratio (r = 0.93; p = 0.0172), rim area (r = −0.97; p = 0.0036), and rim volume (r = −0.95; p = 0.0084) in glaucomatous patients with DM, while in non-diabetic OAG patients changes in the superior mean flow were significantly correlated with cup area (r = −0.30; p = 0.0498), cup volume (r = −0.36; p = 0.0178), and rim volume (r = 0.35; p = 0.0193). Shoshani et al. also demonstrated a different relationship between ocular structure and retrobulbar blood flow in glaucomatous patients with DM compared to those without (Shoshani et al., 2012). 84 OAG patients (20 diabetic and 64 non-diabetic) were assessed with color Doppler imaging for blood flow velocities in the retrobulbar vessels, and with OCT for the circum papillary RNFL thickness. The authors found that diabetic OAG patients had a reduced PSV in the CRA, compared to non-diabetic OAG patients (mean ± standard deviation, SD: 7.6 ± 1.8 cm/s vs 8.9 ± 2.0 cm/s, respectively; p = 0.007). In OAG patients with DM, a significant positive correlation was found between CRA PSV and temporal posterior ciliary artery end diastolic velocity and RNFL thickness (r = 0.501, p = 0.029; r = 0.553, p = 0.019; respectively), while these correlations were weaker and not statistically significant in OAG patients without DM. Taken together, these results suggest a potential role of DM in glaucomatous patients through alterations in ocular blood flow and motivate the need to identify translatable risk factors in patients affected by both diseases.
4.1.8. Cerebrospinal fluid pressure
The cerebrospinal fluid (CSF) in the subarachnoid space surrounding the optic nerve generates a pressure, known as retrolaminar tissue pressure (RLTp), in the optic nerve tissue behind the lamina cribrosa (Fleischman and Berdahl, 2019). The lamina cribrosa in the ONH serves as the boundary between two differentially pressurized compartments, the intraocular space and the retrolaminar space, where the pressure levels equal IOP and RLTp, respectively. Thus, the lamina cribrosa is responsible for the conflicting task of providing structural support to the ONH by withstanding pressure-related mechanical strains, or local deformation, while also allowing the axons an open pathway to leave the eye (Downs and Girkin, 2017).
The pressure difference across the lamina, or translaminar pressure difference (TPD), can then be defined as TPD = IOP–RLTp, and the translaminar pressure gradient (TPG) is calculated as the TPD per unit thickness of lamina cribrosa (Siaudvytyte et al., 2015). In dogs, Morgan et al. found that RLTp is largely determined by the cerebrospinal fluid pressure (CSFp) when CSFp is larger than 1.3±0.6 mmHg (Morgan et al., 1998). For this reason, many studies simply refer to RLTp as CSFp. Furthermore, studies on venous pulsation have established a strong association between CSFp and intracranial pressure (ICP), which led to the hypothesis that there exists a low-resistance connection between the intracranial CSF and the CSF surrounding the optic nerve. Thus, depending on the study, TPD may be defined as the difference between IOP and RLTp, IOP and CSFp or IOP and ICP. However, the validity of these assumptions remains to be fully verified, especially across changes in posture, time of day and disease conditions. Lumbar puncture is the most common technique used to measure CSFp, even though other methods are also available, such as those based on invasive microtransducers or noninvasive transcranial Doppler ultra-sonography (Januleviciene and Siaudvytyte, 2019). Thus, the method and protocol of measurement should be taken into account when evaluating results from studies aimed at assessing the effect of RLTp, or CSFp or ICP, on ocular disease.
The neural, vascular and connective tissue elements which constitute the lamina cribrosa and the optic nerve are very sensitive to alterations in pressure, which may cause biomechanical and/or hemo-dynamic damage in this region and influence the pathophysiology of diseases like glaucoma (Downs and Girkin, 2017; Zhang et al., 2019), central retinal vein occlusion (Morgan et al., 2016a and 2016b), papilledema (Liu et al., 2019) and spaceflight associated neuro-ocular syndrome (National Aeronautics and Space Administration, 2017). For example, thinner laminas and larger laminar displacements have been shown to have a significant influence on the rate of progression of retinal nerve fiber layer thinning (Lee et al., 2015a). Since the pressure difference across the lamina does not depend solely on IOP, ICP variability might, in part, explain differences in severity of glaucomatous nerve damage seen among patients with a given IOP. Specifically, mean ICP was found to be lower in OAG patients (Berdahl et al., 2008a) and may facilitate backward bowing of the lamina typically seen as cupping in certain patients. Also, very low ICP has been shown to contribute to the development of glaucoma in some patients with an IOP that falls within normal range. Berdahl et al. investigated the relationship between ICP and disease in patients with NTG, OAG and OHT. They found that ICP was lowest in NTG, followed by OAG, when compared to healthy controls (8.7 ± 1.2 mmHg, 9.1 ± .0.8 mmHg, and 11.8 ± .0.7 mmHg, respectively). The ICP was found to be higher in OHT (12.6 ± .0.8 mmHg) compared to healthy controls (10.6 ± .0.8 mmHg) (Berdahl et al., 2008b).
A higher TPG may lead to optic nerve damage due to alterations in axonal transportation, lamina deformation, altered blood flow, or a combination of these, ultimately resulting in glaucomatous damage. In fact, TPG is hypothesized to be the primary pressure-related parameter for glaucoma (Burgoyne et al., 2005) since the ONH is located at the junction between the intraocular space and the orbital retrobulbar space. Siaudvytyte et al. and Ren et al. both have studied the effect of TPG on neuroretinal rim area (NRA) in OAG. In their prospective study, Siaudvytyte et al. demonstrated that IOP and NRA was significantly lower in NTG compared to OAG and healthy controls (IOP = 13.7 mmHg, 24.7 mmHg, and 15.9 mmHg, respectively; P < 0.001) and NRA was 0.97 mm2 in NTG, 1.32mm2 in OAG, and 1.79 mm2 in healthy controls; P = 0.003). Additionally, the TPG was found to be highest in OAG (15.7 mmHg) when compared to NTG (6.3 mmHg) and healthy controls (5.4 mmHg; P < 0.001), and ICP was found to be lower in NTG (7.4 mmHg) when compared with OAG (8.9 mmHg) and healthy subjects (10.5 mmHg). Importantly, however, the difference between groups was not statistically significant (P > 0.05) (Siaudvytyte et al., 2014). Ren et al. in their study noted that there was a significant association between NRA and visual field defect with the translaminar cribrosa pressure difference (NRA r = −0.38, p = 0.006; visual field r = 0.38, p = 0.008) (Ren et al., 2011). In addition, 9 out of 22 patients undergoing surgery to reduce ICP for normal pressure hydrocephalus have been shown to develop NTG, which is a 40-fold increase compared to the rate of NTG development in a general elderly population without hydrocephalus (p < 0.001), supporting the theory that an imbalance between IOP and ICP is critical in the development of optic nerve damage (Gallina et al., 2018).
4.1.9. Blood pressure
The relationship between BP and glaucoma is complex and poorly understood. Systemic hypertension and hypotension are both considered risk factors for OAG onset and progression, with contradictory data published in the literature. The relationship between glaucoma and systemic BP is further complicated by the interplay with other known risk factors for glaucoma, such as IOP and OPP. Additionally, BP, IOP, and OPP undergo diurnal fluctuations, and alterations in the circadian rhythm of these variables have been implicated in the pathophysiology of glaucoma. A dysfunctional autoregulation in response to changes in BP, IOP, and OPP has also been suggested to play a major role in the disease onset and progression. This section summarizes the main results relating BP and glaucoma from the published literature.
Targeting the best course for blood pressure has proven challenging in the balance of reducing cardiovascular morbidity and preventing adverse side effects. In the SPRINT trial (Wright et al., 2015), intensive versus standard blood pressure reduction comparisons found achieving systolic blood pressure < 120 mmHg resulted in fewer cardiovascular events and death but significantly higher rates of adverse events when compared to the standard treatment group (target: < 140 mmHg). Currently, the American College of Cardiology suggests approximate targeting of systolic BP < 130 mmHg and diastolic BP < 80 mmHg (Whelton et al., 2018). Specific to glaucoma, several large-scale epidemiologic studies have explored the association between OAG and systemic hypertension and have reported conflicting results. In fact, an elevated BP has been associated with both a decreased and an increased risk of OAG in several large population based studies. Leske et al. evaluated the risk of glaucoma incidence over a 4-year period in 2989 participants of African descent (Leske et al., 2002), and found that the presence of systemic hypertension at baseline halved the risk of developing glaucoma (RR: 0.49, 95%CI 0.29–0.85). On the other hand, other studies showed an increased risk of glaucoma with systemic hypertension. In the Blue Mountains Eye Study conducted on 3654 subjects, the authors found a positive association between OAG and systemic hypertension (odds ratio, OR: 1.56, 95%CI: 1.01–2.40) (Mitchell et al., 1996). Similar results came from the Rotterdam Eye Study performed on 4187 subjects, in which systemic hypertension was associated with a 2 times higher risk of high-pressure glaucoma (OR: 2.33, 95%CI, 0.99–5.47) (Dielemans et al., 1995).
Several mechanisms have been proposed to explain the association between systemic hypertension and glaucoma development and progression. On one hand, the raised BP could directly inflict damage to the microvasculature and impair blood flow to the eye (Flammer et al., 2002; Piltz-seymour et al., 2001). On the other hand, the autoregulation in the posterior ciliary arteries could also be impaired in hypertension (Grunwald et al., 1984b). Additionally, hypertensive patients may have concurrent cardiovascular disease and diabetes mellitus, which may potentially affect the optic nerve head perfusion, and further confound the relationship (Nakamura et al., 2005; Hayreh et al., 1999). Nonetheless, several studies have found a close association between IOP, the main risk factor for OAG, and systemic hypertension (Mitchell et al., 2005; Foster et al., 2003). It is theorized that increased systemic BP leads to an increase in ciliary artery pressure, which can in turn increase the filtration fraction of aqueous humor (Bulpitt et al., 1975) and lead to elevated IOP. These mechanisms may act simultaneously, as was shown in a pilot study from Ciulla et al. (2017). BP, IOP, and retinal capillary blood flow were assessed in 16 OAG patients with systemic hypertension, and 26 without. In the hypertensive OAG patients, increased systolic BP was associated with increased IOP (r = 0.542, p = 0.029), and increased IOP was associated with decreased retinal capillary blood flow (r = −0.607, p = 0.011); these associations were not found in non-hypertensive patients. The authors suggested that systemic hypertension could affect the physiologic relationship between ocular blood flow and IOP inducing glaucomatous damage via a dysregulation of the autoregulatory mechanisms of the ocular vasculature.
Systemic hypotension has also been associated with OAG risk and progression, in particular in patients with NTG. Specifically, excessive nocturnal dipping of BP, either due to medication or prolonged supine posture, has been associated with the development and progression of glaucoma (Costa et al., 2010, 2014; Kaiser et al., 1993; Krasinka et al., 2011; Hayreh et al., 1994; Moore et al., 2008). Costa et al. assessed the IOP, OPP and BP of 29 OAG patients and 24 healthy controls every 2 h over a period of 24 h and found a significantly lower DBP (p < 0.05) and DPP (p < 0.05) at night in the glaucomatous patients (Costa et al., 2010). Charlson et al. investigated the relationship between nocturnal hypotension and visual field progression in 85 NTG patients (Charlson et al., 2014) and found that nocturnal hypotension (nocturnal mean arterial pressure (MAP) of 10 mmHg below the diurnal MAP) was predictive of perimetric disease progression (P < 0.02). Similarly, data from the Maracaibo Aging Study confirmed the critical role of nocturnal hypotension as risk factor for glaucoma. In this study on 93 subjects, extreme decrease in SBP and diastolic BP (DBP) during the night (> 20% compared to the daytime values) significantly increased the risk of glaucoma (systolic dipper OR: 19.78, 95%CI: 2.23–175.50, p = 0.007; diastolic dipper OR: 5.55, 95%CI: 1.04–29.62, p = 0.045) (Melgarejo et al., 2018). In the Singapore Epidemiology of Eye Diseases Study conducted on nearly 10,000 subjects within a multi-ethnic Asian population, individuals within the lowest quartile of systolic BP (SBP < 124 mmHg) were 1.69 times (95%CI: 1.08–2.66) likely to have OAG, compared with mid-range SBP levels (138–153 mmHg, third quartile). Furthermore, the effect of lower SBP on OAG was more pronounced in eyes with IOP ≥21 mmHg (OR: 3.90, 95%CI: 1.24–12.30) (Tham et al., 2018). Several possible mechanisms have been suggested to explain the association between hypotension and glaucomatous damage: (i) a drop in the BP can cause a reduction of the perfusion pressure, and an ischemic damage of the ONH can ensue; and (ii) systemic hypotension may compromise the autoregulation of the ciliary artery circulation, therefore causing an impairment of the vascular supply to the ONH and inducing the glaucomatous damage. The recent finding of a statistically significant correlation between a low systemic BP and vascular dysregulation syndrome in glaucomatous subjects support the latter hypothesis (Binggeli et al., 2018). Finally, it is important to note that aggressive treatment of hypertension could lead to nocturnal hypotension that can be detrimental to the blood supply of the optic nerve and could potentially be an iatrogenic cause for the progression of glaucoma in hypertensive patients via repetitive daily hypoperfusion-reperfusion injuries (Graham et al., 1995).
Vascular regulation is defined as the ability of the vessels to dilate or constrict in response to perfusion pressure changes in order to maintain a constant nutrient supply (Moore et al., 2008). In the eye, dysfunctional regulation in response to changes in IOP, BP and OPP has been indicated as a pathophysiologic mechanism of glaucomatous damage (Moore et al., 2008). Faulty autoregulation of retrobulbar blood flow evaluated via color Doppler imaging has been demonstrated in the central retinal artery of glaucomatous subjects in response to postural changes (Evans et al., 1999). Also, OAG patients showed abnormal autoregulation of the optic nerve head blood flow measured by laser Doppler flowmetry during isometric exercise, compared to healthy subjects (Bata et al., 2019). Larger fluctuations in IOP, BP and OPP have been correlated to glaucomatous damage and progression in several studies (Choi et al., 2007; Lee et al., 2015b; Sehi et al., 2005; Quaranta et al., 2013). The lack of autoregulation and the inability to maintain flow over a wide range of ocular perfusion pressures have been therefore proposed as the cause of the ischemic and glaucomatous damage in susceptible subjects, both in patients with systemic hypertension (Ciulla et al., 2017) and hypotension (Binggelli et al., 2018).
4.1.10. Myopia
Several studies have evaluated the retinal and choroidal vasculature in myopia (Li et al., 2017; Yang et al., 2015). Li et al. used OCTA to image both the superficial and deep vascular plexuses. The Retinal Function Imager was used to image the retinal micro-vessel blood flow velocity. The density of both superficial and deep microvascular plexuses was found to be significantly reduced in the myopia group compared to the controls (p < 0.05). The reduction of the micro-vessel density of the annular zone (0.6–2.5 mm) was 2.1% in the superficial and 2.9% in the deep vascular plexuses. Microvascular densities in both superficial (r = −0.45, p = 0.047) and deep (r = −0.54, p = 0.01) vascular plexuses were negatively correlated with the axial lengths in the myopic eye. However, the retinal micro-vessel blood flow velocity remained unchanged. The authors concluded that the retinal microvascular network alteration may be attributed to ocular elongation that occurs with the progression of myopia. An improved understanding of the retinal microvasculature may help to characterize the underlying pathophysiology of myopia and enable early detection and prevention of myopic retinopathy.
Yang et al. evaluated ocular pulse amplitude, pulse volume, and pulsatile ocular blood flow (calculated from IOP amplitudes) (Yang et al., 2015). The authors showed positive correlations with refractive error and negative correlations with axial length (r = 0.729, r = 0.772, r = 0.781, respectively, all p < 0.001; r = −0.727, r = −0.762, r = −0.771, respectively, all p < 0.001). High myopes demonstrated significantly lower ocular pulse amplitude, pulse volume, and pulsatile ocular blood flow (p < 0.001) compared to low myopes and emmetropes. The authors concluded that changes in axial length in high myopes may influence the choroidal blood flow. It was assumed that the changes are a result of narrowing of the choroidal vessel diameter and increasing rigidity of the choroidal vessel wall. Researchers noted decreased retinal and choroidal blood flow velocities, as well as decreasing mean velocities with increasing refractive error (Grudzińska and Modrzejewska, 2018). These studies suggest that ocular blood flow parameters may be significantly lower in subjects with myopia compared with subjects without refractive error. Importantly, theoretical models have suggested that changes in ocular morphology, for instance characteritistic changes in shape of myopic globes, may result in consequential alterations of retinal hemodynamics (Dziubek et al., 2016).
4.2. Population-based study evidence for glaucoma
Perfusion pressure to the ocular tissues is an important consideration based upon population-based studies in the pathogenesis of glaucoma. OPP represents the driving force of perfusion to intraorbital tissues and is given by the pressure difference between the arterial and venous ends of the ocular circulation. OPP is typically approximated as the difference between two-thirds of the BP measured at the level of the brachial artery and intraocular pressure. Thus, this calculation is based on two major assumptions, namely that (i) the pressure in the ocular arteries equals two-thirds of the pressure measured at the level of the brachial artery (Riva et al., 1986), and (ii) the pressure in the ocular veins equals IOP (Bill, 1985; Mäepea, 1992; Glucksberg and Dunn, 1993). The extent to which these assumptions remain valid across measurement protocols, especially upon changes in posture, has yet to be clarified (Costa et al., 2014). Additionally, OPP is often presented as mean OPP (MOPP = MAP-IOP), systolic OPP (SOPP]SBP-IOP) and diastolic OPP (DOPP = DBP-IOP), where MAP, SBP and DBP are the mean, systolic and diastolic arterial BP, respectively.
OPP represents the interplay between opposing forces of BP at the eye level and IOP within the eye. Importantly, low OPP was identified as an independent risk factor for OAG by the World Glaucoma Association at the 6th annual consensus meeting (Weinreb and Harris, 2009), since many large population-based studies, conducted in numerous ethnic groups, showed a consistency in the relationship of OPP to the prevalence, incidence, and progression of glaucoma. OPP is influenced by the resistance to flow, which is a function of the vessel caliber and/or vessel tone (Caprioli et al., 2010). When autoregulatory mechanisms are intact, in spite of changes in OPP, the body can maintain stable ocular blood flow. However, in OAG patients, fluctuations in systemic BP or IOP alter the flow of blood to the optic nerve head and retina (Harris et al., 2001), thereby setting the premises for perfusion insults. While significant evidence has constantly demonstrated the potential role of OPP in glaucoma, the differing statistical approaches, methodological limitations and variable patient populations add complexity to this hemodynamic biomarker. Herein, evidence of the importance of OPP in glaucoma from population-based studies are evaluated in their relationship to the prevalence, incidence and progression of glaucoma (Table 1) with a critical analysis of methods, statistical approaches, and subsequent limitations.
Table 1.
Population-based studies evaluating the relationship between blood pressure and ocular perfusion pressures and glaucoma prevalence, incidence and progression. DBP: diastolic blood pressure; DOPP: diastolic ocular perfusion pressure; IOP: intraocular pressure; MOPP: mean ocular perfusion pressure; N: number of study subjects; NTG: normal tension glaucoma; OAG: open-angle glaucoma; OPPs: ocular perfusion pressures; POAG: primary open-angle glaucoma; SOPP: systolic ocular perfusion pressure.
| Study | N | Findings | |
|---|---|---|---|
| Prevalence | Baltimore Eye Survey (1995) | 5308 (African and European descent) | Low DOPP and OAG risk |
| Barbados Eye Study (1995) | 4314 (African descent) | Low DOPP and OAG risk | |
| Egna-Neumarkt Study (2000) | 4297 (European descent) | Low DOPP and high-pressure OAG | |
| Proyecto (2001) | 4774 (Latin descent) | Low DOPP and OAG risk | |
| Rotterdam Study (2007) | 5317 (European descent) | Low DOPP and hypertensive OAG risk (patients treated for systemic hypertension) | |
| Beijing Eye Study (2009) | 3251 (Asian descent, Chinese) | No statistically significant association between OPPs (MOPP, DOPP, SOPP) and OAG risk | |
| Los Angeles Latino Eye Study (2010) | 6130 (Latin descent) | Low MOPP, DOPP, SOPP and OAG risk | |
| Singapore Malay Eye Study (2010) | 3280 (Asian descent, Malay) | Low MOPP and DOPP and OAG risk | |
| Thessaloniki Eye Study (2013) | 2554 (European descent) | Low DOPP and OAG risk (patients treated for systemic hypertension) | |
| South India Study (2014) | 208 (Asian descent, Indian) | Low MOPP and OAG risk (patients treated for systemic hypertension) | |
| The Handan Eye Study (2016) | 6830 (Asian descent, Han Chinese) | Low MOPP, DOPP and SOPP and OAG risk | |
| Nigeria National Blindness and Visual Impairment Survey (2016) | 13,591 (African descent) | Low MOPP and OAG risk | |
| Cantor et al. (2018) | 1272 (Latin descent) | Low MOPP and DOPP, high SOPP, high DBP and OAG risk (patients treated for systemic hypertension) | |
| Singapore Epidemiology of Eye Diseases Study (2018) | 9877 participants (Asian descent, multiethnic) | Low and high SOPP and OAG risk | |
| Incidence | Barbados Eye Study (mean follow up: 9 years) (2008) | 3222 (African descent) | Low MOPP, DOPP, and SOPP and incident OAG |
| Rotterdam Study (mean follow up: 9.8 years) (2011) | 3882 (European descent) | Low MOPP and incident OAG (no statistical significance after IOP adjustment) | |
| Rotterdam Study (mean follow up: 12.1 years) (2017) | 3939 (European descent) | DBP not associated with incident OAG (no analysis for OPPs) | |
| Progression | Early Manifest Glaucoma Trial (mean follow up: 8 years) (2007) | 255 (early OAG) | Lower baseline SOPP and OAG perimetric and structural progression |
| Low-pressure Glaucoma Treatment Study (mean follow up: 40.6 months) (2012) | 127 (NTG) | Lower MOPP during follow-up and perimetric progression | |
| Jin at al. (mean follow up: 21.2 years) (2017) | 72 (NTG) | Lower MOPP and perimetric progression |
4.2.1. Prevalence
The prevalence of OAG has been shown to be strongly associated with a low OPP across multiple ethnic groups. Specifically, a low OPP has been identified as a risk factor for glaucoma prevalence in populations of European, African, Latin, and Asian descent (where appropriate, the terms presented by the original source are retained: e.g., OAG vs. POAG and black vs. African descent).
In persons of African and Caucasian descent, several large population-based studies have shown the association between OAG and a lower level of DOPP. Specifically, the Egna-Neumarkt Study (Bonomi et al., 2000) involved 4297 subjects of European descent from the region of Egna-Neumarkt in South Tyrol (Italy). In this population, sex-adjusted OR and 95% CI were calculated to evaluate the risk of primary OAG (OAG; IOP≥22 mmHg) and NTG (IOP < 22 mmHg) in relation to different levels of DOPP. Lower levels of DOPP were associated with an increase in frequency of high pressure OAG, but not low pressure NTG. It is important to note, however, that the sample size of NTG (n = 20) was less than half that of the OAG group (n = 54). A closer look at the sex-adjusted odds ratios may provide more discernment. DOPP was found to be protective of increased risk of OAG (DOPP < 68 mmHg, OR: 1; DOPP 68–76 mmHg, OR: 0.33, 95%CI: 0.17–0.67; DOPP > 76 mmHg, OR: 0.29, 95%CI: 0.14–0.58; chi-square trend: 14.05, P < 0.001). Perhaps due to the limited sample size, this relationship was weaker and non-significant in persons with NTG (DOPP < 68 mmHg, OR: 1; DOPP 68–76 mmHg, OR: 0.83, 95%CI: 0.29–2.41; DOPP > 76 mmHg, OR: 0.84, 95%CI: 0.29–2.45; chi-square trend: 0.11, P = 0.742). Similarly, in the Barbados Eye Study (Leske et al., 1995) on 4314 black participants, the prevalence of OAG was associated with a low (< 52.3 mmHg) versus a high (> 72.0 mmHg) level of DOPP (OR: 3.29, 95%CI: 2.06–5.28, P < 0.05), computed by logistic regression models that adjusted for gender, age, body mass index, history of cataract, and family history of glaucoma. These results were also confirmed in the 5308 white and black subjects, age ≥40 years, who participated in the Baltimore Eye Survey study (Tielsch et al., 1995b), in which participants with DOPP < 30 mmHg were found to have a race-adjusted OAG risk 6 times higher (OR: 6.22, 95%CI: 2.15–17.94) compared to subjects with a DOPP≥50 mmHg. While both systolic and diastolic BP were also associated with OAG, older subjects had a stronger association than younger subjects. Accounting for age’s total physiological impact on these relationships beyond standard statistical adjustments may provide clarity and allow for calculation and specificity of individualized risk.
In Hispanic populations, lower OPP has also been confirmed to be an important consideration in determining the risk for glaucoma. The Proyecto Ver Study (Quigley et al., 2001), a population based study of 4774 Hispanic participants, revealed a 4-fold increase in OAG prevalence in subjects with DOPP lower than 50 mmHg. Indeed, the lower the level of DOPP, the higher the OAG risk (X2 = 28.8; P = 0.001). Contrasting the Baltimore Eye Survey study (Tielsch et al., 1995), BP was not found to be a significant determinant of OAG risk. It is important to consider the different patient populations and their unique characteristics (i.e., percentage of OAG participants between age 40–59 in Proyecto Ver Study = 17%; Baltimore Eye Survey study = 24.8%). The Los Angeles Latino Eye Study (LALES) (Memarzadeh et al., 2010), conducted in 6130 adult Latinos, also found the importance of OPP in determining OAG prevalence. Interestingly, not only low diastolic but also systolic and mean OPPs (DOPP≤40 mmHg, OR: 1.9, 95%CI: 1.1–3.4; SOPP≤80 mmHg, OR: 2.5, 95%CI: 1.2–5.2; MOPP≤50 mmHg, OR: 3.6, 95%CI: 1.5–8.3) were associated with a higher OAG prevalence. These results were calculated by logistic regression models adjusted for age, IOP, history of glaucoma therapy, and presence and treatment of systemic hypertension. Additionally, low diastolic and high systolic (and mean arterial) BP were also found to be associated with a higher prevalence of OAG. Mathematical modeling and AI approaches that more comprehensively account for relevant patient characteristics (e.g., age, gender, hypertensive status) could help overcome comparative limitations in understanding the differing biomarker outcomes of these important studies.
The influence of OPP in Asian populations has also shown significant associations with glaucoma prevalence. In the Singapore Malay Eye Study (Zheng et al., 2010), conducted on 3280 ethnic Malays, lower levels of MOPP (MOPP≤46 mmHg, OR: 1.73, 95%CI: 1.05–3.15; P = 0.01) and DOPP (DOPP≤56 mmHg, OR: 1.75, 95%CI: 1.02–3.01; P = 0.002) were found to be independent risk factors for OAG. Confounding the estimation of risk, the odds ratio between quartiles of MOPP and DOPP were inconsistent with age grouping (below or ≥ age 60 years). For example, younger subjects were found to be at a higher risk in the lowest quartiles of MOPP and DOPP and the influence of IOP on risk differed between the four quartiles of both perfusion pressure calculations. The Handan Eye Study (Liang et al., 2016) conducted in 6830 Han Chinese subjects found SOPP, DOPP, and MOPP evaluated over 24-h to be constantly lower among subjects with a diagnosis of OAG compared to OAG suspect. Based on a general linear model adjusted for age, sex, and mean 24-h IOP, the authors found all calculations of OPP to be lower in OAG: (MOPP, OAG: 50.0 ± 9.2 (mean ± SD) mmHg; suspected OAG: 54.1 ± 8.1 mmHg; P = 0.008; SOPP, OAG: 80.1 ± 15.1; suspected OAG: 86.4 ± 12.6 mmHg; P = 0.021; DOPP, OAG: 34.9 ± 7.8; suspected OAG: 38.0 ± 7.2 mmHg; P = 0.009). In contrast to the aforementioned population-based studies in Asian populations, the Beijing Eye Study (Xu et al., 2009) conducted on 3251 Chinese subjects found no statistically significant association between the prevalence of OAG and OPPs (MOPP: P = 0.28; SOPP: P = 0.38; DOPP: P = 0.29). Possible explanations of the discrepancies among these results were differences in the glaucoma definition, levels of IOP, and glaucoma treatments in the study population.
4.2.2. Incidence
Fewer studies have investigated the relationship between glaucoma incidence and perfusion pressure. When examining the available data, race and statistical adjustment for IOP were defining factors in determining outcomes. The two longitudinal studies that investigated the relationship between perfusion pressure levels and glaucoma occurred in African and Caucasian descent populations via the Barbados Eye Study and Rotterdam Study, respectively.
In the Barbados Eye Study (Leske et al., 2008), 3222 black participants of African descent were followed for a period of 9 years to assess risk factors for the incidence of OAG. The results showed that the lower the level of SOPP, DOPP, and/or MOPP, the higher the risk for OAG. Specifically, in the categories with the lowest levels of perfusion pressures, the RR of OAG based on Cox regression models at least doubled (SOPP≤98 mmHg, RR: 2.00, 95%CI: 1.1–3.5; P = 0.05; DOPP≤53 mmHg, RR: 2.1, 95%CI: 1.2–3.9; P = 0.05; MOPP≤40 mmHg, RR: 2.6, 95%CI: 1.4–4.6; P = 0.05). Conflicting results were obtained in a Caucasian population of 3882 participants assessed in the Rotterdam Study (Ramdas et al., 2011) during a mean follow-up period of 9.8 years. A multivariate Cox proportional hazard model found a statistically significant association between MOPP and incident OAG if not adjusted for IOP (HR: 0.968, 95%CI: 0.945–0.992; P = 0.010), while the association was not statistically significant with the adjustment for IOP (HR: 0.995, 95%CI: 0.971–1.019; P = 0.675). These results were particularly important because they highlighted that OPP did not represent an independent risk factor for OAG incidence, but suggested risk as a derivative of IOP. The failure of agreement between studies may be linked to multiple aspects of patient characteristics including hypertensive status and gender (Barbados Eye Study, Leske et al., 2008: baseline hypertension of persons at risk = 52.4%; males = 41%; Rotterdam Study, Ramdas et al., 2011: incident OAG taking antihypertensives = 27.5%; males = 50.5%). The inability to identically match patient criteria and account for differing approaches to data presentation may therefore hide important agreement between studies that are otherwise considered at conflict.
4.2.3. Progression
Data on OPP and its relationship with glaucoma progression is severely lacking in the literature. Despite the limited data, the 3 long-term studies currently available show agreement in OPP as a risk factor for glaucoma progression. Specifically, in the Early Manifest Glaucoma Trial (Leske et al., 2007) patients with early OAG randomized to treatment with argon laser trabeculoplasty and betaxolol (129) or with no immediate treatment (126) were evaluated every 3 months for a median follow-up period of 8 years. Glaucoma progression was defined by perimetric and photographic disc criteria (overall progression: 67% when follow-up ended). In all study subjects, a lower baseline SOPP was predictive of disease progression (SOPP≤125 mmHg, HR: 1.42, 95%CI: 1.04–1.94, P = 0.0268) (note: abstract of article appears to list systolic BP value as SOPP value: ≤125 mmHg vs. ≤160 mmHg; note Table 2 from Leske et al., 2007). The analysis of BP as a predictor is hard to compare to other data due to the unique and complex approach of the statistical models. The relationship between visual field progression and level of perfusion pressure was also investigated in NTG patients.
Table 2.
Association of Diastolic Perfusion Pressure Status With Open-angle Glaucoma, and Pseudoexfoliative Glaucoma in the Thessaloniki Eye Study. CI: confidence interval; DPP: diastolic perfusion pressure; N: number of subjects; OAG: open-angle glaucoma; OR: odds ratio; PEXG: pseudoexfoliative glaucoma; POAG: primary open-angle glaucoma. With permission from the publisher and authors (Topouzis et al., 2013).
| Effect | In Subjects Without Antihypertensive Treatment | In Subjects With Antihypertensive Treatment | ||||||
|---|---|---|---|---|---|---|---|---|
| N | OR | 95%CI | P Value | N | OR | 95%CI | P Value | |
| Association with OAG | ||||||||
| DPP(per 10 mm Hg) (unadjusted) | 1.039 | 0.72 | 0.57−9.92 | .008 | 1212 | 0.78 | 0.66−0.93 | .006 |
| DPP (per 10 mm Hg) (adjusted) | 1.05 | 0.81–1.35 | .731 | 0.83 | 0.68–1.01 | .062 | ||
| Association with POAG | ||||||||
| DPP (per 10 mm Hg) (unadjusted) | 922 | 0.80 | 0.59–1.07 | .13 | 1060 | 0.69 | 0.56–0.85 | < 0.001 |
| DPP (per 10 mm Hg) (adjusted) | 0.98 | 0.72–1.33 | .891 | 0.78 | 0.62–0.97 | .028 | ||
| Association with PEXG | ||||||||
| DPP (per 10 mm Hg) (unadjusted) | 117 | 0.76 | 0.51–1.13 | .18 | 152 | 1.10 | 0.79–1.52 | .58 |
| DPP (per 10 mm Hg) (adjusted) | 1.39 | 0.81–2.37 | .235 | 0.90 | 0.58–1.41 | .653 | ||
In the Low-pressure Glaucoma Treatment Study (De Moraes et al., 2012), 253 eyes of 127 NTG subjects treated with alpha2 adrenergic agonist or beta-adrenergic antagonist were evaluated for a mean follow-up period of 40.6 ± 12 months. Lower MOPP during the follow up period was found to be associated with an increased risk of perimetric progression based on the Cox proportional hazards multivariate model using a backward elimination approach based on likelihood ratios (HR: 1.21 per mmHg lower, 95%CI: 1.12–1.31; P < 0.001).
In the only other associated long-term observational study, Jin and Noh (2017)followed the course of 72 NTG patients (mean follow-up period: 21.2 ± 1.1 years) treated with IOP-lowering medications and found that lower levels of MOPP were a significant risk factor for visual field progression. Data were defined by the mean deviation value, both in the univariate (RR: 1.94, 95%CI: 0.97–3.12; P = 0.027) and multivariate models (RR: 2.02, 95%CI: 0.84–3.92; P = 0.004). The differing and highly complex statistical study designs and differing treatment regimens plague progression outcomes and point to the need for more advanced biomarker modeling. Significantly more research is needed with careful study designs to understand OPP and its specific role in glaucoma progression, both structurally and functionally.
4.2.4. Confounding considerations
Due to the fact that ocular perfusion pressure involves the interplay between two opposing fluid pressures, BP and IOP, it is important to model how IOP and BP, independently or together, affect the results of the population-based studies. The Thessaloniki Eye Study (TES) provides insight into this complex relationship. The TES is a cross-sectional population-based study conducted in 2554 subjects in Thessaloniki, the major urban center in Northern Greece. There are two cross-sectional studies (2006 and 2013) of importance in the study of glaucoma and its relation to BP and perfusion pressure status (defined as OPP with/without antihypertensive treatment). The 2006 TES (Topouzis et al., 2006) enrolled subjects without evidence of OAG, while the 2013 TES enrolled subjects with and without OAG. In the 2006 TES (Topouzis et al., 2006), 232 subjects without OAG underwent assessment of the optic nerve head structure and BP. Hypertension was defined as SBP≥140 mmHg and/or DBP≥90 mmHg. Both low DBP and anti-hypertensive treatment were found to be independently associated with optic disc structural changes. Specifically, a significant positive correlation was found between low DBP (< 90 mmHg) and decreased rim area (P = 0.0028), increased cup area (P = 0.0028) and increased cupto-disc ratio (C/D) (P = 0.0029). Antihypertensive therapy was also positively correlated with C/D (P = 0.045) and cup area (0.03). A subgroup analysis was then performed that subdivided subjects into four categories: low DBP without antihypertensive treatment, low DBP with antihypertensive treatment, high DBP with antihypertensive treatment, and high DBP without antihypertensive treatment. Only patients with low DBP on antihypertensive medications had a significant positive association with decreased rim area, increased cup area, and C/D. Additionally, a significant correlation was found between low OPP and decreased rim area (P = 0.045), increased cup area (P = 0.045), and increased C/D (P = 0.035). These results demonstrate the importance of the BP status as an independent risk factor in determining OAG risk. In the 2013 analysis (Topouzis et al., 2013) the role of OPP status was investigated in 135 OAG patients (94 OAG and 41 pseudoexfoliative glaucoma). In this population, DOPP was found to be significantly associated with OAG only in subjects using antihypertensive treatment (OR: 0.78 per 10 mmHg, 95%CI: 0.62–0.97; P = 0.028). The association between DOPP and OAG was not statistically significant in subjects not using antihypertensive treatment (OR: 0.98 per 10 mmHg, 95%CI: 0.72–1.33; P = 0.891) (Table 2). Therefore, in the 2013 TES the authors confirmed the association between low DOPP and OAG in subjects who use antihypertensive treatment.
The relationship of BP, OPP, and OPP status has been identified in other large studies. South India Study (Deb et al., 2014) conducted in 208 Indian subjects (108 with systemic hypertension and 100 healthy controls) found a higher MOPP to be a protective factor against glaucoma risk in hypertensive patients. Specifically, the authors found a decreased OAG risk in subjects with hypertension for every mmHg increase in MOPP (OR: 0.69, 95%CI: 0.55–0.87; P < 0.01). Similarly, in the Rotterdam Study (Hulsman et al., 2007) on 5317 participants, only in subjects under treatment for systemic hypertension was a low DOPP (< 50 mmHg) positively associated with hypertensive OAG (OR: 4.68, 95%CI: 1.29–17.01); interestingly, the association with NTG was inverted (OR: 0.25, 95%CI: 0.10–0.63). Recently, Cantor et al. (2018) performed a cross sectional study on 1272 hypertensive patients in six Colombian cities. Patients had to be treated for systemic hypertension for at least 12 months to be included in the study population. The authors found that 131 patients (10.3%) had suspect OAG, and 65 (5.1%) had confirmed OAG. An increased risk of OAG was associated with OPP and DBP. Specifically, a 2 times higher risk of OAG was associated with lower levels of MOPP and DOPP (OPP < 40 mmHg: OR: 2.1, 95%CI: 1.1–4.1; DOPP < 50 mmHg: OR: 2.2, 95%CI: 1.0–5.1), and a higher level of SOPP (SOPP > 130 mmHg) almost tripled the risk (OR: 2.7, 95%CI: 1.1–6.4; P < 0.05). Interestingly, the authors (using p < 0.10) identified OAG risk associated with higher DBP (> 90 mmHg: OR: 2.2, 95%CI: 0.9–5.5, P = 0.08). These results were obtained after multinomial logistic regression adjusted for gender, age, diabetes mellitus, antihypertensive drugs and IOP level.
The importance and differential in outcomes of adjusting for IOP is highlighted in relation to both BP and OPP. As mentioned above, in the Rotterdam Study (Hulsman et al., 2007), the association between the MOPP and the OAG incidence was statistically significant only if not adjusted for IOP. A new analysis on the population of the Rotterdam Study (Springelkamp et al., 2017) (mean follow up: 12.1 years) evaluated glaucoma in terms of functional (visual field loss) and/or structural damage. Baseline IOP was positively correlated with an increased risk of incidence of all glaucoma outcomes (functional: RR: 1.14, 95%CI: 1.10–1.19; structural: RR: 1.11, 95%CI: 1.08–1.15; combined: RR: 1.18, 95%CI: 1.11–1.24; P < 0.001), while baseline DBP was not shown to significantly increase risk (functional: RR: 1.00, 95% CI: 0.98–1.01; P = 0.81; structural: RR: 1.00, 95%CI: 0.99–1.01; P = 0.58; combined: RR: 0.99, 95%CI: 0.96–1.01; P = 0.30). Recently, the Singapore Epidemiology of Eye Diseases Study (Tham et al., 2018), conducted in a multi-ethnic Asian population of 9877 participants found SOPP associated with OAG. Specifically, adjusting for sex, age, race, diabetes mellitus, BMI, history of smoking, treatment for systemic hypertension, IOP and ocular hypotensive treatment, only the lowest quartile (< 110 mmHg, OR: 1.85; 95%CI: 1.16–2.95; P = 0.009) and highest quartile (> 137 mmHg, OR: 1.72, 95%CI: 1.13–2.61; P = 0.011) levels of SOPP were associated with OAG. These adjustments for covariates eliminated the statistical significance for both low MOPP and low DOPP. The Nigeria National Blindness and Visual Impairment Survey (Kyari et al., 2016) investigated the risk factors associated with glaucoma prevalence on 13,591 participants. A higher MOPP was associated with a decreased risk of OAG (OR: 0.96, 95%CI: 0.95–0.97; P < 0.001) only in univariate analysis, while the statistical significance was lost in multivariate analysis (after adjusting for sex, age, ethnicity, literacy, residence, systemic BP, body mass index, ocular axial length, and IOP). Higher IOP was found to increase risk (multivariable: OR: 1.22, 95%CI: 1.18–1.25; P < 0.001; univariable: OR: 1.21, 95%CI: 1.18–1.23; P < 0.001) in both statistical approaches. This highlights the importance of accounting for multiple confounding variables that may either mask or amplify individual study outcomes and points to the need for innovative modeling to determine multi-input inclusive risk.
In conclusion, numerous large population-based studies conducted across multiple genetic regions over the last three decades have shown a consistent strong association between OPP, BP and glaucoma prevalence while these relationships in glaucoma incidence and progression are less well-defined. Several confounding factors currently limit specificity in understanding overall risk including: differing study population characteristics (age, gender, race, etc.), hypertensive status and the use of systemic anti-hypertensive medications, differing patient groupings, accounting for IOP and constrained statistical approaches. The diverse results and lack of consensus among studies points to the need for more advanced approaches to data interpretation that will account for multi-input variables and demographics across diverse data sets.
4.3. Longitudinal evidence of directly measured blood flow biomarkers
The outcomes of numerous clinical studies reviewed below have linked impaired ocular blood flow (as directly assessed via multiple imaging modalities) with glaucoma progression, both structurally and functionally. Additional longitudinal studies investigating the relationship between directly measured biomarkers of ocular blood flow and glaucoma progression are greatly needed to confirm this association. The historical hurdles to conduct a large longitudinal study of ocular blood flow and glaucoma progression include lack of gold standards for hemodynamic assessment, significant cost, the lengthy time required to observe glaucoma progression, lack of current therapies to treat ocular vascular deficiencies, adherence to IOP paradigms, and the limited number of researchers and expertise available to monitor these chronic events. Perhaps the most pivotal consideration in review of the data is the question of whether altered hemodynamic biomarkers occur as a primary insult or secondary to IOP-influenced tissue loss. To date, no study or statistical approach has been able to provide a definitive answer; such a conclusion would forever transform glaucoma patient management. In this section, data from longitudinal studies that have explored the relationship between alterations in ocular blood flow and glaucoma progression are reviewed, with a specific focus on methodological limitations and the need for innovative approaches to fill in the gaps of understanding between vascular health and glaucoma pathophysiology.
4.3.1. Retrobulbar vascular bed
The retrobulbar vascular bed is one of the most studied ocular vascular beds that has shown an association between glaucomatous functional and structural progression. Galassi et al. (2003) and Martínez and Sánchez (2005) conducted two of the first longitudinal studies that investigated the relationship between retrobulbar blood flow and functional glaucoma progression. Galassi et al. investigated the relationship between retrobulbar blood flow and perimetric progression in 44 OAG participants over a period of 7 years (Galassi et al., 2003). Functional progression was defined as irreversible visual field loss in at least 3 consecutive visual field exams, using the classification of Aul-horn. Patients with functional progression (18) had a lower EDV and a higher calculated resistivity index (RI) in the OA compared to those with a stable visual field (P < 0.001). Indeed, a 6-fold increase in the risk of perimetric progression was found in patients with an OA RI ≥ 0.78 (OR: 6.61, 95%CI 1.67–26.1, P = 0.007). Martínez and Sánchez similarly performed a CDI assessment of the RI in the OA and short posterior ciliary arteries (SPCA) of 49 PAOG subjects every 6 months for 36 months (Martínez and Sánchez, 2005). Visual field progression was defined by the occurrence of a new defect or by the deepening or expansion of existing defects confirmed in two additional reliable visual fields performed within 1 month. Results indicated that higher baseline values of OA and SPCA resistivity indices were predictors of functional glaucomatous disease progression. Specifically, the positive likelihood ratio for OA RI > 0.72 was 10.74 (95%CI 2.80–41.21) and for SPCA RI > 0.65 was 7.91(95%CI 2.71–23.11). While both studies highlighted directly assessed biomarkers of retrobulbar blood flow as predictors of functional progression in OAG patients, the level of IOP (an established risk factor for glaucoma onset and progression) was managed differently in the two study populations. Galassi et al. considered an IOP higher than 23 mmHg as one of the criteria for glaucoma diagnosis, and all patients started an IOP-lowering treatment after the baseline CDI assessment. Conversely, only patients with a perimetric disease progression confirmed by three visual field examinations were treated with standard topical therapy by Martínez and Sánchez. Interestingly, the authors of the latter study found that IOP was not associated with an increased risk of disease progression. A possible explanation claimed by the authors was the primary role of the impaired retrobulbar blood flow independent from IOP. However, it is important to note that ocular hypotensive medications have differing mechanisms of action, some of which may influence ocular blood flow. The differing methodologies governing these two studies are an example of the limitations of using standard comparative statistics to interpret clinical study results. Applying mathematical models to data from these studies yields an approach that accounts for a more comprehensive set of parameters that influence the progression of glaucomatous damage (including therapy mechanisms), and thereby allows for more accurate data comparisons and analyses.
Other clinical studies have also investigated the relationship between retrobulbar blood flow and structural disease progression. Calvo et al. prospectively analyzed 262 suspect glaucoma subjects during a 48-months follow-up period (Calvo et al., 2012). The authors used Moorfields Regression Analysis (MRA) classification of the HRT to evaluate glaucoma conversion. Subjects who progressed to glaucoma (36) had statistically significant lower baseline OA EDV and higher baseline OA RI compared to subjects who did not convert. The OA EDV was 5.76 (SD: 2.7) cm/sec in the group of MRA converters and 7.87 (3.5) cm/sec in the non-converters group (P = 0.001); the OA RI was 0.80 (0.1) in MRA converters and 0.75 (0.1) in the non-converting group (P < 0.001). A positive correlation was found between an OA RI > 0.75 and the development of disease in glaucoma suspects (HR: 3.306, 95%CI 1.448–7.547, P = 0.002). A similar methodology was used by Jimenez-Aragon et al. who performed a five-year longitudinal study in 71 subjects that were also analyzed for structural progression via HRT MRA (Jimenez-Aragon et al., 2013). Levels of OA and CRA EDV and RI significantly differed in subjects who progressed structurally (12) compared to those who did not progress (59). Specifically, the OA EDV was 5.29 (2.83) cm/sec in the progression group and 7.32 (3.01) cm/sec in the non-progression group (P = 0.043); the OA RI was 0.79 (0.05) in the progression group and 0.75 (0.05) in the non-progression group (P = 0.038); the CRA EDV was 1.88 (0.54) cm/sec in the progression group and 2.41 (0.84) cm/sec in the non-progression group (P = 0.034); and the CRA RI was 0.77 (0.06) in the progression group and 0.73 (0.05) in the non-progression group (P = 0.043). The inclusion criteria of the studies’ subjects varied, and thus caution must be used when interpreting and comparing the results. For instance, the subjects of the Calvo study (Calvo et al., 2012) had a normal visual field, IOP≥21 mmHg and a glaucomatous appearance of the optic nerve at baseline. The subjects of the Jimenez-Aragon study (Jimenez-Aragon et al., 2013) included both patients with initial perimetric glaucoma and subjects with a normal perimetry. Subjects with a normal perimetry exhibited either a normal optic nerve head and elevated IOP, or a glaucomatous optic nerve head and normal IOP. The limitations of consensus in specific outcomes may be linked to differing inclusion criteria; new approaches using AI that account for diverse inclusion parameters used for patient selection may help to increase vascular biomarker risk specificity.
The IGPS was specifically designed to determine the relationships between multiple measures of ocular hemodynamics and glaucomatous optic neuropathy progression, both structurally and functionally. Moore et al. investigated simultaneous structural and functional progression in 112 OAG patients examined twice at baseline (for agreement) and every 6 months over a 4-year period (Moore et al., 2017). Functional progression was defined as two consecutive visits with an Advanced Glaucoma Intervention Study score increase ≥2 or visual field mean defect decrease ≥2 from baseline (Humphrey). Structural progression was defined as two consecutive visits with a retinal nerve fiber layer thickness decrease ≥8% and/or horizontal or vertical cup/disc ratio increase by ≥ 0.2 from baseline (assessed by both HRT and OCT). The authors found that lower baseline OA velocities were associated with both functional and structural progression after 4 years (Table 3). Specifically, lower baseline OA PSV (23.42 ± 1.34 cm/s) and EDV (5.41 ± 0.27 cm/s) were found in OAG patients who progressed functionally (n = 35) compared with those who did not progress (n = 77; OA PSV: 26.96 ± 1.18 cm/s, P = 0.031; OA EDV: 5.41 ± 0.27 cm/s, P = 0.005). Similar results were found in patients with structural progression (n = 75), with lower baseline OA PSV (24.25 ± 0.80 cm/s) and EDV (5.81 ± 0.20 cm/s) compared with subjects who did not structurally progress (n = 37; OA PSV: 29.11 ± 2.18 cm/s, P = 0.024; OA EDV: 7.19 ± 0.51 cm/s, P = 0.012). The IGPS analysis also demonstrated a mean OA RI > 0.75 in OAG patients who progressed functionally and structurally after 4 years, confirming the results from Calvo et al. (2012) Importantly, the IGPS study was observational, and its study population were treated with a variety of surgeries and IOP-lowering medications that are difficult to fully account for, even with extensive statistical adjustments.
Table 3.
Baseline retrobulbar blood flow findings associated with structural and functional glaucoma progression after four years. With permission from the publisher and authors (Moore et al., 2017).
| Structural Progression | Functional Progression | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Progressed | Not Progressed | P-value | Progressed | Not Progressed | P-value | |||||
| N | Mean (SE) | N | Mean (SE) | N | Mean (SE) | N | Mean (SE) | |||
| OA PSV | 75 | 24.25 (0.80) | 37 | 29.11 (2.18) | 0.024* | 35 | 23.42 (1.34) | 77 | 26.96 (1.18) | 0.031* |
| OA EDV | 75 | 5.81 (0.20) | 37 | 7.19 (0.51) | 0.012* | 35 | 5.41 (0.27) | 77 | 6.65 (0.29) | 0.005* |
| OA RI | 75 | 0.755 (0.007) | 37 | 0.744 (0.011) | 0.528 | 35 | 0.756 (0.010) | 77 | 0.749 (0.01) | 0.520 |
| CRA PSV | 75 | 8.41 (0.23) | 37 | 8.85 (0.32) | 0.275 | 35 | 8.49 (0.40) | 77 | 8.59 (0.20) | 0.824 |
| CRA EDV | 75 | 2.46 (0.07) | 37 | 2.54 (0.15) | 0.862 | 35 | 2.53 (0.14) | 77 | 2.46 (0.08) | 0.937 |
| CRA RI | 75 | 0.703 (0.007) | 37 | 0.712 (0.012) | 0.464 | 35 | 0.696 (0.014) | 77 | 0.710 (0.01) | 0.341 |
| NPCA PSV | 74 | 7.63 (0.22) | 37 | 7.90 (0.29) | 0.479 | 35 | 7.43 (0.29) | 76 | 7.85 (0.22) | 0.280 |
| NPCA EDV | 74 | 2.51 (0.09) | 37 | 2.61 (0.11) | 0.385 | 35 | 2.53 (0.12) | 76 | 2.55 (0.09) | 0.849 |
| NPCA RI | 74 | 0.666 (0.008) | 37 | 0.668 (0.008) | 0.887 | 35 | 0.658 (0.012) | 76 | 0.671 (0.01) | 0.291 |
| TPCA PSV | 75 | 7.81 (0.20) | 37 | 8.40 (0.30) | 0.095 | 35 | 8.14 (0.34) | 77 | 7.94 (0.19) | 0.588 |
| TPCA EDV | 75 | 2.51 (0.08) | 37 | 2.72 (0.13) | 0.148 | 35 | 2.58 (0.14) | 77 | 2.58 (0.08) | 0.873 |
| TPCA RI | 75 | 0.677 (0.007) | 37 | 0.675 (0.012) | 0.998 | 35 | 0.682 (0.010) | 77 | 0.673 (0.01) | 0.549 |
Denotes a statistically significant difference from baseline to four-year follow-up between patients who progressed functionally or structurally and those who did not progress (p < 0.05).
Note: Baseline NPCA measurements were unable to be obtained in one patient due to poor image quality. Table 3 legend: Ophthalmic Artery (OA), Central Retinal Artery (CRA), Nasal Posterior Ciliary Artery (NPCA), Temporal Posterior Ciliary Artery (TPCA), Peak Systolic Velocity (PSV), End Diastolic Velocity (EDV), Resistive Index (RI), Standard Error (SE).
4.3.2. Other vascular tissues
While the aforementioned studies investigated the relationship between glaucoma progression and retrobulbar perfusion, a smaller number of longitudinal studies have used different imaging techniques to assess the role of ocular hemodynamics in glaucoma progression. Foremost, Tobe et al. evaluated the association between retinal capillary blood flow assessed via confocal scanning laser Doppler and structural progression in 103 OAG patients over an 18-month period (Tobe et al., 2015). A reduction in retinal capillary blood flow in the superior retina was found to be associated with structural progression. Specifically, a greater increase in the OCT-derived cup/disc ratio correlated with a higher percentage of active capillary loss in the superior retina after 3 years (β = 0.4803, SE = 0.1975, P = 0.0170). This analysis suggests local retinal tissue may experience ischemia in addition to a lower supply of perfusion from the retrobulbar blood vessels. A study recently published by Kiyota et al. investigated the association between functional glaucoma progression and optic nerve head blood flow assessed via LSFG (Kiyota et al., 2019). In 508 eyes of 309 OAG patients (avg. follow up 4.7 ± 1.1 years), functional progression was measured in terms of average total deviation and total deviation slope in different sectors. A statistically significant association was found between the baseline superior to temporal LSFG-derived blood flow parameters and the perimetric progression in the corresponding sectors of the visual field. Recently, Shiga et al. using LSFG also identified reduced optic nerve head blood flow as being associated with visual field progression in normotensive preperimetric glaucoma followed for at least 16 months (n = 84) (Shiga et al., 2018). New imaging modalities such as OCTA are providing new insights into vascular insult at the optic nerve head level. In a non-longitudinal cross sectional study of 74 normal, 28 preperimetric, 83 early, 43 moderate, and 45 eyes with severe glaucoma, Kumar et al. found OCTA vascular biomarkers to have better discriminant ability (AUC, sensitivity, and specificity: 0.70, 69.2%, and 72.9%, respectively) than structural parameters between normal and preperimetric glaucomatous eyes (Kumar et al., 2016). One of study limitations was the omission of systemic BP and OPP that could affect results. In a 2017 study by Shoji, significantly faster loss of macula vessel density was found in OAG eyes compared to controls (14-month follow up) and changes in macular vascular density were observed prior to structural glaucomatous progression (Shoji et al., 2017). Comparison and interpretation these data between studies using confocal scanning laser Doppler, LSFG, and OCTA is not possible with statistical approaches as the vascular tissue beds they measure are not the same and the implications of their outcomes measures differ in relation to disease pathophysiology.
As previously discussed, there is racial disparity in glaucoma progression; persons of African descent have been shown to exhibit a higher risk of glaucoma progression (Racette et al., 2003; Leske et al., 2008; Huck et al., 2014). Siesky et al. was the first to identify comparatively lower directly assessed ocular hemodyanimc biomarkers in glaucoma subjects of African descent compared to European counterparts (Siesky et al., 2015). African descent OAG patients had lower OA PSV (P = 0.0001) and EDV (P = 0.0008), lower CRA PSV (P = 0.01), and lower temporal and nasal short posterior ciliary artery PSV (P = 0.0037 and P < 0.0001, respectively) compared to their white counterparts. Importantly, these lower blood flow velocities in the vessels supplying the eye occurred despite significant differences that were found in intraocular pressure or visual field parameters. Building upon this novel study, in 2016 Siesky et al. confirmed reductions in retrobulbar blood flow and retinal capillary in OAG patients of African descent more strongly correlated with changes in the ONH (Table 4) and macular thickness (Table 5) over a period of 4 years compared to European counterparts (Siesky et al., 2016). The authors suggested that ocular vascular health might be a more influential contributing factor in the pathophysiology of OAG in patients of African descent thereby partially accounting for their glaucomatous disease disparity.
Table 4.
Correlations between changes in retrobulbar blood flow velocity with changes in optic nerve head morphology in OAG patients of African and European descent. OAG: open-angle glaucoma. With permission from the publisher and authors (Siesky et al., 2016).
| Technique | Measurement | NPCA | |||||||||
| RI | EDV | ||||||||||
| AD | ED | Comparison AD vs. ED | AD | ED | Comparison AD vs. ED | ||||||
| r | p | r | p | p | r | p | r | p | p | ||
| OCT | Cup area | 0.63 | 0.01 | −0.01 | 0.92 | 0.01 | −0.57 | 0.02 | 0.04 | 0.78 | 0.02 |
| Cup area | 0.64 | 0.00 | −0.05 | 0.68 | 0.00 | −0.47 | 0.05 | 0.08 | 0.57 | 0.05 | |
| TPCA | |||||||||||
| RI | EDV | ||||||||||
| AD | ED | Comparison AD vs. ED | AD | ED | Comparison AD vs. ED | ||||||
| r | p | r | p | p | r | p | r | p | p | ||
| −0.55 | 0.02 | 0.26 | 0.04 | 0.00 | −0.39 | 0.13 | 0.18 | 0.18 | 0.04 | ||
| Cup area | −0.29 | 0.26 | 0.09 | 0.49 | 0.18 | −0.50 | 0.04 | 0.11 | 0.40 | 0.02 |
Bold P-values indicate statistical significance (p < 0.05).
Table 3 legend: AD–African descent; ED–European descent; EDV–end diastolic velocity; HRT-III–Heidelberg retinal tomography-III; NPCA–nasal posterior ciliary artery; OAG–open angle glaucoma; OCT–optical coherence tomography; r-correlation coefficient; RI–resistive index; RNFLT-retinal nerve fiber layer thickness; TPCA–temporal posterior ciliary artery
Table 5.
Correlations between changes in zero blood flow pixels and changes in macular thickness over four years in OAG patients of African and European descent. OAG: open-angle glaucoma. With permission from the publisher and authors (Siesky et al., 2016).
| Retinal Area Zero Blood Flow Pixels |
Macular Region | African Descent (AD) | European Descent (ED) | Comparison AD vs. ED | ||||
|---|---|---|---|---|---|---|---|---|
| n | r | p-value | n | r | p-value | p-value | ||
| Superior Retina | outer superior | 18 | −0.61 | 0.006 | 59 | −0.09 | 0.476 | 0.034 |
| inner superior | 18 | −0.51 | 0.031 | 59 | 0.01 | 0.944 | 0.051 | |
| outer inferior | 18 | −0.76 | 0.000 | 59 | −0.05 | 0.700 | 0.001 | |
| inner inferior | 18 | −0.64 | 0.002 | 59 | −0.21 | 0.120 | 0.060 | |
| outer nasal | 18 | −0.58 | 0.010 | 59 | 0.03 | 0.827 | 0.017 | |
| inner nasal | 18 | −0.50 | 0.032 | 59 | −0.16 | 0.235 | 0.174 | |
| outer temporal | 18 | −0.60 | 0.007 | 59 | −0.19 | 0.150 | 0.083 | |
| inner temporal | 18 | −0.27 | 0.289 | 59 | 0.02 | 0.864 | 0.307 | |
| macular volume | 18 | −0.61 | 0.006 | 59 | −0.12 | 0.361 | 0.044 | |
| Inferior Retina | outer superior | 18 | −0.69 | 0.001 | 57 | 0.04 | 0.743 | 0.002 |
| inner superior | 18 | −0.55 | 0.017 | 57 | −0.15 | 0.281 | 0.109 | |
| outer inferior | 18 | −0.80 | 0.000 | 57 | −0.10 | 0.452 | 0.001 | |
| inner inferior | 18 | −0.65 | 0.003 | 57 | −0.17 | 0.211 | 0.039 | |
| outer nasal | 18 | −0.66 | 0.002 | 57 | −0.21 | 0.124 | 0.049 | |
| inner nasal | 18 | −0.54 | 0.020 | 57 | −0.05 | 0.708 | 0.059 | |
| outer temporal | 18 | −0.58 | 0.010 | 57 | 0.06 | 0.670 | 0.014 | |
| inner temporal | 18 | −0.24 | 0.344 | 57 | −0.04 | 0.780 | 0.479 | |
| macular volume | 18 | −0.64 | 0.004 | 57 | −0.07 | 0.629 | 0.019 | |
Bold P-values indicate statistical significance (p < 0.05).
Table 5 legend: n-number of participants; r-correlation coefficient.
All of these longitudinal studies demonstrate the potential importance of directly assessing hemodynamic biomarkers in predicting glaucoma progression. However, several significant limitations plague our full understanding of these relationships. Do high IOP and/or tissue loss result in the observed lower perfusion biomarkers, or is vascular insult primary for disease progression in certain individuals? While these data provide strong evidence that hemodynamic alterations may be primary in certain individuals, a lack of large population-based studies with comparative controls prohibits certainty. Further complicating consensus are difficulties in comparisons of patient cohorts, disease progression outcomes, diverse imaging modalities of differing tissue beds, data omissions, and concurrent disease treatments that may affect hemodynamics. A purposefully designed comprehensive AI-based model informed by mechanistic hypotheses while accounting for all of these factors may be the only realistic method to provide the definitive answer of primary versus secondary vascular involvement in glaucoma progression.
5. Role of the vasculature in other ocular diseases
Alterations in hemodynamic factors have been associated with many pathologic conditions that affect ocular tissues and visual function in addition to glaucoma (Prada et al., 2019). In particular, impairments in blood flow, vessel geometry, vascular mechanical properties, vascular functionality, and BP have been observed in patients with AMD, DR, RVO, RAO, and NAION. In many cases, these impairments result in insult to the retinal tissues and ganglion cells.
When studying diseases of the retina, it is important to consider its unique structure and vascular supply. The retina is a well-organized, layered structure with a vascular supply that also exhibits a characteristic layered pattern. The retina is supplied by three major capillary plexi: the superficial capillary plexus (SCP), intermediary capillary plexus, and DCP. These three capillary layers are differentiated well at the macula but are not in the peripheral retina. The foveal region in the center of the macula lacks characteristic blood vessels and is termed the foveal avascular zone (FAZ). This region of the retina (which measures 0.5 mm in diameter) requires the diffusion of oxygen from the choroid, the vitreous, and the outer macular rim. Alterations in the vasculature that supply and drain the capillary plexi of the retinal layers have been shown to contribute to the development and progression of retinal diseases including AMD and DR.
5.1. Age related macular degeneration
AMD is the leading cause of blindness in adults in developed countries in individuals over 65 years of age (Pascolini et al., 2004; Hyman, 1987). AMD can be classified as one of two types: non-neovascular (or dry) AMD and neovascular (or wet) AMD (Iroku-Malize and Kirsch, 2016). Dry AMD is characterized by deposition of drusen and pathologic changes in the retinal pigment epithelium (RPE) in the macular region. In later stages, it progresses to areas of geographic atrophy with irreversible damage to the RPE and photoreceptors (Bandello et al., 2017). Wet AMD is characterized by choroidal neovascularization that leads to exudation with or without hemorrhage. The exudation eventually leads to fibrotic scar formation and disruption of the architecture of the RPE and photoreceptors. AMD exclusively affects the macula and, therefore, leads to the loss of central vision.
Alterations in both the choroidal and retinal vasculature are believed to play an important role in AMD, as indicated by decreased vascular density in the choriocapillaries, SCP and DCP of 69.2%, 33.3% and 17.3% in eyes with early AMD compared to 75.8%, 40.5% and 20.8% in healthy eyes (p = 0.006, p < 0.001, p = 0.001, respectively) (Lee et al., 2018). Specifically, a reduced blood supply to the choroid likely decreases the amount of oxygen available to supply the FAZ, making it more susceptible to ischemia. It has also been demonstrated that choroidal blood velocity, blood volume and blood flow decreased progressively with an increase in the severity of AMD (p = 0.047, p = 0.02 and p = 0.003, respectively) (Grunwald et al., 2005). The pathogenesis of both dry and wet AMD are still poorly understood, and it has been proposed that photoreceptor and RPE ischemia secondary to poor choroidal perfusion contributes to both classifications of the disease (Friedman, 1997). Ocular imaging techniques, such as OCTA and FA, have been utilized to measure biomarkers of ocular blood flow and choroidal neovascularization in order to help determine the influence of vascular alterations in the pathogenesis of both forms of AMD (Albert et al., 2008; Moult et al., 2014; Novais et al., 2016). Geographic atrophy is characterized by outer retinal and choroidal atrophy, specifically the photoreceptor layer, RPE, and choriocapillaris. These three layers are closely linked anatomically and functionally, and the sequence of events that end up in atrophy is important in the understanding of the pathogenesis of the disease. Recent studies in advanced AMD have shown that loss of choriocapillaris might precede overt RPE atrophy. OCTA showed atrophy of choriocapillaris underneath and beyond the region of photoreceptors and RPE loss, in agreement with previous histopathologic studies. This reduced choriocapillaris flow beyond the borders of geographic atrophy seen on OCTA suggests a primary vascular cause in geographic atrophy (McLeod et al., 2009; Arya et al., 2018; Moreira-Neto et al., 2018). Additionally, the evolution of OCTA technology suggests that CNV seems to originate from regions of severe choriocapillaris alteration (Choi et al., 2015; Schneider et al., 2018).
5.2. Diabetic retinopathy
DR is a leading cause of visual impairment in individuals between the ages of 25 and 75, affecting approximately 4.2 million people worldwide (Hendrick et al., 2015). Most patients who develop DR do not experience symptoms until the very late stages, and since vision threatening complications can occur rapidly in these patients, it is crucial for diabetic patients to be screened for the development of retinal disease (Fraser and D’Amico, 2018). Furthermore, screening is important because therapy may be introduced to both ameliorate symptoms and slow disease progression. Although the pathogenesis of DR is multifactorial, it is primarily due to chronic hyperglycemia leading to pericyte loss around the capillary network and subsequent retinal ischemia. Loss of pericytes leads to increased vascular permeability and capillary loss (Mizutani et al., 1996). Another vascular alteration contributing to DR is retinal microthrombosis, as indicated by a doubling of the number of capillary segments with colocalized immunostaining for factor XIII and GPIIIa in diabetic eyes when compared to nondiabetic eyes (p = 0.02). This leads to retinal capillary occlusion and damage (Boeri et al., 2001). Retinal capillary occlusion and damage lead to ischemia, which induces the release of growth factors (e.g., vascular endothelial growth factor, VEGF) and the promotion of neovascularization. These vascular changes can be detected using OCTA at both the non-proliferative and proliferative stages of the disease; diabetic patients without clinical retinopathy showed consistently higher perfused capillary density compared to healthy controls (36.6% ± 3.30% vs. 33.6% ± 3.98%, p = 0.034), whereas diabetic patients with either nonproliferative or proliferative DR demonstrated progressively decreasing perfused capillary density (Rosen et al., 2019; Zeng et al., 2019). These findings reveal that OCTA can be implemented as a clinical tool for earlier objective detection of preclinical DR. OCTA has also been utilized to associate decreased deep perifoveal vessel density (adjusted mean: 51.93 vs. 49.89 vs. 47.96, p-trend = 0.005), increased superficial (adjusted mean: 235.0 μm vs. 303.4 μm vs. 400.9 μm, p-trend = 0.003) and deep (adjusted mean: 333.1 μm vs. 513.3 μm vs. 530.2 μm, p-trend = 0.001) FAZ area, and decreased retinal sensitivity (adjusted mean: 25.12 dB vs. 22.34 dB vs. 20.67 dB, p-trend = 0.003) with increasing severity of DR (Tsai et al., 2019). These results were recently supported with DR eyes showing decreased choriocapillaris whole-image capillary perfusion density (62.6 ± 6.1 vs. 68.4 ± 5.1, p < 0.003), decreased retinal whole-image capillary perfusion density (46.9 ± 5.1 vs. 50.7 ± 5.6, p < 0.04), and increased FAZ area (0.307 ± 0.133 mm2 vs. 0.184 ± 0.058 mm2, p = 0.008). Additionally, following 12 months of anti-VEGF treatment in these DR eyes, no changes to retinal capillary or choriocapillaris capillary perfusion density and FAZ area were observed, indicating the treatment’s efficacy in preventing disease progression (Conti et al., 2019). These findings demonstrate that OCTA can be useful to monitor the progression of DR and the efficacy of treatment.
5.3. Retinal vein occlusion
RVO involves the formation of a thrombus within a retinal vein that inhibits blood flow from the retina, leading to the subsequent death of the retinal ganglion cells and corresponding vision loss. RVO represents the second most common cause of visual loss from retinal vascular disease (Cugati et al., 2006). The disease is classified into 3 distinct forms depending on the location of the occlusion in the retina. CRVO, the most common of the three forms, occurs when the CRV is occluded. The CRV runs in parallel with the CRA in the optic nerve sheath where it gives off multiple collateral vessels before crossing through the lamina cribrosa. These vessels are particularly susceptible to compression from increased IOP, mechanical stretching of the lamina cribrosa, and posterior bowing of the lamina. There are two types of CRVO: ischemic CRVO and non-ischemic CRVO. Non-ischemic CRVO displays less than 10 disc areas of retinal capillary non-perfusion and likely results from an occlusion of the CRV at a more posterior location, which allows for collateral channels to compensate in venous drainage, resulting in less visual field loss (Oellers et al., 2018). Ischemic CRVO displays 10 or more disc areas of retinal capillary nonperfusion and likely occurs at a more anterior location of the CRV, leading to a higher degree of visual field loss. OCTA has been implemented to further elucidate the vascular differences between the two types of CRVO, with the ischemic type demonstrating decreased flow in the SCP (1.014 ± 0.264 vs. 1.279 ± 0.19, p = 0.003), DCP (0.873 ± 0.442 vs. 1.152 ± 0.32, p = 0.046) and choriocapillaris (0.79 ± 0.327 vs. 1.424 ± 0.51, p < 0.001), and decreased vascular density in the SCP (44.24 ± 2.13 vs. 46.58 ± 4.13, p = 0.028) and DCP (45.28 ± 3.5 vs. 49.32 ± 3.94, p = 0.003) in comparison to the non-ischemic type (Khodabandeh et al., 2018).
Branched retinal vein occlusion (BRVO) occurs when a distal retinal vein is occluded due to the compression from retinal arterioles at arteriovenous crossing points. These occlusions are often associated with sclerotic retinal arterioles, degenerative changes within venous walls, and hypercoagulability (Bowers et al., 1987; Jaulim et al., 2013). OCTA has been utilized to demonstrate that BRVO eyes have a reduction rate in SCP mean vessel density of 13.88% and in DCP mean vessel density of 24.60% in comparison to healthy controls. These findings reveal that the DCP is more susceptible than the SCP to ischemic damage in BRVO (Kim J.T. et al., 2019). Hemiretinal vein occlusion, the least common of the three forms, occurs when a vein that drains the superior or inferior hemiretina (often the superior or inferior branch of the central retinal vein) is occluded.
RAO, which is much less common than RVO, involves thrombosis within a retinal artery that inhibits the blood flow to retinal ganglion cells, causing cell death and vision loss. Similar to RVO, RAO is classified as central RAO (CRAO) or branched RAO (BRAO). CRAO involves an occlusion of the CRA after branching off from the ophthalmic artery and entering the orbit, and represents an ocular emergency (Yuzurihara and Iijima, 2004). An occlusion of any of the 4 main branches of the CRA that supply the inner layers of the retina is considered a BRAO.
5.4. Non-arteritic ischemic optic neuropathy
Lastly, NAION is the most common clinical presentation of ischemic damage to the optic nerve and the most common form of persistent monocular vision loss in individuals over 50 years of age (Berry et al., 2017). The field defect is almost always unilateral, altitudinal, and involves the area of central fixation. NAION is believed to be caused by impaired blood flow in the prelaminar area of the optic nerve, although the exact mechanism is poorly understood. The occlusion is believed to be located in the branches of the peripapillary choroidal arterial system (Ropper et al., 2014). Furthermore, blood flow velocities of the nasal posterior ciliary artery (p < 0.05), CRA (p < 0.001), and capillaries at both the temporal (−20.2% and −18.5%) and nasal (−12.8% and −12.4%) sites of the optic nerve head have shown to be considerably reduced in NAION patients (Kaup et al., 2006; Collignon-Robe et al., 2004). The lack of sufficient blood flow to the optic nerve results in an abrupt and painless onset of vision loss in these patients. Several recent studies have examined the retinal vasculature in NAION. Gaier et al. evaluated OCTA findings in patients with NAION. They concluded that if the eyes are affected acutely, OCTA revealed a reduction in the signal from the major retinal vessels. Additionally, a dilation of patent superficial capillaries in the peripapillary area was also seen and the peripapillary choriocapillaris was obscured by edema. In contrast, eyes that were non-acutely affected showed attenuation of patent capillaries. The degree of dilation of the superficial microvasculature in the acute phase and attenuation in the non-acute phase each correlated inversely with visual field performance (Gaier et al., 2018). This was confirmed in other studies, as well (Mastropasqua et al., 2018). In addition to deficient peripapillary choroidal vasculature present in NAION, the vasculature has a different pattern than in nonischemic disc edema and can cause corresponding visual field deficits (Gandhi et al., 2018). OCTA provides detailed visualization of the peripapillary and macular retinal capillary rarefaction, which correlates with VF and visual acuity loss. OCTA could be a useful tool for quantifying and monitoring ischemia in NAION (Augstburger et al., 2018). OCTA reveals a dynamic shift in the superficial capillary network of the optic nerve head with strong functional correlates in both the acute and non-acute phases of NAION.
5.5. Summary
Research has revealed significant evidence of correlations between ocular disease and vascular and hemodynamic alterations. Despite the advancement of ocular imaging techniques that has led to the availability of massive amounts of data, the interpretation of the imaging data remains challenging. One of the reasons for this is that imaging techniques yield measurements that include the complex interaction among factors that are both local to the eye and systemic. Furthermore, the cause and effect relationships giving rise to the correlations revealed from the imaging devices remain poorly understood, which hinders the early diagnosis of these ocular diseases and their effective treatments. Given the complexity of the physiological and hemodynamic mechanisms involved in these diverse ocular diseases, mathematical modeling, machine learning, deep learning, and AI techniques offer new methods that must be combined with ocular imaging data to enhance the interpretation of ocular imaging biomarkers and the understanding of individual disease susceptibility. Together, these tools will be instrumental in helping elucidate whether ocular blood flow alterations are primary or secondary to disease.
6. Mathematical models for ocular blood flow and glaucoma
The previous sections highlighted the complexity of disentangling interactions among multiple factors, including blood flow and BP, that concur to determine pathological conditions in a given individual. While providing invaluable data on human subjects, clinical and population-based studies are limited as to which procedures can be performed to isolate the action of each factor and determine its relative contribution in health and disease. For example, BP is the main driving force of blood flow, but it also contributes to the secretion of aqueous humor and cerebrospinal fluid which, in turn, generates pressures inside the eye globe and within the optic nerve that tend to impede blood flow through the exposed vessels (Arciero et al., 2019). To further complicate the scenario, the extent to which these pressures are sensitive to each other is regulated by multiple control mechanisms, whose effectiveness and functionality may vary among individuals as well as in health and disease. From this perspective, mathematical modeling can complement standard statistical methods by providing a virtual laboratory based on fundamental principles of physics and physiology where conjectures formulated on the basis of clinical and experimental observations can be simulated and tested at minimal cost and in a controlled environment.
Historical Perspective.
Mathematical modeling has been used for centuries as a tool to investigate complex phenomena and to test theories and conjectures in various disciplines. One of the most celebrated examples is Newton’s Second Law published in 1687 (Newton, 1687), which stems from experimental observations that when a constant force acts on a massive body, it causes it to change its velocity, or accelerate, at a constant rate. This fundamental principle of physics can be utilized as a practical tool in real applications only after it is translated from a statement into a mathematical formula, the renowned F = m a, where the symbols F, m and a represent the factors at play, namely the resultant of forces, mass and acceleration, respectively. Newton’s laws have set the foundations for quantitative approaches to scientific investigations and constitute, to date, pivotal milestones for engineering and technology.
Newton’s laws are also widely used to identify the fundamental physical principles governing the behavior of living systems observed experimentally. For example, in vascular physiology, engineering-type studies of blood flow were pioneered by the French physicist and physiologist Jean Léonard Marie Poiseuille in the 19th century (Poiseuille, 1835, 1846) who studied the flow in capillaries by means of experiments performed on glass tubes. As a result of these experiments, he derived an empirical relationship where the rate Q at which fluid passes through the tube increases proportionately to the pressure difference Δp applied at the ends of the tube as well as being proportional to the fourth power of the diameter D of the tube, namely Q ∝ D4 p. Thanks to the work of Stokes, the experimental relationship established by Poiseuille was explained and derived on the basis of Newton’s laws applied to the motion of fluids (Stokes, 1845). Poiseuille’s law continues to be widely used in modeling blood flow in various organs (Ottesen et al., 2004; Formaggia et al., 2010; Fung et al., 2013). However, Poiseuille’s law is limited by numerous simplifying assumptions, including that (i) vessels are straight cylindrical tubes of circular cross-section, (ii) vessel walls are rigid and impermeable, (iii) blood is a Newtonian viscous fluid, and (iv) fluid flow is isothermal, laminar, and not subject to entrance effects (Secomb, 2016b). Details on the derivation of Poiseuille’s law on the basis of physical principles and its simplifying assumptions can be found in Chapters 10 and 15 of (Sacco et al., 2019). Although several of these assumptions are violated within the circula-tory system, Poiseuille’s Law is a key result that is used in hemodynamic modeling with some adaptations. For example, in vessels of diameter less than 300 μm, resistance cannot be calculated simply using Poiseuille’s Law since the relative apparent viscosity is shown to decrease with tube diameter. This phenomenon, known as the Fahraeus-Lindqvist effect, can be accounted for in models by using an empirical relationship between relative apparent viscosity and vessel diameter and hematocrit (Secomb, 2016b).
The work by Womersley (Womersley et al., 1955) and McDonald (1955) in the 1950s on the time-dependent motion of blood in an elastic artery driven by a fluctuating pressure gradient is considered to be the work that triggered the modern era of theoretical hemodynamics (Secomb, 2016b). A textbook by McDonald is a standard reference in the field of biorheology, and additional information on the history of the foundational work in hemodynamics is provided in multiple references (McDonald, 1974; Secomb, 2016b).
The foundation for the theory of oxygen transport to tissue was provided in the early 20th century by the Danish physiologist August Krogh (1919). Krogh aimed to determine the rate at which oxygen was used up by tissue and the average distance that oxygen had to travel from a capillary to the tissue. Thus, he took numerous measurements in striated muscles of mammals, since capillaries were arranged very regularly along the muscle fibers. He proposed that oxygen is supplied to tissue via passive diffusion solely from capillaries and developed a simple model (known as the Krogh tissue cylinder) that described a cylindrical region of tissue supplied by a single capillary. Krogh and mathematician Erlang together formulated a differential equation governing oxygen diffusion and uptake in the Krogh tissue cylinder (Popel, 1989). As new technology has been developed in recent decades to review Krogh’s model, several studies suggest a more complex nature of oxygen delivery to tissue not solely dependent on capillaries. Regardless of its potential limitations, the Krogh model and the Krogh-Erlang equation have formed the basis for most estimates of oxygen transport for the last 100 years.
Motivation for mathematical models.
This brief historical outline shows how models inspired by data (known as data-driven models) and models based on physical principles (known as mechanism-driven models) have successfully complemented each other to advance the understanding and knowledge of complex systems. While data-driven models identify correlations among factors, mechanism-driven models formalize cause-effect relationships in mathematical equations that can serve as a virtual laboratory to investigate how different factors, considered individually or in combination, influence the system. Given these two approaches, mechanism-driven modeling seems to be both a natural and ideal approach for addressing the outstanding questions and controversies illustrated in the previous sections concerning the complex multifactorial interactions influencing ocular biomechanics, hemodynamics and oxygenation.
Ocular biomechanics has received considerable attention in the last decades with the development of mathematical models that can be used to determine tissue strains and stresses due to IOP and CSFp (Ethier et al., 2004; Boote et al., 2019). Remarkably, computational models have been capable of predicting the collagen fibril architecture in the optic nerve head and in corneo-scleral shells as a result of remodeling (Grytz et Meschke, 2010; Grytz et al., 2011) and have enabled the quantification of tissue biomechanical responses to IOP and CSFp via imaging methods (Fazio et al., 2018). Theoretical studies of the impact of ocular mechanics on circulation and oxygenation have begun only recently (Arciero et al., 2013, 2019; Guidoboni et al., 2014). Despite the short timeframe over which these models have been developed, major findings have already been made regarding the impact of IOP, mean arterial pressure, vessel network structure, and flow regulation mechanisms on blood flow and oxygenation of the eye. Figs. 1 and 2 and the sections that follow summarize the main directions that modeling efforts have taken to relate hemodynamics, biomechanics, and oxygen transport in various components of the eye.
Fig. 1.
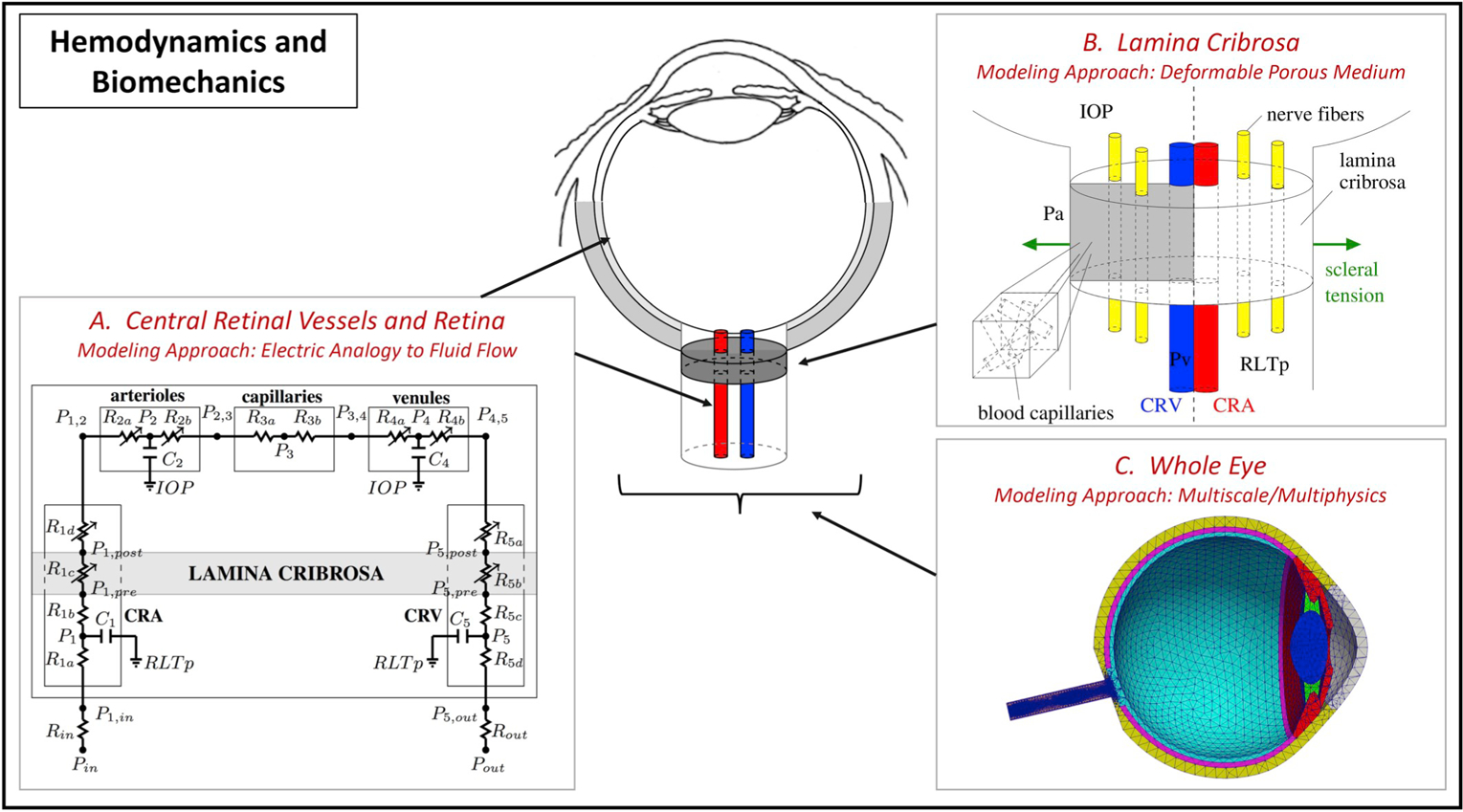
Overview of mechanism-driven models to study the interaction between hemodynamics and biomechanics in A) the central retinal vessels and retina by means of electric analogy to fluid flow. Image reproduced from (Guidoboni et al., 2014b), with permission (Image copyright holder: Association for Research in Vision and Ophthalmology), B) the lamina cribrosa in the optic nerve head by means of deformable porous medium model. Image reproduced from (Prada, 2016), with permission, and C) the whole eye by means of multiscale/Multiphysics model. Image reproduced from (Sala, 2019), with permission.
Fig. 2.

Overview of mechanism-driven models to study the interaction between hemodynamics and oxygenation in A) the retina by means of Krogh cylinder model. Image taken directly from (Carichino et al., 2016), with permission, B) the central retinal vessels and retina by means of advection-diffusion-reaction problem through the retinal vasculature and tissue layers (Causin et al., 2016) (Reprinted by permission from Springer Nature), and C) the retina by means of Green’s function method (Fry et al., 2018).
6.1. Models incorporating hemodynamics and biomechanics: clinically relevant findings
Ocular hemodynamics and biomechanics are strongly intertwined, as schematically represented in Fig. 3. The eye is a pressurized ambient and, as a consequence, the ocular vasculature must function while withstanding the load exerted by external pressures on it. Specifically, intraocular vascular segments are exposed to IOP, whereas blood vessels running through the optic nerve canal are exposed to RLTp, which is related to CSFp and ICP as discussed in Section 4.1.8. Abnormal elevations in these external pressures would lead to a compression of blood vessels, thereby posing a challenge to blood flow through them. The vasculature can counter this challenge by means of active and passive mechanisms. Active mechanisms are triggered by biochemical and biomechanical stimuli that cause vasoactive vessels to constrict or dilate in order to maintain a relatively constant blood supply while meeting the metabolic demand of the tissue. Passive mechanisms stem from the pressure feedback that occurs in hydraulic networks, where vessel constrictions in some portion of the network cause the BP to increase upstream of the constriction and decrease downstream. Furthermore, the ability of a compliant vessel to withstand a certain level of external pressure strongly depends on the pressure level inside the vessel itself: the larger the internal pressure, the higher the external pressure must be to constrict the vessel. Ultimately, hemodynamic measurements of the ocular vasculature in vivo will be the result of the intertwined action of BP driving the flow, IOP and RLTp impeding the flow, and regulatory mechanisms modulating the flow.
Fig. 3.
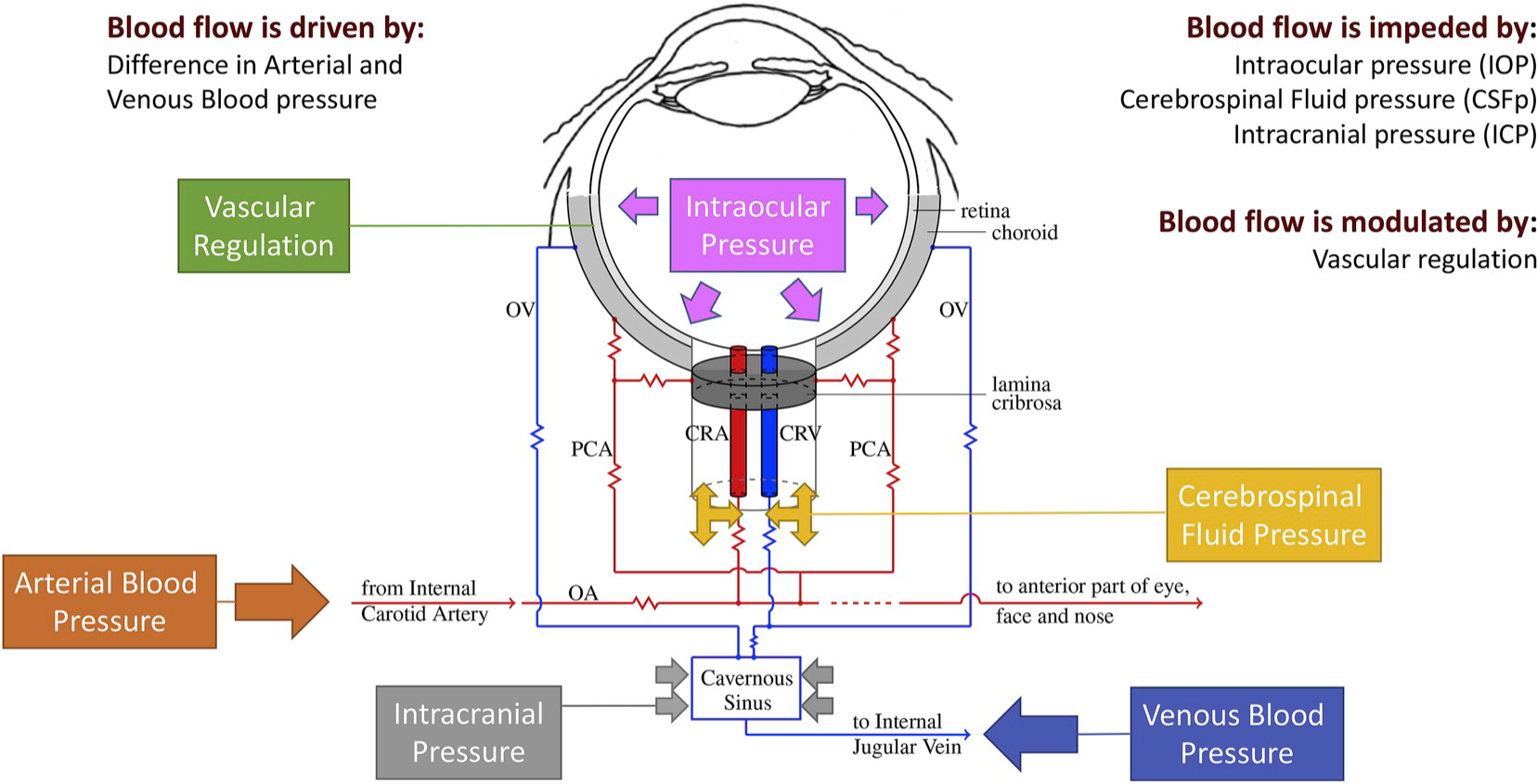
Schematic representation of competing mechanisms influencing ocular circulation. Blood flow is driven by the difference between arterial and venous pressures, impeded by external pressures acting upon the vasculature (e.g. intraocular pressure, cerebrospinal fluid pressure and intracranial pressure), and modulated by vascular regulation. In vivo measurements are the result of a complex interaction among these mechanisms. Abbreviations: OA: Ophthalmic Artery; OV: Ophthalmic Vein; CRA: Central Retinal Artery; CRV: Central Retinal Vein; PCA: Posterior Ciliary Artery.
6.1.1. Retinal vasculature and central retinal vessels
It is quite challenging to disentangle the contributions of BP, IOP, RLTp and vascular regulation in each patient by means of a single clinical measurement. Let us consider the retinal vasculature as an example. Since the retina is in direct contact with IOP, it seems very likely that changes in IOP would lead to changes in retinal blood flow and velocity. However, clinical evidence of such an impact is not unanimous, as discussed in Section 4.3. Specifically, several clinical studies showed a decrease in retinal and retrobulbar blood flow and velocity when IOP was increased (Alagoz et al., 2008; Findl et al., 1997; Galassi et al., 2008; Harris et al., 1996; Huber-van der Velden et al., 2012; Koz et al., 2007; Synder et al., 2004; Trible et al., 1994), whereas other studies did not find any significant changes in retinal and retrobulbar hemodynamics with changes in IOP (Akarsu et al., 2004; Arikan et al., 2006; Cantor, 2001; Erkin et al., 2004; Fuchsjager-Mayrl et al., 2010; Harris et al., 2009; Hommer et al., 2012; Inan et al., 2003; James, 1994; Kaup et al., 2004; Poinoosawmy et al., 2002; Stankiewicz et al., 2011). The resolution of apparent inconsistencies among findings reported by clinical and experimental studies can be addressed via mechanism-driven models.
The first mathematical model that simultaneously accounts for blood flow in the retinal vasculature and in the central retinal vessels, blood flow autoregulation, BP and biomechanical action of IOP on the retinal vasculature was proposed by Guidoboni et al. (2014b). The model leverages an electric circuit analogy to fluid flow, where the blood flowing through the retinal vasculature is modeled as a current flowing through an electric circuit (Sacco et al., 2019). In this modeling analogy, electric currents and electric potentials represent blood flow rates and BPs, while resistors and capacitors are utilized to model hydraulic resistances and vessel compliance. Variable resistances describe active and passive diameter changes due to vascular regulation and IOP-induced compression. A schematic of the model is reported in Fig. 1(A). The model was utilized by Cassani et al. to predict the variance in the hemodynamic outcomes following trabeculectomy among patients depending on BP and autoregulatory capacity (Cassani et al., 2014). More broadly, the model predicts that a patient’s IOP shifts the plateau of vascular regulation to occur within a different range of OPP and may induce drastic blood flow reductions due to venous collapsibility in patients with low BP, making them more susceptible to ischemic damage. For example, the model showed that in individuals with an IOP greater than 21 mmHg, individuals with low BP would experience venous collapse at lower IOP values when compared to individuals with normal or high BP. These findings were confirmed on a population-based study including nearly 10,000 people (20,000 eyes) in a multi-ethnic Asian population (Singapore Epidemiology of Eye Diseases study, previously discussed), where the effect of lower systolic BP on glaucoma was found to be more pronounced in eyes with IOP ≥ 21 mmHg, and individuals with the lowest values of systolic BP (< 124 mmHg) were 1.69 times more likely to exhibit glaucoma (Tham et al., 2018). Thus, modeling is being used to provide a testable mechanistic explanation for why individuals with low BP and high IOP are at higher risk for glaucoma.
In addition, the model clarified that the same values of single ocular measurements may have very different clinical implications in individuals presenting different combinations of IOP and systolic and diastolic BPs (SBP/DBP) (Guidoboni et al., 2014b). Model simulations showed that a peak systolic velocity in the central retinal artery of 10 cm/s corresponds to adequate retinal perfusion for a subject with IOP = 15 mmHg and SBP/DBP = 120/80 mmHg but corresponds to low perfusion for a subject with IOP = 15 mmHg and SBP/DBP = 140/90 mmHg. In this way, modeling is used to emphasize an important point that assumed thresholds for health based on isolated clinical measures do not always communicate the entirety of the situation. Rather, such thresholds should be set based on a more comprehensive set of conditions of a patient, which modeling can help define.
One of the major novelties introduced in (Guidoboni et al., 2014b) is the mathematical description of retinal venules and veins as Starling resistors, namely tubes that can collapse when the transmural pressure difference becomes negative (Pedley, 1996). While novel in the context of the mathematical modeling of retinal blood flow, the concept of Starling resistor was first introduced in 1912 by the English physiologist Ernest Starling in his studies on the heart (Knowlton and Starling, 1912) and then utilized by several authors to account for vessel collapsibility in the vascular system and in the airways, see e.g. (Chen et al., 2019; Pedley, 1996; De Simone et al., 2017). Interestingly, depending on the regime of motion and the levels of internal and external pressures, collapsible tubes may exhibit self-excited oscillations and give rise to the so-called “waterfall effect” wherein, subsequent to collapse, the flow through the tube becomes independent of the downstream pressure (Conrad, 1969; Bertram, 1995). When a collapsible tube is included in a hydraulic network, the tube collapse affects the pressure distribution throughout the whole network; specifically, a collapse leads to an increase in pressure for the network sections located upstream of the collapse and a decrease in pressure for the downstream sections (Sacco et al., 2019).
The model in (Guidoboni et al., 2014b) showed that the collapsibility of retinal venules plays an important role in determining the hemodynamic response of the retinal vascular bed to IOP elevation. Fig. 4(A–E) reports the BP in the retinal microcirculation and the central retinal vessels for an individual with a mean arterial pressure of 93.3 mmHg (corresponding to SBP/DBP = 120/80 mmHg) and IOP ranging between 15 and 45 mmHg when regulatory mechanisms are functional (red circles) or impaired (blue dashed curve). Model simulations show that increased IOP induces a significant increase in the intraluminal BP in all vascular compartments upstream of the CRV, as reported in Fig. 4(A–D), thereby confirming the experimental results on cats reported by Glucksberg and Dunn (1993) and Attariwala et al. (1994). This pressure increase does not appear to differ significantly in the case of functional or impaired regulation. Fig. 4(F) displays the predicted changes in retinal blood flow upon IOP elevation. Model simulations show that active regulatory mechanisms allow for a wider plateau as IOP increases, as expected. However, even in the absence of active regulation, retinal blood flow does not exhibit a catastrophic collapse with IOP elevation, thereby pointing to some intrinsic hydraulic mechanisms that are capable of sustaining flow despite lack of active regulation.
Fig. 4.
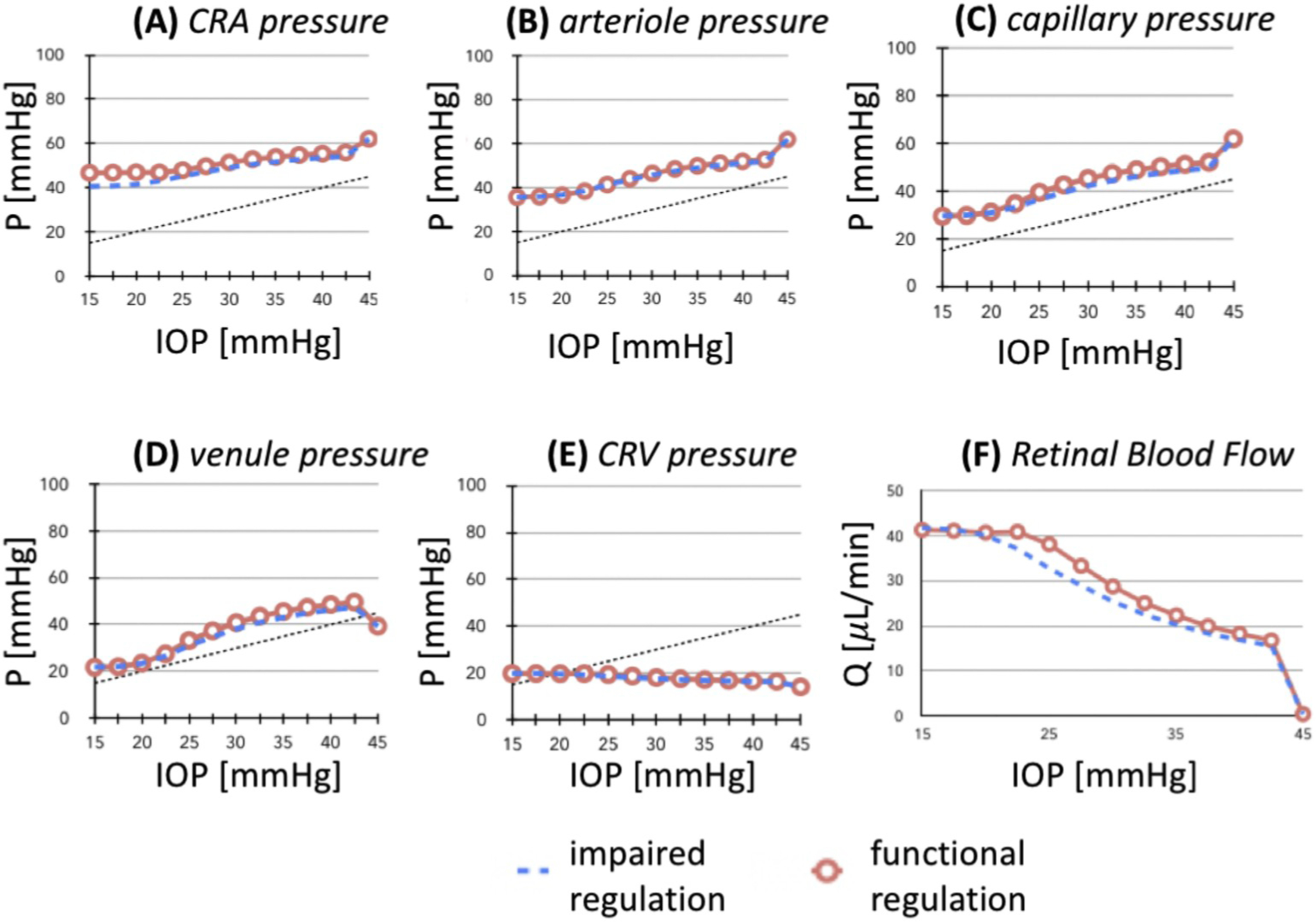
Simulation of hemodynamic variables predicted in the case of systolic and diastolic blood pressures equal to 120 mmHg and 80 mmHg, respectively, and intraocular pressure (IOP) varying between 15 mmHg and 45 mmHg. Image taken directly from (Chiaravalli, 2018), with permission. Intraluminal blood pressure (P) is predicted to increase with IOP in A) central retinal artery (CRA), B) arterioles, C) capillaries and D) venules. Panel E) shows that intraluminal pressure does not increase in the central retinal vein (CRV) located downstream of the lamina cribrosa. Predicted total retinal blood flow (Q) is portrayed in panel F). Results are reported in the case of functional active regulation (red circles) and impaired active regulation (blue lines). Overall, the results indicate the presence of a feedback hydraulic mechanism due to venous collapse that yields an intraluminal pressure increase upstream of the CRV, thereby aiding the retinal vasculature to better withstand IOP elevation.
When extending the retinal circulation model proposed in (Guidoboni et al., 2014b) to include other vascular beds (i.e., retina, choroid, optic nerve head, ciliary body) and their connections with cerebral and systemic circulations, Salerni et al. found that the passive regulatory mechanism in response to IOP elevation holds in the retinal vasculature but does not occur in the other ocular vascular beds (Salerni et al., 2018, 2019). These predictions point to a purely mechanical feedback mechanism due to the collapsibility of the retinal venules exposed to IOP to help maintain a relatively constant level of blood flow through the retina even in the case of impaired active regulation; a similar mechanism is not present in the choroid and ciliary body due to their different architectures in venous drainage. This mechanism may also play a crucial role in determining differential responses to microgravity conditions (Salerni et al., 2019). The ability to simulate and evaluate the flow conditions in the venous segments of the eye is a major contribution in ophthalmology, since the veins are known to play a very important role in ocular physiology but are very difficult to measure in vivo.
Changes in CSFp might also affect the hemodynamics in the central retinal vessels and, consequently, in the retinal vasculature, as schematized in Fig. 3. This delicate aspect remains, to date, widely under-studied. A first step in this direction is provided by the work of Carichino et al. (2014) who developed a multiscale model coupling the compliance of the arterial segments, the collapsibility of the venous vessels and the deformation of the lamina cribrosa due to IOP, CSFp and scleral tension, as proposed in (Guidoboni et al., 2014a). The mathematical model results suggest that changes in vascular resistances due to changes in CSFp are much smaller than those due to the direct action of IOP on retinal venules. The relationship between IOP, CSFp and blood flow is further complicated by the fact that all three factors are influenced by the level of BP, but perhaps each to a different extent. A first mathematical formalization of the connections between the flows of blood and aqueous humor in the eyes, the flows of blood, cerebrospinal fluid and interstitial fluid in the brain, and their interactions has recently been proposed by Salerni et al. (2019). The model proved capable of simulating the relationships among IOP, CSFp and BP reported in major clinical and population-based studies, while capturing the relationship between choroidal venous pressure and IOP reported in the seminal work by Bill (1963). Further clinical, experimental and modeling studies are required to deepen the current understanding of the relationship between IOP, CSFp, BP and blood flow.
6.1.2. Optic nerve head vasculature
The modeling of optic nerve head hemodynamics has only started in the recent years. In the work by Chuangsuwanich et al., two-dimensional capillary networks were artificially generated to identify and rank the morphologic factors influencing the hemodynamics and oxygen concentrations in the microvasculature of the lamina cribrosa (Chuangsuwanich et al., 2016). Model simulations show that the imposed arterial and venous pressures and the diameter of the lamina cribrosa are the factors that influence hemodynamics and oxygenation the most. In particular, higher oxygen concentrations across the network were predicted for smaller diameters of the lamina cribrosa, higher arterial pressure and lower venous pressure. Since the vessels are assumed to be rigid tubes, this model cannot be used to predict the influence of external pressures on hemodynamics. Thus, in order to investigate the role of IOP on the perfusion of the lamina cribrosa, it is necessary to relax the rigid tube assumption and let the blood vessel deform under the action of transmural pressure differences. However, from the mathematical perspective, resolving blood flow and oxygen transport through a network of compliant and collapsible vessels is extremely challenging.
In order to avoid the extreme difficulty in accounting for the fine details of the microvascular network, Causin et al. proposed a modeling approach based on the equations of poroelasticity (Causin et al., 2014). This approach was further extended to include structural viscoelasticity (Bociu et al., 2016; Prada, 2016). In this modeling approach, the lamina cribrosa is described as a deformable porous medium composed of a deformable solid (comprising collagen, elastin, extracellular matrix and neural tissue) and an interconnected vascular porous space filled by blood, as depicted in Fig. 1(B). This modeling approach has led to the insight that reduced structural viscoelasticity due to aging or disease may compromise the ability of ocular tissues to maintain perfusion upon sudden changes in IOP. Sudden changes in IOP are physiological and they occur every time we blink or rub our eyes, as confirmed via IOP telemetry in non-human primates (Downs, 2015). Thus, the model led to the formulation of the hypothesis that even physiological changes in IOP might induce pathological changes in the hemodynamics of the optic nerve head if the viscoelasticity provided by the collagen fibers is not intact (Verri et al., 2018; Bociu et al., 2019). Consistent with this hypothesis, viscoelastic changes in ocular tissues have been associated with glaucoma in studies conducted in rabbits and monkeys (Downs et al., 2003). Such a hypothesis may help provide a new paradigm to understand the causes of disc hemorrhages in some glaucoma patients. Interestingly, the model predicts similar susceptibility to hemodynamic insult when individuals with reduced tissue viscoelasticity are exposed to sudden changes in gravitational acceleration, such as those experienced by astronauts during missions. These considerations are particularly relevant for the optic nerve head tissue, whose pathological changes have been associated with the visual impairments affecting many crew members during and after long-duration space flights (National Aeronautics and Space Administration, 2017).
Recently, Sala et al. integrated in a single mathematical model the coupling between the blood flow within the lamina cribrosa, described as a three-dimensional porous medium, and the blood flow in the retinal, choroid and retrobulbar circulation, described as a lumped parameter network (Sala et al., 2018a, 2018c; Sala, 2019a). A schematic of the coupling is reported in Fig. 5. The model also includes a realistic ocular geometry, as represented in Fig. 1(C). This multiscale model has been used to quantify the effect of IOP, CSFp pressure and BP on the hemodynamic and biomechanic responses of the lamina cribrosa. Simulations suggest that similar TPD levels lead to similar stress and strain distributions within the lamina but noticeably different perfusion velocities. This model is currently available as a web-based application for training and research purposes (Sala et al., 2018b, 2019b; Sala, 2019a).
Fig. 5.
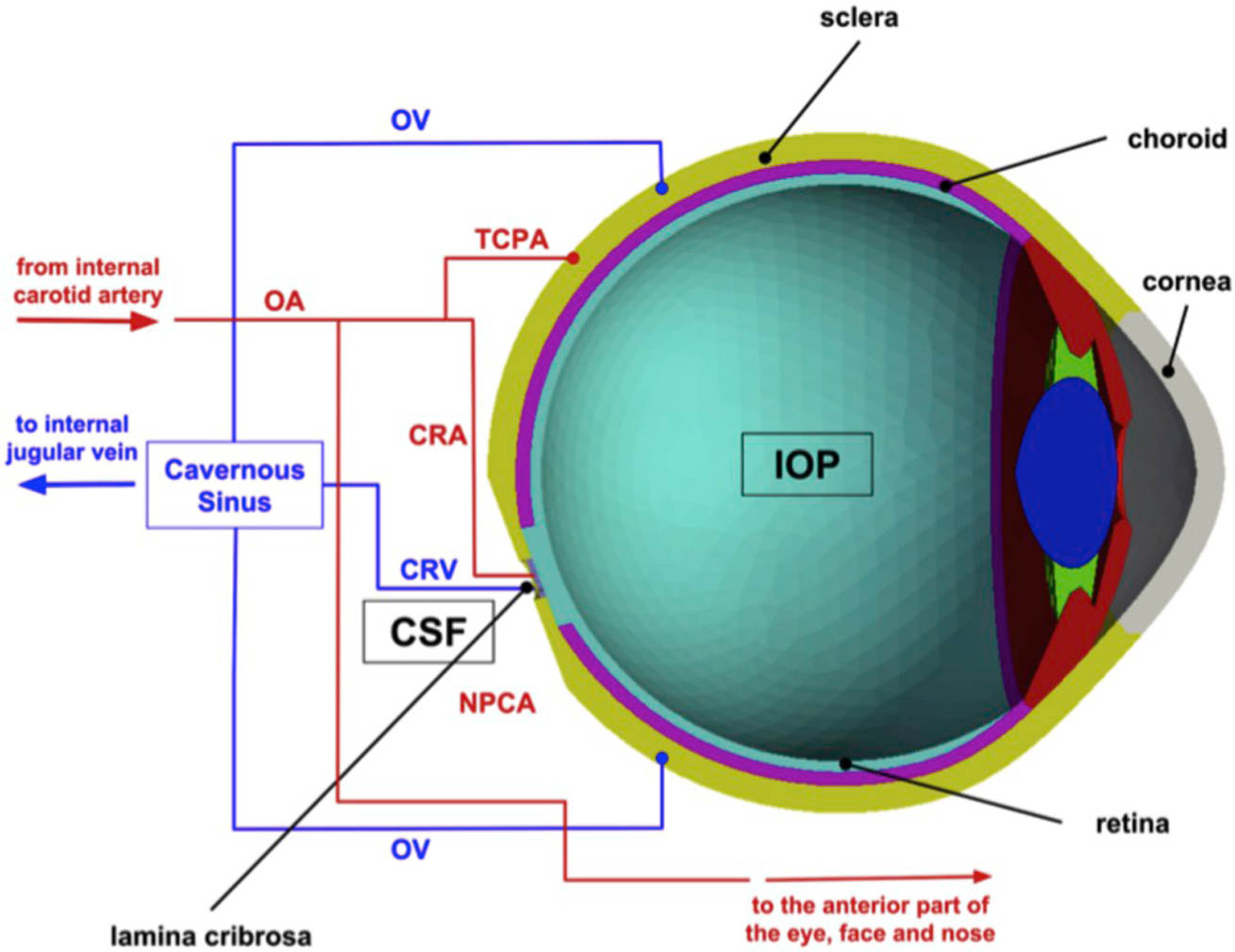
Schematic representation of an integrated mathematical model coupling the blood flow within the lamina cribrosa, described as a three-dimensional porous medium, with the blood flow in the retinal, choroid and retrobulbar circulation, described as a lumped parameter network. Image reproduced from (Sala et al., 2018b), with permission. The model is also available as a web-based app for training and research purposes (_S1_Reference281Sala et al. 2018b, 2019a; Sala, 2019b).
6.2. Models incorporating hemodynamics and oxygen transport: clinically relevant findings
6.2.1. Flow regulation
One of the most important aspects of the vasculature is its intrinsic ability to regulate flow via the constriction or relaxation of vascular smooth muscle in response to factors such as pressure, flow, and metabolites. Thus, to create a more realistic representation of blood flow in the eye, mathematical models (Arciero et al., 2008, 2013; Carlson et al., 2008) have been developed that consist of vasoactive segments that can regulate flow as opposed to rigid tubes. For example, Arciero et al. (2013) adapted a previous vascular wall mechanics model (Carlson et al., 2008; Arciero et al., 2008) to simulate the effects of pressure, shear stress, conducted metabolic responses, and carbon dioxide on retinal blood flow. The model accounts for both passive and active components of wall tension, where passive tension is assumed to result from structural components of the vessel and active tension is generated by the contraction of vascular smooth muscle. The degree of smooth muscle activation is modeled as a linear combination of the myogenic (pressure), shear, conducted metabolic, and carbon dioxide responses. In (Arciero et al., 2013), these four vascular responses are modeled in the context of a representative segment model where the retinal microcirculation is modeled as compartments of large arterioles, small arterioles, capillaries, small venules, and large venules connected in series that each contain identical parallel-arranged segments. Oxygen transport is simulated using a Krogh cylinder model (Fig. 2(A)) in which oxygen is provided via diffusion from a vessel to a cylindrical region of tissue surrounding it according to the following equation:
where k is the diffusion coefficient, P is the partial pressure of oxygen at radial distance r within the tissue cylinder, and M0 is the tissue oxygen demand of the retinal ganglion cells in the inner retina per tissue volume (often assumed to be a constant value). The solution of this diffusion equation yields the partial pressure of oxygen as a function of tissue cylinder radius and position (x) along the vascular pathway. Concurrently, the conservation of mass equation must be solved (equating oxygen flux with oxygen consumption rate):
where x is distance along the network, Q is the blood flow in each compartment, c0i s the oxygen carrying capacity of red blood cells at 100% saturation, HD is discharge hematocrit, S(x) is blood oxygen saturation and is the oxygen consumption per vessel length (where rt is the radius of the tissue cylinder and rv is the radius of the vessel). The solution of this equation gives the oxygen saturation (S) at any point (x) along the vascular pathway, providing an important indication of the oxygenation of retinal tissue. Predictions from this model revealed that the conducted metabolic and carbox dioxide responses were critical for the autoregulation of blood flow. Strikingly, the model predicts that the ability to autoregulate is reduced by 95% if both the metabolic and carbon dioxide responses are impaired. The model also predicts that autoregulation is impaired at decreased perfusion pressures due to elevated IOP, providing important theoretical evidence of how blood flow impairments may contribute to OAG (Fig. 6(A)).
Fig. 6.
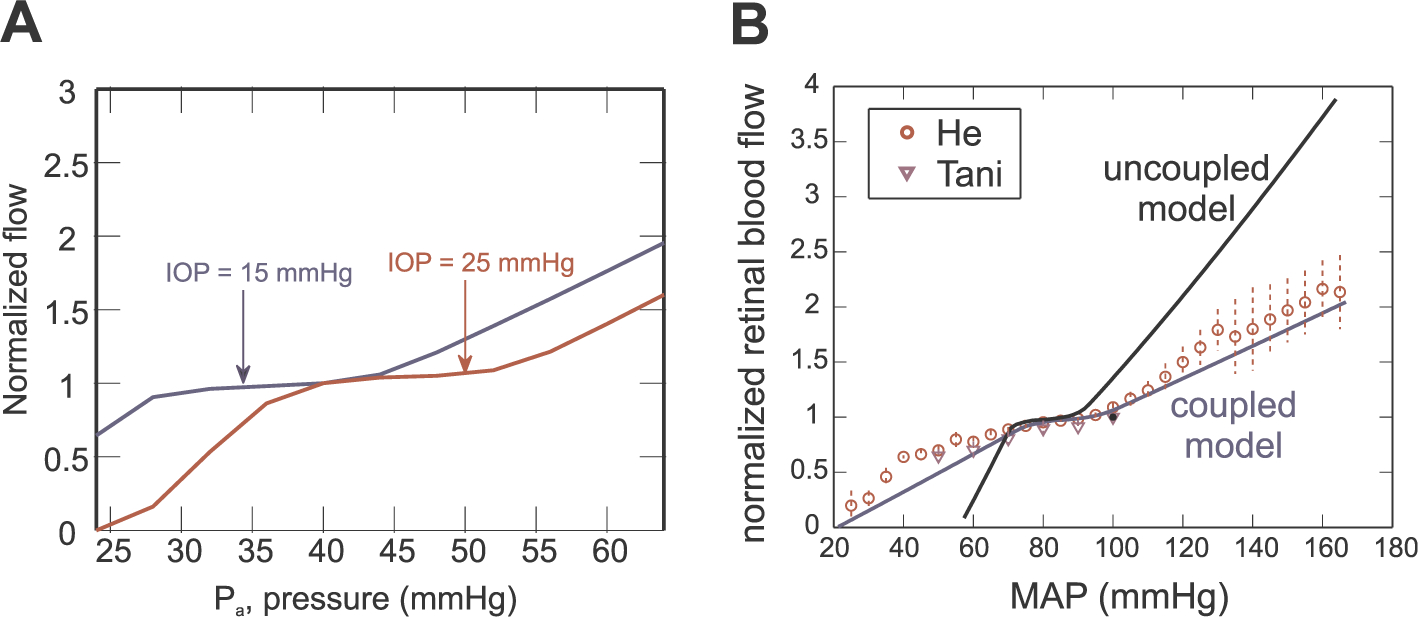
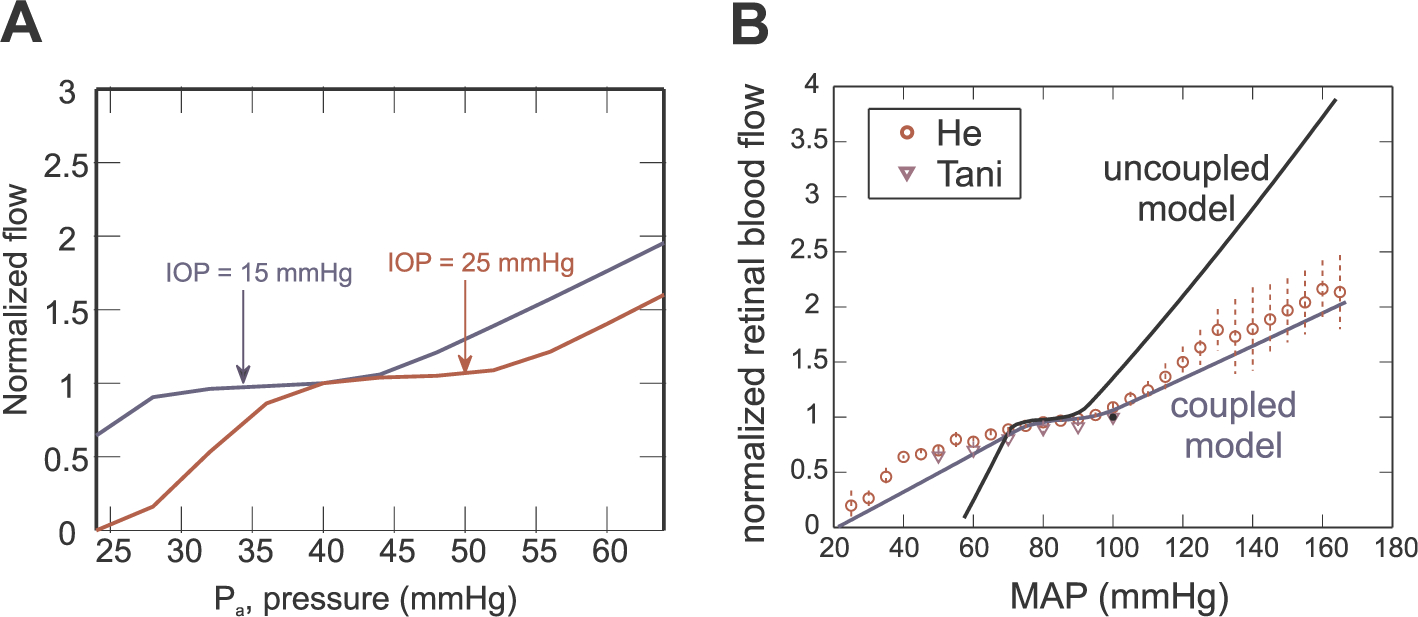
A) Model predicted autoregulation curves for IOP = 15 mmHg (control, blue) and IOP = 25 mmHg (elevated, red). Model predictions show autoregulation fails to operate over its expected pressure range when IOP is increased. Figure reproduced from (Arciero et al., 2013) with permission. B) Model predicted autoregulation curves predicted by the coupled model (blue) (Cassani et al., 2015) and the uncoupled model (black) (Arciero et al., 2013). Model predictions are compared with data from (He et al., 2012, 2013; Tani et al., 2014) as mean arterial pressure (MAP) is varied. Figure reproduced from (Cassani et al., 2015) with permission.
The model developed by Arciero et al. provides a central foundation upon which numerous investigations, theories, and models have been built (Arciero et al., 2013). For example, Cassani et al. (2015) coupled the effects of the pressure in the CRA and CRV to the mechanistic description of flow regulation in the retinal microcirculation (Arciero et al., 2013). By including more specific mechanistic effects of IOP, such as the deformation of the lamina cribrosa and vessel tone of the venous compartments from IOP, the coupled model provides a more accurate representation of blood flow to the retina. As seen in Fig. 6(B), the coupled model also more closely matches experimental data collected by He et al., 2012, 2013 and Tani et al. (2014) for a wider range of pressures. Cassani et al. (2016) applied this coupled model of flow regulation (Cassani et al., 2015) to study the response of blood flow to changes in tissue oxygen demand. Flow is predicted to increase by 23% in response to a 50% increase in tissue oxygen demand when all regulation mechanisms are active. This significant response in flow is inhibited if the conducted metabolic and carbon dioxide responses are inactive, again highlighting the importance of these two responses in regulating retinal blood flow. The model also predicts that vascular regulatory mechanisms may respond differently to different levels of oxygen demand since flow is not reduced in the same ratio when oxygen demand is decreased by 50%. Importantly, the model does not distinguish between an inner and outer retina, and thus some of the metabolic flow levels observed clinically for flicker and light-and-dark studies cannot yet be replicated by this version of the mathematical model. Neverthless, extensions of this model can be used to determine possible explanations for the observation of higher venous oxygenation in dark (Hardarson et al., 2009).
In a highly transformative study in 2016, the model developed by Arciero et al. (2013) was applied to a set of oximetry data obtained from healthy individuals and glaucoma patients (Fig. 7(A)) to propose possible explanations for the clincially observed increases in venous saturation among advanced glaucoma patients (Carichino et al., 2016). The model was used to show that a decrease in retinal tissue oxygen demand, an impairment in flow autoregulation, or altered capillary density can each lead to increases in venous saturation. This outcome challenged typical clinical thought that increased venous saturation results primarily from decreased oxygen demand. In the modeling study, patient-specific simulations were run using clinical measurements of IOP, BP and arterial oxygen (O2) saturation obtained from healthy individuals, glaucoma patients with elevated IOP (OAG) and glaucoma patients without elevated IOP (NTG). The model was used to predict the level of O2 demand that would be necessary to yield the clinically-measured value of venous O2 saturation for each specific individual in the study. The model predictions suggested that the increased saturation can be explained by decreased O2 demand in OAG patients, but not in NTG patients (Fig. 7(B)). Importantly, this insight on different mechanisms at play in different patient groups could not be achieved by means of simple statistical analysis of measured data, since both groups showed the same increase in venous O2 saturation with respect to healthy controls (Fig. 7(A)). Further simulations reported in (Carichino et al., 2016) suggest that impaired autoregulation is likely to cause the increased venous O2 saturation measured in NTG patients. If confirmed on a larger dataset, these findings could lead to a major breakthrough in glaucoma management and care that differentiates clinical approaches for OAG and NTG patients. While precise therapeutic protocols to improve autoregulation have not been established, multiple studies have shown that treatment with medications such as dorzolamide-timolol and brimonidine-timolol have successfully improved retinal vascular autoregulation in glaucoma patients who were exhibiting symptoms of autoregulation dysfunction (Feke et al., 2013). Studies of the cerebral microcirculation have shown cerebral auto-regulation assessments may have prognostic value, may indicate pathological states, and may have the potential to influence therapy (Donnelly et al., 2015). Thus, pharmacological manipulation of auto-regulation is hypothesized to be a promising technique in many tissue environments, including the retina.
Fig. 7.
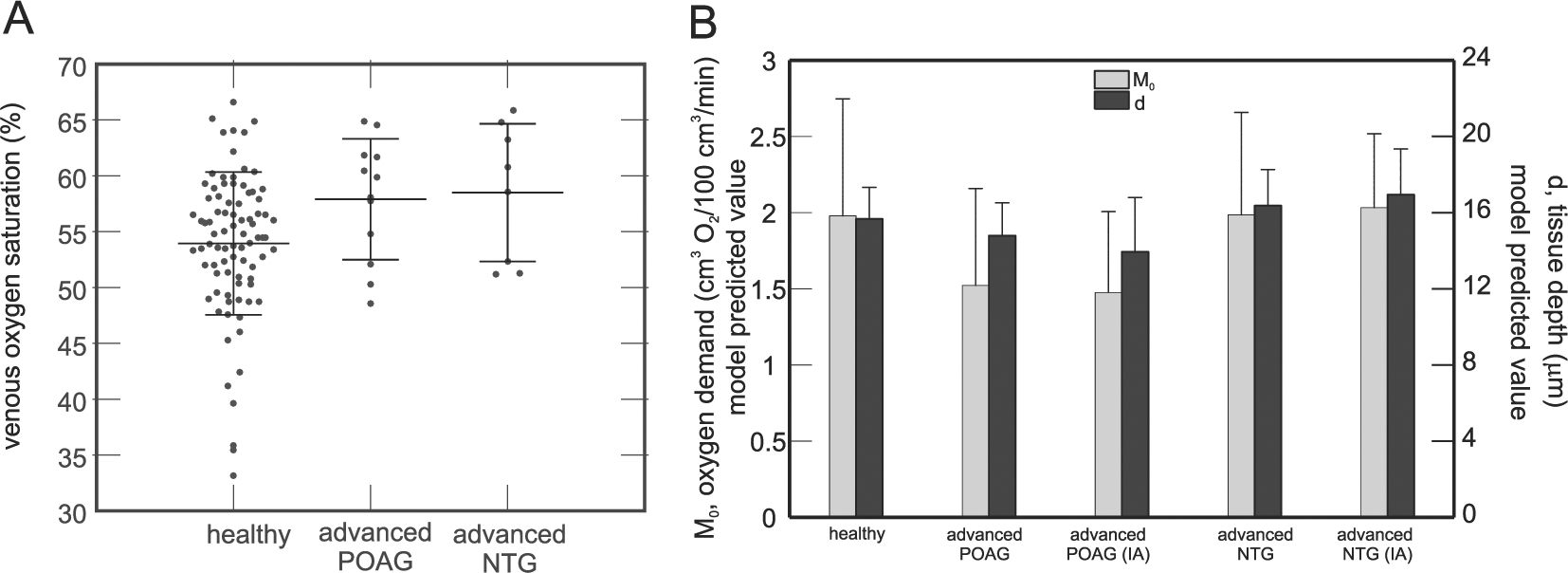
A) Clinical data showing increased venous O
6.2.2. Retinal tissue geometry and oxygen transport
Since impaired blood flow and oxygenation have been identified as important factors contributing to the onset and progression of glaucoma, improved models that account for more accurate depictions of the retinal vasculature and oxygen transport throughout the network are needed. Causin et al. (2016) and Fry et al. (2018) developed two different approaches for providing more accurate predictions of flow and oxygenation in the retina. Causin et al. coupled a blood flow mechanics and oxygen transport model in the retina while also accounting for the three-dimensional structure of the retina (see Fig. 2(B)) (Causin et al., 2016). In particular, their 6-layer model included three layers for the inner retina and three layers for the outer retina. Arteriolar and venous networks were modeled as fractal trees, and capillary beds were represented as a set of parallel pipes in two distinct tissue layers. Oxygen transport and distribution was described by a nonlinear diffusion-reaction system of partial differential equations. The consumption rate among the retinal layers was not uniform; the consumption rates were chosen based on the roles of each layer (e.g., maximal oxygen consumption was assumed in the outer retinal layers at the level of the photoreceptors). Using this network model, Causin et al. predicted the effects of changes in BP, plasma viscosity, metabolic consumption rate, and arterial oxygen permeability on tissue and blood oxygen saturation levels (Causin et al., 2016). Ultimately, the model was used to show that changes in systemic BP greatly impacts blood and tissue oxygen profiles, plasma viscosity and metabolic consumption rates strongly influence oxygen tension at the retinal ganglion cell level, and arterial oxygen permeability impacts oxygen saturation in retinal arterioles. Although the model does not include blood flow regulation mechanisms, it provides an important method that couples a description of the retinal microcirculation with the surrounding tissue.
Fry et al. developed a mathematical model that provides spatially accurate predictions of oxygen levels within retinal blood and tissue (Fry et al., 2018). Confocal microscopy images of the mouse retina were used to create realistic network structures and connections of the retinal arterioles and venules. These vessels, in addition to capillary meshes, were distributed non-uniformly among three distinct retinal layers at varying depths (superficial, intermediate, and deep layers). To predict the oxygen delivery and distribution in such an irregular arrangement of vessels, a mathematical model was developed using numerical methods based on a Green’s function approach (Fig. 2(C)). More precisely, the Green’s function approach, originally developed by Secomb et al. represents vessels as discrete oxygen sources and tissue regions as oxygen sinks (Secomb et al., 2004; Secomb, 2016a). The oxygen concentration at each tissue point is calculated by summing the oxygen fields (called Green’s functions) produced by each of the surrounding blood vessels. A great benefit of this model is its ability to generate a spatial representation of oxygenation in the retina, which has led to important insight into the reliability of hypoxic indicators. More specifically, Fry et al. showed that average vessel PO2 or isolated tissue PO2 measurements are not sufficient indicators of tissue regions at risk of hypoxia. The model simulations in a heterogeneous arteriolar network showed widespread values of PO2; in fact, the standard deviation of vessel PO2 increased as oxygen demand was increased, indicating that the average value of PO2 is a poor indicator of network oxygenation. The heterogeneity of pathways leads to many non-equivalent flow pathways, and the model predicts that some terminal arterioles will have abnormally low PO2 levels. This would create areas at risk of hypoxia that would not be observed if average vessel PO2 were the sole indicator of oxygenation or if measurements were not taken over the entire spatial region. This mathematical model has even higher potential for relating hemodynamics, vascular network structure, and vision function once the model is adapted to include flow regulation mechanisms within the heterogeneous network structure of the human retina.
7. Perspective and future directions
The recent years have witnessed impactful advances in imaging technologies for ophthalmology and yet several controversies remain unresolved regarding how specific risk factors combine to determine overall risk of disease for a given individual. The identification of cases where hemodynamic factors are primary to the pathogenesis of ocular disease bears particular urgency, since this realization would enable targeted therapeutic avenues which are currently unpractical due to the lack of consistent and convincing data. To meet this challenge, the field should advance on multiple fronts, including the design of additional longitudinal studies to assess the relationship between hemodynamic biomarkers and disease progression across diverse patient cohorts and the development of novel methodologies for data analysis that integrate statistical methods with mechanism-driven models. Particular attention must be paid to the technologies and protocols utilized for data acquisition. Conclusions derived from data analysis are only as reliable as the data they are based upon. This review article outlined several limitations in imaging technologies and assumptions that have direct and detrimental effects on measurements. Addressing these limitations and validating the underlying assumptions is of utmost importance in order to avoid the misinterpretation of collected data and illustrates the need for collaborative effort of the medical, engineering, physical and mathematical communities.
Mechanism-driven mathematical models are based on physical laws and clinical observations in order to provide an accurate and relevant tool for clinicians. This review provided numerous examples of how these models can be used to simulate the hemodynamic interactions in the vasculature of the eye and generate testable hypotheses that advance the current understanding of ocular physiology while aiding the design of future clinical and experimental studies. Theoretical predictions obtained via mechanism-driven models can also be used as a guide for a more effective statistical analysis of real data, as exemplified in (Carichino et al., 2016). Individual variability of model parameters and the influence of such variability on specific model outputs can be accounted for by means of sensitivity analysis and uncertainty quantification. An example can be found in the work by Szopos et al. (2016), where uncertainty in the model parameters is included in a mechanism-driven model of aqueous humor flow and intraocular pressure to predict the efficacy of different types of ocular hypotensive medications on different individuals, thereby moving forward towards individualized glaucoma management. However, it remains very challenging to explicitly account for every individual characteristic (e.g. age, gender, race, BMI) in a mechanistic way, since the cause-effect relationship among such characteristics and specific model parameters (e.g. vessel wall stiffness, geometry of blood vessels) are still mostly unknown (Guidoboni et al., 2013).
Ultimately, the construction of mechanism-driven mathematical models of ocular blood flow that will have the most potential to impact ocular physiology and pathology must involve multiple components: (i) fundamental fluid and solid mechanics laws describing interactions and behavior of blood flow, vessels, and oxygen; (ii) parameters determined from statistical optimization of clinical measurements; and (iii) statistical modeling to enhance model parameters and components for widespread patient-specific data. The development of such comprehensive mechanism-driven models is a very difficult task and often involves several simplifying assumptions. As interdisciplinary teams strive to create the most informative and representative models of the ocular circulation, the main challenge in the next decades will be to develop a unified modeling framework that simultaneously describes: (i) the interconnections between different vascular beds, including retinal, choroidal, ciliary and retrobulbar circulations; (ii) the coupling between phenomena occurring at different scales in space (e.g., scales ranging from the motion of ions across cellular membranes to the macroscopic change of arteriole diameter) and time (e.g., the hemodynamic changes due to the circadian rhythm and slow changes due to tissue growth and remodeling); and (iii) the interplay between the deterministic laws of nature governing the fluid dynamics of blood flow and the intrinsic variability of biophysical and geometrical characteristics among individuals.
AI represents a new wave in science and technology that is leading to the development of automated tools for the diagnosis, screening, and monitoring of ocular diseases. A key goal of AI is to provide more efficient, accurate, and sensitive methods for interpreting the numerous sets of data that are obtained in the clinic. AI can be implemented using multiple approaches, including machine learning and deep learning with convolutional neural networks. Machine learning combines basic rules with sample images from healthy and disease images to develop statistical algorithms that can determine differences between the images and ultimately detect cases of disease. Deep learning involves repetition of algorithms and self-correction by adjusting parameters for the algorithm to decrease errors in diagnoses. In this way, deep learning approaches a more authentic idea of “artificial intelligence” or “thinking.” Already, machine learning and deep learning have been applied to fundus photographs, OCT and visual fields to obtain successful classifications of diabetic retinopathy, retinopathy of prematurity, glaucoma, and AMD (Ting et al., 2019; Hogarty et al., 2019). For example, Kim et al. (2017) and Raghavendra et al. (2018) developed neural networks capable of distinguishing between normal and glaucoma patients based on fundus images that yielded an accuracy equivalent to human experts. As AI becomes more prevalent in medicine, it is predicted that high-powered machine learning algorithms will be built into various imaging machines (e.g., OCT) so that the use of AI can become widespread at the bedside.
Such use of science and technology has great potential for the practice of medicine. Improved and earlier diagnosis as a result of the use of AI would provide major benefits to all persons suffering from ophthalmic disease. However, AI also has considerable limitations. For example, if the training set of images is too small or not representative of diverse patient populations (in terms of age, ethnicity, etc.), the outcomes predicted by AI may be biased and not fully reliable. However, if the training set of images is too large, the training process becomes less efficient and could overfit the machine learning classifiers to the training dataset (Hogarty et al., 2019). Also, AI techniques represent a “black-box” approach that does not inform as to why the algorithm arrived to certain conclusions; thus, AI results must be confirmed or rejected by clinicians who exhibit a more complete understanding of image subtleties and patient history and conditions. Furthermore, the predictions made by deep learning (AI) systems may seem significant to a computer scientist but may not be interpreted in the same way by an ophthalmologist (Ting et al., 2019). In an effort to provide users with more confidence on how to use and when to trust AI results, the computer science discipline is now directing considerable effort to the development of Explainable AI, which is meant to explain how the AI algorithm arrived to a certain conclusion. It is important to emphasize, though, that the transparency provided by current Explainable AI relates to an explanation about inner decision rules implemented in the computer algorithm but not an explanation for why the disease is present and progressing. In sum, statistical, machine learning, deep learning, and AI represent very exciting and powerful predictive techniques for the clinic but are limited to the information already contained in the data set. Mechanism-driven modeling, on the other hand, can take measured data (e.g., IOP or systemic blood pressure) and estimate factors that are of physiological interest but that may be difficult to measure (e.g., functionality of blood flow regulation or oxygen metabolic demand). Without enhancing the measurable dataset to gain knowledge of the mechanisms causing observed outcomes, new and improved therapies for disease cannot be developed.
Thus, the necessary recipe for revolutionizing ophthalmic care must be a unique combination of clinical measurements, statistical approaches, AI methods and mechanistic mathematical modeling. In fact, the first study of this type has been recently proposed (Guidoboni et al., 2019.). Data-driven models can handle an enormous amount of data and generate algorithms that indicate scenarios of ocular disease based on given inputs; mechanisms-driven models can be used not only to confirm or reject such predictions but also will reveal the factors or mechanisms that lead to the disease. In this way, mechanism-driven models and data-driven models are truly complementary tools that will transform the prognosis, diagnosis, and treatment of ocular diseases. Importantly, it is also essential to strive for the continuous development and improvement of imaging modalities with high resolution and high reliability that are also non-invasive and user friendly. These targeted improvements will help ensure higher quality of clinical research data, which are the essential inputs to any data analysis. By simultaneously improving data imaging and analysis techniques, it will be possible to deliver more precise individualized diagnosis, targeted therapies, and improved patient outcomes.
Acknowledgments
The authors wish to acknowledge Nicholas Kalafatis, BS, for his assistance and contribution to completing the manuscript.
Funding
Supported by NIH grant (NIH 1 R01 EY030851–01), NSF-DMS 1853222/1853303, NSF DMS-1654019, and NSF DMS-1852146.
Abbreviations
- AI
Artificial Intelligence
- AMD
Age-Related Macular Degeneration
- BMI
Body Mass Index
- BP
Blood Pressure
- BRAO
Branched Retinal Artery Occlusion
- C/D
RatioCup-to-Disc Ratio
- CDI
Color Doppler Imaging
- CI
Confidence Intervals
- CRA
Central Retinal Artery
- CRAO
Central Retinal Artery Occlusion
- CRV
Central Retinal Vein
- CRVO
Central Retinal Vein Occlusion
- CSF
Cerebrospinal Fluid
- CSFp
Cerebrospinal Fluid Pressure
- DBP
Diastolic Blood Pressure
- DCP
Deep Capillary Plexus
- DH
Disc Hemorrhage
- DM
Diabetes Mellitus
- DMT2
Diabetes Mellitus Type 2
- DOPP
Diastolic Ocular Perfusion Pressure
- DR
Diabetic Retinopathy
- EDV
End Diastolic Velocity
- FA
Fluorescein Angiography
- FAZ
Foveal Avascular Zone
- HR
Hazard Ratio
- HRT
Heidelberg Retinal Tomography
- ICP
Intracranial Pressure
- IGPS
Indianapolis Glaucoma Progression Study IOP Intraocular Pressure
- LSFG
Laser Speckle Flowgraphy
- MAP
Mean Arterial Pressure
- MOPP
Mean Ocular Perfusion Pressure
- MRA
Moorfields Regression Analysis
- NAION
Non-Arteritic Ischemic Optic Neuropathy NRA Neuro-retinal Rim Area
- NTG
Normal-Tension Glaucoma
- NW
Normal Weight
- OA
Ophthalmic Artery
- OAG
Open-Angle Glaucoma
- OB
Obesity
- OCT
Optical Coherence Tomography
- OCTA
Optical Coherence Tomography Angiography OHT Ocular Hypertension
- ONH
Optic Nerve Head
- OPP
Ocular Perfusion Pressure
- OR
Odds Ratio
- OW
Overweight
- PSV
Peak Systolic Velocity
- RAO
Retinal Artery Occlusion
- RI
Resistivity Index
- RLTp
Retrolaminar Tissue Pressure
- RNFL
Retinal Nerve Fiber Layer
- RPCP
Retinal Peripapillary Capillary Plexus
- RPE
Retinal Pigmented Epithelium
- RR
Relative Risk Ratio
- RVO
Retinal Vein Occlusion
- SBP
Systolic Blood Pressure
- SCP
Superficial Capillary Plexus
- SD
Standard Deviation
- SOPP
Systolic Ocular Perfusion Pressure
- SPCA
Short Posterior Ciliary Arteries
- SVP
Superficial Vascular Plexus
- TES
Thessaloniki Eye Study
- TPD
Translaminar Pressure Difference
- TPG
Translaminar Pressure Gradient
Footnotes
Declaration of competing interest
There are no conflicts of interest.
References
- Aizawa N, Nitta F, Kunikata H, Sugiyama T, Ikeda T, Araie M, Nakazawa T, 2014. Laser speckle and hydrogen gas clearance measurements of optic nerve circulation in albino and pigmented rabbits with or without optic disc atrophy. Invest. Ophthalmol. Vis. Sci 55 (12), 7991–7996. [DOI] [PubMed] [Google Scholar]
- Akarsu C, Yilmaz S, Taner P, Ergin A, 2004. Effect of bimatoprost on ocular circulation in patients with open-angle glaucoma or ocular hypertension. Graefes Arch. Clin. Exp. Ophthalmol 242, 814–818. [DOI] [PubMed] [Google Scholar]
- Alagoz G, Gurel K, Bayer A, Serin D, Celebi S, Kukner S, 2008. A comparative study of bimatoprost and travoprost: effect on intraocular pressure and ocular circulation in newly diagnosed glaucoma patients. Ophthalmologica 222, 88–95. [DOI] [PubMed] [Google Scholar]
- Albert DM, Miller JW, Azar DT, Blodi BA, 2008. Albert & Jakobiec’s Principles & Practice of Ophthalmology, third ed. Saunders Elsevier, Pennsylvania. [Google Scholar]
- Alshawa L, Harris A, Gross J, Snyder A, Rao A, Siesky B, 2017. Primary open-angle glaucoma in patients of Middle Eastern descent. Saudi J. Ophthalmol 31 (4), 209–210. 10.1016/j.sjopt.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aref AA, Maleki S, Tan O, Huang D, Varma R, Shahidi M, 2019. Relating glaucomatous visual field loss to retinal oxygen delivery and metabolism. Acta Ophthalmol. 97 (7), e968–e972. [DOI] [PubMed] [Google Scholar]
- Arikan OK, Akarsu C, Unal B, Ergin A, Koc C, 2006. Effect of oxymetazoline nasal spray on intraocular pressure and retrobulbar hemodynamics. J. Otolaryngol 35, 30–35. [DOI] [PubMed] [Google Scholar]
- Arciero JC, Carlson BE, Secomb TW, 2008. Roles of oxygen-dependent ATP release by red blood cells and conducted responses in metabolic regulation of blood flow. Am. J. Physiol. Heart Circ. Physiol 295, H1562–H1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arciero J, Harris A, Siesky B, Amireskandari A, Gershuny V, Pickrell A, Guidoboni G, 2013. Theoretical analysis of vascular regulatory mechanisms contributing to retinal blood flow autoregulation. Invest. Ophthalmol. Vis. Sci 54 (8), 5584–5593. 10.1167/iovs.12-11543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arciero J, Carichino L, Cassani S, Guidoboni G, 2019. Mathematical Modeling of Blood Flow in the Eye. In: Guidoboni G, Harris A, Sacco R (Eds.), Ocular Fluid Dynamics. Anatomy, Physiology, Imaging Techniques, and Mathematical Modeling Modeling and Simulation in Science, Engineering, and Technology Springer-Birkhauser, New York. [Google Scholar]
- Arya M, Sabrosa AS, Duker JS, Waheed NK, 2018. Choriocapillaris changes in dry age-related macular degeneration and geographic atrophy: a review. Eye Vis (Lond). 5, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asejczyk-Widlicka M, Krzyzanowska-Berkowska P, Sander BP, Iskander DR, 2015. Age-related changes in ocular blood velocity in suspects with glaucomatous optic disc appearance. Comparison with healthy subjects and glaucoma patients. PloS One 10 (7), e0134357. 10.1371/journal.pone.0134357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attariwala R, Giebs CP, Glucksberg MR, 1994. The influence of elevated intraocular pressure on vascular pressures in the cat retina. Invest. Ophthalmol. Vis. Sci 35 (3), 1019–1025. [PubMed] [Google Scholar]
- Augstburger E, Zéboulon P, Keilani C, Baudouin C, Labbé A, 2018. Retinal and choroidal microvasculature in nonarteritic anterior ischemic optic neuropathy: an optical coherence tomography angiography study. Invest. Ophthalmol. Vis. Sci 59 (2), 870–877. [DOI] [PubMed] [Google Scholar]
- Baek SU, Kim YK, Ha A, Kim YW, Lee J, Kim JS, Jeoung JW, Park KH, 2019. Diurnal change of retinal vessel density and mean ocular perfusion pressure in patients with open-angle glaucoma. PloS One 14 (4), e0215684. 10.1371/journal.pone.0215684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandello F, Sacconi R, Querques L, Corbelli E, Cicinelli MV, Querques G, 2017. Recent advances in the management of dry age-related macular degeneration: a review. F1000Res 6, 245. 10.12688/f1000research.10664.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks HT, Bekele-Maxwell K, Bociu L, Noorman M, Guidoboni G, 2017. Sensitivity analysis in poro-elastic and poro-visco-elastic models. Q. Appl. Math 75, 697–735. [Google Scholar]
- Bertram CD, 1995. The dynamics of collapsible tubes. Symp. Soc. Exp. Biol 49, 253–264. [PubMed] [Google Scholar]
- Bharadwaj AS, Appukuttan B, Wilmarth PA, Pan Y, Stempel AJ, Chipps TJ, Benedetti EE, Zamora DO, Choi D, David LL, Smith JR, 2013. Role of the retinal vascular endothelial cell in ocular disease. Prog. Retin. Eye Res 32, 102–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bata AM, Fondi K, Witkowska KJ, Werkmeister RM, Hommer A, Vass C, Resch H, Schmidl D, Popa-Cherecheanu A, Chua J, Garhöfer G, Schmetterer L, 2019. Optic nerve head blood flow regulation during changes in arterial blood pressure in patients with primary open-angle glaucoma. Acta Ophthalmol. 97 (1), e36–e41. 10.1111/aos.13850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazvand F, Mirshahi R, Fadakar K, Faghihi H, Sabour S, Ghassemi F, 2017. The quantitative measurements of vascular density and flow area of optic nerve head using optical coherence tomography angiography. J. Glaucoma 26 (8), 735–741. 10.1097/IJG.0000000000000722. [DOI] [PubMed] [Google Scholar]
- Bengtsson B, Heijl A, 2016. Lack of Visual Field Improvement After Initiation of Intraocular Pressure Reducing Treatment in the Early Manifest Glaucoma Trial. Invest Ophthalmol Vis Sci. 57 (13), 5611–5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdahl JP, Allingham RR, Johnson DH, 2008a. Cerebrospinal fluid pressure is decreased in primary open-angle glaucoma. Ophthalmology 115, 763–768. 10.1016/j.ophtha.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Berdahl JP, Fautsch MP, Stinnett SS, Allingham RR, 2008b. Intracranial pressure in primary open angle glaucoma, normal tension glaucoma, and ocular hypertension: a case-control study. Invest. Ophthalmol. Vis. Sci 49 (12), 5412–5418. 10.1167/iovs.08-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry S, Lin WV, Sadaka A, Lee AG, 2017. Nonarteritic anterior ischemic optic neuropathy: cause, effect, and management. Eye Brain 9, 23–28. 10.2147/EB.S125311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram B, Hoberg A, Wolf S, Schulte K, Reim M, 1991. Video fluorescein angiography studies in acute anterior ischemic optic neuropathy. Klin Monbl Augenheilkd 199 (6), 419–423. [DOI] [PubMed] [Google Scholar]
- Bill A, 1963. The uveal venous pressure. Arch. Ophthalmol 69, 780–782. [DOI] [PubMed] [Google Scholar]
- Bill A, 1985. Some aspects of the ocular circulation. Friedenwald Lecture. Invest Ophthalmol Vis Sci 26, 410–424. [PubMed] [Google Scholar]
- Binggeli T, Schoetzau A, Konieczka K, 2018. In glaucoma patients, low blood pressure is accompanied by vascular dysregulation. EPMA J. 9 (4), 387–391. 10.1007/s13167-018-0155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bociu L, Guidoboni G, Sacco R, Webster JT, 2016. Analysis of nonlinear poro-elastic and poro-visco-elastic models. Arch. Ration. Mech. Anal 222 (3), 1445–1519. [Google Scholar]
- Bociu L, Guidoboni G, Sacco R, Verri M, 2019. On the role of compressibility in poroviscoelastic models. Math. Biosci. Eng 16 (5), 6167–6208. 10.3934/mbe.2019308. [DOI] [PubMed] [Google Scholar]
- Boeri D, Maiello M, Lorenzi M, 2001. Increased prevalence of microthromboses in retinal capillaries of diabetic individuals. Diabetes 50 (6), 1432–1439. [DOI] [PubMed] [Google Scholar]
- Bonomi L, Marchini G, Marraffa M, Bernardi P, Morbio R, Varotto A, 2000. Vascular risk factors for primary open angle glaucoma: the Egna-Neumarkt Study. Ophthalmology 107 (7), 1287–1293. [DOI] [PubMed] [Google Scholar]
- Boote C, Sigal IA, Grytz R, Hua Y, Nguyen TD, Girard MJA, 2019. Scleral structure and biomechanics. Prog. Retin. Eye Res 11, 100773. 10.1016/j.preteyeres.2019.100773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers DK, Finkelstein D, Wolff SM, Green WR, 1987. Branch retinal vein occlusion. A clinicopathologic case report. Retina 7 (4), 252. [DOI] [PubMed] [Google Scholar]
- Budenz DL, Huecker JB, Gedde SJ, et al. , 2017. Thirteen-year follow-up of optic disc hemorrhages in the ocular hypertension treatment study. Am. J. Ophthalmol 174, 126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulpitt CJ, Hodes C, Everitt MG, 1975. Intraocular pressure and systemic blood pressure in the elderly. Br. J. Ophthalmol 59 (12), 717–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne CF, Crawford DJ, Bellezza AJ, Suh JK, Hart RT, 2005. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog. Retin. Eye Res 24 (1), 39–73. [DOI] [PubMed] [Google Scholar]
- Calvo P, Ferreras A, Polo V, Güerri N, Seral P, Fuertes-Lazaro I, Pablo LE, 2012. Predictive value of retrobulbar blood flow velocities in glaucoma suspects. Invest. Ophthalmol. Vis. Sci 53 (7), 3875–3884. 10.1167/iovs.11-8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JP, Zhang M, Hwang TS, Bailey ST, Wilson DJ, Jia Y, Huang D, 2017. Detailed vascular anatomy of the human retina by projection-resolved optical coherence tomography angiography. Sci. Rep 7, 42201. 10.1038/srep42201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor LB, 2001. The effect of trabeculectomy on ocular hemodynamics. Trans. Am. Ophthalmol. Soc 99, 241–252. [PMC free article] [PubMed] [Google Scholar]
- Cantor E, Méndez F, Rivera C, Castillo A, Martínez-Blanco A, 2018. Blood pressure, ocular perfusion pressure and open-angle glaucoma in patients with systemic hypertension. Clin. Ophthalmol 12, 1511–1517. 10.2147/OPTH.S165747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli J, Coleman AL, Blood Flow in Glaucoma Discussion, 2010. Blood pressure, perfusion pressure, and glaucoma. Am. J. Ophthalmol 149 (5), 704–712. 10.1016/j.ajo.2010.01.018. [DOI] [PubMed] [Google Scholar]
- Carichino L, Guidoboni G, Siesky B, Amireskandari A, Januleviciene I, Harris A, 2014. Effect of intraocular pressure and cerebrospinal fluid pressure on the blood flow in the central retinal vessels. In: Causin P, Guidoboni G, Sacco R, Harris A (Eds.), Integrated Multidisciplinary Approaches in the Study and Care of the Human Eye. Kugler Publications, Amsterdam, Netherlands, pp. 161–175 pp. 161–175. [Google Scholar]
- Carichino L, Harris A, Guidoboni G, Siesky B, Pinto LA, Vandewalle E, Olafsdottir OB, Hardarson SH, Van Keer K, Stalmans I, Stefánsson E, Arciero J, 2016. A theoretical investigation of the increase in venous oxygen saturation levels in advanced glaucoma patients. J Model Ophthalmol 1 (1), 64–87. [Google Scholar]
- Carichino L, Harris A, Lapin S, Guidoboni G, Cassani S, De Silvestri A, Tinelli C, Milano G, Siesky B, Verticchio Vercellin AC, 2019. Waveform parameters of retrobulbar vessels in glaucoma patients with different demographics and disease severity. Eur. J. Ophthalmol 7 10.1177/1120672119848259.1120672119848259. [DOI] [PubMed] [Google Scholar]
- Carlson BE, Arciero JC, Secomb TW, 2008. Relative influence of myogenic, shear-dependent, and conducted responses on vascular autoregulation. Am. J. Physiol. Heart Circ. Physiol 295, H1572–H1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassani S, Guidoboni G, Janulviciene I, Carichino L, Siesky B, Tobe LA, Amireskandari A, Baikstiene DP, Harris A, 2014. Effect of trabeculectomy on retinal hemodynamics: mathematical modeling of clinical data. In: Causin P, Guidoboni G, Sacco R, Harris A (Eds.), Integrated Multidisciplinary Approaches in the Study and Care of the Human Eye. Kugler Publications, Amsterdam, Netherlands, pp. 161–175. [Google Scholar]
- Cassani S, Harris A, Siesky B, Arciero JC, 2015. Theoretical analysis of the relationship between changes in retinal blood flow and ocular perfusion pressure. J. Coupled Syst. Multiscale Dyn 3 (1), 38–46. [Google Scholar]
- Cassani S, Arciero JC, Guidoboni G, Siesky B, Harris A, 2016. Theoretical predictions of metabolic flow regulation in the retina. J Model Ophthalmol 2, 70–78. [Google Scholar]
- Causin P, Guidoboni G, Harris A, Prada D, Sacco R, Terragni S, 2014. A poroelastic model for the perfusion of the lamina cribrosa in the optic nerve head. Math. Biosci 257, 33–41. 10.1016/j.mbs.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Causin P, Guidoboni G, Malgaroli F, Sacco R, Harris A, 2016. Blood flow mechanics and oxygen transport and delivery in the retinal microcirculation: multiscale mathematical modeling and numerical simulation. Biomech. Model. Mechanobiol 15 (3), 525–542. 10.1007/s10237-015-0708-7. [DOI] [PubMed] [Google Scholar]
- Charlson ME, de Moraes CG, Link A, Wells MT, Harmon G, Peterson JC, Ritch R, Liebmann JM, 2014. Nocturnal systemic hypotension increases the risk of glaucoma progression. Ophthalmology 121 (10), 2004–2012. 10.1016/j.ophtha.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Menon D, Kavanagh B, 2019. Impact of altered airway pressure on intracranial pressure, perfusion, and oxygenation: a narrative review. Crit. Care Med 47 (2), 254–263. 10.1097/CCM.0000000000003558. [DOI] [PubMed] [Google Scholar]
- Cheung N, Mitchell P, Wong TY, 2010. Diabetic retinopathy. Lancet 376 (9735), 124–136. 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- Chiaravalli G, 2018. A Virtual Laboratory for Retinal Physiology: a Theoretical Study of Retinal Oxygenation in Healthy and Disease (MS Thesis, Politecnico di Milano, Italy). .
- Chiaravalli G, Guidoboni G, Sacco R, Ciulla T, Harris A, 2019. A theoretical study of vascular configurations of retinal capillary plexi based on OCTA data. Invest. Ophthalmol. Vis. Sci 60 (9), 149. [Google Scholar]
- Choi W, Moult EM, Waheed NK, Adhi M, Lee B, Lu CD, et al. , 2015. Ultrahigh-speed, swept-source optical coherence tomography angiography in nonexudative age-related macular degeneration with geographic atrophy. Ophthalmology 122, 2532–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra V, Varma R, Francis BA, Wu J, Torres M, Azen SP, 2008. Type 2 diabetes mellitus and the risk of open-angle glaucoma the Los Angeles Latino eye study. Ophthalmology 115, 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuangsuwanich T, Birgersson KE, Thiery A, Thakku SG, Leo HL, Girard MJ, 2016. Factors influencing lamina cribrosa microcapillary hemodynamics and oxygen concentrations. Invest. Ophthalmol. Vis. Sci 57 (14), 6167–6179. 10.1167/iovs.16-20167. [DOI] [PubMed] [Google Scholar]
- Ciulla L, Harris A, Geng J, Ip C, Serrano P, Gross J, Siesky B, 2017. The role of hypertension in retinal blood flow alterations in open-angle glaucoma patients. Acta Ophthalmol. 95 (8), e794–e795. 10.1111/aos.13365. [DOI] [PubMed] [Google Scholar]
- Collignon-Robe NJ, Feke GT, Rizzo JF 3rd, 2004. Optic nerve head circulation in nonarteritic anterior ischemic optic neuropathy and optic neuritis. Ophthalmology 111 (9), 1663–1672. [DOI] [PubMed] [Google Scholar]
- Conrad WA, 1969. Pressure-flow relationships in collapsible tubes. IEEE Trans. Biomed. Eng 16 (4), 284–295. [DOI] [PubMed] [Google Scholar]
- Conti FF, Song W, Rodrigues EB, Singh RP, 2019. Changes in retinal and choriocapillaris density in diabetic patients receiving anti-vascular endothelial growth factor treatment using optical coherence tomography angiography. Int J Retina Vitreous 5, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa VP, Jimenez-Roman J, Carrasco FG, Lupinacci A, Harris A, 2010. Twenty-four-hour ocular perfusion pressure in primary open-angle glaucoma. Br. J. Ophthalmol 94 (10), 1291–1294. [DOI] [PubMed] [Google Scholar]
- Costa VP, Harris A, Anderson D, Stodtmeister R, Cremasco F, Kergoat H, Lovasik J, Stalmans I, Zeitz O, Lanzl I, Gugleta K, Schmetterer L, 2014. Ocular perfusion pressure in glaucoma. Acta Ophthalmol. 92 (4), e252–e266. 10.1111/aos.12298. [DOI] [PubMed] [Google Scholar]
- Cousins CC, Chou JC, Greenstein SH, Brauner SC, Shen LQ, Turalba AV, Houlihan P, Ritch R, Wiggs JL, Knepper PA, Pasquale LR, 2019. Resting nailfold capillary blood flow in primary open-angle glaucoma. Br. J. Ophthalmol 103 (2), 203–207. 10.1136/bjophthalmol-2018-311846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creager MA, Lüscher TF, Cosentino F, Beckman JA, 2003. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part I. Circulation 108 (12), 1527–1532. [DOI] [PubMed] [Google Scholar]
- Cugati S, Wang JJ, Rochtchina E, Mitchell P, 2006. Ten-year incidence of retinal vein occlusion in an older population: the Blue Mountains Eye Study. Arch. Ophthalmol 124 (5), 726–732. [DOI] [PubMed] [Google Scholar]
- Cursiefen C, Wisse M, Cursiefen S, Junemann A, Martus P, Korth M, 2000. Migraine and tension headache in high-pressure and normal-pressure glaucoma. Am. J. Ophthalmol 129, 102–104. [DOI] [PubMed] [Google Scholar]
- de Carlo TE, Romano A, Waheed NK, Duker JS, 2015. A review of optical coherence tomography angiography (OCTA). Int J Retina Vitreous 1, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Moraes CG, Liebmann JM, Greenfield DS, Gardiner SK, Ritch R, Krupin T, Low-pressure Glaucoma Treatment Study Group, 2012. Risk factors for visual field progression in the low-pressure glaucoma treatment study. Am. J. Ophthalmol 154 (4), 702–711. 10.1016/j.ajo.2012.04.015. [DOI] [PubMed] [Google Scholar]
- De Simone D, Ranieri R, Bonavita V, 2017. Starling resistors, autoregulation of cerebral perfusion and the pathogenesis of idiopathic intracranial hypertension. Panminerva Med. 59 (1), 76–89. 10.23736/S0031-0808.16.03248-1. [DOI] [PubMed] [Google Scholar]
- de Voogd S, Ikram MK, Wolfs RC, Jansonius NM, Witteman JC, Hofman A, de Jong PT, 2006. Is diabetes mellitus a risk factor for open-angle glaucoma? The Rotterdam Study. Ophthalmology 113 (10), 1827–1831. [DOI] [PubMed] [Google Scholar]
- Deb AK, Kaliaperumal S, Rao VA, Sengupta S, 2014. Relationship between systemic hypertension, perfusion pressure and glaucoma: a comparative study in an adult Indian population. Indian J. Ophthalmol 62 (9), 917–922. 10.4103/0301-4738.143927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dielemans I, de Jong PT, Stolk R, Vingerling JR, Grobbee DE, Hofman A, 1996. Primary open-angle glaucoma, intraocular pressure, and diabetes mellitus in the general elderly population. The Rotterdam Study. Ophthalmology 103, 1271–1275. [DOI] [PubMed] [Google Scholar]
- Dielemans I, Vingerling JR, Algra D, Hofman A, Grobbee DE, de Jong PTVM, 1995. Primary open-angle glaucoma, intraocular pressure, and systemic blood pressure in the general elderly population. The Rotterdam Study. Ophthalmology 102 (1), 54–60. [DOI] [PubMed] [Google Scholar]
- Doganay S, Evereklioglu C, Er H, Turkoz Y, Sevinc A, Mehmet N, Savli H, 2002. Comparison of serum NO, TNF-alpha, IL-1beta, sIL-2R, IL-6 and IL-8 levels with grades of retinopathy in patients with diabetes mellitus. Eye (Lond). 16 (2), 163–170. [DOI] [PubMed] [Google Scholar]
- Donnelly J, Aries M, Czosnyka M, 2015. Further understanding of cerebral autoregulation at the bedside: possible implications for future therapy. Expert Rev. Neurother 15 (2), 169–185. 10.1586/14737175.2015.996552. [DOI] [PubMed] [Google Scholar]
- Downs JC, Suh JK, Thomas KA, Bellezza AJ, Burgoyne CF, Hart RT, 2003. Viscoelastic characterization of peripapillary sclera: material properties by quadrant in rabbit and monkey eyes. J. Biomech. Eng 125 (1), 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs JC, Suh JK, Thomas KA, Bellezza AJ, Hart RT, Burgoyne CF, 2005. Viscoelastic material properties of the peripapillary sclera in normal and early-glaucoma monkey eyes. Invest. Ophthalmol. Vis. Sci 46, 540–546. [DOI] [PubMed] [Google Scholar]
- Downs JC, 2015. IOP telemetry in the nonhuman primate. Exp. Eye Res 141, 91–98. 10.1016/j.exer.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs JC, Girkin CA, 2017. Lamina cribrosa in glaucoma. Curr. Opin. Ophthalmol 28 (2), 113–119. 10.1097/ICU.0000000000000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drance SM, Sweeney VP, Morgan RW, Feldman F, 1973. Studies of factors involved in the production of low-tension glaucoma. Arch. Ophthalmol 89, 457–465. [DOI] [PubMed] [Google Scholar]
- Dziubek A, Guidoboni G, Harris A, Hirani AH, Rusjan E, Thistleton W, 2016. Effect of ocular shape and vascular geometry on retinal hemodynamics: a computational model. Biomech. Model. Mechanobiol 15 (4), 893–907. [DOI] [PubMed] [Google Scholar]
- Ethier CR, Johnson M, Ruberti J, 2004. Ocular biomechanics and biotransport. Annu. Rev. Biomed. Eng 6, 249–273. [DOI] [PubMed] [Google Scholar]
- Erkin EF, Tarhan S, Kayikcioglu OR, Deveci H, Guler C, Goktan C, 2004. Effects of betaxolol and latanoprost on ocular blood flow and visual fields in patients with primary open-angle glaucoma. Eur. J. Ophthalmol 14, 211–219. [DOI] [PubMed] [Google Scholar]
- Evans DW, Harris A, Garrett M, Chung HS, Kagemann L, 1999. Glaucoma patients demonstrate faulty autoregulation of ocular blood flow during posture change. Br. J. Ophthalmol 83 (7), 809–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan BJ, Bailey JC, Igo RP Jr., Kang JH, Boumenna T, Brilliant MH, Budenz DL, Fingert JH, Gaasterland T, Gaasterland D, Hauser MA, Kraft P, Lee RK, Lichter PR, Liu Y, Moroi SE, Myers JS, Pericak-Vance MA, Realini A, Rhee DJ, Richards JE, Ritch R, Schuman JS, Scott WK, Singh K, Sit AJ, Vollrath D, Weinreb RN, Wollstein G, Zack DJ, Haines JL, Pasquale LR, Wiggs JL, 2019. Association of a primary open-angle glaucoma genetic risk score with earlier age at diagnosis. JAMA Ophthalmol. 10.1001/jamaophthalmol.2019.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazio MA, Clark ME, Bruno L, Girkin CA, 2018. In vivo optic nerve head mechanical response to intraocular and cerebrospinal fluid pressure: imaging protocol and quantification method. Sci Rep. 8 (1), 12639. 10.1038/s41598-018-31052-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feke G, Rhee D, Turalba A, Pasquale L, 2013. Effects of dorzolamide-timolol and brimonidine-timolol on retinal vascular autoregulation and ocular perfusion pressure in primary open angle glaucoma. J. Ocul. Pharmacol. Therapeut 29 (7), 639–645. [DOI] [PubMed] [Google Scholar]
- Fercher AF, Briers JD, 1981. Flow visualization by means of single-exposure speckle photography. Optic Commun. 37, 326–330. 10.1016/0030-4018(81)90428-4. [DOI] [Google Scholar]
- Ferdinand KC, 2008. Cardiovascular disease in blacks: can we stop the clock? J Clin Hypertens (Greenwich) 10 (5), 382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findl O, Strenn K, Wolzt M, Menapace R, Vass C, Eichler HG, Schmetterer L, 1997. Effects of changes in intraocular pressure on human ocular haemodynamics. Curr. Eye Res 16, 1024–1029. [DOI] [PubMed] [Google Scholar]
- Finkelstein EA, Khavjou OA, Thompson H, Trogdon JG, Pan L, Sherry B, Dietz W, 2012. Obesity and severe obesity forecasts through 2030. Am. J. Prev. Med 42 (6), 563–570. 10.1016/j.amepre.2011.10.026. [DOI] [PubMed] [Google Scholar]
- Flammer J, 1985. Psychophysics in glaucoma. A modified concept of the disease. In: Greeve EL, Leydhecker W, Raitta C (Eds.), Proceedings of the European Glaucoma Society. Second European Glaucoma Symposium, pp. 11–17. [Google Scholar]
- Flammer J, Orgül S, Costa VP, Orzalesi N, Krieglstein GK, Serra LM, Renard JP, Stefánsson E, 2002. The impact of ocular blood flow in glaucoma. Prog. Retin. Eye Res 21 (4), 359–393 submitted for publication. [DOI] [PubMed] [Google Scholar]
- Flammer J, Konieczka K, Flammer AJ, 2013. The primary vascular dysregulation syndrome: implications for eye diseases. EPMA J. 4 (1), 14. 10.1186/1878-5085-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischman D, Berdahl J, 2019. Anatomy and physiology of the cerebrospinal fluid. In: Guidoboni G, Harris A, Sacco R (Eds.), Ocular Fluid Dynamics. Anatomy, Physiology, Imaging Techniques, and Mathematical Modeling Modeling and Simulation in Science, Engineering, and Technology Springer-Birkhauser, New York. [Google Scholar]
- Formaggia L, Quarteroni A, Veneziani A, 2010. Cardiovascular Mathematics: Modeling and Simulation of the Circulatory System. Springer Science & Business Media. [Google Scholar]
- Foster PJ, Machin D, Wong TY, Ng TP, Kirwan JF, Johnson GJ, Khaw PT, Seah SK, 2003. Determinants of intraocular pressure and its association with glaucomatous optic neuropathy in Chinese Singaporeans: the Tanjong Pagar Study. Invest. Ophthalmol. Vis. Sci 44 (9), 3885–3891. [DOI] [PubMed] [Google Scholar]
- Fouquet S, Vacca O, Sennlaub F, Paques M, 2017. The 3D retinal capillary circulation in pigs reveals a predominant serial organization. Invest. Ophthalmol. Vis. Sci 58 (13), 5754–5763. 10.1167/iovs.17-22097. [DOI] [PubMed] [Google Scholar]
- Fraser CE D’Amico DJ Diabetic Retinopathy: Classification and Clinical Features. https://www.uptodate.com/contents/diabetic-retinopathy-classification-and-clinical-features/print, Accessed date: 24 September 2019.
- Friedman DS, Wolfs RC, O’Colmain BJ, Klein BE, Taylor HR, West S, Leske MC, Mitchell P, Congdon N, Kempen J, Eye Diseases Prevalence Research Group, 2004. Prevalence of open-angle glaucoma among adults in the United States. Arch. Ophthalmol 122 (4), 532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman E, 1997. A hemodynamic model of the pathogenesis of age-related macular degeneration. Am. J. Ophthalmol 124 (5), 677–682. [DOI] [PubMed] [Google Scholar]
- Fry BC, Coburn BE, Whiteman S, Harris A, Siesky B, Arciero JC, 2018. Predicting retinal tissue oxygenation using an image-based theoretical model. Math. Biosci 305, 1–9. 10.1016/j.mbs.2018.08.005. [DOI] [PubMed] [Google Scholar]
- Fuchsjager-Mayrl G, Georgopoulos M, Hommer A, Weigert G, Pemp B, Vass C, Garhöfer G, Schmetterer L, 2010. Effect of dorzolamide and timolol on ocular pressure: blood flow relationship in patients with primary open-angle glaucoma and ocular hypertension. Invest. Ophthalmol. Vis. Sci 51, 1289–1296. [DOI] [PubMed] [Google Scholar]
- Fung YC, 2013. Biomechanics: Circulation. Springer Science & Business Media. [Google Scholar]
- Furlanetto RL, De Moraes CG, Teng CC, Liebmann JM, Greenfield DS, Gardiner SK, Ritch R, Krupin T, Low-Pressure Glaucoma Treatment Study Group, 2014. Risk factors for optic disc hemorrhage in the low-pressure glaucoma treatment study. Am. J. Ophthalmol 157 (5), 945–952. 10.1016/j.ajo.2014.02.009. [DOI] [PubMed] [Google Scholar]
- Gaier ED, Wang M, Gilbert AL, Rizzo JF 3rd, Cestari DM, Miller JB, 2018. Quantitative analysis of optical coherence tomographic angiography (OCT-A) in patients with non-arteritic anterior ischemic optic neuropathy (NAION) corresponds to visual function. PloS One 13 (6), e0199793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galassi F, Sodi A, Ucci F, Renieri G, Pieri B, Baccini M, 2003. Ocular hemodynamics and glaucoma prognosis: a color Doppler imaging study. Arch. Ophthalmol 121 (12), 1711–1715. [DOI] [PubMed] [Google Scholar]
- Galassi F, Giambene B, Corvi A, Falaschi G, Menchini U, 2008. Retrobulbar hemodynamics and corneal surface temperature in glaucoma surgery. Int. Ophthalmol 28, 399–405. [DOI] [PubMed] [Google Scholar]
- Gallina P, Savastano A, Becattini E, Orlandini S, Scollato A, Rizzo S, Carreras G, Di Lorenzo N, Porfirio B, 2018. Glaucoma in patients with shunt-treated normal pressure hydrocephalus. J. Neurosurg 129 (4), 1078–1084. 10.3171/2017.5.JNS163062. [DOI] [PubMed] [Google Scholar]
- Gandhi U, Chhablani J, Badakere A, Kekunnaya R, Rasheed MA, Goud A, Chhablani PP, 2018. Optical coherence tomography angiography in acute uni-lateral nonarteritic anterior ischemic optic neuropathy: a comparison with the fellow eye and with eyes with papilledema. Indian J. Ophthalmol 66 (8), 1144–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner, et al. , 2019. Increased optic nerve head capillary blood flow in early primary open-angle glaucoma. Invest. Ophthalmol. Vis. Sci 60 (8), 3110–3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser P, Flammer J, 1987. Influence of vasospasm on visual function. Doc. Ophthalmol 66 (1), 3–18. [DOI] [PubMed] [Google Scholar]
- Gerber AL, Harris A, Siesky B, Lee E, Schaab TJ, Huck A, Amireskandari A, 2015. Vascular dysfunction in diabetes and glaucoma: a complex relationship reviewed. J. Glaucoma 24 (6), 474–479. 10.1097/IJG.0000000000000137. [DOI] [PubMed] [Google Scholar]
- Gilmore ED, Hudson C, Nrusimhadevara RK, et al. , 2008. Retinal arteriolar hemodynamic response to a combined isocapnic hyperoxia and glucose provocation in early sight-threatening diabetic retinopathy. Invest. Ophthalmol. Vis. Sci 49 (2), 699–705. [DOI] [PubMed] [Google Scholar]
- Glucksberg MR, Dunn R, 1993. Direct measurement of retinal microvascular pressures in the live, anesthetized cat. Microvasc. Res 45 (2), 158–165. [DOI] [PubMed] [Google Scholar]
- Gordon MO, Beiser JA, Kass MA, 2008. Is a history of diabetes mellitus protective against developing primary open-angle glaucoma? Arch. Ophthalmol 126, 280–281. [DOI] [PubMed] [Google Scholar]
- Graham SL, Drance SM, Wijsman K, Douglas GR, Mikelberg FS, 1995. Ambulatory blood pressure monitoring in glaucoma. The nocturnal dip. Ophthalmology 102 (1), 61–69. [DOI] [PubMed] [Google Scholar]
- Gramer G, Weber BH, Gramer E, 2015. Migraine and Vasospasm in Glaucoma: Age-Related Evaluation of 2027 Patients With Glaucoma or Ocular Hypertension. Invest Ophthalmol Vis Sci 56 (13), 7999–8007. [DOI] [PubMed] [Google Scholar]
- Grudzińska E, Modrzejewska M, 2018. Modern diagnostic techniques for the assessment of ocular blood flow in myopia: current state of knowledge. J Ophthalmol 2018, 4694789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald JE, DuPont J, Riva CE, 1996. Retinal haemodynamics in patients with early diabetes mellitus. Br. J. Ophthalmol 80 (4), 327–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald JE, Metelitsina TI, Dupont JC, Ying GS, Maguire MG, 2005. Reduced foveolar choroidal blood flow in eyes with increasing AMD severity. Invest. Ophthalmol. Vis. Sci 46 (3), 1033–1038. [DOI] [PubMed] [Google Scholar]
- Grunwald JE, Riva CE, Brucker AJ, Sinclair SH, Petrig BL, 1984a. Altered retinal vascular response to 100% oxygen breathing in diabetes mellitus. Ophthalmology 91 (12), 1447–1452. [DOI] [PubMed] [Google Scholar]
- Grunwald JE, Riva CE, Stone RA, Keates EU, Petrig BL, 1984b. Retinal autoregulation in open-angle glaucoma. Ophthalmology 91 (12), 1690–1694. [DOI] [PubMed] [Google Scholar]
- Grytz R, Meschke G, 2010. A computational remodeling approach to predict the physiological architecture of the collagen fibril network in corneo-scleral shells. Biomech. Model. Mechanobiol 9 (2), 225–235. 10.1007/s10237-009-0173-2. [DOI] [PubMed] [Google Scholar]
- Grytz R, Meschke G, Jonas JB, 2011. The collagen fibril architecture in the lamina cribrosa and peripapillary sclera predicted by a computational remodeling approach. Biomech. Model. Mechanobiol 10 (3), 371–382. 10.1007/s10237-010-0240-8. [DOI] [PubMed] [Google Scholar]
- Guidoboni G, Harris A, Arciero JC, Siesky BA, Amireskandari A, Gerber AL, Huck AH, Kim NJ, Cassani S, Carichino L, 2013. Mathematical modeling approaches in the study of glaucoma disparities among people of African and European Descents. J. Coupled Syst. Multiscale Dynam 1 (1), 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidoboni G, Harris A, Carichino L, Arieli Y, Siesky B, 2014a. Effect of intraocular pressure on the hemodynamics of the central retinal artery: a mathematical model. Math. Biosci. Eng 11 (3), 523–546. 10.3934/mbe.2014.11.523. [DOI] [PubMed] [Google Scholar]
- Guidoboni G, Harris A, Cassani S, Arciero J, Siesky B, Amireskandari A, Tobe L, Egan P, Januleviciene I, Park J, 2014b. Intraocular pressure, blood pressure, and retinal blood flow autoregulation: a mathematical model to clarify their relationship and clinical relevance. Invest. Ophthalmol. Vis. Sci 55 (7), 4105–4118. 10.1167/iovs.13-13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidoboni G, Chong R, Marazzi N, Chee M, Wellington J, Lichtenegger E, Cheng C, Harris A, 2019. A Mechanism-Driven Algorithm for Artificial Intelligence in Ophthalmology: Understanding Glaucoma Risk Factors in the Singapore Eye Diseases Study. (accepted abstract for ARVO 2020 Annual Meeting).
- Guthauser U, Flammer J, Mahler F, 1988. The relationship between digital and ocular vasospasm. Graefes Arch. Clin. Exp. Ophthalmol 226 (3), 224–226. [DOI] [PubMed] [Google Scholar]
- Ha A, Kim YK, Baek SU, Park KH, Jeoung JW, 2019. Optic disc microemorrhage in primary open-angle glaucoma: clinical implications for visual field progression. Invest. Ophthalmol. Vis. Sci 60 (6), 1824–1832. 10.1167/iovs.19-26673. [DOI] [PubMed] [Google Scholar]
- Hammer M, Vilser W, Riemer T, Mandecka A, Schweitzer D, Kuhn H, Dawczynski J, Liemt F, Strobel J, 2009. Diabetic patients with retinopathy show increased retinal venous oxygen saturation. Graefes Arch. Clin. Exp. Ophthalmol 247 (8), 1025–1030. 10.1007/s00417-009-1078-6. [DOI] [PubMed] [Google Scholar]
- Hardarson SH, Basit S, Jonsdottir TE, Eysteinsson T, Halldorsson GH, Karlsson RA, Beach JM, Benediktsson JA, Stefansson E, 2009. Oxygen saturation in human retinal vessels is higher in dark than in light. Invest. Ophthalmol. Vis. Sci 50 (5), 2308–2311. 10.1167/iovs.08-2576. [DOI] [PubMed] [Google Scholar]
- Harris A, Joos K, Kay M, Evans D, Shetty R, Sponsel WE, Martin B, 1996. Acute IOP elevation with scleral suction: effects on retrobulbar haemodynamics. Br. J. Ophthalmol 80, 1055–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A, Chung HS, Ciulla TA, Kagemann L, 1999. Progress in measurement of ocular blood flow and relevance to our understanding of glaucoma and age-related macular degeneration. Prog. Retin. Eye Res 18 (5), 669–687. [DOI] [PubMed] [Google Scholar]
- Harris A, Harris M, Biller J, Garzozi H, Zarfty D, Ciulla TA, Martin B, 2000. Aging affects the retrobulbar circulation differently in women and men. Arch. Ophthalmol 118 (8), 1076–1080. [DOI] [PubMed] [Google Scholar]
- Harris A, Jonescu-Cuypers C, Martin B, Kagemann L, Zalish M, Garzozi HJ, 2001. Simultaneous management of blood flow and IOP in glaucoma. Acta Ophthalmol. Scand 79 (4), 336–341. [DOI] [PubMed] [Google Scholar]
- Harris A, Siesky B, Zarfati D, Haine CL, Catoira Y, Sines DT, McCranor L, Garzozi HJ, 2007. Relationship of cerebral blood flow and central visual function in primary open-angle glaucoma. J. Glaucoma 16 (1), 159–163. [DOI] [PubMed] [Google Scholar]
- Harris A, Kagemann L, Ehrlich R, Rospigliosi C, Moore D, Siesky B, 2008. Measuring and interpreting ocular blood flow and metabolism in glaucoma. Can. J. Ophthalmol 43 (3), 328–336. 10.3129/i08-051. [DOI] [PubMed] [Google Scholar]
- Harris A, Garzozi HJ, McCranor L, Rechtman E, Yung CW, Siesky B, 2009. The effect of latanoprost on ocular blood flow. Int. Ophthalmol 29, 19–26. [DOI] [PubMed] [Google Scholar]
- Harris A, Jonescu-Cuypers CP, Kagemann L, Ciulla TA, Krieglstein GK, 2010. Atlas of Ocular Blood Flow, second ed. Butterworth-Heinemann Elsevier, Pennsylvania. [Google Scholar]
- Harris A, Siesky B, Wirostko B, 2013. Cerebral blood flow in glaucoma patients. J. Glaucoma 22 (Suppl. 5), S46–S48. 10.1097/IJG.0b013e3182934b6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayreh SS, 1999. The role of age and cardiovascular disease in glaucomatous optic neuropathy. Surv. Ophthalmol 43 (Suppl. 1), S27–S42. [DOI] [PubMed] [Google Scholar]
- Hayreh SS, Zimmerman MB, Podhajsky P, Alward WL, 1994. Nocturnal arterial hypotension and its role in optic nerve head and ocular ischemic disorders. Am. J. Ophthalmol 117 (5), 603–624. [DOI] [PubMed] [Google Scholar]
- Hayreh SS, 2001a. The blood supply of the optic nerve head and the evaluation of it -myth and reality. Prog. Retin. Eye Res 20 (5), 563–593 submitted for publication). [DOI] [PubMed] [Google Scholar]
- Hayreh SS, 2001b. Blood flow in the optic nerve head and factors that may influence it. Prog. Retin. Eye Res 20 (5), 595–624. [DOI] [PubMed] [Google Scholar]
- He Z, Lim JK, Nguyen CT, Vingrys AJ, Bui BV, 2013. Coupling blood flow and neural function in the retina: a model for homeostatic responses to ocular perfusion pressure challenge. Phys. Rep 1 (3), e00055. 10.1002/phy2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Nguyen CT, Armitage JA, Vingrys AJ, Bui BV, 2012. Blood pressure modifies retinal susceptibility to intraocular pressure elevation. PloS One 7 (2), e31104. 10.1371/journal.pone.0031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijl A, Leske MC, Bengtsson B, Hyman L, Hussein M, Early Manifest Glaucoma Trial Group, 2002. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch. Ophthalmol 120 (10), 1268–1279. [DOI] [PubMed] [Google Scholar]
- Hendrick AM, Gibson MV, Kulshreshtha A, 2015. Diabetic retinopathy. Prim Care 42 (3), 451–464. 10.1016/j.pop.2015.05.005. [DOI] [PubMed] [Google Scholar]
- Hennis A, Wu SY, Nemesure B, Leske MC, 2003. Hypertension, diabetes and longitudinal changes in intraocular pressure. Ophthalmology 110, 908–914. [DOI] [PubMed] [Google Scholar]
- Hogarty D, Mackey D, Hewitt A, 2019. Current state and future prospects of artificial intelligence in ophthalmology: a review. Clin. Exp. Ophthalmol 47, 128–139. [DOI] [PubMed] [Google Scholar]
- Hommer A, Sperl P, Resch H, Popa-Cherecheanu A, Qiao C, Schmetterer L, Garhöfer G, 2012. A double-masked randomized crossover study comparing the effect of latanoprost/timolol and brimonidine/timolol fixed combination on intraocular pressure and ocular blood flow in patients with primary open-angle glaucoma or ocular hypertension. J. Ocul. Pharmacol. Therapeut 2012 (28), 569–575. [DOI] [PubMed] [Google Scholar]
- Huber-van der Velden KK, Lux A, Severing K, Klamann MK, Winterhalter S, Remky A, 2012. Retrobulbar hemodynamics before and after oculopression with and without dorzolamide. Curr. Eye Res 37, 719–725. [DOI] [PubMed] [Google Scholar]
- Huck A, Harris A, Siesky B, Kim N, Muchnik M, Kanakamedala P, Amireskandari A, Abrams-Tobe L, 2014. Vascular considerations in glaucoma patients of African and European descent. Acta Ophthalmol. 92 (5), e336–e340. 10.1111/aos.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsman CA, Vingerling JR, Hofman A, Witteman JC, de Jong PT, 2007. Blood pressure, arterial stiffness, and open-angle glaucoma: the Rotterdam study. Arch. Ophthalmol 125 (6), 805–812. [DOI] [PubMed] [Google Scholar]
- Hutchins K, Harris A, Thomas J, Alkhairy S, Verticchio Vercellin AC, Shah A, Siesky B, 2018. Alzheimer’s disease and primary open-angle glaucoma associated with vascular health in patients of African descent. Acta Ophthalmol. 96 (8), e1031. 10.1111/aos.13739. [DOI] [PubMed] [Google Scholar]
- Hyman L, 1987. Epidemiology of eye disease in the elderly. Eye (Lond). 1 (Pt 2), 330–341. [DOI] [PubMed] [Google Scholar]
- Inan UU, Ermis SS, Yucel A, Ozturk F, 2003. The effects of latanoprost and brimonidine on blood flow velocity of the retrobulbar vessels: a 3-month clinical trial. Acta Ophthalmol. Scand 81, 155–160. [DOI] [PubMed] [Google Scholar]
- Iroku-Malize T, Kirsch S, 2016. Eye conditions in older adults: age-related macular degeneration. FP Essent 445, 24–28. [PubMed] [Google Scholar]
- James CB, 1994. Effect of trabeculectomy on pulsatile ocular blood flow. Br. J. Ophthalmol 78, 818–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Januleviciene I, Sliesoraityte I, Siesky B, Harris A, 2008. Diagnostic compatibility of structural and haemodynamic parameters in open-angle glaucoma patients. Acta Ophthalmol. 86 (5), 552–557. [DOI] [PubMed] [Google Scholar]
- Januleviciene I, Siaudvytyte L, 2019. Instruments to measure and visualize geometrical and functional parameters related to the fluid-dynamics of cerebrospinal fluid in the eye. In: Guidoboni G, Harris A, Sacco R (Eds.), Ocular Fluid Dynamics. Anatomy, Physiology, Imaging Techniques, and Mathematical Modeling. Modeling and Simulation in Science, Engineering, and Technology Springer-Birkhauser, New York. [Google Scholar]
- Jaulim A, Ahmed B, Khanam T, Chatziralli IP, 2013. Branch retinal vein occlusion: epidemiology, pathogenesis, risk factors, clinical features, diagnosis, and complications. An update of the literature. Retina 33 (5), 901–910. 10.1097/IAE.0b013e3182870c15. [DOI] [PubMed] [Google Scholar]
- Jimenez-Aragon F, Garcia-Martin E, Larrosa-Lopez R, Artigas-Martín JM, Seral-Moral P, Pablo LE, 2013. Role of color Doppler imaging in early diagnosis and prediction of progression in glaucoma. BioMed Res. Int 2013, 871689. 10.1155/2013/871689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SW, Noh SY, 2017. Long-term clinical course of normal-tension glaucoma: 20 Years of experience. J Ophthalmol 2017, 2651645. 10.1155/2017/2651645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen CM, Bek T, 2016. Lack of differences in the regional variation of oxygen saturation in larger retinal vessels in diabetic maculopathy and proliferative diabetic retinopathy. Br. J. Ophthalmol 101 (6), 752–757. 10.1136/bjophthalmol-2016-308894. [DOI] [PubMed] [Google Scholar]
- Jorgensen CM, Hardarson SH, Bek T, 2014. The oxygen saturation in retinal vessels from diabetic patients depends on the severity and type of vision-threatening retinopathy. Acta Ophthalmol. 92 (1), 34–39. 10.1111/aos.12283. [DOI] [PubMed] [Google Scholar]
- Kaiser HJ, Flammer J, Graf T, Stümpfig D, 1993. Systemic blood pressure in glaucoma patients. Graefes Arch. Clin. Exp. Ophthalmol 231 (12), 677–680. [DOI] [PubMed] [Google Scholar]
- Kanakamedala P, Harris A, Siesky B, Tyring A, Muchnik M, Eckert G, Abrams Tobe L, 2014. Optic nerve head morphology in glaucoma patients of African descent is strongly correlated to retinal blood flow. Br. J. Ophthalmol 98 (11), 1551–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaskan B, Ramezani K, Harris A, Siesky B, Olinde C, WuDunn D, Eikenberry J, Tobe LA, Racette L, 2016. Differences in ocular blood flow between people of african and European descent with healthy eyes. J. Glaucoma 25 (9), 709–715. 10.1097/IJG.0000000000000509. [DOI] [PubMed] [Google Scholar]
- Kaup M, Plange N, Arend KO, Remky A, 2006. Retrobulbar haemodynamics in non-arteritic anterior ischaemic optic neuropathy. Br. J. Ophthalmol 90 (11), 1350–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaup M, Plange N, Niegel M, Remky A, Arend O, 2004. Effects of brinzolamide on ocular haemodynamics in healthy volunteers. Br. J. Ophthalmol 88, 257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodabandeh A, Shahraki K, Roohipoor R, Riazi-Esfahani H, Yaseri M, Faghihi H, Bazvand F, 2018. Quantitative measurement of vascular density and flow using optical coherence tomography angiography (OCTA) in patients with central retinal vein occlusion: can OCTA help in distinguishing ischemic from non-ischemic type? Int J Retina Vitreous 4, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Cho K, Oh S, 2017. Development of machine learning models for diagnosis of glaucoma. PloS One 12, e0177726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton FP, Starling EH, 1912. The influence of variations in temperature and blood pressure on the performance of the isolated mammalian heart. J. Physiol 44 (3), 206–219. 10.1113/jphysiol.1912.sp001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JA, Lee EJ, Kim TW, 2019. Evaluation of parapapillary choroidal microvasculature dropout and progressive retinal nerve fiber layer thinning in patients with glaucoma. JAMA Ophthalmol 137 (7), 810–816. 10.1001/jamaophthalmol.2019.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JT, Chun YS, Lee JK, Moon NJ, Yi DY, 2019. Comparison of vessel density reduction in the deep and superficial capillary plexuses in branch retinal vein occlusion. Ophthalmologica 1–9. [DOI] [PubMed] [Google Scholar]
- Kim SH, Park KH, The relationship between recurrent optic disc hemorrhage and glaucoma progression, 2006. Ophthalmology 113 (4), 598–602. [DOI] [PubMed] [Google Scholar]
- Kim KE, Park KH, 2017. Optic disc hemorrhage in glaucoma: pathophysiology and prognostic significance. Curr. Opin. Ophthalmol 28 (2), 105–112. [DOI] [PubMed] [Google Scholar]
- Kim YK, Choi HJ, Jeoung JW, Park KH, Kim DM, 2014. Five-year incidence of primary open-angle glaucoma and rate of progression in health center-based Korean population: the gangnam eye study. PloS One 9 (12), e114058. 10.1371/journal.pone.0114058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyota N, Shiga Y, Yasuda M, Aizawa N, Omodaka K, Tsuda S, Kunikata H, Nakazawa T, 2019. Sectoral differences in the association of optic nerve head blood flow and glaucomatous visual field defect severity and progression. Invest. Ophthalmol. Vis. Sci 60 (7), 2650–2658. 10.1167/iovs.19-27230. [DOI] [PubMed] [Google Scholar]
- Klein BE, Klein R, Jensen SC, 1994. Open-angle glaucoma and older-onset diabetes. The Beaver Dam eye study. Ophthalmology 101, 1173–1177. [DOI] [PubMed] [Google Scholar]
- Kohmoto R, Sugiyama T, Ueki M, Kojima S, Maeda M, Nemoto E, Tokuoka S, Ikeda T, 2019. Correlation between laser speckle flowgraphy and optical coherence tomography angiography measurements in normal and glaucomatous eyes. Clin. Ophthalmol 13, 1799–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konieczka K, Ritch R, Traverso CE, Kim DM, Kook MS, Gallino A, Golubnitschaja O, Erb C, Reitsamer HA, Kida T, Kurysheva N, Yao K, 2014. Flammer syndrome. EPMA J. 5 (1), 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koustenis A Jr., Harris A, Gross J, Januleviciene I, Shah A, Siesky B, 2017. Optical coherence tomography angiography: an overview of the technology and an assessment of applications for clinical research. Br. J. Ophthalmol 101 (1), 16–20. 10.1136/bjophthalmol-2016-309389. [DOI] [PubMed] [Google Scholar]
- Koz OG, Ozsoy A, Yarangumeli A, Kose SK, Kural G, 2007. Comparison of the effects of travoprost, latanoprost and bimatoprost on ocular circulation: a 6-month clinical trial. Acta Ophthalmol. Scand 85, 838–843. [DOI] [PubMed] [Google Scholar]
- Krasinska B, Karolczak-Kulesza M, Krasinski Z, Pawlaczyk-Gabriel K, Niklas A, Głuszek J, Tykarski A, 2011. A marked fall in nocturnal blood pressure is associated with the stage of primary open-angle glaucoma in patients with arterial hypertension. Blood Pres. 20 (3), 171–181. 10.3109/08037051.2010.538964. [DOI] [PubMed] [Google Scholar]
- Krogh A, 1919. The number and distribution of capillaries in muscles with calculations of the oxygen pressure head necessary for supplying the tissue. J. Physiol. (London) 52, 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar RS, Anegondi N, Chandapura RS, Sudhakaran S, Kadambi SV, Rao HL, Aung T, Sinha Roy A, 2016. Discriminant function of optical coherence tomography angiography to determine disease severity in glaucoma. Invest. Ophthalmol. Vis. Sci 57 (14), 6079–6088. 10.1167/iovs.16-19984. [DOI] [PubMed] [Google Scholar]
- Kur J, Newman EA, Chan-Ling T, 2012. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog. Retin. Eye Res 31 (5), 377–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyari F, Abdull MM, Wormald R, Evans JR, Nolan W, Murthy GVS, Gilbert CE, Nigeria National Blindness and Visual Impairment Study Group, 2016. Risk factors for open-angle glaucoma in Nigeria: results from the Nigeria national blindness and visual impairment Survey. BMC Ophthalmol. 16, 78. 10.1186/s12886-016-0264-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Ahn J, Yun C, Kim SW, Oh J, 2018. Variation of retinal and choroidal vasculatures in patients with age-related macular degeneration. Invest. Ophthalmol. Vis. Sci 59 (12), 5246–5255. 10.1167/iovs.17-23600. [DOI] [PubMed] [Google Scholar]
- Lee CY, Liu CH, Chen HC, Sun CC, Yao YP, Chao SC, 2019. Correlation between basal macular circulation and following glaucomatous damage in progressed high-tension and normal-tension glaucoma. Ophthalmic Res. 62 (1), 46–54. 10.1159/000499695. [DOI] [PubMed] [Google Scholar]
- Lee E, Harris A, Siesky B, Schaab T, McIntyre N, Tobe LA, Ling J, 2014. The influence of retinal blood flow on open-angle glaucoma in patients with and without diabetes. Eur. J. Ophthalmol 24 (4), 542–549. 10.5301/ejo.5000419. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Kim TW, Kim M, Kim H, 2015a. Influence of lamina cribrosa thickness and depth on the rate of progressive retinal nerve fiber layer thinning. Ophthalmology 122 (4), 721–729. 10.1016/j.ophtha.2014.10.007. [DOI] [PubMed] [Google Scholar]
- Lee J, Choi J, Jeong D, Kim S, Kook MS, 2015b. Relationship between daytime variability of blood pressure or ocular perfusion pressure and glaucomatous visual field progression. Am. J. Ophthalmol 160 (3), 522–537. 10.1016/j.ajo.2015.05.034.e1. [DOI] [PubMed] [Google Scholar]
- Leitgeb RA, Werkmeister RM, Blatter C, Schmetterer L, 2014. Doppler optical coherence tomography. Prog. Retin. Eye Res 41, 26–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leske MC, Connell AM, Wu SY, Hyman LG, Schachat AP, 1995. Risk factors for open-angle glaucoma: the Barbados eye study. Arch. Ophthalmol 113 (7), 918–924. 10.1001/archopht.1995.01100070092031. [DOI] [PubMed] [Google Scholar]
- Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E, EMGT Group, 2003. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch. Ophthalmol 121 (1), 48–56. [DOI] [PubMed] [Google Scholar]
- Leske MC, Heijl A, Hyman L, Bengtsson B, Dong L, Yang Z, EMGT Group, 2007. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology 114 (11), 1965–1972. 10.1016/j.ophtha.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Leske MC, Wu SY, Hennis A, Honkanen R, Nemesure B, 2008. Risk factors for incident open-angle glaucoma: the Barbados Eye Studies. Ophthalmology 115 (1), 85–93. 10.1016/j.ophtha.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Leske MC, Wu SY, Nemesure B, Hennis A, 2002. Incident open-angle glaucoma and blood pressure. Arch. Ophthalmol 120 (7), 954–959. 10.1001/archopht.120.7.954. [DOI] [PubMed] [Google Scholar]
- Li M, Yang Y, Jiang H, et al. , 2017. Retinal microvascular network and microcirculation assessments in high myopia. Am. J. Ophthalmol 174, 56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang YB, Zhou Q, Friedman DS, Guo LX, Sun LP, Zong QF, Yang XD, Wang NL, 2016. A population-based assessment of 24-hour ocular perfusion pressure among patients with primary open angle glaucoma: the handan eye study. Asia Pac J Ophthalmol (Phila). 5 (2), 127–132. 10.1097/APO.0000000000000155. [DOI] [PubMed] [Google Scholar]
- Lin SC, Pasquale LR, Singh K, 2018. The association between body mass index and open-angle glaucoma in a South Korean population-based sample. J. Glaucoma 27 (3), 239–245. 10.1097/IJG.0000000000000867. [DOI] [PubMed] [Google Scholar]
- Liu W, Ling J, Chen Y, Wu Y, Lu P, 2017. The association between adiposity and the risk of glaucoma: a meta-analysis. J Ophthalmol 2017, 9787450. 10.1155/2017/9787450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KC, Fleischman D, Lee AG, Killer HE, Chen JJ, Bhatti MT, 2019. Current concepts of cerebrospinal fluid dynamics and the translaminar cribrosa pressure gradient: a paradigm of optic disc disease. Surv. Ophthalmol S0039–6257 (19), 30251–30256. 10.1016/j.survophthal.2019.08.005.pii. [DOI] [PubMed] [Google Scholar]
- Mäepea O, 1992. Pressures in the anterior arteries, choroidal veins and choriocapillaris. Exp. Eye Res 54, 731–736. [DOI] [PubMed] [Google Scholar]
- Martínez A, Sánchez M, 2005. Predictive value of colour Doppler imaging in a prospective study of visual field progression in primary open-angle glaucoma. Acta Ophthalmol. Scand 83 (6), 716–722. [DOI] [PubMed] [Google Scholar]
- Mastropasqua R, Agnifili L, Borrelli E, Fasanella V, Brescia L, Di Antonio L, Mastropasqua L, 2018. Optical coherence tomography angiography of the peripapillary retina in normal-tension glaucoma and chronic nonarteritic anterior ischemic optic neuropathy. Curr. Eye Res 43 (6), 778–784. [DOI] [PubMed] [Google Scholar]
- Matsunaga D, Yi J, Puliafito CA, Kashani AH, 2014. OCT angiography in healthy human subjects. Ophthalmic Surg Lasers Imaging Retina 45 (6), 510–515. 10.3928/23258160-20141118-04. [DOI] [PubMed] [Google Scholar]
- May CA, Neuhaler W, Lutjen-Drecoll E, 2004. Immunohistochemical classification and functional morphology of human choroidal ganglion cell. Invest. Ophthalmol. Vis. Sci 45 (2), 361–367. [DOI] [PubMed] [Google Scholar]
- McDonald D, 1955. The relation of pulsatile pressure to flow in arteries. J. Physiol 127, 533–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald D, 1974. Blood Flow in Arteries, Second. Edward Arnold, London. [Google Scholar]
- McLeod DS, Grebe R, Bhutto I, Merges C, Baba T, Lutty GA, 2009. Relationship between RPE and choriocapillaris in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci 50 (10), 4982–4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melgarejo JD, Lee JH, Petitto M, Yépez JB, Murati FA, Jin Z, Chávez CA, Pirela RV, Calmón GE, Lee W, Johnson MP, Mena LJ, Al-Aswad LA, Terwilliger JD, Allikmets R, Maestre GE, De Moraes CG, 2018. Glaucomatous optic neuropathy associated with nocturnal dip in blood pressure: findings from the Maracaibo aging study. Ophthalmology (6), 807–814. 10.1016/j.ophtha.2017.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memarzadeh F, Ying-Lai M, Chung J, Azen SP, Varma R, Los Angeles Latino Eye Study Group, 2010. Blood pressure, perfusion pressure, and open-angle glaucoma: the Los Angeles Latino eye study. Invest. Ophthalmol. Vis. Sci 51 (6), 2872–2877. 10.1167/iovs.08-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P, Lee AJ, Wang JJ, Rochtchina E, 2005. Intraocular pressure over the clinical range of blood pressure: blue Mountains eye study findings. Am. J. Ophthalmol 140 (1), 131–132. [DOI] [PubMed] [Google Scholar]
- Mitchell P, Smith W, Attebo K, Healey PR, 1996. Prevalence of open-angle glaucoma in Australia. The blue Mountains eye study. Ophthalmology 103 (10), 1661–1669. [DOI] [PubMed] [Google Scholar]
- Mitchell P, Smith W, Chey T, Healey PR, 1997. Open angle glaucoma and diabetes: the Blue Mountains eye study, Australia. Ophthalmology 104, 712–718. [DOI] [PubMed] [Google Scholar]
- Mizutani M, Kern TS, Lorenzi M, 1996. Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. J. Clin. Invest 97 (12), 2883–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D, Harris A, Wudunn D, Kheradiya N, Siesky B, 2008. Dysfunctional regulation of ocular blood flow: a risk factor for glaucoma? Clin. Ophthalmol 2 (4), 849–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore NA, Harris A, Wentz S, Verticchio Vercellin AC, Parekh P, Gross J, Hussain RM, Thieme C, Siesky B, 2017. Baseline retrobulbar blood flow is associated with both functional and structural glaucomatous progression after 4 years. Br. J. Ophthalmol 101 (3), 305–308. 10.1136/bjophthalmol-2016-308460. [DOI] [PubMed] [Google Scholar]
- Moreira-Neto CA, Moult EM, Fujimoto JG, Waheed NK, Ferrara D, 2018. Choriocapillaris loss in advanced age-related macular degeneration. J Ophthalmol 2018, 8125267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan WH, Yu DY, Alder VA, Cringle SJ, Cooper RL, House PH, Constable IJ, 1998. The correlation between cerebrospinal fluid pressure and retrolaminar tissue pressure. Invest. Ophthalmol. Vis. Sci 39 (8), 1419–1428. [PubMed] [Google Scholar]
- Morgan WH, Hazelton ML, Yu DY, 2016a. Retinal venous pulsation: expanding our understanding and use of this enigmatic phenomenon. 2016 Nov. Prog. Retin. Eye Res 55, 82–107. 10.1016/j.preteyeres.2016.06.003. Epub 2016 Jul 11. [DOI] [PubMed] [Google Scholar]
- Morgan WH, Balaratnasingam C, Lind CR, Colley S, Kang MH, House PH, Yu DY, 2016b. Cerebrospinal fluid pressure and the eye. Br. J. Ophthalmol 100 (1), 71–77. 10.1136/bjophthalmol-2015-306705. [DOI] [PubMed] [Google Scholar]
- Moult E, Choi W, Waheed NK, Adhi M, Lee B, Lu CD, Jayaraman V, Potsaid B, Rosenfeld PJ, Duker JS, Fujimoto JG, 2014. Ultrahigh-speed swept-source OCT angiography in exudative AMD. Ophthalmic Surg Lasers Imaging Retina 45 (6), 496–505. 10.3928/23258160-20141118-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mursch-Edlmayr A, Luft N, Podkowinski D, Ring M, Schmetterer L, Bolz M, 2018. Laser speckle flowgraphy derived characteristics of optic nerve head perfusion in normal tension glaucoma and healthy individuals: a Pilot study. Sci. Rep 8 (1), 5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Kanamori A, Negi A, 2005. Diabetes mellitus as a risk factor for glaucomatous optic neuropathy. Ophthalmologica 219 (1), 1–10. [DOI] [PubMed] [Google Scholar]
- National Aeronautics and Space Administration (NASA), 2017. Evidence Report. Risk of Spaceflight Associated Neuro-Ocular Syndrome (SANS). . https://humanresearchroadmap.nasa.gov/evidence/reports/SANS.pdf, Accessed date: 25 September 2019. [Google Scholar]
- Nelson DA, Krupsky S, Pollack A, Aloni E, Belkin M, Vanzetta I, Rosner M, Grinvald A, 2005. Special report: noninvasive multi-parameter functional optical imaging of the eye. Ophthalmic Surg. Laser. Imag 36 (1), 57–66. [PubMed] [Google Scholar]
- Newton I, 1687. Philosophiae Naturalis Principia Mathematica. Reg. Soc Praeses, London. [Google Scholar]
- Ng A, Harris A, Verticchio Vercellin A, Shah A, Kawiecki RM, Patel PR, Siesky B, 2018. Changes in structural parameters predict functional progression of open angle glaucoma in overweight patients. Invest. Ophthalmol. Vis. Sci 59 (9), 4060. [Google Scholar]
- Ngo S, Harris A, Siesky B, Schroeder A, Eckert G, Holland S, 2013. Blood pressure, ocular perfusion pressure, and body mass index in glaucoma patients. Eur. J. Ophthalmol 23 (5), 664–669. 10.5301/ejo.5000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura H, Shimokata H, Ando F, Miyake Y, Kuzuya F, 1999. Age-related changes in intraocular pressure in a large Japanese population: a cross-sectional and longitudinal study. Ophthalmology 106 (10), 2016–2022. [DOI] [PubMed] [Google Scholar]
- Novais EA, Adhi M, Moult EM, Louzada RN, Cole ED, Husvogt L, Lee B, Dang S, Regatieri CV, Witkin AJ, Baumal CR, Hornegger J, Jayaraman V, Fujimoto JG, Duker JS, Waheed NK, 2016. Choroidal neovascularization analyzed on ultrahigh-speed swept-source optical coherence tomography angiography compared to spectral-domain optical coherence tomography angiography. Am. J. Ophthalmol 164, 80–88. 10.1016/j.ajo.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermeyer Z, Emanuel E, 2016. Predicting the future – big data, machine learning, and clinical medicine. N. Engl. J. Med 375 (13), 1216–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oellers P, Hahn P, Fekrat S, 2018. Central retinal vein occlusion. In: Schachat AP (Ed.), Ryan’s Retina, sixth ed. Elsevier, Edinburgh, pp. 1166–1179. [Google Scholar]
- Olafsdottir OB, Vandewalle E, Abegão Pinto L, Geirsdottir A, De Clerck E, Stalmans P, Gottfredsdottir MS, Kristjansdottir JV, Van Calster J, Zeyen T, Stefánsson E, Stalmans I, 2014. Retinal oxygen metabolism in healthy subjects and glaucoma patients. Br. J. Ophthalmol 98 (3), 329–333. 10.1136/bjophthalmol-2013-303162. [DOI] [PubMed] [Google Scholar]
- Osamura H, Shiba T, Itokawa T, Matsumoto T, Hori Y, 2017. Relationships among ocular blood flow shown by laser speckle flowgraphy, retinal arteriosclerotic change, and chorioretinal circulation time obtained by fluorescein angiography. J Ophthalmol 2017, 2969064. 10.1155/2017/2969064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottesen JT, Olufsen MS, Larsen JK, 2004. Applied Mathematical Models in Human Physiology. Mathematical Modeling and Computation Society for Industrial and Applied Mathematics. [Google Scholar]
- Pascolini D, Mariotti SP, Pokharel GP, Pararajasegaram R, Etya’ale D, Negrel AD, Resnikoff S, 2004. 2002 global update of available data on visual impairment: a compilation of population-based prevalence studies. Ophthalmic Epidemiol. 11 (2), 67–115. [DOI] [PubMed] [Google Scholar]
- Pasquale L, Jonas J, Anderson D, 2009. Anatomy and physiology. In: Weinreb RN, Harris A (Eds.), Ocular Blood Flow in Glaucoma, first ed. Kugler, Amsterdam, pp. 5–9. [Google Scholar]
- Pasquale LR, Willett WC, Rosner BA, Kang JH, 2010. Anthropometric measures and their relation to incident primary open-angle glaucoma. Ophthalmology 117 (8), 1521–1529. 10.1016/j.ophtha.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedley TJ, Brook BS, Seymour RS, 1996. Blood pressure and flow rate in the giraffe jugular vein. Philos. Trans. R. Soc. Lond. B Biol. Sci 351 (1342), 855–866. [DOI] [PubMed] [Google Scholar]
- Philip S, Najafi A, Tantraworasin A, Pasquale LR, Ritch R, 2019. Nailfold capillaroscopy of resting peripheral blood flow in exfoliation glaucoma and primary open-angle glaucoma. JAMA Ophthalmol 137 (6), 618–625. 10.1001/jamaophthalmol.2019.0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piltz-seymour JR, Grunwald JE, Hariprasad SM, Dupont J, 2001. Optic nerve blood flow is diminished in eyes of primary open-angle glaucoma suspects. Am. J. Ophthalmol 132 (1), 63–69. [DOI] [PubMed] [Google Scholar]
- Plange N, Kaup M, Remky A, Arend KO, 2008. Prolonged retinal arteriovenous passage time is correlated to ocular perfusion pressure in normal tension glaucoma. Graefes Arch. Clin. Exp. Ophthalmol 246 (8), 1147–1152. 10.1007/s00417-008-0807-6. [DOI] [PubMed] [Google Scholar]
- Poinoosawmy D, Indar A, Bunce C, Garway-Heath DF, Hitchings RA, 2002. Effect of treatment by medicine or surgery on intraocular pressure and pulsatile ocular blood flow in normal-pressure glaucoma. Graefes Arch. Clin. Exp. Ophthalmol 240, 721–726. [DOI] [PubMed] [Google Scholar]
- Poiseuille JLM, 1835. Recherches sur les causes du mouvement du sang dans les vaisseaux capillaires. C. R. Acad. Sci 6, 554–560. [Google Scholar]
- Poiseuille JLM, 1846. Recherches expérimentales sur le mouvement des liquides dans les tubes de très-petits diamètres. In: Mémoires presentés par divers savants à l’Académie Royale des Sciences de l’Institut de France, vol. 9. pp. 433–544. [Google Scholar]
- Popel AS, 1989. Theory of oxygen transport to tissue. Crit. Rev. Biomed. Eng 17 (3), 257–321. [PMC free article] [PubMed] [Google Scholar]
- Pourcelot L, 1974. Applications of cliniques de l’examinen Doppler transcutane. INSERM 34, 213–240. [Google Scholar]
- Pournaras CJ, Rungger-Brändle E, Riva CE, Hardarson SH, Stefansson E, 2008. Regulation of retinal blood flow in health and disease. Prog. Retin. Eye Res 27 (3), 284–330. [DOI] [PubMed] [Google Scholar]
- Prada D, 2016. A Hybridizable Discontinuous Galerkin Method for Nonlinear Porous Media Viscoelasticity with Applications in Ophthalmology. PhD Thesis. Indiana University Purdue University Indianapolis, Indianapolis, IN, USA. [Google Scholar]
- Prada D, Rowe L, Hajrasouliha A, Ciulla T, Januleviciene I, Chiaravalli G, Guidoboni G, Harris A, 2019a. Pathological consequences of vascular alterations in the eye. In: Guidoboni G, Harris A, Sacco R (Eds.), Ocular Fluid Dynamics. Anatomy, Physiology, Imaging Techniques, and Mathematical Modeling. Modeling and Simulation in Science, Engineering, and Technology Springer-Birkhäuser, New York. [Google Scholar]
- Prada D, Sacco R, Cockburn B, Bociu L, Webster J, Siesky B, Harris A, Guidoboni G, 2016. Influence of tissue viscoelasticity on the optic nerve head perfusion: a mathematical model. Invest. Ophthalmol. Vis. Sci 57 (12), 3558. [Google Scholar]
- Prada D, Harris A, Guidoboni G, Rowe L, Verticchio Vercellin AC, Mathew A, 2019b. Vascular anatomy and physiology of the eye. In: Guidoboni G, Harris A, Sacco R (Eds.), Ocular Fluid Dynamics. Anatomy, Physiology, Imaging Techniques, and Mathematical Modeling. Modeling and Simulation in Science, Engineering, and Technology Springer-Birkhäuser, New York. [Google Scholar]
- Quaranta L, Katsanos A, Russo A, Riva I, 2013. 24-hour intraocular pressure and ocular perfusion pressure in glaucoma. Surv. Ophthalmol 58 (1), 26–41. 10.1016/j.survophthal.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Quigley HA, West SK, Rodriguez J, Munoz B, Klein R, Snyder R, 2001. The prevalence of glaucoma in a population-based study of Hispanic subjects: proyecto VER. Arch. Ophthalmol 119 (12), 1819–1826. 10.1001/archopht.119.12.1819. [DOI] [PubMed] [Google Scholar]
- Racette L, Wilson MR, Zangwill LM, Weinreb RN, Sample PA, 2003. Primary open-angle glaucoma in blacks: a review. Surv. Ophthalmol 48 (3), 295–313. [DOI] [PubMed] [Google Scholar]
- Raghavendra U, Fujita H, Bhandary S, Gudigar A, Tan J, Acharya U, 2018. Deep convolution neural network for accurate diagnosis of glaucoma using digital fundus images. Inf. Sci 441, 41–49. [Google Scholar]
- Ramdas WD, Wolfs RC, Hofman A, de Jong PT, Vingerling JR, Jansonius NM, 2011. Ocular perfusion pressure and the incidence of glaucoma: real effect or artifact? The Rotterdam Study. Invest. Ophthalmol. Vis. Sci 52 (9), 6875–6881. 10.1167/iovs.11-7376. [DOI] [PubMed] [Google Scholar]
- Ramm L, Jentsch S, Peters S, Augsten R, Hammer M, 2014. Investigation of blood flow regulation and oxygen saturation of the retinal vessels in primary open-angle glaucoma. Graefes Arch. Clin. Exp. Ophthalmol 252 (11), 1803–1810. 10.1007/s00417-014-2766-4. [DOI] [PubMed] [Google Scholar]
- Ren R, Wang N, Zhang X, Cui T, Jonas JB, 2011. Trans-lamina cribrosa pressure difference correlated with neuroretinal rim area in glaucoma. Graefes Arch. Clin. Exp. Ophthalmol 249 (7), 1057–1063. 10.1007/s00417-011-1657-1. [DOI] [PubMed] [Google Scholar]
- Rim TH, Lee SY, Bae HW, Seong GJ, Kim SS, Kim CY, 2018. Dec. Increased risk of open-angle glaucoma among patients with diabetes mellitus: a 10-year follow-up nationwide cohort study. Acta Ophthalmol. 96 (8), e1025–e1030. 10.1111/aos.13805. Epub 2018 Jun 4. [DOI] [PubMed] [Google Scholar]
- Riva CE, Grunwald JE, Petrig BL, 1986. Autoregulation of human retinal blood flow. An investigation with Doppler velocimetry. Invest. Ophthalmol. Vis. Sci 27, 1706–1712. [PubMed] [Google Scholar]
- Ropper AH, Samuels MA, Klein JP, 2014. Chapter 13. Disturbances of Vision. in: Adams & Victor’s Principles of Neurology, tenth ed. McGraw-Hill, New York. [Google Scholar]
- Rosen RB, Andrade Romo JS, Krawitz BD, Mo S, Fawzi AA, Linderman RE, Carroll J, Pinhas A, Chui TYP, 2019. Earliest evidence of preclinical diabetic retinopathy revealed using optical coherence tomography angiography perfused capillary density. Am. J. Ophthalmol 203, 103–115. 10.1016/j.ajo.2019.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusia D, Harris A, Pernic A, Williamson KM, Moss AM, Shoshani YZ, Siesky B, 2011. Feasibility of creating a normative database of colour Doppler imaging parameters in glaucomatous eyes and controls. Br J Ophthalmol 95 (9), 1193–1198. [DOI] [PubMed] [Google Scholar]
- Sacco R, Guidoboni G, Mauri AG, 2019. A Comprehensive Physically-Based Approach to Modeling in Bioengineering and Life Sciences, First ed. Academic Press, London. [Google Scholar]
- Sala L, Prud’homme C, Guidoboni G, Szopos M, 2018a. Towards a full model for ocular biomechanics, fluid dynamics, and hemodynamics. J. Model. Ophtalmol 2 (2), 7–13. [Google Scholar]
- Sala L, Prud’homme C, Guidoboni G, Szopos M, 2018b. Ocular mathematical virtual simulator: a hemodynamical and biomechanical study towards clinical applications. J. Coupled Syst. Multiscale Dynam 6 (3), 241–247. [Google Scholar]
- Sala L, Prud’homme C, Guidoboni G, Szopos M, Siesky BA, Harris A, 2018c. Analysis of IOP and CSF alterations on ocular biomechanics and lamina cribrosa hemodynamics. Invest. Ophthalmol. Vis. Sci 59 (9), 4475. [Google Scholar]
- Sala L, 2019a. Modélisation mathématique et simulation de flux sanguins oculaires et leur interactions. PhD Thesis. Université de Strasbourg, Strasbourg, France. [Google Scholar]
- Sala L, Prud’homme C, Guidoboni G, Szopos M, Verticchio Vercellin AC, Siesky BA, Harris A, 2019b. Analysis of IOP and CSF alterations on ocular biomechanics and lamina cribrosa hemodynamics. Invest. Ophthalmol. Vis. Sci 60 (9), 4277.31618764 [Google Scholar]
- Salerni F, Repetto R, Harris A, Pinsky P, Prud’homme C, Szopos M, Guidoboni G, 2018. Mathematical modeling of ocular and cerebral hemo-fluid dynamics: application to VIIP. J Model Ophthalmol 2 (2), 64–68. [Google Scholar]
- Salerni F, Repetto R, Harris A, Pinsky P, Prud’homme C, Szopos M, Guidoboni G, 2019. Biofluid modeling of the coupled eye-brain system and insights into simulated microgravity conditions. PloS One 14 (8), e0216012. 10.1371/journal.pone.0216012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato EA, Ohtake Y, Shinoda K, et al. , 2006. Decreased blood flow at neuroretinal rim of optic nerve head corresponds with visual field deficit in eyes with normal tension glaucoma. Graefes Arch. Clin. Exp. Ophthalmol 244 (7), 795–801. [DOI] [PubMed] [Google Scholar]
- Schneider EW, Fowler SC, 2018. Optical coherence tomography angiography in the management of age-related macular degeneration. Curr. Opin. Ophthalmol 29 (3), 217–225. [DOI] [PubMed] [Google Scholar]
- Schulte K, Wolf S, Arend O, Harris A, Henle C, Reim M, 1996. Retinal hemodynamics during increased intraocular pressure. Ger. J. Ophthalmol 5 (1), 1–5. [PubMed] [Google Scholar]
- Secomb TW, 2016a. A Green’s function method for simulation of time-dependent solute transport and reaction in realistic microvascular geometries. Math. Med. Biol 33 (4), 475–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secomb T, 2016b. Hemodynamics. Comp. Physiol 6 (2), 975–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secomb TW, Hsu R, Park EY, Dewhirst MW, 2004. Green’s function methods for analysis of oxygen delivery to tissue by microvascular networks. Ann. Biomed. Eng 32 (11), 1519–1529. [DOI] [PubMed] [Google Scholar]
- Sehi M, Flanagan JG, Zeng L, Cook RJ, Trope GE, 2005. Anterior optic nerve capillary blood flow response to diurnal variation of mean ocular perfusion pressure in early untreated primary open-angle glaucoma. Invest. Ophthalmol. Vis. Sci 46 (12), 4581–4587. [DOI] [PubMed] [Google Scholar]
- Shiga Y, Aizawa N, Tsuda S, Yokoyama Y, Omodaka K, Kunikata H, Yasui T, Kato K, Kurashima H, Miyamoto E, Hashimoto M, Nakazawa T, 2018. Preperimetric glaucoma prospective study (PPGPS): predicting visual field progression with basal optic nerve head blood flow in normotensive PPG eyes. Transl. Vis. Sci. Technol 7 (1), 11. 10.1167/tvst.7.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki T, Hirooka K, Nakano Y, Nitta E, Ukegawa K, Tsujikawa A, 2018. Oxygen venular saturation correlates with a functional loss in primary open-angle glaucoma and normal-tension glaucoma patients. Acta Ophthalmol. 96 (3), e304–e308. 10.1111/aos.13575. [DOI] [PubMed] [Google Scholar]
- Shoji T, Zangwill LM, Akagi T, Saunders LJ, Yarmohammadi A, Manalastas PIC, Penteado RC, Weinreb RN, 2017. Progressive macula vessel density loss in primary open-angle glaucoma: a longitudinal study. Am. J. Ophthalmol 182, 107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoshani Y, Harris A, Shoja MM, Arieli Y, Ehrlich R, Primus S, Ciulla T, Cantor A, Wirostko B, Siesky B, 2012. Impaired ocular blood flow regulation in patients with open-angle glaucoma and diabetes. Clin. Exp. Ophthalmol 40 (7), 697–705. 10.1111/j.1442-9071.2012.02778.x. [DOI] [PubMed] [Google Scholar]
- Siaudvytyte L, Januleviciene I, Daveckaite A, Ragauskas A, Bartusis L, Kucinoviene J, Siesky B, Harris A, 2015. Literature review and meta-analysis of translaminar pressure difference in open-angle glaucoma. Eye (Lond). 29 (10), 1242–1250. 10.1038/eye.2015.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siaudvytyte L, Januleviciene I, Ragauskas A, Bartusis L, Meiliuniene I, Siesky B, Harris A, 2014. The difference in translaminar pressure gradient and neuroretinal rim area in glaucoma and healthy subjects. J Ophthalmol 2014, 937360. 10.1155/2014/937360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siesky B, Harris A, Racette L, Cantor LB, Tobe LA, Catoira-Boyle Y, Yung CWR, WuDunn D, Beach J, 2013. Retinal oximetry in primary open-angle glaucoma: differences in patients of African and European descent. Invest. Ophthalmol. Vis. Sci 54 (15), 4471 ARVO, May 5–9, 2013. Seattle, Washington.23737476 [Google Scholar]
- Siesky B, Harris A, Racette L, Abassi R, Chandrasekhar K, Tobe LA, Behzadi J, Eckert G, Amireskandari A, Muchnik M, 2015. Differences in ocular blood flow in glaucoma between patients of African and European descent. J. Glaucoma 24 (2), 117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siesky B, Harris A, Carr J, Verticchio Vercellin A, Hussain RM, Parekh Hembree P, Wentz S, Isaacs M, Eckert G, Moore NA, 2016. Reductions in retrobulbar and retinal capillary blood flow strongly correlate with changes in optic nerve head and retinal morphology over 4 Years in open-angle glaucoma patients of african descent compared with patients of European descent. J. Glaucoma 25 (9), 750–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sines D, Harris A, Siesky B, Januleviciene I, Haine CL, Yung CW, Catoira Y, Garzozi HJ, 2007. The response of retrobulbar vasculature to hypercapnia in primary open-angle glaucoma and ocular hypertension. Ophthalmic Res. 39 (2), 76–80. [DOI] [PubMed] [Google Scholar]
- Sommer A, Tielsch JM, Katz J, Quigley HA, Gottsch JD, Javitt JC, Martone JF, Royall RM, Witt KA, Ezrine S, 1991. Racial differences in the cause-specific prevalence of blindness in east Baltimore. N. Engl. J. Med 325 (20), 1412–1417. [DOI] [PubMed] [Google Scholar]
- Spaide RF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G, 2018. Optical coherence tomography angiography. Prog. Retin. Eye Res 64, 1–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springelkamp H, Wolfs RC, Ramdas WD, Hofman A, Vingerling JR, Klaver CC, Jansonius NM, 2017. Incidence of glaucomatous visual field loss after two decades of follow-up: the Rotterdam Study. Eur. J. Epidemiol 32 (8), 691–699. 10.1007/s10654-017-0270-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalmans I, Vandewalle E, Anderson DR, Costa VP, Frenkel RE, Garhofer G, Grunwald J, Gugleta K, Harris A, Hudson C, Januleviciene I, Kagemann L, Kergoat H, Lovasik JV, Lanzl I, Martinez A, Nguyen QD, Plange N, Reitsamer HA, Sehi M, Siesky B, Zeitz O, Orgül S, Schmetterer L, 2011. Use of colour Doppler imaging in ocular blood flow research. Acta Ophthalmol. 89 (8), e609–e630. 10.1111/j.1755-3768.2011.02178.x. [DOI] [PubMed] [Google Scholar]
- Stankiewicz A, Misiuk-Hojlo M, Grabska-Liberek I, Romanowska-Dixon B, Wierzbowska J, Wasyluk J, Mulak M, Szuścik I, Sierdziński J, Ehrlich R, Siesky B, Harris A, 2011. Intraocular pressure and ocular hemodynamics in patients with primary open-angle glaucoma treated with the combination of morning dosing of bimatoprost and dorzolamide hydrochloride. Acta Ophthalmol. 89, e57–63. [DOI] [PubMed] [Google Scholar]
- Stefánsson E, Pedersen DB, Jensen PK, la Cour M, Kiilgaard JF, Bang K, Eysteinsson T, 2005. Optic nerve oxygenation. Prog. Retin. Eye Res 24 (3), 307–332 Epub 2004 Dec 20. [DOI] [PubMed] [Google Scholar]
- Stefánsson E, Olafsdottir OB, Einarsdottir AB, Eliasdottir TS, Eysteinsson T, Vehmeijer W, Vandewalle E, Bek T, Hardarson SH, 2017. Retinal oximetry discovers novel biomarkers in retinal and brain diseases. Invest. Ophthalmol. Vis. Sci 58 (6), BIO227–BIO233. 10.1167/iovs.17-21776. [DOI] [PubMed] [Google Scholar]
- Stokes GG, 1845. On the theories of the internal friction of fluids in motion, and of the equilibrium and motion of elastic solids. Trans. Camb. Phil. Soc 8, 287–341. [Google Scholar]
- Sugiyama K, Tomita G, Kitazawa Y, Onda E, Shinohara H, Park KH, 1997. The associations of optic disc hemorrhage with retinal nerve fiber layer defect and peripapillary atrophy in normal-tension glaucoma. Ophthalmology 104, 1926–1933. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Schwartz B, Takamoto T, Azuma I, 2000. Evaluation of the circulation in the retina, peripapillary choroid and optic disk in normal-tension glaucoma. Ophthalmic Res. 32 (2–3), 79–86. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Utsunomiya K, Ota H, Ogura Y, Narabayashi I, Ikeda T, 2006. Comparative study of cerebral blood flow in patients with normal-tension glaucoma and control subjects. Am. J. Ophthalmol 141 (2), 394–396. [DOI] [PubMed] [Google Scholar]
- Synder A, Augustyniak E, Laudanska-Olszewska I, Wesolek- Czernik A, 2004. Evaluation of blood-flow parameters in extra- ocular arteries in patients with primary open-angle glaucoma before and after surgical treatment. Klin. Oczna 106, 206–208. [PubMed] [Google Scholar]
- Szopos M, Cassani S, Guidoboni G, Prud’homme C, Sacco R, Siesky B, Harris A, 2016. Mathematical modeling of aqueous humor flow and intraocular pressure under uncertainty: towards individualized glaucoma management. J. Model. Ophthalmol 1 (2), 29–39. [Google Scholar]
- Tani T, Nagaoka T, Nakabayashi S, Yoshioka T, Yoshida A, 2014. Autoregulation of retinal blood flow in response to decreased ocular perfusion pressure in cats: comparison of the effects of increased intraocular pressure and systemic hypotension. Invest. Ophthalmol. Vis. Sci 55 (1), 360–367. 10.1167/iovs.13-12591. [DOI] [PubMed] [Google Scholar]
- Tham YC, Lim SH, Gupta P, Aung T, Wong TY, Cheng CY, 2018. Inter-relationship between ocular perfusion pressure, blood pressure, intraocular pressure profiles and primary open-angle glaucoma: the Singapore Epidemiology of Eye Diseases study. Br. J. Ophthalmol 102 (10), 1402–1406. 10.1136/bjophthalmol-2017-311359. [DOI] [PubMed] [Google Scholar]
- Tielsch JM, Katz J, Quigley HA, Javitt JC, Sommer A, 1995a. Diabetes, intraocular pressure, and primary open-angle glaucoma in the Baltimore Eye Survey. Ophthalmology 102, 48–53. [DOI] [PubMed] [Google Scholar]
- Tielsch JM, Katz J, Sommer A, Quigley HA, Javitt JC, 1995b. Hypertension, perfusion pressure, and primary open-angle glaucoma. A population-based assessment. Arch. Ophthalmol 113, 216–221. [DOI] [PubMed] [Google Scholar]
- Ting DSW, Pasquale LR, Peng L, Campbell JP, Lee AY, Raman R, Tan GSW, Schmetterer L, Keane PA, Wong TY, 2019. Artificial intelligence and deep learning in ophthalmology. Br. J. Ophthalmol 103 (2), 167–175. 10.1136/bjophthalmol-2018-313173. Epub 2018 Oct 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobe LA, Harris A, Schroeder A, Gerber A, Holland S, Amireskandari A, Kim NJ, Siesky B, 2013. Retinal oxygen saturation and metabolism: how does it pertain to glaucoma? An update on the application of retinal oximetry in glaucoma. Eur. J. Ophthalmol 23 (4), 465–472. 10.5301/ejo.5000289. [DOI] [PubMed] [Google Scholar]
- Tobe LA, Harris A, Hussain RM, Eckert G, Huck A, Park J, Egan P, Kim NJ, Siesky B, 2015. The role of retrobulbar and retinal circulation on optic nerve head and retinal nerve fibre layer structure in patients with open-angle glaucoma over an 18-month period. Br. J. Ophthalmol 99 (5), 609–612. 10.1136/bjophthalmol-2014-305780. [DOI] [PubMed] [Google Scholar]
- Topouzis F, Coleman AL, Harris A, Jonescu-Cuypers C, Yu F, Mavroudis L, Anastasopoulos E, Pappas T, Koskosas A, Wilson MR, 2006. Association of blood pressure status with the optic disk structure in non-glaucoma subjects: the Thessaloniki eye study. Am. J. Ophthalmol 142 (1), 60–67. [DOI] [PubMed] [Google Scholar]
- Topouzis F, Wilson MR, Harris A, Founti P, Yu F, Anastasopoulos E, Pappas T, Koskosas A, Salonikiou A, Coleman AL, 2013. Association of open-angle glaucoma with perfusion pressure status in the Thessaloniki Eye Study. Am. J. Ophthalmol 155 (5), 843–851. 10.1016/j.ajo.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Trible JR, Sergott RC, Spaeth GL, Wilson RP, Katz LJ, Moster MR, Schmidt CM, 1994. Trabeculectomy is associated with retrobulbar hemodynamic changes. A color Doppler analysis. Ophthalmology 101, 340–351. [DOI] [PubMed] [Google Scholar]
- Tsai ASH, Gan ATL, Ting DSW, Wong CW, Teo KYC, Tan ACS, Lee SY, Wong TY, Tan GSW, Gemmy Cheung CM, 2019. Diabetic macular ischemia: correlation of retinal vasculature changes by optical coherence tomography angiography and functional deficit. Retina. 10.1097/IAE.0000000000002721. ([Epub ahead of print]). [DOI] [PubMed] [Google Scholar]
- Van Melkebeke L, Barbosa-Breda J, Huygens M, Stalmans I, 2018. Optical Coherence Tomography Angiography in Glaucoma: A Review. Ophthalmic Res. 60 (3), 139–151. [DOI] [PubMed] [Google Scholar]
- Verri M, Guidoboni G, Bociu L, Sacco R, 2018. The role of structural viscoelasticity in deformable porous media with incompressible constituents: applications in biomechanics. Math. Biosci. Eng 15 (4), 933–959. 10.3934/mbe.2018042. [DOI] [PubMed] [Google Scholar]
- Volaco A, Cavalcanti AM, Filho RP, Précoma DB, 2018. Socioeconomic status: the missing link between obesity and diabetes mellitus? Curr. Diabetes Rev 14 (4), 321–326. 10.2174/1573399813666170621123227. [DOI] [PubMed] [Google Scholar]
- Wang JJ, Mitchell P, Smith W, 1997. Is there an association between migraine headache and open-angle glaucoma: findings from the Blue Mountains Eye Study. Ophthalmology 104 (10), 1714–1719. [DOI] [PubMed] [Google Scholar]
- Weinreb RN, Harris A, 2009. Ocular Blood Flow in Glaucoma: Consensus Series 6. Kugler Publications, Netherlands. [Google Scholar]
- Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr., Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr., 2018. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American heart association task force on clinical practice guidelines. Hypertension 71 (6), 1269–1324. [DOI] [PubMed] [Google Scholar]
- WHO, 2019. Diabetes. https://www.who.int/news-room/fact-sheets/detail/diabetes, Accessed date: 25 September 2019.
- Womersley J, 1955. Method for the calculation of velocity, rate of flow and viscous drag in arteries when the pressure gradient is known. J. Physiol 127, 553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SY, Leske MC, 1997. Associations with intraocular pressure in the Barbados eye study. Arch. Ophthalmol 115 (12), 1572–1576. [DOI] [PubMed] [Google Scholar]
- SPRINT Research Group, Wright JT Jr., Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr., Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT, 2015. A randomized trial of intensive versus standard blood-pressure control. N. Engl. J. Med 373 (22), 2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Wang YX, Jonas JB, 2009. Ocular perfusion pressure and glaucoma: the Beijing eye study. Eye (Lond). 23 (3), 734–736. 10.1038/eye.2008.342. [DOI] [PubMed] [Google Scholar]
- Yang YS, Koh JW, 2015. Choroidal blood flow change in eyes with high myopia. Kor. J. Ophthalmol 29 (5), 309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuzurihara D, Iijima H, 2004. Visual outcome in central retinal and branch retinal artery occlusion. Jpn. J. Ophthalmol 48 (5), 490–492. [DOI] [PubMed] [Google Scholar]
- Zeng Y, Cao D, Yu H, Yang D, Zhuang X, Hu Y, Li J, Yang J, Wu Q, Liu B, Zhang L, 2019. Early retinal neurovascular impairment in patients with diabetes without clinically detectable retinopathy. Br. J. Ophthalmol 10.1136/bjophthalmol-2018-313582. [DOI] [PubMed] [Google Scholar]
- Zhang HR, 1994. Scanning electron-microscopic study of corrosion casts on retinal and choroidal angioarchitecture in man and animals. Prog. Retin. Eye Res 13, 243–270. [Google Scholar]
- Zhang J, Stringer MD, 2010. Ophthalmic and facial veins are not valveless. Clin. Exp. Ophthalmol 38 (5), 502–510. 10.1111/j.1442-9071.2010.02325.x. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Li J, Li X, Wang N, 2019. Pathological consequences of reduced cerebrospinal fluid pressure: experimental studies. In: Guidoboni G, Harris A, Sacco R (Eds.), Ocular Fluid Dynamics. Anatomy, Physiology, Imaging Techniques, and Mathematical Modeling. Modeling and Simulation in Science, Engineering, and Technology Springer-Birkhauser, New York. [Google Scholar]
- Zhao D, Cho J, Kim MH, Friedman DS, Guallar E, 2015. Diabetes, fasting glucose, and the risk of glaucoma: a meta-analysis. Ophthalmology 122 (1), 72–78. 10.1016/j.ophtha.2014.07.051. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Wong TY, Mitchell P, Friedman DS, He M, Aung T, 2010. Distribution of ocular perfusion pressure and its relationship with open-angle glaucoma: the Singapore Malay eye study. Invest. Ophthalmol. Vis. Sci 51 (7), 3399–3404. 10.1167/iovs.09-4867. [DOI] [PubMed] [Google Scholar]
- Zilia, 2019. Technology: Non-invasive Ocular Oximetry Tools. https://ziliahealth.com/technology-non-invasive-ocular-oximetry-tools/, Accessed date: 24 September 2019.


