Abstract
Background
Undifferentiated pleomorphic sarcoma (UPS), also known as malignant fibrous histiocytoma (MFH), hardly originates from the colorectum.
Case presentation
We reported a 65-year-old female presented with UPS in the descending colon. Computed tomography (CT) revealed an irregularly thickened descending colon. On colonoscopy examination, an ulcerative tumour was identified. The patient received radical resection of the left colon and partial enterectomy. The resected tumor was ulcerative, 10 cm × 8 cm × 5 cm in size, and infiltrated the serosa layer. Postsurgical pathology showed that the tumor was high-graded UPS in the colon with large amounts of necrotic tissues.
Conclusions
UPS in the large intestine is a rare malignant tumor with a poor prognosis and unknown pathogenesis. The main treatment for UPS is early complete resection. Postsurgery adjuvant radiotherapy or chemotherapy can be attempted.
Keywords: Undifferentiated pleomorphic sarcoma, Malignant fibrous histiocytoma, Case report, Colon
Background
Sarcomas are heterogeneous malignant tumors originating from the mesenchymal tissues, only accounting for 1% of malignancies in adults [1]. Undifferentiated pleomorphic sarcoma (UPS), also known as malignant fibrous histiocytoma (MFH), accounts for 28% of all soft-tissue sarcomas and usually occurs in the extremities and retroperitoneum [2, 3]. Currently, the pathogenesis of UPS is not completely understood. However, it has been proposed that some predisposing factors are involved in the occurrence of UPS, including genetic abnormalities, chemoradiotherapy stimulation, chronic irritation, and lymphedema [4]. In addition, UPS is more common in male patients aged between 60 and 80 [5]. UPS is more aggressive with strong regional invasiveness and distant metastasis. It has been reported that the prognosis of UPS is poor due to late diagnosis and a lack of effective treatments [6]. Especially, the prognosis of intra-abdominal UPS is poorer than those in the extremities [6]. However, UPS in the large intestine is extremely rare. In this study, we report a rare case of high-grade UPS in the colon.
Case presentation
This study was approved by the Institutional Review Board and the Ethics Committee of the Second Hospital of Jilin University, Changchun, China. Informed consent was obtained for the publication of this case. The relevant medical details are represented in Table 1.
Table 1.
Relevant medical details
| Medical history | 1. Hypertension for about 10 years, with the highest blood pressure of 180/100 mmHg |
| 2. Cerebral infarction for 10 years with no obvious sequelae | |
| 3. Coronary heart disease for about 1 year | |
| Major complaints | 1. Fever and fatigue for 1 week with the highest body temperature of 39.4 °C |
| 2. Bloody stool, abdominal distention, and decreased exhaustion and defecation 1 month earlier | |
| 3. The abdominal distention increased gradually during recent 1 month | |
| Physical examination | 1. Pale eyelids, thickened breath sounds of both lungs, and a drum sound in the abdomen through abdominal perfusion |
| 2. A 5 cm × 5 cm sized mass could be touched in the left abdomen with mild tenderness | |
| Biochemical examination | White blood cells (29.3 × 109/L, normal: 3.5–9.5 × 109/L); carbohydrate antigen 125 (CA125, 49.3 U/mL, normal: 0–35 U/mL); hemoglobin (75 g/L, normal: 115–150 g/L) |
| Computer tomography (CT) | Bilateral pleural effusion, pericardial effusion, pelvic fluid, and an irregularly thickened wall of the descending colon |
| Colonoscopy | An ulcerative tumor in the descending colon, which invaded the wall of the descending colon circularly |
A 65-year-old female came to the Respiratory Department of the Second Hospital of Jilin University due to fever and fatigue for 1 week with the highest body temperature of 39.4 °C. The patient denied any symptoms of cough, expectoration, chest tightness, or shortness of breath. The body temperature of the patient still ranged from 38.0 to 39.0 °C after taking oral anti-inflammatory drugs. The relevant medical details of the patient are summarized in Table 1, including medical history, major complaints, physical examination, biochemical results, computer tomography (CT) examination, and colonoscopy.
CT imaging revealed an irregularly thickened descending colon (Fig. 1A). The patient received anti-infective, antipyretic, and other symptomatic supportive treatments. During colonoscopy examination, an ulcerative tumor was found in the descending colon (Fig. 1B), which obstructed further colonoscopy examination. No pathological biopsy was performed during colonoscopy due to the Aspirin medication history of the patient. Antiplatelet therapy was usually reckoned as a contraindication for biopsy in our local guidelines because endoscopists believe that antiplatelet therapy can increase bleeding risk during this procedure [7].
Fig. 1.
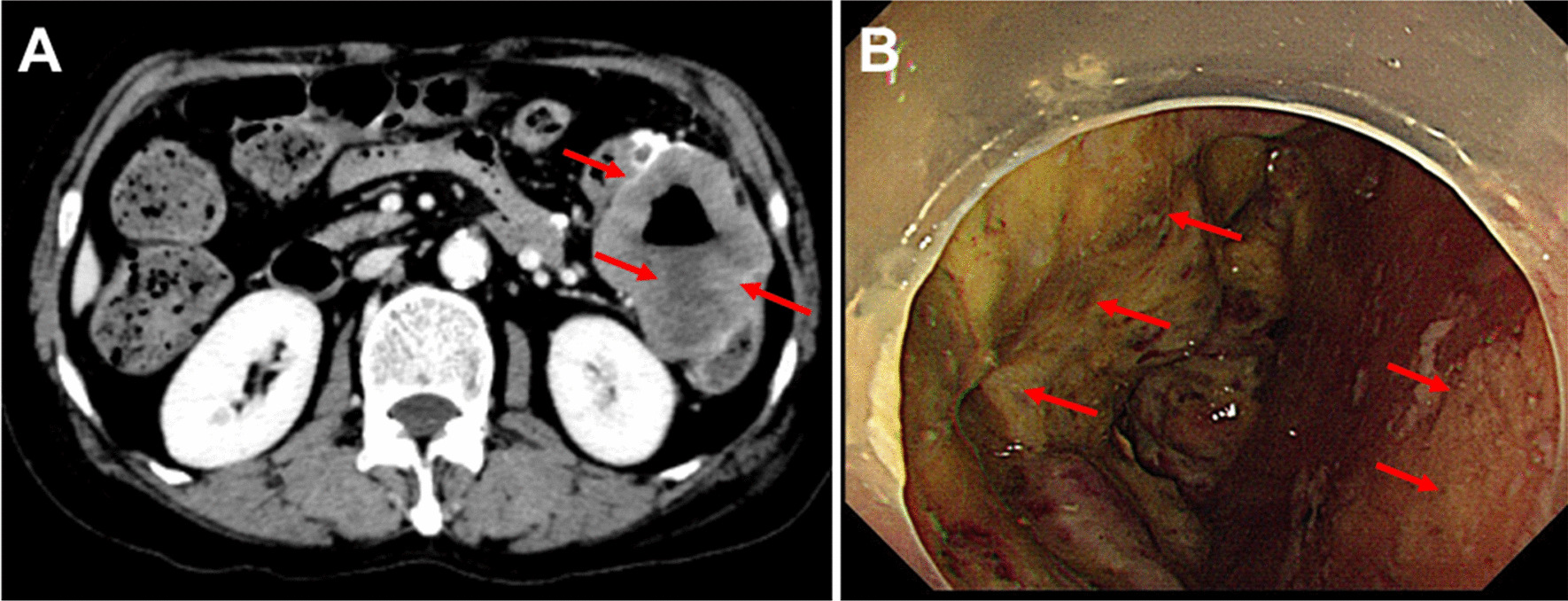
Abdominal CT and electric colonoscopy examinations. A Abdominal CT examination indicates the tumor (red arrows) in the descending colon. B Colonoscopy shows the ulcerative tumor (red arrows), which has invaded the descending colon wall
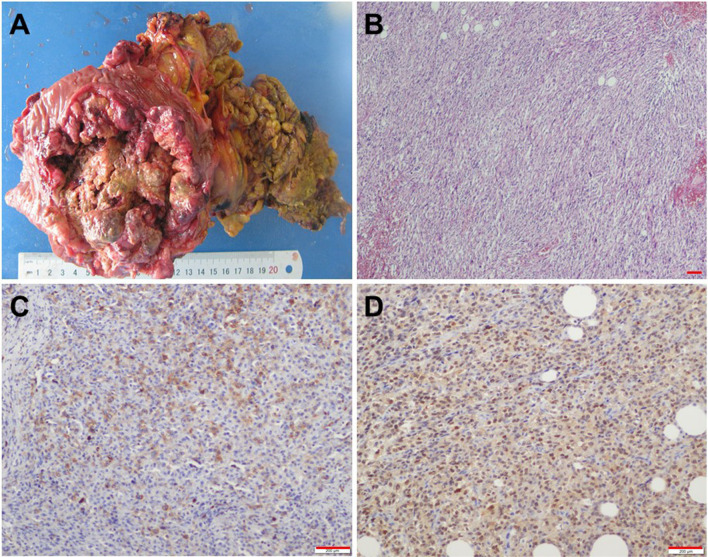
The symptoms in the lung of the patient eased gradually after symptomatic treatment and the body temperature was normalized. The patient was transferred to the Department of General Surgery for laparotomy. An ulcerative tumor in the splenic curvature of the colon was found during the surgery. The tumor invaded the small intestine, which was 10 cm away from the ligament of Treitz. The patient received radical resection of the left colon, and the transverse colon and sigmoid colon were anastomosed end to end. Furthermore, the small intestine and its mesangium invaded by the tumor were dissected, and the small intestines on both sides were anastomosed end-to-end. The resected tumor was ulcerative, 10 cm × 8 cm × 5 cm in size, and infiltrated in the serosa layer (Fig. 2A). Microscope CX31 (Olympus, Japan) and the Microscopic Image Analysis Software 11.0 were used for histopathological analysis. The measured resolution for all the microscopic images was 0.5 nm. Postsurgical pathology showed that the tumor was high-graded UPS in the colon with large necrotic tissues (Fig. 2B). The serosa layer of the colon was filled with fibrous hyperplasia, vessel hyperplasia, inflammatory cell infiltration, and necrotic tissues. The mucous layer of the colon was filled with inflammatory cells. Further immunohistochemical analysis showed that the tumor cells were positively stained for α1-antichymotrypsin (Fig. 2C) and vimentin (Fig. 2D), which was consistent with the characteristics of UPS. The patient recovered well and was discharged from our department on the 10th-day after surgery and received no further treatment. One year follow-up was performed, and CT and colonoscopy did not reveal any signs of local recurrence or distant metastasis.
Fig. 2.
Pathological examination and immunohistochemical staining of the tumor. A The resected tumor is ulcerative, 10 cm × 8 cm × 5 cm in size, and has infiltrated into the serosa layer. B Histopathology shows that the tumor is high-graded UPS in the colon with fibrous hyperplasia, necrotic tissues, and inflammatory cells. C Immunohistochemical examination shows that tumor cells are positive for α1-antichymotrypsin. D Immunohistochemical examination shows that tumor cells exhibit marked positivity for vimentin. Scale bars = 200 µm
Discussion and conclusions
First found and named by Ozzello et al. in 1963 [8], MFH includes four histological subtypes: myxoid, inflammatory, storiform-pleomorphic, and giant-cell [9]. In 2002, the World Health Organization redefined the MFH classification. The subtypes of storiform-pleomorphic MFH, giant-cell MFH, and inflammatory MFH were classified into fibrous histiocytic tumors and replaced by UPS and UPS with giant cells. Another subtype of myxoid MFH was defined as the myofibroblastic category and renamed to myxofibrosarcoma [10].
In order to further explore UPS, we searched the literature on PubMed, updated to May 01, 2021, using keywords including “Undifferentiated pleomorphic sarcoma,” “malignant fibrous histiocytoma,” “colon,” “rectum,” and their variants. Studies were included per the following inclusion criteria: (1) clinical features of the patient available; (2) primary UPS of the colon or rectum and confirmed by histology; (3) case reports. Studies with duplicate data or data not relevant to UPS were excluded. After the screening, only 20 cases were included (Table 2) [11–30]. The characteristics of UPS were analyzed in this study.
Table 2.
The UPS of colorectum in the literature
| Author | Age (year) | Sex | Tumor location | Longitude diameter (cm) | Symptoms | Surgery | Adjuvant therapy | Follow-up |
|---|---|---|---|---|---|---|---|---|
| Sewell et al. [11] | 74 | M | Transverse colon | 8.5 | Anorexia | Yes | No | 12 months |
| Diarrhea | No recurrence or metastasis | |||||||
| Levinson and Tsang [12] | 17 | M | Transverse and sigmoid colon | 10, 8 | Abdominal pain | Yes | NA | NA |
| Rubbini et al. [13] | 60 | M | Sigmoid colon | 7 | Bloody stool | Yes | Chemotherapy | 53 months |
| Dead, liver metastasis | ||||||||
| Baratz et al. [14] | 73 | M | Transverse colon | 15 | Anorexia | Yes | No | 6 months |
| Anemia | No recurrence or metastasis | |||||||
| Waxman et al. [15] | 52 | F | Sigmoid colon | 7.5 | Abdominal pain | Yes | No | 9 months |
| Dead, local recurrence | ||||||||
| Satake and Matsuyama [16] | 62 | M | Ascending and transverse colon | 17, 19 | Abdominal mass | No | NA | NA |
| Katz et al. [17] | 62 | F | Cecum | 2 | Abdominal pain | Yes | No | 3 months |
| No recurrence or metastasis | ||||||||
| Murata et al. [18] | 50 | M | Ascending colon | 9.5 | Abdominal distention | Yes | Chemotherapy | 10 months |
| No recurrence or metastasis | ||||||||
| Huang and Wei [19] | 12 | M | Ascending colon | 3.5 | Abdominal pain | Yes | No | 16 months |
| No recurrence or metastasis | ||||||||
| Makino et al. [20] | 72 | M | Transverse colon | 7 | Abdominal pain | Yes | No | 4 months |
| Dead, local recurrence | ||||||||
| Hiraoka et al. [21] | 64 | M | Cecum | 5 | Abdominal distention | Yes | No | 4 months |
| Dead, lymph nodes metastasis | ||||||||
| Udaka et al. [22] | 47 | M | Ascending colon | 7 | Abdominal mass | Yes | No | 13 months |
| No recurrence or metastasis | ||||||||
| Gupta and Malani [23] | 46 | F | Cecum and ascending colon | 17 | Abdominal distention | Yes | No | 36 months |
| Abdominal mass | No recurrence or metastasis | |||||||
| Okubo et al. [24] | 66 | M | Ascending colon | 14.5 | Abdominal pain | Yes | No | 33 months |
| No recurrence or metastasis | ||||||||
| Kawashima et al. [25] | 50 | F | Descending colon | 10 | Abdominal pain | Yes | No | 7 years |
| No recurrence or metastasis | ||||||||
| Ji et al. [26] | 68 | F | Ascending colon | 8 | Fever | Yes | Radiotherapy | 5 years |
| Dead, local recurrence | ||||||||
| Bosmans et al. [27] | 73 | M | Sigmoid colon | 3.5 | Anemia | Yes | No | 22 months |
| No recurrence or metastasis | ||||||||
| Wang et al. [28] | 55 | M | Sigmoid colon | 6.0 | Abdominal pain | Yes | No | 5 months |
| Dead, local recurrence | ||||||||
| Fu et al. [29] | 70 | M | Cecum | 12 | Abdominal pain | Yes | No | 1 month |
| Dead, lung metastasis | ||||||||
| Singh et al. [30] | 55 | M | Rectum | 2.5 | Perineal pain | Yes | Chemotherapy | 46 months |
| Radiotherapy | No recurrence or metastasis |
M, male; F, female; NA, not applicable
According to the studies in Table 2 and the case in this study, UPS in the colorectum mainly occurs in male patients with a male to female ratio of 2.5:1. The patients' age ranges from 12 to 74 years, with an average of 56.81 ± 16.65. Although UPS could occur in any part of the colorectum, only one case was reported to be originated from the rectum. Most cases were diagnosed as a large tumor with a 2–19 cm diameter, with a median of 7.75 cm. The main symptoms of UPS originating from the large intestine include abdominal pain, abdominal distention, anorexia, diarrhea, anemia, fever, and perineal pain.
UPSs in the colon are mesenchymal tumors. Therefore, most of UPSs originate from deep fascia or muscularis and grow out of the intestinal lumen [27]. Usually, clinical manifestations of UPSs in the colon are not completely specific. In this case, the main clinical presentations are similar to colorectal cancer, include bloody stools, fatigue, and reduced bowel movement frequency, which may be caused by tumor hemorrhage and inevitable tumor outgrow. In addition, due to the intact of the colonic mucosa, nothing abnormal can be observed in colonoscopy even UPSs in the colon have already occurred. However, if the tumor is sufficiently large and has infiltrated the mucosa layer, a colonoscopy examination is useful. For example, in this case, colonoscopy indicated an ulcerative tumor that prevented further colonoscopy examination. To date, the diagnosis of UPS in the colon remains highly challenging due to the lack of effective early cancer screening strategies [26]. Histopathology is still the gold standard for UPS diagnosis. Microscopically, the histological characteristics of UPS include the complexity of cell components, pleomorphism of tumor cells, and the diversity of tissue structure [27]. Tumor tissues often include fibroblasts, histone cells, giant cells, xanthoma cells, and inflammatory cells [27]. Although immunohistochemical stains could be useful for UPS diagnosis, no reproducible immunophenotype or protein expression can be used in more specific subclassification [31]. More specifically, some special staining can be used to exclude other tumors. For example, the pleomorphic liposarcoma is positive for SMA, S-100 protein, keratins, and desmin, while the pleomorphic leiomyosarcoma and pleomorphic rhabdomyosarcoma are only positive for desmin [31]. However, UPSs are frequently positive for vimentin, actin, CD68, α1-antitrypsin, and α1-antichymotrypsin [31].
Radical surgery is the primary treatment for UPS in the colorectum. However, UPS in the colorectum often has exogenous growth that infiltrates the surrounding tissues. Therefore, extensive or radical excision will not prevent possible local recurrence or distant metastasis. The effects of postsurgical chemotherapy or radiotherapy are still unclear. Among the 20 cases in earlier studies and the 1 case in this study, 20 patients received radical surgery, 2 patients received postsurgical chemotherapy, 1 patient received postsurgical radiotherapy, and 1 patient received postsurgical chemotherapy and radiotherapy. Of the 19 patients followed up after surgery, 7 had local recurrences or distant metastasis. The follow-up time was generally short, and the follow-up data of some patients were lost. The 6 months, 1 year, 2 years, and 5 years survival rates of patients with UPS in the large intestine were 77.78% (14/18), 75.00% (12/16), 63.64% (7/11), and 12.50% (1/8), respectively.
UPS in the colorectum is a rare malignant tumor with a poor prognosis and unknown pathogenesis. Nearly half of the patients with UPS died of postoperative recurrences or metastasis. The primary treatment for UPS is early complete resection of the tumor. Adjuvant radiotherapy and/or chemotherapy can be attempted after the surgery with individual efficacy.
Acknowledgements
Not applicable.
Abbreviations
- UPS
Undifferentiated pleomorphic sarcoma
- MFH
Malignant fibrous histiocytoma
- CT
Computed tomography
- CEA
Carcinoembryonic antigen
Authors' contributions
XH revised the second and third version of this study and helped the English editing. LZ and YM wrote the first version of the article. GL, GZ, and HH participated in the conception and design of the study and the drafting of the article. JL and SW designed the study and revised the manuscript. All authors reviewed and approved the final version of the article.
Funding
This study was supported by grants from the Youth Program of the National Natural Science Foundation of China (#32000953), the Department of Finance of Jilin Province (#3D5197434429), the Education Project of Jilin University (#419070600046), and the Health Commission of Jilin Province (#2021LC09). The funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing this manuscript.
Availability of data and materials
All data generated or analyzed are included in this published article.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee and Institutional Review Board of the Second Hospital of Jilin University, Changchun, China. Written informed consent was obtained from the patient.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Competing interests
All authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xu Han and Linxian Zhao contributed equally to this work
Contributor Information
Shu Wang, Email: 32199783@qq.com.
Jiannan Li, Email: jnli@ciac.ac.cn.
References
- 1.Fletcher CD, Unni KK, Mertens F. Pathology and genetics of tumours of soft tissue and bone; 2002.
- 2.Lee JH, Kang DB, Park WC. Primary undifferentiated pleomorphic sarcoma of the colon mesentery. Ann Coloproctol. 2019;35(3):152–154. doi: 10.3393/ac.2018.03.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cormier JN, Pollock RE. Soft tissue sarcomas. CA Cancer J Clin. 2004;54(2):94–109. doi: 10.3322/canjclin.54.2.94. [DOI] [PubMed] [Google Scholar]
- 4.Azizi R, Mahjoubi B, Shayanfar N, Anaraki F, Zahedi-Shoolami L. Malignant fibrous histiocytoma of rectum: report of a case. Int J Surg Case Rep. 2011;2(6):111–113. doi: 10.1016/j.ijscr.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nascimento AF, Raut CP. Diagnosis and management of pleomorphic sarcomas (so-called "MFH") in adults. J Surg Oncol. 2008;97(4):330–339. doi: 10.1002/jso.20972. [DOI] [PubMed] [Google Scholar]
- 6.Salemis NS, Gourgiotis S, Tsiambas E, Panagiotopoulos N, Karameris A, Tsohataridis E. Primary intra-abdominal malignant fibrous histiocytoma: a highly aggressive tumor. J Gastrointest Cancer. 2010;41(4):238–242. doi: 10.1007/s12029-010-9153-0. [DOI] [PubMed] [Google Scholar]
- 7.Kimchi NA, Broide E, Scapa E, Birkenfeld S. Antiplatelet therapy and the risk of bleeding induced by gastrointestinal endoscopic procedures. Digestion. 2007;75(1):36–45. doi: 10.1159/000101565. [DOI] [PubMed] [Google Scholar]
- 8.Ozzello L, Stout AP, Murray MR. Cultural characteristics of malignant histiocytomas and fibrous xanthomas. Cancer. 1963;16:331–344. doi: 10.1002/1097-0142(196303)16:3<331::AID-CNCR2820160307>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 9.Sw W, Fm E. Malignant fibrous histiocytoma: an a ysis of 200 cases. Cancer Biol Ther. 1978;41(6):2250–2266. doi: 10.1002/1097-0142(197806)41:6<2250::aid-cncr2820410626>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 10.Lee JH, Kang DB, Park WC. Primary undifferentiated pleomorphic sarcoma of the colon mesentery. Ann Coloproctol. 2019;35:152–154. doi: 10.3393/ac.2018.03.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sewell R, Levine BA, Harrison GK, Tio F, Schwesinger WH. Primary malignant fibrous histiocytoma of the intestine: intussusception of a rare neoplasm. Dis Colon Rectum. 1980;23(3):198–201. doi: 10.1007/BF02587627. [DOI] [PubMed] [Google Scholar]
- 12.Levinson MM, Tsang D. Multicentric malignant fibrous histiocytomas of the colon: report of a case and review of the subject. Diseases Colon Rectum. 1982;25(4):327–331. doi: 10.1007/BF02553607. [DOI] [PubMed] [Google Scholar]
- 13.Rubbini M, Marzola A, Spanedda R, Scalco GB, Zamboni P, Guerrera C, Donini I. Primary malignant fibrous histiocytoma of the sigmoid colon: a case report. Ital J Surg Sci. 1983;13(4):299–302. [PubMed] [Google Scholar]
- 14.Baratz M, Ostrzega N, Michowitz M, Messer G. Primary inflammatory malignant fibrous histiocytoma of the colon. Dis Colon Rectum. 1986;29(7):462–465. doi: 10.1007/BF02561588. [DOI] [PubMed] [Google Scholar]
- 15.Waxman M, Faegenburg D, Waxman JS, Janelli DE. Malignant fibrous histiocytoma of the colon associated with diverticulitis. Dis Colon Rectum. 1983;26(5):339–343. doi: 10.1007/BF02561712. [DOI] [PubMed] [Google Scholar]
- 16.Satake T, Matsuyama M. Cytologic features of ascites in malignant fibrous histiocytoma of the colon. Acta Pathol Jpn. 1988;38(7):921–928. doi: 10.1111/j.1440-1827.1988.tb02363.x. [DOI] [PubMed] [Google Scholar]
- 17.Katz RN, Waye JD, Batzel EL, Reiner MA, Freed JS. Malignant fibrous histiocytoma of the gastrointestinal tract in a patient with neurofibromatosis. Am J Gastroenterol. 1990;85(11):1527–1530. [PubMed] [Google Scholar]
- 18.Murata I, Makiyama K, Miyazaki K, Kawamoto AS, Yoshida N, Muta K, Itsuno M, Hara K, Nakagoe T, Tomita M. A case of inflammatory malignant fibrous histiocytoma of the colon. Gastroenterol Jpn. 1993;28(4):554–563. doi: 10.1007/BF02776955. [DOI] [PubMed] [Google Scholar]
- 19.Huang Z, Wei K. Malignant fibrous histiocytoma of the ascending colon in a child. Am J Gastroenterol. 1993;88(6):972–973. [PubMed] [Google Scholar]
- 20.Makino M, Kimura O, Kaibara N. Radiation-induced malignant fibrous histiocytoma of the transverse colon: case report and review of the literature. J Gastroenterol. 1994;29(6):767–771. doi: 10.1007/BF02349285. [DOI] [PubMed] [Google Scholar]
- 21.Hiraoka N, Mukai M, Suzuki M, Maeda K, Nakajima K, Hashimoto M, Hosoda Y, Hata J. Malignant fibrous histiocytoma of the cecum: report of a case and review of the literature. Pathol Int. 1997;47(10):718–724. doi: 10.1111/j.1440-1827.1997.tb04448.x. [DOI] [PubMed] [Google Scholar]
- 22.Udaka T, Suzuki Y, Kimura H, Miyashita K, Suwaki T, Yoshino T. Primary malignant fibrous histiocytoma of the ascending colon: report of a case. Surg Today. 1999;29(2):160–164. doi: 10.1007/BF02482242. [DOI] [PubMed] [Google Scholar]
- 23.Gupta C, Malani AK. Primary malignant fibrous histiocytoma of the colon. Clin Gastroenterol Hepatol. 2006;4(6):28. doi: 10.1016/j.cgh.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Okubo H, Ozeki K, Tanaka T, Matsuo T, Mochinaga N. Primary malignant fibrous histiocytoma of the ascending colon: report of a case. Surg Today. 2005;35(4):323–327. doi: 10.1007/s00595-004-2915-1. [DOI] [PubMed] [Google Scholar]
- 25.Kawashima H, Ikeue S, Takahashi Y, Kashiyama M, Hara T, Yamazaki S, Hirao M, Okamoto K. Primary malignant fibrous histiocytoma of the descending colon. Surg Today. 1997;27(9):851–854. doi: 10.1007/BF02385277. [DOI] [PubMed] [Google Scholar]
- 26.Ji W, Zhong M, You Y, Hu K, Wu B. Primary malignant fibrous histiocytoma of the colon: a case report and review of the literature. Mol Clin Oncol. 2016;4(6):1006–1008. doi: 10.3892/mco.2016.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bosmans B, de Graaf EJR, Torenbeek R, Tetteroo GWM. Malignant fibrous histiocytoma of the sigmoid: a case report and review of the literature. Int J Colorectal Dis. 2007;22(5):549–552. doi: 10.1007/s00384-006-0162-1. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y-J, Tang S-S, Zhao Y. Contrast-enhanced sonographic appearance of malignant fibrous histiocytoma in the sigmoid colon: A case report. J Clin Ultrasound. 2012;40(7):439–442. doi: 10.1002/jcu.20862. [DOI] [PubMed] [Google Scholar]
- 29.Fu D-L, Yang F, Maskay A, Long J, Jin C, Yu X-U, Xu J, Zhou Z-W, Ni Q-X. Primary intestinal malignant fibrous histiocytoma: two case reports. World J Gastroenterol. 2007;13(8):1299–1302. doi: 10.3748/wjg.v13.i8.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh DR, Aryya NC, Sahi UP, Shukla VK. Malignant fibrous histiocytoma of the rectum. Eur J Surg Oncol. 1999;25(4):447–448. doi: 10.1053/ejso.1999.0677. [DOI] [PubMed] [Google Scholar]
- 31.Soini Y, Autio-Harmainen H. Tumor cells of malignant fibrous histiocytomas express mRNA for laminin. Am J Pathol. 1991;139(5):1061–1068. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed are included in this published article.