Abstract
OBJECTIVE
Pregnant people are at increased risk of COVID-19–related morbidity and mortality, and vaccination presents an important strategy for preventing negative outcomes. However, pregnant people were not included in vaccine trials, and there are limited data on COVID-19 vaccines during pregnancy. The objectives of this systematic review were to identify the safety, immunogenicity, effectiveness, and acceptance of COVID-19 vaccination among pregnant people in the United States.
DATA SOURCES
Four databases (PubMed, Web of Science, CINAHL, and Google Scholar) were used to identify eligible studies published from January 1, 2020 through February 6, 2022.
STUDY ELIGIBILITY CRITERIA
Inclusion criteria were peer-reviewed empirical research conducted in the United States, publications in English, and research addressing 1 of the following topics: safety, immunogenicity, effectiveness, and acceptance of COVID-19 vaccination among pregnant people.
METHODS
A narrative synthesis approach was used to synthesize findings. Critical appraisal was done using the JBI (formerly Joanna Briggs Institute) tool.
RESULTS
Thirty-two studies were identified. Most studies (n=24) reported the use of Pfizer and Moderna COVID-19 vaccines among pregnant people; only 6 reported the Janssen vaccine. Of the 32 studies, 11 examined COVID-19 vaccine safety, 10 investigated immunogenicity and effectiveness, and 11 assessed vaccine acceptance among pregnant people. Injection-site pain and fatigue were the most common adverse events. One case study reported immune thrombocytopenia. COVID-19 vaccination did not increase the risk of adverse pregnancy or neonatal outcomes compared with unvaccinated pregnant people. After COVID-19 vaccination, pregnant people had a robust immune response, and vaccinations conferred protective immunity to newborns through breast milk and placental transfer. COVID-19 vaccine acceptance was low among pregnant people in the United States. African American race, Hispanic ethnicity, younger age, low education, previous refusal of the influenza vaccine, and lack of provider counseling were associated with low vaccine acceptance.
CONCLUSION
Peer-reviewed studies support COVID-19 vaccine safety and protective effects on pregnant people and their newborns. Future studies that use rigorous methodologies and include diverse populations are needed to confirm current findings. In addition, targeted and tailored strategies are needed to improve vaccine acceptance, especially among minorities.
Key words: COVID-19 vaccine, immunogenicity, messenger RNA vaccine, neonatal outcomes, pregnancy, pregnancy outcomes, vaccine acceptance, vaccine effectiveness, vaccine hesitancy, vaccine safety
AJOG MFM at a Glance.
Why was this study conducted?
Pregnant people are at increased risk of COVID-19–related morbidity and mortality. There are limited data regarding the safety, effectiveness, and acceptance of COVID-19 vaccination among pregnant people in the United States.
Key findings
Peer-reviewed studies support COVID-19 vaccines’ safety and effectiveness in pregnant people and their fetuses or neonates; however, vaccine acceptance was low, especially among minorities.
What does this add to what is known?
This systematic review explored the safety, effectiveness, and acceptance of COVID-19 vaccination among pregnant people in the United States. The safety and effectiveness of the COVID-19 vaccine among pregnant people are similar to those reported in the general population. However, pregnant people exhibited vaccine hesitancy because of fear of vaccine side effects and risks to the fetus or neonate.
Introduction
Pregnant people are at increased risk of COVID-19–related morbidity and mortality. The heightened morbidities are noted in terms of an increased risk of preterm birth,1 , 2 increased need for intensive care unit (ICU) admission and invasive ventilation, and death.3, 4, 5 Vaccination presents an important strategy to prevent negative outcomes in this population. The Centers for Disease Control and Prevention, American College of Obstetricians and Gynecologists, and the Society for Maternal-Fetal Medicine recommend that pregnant people receive COVID-19 vaccines.6, 7, 8
Because pregnant people were not included in the COVID-19 vaccine trials, there are limited data on vaccination safety and pregnancy outcomes compared with the general population.9 , 10 The lack of safety and efficacy data means that pregnant people are left with 2 options: get the vaccine, with limited safety and efficacy data, or skip the vaccine, thus leaving themselves and their fetuses vulnerable to adverse effects of COVID-19. Reviews of recent studies indicate that COVID-19 vaccination during pregnancy produces immune responses and does not cause major adverse effects and negative pregnancy or neonatal outcomes.11 , 12 Although there has been exponential growth in research on COVID-19 vaccination during pregnancy, many of these reviews included vaccines that are not authorized in the United States.11 , 12 Furthermore, these reviews included studies conducted in international settings where vaccine availability, vaccine guidelines, and healthcare systems differ from those of the United States. In addition, none of the reviews provided information about the acceptance and uptake of COVID-19 vaccines among pregnant people. Therefore, there is an urgent need for a clear understanding of the safety, efficacy, and acceptance of COVID-19 vaccination during pregnancy so that pregnant people may be supported in making the best decision for their individual situations.
Objective
The objective of this systematic review was to identify and synthesize what is known about COVID-19 vaccination among pregnant people in the United States, including safety, effectiveness, acceptance, hesitancy, and uptake.
Methods
The review protocol was registered with the International Prospective Register of Systematic Reviews under CRD42021286726 (at https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=286726). The Population, Intervention, Comparison, and Outcome framework was used to organize this review.13 The population of interest was pregnant people in the United States. The intervention included COVID-19 vaccinations. The outcomes were safety, immunogenicity, effectiveness, and acceptance of the COVID-19 vaccinations. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines were used to direct the methodology of this systematic review.14
Information sources and search strategy
A literature search was conducted to include studies published from January 1, 2020 through February 6, 2022. Sources included the following databases: PubMed, Web of Science, CINAHL, and Google Scholar. The key terms included in the search were “pregnant OR pregnancy OR pregnant women” and “COVID-19 vaccine OR COVID-19 vaccination.” Search results from each database were exported to EndNote (Clarivate, Philadelphia, PA). The full details of the search strategy are available in Supplementary Table S1.
Study selection and data extraction
Studies were included if they were peer-reviewed empirical studies conducted in the United States from January 1, 2020 through February 6 2022, published in English, and addressed at least 1 of the following topics: (1) safety, immunogenicity, and effectiveness of COVID-19 vaccination in pregnant people or (2) attitudes, beliefs, perceptions, acceptance, or hesitancy of pregnant people toward COVID-19 vaccination. The exclusion criteria included nonempirical and non–peer-reviewed research, studies published only as abstracts, literature reviews, commentaries, editorials, animal-model studies, studies not examining COVID-19 vaccination in pregnant people, studies published in non-English languages, and studies conducted outside the United States. Research conducted outside the United States was excluded because of the difference in vaccine availability, health advisories, and healthcare system structures. The 2-year time frame was used because the first case of COVID-19 was reported in the United States in January 2020, and vaccination began in December 2020.
Initial screening of all abstracts and titles was conducted by S.R. and checked by another author (H.N.Y.) to determine whether to include or exclude studies on the basis of the inclusion criteria. All full-text screening disagreements were reconciled through discussion between the authors (S.R., R.H.S., R.L.T., and H.N.Y.) to achieve mutual consensus before moving to full-text review.
Assessment of risk of bias
Critical appraisals of included studies were conducted to evaluate the methodological quality of research, that is, to what extent a study was designed, conducted, analyzed, interpreted, and reported to avoid systematic errors.15 Appraisals focused on methodological domains through which bias may have been introduced into the results.15 All studies identified as meeting the inclusion criteria were assessed for risk of bias by using the JBI (formerly Joanna Briggs Institute) critical appraisal checklist for cohort studies, case-control, case report, case series, quasi-experimental (prepost), and cross-sectional studies.16 The checklist response options included: “Yes” (the criteria are clearly identifiable through the report description), “Unclear” (the criteria are not clearly identified in the report), and “No” (the criteria are not identifiable). On the basis of the number (%) of “Yes” responses, the risk of bias was ranked as “high” (≤49%), “moderate” (50%–69%), and “low” (≥70%).16 Two independent reviewers (S.R. and H.N.Y.) conducted the appraisals, and both reviewers were blinded to each other's quality appraisal reviews. After independent review, the results were collected by the first reviewer (S.R.), and discrepancies were discussed with a third reviewer (R.L.T.). There were no exclusions made on the basis of a minimum threshold.
Data synthesis
A standard data extraction form was used to collect the following information: study author(s) and year published, study title, study design, study setting, participants, COVID-19 vaccine type, outcomes, and conclusion(s). Data extraction and data synthesis were initially conducted by the first reviewer (S.R.) but discussed regularly with the review team (R.H.S., R.L.T., and H.N.Y.) to obtain agreement on all included studies and resolve any disagreements. A narrative synthesis approach was used to analyze studies included in this review.17 This approach synthesizes findings from multiple sources and primarily uses words and text to summarize and explain findings17; it is used when meta-analysis is not feasible because of high heterogeneity across studies.
Results
Study selection
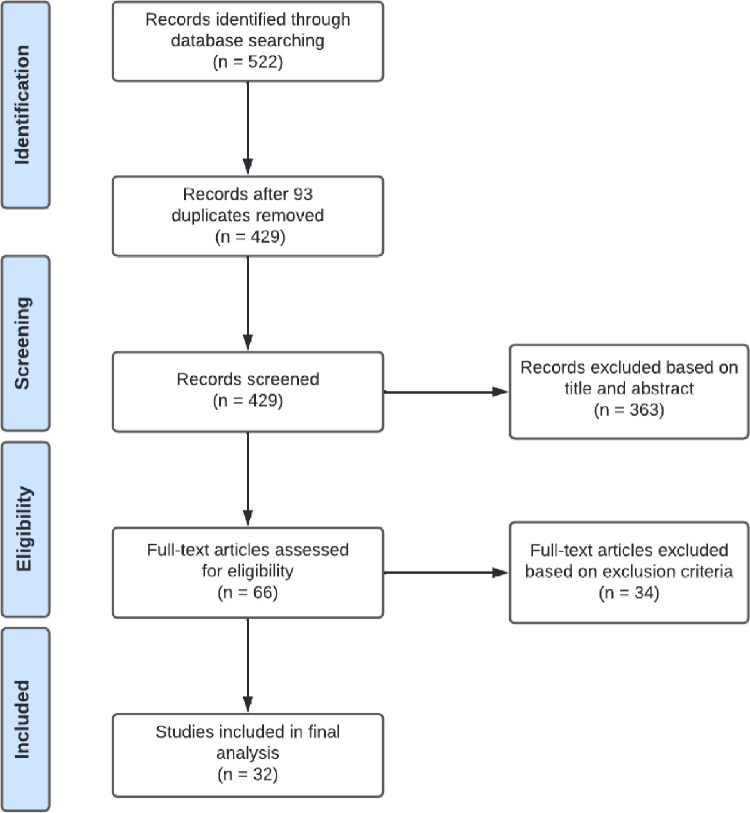
A total of 522 studies were obtained from PubMed, Web of Science, and CINAHL and imported into EndNote software. Removal of 93 duplicates yielded 429 studies. Of those, 363 studies were removed on the basis of exclusion criteria during the title and abstract screening. The remaining 66 studies were screened for full-text review. Of these, 34 were excluded for not meeting the eligibility criteria. Therefore, 32 studies were included in the review (Figure 1 ).
Figure 1.
PRISMA flow diagram of the included studies
The PRISMA flow diagram for the systematic review detailing the database searches, the number of abstracts screened, full texts retrieved, and the final studies included in the analysis.
PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Rawal. COVID-19 vaccination among pregnant people in the United States. Am J Obstet Gynecol MFM 2022.
Study characteristics
The characteristics of included studies are described in the Table 1 . All of the included studies used observational study designs; there were 15 cohort, 10 cross-sectional, 4 case report, 1 prepost, 1 case-control, and 1 case series study. No randomized controlled trials were identified. Seven studies used COVID-19 vaccination registries and had sample sizes ranging from 2002 to 135,968; the remaining 25 had sample sizes <1030. Twenty-four studies reported the use of Pfizer (Pfizer, Inc., New York, NY and BioNTech, Germany) and Moderna (ModernaTX, Inc.; Cambridge, MA) COVID-19 vaccines among pregnant people; 6 reported the Janssen (Janssen Biotech, Inc.; a Janssen Pharmaceutical Company of Johnson & Johnson, Horsham, PA) vaccine. Only 1 study reported the use of COVID-19 vaccine boosters in pregnant people. Five studies compared vaccinated pregnant people with vaccinated nonpregnant people, and 5 studies compared vaccinated pregnant people with unvaccinated pregnant people.
Table 1.
Characteristics of included studies (n=32)
| COVID-19 vaccine safety | |||||||
|---|---|---|---|---|---|---|---|
| Author(s), year | Study title | Study design | Study setting | Participants (n) | COVID-19 vaccine types, % received | Outcomes | Conclusions |
| Bennett et al,18 2021 | Newly diagnosed immune thrombocytopenia in a pregnant patient after coronavirus disease 2019 vaccination | Case report | Hospital in Ohio |
Vaccinated pregnant woman at the first trimester of pregnancy (n=1) |
Moderna mRNA-1273 Patient received first dose only. |
Vaccine side effects: ITP occurred 13 d after COVID-19 vaccination. ITP was resolved by oral corticosteroids and patient was discharged on the fourth day of hospitalization with no complications. |
COVID-19 vaccination benefits outweigh the risk of infection in pregnancy. Pregnant women should be included in clinical trials. |
| Kachikis et al,19 2021 | Short-term reactions among pregnant and lactating individuals in the first wave of the COVID-19 vaccine rollout | Cohort study | Online registry in the United States | Pregnant (n=7809), lactating (n=6815), and neither pregnant nor lactating women planning pregnancy (n=2901) | Pfizer-BioNTech BNT162b2: 61.9% Moderna mRNA-1273: 37.8% Janssen JNJ-78436735: 0.23% 85.9% of all participants received both doses. |
Vaccine side effects: Women who received vaccine experienced pain at injection site (91.4%) and fatigue (31.3%). Pregnancy outcomes: 0.7% of pregnant women reported miscarriages at the time of their second vaccine dose. |
COVID-19 vaccines were well-tolerated among pregnant women. |
| Kadali et al,20 2021 | Adverse effects of COVID-19 messenger RNA vaccines among pregnant women: a cross-sectional study on healthcare workers with detailed self-reported symptoms | Cross-sectional survey | Online survey of US adults | Vaccinated pregnant HCWs (n=38) and nonpregnant HCWs (n=991) | Pfizer-BioNTech BNT162b2: 52.6% Moderna mRNA-1273: 47.4% About 31 of 38 (81.58%) of the pregnant HCWs received both doses of the mRNA vaccine. |
Vaccine side effects: The vaccine side effects experienced by pregnant HCWs were minor and included sore arm (93%) and itching (5%). The side effects seemed to be similar (with no significant statistical difference) to those observed in nonpregnant HCWs. |
COVID-19 vaccine side effects and safety were comparable between pregnant and nonpregnant HCWs. |
| Kharbanda et al,21 2021 | Spontaneous abortion following COVID-19 vaccination during pregnancy | Case-control surveillance of Vaccine Safety Datalink | 8 health systems (5 Kaiser Permanente health systems; Denver Health; HealthPartners; and Marshfield Clinic in Washington, California, Colorado, Wisconsin |
Pregnant women (n=105,446) | Pfizer-BioNTech BNT162b2: received ≥1 doses (7.80%) Moderna mRNA-1273: received ≥1 doses (6.0%) Janssen JNJ-78436735: 0.50% |
Pregnancy outcomes: A total of 13,160 miscarriages and 92,286 ongoing pregnancies were identified. Spontaneous abortions were not associated with increased odds of exposure to COVID-19 vaccine in the previous 28 d compared with ongoing pregnancies (aOR, 1.02; 95% CI, 0.96–1.08). Results were consistent for mRNA-1273 and BNT162b2 and by gestational age group. |
Among women with miscarriages, the odds of COVID-19 vaccine exposure were not increased in the previous 28 d compared with women with ongoing pregnancies. |
| Lipkind et al,22 2022 | Receipt of COVID-19 vaccine during pregnancy and preterm or small-for-gestational-age at birth - eight integrated healthcare organizations, United States, December 15, 2020-July 22, 2021 | Cohort study | 8 health systems (5 Kaiser Permanente health systems; Denver Health; HealthPartners; and Marshfield Clinic in Washington, California, Colorado, Wisconsin |
Unvaccinated pregnant women (n=36,015) and vaccinated pregnant women (n=10,064) | Pfizer-BioNTech BNT162b2: received ≥1 doses (54.40 %) Moderna mRNA-1273: received ≥1 doses (41.40%) Janssen JNJ-78436735: 4.20% |
Pregnancy outcomes: Prevalence of preterm birth and SGA neonates were 6.6 and 8.2/100 live births, respectively. COVID-19 vaccination during pregnancy was not significantly associated with increased risk for preterm birth overall (aHR, 0.91; 95% CI, 0.82–1.01; P=.06) or SGA neonates (aHR, 0.95; 95% CI, 0.87–1.03; P=.24). |
COVID-19 vaccination during pregnancy is not associated with negative neonatal outcomes when compared with unvaccinated pregnant women. |
| Nakahara et al,23 2022 | Safety-related outcomes of novel mRNA COVID-19 vaccines in pregnancy | Cohort study | Ochsner Health System in Louisiana and Mississippi | Unvaccinated women (n=166) and vaccinated pregnant women (n=83) | mRNA vaccine (type not stated) | Pregnant individuals were more likely to report fever (4.80% vs 0.60%; P=.04) and gastrointestinal symptoms (4.80% vs 0%; P=.01). Frequency of complaint following vaccine administration was not different between pregnant and nonpregnant persons (18.10% vs 16.90%, P=.20). |
Side effects following COVID-19 vaccination were similar between pregnant and nonpregnant individuals. |
| Shanes et al,24 2021 | Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination in pregnancy | Cohort study | Hospital in Chicago |
Unvaccinated pregnant (n=116) and vaccinated pregnant women (n=84) | mRNA vaccine (type not stated) | Pregnancy outcomes: Placental examination in vaccinated women showed no increased incidence of placental injuries compared with the control group. |
There were no observed adverse pregnancy outcomes and placental injuries in vaccinated pregnant women. |
| Shimabukuro et al,25 2021 | Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons | Cohort study | COVID-19 Vaccine Pregnancy Registry in the United States | Vaccinated pregnant women (n=35,691) |
Pfizer-BioNTech BNT162b2: 53.9% Moderna mRNA-1273: 46.10% |
Vaccine side effects: Injection-site pain reported. Pregnancy outcomes: No neonatal deaths were reported. There were 12.60% of spontaneous abortions, 9.40% of preterm births, and 3.20% of SGA neonates. |
Preliminary findings did not show any major safety issues among pregnant mRNA vaccine recipients. |
| Theiler et al,26 2021 | Pregnancy and birth outcomes after SARS-CoV-2 vaccination in pregnancy | Cohort study | Mayo Clinic Health System in Minnesota and Wisconsin | Unvaccinated pregnant women (n=1862) and vaccinated pregnant women (n=140) |
Pfizer-BioNTech BNT162b2: 90.70% Moderna mRNA-1273: 8.57% Janssen JNJ-78436735: 0.71%. 73.60% of pregnant women completed both doses of vaccination before delivery. |
Pregnancy outcomes: Thromboembolic events, gestational hypertension, and preeclampsia risk were similar between vaccinated and unvaccinated pregnant women. Neonatal outcomes: Preterm birth and neonatal birthweight in pregnant vaccinated people were similar to those of unvaccinated pregnant women. |
Vaccinated pregnant women were less likely to experience COVID-19 infection than unvaccinated pregnant women. Vaccination during pregnancy was not associated with increased pregnancy or delivery complications. |
| Trostle et al,27 2021 | COVID-19 vaccination in pregnancy: early experience from a single institution | Cohort study | Academic medical center in New York | Vaccinated pregnant women (n=424) |
mRNA vaccine: 100%. Of those, 82.10% received both doses and 17.90% received only 1 dose. |
Pregnancy outcomes: Nine women had spontaneous abortions, 3 terminated their pregnancies, and 327 had ongoing pregnancies. There were no stillbirths. Neonatal outcomes: The rate of preterm birth was 5.90%. There were 15.30% of neonates requiring admission to the NICU). Amount of SGA neonates (per WHO standards) was 12.20%. |
The rate of spontaneous abortion in this study was within the expected rate of 10%, and preterm birth rate of 5.9% was below the national average of 9.50%. The 12.20% rate of SGA neonates was near the expected value. |
| Zauche et al,28 2021 | Receipt of mRNA Covid-19 vaccines and risk of spontaneous abortion | Cohort study | COVID-19 vaccine pregnancy registry in the United States | Vaccinated pregnant women (n=2456) |
Pfizer-BioNTech BNT162b2: 52.70% Moderna mRNA-1273: 47.30% |
Pregnancy outcomes: The cumulative risk of spontaneous abortion from 6 to <20 wk of gestation was 14.10% (95% CI, 12.10–16.10) in the primary analysis and 12.80% (95% CI, 10.80–14.80) in an analysis using direct maternal age standardization to the reference population. |
The risk of spontaneous abortion after mRNA COVID-19 vaccination is consistent with the expected risk of spontaneous abortion. The mRNA COVID-19 vaccination is safe in pregnancy. |
| COVID-19 vaccine immunogenicity and effectiveness | |||||||
|---|---|---|---|---|---|---|---|
| Author(s), year | Study title | Study design | Study setting |
Participants (n) | COVID-19 vaccine type, % received | Outcomes | Conclusions |
| Atyeo et al,29 2021 | COVID-19 mRNA vaccines drive differential antibody Fc-functional profiles in pregnant, lactating, and nonpregnant women | Cohort study | Tertiary care centers in the United States | Vaccinated, pregnant (n=84), lactating (n=31), and nonpregnant (n=16) age-matched controls | Both doses of Pfizer-BioNTech BNT162b2 or Moderna mRNA-1273 | Vaccine-specific antibody levels were lower than those of nonpregnant women after the first vaccine dose, which normalized after the second dose. | There is a need to administer both doses of the COVID-19 vaccine in pregnant people to ensure full immunity is attained. |
| Collier et al,30 2021 | Immunogenicity of COVID-19 mRNA vaccines in pregnant and lactating women | Cohort study | Hospital in Massachusetts |
Pregnant (n=30), lactating (n=16), and neither pregnant nor lactating women (n=57) who were vaccinated or had had confirmed COVID-19 infection in the past | Both doses of Pfizer-BioNTech BNT162b2 or Moderna mRNA-1273 | Pregnant, lactating, and nonpregnant women who were vaccinated developed antibody responses and T-cell responses against COVID-19 infection. | Pregnant and nonpregnant vaccinated women developed antibody responses and T-cell responses against SARS-CoV-2 variants. |
| Gill and Jones,31 2021 | Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies in neonatal cord blood after vaccination in pregnancy |
Case study | Hospital in Minnesota |
Pregnant woman vaccinated in the third trimester of pregnancy (n=1) |
Both doses of Pfizer-BioNTech BNT162b2 mRNA vaccine | Uncomplicated spontaneous vaginal delivery of a female neonate occurred at term. The patient's blood and neonatal cord blood were evaluated for SARS-CoV-2–specific antibodies. Both the patient and the neonate were positive for antibodies. There was transplacental transfer of neutralizing SARS-CoV-2 antibodies. |
This is the first case report documenting transplacental transfer of neutralizing SARS-CoV-2 antibodies after vaccination in the third trimester of pregnancy. |
| Gray et al,32 2021 | Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study |
Cohort study | Academic medical centers in Massachusetts |
Vaccinated pregnant (n=84), lactating (n=31), and nonpregnant women (n=16) |
Pfizer-BioNTech BNT162b2: 49% Moderna mRNA-1273: 51% |
Vaccines created robust humoral immunity in pregnant and lactating women, with immunogenicity similar to that of nonpregnant women (pregnant: median, 5.59; IQR, 4.68–5.89; lactating: median, 5.74; IQR, 5.06–6.22; nonpregnant: median, 5.62; IQR, 4.77–5.98; P=.24). Vaccine-generated antibodies were present in all umbilical cord blood and breast milk samples. |
COVID-19 mRNA vaccines generated immunity in pregnant and lactating women, with immunogenicity similar to that observed in nonpregnant women. Immune transfer to neonates occurred via placental transfer and breast milk. |
| Mangat and Milosavljevic,33 2021 | BNT162b2 vaccination during pregnancy protects both the mother and infant: anti-SARS-CoV-2 S antibodies persistently positive in an infant at 6 months of age |
Case study | Mayo Clinic Health System | Pregnant woman vaccinated with 2 doses of COVID-19 vaccine at 22 and 26 wk of gestation (n=1) |
Both doses of Pfizer-BioNTech BNT162b2 mRNA vaccine | At 33 wk of gestation, a preterm neonate was delivered via emergency cesarean delivery. To evaluate for SARS-CoV-2–specific antibodies, a serologic test was done on the newborn at 6 wk, 3 mo, and 6 mo. Positive anti–SARS-CoV-2 S antibodies were detected in the infant at 6 wk, 3 mo, and 6 mo of age. |
There was transplacental transfer of neutralizing SARS-CoV-2 antibodies after vaccination during pregnancy, and the immune response persisted at the infant's 6 mo of age. |
| Mithal et al,34 2021 | Cord blood antibodies following maternal coronavirus disease 2019 vaccination during pregnancy | Case series | Hospital in Chicago |
Vaccinated pregnant women (n=27) |
Pfizer-BioNTech BNT162b2: 64% Moderna mRNA-1273: 18% Unknown: 14% |
Maternal plasma and cord blood testing showed that 96.29% had a positive SARS-CoV-2 IgG test at the time of delivery. Of 28 neonates, 25 had positive IgG tests. The observed mean IgG transfer ratio demonstrated that infant antibody levels were about equal to the maternal levels. |
Pregnant women who received a COVID-19 mRNA vaccine during the third trimester had transplacental transfer of IgG to the infant. |
| Paul and Chad,35 2021 | Newborn antibodies to SARS-CoV-2 detected in cord blood after maternal vaccination - a case report | Case study | Hospital in Florida | Vaccinated pregnant woman (n=1) | Single dose of Moderna mRNA-1273 | COVID-19–naïve mother who had received a single dose of mRNA vaccine 3 wk before delivery delivered an infant with SARS-CoV-2 IgG antibodies detectable in cord blood. | SARS-CoV-2 IgG antibodies are detectable in a newborn's cord blood sample after only a single dose of the Moderna vaccine. Thus, there is potential for protection and infection risk reduction from SARS-CoV-2 with maternal vaccination. |
| Prabhu et al,36 2021 | Antibody response to coronavirus disease 2019 (COVID-19) messenger rna vaccination in pregnant women and transplacental passage into cord blood | Cross-sectional study | Academic medical center in New York | Vaccinated pregnant women (n=122) | Pfizer-BioNTech BNT162b2: 69.67% Moderna mRNA-1273: 30.32% Single dose of the COVID-19 vaccine received by 55 and both doses by 67 participants. |
Cord blood testing of vaccinated pregnant women showed antibody production. Maternal antibody production started on the 5th day and transfer of immunity to the neonate on the 16th day after first vaccination. Maternal IgG-level increment was statistically significant. The association of maternal IgG levels with cord blood IgG levels was also statistically significant. |
Pregnant women who received a COVID-19 mRNA vaccine had an immune response, and there was transplacental transfer of IgG to the neonate. |
| Trostle et al,37 2021 | High antibody levels in cord blood from pregnant women vaccinated against COVID-19 | Cohort study | Academic medical center in New York | Vaccinated pregnant women (n=36) | Pfizer-BioNTech BNT162b2: 72% Moderna mRNA-1273: 28% |
Cord blood testing after delivery showed transplacental antibody transfer, with cord blood specimens having high levels of anti-S antibodies. |
COVID-19 vaccination during pregnancy confers high levels of antibody transfer in the neonates, suggesting immune protection against SARS-CoV-2. |
| Yang et al,38 2021 | Association of gestational age at COVID-19 vaccination, history of sars-cov-2 infection, and a vaccine booster dose with maternal and umbilical cord antibody levels at delivery |
Cohort study | Medical center in New York | Vaccinated pregnant women (n=1359) | Pfizer-BioNTech BNT162b2: 75.42% Booster: 1.80% Moderna mRNA-1273: 22.15% Janssen JNJ-78436735: 2.43% Booster: 0.70% |
The highest maternal and umbilical cord blood IgG antibody levels occurred with early-third-trimester vaccination. However, neonates born to women fully vaccinated early in the first trimester had similar or higher cord IgG levels than neonates born to women who were vaccinated in the third trimester but not fully vaccinated before delivery. |
A complete COVID-19 vaccination course and a third-trimester booster dose were associated with the highest maternal and umbilical cord antibody levels. |
| COVID-19 vaccine acceptance | |||||||
|---|---|---|---|---|---|---|---|
| Author(s), year | Study title | Study design | Study setting | Participants (n) | COVID-19 vaccine types, % received | Outcomes | Conclusions |
| Ahlers-Schmidt et al,39 2020 |
Concerns of women regarding pregnancy and childbirth during the COVID-19 pandemic | Cohort study | Sedgwick County prenatal programs in Kansas | Pregnant (n=46) and postpartum women (n=68) enrolled in prenatal programs | Not stated | Vaccine acceptance: If a COVID-19 vaccine became available, 47.80% (n=54) were interested in receiving it, 23% were not, and 29.20% were unsure. Concerns were side effects/ sickness (55.90%), cost (5.10%), and the perception of it being unnecessary (3.40%). |
More than half of the participants would not receive or were unsure of receiving COVID-19 vaccination. |
| Battarbee et al,40 2022 | Attitudes toward COVID-19 illness and COVID-19 vaccination among pregnant women: a cross-sectional multicenter study during August-December 2020 | Cross-sectional survey study | Salt Lake City, UT; Birmingham, AL; and New York, NY | Pregnant women (n=915) | Not stated | Vaccine acceptance: 41% of pregnant women were willing to get a COVID-19 vaccine. The major concern was vaccine safety (82%). Receipt of the influenza vaccine in the past year was associated with higher odds of vaccine acceptance (aOR, 2.10; 95% CI, 1.50–3.00). Black and Hispanic women had lower odds of accepting a vaccine than White women (aOR, 0.40; 95% CI, 0.20–0.60 for both). |
More than half of the pregnant participants were unwilling to receive vaccination. Minorities and those without previous influenza vaccination were less likely to accept the COVID-19 vaccine. |
| Desai et al,41 2021 | COVID-19 vaccine acceptance in pregnancy | Cross-sectional survey study | Perinatal Center at the Pomona Valley Hospital in California | Pregnant women (n=124) | Not stated | Vaccine uptake: Pregnant women who had received the annual influenza vaccine were significantly more likely to get the COVID-19 vaccine (50% vs 9.70%; P<.05). Those who had previously discussed the COVID-19 vaccine with a physician were significantly more likely to receive the vaccine (45.80% vs 26%; P=.04). |
Pregnant women who discussed the COVID-19 vaccine with a healthcare provider were statistically more willing to receive the vaccine. |
| Hirshberg et al,42 2021 | Offering on-site COVID-19 vaccination to high-risk obstetrical patients: initial findings | Prepost study | Obstetrical clinic at a single academic medical center in Missouri and Illinois |
High-risk obstetrical patients (n=93) | Pfizer-BioNTech BNT162b2 vaccine | Vaccine uptake: Of 32 eligible patients counseled before on-site vaccine availability, 1 (3%) received vaccination off-site. Of 55 eligible patients counseled after on-site vaccine availability, 2 (3%) received on-site vaccination, and 4 (7%) proceeded with vaccination off-site. On-site vaccination availability did not significantly increase vaccination rates (3% vs 11%; P=.22). |
Vaccine hesitancy, not availability, is a critical driver of low vaccination rates in high-risk obstetrical patients. |
| Huddleston et al,43 2021 | COVID-19 vaccination patterns and attitudes among american pregnant individuals |
Cross-sectional survey study | Online survey of US pregnant women | Pregnant women at <10 weeks’ gestation (n=2506) | Not stated | Vaccine acceptance: Among the unvaccinated, only 35.70% reported vaccine acceptance. Predictors of lower odds of vaccination were Black race and being counseled not to vaccinate by a provider. |
There was substantial vaccine hesitancy among unvaccinated respondents. |
| Levy et al,44 2021 | Acceptance of COVID-19 vaccination in pregnancy: a survey study |
Cross-sectional survey study | Single ultrasound unit in New York | Pregnant women (n=653) | Not stated | Vaccine acceptance: 58.30% of pregnant women reported vaccine acceptance. Among those who declined vaccination, common concerns were risk to the fetus or neonate (45.80%) and vaccine side effects (17.70%). African American race, Hispanic ethnicity, low education, and declining the influenza vaccine were associated with nonacceptance of COVID-19 vaccination in pregnancy. |
The COVID-19 vaccine acceptance rate of 58.4% was consistent with the acceptance of other recommended vaccines in pregnancy (DTaP, influenza) and is associated with patient characteristics and vaccine history. |
| Razzaghi et al,45 2021 | COVID-19 vaccination coverage among pregnant women during pregnancy —eight integrated healthcare organizations, United States, December 14, 2020–May 8, 2021 | Cohort study | 8 health systems (5 Kaiser Permanente health systems; Denver Health; HealthPartners; and Marshfield Clinic in Washington, California, Colorado, Wisconsin | Total population in the registry (N=135,968) Pregnant women who received ≥1 dose of COVID-19 vaccination during pregnancy (n=22,197) |
Pfizer-BioNTech BNT162b2: 8.7% Moderna mRNA-1273: 7.0% Janssen JNJ-78436735: 0.6% |
Vaccine uptake: 16.3% of pregnant women identified in CDC's Vaccine Safety Datalink had received ≥1 dose of a COVID-19 vaccine during pregnancy. Vaccination was lowest among Hispanic (11.90%), Black (6%), and women aged 18–24 y (5.50%). Concerns were limited safety data in pregnancy and possibility of harm to the fetus. |
COVID-19 vaccination coverage is low among pregnant women. |
| Sutton et al,46 2021 | COVID-19 vaccine acceptance among pregnant, breastfeeding, and nonpregnant reproductive-aged women | Cross-sectional online survey study | Healthcare institution in New York | Pregnant (n=216), nonpregnant (n=656), and breastfeeding women (n=122) (including patients, providers, and staff) at a healthcare institution | Not stated | Vaccine acceptance: Pregnant women had the lowest rate of vaccine acceptance (44.30%; P<.05) compared with other groups. Nonpregnant women were most likely to accept vaccination (n=457, 76.20%; P<.05), with breastfeeding women being the second most likely (55.20%). Working in healthcare was not associated with vaccine acceptance. |
Pregnant respondents were more likely to decline vaccination than nonpregnant and breastfeeding women. |
| Sznajder et al,47 2022 | Covid-19 vaccine acceptance and associated factors among pregnant women in Pennsylvania 2020 | Cross-sectional online survey study | Academic medical center in Pennsylvania | Pregnant women (n=196) | Not stated | Vaccine acceptance: 65% of pregnant respondents were willing to receive the COVID-19 vaccine. Being employed full-time (aOR, 2.22; 95% CI, 1.02–4.81), being overloaded/stressed (aOR, 2.18; 95% CI, 1.02–4.68), and having had an influenza vaccine in the past year (aOR, 4.82; 95% CI, 2.17–10.72) were significantly associated with COVID-19 vaccine acceptance. |
Factors associated with COVID-19 vaccine acceptance included having had an influenza vaccine in the previous year, being employed full-time, and a general feeling of being overloaded. |
| Townsel et al,48 2021 | COVID-19 vaccine hesitancy among reproductive-aged female tier 1A healthcare workers in a United States Medical Center | Cross-sectional online survey study | Academic medical center in Michigan | Pregnant (n=245), TTC (n=891), and breastfeeding (n=177) female employees at a medical center | Not stated | Vaccine acceptance: Pregnant participants were 6 times more likely to delay and twice as likely to decline COVID-19 vaccination (P<.05) compared with other women of reproductive age. The highest rates of concern were observed for safety and effectiveness of the vaccine. |
Pregnant women had significantly higher rates of declining or delaying COVID-19 vaccination than other women of reproductive age. |
| Wang et al,49 2022 | Perceptions and knowledge of COVID-19 vaccine safety and efficacy among vaccinated and nonvaccinated obstetrical healthcare workers |
Cross-sectional online survey study | Tertiary care institution in Pennsylvania | Vaccinated pregnant HCWs (n=65) and nonvaccinated pregnant HCWs (n=18) |
At least 1 dose of Pfizer-BioNTech BNT162b2 or Moderna mRNA-1273: 78.30% |
Vaccine acceptance: Vaccine receipt was 16.90%. Pregnancy status influenced 8/18 (44.4%) nonvaccinated HCWs to not receive the COVID-19 vaccine, but influenced 1/65 (1.50%) vaccinated HCWs to receive the vaccine. |
Pregnancy status, especially the uncertainty of COVID-19 vaccination safety in pregnancy, was a major reason for vaccine refusal among nonvaccinated HCWs. |
aHR, adjusted hazard ratio; aOR, adjusted odds ratio; CDC, Centers for Disease Control and Prevention; CI, confidence interval; DtaP, diphtheria, tetanus, and acellular pertussis; HCW, healthcare worker; IgG, immunoglobulin G; IQR, interquartile range; ITP, immune thrombocytopenia; mRNA, messenger RNA; NICU, neonatal intensive care unit; SGA, small for gestational age; TTC, trying to conceive; WHO, World Health Organization.
Rawal. COVID-19 vaccination among pregnant people in the United States. Am J Obstet Gynecol MFM 2022.
Risk of bias of included studies
Critical appraisals showed that 16 studies had a low risk of bias, 14 had moderate risk, and 2 exhibited high risk. One case-control study included in this review did not match participants, and only 7 studies controlled for confounders. Three studies were purely descriptive, and 2 studies did not explain which statistical test was used to compare differences in observations before and after an intervention. Cross-sectional studies assessing vaccine acceptance did not use valid and reliable instruments to measure acceptance. Additional details regarding the risk of bias are summarized in Supplementary Tables S2 to S7.
Synthesis of results
COVID-19 vaccine safety
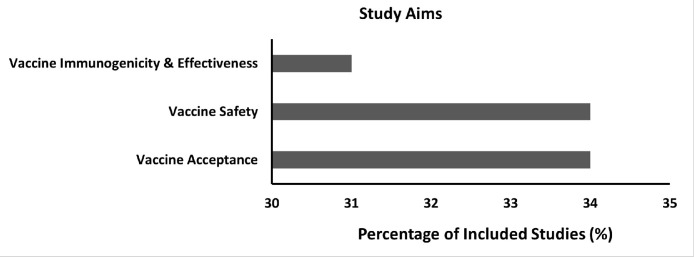
Eleven of the 32 (34%) studies (Figure 2 ) discussed COVID-19 vaccination–related side effects in pregnant people,18, 19, 20 , 23 , 25 , 32 pregnancy outcomes (gestational hypertension, preeclampsia, thromboembolism, placental injuries, miscarriage, and stillbirth),21 , 24, 25, 26, 27, 28 and neonatal outcomes (preterm birth, congenital anomalies, small size for gestational age, neonatal ICU admission, and neonatal death).22 , 25 ,– 27
Figure 2.
Study aims and percentage of included studies
Rawal. COVID-19 vaccination among pregnant people in the United States. Am J Obstet Gynecol MFM 2022.
Included studies that evaluated pregnancy and neonatal outcomes following COVID-19 vaccination did not demonstrate harmful effects with respect to pregnancy,21 , 24, 25, 26, 27, 28 fetal development,25, 26, 27 or neonatal outcomes.22 , 25, 26, 27 There were no statistical differences in pregnancy outcomes such as gestational hypertension (P=.60), preeclampsia (P=1.00), and thromboembolism incidence (P=1.00) between vaccinated and unvaccinated pregnant people.26 There were no placental injuries24 and no stillbirths.26 , 27 The miscarriage rates after receiving COVID-19 vaccination ranged from 6.50% to 14.10%,25 , 27 , 28 similarly to the 11% to 16% expected rate of miscarriage in the general population.50 , 51 With respect to newborns, there was no increased risk of adverse neonatal outcomes because of COVID-19 vaccination during pregnancy. No neonatal deaths were reported in the included studies.25, 26, 27 Rates of other neonatal outcomes including preterm birth (9.40%, 5.90%),22 , 25 , 27 congenital anomalies (2.20%, 1.20%),25 , 27 small size for gestational age (3.20%, 12.20%),22 , 25 , 27 and neonatal ICU admission (0.70%, 15.30%)26 , 27 following COVID-19 vaccination were similar to the expected rates of neonatal outcomes in the unvaccinated population.52, 53, 54, 55, 56
Side effects reported in pregnant people were similar to those observed in the general population, and the most common side effects included injection-site pain,19 , 20 , 25 injection-site soreness,20 , 21 fevers or chills,19 , 20 , 25 , 32 fatigue,19 , 20 and itching.20 Immune thrombocytopenia (ITP) was reported in a case study.18 Studies showed that the incidence of side effects (injection-site pain, injection-site soreness, and fatigue) was higher with the second dose of vaccination than with the first dose.19 , 25 , 32
COVID-19 vaccine immunogenicity and effectiveness
Ten of the 32 (31%) studies (Figure 2) in pregnant people examined the immunogenicity or the ability of the COVID-19 vaccine to elicit an immune response.32 , 26 , 30 , 31 , 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44 These studies demonstrated that COVID-19 vaccination during pregnancy produced a robust immune response, and the antibody production was similar to that of nonpregnant people.30 , 32 These antibodies were also found in umbilical cord blood,32, 33, 34, 35, 36, 37, 38 which means COVID-19 vaccination during pregnancy may convey some immunity to neonates against COVID-19. In addition, the highest maternal and umbilical cord antibody levels were achieved through the completion of a full vaccination series and a booster dose.38
Regarding the strength of the vaccine, immunity produced by the COVID-19 vaccination was found to be significantly stronger than that obtained after natural infection with the virus (P<.05).32 There was a rapid immunologic response following the first dose of the vaccine, and administration of the second dose further increased the antibody level among vaccinated pregnant people.32 Similar results were observed in an age-matched cohort study where pregnant people had lower antibody levels after the first dose, but by follow-up after the second dose, the achieved immune responses were comparable to those of nonpregnant people.29 With regard to effectiveness, COVID-19 vaccination was effective in preventing COVID-19 infection among pregnant people. A study showed that only 9 of 2136 (0.40%) and 3 of 1822 (0.20%) pregnant people experienced COVID-19 infection >14 days after the first Pfizer-BioNTech and Moderna vaccine, respectively.25 Another study that compared vaccinated and unvaccinated pregnant people showed that vaccination significantly reduced the risk of future COVID-19 infection (P<.05).26
COVID-19 vaccine acceptance
Eleven of the 32 (34%) studies (Figure 2) examined pregnant people's acceptance or uptake of COVID-19 vaccination.39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49 Overall, COVID-19 vaccine acceptance rates ranged between 3% and 65%. Studies conducted before the COVID-19 vaccine became available in the United States showed that 41%40 and 47.80%39 of pregnant people would be interested in receiving it. Vaccine-hesitant pregnant people had concerns about side effects, sickness, allergy to the vaccine, and a perception that the vaccine is unnecessary.39 A study reported 65% vaccine acceptance among pregnant people; this study had a sample consisting of people with higher education and greater income47 compared with other studies.39 , 43 , 44 The vaccine acceptance rate did not improve after the COVID-19 vaccine became available in the United States. Studies conducted after the vaccine became available showed acceptance rates of 3%,42 16.30%,45 35.70%,43 44.30%,46 and 58.30%.44
Seven of the 11 vaccine acceptance studies examined factors that were associated with vaccine acceptance. Pregnant people's receipt of the influenza vaccine in the previous year and communication with a medical professional about vaccines were associated with increased likelihood of COVID-19 vaccine acceptance.29 , 41 , 47 In contrast, pregnant people's previous refusal of the seasonal influenza vaccine,40 , 44 lack of provider counseling,43 younger age,29 , 45 African American race,40 , 43, 44, 45 , 48 Hispanic ethnicity,40 , 43, 44, 45 , and low education43 were associated with refusal of vaccination. Frequently cited concerns included safety and effectiveness of COVID-19 vaccination, fears of birth defects, unknown long-term health effects on children, and risk of pregnancy loss.44 , 48 , 49
Comment
Principal findings
This study reviewed the available literature on COVID-19 vaccination among pregnant people in the United States. Peer-reviewed observational studies support the assertion that the COVID-19 vaccine is safe during pregnancy and provides protective effects for both pregnant people and their newborns. Most of the reported side effects such as injection-site pain, soreness, fever or chills, and fatigue were not severe and were similar to those reported in the general population. ITP was reported in 1 case study.18 This very rare event has an incidence ranging from 1 case per 26,000 to 1 case per 127,000 doses,57 and may be resolved by oral corticosteroids without subsequent complications.18
The protective effects of COVID-19 vaccines in pregnant people were similar to those observed in the general population. Pregnant people had a robust immune response after vaccination, with immunogenicity equivalent to that observed in nonpregnant people.32 The vaccines also conferred protective immunity to newborns through breast milk and placental transfer.27 , 32 , 35 This demonstrates that COVID-19 vaccination in pregnancy likely has a dual benefit: both the mother and newborn receive antibodies. Supported by studies that demonstrated the efficient maternofetal transplacental transfer of anti–COVID-19 antibodies,58 , 59 Israel placed pregnant people on its vaccine priority list.60 The United States has not formally prioritized COVID-19 vaccination for pregnant people, which may ultimately contribute to poorer maternal and fetal outcomes in the United States. Even though COVID-19 vaccination is beneficial during both pregnancy and lactation, it may be most beneficial during pregnancy because higher levels of antibodies were found in early milk than in later milk.30
Although randomized controlled clinical trials involving pregnant people are lacking, data from all observational studies indicate that pregnant people tolerate COVID-19 vaccines well. Major adverse events have not been reported for mothers and fetuses or neonates, and the scientific understanding of the vaccine's mechanism of action does not raise theoretical safety concerns.21 , 25 , 28 , 45 Studies of COVID-19 vaccines authorized in the United States show that the vaccine virus does not cross the placenta.34 , 59 Only protective antibodies produced in the vaccinated mother's body are transferred to the neonates through breast milk or placental transfer.32 , 35 , 37 COVID-19 vaccine safety and effectiveness are important factors in achieving population immunity; however, wider acceptance of vaccines is crucial for achieving sufficient immunization coverage.
Current research indicates a low acceptance of COVID-19 vaccination among pregnant people in the United States. Specifically, Black and Latinx people have shown less trust in the vaccine, citing fear of side effects and risks to the fetus or neonate.39 , 44 , 48 , 49 The lack of trust in the COVID-19 vaccine and vaccine refusal may stem from long-standing medical distrust among various communities caused by historic misdeeds (eg, the Tuskegee Syphilis Study).61 Contemporary healthcare encounters may also cultivate distrust of healthcare professionals and researchers. A 2020 Kaiser Family Foundation survey of 1700 US adults showed that 45% of Black patients reported at least 1 of 6 types of negative experiences with a healthcare professional, and 36% believed they would have received better care if they were of different race or ethnicity.62
Low acceptance of vaccines could be addressed by forming partnerships between healthcare and trusted community-based organizations (CBOs). Collaborations with trusted CBOs can contribute to developing and delivering accurate, consistent, and transparent messaging to effectively promote vaccine acceptance and other positive health behaviors.63, 64, 65 Virtual town hall meetings hosted by community leaders and local healthcare providers (HCPs) can engage communities in discussions regarding COVID-19 vaccines.66 Targeted messages conveyed through multiple languages that focus on vaccine safety, efficacy, and vaccines’ ability to confer protective immunity to neonates may alleviate fear and increase the likelihood of vaccination.67 HCPs discussing risk and benefit information with pregnant people during routine visits may be another strategy to alleviate fear and reduce vaccine hesitancy. Previous research has shown that vaccine communication comprising education and recommendations from HCPs bolstered Tdap and influenza vaccine acceptance among pregnant people.68, 69, 70 Given what is known about COVID-19 vaccine safety and effectiveness, HCPs can use available data to educate and empower pregnant people to make informed decisions. In addition, HCPs who have received the COVID-19 vaccine when they were pregnant may be positioned to share their credible vaccination experiences. A national recommendation endorsing COVID-19 vaccine administration during pregnancy, with additional support and reinforcement by HCPs, may improve vaccine uptake by pregnant people.
Strengths and limitations
This systematic review explored COVID-19 vaccination among pregnant people in the United States and included all peer-reviewed empirical studies published so far on this topic. However, certain limitations of the present study should be acknowledged. First, all studies included in this review were observational, nonrandomized, and lacked long-term safety and effectiveness data. Thus, the evidence presented in this review may be limited because of previous study designs. Second, studies included in this review were not excluded on the basis of critical appraisals of the research (ie, risk-of-bias assessments). It was considered important to include all studies irrespective of the risk of bias to obtain a more comprehensive picture of relevant research pertaining to the aim of this review. However, it is acknowledged that the lack of a minimum threshold may hold some limitations for the findings. Lastly, the evidence presented in this review may be limited for the Janssen COVID-19 vaccine, given that only 6 studies reported the use of the Janssen COVID-19 vaccine among pregnant people.
Conclusions and implications
Peer-reviewed studies support COVID-19 vaccine safety and protective effects on pregnant people and their newborns. Future studies that use rigorous methodologies and include diverse populations (eg, minorities and rural residents) are needed to confirm current findings and examine the effectiveness of COVID-19 vaccines and boosters against emerging SARS-CoV-2 variants during pregnancy. In addition, targeted and tailored strategies may help improve vaccine acceptance among pregnant people, especially vulnerable populations.
Footnotes
The authors report no conflict of interest.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
The study was registered under the The International Prospective Register of Systematic Reviews (under registration number: CRD42021286726, registration date: November 01, 2021)
The abstract has been submitted to the upcoming meeting of The Professional Society for Health Economics and Outcomes Research 2022, to be held at National Harbor, MD, May 15–18, 2022.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajogmf.2022.100616.
Appendix. Supplementary materials
References
- 1.Woodworth KR, Olsen EOM, Neelam V, et al. Birth and infant outcomes following laboratory-confirmed SARS-CoV-2 infection in pregnancy - SET-NET, 16 jurisdictions, March 29-October 14, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1635–1640. doi: 10.15585/mmwr.mm6944e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khalil A, Von Dadelszen P, Draycott T, Ugwumadu A, O'Brien P, Magee L. Change in the incidence of stillbirth and preterm delivery during the COVID-19 pandemic. JAMA. 2020;324:705–706. doi: 10.1001/jama.2020.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zambrano LD, Ellington S, Strid P, et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641–1647. doi: 10.15585/mmwr.mm6944e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lokken EM, Huebner EM, Taylor GG, et al. Disease severity, pregnancy outcomes, and maternal deaths among pregnant patients with severe acute respiratory syndrome coronavirus 2 infection in Washington State. Am J Obstet Gynecol. 2021;225 doi: 10.1016/j.ajog.2020.12.1221. :77.e1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crovetto F, Crispi F, Llurba E, et al. Impact of severe acute respiratory syndrome coronavirus 2 infection on pregnancy outcomes: a population-based study. Clin Infect Dis. 2021;73:1768–1775. doi: 10.1093/cid/ciab104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American College of Obstetricians and Gynecologists. ACOG and SMFM recommend COVID-19 vaccination for pregnant individuals. 2021. Available at:https://www.acog.org/news/news-releases/2021/07/acog-smfm-recommend-covid-19-vaccination-for-pregnant-individuals. Accessed November 30, 2021.
- 7.Centers for Disease Control and Prevention. COVID-19 vaccines while pregnant or breastfeeding. 2021. Available at:https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html. Accessed November 30, 2021.
- 8.Rasmussen SA, Jamieson DJ. Pregnancy, postpartum care, and COVID-19 vaccination in 2021. JAMA. 2021;325:1099–1100. doi: 10.1001/jama.2021.1683. [DOI] [PubMed] [Google Scholar]
- 9.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pogue K, Jensen JL, Stancil CK, et al. Influences on attitudes regarding potential COVID-19 vaccination in the United States. Vaccines. 2020;8:582. doi: 10.3390/vaccines8040582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu W, Sivajohan B, McClymont E, et al. Systematic review of the safety, immunogenicity, and effectiveness of COVID-19 vaccines in pregnant and lactating individuals and their infants. Int J Gynaecol Obstet. 2022;156:406–417. doi: 10.1002/ijgo.14008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falsaperla R, Leone G, Familiari M, Ruggieri M. COVID-19 vaccination in pregnant and lactating women: a systematic review. Expert Rev Vaccines. 2021;20:1619–1628. doi: 10.1080/14760584.2021.1986390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richardson WS, Wilson MC, Nishikawa J, Hayward RS. The well-built clinical question: a key to evidence-based decisions. ACP J Club. 1995;123:A12–A13. [PubMed] [Google Scholar]
- 14.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Assessing risk of bias in a randomized trial. 2019. Available at:https://onlinelibrary.wiley.com/doi/10.1002/9781119536604.ch8. Accessed November 30, 2021.
- 16.The Joanna Briggs Institute. Checklist for Systematic Reviews and Research syntheses. 2017. Available at:https://jbi.global/sites/default/files/2019-05/JBI_Critical_Appraisal-Checklist_for_Systematic_Reviews2017_0.pdf. Accessed December 1, 2021.
- 17.Popay J, Roberts H, Sowden A, Petticrew M, Arai L, Rodgers M, Britten N, Roen K, Duffy S. Guidance on the conduct of narrative synthesis in systematic reviews. A product from the ESRC methods programme Version. 2006;1(1):b92.
- 18.Bennett C, Chambers LM, Son J, Goje O. Newly diagnosed immune thrombocytopenia in a pregnant patient after coronavirus disease 2019 vaccination. J Obstet Gynaecol Res. 2021;47:4077–4080. doi: 10.1111/jog.14978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kachikis A, Englund JA, Singleton M, Covelli I, Drake AL, Eckert LO. Short-term reactions among pregnant and lactating individuals in the first wave of the COVID-19 vaccine rollout. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.21310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kadali RAK, Janagama R, Peruru SR, et al. Adverse effects of COVID-19 messenger RNA vaccines among pregnant women: a cross-sectional study on healthcare workers with detailed self-reported symptoms. Am J Obstet Gynecol. 2021;225:458–460. doi: 10.1016/j.ajog.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kharbanda EO, Haapala J, DeSilva M, et al. Spontaneous abortion following COVID-19 vaccination during pregnancy. JAMA. 2021;326:1629–1631. doi: 10.1001/jama.2021.15494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lipkind HS, Vazquez-Benitez G, DeSilva M, et al. Receipt of COVID-19 vaccine during pregnancy and preterm or small-for-gestational-age at birth - eight integrated health care organizations, United States, December 15, 2020-July 22, 2021. MMWR Morb Mortal Wkly Rep. 2022;71:26–30. doi: 10.15585/mmwr.mm7101e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakahara A, Biggio JR, Elmayan A, Williams FB. Safety-related outcomes of novel mRNA COVID-19 vaccines in pregnancy. Am J Perinatol. 2022 doi: 10.1055/a-1745-1168. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Shanes ED, Otero S, Mithal LB, Mupanomunda CA, Miller ES, Goldstein JA. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination in pregnancy: measures of immunity and placental histopathology. Obstet Gynecol. 2021;138:281–283. doi: 10.1097/AOG.0000000000004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimabukuro TT, Kim SY, Myers TR, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384:2273–2282. doi: 10.1056/NEJMoa2104983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Theiler RN, Wick M, Mehta R, Weaver AL, Virk A, Swift M. Pregnancy and birth outcomes after SARS-CoV-2 vaccination in pregnancy. Am J Obstet Gynecol MFM. 2021;3 doi: 10.1016/j.ajogmf.2021.100467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trostle ME, Limaye MA, Avtushka V, Lighter JL, Penfield CA, Roman AS. COVID-19 vaccination in pregnancy: early experience from a single institution. Am J Obstet Gynecol MFM. 2021;3 doi: 10.1016/j.ajogmf.2021.100464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zauche LH, Wallace B, Smoots AN, et al. Receipt of mRNA Covid-19 vaccines and risk of spontaneous abortion. N Engl J Med. 2021;385:1533–1535. doi: 10.1056/NEJMc2113891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atyeo C, DeRiso EA, Davis C, et al. COVID-19 mRNA vaccines drive differential antibody Fc-functional profiles in pregnant, lactating, and nonpregnant women. Sci Transl Med. 2021;13:eabi8631. doi: 10.1126/scitranslmed.abi8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collier AY, McMahan K, Yu J, et al. Immunogenicity of COVID-19 mRNA vaccines in pregnant and lactating women. JAMA. 2021;325:2370–2380. doi: 10.1001/jama.2021.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gill L, Jones CW. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies in neonatal cord blood after vaccination in pregnancy. Obstet Gynecol. 2021;137:894–896. doi: 10.1097/AOG.0000000000004367. [DOI] [PubMed] [Google Scholar]
- 32.Gray KJ, Bordt EA, Atyeo C, et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021;225 doi: 10.1016/j.ajog.2021.03.023. :303.e1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mangat C, Milosavljevic N. BNT162b2 vaccination during pregnancy protects both the mother and infant: anti-SARS-CoV-2 S antibodies persistently positive in an infant at 6 months of age. Case Rep Pediatr. 2021;2021 doi: 10.1155/2021/6901131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mithal LB, Otero S, Shanes ED, Goldstein JA, Miller ES. Cord blood antibodies following maternal coronavirus disease 2019 vaccination during pregnancy. Am J Obstet Gynecol. 2021;225:192–194. doi: 10.1016/j.ajog.2021.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paul G, Chad R. Newborn antibodies to SARS-CoV-2 detected in cord blood after maternal vaccination - a case report. BMC Pediatr. 2021;21:138. doi: 10.1186/s12887-021-02618-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prabhu M, Murphy EA, Sukhu AC, et al. Antibody response to coronavirus disease 2019 (COVID-19) messenger RNA vaccination in pregnant women and transplacental passage into cord blood. Obstet Gynecol. 2021;138:278–280. doi: 10.1097/AOG.0000000000004438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trostle ME, Aguero-Rosenfeld ME, Roman AS, Lighter JL. High antibody levels in cord blood from pregnant women vaccinated against COVID-19. Am J Obstet Gynecol MFM. 2021;3 doi: 10.1016/j.ajogmf.2021.100481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang YJ, Murphy EA, Singh S, et al. Association of gestational age at coronavirus disease 2019 (COVID-19) vaccination, history of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, and a vaccine booster dose with maternal and umbilical cord antibody levels at delivery. Obstet Gynecol. 2022;139:373–380. doi: 10.1097/AOG.0000000000004693. [DOI] [PubMed] [Google Scholar]
- 39.Ahlers-Schmidt CR, Hervey AM, Neil T, Kuhlmann S, Kuhlmann Z. Concerns of women regarding pregnancy and childbirth during the COVID-19 pandemic. Patient Educ Couns. 2020;103:2578–2582. doi: 10.1016/j.pec.2020.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Battarbee AN, Stockwell MS, Varner M, et al. Attitudes toward COVID-19 illness and COVID-19 vaccination among pregnant women: a cross-sectional multicenter study during August-December 2020. Am J Perinatol. 2022;39:75–83. doi: 10.1055/s-0041-1735878. [DOI] [PubMed] [Google Scholar]
- 41.Desai P, Kaur G, Dong F, Rodriguez MH. COVID-19 vaccine acceptance in pregnancy. Neonatol Today. 2021;16:11–15. [Google Scholar]
- 42.Hirshberg JS, Huysman BC, Oakes MC, et al. Offering onsite COVID-19 vaccination to high-risk obstetrical patients: initial findings. Am J Obstet Gynecol MFM. 2021;3 doi: 10.1016/j.ajogmf.2021.100478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huddleston HG, Jaswa EG, Lindquist KJ, et al. COVID-19 vaccination patterns and attitudes among American pregnant individuals. Am J Obstet Gynecol MFM. 2022;4 doi: 10.1016/j.ajogmf.2021.100507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levy AT, Singh S, Riley LE, Prabhu M. Acceptance of COVID-19 vaccination in pregnancy: a survey study. Am J Obstet Gynecol MFM. 2021;3 doi: 10.1016/j.ajogmf.2021.100399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Razzaghi H, Meghani M, Pingali C, et al. COVID-19 vaccination coverage among pregnant women during pregnancy - eight integrated health care organizations, United States, December 14, 2020-May 8, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:895–899. doi: 10.15585/mmwr.mm7024e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sutton D, D'Alton M, Zhang Y, et al. COVID-19 vaccine acceptance among pregnant, breastfeeding, and nonpregnant reproductive-aged women. Am J Obstet Gynecol MFM. 2021;3 doi: 10.1016/j.ajogmf.2021.100403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sznajder KK, Kjerulff KH, Wang M, Hwang W, Ramirez SI, Gandhi CK. Covid-19 vaccine acceptance and associated factors among pregnant women in Pennsylvania 2020. Prev Med Rep. 2022;26 doi: 10.1016/j.pmedr.2022.101713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Townsel C, Moniz MH, Wagner AL, et al. COVID-19 vaccine hesitancy among reproductive-aged female tier 1A healthcare workers in a United States Medical Center. J Perinatol. 2021;41:2549–2551. doi: 10.1038/s41372-021-01173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang T, Krishnamurti T, Bernard M, Lopa S, Quinn B, Simhan H. Perceptions and knowledge of COVID-19 vaccine safety and efficacy among vaccinated and non-vaccinated obstetric healthcare workers. Behav Med. 2022 doi: 10.1080/08964289.2021.2023456. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 50.Mukherjee S, Velez Edwards DR, Baird DD, Savitz DA, Hartmann KE. Risk of miscarriage among black women and white women in a US Prospective Cohort Study. Am J Epidemiol. 2013;177:1271–1278. doi: 10.1093/aje/kws393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Magnus MC, Wilcox AJ, Morken NH, Weinberg CR, Håberg SE. Role of maternal age and pregnancy history in risk of miscarriage: prospective register based study. BMJ. 2019;364:l869. doi: 10.1136/bmj.l869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferré C, Callaghan W, Olson C, Sharma A, Barfield W. Effects of maternal age and age-specific preterm birth rates on overall preterm birth rates - United States, 2007 and 2014. MMWR Morb Mortal Wkly Rep. 2016;65:1181–1184. doi: 10.15585/mmwr.mm6543a1. [DOI] [PubMed] [Google Scholar]
- 53.Centers for Disease Control and Prevention. Percentage of births born preterm by state. 2018. Available at:https://www.cdc.gov/nchs/pressroom/sosmap/preterm_births/preterm.htm. Accessed December 1, 2021.
- 54.Boghossian NS, Geraci M, Edwards EM, Horbar JD. Morbidity and mortality in small for gestational age infants at 22 to 29 weeks’ gestation. Pediatrics. 2018;141 doi: 10.1542/peds.2017-2533. [DOI] [PubMed] [Google Scholar]
- 55.Francis A, Hugh O, Gardosi J. Customized vs INTERGROWTH-21st standards for the assessment of birthweight and stillbirth risk at term. Am J Obstet Gynecol. 2018;218:S692–S699. doi: 10.1016/j.ajog.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 56.Centers for Disease Control and Prevention (CDC) Update on overall prevalence of major birth defects - Atlanta, Georgia, 1978-2005. MMWR Morb Mortal Wkly Rep. 2008;57:1–5. [PubMed] [Google Scholar]
- 57.Pai M, Chan B, Stall NM, et al. Vaccine-induced immune thrombotic thrombocytopenia (VITT) following adenovirus vector COVID-19 vaccination. Science Briefs of the Ontario COVID-19 Science Advisory Table, 2(17):1–7.
- 58.Rottenstreich A, Zarbiv G, Oiknine-Djian E, Zigron R, Wolf DG, Porat S. Efficient maternofetal transplacental transfer of anti- severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike antibodies after antenatal SARS-CoV-2 BNT162b2 messenger RNA vaccination. Clin Infect Dis. 2021;73:1909–1912. doi: 10.1093/cid/ciab266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beharier O, Mayo RP, Raz T, et al. Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine. J Clin Investig. 2021;131 doi: 10.1172/JCI154834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vaccinating Women who are Planning a Pregnancy, Pregnant or Breastfeeding with the COVID-19 Vaccine-Clarification [Press release]. Ministry of Health, 2021. Available at:https://www.gov.il/en/departments/news/28012021-03. Accessed December 10, 2021.
- 61.Shavers VL, Lynch CF, Burmeister LF. Knowledge of the Tuskegee study and its impact on the willingness to participate in medical research studies. J Natl Med Assoc. 2000;92:563–572. [PMC free article] [PubMed] [Google Scholar]
- 62.Hamel L, Lopes L, Muñana C, Artiga S, Brodie M. Kaiser Family Foundation; 2020. The undefeated survey on race and health.https://www.kff.org/report-section/kff-the-undefeated-survey-on-race-and-health-main-findings/ Available at: Accessed February 6, 2022. [Google Scholar]
- 63.Quinn SC, Andrasik MP. Addressing vaccine hesitancy in BIPOC communities—toward trustworthiness, partnership, and reciprocity. N Engl J Med. 2021;385:97. doi: 10.1056/NEJMp2103104. [DOI] [PubMed] [Google Scholar]
- 64.Cowell AJ, Farrelly MC, Chou R, Vallone DM. Assessing the impact of the national ‘truth’ antismoking campaign on beliefs, attitudes, and intent to smoke by race/ethnicity. Ethn Health. 2009;14:75–91. doi: 10.1080/13557850802257715. [DOI] [PubMed] [Google Scholar]
- 65.Carson SL, Gonzalez C, Lopez S, et al. Reflections on the importance of community-partnered research strategies for health equity in the era of COVID-19. J Health Care Poor Underserved. 2020;31:1515–1519. doi: 10.1353/hpu.2020.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wagner EF, Langwerden RJ, Morris SL, et al. Virtual town halls addressing vaccine hesitancy among racial/ethnic minorities: preliminary findings. J Am Pharm Assoc (2003) 2022;62:317–325. doi: 10.1016/j.japh.2021.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Feifer RA, Bethea L, White EM. Racial disparities in COVID-19 vaccine acceptance: building trust to protect nursing home staff and residents. J Am Med Dir Assoc. 2021;22:1853–1855. doi: 10.1016/j.jamda.2021.07.006. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Strassberg ER, Power M, Schulkin J, et al. Patient attitudes toward influenza and tetanus, diphtheria and acellular pertussis vaccination in pregnancy. Vaccine. 2018;36:4548–4554. doi: 10.1016/j.vaccine.2018.05.121. [DOI] [PubMed] [Google Scholar]
- 69.Yuen CYS, Tarrant M. Determinants of uptake of influenza vaccination among pregnant women - a systematic review. Vaccine. 2014;32:4602–4613. doi: 10.1016/j.vaccine.2014.06.067. [DOI] [PubMed] [Google Scholar]
- 70.Myers KL. Predictors of maternal vaccination in the United States: an integrative review of the literature. Vaccine. 2016;34:3942–3949. doi: 10.1016/j.vaccine.2016.06.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.