Abstract
Heat stress (HS) is an important environmental stress factor affecting poultry production on a global scale. With the rise in ambient temperature and increasing effects of global warming, it becomes pertinent to understand the effects of HS on poultry production and the strategies that can be adopted to mitigate its detrimental impacts on the performance, health, welfare, immunity, and survival of birds. Amino acids (AAs) have been increasingly adopted as nutritional modifiers in animals to ameliorate the adverse effects of HS. They are essential for protein synthesis, growth, maintenance, reproduction, immunity, stress response, and whole-body homeostasis. However, HS tends to adversely affect the availability, transport, absorption, and utilization of these AAs. Studies have investigated the provision of these AAs to poultry during HS conditions, and variable findings have been reported. Taurine, L-theanine, and L-citrulline are non-essential amino acids that are increasingly gaining attention as nutritional supplements in HS animals. Similarly, betaine is an amino acid derivative that possesses favorable biological properties which contributes to its role as a functional additive during HS. Of particular note, taurine is negligible in plants, while betaine, L-theanine, and L-citrulline can be found in selected plants. These nutrients are barely found in feed ingredients, but their supply has been shown to elicit important physiological roles including anti-stress effects, anti-oxidative, anti-inflammatory, gut promoting, and immunomodulatory functions. The present review provides information on the use of these nutritionally and physiologically beneficial nutrients as functional additives to poultry diets during HS conditions. Presently, although several studies have reported on the positive effects of these additives in human and murine studies, however, there is limited information regarding their utilization during heat stress in poultry nutrition. Therefore, this review aims to expound on the functional properties of these nutrients, their potentials for HS alleviation, and to stimulate further researches on their biological roles in poultry nutrition.
Keywords: Amino acids, Antioxidant, Heat Stress, Immunity, Inflammation, Nutrition, Performance, Poultry
Background
In poultry production, several factors including the environment, nutrition, management, pathogens, and disease conditions can induce stress [1]. Since the 1800s, it has been reported that ambient temperature has risen by 1.0 °C, with the tendency to increase by 1.5 °C between 2030 and 2052 [2]. The increase in diurnal temperature sponsored by climate change and its antecedent of global warming on the earth’s surface has gained increasing concern and intensified investigations in the search for heat stress (HS) mitigation strategies. Alongside this, HS management has become a persistent challenge due to the rise in the human population, the higher number of production animals, and their higher metabolic activity due to genetic improvements [3].
In the tropical and sub-tropical regions of the world, HS is a major environmental stressor affecting poultry production. When there is a negative balance between the amount of heat generated by the animal and the amount of heat dissipated to its environment, these results to HS [4]. This negative balance is aggravated by other contributing factors such as sunshine, thermal irradiation, air temperature, relative humidity, housing condition, ventilation, stocking density, management conditions, environmental control systems, production systems, and animal characteristics (that is, the age, species, gender, metabolic rate, activity, and thermoregulatory mechanisms), which affects the animal’s responsiveness to HS [5–7]. In addition, HS regulates the concentration of free amino acids, altering the amino acid metabolism in poultry [8]. Consequently, during HS, there is an imminent need to devise useful strategies that would aid overcome stress effects in farm animals. Alongside environmental improvements to poultry housing, dietary manipulation is an important machinery that can contribute to alleviating the negative impacts of HS [9]. It had been revealed that despite improvements in management technology, nutritional manipulation is considered an effective strategy for HS alleviation in poultry [6]. Nutritional manipulation involves the inclusion/supplementation of functional additives (supplements) with beneficial properties to poultry diets [10]. It is an acceptable practice that involves the inclusion of vitamins, minerals, amino acids, phytogenes, growth promoters, antioxidants, nutraceuticals, herbs, probiotics, etcetera in poultry nutrition [11, 12].
Amino acids (AAs) serve as building blocks for the synthesis of proteins, bioactive peptides, and low-molecular weight metabolites, which regulate physiological functions in animals [13]. These AAs are useful candidates for feed adjustments since they play essential roles in animal metabolism and stress alleviation. The supplementation of functional AAs to low protein diets has been applied to promote livestock production and produce high-quality animal protein supply [13]. Asides from the conventional essential and nonessential amino acids, some non-standard amino acids (NSAA) have increasingly been utilized as feed additives in poultry nutrition. These NSAAs are typically non-proteinogenic, non-essential, and probable derivatives of secondary metabolites of the conventional AAs, with multi-beneficial effects. Although there are reports on AA utilization in poultry under stress conditions, there is limited information on the potentials of these functional NSAAs in poultry nutrition. This knowledge is considered important in the development of effective feeding strategies for poultry during HS conditions.
The NSAAs are involved in regulating the normal physiology of animals including affording protection from stressful conditions. Firstly is taurine, a non-protein AA whose requirement is likely increased during stress conditions to become a semi-essential AA [14]. Taurine is involved in numerous biological processes including anti-inflammatory, anti-oxidation, bile acid conjugation, membrane stability, osmoregulation, regulation of cellular calcium flux, and immunomodulation [15, 16]. Taurine has been shown to afford protective effects under stress models such as HS, endotoxin challenge, high stocking density, and in cases of toxicity [14]. Secondly, L-theanine, a naturally occurring AA in green tea leaves is widely used as a therapeutic agent, having functional roles in nervous regulation, antioxidation, and immunity [17]. Interestingly, studies have shown that L-theanine is a potent anti-stressor since it can decrease the concentration of stress hormones such as corticosterone, dopamine, and noradrenaline [18]. Thirdly, L-citrulline, a non-essential alpha-amino acid, is a crucial metabolite of the urea cycle [19] and is considered conditionally essential for gut functions [20]. It is involved in various physiological events, including arginine synthesis, nitrogen balance, anabolic processes, protein synthesis, growth and development, intestinal homeostasis, and muscle performance [21, 22]. Recent findings have expounded on the role of L-citrulline in affording thermotolerance to HS birds [23, 24]. Lastly is betaine which has been widely used as a highly valuable feed additive, and it is involved in various metabolic roles including protein synthesis, energy metabolism, anti-oxidation, osmoprotection, and methionine sparing [25]. Although not an amino acid, betaine is endogenously synthesized through choline metabolism, and as such, acts functionally as a vitamin or pro-vitamin [26, 27]. During HS, betaine supplementation provides beneficial effects against osmotic stress and dehydration, promoting survival [28]. Therefore, in the present review, these four feed additives are discussed explicitly. This article describes the functional roles and useful applications of these nutrients as anti-stressors and their biological actions in restoring health. Specifically, the beneficial application of these nutrients as feed supplements in poultry production and their role in alleviating the adverse effects of HS is discussed.
Heat stress in poultry production
Poultry are endothermic homeotherms, since their body temperature is maintained within a narrow range of 40.0 to 42.2 °C [29]. High environmental temperature above the comfort zone initiates an irreversible cascade of thermoregulatory events that negatively affect their production and survival [30, 31]. Poultry birds are highly sensitive to high ambient temperatures since a large proportion of their body surface is covered with feathers and they do not possess sweat glands like most mammals for heat dissipation [32]. The thermoneutral temperature for most poultry species is around 18-20 °C, and at temperatures exceeding 30 °C, the birds are susceptible to HS [29]. The optimum temperature for laying hens lies between 19-22 °C and 18-22 °C for growing broilers [33]. In a bid to meet protein demand, increase feed efficiency, productivity, and disease resistance, poultry birds have been subjected to years of rigorous breeding and selection, which has yielded highly productive breeds for both the meat and egg production industries. Modern-day broilers have been intensively bred for high feed conversion efficiency, accelerated muscle growth, and high rate of mitochondrial metabolism but they possess a limited capacity for heat tolerance [34]. Laying hens produce high metabolic heat with their increased rate of egg formation, increasing their vulnerability to HS [35]. As such, present-day chickens are highly susceptible to HS since they have greater metabolic activity, high core body temperature, narrow comfort zones, and minimal heat dissipation [36].
HS is typically encountered as “acute HS”, which refers to the rapid exposure to high environmental temperatures and humidity over a short duration, or “chronic HS” which is regarded as exposure to high environmental temperature and humidity over a long duration, usually ranging from days to weeks [37, 38]. When birds are exposed to HS, they initiate the stress response which involves the immediate activation of the sympathetic nervous system (SAM) responsible for the “fight-or-flight response”, as well as the activation of the hypothalamo–pituitary–adrenal (HPA) axis, which is responsible for the secretion of glucocorticoids (mainly corticosterone in poultry) [39, 40]. Thus, the integration of the SAM and the HPA axis forms the two major components involved in stress response [41].
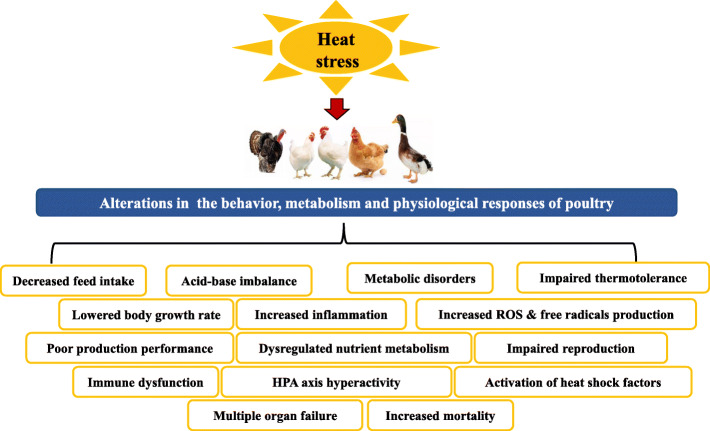
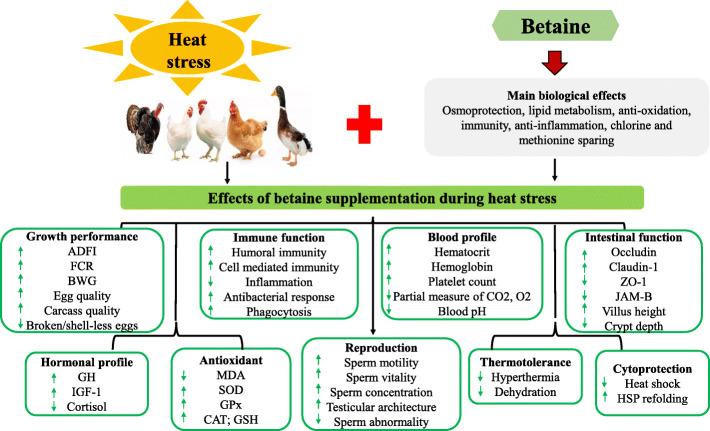
HS exposure causes severe changes to the physiology, nutrition, immunity, behavior, growth, and production traits of poultry, culminating in huge economic losses [31]. Under normal conditions, cellular ROS production occurs during ATP generation, however, HS exposure tends to induce the production of excessive free radicals, and a decrease in the enzyme activity and concentration of anti-oxidative parameters [42]. During chronic HS, increased ROS in skeletal muscle was evident due to increased oxygen consumption and the elevation of mitochondrial membrane potential at state 4 [43]. This imbalance causes oxidative stress, with resultant effects of lipid peroxidation and cellular damage [44]. More so, HS decreases the relative weights of lymphoid organs and alters immunoglobulin levels, which further predisposes the animals to infections [45]. Alongside having a diminished humoral immunity, immune cells such as lymphocytes, macrophages, and antibody responses are compromised in HS birds [32]. HS diminishes the growth performance and intestinal barrier function but promotes inflammation in birds [46]. Alhenaky [47] reported that HS increased serum corticosterone, systemic cytokines, lipopolysaccharide contents, and intestinal permeability to Salmonella spp. and endotoxins, causing increased mortality of birds. Elevated levels of corticosterone during HS alters the heterophil:lymphocyte ratio, immune functions, digestibility, glucose homeostasis, and nutrient metabolism [45, 48, 49]. Altogether, HS increases the body temperature, reduces feed intake and production indices, depresses immunity, acid-base imbalance, endocrine dysfunction, impairment in reproduction, blood pH and electrolyte imbalance, impairs nutrient digestibility, alterations to the gut microbiota, gastrointestinal dysfunctions, and increases mortality (Fig. 1, [24, 50, 51]). Detailed reviews on HS in poultry has been previously published [36, 38, 50, 51].
Fig. 1.
Effects of heat stress on the behavior and physiological responses of poultry
Amino acids as nutritional modifiers during heat stress
Exposure to HS directly causes alterations (either an increase or decrease) in the levels of certain amino acids within the plasma, brain, and ileum tissues of poultry [52]. In a meta-analysis study, it was identified that HS increased the alanine, lysine, methionine, threonine, and serine but significantly decreased the cysteine, proline, and histidine concentrations in the plasma. Likewise, the brain concentration of glutamic acid, leucine, lysine, methionine, proline, threonine, valine, isoleucine, and histidine was increased by HS [52]. It is understood that during HS, birds increase their plasma volume to allow for heat dissipation via evaporative cooling. This may result in the dilution of blood components including AAs, lowering their concentration [53]. In addition, AA consumption and retention are lowered, and the dynamics of AA transporters are altered by HS in various tissues [54]. HS affects protein and amino acid metabolism via increased muscle protein breakdown during liver gluconeogenesis [55].
Excessive protein intake and metabolism exacerbates ionic imbalance and HS in poultry, thus it is recommended to offer low protein diets enriched with supplemented AAs to HS birds [56]. A strategy to promote poultry performance during HS is via improving their access to limiting nutrients while diminishing heat increment through the feed. It had been suggested that the supplementation of free AA should be in such a manner as to achieve an ideal balance beyond the requirement, which would promote effective protein supply and compensate for decreased feed intake during HS [57]. In addition, Suganya et al. [58] reported that the proportion of critical amino acids supplied during HS should be increased by 5-10% compared to normal conditions. It was shown that feeding low protein diet (207, 193.5, and 175.5 g/kg at the starter, grower, and finisher phases (90% of the Ross 308 recommendations for CP)) fortified with AAs including valine, lysine, methionine, and threonine, supported the growth of cyclic HS broilers equivalent to birds fed with a standard CP diet [44]. Altogether, studies have shown that supplying adequate amounts of both essential and non-essential AAs can alleviate the detrimental effects of HS and promote production performance in poultry [59, 60].
The utilization of non-standard amino acids and betaine as functional additives during heat stress
Taurine
Taurine (2-aminoethanesulfonic acid) is a sulfur-containing β-amino acid [Fig. 2], which is highly abundant in the brain, heart, liver, kidney, and skeletal muscles of animals [61, 62]. It has been implicated in a myriad of functions such as cellular homeostasis, stress response, anti-oxidation, neuroprotection, energy metabolism, ion movement, calcium signaling, osmoregulation, cytoprotection, and mitochondrial functions [16, 61, 63, 64]. The utilization of taurine to improve poultry growth and performance has proved quite variable and inconclusive [65–67]. Interestingly, taurine is widely gaining research interest following the discovery that taurine inclusion in poultry nutrition elicits protective effects under different stressors including environmental, nutritional, technological, and biological stressors [1, 14].
Fig. 2.
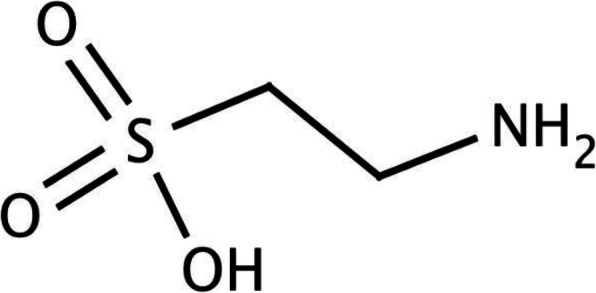
Chemical structure of Taurine
The utilization of taurine during HS conditions in poultry has yielded significant and remarkable findings with regards to HS adaptation, thermotolerance, anti-inflammation, antioxidation, and improved production performance (Table 1). It was shown that pre-feeding broilers with taurine (0.1% taurine) from 2 weeks of age, improved the final BW during HS (34 °C, 60% RH for 3-5 weeks) [68]. In contrast, some studies have reported that taurine supplementation did not alleviate HS depression of the BW, ADFI, and ADG in broilers [70, 72]. In 6 d old chicks, central administration of taurine induced anorexia and dose-dependent hypothermia via a GABAAR-dependent mechanism under thermoneutral conditions, whereas during HS, central taurine increased the glucose and uric acid levels, but decreased the sodium and calcium concentrations [69]. A study on quails fed with dietary taurine (2.5 or 5 g/kg of diet) reared under HS (34 ± 2 °C, HS for 8 h/d; 12 weeks) showed that higher taurine concentrations improved the feed intake, egg production, and apparent digestibility [74]. Also, taurine decreased the MDA concentrations but upregulated the gene expressions of nutrient transporters in the ileum such as PEPT1, EAAT3, CAT2, SGLT1, SGLT5, GLUT2, and GLUT5 during HS conditions [74]. Taurine alleviated HS impairment of the intestinal morphology in broilers by increasing the villus height but decreased the crypt depth in broilers' intestines [70]. Moreover, HS increment of intestinal hormones such as SS, PYY, ghrelin, and CCK in the duodenum, was further amplified with taurine supplementation, resulting in lowered feed intake due to the excessive release of these anorexic gut hormones [70]. These intestinal anorexic hormones inhibit appetite via impeding intestinal movements and inducing satiety [98]
Table 1.
Summary of the functional roles of taurine, L-theanine, L-citrulline and betaine in poultry species
| Animal model | Dosage and administration | Stress model/study design | Major findings | Reference |
|---|---|---|---|---|
| Taurine | ||||
| One-day-old Ross broilers | 0.1% taurine was added to drinking water from 2 weeks of age | The treatments consisted of control birds which were reared at 24 °C and HS group at 34 °C, 60% RH from 3 to 5 weeks of age | Taurine lowered the gene and protein expressions of heat shock proteins in the liver and muscle tissues of HS broilers | [68] |
| Julia layer chicks (6 days old) | Taurine was dissolved in 0.85% saline containing 0.1% Evans blue for intracerebroventricular (ICV) injection | Chicks were ICV injected with either saline or 5 μmol taurine and exposed to either 35 ± 1 °C or 30 ± 1 °C for 60 mins in temperature controlled chambers | Central taurine induced dose-dependent hypothermia and inhibited food intake under control temperature. During heat stress, central taurine altered the plasma metabolites suggesting that brain taurine may supply energy and protect against oxidative stress during high temperature in chicks. | [69] |
| Male Arbor Acres broilers | Basal diet was supplemented with 5 g/kg taurine | Broilers were raised at thermoneutrality, 22 °C or under consistent HS at 32 °C, 55 ± 5% RH from 28 days of age | Taurine had no positive effects on the growth performance of HS birds but it alleviated the adverse impacts of HS on the jejunal morphology, increased duodenal somatostatin and peptide YY hormones, and upregulated the intestinal expression of appetite related genes in HS broilers | [70] |
| Male Arbor Acres broiler chicks | Broilers were fed the basal diet supplemented with 5.00 g/kg taurine | At 28 days old, broilers were subjected to thermoneutral (22 °C), consistent HS, (32 °C), or HS + Taurine (consistent 32 °C, basal diet + 5.00 g/kg taurine) and 55 ± 5% RH for a 14-day trial | Taurine decreased reactive oxygen species and malonaldehyde but increased nuclear factor erythroid 2-related factor 2 (Nrf2), NAD(P) H quinone dehydrogenase 1 and heme oxygenase 1 expression in HS broilers. Taurine also alleviated mitochondrial damage and improved the breast meat quality in chronic HS broilers | [71] |
| Male Arbor Acres broilers | Broilers were fed basal diet supplemented with 5.00 g/kg taurine from 28 days old | Broilers were randomly distributed to positive control (22 °C, basal diet), HS, (constant 32 °C, basal diet), or heat stress + taurine, 55 ± 5% RH for 14 d. | Taurine supplementation did not alleviate the high cloacal temperature in chronic HS broilers. Taurine improved the carcass by facilitating lipolysis for energy, enhancing protein synthesis, and suppressing protein degradation in the breast muscles in HS broilers | [72] |
| Male Arbor Acres broilers | Experimental diet was supplemented with 5 g/kg taurine | Broilers were grouped as control group (22 °C), HS group (32 °C) and HS + taurine fed group | Taurine supplementation attenuated breast muscle loss induced by chronic HS via reversing endoplasmic reticulum stress induced apoptosis and suppressing protein catabolism. Taurine moderated the decreases in breast muscle mass and yield in chronic HS broilers. | [73] |
| Laying Japanese quails (Coturnix coturnix japonica) at 5 weeks of age | Taurine was supplemented at 2.5 or 5 g/kg diet | Animals were housed in temperature-controlled rooms for 12 weeks at either 22 ± 2 °C for 24 h per day considered as thermoneutral or under 34 ± 2 °C for 8 h per day, followed by 22 °C for 16 h considered as heat-stress (HS). | Taurine supplementation, especially at higher dose(5 g/kg) improved the production performance, nutrient digestion, and ileal nutrient transport in HS quails | [74] |
| L-Theanine | ||||
| Male WENS yellow-feathered broilers | Birds received a corn soybean meal basal diet in mash form or a basal diet supplemented with 800 mg/kg L-theanine | At 24, 25 and 26 days of age, birds were intra-abdominally injected with 0.2 mL sterile saline or LPS (Escherischia coli serotype O127:B8) dissolved in saline at adequate doses of 600 mg/kg BW | L-theanine exerted a protective role on the growth performance of LPS-challenged broilers and attenuated LPS-induced immune stress | [17] |
| Arbor Acre broilers | Basal diets were supplemented with different concentrations of L-theanine at 0, 100, 200 and 300 mg/kg feed | Experimental birds were divided into 4 treatments groups of control and 3 levels of L-theanine supplementation | Supplementation of L-theanine up to 200 mg/kg enhanced the growth performance, meat quality, immune response, and anti-oxidant status of broilers but reduced the total serum cholesterol levels. However, higher dose up to 300 mg/kg L-theanine may pose deleterious effects on the performance and health of birds. | [75] |
| Arbor Acre broilers | L-theanine was mixed in basal diets at different concentrations at 0, 100, 200, and 300 mg/kg feed | Experimental treatments included control (basal diet); basal diet + 100mg L-theanine/kg diet; basal diet + 200mg L-theanine/kg diet; and basal diet + 300mg L-theanine/kg diet | L-theanine promoted immune and growth responses by favoring the abundance of beneficial gut microbes and downregulating the expression of inflammatory mediators | [76] |
| Yellow-feathered broilers | L-theanine was provided at four different levels of 100, 200, 400 and 800 mg/kg | Broilers were grouped as the control group (basal diet); antibiotic group; and four L-theanine test groups at different levels of 100, 200, 400 and 800 mg/kg diet. The test period was 49 d. | L-theanine had no adverse effects on the production performance and immune organ index of yellow feather broilers at different production stages | [77] |
| Egg laying chickens | L-theanine was provided as 200 mg/kg in the basal diets | The ambient room temperature of experimental chickens was controlled at 32 ± 3 °C, and 70% RH. The trial period lasted for 28 d | L-theanine had no significant effect on the growth performance of chickens. However, L-theanine increased the catalase activity but reduced malondialdehyde content in various tissues providing antioxidant effects | [78] |
| Chaohu ducks | Basal diets were supplemented with 0 (control), 300, 600, 900 and 1500 mg/kg of L-theanine | Room temperature was between 27 to 36 °C, with 70% RH. Lighting period was 23 h/d and the trial lasted for 28 d | L-theanine yielded significant improvements in immune function and jejunum morphology and antioxidant capacity of ducks. The optimum inclusion levels of L-theanine was 600 to 900 mg/kg based on the current experimental condition | [79] |
| Male Ross 308 broilers | Dietary L-theanine was supplemented at 600 mg/kg of diet | Broilers were subjected to 3 protocols of 0-h transport (control group), 3-h transport, and 3-h transport + dietary L-theanine supplementation | L-theanine alleviated transport-stress-impairment of immune organ indexes and meat quality of broilers. L-theanine reversed the detrimental effects of transport stress on muscle antioxidant capacity and glycolysis metabolism. | [80] |
| Betaine | ||||
| Hens and roosters of Mandarah strain | Dietary supplementation of 1000 mg/kg betaine | Control conditions: 22–24 °C; 45–55% RH or Chronic HS at 38 ± 1 °C; 55–65% RH from 11:00 to 15:00 h for 3 weeks | Betaine supplementation during chronic HS improved the body weight gain, survival rate, laying rate, egg mass and feed intake | [81] |
| Mandarah (a dual-purpose breed) chickens | Basal diet supplemented with 1000 mg/kg of betaine | Thermoneutral conditions of 22–24 °C, 45–55% RH or chronic HS (38 ± 1 °C; 55–65% RH) for three successive days a week, from 11:00 to 15:00 h | Betaine supplementation alleviated the adverse effects of chronic HS by improving the semen characteristics, fertility, physiological, haematological indices, antioxidant status, wellbeing, and intestinal DNA functions of breeder roosters | [82] |
| Male White Ross breed broiler chickens | Daily oral administration of betaine hydrochloride at 250 mg/kg for 42 d | Hot dry season having dry-bulb temperature (28.33-35.67 °C), relative humidity (69.0-93.0%), and THI (27.85-36.1) | Betaine and its co-administration with ascorbic acid decreased fearfulness in birds and increased antioxidant enzymes activities of SOD and GPx activity in broiler chickens | [83] |
| Female Japanese quails | Dietary supplementation of betaine at 2 g/kg of feed | Dry season conditions with highest mean dry-bulb temperature of 32.0 to 32.1 °C, highest mean THI of 85.4-85.5 and highest mean RH of 79.6% | Dietary enrichment with betaine and ascorbic acid improved activities of serum sex and stress hormones, and erythrocytic parameters of Japanese quails during thermally stressful dry season. | [84] |
| Day-old broiler chicks | Betaine (Betafin.), was administered at the dose rate of 50 g/50 kg of feed from the first day of experiment | Hot summer season (average temperature, 34.6 °C) where HS birds were managed without using desert cooler | Betafin improved the growth performance and immunity of birds during heat stress | [85] |
| Ross 308 male broiler chickens | 1 g/kg feed of betaine was added in powder form on top of the basal diets | HS chickens were housed in a chambers at 34 °C for 8 h (9:00–17:00 h). | Dietary betaine improved the growth performance (ADG, EPI, FCR) and humoral immunity against NDV and infectious bronchitis virus in heat-stressed broilers | [86] |
| Yellow-feathered male broilers (Huaixiang chickens) | Basal diets supplemented with 500, 1000, 2000 mg/kg betaine | Birds were exposed to thermoneutral conditions of 26 ± 1 °C or cyclic HS of 32 ± 1 °C for 8 h/d from 9:00 to 17:00 h with 65–75% RH | Dietary betaine alleviated the impacts of long term HS on the growth performance, digestive function, and carcass traits in indigenous yellow-feathered broilers. | [87] |
| Meat-type ducks | Betaine was supplemented at 700, 1000 and 1300 ppm betaine | From 22 to 42 days of age, heat wave was applied at 11:00 to 17:00 h, 33 to 43 °C, and 70% RH, followed by maintaining at 22 to 26 °C from 17:00 to 11:00 h, 50% RH | Betaine supplementation had beneficial effects on the short chain fatty acid levels, hematological parameters, and body weight of heat stressed ducks. | [88] |
| Hy-line Brown laying hens | 3.0 or 6.0 g/kg of purified betaine was supplemented to the basal diet at the expense of celite. | Hens were raised during the hot season with 25.8 ± 2.0 °C average daily room temperature, 74.8 ± 7.3% RH, and heat stress index of 76 | Dietary betaine improved the hen-day egg production, decreased the broken and shell-less egg production and selectively modified the jejunal tight junction-related genes in laying hens raised under hot environment | [89] |
| Male broiler chickens (Cobb × Cobb) | Betaine (Betafin®) was supplied in the drinking water (50 g/kg) or feed (100 g/kg) | Broilers were subjected to 34 ± 1 °C, 75% RH for 4 h in environmental chambers at 35 day, then increased to 36 ± 1 °C for 4 h/d from d 36 to 41. | Study revealed that birds supplemented with betaine via drinking water had better resistance against high ambient temperatures than birds fed betaine in diets | [28] |
| L-Citrulline | ||||
| Ross 308 broiler chickens | Dietary supplementation with 1% L-Citrulline of basal diet | Broilers were subjected to two environments, either thermoneutral at 24 °C or HS at 35 °C for 5 h, 60% RH | L-Citrulline affected the body temperature, antioxidant status, heat shock response and nitric oxide regeneration of broilers during HS and at thermoneutrality | [24] |
| Hy-Line Brown laying hens | Dietary addition at 0.25%, 0.5%, and 1% L-citrulline to basal diets | Summer season with average daily minimum and maximum temperatures of 25.02 °C and 31.01 °C | Dietary L-Citrulline did not influence the production performance, and rectal temperature of laying hen. L-Citrulline modulated systemic arginine metabolism, nitric oxide synthesis, and antioxidant defences of laying hens | [90] |
| Hy-Line Brown laying hens | Diets were offered as a reduced protein diet deficient in Arginine supplemented with 0.35% L-Citrulline at the expense of wheat | Birds were fed commercial diets 16 to 20 weeks of age and experimental diets started from 21 to 40 weeks of age. | Supplementation of either L-Citrulline to reduced protein diets did not affect the egg quality, protein and energy digestibilities of hens but tended to increase the Haugh unit and lower the shell breaking strength of eggs | [91] |
| KUB Chicks | Oral administration of L-citrulline at 3.75, 7.5 and 15 mmol/kg body weight. | 5 days old chicks received L-Citrulline orally | L-Citrulline did not influence the feed intake, body temperature or plasma metabolites in chicks. | [92] |
| Male layer chicks (Julia) | Chicks received oral administration of L-Cit (15 mmol/10 mL/kg body weight) as single or double doses | Birds were exposed to HS (35 ± 1 °C) or thermoneutral temperature (30 ±1 °C) for 180 mins. | Single L-citrulline administration caused persistent hypothermia and lowered plasma glucose without affecting food intake. Dual administration of L-Citrulline afforded thermotolerance without a significant change in plasma nitric oxide of chicks | [23] |
| Male layer chicks (Julia) | L-Citrulline was administered as i.c.v. injection at 1 μmol/10 μL dosage. Orally administered L-citrulline was at 3.75, 7.5 or 15 mmol/10 mL/kg body weight | Exp. 1 was an intracerebroventricular (i.c.v.) injection while Exp. 2 was the oral administration of L-citrulline | Central citrulline did not alter body temperature, whereas, peripheral L-citrulline had a hypothermic effect in a dose responsive manner. Rectal temperature was decreased at 30, 60 and 120 mins after injection of the highest dose of L-Citrulline. | [93] |
| Male layer chicks (Julia) | Oral administration of watermelon rind extract (1.6 mL) or L-Cit (15 mmol/10 mL) | Chickens were treated with dual oral administration of (1.6 mL) watermelon rind extract or L-Cit (15 mmol/10 mL and exposed to high ambient temperature (35 ± 1 °C, 2 h) for 120 mins | Watermelon rind extract reduced rectal temperatures under control and heat stressed conditions in a similar fashion as high L-citrulline treatment | [94] |
| Male layer chicks (Julia) | Watermelon rind dried powder (WRP) was mixed with commercial starter diet to prepare a 9% WRP mash diet. | WRP mash diet was fed to 3- to 15-day-old chicks | Chronic supplementation of the WRP mash diet increased plasma L-citrulline levels, but did not affect the body temperature in chicks | [95] |
| Layer chicks | Oral administration of either a medium containing L-Citrulline producing live bacteria and 277 mmol/L L-Citrulline or an equimolar amount of L-Citrulline | In Exp. 1, chicks were orally administered treatments at 7-day-old and in Exp. 2, chicks were subjected to chronic treatment from 7 to 13 days of age | Acute or chronic administration of the media containing L-citrulline-producing live bacteria decreased the rectal and surface body temperatures of chicks, but an equimolar amount of L-citrulline elicited no changes | [96] |
| Ross 308 cockerels | L-citrulline was supplemented to low protein deficient in Arg at two levels of 0.238% and 0.476% L-Citrulline | Dietary treatments included eight groups assigned as normal-protein diet; low-protein diet deficient in Arginine (LP) and LP with two levels of either Arginine (0.238% and 0.476%), guanidinoacetic acid (0.309% and 0.618%) or Citrulline (0.238% and 0.476%). | L-Citrulline supplementation to low protein diets increased the body weight gain, carcass yield, bone length, diameter and ash but did not increase the ileal energy or nitrogen digestibility | [97] |
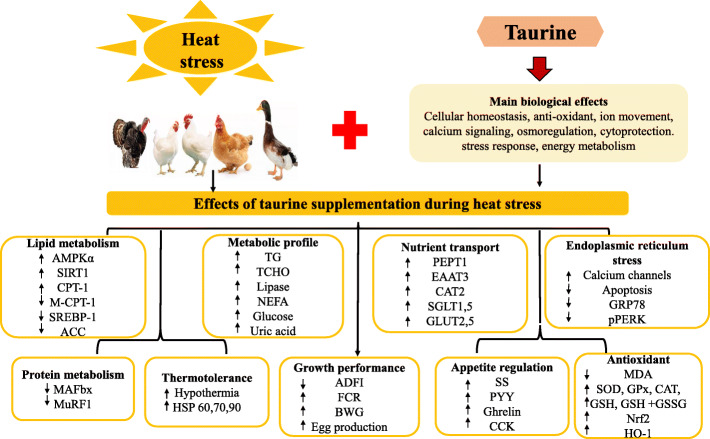
Taurine supplementation at 5.00 g/kg of basal diet was investigated on the growth performance and carcass characteristics in chronic HS broilers. Taurine increased the breast muscle proportion but decreased MAFbx, MuRF1, ACC, and M-CPT1 genes in breast muscle during HS. Also, taurine enhanced lipase activity in the abdominal fat, and serum NEFA concentration suggesting that under chronic HS, taurine supplementation can significantly promote lipolysis and protein synthesis, while suppressing protein degradation in breast muscles tissues [72]. Taurine supplementation to HS broilers (32 °C, basal diet + 5g/kg for 2 weeks) attenuated HS-induced breast muscle loss via suppressed protein catabolism but significantly improved the breast muscle mass and yield [73]. Additionally, taurine had been shown to influence lipid metabolism in broilers via increasing the hepatic expression of AMPKα, SIRT1, and CPT-1 but decreased SREBP-1 expression, and further altered the serum TG, TCHO, and hepatic lipase activity [99].
In several studies, taurine acts to attenuate inflammation and oxidative stress. At the molecular level, taurine modulated endoplasmic reticulum (ER) stress, Ca2+ homeostasis, and neuronal activity [62]. HS can induce oxidative damage and mitochondrial impairment in tissues, however, taurine is a potent antioxidant that functions to promote the antioxidant defense mechanisms and attenuate ROS generation [71]. Taurine is a non-enzymatic, free radical scavenger that possesses antioxidant properties arising from its sulfur moiety [100, 101]. It is known that apart from scavenging ROS directly, taurine can also act to enhance the cellular antioxidant enzyme activities for SOD, GPx, and catalase [102]. Taurine supplementation reduced myocardial MDA, preserved GSH levels, and normalized the GSH + GSSG levels, preventing damage from oxidative stress [101]. In addition, taurine activated the Nrf2 pathway (a major regulator of cellular defenses against oxidative stress) and enhanced HMOX1 expression to combat redox imbalance and alleviate oxidative impairment [103]. Taurine supplemented broilers exposed to chronic HS (consistent 32 °C, 55 ± 5% RH; basal diet + 5.00 g/kg; 14 d) showed decreased ROS and MDA production. Taurine alleviated HS-induced structural damage of the mitochondria and upregulated Nrf2, NAD(P)H, and HO-1 expression in the breast muscle, affording mitochondrial protection and improving the redox status of HS broilers [71]. Also, during HS, taurine reversed ER stress by mediating the expressions of Ca2+ channels, stress factors, and apoptotic factors in the breast muscle of broilers [73]. Additionally, taurine can afford thermotolerance by regulating the expression of heat shock genes and proteins [14]. Taurine downregulated HS-induced increment in the mRNA expression of HSP 60, 70, and 90 and protein expressions of HSP 60 and HSP 70 in hepatic tissues of broilers [68]. Summarily, the protective effects of taurine during HS are depicted in Fig. 3.
Fig. 3.
Effects of taurine supplementation during heat stress in animals
L-Theanine
L-Theanine, chemically known as 2-amino-4-(ethylcarbamoyl) butyric acid, is a non-protein amino acid (Fig. 4) that can be obtained from glutamic acid [104]. It is naturally found in tea plants and constitutes a major component of green teas (Camellia sinensis) [105]. It is synthesized in the roots and accumulates in tea leaves, with an average content of 1.2 to 6.2 mg/g fresh weight, 1 to 2.5% of total leaf weight, and represents about 50% of total free amino acids in teas [106]. Research on L-theanine has gained attention due to its numerous health benefits, including its growth promotion, anti-apoptosis, anti-oxidation, anti-stress, anti-anxiety, anti-carcinogenic, neuroprotection, antimicrobial, immunomodulation, and anti-inflammatory functions [18, 107–110]. It can cross the blood-brain barrier to afford neuroprotection against oxidative stress [111]. L-Theanine has been reported as basically non-toxic, with no adverse effect on the physiological and histopathological characteristics in mammals [105]. It is widely used as a functional ingredient and dietary supplement for humans or animals [112].
Fig. 4.
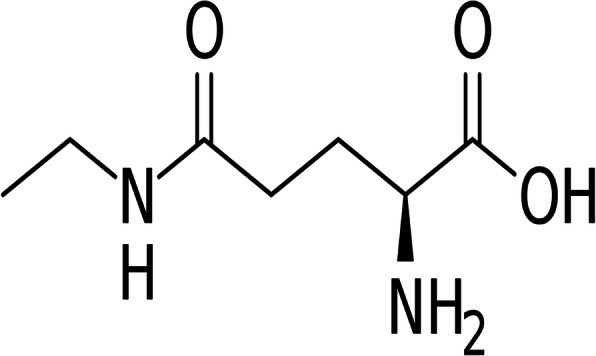
Chemical structure of L-theanine
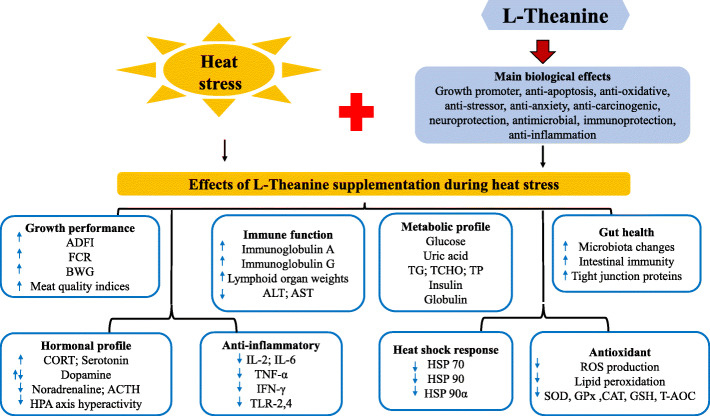
In human and murine models, L-theanine has been applied using various acute and chronic stress conditions [113]. L-Theanine elicits anti-stress effects by attenuating the activation of the sympathetic nervous system [114]. Serum corticosterone level was lowered in L-theanine treated rats subjected to water immersion stress [115]. In mice subjected to chronic restraint stress, L-theanine treatment (2 and 4 mg/kg) elicited protective effects by reversing the extent of cognitive impairment, oxidative damage, and the abnormal concentration of corticosterone, norepinephrine, and dopamine in the serum and brain tissues [116]. In poultry, variations in circulating corticosterone serve as physiological stress indicators [18], since it increases during exposure to stress stimuli, however, prolonged elevation in plasma corticosterone and activation of the HPA axis results in deleterious consequences [117]. Therefore, these stress and fatigue-relieving effects of L-theanine pose potential benefits in poultry production (Fig. 5) [51].
Fig. 5.
Effects of L-Theanine supplementation during heat stress in animals
Presently, there are limited reports on L-theanine application in livestock and poultry production [18], and a handful on its utilization for HS alleviation in poultry, creating an existing knowledge gap in poultry nutrition researches. Dietary supplemented L-theanine optimized at 600 to 900 mg/kg in duck’s feed had positive effects on the growth performance, antioxidant capacity, and jejunal morphology of birds [79]. Also, L-theanine improved the protein metabolism, immune function, and antioxidant capacity in the small intestine and liver of roosters [118]. Serum metabolites such as GLU, UA, TG, TCHO, low-density lipoprotein cholesterol, insulin, IL-2, and IL-6 were reduced, while the total protein, globulin, IgA, and IgG were increased [79]. Supplementation of L-theanine in broiler’s diet improved the expression of tight junction proteins including ZO-1, occludin, and claudin-3 [76]. Also, it was shown that L-theanine positively influenced gut health and growth responses in broilers via two routes; firstly by favoring an increase beneficial microbes (Lactobacillus), and reducing pathogenic microbes (Clostridium), and secondly by mediating the intestinal immune response via downregulating TLR-2, TLR-4, TNF-α, IFN- γ, and IL-2 genes [76]. In addition, L-theanine with L-glutamine synergistically alleviated intestinal stress by increasing the intestinal villi length and crypt depth during E. coli infection [119].
Supplementing L-theanine to young roosters promoted HS tolerance (35 oC, 70% RH), growth performance, and immune organ index at concentrations above 200 mg/kg [118]. In laying chickens exposed to HS, L-theanine showed no significant effects on the growth performance, but promoted the CAT activity and decreased the MDA content in various tissues and organs [78]. In another study, it was shown that dietary L-theanine (600 mg/kg) supplementation improved the FCR and BW of broilers, and mitigated transport stress by increasing the lymphoid organ index (thymus, spleen, and bursa of Fabricius), muscle antioxidant activity (T-AOC, CAT, and GSH-PX) and meat quality indices (decreased drip loss, muscle MDA, PC, and lactate contents) [80]. Studies from other animals also validate the efficacy of L-theanine as an anti-stress agent. L-theanine (100 or 200 mg/kg BW) treatment in mice subjected to whole-body HS (42 °C, 60% RH for 2 h) prevented the upregulation of heat shock proteins (HSP70, HSP90, and HSP90α), minimized heat-induced liver damage, oxidative stress (GSH, T-SOD, CAT, and MDA levels), inflammatory responses, and reversed HPA axis hyperactivity (lowered plasma ACTH and CORT levels) [120]. It is understood that L-theanine antagonism of the glutamate receptor allows for its inhibition of the HPA axis hyperactivity [120]. In HS mice, L-theanine increased the feed intake and body weight, enhanced SOD, CAT, GSH-Px activities in the liver and jejunum, and reduced the serum contents of ALT, AST, TNF-α, IL-6, and IFN-γ [121].
L-Theanine elicits its anti-oxidative effects via attenuating ROS production, decreasing lipid peroxidation, and promoting glutathione concentration [122]. It can regulate both the non-enzymatic and enzymatic anti-oxidative activities via transcriptional, post-transcriptional, and post-translational mechanisms [123]. In a rat model of cerebral ischemia/reperfusion injury, L-theanine treatment afforded neuroprotective effects by inhibiting HO-1 expression but activated the ERK1/2 pathway in the hippocampus [124]. In another study conducted to investigate the protective effects of L-Theanine in D-galactose-induced senescent rats, it was revealed that L-theanine reversed the imbalance in oxidative stress (increased SOD, CAT, GPx, lowered MDA), inflammatory responses (lowered IL-1β, IL-6, TNF-a; but increased IL-4 and IL-10), liver aging and inhibited the phosphorylation of FoxO1 and NF-κB (p65) in liver tissues [125]. L-theanine supplementation downregulated the colonic mRNA expression of iNOS, COX-2, and TLR-2/-4/6/-9; upregulated ZO-1 and claudin-1 expression; decreased pro-inflammatory factors (IL-1β, IL-6, TNF-α, MCP-1, and MPO) and also reversed the imbalance in GSH and MDA elicited during DSS induced colitis [126]. Altogether, it is evident that L-theanine improves intestinal absorption, reduces inflammation and oxidative damage, and attenuates tissue and organ damages during HS (Table 1).
L-Citrulline
L-Citrulline (C6H13N3O3)6, a neutral, non-essential, non-protein amino acid, is a metabolic intermediate in the urea cycle (Fig. 6) [19, 127]. Recent evidence has pointed out its almost critical role in pharmaconutrition [128]. In several studies, L-citrulline has been demonstrated in protein synthesis, intestinal homeostasis, nitrogen balance, growth and development, anti-oxidation, gut barrier function, muscle performance, intestinal digestion and absorption, renal function, exercise performance, blood pressure, vasodilation, anti-inflammation, L-arginine synthesis, and nitric oxide production [22, 127–131]. L-Citrulline is naturally occurring in living organisms, but it is largely found in watermelon (Citrullus vulgaris), and variable quantities are present in cucumbers, pumpkins, squash, bitter melons, muskmelons, and gourds [129]. It can be derived directly from L-arginine through the action of nitric oxide synthases resulting in nitric oxide production or via conversion by arginase into ornithine in the urea cycle to deliver L-citrulline [21, 132]. L-Citrulline has been described as well tolerated, with no side effects [133].
Fig. 6.
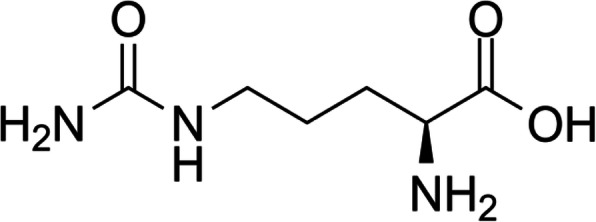
Chemical structure of L-citrulline
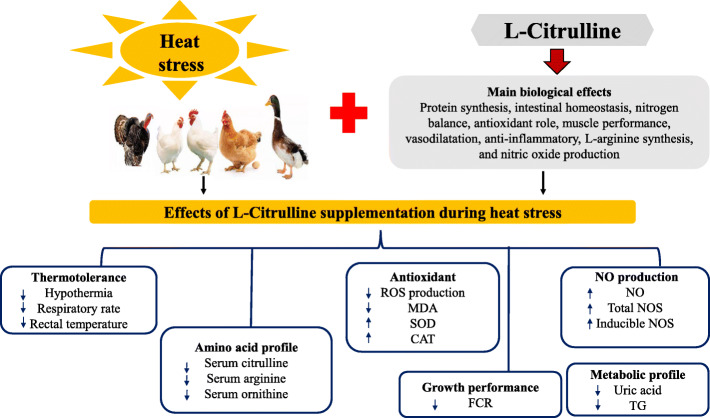
Recently, L-citrulline has been revealed as an efficacious nutritional supplement, capable of affording thermotolerance during HS (Table 1). In chicks and broilers, L-citrulline supplementation was reported to elicit hypothermic effects and thermotolerance [23, 24, 93, 134, 135]. Chowdhury [134] reports L-citrulline as an HS biomarker AA, since its administration was found to induce hypothermia and more so, the systemic concentration of L-citrulline was severely lowered under HS exposure in chickens [136]. Dietary supplementation of 1% L-citrulline to broiler chickens subjected to thermoneutral (24 °C) or HS (35 °C, 60% RH for 5 h) significantly decreased the core body temperature, promoted the antioxidant defenses, and preserved hypothalamic heat shock responses [24]. Orally administered L-citrulline (10 mL/kg body weight) reduced body temperature effectively, validating its hypothermic role in chicks [93]. Similar experiments using watermelon rinds, an agricultural biowaste rich in L-citrulline have corroborated these findings [94, 95]. Also, the utilization of L-citrulline producing live bacterial media persistently lowered the body (surface and rectal) temperature of chicks [96].
In laying hens, dietary L-citrulline supplementation (0.28% L-citrulline) to low protein diets (13% crude protein) did not influence the egg quality, protein digestibility, and energy digestibility of hens, but tended to increase the Haugh unit and lower the egg shell breaking strength [91]. During the summer season, L-citrulline did not affect several parameters related to egg production, egg quality, and plasma biochemistry in laying hens except for an increase in the eggshell index and decreased plasma TG [90]. However, L-citrulline modulated the free serum levels of key AAs involved in arginine metabolism, such as arginine, citrulline, and ornithine [90]. Also, in layer chicks, L-citrulline treatment did not affect the food intake but significantly decreased the plasma glucose levels [23]. Therefore, L-citrulline can be proposed as a novel nutritional candidate that can assist poultry to cope with HS (Fig. 7). In contrast with the findings discussed above, during cyclical HS (33.6 to 38.3 °C), Kvidera [137] reported no significant effects of L-citrulline supplementation (0.13 g/kg BW) on production parameters, although, L-citrulline modestly affected the thermal response by decreasing the respiratory rate, with a tendency to decrease the rectal temperature. In another study, dietary supplementation of 1% L-citrulline did not influence the rectal temperature, feed intake, and body weight but lowered the respiratory rate of sows and pre-weaning mortality of piglets [138]. Similarly, 1% L-citrulline fed to laying hens did not influence the rectal temperature of hens during summer [90]. The reasons for this disparity in findings are yet to be ascertained but point to the differential effects of L-citrulline on body temperature responses which may be dependent on ambient temperature, age and species of birds, and route of administration.
Fig. 7.
Effects of L-citrulline supplementation during heat stress in animals
L-citrulline has also been shown to exhibit antioxidative and anti-inflammatory properties. L-citrulline is one of the most potent scavengers of hydroxyl radicals effectively protecting DNA and metabolic enzymes from oxidative injuries [21] and in conditions of nitrosative stress [139]. As a potent antioxidant, L-citrulline acts to scavenge hydroxyl radicals at the rate of 3.9 × 109 M-1 s-1 [140]. Oral co-administration of L-arginine and L-citrulline lowered superoxide production and oxidation-sensitive genes, Elk-1 and p-CREB expression [141]. Endothelial p67phox expression (an important component of the NADH/NADPH oxidase system) was attenuated by L-citrulline supplementation under high glucose conditions [142]. Also, dietary L-citrulline (2.5 g/kg BW, 6 weeks) provided protective effects to NAFLD mice by lowering markers of liver damage such as hepatic TG, TLR-4 mRNA expression, neutrophils counts, and plasminogen activator inhibitor-1 protein levels, but promoted the duodenal protein expression of tight junction proteins, occludin and ZO-1 [143]. L-citrulline (200 mg/kg/d) modulated immune functions in infantile rats through lowered neutrophil to lymphocyte ratio, increased IL-10 and TGF-β1 production, and upregulated expression of SIRT-1, which plays a regulatory role in the immune system [144].
Furthermore, L-citrulline can stimulate muscle function under stressful conditions [145]. The ability of L-citrulline to maintain muscle mass during protein deficiencies via stimulation of protein synthesis and improvements in muscle functionality has been reported [146–148]. In old malnourished rats, Osowska [149] reported that L-citrulline supplementation increased protein synthesis and muscle protein content, probably by increasing whole-body nitrogen availability, ultimately improving the nutritional status. L-Citrulline supplementation (1 g/kg/d) to food-restricted rats restored the muscle protein synthesis, increased muscle strength, and the maximum tetanic force [150]. In muscle-wasting conditions, Ham [151] demonstrated that L-citrulline could protect protein metabolism and skeletal muscle cell size, independent of L-arginine metabolism. Similarly, Villareal [152] found that L-citrulline supplementation upregulated PGC-1α levels resulting in increased skeletal muscle weights during exercise in rat models. In aged malnourished rats, L-citrulline (5 g/kg/d) supplementation led to higher insulin, liver, and muscle protein mass, higher muscle protein synthesis rate, and improved muscle protein metabolism [149]. More so, it had been reported that L-citrulline regulates muscle protein synthesis via mechanisms of the mTOR pathway [7, 153] and S6K-1 phosphorylation in LPS-treated rats [148]. At present, extensive studies have been conducted on the efficacy of L-citrulline to promote muscle functions and protein metabolism in humans and murine models [7, 130, 131, 146, 153], but this still remains a research gap yet to be explored in poultry species.
Betaine
Betaine (also known as trimethylglycine betaine) is an amino acid derivative, that is stable, non-toxic, and present in plants, animal tissues, and foods such as wheat, sugar beets, spinach, and aquatic invertebrates [154]. It is a nutritional additive categorized under the functional group of vitamins, pro-vitamins, or chemical substances that have similar effects [27]. Betaine’s structure consists of three methyl groups, from which it can donate methyl groups to the folate pool and also to homocysteine for methionine formation [155] (Fig. 8). Betaine can be synthesized from the oxidation of choline by choline oxidase, and it can also be derived from some feed ingredients as natural sources of betaine [156, 157]. However, the amount and rate of choline synthesis in most animals are inadequate to meet biological demands [158]. In addition, the bioavailability of betaine in feed ingredients such as grains and wheat bran is low [154]. Betaine inclusion in poultry feeds has several benefits (Fig. 9), such as choline and methionine sparing, carcass fat reduction and cell osmoregulation, improved nutrient digestibility, increased growth performance, and feed conversion efficiency in broilers, meat ducks, quails, and turkeys [155, 159, 160]. It is also involved in several biological processes including osmoprotection, lipid metabolism, antioxidation, anti-inflammation, and immunity [159, 161, 162].
Fig. 8.
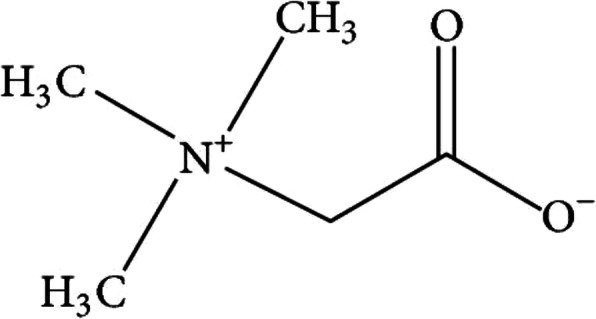
Chemical structure of betaine
Fig. 9.
Effects of betaine supplementation during heat stress in animals
Betaine supplemented in drinking water (50 g/kg) or feed (100 g/kg) of HS broilers (36 ± 1 °C, 75% RH; 4 h/d) did not affect the weight gain, feeding and feed/gain ratio, however, the betaine treated chicks were less hyperthermic [28]. Also, in broilers exposed to cyclic temperature conditions (9 h at 28–29 °C and 14 h at 22–24 °C for 2 d), betaine supplementation did not affect the growth or meat quality indices [163]. In contrast, dietary betaine has been shown to improve the average daily gain, European performance index, and humoral immunity of HS (34 °C for 8 h) broilers [86]. It had been suggested that the benefits of betaine may likely be more pronounced when broilers are exposed to temperatures > 32 °C [163], and during stress conditions that affect cell osmolarity [164]. Betaine improved the immune status of HS birds by boosting both humoral and cell-mediated immunity [85, 165]. Klasing [166] explained that betaine’s involvement in promoting cell-mediated immune responses was due to its role in facilitating nitric oxide release from heterophils and macrophages. In addition, betaine was able to decrease inflammation and increase antibacterial response via regulating the osmotic pressure, and phagocytotic activity of monocytes/macrophages [167]. Exposing poultry to HS increases panting for evaporative cooling, water, and electrolyte K+ and Na+ excretion, and reduces the amount of ionized calcium and bicarbonate levels in the blood [159]. These changes negatively affect the birds, causing acid-base perturbations (known as respiratory alkalosis) [168]. It was reported that the zwitterionic structure of betaine allows for its osmolytic, cytoprotective, and methyl donating functions [157, 169]. Therefore, betaine can alleviate osmotic damage to tissues by preventing dehydration, vascular permeability, loss of blood plasma, and maintaining normal cell volume during hyperthermia [170]. Betaine supplementation to HS ducks (33 to 43 °C; 70% RH, 4 h, d 21-42 ) produced greater BW, improved blood biomarkers, increased the blood electrolyte concentrations, reduced blood gas profiles, and lowered blood pH [88, 171]. Additionally, in female quails, betaine (2 g/kg) promoted the RBC counts but decreased the cortisol, MCV, and MCH, further validating its role in improving erythrocytic parameters [84] and the immune status of HS birds.
Betaine improved performance parameters including the feed intake, egg weight, FCR, protein, energy efficiency ratios, and certain egg quality traits in quails raised at high temperature [172]. Betaine directly promoted the secretion of GH and IGF-1, ameliorating HS depression of growth performance [173]. It was reported that betaine improved the carcass quality, growth, and feed efficiency in ducks, where the dietary methionine levels were not marginally limiting [174]. Similarly, betaine alleviated the adverse effects of long-term HS (32 ± 1 °C, 65-75% RH for 8 h/d; 10 weeks) by improving the BWG, feed intake, nitrogen retention, and intestinal epithelial morphology [87]. In laying hens, dietary betaine decreased the broken and shell-less egg production during HS but it selectively influenced the expression of jejunal tight junction genes [89]. More so, betaine was shown to increase the villus height, villus height to crypt depth ratio, and the protein expression of tight junction proteins in the small intestine of piglets [161]. These studies demonstrate the efficacy of betaine to improve growth performance, carcass quality, immunity, and gut functions during HS conditions (Table 1).
Betaine plays a crucial role in maintaining the antioxidant defense and redox balance during HS. This effect has been attributed to its ability to attenuate mitochondrial lipid peroxidation [175], its protection of the mitochondrial complex [176], modification of cysteine supply in the transsulfuration pathway for GSH synthesis, as well as its regulation of the methionine−homocysteine cycle (to increase methionine and S-adenosylmethionine which forms a protective membrane around cells) [162]. Daily oral supply of betaine (250 mg/kg) induced SOD and GPx activities in HS broilers [83]. Also, the SOD and GPx activity, CAT, SOD2, and GPx mRNA expression were modulated by dietary betaine supplementation [25]. During the hot-dry season (THI = 27.85-36.1), oral betaine (250 mg/kg) increased GPx and SOD activity, while betaine alone or in combination with ascorbic acid decreased the tonic immobility in broilers [83]. Moreover, the discovery that betaine can decrease fearfulness in broilers was related to its antioxidant-promoting capacity [83]. Dietary betaine (1000 mg/kg) supplemented to HS broilers (34 °C, 8 h for 21 d ) increased the GSH, SOD, GPx activity, but lowered the MDA content in breast muscle of HS broilers [177]. Importantly, betaine also functions in chaperoning, cytoprotection, protein stability, and the cellular regulation of transcription factors [178, 179]. Betaine stabilizes cellular proteins against heat-induced denaturation, as such attenuating and/or inhibiting the induction of heat shock proteins [178]. Diamant [180] showed that betaine increased the rate of HSPs refolding proteins by 30% to 50%, and activated protein disaggregation by 2.5 fold, stabilizing the end product and chaperone structure.
Betaine has also been used in combination with other bioactive substances. Dietary betaine (1000 mg/kg betaine) improved the laying rate, and feed intake of chronic HS hens (38 ± 1 °C; 55% to 65% RH), and demonstrated synergistic effects in combination with Vitamins C and E for HS alleviation [81]. In quails reared during the dry season (THI = 69.8-91.0), dietary betaine and ascorbic acid (Bet (2 g/kg) + AA (200 mg/kg)) enhanced testicular architecture, gonadotropins secretion, sperm motility, gonadal sperm concentration, antioxidant status, but lowered the total sperm abnormalities [181]. In a similar study, Attia [82] showed that the supply of betaine alone (1000 mg/kg), or with vitamin C (200 mg/kg) or vitamin E (150 mg/kg) to chronic HS roosters (38 ± 1 °C; 55–65% RH for 3 d/week) completely reversed the adverse effects of HS on the sperm concentration, sperm livability, semen pH, and fertility, and improved the seminal total antioxidant capacity. Also, in female Japanese quails reared under hot conditions, betaine (2 g/kg) promoted the estradiol levels but decreased the cortisol, further validating the role of betaine in improving serum sex and stress hormones [84]. Betaine enhanced the testicular antioxidant defense and accelerated germinal epithelium regeneration during HS [182]. These improvements of testicular functions by betaine and ascorbic acid are due to the attenuation of oxidative stress and enhanced responsiveness of the hypothalamic-pituitary-gonadal axis for gonadotropin release, and spermatogenesis [82, 164, 181]. Hence, betaine can be utilized as an important additive for inclusion in breeding programs, especially in hot climates since it can exert both antioxidant and protective effects on the reproductive functions of birds.
Conclusion
HS in poultry is accompanied by a cascade of detrimental effects impacting the behavioral, neuroendocrine, and physiological responses. This review discusses the protective effects of some non-standard amino acids and betaine, and their mechanisms of action in alleviating the detrimental impacts of HS in poultry. These nutrients have been reported to elicit anti-inflammatory, antioxidant, immunomodulatory, and gut-promoting functions in poultry during stress exposure. Commonly utilized as dietary supplements, the incorporation of these nutrients into poultry diets is now considered as a functional additive necessary to improve poultry performance and overall health. It is important to understand that the duration and intensity of HS, poultry nutrition, and variability in HS condition are important considerations when using these nutrients for HS mitigation. Nonetheless, this review provides a better perspective on the less considered AAs that provide functional roles in HS management for poultry and livestock production.
Future perspectives
Presently, the information available on the actions of taurine, L-theanine, L-citrulline and betaine during HS in poultry are gradually emerging and nonetheless controversial. An understanding of the underlying mechanisms through which they elicit their multiple biological effects is still open for further investigations. Therefore, this creates an existing research gap in poultry nutrition to optimize the utilization and adoption of these nutrients not only under heat stress conditions but also for commercial production practices. More so, it will be interesting to uncover their synergism, imbalance, and antagonistic potentials, when used with the standard AAs for poultry nutrition. Recent advances in biotechnology, molecular biology, and omics technology would together pave ways to strengthen researches in these areas. These tools will be vital in understanding their features, interactions, cross-reactivity, mechanism of action, functional roles, and optimization, ultimately increasing their utilization in health and disease states.
Abbreviations
- AAs
Amino acids
- EAA
Essential AA
- NEAA
Non-essential AA
- NSAA
Non-standard Amino acids
- NAFLD ACC
Acetyl CoA carboxylase
- FAS
Fatty acid synthase
- HDL-C
High-density lipoprotein cholesterol
- HL
Hepatic lipase
- HSL
Hormone-sensitive triglyceride lipase
- HS
Heat stress
- LDL-C
Low-density lipoprotein cholesterol
- LPL
Lipoprotein lipase
- NEFA
Nonesterified fatty acid
- TC
Total cholesterol
- TG
Triglycerides
- TL
Total lipase
- CAT
Catalase
- GSH
Glutathione
- GPX
Glutathione peroxidase
- MDA
Malonaldehyde
- SOD
Superoxide dismutase
- T-AOC
Total antioxidant capacity
- HO-1
Heme oxygenase-1
- NRF2
Nuclear factor erythroid 2–related factor 2
- AMPKα
Adenosine monophosphate–activated protein kinase alpha
- ApoA1
Apolipoprotein A1
- SIRT1
Sirtuin 1
- CPT-1
Carnitine palmitoyltransferase 1
- FAS
Fatty acid synthase
- PPARα
Peroxisome proliferator-activated receptor α
- SREBP-1
Sterol regulatory element-binding protein-1
- MAFbx
Muscle atrophy Fbox protein
- MuRF1
Muscle ring-finger protein-1
- M-CPT
Muscular isoform of carnitine palmitoyl transferase 1
- NAFLD
Non-alcoholic fatty liver disease
- ZO-1
-
Zonula occludens-1, HMOX1
Heme oxygenase 1 gene
- Ig
Immunoglobulin
- iNOS
inducible nitric oxide synthase
- COX-2
Cyclooxygenase
- TLRs
Toll-like receptors
- DSS
Dextran sulfate sodium
- GABAAR
Gamma-aminobutyric acid receptor
Authors’ contributions
VAU conceptualized and drafted the manuscript. EOO and FKA contributed in literature review. JZ, XW, HJ, OMO, and HL critically reviewed, edited, and approved the final version of the manuscript. All authors have read and agreed to publish this manuscript.
Funding
This work was supported by National Key Research Program of China (2016YFD0500510), the China Agriculture Research System (CARS-40-K09), National Natural Science Foundation of China (31772619) and Key Technology Research and Development Program of Shandong province (2019JZZY020602).
Availability of data and materials
Not applicable
Declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
References
- 1.Surai PF, Fisinin VI. Vitagenes in poultry production: Part 1. Technological and environmental stresses. World's Poult Sci J. 2019;72(4):721–734. doi: 10.1017/s0043933916000714. [DOI] [Google Scholar]
- 2.IPCC. Climate Change: Special report; Summary for Policymakers. IPCC (Intergovernmental Panel on Climate Change). 2018:1-24. www .ipcc .ch/ site/ assets/ uploads/ sites/ 2/ 2019/ 05/ SR15 _SPM _version _report _LR .pdf. assessed 5.2.2021.
- 3.Becker CA, Collier RJ, Stone AE. Invited review: Physiological and behavioral effects of heat stress in dairy cows. J Dairy Sci. 2020;103(8):6751–6770. doi: 10.3168/jds.2019-17929. [DOI] [PubMed] [Google Scholar]
- 4.Renaudeau D, Collin A, Yahav S, de Basilio V, Gourdine JL, Collier RJ. Adaptation to hot climate and strategies to alleviate heat stress in livestock production. Animal. 2012;6(5):707–728. doi: 10.1017/s1751731111002448. [DOI] [PubMed] [Google Scholar]
- 5.Rostagno MH. Effects of heat stress on the gut health of poultry. J Anim Sci. 2020;98(4):1–9. doi: 10.1093/jas/skaa090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdel-Moneim A-ME, Shehata AM, Khidr RE, Paswan VK, Ibrahim NS, El-Ghoul AA, et al. Nutritional manipulation to combat heat stress in poultry – A comprehensive review. J Therm Biol. 2021;98:102915. doi: 10.1016/j.jtherbio.2021.102915. [DOI] [PubMed] [Google Scholar]
- 7.Le Plenier S, Walrand S, Noirt R, Cynober L, Moinard C. Effects of leucine and citrulline versus non-essential amino acids on muscle protein synthesis in fasted rat: a common activation pathway. Amino Acids. 2012;43(3):1171–1178. doi: 10.1007/s00726-011-1172-z. [DOI] [PubMed] [Google Scholar]
- 8.Ito K, Bahry MA, Hui Y, Furuse M, Chowdhury VS. Acute heat stress up-regulates neuropeptide Y precursor mRNA expression and alters brain and plasma concentrations of free amino acids in chicks. Comp Biochem Physiol A Mol Integr Physiol. 2015;187:13–19. doi: 10.1016/j.cbpa.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Nawab A, Ibtisham F, Li G, Kieser B, Wu J, Liu W, et al. Heat stress in poultry production: Mitigation strategies to overcome the future challenges facing the global poultry industry. J Therm Biol. 2018;78:131–139. doi: 10.1016/j.jtherbio.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Olgun O, Abdulqader AF, Karabacak A. The importance of nutrition in preventing heat stress at poultry. World's Poult Sci J. 2021:77(3):661–78. 10.1080/00439339.2021.1938340.
- 11.Abd El-Hack ME, Abdelnour SA, Taha AE, Khafaga AF, Arif M, Ayasan T, et al. Herbs as thermoregulatory agents in poultry: An overview. Sci. Total Environ. 2020;703:134399. doi: 10.1016/j.scitotenv.2019.134399. [DOI] [PubMed] [Google Scholar]
- 12.Alagawany M, Abd El-Hack ME, Saeed M, Naveed M, Arain MA, Arif M, et al. Nutritional applications and beneficial health applications of green tea and l-theanine in some animal species: A review. J Anim Physiol Anim Nutr (Berl) 2020;104(1):245–256. doi: 10.1111/jpn.13219. [DOI] [PubMed] [Google Scholar]
- 13.Le Floc’h N, Wessels A, Corrent E, Wu G, Bosi P. The relevance of functional amino acids to support the health of growing pigs. Anim. Feed Sci. Technol. 2018;245:104–116. doi: 10.1016/j.anifeedsci.2018.09.007. [DOI] [Google Scholar]
- 14.Surai PF, Kochish II, Kidd MT. Taurine in poultry nutrition. Anim Feed Sci. Technol. 2020;260:114339. doi: 10.1016/j.anifeedsci.2019.114339. [DOI] [Google Scholar]
- 15.Wang FR, Dong XF, Tong JM, Zhang XM, Zhang Q, Wu YY. Effects of dietary taurine supplementation on growth performance and immune status in growing Japanese quail (Coturnix coturnix japonica) Poult Sci. 2009;88(7):1394–1398. doi: 10.3382/ps.2009-00022. [DOI] [PubMed] [Google Scholar]
- 16.Salze GP, Davis DA. Taurine: a critical nutrient for future fish feeds. Aquaculture. 2015;437:215–229. doi: 10.1016/j.aquaculture.2014.12.006. [DOI] [Google Scholar]
- 17.Li R, Song Z, Zhao J, Huo D, Fan Z, Hou DX, et al. Dietary L-theanine alleviated lipopolysaccharide-induced immunological stress in yellow-feathered broilers. Anim Nutr. 2018;4(3):265–272. doi: 10.1016/j.aninu.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saeed M, Khan MS, Kamboh AA, Alagawany M, Khafaga AF, Noreldin AE, et al. L-theanine: an astounding sui generis amino acid in poultry nutrition. Poult Sci. 2020;99(11):5625–5636. doi: 10.1016/j.psj.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allerton TD, Proctor DN, Stephens JM, Dugas TR, Spielmann G, Irving BA. L-Citrulline Supplementation: Impact on cardiometabolic health. Nutrients. 2018;10(7):921. doi: 10.3390/nu10070921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaore SN, Amane HS, Kaore NM. Citrulline: pharmacological perspectives and its role as an emerging biomarker in future. Fundam Clin Pharmacol. 2013;27(1):35–50. doi: 10.1111/j.1472-8206.2012.01059.x. [DOI] [PubMed] [Google Scholar]
- 21.Moinard C, Cynober L. Citrulline: a new player in the control of nitrogen homeostasis. J Nutr. 2007;137(6 Suppl 2):1621S–1625S. doi: 10.1093/jn/137.6.1621S. [DOI] [PubMed] [Google Scholar]
- 22.Xu XM, Wu CM, Jia G, Zhao H, Chen XL, Wang J, et al. Citrulline: Modulation on protein synthesis, intestinal homeostasis and antioxidant status. Int J Nutr Sci. 2019;4(2):1033. [Google Scholar]
- 23.Chowdhury VS, Han G, Bahry MA, Tran PV, Do PH, Yang H, et al. L-Citrulline acts as potential hypothermic agent to afford thermotolerance in chicks. J Therm Biol. 2017;69:163–170. doi: 10.1016/j.jtherbio.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Uyanga VA, Wang M, Tong T, Zhao J, Wang X, Jiao H, et al. L-Citrulline influences the body temperature, heat shock response and nitric oxide regeneration of broilers under thermoneutral and heat stress condition. Front. Physiol. 2021;12(1283):671691. doi: 10.3389/fphys.2021.671691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cai Y, Deng M, Zhang Q, Liu Z, Wang L, Sheng W, et al. Effects of dietary betaine supplementation on biochemical parameters of blood and testicular oxidative stress in Hu sheep. Theriogenology. 2021;164:65–73. doi: 10.1016/j.theriogenology.2021.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Arumugam MK, Paal MC, Donohue TM Jr, Ganesan M, Osna NA, Kharbanda KK. Beneficial effects of betaine: A comprehensive review. Biology (Basel). 2021;10(6):456. 10.3390/biology10060456. [DOI] [PMC free article] [PubMed]
- 27.EFSA FEEDAP Panel Scientific Opinion on the safety and efficacy of betaine anhydrous as a feed additive for all animal species based on a dossier submitted by Trouw Nutritional International B.V. EFSA J. 2013;11(5):3211–3219. doi: 10.2903/j.efsa.2013.3211. [DOI] [Google Scholar]
- 28.Zulkifli I, Mysahra SA, Jin LZ. Dietary supplementation of betaine (betafin(r)) and response to high temperature stress in male broiler chickens. Asian-Australas J Anim Sci. 2004;17(2):244-9. 10.5713/ajas.2004.244.
- 29.Kilic I, Simsek E. The effects of heat stress on egg production and quality of laying hens. J Anim Vet Adv. 2013;12(1):42–7. 10.3923/javaa.2013.42.47.
- 30.Oke OE. Evaluation of physiological response and performance by supplementation of Curcuma longa in broiler feed under hot humid tropical climate. Trop Anim Health Prod. 2018;50(5):1071–1077. doi: 10.1007/s11250-018-1532-8. [DOI] [PubMed] [Google Scholar]
- 31.Alagawany M, Elnesr SS, Farag MR, Abd El-Hack ME, Barkat RA, Gabr AA, et al. Potential role of important nutraceuticals in poultry performance and health - A comprehensive review. Res Vet Sci. 2021;137:9–29. doi: 10.1016/j.rvsc.2021.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Lara L, Rostagno M. Impact of heat stress on poultry production. Animals. 2013;3:356–69. 10.3390/ani3020356. [DOI] [PMC free article] [PubMed]
- 33.Charles DR. Responses to the thermal environment. In: Poultry environment problems, a guide to solutions. Nottingham, United Kingdom: Nottingham University Press; 2002. [Google Scholar]
- 34.He XF, Lu Z, Ma B, Zhang L, Li JL, Jiang Y, et al. Effects of chronic heat exposure on growth performance, intestinal epithelial histology, appetite-related hormones and genes expression in broilers. J Sci Food Agric. 2018;98(12):4471–4478. doi: 10.1002/jsfa.8971. [DOI] [PubMed] [Google Scholar]
- 35.Blem CR. Energy balance: Whittow GC. Sturkie’s Avian Physiol Acad Press; 2000: 327–341.
- 36.Emami N, Jung US, Voy B, Dridi S. Radical Response: Effects of heat stress-induced oxidative stress on lipid metabolism in the avian liver. Antioxidants. 2020;10:35. doi: 10.3390/antiox10010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akbarian A, Michiels J, Degroote J, Majdeddin M, Golian A, De Smet S. Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. J Anim Sci Biotechnol. 2016;7:37. doi: 10.1186/s40104-016-0097-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farag MR, Alagawany M. Physiological alterations of poultry to the high environmental temperature. J Therm Biol. 2018;76:101–106. doi: 10.1016/j.jtherbio.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 39.Yoshidome K, Fukano N, Ouchi Y, Tomonaga S, Cockrem JF, Bungo T. The use of behavioral tests of fearfulness in chicks to distinguish between the Japanese native chicken breeds. Tosa-Kukin and Yakido Anim Sci J. 2021;92(1):e13507. doi: 10.1111/asj.13507. [DOI] [PubMed] [Google Scholar]
- 40.Goldstein DS. Adrenal responses to stress. Cell Mol Neurobiol. 2010;30(8):1433–1440. doi: 10.1007/s10571-010-9606-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Godoy LD, Rossignoli MT, Delfino-Pereira P, Garcia-Cairasco N, de Lima Umeoka EH. A comprehensive overview on stress neurobiology: Basic concepts and clinical implications. Front Behav Neurosci. 2018;12(127):00127. doi: 10.3389/fnbeh.2018.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song J, Lei X, Luo JX, Everaert N, Zhao GP, Wen J, et al. The effect of Epigallocatechin-3-gallate on small intestinal morphology, antioxidant capacity and anti-inflammatory effect in heat-stressed broilers. Anim Physiol Anim Nutr (Berl) 2019;103(4):1030–1038. doi: 10.1111/jpn.13062. [DOI] [PubMed] [Google Scholar]
- 43.Azad MAK, Kikusato M, Sudo S, Amo T, Toyomizu M. Time course of ROS production in skeletal muscle mitochondria from chronic heat-exposed broiler chicken. Comp Biochem Physiol, Mol Amp; Integr Physiol. 2010;157(3):266–271. doi: 10.1016/j.cbpa.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 44.Amiri M, Ghasemi HA, Hajkhodadadi I, Farahani AHK. Efficacy of guanidinoacetic acid at different dietary crude protein levels on growth performance, stress indicators, antioxidant status, and intestinal morphology in broiler chickens subjected to cyclic heat stress. Anim Feed Sci Technol. 2019;254:114208. 10.1016/j.anifeedsci.2019.114208.
- 45.Calefi AS, de Siqueira A, Namazu LB, Costola-de-Souza C, Honda BBT, Ferreira AJP, et al. Effects of heat stress on the formation of splenic germinal centres and immunoglobulins in broilers infected by Clostridium perfringens type A. Vet Immunol Immunopathol. 2016;171:38–46. doi: 10.1016/j.vetimm.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 46.Liu L, Ren M, Ren K, Jin Y, Yan M. Heat stress impacts on broiler performance: a systematic review and meta-analysis. Poult Sci. 2020;99(11):6205–11. doi: 10.1016/j.psj.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alhenaky A, Abdelqader A, Abuajamieh M, Al-Fataftah AR. The effect of heat stress on intestinal integrity and Salmonella invasion in broiler birds. J Therm Biol. 2017;70:9–14. doi: 10.1016/j.jtherbio.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 48.Binsiya TK, Veerasamy S, Bagath M, Krishnan G, Iqbal H, Manimaran A, et al. Significance of hypothalamic-pituitary-adrenal axis to adapt to climate change in livestock. Int Res J Agric Food Sci. 2017;2:1–20. [Google Scholar]
- 49.Scanes CG. Biology of stress in poultry with emphasis on glucocorticoids and the heterophil to lymphocyte ratio. Poult Sci. 2016;95(9):2208–2215. doi: 10.3382/ps/pew137. [DOI] [PubMed] [Google Scholar]
- 50.Kumar M, Ratwan P, Dahiya SP, Nehra AK. Climate change and heat stress: Impact on production, reproduction and growth performance of poultry and its mitigation using genetic strategies. J Therm Biol. 2021;97:102867. doi: 10.1016/j.jtherbio.2021.102867. [DOI] [PubMed] [Google Scholar]
- 51.Saeed M, Abbas G, Alagawany M, Kamboh AA, Abd El-Hack ME, Khafaga AF, et al. Heat stress management in poultry farms: A comprehensive overview. J Therm Biol. 2019;84:414–425. doi: 10.1016/j.jtherbio.2019.07.025. [DOI] [PubMed] [Google Scholar]
- 52.Jafari MJ, Iranpour S, Gharavandi S, Tehrani BJ, Askari M, Omidi A, et al. The effects of heat stress exposure on free amino acid concentrations within the plasma and the brain of heat-exposed chicks: A systematic review and meta-analysis. J Therm Biol. 2021;97:102872. 10.1016/j.jtherbio.2021.102872. [DOI] [PubMed]
- 53.Gonzalez-Esquerra R, Leeson S. Physiological and metabolic responses of broilers to heat stress - implications for protein and amino acid nutrition. World’s Poult Sci J. 2019;62(2):282–295. doi: 10.1079/wps200597. [DOI] [Google Scholar]
- 54.Habashy WS, Milfort MC, Adomako K, Attia YA, Rekaya R, Aggrey SE. Effect of heat stress on amino acid digestibility and transporters in meat-type chickens. Poult Sci. 2017;96(7):2312–2319. doi: 10.3382/ps/pex027. [DOI] [PubMed] [Google Scholar]
- 55.Ma B, Zhang L, Li J, Xing T, Jiang Y, Gao F. Heat stress alters muscle protein and amino acid metabolism and accelerates liver gluconeogenesis for energy supply in broilers. Poult Sci. 2021;100(1):215–223. doi: 10.1016/j.psj.2020.09.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Donald DB, William DW. Commercial chicken meat and egg production. 6th ed: XCVI, 1365. New York: Kluwer Academic Publishers; 2002. 10.1007/978-1-4615-0811-3.
- 57.Zarate AJ, Moran ET, Burnham DJ. Exceeding essential amino acid requirements and improving their balance as a means to minimize heat stress in broilers. J Appl Poult Res. 2003;12(1):37–44. doi: 10.1093/japr/12.1.37. [DOI] [Google Scholar]
- 58.Suganya T, Senthilkumar S, Deepa K, Amutha R. Nutritional management to alleviate heat stress in broilers. Int J Sci Environ Technol. 2015;4(3):661–666. [Google Scholar]
- 59.Han G, Ouchi Y, Hirota T, Haraguchi S, Miyazaki T, Arakawa T, et al. Effects of l-leucine in ovo feeding on thermotolerance, growth and amino acid metabolism under heat stress in broilers. Animal. 2020;14(8):1701–1709. doi: 10.1017/S1751731120000464. [DOI] [Google Scholar]
- 60.Wu QJ, Jiao C, Liu ZH, Cheng BY, Liao JH, Zhu DD, et al. Effect of glutamine on the growth performance, digestive enzyme activity, absorption function, and mRNA expression of intestinal transporters in heat-stressed chickens. Res Vet Sci. 2021;134:51–57. doi: 10.1016/j.rvsc.2020.12.002. [DOI] [PubMed] [Google Scholar]
- 61.Ito T, Kimura Y, Uozumi Y, Takai M, Muraoka S, Matsuda T, et al. Taurine depletion caused by knocking out the taurine transporter gene leads to cardiomyopathy with cardiac atrophy. J Mol Cell Cardiol. 2008;44(5):927–937. doi: 10.1016/j.yjmcc.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 62.Jakaria M, Azam S, Haque ME, Jo SH, Uddin MS, Kim IS, et al. Taurine and its analogs in neurological disorders: Focus on therapeutic potential and molecular mechanisms. Redox Biol. 2019;24:101223. doi: 10.1016/j.redox.2019.101223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lotsch J, Hummel T, Warskulat U, Coste O, Haussinger D, Geisslinger G, et al. Congenital taurine deficiency in mice is associated with reduced sensitivity to nociceptive chemical stimulation. Neuroscience. 2014;259:63–70. doi: 10.1016/j.neuroscience.2013.11.037. [DOI] [PubMed] [Google Scholar]
- 64.Li F, Abatan OI, Kim H, Burnett D, Larkin D, Obrosova IG, et al. Taurine reverses neurological and neurovascular deficits in Zucker diabetic fatty rats. Neurobiol Dis. 2006;22(3):669–676. doi: 10.1016/j.nbd.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 65.Blair R, Jacob JP, Gardiner EE. Lack of an effect of taurine supplementation on the incidence of sudden death syndrome in male broiler chicks. Poult Sci. 1991;70(3):554–560. doi: 10.3382/ps.0700554. [DOI] [PubMed] [Google Scholar]
- 66.Yuan JM, Wang ZH. Effect of taurine on intestinal morphology and utilisation of soy oil in chickens. Br Poult Sci. 2010;51(4):540–545. doi: 10.1080/00071668.2010.506984. [DOI] [PubMed] [Google Scholar]
- 67.Shim KS, Park GH, Na CS, Ji JR, Choe HS. Effects of taurine supplementation on performance, egg quality, blood parameter and liver lipid and lipid peroxidation levels of laying hens fed high fat diet. Korean J Poult Sci. 2010;37(4):337–345. doi: 10.5536/kjps.2010.37.4.337. [DOI] [Google Scholar]
- 68.Belal SA, Kang DR, Cho ESR, Park GH, Shim KS. Taurine reduces heat stress by regulating the expression of heat shock proteins in broilers exposed to chronic heat. Brazilian J Poult Sci. 2018;20:479–486. doi: 10.1590/1806-9061-2017-0712. [DOI] [Google Scholar]
- 69.Elhussiny MZ, Tran PV, Pham CV, Nguyen LTN, Haraguchi S, Gilbert ER, et al. Central GABAA receptor mediates taurine-induced hypothermia and possibly reduces food intake in thermo-neutral chicks and regulates plasma metabolites in heat-exposed chicks. J Therm Biol. 2021;98:102905. doi: 10.1016/j.jtherbio.2021.102905. [DOI] [PubMed] [Google Scholar]
- 70.He X, Lu Z, Ma B, Zhang L, Li J, Jiang Y, et al. Effects of dietary taurine supplementation on growth performance, jejunal morphology, appetite-related hormones, and genes expression in broilers subjected to chronic heat stress. Poult Sci. 2019;98(7):2719–2728. doi: 10.3382/ps/pez054. [DOI] [PubMed] [Google Scholar]
- 71.Lu Z, He X, Ma B, Zhang L, Li J, Jiang Y, et al. Dietary taurine supplementation improves breast meat quality in chronic heat-stressed broilers via activating the Nrf2 pathway and protecting mitochondria from oxidative attack. J Sci Food Agric. 2019;99(3):1066–1072. doi: 10.1002/jsfa.9273. [DOI] [PubMed] [Google Scholar]
- 72.Lu Z, He XF, Ma BB, Zhang L, Li JL, Jiang Y, et al. The alleviative effects and related mechanisms of taurine supplementation on growth performance and carcass characteristics in broilers exposed to chronic heat stress. Poult Sci. 2019;98(2):878–886. doi: 10.3382/ps/pey433. [DOI] [PubMed] [Google Scholar]
- 73.Ma B, Zhang L, Li J, Xing T, Jiang Y, Gao F. Dietary taurine supplementation ameliorates muscle loss in chronic heat stressed broilers via suppressing the pERK signaling and reversing endoplasmic reticulum-stress-induced apoptosis. J Sci Food Agric. 2020;101:10835. doi: 10.1002/jsfa.10835. [DOI] [PubMed] [Google Scholar]
- 74.Orhan C, Kucuk O, Sahin N, Tuzcu M, Sahin K. Effects of taurine supplementation on productive performance, nutrient digestibility and gene expression of nutrient transporters in quails reared under heat stress. J Therm Biol. 2020;92:102668. doi: 10.1016/j.jtherbio.2020.102668. [DOI] [PubMed] [Google Scholar]
- 75.Saeed M, Yatao X, Hassan FU, Arain MA, Abd El-Hack ME, Noreldin AE, et al. Influence of graded levels of l-theanine dietary supplementation on growth performance, carcass traits, meat quality, organs histomorphometry, blood chemistry and immune response of broiler chickens. Int J Mol Sci. 2018;19(2):462. doi: 10.3390/ijms19020462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saeed M, Yatao X, Tiantian Z, Qian R, Chao S. 16S ribosomal RNA sequencing reveals a modulation of intestinal microbiome and immune response by dietary L-theanine supplementation in broiler chickens. Poult Sci. 2019;98(2):842–854. doi: 10.3382/ps/pey394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wen H. Effects of l-theanine on performance and immune function of yellow-feathered broilers. Chin J Anim Nutr. 2012;24(10):1946-54.
- 78.Wang LW, Cai HY, China Animal Husbandry & Veterinary Medicine. Effects of tea polyphenol and l-theanine on growth performance and antioxidant performance of egg-laying chickens under heat stress. China Anim Husband Vet Med. 2013;40:76-80.
- 79.Zhang C, Chen KK, Zhao XH, Wang C, Geng ZY. Effect of l-theanine on the growth performance, immune function, and jejunum morphology and antioxidant status of ducks. Animal. 2019;13(6):1145–1153. doi: 10.1017/S1751731118002884. [DOI] [PubMed] [Google Scholar]
- 80.Zhang C, Geng Z, Chen K, Zhao X, Wang C. L-theanine attenuates transport stress-induced impairment of meat quality of broilers through improving muscle antioxidant status. Poult Sci. 2019;98:4648–4655. doi: 10.3382/ps/pez164. [DOI] [PubMed] [Google Scholar]
- 81.Attia YA, Abd El-Hamid Ael H, Abedalla AA, Berika MA, Al-Harthi MA, Kucuk O, et al. Laying performance, digestibility and plasma hormones in laying hens exposed to chronic heat stress as affected by betaine, vitamin C, and/or vitamin E supplementation. SpringerPlus. 2016;5(1):1619. doi: 10.1186/s40064-016-3304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Attia YA, El-Naggar AS, Abou-Shehema BM, Abdella AA. Effect of supplementation with trimethylglycine (betaine) and/or vitamins on semen quality, fertility, antioxidant status, dna repair and welfare of roosters exposed to chronic heat stress. Animals. 2019;9(8):547. doi: 10.3390/ani9080547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Egbuniwe IC, Ayo JO, Kawu MU, Mohammed A. Effects of betaine and ascorbic acid on tonic immobility, superoxide dismutase and glutathione peroxidase in broiler chickens during the hot-dry season. J Vet Behav. 2016;12:60–65. doi: 10.1016/j.jveb.2015.11.001. [DOI] [Google Scholar]
- 84.Egbuniwe IC, Uchendu CN, Obidike IR. Ameliorative effects of betaine and ascorbic acid on endocrine and erythrocytic parameters of sexually-maturing female Japanese quails during the dry season. J Therm Biol. 2021;96:102812. doi: 10.1016/j.jtherbio.2020.102812. [DOI] [PubMed] [Google Scholar]
- 85.Farooqi H, Khan M, Khan M, Rabbani M, Pervez K, Khan J. Evaluation of betaine and vitamin c in alleviation of heat stress in broilers. Int J Agri Biol. 2005;7(5):744–746. [Google Scholar]
- 86.Ghasemi HA, Nari N. Effect of supplementary betaine on growth performance, blood biochemical profile, and immune response in heat-stressed broilers fed different dietary protein levels. J Appl Poult Res. 2020;29(2):301–313. doi: 10.1016/j.japr.2019.11.004. [DOI] [Google Scholar]
- 87.Liu W, Yuan Y, Sun C, Balasubramanian B, Zhao Z, An L. Effects of dietary betaine on growth performance, digestive function, carcass traits, and meat quality in indigenous yellow-feathered broilers under long-term heat stress. Animals. 2019;9(8):506. doi: 10.3390/ani9080506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Park SO, Kim WK. Effects of betaine on biological functions in meat-type ducks exposed to heat stress. Poult Sci. 2017;96(5):1212–1218. doi: 10.3382/ps/pew359. [DOI] [PubMed] [Google Scholar]
- 89.Shin JE, Kim JH, Goo D, Han GP, Pitargue FM, Kang HK, et al. Effect of dietary supplementation of betaine on productive performance, egg quality and jejunal tight junction-related gene expression in laying hens raised under hot environmental conditions. Livest Sci. 2018;214:79–82. doi: 10.1016/j.livsci.2018.05.013. [DOI] [Google Scholar]
- 90.Uyanga VA, Jiao H, Zhao J, Wang X, Lin H. Dietary L-citrulline supplementation modulates nitric oxide synthesis and anti-oxidant status of laying hens during summer season. J Anim Sci Biotechnol. 2020;11:103. 10.1186/s40104-020-00507-5. [DOI] [PMC free article] [PubMed]
- 91.Dao HT, Sharma NK, Bradbury EJ, Swick RA. Response of laying hens to l-arginine, l-citrulline and guanidinoacetic acid supplementation in reduced protein diet. Anim Nutr. 2021;7(2):460–471. doi: 10.1016/j.aninu.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Erwan E, Adelina T, Koto A, Maslami V. The potency of oral administration of l-citrulline as anti heat stress agent in KUB chickens. J World Poult Res. 2020;10(1):36–40. doi: 10.36380/jwpr.2020.5. [DOI] [Google Scholar]
- 93.Chowdhury VS, Shigemura A, Erwan E, Ito K, Bahry MA, Tran PV, et al. Oral administration of l-citrulline, but not l-arginine or l-ornithine, acts as a hypothermic agent in chicks. J Poult Sci. 2015;52:331–335. doi: 10.2141/jpsa.0150014. [DOI] [Google Scholar]
- 94.Nguyen LTN, Eltahan HM, Pham CV, Han G, Chowdhury VS, Furuse M. Oral administration of watermelon rind extract to induce hypothermia in chicks. J Poult Sci. 2020;57(1):37–44. doi: 10.2141/jpsa.0190054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nguyen LTN, Han G, Yang H, Ikeda H, Eltahan HM, Chowdhury VS, et al. Dried watermelon rind mash diet increases plasma l-citrulline level in chicks. J Poult Sci. 2019;56(1):65–70. doi: 10.2141/jpsa.0180018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tran PV, Do PH, Han G, Bahry MA, Yang H, Chowdhury VS, et al. Oral administration of a medium containing l-citrulline-producing live bacteria reduces body temperature in chicks. J Poult Sci. 2019;56:285–289. doi: 10.2141/jpsa.0180136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dao HT, Sharma NK, Bradbury EJ, Swick RA. Response of meat chickens to different sources of arginine in low-protein diets. J Anim Physiol Anim Nutr (Berl) 2021;105(4):731–746. doi: 10.1111/jpn.13486. [DOI] [PubMed] [Google Scholar]
- 98.Song Z, Liu L, Sheikhahmadi A, Jiao H, Lin H. Effect of heat exposure on gene expression of feed intake regulatory peptides in laying hens. J Biomed Biotechnol. 2012;2012:484869. doi: 10.1155/2012/484869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Han HL, Zhang JF, Yan EF, Shen MM, Wu JM, Gan ZD, et al. Effects of taurine on growth performance, antioxidant capacity, and lipid metabolism in broiler chickens. Poult Sci. 2020;99(11):5707–5717. doi: 10.1016/j.psj.2020.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Seify M, Zarabadipour M, Ghaleno LR, Alizadeh A, Rezazadeh VM. The anti-oxidant roles of Taurine and Hypotaurine on acrosome integrity, HBA and HSPA2 of the human sperm during vitrification and post warming in two different temperature. Cryobiology. 2019;90:89–95. doi: 10.1016/j.cryobiol.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 101.Oudit GY, Trivieri MG, Khaper N, Husain T, Wilson GJ, Liu P, et al. Taurine supplementation reduces oxidative stress and improves cardiovascular function in an iron-overload murine model. Circulation. 2004;109(15):1877–1885. doi: 10.1161/01.CIR.0000124229.40424.80. [DOI] [PubMed] [Google Scholar]
- 102.Shao X, Hu Z, Hu C, Bu Q, Yan G, Deng P, et al. Taurine protects methamphetamine-induced developmental angiogenesis defect through antioxidant mechanism. Toxicol Appl Pharmacol. 2012;260(3):260–270. doi: 10.1016/j.taap.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 103.Sun Jang J, Piao S, Cha Y-N, Kim C. Taurine chloramine activates Nrf2, increases HO-1 expression and protects cells from death caused by hydrogen peroxide. J Clin Biochem Nutr. 2009;45(1):37–43. doi: 10.3164/jcbn.08-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen Z, Wang Z, Yuan H, He N. From Tea Leaves to Factories: A review of research progress in l-theanine biosynthesis and production. J Agric Food Chem. 2021;69(4):1187–1196. doi: 10.1021/acs.jafc.0c06694. [DOI] [PubMed] [Google Scholar]
- 105.Adhikary R, Mandal V. l -theanine: A potential multifaceted natural bioactive amide as health supplement. Asian Pac J Trop Biomed. 2017;7(9):842–848. doi: 10.1016/j.apjtb.2017.08.005. [DOI] [Google Scholar]
- 106.Mu W, Zhang T, Jiang B. An overview of biological production of L-theanine. Biotechnol Adv. 2015;33(3-4):335–342. doi: 10.1016/j.biotechadv.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 107.Ben P, Zhang Z, Zhu Y, Xiong A, Gao Y, Mu J, et al. l-Theanine attenuates cadmium-induced neurotoxicity through the inhibition of oxidative damage and tau hyperphosphorylation. Neurotoxicology. 2016;57:95–103. doi: 10.1016/j.neuro.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 108.Chatterjee S, Chatterjee A, Roy S, Bera B, Bandyopadhyay SK. L-Theanine healed NSAID-induced gastric ulcer by modulating pro/antioxidant balance in gastric ulcer margin. J Nat Med. 2014;68(4):699–708. doi: 10.1007/s11418-014-0852-x. [DOI] [PubMed] [Google Scholar]
- 109.Gong Z, Liu Q, Lin L, Deng Y, Cai S, Liu Z, et al. L-Theanine prevents ETEC-induced liver damage by reducing intrinsic apoptotic response and inhibiting ERK1/2 and JNK1/2 signaling pathways. Eur J Pharmacol. 2018;818:184–190. doi: 10.1016/j.ejphar.2017.10.050. [DOI] [PubMed] [Google Scholar]
- 110.Hwang YP, Jin SW, Choi JH, Choi CY, Kim HG, Kim SJ, et al. Inhibitory effects of l-theanine on airway inflammation in ovalbumin-induced allergic asthma. Food Chem Toxicol. 2017;99:162–169. doi: 10.1016/j.fct.2016.11.032. [DOI] [PubMed] [Google Scholar]
- 111.Takeshima M, Miyazaki I, Murakami S, Kita T, Asanuma M. L-Theanine protects against excess dopamine- induced neurotoxicity in the presence of astrocytes. J Clin Biochem Nutr. 2016;59(2):93–99. doi: 10.3164/jcbn.16-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dramard V, Kern L, Hofmans J, Reme CA, Nicolas CS, Chala V, et al. Effect of L-theanine tablets in reducing stress-related emotional signs in cats: an open-label field study. Ir Vet J. 2018;71:21. doi: 10.1186/s13620-018-0130-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lopes Sakamoto F, Metzker Pereira Ribeiro R, Amador Bueno A, Oliveira Santos H. Psychotropic effects of L-theanine and its clinical properties: From the management of anxiety and stress to a potential use in schizophrenia. Pharmacol Res. 2019;147:104395. doi: 10.1016/j.phrs.2019.104395. [DOI] [PubMed] [Google Scholar]
- 114.Kimura K, Ozeki M, Juneja LR, Ohira H. L-Theanine reduces psychological and physiological stress responses. Biol Psychol. 2007;74(1):39–45. doi: 10.1016/j.biopsycho.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 115.Tamano H, Fukura K, Suzuki M, Sakamoto K, Yokogoshi H, Takeda A. Preventive effect of theanine intake on stress-induced impairments of hippocamapal long-term potentiation and recognition memory. Brain Res Bull. 2013;95:1–6. doi: 10.1016/j.brainresbull.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 116.Tian X, Sun L, Gou L, Ling X, Feng Y, Wang L, et al. Protective effect of l-theanine on chronic restraint stress-induced cognitive impairments in mice. Brain Res. 2013;1503:24–32. doi: 10.1016/j.brainres.2013.01.048. [DOI] [PubMed] [Google Scholar]
- 117.Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5(7):374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- 118.Wang L. Effects of green tea extract on growth performance, immunity and antioxidation of laying Rooster. Masters Dissertation: Anhui Agricultural University; 2013. [Google Scholar]
- 119.Liu A, Gong Z, Lin L, Xu W, Zhang T, Zhang S, et al. Effects of l-theanine on glutamine metabolism in enterotoxigenic Escherichia coli (E44813)-stressed and non-stressed rats. J Funct Foods. 2020;64:103670. 10.1016/j.jff.2019.103670.
- 120.Wang D, Cai M, Wang T, Zhao G, Huang J, Wang H, et al. Theanine supplementation prevents liver injury and heat shock response by normalizing hypothalamic-pituitaryadrenal axis hyperactivity in mice subjected to whole body heat stress. J Funct Foods. 2018;45:181–189. doi: 10.1016/j.jff.2018.04.001. [DOI] [Google Scholar]
- 121.Chen G, Liu L, Yi X, Li L, Lin L, Xiao W. L-theanine attenuates tissue and oxidative damages in heat stressed mice. J Tea Sci. 2017;37(1):17–24. [Google Scholar]
- 122.Saeed M, Abd El-Hac ME, Alagawany M, Naveed M, Arain MA, Arif M, et al. Phytochemistry, modes of action and beneficial health applications of green tea (Camellia sinensis) in humans and animals. Int J Pharmacol. 2017;13(7):698–708. doi: 10.3923/ijp.2017.698.708. [DOI] [Google Scholar]
- 123.Deng Y, Xiao W, Chen L, Liu Q, Liu Z, Gong Z. In vivo antioxidative effects of l-theanine in the presence or absence of Escherichia coli-induced oxidative stress. J Funct Foods. 2016;24:527–536. doi: 10.1016/j.jff.2016.04.029. [DOI] [Google Scholar]
- 124.Zhao J, Zhao X, Tian J, Xue R, Luo B, Lv J, et al. Theanine attenuates hippocampus damage of rat cerebral ischemia-reperfusion injury by inhibiting HO-1 expression and activating ERK1/2 pathway. Life Sci. 2020;241:117160. doi: 10.1016/j.lfs.2019.117160. [DOI] [PubMed] [Google Scholar]
- 125.Zeng L, Lin L, Peng Y, Yuan D, Zhang S, Gong Z, et al. l-Theanine attenuates liver aging by inhibiting advanced glycation end products in d-galactose-induced rats and reversing an imbalance of oxidative stress and inflammation. Exp Gerontol. 2020;131:110823. doi: 10.1016/j.exger.2019.110823. [DOI] [PubMed] [Google Scholar]
- 126.Wang D, Cai M, Wang T, Liu T, Huang J, Wang Y, et al. Ameliorative effects of L-theanine on dextran sulfate sodium induced colitis in C57BL/6J mice are associated with the inhibition of inflammatory responses and attenuation of intestinal barrier disruption. Food Res Int. 2020;137:109409. doi: 10.1016/j.foodres.2020.109409. [DOI] [PubMed] [Google Scholar]
- 127.Curis E, Nicolis I, Moinard C, Osowska S, Zerrouk N, Benazeth S, et al. Almost all about citrulline in mammals. Amino Acids. 2005;29(3):177–205. doi: 10.1007/s00726-005-0235-4. [DOI] [PubMed] [Google Scholar]
- 128.Cynober L, Moinard C, De Bandt JP. The 2009 ESPEN Sir David Cuthbertson. Citrulline: a new major signaling molecule or just another player in the pharmaconutrition game? Clin Nutr. 2010;29(5):545–551. doi: 10.1016/j.clnu.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 129.Kaore SN, Kaore NM. Citrulline: Pharmacological perspectives and role as a biomarker in diseases and toxicities. Gupta R. Biomarkers Toxicol. 2014. 883-905. https://doi.org/10.1016/B978-0-12-404630-6.00053-1 .
- 130.Gonzalez AM, Trexler ET. Effects of citrulline supplementation on exercise performance in humans: a review of the current literature. J Strength Cond Res. 2020;34(5):1480–1495. doi: 10.1519/jsc.0000000000003426. [DOI] [PubMed] [Google Scholar]
- 131.Uyanga VA, Amevor FK, Liu M, Cui Z, Zhao X, Lin H. Potential implications of citrulline and quercetin on gut functioning of monogastric animals and humans: A comprehensive review. Nutrients. 2021;13(11):3782. doi: 10.3390/nu13113782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Luiking YC, Engelen MP, Deutz NE. Regulation of nitric oxide production in health and disease. Curr Opin Clin Nutr Metab Care. 2010;13(1):97–104. doi: 10.1097/MCO.0b013e328332f99d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Breuillard C, Cynober L, Moinard C. Citrulline and nitrogen homeostasis: an overview. Amino Acids. 2015;47(4):685–691. doi: 10.1007/s00726-015-1932-2. [DOI] [PubMed] [Google Scholar]
- 134.Chowdhury VS. Heat stress biomarker amino acids and neuropeptide afford thermotolerance in chicks. J Poult Sci. 2019;56(1):1–11. 10.2141/jpsa.0180024. [DOI] [PMC free article] [PubMed]
- 135.Chowdhury VS, Han G, Yang H, Ikeda H, Bungo T, Furuse M. L-Citrulline and L-leucine-mediated thermoregulation affords thermotolerance. Proc Jpn SocAnimal Nutrition and Metabolism. 2017;62(2):1-11.
- 136.Chowdhury VS, Tomonaga S, Ikegami T, Erwan E, Ito K, Cockrem JF, et al. Oxidative damage and brain concentrations of free amino acid in chicks exposed to high ambient temperature. Comp Biochem Physiol A Mol Integr Physiol. 2014;169:70–76. doi: 10.1016/j.cbpa.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 137.Kvidera SK, Mayorga EJ, Seibert JT, Ross JW, Rhoads RP, Horst EA, et al. Effect of supplemental citrulline on thermal and production parameters during heat stress in growing pigs. J Anim Sci. 2016;94(suppl_5):476–477. doi: 10.2527/jam2016-0995. [DOI] [Google Scholar]
- 138.Liu F, de Ruyter EM, Athorn RZ, Brewster CJ, Henman DJ, Morrison RS, et al. Effects of L-citrulline supplementation on heat stress physiology, lactation performance and subsequent reproductive performance of sows in summer. J Anim Physiol Anim Nutr (Berl) 2019;103(1):251–257. doi: 10.1111/jpn.13028. [DOI] [PubMed] [Google Scholar]
- 139.Winnica D, Que LG, Baffi C, Grasemann H, Fiedler K, Yang Z, et al. L-citrulline prevents asymmetric dimethylarginine-mediated reductions in nitric oxide and nitrosative stress in primary human airway epithelial cells. Clin Exp Allergy. 2017;47(2):190–199. doi: 10.1111/cea.12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Coles KE. Investigation into the antioxidant capacity of L-arginine and L-citrulline in relation to their vascular protective properties. Ann Harbor: Doctoral dissertation, Cardiff University; 2007.
- 141.Hayashi T, Juliet PA, Matsui-Hirai H, Miyazaki A, Fukatsu A, Funami J, et al. L-Citrulline and l-arginine supplementation retards the progression of high-cholesterol-diet-induced atherosclerosis in rabbits. Proc Natl Acad Sci U S A. 2005;102(38):13681–13686. doi: 10.1073/pnas.0506595102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tsuboi T, Maeda M, Hayashi T. Administration of L-arginine plus L-citrulline or L-citrulline alone successfully retarded endothelial senescence. PLoS ONE. 2018;13(2):e0192252. doi: 10.1371/journal.pone.0192252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sellmann C, Jin CJ, Engstler AJ, De Bandt J-P, Bergheim I. Oral citrulline supplementation protects female mice from the development of non-alcoholic fatty liver disease (NAFLD) Eur J Nutr. 2017;56(8):2519–2527. doi: 10.1007/s00394-016-1287-9. [DOI] [PubMed] [Google Scholar]
- 144.Lee YC, Su YT, Liu TY, Tsai CM, Chang CH, Yu HR. L-Arginine and L-Citrulline supplementation have different programming effect on regulatory T-cells function of infantile rats. Front Immunol. 2018;9:2911. doi: 10.3389/fimmu.2018.02911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Faure C, Raynaud-Simon A, Ferry A, Dauge V, Cynober L, Aussel C, et al. Leucine and citrulline modulate muscle function in malnourished aged rats. Amino Acids. 2012;42(4):1425–1433. doi: 10.1007/s00726-011-0841-2. [DOI] [PubMed] [Google Scholar]
- 146.Breuillard C, Goron A, Moinard C. Citrulline and skeletal muscle. Nutrition and Skeletal Muscle: Elsevier Inc; 2019. p. 329–34.
- 147.Giannesini B, Le Fur Y, Cozzone PJ, Verleye M, Le Guern ME, Bendahan D. Citrulline malate supplementation increases muscle efficiency in rat skeletal muscle. Eur J Pharmacol. 2011;667(1-3):100–104. doi: 10.1016/j.ejphar.2011.05.068. [DOI] [PubMed] [Google Scholar]
- 148.Kuci O, Verlaan D, Vicente C, Nubret E, Le Plenier S, De Bandt JP, et al. Citrulline and muscle protein homeostasis in three different models of hypercatabolism. Clin Nutr. 2020;39(3):917–927. doi: 10.1016/j.clnu.2019.03.036. [DOI] [PubMed] [Google Scholar]
- 149.Osowska S, Duchemann T, Walrand S, Paillard A, Boirie Y, Cynober L, et al. Citrulline modulates muscle protein metabolism in old malnourished rats. Am J Physiol Endocrinol Metab. 2006;291(3):E582–E586. doi: 10.1152/ajpendo.00398.2005. [DOI] [PubMed] [Google Scholar]
- 150.Ventura G, Noirez P, Breuillé D, Godin JP, Pinaud S, Cleroux M, et al. Effect of citrulline on muscle functions during moderate dietary restriction in healthy adult rats. Amino Acids. 2013;45(5):1123–1131. doi: 10.1007/s00726-013-1564-3. [DOI] [PubMed] [Google Scholar]
- 151.Ham DJ, Gleeson BG, Chee A, Baum DM, Caldow MK, Lynch GS, et al. L-Citrulline protects skeletal muscle cells from cachectic stimuli through an inos-dependent mechanism. PLoS ONE. 2015;10(10):e0141572. doi: 10.1371/journal.pone.0141572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Villareal MO, Matsukawa T, Isoda H. L-Citrulline supplementation-increased skeletal muscle PGC-1alpha expression is associated with exercise performance and increased skeletal muscle weight. Mol Nutr Food Res. 2018;62(14):e1701043. 10.1002/mnfr.201701043. [DOI] [PMC free article] [PubMed]
- 153.Cynober L, de Bandt JP, Moinard C. Leucine and Citrulline: Two major regulators of protein turnover. World Rev Nutr Diet. 2013;105:97–105. [DOI] [PubMed]
- 154.Park JH, Kim IH. The effects of betaine supplementation in diets containing different levels of crude protein and methionine on the growth performance, blood components, total tract nutrient digestibility, excreta noxious gas emission, and meat quality of the broiler chickens. Poult Sci. 2019;98(12):6808–6815. doi: 10.3382/ps/pez412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kidd MT, Ferket PR, Garlich JD. Nutritional and osmoregulatory functions of betaine. World's Poult Sci J. 1997;53(2):125–139. doi: 10.1079/WPS19970013. [DOI] [Google Scholar]
- 156.Ahmed M, Ismail Z, Abdel-Wareth A. Application of betaine as feed additives in poultry nutrition – a review. J Exp Appl Anim Sci. 2018;2:266–272. doi: 10.20454/jeaas.2018.1428. [DOI] [Google Scholar]
- 157.Ratriyanto A, Mosenthin R. Osmoregulatory function of betaine in alleviating heat stress in poultry. J Anim Physiol Anim Nutr (Berl) 2018;102(6):1634–1650. doi: 10.1111/jpn.12990. [DOI] [PubMed] [Google Scholar]
- 158.Dilger RN, Garrow TA, Baker DH. Betaine can partially spare choline in chicks but only when added to diets containing a minimal level of choline. J Nutr. 2007;137(10):2224–2228. doi: 10.1093/jn/137.10.2224. [DOI] [PubMed] [Google Scholar]
- 159.Metzler-Zebeli BU, Eklund M, Mosenthin R. Impact of osmoregulatory and methyl donor functions of betaine on intestinal health and performance in poultry. World's Poult Sci J. 2009;65(3):419–442. doi: 10.1017/S0043933909000300. [DOI] [Google Scholar]
- 160.Ratriyanto A, Indreswari R, Nuhriawangsa AMP. Effects of dietary protein level and betaine supplementation on nutrient digestibility and performance of japanese quails. Revista Brasileira de Ciência Avícola. 2017;19:445–454. doi: 10.1590/1806-9061-2016-0442. [DOI] [Google Scholar]
- 161.Wang H, Li S, Xu S, Feng J. Betaine improves growth performance by increasing digestive enzymes activities, and enhancing intestinal structure of weaned piglets. Anim Feed Sci Technol. 2020;267:114545. 10.1016/j.anifeedsci.2020.114545.
- 162.Zhang M, Zhang H, Li H, Lai F, Li X, Tang Y, et al. Antioxidant mechanism of betaine without free radical scavenging ability. J Agric Food Chem. 2016;64(42):7921–7930. doi: 10.1021/acs.jafc.6b03592. [DOI] [PubMed] [Google Scholar]
- 163.Downing JA, Kerr MJ, Hopkins DL. The effects of pre-transport supplementation with electrolytes and betaine on performance, carcass yield and meat quality of broilers in summer and winter. Livestock Sci. 2017;205:16–23. doi: 10.1016/j.livsci.2017.09.006. [DOI] [Google Scholar]
- 164.Cabezon FA, Stewart KR, Schinckel AP, Barnes W, Boyd RD, Wilcock P, et al. Effect of natural betaine on estimates of semen quality in mature AI boars during summer heat stress. Anim Reprod Sci. 2016;170:25–37. doi: 10.1016/j.anireprosci.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 165.Hassan RA, Ebeid TA, Abd El-Lateif AI, Ismail NB. Effect of dietary betaine supplementation on growth, carcass and immunity of New Zealand White rabbits under high ambient temperature. Livest Sci. 2011;135(2-3):103–109. doi: 10.1016/j.livsci.2010.06.132. [DOI] [Google Scholar]
- 166.Klasing KC, Adler KL, Remus JC, Calvert CC. Dietary betaine increases intraepithelial lymphocytes in the duodenum of coccidia-infected chicks and increases functional properties of phagocytes. J Nutr. 2002;132(8):2274–2282. doi: 10.1093/jn/132.8.2274. [DOI] [PubMed] [Google Scholar]
- 167.Lu J-F, Luo S, Jin T-C, Wang L-C, Yang G-J, Lu X-J, et al. Betaine protects ayu (Plecoglossus altivelis) against Vibrio anguillarum infection in salinity by regulating the immunomodulatory activity of monocytes/macrophages. Aquaculture. 2021;536:736482. 10.1016/j.aquaculture.2021.736482.
- 168.Sayed MA, Downing J. The effects of water replacement by oral rehydration fluids with or without betaine supplementation on performance, acid-base balance, and water retention of heat-stressed broiler chickens. Poult Sci. 2011;90(1):157–167. doi: 10.3382/ps.2009-00594. [DOI] [PubMed] [Google Scholar]
- 169.Yang J, Cai N, Zhai H, Zhang J, Zhu Y, Zhang L. Natural zwitterionic betaine enables cells to survive ultrarapid cryopreservation. Sci Rep. 2016;6(1):37458. doi: 10.1038/srep37458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cronjé PB. Heat stress in livestock—The role of the gut in its aetiology and a potential role for betaine in its alleviation. Conf Recent Adv Anim Nutr Australia. 2005:15:107–22.
- 171.Park BS, Park SO. Effects of feeding time with betaine diet on growth performance, blood markers, and short chain fatty acids in meat ducks exposed to heat stress. Livest Sci. 2017;199:31–36. doi: 10.1016/j.livsci.2017.03.003. [DOI] [Google Scholar]
- 172.Ratriyanto A, Prastowo S. Floor space and betaine supplementation alter the nutrient digestibility and performance of Japanese quail in a tropical environment. J Therm Biol. 2019;83:80–86. doi: 10.1016/j.jtherbio.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 173.Lakhani P, Kumar P, Alhussien MN, Lakhani N, Grewal S, Vats A. Effect of betaine supplementation on growth performance, nutrient intake and expression of IGF-1 in Karan Fries heifers during thermal stress. Theriogenology. 2020;142:433–440. doi: 10.1016/j.theriogenology.2019.10.021. [DOI] [PubMed] [Google Scholar]
- 174.Wang YZ, Xu ZR, Feng J. The effect of betaine and dl-methionine on growth performance and carcass characteristics in meat ducks. Anim Feed Sci Technol. 2004;116(1):151–159. doi: 10.1016/j.anifeedsci.2004.05.003. [DOI] [Google Scholar]
- 175.Ganesan B, Rajesh R, Anandan R, Dhandapani N. Biochemical studies on the protective effect of betaine on mitochondrial function in experimentally induced myocardial infarction in rats. J Health Sci. 2007;53:671–681. doi: 10.1248/jhs.53.671. [DOI] [Google Scholar]
- 176.Khodayar MJ, Kalantari H, Khorsandi L, Rashno M, Zeidooni L. Betaine protects mice against acetaminophen hepatotoxicity possibly via mitochondrial complex II and glutathione availability. Biomed Pharmacother. 2018;103:1436–1445. doi: 10.1016/j.biopha.2018.04.154. [DOI] [PubMed] [Google Scholar]
- 177.Wen C, Chen YP, Leng ZX, Ding LR, Wang T, Zhou YM. Dietary betaine improves meat quality and oxidative status of broilers under heat stress. J Sci Food Agric. 2019;99(2):620–623. doi: 10.1002/jsfa.9223. [DOI] [PubMed] [Google Scholar]
- 178.Dangi SS, Dangi SK, Chouhan VS, Verma MR, Kumar P, Singh G, et al. Modulatory effect of betaine on expression dynamics of HSPs during heat stress acclimation in goat (Capra hircus) Gene. 2016;575(2 Pt 2):543–550. doi: 10.1016/j.gene.2015.09.031. [DOI] [PubMed] [Google Scholar]
- 179.Figueroa-Soto CG, Valenzuela-Soto EM. Glycine betaine rather than acting only as an osmolyte also plays a role as regulator in cellular metabolism. Biochimie. 2018;147:89–97. doi: 10.1016/j.biochi.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 180.Diamant S, Eliahu N, Rosenthal D, Goloubinoff P. Chemical chaperones regulate molecular chaperones in vitro and in cells under combined salt and heat stresses. J Biol Chem. 2001;276(43):39586–39591. doi: 10.1074/jbc. [DOI] [PubMed] [Google Scholar]
- 181.Egbuniwe IC, Uchendu CN, Obidike IR. Effects of betaine and ascorbic acid supplementation on serum gonadotropin, testicular histological analysis and sperm quality in male Japanese quails during the dry season. Theriogenology. 2020;158:391–405. doi: 10.1016/j.theriogenology.2020.09.029. [DOI] [PubMed] [Google Scholar]
- 182.Shadmehr S, Fatemi Tabatabaei SR, Hosseinifar S, Tabandeh MR, Amiri A. Attenuation of heat stress-induced spermatogenesis complications by betaine in mice. Theriogenology. 2018;106:117–126. doi: 10.1016/j.theriogenology.2017.10.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable