Abstract
Background
The impact of the COVID-19 pandemic on surgical care delivery in low- and middle-income countries (LMIC) has been challenging to assess due to a lack of data. This study examines the impact of COVID-19 on pediatric surgical volumes at four LMIC hospitals.
Methods
Retrospective and prospective pediatric surgical data collected at hospitals in Burkina Faso, Ecuador, Nigeria, and Zambia were reviewed from January 2019 to April 2021. Changes in surgical volume were assessed using interrupted time series analysis.
Results
6078 total operations were assessed. Before the pandemic, overall surgical volume increased by 21 cases/month (95% CI 14 to 28, p < 0.001). From March to April 2020, the total surgical volume dropped by 32%, or 110 cases (95% CI − 196 to − 24, p = 0.014). Patients during the pandemic were younger (2.7 vs. 3.3 years, p < 0.001) and healthier (ASA I 69% vs. 66%, p = 0.003). Additionally, they experienced lower rates of post-operative sepsis (0.3% vs 1.5%, p < 0.001), surgical site infections (1.3% vs 5.8%, p < 0.001), and mortality (1.6% vs 3.1%, p < 0.001).
Conclusions
During the COVID-19 pandemic, children’s surgery in LMIC saw a sharp decline in total surgical volume by a third in the month following March 2020, followed by a slow recovery afterward. Patients were healthier with better post-operative outcomes during the pandemic, implying a widening disparity gap in surgical access and exacerbating challenges in addressing the large unmet burden of pediatric surgical disease in LMICs with a need for immediate mitigation strategies.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00268-022-06503-2.
Introduction
On March 11th, 2020, the COVID-19 outbreak was declared a global pandemic by the World Health Organization [1]. In the following months, there was an unprecedented impact on the provision of surgical care worldwide [2, 3]. As healthcare systems triaged and scrambled for resources during the surges, many surgical activities across different subspecialties were deemed non-essential and put on hold or canceled [4–11]. The additional obstacles to surgical care access have resulted in increased backlogs with substantial economic impact [12–14]. These problems are likely further exacerbated in low- and middle-income countries (LMIC), where the proportion of unmet surgical needs is highest [15, 16].
With children making up about half of the population in LMIC, it has been estimated that at least 6000 children likely died every month during the pandemic due to the collateral effects on health systems [17, 18]. The impact on surgery due to COVID-19 is underreported and poorly characterized. There have been limited published data from LMIC that describe the growing disease burden due to the pandemic [19]. Further data are needed to help address the unmet surgical burden in these regions in order to inform advocacy and mitigation strategies.
Materials and methods
Data collection
Kids Operating Room (KidsOR) is a non-governmental organization dedicated to building pediatric operating rooms in LMIC hospitals [20]. As part of the effort, the charity has helped strengthen pediatric surgical data capacity to promote advocacy, quality improvement, and research. Based on a consensus-driven data collection protocol, retrospective and prospective data were collected at partner hospitals (Online Supplementary Table 1). All data were collected and stored on a secure, web-based platform Research Electronic Data Capture (REDCap) [21, 22]. All patients < 18 years old that had operations entered in the database from January 1, 2019, to April 30, 2021, were included. Participants provided informed consent for the collection of data, and the study has been approved by the Institution Review Boards of the University of California, San Francisco (#19-29663).
This multicenter study involved the following partner hospitals: Centre Hospitalier Universitaire Pédiatrique Charles De Gaulle in Ouagadougou, Burkina Faso (“Burkina Faso” hereafter), Hospital de los Valles in Quito, Ecuador (“Ecuador” hereafter), National Hospital in Abuja, Nigeria (“Nigeria” hereafter), and University Teaching Hospital in Lusaka, Zambia (“Zambia” hereafter). Burkina Faso, Nigeria, and Zambia are public tertiary referral hospitals, while Ecuador is private. Out of eight total sites in the database, these four sites were selected based on the criteria of having at least 12 months of data entered in the database before and after March 2020, consistent data collection without gaps during the study period, and signed Memorandum of Understanding (MOU) with KidsOR.
Data analysis
An interrupted time-series analysis (ITSA) was performed using the two ordinary least-squares regression-based approaches Newey–West standard errors to assess monthly changes in surgical volume, in total and by elective and emergency cases [23]. ITSA was chosen due to its statistical utility in assessing the impact of an event on a population level with a clearly defined time periods that allow for comparing pre- and post-time series data [24]. Pre-COVID-19 period (“pre-COVID” hereafter) was defined as data entered in the 14-month period from January 1, 2019, to February 28, 2020, and post-COVID-19 (“post-COVID” hereafter) in the 14-month period from March 1, 2020, to April 30, 2021. Through ITSA, we were able to measure: (a) the baseline estimate of surgical volume at the start of the study period, (b) monthly trend during the pre-COVID period, (c) immediate change associated with the COVID-19 pandemic from March to April 2020, (d) monthly trend during the post-COVID period, and (e) differences in slope for pre- and post-COVID trends (Online Supplementary Table 2). To ensure that the ITSA models accounted for autocorrelation, we used the Cumby-Huizinga test to identify statistically significant lags, and adjustments were made accordingly.
Demographic and clinical characteristics were compared pre- and post-COVID using bivariate statistical analyses such as Wilcoxon rank-sum for medians and Pearson’s Chi-square test for categorical variables. Conclusions on statistical significance were made based on p-values with a preset level of significance p < 0.05. All statistical analyses were done on STATA 17 (StataCorp, College Station, TX, USA).
Results
Demographic & clinical characteristics
Across all participating sites, 6078 cases were recorded from January 2019 to April 2021, of which 2758 (45%) were pre-COVID and 3320 (55%) were post-COVID (Table 1). The median age of children who underwent surgery was 3.3 years pre-COVID and decreased to 2.7 years post-COVID (p < 0.001). Specifically, more neonates (9.4% vs 14.5%, p < 0.001) and fewer adolescents (12.7% vs 9.5%, p < 0.001) underwent surgery during the post-COVID period. In terms of socioeconomic data, 9.2% (561/6078) of the patients’ families reported their annual income and the median differed pre- and post-COVID ($750 vs. $1350 in United States dollars, p = 0.072), though this did not reach statistical significance.
Table 1.
Demographic characteristics pre- vs post-COVID
| Pre N = 2758 |
Post N = 3320 |
Total N = 6078 |
p-value | |
|---|---|---|---|---|
| Sex | 0.39 | |||
| Female | 925 (33.8%) | 1079 (32.7%) | 2004 (33.2%) | |
| Male | 1815 (66.2%) | 2220 (67.3%) | 4035 (66.8%) | |
| Age (year) | 3.3 (0.8–8.6) | 2.7 (0.4–7.4) | 3.0 (0.6–7.9) | < 0.001* |
| Age group | ||||
| Neonate | 254 (9.4%) | 326 (14.5%) | 580 (11.7%) | < 0.001* |
| Infant | 824 (30.4%) | 650 (28.9%) | 1474 (29.7%) | 0.223 |
| Young child | 709 (26.2%) | 614 (27.3%) | 1323 (26.7%) | 0.4 |
| School age | 575 (21.2%) | 448 (19.9%) | 1023 (20.6%) | 0.24 |
| Adolescent | 345 (12.7%) | 215 (9.5%) | 560 (11.3%) | < 0.001* |
| Sites | ||||
| Burkina Faso | 1282 (46.5%) | 1822 (54.9%) | 3104 (51.1%) | < 0.001* |
| Ecuador | 296 (10.7%) | 220 (6.6%) | 516 (8.5%) | < 0.001* |
| Nigeria | 354 (12.8%) | 430 (13.0%) | 784 (12.9%) | 0.893 |
| Zambia | 826 (29.9%) | 848 (25.5%) | 1674 (27.5%) | < 0.001* |
| Annual income ($) | 750 (75–5592) | 1350 (270–5592) | 750 (75–5592) | 0.072 |
Data are presented as n (%) for categorical measures and median (interquartile range) for continuous measures
*Statistically significant at alpha of p < 0.05
Regarding clinical characteristics (Table 2), a statistically significant decrease in elective surgery from 55 to 52% and increase in emergency surgery from 45 to 48% occurred from pre- to post-COVID periods (p = 0.016). Additionally, postoperative outcomes also improved from pre- to post-COVID, with lower rates of sepsis (1.5% vs 0.3%, p < 0.001), surgical site infections (5.8% vs 1.3%, p < 0.001), and mortality (3.1% vs 1.6%, p < 0.001). The average length of stay decreased from 4.1 to 3.5 days (p = 0.009), and the rate of reoperations dropped from 19.7 to 16.0% (p < 0.001). Safety checklist usage also decreased from 44.9 to 41.0% (p = 0.003). Patients classified as American Society of Anesthesiologists (ASA) status I increased from pre- to post-COVID (65.6% vs. 69.3%, p = 0.003) while those that were ASA III decreased (7.8% vs. 4.5%, p < 0.001). In terms of diagnoses, there were statistically significant increases in those categorized as general surgery (32% vs 34%, p = 0.048) and “other” (10% vs 17%, p < 0.001), and decreases in infection (19% vs 16%, p < 0.001) and trauma (16% vs 12%, p < 0.001).
Table 2.
Demographic characteristics pre- vs post-COVID
| Pre N = 2758 |
Post N = 3320 |
Total N = 6078 |
p-value | |
|---|---|---|---|---|
| Type of surgery | 0.016* | |||
| Emergency | 1238 (45.2%) | 1599 (48.3%) | 2837 (46.9%) | |
| Elective | 1501 (54.8%) | 1711 (51.7%) | 3212 (53.1%) | |
| Disease category | ||||
| Congenital | 498 (18.1%) | 543 (16.4%) | 1041 (17.1%) | 0.081 |
| General | 869 (31.5%) | 1125 (33.9%) | 1994 (32.9%) | 0.048* |
| Infection | 514 (18.7%) | 515 (15.5%) | 1029 (17.0%) | 0.001* |
| Oncology | 147 (5.3%) | 177 (5.3%) | 324 (5.3%) | 0.995 |
| Trauma | 447 (16.2%) | 390 (11.8%) | 837 (13.8%) | < 0.001* |
| Other | 280 (10.2%) | 565 (17.0%) | 845 (13.9%) | < 0.001* |
| ASA class | ||||
| I | 1723 (65.6%) | 2173 (69.3%) | 3896 (67.6%) | 0.003* |
| II | 671 (25.6%) | 796 (25.4%) | 1467 (25.5%) | 0.882 |
| III | 206 (7.8%) | 140 (4.5%) | 346 (6.0%) | < 0.001* |
| IV | 25 (1.0%) | 26 (0.8%) | 51 (0.9%) | 0.62 |
| Safety checklist | 1232 (44.9%) | 1354 (41.0%) | 2586 (42.8%) | 0.003* |
| Pre-op sepsis | 312 (11.4%) | 206 (6.2%) | 518 (8.6%) | < 0.001* |
| Post-op sepsis | 42 (1.5%) | 10 (0.3%) | 52 (0.9%) | < 0.001* |
| Surgical site infection | 160 (5.8%) | 43 (1.3%) | 203 (3.4%) | < 0.001* |
| Reoperation | 539 (19.7%) | 526 (16.0%) | 1065 (17.7%) | < 0.001* |
| LOS (days) | 4.1 (3.7–4.5) | 3.5 (3.2–3.7) | 3.7 (3.5–4.0) | 0.009* |
| Mortality | 75 (3.1%) | 50 (1.6%) | 125 (2.3%) | < 0.001* |
Data are presented as n (%) for categorical measures and median (interquartile range) for continuous measures
*Statistically significant at alpha of p < 0.05
Surgical volume
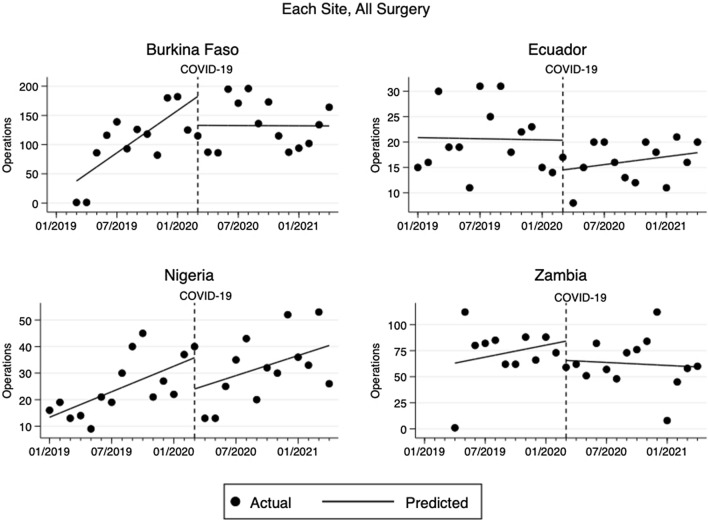
Burkina Faso accounted for nearly half of the total surgical volume (51%), followed by Zambia (30%), Nigeria (13%), and Ecuador (9%). From January 2019 to February 2020, the surgical volume across the sites altogether increased by 21 cases/month (95% CI 13.5 to 28.2, p < 0.001) from a baseline of 55 cases/month (Fig. 1). With the COVID-19 pandemic, the surgical volume dropped by 110 cases (95% CI − 196 to − 24, p = 0.014), which was a 32% decrease, from March 2020 to April 2020. In the subsequent post-COVID period, the surgical volume recovered at a rate of 1 additional case/month (95% CI − 6.3 to 8.2, p = 0.784). This reduction in the rate of change from pre-COVID trend by 20 cases/month (95% CI − 30 to − 10, p < 0.001) was statistically significant. Similar patterns in total surgical volume emerged individually at each site (Fig. 2). Of note, the surgical volume returned to pre-COVID level in Nigeria only.
Fig. 1.
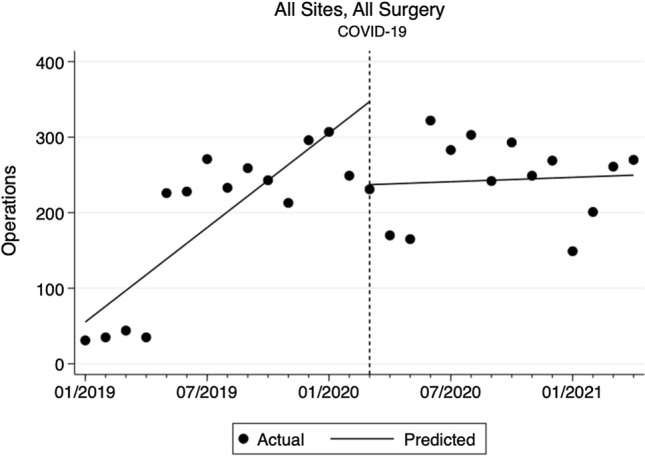
Changes in monthly total surgical volume at all sites pre- and post-COVID
Fig. 2.
Changes in monthly total surgical volume at each site pre- and post-COVID
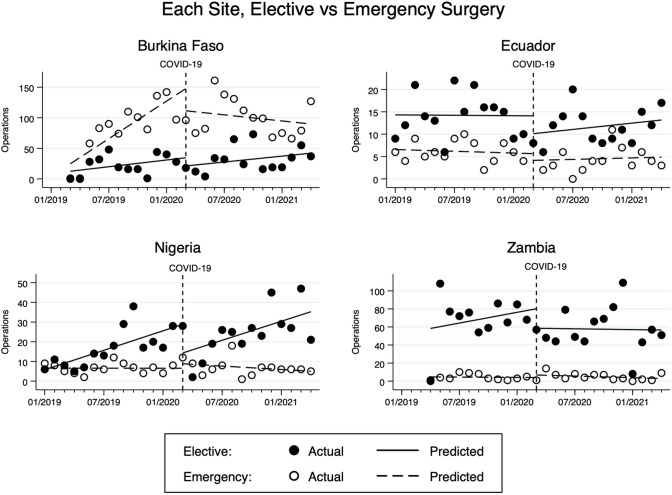
The change in the type of surgical cases also differed (Fig. 3). Both elective and emergency surgical volume increased by 10 cases/month (95% CI 6 to 14, p < 0.001) and 11 cases/month (95% CI 8 to 14, p < 0.001), respectively, during pre-COVID. In the immediate month following COVID-19 from March to April 2020, the volume dropped by 73 cases (95% CI − 117 to − 30, p = 0.002) for elective surgery and 36 cases (95% CI − 71 to − 1, p = 0.043) for emergency surgery. During the post-COVID period, the elective surgical volume recovered at a rate of 3 cases/month (95% CI − 2 to 8, p = 0.175), while emergency surgical volume decreased by 2 cases/month (95% CI − 6 to 2, p = 0.22). Although these post-COVID trends were not statistically significant, the reductions in the rate of change from the pre-COVID trend by 7 cases/month (95% CI − 13 to − 1, p = 0.038) for elective surgery and by 13 cases/month (95% CI − 30 to − 10, p < 0.001) for emergency surgery were statistically significant. On further sub-analysis at each site (Fig. 4), similar trends and changes were again found for elective and emergency surgeries, but lacked statistical significance.
Fig. 3.
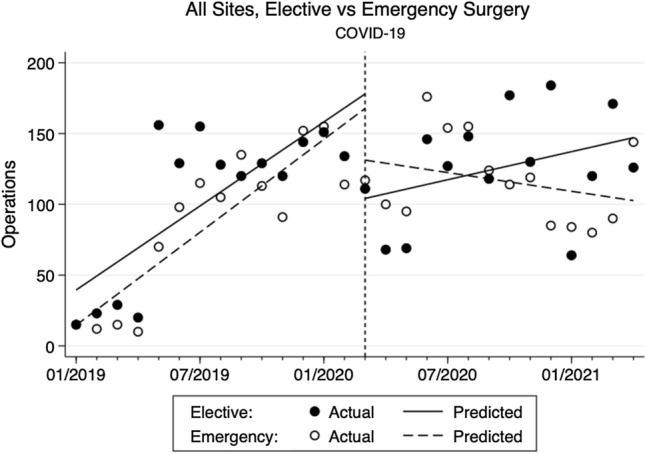
Changes in monthly elective and emergency surgical volume at all sites pre- and post-COVID
Fig. 4.
Changes in monthly elective and emergency surgical volume at each site pre- and post-COVID
Discussion
This study investigated the effects of the COVID-19 pandemic on the pediatric surgical volume in four hospitals in Burkina Faso, Nigeria, Ecuador, and Zambia. There were several statistically significant changes in the demographic and clinical characteristics of the patients from pre- to post-COVID. For instance, the median age of patients decreased by 0.6 years, and further sub-analysis by age groups revealed more neonates and fewer adolescents presenting during the pandemic. This was likely due to neonates already being present in the hospital following their births and neonatal care being prioritized in tertiary referral hospitals in LMIC as other available healthcare facilities may not have the capacity to provide neonatal surgical care. For adolescents, the decrease in the presentation may reflect a reduction in road traffic injuries, the major contributor of surgical morbidity and mortality in this age group in LMIC, due to lockdown policies seen worldwide [25–27]. Additionally, median family annual income was $600 higher during the pandemic, which suggests that poorer patients were less likely to seek care during the pandemic. This difference approached statistical significance and may have been underpowered as only a small proportion of patient families volunteered socioeconomic information.
Clinically, patients who presented for surgery during the pandemic were healthier with lower ASA class and had better outcomes with > 50% reductions in post-operative sepsis, surgical site infection, mortality, and smaller reductions in the rate of reoperation and length of stay. These improvements likely reflect a change in the patient population where sicker patients may not be getting the needed care due to increased barriers to access healthcare during the pandemic [28, 29]. Our findings add to the growing evidence for hidden morbidity and excess mortality during the pandemic from patients delaying care and potentially dying at home, especially in rural and underserved areas [30, 31].
As for surgical volume, there were sharp declines in the immediate month following the pandemic's start from March to April 2020. This finding contrasts with the steady rise in the total number of operations at all sites during the pre-COVID period, which could be due to KidsOR and its efforts to increase surgical capacities at these sites by providing dedicated surgical rooms or specialized equipment. By case type, elective surgeries fell sharply in the months following the pandemic, consistent with cancellations and postponements seen worldwide. Importantly, these findings address the limitations in international studies that found the highest rates of elective surgery cancellations in HIC likely due to limited data and participation from LMIC [8]. On the other hand, although emergency surgical volume similarly decreased, it has continued to decrease during the post-COVID period. This finding may be consistent with those in other studies where hospitals worldwide that experienced decreases in emergent surgeries during the pandemic [32–37]. Again, we believe this may be due to sicker patients avoiding medical care or facing insurmountable challenges in accessing care during the pandemic, but more research is needed.
Additionally, the overall surgical volume has been slow to recover in the subsequent months during the pandemic. For all sites, the total number of surgeries has not rebounded back to pre-COVID volume more than a year after the start of the pandemic. Although this study did not directly measure delayed or canceled operations, this finding likely exacerbates the pre-existing pediatric surgical backlogs in LMIC [38–40]. Furthermore, the slow recovery rate in surgical volume may be generalizable to other locations in Sub-Saharan Africa and other LMIC. Greater human and physical resources in HIC allow for healthcare systems in HIC to manage COVID-19 surges. Meanwhile, healthcare systems have been overwhelmed in LMIC and worsened by inequities in resources such as personal protective equipment and vaccines [41–45]. The findings of our study are particularly concerning as many LMIC have reported surges of COVID cases in recent months [46]. Lastly, the lack of easily accessible data in LMIC has made it difficult to assess the true impact of COVID-19 and track trends. These factors all combine to not only prolong the reduction in surgical capacity in LMIC but also make it difficult to coordinate and plan recovery strategies.
Given these findings, innovative strategies are needed to mitigate the impact. On a community level, outreach programs to increase access to healthcare providers or transportation policies designed for patients with acute medical needs could help address the hidden morbidity. Although widely used in HIC during the pandemic, telehealth has been underutilized in LMIC and should be further utilized to increase healthcare access [47–49].
On a broader level, strategies such as task sharing that had been adopted in LMIC to help bridge the gaps in surgical care could be expanded [50, 51]. For example, in pediatric surgery, task sharing may be a feasible alternative to help meet surgical demand for certain simple procedures such as hernia repairs, which medical officers can perform. The impact on the surgical and anesthesia workforce also needs to be considered, especially given that a substantial amount of the surgical workforce has been infected or died in LMIC [52, 53]. Provider shortages will likely continue due to high-risk work environments in these areas [54]. In the short-term, solutions such as fast-tracking medical and nursing training as well as virtual didactics have been suggested [55, 56].
Global partnerships can also play a substantial role in recovery from the pandemic. For instance, KidsOR has dedicated support for infrastructure, workforce strengthening, and local data platforms in partnership with multiple stakeholders, including hospitals, universities, and regional professional organizations. Similarly, other organizations such as Smile Train and Lifebox have continued to provide funding, resources, and training to ensure the continued provision of safe surgical care in LMIC [57, 58]. As the pandemic continues, long-term and systemic strategies on a global scale will need to be employed.
Our study has several limitations. First, the retrospective nature of the analyses did not allow us to control for potential confounding factors, and therefore, associations that other local and regional events may have influenced. For example, the initial lockdown period was used as the sole cut-off between pre- and post-COVID periods without factoring in subsequent partial lockdowns and fluctuations in pandemic intensity. Second, the participating study sites have differences such as the type of hospital (e.g., public vs. private), patient population (e.g., urban vs. rural), and overall surgical volume (e.g., high in Burkina Faso vs. low in Ecuador). Given these differences, direct comparisons by type of surgery and between the sites could not be made. Third, the ITSA model lacked statistical significance at the individual site level and by case type due to fewer cases on stratification. Fourth, there was significant variation in data availability on socioeconomic variables due to the sensitive nature of these questions, such that only 9% of the participants responded with their annual incomes. Lastly, the inclusion criteria for the sites required consistent data collection from 2019 to 2021, and as such, four of the partner hospitals with missing data were excluded. These sites may have been affected by the pandemic more and may not have had the capacity to enter data in a timely fashion.
Conclusion
The decreases in surgical volume without evidence of sustained recovery across multiple LMIC hospitals demonstrates the persistent effects of the pandemic and provides evidence that the collateral damage of the pandemic on health services has extended to children’s surgery. Healthier and wealthier patients undergoing surgery with improved outcomes post-pandemic also raise concerns about hidden morbidity and mortality at the population level, especially among those with less resources. Overall, these findings call for a renewed commitment to equity, global partnerships, and innovative resource mobilization to mitigate the pandemic's impact on surgical services for children now and for future public health crises.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank our data assistants Hippolyte Tiendrébéogo and Joseph Bonkoungou at the Charles de Gaulle Paediatric Teaching Hospital in Burkina Faso, Michelle Andrade from Hospital de los Valles in Ecuador, Nancy Ukwu from National Hospital Abuja in Nigeria, and Kalota Seith, MBBS, from University Teaching Hospital of Lusaka in Zambia. Statistical support was expertly provided by Amy Shui from the Biostatistics Core, UCSF Department of Surgery as well as Zachary Matthay, MD, a resident physician at UCSF Department of Surgery.
Paul Park
is a fourth-year medical student at the University of California, San Francisco (UCSF). He became interested in addressing health inequities worldwide as an undergraduate studying public health at UC Berkeley. Prior to medical school, he completed a Master of Science in global health at UCSF. He became interested in surgery after his third-year clerkship at San Francisco General Hospital and pursued a research gap year on global pediatric surgery in working with Dr. Doruk Ozgediz at the Center for Health Equity in Surgery and Anesthesia at UCSF and the international charity organization Kids Operating Room (KidsOR).
Author contributions
PP, RL, GK, AY, EB, and DO designed the research. PP conducted the data analysis, produced the figures, tables, and supplementary material, and drafted the initial manuscript with input from all authors. BB, BK, TT, EA, MO, MU, JD, DC, and DO are all principal investigators at their respective institutions and oversaw the data collection process. All authors contributed to and approved the final version of the manuscript. PP is the first author. DO is the last author.
Funding
No funding was provided for this study.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Participants provided informed consent for the collection of data, and the study has been approved by the Institution Review Boards of the University of California, San Francisco (#19-29663).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Bio Medica Atenei Parm. 2020;91:157. doi: 10.23750/ABM.V91I1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kibbe MR. Surgery and COVID-19. JAMA—J Am Med Assoc. 2020;324:1151–1152. doi: 10.1001/jama.2020.15191. [DOI] [PubMed] [Google Scholar]
- 3.COVIDSurg Collaborative Global guidance for surgical care during the COVID-19 pandemic. Br J Surg. 2020;107:1097. doi: 10.1002/BJS.11646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brindle ME, Gawande A. Managing COVID-19 in surgical systems. Ann Surg. 2020 doi: 10.1097/SLA.0000000000003923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saraswathula A, Gourin CG, Stewart CM. National trends in US otolaryngology surgical volume during the early COVID-19 pandemic. JAMA Otolaryngol Neck Surg. 2021;147:397–399. doi: 10.1001/JAMAOTO.2020.5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pickens RC, Kao AM, Williams MA, et al. Pediatric surgical reentry strategy following the COVID-19 pandemic: a tiered and balanced approach. Am Surg. 2021 doi: 10.1177/00031348211011125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rheumatology TL. Too long to wait: the impact of COVID-19 on elective surgery. Lancet Rheumatol. 2021;3:e83. doi: 10.1016/S2665-9913(21)00001-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nepogodiev D, Omar OM, Glasbey JC, et al. Elective surgery cancellations due to the COVID-19 pandemic: global predictive modelling to inform surgical recovery plans. Br J Surg. 2020;107:1440–1449. doi: 10.1002/BJS.11746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okuno T, Takada D, Shin J, et al. Surgical volume reduction and the announcement of triage during the 1st wave of the COVID-19 pandemic in Japan: a cohort study using an interrupted time series analysis. Surg Today. 2021;1:1–8. doi: 10.1007/S00595-021-02286-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen TC, Thourani VH, Nissen AP, et al. The effect of COVID-19 on adult cardiac surgery in the United States in 717,103 patients. Ann Thorac Surg. 2021 doi: 10.1016/J.ATHORACSUR.2021.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prasad NK, Englum BR, Turner DJ, et al. A nation-wide review of elective surgery and COVID-surge capacity. J Surg Res. 2021;267:211. doi: 10.1016/J.JSS.2021.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Best MJ, McFarland EG, Anderson GF, Srikumaran U. The likely economic impact of fewer elective surgical procedures on US hospitals during the COVID-19 pandemic. Surgery. 2020;168:962. doi: 10.1016/J.SURG.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Lancet Rheumatology Too long to wait: the impact of COVID-19 on elective surgery. Lancet Rheumatol. 2021;3:e83. doi: 10.1016/S2665-9913(21)00001-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carr A, Smith JA, Camaradou J, Prieto-Alhambra D. Growing backlog of planned surgery due to covid-19. BMJ. 2021 doi: 10.1136/BMJ.N339. [DOI] [PubMed] [Google Scholar]
- 15.Shrime MG, Bickler SW, Alkire BC, Mock C. Global burden of surgical disease: an estimation from the provider perspective. Lancet Glob Heal. 2015;3:S8–S9. doi: 10.1016/S2214-109X(14)70384-5. [DOI] [PubMed] [Google Scholar]
- 16.Alkire BC, Raykar NP, Shrime MG, et al. Global access to surgical care: a modelling study. Lancet Glob Heal. 2015;3:e316–e323. doi: 10.1016/S2214-109X(15)70115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mullapudi B, Grabski D, Ameh E, et al. Estimates of number of children and adolescents without access to surgical care. Bull World Health Organ. 2019;97:254–258. doi: 10.2471/BLT.18.216028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberton T, Carter ED, Chou VB, et al. Early estimates of the indirect effects of the COVID-19 pandemic on maternal and child mortality in low-income and middle-income countries: a modelling study. Lancet Glob Heal. 2020;8:e901–e908. doi: 10.1016/S2214-109X(20)30229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Usuzaki T, Chiba S, Shimoyama M, et al. A disparity in the number of studies related to COVID-19 and SARS-CoV-2 between low- and middle-income countries and high-income countries. Int Health. 2021;13:379–381. doi: 10.1093/INTHEALTH/IHAA088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.What we do. KidsOR. https://www.kidsor.org/what-we-do/. Accessed 13 Mar 2020
- 21.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)-a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linden A. Conducting interrupted time-series analysis for single- and multiple-group comparisons. Stata J. 2015;15:480–500. doi: 10.1177/1536867X1501500208. [DOI] [Google Scholar]
- 24.Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. 2017;46:348–355. doi: 10.1093/IJE/DYW098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vandoros S. COVID-19, lockdowns and motor vehicle collisions: empirical evidence from Greece. Inj Prev. 2021 doi: 10.1136/INJURYPREV-2020-044139. [DOI] [PubMed] [Google Scholar]
- 26.Huang W, Lin Q, Xu F, Chen D. Effect of COVID-19 on epidemiological characteristics of road traffic injuries in Suzhou: a retrospective study. BMC Emerg Med. 2021;21(1):1–6. doi: 10.1186/S12873-021-00483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saladié Ò, Bustamante E, Gutiérrez A. COVID-19 lockdown and reduction of traffic accidents in Tarragona province. Spain Transp Res Interdiscip Perspect. 2020;8:100218. doi: 10.1016/J.TRIP.2020.100218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Núñez A, Sreeganga SD, Ramaprasad A. Access to Healthcare during COVID-19. Int J Environ Res Public Health. 2021;18:1–12. doi: 10.3390/IJERPH18062980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Assefa N, Sié A, Wang D, et al. Reported barriers to healthcare access and service disruptions caused by COVID-19 in Burkina Faso, Ethiopia, and Nigeria: a telephone survey. Am J Trop Med Hyg. 2021;105:323–330. doi: 10.4269/AJTMH.20-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stokes AC, Lundberg DJ, Bor J, et al. Association of health care factors with excess deaths not assigned to COVID-19 in the US. JAMA Netw Open. 2021;4:e2125287–e2125287. doi: 10.1001/JAMANETWORKOPEN.2021.25287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woolf SH, Chapman DA, Sabo RT, Zimmerman EB. Excess deaths from COVID-19 and other causes in the US, March 1, 2020, to January 2, 2021. JAMA. 2021;325:1786–1789. doi: 10.1001/JAMA.2021.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Connell RM, Khan MA, Amir M, et al. The impact of COVID-19 on emergency general surgery admissions and operative volumes: a single centre experience. Surgeon. 2020 doi: 10.1016/j.surge.2020.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De FO, D’Ascenzo F, Angelini F, et al. Reduced rate of hospital admissions for ACS during Covid-19 outbreak in northern Italy. N Engl J Med. 2020;383:88–89. doi: 10.1056/NEJMC2009166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pikoulis E, Koliakos N, Papaconstantinou D, et al. The effect of the COVID pandemic lockdown measures on surgical emergencies: experience and lessons learned from a Greek tertiary hospital. World J Emerg Surg. 2021 doi: 10.1186/S13017-021-00364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McLean RC, Young J, Musbahi A, et al. A single-centre observational cohort study to evaluate volume and severity of emergency general surgery admissions during the COVID-19 pandemic: is there a “lockdown” effect? Int J Surg. 2020;83:259–266. doi: 10.1016/J.IJSU.2020.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matthay ZA, Kornblith AE, Matthay EC, et al. The DISTANCE study: determining the impact of social distancing on trauma epidemiology during the COVID-19 epidemic—an interrupted time-series analysis. J Trauma Acute Care Surg. 2021;90:700. doi: 10.1097/TA.0000000000003044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greenberg AL, Schwartz H, Collins CR, et al. Emergency general surgery utilization and disparities during COVID-19: an interrupted time-series analysis. Trauma Surg Acute Care Open. 2021;6:e000679. doi: 10.1136/TSACO-2021-000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yousef Y, Lee A, Ayele F, Poenaru D. Delayed access to care and unmet burden of pediatric surgical disease in resource-constrained African countries. J Pediatr Surg. 2019;54:845–853. doi: 10.1016/j.jpedsurg.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 39.Smith ER, Concepcion TL, Shrime M, et al. Waiting too long: the contribution of delayed surgical access to pediatric disease burden in Somaliland. World J Surg. 2019;443(44):656–664. doi: 10.1007/S00268-019-05239-W. [DOI] [PubMed] [Google Scholar]
- 40.Poenaru D, Pemberton J, Cameron BH. The burden of waiting: DALYs accrued from delayed access to pediatric surgery in Kenya and Canada. J Pediatr Surg. 2015;50:765–770. doi: 10.1016/J.JPEDSURG.2015.02.033. [DOI] [PubMed] [Google Scholar]
- 41.Dondorp AM, Hayat M, Aryal D, et al. Respiratory support in COVID-19 patients, with a focus on resource-limited settings. Am J Trop Med Hyg. 2020;102:1191. doi: 10.4269/AJTMH.20-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hopman J, Allegranzi B, Mehtar S. Managing COVID-19 in low- and middle-income countries. JAMA. 2020;323:1549–1550. doi: 10.1001/JAMA.2020.4169. [DOI] [PubMed] [Google Scholar]
- 43.Adesunkanmi AO, Ubom AE, Olasehinde O, et al. Impact of COVID-19 on the cost of surgical and obstetric care: experience from a Nigerian teaching hospital and a review of the Nigerian situation. Pan Afr Med J. 2020;37:15. doi: 10.11604/PAMJ.SUPP.2020.37.15.25935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rouw A, Wexler A, Kates J, Michaud J. Global COVID-19 vaccine access: a snapshot of inequality. San Francisco: KFF; 2021. [Google Scholar]
- 45.Rouw A, Wexler A, Kates J, Michaud J. Tracking global COVID-19 vaccine equity. San Francisco: KFF; 2021. [Google Scholar]
- 46.Johns Hopkins Coronavirus Resource Center (2021) COVID-19 Map. https://coronavirus.jhu.edu/map.html. Accessed 3 Aug 2021
- 47.Doraiswamy S, Abraham A, Mamtani R, Cheema S. Use of telehealth during the COVID-19 pandemic: scoping review. J Med Internet Res. 2020 doi: 10.2196/24087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim T, Zuckerman JE. Realizing the potential of telemedicine in global health. J Glob Health. 2019 doi: 10.7189/JOGH.09.020307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoffer-Hawlik MA, Moran AE, Burka D, et al. Leveraging telemedicine for chronic disease management in low- and middle-income countries during Covid-19. Glob Heart. 2020;15:63. doi: 10.5334/GH.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robertson FC, Lippa L, Broekman MLD. Editorial. Task shifting and task sharing for neurosurgeons amidst the COVID-19 pandemic. J Neurosurg. 2020;133:5–7. doi: 10.3171/2020.4.JNS201056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ashengo T, Skeels A, Hurwitz EJH, et al. Bridging the human resource gap in surgical and anesthesia care in low-resource countries: a review of the task sharing literature. Hum Resour Heal. 2017;151(15):1–11. doi: 10.1186/S12960-017-0248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bergström S, McPake B, Pereira C, Dovlo D. Disease control priorities, third ed (volume 1) essential surgery. Washington, DC: The World Bank; 2015. Workforce innovations to expand the capacity for surgical services. [PubMed] [Google Scholar]
- 53.Erdem H, Lucey DR. Healthcare worker infections and deaths due to COVID-19: a survey from 37 nations and a call for WHO to post national data on their website. Int J Infect Dis. 2021;102:239. doi: 10.1016/J.IJID.2020.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McMahon DE, Peters GA, Ivers LC, Freeman EE. Global resource shortages during COVID-19: Bad news for low-income countries. PLoS Negl Trop Dis. 2020;14:e0008412. doi: 10.1371/JOURNAL.PNTD.0008412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eyawo O, Viens AM, Ugoji UC. Lockdowns and low- and middle-income countries: building a feasible, effective, and ethical COVID-19 response strategy. Glob Heal. 2021;17(1):1–5. doi: 10.1186/S12992-021-00662-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mazingi D, Ihediwa G, Ford K, et al. Mitigating the impact of COVID-19 on children’s surgery in Africa. BMJ Glob Heal. 2020;5:e003016. doi: 10.1136/BMJGH-2020-003016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lifebox (2020) Lifebox global response to COVID-19. https://www.lifebox.org/covid/covid-19/. Accessed 3 Nov 2021
- 58.Smile Train (2021) Impact of the COVID-19 pandemic on perioperative providers and resources in LMICs. https://www.smiletrain.org/sites/default/files/2021-06/regional-brief-special-report-smile-train.pdf. Accessed 3 Nov 2021
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.